Abstract
Introduction
The question of whether periodontal therapy is an effective strategy for achieving glycemic control in people with type 2 diabetes mellitus (T2DM) and periodontitis continues to be open to debate. To clarify this issue, we conducted a systematic review and meta-analysis.
Methods
A systematic literature search of randomized controlled trials (RCTs) was carried out by searching four electronic databases and four journals up to April 2020. RCTs that evaluated the effect of periodontal therapy on glycemic control in people with T2DM were included.
Results
A total of 23 RCTs were included in this systematic review and meta-analysis. We found that after 3 and 6 months, periodontal therapy could significantly reduce glycosylated hemoglobin (HbA1c) level (3-month: weighted mean difference [WMD] − 0.514, 95% confidence interval [CI] − 0.730, − 0.298, p = 0.000; 6-month: WMD − 0.548, 95% CI − 0.859, − 0.238, p = 0.000). However, huge heterogeneity existed. Further analyses on 11 potential sources of heterogeneity found that baseline HbA1c of the included studies was the most significant factor causing heterogeneity. The benefit of periodontal therapy on glycemic control was much more obvious in studies with a higher baseline HbA1c level than in those with a lower baseline HbA1c level.
Conclusions
Periodontal therapy significantly contributed to glycemic control in T2DM patients, especially in patients with higher baseline HbA1c level.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01000-6.
Keywords: Blood glucose; Diabetes mellitus, type 2; Meta-analysis; Periodontitis; Scaling and root planing; Systematic review
Key Summary Points
| Why carry out this study? |
| Patients with diabetes have a two- to threefold higher risk for developing chronic periodontitis. |
| Periodontitis causes systematic inflammation which in turn may have an adverse effect on the glycemic control of patients with diabetes. |
| We hypothesize that periodontal treatment may contribute to the suppression of systemic inflammation and have a hypoglycemic effect in patients with diabetes. |
| What was learned from the study? |
| Our analysis reveals a significant benefit of periodontal treatment on glycemic control in people with type 2 diabetes mellitus (T2DM) and periodontitis. |
| The clinical benefit was mainly found in studies with a high baseline glycosylated hemoglobin (HbA1c) level; studies that included patients with good glycemic control at baseline seemed not to benefit from the periodontal treatment. |
| Patients suffering from T2DM should self-check their own oral hygiene and periodontal status and receive routine periodontal therapy at least once a year, especially patients with poor glycemic control. |
| Future randomized controlled studies on this topic should investigate where the threshold of baseline HbA1c lies that maximizes the benefits of periodontal therapy on glycemic control. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to:10.6084/m9.figshare.13526639.
Introduction
Type 2 diabetes mellitus (T2DM) is considered to be a major public health problem, with about 425 million adults suffering from T2DM worldwide. According to the statistics published in 2017 by International Diabetes Federation, this number may reach 700 million in 2045. Periodontitis (PD) is a major dental disease, with a global prevalence of about 40–75% according to the World Health Organization. As the world’s population ages, periodontal disease will become an even more important health care issue. There is a bidirectional relationship between T2DM and PD [1]. Patients with diabetes have a two- to threefold higher risk of developing chronic PD than their non-diabetic counterparts [2], and those who also have elevated glycosylated hemoglobin (HbA1c) have a significantly higher prevalence of PD and suffer more from tooth loss [3, 4]. Studies have confirmed that acute and chronic inflammations may lead to elevated HbA1c levels, which indicates that PD may have an adverse effect on the glycemic control of patients with diabetes [5].
Periodontal therapy may contribute to a reduction in systemic inflammation and better glycemic control of patients with diabetes. Based on this hypothesis, numerous small sample-sized randomized clinical trials (RCTs) have been conducted. Systematic reviews of these RCTs arrived at the conclusion that periodontal therapy results in better glycemic control [6–8]. In 2013, the Diabetes and Periodontal Therapy Trial (DPTT) [9] was conducted to assess whether periodontal therapy influences glycemic control. A total of 514 patients with T2DM were enrolled in the DPTT, and the conclusion of the authors was that periodontal therapy did not improve glycemic control in patients with T2DM. Subsequently, in 2015, the Cochrane Library published a systematic review which included the DPTT [10] and drew the conclusion that the evidence for periodontal therapy improving glycemic control is of low quality and that the mean reduction in HbA1c was only 0.29%. This conclusion was not in agreement with the previous meta-analysis. However, D'Aiuto et al. recently published a well-designed RCT which included 264 participants and found that intensive periodontal therapy significantly reduced HbA1c level (− 0.6%) in T2DM patients [11]. This study complicated an already divergent story.
With the aim to clarify whether periodontal therapy could benefit T2DM patients in terms of glycemic control, we have re-evaluated the published RCTs on this topic by mean of a meta-analysis and explored the factors that might influence the treatment benefit of periodontal therapy on glycemic control.
Methods
The protocol of the present systematic review was registered in PROSPERO (CRD42018089993). All procedures were carried out following this protocol and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [12]. Two authors independently performed study selection, quality assessment and data extraction. Any disagreements were resolved by consensus discussion.
Data Sources and Literature Search
The searching strategy was a combination of electronic and manual searches. The following electronic databases were searched without language limitation up to April 2020: MEDLINE, EMBASE, Chinese BioMedical Literature Database and China National Knowledge Infrastructure. MeSH terms with free text words were combined when conducting the electronic searches. The MeSH terms used in the electronic searches for PD were: “periodontal diseases” and “periodontitis”; the free text words were “(periodont$ or gingivitis or gingiva$ or gum$).mp.”. The MeSH term used in the electronic searches for T2DM was “diabetes mellitus, type 2”; free text words were “[(type 2) adj4 (DM or diabet$) or T2DM or DMT2 or NIDDM or T2D].mp.”. The MeSH terms used for Periodontal therapy were “dental scaling,” “periodontal debridement,” “root planing” and “subgingival curettage”; free text words were “(scaling or debridement) or (root planing) or curettage or SRP or (scaling and root planing).mp.”.
The titles and abstracts were initially scanned to identify any eligible studies. The full texts of the possibly eligible studies were obtained for final judgment. Manual searches were made, including the reference lists of included studies, of the following journals: Journal of Periodontology, Journal of Periodontology Research, Journal of Clinical Periodontology and Journal of Dental Research.
Study Selection
Inclusion criteria For inclusion in the meta-analysis the study design had to be that of a RCT that investigated whether periodontal therapy would help control the HbA1c level. Participants should be clinically diagnosed with PD and T2DM. The intervention group should undergo periodontal therapy. Periodontal therapy should include scaling and root planing (SRP). SRP combined with periodontal surgery, extraction of teeth beyond repair (“hopeless teeth”), oral hygiene instruction, local or systematic use of antibiotics, routine rinsing with mouthwash, among others, were also regarded as periodontal therapy. The control group should not receive SRP. Studies in which the control group received supragingival scaling or extraction of hopeless teeth were also included. The outcome was required to be HbA1c level. Both change in HbA1c (ΔHbA1c, preferred) or endpoint HbA1c level (absolute HbA1c level) were included. Studies should also report the mean periodontal probing depth (PPD) at baseline and at the end of follow-up (for evaluating the impact of baseline PD severity and periodontal therapy responses with metabolic control). The follow-up period was required to be 3 and/or 6 months or longer.
Exclusion criteria Non-randomized controlled trials would be excluded. Studies that included persons with type 1 diabetes or pre-diabetes would be excluded. Studies whose intervention did not include SRP would be excluded. Studies whose control group received potential SRP-based periodontal therapy, such as routine dental care, or who received antibiotics would be excluded. Studies with insufficient data or unavailable data would be excluded. Studies comparing different types of periodontal therapy, such as comparing SRP + doxycycline versus SRP alone, would be disregarded. Studies that did not report mean PPD would be excluded.
Methodological Quality Assessment and Data Extraction
The modified Jadad scoring system (scoring from 0 to 7) was utilized [13]. The score in this system is based on four criteria: “randomization,” “concealment of allocation,” “double blinding” and “withdraws and dropouts.” A Jadad score of < 4 is regarded as highly biased. The extracted data included investigator; basic characteristics of study, including country, mean age, gender and follow-up period; baseline information, including mean HbA1c level, duration of T2DM and mean PPD; inclusion and exclusion criteria of the original study; interventions of treatment group and control group; and T2DM treatment modification during the study.
