Abstract
Introduction
STELLA-LONG TERM is a post-marketing surveillance study evaluating the safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus.
Methods
Patients were classified by age at ipragliflozin initiation (< 65 and ≥ 65 years), and elderly patients were subclassified by baseline body mass index (BMI) < 25.0 or ≥ 25.0 kg/m2. Incidence of adverse drug reactions (ADRs) and effectiveness were evaluated over 3 years.
Results
Among 11,051 patients, 7894 (71.4%) were aged < 65 years and 3157 (28.6%) ≥ 65 years. The 3-year ADR incidence was similar in patients aged ≥ 65 (19.04%) and < 65 years (19.36%; P = 0.701). Serious ADRs were more frequent in the subgroup ≥ 65 years (2.79% vs 1.55%; P < 0.001). In terms of ADRs of special interest, a significantly greater proportion of elderly patients had skin complications (2.22% vs 1.62%, P = 0.033), renal disorders (2.28% vs 1.51%, P = 0.005), hypoglycemia (0.73% vs 0.43%, P = 0.048), or malignant tumors (1.01% vs 0.24%, P < 0.001), while the incidence of polyuria/pollakiuria (5.97% vs 4.47%, P = 0.002) and hepatic disorders (1.39% vs 0.73%, P = 0.004) was significantly higher in non-elderly than elderly patients. In patients aged ≥ 65 years, the incidence of ADRs was higher when baseline BMI was ≥ 25 kg/m2 versus < 25 kg/m2 (24.40% vs 17.68%; P < 0.001). Glycosylated hemoglobin, fasting blood glucose, and body weight significantly decreased from baseline in both age groups at each evaluation up to 3 years (all P < 0.001).
Conclusions
Ipragliflozin was well tolerated and effective for 3 years in routine clinical use in elderly and non-elderly patients, although elderly patients had a higher rate of serious ADRs. No new safety concerns were identified.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT02479399.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01042-w.
Keywords: Elderly, Ipragliflozin, Japan, Post-marketing surveillance, Sodium-glucose cotransporter 2 inhibitor, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| The STELLA-LONG TERM observational study investigated the safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes during 3 years of real-world use. |
| This prespecified subgroup analysis investigated outcomes in STELLA-LONG TERM among patients aged < 65 years and ≥ 65 years, since elderly people represent a high proportion of the Japanese population. |
| What was learned from this study? |
| Long-term ipragliflozin therapy was well tolerated and effective in all elderly patients, including those in the 75 years and older age group and those with a baseline body mass index < 25 kg/m2. |
| No new safety concerns were identified, but the incidence of serious adverse drug reactions was significantly higher in elderly than non-elderly patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14124275.
Introduction
Ipragliflozin was the first sodium–glucose cotransporter 2 (SGLT2) inhibitor to be approved in Japan for the treatment of patients with type 2 diabetes mellitus [1]. The approval was based on several randomized controlled trials in which ipragliflozin, as monotherapy or in combination with other antidiabetic agents, improved glycemic control [1–7].
The average age of patients with diabetes in Japan is increasing. In a 2018 survey, 72.5% of patients who were strongly suspected of having diabetes were aged ≥ 65 years and 33.5% were aged ≥ 75 years [8]. Given the high proportion of elderly people in Japan, and concerns raised regarding the safety of SGLT2 inhibitors in elderly patients, it was deemed necessary to confirm the safety of ipragliflozin in elderly Japanese patients [9]. The STELLA-ELDER study showed that 16.9% of patients aged ≥ 65 years who were receiving ipragliflozin during routine clinical practice developed adverse drug reactions (ADRs) over 1 year of treatment, with most of these events developing within the first 30 days of treatment [9, 10].
STELLA-LONG TERM (Specified drug use resulTs survEy of ipragLifLozin treAtment in type 2 diabetic patients: LONG-TERM use) was a 3-year post-marketing surveillance study designed to investigate the safety and effectiveness of long-term ipragliflozin therapy in routine clinical practice in Japan. Several subgroup analyses were conducted, including the comparison of effectiveness and safety in elderly (aged ≥ 65 years) versus non-elderly patients.
Subgroup analyses of STELLA-LONG TERM using 3- and 12-month interim data showed significant decreases from baseline in mean glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and body weight with ipragliflozin in both elderly and non-elderly patients [11, 12]. The incidence of ADRs was similar in the two age groups (≥ 65 and < 65 years); however, significantly more elderly than non-elderly patients experienced a serious ADR at 3 months (0.8% vs 0.5%, P = 0.019) [12] and 12 months (1.4% vs 0.8%, P = 0.002) [11], and no new safety concerns were identified compared with pre-approval clinical trials.
Here, we report the subgroup analysis of the safety and effectiveness of ipragliflozin in elderly versus non-elderly Japanese patients from the final, 3-year results of the STELLA-LONG TERM study.
Methods
STELLA-LONG TERM was a 3-year, observational, multicenter post-marketing surveillance study in Japanese patients with type 2 diabetes mellitus (ClinicalTrials.gov identifier NCT02479399). The study design and methods have been previously described [13]. Briefly, data from patients who were first prescribed ipragliflozin between July 17, 2014 and October 16, 2015 at participating clinical sites were included. Patients received ipragliflozin 50 mg once daily, before or after breakfast. Increasing the dose to 100 mg once daily was permitted if the treating physician judged the efficacy of the lower dose to be insufficient. A lower dose was also permitted, with caution advised in patients with severe hepatic impairment.
This study was conducted in compliance with Japanese Good Post-marketing Study Practice (GPSP) regulations and the study protocol was approved by the Ministry of Health, Labour and Welfare of the Japanese government. All medical institutions that agreed to provide data signed a contract with Astellas Pharma Inc. The review board that approved the study waived the need for consent, as anonymous data were collected via electronic survey forms from clinical settings.
Assessments
Data in this analysis were compared in two age categories: elderly (≥ 65 years) versus non-elderly (< 65 years), and non-elderly versus two elderly subgroups (65 to < 75 years and ≥ 75 years). Data were also compared between patients with a baseline body mass index (BMI) of < 25 versus ≥ 25 kg/m2. Safety data collected included the incidence of ADRs [categorized according to Medical Dictionary for Drug Regulatory Activities—Japanese translation (MedDRA/J) version 22.0] and ADRs of special interest during treatment, with ADRs defined as adverse events (AEs) for which a causal relationship to ipragliflozin could not be excluded.
In addition, changes in vital signs and laboratory parameters were monitored in the safety analysis population, including heart rate, white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin, hematocrit, blood urea nitrogen (BUN), serum albumin, serum creatinine, sodium, chloride, potassium, calcium, phosphorus, magnesium, serum ketone body levels, fasting C-peptide, estimated glomerular filtration rate (eGFR), pH, urinary albumin, and urinary creatinine levels.
Effectiveness outcomes included changes from baseline in HbA1c, FPG, and body weight. Changes in HbA1c were also compared in patients with a baseline level of < 8% versus ≥ 8%. Additionally, changes in vital signs and laboratory parameters, including fasting insulin, BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (γ-GTP), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, uric acid, and total bilirubin were analyzed in the effectiveness analysis population.
Data were collected at baseline and 1, 3, 6, 12, 24, and 36 months after treatment was initiated.
Statistical Analysis
The rationale for sample size calculations for the primary analysis has been defined previously [13]. The safety analysis population included patients who received at least one dose of ipragliflozin, had no registration violations, and had data available for at least one visit after baseline. The effectiveness analysis set was the safety population excluding patients who were noncompliant with treatment, had unclear efficacy assessment data or missing baseline or post-baseline data for HbA1c, serum fasting insulin, or FPG.
