Abstract
Introduction
The relative efficacy and safety of once-daily oral semaglutide vs. injectable glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in subjects with type 2 diabetes (T2D) inadequately controlled on basal insulin were assessed using network meta-analysis (NMA).
Methods
A systematic literature review (SLR) was performed to identify randomised controlled trials of GLP-1 RAs in this population. Data at 26 ± 4 weeks were extracted for efficacy and safety outcomes feasible for the NMA: change from baseline in glycated haemoglobin (HbA1c), weight and blood pressure; HbA1c target levels (< 7.0% and ≤ 6.5%); composite endpoint; incidence of nausea, vomiting or diarrhoea. Comparators of interest were all licensed doses of dulaglutide, exenatide, liraglutide, lixisenatide and once-weekly injectable semaglutide.
Results
The NMA included seven trials. Once-daily oral semaglutide 14 mg was associated with significantly greater HbA1c reductions vs. most comparators (treatment differences: − 0.42 to − 1.32%); differences vs. once-weekly injectable semaglutide (0.5 mg and 1 mg doses) were not statistically significant. Once-daily oral semaglutide 14 mg was associated with significantly greater weight reductions vs. exenatide 2 mg and lixisenatide 20 μg (− 2.21 and − 2.39 kg respectively); non-statistically significant weight reductions in favour of once-daily oral semaglutide 14 mg were observed vs. all other comparators except once-weekly injectable semaglutide 1 mg. Similar trends were observed for the proportion of subjects achieving HbA1c < 7.0% and ≤ 6.5% and the composite endpoint. Once-daily oral semaglutide 14 mg was associated with similar odds of experiencing nausea, vomiting or diarrhoea vs. all comparators.
Conclusion
Once-daily oral semaglutide 14 mg, as an add-on to basal insulin, is an efficacious treatment for reducing HbA1c and weight and meeting glycaemic targets at 26 ± 4 weeks. Once-daily oral semaglutide 14 mg also offers the option of an oral treatment with similar or better efficacy and similar tolerability vs. most injectable GLP-1 RAs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01034-w.
Keywords: Basal insulin, GLP-1, Glycaemic control, Network meta-analysis, Semaglutide, Systematic review, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| Currently, there are no head-to-head trials comparing once-daily oral semaglutide with injectable glucagon-like peptide-1 receptor agonists (GLP-1 RAs) as an add-on to insulin therapy |
| Given the progressive nature of type 2 diabetes (T2D), comparative data in people with advanced disease are highly valuable to guide clinical decision-making for this population |
| This study sought to assess the relative efficacy and safety of once-daily oral semaglutide vs. injectable GLP-1 RAs in people with T2D inadequately controlled on basal insulin, using network meta-analysis |
| What was learned from the study? |
| Once-daily oral semaglutide 14 mg, as an add-on to basal insulin, is an efficacious treatment that offers people with T2D the option of an oral treatment with similar or better efficacy and similar tolerability compared with most injectable GLP-1 RAs |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.13953326.
Introduction
Type 2 diabetes (T2D) is a chronic, progressive disease characterised by hyperglycaemia (i.e. elevated levels of blood glucose) and is associated with microvascular and macrovascular complications [1], which can reduce life expectancy, impair quality of life and increase treatment costs associated with diabetes [2–4].
Due to the progressive nature of T2D, treatment is often intensified over time to achieve adequate glycaemic control; the recommended target glycated haemoglobin (HbA1c) levels for many people are either < 7.0% (53 mmol/l) or ≤ 6.5% (48 mmol/mol) [5–9]. For those who do not meet their glycaemic targets after initial metformin treatment, current American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) guidelines recommend intensifying treatment with sulfonylureas (SU), thiazolidinediones (TZD), dipeptidyl peptidase-4 inhibitors (DPP-4i), sodium-glucose co-transporter-2 inhibitors (SGLT-2is) or glucagon-like peptide 1 receptor agonists (GLP-1 RAs) [10].
Despite intensive non-insulin therapy, some people require insulin treatment, typically basal insulin, to achieve and maintain target HbA1c levels [7, 10, 11]. However, a significant proportion of people receiving basal insulin across Europe (78.1%) and the USA (72.2%) still have inadequate glycaemic control at 3 and 24 months post-initiation, respectively [12]. Treatment can be further intensified by adding rapid-acting bolus insulin, other oral anti-diabetic drugs or injectable GLP-1 RA [7, 10, 11]. The combination treatment of GLP-1 RAs and basal insulin has demonstrated robust glycaemic control without an increase in hypoglycaemia or weight gain, compared with the addition of mealtime insulin [10, 13, 14].
Once-daily oral semaglutide is the first GLP-1 RA in a tablet formulation for the treatment of T2D in adults. The Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) phase 3 clinical trial programme provided extensive evidence on the efficacy and safety of once-daily oral semaglutide in a broad population with T2D, ranging from early to late disease stage [15–19]. The PIONEER 8 study demonstrated the efficacy of once-daily oral semaglutide as an add-on to insulin therapy in people with T2D who were uncontrolled on insulin (with or without metformin); once-daily oral semaglutide provided superior reductions in HbA1c levels and body weight vs. placebo, enabling up to 54% of people with T2D to achieve HbA1c < 7.0% at 52 weeks [19]. Better glycaemic control was achieved with both doses of once-daily oral semaglutide (7 and 14 mg) vs. placebo at 26 and 52 weeks, despite lower total daily insulin dosages relative to baseline [19].