Data Analysis
The software STATA version 14.0 (StataCorp LLC, College Station, TX, USA) was utilized for meta-analysis. The weighted mean difference (WMD) with 95% confidence interval (CI) was utilized for pooling the data. Only follow-up results for the same time period would be pooled. The significance was determined by two-sided α value with a cutoff p value of 0.05. All meta-analyses were performed using the random-effects model. Cochran’s Q test and I2 static were used for detecting statistical heterogeneity among studies. Low heterogeneity was assumed at p > 0.10 and I2 < 50%; otherwise, high heterogeneity was assumed. To investigate possible sources of heterogeneity, meta-regression analysis and subgroup analysis were performed. The variables for meta-regression and subgroup analysis included sample size, Jadad scores, baseline HbA1c of intervention group, whether ΔHbA1c was taken as outcome, whether intervention group received periodontal surgery/extraction of hopeless teeth, whether control group received supragingival scaling/extraction of hopeless teeth and whether the study was published in Chinese. The influence test was applied by deleting every single study in turn to test whether the results were stable. The publication bias would be detected by Egger’s test and Begg’s test if a meta-analysis included more than ten studies [14]; we considered that no publication bias existed when both test results satisfied p > 0.05.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Results of Search and Characteristics of Included Studies
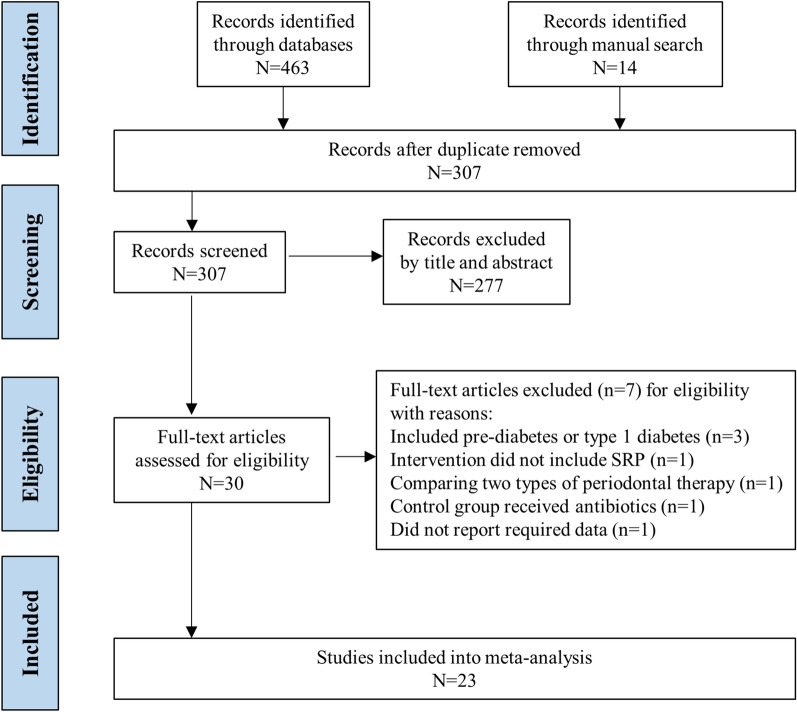
The search of the four electronic database identified 463 studies; manual searching identified 14 studies. A total of 307 studies were retained from the primary searches after the removal of duplicate studies. After title and abstract selection, 30 publications were retained for full-text review. After full-text evaluation, a total of 23 RCTs [9, 11, 15–35] were included in the present study. Figure 1 shows the PRISMA diagram of the study selection process. The studies included in the meta-analysis are summarized in Table 1. In brief, among the included RCTs, 11 studies were considered to have low bias (Jadad ≥ 4); six studies included more than 100 participants; the intervention group in eight studies received periodontal surgery or extraction of hopeless teeth in addition to SRP; the intervention group in five studies received antibiotics; the control group in five studies received supra-gingival scaling or extraction of hopeless teeth; 18 studies reported a change in T2DM treatment during the RCT; and six studies were published in Chinese. Among these 23 RCTs, 19 and ten RCTs reported the HbA1c change after periodontal therapy with a follow-up period of 3 and 6 months, respectively. The meta-analyses and further analyses were conducted according to the follow-up period.
Fig. 1.
Flow chart of study selection. SRP Scaling and root planing
Table 1.
Characteristics of included randomized controlled trials
| First author/year/study reference | Basic characteristics of study | Baseline T2DM/PD information (mean ± SD) | Inclusion/exclusion criteria | Treatment on PD and T2DM treatment change |
|---|---|---|---|---|
| Bukleta 2018 [15] |
Kosovo Mean age ± SD (years): 59.49 ± 10.82 Gender (male/female): 50/50 Follow-up: 3 months Jadad score: 2 |
HbA1c level (%): Int: 9.59 ± 2.5; Ctr: 8.82 ± 3.01 T2DM history (year): NA Mean PPD (mm): Int: 6.52 ± 0.82; Ctr: 6.60 ± 1.47 |
Inclusion: diagnosed with T2DM; had a baseline HbA1c ≥ 6.5%, had at least 10 teeth (excluding third molars); had a clinical diagnosis of periodontal disease with at least one site with a PPD ≥ 5 mm, two teeth with CAL ≥ 6 mm; no modification in the pharmacological treatment of T2DM during the study period. All the patients had at least one tooth needing extraction |
Int: full-mouth SRP using an ultrasonic device and periodontal curettes for the mechanical debridement. Extraction of hopeless teeth. Using mouthwash for 3 weeks (n = 50) Ctr: Extraction of hopeless teeth. Using mouthwash for 3 weeks (n = 50) T2DM treatment change: patients with modification in the pharmacological treatment of T2DM during the study period were excluded |
| Chen 2012 [16] |
China Mean age ± SD (years): Int: 59.86 ± 9.48; Ctr: 63.2 ± 8.51 Gender (male/female): Int: 23/19; Ctr: 17/24 Follow-up: 3 months; 6 months Jadad score: 6 |
HbA1c level (%): Int: 7.31 ± 1.23; Ctr: 7.25 ± 1.49 T2DM history (year): Int: 8.69 ± 5.25; Ctr: 9.56 ± 6.02 Mean PPD (mm): Int: 2.66 ± 0.68; Ctr: 2.47 ± 0.57 |
Inclusion: diagnosed with T2DM for > 1 year, with no change in their diabetic treatment plan in the previous 2 months and with no major diabetic complication. Diagnosed with chronic PD (with a ≥ 1 mm mean CAL with 16 teeth) Exclusion: (1) the presence of a systemic disease other than diabetes that could influence the course of the periodontal disease; (2) systemic antibiotic administration within the previous 3 months; (3) pregnancy or lactation; (4) refusal to provide written informed consent; (5) had an active infection other than PD; or (6) had received periodontal treatment in the previous 12 months |
Int: SRP at baseline and additional subgingival debridement at the 3-month follow-up. SRP was completed within 24 h, using standard rigid periodontal curets and ultrasonic instrumentation (n = 42) Ctr: no treatment measure or formal OHI until the end of the study (n = 41) T2DM treatment change: participants were instructed to continue with their medication and lifestyle |
| D'Aiuto 2018 [11] |
UK Mean age ± SD (years): Int: 58.2 ± 9.7; Ctr: 55.5 ± 10.0 Gender (male/female): Int: 82/51; Ctr: 83/48 Follow-up: 6 months; 12 months Jadad score: 6 |
HbA1c level (%): Int: 8.1 ± 1.7; Ctr: 8.1 ± 1.7 T2DM history (year): Int: 8.3 ± 7.4; Ctr: 8.7 ± 8.4 Mean PPD (mm): Int: 3.9 ± 0.7; Ctr: 3.9 ± 0.8 |
Inclusion: diagnosed with T2DM for > 6 months; with moderatetosevere PD (≥ 20 periodontal pockets with PPD of > 4 mm and marginal alveolar bone loss of > 30%), and at least 15 teeth Exclusion: with uncontrolled sy0stemic diseases other than diabetes (cardiovascular diseases [including hypertension], liver diseases, pulmonary diseases, end stage renal failure, or neoplasm); hepatitis B or HIV infection; chronic treatment (> 2 weeks) with drugs known to affect periodontal tissues (phenytoin or ciclosporin); chronic systemic antibiotic treatment; pregnancy or lactation |
Int: OHI. Extraction of hopeless teeth. Initial single session of whole mouth SRP. After initial SRP, received root scaling every 3 months. Twp months after first SRP, received additional root scaling or periodontal surgery (n = 133) Ctr: OHI. Extraction of hopeless teeth. Initial single session of whole mouth SGS. After initial SGS, received SGS every 3 months. If SRP were needed, withdrew (n = 131) T2DM treatment change: mo significant lifestyles or diets changes during study. Medication change was similar between groups during the trail |
| Engebretson 2013 [9] |
USA Mean age ± SD (years): Int: 56.7 ± 10.5; Ctr: 57.9 ± 9.6 Gender (male/female): Int: 143/114; Ctr: 134/123 Follow-up: 3 months; 6 months Jadad score: 6 |
HbA1c level (%): Int: 7.84 ± 0.65; Ctr: 7.78 ± 0.60 T2DM history (year): Int: 12.3 ± 8.2; Ctr: 11.3 ± 8.4 Mean PPD (mm): Int: 3.28; Ctr: 3.26 |
Inclusion: age > 35 years.; diagnosed with T2DM of > 3 months HbA1c between 7.0 to 9.0%; no changes in diabetes medications within the last 3 months; not pregnant; diagnosed with moderate to advanced PD (CAL and PPD > 5 mm in ≥ 2 quadrants); at least 16 natural teeth; n periodontal treatment in the prior 6 months Exclusion criteria: limited life expectancy; diabetes related emergency within 30 days; use of nonsteroidal anti-inflammatory drugs other than daily low-dose aspirin; use of immune-suppressive medications; antibiotic use (> 7 days within 30 days of enrollment); dialysis; risk of bleeding complications; or heavy alcohol consumption |
Int: SRP for > 160 min using curettes and ultrasonic instruments during ≥ 2 sessions completed within 42 days of the baseline visit. OHI after treatment. Used mouthwash twice daily for 2 weeks. Three and 6 months after the baseline visit, SRP and OHI were given during a single session (n = 257) Ctr: OHI at the baseline and 3- and 6-month visits. Following their 6-month visit, SRP were given (n = 257) T2DM treatment change: participants agreed to not change diabetes medications during the trial unless medically indicated. Medication change was similar between groups during the trail |
| Fu 2014 [17] |
China Mean age (years): 58.6 (range 40–67) Gender (male/female): 67/53 Follow-up: 6 months Jadad score: 1 |
HbA1c level (%): Int: 9.46 ± 1.21; Ctr: 9.53 ± 1.14 T2DM history (year): NA Mean PPD (mm): Int: 5.73 ± 0.39; Ctr: 5.77 ± 0.44 |
Inclusion: diagnosed with T2DM and PD Exclusion: with uncontrolled systemic diseases other than diabetes |
Int: SRP. Used mouthwash and oral antibiotics pre- and post-SRP. Details of SRP were NA (n = 60) Ctr: None (n = 60) T2DM treatment change: both groups received standard T2DM treatment, including diet, exercise, and insulin (details NA) |
| Fu 2014 [18] |
China Mean age ± SD (years): Int: 51.6 ± 3.8; Ctr: 50.3 ± 3.9 Gender (male/female): Int: 17/14; Ctr: 15/16 Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 9.88 ± 0.86; Ctr: 9.91 ± 0.78 T2DM history (year): Int: 3.35 ± 1.43; Ctr: 3.21 ± 1.32 Mean PPD (mm): Int: 5.76 ± 0.51; Ctr: 5.68 ± 0.47 |
