Abstract
Objective:
Slowed processing speed and executive dysfunction are associated with poor outcomes in Late Life Depression (LLD), though it is unclear why. We investigated whether these variables interfere with the development of positive treatment expectancies in an antidepressant trial.
Methods:
Depressed older subjects were randomized to Open (intended to increase patient expectancy) or Placebo-controlled (termed ‘Hidden,’ intended to decrease expectancy) administration of antidepressant medication for 8 weeks. Analysis of covariance analyzed the between-group difference on expectancy (Credibility and Expectancy Scale [CES]) and depression (Hamilton Rating Scale for Depression [HRSD], Clinical Global Impressions [CGI] Severity). Moderator analyses examined whether these Open vs. Hidden differences varied based on higher vs. lower processing speed and executive function.
Results:
Among the 108 participants, a significant between-group difference was observed on expectancy (effect size [ES, Cohen’s d]=0.51 on CES Item 2; ES=0.64 on Item 4), indicating the manipulation was effective. Processing speed as measured by the Stroop Color-Word Test (number color-words named in congruent condition) was a significant moderator of the Open vs. Hidden effect on expectancy. Depressive symptom improvement was greater on average for Open vs. Hidden participants who received active drug (CGI-severity ES=1.25, HRSD ES=0.41), but no neurocognitive moderators of the between-group difference reached statistical significance.
Conclusions:
Slowed processing speed impairs the development of expectancies in antidepressant trials for LLD, which may help explain lower antidepressant response among older adults. Future studies may address whether interventions to optimize treatment expectancies are capable of improving treatment outcomes.
Keywords: late life depression, executive dysfunction, processing speed, antidepressants, expectancy
INTRODUCTION
Cognitive performance deficits (e.g., decreased processing speed, executive dysfunction) and white matter hyperintensities (WMH) on T2-weighted magnetic resonance imaging (MRI) scans are common in Late Life Depression (LLD) and portend poor outcomes (1,2). To account for these data, the Vascular Depression model proposes that lesions to deep white matter tracts disconnect prefrontal cortex (PFC) from striatal and limbic areas, disrupting reciprocal modulation between these regions, and causing depressive symptoms, cognitive impairment, and treatment resistance (3). While this model may successfully predict a patient’s course of illness given baseline characteristics, it does not provide an explanation for why neurocognitive deficits should be associated with antidepressant non-response. Known mechanisms of action for Selective Serotonin Reuptake Inhibitors (SSRIs) are independent of frontostriatal circuits (e.g., salutary modulation of hyperactive limbic structures (4), stimulation of neurogenesis (5)), and SSRIs are effective for the treatment of large vessel post-stroke depression (6).
In considering antidepressant non-response in LLD, it bears remembering that a large proportion of observed treatment response is generated by placebo effects rather than by specific medication effects (7). In clinical trials for Major Depressive Disorder (MDD), placebo effects appear to be driven by patients’ expectancies of improvement (8), which are generated by the provision of verbal information regarding whether a treatment will be helpful. Our group has reported that active comparator-type studies, in which participants are assured of receiving active medication for their condition, have medication response rates nearly double those of LLD patients enrolled in placebo-controlled studies, who are aware they may be receiving placebo (9). Additionally, we have found that participants over 55 years of age are less responsive to experimental manipulations of expectancy and demonstrate smaller magnitudes of placebo effects in antidepressant clinical trials (10). Applying these data to antidepressant non-response in LLD, the possibility arises that cognitive impairment and cerebrovascular lesions may interfere not with the specific effects of medication, but rather with the ability to generate expectancies.
A widely-used experimental paradigm for examining expectancy in clinical trials compares the outcome difference between Open (overt) and ‘Hidden’ (covert) treatment administration (11). Hidden treatments, of which the patient is completely unaware or less certain of receiving, are less effective on outcomes than openly administered treatments, as we have previously reported in the case of active-comparator vs. placebo-controlled antidepressant trials in LLD (9). This Open-Hidden difference is a measure of the expectancy effect contribution to outcome. Previously, we implemented a version of this paradigm in which we found a large Open-Hidden difference in both expectancy and depressive symptom improvement among depressed younger adults randomized to open antidepressant medication vs. placebo-controlled (i.e., Hidden) administration of medication (10). Here, we apply the Open-Hidden antidepressant paradigm to test the hypotheses that expectancy and depressive symptom change will be greater in depressed older adults with relatively better compared to worse neurocognitive health.
Cognition and white matter integrity were assessed in depressed outpatients 60 years of age or older using a neuropsychological test battery focused on processing speed and executive function complemented by structural MRI and diffusion tensor imaging. This manuscript concerns the cognitive performance data, whereas a forthcoming paper will report the MRI studies performed. After baseline evaluation, participants then were randomized to Open medication (i.e., 100% chance of receiving active medication) or placebo-controlled administration of medication (i.e., “Hidden,” perceived 50% chance of receiving active medication, but in reality nearly all received medication) for an 8-week duration trial. The primary outcomes of interest were the Open-Hidden difference in patient expectancy immediately following randomization (prior to medication treatment) and the Open-Hidden difference in depressive symptoms at 8 weeks. We hypothesized that the Open-Hidden difference in patient expectancy and antidepressant outcome would be significantly less in LLD patients having slowed processing speed and executive function.
METHOD
Subjects
This study was conducted in the Clinic for Aging, Anxiety, and Mood Disorders at the New York State Psychiatric Institute (NYSPI), approved by the NYSPI Institutional Review Board, and registered on Clinicaltrials.gov (NCT01931202). Eligible subjects were men and women aged 60-90 years old who met Diagnostic and Statistical Manual IV (DSM-IV) criteria for non-psychotic MDD, had a 24-item HRSD score ≥ 16, and were willing to and capable of providing informed consent and complying with study procedures. Subjects were excluded from participation for a current comorbid Axis I DSM IV disorder (other than Nicotine Dependence, Adjustment Disorder, or Anxiety Disorders), substance abuse or dependence within the past 12 months, lifetime psychosis or mania, or probable Alzheimer’s dementia, Vascular Dementia, or Parkinson’s disease. Other exclusion criteria included Mini Mental Status Examination < 24, significant suicidality, adverse reaction or non-response to escitalopram and duloxetine, current treatment with psychotherapy, antidepressants, anti-psychotics, or mood stabilizers, Clinical Global Impressions (CGI) Severity score of 7 at baseline, or acute, severe, or unstable medical illness.