Several treatment options for T2D are currently available, thereby requiring that decision-makers understand the relative clinical benefits of each treatment to provide the best recommendations within budgetary constraints. Currently, there is no evidence from direct head-to-head trials between once-daily oral semaglutide and injectable GLP-1 RAs as an add-on to insulin therapy. Given the progressive nature of T2D, comparative evidence demonstrating the clinical benefits of once-daily oral semaglutide in people with advanced T2D would be valuable to guide clinical decision-making for this population. Network meta-analysis (NMA) allows the use of all available trial data by combining direct and indirect evidence. Thus, the objective of this study was to conduct a systematic literature review (SLR) and an NMA to assess the relative efficacy and safety of GLP-1 RAs as an add-on to insulin therapy in people with T2D inadequately controlled on basal insulin.
Methods
Systematic Literature Review
An SLR was performed, in accordance with the Centre for Reviews and Dissemination and the Cochrane Collaboration guidelines [20, 21], to identify randomised controlled trials on all currently used T2D pharmacotherapies and patient populations. Methodology and results presented herein are specific to studies reporting the efficacy and safety of once-daily oral semaglutide and injectable GLP-1 RAs in people with T2D inadequately controlled on basal insulin.
Study selection criteria were defined in terms of population, interventions, comparisons, outcomes and study design (PICOS) to ensure that potentially relevant studies were selected systematically to minimise bias [20, 21]. The search strategy and PICOS criteria are reported in the supplementary material. Briefly, a database search of MEDLINE®, EMBASE and the Cochrane Library (Tables S1–S4) was performed via the Ovid platform on 5 April 2016 and updated on 9 July 2019. Conference proceedings were searched including the EASD, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), the International Diabetes Federation and the ADA Scientific Sessions. Studies were screened independently by two reviewers against the PICOS selection criteria for inclusion in the SLR (Table S5). To provide a robust summary of relative treatment effects, an analysis was conducted to assess the degree of heterogeneity between the trials included in the network in terms of participants, study design, interventions and outcomes (full analysis available in supplementary material) [22]. A quality assessment of the trials was also conducted using the Cochrane Risk of Bias Assessment [21].
Network Meta-analysis
An NMA was performed, in accordance with guidance from the National Institute for Health and Care Excellence (NICE) and ISPOR Good Research Practise Task Force [23–26], to assess the relative efficacy and safety of once-daily oral semaglutide compared with injectable GLP-1 RAs for the treatment of T2D as an add-on to insulin therapy. In the analysis, the primary intervention of interest was once-daily oral semaglutide (7 and 14 mg doses). The primary comparators of interest were all licensed doses of injectable GLP-1 RAs approved for the treatment of T2D—once-weekly dulaglutide, twice-daily exenatide, once-weekly exenatide extended-release, once-daily liraglutide, once-daily lixisenatide, and once-weekly injectable semaglutide. Albiglutide was included in the SLR but was subsequently excluded as it was withdrawn from the market in 2017 [27] and is no longer a relevant comparator.
The add-on to insulin population was defined as subjects with T2D inadequately controlled on insulin. To ensure the comparator trials chosen for inclusion in the NMA matched the index trial (i.e. PIONEER 8) in terms of population and background of insulin therapy, the definition was closely aligned with the population in PIONEER 8—people with T2D inadequately controlled on insulin (basal, basal-bolus or premix regimens) with or without metformin. As there were very few trials that included the mixed insulin background, and 10 of the 12 trials included basal insulin as the background therapy, the base case was chosen based upon this population. Of note, the PIONEER 8 trial used two different estimands. The treatment policy estimand evaluated the treatment effect for all randomised subjects regardless of trial product discontinuation and use of rescue medication (data analysed using multiple imputation), whereas the trial product estimand evaluated the treatment effect for all randomised subjects under the assumption that they remained on the trial product for the entire planned duration of the trial and did not use rescue medication (data analysed using a mixed model for repeated measures) [28]. To allow for robust comparisons with trials reporting data without the use of rescue medication, the trial product estimand from PIONEER 8 was used for this NMA. The same statistical approach (i.e. mixed model for repeated measures) was used in the majority of the included comparator trials, which further supports the use of the trial product estimand from the PIONEER 8 trial.
A feasibility assessment was conducted to assess the quality of the studies and to ensure that study populations, treatments, outcomes and time points were aligned to provide robust analysis and clinically meaningful results. It was considered feasible to examine all trials identified in the SLR for data on at least one of the following efficacy outcomes: change from baseline in HbA1c, systolic blood pressure (SBP) and body weight; proportion of people with T2D achieving HbA1c < 7% or ≤ 6.5%; and a composite endpoint defined as HbA1c < 7% without weight gain or hypoglycaemia. This composite endpoint was included in the analysis as it is increasingly used as a primary or secondary outcome in T2D trials to address the multiple goals recommended by current ADA guidelines [10, 29]. The following safety outcomes were also assessed in the NMA: incidence of nausea, vomiting or diarrhoea. Feasibility was assessed at two time points: 26 ± 4 weeks and 52 ± 4 weeks; no analysis was feasible at 52 ± 4 weeks as only two studies [30, 31] reported data at these time points.
Statistical Analysis
Analyses of continuous outcomes (using a normal likelihood, identity link, shared parameter model) and dichotomous outcomes (using a binomial likelihood, logit link model) feasible for assessment were implemented in WinBUGS (MRC Biostatistics Unit, Cambridge, UK) [32] using a Bayesian approach involving the formal combination of a prior probability distribution and a likelihood distribution, and three Markov Monte-Carlo chains (10,000 iterations). The fixed-effects model was chosen for all the base case analyses. A random-effects analysis was not conducted as there were no pairs of treatments in the evidence network supported by more than one trial, which would result in poor estimates of the distribution of intervention effects [33]. Assessment of the convergence and autocorrelation was performed by analysing history and density plots, and Brooks-Gelman-Rubin diagnostic plots [33].