Inclusion: diagnosed with T2DM for > 12 months and did not change T2DM medication in last 3 months; with PD (≥ 6 sites with PPD of > 4 mm and CAL of > 4 mm), and at least 15 teeth Exclusion: with uncontrolled systemic diseases other than diabetes; with sever diabetes complications; chronic systemic antibiotic treatment |
Int: SRP. Used oral antibiotics pre- and post-SRP (roxithromycin, 0.2 g qid; metronidazole, 0.4 g tid). Details of SRP were NA (n = 31) Ctr: None (n = 31) T2DM treatment change: both groups received standard T2DM treatment, including oral repaglinide (1 mg tid) and metformin (0.5 g tid) |
| Gu 2011 [19] |
China Age (year): 60–70 Gender (male/female): 38/22 Follow-up: 6 months Jadad score: 4 |
HbA1c level (%): Int: 9.46 ± 1.21; Ctr: 9.53 ± 1.14 T2DM history (year): NA Mean PPD (mm): Int: 5.94 ± 1.02; Ctr: 6.08 ± 0.92 |
Inclusion: diagnosed with T2DM for > 12 months and did not change T2DM medication recently; baseline HbA1c level between 8 and 12%; without systemic diseases other than diabetes; with PD (≥ 6 sites with PPD of > 5 mm); radiograph showed multiple teeth showed alveolar bone loss of > 1/3 of root; at least 15 teeth; did not use antibiotics in the last 3 months; did not receive periodontal therapy in last 6 months |
Int: OHI. Extraction of hopeless teeth. SRP. Used mouthwash for 2 weeks post SRP. Details of SRP were NA (n = 30) Ctr: None (n = 30) T2DM treatment change: participants did not change diabetes medications during the trial |
| Hao 2016 [20] |
China Mean age ± SD (years): Int: 61.06 ± 10.26; Ctr: 63.59 ± 8.97 Gender (male/female): Int: 18/11; Ctr: 16/14 Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 9.07 ± 1.54; Ctr: 9.21 ± 1.59 T2DM history (year): Int: 7.65 ± 5.33; Ctr: 6.85 ± 4.67 Mean PPD (mm): Int: 4.12 ± 0.70; Ctr: 4.05 ± 0.63 |
Inclusion: diagnosed with T2DM for > 12 months and did not change T2DM medication in last 2 months; with PD (≥ 2 teeth with PPD of > 5 mm and CAL of > 2 mm), and at least 16 teeth Exclusion: with uncontrolled systemic diseases other than diabetes; with severe diabetes complications; antibiotic use (> 7 days within 30 days of enrollment); received periodontal therapy in last 6 months; pregnancy or lactation |
Int: OHI and SRP. Details of SRP were NA (n = 30) Ctr: None (n = 30) T2DM treatment change: both groups received standard T2DM treatment, including diet, exercise, and medications (details NA) |
| Katagiri 2009 [21] |
Japan Mean age ± SD (years): Int: 60.3 ± 9.9 Ctr: 59.0 ± 4.8 Gender (male/female): Int: 21/11; Ctr: 6/11 Follow-up: 3 months; 6 months Jadad score: 2 |
HbA1c level (%): Int: 7.2 ± 0.9; Ctr: 6.9 ± 0.9 T2DM history (year): Int: 11.3 ± 6.4; Ctr: 8.8 ± 7.5 Mean PPD (mm): Int: 3.0 ± 0.9; Ctr: 2.8 ± 0.9 |
Inclusion: age between 39–75 years, HbA1c between 6.5–10.0%; without severe diabetic complications; no evidence of systemic diseases; no systemic antibiotics during the preceding 3 months; no pregnancy or lactation; no allergy to tetracycline; no smoking; and no modifications in the treatment of diabetes in last 2 months; at least 11 teeth, with PD (≥ 2 teeth with PPD of > 4 mm); no periodontal treatment in last 6 months |
Int: SRP was done using ultrasonic instrumentation. The SRP was completed over the course of four visits within 2 months. 10 mg of minocycline ointment was administered topically in every periodontal pocket at the end of each visit. OHI (n = 32) Ctr: OHI (n = 17) T2DM treatment change: the doses and kinds of anti-diabetic drugs, and methods of diet and exercise were not changed |
| Kaur 2015 [22] |
India Mean age ± SD (years): Int: 51.82 ± 5.85; Ctr: 52.94 ± 6.03 Gender (male/female): Int: 22/28; Ctr: 26/24 Follow-up: 3 months; 6 months Jadad score: 2 |
HbA1c level (%): (a): Int: 6.03 ± 0.47 Ctr: 5.94 ± 0.75 (b): Int: 9.99 ± 2.02 Ctr: 9.81 ± 2.24 T2DM history (year): Int: 8.57 ± 6.39; Ctr: 7.05 ± 4.43 Mean PPD (mm): (a): Int: 2.82 ± 0.44 Ctr: 2.92 ± 0.58 (b): Int: 3.08 ± 0.45 Ctr: 3.23 ± 0.48 |
Inclusion: diagnosed with T2DM for > 12 months; no change in T2DM medication use during the 2 months before the study or during the study; age between 45 and 60 years; presence of ≥ 12 teeth (excluding third molars); diagnosed as moderate (> 2 sites with CAL > 4 mm and > 1 site with PPD > 4 mm) or severe (> 2 sites with CAL > 6 mm and > 1 site with PPD > 5 mm) generalized chronic PD Exclusion: had systemic disease that could influence the course of periodontal disease; pregnant or lactating; current or ex-smokers; major diabetic complications; a history of systemic antibiotic use within the previous 3 months or periodontal treatment during the previous 6 months |
Int: OHI. Four SRP sessions over a maximum of 2 weeks, using Gracey curettes and an ultrasonic scaler. Additional supportive SRP was done when necessary (n = 50) Ctr: None (n = 50) T2DM treatment change: no change in T2DM medication use during the 2 months before the study or during the study |
| Kiran 2005 [23] |
Turkey Age (year): Int: 55.95 ± 11.21; Ctr: 52.82 ± 12.27 Gender (male/female): Int: 10/12; Ctr: 8/14 Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 7.31 ± 0.74; Ctr: 7.00 ± 0.72 T2DM history (year): Int: 9.32 ± 8.36; Ctr: 8.05 ± 5.90 Mean PPD (mm): Int: 2.29 ± 0.49; Ctr: 2.24 ± 0.70 |
Inclusion: diagnosed with T2DM with HbA1c values between 6 and 8%; creatinine values < 1.4 mg/dl; liver function tests were not up to threefold the normal range; no major diabetic complications; no history of systemic antibiotic administration within the last 3 months; no periodontal treatment 6 months prior to the study |
Int: OHI and SRP. 9 patients had 9 teeth with periapical lesions. 4 teeth were extracted and 5 had root canal treatment (n = 22) Ctr: OHI. 5 patients had 5 teeth with periapical lesions where they received root canal treatment (n = 22) T2DM treatment change: no change in the medication or diet was made for both groups during the study period. None of the groups received any additional guidance in managing their diabetic status |
| Li 2011 [24] |
China Mean age ± SD (years): Int: 58.7 ± 10.73; Ctr: 62.4 ± 9.18 Gender (male/female): Int: 51/39; Ctr: 19/26 Follow-up: 3 months; 6 months Jadad score: 1 |
HbA1c level (%): Int: 7.33 ± 1.37; Ctr: 7.25 ± 1.54 T2DM history (year): NA Mean PPD (mm): Int: 2.48 ± 0.56; Ctr: 2.62 ± 0.68 |
Inclusion: diagnosed with T2DM for > 12 months and did not change T2DM medication in last 2 months; with PD and at least 15 teeth; no periodontal treatment in last 12 months; did not use antibiotics in the last 3 months Exclusion: with systemic diseases other than diabetes; with severe diabetes complications; with acute infection; pregnancy or lactation |
Int: OHI. Extraction of hopeless teeth. SRP at baseline and additional SRP at the 3-month follow-up. First SRP was done within 24 h (n = 90) Ctr: OHI (n = 45) T2DM treatment change: NA |
| Makaky 2019 [25] |
Egypt Mean age ± SD (years): Int: 52.95 ± 6.523; Ctr: 52.23 ± 7.028 Gender (male/female): Int: 18/26; Ctr: 20/24 Follow-up: 3 months Jadad score: 4 |
HbA1c level (%): Int: 8.12 ± 0.74; Ctr: 8.21 ± 0.71 T2DM history (year): NA Mean PPD (mm): Int: 4.99 ± 0.75; Ctr: 5.01 ± 0.83 |
Inclusion: diagnosed with T2DM for > 5 years with HbA1c level from 7 to 9%; no change in diabetes treatment over the previous 3 months; diagnosis with chronic PD (> 4 teeth with one site with a CAL > 3 mm and PPD > 4 mm); 30% sites with CAL and pocket depth PD > 4 mm; at least 6 teeth; age from 40 to 70 years Exclusion: pregnancy; alcoholism and smoking; presence of any systemic disorders other than hypertension and T2DM; with T2DM major complications; antimicrobial therapies in the last 6 months; periodontal therapies in the last 6 months; allergy to metronidazole and amoxicillin |
Int: OHI. SRP using ultrasonic apparatus in a single 2-h session. After SRP, using systemic antibiotics for 2 weeks (metronidazole 400 mg tid and amoxicillin 500 mg tid) (n = 44) Ctr: None (n = 44) T2DM treatment change: NA |
| Mauri-Obradors 2018 [26] |
Spain Mean age ± SD (years): Int: 61 ± 11; Ctr: 62 ± 10 Gender (male/female): Int: 17/25; Ctr: 20/28 Follow-up: 6 months Jadad score: 4 |
HbA1c level (%): Int: 7.65; Ctr: 7.75 T2DM history (year): Int: 10; Ctr: 11 Mean PPD (mm): Int: 3.5; Ctr: 3.0 |
Inclusion: diagnosed with T2DM for > 1.5 years; with generalized chronic PD (> 9 teeth present and > 30% of the sites with a PPD and CAL > 4 mm) Exclusion: antibiotic treatment during the previous 15 days or for periods > 10 days during the last 3 months; SRP within the past 6 months; pregnancy; significant changes in diabetes medication during the course of the study; with other serious systemic disease |
Int: OHI and SRP. Additional SRP when needed (n = 36) Ctr: OHI and SGS. (n = 44) T2DM treatment change: patients had significant changes in diabetes medication during the course of the study were excluded |
| Mizuno 2017 [27] |
Japan Mean age ± SD (years): Int: 61.2 ± 9.2; Ctr: 62.8 ± 12.1 Gender (male/female): Int: 13/7; Ctr: 15/2 Follow-up: 3 months; 6 months Jadad score: 5 |
HbA1c level (%): Int: 7.5 ± 1.7; Ctr: 7.7 ± 1.2 T2DM history (year): NA Mean PPD (mm): Int: 2.4 ± 0.5; Ctr: 2.4 ± 0.7 |
Inclusion: diagnosed with T2DM for > 2 months; diagnosed with mild to advanced chronic PD (> 2 sites with CAL > 3 mm and PPD > 4 mm or 1 site with PPD > 5 mm); age > 30 years Exclusion: pregnancy; inappropriate status for the trials, such as limited life expectancy and diabetes-related emergency; received periodontal treatment in the prior 6 months |
Int: at the baseline visit, receive OHI and one session SGS. Within 42 days after the baseline visit, receive SRP (2–3 sessions, each time > 60 min) using curettes and an ultrasonic instrument (n = 20) Ctr: At the baseline visit, receive OHI and one session SGS (n = 17) T2DM treatment change: of the 30 participants with medication data available, both groups had no protocol defined changes during the study |