Procedures for Manipulating Expectancy
At baseline, subjects were shown a schematic of the study design similar to Figure 1, panel A and informed that participants in this study would be randomized to “Group 1,” which would entail certain administration of antidepressant medication, or “Group 2,” which would entail taking a pill that may be antidepressant medication or placebo. Participants were informed that they had not yet been randomized between these groups, and pre-randomization expectancy and depression scores were measured at this time.
Figure 1.
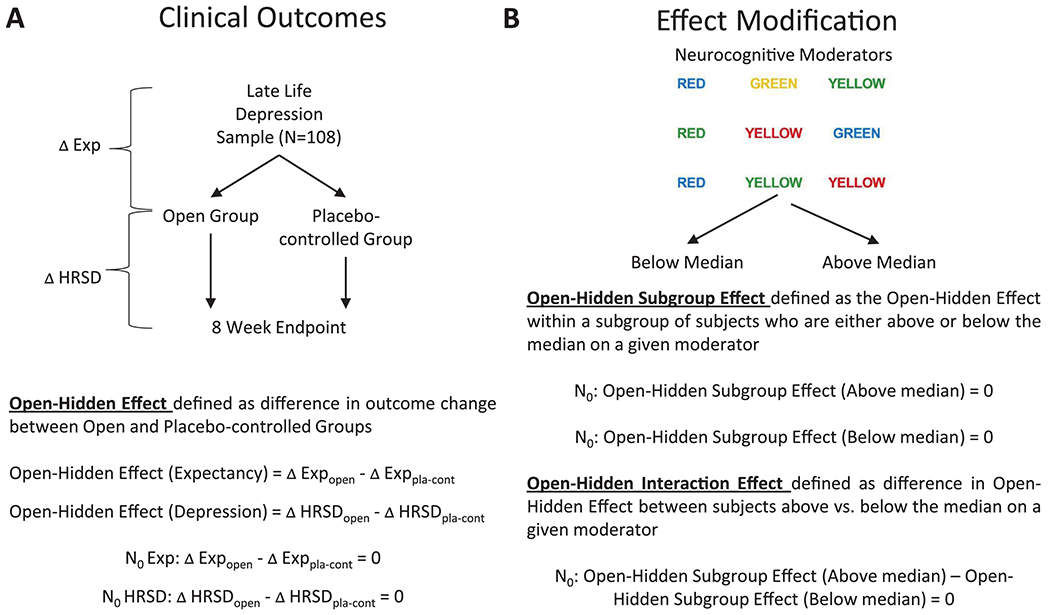
Schematization of the experimental logic in this study. A. First, it was determined whether the probability of receiving active medication influenced patient expectancy and antidepressant outcome. B. Next, we analyzed whether the cognitive moderators of interest influenced the strength of this manipulation.
Approximately one week later, subjects returned for a Week 0 visit during which they were randomized to the Placebo-controlled Group (i.e., ‘Hidden’) or Open Group (100% chance of receiving active treatment). Within the Placebo-controlled Group, subjects were randomized to medication or placebo with a 6:1 ratio favoring medication, which maximized the sample sizes for the primary comparison of interest (i.e., Open medication vs. Hidden medication). Subjects in the Placebo-controlled Group were informed: “You have been randomly assigned to the Placebo-controlled Group of the study. This means that there is a chance you will receive the antidepressant medication escitalopram (or duloxetine, as applicable) for the duration of the study. Escitalopram has been proven effective for the treatment of depression in patients like you. There is also a chance you will receive placebo for the duration of the study. A placebo is a sugar pill that is not specifically effective for depression. Neither you, nor your doctors, will know whether you are receiving escitalopram or placebo. If it can be avoided, please do not reveal to anyone in the study the Group to which you have been assigned.” Subjects in the Open Group were informed: “You have been randomly assigned to the Open Group of the study. This means that there is a 100% chance you will receive the antidepressant medication escitalopram for the duration of the study. Escitalopram has been proven effective for the treatment of depression in patients like you. You will not be receiving any placebo pills for the duration of the study. While you are aware that you are receiving actual antidepressant medication and not placebo, other study personnel do not know whether you are taking escitalopram or placebo. If it can be avoided, please do not reveal to anyone in the study the Group to which you have been assigned.” Post-randomization expectancy was measured with subjects having this additional information.
Antidepressant Treatment
Escitalopram lOmg was the default treatment option for subjects receiving medication, though individuals who had previously failed or had difficulty tolerating escitalopram were begun on duloxetine 30mg per day. Medication dose was titrated to escitalopram 20mg (or duloxetine 90mg) if subjects did not meet remission criteria (HRSD ≤ 7). Subjects returned for 8 weekly visits, and those unable to tolerate the increased dose of medication had their dosage reduced to the maximum previously tolerated dose. As some individuals received placebo in the study, participants were monitored at each visit for the presence of early discontinuation criteria (CGI-Improvement score of 6 or 7 for two consecutive weeks or judgment of the study clinician that discontinuation was merited [i.e., due to suicidal ideation]). If either early discontinuation criterion was met, participants were dropped out into post-protocol open treatment.
Clinical Evaluation and Expectancy Assessment
A Structured Clinical Interview Diagnostic for DSM-IV TR (SCID) was performed at baseline to confirm subject eligibility. The 24-item HRSD was performed at every study visit. Response (≥ 50% decrease in baseline HRSD score) and remission (Week 8 HRSD ≤ 7) were defined for secondary analyses. Other secondary outcomes included weekly Quick Inventory for Depressive Symptomatology (QIDS), CGI-Severity, and CGI-Improvement scales, a rating scale for treatment-emergent side effects, and weekly pill counts (to ensure compliance). The measure used to assess expectancy was a modified version of the Credibility and Expectancy Scale (CES) (12–13). Following our previous work (10), we analyzed CES items 2 (“I believe the chances of my depression being completely better at the end of this study are…”) and 4 (“Compared with now, I think my depression at the end of this study will be…”). Likert response options ranged from 1 to 7, with 1 anchored with ‘Very Poor/Much Worse,’ 4 ‘Unchanged/No Different,’ and 7 ‘Very Good/Much Better.’