Categorical variables are presented as number and proportions (%) and continuous variables as mean ± standard deviation (SD). Patient demographic and clinical data for the different age groups or BMI categories were compared using the chi-squared test, two-sample t test, or one-way analysis of variance as appropriate. Changes from baseline in laboratory variables were analyzed using the one-sample t test. For descriptive purposes only, baseline demographic and ADR data were compared with those from STELLA-ELDER [9].
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Disposition
A total of 11,424 patients were registered from 2431 medical institutions, and survey forms were collected for 11,289 patients. The safety analysis set included 11,051 patients, while the effectiveness analysis set included 8763 patients. Details of reasons for exclusion from the two analysis populations are described in the final report of the overall STELLA-LONG TERM study [14].
Patient Characteristics
In the safety analysis set, 7894 patients were aged < 65 years (mean age 51.2 years) at baseline; 3157 were aged ≥ 65 years (mean age 71.3 years; Table 1) and of these, 752 were aged ≥ 75 years (Supplementary Table S1). Compared with the elderly group (≥ 65 years), a significantly higher proportion of non-elderly patients (< 65 years) were men (63.6% vs 53.5% of elderly patients; P < 0.001), and significantly fewer had HbA1c < 8% (50.1% vs 59.5% of elderly patients; P < 0.001; Table 1). In the effectiveness analysis set, the mean HbA1c at baseline was 8.17% in patients aged < 65 years and 7.77% in patients aged ≥ 65 years (P < 0.001).
Table 1.
Patient baseline characteristics in the safety analysis population (first age category analysis) of STELLA-LONG TERM and in STELLA-ELDER
| STELLA-LONG TERM | STELLA-ELDER [9] | |||
|---|---|---|---|---|
| < 65 years | ≥ 65 years | P valuea | ||
| All, n | 7894 | 3157 | 8505 | |
| Sex, n (%) | ||||
| Male | 5023 (63.6) | 1690 (53.5) | (1) < 0.001 | 4181 (49.2) |
| Female | 2871 (36.4) | 1467 (46.5) | 4324 (50.8) | |
| Age, years | ||||
| Mean ± SD | 51.2 ± 9.0 | 71.3 ± 5.5 | 72.3 ± 5.9 | |
| Median (range) | 52.0 (14–64) | 70.0 (65–95) | ||
| 65 to < 75, n (%) | – | 2405 (76.2) | 5800 (68.2) | |
| ≥ 75, n (%) | – | 752 (23.8) | 2705 (31.8) | |
| Body weight, kg, mean ± SD (n) | 81.92 ± 17.35 (6015) | 67.57 ± 12.01 (2157) | (2) < 0.001 | 67.5 ± 12.9 |
| BMI, kg/m2 | ||||
| Mean ± SD (n) | 29.90 ± 5.44 (5548) | 26.76 ± 4.07 (1944) | (2) < 0.001 | 27.0 ± 4.56 |
| < 25.0, n (%) | 883 (11.2) | 690 (21.9) | (1) < 0.001 | 1762 (20.7) |
| ≥ 25.0, n (%) | 4665 (59.1) | 1254 (39.7) | 3306 (38.9) | |
| Unknown | 2346 (29.7) | 1213 (38.4) | 3437 (40.4) | |
| Duration of diabetes, years | ||||
| Mean ± SD (n) | 7.22 ± 5.74 (5417) | 10.16 ± 7.79 (1831) | (2) < 0.001 | 10.6 ± 7.52 |
| < 5, n (%) | 2124 (26.9) | 469 (14.9) | (1) < 0.001 | 1177 (13.8) |
| ≥ 5, n (%) | 3293 (41.7) | 1362 (43.1) | 4150 (48.8) | |
| Unknown, n (%) | 2477 (31.4) | 1326 (42.0) | 3178 (37.4) | |
| Complications, n (%) | ||||
| Yes | 6603 (83.6) | 2762 (87.5) | (1) < 0.001 | 6917 (81.3) |
| No | 1232 (15.6) | 367 (11.6) | 1477 (17.4) | |
| Unknown | 59 (0.7) | 28 (0.9) | 111 (1.3) | |
| eGFR, mean mL/min/1.73 m2 ± SD (n) | 85.56 ± 19.35 (4762) | 70.56 ± 17.96 (1935) | (2) < 0.001 | 69.7 ± 19.4 |
| HbA1c, % | ||||
| Mean ± SD (n)b | 8.17 ± 1.51 (6413) | 7.77 ± 1.24 (2313) | (2) < 0.001 | 7.84 ± 1.33 (6853) |
| < 8%, n (%) | 3958 (50.1) | 1877 (59.5) | (1) < 0.001 | 4381 (51.5) |
| ≥ 8%, n (%) | 3473 (44.0) | 1009 (32.0) | 2747 (32.3) | |
| Unknown | 463 (5.9) | 271 (8.6) | 1377 (16.2) | |
| Initial dose of ipragliflozin, n (%) | ||||
| 25 mg | 879 (11.1) | 541 (17.1) | –c | 1152 (13.5) |
| 50 mg | 6999 (88.7) | 2613 (82.8) | 7344 (86.3) | |
| 100 mg | 13 (0.2) | 2 (0.1) | 9 (0.1) | |
| Other | 3 (0.04) | 1 (0.03) | 0 | |
| Daily dose of ipragliflozin, mg, mean ± SD (n) | 48.34 ± 8.39 (7894) | 46.73 ± 9.91 (3157) | (2) < 0.001 | |
| Dose changes during treatment, n (%) | ||||
| 25 mg to 25 mg | 602 (7.6) | 419 (13.3) | (1) < 0.001 | 856 (10.1) |
| 25 mg to 50 mg | 242 (3.1) | 102 (3.2) | 263 (3.1) | |
| 50 mg to 50 mg | 6765 (85.7) | 2514 (79.6) | 7168 (84.3) | |
| 50 mg to 100 mg | 121 (1.5) | 41 (1.3) | 78 (0.9) | |
| Other | 164 (2.1) | 81 (2.6) | 140 (1.6) | |
BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, SD standard deviation
aP values across subgroups assessed by (1) chi-squared test or (2) two-sample t test; no statistical comparison between groups was made for specific complications
bMean HbA1c values were from the effectiveness analysis set
cNo P value was calculated when at least one element of the contingency table was < 10
Elderly patients had a longer mean duration of diabetes (10.16 vs 7.22 years; P < 0.001), and a significantly higher proportion of elderly patients had complications at baseline (87.5% vs 83.6%; P < 0.001). Non-elderly patients had a significantly higher mean body weight (81.92 vs 67.57 kg; P < 0.001), BMI (29.90 vs 26.76 kg/m2; P < 0.001), and eGFR (85.56 vs 70.56 mL/min/1.73 m2; P < 0.001) than elderly patients.
Baseline characteristics from STELLA-ELDER [9] are provided in Table 1 for comparison.
Safety
In the safety analysis set, 2129 patients (19.27%) had at least one ADR (Table 2).
Table 2.