| Moeintaghavi 2012 [28] |
Iran Mean age ± SD (years): 50.29 ± 3 Gender (male/female): Int: 9/13; Ctr: 11/7 Follow-up: 3 months Jadad score: 6 |
HbA1c level (%): Int: 8.15 ± 1.18; Ctr: 8.72 ± 2.22 T2DM history (year): NA Mean PPD (mm): Int: 2.31 ± 0.65; Ctr: 2.06 ± 0.24 |
Inclusion: diagnosed with T2DM with HbA1c over 7%, and no major diabetic complications; using glibenclamide and metformin, without insulin administration for T2DM; no systemic antibiotic administration or periodontal treatment within the last 6 months; diagnosed with mild to moderate PD Exclusion: presence of systemic diseases other than T2DM that could influence the course of PD; intake of immunosuppressive drugs, steroids, hydantoin or non-steroidal anti-inflammatory drugs; tobacco use; pregnancy or intention to become pregnant during the study period; fixed orthodontic appliances |
Int: OHI and extraction of unsalvageable teeth. Then SRP by using an ultrasonic device and standard periodontal curettes with no time limitation. No additional periodontal therapy was given (n = 22) Ctr: OHI and extraction of unsalvageable teeth (n = 18) T2DM treatment change: all patients were under strict medical supervision and, as a prerequisite, no additional guidance in managing diabetic status or changes in diet, medication or physical therapy was given |
| Raman 2014 [29] |
Malaysia Age (year): Int: 57.7 ± 9.9; Ctr: 54.6 ± 6.2 Gender (male/female): Int: 11/4; Ctr: 9/8 Follow-up: 3 months Jadad score: 5 |
HbA1c level (%): Int: 7.8 ± 1.5; Ctr: 7.6 ± 1.5 T2DM history (year): NA Mean PPD (mm): Int: 2.56 ± 0.57; Ctr: 2.29 ± 0.69 |
Inclusion: diagnosed with T2DM; diagnosed with moderate to advanced chronic PD, with at least 12 teeth present and with 5 or more sites of PPD > 5 mm and CAL > 4 mm in at least 2 different quadrants which bled on probing Exclusion: a history of systemic antibiotic usage over the previous 4 months; having received non-surgical periodontal treatment within the past 6 months or surgical periodontal treatment within the past 12 months; pregnancy; change of medication for diabetes during the course of the study; current smoker; or history of a cerebrovascular or cardiovascular event within the past 12 months |
Int: OHI. SRP in a single visit using an ultrasonic scaler and Gracey curretes. 0.12% chlorhexidine mouthrinse (tid, 14 days) after SRP (n = 15) Ctr: OHI (n = 17) T2DM treatment change: participants who changed medication for diabetes during the course of the study were excluded |
| Singh 2008 [30] |
India Age (years): NA Gender (male/female): NA Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 7.9 ± 0.7; Ctr: 8.08 ± 0.7 T2DM history (year): NA Mean PPD (mm): Int: 2.67 ± 0.35; Ctr: 2.44 ± 0.26 |
Inclusion: diagnosed with T2DM; no major diabetic complications; no systemic diseases other than T2DM that could influence the course of PD; with moderate to advanced PD (> 30% of the teeth with a PPD > 4 mm) Exclusion: with uncontrolled T2DM; less than 16 teeth; had systemic antibiotic administration in last 3 months; had periodontal treatment in last 6 months |
Int: Extraction of hopeless teeth and SRP (n = 15) Ctr: Extraction of hopeless teeth (n = 15) T2DM treatment change: no change of medication and diet was modified for the participants |
| Telgi 2013 [31] |
India Mean age ± SD (years): NA Gender (male/female): NA Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 7.68 ± 0.63; Ctr: 7.56 ± 0.59 T2DM history (year): NA Mean PPD (mm): Int: 5.05 ± 0.70; Ctr: 5.11 ± 0.57 |
Inclusion: diagnosed with T2DM; blood sugar controlled only with oral hypoglycemic agents; mild to moderate PD (PPD of 4–5 mm); presence of a minimum of 28 teeth; and no systemic antibiotic administration and no periodontal treatment in last 6 months Exclusion: patients with systemic diseases other than T2DM; tobacco and alcohol user; and suffering from oral disease that needed emergency treatment |
Int: SRP. OHI and regularly used 0.12% chlorhexidine mouthwash (qd) (n = 15) Ctr: OHI and regularly used 0.12% chlorhexidine mouthwash (qd) (n = 15) T2DM treatment change: NA |
| Tsobgny-Tsague 2018 [32] |
Cameroon Mean age ± SD (years): Int: 51.2 ± 7.8; Ctr: 51.7 ± 9.9 Gender (male/female): Int: 8/7; Ctr: 5/10 Follow-up: 3 months Jadad score: 5 |
HbA1c level (%): Int: 9.7 ± 1.6; Ctr: 8.9 ± 0.9 T2DM history (year): Int: 5.0 ± 3.86; Ctr: 4.26 ± 3.3 Mean PPD (mm): Int: 3.0 ± 0.4; Ctr: 3.1 ± 0.6 |
Inclusion: poorly controlled T2DM, with moderate to severe chronic PD, and having at least 11 teeth in the mouth Exclusion: periodontal treatment; alteration in diabetes treatment 6 months prior to the study; onset of systemic diseases or an acute condition; use of immunosuppressive medications or other drugs; or presence of conditions able to alter PD clinical features (pregnant women, alcohol users, smokers and acute anemia) |
Int: OHI. A single SRP using ultrasonic device and Gracey’s curettes. Chlorhexidine gluconate 0.2% as mouth wash (10 ml bid for 5 days) (n = 15) Ctr: OHI (n = 15) T2DM treatment change: NA |
| Wang, J 2013 [33] |
China Mean age ± SD (years): 35–74 Gender (male/female): 32/43 Follow-up: 3 months Jadad score: 1 |
HbA1c level (%): Int: 7.57 ± 1.62; Ctr: 7.73 ± 1.74 T2DM history (year): 7.68 ± 1.37 Mean PPD (mm): Int: 4.21 ± 0.65; Ctr: 4.01 ± 0.73 |
Inclusion: diagnosed with T2DM and PD Exclusion: received periodontal treatment in last 6 months; defective prosthesis; edentulous; with severe diseases other than diabetes; mental disorders; use of antibiotics or steroids in last 6 months |
Int: OHI. Initial single session of whole mouth SGS using ultrasonic instrumentation. After 1 week received SRP and took oral antibiotics (amoxicillin and metronidazole) for 3 days (n = 37) Ctr: None (n = 38) T2DM treatment change: NA |
| Wang 2017 [34] |
China Mean age ± SD (years): Int: 61.2 ± 9.2; Ctr: 61.90 ± 6.75 Gender (male/female): Int: 12/7; Ctr: 14/6 Follow-up: 3 months Jadad score: 3 |
HbA1c level (%): Int: 7.63 ± 0.89; Ctr: 7.67 ± 1.32 T2DM history (year): Int: 8.47 ± 3.08; Ctr: 7.70 ± 4.69 Mean PPD (mm): Int: 3.66 ± 0.60; Ctr: 3.85 ± 0.58 |
Inclusion: diagnosed of T2DM for > 12 months with HbA1c from 6.5–10.0%; with chronic PD (more than 30% of teeth with PPD ≥ 5 mm and CAL > 4 mm, or > 60% of teeth with PPD > 4 mm and CAL ≥ 3 mm); at least 15 remaining teeth; body mass index < 30; without periodontal treatment in the previous 6 months; without antibiotic or NSAIDs administration in the 3 months; without serious systemic diseases or complications |
Int: OHI and SRP. Also received extraction of hopeless teeth, and the restoration of balanced occlusion (n = 19) Crt: None (n = 20) T2DM treatment change: to guard against the effects of the periodontal intervention on glycemic control and adipokines, no changes in medications or diet were made for any subject during the study period |
| Zhang 2013 [35] |
China Mean age ± SD (years): Int: 60.4 ± 9.77; Ctr: 62.7 ± 10.7 Gender (male/female): Int: 21/28; Ctr: 10/12 Follow-up: 3 months Jadad score: 4 |
HbA1c level (%): Int: 7.68 ± 1.22; Ctr: 7.38 ± 1.30 T2DM history (year): Int: 8.63 ± 4.20; Ctr: 7.29 ± 5.61 Mean PPD (mm): Int: 2.50 ± 0.45; Ctr: 2.43 ± 0.47 |
Inclusion: diagnosed to have T2DM > 1 year with HbA1c > 5.5%; diagnosed with chronic PD (at least 4 teeth with PPD > 5 mm, CAL > 4 mm, and bleeding on probing distributed in n > 2 quadrants); at least 16 teeth; age 35–80 years Exclusion: accompanied with other systemic immune diseases; administered with antibiotics, immunomodulators, contraceptives, or any other form of hormone within the past 3 months; underwent modified T2DM treatment strategy within 3 months; had periodontal treatment within the past 12 months; needed extraction or endodontic treatment; smokers; pregnant or lactating women |
Int: OHI and SRP (including manual curettage). SRP were completed within 2 weeks after the baseline evaluation (n = 49) Ctr: None (n = 22) T2DM treatment change: the recruited patients were instructed to maintain their previous diet and exercise habits and to inform the authors whenever their glucose control treatment was changed. Patients were dropped from the study if their diabetes treatment scheme was changed |
CAL clinical attachment loss, Ctr: control group, Int: intervention group, HbA1c glycosylated hemoglobin, HIV human immunodeficiency virus, NA not available, NSAIDs nonsteroidal anti-inflammatory drugs, OHI oral hygiene instruction, PD periodontitis, PPD pocket probing depth, qid four time a day, SD standard deviation SGS supragingival scaling, SRP scaling and root planing,T2DM type 2 diabetes mellitus, tid three times a day
Results of Pooled Data
Meta-analysis of 3-Month Follow-Up Data
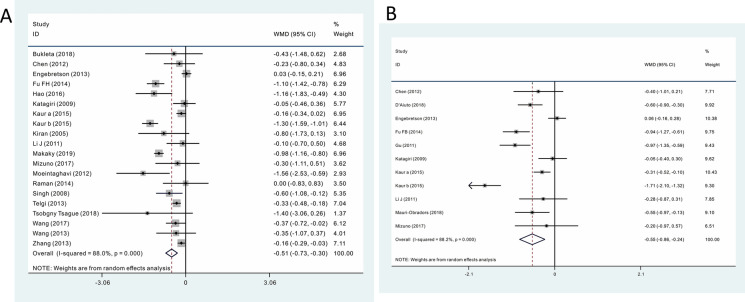
A total of 19 RCTs with 1660 participants were included in the meta-analysis of results from the 3-month follow-ups. Of these 1660 participants, 896 received periodontal therapy, and 764 served as controls. Among these 19 studies, one study [22] performed a subgroup analyses; consequently, a total of 20 comparisons were included in the meta-analysis. Pooled results showed periodontal therapy decreased the HbA1c level by 0.514% (WMD − 0.514, 95% CI − 0.730, − 0.298; p = 0.000, Fig. 2a). Influence analysis demonstrated that the pooled results were stable (Electronic Supplementary Material [ESM] Fig. 1a). No significant publication bias was detected (Begg = 0.206, Egger = 0.126). However, the result sshowed significant heterogeneity (p = 0.000; I2 = 88.0%).