By design, the study physicians who conducted randomizations and clinical treatment as well as research assistants coordinating study visits were aware of participants’ Group assignments. It was intended for participants’ clinical interactions to replicate insofar as was possible an openly administered vs. a placebo-controlled treatment, thereby enhancing the magnitude of the expectancy manipulation. However, raters for all rater-administered measures (i.e., HRSD, the primary depression outcome measure) were blinded to participant’s Group assignment. Participants were asked not to reveal their Group assignments to raters, raters did not participate in the clinical care of the participant, and raters did not attend laboratory meetings where participants were discussed. In addition, the second randomization within the Placebo-controlled Group was blinded, and neither subjects nor outcome assessors were aware of the 6:1 randomization schedule or the specific treatment assignment to medication or placebo.
Speed of Processing and Executive Function Assessment
All neurocognitive tasks were measured at baseline. The Stroop Color-Word Test (14), specifically reading color-names printed in inconsistent colors (termed ‘Stroop Color-Word’ here), was used to measure response inhibition (a constituent executive function). Responses from the two congruent conditions of the Stroop Color-Word Test were used as measures of processing speed (i.e., reading color-names [Stroop Word] and naming colors [Stroop Color]). Psychomotor speed was measured with the Digit Symbol subtest from the WAIS-III (15), and executive function measurement was supplemented by the Initiation/Perseveration (I/P) subtest of the Mattis Dementia Rating Scale (DRS) (16).
Data Analysis
As schematized in Figure 1, panel A, participants were randomized to open vs. placebo-controlled (i.e., Open vs. Hidden) administration of antidepressant medication, and analysis of covariance (ANCOVA) was used to analyze the resulting between-group differences (i.e., Open-Hidden Effect) on patient expectancy and depressive symptom scores. For each expectancy measure (i.e., CES items 2 and 4), the outcome modeled was the change from baseline to post-randomization (Week 0) with predictors including baseline expectancy and group. Encompassing all subjects (including those randomized to placebo), this model provides baseline adjusted estimates of change within group, i.e. the difference from pre- to post-randomization for the Open group (ΔExpOpen), the difference from pre- to post-randomization for the Hidden group (ΔExpPla-cont), and the randomization effect between groups (Open-Hidden Effect: ΔExpOpen - ΔExpPla-cont). Similar analyses were conducted for depressive symptom outcomes, with the exception that the change in HRSD scores in the Hidden group was restricted to subjects receiving antidepressant medication (not placebo).
Next, we evaluated whether the Open-Hidden Effects observed were modified by higher or lower scores on cognitive variables (Figure 1, panel B). The primary cognitive measure (Stroop Color-Word Test) was used to define subgroups of participants using median splits on processing speed (Stroop Color and Stroop Word congruent conditions) and response inhibition (Stroop Color-Word incongruent condition). The primary outcome measures for expectancy were CES scores, and change on the HRSD was defined a priori as the primary clinical outcome measure. ANCOVA models similar to those described above but additionally including an interaction between group (Open vs. Hidden) and the cognitive measure were used to examine within- and between-group differences on patient expectancy and depressive symptom scores within each median split category (i.e., above and below median). Contrasts performed from the model comprised: (1) the Open-Hidden Effect in subjects above the median on a given cognitive moderator (Subgroup Effect), (2) the Open-Hidden Effect in subjects below the median on a given cognitive moderator (Subgroup Effect), and (3) the Open-Hidden Effect difference between median split categories (Interaction Effect). Finally, the above analyses were repeated for secondary depression outcomes (CGI Severity and Improvement) as well as secondary measures of cognition (Digit Symbol Test, Wechsler Test of Adult Reading [WTAR], Logical Memory, and DRS). Effect size (ES) was measured using Cohen’s d.
RESULTS
Subject Disposition and Characteristics
The final CONSORT diagram for the study is shown in Figure 2. One hundred eight randomized subjects were available for analyses of expectancy, and 100 (i.e., those receiving antidepressant medication as compared to placebo) were available for analysis of depression outcomes. Depressive symptom scores, cognitive data, and demographic information for participants are provided in Table 1 and did not differ significantly at baseline across the study groups. Age was negatively correlated with cognitive performance on all measures except WTAR (r = −0.36, n = 106, p = 0.01 for Stroop Color-Word; r = - 0.35, n = 106, p < 0.001 for DRS I/P subscale; r = −0.27, n = 107, p = 0.004 on Digit Symbol; and r = −0.24, n = 108, p = 0.01 on WMS-Delayed).
Figure 2.
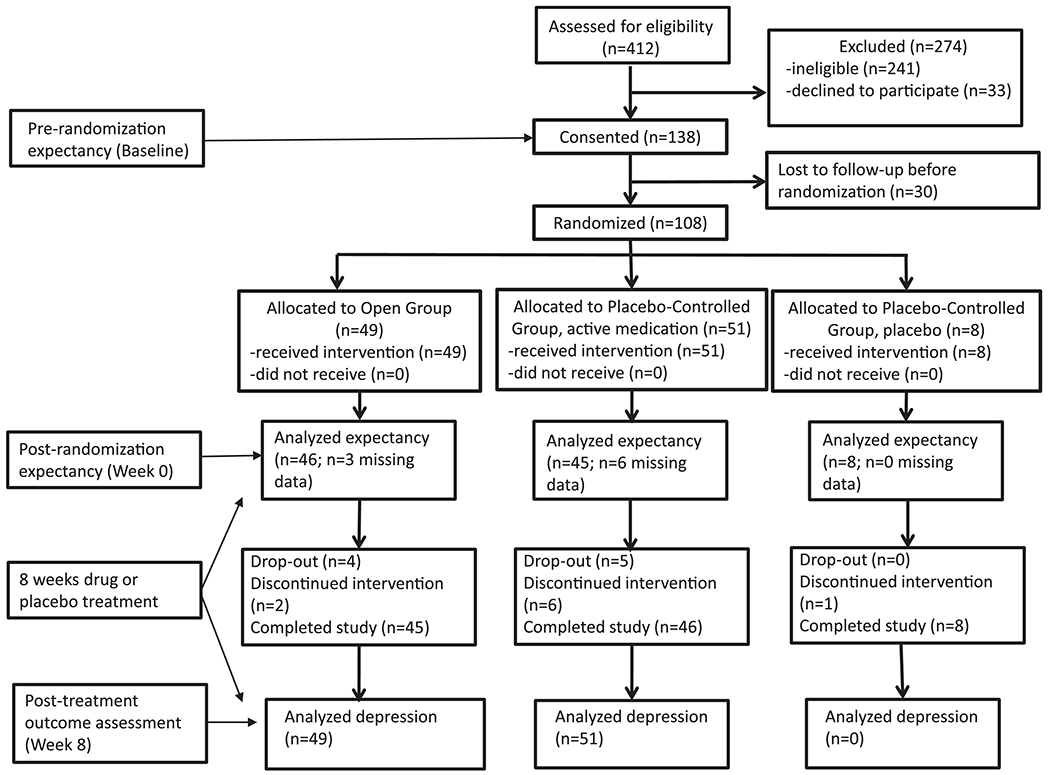
CONSORT flow diagram with timing of study assessments.