Adverse drug reactions in the safety analysis population (first age category analysis) of STELLA-LONG TERM, in pre-approval clinical trials, and STELLA-ELDER
| ADRs, n (%) | Pre-approval (n = 1669) [2–7] | STELLA-LONG TERM | STELLA-ELDER (n = 8505) [9] | |||
|---|---|---|---|---|---|---|
| All (n = 11,051) | < 65 years (n = 7894) | ≥ 65 years (n = 3157) | P valuea | |||
| Any ADR | 549 (32.89) | 2129 (19.27) | 1528 (19.36) | 601 (19.04) | 0.701 | 1438 (16.9) |
| Serious ADR | 14 (0.8) | 210 (1.90) | 122 (1.55) | 88 (2.79) | < 0.001 | 127 (1.5) |
| ADRs of special interest | ||||||
| Polyuria/pollakiuria | 168 (10.0) | 612 (5.54) | 471 (5.97) | 141 (4.47) | 0.002 | 170 (2.0) |
| Volume depletion | 73 (4.5) | 243 (2.20) | 167 (2.12) | 76 (2.41) | 0.345 | 266 (3.1) |
| Skin complications | 59 (4.0) | 198 (1.79) | 128 (1.62) | 70 (2.22) | 0.033 | 269 (3.2) |
| Renal disorder | 76 (4.8) | 191 (1.73) | 119 (1.51) | 72 (2.28) | 0.005 | 118 (1.4) |
| Urinary tract infection | 29 (1.8) | 170 (1.54) | 115 (1.46) | 55 (1.74) | 0.271 | 118 (1.4) |
| Genital infection | 32 (2.0) | 161 (1.46) | 126 (1.60) | 35 (1.11) | 0.053 | 166 (2.0) |
| Hepatic disorder | 17 (1.0) | 133 (1.20) | 110 (1.39) | 23 (0.73) | 0.004 | 19 (0.2) |
| Cardiovascular disease | 16 (1.0) | 67 (0.61) | 50 (0.63) | 17 (0.54) | 0.562 | 24 (0.3) |
| Hypoglycemia | 22 (1.4) | 57 (0.52) | 34 (0.43) | 23 (0.73) | 0.048 | 58 (0.7) |
| Malignant tumor | 4 (0.2) | 51 (0.46) | 19 (0.24) | 32 (1.01) | < 0.001 | 11 (0.1) |
| Cerebrovascular disease | 4 (0.2) | 48 (0.43) | 29 (0.37) | 19 (0.60) | 0.090 | 36 (0.4) |
| Ketone body related events | 11 (1.0) | 7 (0.06) | 6 (0.08) | 1 (0.03) | –b | 2 (0.02) |
| Fracture | 0 | 4 (0.04) | 4 (0.05) | 0 | –b | 2 (0.02) |
| Lower limb amputation | 0 | 0 | 0 | 0 | –b | 0 |
No. of patients (%) are shown
ADR adverse drug reaction
aChi-squared test for difference between BMI subgroups
bNo P value was calculated when at least one element of the contingency table was < 10
In the first age category analysis (< 65 vs ≥ 65 years), the incidence of ADRs was similar between non-elderly and elderly patients (19.36% vs 19.04%, P = 0.701). A significantly greater proportion of elderly than non-elderly patients had a serious ADR (2.79% vs 1.55%, P < 0.001). When assessing ADRs of special interest, a significantly greater proportion of elderly patients had skin complications (2.22% vs 1.62%, P = 0.033), renal disorders (2.28% vs 1.51%, P = 0.005), hypoglycemia (0.73% vs 0.43%, P = 0.048), or malignant tumors (1.01% vs 0.24%, P < 0.001). The incidence of polyuria/pollakiuria (5.97% vs 4.47%, P = 0.002) and hepatic disorders (1.39% vs 0.73%, P = 0.004) was significantly higher in non-elderly than elderly patients.
ADR data from STELLA-ELDER are provided in Table 3 for comparison [9].
Table 3.
Adverse drug reactions by BMI and age in the safety analysis population
| Elderly patients, by BMI category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 65 years (n = 1944) | 65 to < 75 years (n = 1536) | ≥ 75 years (n = 408) | |||||||
| BMI | P valuea | BMI | P valuea | BMI | P valuea | ||||
| < 25 kg/m2 (n = 690) | ≥ 25 kg/m2 (n = 1254) | < 25 kg/m2 (n = 515) | ≥ 25 kg/m2 (n = 1021) | < 25 kg/m2 (n = 175) | ≥ 25 kg/m2 (n = 233) | ||||
| Any ADR | 122 (17.68) | 306 (24.40) | < 0.001 | 102 (19.81) | 258 (25.27) | 0.017 | 20 (11.43) | 48 (20.60) | 0.014 |
| Serious ADR | 19 (2.75) | 47 (3.75) | 0.247 | 15 (2.91) | 39 (3.82) | 0.362 | 4 (2.29) | 8 (3.43) | –b |
| Patients with a BMI < 25 kg/m2, by age | ||||||
|---|---|---|---|---|---|---|
| < 65 years (n = 883) | ≥ 65 years (n = 690) | P valuea | 65 to < 75 years (n = 515) | ≥ 75 years (n = 175) | P valuea | |
| ADR | 159 (18.01) | 122 (17.68) | 0.867 | 102 (19.81) | 20 (11.43) | 0.043 |
| Serious ADR | 12 (1.36) | 19 (2.75) | 0.048 | 15 (2.91) | 4 (2.29) | –b |
No. of patients (%) are shown
ADR adverse drug reaction, BMI body mass index
aChi-squared test for difference between BMI subgroups
bNo P value was calculated when at least one element of the contingency table was < 10
Supplementary Table S2 summarizes the ADRs and ADRs of special interest according to the second age category analysis (patients aged < 65, 65 to < 75, and ≥ 75 years). The incidence of any ADR was lower in patients aged ≥ 75 years than in those aged < 65 or 65 to < 75 years (P = 0.035), while the incidence of serious ADRs was significantly higher in patients aged 65 to < 75 or ≥ 75 years than in those aged < 65 years (P < 0.001).
Elderly patients (≥ 65 years) with a BMI < 25 kg/m2 had a significantly lower incidence of ADRs than those with a BMI ≥ 25 kg/m2. In the ≥ 65 years age group, the incidence of ADRs was 17.68% in patients with a BMI of < 25 kg/m2 and 24.40% in those with a BMI of ≥ 25 kg/m2 (P < 0.001); respective values were 19.81% and 25.27% (P = 0.017) in the 65 to < 75 years age group, and 11.43% and 20.60% (P = 0.014) in the ≥ 75 years group. Among patients with a baseline BMI < 25 kg/m2, a significantly greater proportion of elderly vs non-elderly patients in the first age category analysis (≥ 65 vs < 65 years) had a serious ADR (2.75% vs 1.36%, P = 0.048; Table 3). Within the BMI < 25 kg/m2 group, patients aged ≥ 75 years had a significantly lower incidence of ADRs than those aged < 65 or 65 to < 75 years (P = 0.043; Table 3).
Both non-elderly and elderly patients (< 65 vs ≥ 65 years; first age category analysis) in the safety analysis cohort showed significant decreases from baseline to 3 years in WBC count (− 275.0 ± 1447.8/µL and − 148.7 ± 1323.7/µL, both P < 0.05) and eGFR (− 3.18 ± 12.24 mL/min/1.73 m2 and − 2.21 ± 10.74 mL/min/1.73 m2, both P < 0.001; Table 4).
Table 4.