Fig. 2.
Meta-analyses of the impact of periodontal therapy on glycemic control. a 3-month follow-up results, b 6-month follow-up results. CI Confidence interval, WMD weighted mean difference
Meta-analysis of 6-Month Follow-Up Data
A total of ten RCTs (11 comparisons) with 1441 participants were included in the meta-analysis of results from the 6-month follow-ups. Of these 1441 participants, 749 received periodontal therapy, and 692 served as controls. Pooled results showed that periodontal therapy decreased the HbA1c level by 0.548% (WMD − 0.548, 95% CI − 0.859, − 0.238; p = 0.000; Fig. 2b). No publication bias was detected (Begg = 0.815; Egger = 0.930). Influence analysis found that Kuar et al.’s study [22] had a significant impact on the width of the 95% confidence interval. Deleting this study partially decreased the effect size (WMD − 0.430, 95% CI − 0.676, − 0.184; p = 0.000) and heterogeneity (p = 0.000; I2 = 79.1%), but did not change the significance of the meta-analysis, indicating that the pooled result was relative stable (ESM Fig. 1b). Similar to the results of the 3-month follow-ups, significant heterogeneity (p = 0.000; I2 = 88.2%) existed among the included studies.
Results of Meta-regression Analyses
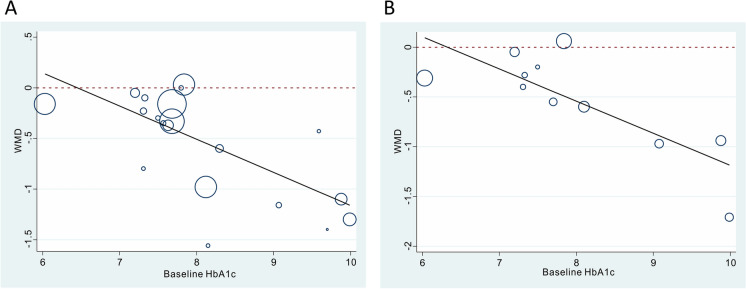
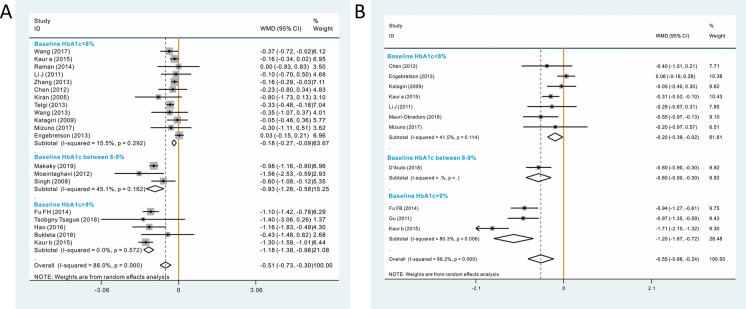
Since a very significant heterogeneity was found among included the included studies during both the 3- and 6-month follow-up periods, we performed meta-regression and subgroup analyses on 11 candidate factors that possibly cause heterogeneity with the aim to explore the source of this heterogeneity. As shown in Table 2, among the 11 covariates tested, baseline HbA1c level was the most significant covariate that could explain between-study heterogeneity. Specifically, studies with a higher baseline HbA1c level obtained a greater reduction in HbA1c after periodontal therapy (Fig. 3). Baseline HbA1c level explained 90.05 and 80.90% of between-study heterogeneity in the 3- and 6-month follow-up results, respectively. Based on these results, we performed the subgroup analysis (Fig. 4).
Table 2.
Results of subgroup analysis and meta-regression
| Subgroup analysis (heterogeneity explained and p valuea) | Category | NOC | Pooled results | Heterogeneity | ||
|---|---|---|---|---|---|---|
| WMD (95% CI) | p value | I2 | p value | |||
| 3-month follow-up | ||||||
| Total | – | 20 | − 0.514 (− 0.730, − 0.298) | 0.000 | 88.0% | 0.000 |
| Baseline HbA1c (%) (90.95%; p = 0.000) | < 8 | 12 | − 0.178 (− 0.267, − 0.090) | 0.000 | 15.5% | 0.292 |
| 8–9 | 3 | − 0.929 (− 1.278, − 0.581) | 0.000 | 45.1% | 0.162 | |
| ≥ 9 | 5 | − 1.179 (− 1.379, − 0.979) | 0.000 | 0.0% | 0.572 | |
| Baseline PPD (mm) (10.47%; p = 0.219) | < 3 | 9 | − 0.266 (− 0.440, − 0.092) | 0.003 | 36.8% | 0.124 |
| 3–4 | 5 | − 0.510 (− 1.129, 0.110) | 0.107 | 93.5% | 0.000 | |
| ≥ 4 | 6 | − 0.755 (− 1.139, − 0.371) | 0.000 | 87.7% | 0.000 | |
| Reduction of PPD after periodontal therapy (mm) (35.29%; p = 0.015) | < 0.5 | 8 | − 0.312 (− 0.560, − 0.064) | 0.014 | 64.5% | 0.006 |
| 0.5–1 | 9 | − 0.475 (− 0.814, − 0.137) | 0.006 | 92.1% | 0.000 | |
| ≥ 1 | 3 | − 1.120 (− 1.404, − 0.836) | 0.000 | 0.0% | 0.933 | |
| Jadad score (− 4.99%; p = 0.696) | ≥ 4 | 9 | − 0.471 (− 0.835, − 0.107) | 0.011 | 90.5% | 0.000 |
| < 4 | 11 | − 0.554 (− 0.843, − 0.264) | 0.000 | 85.9% | 0.000 | |
| Sample size (14.04%; p = 0.091) | ≥ 100 | 3 | 0.008 (− 0.158, 0.175) | 0.922 | 0.0% | 0.653 |
| < 100 | 17 | − 0.584 (− 0.816, − 0.351) | 0.000 | 87.8% | 0.000 | |
| ΔHbA1c as outcome (0.06%; p = 0.325) | Yes | 9 | − 0.415 (− 0.700, − 0.131) | 0.004 | 93.4% | 0.000 |
| No | 11 | − 0.640 (− 0.946, − 0.334) | 0.000 | 56.0% | 0.012 | |
| Treatment group received periodontal surgery or tooth extraction (− 6.37%; p = 0.738) | Yes | 5 | − 0.499 (− 0.759, − 0.239) | 0.000 | 0.0% | 0.693 |
| No | 15 | − 0.497 (− 0.746, − 0.249) | 0.000 | 91.0% | 0.000 | |
| Treatment group received antibiotics (20.09%; p = 0.106) | Yes | 3 | − 0.956 (− 1.205, − 0.707) | 0.000 | 42.1% | 0.178 |
| No | 17 | − 0.420 (− 0.620, − 0.221) | 0.000 | 80.9% | 0.000 | |
| Control group received supragingival scaling or tooth extraction (− 6.04%; p = 0.877) | Yes | 3 | − 0.509 (− 0.894, − 0.124) | 0.010 | 0.0% | 0.812 |
| No | 17 | − 0.522 (− 0.756, − 0.287) | 0.000 | 89.8% | 0.000 | |
| Reported no significant change in T2DM treatment during trial (− 7.28%; p = 0.914) | Yes | 15 | − 0.508 (− 0.759, − 0.256) | 0.000 | 86.9% | 0.000 |
| No | 5 | − 0.538 (− 0.988, − 0.087) | 0.019 | 88.2% | 0.000 | |
| Published in Chinese (1.89%; p = 0.383) | Yes | 4 | − 0.708 (− 1.241, − 0.176) | 0.000 | 72.8% | 0.000 |
| No | 16 | − 0.464 (− 0.697, − 0.231) | 0.009 | 88.7% | 0.012 | |
| 6-month follow-up | ||||||
| Total | – | 11 | − 0.548 (− 0.859, − 0.238) | 0.000 | 88.1% | 0.000 |
| Baseline HbA1c (%) (80.90%; p = 0.001) (85.62%; p = 0.002)b | < 8 | 7 | − 0.205 (− 0.389, − 0.021) | 0.029 | 41.5% | 0.114 |
| 8–9 | 1 | − 0.600 (− 0.900, − 0.300) | 0.000 | – | – | |
| ≥ 9 | 3 | − 1.199 (− 1.675, − 0.723) | 0.000 | 80.3% | 0.006 | |
| ≥ 9b | 2 | − 0.953 (− 1.200, − 0.706) | 0.000 | 0.0% | 0.906 | |
| Baseline PPD (mm) (11.67%; p = 0.172) | < 3 | 4 | − 0.309 (− 0.490, − 0.128) | 0.001 | 0.0% | 0.982 |
| 3–4 | 5 | − 0.560 (− 1.143, 0.023) | 0.060 | 93.9% | 0.000 | |
| ≥ 4 | 2 | − 0.953 (− 1.200, − 0.706) | 0.000 | 0.0% | 0.906 | |
| Reduction of PPD after periodontal therapy (mm) (27.88%; p = 0.075) | < 0.5 | 4 | − 0.034 (− 0.222, 0.154) | 0.722 | 0.0% | 0.399 |
| 0.5–1 | 5 | − 0.634 (− 1.119, − 0.149) | 0.010 | 91.3% | 0.000 | |
| ≥ 1 | 2 | − 0.953 (− 1.200, − 0.706) | 0.000 | 0.0% | 0.906 | |
| Jadad score (11.44%; p = 0.168) | ≥ 4 | 6 | − 0.323 (− 0.623, − 0.023) | 0.035 | 66.9% | 0.010 |
| < 4 | 5 | − 0.786 (− 1.315, − 0.257) | 0.004 | 92.8% | 0.000 | |
| Sample size (− 8.78%; p = 0.621) | ≥ 100 | 4 | − 0.440 (− 0.943, 0.062) | 0.086 | 89.5% | 0.000 |
| < 100 | 7 | − 0.613 (− 1.047, − 0.178) | 0.006 | 88.5% | 0.000 | |
| ΔHbA1c as outcome (− 10.10%; p = 0.746) | Yes | 4 | − 0.487 (− 1.107, 0.133) | 0.124 | 36.2% | 0.152 |
| No | 7 | − 0.653 (− 0.861, − 0.445) | 0.000 | 95.1% | 0.000 | |
| Treatment group received periodontal surgery or tooth extraction (− 13.38%; p = 0.920) | Yes | 1 | − 0.600 (− 0.900, − 0.300) | 0.000 | – | – |
| No | 10 | − 0.542 (− 0.890, − 0.195) | 0.002 | 89.3% | 0.000 | |
| Treatment group received antibiotics (− 12.83%; p = 0.886) | Yes | 2 | − 0.497 (− 1.369, 0.375) | 0.002 | 92.5% | 0.000 |
| No | 9 | − 0.560 (− 0.917, − 0.203) | 0.264 | 88.8% | 0.000 | |