Table 1.
Baseline subject characteristics across demographic, clinical, and neurocognitive measures.
| Open Group (n=49) | Placebo-controlled Group—drug (n=51) | Placebo-controlled Group—placebo (n=8) | Between-Group Difference | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Mean ± SD or % | n | Mean ± SD or % | n | Mean ± SD or % | Test Statistic (DF) | p-value |
| Demographicsa | ||||||||
| Age | 49 | 69.2 ± 7.2 | 51 | 70.7 ± 8.4 | 8 | 73.9 ± 7.2 | F(2,105)=1.41 | 0.248 |
| Sex | χ2(2)=0.46 | 0.793 | ||||||
| Male | 17 | 34.7 | 19 | 37.3 | 2 | 25.0 | ||
| Female | 32 | 65.3 | 32 | 62.7 | 6 | 75.0 | ||
| Race | χ2(8)=3.00 | 0.935 | ||||||
| Asian | 1 | 2.0 | 0 | 0.0 | 0 | 0.0 | ||
| Black | 8 | 16.3 | 6 | 11.7 | 1 | 12.5 | ||
| White | 34 | 69.4 | 35 | 68.6 | 5 | 62.5 | ||
| Multiracial | 3 | 6.1 | 6 | 11.7 | 1 | 12.5 | ||
| Do not know | 3 | 6.1 | 4 | 7.8 | 1 | 12.5 | ||
| Ethnicity | χ2(2)=2.16 | 0.339 | ||||||
| Not Hispanic/ Latino | 38 | 77.6 | 45 | 88.2 | 7 | 87.5 | ||
| Hispanic/Latino | 11 | 22.4 | 6 | 11.8 | 1 | 12.5 | ||
| Years of education | 44 | 16.5 ± 2.7 | 42 | 16.2 ± 3.1 | 8 | 17.3 ± 2.1 | F(2,91)=0.53 | 0.593 |
| Baseline Depression measuresb | ||||||||
| 24-item Hamilton Rating Scale for Depression | 49 | 22.9 ± 6.3 | 51 | 23.6 ± 6.1 | 8 | 22.8 ± 4.9 | F(2,105)=0.19 | 0.824 |
| Quick Inventory of Depressive Symptomatology | 49 | 13.0 ± 4.7 | 50 | 13.5 ± 4.5 | 8 | 12.4 ± 4.4 | F(2,104)=0.32 | 0.718 |
| Clinical Global Impressions—Severity | 49 | 4.1 ± 0.6 | 51 | 3.9 ± 0.7 | 8 | 4.1 ± 0.6 | F(2,105)=0.86 | 0.427 |
| Baseline Expectancy measuresc | ||||||||
| Credibility and Expectancy Scale—Item 2 (Better) | 47 | 5.3 ± 1.3 | 46 | 46 5.5 ± 1.2 | 8 | 6.0 ± 1.1 | F(2,98)=0.99 | 0.377 |
| Credibility and Expectancy Scale—Item 4 (Depression) | 47 | 5.9 ± 0.9 | 46 | 6.0 ± 0.9 | 8 | 6.3 ± 1.2 | F(2,98)=0.65 | 0.526 |
| Baseline Cognitive measuresd | ||||||||
| Stroop Word | 49 | 89.7 ± 17.2 | 50 | 89.8 ± 16.0 | 8 | 83.1 ± 14.2 | F(2,104)=0.59 | 0.555 |
| Stroop Color | 49 | 59.0 ± 12.5 | 50 | 58.4 ± 11.4 | 7 | 55.1 ± 7.2 | F(2,103)=0.33 | 0.718 |
| Stroop Color Word | 49 | 32.6 ± 11.0 | 50 | 31.0 ± 9.0 | 7 | 31.7 ± 6.1 | F(2,103)=0.32 | 0.730 |
| Dementia Rating Scale—Initiation/Perseveration subscale score | 48 | 35.7 ± 2.7 | 50 | 35.2 ± 2.9 | 8 | 34.5 ± 5.6 | F(2,103)=0.67 | 0.516 |
| Digit Symbol Test | 49 | 38.9 ± 11.6 | 50 | 40.3 ± 10.3 | 8 | 35.4 ± 7.7 | F(2,104)=0.80 | 0.452 |
| Logical memory (WMS-R) delayed raw score | 49 | 13.2 ± 4.1 | 51 | 12.3 ± 4.8 | 8 | 12.1 ± 5.4 | F(2,105)=0.67 | 0.511 |
| Wechsler Test of Adult Reading (WTAR) | 48 | 42.9 ± 7.3 | 48 | 43.9 ± 6.1 | 7 | 43.7 ± 6.4 | F(2,100)=0.26 | 0.770 |
SD – standard deviation, DF – degrees of freedom.
One subject missing race information was recoded as “Do not know”. 14 subjects missing education.
One subject missing QIDS Depression.
Seven subjects missing baseline expectancy measures.
Two subjects missing baseline Stroop Color Word, Stroop Color, and Dementia Rating Scale—initiation/perseveration subscale score. One subject missing Digit symbol test score. Five subjects missing WTAR.