Changes from baseline to 3 years in vital signs and laboratory parameters in the safety analysis population
| < 65 years | ≥ 65 years | P value for comparison between age groupsa | |||||
|---|---|---|---|---|---|---|---|
| Mean value ± SD | Change from baseline ± SD | Mean value ± SD | Change from baseline ± SD | ||||
| Baseline | 3 years | Baseline | 3 years | ||||
| Heart rate, BPM | 79.2 ± 12.7 (n = 3715) | 77.4 ± 11.7 (n = 2268) | − 0.9 ± 10.3** (n = 1814) | 74.8 ± 10.9 (n = 1478) | 74.1 ± 10.6 (n = 899) | − 0.1 ± 9.6 (n = 742) | 0.081 |
| WBC count, /µL | 7259.1 ± 1995.1 (n = 4242) | 6902.4 ± 1752.4 (n = 2519) | − 275.0 ± 1447.8** (n = 1991) | 6517.8 ± 1667.5 (n = 1768) | 6392.7 ± 1540.0 (n = 1053) | − 148.7 ± 1323.7* (n = 830) | 0.031 |
| RBC count, × 104/μL | 489.8 ± 115.8 (n = 4238) | 504.5 ± 47.8 (n = 2529) | 17.3 ± 32.3** (n = 1988) | 453.0 ± 46.4 (n = 1771) | 471.0 ± 47.2 (n = 1057) | 16.5 ± 31.4** (n = 836) | 0.533 |
| Hemoglobin, g/dL | 14.67 ± 1.50 (n = 4239) | 15.18 ± 1.43 (n = 2538) | 0.53 ± 1.02** (n = 1998) | 13.83 ± 1.42 (n = 1754) | 14.39 ± 1.41 (n = 1052) | 0.45 ± 0.99** (n = 835) | 0.053 |
| Hematocrit, % | 43.80 ± 3.98 (n = 4246) | 45.84 ± 3.94 (n = 2523) | 2.03 ± 2.99** (n = 1979) | 41.72 ± 3.94 (n = 1782) | 43.85 ± 4.20 (n = 1060) | 1.85 ± 3.14** (n = 835) | 0.147 |
| BUN, mg/dL | 14.15 ± 4.11 (n = 4217) | 15.56 ± 4.13 (n = 2512) | 1.34 ± 3.65** (n = 2005) | 16.58 ± 4.98 (n = 1639) | 17.46 ± 4.85 (n = 950) | 1.15 ± 3.98** (n = 747) | 0.232 |
| Serum albumin, g/dL | 4.36 ± 0.37 (n = 2129) | 4.36 ± 0.35 (n = 1285) | 0.01 ± 0.30 (n = 907) | 4.23 ± 0.44 (n = 863) | 4.25 ± 0.36 (n = 512) | − 0.01 ± 0.33 (n = 359) | 0.314 |
| Serum creatinine, mg/dL | 0.721 ± 0.204 (n = 4827) | 0.734 ± 0.214 (n = 2854) | 0.019 ± 0.122** (n = 2316) | 0.780 ± 0.255 (n = 1936) | 0.786 ± 0.257 (n = 1141) | 0.021 ± 0.136** (n = 924) | 0.764 |
| Sodium, mmol/L | 139.9 ± 2.5 (n = 3362) | 140.5 ± 2.3 (n = 1901) | 0.4 ± 2.6** (n = 1487) | 140.5 ± 2.4 (n = 1371) | 141.0 ± 2.4 (n = 779) | 0.5 ± 2.6** (n = 594) | 0.742 |
| Chloride, mmol/L | 102.4 ± 2.9 (n = 3268) | 102.8 ± 2.7 (n = 1877) | 0.4 ± 2.9** (n = 1457) | 103.0 ± 2.8 (n = 1345) | 103.4 ± 2.7 (n = 766) | 0.4 ± 2.8** (n = 584) | 0.899 |
| Potassium, mmol/L | 4.19 ± 0.40 (n = 3537) | 4.22 ± 0.39 (n = 1955) | 0.01 ± 0.39 (n = 1529) | 4.30 ± 0.47 (n = 1503) | 4.28 ± 0.42 (n = 811) | − 0.02 ± 0.42 (n = 616) | 0.219 |
| Calcium, mmol/L | 9.36 ± 0.45 (n = 1067) | 9.31 ± 0.45 (n = 645) | − 0.05 ± 0.42* (n = 424) | 9.29 ± 0.44 (n = 434) | 9.23 ± 0.44 (n = 275) | 0.00 ± 0.44 (n = 172) | 0.146 |
| Phosphorus, mg/dL | 3.29 ± 0.69 (n = 574) | 3.40 ± 0.56 (n = 348) | 0.11 ± 0.51* (n = 211) | 3.17 ± 0.55 (n = 221) | 3.22 ± 0.56 (n = 144) | 0.18 ± 0.58* (n = 75) | 0.333 |
| Magnesium, mg/dL | 2.07 ± 0.24 (n = 171) | 2.20 ± 0.22 (n = 165) | 0.08 ± 0.22* (n = 51) | 2.13 ± 0.22 (n = 91) | 2.28 ± 0.23 (n = 89) | 0.13 ± 0.22* (n = 24) | 0.434 |
| Serum ketone body, μmol/L | 127.79 ± 124.83 (n = 112) | 111.63 ± 174.35 (n = 86) | 0.82 ± 78.22 (n = 28) | 144.54 ± 184.88 (n = 28) | 110.93 ± 99.43 (n = 29) | 13.10 ± 101.09 (n = 9) | –b |
| Fasting C-peptide, ng/mL | 3.595 ± 4.724 (n = 283) | 2.791 ± 1.693 (n = 112) | − 0.848 ± 1.995* (n = 54) | 3.046 ± 2.123 (n = 91) | 2.981 ± 2.021 (n = 36) | − 0.478 ± 1.148 (n = 15) | 0.496 |
| eGFR, mL/min/1.73 m2 | 85.56 ± 19.35 (n = 4762) | 82.46 ± 18.71 (n = 2839) | − 3.18 ± 12.24** (n = 2289) | 70.56 ± 17.96 (n = 1935) | 69.05 ± 17.22 (n = 1140) | − 2.21 ± 10.74** (n = 922) | 0.035 |
| pH | 5.99 ± 0.73 (n = 2904) | 5.93 ± 0.71 (n = 1697) | − 0.05 ± 0.83* (n = 1310) | 5.98 ± 0.76 (n = 946) | 5.96 ± 0.77 (n = 559) | − 0.02 ± 0.87 (n = 421) | 0.508 |
| Urinary albumin, mg/g·Cr | 115.66 ± 413.90 (n = 620) | 81.76 ± 347.36 (n = 332) | − 22.31 ± 247.97 (n = 162) | 89.14 ± 225.92 (n = 160) | 64.78 ± 175.08 (n = 105) | − 38.57 ± 310.31 (n = 55) | 0.695 |
| Urinary creatinine, mg/dL | 116.767 ± 139.480 (n = 472) | 76.143 ± 57.129 (n = 254) | − 27.398 ± 72.246** (n = 116) | 87.824 ± 99.304 (n = 150) | 55.990 ± 37.936 (n = 96) | − 16.975 ± 52.858* (n = 46) | 0.376 |
BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, RBC red blood cell, SD standard deviation, WBC white blood cell
*P < 0.05, **P < 0.001 vs baseline (one-sample t test)
aTwo-sample t test for the comparison of change from baseline between patients aged < 65 years and patients aged ≥ 65 years
bNo P value was calculated when at least one element of the contingency table was < 10
In both non-elderly and elderly patients, significant increases from baseline to 3 years were observed in mean RBC count (both P < 0.001), hemoglobin levels (both P < 0.001), hematocrit (both P < 0.001), BUN (both P < 0.001), serum creatinine (both P < 0.001), sodium (both P < 0.001), chloride (both P < 0.001), and phosphorus (both P < 0.05; Table 4).