Presentation of Results
Results are presented as mean treatment differences or odds ratios (ORs) and an associated 95% credible interval. For continuous outcomes of interest, HbA1c, SBP and body weight, a treatment is favoured if the mean difference is < 0 vs. another treatment. For dichotomous efficacy outcomes, a treatment associated with an OR ≥ 1 (e.g. higher odds for achieving HbA1c < 7% vs. another treatment) is favoured. For dichotomous safety outcomes, where reducing the probability of an adverse event is desirable, a treatment associated with an OR < 1 is favoured. Once-daily oral semaglutide may achieve a numerical reduction/increase vs. a comparator, and if the 95% credible interval excludes the 0.0 (treatment differences) or 1.0 (ORs) the difference is defined as statistically significant. It should be noted that numerical difference is used in this NMA to indicate treatment differences that are not statistically significant. Additionally, the median ranks of each treatment and the surface under the cumulative ranking (SUCRA) are provided. The SUCRA is a numerical summary statistic of cumulative ranking probability plots (i.e. the probability a treatment is among the top n treatments) [34]. A higher SUCRA value indicates an increased possibility that a treatment is in the top rank. A treatment which is certain to be the best will have a SUCRA value of 100% (i.e. treatment is ranked first in all NMA simulations) and a treatment certain to be the worst will have a value of 0% (i.e. treatment is ranked last in all NMA simulations) [34].
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Identified Publications
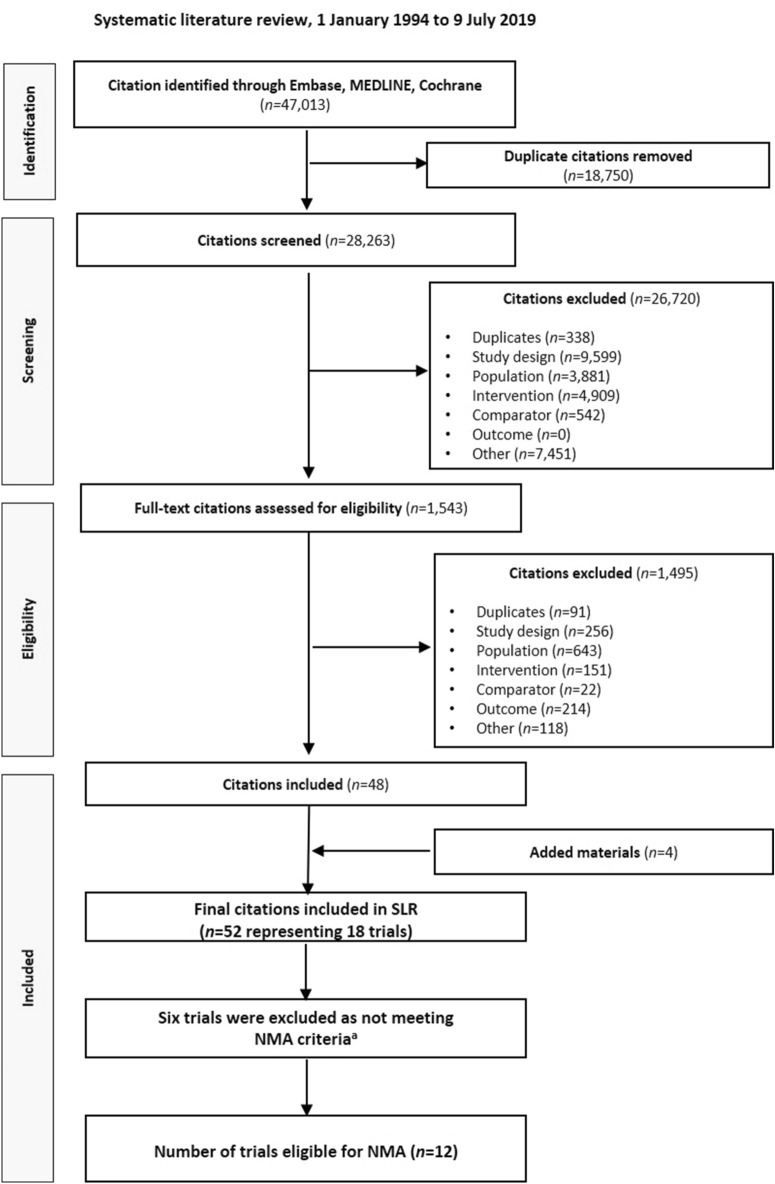
A PRISMA flow diagram of the SLR is shown in Fig. 1. In total, 52 publications reporting on 18 unique trials were included in the SLR. Of these, 15 trials [19, 30, 35–48] were considered as potentially relevant for inclusion in the NMA, and 3 [44, 45, 49] were excluded. The rationale for excluding these trials is detailed in the supplementary material (Table S6). Of these 15 trials, 3 further trials were excluded as not meeting the NMA criteria. Specifically, the GetGoal-Duo2 and Diamant et al. 2014 studies [38, 50] were excluded as they reported results for relevant interventions with only secondary comparators. The insulin glargine treatment arm of the AWARD-4 study [30] was also removed for similar reasons. Furthermore, the LIRA ADD2INSULIN Japan study [46] was excluded as it was conducted in a Japanese-only population and reported data at 36 weeks, whereas other included trials reported data at 24–30 weeks.
Fig. 1.
PRISMA flow diagram of the SLR. NMA network meta-analysis, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review. aThe rationale for excluding the six trials is detailed in Table S37
Overall, the heterogeneity analysis (see supplementary material) indicated the study design and patient characteristics across the 12 included trials [19, 30, 35–37, 39, 41–43, 47, 48, 51] were sufficiently aligned with the PIONEER 8 trial and generalisable to the population of interest to be combined in the analyses. Namely, the mean age of patients varied between 55.0 and 73.0 years, the mean body weight ranged between 66.0 and 94.0 kg, HbA1c levels between 7.6 and 8.5%, the duration of diabetes was 9.0–16.8 years, and background therapy was insulin with or without metformin (Table S7).