As a check of the intended expectancy manipulation, participants were queried following randomization to the Open or Hidden Groups using a multiple-choice questionnaire as to what they believed were their chances of receiving active medication in the study. Eighty percent (39/49) of Open Group participants responded correctly that they had a 100% chance of receiving active medication. Seventy-six percent (45/59) of participants in the Hidden Group responded that they had a 50% chance of receiving active medication.
Antidepressant medication treatment effects
No benefit of antidepressant medication vs. placebo was observed for HRSD change (unstandardized b=−0.28, t(96)=−0.10, p=0.92), after adjusting for pre-randomization HRSD; likely because very few subjects received placebo (n=8). Response rates, defined as at least a 50% decrease in HRSD scores at post-treatment were 25% (2/8) for Placebo-controlled placebo, 47% (24/51) for Placebo-controlled medication, and 51% (25/49) for Open medication (Fisher’s exact test, p = 0.44). Remission rates, defined as achieving post-treatment HRSD score of at least 7, were 13% (1/8) for Placebo-controlled placebo, 31% (16/51) for Placebo-controlled medication, and 45% (22/49) for Open medication (Fisher’s exact test, p=0.13).
Open-Hidden Effects on Expectancy and Depressive Symptoms
Due to missing data on expectancy or depression symptoms at baseline or follow-up, there were 99 (of 108) subjects available for analysis of expectancy and 91 (of 100 on active drug) available for depression. Drop-out or missing data did not differ by group. As shown in Table 2, a significant Open-Hidden Effect was observed on expectancy change scores measured with the CES (+0.63, t(96)=2.76, p=0.007, effect size [ES]=0.51 on Item 2; +0.57, t(96)=3.61, p<0.001, ES=0.64 on Item 4). At post-randomization, Open Group participants reported increased, though not statistically significant, expectancy of improvement (+0.26 points on CES Item 2 and +0.10 points on Item 4). In contrast, Hidden (Placebo-controlled) Group participants reported significant decreases in expectancy on the CES (−0.37, t(96)=−2.40, p=0.019 on Item 2 and −0.47, t(96)=−4.39, p<0.001 on Item 4).
Table 2. Predicted mean change in expectancy and depressive symptom outcomes by group.
Sample size in the Hidden Group differs for the expectancy analyses (upper) and depressive symptom analyses (lower). Subjects receiving both active medication and placebo are included in the expectancy analyses, since these pertain to data acquired before the initiation of study medication. Only subjects receiving active medication are included in the depressive symptom analyses. Effect size (ES) was measured using Cohen’s d.
| Change from Pre- to Post-Randomization | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Open Group | Hidden (both active drug and placebo) | Open-Hidden Effect (Open – Placebo-controlled) | ||||||
| Expectancy | n | Mean ± SE | n | Mean ± SE | Mean difference ± SE | t-value | DF | p-value | Effect size |
| Credibility and Expectancy Scale—Item 2 | 46 | 0.26 ± 0.17 | 53 | −0.37 ± 0.15 | 0.63 ± 0.23 | 2.76 | 96 | 0.007 | 0.51 |
| Credibility and Expectancy Scale—Item 4 | 46 | 0.10 ± 0.11 | 53 | −0.47±0.11 | 0.57 ± 0.16 | 3.61 | 96 | <0.001 | 0.64 |
| Change from Pre- to Post-Treatment | |||||||||
| Depressive Symptoms | Open Group | Hidden (only the active drug) | Open-Hidden Effect (Open – Placebo-controlled) | ||||||
| 24-item Hamilton Rating Scale for Depression | 45 | −11.49 ± 1.18 | 46 | −8.95 ± 1.16 | −2.54 ± 1.66 | 2.35 | 88 | 0.129 | 0.41 |
| Quick Inventory of Depressive Symptomatology | 44 | −6.77 ± 0.76 | 45 | −6.37 ± 0.75 | −0.39±1.08 | −0.36 | 86 | 0.717 | 0.09 |
| Clinical Global Impressions—Severity | 42 | −0.66 ± 0.43 | 44 | 0.11 ± 0.41 | −0.78±0.29 | −2.71 | 83 | 0.008 | 1.25 |
| Clinical Global ImpressionsߞSeverity | 42 | −1.51 ± 0.19 | 44 | −1.02 ± 0.18 | −0.49±0.26 | −1.89 | 83 | 0.062 | 0.47 |
SE=standard error, DF=degrees of freedom, n=sample size.
Table 2 also shows that scores on the primary depression outcome (24-item HRSD) significantly decreased from pre- to post-treatment among participants receiving active drug in both the Open (−11.5, t(88)=−9.77, p<0.0001, ES=1.86) and Hidden Groups (−9.0, t(88) =−7.70, p<.0001, ES=1.45). The difference in the Open-Hidden Effect on HRSD score change at week 8 was moderate (differential HRSD change=−2.54, t(88)=1.53, p=0.13, ES=0.41) but was not statistically significant. On secondary clinical measures, significant depression improvements in favor of the Open Group were found for CGI-Severity (−0.78, t(83)=−2.71, p=0.008, ES=1.25) but not self-reported symptoms (QIDS: −0.39, t(86)=−0.36, p=0.717, d=0.09) or CGI-Improvement (−0.49, t(83)=−1.89, p=0.064, ES=0.47).
Open-Hidden Effect Modification by Neurocognitive Variables
Median splits were used to categorize performance into better and worse functioning groups on the primary neurocognitive measure (Stroop Color Word Test) and secondary measures at baseline. These medians were 60 for Stroop Color (range 20-59 below median, 60-95 above median), 90 for Stroop Word (range 43-89 below median, 90-139 above median), 33 for Stroop Color-Word (range 12-32 below median, 33-64 above median), 37 for DRS I/P (range 21-36 below median, 37-37 above median), 41 for Digit Symbol (range 12-40 below median, 41-69 above median), 46 for WTAR (range 24-45 below median, 46-50 above median), and 14 for WMS-Delayed (range 1-13 below median, 14-21 above median).
The differential effect between subgroups (i.e. the Open-Hidden Interaction Effect) on expectancy (CES item 2) reached statistical significance for speed of processing as measured by the Stroop Word score (F(1,93)=4.5, p=0.037). Subjects with Stroop Word scores above the sample median (n=21 in the Open Group; n=28 in the Placebo-controlled Group) demonstrated significantly higher CES item 2 scores following randomization to the Open Group (diff=1.08, t(93)=3.38, p=0.0011) compared to subjects with scores below the median (diff=0.13, t(93)=0.41, p=0.68). Significant differential processing speed subgroup effects were not observed on the CES item 4 expectancy measure. Likewise, the Open-Hidden Interaction Effect on expectancy was not significant for speed of processing as measured by the Stroop Color score (F(1,92)=3.59, p=0.061), and neither response inhibition nor secondary neurocognitive variables were found to be significant moderators of expectancy change.
No primary or secondary neurocognitive variable was found to be a significant moderator of depressive symptom change.
DISCUSSION
This study evaluated whether neurocognitive function moderates the formation of expectancies during antidepressant treatment. Similar to our findings in younger adults (10, 21), we found that randomization to open as opposed to placebo-controlled antidepressant treatment was associated with significant and positive expectancy change. On some measures, cognitive health influenced the strength of this change, as a greater Open-Hidden Effect on expectancy occurred among older adults with faster as compared to slower processing speed. Effects of the Open vs. Hidden manipulation on depressive symptom change were less marked, and none of the neurocognitive variables examined were found to be significant moderators of the Open-Hidden Effect on depression.
While executive dysfunction is often cited as a primary negative prognostic factor for the treatment of depressed older adults (22), it is decreased processing speed that more accurately may be termed “the core cognitive deficit” in LLD (23). Decreased processing speed has been repeatedly found in patients with LLD relative to healthy controls (24) and in many cases (25–26), though not all (27) persists despite otherwise effective antidepressant treatment. Most neuropsychological tests, including those for executive function, are timed, so slowed speed of processing results in poor performance across domains. Consequently, slowed processing speed has been found not only to mediate the effects of executive dysfunction on daily functioning (28), but also to determine performance on measures of verbal reasoning, fluency, and working memory (29).
Generating and maintaining expectancies require understanding complex verbal information presented by a clinician about the nature of the treatment being prescribed, its anticipated effects and side effects, and the time course of improvement (30). Salthouse (1996) pointed out that cognitive performance is degraded by slowed processing speed because relatively earlier products of computation may be lost or forgotten before later processing steps are complete (29). In such fashion older adults with slowed processing speed may have difficulty synthesizing an informed consent disclosure and generating appropriate treatment expectancies. We did not find evidence that processing speed significantly moderated the effects of our group manipulation on depressive symptom change, but a previous literature has highlighted links between higher baseline treatment expectancies and improved antidepressant treatment outcome (31–35). Additional studies in which expectancy has been experimentally manipulated support causal inferences that more positive expectancy leads to greater improvement in depressive symptoms (10, 21).
A statistically significant result for neurocognitive health moderating the experimentally-induced change in expectancy or depressive symptoms only was found for one of the six primary tests performed. Thus, interpretations of the study findings must be undertaken cautiously and repetition by future studies conducted to determine whether the results hold. However, even a single positive finding bearing on the propensity of an individual to experience expectancy-based placebo effects is intriguing, since previous research largely has failed to identify such characteristics. Patient characteristics such as neuroticism, suggestibility, introversion, intelligence, and self-esteem have not been found to have significant associations with response to placebo (36), giving rise to the term “the elusive placebo reactor” as early as the 1960s (37). More recently, activation of the mu-opioid system (38) and functional polymorphisms modulating monoaminergic tone (39) have been found to distinguish depressed individuals responding to placebo. If further studies bear out the results found here, it may be the case that processing speed, and neurocognitive health more broadly, may be included among the brain capacities linked to placebo response, facilitating a mechanistic understanding of how expectancies lead to symptom change.
Though still speculative, the study results raise the possibility that for individuals with slowed processing speed, LLD treatment may be enhanced by developing methods of restoring the expectancy-related component of medication response. Cognitive remediation strategies such as computerized cognitive training (CCT) have been shown to positively affect processing speed, among other cognitive functions. For example, large scale studies like the IMPACT (40) and ACTIVE (41) studies showed improvements in cognition including measures of processing speed, memory, and global cognition in healthy adults randomized to CCT. Pairing such interventions with antidepressant medications may be a safe, feasible, and effective way to enhance treatment response. Consistent with this possibility are data from a study of older adults with age-related cognitive decline, who received cognitive training combined with either vortioxetine or placebo (42). Participants assigned to cognitive training plus vortioxetine demonstrated a larger increase in global cognitive performance, encompassing measures of processing speed, executive function, including inhibition and set shifting, and working memory.
Lastly, these findings should be interpreted in light of several limitations, among which is the relatively limited sample size. While N=108 is a reasonable number of subjects for a mechanistically oriented study, it did not provide sufficient power to detect effects on HRSD scores of the magnitude effect sizes observed (i.e., d~0.4). Second, we recruited a sample of depressed older adults from the general community and did not have specific inclusion thresholds for slowed processing speed or executive dysfunction, which may have resulted in limited variability on these moderators. Third, we tested whether multiple cognitive variables served as effect modifiers on the Open-Hidden difference in expectancy and depression scores, and it is possible that Type I error influenced the results reported.
Table 3. Selected moderator analyses for primary and secondary cognitive variables of interest.
Moderators listed on the left column were dichotomized using a median split, and re-organized as ‘Better Function’ or ‘Worse Function’ for ease of reference since scores above or below the median may represent better or worse performance depending on the measure. Open-Hidden subgroup effect refers to the predicted mean difference between the Open versus Hidden administration of antidepressant medication within the “Better Function” and “Worse Function” moderator group. Better-Worse Difference refers to the interaction effect between the selected moderator and randomization group. The interaction effect is defined as the difference between the predicted mean differences (Open-Hidden Subgroup Effect) in the Better versus the Worse subgroup. Effect size (ES) was measured using Cohen’s d.
| Expectancy Outcome (CES Item 2) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Better Function | Worse Function | Better-Worse Difference | |||||||||||||
| Moderator | Open-Hidden Subgroup Effect | SE | t-value | p-value | Effect size | Open-Hidden Subgroup Effect | SE | t-value | p-value | Effect size | Open-Hidden Subgroup Effect | t-value | df | p-value | Effect size |
| Primary | |||||||||||||||
| Processing Speed | |||||||||||||||
| Stroop Color | 1.02 | 0.32 | 3.21 | 0.002 | 0.82 | 0.16 | 0.32 | 0.50 | 0.617 | 0.13 | 0.85 | 1.89 | 92 | 0.061 | 0.69 |
| Stroop Word | 1.08 | 0.32 | 3.38 | 0.001 | 0.88 | 0.13 | 0.32 | 0.41 | 0.682 | 0.10 | 0.95 | 2.12 | 93 | 0.037 | 0.77 |
| Response Inhibition | |||||||||||||||
| Stroop Color-Word | 0.67 | 0.33 | 2.01 | 0.048 | 0.54 | 0.56 | 0.32 | 1.74 | 0.086 | 0.45 | 0.11 | 0.23 | 92 | 0.816 | 0.09 |
| Secondary Digit Symbo1 | 0.76 | 0.34 | 2.26 | 0.026 | 0.62 | 0.47 | 0.31 | 1.50 | 0.136 | 0.38 | 0.30 | 0.65 | 93 | 0.518 | 0.24 |
| WMS | 0.82 | 0.32 | 2.56 | 0.012 | 0.67 | 0.49 | 0.33 | 1.49 | 0.139 | 0.40 | 0.33 | 0.72 | 94 | 0.475 | 0.227 |
| WTAR | 1.06 | 0.33 | 3.23 | 0.002 | 0.86 | 0.29 | 0.34 | 0.85 | 0.396 | 0.23 | 0.77 | 1.64 | 89 | 0.105 | 0.63 |
| DRS | 0.51 | 0.28 | 1.79 | 0.077 | 0.41 | 1.07 | 0.39 | 2.74 | 0.007 | 0.87 | −0.56 | −1.17 | 92 | 0.244 | 0.46 |
| Depression Outcomes (HRSD) | |||||||||||||||
| Better Function | Worse Function | Better-Worse Difference | |||||||||||||
| Moderator | Open-Hidden Subgroup Effect | SE | t-value | p-value | Effect size | Open-Hidden Subgroup Effect | SE | t-value | p-value | Effect size | Open-Hidden Subgroup Effect | t-value | df | p-value | Effect size |
| Primary | |||||||||||||||
| Processing Speed | |||||||||||||||
| Stroop Col | −3.69 | 2. | − | 0.10 | 0.6 | −1.33 | 2. | − | 0.60 | 0.2 | −2.35 | − | 0.49 | ||
| Stroop Word | −3.80 | 2.29 | − | 0.166 | 0.62 | −1.20 | 2. | − | 0.63 | 0.2 | −2.60 | − | 0.44 | ||
| Response Inhibition | |||||||||||||||
| Stroop Color | −1.39 | 2.40 | −0.58 | 0.565 | 0.22 | −3.32 | 2.34 | −1.42 | 0.160 | 0.56 | 1.94 | 0.58 | 85 | 0.564 | 0.31 |
| Secondary | |||||||||||||||
| Digit Symbol | −4.23 | 2.35 | −1.80 | 0.076 | 0.9 | −0.80 | 2.43 | −1.42 | 0.741 | 0.13 | −3.42 | −1.01 | 85 | 0.31 | |
| WMS | −2.28 | 2.32 | −0.98 | 0.328 | 0.37 | −1.37 | 2.35 | −0.561 | 0.22 | 0.091 | − | 0.783 | 0.78 | ||
| WTAR | −3.56 | 2.36 | −1.51 | 0.135 | 0.58 | −1.30 | 2.43 | −0.54 | 0.594 | 0.21 | −2.26 | −0.67 | 83 | 4 | 0.37 |
| DRS | −3.18 | 2.04 | −1.56 | 0.212 | 0.52 | −1.89 | 2.87 | −0.31 | 0.757 | 0.14 | −2.30 | −0.65 | 84 | 5 | 7 |
CES=Credibility and Expectancy Scale, item 2: “I believe the chances of my depression being completely better at the end of this study are…”, 1=very poor to 7=very good. WMS=Logical Memory WMS-R Delayed raw score, Digit Symbol=subtest from the WAIS-III, WTAR=Wechsler Test of Adult Reading, DRS= Initiation/Perseveration (I/P) subtest of the Mattis Dementia Rating Scale. SE=standard error of the corresponding subgroup effect. DF=degrees of freedom for all t-tests.
HIGHLIGHTS.
1). What is the primary question addressed by this study?
This study investigated whether cognitive impairment in depressed older adults interferes with the development of expectancies about whether and how much an individual will improve in an antidepressant clinical trial.
2). What is the main finding of this study?
Depressed older adults with slowed processing speed had difficulty generating appropriate treatment expectancies when presented verbal information about their likelihood of receiving antidepressant medication.
3). What is the meaning of the finding?
Disrupting expectancies, which have previously been shown to be positively associated with depressive symptom change, may help explain why depressed older adults with slowed processing speed fail to demonstrate as much improvement when treated with antidepressant medication. Interventions to improve processing speed or otherwise restore appropriate expectancies may be useful therapeutic strategies.
Acknowledgement:
This study was funded by National Institute for Mental Health R01 MH102293 (Rutherford).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES/CONFLICT OF INTEREST
Dr. Rutherford had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Rutherford, Brown, Brickman, He, Sneed, Wall, and Roose, Ms. O’Boyle and Choi, and Mr. Maas and Choi have no disclosures or conflicts of interest to report. This paper has not been previously presented.
Contributor Information
Bret R Rutherford, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute, 1051 Riverside Drive, Box 98, New York, NY 10032.
C. Jean Choi, Division of Mental Health Data Science, New York State Psychiatric Institute.
Jongwoo Choi, Division of Mental Health Data Science, New York State Psychiatric Institute.
Ben Maas, Columbia University Vagelos College of Physicians and Surgeons.
Xiaofu He, New York State Psychiatric Institute.
Kaleigh O’Boyle, New York State Psychiatric Institute.
Joel Sneed, Queens College of the City University of New York.
Patrick J. Brown, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
Adam Brickman, Columbia University Vagelos College of Physicians and Surgeons.
Melanie M. Wall, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
Steven P. Roose, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
REFERENCES
- 1.Butters MA, Whyte E, Nebes RD, Begley AE, Dew MA, Muisant BH, Zmuda MD, Bhalia R, Meltzer CC, Pollock BG, Reynolds CF 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004; 61:587–595. [DOI] [PubMed] [Google Scholar]
- 2.Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry 1999; 7:309–316. [PubMed] [Google Scholar]
- 3.Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biological Psychiatry 2008; 64:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fmri study. Biol Psychiatry 2001; 50:651–658. [DOI] [PubMed] [Google Scholar]
- 5.Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatr 2011; 16:738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA 2008; 299:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutherford BR, Roose SP. A Model of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry 2013; 170:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull. 2004; 30:324–340. [DOI] [PubMed] [Google Scholar]
- 9.Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design makes a difference: antidepressant response rates in placebo-controlled versus comparator trials in late life depression. Am J Geri Psychiatry 2008; 16:65–73. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford BR, Wall MM, Brown PJ, Choo TH, Wager TD, Peterson BS, Chung S, Kirsch I, Roose SP. Patient Expectancy as a Mediator of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry 2017; 174:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colloca L, Benedetti F, Porro CA. Experimental designs and brain mapping approaches for studying the placebo analgesic effect. Eur J Appl Physiol 2008; 102:371 –380. [DOI] [PubMed] [Google Scholar]
- 12.Borkovec TD, Nau SD. Credibility of Analogue Therapy Rationales. J Behav Ther Exp Psychiat 1972; 3:257–260. [Google Scholar]
- 13.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiat 2000; 31:73–86. [DOI] [PubMed] [Google Scholar]
- 14.Golden CJ, Freshwater SM. Stroop color and word test: a manual for clinical and experimental uses. Chicago: Stoelting, 2002. [Google Scholar]
- 15.Wechsler D WAIS-III administration and scoring manual. San Antonio (TX): Psychological Corporation, 1997. [Google Scholar]
- 16.Mattis S Dementia Rating Scale: professional manual. Odessa (FL): Psychological Assessment Resources Inc., 1988. [Google Scholar]
- 17.Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert F, Muraskin J, Roose SP. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res 2011; 193: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman AM, Tosto G, Gutierrez J, Andrews H, Gu Y, Narkhede A, Mayeux R. An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology 2018; 91(15), e1402–e1412. doi: 10.1212/wnl.0000000000006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2008; 23 Suppl 1: S208–219. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford BR, Marcus SM, Wang P, Sneed JR, Pelton GH, Devanand DP, Duan N, Roose SP. A Randomized, Prospective Pilot Study of Patient Expectancy and Antidepressant Outcome. Psychol Med 2013; 43:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosedahl E. Recovery in geriatric depression. Arch Gen Psychiatry 1996; 53:305–312. [DOI] [PubMed] [Google Scholar]
- 23.Sheline YI, Barch DM, Garcia K, et al. Cognitive Function in Late Life Depression: Relationships to Depression Severity, Cerebrovascular Risk Factors and Processing Speed. Biol Psychiatry 2006; 60:58–65. [DOI] [PubMed] [Google Scholar]
- 24.Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP. A Meta-Analysis of Executive Dysfunction and Antidepressant Treatment Response in Late-Life Depression. Am J Geriatr Psychiatry 2016; 24:31 –41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomized longitudinal study. Lancet Psychiatry 2016; 3:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant B, Zmuda MD, Reynolds CF 3rd. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized double-blind clinical trial with nortriptyliine and paroxetine. J Psychiatr Res 2003; 37:99–108. [DOI] [PubMed] [Google Scholar]
- 27.McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The Effects of Vortixetine on Cognitive Function in Patients with Major Depressive Disorder: A Meta-Analysis of Three Randomized Controlled Trials. Int J Neuropsychopharmacol 2016; 19:pyw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown PJ, Liu X, Sneed JR, Pimontel MA, Devanand DP, Roose SP. Speed of Processing and Depression Affect Function in Older Adults with Mild Cognitive Impairment. Am J Geri Psychiatry 2013; 21:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salthouse TA. The Processing-Speed Theory of Adult Age Differences in Cognition. Psychol Rev 1996; 103:403–428. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford BR, Wager TD, Roose SP. Expectancy and the Treatment of Depression: A Review of Experimental Methodology and Effects on Patient Outcome. Curr Psychiatry Rev 2010; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutherford BR, Sneed JR, Roose SP. Does study design affect outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother and Psychosom 2009; 78:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 2009; 19:34–40. [DOI] [PubMed] [Google Scholar]
- 33.Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, Lanctot KL. Does Inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry 2010; 71:270–279. [DOI] [PubMed] [Google Scholar]
- 34.Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. J Clin Psychiatry 2004; 65:1174–1179. [DOI] [PubMed] [Google Scholar]
- 35.Meyer B, Pilkonis PA, Krupnick JL, Egan MK, Simmens SJ, Sotsky SM. Treatment expectancies, patient alliance, and outcome: further analyses from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol 2002; 70:1051–1055 [PubMed] [Google Scholar]
- 36.Shapiro AK, Morris LA: The placebo effect in medical and psychological therapies, in Handbook of Psychotherapy and Behavior Change: An Empirical Analysis. Edited by Garfield SL, Bergins AE. New York, Aldine Publishing, 1978, pp 477–536. [Google Scholar]
- 37.Liberman RP: The elusive placebo reactor. Neuropsychopharmacology 1967; 5:557–566. [Google Scholar]
- 38.Pecina M, Bohnert ASB, Sikora M, et al. Association between Placebo0Activated Neural Systems and Antidepressant Responses: Neurochemistry of Placebo Effects in Major Depression. JAMA Psychiatry 2015; 72:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuchter AF, McCracken JT, Hunter AM, Cook IA, Alpert JE. Monoamine oxidase a and catechol-o-methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J Clin Psychopharmacol 2009; 29:372–377. [DOI] [PubMed] [Google Scholar]
- 40.Smith GE, Housen P, Yaffe K, et al. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J Am Geriatr Soc 2009; 57:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ball K, Berch DB, Helmers KF, et al. Effects of Cognitive Training Interventions With Older Adults: A Randomized Controlled Trial. JAMA 2002; 288:2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenze EJ, Stevens A, Waring JD, et al. Augmenting Computerized Cognitive Training with Vortioxetine for Age-Related Cognitive Decline: A Randomized Controlled Trial. Am J Psychiatry 2020; 177:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]