When comparing the magnitude of change between the two age groups, WBC count (P < 0.05), and eGFR (P < 0.05) were significantly more reduced in non-elderly (< 65 years) than in elderly patients (≥ 65 years; Table 4).
Changes from baseline in vital signs and laboratory parameters in patients aged 65 to < 75 years and ≥ 75 years are shown in Supplementary Table S3.
Effectiveness
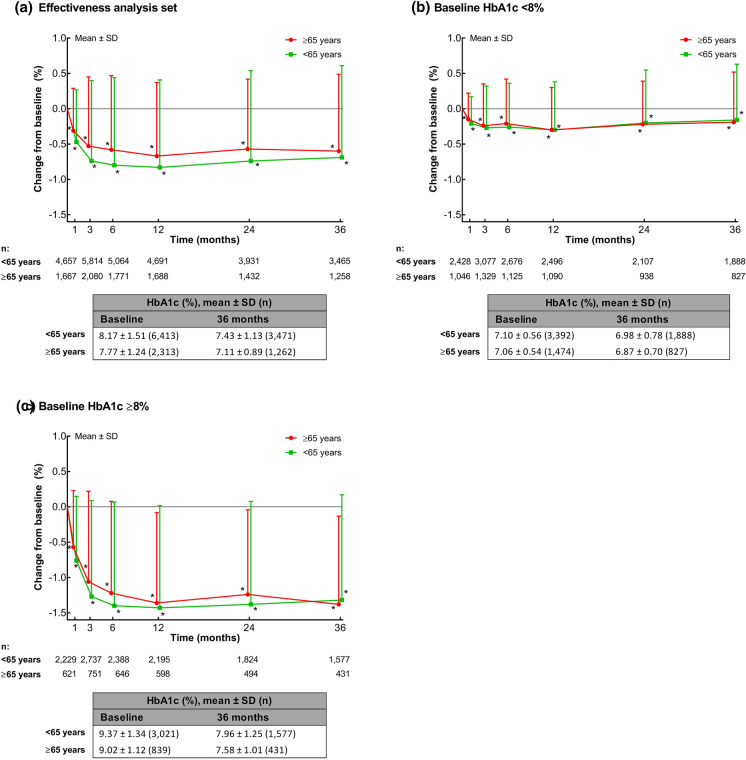
Over the 3 years, mean HbA1c was significantly decreased from baseline (P < 0.001) at each time point in non-elderly and elderly patients for first age category analysis (Fig. 1a), as well as in the subcategories of elderly patients aged 65 to < 75 years and ≥ 75 years (Supplementary Fig. S1). At 3 years, the mean ± SD change from baseline in HbA1c was − 0.69 ± 1.30% in patients aged < 65 years, and − 0.60 ± 1.09% in patients aged ≥ 65 years. When analyzed by baseline HbA1c category, mean HbA1c was significantly decreased from baseline at each time point in patients with baseline HbA1c < 8% and ≥ 8%, in both non-elderly and elderly patients (Fig. 1b, c).
Fig. 1.
Mean ± standard deviation (SD) changes in glycated hemoglobin (HbA1c) by age in a the effectiveness analysis population, b patients with baseline HbA1c < 8%, and c patients with baseline HbA1c ≥ 8%. Tables show the absolute mean ± SD values at baseline and 3 years. *P < 0.001 vs baseline (one-sample t test)
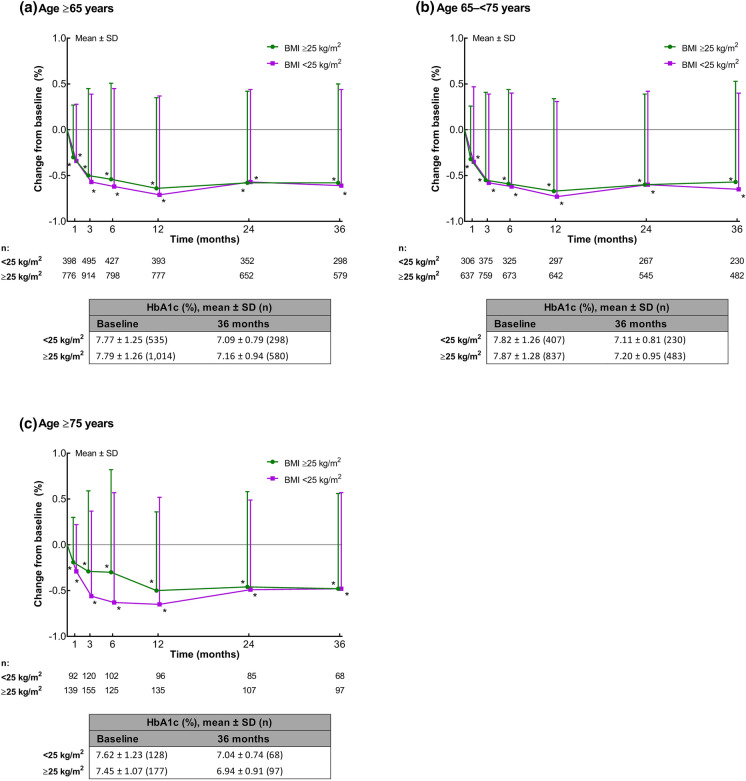
When data for elderly patients were analyzed according to baseline BMI (< 25 vs ≥ 25 kg/m2), in both BMI categories, mean HbA1c was significantly (P < 0.001) reduced from baseline at each time point in patients aged ≥ 65 years (Fig. 2a), 65 to < 75 years (Fig. 2b), and ≥ 75 years (Fig. 2c). In patients aged ≥ 65 years, the mean ± SD change in HbA1c from baseline at 3 years was − 0.61 ± 1.05% in those with a baseline BMI < 25 kg/m2 and − 0.58 ± 1.08% in those with a baseline BMI ≥ 25 kg/m2.
Fig. 2.
Mean ± standard deviation (SD) changes in glycated hemoglobin (HbA1c) by BMI (< 25 vs ≥ 25 kg/m2) in elderly patients: a patients aged ≥ 65 years, b patients aged 65 to < 75 years, and c patients aged ≥ 75 years. Tables show the absolute mean ± SD values at baseline and 3 years. *P < 0.001 vs baseline (one-sample t test)
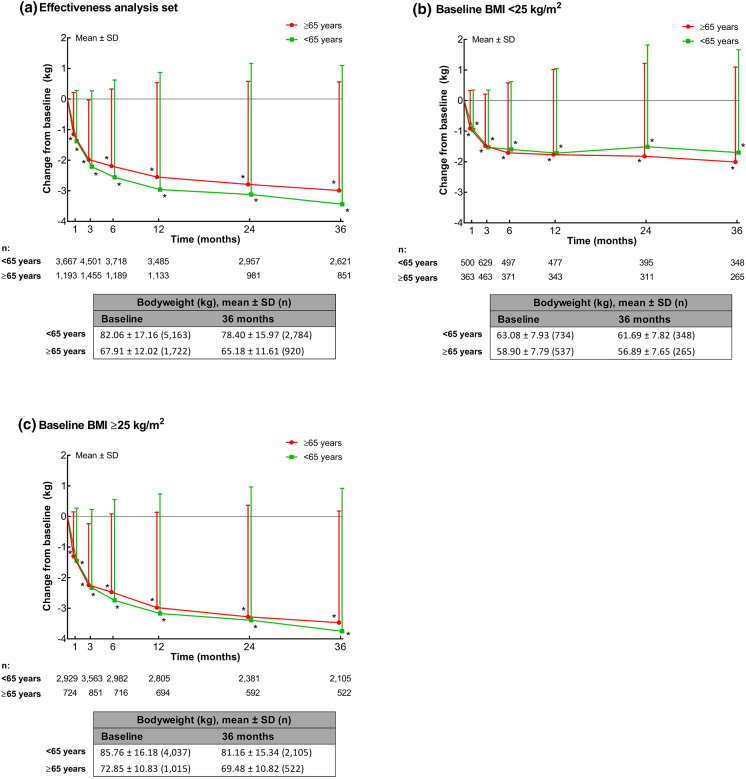
Over the 3-year study, mean body weight was significantly (P < 0.001) reduced from baseline at each time point in patients aged < 65 and ≥ 65 years (Fig. 3a), and in those aged 65 to 75 years and ≥ 75 years (Supplementary Fig. S2a). After 3 years, the mean ± SD change in body weight was − 3.34 ± 4.54 kg in patients aged < 65 years and − 2.99 ± 3.55 kg in patients aged ≥ 65 years.