| Control group received supragingival scaling or tooth extraction (− 11.89%; p = 0.809) | Yes | 3 | − 0.548 (− 0.782, − 0.314) | 0.006 | 91.6% | 0.000 |
| No | 8 | − 0.573 (− 0.979, − 0.167) | 0.000 | 0.0% | 0.640 | |
| Reported no significant change in T2DM treatment during trial (− 8.19%; p = 0.642) | Yes | 10 | − 0.571 (− 0.901, − 0.241) | 0.001 | 89.4% | 0.000 |
| No | 1 | − 0.280 (− 0.869, 0.309) | 0.351 | – | – | |
| Published in Chinese (− 2.68%; p = 0.445) | Yes | 3 | − 0.471 (− 0.840, − 0.101) | 0.013 | 53.3% | 0.118 |
| No | 8 | − 0.801 (− 1.152, − 0.450) | 0.000 | 89.4% | 0.000 | |
CI Confidence inteval, NOC number of comparisons, WMD weighted mean difference
aCalculated by single variable meta-regression
bCalculated after deleting Kaur et al.’s [22] study
Fig. 3.
Single variable meta-regression on the relationship between baseline glycosylated hemoglobin (HbA1c) level and post-treatment HbA1c change. a 3-month follow-up results, b 6-months follow-up results
Fig. 4.
Subgroup analysis based on baseline HbA1c level. a 3-month follow-up results, b 6-months follow-up results
Subgroup Analysis of 3-Month Follow-Up Data
Baseline HbA1c Level < 8%
This subanalysis included 13 studies (7 were highly biased) with 1198 participants (662 and 536 in the intervention group and control group, respectively), with a lower baseline HbA1c level (< 8%),. Periodontal therapy offered a limited but statistically significant benefit in terms of HbA1c reduction (WMD − 0.178, 95%CI − 0.267, − 0.090; p = 0.000). No heterogeneity was detected (p = 0.292; I2 = 15.5%). No significant publication bias was detected (Begg = 0.537; Egger = 0.075). Influence analysis (ESM Fig. 2a) showed that deleting Telgi et al.’s study [31] resulted in a reduction of effect size (WMD − 0.133, 95% CI − 0.216, − 0.050; p = 0.002), whereas deleting Engebretson et al.’s study [9] increased effect size (WMD − 0.220, 95% CI − 0.300, − 0.14; p = 0.000); however, all results were significant, indicating that the pooled result was relatively stable.
Baseline HbA1c Level Between 8 and 9%
This subanalysis included three studies (1 was highly biased) with 158 participants (81 and 77 in the intervention group and control group, respectively), with a baseline HbA1c between 8 and 9%. Periodontal therapy was able to provide a decrease in HbA1c of 0.929% (WMD − 0.929, 95%CI − 1.278, − 0.581; p = 0.000). No significant heterogeneity was detected (p = 0.162; I2 = 45.1%). Influence analysis showed that deleting Makaky et al.’s study [25] increased the width of the 95% confidence interval, but the WMD did not change significantly and was still significant (WMD − 0.983, 95% CI − 1.904, − 0.061; p = 0.037), indicating that the pooled result was relative stable (ESM Fig. 2b).
Baseline HbA1c Level ≥ 9%
This subanalysis included five studies (4 were highly biased) with 304 participants (153 and 151 in the intervention group and control group, respectively). The result indicated that periodontal therapy was able to provide an even more significant benefit in terms of glycemic control for the poorly controlled T2DM patients (WMD − 1.179, 95% CI − 1.379, − 0.979; p = 0.000). No significant heterogeneity was detected (p = 0.572; I2 = 0.0%). Influence analysis showed that the pooled result was stable (ESM Fig. 2c).
Subgroup Analysis of 6-Month Follow-Up Data
Baseline HbA1c Level < 8%
This subanalysis included eight studies (3 were highly biased) with 945 patients (499 and 446 in the intervention group and control group, respectively), with a baeline HbA1c of < 8. The pooled results showed that periodontal therapy was able to provide a decrease in HbA1c of 0.215% (WMD − 0.215, 95% CI − 0.374, − 0.056; p = 0.000). No heterogeneity was detected (p = 0.149; I2 = 34.9%). Influence analysis (ESM Fig. 3a) showed that the pooled result was stable. Deleting Engebretson et al.’s study [9] had no impact on the significance of the pooled results, but did increase effect size and decrease the width of the 95% confidence interval (WMD − 0.334, 95% CI − 0.472, − 0.196; p = 0.000).
Baseline HbA1c Level Between 8 and 9%
This subanalysis included only one study [11] with 264 patients (133 and 131 in the intervention group and control group, respectively) that reported both 6- and 12-month follow-up results. For the 12-month follow-up result, a significant reduction of HbA1c was observed (WMD − 0.6, 95% CI − 0.9, − 0.3; p = 0.000). Since only one study was included, no other analysis was performed.
Baseline HbA1c Level ≥ 9%
This subanalysis included three studies (2 were highly biased) with 232 patients (117 and 115 in the intervention group and control group, respectively), with baseline HbA1c of > 9%. Periodontal therapy was able to achieve a very significant reduction of HbA1c level (WMD − 1.199, 95% CI − 1.675, − 0.723; p = 0.006). Significant heterogeneity was detected (p = 0.006; I2 = 80.3%). Deleting Kaur et al.’s study [22] was able to significantly reduce the heterogeneity (p = 0.906; I2 = 0.0%), indicating that Kaur et al.’s study is the source of heterogeneity. Deleting this study did not influence the significance of the pooled result and had only had a little impact on the effect size (WMD − 0.953, 95% CI − 1.200, − 0.706; p = 0.000), indicating that the results were stable (ESM Fig. 3b). However, due to there being only three studies were included, the reason why Kaur et al.’s study influenced the heterogeneity remains unclear, although it may be due to the other two studies being published in Chinese.
Subgroup Analyses of Other Covariates
In addition to analyzing the baseline HbA1c level, we also performed subgroup analysis on the other ten covariates (Table 2). Subgroup analysis based on these covariates still showed significant heterogeneity, indicating they were not the major source of between-study heterogeneity.
Reduction of PPD after periodontal therapy, which is a reflection of the responses of infective periodontal tissue to treatment, could also explain the heterogeneity at a certain level (3 months: 35.29%; 6 months: 27.88%). Accordingly, our results indicate that studies with a higher PPD reduction also showed a greater decrease in HbA1c level (ESM Fig. 4). Baseline PPD level may also reflect active signs of gingival inflammation in PD. As shown in Table 2, studies with a higher baseline PPD tended to show a more obvious reduction in HbA1c level (ESM Fig. 5). However, baseline PPD level could only explain around 10% of the between-study heterogeneity. Another possible explanation for the correlation between baseline PPD level and HbA1c reduction may be the correlation between baseline PPD level and baseline HbA1c level.