However, five trials [30, 37, 42, 47, 48] in the base case network were considered as potential outliers (i.e. differed significantly from other trials to be considered in the statistical analysis) and were consequently excluded. The GetGoal-O trial was conducted exclusively in elderly subjects [37], and the GetGoal-L-C and GetGoal-L-Asia trials were conducted primarily in an Asian population [47, 48]. The GetGoal-Duo 1 trial included subjects who were newly initiated on basal insulin and only included if they were uncontrolled after 12 weeks [42]. Finally, the AWARD-4 trial included subjects who received mixed insulins [30].
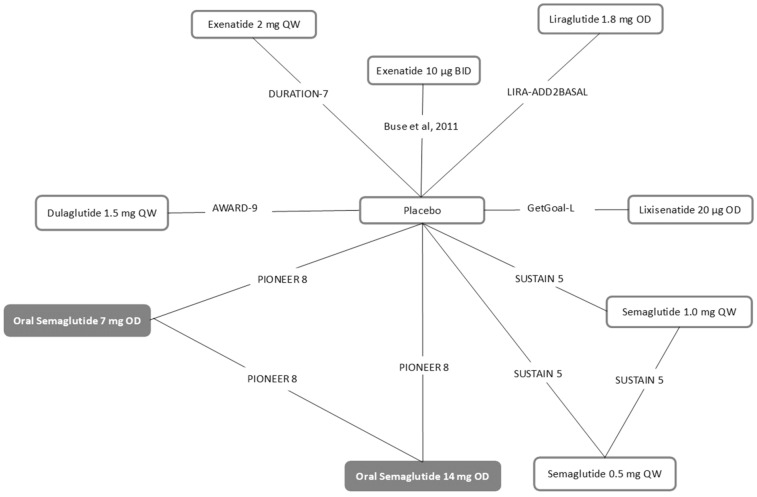
In total, seven trials [19, 35, 36, 39, 41, 43, 51] were considered in the NMA and formed a connected network (Fig. 2) comparing once-daily oral semaglutide (7 and 14 mg doses) with once-weekly dulaglutide 1.5 mg, once-weekly exenatide 2 mg, twice-daily exenatide 10 μg, once-daily liraglutide 1.8 mg, once-daily lixisenatide 20 μg and once-weekly injectable semaglutide (0.5 and 1.0 mg doses). All seven trials reported on at least one outcome of interest at 24–30 weeks' follow-up (Tables S8–S12). Therefore, it was decided to analyse each efficacy outcome assessed at the 6-month (26 ± 4 weeks) follow-up. Overall, the level of response to treatment within the 26 ± 4 week target range was deemed unlikely to vary considerably; 8 of the 12 trials reported data at either 24 or 26 weeks.
Fig. 2.
Base case evidence network for six outcomesa (seven studies). BID twice daily, OD once daily, QW once weekly. aOutcomes included: change from baseline in HbA1c and body weight, proportion of subjects achieving HbA1c < 7%, incidence of nausea, vomiting and diarrhoea. Base case networks for systolic blood pressure, proportion of subjects achieving HbA1c < 6.5% and composite endpoint are presented in Fig. S2–S4
The quality assessment (Fig. S1) indicated that the highest risk of bias across the studies was associated with elements of study blinding and reporting. Additionally, the risk of bias was considered low or unclear across the studies for the remaining elements of bias assessment.
Network Meta-analysis Results
Seven trials were included in the analyses; the overall evidence network is shown in Fig. 2. The evidence networks for the proportion of subjects achieving HbA1c ≤ 6.5% and the proportion of subjects achieving the composite endpoint and SBP are available in the supplementary material (Figs. S2–S4). Base case results of the NMA for once-daily oral semaglutide 14 mg are presented in Fig. 3 for efficacy outcomes and in Fig. 4 for safety outcomes. The full matrices of relative treatment effect results are presented in the supplementary material (Tables S13–S21). The SUCRA and median ranks for each outcome are presented in the supplementary material (Tables S22 and S23). Results for the once-daily oral semaglutide 7 mg dose are available in the supplementary material (Table S24). Additionally, the estimated absolute treatment effects for each outcome are available in the supplementary material (Table S25).
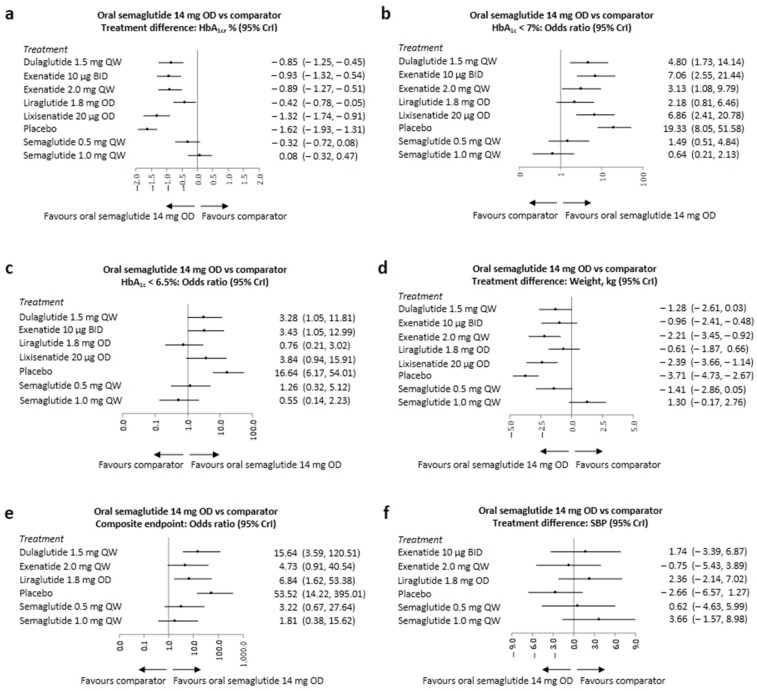
Fig. 3.
Forest plots of the NMA results—once-daily oral semaglutide 14 mg vs. comparator for efficacy outcomes. BID twice-daily, CrI credible interval, HbA1c glycated haemoglobin, NMA network meta-analysis, OD once-daily, QW once-weekly, SBP systolic blood pressure. Treatment differences are considered significant when the 95% CrI excludes the null value. Odds ratios are considered significant when the 95% CrI excludes 1. The NMA results are presented as forest plots for: a change from baseline in HbA1c; b proportion of subjects achieving target HbA1c < 7%; c HbA1c ≤ 6.5%; d change from baseline in body weight; e proportion of subjects achieving composite endpoint; f change from baseline in SBP. X-axis is presented as a logarithmic scale for graphs displaying odds ratio data
Fig. 4.
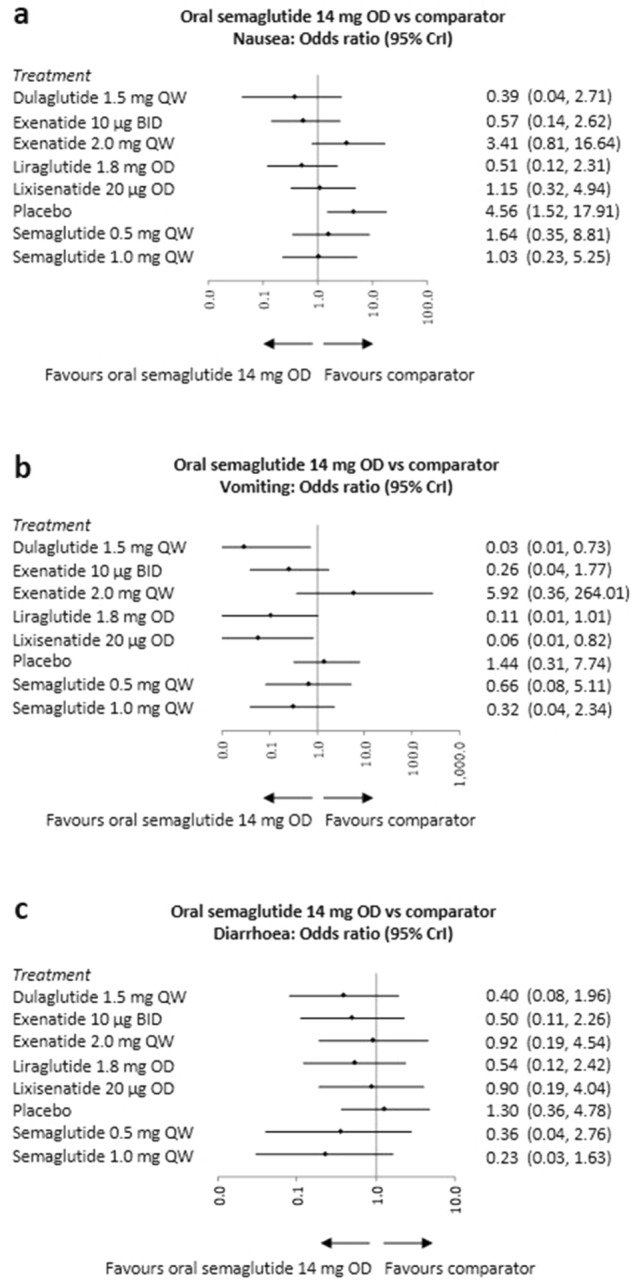
Forest plots of the NMA results—once daily oral semaglutide 14 mg vs. comparator for safety outcomes. BID twice-daily, CrI credible interval, NMA network meta-analysis, OD once-daily, QW once-weekly. Odds ratios are considered significant when the 95% CrI excludes 1. The NMA results are presented as forest plots for incidence of: a nausea; b vomiting; c diarrhoea. X-axis is presented as a logarithmic scale for graphs displaying odds ratio data
Glycaemic Control
Change from Baseline in HbA1c
All seven studies reported data on the change in HbA1c from baseline at 26 ± 4 weeks (Table S8). Once-daily oral semaglutide 14 mg was associated with a significantly greater reduction in HbA1c vs. once-weekly dulaglutide 1.5 mg, twice-daily exenatide 10 μg, once-weekly exenatide 2 mg, once-daily liraglutide 1.8 mg and once-daily lixisenatide 20 μg (Fig. 3a and Table S13). Reductions in HbA1c for once-daily oral semaglutide 14 mg were numerically greater, although not statistically significant, vs. once-weekly injectable semaglutide 0.5 mg. Once-weekly injectable semaglutide 1 mg was associated with numerically greater, but not statistically significant, HbA1c reductions vs. once-daily oral semaglutide 14 mg. Once-daily oral semaglutide 14 mg achieved a median rank of second and a SUCRA value of 92.0% (Tables S22 and S23), indicating that this treatment is among the best in this network.
Proportion of Subjects Achieving HbA1c < 7% or ≤ 6.5%
Data on the proportion of subjects achieving HbA1c < 7% or ≤ 6.5% at 26 ± 4 weeks were reported in seven and six studies, respectively (Table S9). Once-daily oral semaglutide 14 mg was associated with significantly higher odds of achieving HbA1c < 7% vs. once-weekly dulaglutide 1.5 mg, twice-daily exenatide 10 μg, once-weekly exenatide 2 mg and once-daily lixisenatide 20 μg (Fig. 3b and Table S14). The odds of achieving HbA1c < 7% with once-daily oral semaglutide 14 mg were numerically higher, although not statistically significant, vs. once-daily liraglutide 1.8 mg and once-weekly injectable semaglutide 0.5 mg. Once-weekly injectable semaglutide 1 mg was associated with numerically higher, although not statistically significant, odds of achieving HbA1c < 7% vs. once-daily oral semaglutide 14 mg.