Fig. 3.
Mean ± standard deviation (SD) changes in body weight by age in a the effectiveness analysis population, b patients with a baseline BMI < 25 kg/m2, and c patients with a baseline BMI ≥ 25 kg/m2. Tables show the absolute mean ± SD values at baseline and 3 years. *P < 0.001 vs baseline (one-sample t test)
When analyzed by baseline BMI category, mean body weight was significantly reduced from baseline at all time points in patients with a baseline BMI < 25 kg/m2 and ≥ 25 kg/m2 across all age categories (< 65 and ≥ 65 years, Fig. 3b, c; and < 65, 65 to < 75 years and ≥ 75 years, Supplementary Fig. S2b and c).
In the first age category analysis, mean ± SD FPG was significantly (P < 0.001) reduced from baseline to 3 years by − 29.3 ± 50.5 mg/dL in patients aged < 65 years and − 27.5 ± 49.1 mg/dL in patients aged ≥ 65 years (Table 5). In both non-elderly and elderly patients in the first age category effectiveness analysis, there were also significant reductions from baseline in fasting insulin, BMI, waist circumference, SBP, DBP, uric acid, and liver function markers (AST, ALT, γ-GTP, and ALP), as well as significant improvements in lipid parameters (triglycerides, total cholesterol, LDL-C, and HDL-C; Table 5). The changes from baseline were significantly greater for patients aged < 65 years than ≥ 65 years for AST (P < 0.05) and ALT (P < 0.001); there were no other between-group differences.
Table 5.
Changes from baseline to 3 years in vital signs and laboratory parameters in the effectiveness analysis population
| < 65 years | ≥ 65 years | P value for comparison between age groupsa | |||||
|---|---|---|---|---|---|---|---|
| Mean value ± SD | Change from baseline ± SD | Mean value ± SD | Change from baseline ± SD | ||||
| Baseline | 3 years | Baseline | 3 years | ||||
| FPG, mg/dL | 167.7 ± 59.6 (n = 3692) | 136.3 ± 37.6 (n = 2038) | − 29.3 ± 50.5** (n = 1745) | 164.7 ± 56.6 (n = 1455) | 135.2 ± 37.6 (n = 788) | − 27.5 ± 49.1** (n = 700) | 0.418 |
| Fasting insulin, μU/mL | 13.60 ± 9.51 (n = 411) | 11.64 ± 8.58 (n = 181) | − 2.87 ± 8.24** (n = 96) | 11.42 ± 9.60 (n = 151) | 8.30 ± 4.38 (n = 85) | − 3.45 ± 8.30* (n = 44) | 0.699 |
| BMI, kg/m2 | 29.93 ± 5.37 (n = 4771) | 28.62 ± 4.97 (n = 2581) | − 1.27 ± 1.66** (n = 2453) | 26.83 ± 4.08 (n = 1552) | 25.82 ± 3.98 (n = 838) | − 1.19 ± 1.42** (n = 787) | 0.203 |
| Waist circumference, cm | 98.75 ± 12.64 (n = 995) | 94.27 ± 12.19 (n = 472) | − 2.82 ± 4.65** (n = 373) | 92.83 ± 10.13 (n = 397) | 89.88 ± 10.25 (n = 234) | − 2.99 ± 4.67** (n = 190) | 0.666 |
| SBP, mmHg | 133.0 ± 15.2 (n = 5245) | 128.8 ± 13.1 (n = 3031) | − 4.4 ± 14.7** (n = 2766) | 134.3 ± 14.9 (n = 1899) | 129.9 ± 13.0 (n = 1099) | − 4.2 ± 14.6** (n = 1011) | 0.682 |
| DBP, mmHg | 79.5 ± 10.2 (n = 5178) | 76.9 ± 9.4 (n = 3017) | − 2.8 ± 10.1** (n = 2731) | 74.1 ± 9.8 (n = 1888) | 72.0 ± 9.1 (n = 1093) | − 2.4 ± 9.9** (n = 1001) | 0.342 |
| AST, U/L | 31.2 ± 20.3 (n = 4271) | 25.7 ± 12.8 (n = 2440) | − 4.6 ± 14.9** (n = 2104) | 27.2 ± 16.2 (n = 1542) | 24.4 ± 13.6 (n = 906) | − 3.0 ± 15.6** (n = 771) | 0.010 |
| ALT, U/L | 41.8 ± 31.1 (n = 4330) | 31.9 ± 21.5 (n = 2460) | − 8.5 ± 22.6** (n = 2135) | 28.0 ± 19.2 (n = 1552) | 23.3 ± 14.8 (n = 909) | − 5.3 ± 16.2** (n = 778) | < 0.001 |
| ALP, U/L | 243.9 ± 83.3 (n = 2303) | 228.7 ± 70.4 (n = 1338) | − 10.0 ± 54.4** (n = 1035) | 248.6 ± 84.5 (n = 847) | 234.6 ± 70.9 (n = 495) | − 12.9 ± 58.6** (n = 382) | 0.380 |
| γ-GTP, U/L | 62.1 ± 72.8 (n = 3987) | 46.7 ± 55.3 (n = 2286) | − 12.1 ± 47.8** (n = 1938) | 49.2 ± 64.6 (n = 1435) | 35.6 ± 39.7 (n = 835) | − 11.8 ± 39.0** (n = 709) | 0.905 |
| Total cholesterol, mg/dL | 199.0 ± 40.1 (n = 2728) | 192.8 ± 36.0 (n = 1455) | − 3.0 ± 33.9* (n = 1210) | 190.4 ± 38.6 (n = 993) | 187.6 ± 32.6 (n = 528) | − 2.9 ± 28.6* (n = 446) | 0.944 |
| LDL-C, mg/dL | 116.2 ± 32.4 (n = 3913) | 110.1 ± 28.4 (n = 2303) | − 5.8 ± 30.4** (n = 1882) | 109.9 ± 29.9 (n = 1430) | 106.0 ± 27.6 (n = 833) | − 4.6 ± 26.4** (n = 691) | 0.347 |
| HDL-C, mg/dL | 49.9 ± 13.0 (n = 4132) | 53.6 ± 16.3 (n = 2368) | 3.2 ± 8.7** (n = 2018) | 53.5 ± 14.7 (n = 1448) | 56.1 ± 14.3 (n = 853) | 3.0 ± 10.3** (n = 719) | 0.580 |
| Triglycerides, mg/dL | 208.5 ± 198.9 (n = 4322) | 183.2 ± 154.1 (n = 2475) | − 15.1 ± 148.9** (n = 2122) | 163.5 ± 126.4 (n = 1532) | 147.1 ± 79.0 (n = 893) | − 12.4 ± 86.9** (n = 759) | 0.633 |
| Uric acid, mg/dL | 5.40 ± 1.31 (n = 3808) | 5.12 ± 1.20 (n = 2199) | − 0.23 ± 1.01** (n = 1810) | 5.17 ± 1.33 (n = 1369) | 4.89 ± 1.21 (n = 795) | − 0.26 ± 1.03** (n = 655) | 0.473 |
| Total bilirubin, mg/dL | 0.637 ± 0.256 (n = 1906) | 0.650 ± 0.257 (n = 1145) | 0.030 ± 0.208** (n = 891) | 0.627 ± 0.238 (n = 764) | 0.654 ± 0.228 (n = 452) | 0.026 ± 0.185* (n = 351) | 0.754 |
γ-GTP gamma-glutamyl transpeptidase, ALT alanine aminotransferase, ALP alkaline phosphatase, AST aspartate aminotransferase, BMI body mass index, DBP diastolic blood pressure, FPG fasting plasma glucose, HDL-C high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, SD standard deviation, SBP systolic blood pressure
*P < 0.05, **P < 0.001 vs baseline (one-sample t test)
aTwo-sample t test for the comparison of change from baseline between patients aged < 65 years and patients aged ≥ 65 years
Changes in vital signs and laboratory markers in the effectiveness analysis population in patients aged 65 to < 75 years and ≥ 75 years are shown in Supplementary Table S4.