We also analyzed the added value of periodontal surgery/tooth extraction or antibiotics when providing periodontal therapy. Our results show that additional periodontal surgery/tooth extraction did not influence the pooled results. Regarding the use of antibiotics, in the 3-month follow-up subanalysis we found that studies which used antibiotics showed a more obvious reduction in HbA1c compared to those in which antibiotics were not used (− 0.956 vs. − 0.420), whereas in the 3-month follow-up results there were no significant differences (− 0.497 vs. − 0.560). These results indicate that the added value of antibiotics requires further investigation. Regarding the control group, pooled results suggest that in studies in which supragingival scaling/tooth extraction was selected as the control intervention, this intervention did not affect the HbA1c reduction compared to studies whose control group received oral hygiene instruction/no intervention.
T2DM treatment, including medication, insulin and lifestyle management, is the most important factor that influences glycemic control and one which may have a profound short-term influence on HbA1c levels. Among the studies included in this meta-analysis, 18 studies reported that there were no significant changes in T2DM treatment, whereas five studies did not describe this aspect of the study. Regarding the 3-month follow-up results, the subgroup analyses found that there were no significant differences between the studies that reported a change in T2DM treatment and those that did not.
We also separately analyzed those studies that took ΔHbA1c value or absolute endpoint HbA1c value as the outcome and found that, in some cases, the studies using ΔHbA1c value as the outcome, which is considered to be a more accurate index, had a smaller effect size. Jadad score and sample size are two covariates that were closely related to the reliability and quality of the original evidence. Subgroup analysis provided the information that studies with a higher Jadad score and larger sample size tended to obtain a smaller effect size. Since we included articles published in Chinese, we also did a subgroup analysis for language and found the language in which the study was published did not influence the significance of pooled results.
Discussion
Inflammation of periodontal tissue leads to local vascular proliferation, the release of proinflammatory factors, abnormal glucolipid metabolism and insulin resistance. A very recent review showed that periodontal therapy was capable of reducing the level of serum inflammatory factors, which in turn might benefit the glycemic control [36]. Glycemic control is a critical aspect of the comprehensive therapy regimen of patients with diabetes. It is crucial to regulate the level of blood glucose at an appropriate degree in order to prevent the development of diabetes-related complications, such as micro- and macroangiopathy and central and peripheral nervous system lesions. According to the UK Prospective Diabetes Study (UKPDS) [37], a 1% reduction in the HbA1c level of diabetes patients leads to a 35% decrease of the risk for diabetic microangiopathy, and this correlation is considered to be linear; with respect to the whole study population, a 0.2% reduction of the mean of HbA1c resulted in a 10% decrease in mortality. In addition to the UKPDS study, both the DCCT/EDIC study [38] and the Steno-2 study [39] have shown that reductions in HbA1c contribute to a decreased risk of diabetic nephropathy, retinopathy and neuropathy. To date, there is no evidence that quantifies the “threshold” of HbA1c reduction for these benefits; thus it is expected that any, even minor, reduction in HbA1c lowers the risk of diabetic complications. These results show that even a limited reduction in the HbA1c level significantly benefits diabetes patients.
We found that periodontal therapy had a significantly beneficial effect on glycemic control and that this clinical benefit was nearly all contributed by participants with a high baseline HbA1c level. In our meta-analyses, periodontal therapy was of limited clinical significance in studies where the baseline HbA1c level was < 8%, with only about a 0.2% reduction in HbA1c in the pooled results. In comparison, the reduction in HbA1c was nearly 1% in those studies in which the baseline HbA1c level was > 8%. The difference in the effect of periodontal therapy on glycemic control may possibly be explained by the fact that patients with poor glycemic control suffer an excessive inflammation reaction due to angiopathy and poor healing, with periodontal therapy consequently resulting in a more significant reduction of serum inflammatory factors as well as a possible remission of glucolipid metabolism and insulin resistance. These results may suggest how periodontal therapy can be applied in the clinical setting to control the HbA1c level of diabetes patients by indicating the “threshold” of baseline HbA1c level that would provide the most benefit.
Recent relevant studies also support our results. Nishioka et al. [40] found that for individuals with borderline diabetes (baseline HbA1c 5.6%), periodontal therapy had no significant effect on markers related to insulin and glucose metabolism. Quintero et al. [41] found that periodontal therapy had the greatest impact on HbA1c reduction in patients with an HbA1c > 9%, no matter how the SRP was done, namely in multiple sessions quadrant-by-quadrant or within 24 h (one stage). Koromantzos et al. [42] used a multivariate linear regression analysis model, with change in HbA1c from baseline to the 6-month follow-up visit as the dependent variable and baseline HbA1c level, group, use of oral hypoglycemic agents, insulin change, age, gender, body mass index and smoking status as the independent variables, and showed that only periodontal treatment and baseline HbA1c level significantly and independently predicted the variance in HbA1c decline over 6 months. Taken together, not only our study results but also those from the recent relevant studies support the possible benefits of periodontal therapy for poorly controlled T2DM patients, rather than the well-controlled ones, in terms of glycemic control.
We also found that reduction of PPD after periodontal therapy and baseline PPD level of the included studies might influence the pooled results. However, factors related to PPD were not the major source of heterogeneity. The studies included in this meta-analysis also performed regression analysis on the relationship between PPD and HbA1c reduction. Engebretson et al. [9] performed a post hoc subgroup comparison of treatment groups by response tertiles (PPD reduction) and also found no significant between-group differences in change in HbA1c levels at any point. D'Aiuto et al. [11] found that between-group differences in HbA1c were correlated with PPD (R = 0.2; Spearman-rank correlation test, p = 0·0074; β coefficient 0.28, 95% CI 0·08, 0·48); however, Moeintaghavi et al. [28] applied logistic regression analysis and found that periodontal therapy was associated with a decrease in HbA1c, regardless of pre-treatment pocket depth (p = 0.005). The above results suggest that future studies on this topic should focus on the impact of baseline PPD and PPD reduction on the effect of periodontal therapy on glycemic control.
T2DM management (drugs, insulin and lifestyle management) and T2DM comorbidities (nephropathy, ocular complication, foot ulcer, peripheral neuropathy, cardiovascular diseases, among others) are widely acknowledged to impact the HbAc1 level. In terms of T2DM management, among the 23 RCTs included in this meta-analysis, 18 reported that there were no significant changes in T2DM treatment, while five studies did not describe this issue. The subgroup analyses found that there were no significant differences between the studies which reported T2DM treatment change and those that did not. In terms of T2DM comorbidities, none of the included studies reported how T2DM comorbidities influence the effect of periodontal treatment on HbA1c control. Also, there was no direct evidence proving that periodontal therapy had an impact on the incidence of T2DM comorbidities. Six included studies reported that there was a significant change in the serum lipid profile (related to cardiovascular disease) after periodontal treatment [9, 11, 16, 23, 28, 29, 33]. D'Aiuto et al. reported that periodontal therapy improved kidney function and vascular function and lowered the estimated cardiovascular disease risk [11]. Future trials on this topic should focus on these two aspects.
Many meta-analyses have been performed on this topic. However, to our knowledge, the present meta-analysis is the most comprehensive and in-depth study on this topic to date. Another very recent meta-analysis [43] on this topic only included nine RCTs, with the authors concluding that SRP is effective in reducing HbA1c; their pooled result was − 0.56% (p < 0.01), which is similar to our results. There are several differences between the systematic review by Simpson et al. [10] on this same topic based on the Cochrane database and the present work. Simpson et al. [10] included 14 studies but only performed one subgroup analysis for antimicrobics. In contrast, we included a total of 23 studies and performed 11 subgroup analyses based on the covariates. We also performed other analyses, including an analysis on the influence of test and meta-regression to avoid bias, which provided sufficient information to draw conclusions.
There are several limitations to the present meta-analysis. The DPTT study [6] actually involved participants with a high HbA1c level (≥ 8%: 195/514) but the authors did not report stratified results. Based on information from other DPTT-related reports [44, 45], it is likely that the treatment benefit was not associated with the baseline HbA1c level as the authors performed the subgroup analysis on other indexes, and it is highly unlikely that they would ignore important information on HbA1c level. The authors of another well-designed RCT [11] also did not report a correlation between baseline HbA1c and HbA1c reduction after periodontal therapy. In the present study, only Kaur et al.’s study [22] specifically investigated the relationship between baseline HbA1c level and the effect of periodontal therapy on glycemic control. These authors arrived at the same conclusion as we did based on our meta-analysis. However, the 6-month follow-up results, including those from Kaur et al.’s study, cause instability and high heterogeneity of the meta-analyses. This indicates that there must be a sufficient number of well-designed RCTs that are sufficiently similar and include results directly related to the baseline HbA1c, so that the subsequent systematic review and meta-analysis can correctly deduce the results for pooling with other studies. Also, the methodological quality of the included studies aimed at a high baseline HbA1c level was relatively low and the sample sizes were small, thus making the pooled results less convincible.
Our present study has primarily demonstrated that periodontal therapy significantly contributed to the glycemic control of T2DM patients, especially in patients with a higher baseline HbA1c level. Regarding the clinical implications, we recommend that general practitioners, endocrinologists and patients with T2DM focus much more on all aspects of oral hygiene and periodontal status. Gum bleeding/redness/swelling, tooth loosening and bad breath are common symptoms of periodontitis. We recommend that general practitioners and endocrinologists should consider a brief oral examination of patients with T2DM at each visit, focusing on the above periodontitis-related symptoms; this is particularly relevant for the patients with poor glycemic control. We recommend that patients suffering from T2DM should self-check their own oral hygiene and periodontal status and receive routine periodontal therapy at least once a year.
Conclusions
In conclusion, periodontal therapy might be effective for reducing the HbA1c level of patients with diabetes. The effects of periodontal therapy significantly rely on the HbA1c level prior to periodontal therapy. Our results also indicate that future studies should perform subgroup analysis based on baseline HbA1c level to investigate how different baseline HbA1c levels affect the extent to which periodontal therapy can reduce the HbA1c level and to identify where the “threshold” of baseline HbA1c level is that maximizes the benefits of periodontal therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Analysis of factors impacting the effect of periodontal therapy on glycemic control. a 3-Month follow-up results, b 6-month follow-up results (TIFF 692 kb)
Supplementary Fig. 2 Analysis of HbA1c change after 3 months. a Baseline HbA1c < 8%, b baseline HbA1c between 8 and 9%, c baseline HbA1c ≥ 9% (TIFF 443 kb)
Supplementary Fig. 3 Analysis of HbA1c change after 6 months. a Baseline HbA1c < 8%, b baseline HbA1c ≥ 9% (TIFF 379 kb)
Supplementary Fig. 4. Single variable meta-regression on the relationship between PPD reduction and post-treatment HbA1c change. a 3-month follow-up results. b 6-month follow-up results (TIFF 226 kb)
Supplementary Fig. 5 Single variable meta-regression on the relationship between baseline PPD and post-treatment HbA1c change. a 3-month follow-up results, b 6-month follow-up results (TIFF 234 kb)
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by National Nature Science Foundation of China (81972538, 81672669, 81972546) and Graduate Student’s Research and Innovation Fund of Sichuan University (Grant number: 2018YJSY106).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ya-fei Chen, Qi Zhan, Chen-zhou Wu, Yi-hang Yuan, Wen Chen, Fan-yuan Yu, Yi Li and Long-jiang Li have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Footnotes
The original online version of this article was revised due to update of Co corresponding author Qi Zhan.
Change history
3/27/2021
A Correction to this paper has been published: 10.1007/s13300-021-01036-8
Contributor Information
Qi Zhan, Email: 2016151642005@stu.scu.edu.cn.
Long-jiang Li, Email: lilongjiang63@163.com.
References
- 1.Wu C-Z, Yuan Y-H, Liu H-H, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. doi: 10.1186/s12903-020-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chee B, Park B, Bartold PM. Periodontitis and type II diabetes: a two-way relationship. Int J Evid Based Healthc. 2013;11(4):317–329. doi: 10.1111/1744-1609.12038. [DOI] [PubMed] [Google Scholar]
- 3.Chiu SY, Lai H, Yen AM, Fann JC, Chen LS, Chen HH. Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: a community-based study of 5,885 Taiwanese aged 35–44 years (KCIS No. 32) Acta Diabetol. 2015;52(1):123–131. doi: 10.1007/s00592-014-0612-0. [DOI] [PubMed] [Google Scholar]
- 4.Demmer RT, Holtfreter B, Desvarieux M, et al. The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP) Diabetes Care. 2012;35(10):2036–2042. doi: 10.2337/dc11-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sammalkorpi K. Glucose intolerance in acute infections. J Intern Med. 1989;225(1):15–19. doi: 10.1111/j.1365-2796.1989.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 6.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol. 2013;40(Suppl 14):S153–S163. doi: 10.1111/jcpe.12084. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. 2015;16:291. doi: 10.1186/s13063-015-0810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016;17(1):31. doi: 10.1186/s12903-016-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310(23):2523–2532. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;11:004714. doi: 10.1002/14651858.CD004714.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Aiuto F, Gkranias N, Bhowruth D, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 15.Bukleta D, Krasniqi S, Beretta G, et al. Impact of combined nonsurgical and surgical periodontal treatment in patients with type 2 diabetes mellitus—a preliminary report randomized clinical study. Biomed Res. 2018;29:633–639. doi: 10.4066/biomedicalresearch.29-17-644. [DOI] [Google Scholar]
- 16.Chen L, Luo G, Xuan D, et al. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol. 2012;83(4):435–443. doi: 10.1902/jop.2011.110327. [DOI] [PubMed] [Google Scholar]
- 17.Fu FB, Xiang J. Effect of periodontal therapy on glycemic control of type 2 diabetic patients with periodontitis. Shanxi Med. 2014;43(6):688–689. [Google Scholar]
- 18.Fu FH, Zhang Z, Chen Q. Chinical research about the effect of periodontal therapy on the level of plasma glucose in diabetic patients with periodontitis. Chin J New Clin Med. 2014;7(2):124–127. [Google Scholar]
- 19.Gu J, Lin S, Zhou M. The effect of periodontal therapy on the type 2 diabetic patients with severe chronic periodontitis. Chin J Geriatr Dent. 2011;9(6):339–341. [Google Scholar]
- 20.Hao S, Zhou Y, Liu Z, Wu W. Effect of non surgical periodontal therpy on serum carbohydrate and lipoproteins in patients with chronic periodontitis and type 2 diabetes mellitus. Beijing J Stomat. 2016;24(4):225–228. [Google Scholar]
- 21.Katagiri S, Nitta H, Nagasawa T, et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract. 2009;83(3):308–315. doi: 10.1016/j.diabres.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Kaur PK, Narula SC, Rajput R, RKS, Tewari S. Periodontal and glycemic effects of nonsurgical periodontal therapy in patients with type 2 diabetes stratified by baseline HbA1c. J Oral Sci 2015;57(3):201–11. [DOI] [PubMed]
- 23.Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32(3):266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chen L, Liu F, Zhang J. Effect of non-surgical therapy on control metabolic level in type 2 diabetes mellitus with chronic periodontitis. Guang zhou ya bing fang zhi. 2011;19(6):305–308. [Google Scholar]
- 25.El-Makaky Y, Shalaby HK. The effects of non-surgical periodontal therapy on glycemic control in diabetic patients: a randomized controlled trial. Oral Dis 2019;26. 10.1111/odi.13256. [DOI] [PubMed]
- 26.Mauri-Obradors E, Merlos A, Estrugo-Devesa A, Jané-Salas E, López-López J, Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized controlled trial. J Clin Periodontol. 2018;45(3):345–353. doi: 10.1111/jcpe.12858. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno H, Ekuni D, Maruyama T, et al. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial. PLoS One. 2017;12(11):e0188171. doi: 10.1371/journal.pone.0188171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J. 2012;57(1):31–37. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 29.Raman RP, Taiyeb-Ali TB, Chan SP, Chinna K, Vaithilingam RD. Effect of nonsurgical periodontal therapy verses oral hygiene instructions on type 2 diabetes subjects with chronic periodontitis: a randomised clinical trial. BMC Oral Health. 2014;14:79. doi: 10.1186/1472-6831-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Countr. 2008;28(2):38–44. doi: 10.4103/0973-3930.43097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telgi RL, Tandon V, Tangade PS, Tirth A, Kumar S, Yadav V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci. 2013;43(4):177–182. doi: 10.5051/jpis.2013.43.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsobgny-Tsague NF, Lontchi-Yimagou E, Nana ARN, et al. Effects of nonsurgical periodontal treatment on glycated haemoglobin on type 2 diabetes patients (PARODIA 1 study): a randomized controlled trial in a sub-Saharan Africa population. BMC Oral Health. 2018;18(1):28. doi: 10.1186/s12903-018-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wang W, Wu I, Sun D. Effect of periodontal treatment on blood glucose and blood lipid in diabetic and periodontitis patients. J Clin Stomatol. 2013;29(3):177–179. [Google Scholar]
- 34.Wang S, Liu J, Zhang J, et al. Glycemic control and adipokines after periodontal therapy in patients with type 2 diabetes and chronic periodontitis. Braz Oral Res. 2017;31:e90. doi: 10.1590/1807-3107bor-2017.vol31.0090. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Li C, Shang S, Luo Z. Scaling and root planing with enhanced root planing on healthcare for type 2 diabetes mellitus: a randomized controlled clinical trial. J Dent Sci. 2013;8(3):272–280. doi: 10.1016/j.jds.2012.10.009. [DOI] [Google Scholar]
- 36.Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J Clin Periodontol. 2018;45(2):167–187. doi: 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- 37.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 38.Schade DS, Lorenzi GM, Braffett BH, et al. Hearing impairment and type 1 diabetes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) cohort. Diabetes Care. 2018;41(12):2495–2501. doi: 10.2337/dc18-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 40.Nishioka S, Maruyama K, Tanigawa T, et al. Effect of non-surgical periodontal therapy on insulin resistance and insulin sensitivity among individuals with borderline diabetes: a randomized controlled trial. J Dent. 2019;85:18–24. doi: 10.1016/j.jdent.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Quintero AJ, Chaparro A, Quirynen M, et al. Effect of two periodontal treatment modalities in patients with uncontrolled type 2 diabetes mellitus: a randomized clinical trial. J Clin Periodontol. 2018;45(9):1098–1106. doi: 10.1111/jcpe.12991. [DOI] [PubMed] [Google Scholar]
- 42.Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38(2):142–147. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 43.Baeza M, Morales A, Cisterna C, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci Revista FOB. 2020;28:e20190248. doi: 10.1590/1678-7757-2019-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelato MC, Schoenfeld E, Hou W, et al. Changes in diabetes medications in the Diabetes and Periodontal Therapy Trial and their effect on hemoglobin A1c (HbA1c) Contemp Clin Trials. 2016;50:21–27. doi: 10.1016/j.cct.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Geisinger ML, Michalowicz BS, Hou W, et al. Systemic inflammatory biomarkers and their association with periodontal and diabetes-related factors in the diabetes and periodontal therapy trial. A randomized controlled trial. J Periodontol. 2016;87(8):900–913. doi: 10.1902/jop.2016.150727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Analysis of factors impacting the effect of periodontal therapy on glycemic control. a 3-Month follow-up results, b 6-month follow-up results (TIFF 692 kb)
Supplementary Fig. 2 Analysis of HbA1c change after 3 months. a Baseline HbA1c < 8%, b baseline HbA1c between 8 and 9%, c baseline HbA1c ≥ 9% (TIFF 443 kb)
Supplementary Fig. 3 Analysis of HbA1c change after 6 months. a Baseline HbA1c < 8%, b baseline HbA1c ≥ 9% (TIFF 379 kb)
Supplementary Fig. 4. Single variable meta-regression on the relationship between PPD reduction and post-treatment HbA1c change. a 3-month follow-up results. b 6-month follow-up results (TIFF 226 kb)
Supplementary Fig. 5 Single variable meta-regression on the relationship between baseline PPD and post-treatment HbA1c change. a 3-month follow-up results, b 6-month follow-up results (TIFF 234 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.