Once-daily oral semaglutide 14 mg was also associated with significantly higher odds of achieving HbA1c ≤ 6.5% vs. once-weekly dulaglutide 1.5 mg and twice-daily exenatide 10 μg (Fig. 3c and Table S15). The odds of achieving HbA1c ≤ 6.5% with once-daily oral semaglutide 14 mg were numerically higher, although not statistically significant, vs. once-daily lixisenatide 20 μg and once-weekly injectable semaglutide 0.5 mg. Once-weekly injectable semaglutide 1 mg and once-daily liraglutide 1.8 mg were associated with numerically higher odds of achieving HbA1c ≤ 6.5% vs. once-daily oral semaglutide 14 mg. For both HbA1c target outcomes, once-daily oral semaglutide 14 mg achieved median rankings of second and third and SUCRA values of 87.6% and 76.0%, respectively (Tables S22 and S23), indicating that this treatment is among the best within this network.
Body Weight
All seven studies reported data on the change from baseline in body weight at 26 ± 4 weeks (Table S10). Once-daily oral semaglutide 14 mg was associated with a significantly greater reduction in weight vs. once-weekly exenatide 2 mg and once-daily lixisenatide 20 μg (Fig. 3d and Table S16). Reductions in weight with once-daily oral semaglutide 14 mg were numerically greater, although not statistically significant, vs. all other comparators, except for once-weekly injectable semaglutide 1 mg, which was associated with a numerically greater, although not statistically significant, reduction in body weight vs. once-daily oral semaglutide 14 mg. Once-daily oral semaglutide 14 mg had a median rank of second and a SUCRA value of 85.4% (Tables S22 and S23), indicating that this treatment is among the best within this network.
Proportion of Subjects Achieving the Composite Endpoint
Five studies reported data on the proportion of subjects achieving the composite endpoint, defined as HbA1c < 7% without weight gain and hypoglycaemia at 26 ± 4 weeks (Table S9). Once-daily oral semaglutide 14 mg was associated with significantly higher odds of achieving the composite endpoint vs. once-weekly dulaglutide 1.5 mg and once-daily liraglutide 1.8 mg (Fig. 3e and Table S17). The odds of achieving the composite endpoint with once-daily oral semaglutide 14 mg were numerically higher, but not statistically significant, vs. 0.5 mg and 1 mg doses of once-weekly injectable semaglutide. Once-daily oral semaglutide 14 mg had a median rank of first and a SUCRA value of 95.0% (Tables S22 and S23), indicating that this treatment is likely to be the best in this network.
Systolic Blood Pressure
Five studies reported data on the change from baseline in SBP at 26 ± 4 weeks (Table S11). The change in SBP with once-daily oral semaglutide 14 mg was not significantly different from those observed with the injectable GLP-1 RA comparators (Fig. 3F and Table S18).
Incidence of Nausea, Vomiting and Diarrhoea
All seven studies reported data on the incidence of nausea, vomiting and diarrhoea at 26 ± 4 weeks (Table S12). Once-daily oral semaglutide 14 mg was not associated with significantly different odds of experiencing nausea or diarrhoea vs. GLP-1 RA comparators. The odds of vomiting with once-daily oral semaglutide 14 mg were significantly lower vs. once-weekly dulaglutide 1.5 mg and once-daily lixisenatide 20 μg (Fig. 4 and Tables S19–S21).
Discussion
The aim of this study was to assess the relative efficacy and safety of once-daily oral semaglutide (7 and 14 mg) compared with injectable GLP-1 RAs as an add-on to insulin therapy in people with T2D inadequately controlled on basal insulin.
The analyses indicated that once-daily oral semaglutide 14 mg was associated with a significantly greater reduction in HbA1c at 26 ± 4 weeks vs. most injectable GLP-1 RA comparators; differences vs. once-weekly injectable semaglutide (0.5 mg and 1 mg doses) were not statistically significant. Once-daily oral semaglutide 14 mg was also associated with significantly greater reductions in body weight vs. once-weekly exenatide 2 mg and once-daily lixisenatide 20 μg; non-statistically significant weight reductions in favour of once-daily oral semaglutide 14 mg were observed vs. all other comparators except once-weekly injectable semaglutide 1 mg. Similar results were observed for the proportion of people achieving glycaemic treatment targets at 26 ± 4 weeks. These findings are supported by treatment ranks and SUCRA values, indicating that once-daily oral semaglutide 14 mg is the second-best treatment, after once-weekly injectable semaglutide 1 mg, for reducing HbA1c levels and body weight at 26 ± 4 weeks. Based on treatment ranks and SUCRA values, once-daily oral semaglutide 14 mg is likely to be among the best treatments in the evidence network for achieving HbA1c < 7% without weight gain and hypoglycaemia (i.e. the composite outcome).
Balance between efficacy improvements and associated risk of adverse events is warranted for effective treatment. In this study, increased efficacy was achieved without an increase in adverse events. Once-daily oral semaglutide 14 mg was associated with a significantly lower probability of vomiting compared with once-weekly dulaglutide 1.5 mg and once-daily lixisenatide 20 μg. Similar odds of experiencing nausea and diarrhoea were observed when compared with all other GLP-1 RA comparators.
To our knowledge, this is the first NMA comparing the relative efficacy and safety of once-daily oral semaglutide vs. injectable GLP-1 RAs as an add-on to insulin therapy in people with T2D inadequately controlled on basal insulin. A recently published NMA by Nuhoho et al. compared the relative efficacy and safety of once-daily oral semaglutide 14 mg vs. injectable GLP-1 RAs in people with T2D inadequately controlled on 1–2 oral anti-diabetic drugs [52]. While the target population in this NMA is different from the one considered by Nuhoho et al., findings in terms of GLP-1 RAs’ relative efficacy and ranking are consistent between the two studies, indicating that once-daily oral semaglutide is an efficacious add-on therapy for a broad population with T2D, including people with advanced disease.
Findings from this NMA are robust, based on the quality and homogeneity of the trials included in the network, and the alignment of the methodology with guidelines from NICE, ISPOR and the Cochrane Institute [21, 23–26]. Furthermore, all randomised controlled trials included in the NMA were identified in an SLR to ensure that all available evidence was considered.
This NMA was subject to some limitations. There were some differences in the time points reported in the individual studies, which were addressed by using the well-accepted method of applying a time point window (26 ± 4 weeks) into the analyses [53–55]. Differences in the insulin titration rules were also observed across the studies, with various insulin algorithms being used such as treat-to-target, Initiate Insulin by Aggressive Titration and Education, titration at the investigators’ discretion, or capping of basal insulin dose at a level equal to the pre-study level. Consequently, there may be variability in the placebo effect observed in different studies, which may impact treatment outcomes. The relatively small sample size of the once-daily oral semaglutide arms in the network compared with the injectable GLP-1 RAs arms may also be a limitation of the NMA. Furthermore, as it was not possible to conduct a random-effects analysis, the fixed-effects model was chosen for the base case analyses; this approach does not allow an estimate of heterogeneity between studies and may represent a limitation to this NMA given that heterogeneity within the data sets of each analysis is inevitable. Also, as there was only one study linking each treatment in the evidence network, there was no opportunity to assess for inconsistency in the NMA. Finally, while bias was considered low across the studies, inherent publication bias as well as time lag and language bias were assumed, as with any analysis based on published data [56]. While these differences made the comparisons between included trials challenging, such limitation is common in all NMAs; thus, caution is advised when interpreting NMA results.
Conclusion
Once-daily oral semaglutide 14 mg as an add-on to basal insulin is an efficacious treatment for reducing HbA1c and body weight and increasing the proportion of people with T2D meeting glycaemic treatment targets at 26 ± 4 weeks. Based on the safety outcomes assessed, once-daily oral semaglutide 14 mg has a similar tolerability profile to injectable GLP-1 RAs in this population. In addition, once-daily oral semaglutide offers people with T2D the option of an oral treatment which may reduce the injection burden in a population requiring GLP-1 RAs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal’s Rapid Service fee were funded by Novo Nordisk.
Editorial and Other Assistance
Editorial assistance in the preparation of this manuscript was provided by Sonia Alesso of DRG Abacus Part of Clarivate. This was funded by Novo Nordisk.
Authorship
The SLR and feasibility assessment were conducted by Palvi Gupta of DRG Abacus Part of Clarivate and Jatin Gupta of CONTINUUM India Ltd. The NMA was conducted by Michelle Orme of ICERA Consulting Ltd. All named authors meet the International Committee of Medical Journal Editors (ICMJE) uniform requirements for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval for the version to be published. All authors had full access to all the data in this study and take complete responsibility for the integrity and accuracy of the data analysis.
Disclosures
Barrie Chubb is an employee and stakeholder of Novo Nordisk. Palvi Gupta is an employee of DRG Abacus Part of Clarivate. Jatin Gupta was an employee of DRG Abacus Part of Clarivate at the time the NMA and manuscript were developed and is currently an employee of CONTINUUM India Limited. Solomon Nuhoho was an employee of Novo Nordisk at the time the NMA and manuscript were developed. Klaus Kallenbach is an employee of Novo Nordisk. Michelle Orme is an outcomes research consultant with ICERA Consulting Ltd.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analysed during this study are included in this published article as supplementary information files.
References
- 1.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 2.Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the US. Diabetes Care. 2002;25(3):476–481. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 3.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore DJ, Gregory JM, Kumah-Crystal YA, Simmons JH. Mitigating micro-and macro-vascular complications of diabetes beginning in adolescence. Vasc Health Risk Manag. 2009;5:1015–1031. doi: 10.2147/vhrm.s4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018:dci180033. [DOI] [PMC free article] [PubMed]
- 6.International Diabetes Federation (IDF). Clinical practice recommendations for managing type 2 diabetes in primary care, 2017. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html. Accessed 3 Mar 2021.
- 7.American Diabetes Association Introduction: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S1–S2. doi: 10.2337/dc20-Sint. [DOI] [PubMed] [Google Scholar]
- 8.American Association of Clinical Endocrinologists & American College of Endocrinology (AACE/ACE). Comprehensive type 2 diabetes management algorithm 2019. https://pro.aace.com/disease-state-resources/diabetes/clinical-practice-guidelines-treatment-algorithms/comprehensive. Accessed 3 Mar 2021.
- 9.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology—clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2015;21(Suppl 1):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 11.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25(1):69–100. doi: 10.4158/CS-2018-0535. [DOI] [PubMed] [Google Scholar]
- 12.Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19(8):1155–1164. doi: 10.1111/dom.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 14.Giorgino F, Bonadonna RC, Gentile S, Vettor R, Pozzilli P. Treatment intensification in patients with inadequate glycemic control on basal insulin: rationale and clinical evidence for the use of short-acting and other glucagon-like peptide-1 receptor agonists. Diabetes Metab Res Rev. 2016;32(6):497–511. doi: 10.1002/dmrr.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 16.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. J Am Med Assoc. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–2271. doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centre for Reviews and Dissemination. Systematic Reviews. 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 3 Mar 2021.
- 21.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.2 [updated September 2009]. https://training.cochrane.org/cochrane-handbook-systematic-reviews-interventions. Accessed 3 Mar 2021.
- 22.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, vol. 5.0.1. https://training.cochrane.org/cochrane-handbook-systematic-reviews-interventions. Accessed 3 Mar 2021.
- 23.Dias S, Welton N, Sutton A, Ades A. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011. https://www.ncbi.nlm.nih.gov/books/NBK310366/. Accessed 3 Mar 2021. [PubMed]
- 24.Dias S, Welton N, Sutton A, Caldwell D, Lu G, Ades A. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. 2011; last updated April 2014. http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.final_.15April2014.pdf. Accessed 3 Mar 2021. [PubMed]
- 25.Dias S, Welton N, Sutton A, Valdwell D, Guobing L, Ades A. NICE DSU technical support document 3. Heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011. https://www.ncbi.nlm.nih.gov/books/NBK395886/pdf/Bookshelf_NBK395886.pdf. Accessed 3 Mar 2021.
- 26.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency. Albiglutide withdrawal. 2018. https://www.ema.europa.eu/en/documents/assessment-report/public-statement-eperzan-withdrawal-marketing-authorisation-european-union_en.pdf. Accessed 3 Mar 2021.
- 28.Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203–2210. doi: 10.1111/dom.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einarson TR, Garg M, Kaur V, Hemels ME. Composite endpoints in trials of type-2 diabetes. Diabetes Obes Metab. 2014;16(6):492–499. doi: 10.1111/dom.12226. [DOI] [PubMed] [Google Scholar]
- 30.Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 31.Novo Nordisk. Data on file. PIONEER 8. Insulin add-on, version 1.0; 12 February 2019. Efficacy and safety of oral semaglutide versus placebo in subjects with type 2 diabetes mellitus treated with insulin: a 52-week, randomised, double-blind, placebo-controlled trial. 2019.
- 32.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 33.Welton NJ, Sutton AJ, Cooper NJ, Abrams KR, Ades AE. Evidence synthesis for decision making in healthcare. 1. New York: Wiley; 2012. pp. 124–126. [Google Scholar]
- 34.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056–1064. doi: 10.1111/dom.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 37.Dailey GE, Dex TA, Roberts M, Liu M, Meneilly GS. Efficacy and safety of lixisenatide as add-on therapy to basal insulin in older adults with type 2 diabetes in the GetGoal-O study. J Diabetes. 2019;11(12):971–981. doi: 10.1111/1753-0407.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 39.Guja C, Frías JP, Somogyi A, et al. Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: the DURATION-7 randomized study. Diabetes Obes Metab. 2018;20(7):1602–1614. doi: 10.1111/dom.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzili P, Norwood P, Jodar E, et al. Improved glyacemic control and weight loss with once weekly dulaglutide versus placebo, both added to titrated daily insulin glargine in type 2 diabetes patients (AWARD-9). Abstract 145. In: Presented at 52nd Annual Meeting of the European Association for the Study of Diabetes. Munich, 16 September 2016.
- 41.Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36(9):2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36(9):2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317–2325. doi: 10.2337/dc14-0001. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstock J, Nino AJ, Soffer J, et al. Near-normoglycemia, with meaningful discontinuations of prandial insulin, by adding weekly albiglutide (Albi) to uncontrolled basal/bolus insulin-treated type 2 diabetes (T2DM) Diabetes. 2018;67(Supplement 1):1073-P. [Google Scholar]
- 46.Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565–573. doi: 10.1111/jdi.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab. 2012;14(10):910–917. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W, Min K, Zhou Z, et al. Efficacy and safety of lixisenatide in a predominantly Asian population with type 2 diabetes insufficiently controlled with basal insulin: the GetGoal-L-C randomized trial. Diabetes Obes Metab. 2018;20(2):335–343. doi: 10.1111/dom.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 50.Rosenstock J, Hanefeld M, Gentile S. Advancing basal insulin glargine with prandial lixisenatide QD vs insulin glulisine QD or TID in T2DM: the GetGoal-Duo2 evidence-based trial ( NCT01768559) Diabetes. 2015;64:LB27–LB28. [Google Scholar]
- 51.Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9) Diabetes Obes Metab. 2017;19(7):1024–1031. doi: 10.1111/dom.12937. [DOI] [PubMed] [Google Scholar]
- 52.Nuhoho S, Gupta J, Hansen BB, Fletcher-Louis M, Dang-Tan T, Paine A. Orally administered semaglutide versus GLP-1 RAs in patients with type 2 diabetes previously receiving 1–2 oral antidiabetics: systematic review and network meta-analysis. Diabetes Ther. 2019;10(6):2183–2199. doi: 10.1007/s13300-019-00706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnett AH, Orme ME, Fenici P, Townsend R, Wygant G, Roudaut M. Systematic review and network meta-analysis to compare dapagliflozin with other diabetes medications in combination with metformin for adults with type 2 diabetes. Intern Med. 2014;S6:006. [Google Scholar]
- 54.Kayaniyil S, Lozano-Ortega G, Bennett HA, et al. A network meta-analysis comparing exenatide once weekly with other GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus. Diabetes Ther. 2016;7(1):27–43. doi: 10.1007/s13300-016-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orme ME, Nguyen H, Lu JY, Thomas SA. Comparative effectiveness of glycemic control in patients with type 2 diabetes treated with GLP-1 receptor agonists: a network meta-analysis of placebo-controlled and active-comparator trials. Diabetes Metab Syndr Obes. 2017;10:111–122. doi: 10.2147/DMSO.S116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. doi: 10.1136/bmj.d7762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article as supplementary information files.