Discussion
In this subgroup analysis of the large, 3-year STELLA-LONG TERM post-marketing surveillance study, ipragliflozin treatment was well tolerated and effective in both elderly and non-elderly Japanese patients with type 2 diabetes. Of the > 11,000 patients included in STELLA-LONG TERM, approximately 29% were aged ≥ 65 years. As may be expected, compared with non-elderly patients, elderly patients had a significantly longer duration of diabetes at baseline, were more likely to have complications, and had a significantly lower mean body weight, BMI, and eGFR. Additionally, a significantly greater proportion of elderly than non-elderly patients had an HbA1c < 8% at baseline.
In comparison with the 1-year STELLA-ELDER Japanese post-marketing study, elderly patients in STELLA-LONG TERM had a numerically similar baseline mean body weight, BMI, eGFR, duration of diabetes, and proportion of patients with an HbA1c ≥ 8%; whereas the proportion of patients with complications at baseline was numerically higher among elderly patients in the current study than those in STELLA-ELDER [10].
In the current study, the incidence of ADRs was similar in patients aged < 65 years (19.36%) and in those aged ≥ 65 years (19.0%). These proportions are greater than those reported in the 1-year interim results of this study, when 14.8% of non-elderly and 14.2% of elderly patients had at least one ADR [11]; the latter incidence in elderly patients was lower than that after 1 year of treatment in STELLA-ELDER [10]. A significantly greater proportion of elderly versus non-elderly patients had a serious ADR in both this final 3-year analysis (2.79% vs 1.55%) and the 1-year analysis (1.4% vs 0.8%) of STELLA-LONG TERM [11]. The most likely explanation for the difference in ADR rates between STELLA-ELDER and the current study lies in the timing of the two studies. Ipragliflozin was approved in Japan in April 2014 and the registration period for the STELLA-ELDER study was the first 3 months after ipragliflozin was introduced. The Japanese Diabetes Society (JDS) published recommendations on the proper use of SGLT2 inhibitors in June 2014 (updated again in 2020 [15]), after registration for STELLA-ELDER had begun. Patients were registered in the STELLA-LONG TERM between July 2014 and October 2015, after publication of the JDS recommendations, which probably influenced prescribing practices, particularly in elderly patients.
When assessing ADRs of special interest, in both this analysis and the 1-year analysis, the incidence of skin complications, renal disorder, and hypoglycemia was higher in elderly than in non-elderly patients, while polyuria/pollakiurua and hepatic disorders were more common in non-elderly patients [11].
In this final report, the incidence of malignant tumors was significantly higher in elderly patients, and the incidence was higher than that observed in the 1-year interim report [11]. Over 3 years in the overall safety analysis population, the incidence of malignant tumors reported as an ADR was 0.46%; 0.24% in patients aged < 65 years, and 1.01% in those aged ≥ 65 years (P < 0.001), whereas in the 1-year analysis, malignant tumors were diagnosed in 0.2% of patients aged < 65 years and 0.3% of those aged ≥ 65 years (P = 0.079) [11]. The increase in incidence from 1 to 3 years is not surprising, given that in the general population the incidence of malignant tumors increases with a longer period of observation. Although it is difficult to compare studies with different designs, the main report of the final STELLA-LONG TERM compared the incidence of malignant tumors in the safety population with that in the J-DOIT3 study [16] and found that it was lower in STELLA-LONG TERM than in Japanese patients receiving conventional standard care for type 2 diabetes [14].
The higher incidence of hypoglycemia in the elderly patients in this trial may possibly be explained by these patients having a lower BMI than non-elderly patients. Kidney function often deteriorates with age, which may explain the higher incidence of renal disorders in the elderly patients in this trial. On the other hand, data from international clinical trials suggest that SGLT2 inhibitors have renoprotective effects, which may benefit elderly patients [17–19]. Further research is warranted to confirm this effect in Japanese patients [19]. Such research should include a comparator agent so that the effect of SGLT2 inhibitors on renal function in elderly Japanese patients can be confirmed.
Compared with younger individuals, elderly patients are also more prone to particular skin conditions, such as pruritus, infections, and eczema [20, 21], and these may be exacerbated by medical comorbidities such as diabetes or by concomitant medications [20]. This may explain the higher incidence of skin complications in the elderly patients in this trial.
The treatment of frail older patients with diabetes must be carefully managed [22, 23]. Compared with elderly patients without diabetes, sarcopenia/muscle mass loss appears to be accelerated in elderly patients with diabetes [24]; thus, we paid careful attention to older patients with a lower BMI. In elderly patients in this study, the incidence of ADRs was significantly lower in patients with a baseline BMI < 25 kg/m2 than ≥ 25 kg/m2. Among elderly patients with a BMI < 25 kg/m2, the incidence of ADRs was significantly lower in patients aged ≥ 75 years than in those aged 65 to < 75 years, suggesting that there is no increased risk of ADRs among the older frail population.
Statistically significantly greater decreases in WBC and eGFR were observed in non-elderly compared with elderly patients in this study. However, in both cohorts, mean values at the study endpoint were still well above those that would indicate even mild renal impairment or leukopenia. Further, studies of some other SGLT2 inhibitors (empagliflozin [25], tofogliflozin [26]) in Japanese patients have also shown decreases in eGFR.
In addition to confirming the long-term safety of ipragliflozin in a real-world setting, this trial showed ipragliflozin to be effective in both elderly and non-elderly patients. Throughout 3 years of treatment, mean HbA1c and body weight were reduced from baseline at every measured time point in both age groups. These significant reductions in mean HbA1c were observed regardless of whether baseline HbA1c was < 8% or ≥ 8%. Significant reductions in mean HbA1c were also recorded in those aged ≥ 75 years, regardless of whether their baseline BMI was < 25 kg/m2 or ≥ 25 kg/m2.
This study was not without limitations. The main objective of post-marketing surveillance studies is to collect real-world data on the safety and effectiveness of a drug from as many patients as possible. As such as study, STELLA-LONG TERM had a single-arm, observational methodology and was carried out in routine clinical practice; therefore, it lacked a control arm and the results may have been influenced by variables other than the study treatment (such as the use of other antidiabetic drugs or selection bias in the use of ipragliflozin in elderly patients). Further, patients who completed or discontinued the study were not followed up, and these missing data need to be taken into account when interpreting our results. Finally, we did not specifically investigate factors relevant to sarcopenia or frailty in the elderly subgroup.
Conclusion
In this subgroup analysis of the final results of the 3-year STELLA-LONG TERM trial, ipragliflozin was well tolerated and effective in both elderly and non-elderly patients, and no new safety concerns were identified. The incidence of serious ADRs was significantly higher in elderly than non-elderly patients. Additionally, long-term ipragliflozin therapy was well tolerated and effective in all elderly patients, including those aged ≥ 75 years and with a baseline BMI < 25 kg/m2.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This manuscript and the journal’s Rapid Service fee was funded by Astellas Pharma Inc.
Medical Writing and Editorial Assistance
The authors thank all the participants in this study. This study was funded by Astellas Pharma Inc. Medical writing support was provided by Toni Dando and Catherine Rees, of inScience Communications, Springer Healthcare, and was funded by Astellas Pharma Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
I Nakamura contributed to the study design, study conduct, data collection, data analysis and data interpretation. H Maegawa, K Tobe, and S Uno contributed to the study design, data analysis, and data interpretation. All authors contributed to writing manuscript and approved the final draft for submission.
Prior Presentation
The data included in this manuscript were presented, in part, at the 35th Annual Meeting of the Japan Society of Diabetic Complications in December 2020.
Disclosures
Ichiro Nakamura and Satoshi Uno are employees of Astellas Pharma Inc. Hiroshi Maegawa has received lecture fees from MSD K.K., Sanofi K.K., Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sumitomo Dainippon Pharma Co. Ltd.; research support from Astellas Pharma Inc., AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd., Sunstar Inc., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd., Nissan Chemical Corporation and MIKI Corporation; grants from Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kowa Pharmaceutical Co. Ltd., Taisho Pharma Co. Ltd., Ono Pharmaceutical Co. Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co. Ltd., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Bayer Yakuhin Ltd., Teijin Pharma Limited, Shionogi & Co. Ltd., Novartis Pharma K.K. and Nipro Corporation. Kazuyuki Tobe received lecture fees from MSD K.K., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co. Ltd.; grants from Daiichi Sankyo Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Eli Lilly Japan K.K., Asahi Kasei Pharma Corporation, The Mitsubishi Foundation, and Suntory Global Innovation Center Ltd.
Compliance with Ethics Guidelines
This study was conducted in compliance with Japanese Good Post-marketing Study Practice (GPSP) regulations and the study protocol was approved by the Ministry of Health, Labour and Welfare of the Japanese government. All medical institutions that agreed to provide data signed a contract with Astellas Pharma Inc. The review board that approved the study waived the need for consent, as anonymous data were collected via electronic survey forms from clinical settings.
Data Availability
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Referencess
- 1.Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74(5):611–617. doi: 10.1007/s40265-014-0204-x. [DOI] [PubMed] [Google Scholar]
- 2.Kadokura T, Akiyama N, Kashiwagi A, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106(1):50–56. doi: 10.1016/j.diabres.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6(2):125–138. doi: 10.1007/s13340-014-0184-9. [DOI] [Google Scholar]
- 4.Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17(3):304–308. doi: 10.1111/dom.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6(1):8–18. doi: 10.1007/s13340-014-0164-0. [DOI] [Google Scholar]
- 6.Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study) Diabetol Int. 2015;6(2):104–116. doi: 10.1007/s13340-014-0182-y. [DOI] [Google Scholar]
- 7.Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152–160. doi: 10.1111/dom.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health Labour and Welfare. National health and nutrition survey (in Japanese). 2018. https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html. Accessed 8 July 2020.
- 9.Yokote K, Terauchi Y, Nakamura I, Sugamori H. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17(15):1995–2003. doi: 10.1080/14656566.2016.1219341. [DOI] [PubMed] [Google Scholar]
- 10.Terauchi Y, Yokote K, Nakamura I, Sugamori H. Safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): interim results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17(4):463–471. doi: 10.1517/14656566.2016.1145668. [DOI] [PubMed] [Google Scholar]
- 11.Maegawa H, Tobe K, Nakamura I, Uno S. Safety and effectiveness of ipragliflozin in elderly versus non-elderly Japanese type 2 diabetes mellitus patients: 12 month interim results of the STELLA-LONG TERM study. Curr Med Res Opin. 2019;35(11):1901–1910. doi: 10.1080/03007995.2019.1647503. [DOI] [PubMed] [Google Scholar]
- 12.Maegawa H, Tobe K, Tabuchi H, Nakamura I, Uno S. Safety and efficacy of ipragliflozin in elderly versus non-elderly Japanese patients with type 2 diabetes mellitus: a subgroup analysis of the STELLA-LONG TERM study. Expert Opin Pharmacother. 2018;19(4):327–336. doi: 10.1080/14656566.2018.1434145. [DOI] [PubMed] [Google Scholar]
- 13.Maegawa H, Tobe K, Tabuchi H, Nakamura I. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17(15):1985–1994. doi: 10.1080/14656566.2016.1217994. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura I, Maegawa H, Tobe K, Uno S. Real-world evidence for long-term safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus: final results of a 3-year post-marketing surveillance study (STELLA-LONG TERM) Expert Opin Pharmacother. 2020 doi: 10.1080/14656566.2020.1817388. [DOI] [PubMed] [Google Scholar]
- 15.Committee on the Proper Use of SGLT2 Inhibitors Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig. 2020;11(1):257–261. doi: 10.1111/jdi.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–964. doi: 10.1016/S2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 17.Kitada M, Hirai T, Koya D. Significance of SGLT2 inhibitors: lessons from renal clinical outcomes in patients with type 2 diabetes and basic researches. Diabetol Int. 2020;11:245–251. doi: 10.1007/s13340-020-00444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A, Node K. Promising roles of sodium–glucose co-transporter 2 inhibitors in heart failure prevention and treatment. Diabetol Int. 2020;11:252–260. doi: 10.1007/s13340-020-00445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watada H. Current understanding of the effect of sodium–glucose co-transporter-2 inhibitors in Asian patients with diabetes mellitus. Diabetol Int. 2020;11:242–244. doi: 10.1007/s13340-020-00443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos I. Comparative study of dermatological diseases of the elderly in relation to the rest population. Clin Cosmet Investig Dermatol. 2020;13:173–178. doi: 10.2147/CCID.S242294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalcin B, Tamer E, Toy GG, Oztas P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45(6):672–676. doi: 10.1111/j.1365-4632.2005.02607.x. [DOI] [PubMed] [Google Scholar]
- 22.Abdelhafiz AH, Koay L, Sinclair AJ. The effect of frailty should be considered in the management plan of older people with type 2 diabetes. Future Sci OA. 2016;2(1):FSO102. doi: 10.4155/fsoa-2015-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki E, Goto A, Kondo T, et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol Int. 2020;11:165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaku K, Chin R, Naito Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020;19(2):211–221. doi: 10.1080/14740338.2020.1694659. [DOI] [PubMed] [Google Scholar]
- 26.Utsunomiya K, Kakiuchi S, Senda M, et al. Safety and effectiveness of tofogliflozin in Japanese patients with type 2 diabetes mellitus: results of 24-month interim analysis of a long-term post-marketing study (J-STEP/LT) J Diabetes Investig. 2020;11(4):906–916. doi: 10.1111/jdi.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx