Abstract
Purpose
To compare the efficacy and tolerability of mirabegron and solifenacin in pediatric patients with idiopathic overactive bladder (OAB) and to identify factors affecting OAB symptom improvement after treatment.
Materials and Methods
We retrospectively reviewed 103 patients (5–15 years old) who visited our hospital with OAB symptoms between July 2017 and March 2019. All participants had received solifenacin or mirabegron. Those who had secondary OAB or who did not complete the frequency-volume chart either before or after treatment were excluded. The age-adjusted bladder capacity ratio was used to evaluate bladder capacity. Efficacy was assessed on the basis of patient reports and changes in the frequency-volume chart, and ≥90% reduction was regarded as “responding to medication.” Tolerability was assessed by obtaining reports from patients about the adverse effects of the drug.
Results
After the exclusion of 58 patients, 45 patients (29 in solifenacin-group and 16 in mirabegron-group) were included in the primary analysis. The age-adjusted bladder capacity ratio increased from 0.71 to 0.96 (p<0.001) and from 0.57 to 0.97 (p=0.002) after solifenacin and mirabegron use, respectively. Decreased bladder capacity before medication was associated with responding to medication (odds ratio, 7.41; p=0.044). There was no significant difference in efficacy between the two drugs. Drug-induced adverse effects were reported in only 3 (10.3%) of the solifenacin-treated patients.
Conclusions
Mirabegron showed comparable efficacy to solifenacin in pediatric patients with idiopathic OAB. Additionally, only few adverse effects were reported, suggesting that mirabegron can be a safe alternative for the treatment of idiopathic pediatric OAB.
Keywords: Child; Enuresis; Urinary bladder, overactive
Graphical Abstract

INTRODUCTION
Overactive bladder (OAB) is a common symptom in pediatric patients, with an overall prevalence of less than 17% [1,2,3]. Pediatric OAB patients must be managed aggressively because OAB affects health-related quality of life [4]. Treatment includes urotherapy and pharmacotherapy, and antimuscarinics are the first pharmaceutical option [5]. However, these drugs have a poor response rate and cause adverse effects, such as dry mouth, constipation, and urinary retention. In addition, prolonged use of antimuscarinics can lead to adverse effects on the central nervous system, such as cognitive and memory dysfunction. Therefore, most parents reject the use of these drugs, causing the majority of pediatric OAB patients to discontinue antimuscarinic use [6,7].
Mirabegron, a β3-adrenoreceptor agonist, is a new drug for controlling OAB symptoms. It has been used as an alternative to antimuscarinics in patients with OAB because it increases bladder capacity without impairing detrusor contractility [8]. Studies comparing mirabegron with antimuscarinics in adults with OAB have shown that mirabegron has similar efficacy to antimuscarinics but has better tolerability [9,10]. However, only a few studies have investigated these properties in pediatric OAB patients; therefore, mirabegron is currently not licensed for pediatric use [11,12,13]. Hence, the primary objective of this study was to compare the efficacies of mirabegron and solifenacin in pediatric OAB patients. The secondary objectives were to evaluate mirabegron tolerability and identify factors contributing to symptom improvement in pediatric OAB patients after treatment.
MATERIALS AND METHODS
This was a retrospective observational study approved by Institutional Review Board of the Ulsan University Hospital (approval number: UUH 2020-03-010), which waived the need for informed consent. We reviewed 103 pediatric patients, aged between 5 and 15 years, who visited Ulsan University Hospital with OAB symptoms from July 2017 to March 2019. OAB was diagnosed on the basis of what patients and/or parents described. Inclusion criteria were having taken an OAB medicine (solifenacin 5 mg or mirabegron 50 mg) and having completed a frequency-volume chart (FVC) both before and after medication use. Exclusion criteria included secondary OAB, lack of a complete FVC either before or after medication use, and loss to follow-up. We evaluated age, sex, medication (solifenacin 5 mg, mirabegron 50 mg), previous history of antimuscarinic use, incontinence, nocturnal enuresis, duration of symptoms, duration of medication, medication adverse effects, and FVC factors (maximal voided volume [MVV], maximal voided volume during daytime [MVVDT]). To overcome the age-dependent change in bladder capacity, the change was expressed as an age-adjusted bladder capacity ratio (aBCR) by using measured actual bladder capacity (MVV and 1.25×MVVDT) divided by the expected bladder capacity (eBC) for age. The eBC was calculated by using the following formula: “bladder capacity (mL)=(age+1)×30” [14]. Children with a BCR <0.65 were considered to have decreased bladder capacity [15].
Efficacy was assessed by reports from patients and/or parents and by changes in the aBCR as estimated from FVCs. To evaluate OAB symptom relief, patients and parents reported efficacy as complete dryness, improvement (90%–99% reduction), partial improvement (50%–89% reduction), or failure (<50% reduction). A reduction of more than 90% was regarded as “responding to medication” [15]. Tolerability was also assessed by obtaining reports from patients and parents about drug adverse effects. Adverse effects were described by using the common terminology criteria for adverse events (CTCAE), version 5.0.
We hypothesized that, as in adults, mirabegron would have similar efficacy but fewer adverse effects than solifenacin in pediatric OAB patients. Wilcoxon's signed-rank test was used to compare matched variables before and after treatment. Continuous variables were presented as medians with interquartile ranges (IQRs) and categorical variables as frequencies with percentages. The chi-square test or Fisher's exact test was used to evaluate differences in the categorical variables, and the independent t-test was used to compare the means of continuous variables. Pearson's correlation coefficient and linear regression analysis were used to calculate correlations between baseline aBCR and the rate of change after medication. Multivariate binary logistic regression analysis was used to assess the factors affecting medication response. Kaplan-Meier analysis was performed to compare drug maintenance according to medications, and the Cox proportional hazards method was used to determine predictive factors for medication discontinuation. A p<0.05 indicated statistical significance. SPSS version 25.0.0 (IBM Corp., Armonk, NY, USA) was used.
RESULTS
1. Patient characteristics
A total of 103 patients were enrolled, of whom 58 were excluded because 38 were patients with secondary OAB, 14 did not have an FVC before or after medication, and 6 were lost to follow-up (Fig. 1). The remaining 45 patients were included in the primary analysis. The median age at first visit was 7.1 years (IQR: 6.0–9.4) and 22 (48.9%) of the patients were male. Twenty-nine patients were enrolled in the solifenacin group and 16 in the mirabegron group. The patients in the mirabegron group were older, had a longer duration of symptoms, and had a lower aBCR before starting medication than did the patients in the solifenacin group (Table 1).
Fig. 1. Overview of the study population.
Table 1. Patient characteristics.
| Variable | Solifenacin 5 mg (n=29) | Mirabegron 50 mg (n=16) | p-value |
|---|---|---|---|
| Age (y) | |||
| Median | 6.6 | 9.8 | |
| Range | 5.0–14.7 | 5.6–12.3 | |
| IQR | 5.6–7.1 | 8.3–11.0 | |
| Sex, male (%) | 41.4 | 62.5 | 0.175 |
| Previous drug history, n (%) | 5 (17.2) | 2 (12.5) | >0.999a |
| Incontinence, n (%) | 12 (41.4) | 3 (18.8) | 0.105 |
| Nocturnal enuresis, n (%) | 19 (65.5) | 16 (100.0) | 0.008a |
| Duration of symptom (mo) | 0.005 | ||
| Median | 6.0 | 12.0 | |
| Range | 1.0–18.0 | 1.0–36.0 | |
| IQR | 6.0–12.0 | 12.0–24.0 | |
| Duration of medication (d) | 0.570 | ||
| Median | 193 | 239 | |
| Range | 30–772 | 72–466 | |
| IQR | 158–351 | 114–328 | |
| Frequency | 0.261 | ||
| Median | 7 | 6 | |
| Range | 4–15 | 4.5–10 | |
| IQR | 5.5–8.5 | 5–7.5 | |
| aBCR (MVV/eBC) | 0.015 | ||
| Median | 0.60 | 0.56 | |
| Range | 0.37–1.43 | 0.27–0.76 | |
| IQR | 0.52–0.82 | 0.44–0.64 | |
| Decreased bladder capacity, n (%) | 15 (51.7) | 13 (81.3) | 0.051 |
| aBCR (1.25×MVVDT/eBC) | 0.072 | ||
| Median | 0.66 | 0.55 | |
| Range | 0.11–1.46 | 0.29–0.95 | |
| IQR | 0.57–0.95 | 0.49–0.69 | |
| Decreased bladder capacity, n (%) | 13 (44.8) | 12 (75.0) | 0.051 |
IQR, interquartile range; aBCR, age-adjusted bladder capacity ratio; MVV, maximal voided volume; eBC, expected bladder capacity by using age; MVVDT, maximal voided volume during daytime.
a:Fisher's exact test.
2. Clinical outcomes
After solifenacin treatment, the aBCR obtained by using both MVV and MVVDT improved significantly from 0.68 to 0.87 (MVV, p<0.001) and from 0.71 to 0.96 (MVVDT, p<0.001), respectively (Fig. 2A, B). The proportion of patients with decreased bladder capacity was also reduced from 51.7% to 24.1% and from 44.8% to 13.8%, respectively (Supplementary Table 1). Similar to solifenacin, mirabegron improved aBCR (MVV and MVVDT) from 0.54 to 0.83 (MVV, p=0.007) and from 0.57 to 0.97 (MVVDT, p=0.002) and reduced the proportion of patients with decreased bladder capacity from 81.3% to 31.3% and from 75.0% to 18.8%, respectively (Fig. 2A, B, and Supplementary Table 1). However, the changes in aBCR obtained by using MVV and MVVDT before and after treatment did not differ significantly between the solifenacin and mirabegron groups (MVV: 0.19±0.19 vs. 0.30±0.37, p=0.194; MVVDT: 0.25±0.31 vs. 0.40±0.42, p=0.198) (Fig. 2C). Fig. 3 represents a scatter plot of the aBCR (MVVDT/eBC) change rate before and after treatment versus baseline aBCR (MVVDT/eBC), showing a moderate negative correlation (r =−0.578). Linear regression analysis was carried out using the following formula: Y=−1.69X+2.73 (Y=rate of change in aBCR after medication, X =baseline 1.25×MVVDT/eBC, r2=0.334, p<0.001).
Fig. 2. Differences in bladder capacity before and after treatment according to drug used. The aBCR uses (A) MVV or (B) MVVDT divided by eBC. (C) Changes in aBCRs obtained using both MVV and MVVDT before and after medication between two drugs. aBCR, age-adjusted bladder capacity ratio; MVV, maximal voided volume; MVVDT, maximal voided volume during daytime; eBC, expected bladder capacity by using age.
Fig. 3. Correlation between baseline aBCR (MVVDT/eBC) and change rate after treatment. aBCR, age-adjusted bladder capacity ratio; MVVDT, maximal voided volume during daytime; eBC, expected bladder capacity by using age.

Assessment of symptom relief revealed that incontinence improved in all patients in both treatment groups. Urgency and nocturnal enuresis also improved in both groups, but there was no significant difference in the response to medication between solifenacin 5 mg and mirabegron 50 mg (69.0% vs. 81.3%, p=0.491, and 63.2% vs. 87.5%, p=0.233) (Supplementary Table 2). Multivariate binary logistic regression analysis showed that decreased bladder capacity before treatment was associated with improvement in OAB symptoms after medication (odds ratio [OR], 7.41; 95% confidence interval [CI], 1.05–52.18; p=0.044) (Table 2).
Table 2. Results of multivariate binary logistic regression analysis for determining factors associated with improvement in OAB symptoms after medication.
| Variable | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Decreased bladder capacity | Yes vs. no | 7.41 | 1.05–52.18 | 0.044 |
| Medication | Mirabegron vs. solifenacin | 0.20 | 0.01–3.17 | 0.251 |
| Sex | Female vs. male | 0.41 | 0.08–2.13 | 0.287 |
| Age | Continuous variable | 0.82 | 0.45–1.51 | 0.523 |
| Duration of medication | Continuous variable | 0.99 | 0.99–1.01 | 0.385 |
| Duration of symptom | Continuous variable | 1.07 | 0.95–1.20 | 0.270 |
OAB, overactive bladder; OR, odds ratio; CI, confidence interval.
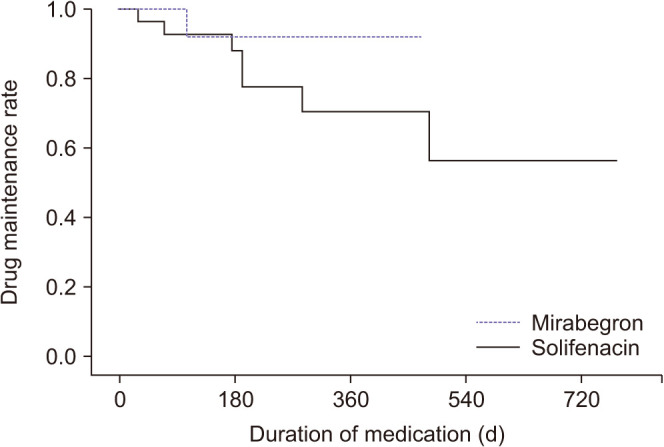
Drug-induced adverse effects were reported in only three patients (10.3%) in the solifenacin group. These three patients reported adverse effects of CTCAE grade 2 (abdominal cramps in 1, anorexia in 1, and nausea in 1). There were no cases of hypertension, hypotension, or voiding difficulty. The medication (mirabegron vs. solifenacin) did not make a significant difference in drug maintenance, which was 92.3% and 88.1% at 6 months after treatment, respectively (OR, 0.30; 95% CI, 0.04–2.48; p=0.263) (Fig. 4).
Fig. 4. Kaplan-Meier analysis of the relationship between drug maintenance and medications (solifenacin vs. mirabegron).
DISCUSSION
Antimuscarinic agents have been used as first-line treatment for idiopathic OAB; however, because of their short- and long-term adverse effects, new alternatives have been investigated [16]. Mirabegron, an alternative to antimuscarinics, has comparable efficacy with favorable tolerability [17]. It has, therefore, been recommended as a medication for adult idiopathic OAB, but more studies are needed to investigate its efficacy and tolerability in pediatric OAB patients [9,10]. This study had the strength that the effects of mirabegron and solifenacin were directly compared in pediatric patients, including patients administered only 5 mg solifenacin.
In this study, participants in the mirabegron group were older than those in the solifenacin group, and an important factor for drug selection in pediatric patients is whether they can swallow a tablet. Solifenacin can be prescribed as a powder; therefore, it can be administered to younger children who are unable to swallow a tablet. Mirabegron can only be prescribed to older children (around 9 years old), as a powdered formulation is not available [8,18]. Owing to the age difference between the two treatment groups, the factors affected by age (e.g., bladder capacity) were first adjusted and then compared. We adjusted for bladder capacity using the ratio of measured MVV to eBC, which is a widely used method for evaluating bladder volume [13].
Most children with OAB have nocturnal enuresis. In these patients, the first morning voided volume does not represent a full bladder owing to unconscious bladder emptying during sleep; therefore, their bladder capacity is predicted to be less than that of children without nocturnal enuresis [19]. In the present study, the aBCR (MVV/eBC) differed between patients with and without nocturnal enuresis (0.58±0.17 vs. 0.84±0.31, p=0.042), but the aBCR (MVVDT/eBC) did not (0.52±0.18 vs. 0.56±0.26, p=0.746) (Supplementary Table 3). In this respect, MVVDT (except for the first morning voided volume) can be used for more accurate evaluation when analyzing bladder capacity in patients with nocturnal enuresis.
Previous mirabegron studies in pediatric OAB evaluated only changes in symptoms [12,20] or in bladder capacity without considering patient age [11]. This study used age-adjusted bladder capacity and compared its change in pediatric OAB patients taking either solifenacin or mirabegron. Both drugs significantly increased the age-adjusted bladder capacity, 1.35 times and 1.70 times, which reduced the rate of decreased bladder capacity by 31% and 56%, respectively. This effect depended on bladder capacity before treatment; for instance, the lower the bladder capacity before medication, the better the treatment effect. Interestingly, as the pre-treatment bladder capacity approached the age-eBC, both solifenacin and mirabegron were ineffective, suggesting that these drugs have little effect in patients with normal bladder capacity. This study showed no significant difference in the ability of the two drugs to increase bladder capacity.
In addition to an increase in bladder capacity, improvement in OAB symptoms was also observed. This study showed that incontinence improved in all patients, and urgency and nocturnal enuresis also improved in 65% and 80% of cases, respectively. These effects were not significantly different between children administered solifenacin 5 mg and those administered mirabegron 50, which is in line with the results of a previous meta-analysis in adult OAB patients [8,10]. The results showed that OAB symptoms are more likely to improve if bladder capacity before treatment is already reduced, as these drugs act in patients with decreased bladder capacity. Similar results were found in a previous study based on urodynamic study. The study showed that lower bladder capacity at the first sensation to void correlated with better improvement in OAB symptoms after taking mirabegron, suggesting a correlation between bladder capacity before medication and improvement in OAB symptoms [21].
In the present study, the incidence of adverse effects was low in both treatment groups, but owing to the small sample size, this finding should be reviewed cautiously. In addition, only short-term adverse effects could be evaluated as the duration of drug administration was less than 6 to 8 months. In the case of solifenacin, most adverse effects were mild (CTCAE grade 2) gastrointestinal problems (e.g., nausea and vomiting). This finding is similar to that of a Korean prospective study, confirming the tolerability of solifenacin 5 mg in pediatric idiopathic OAB patients [18]. Patients taking mirabegron did not report any adverse effects. Despite these results, there was no significant difference in drug maintenance, possibly due to the mild adverse effects in the solifenacin group and the drug tolerability observed in both groups.
Limitations of the present study included its retrospective design and small sample size owing to the fact that in Korea solifenacin and mirabegron are used off-label for pediatric patients. Therefore, because only a few studies have investigated the effects of mirabegron in pediatric OAB patients, it is difficult to conduct prospective studies. In addition, we could not show improvement in OAB symptoms by using objective methods such as the patient's perception of bladder condition scale. Thus, the results were based on the degree of improvement described by parents and children, which could not be compared numerically. Third, we did not use an objective questionnaire to assess the tolerability of each medication; therefore, it is likely that adverse events were underreported owing to the lack of an adverse effect profile questionnaire. Finally, this study did not assess patient quality of life, which is seriously impacted by OAB [22].
CONCLUSIONS
Mirabegron has similar efficacy to solifenacin in pediatric patients with idiopathic OAB. In particular, OAB symptoms improved more effectively when the bladder capacity was already impaired before treatment. Additionally, mirabegron caused only a few adverse effects, suggesting it is a safe alternative for the treatment of pediatric patients with idiopathic OAB.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Seong Cheol Kim.
- Data acquisition: Myungchan Park, Chongsok Chae, Ji Hyung Yoon, and Sejun Park.
- Statistical analysis: Seong Cheol Kim.
- Data analysis and interpretation: Kyung Hyun Moon, Sang Hyeon Cheon, Taekmin Kwon, Seong Cheol Kim, and Sungchan Park.
- Drafting of the manuscript: Seong Cheol Kim.
- Critical revision of the manuscript: Seong Cheol Kim and Sungchan Park.
- Supervision: Seong Cheol Kim and Sungchan Park.
- Approval of the final manuscript: Seong Cheol Kim and Sungchan Park.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4111/icu.20200380.
The difference in bladder capacity between before and after medication according to drug useda
Subjective treatment outcomes reported by patients and/or parents
Difference in baseline aBCR according to nocturnal enuresis
References
- 1.Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. Prevalence and associated factors of overactive bladder in Korean children 5–13 years old: a nationwide multicenter study. Urology. 2009;73:63–67. doi: 10.1016/j.urology.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Franco I. Overactive bladder in children. Nat Rev Urol. 2016;13:520–532. doi: 10.1038/nrurol.2016.152. [DOI] [PubMed] [Google Scholar]
- 3.Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children's Continence Society. J Urol. 2014;191:1863–1865.e13. doi: 10.1016/j.juro.2014.01.110. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo L, Thorpe A, Warner J, Sidhu M. The cost-effectiveness of solifenacin vs fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release and tolterodine immediate-release in the treatment of patients with overactive bladder in the UK National Health Service. BJU Int. 2010;106:506–514. doi: 10.1111/j.1464-410X.2009.09160.x. [DOI] [PubMed] [Google Scholar]
- 5.Radmayr C, Bogaert G, Dogan HS, Kočvara R, Nijman JM, Stein R, et al. EAU guidelines on paediatric urology [Internet] Arnhem: European Association of Urology; 2019. [cited 2020 Mar 20]. Available from: http://uroweb.org/guideline/paediatric-urology/ [Google Scholar]
- 6.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–459. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 8.Andersson KE. Prospective pharmacologic therapies for the overactive bladder. Ther Adv Urol. 2009;1:71–83. doi: 10.1177/1756287209103937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65:755–765. doi: 10.1016/j.eururo.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Kelleher C, Hakimi Z, Zur R, Siddiqui E, Maman K, Aballéa S, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. 2018;74:324–333. doi: 10.1016/j.eururo.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Blais AS, Nadeau G, Moore K, Genois L, Bolduc S. Prospective pilot study of mirabegron in pediatric patients with overactive bladder. Eur Urol. 2016;70:9–13. doi: 10.1016/j.eururo.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Morin F, Blais AS, Nadeau G, Moore K, Genois L, Bolduc S. Dual therapy for refractory overactive bladder in children: a prospective open-label study. J Urol. 2017;197:1158–1163. doi: 10.1016/j.juro.2016.11.101. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Lee YS, Lee CN, Kim SH, Kim SW, Han SW. Efficacy and safety of mirabegron, a β3-adrenoceptor agonist, for treating neurogenic bladder in pediatric patients with spina bifida: a retrospective pilot study. World J Urol. 2019;37:1665–1670. doi: 10.1007/s00345-018-2576-0. [DOI] [PubMed] [Google Scholar]
- 14.Hjalmas K, Arnold T, Bower W, Caione P, Chiozza LM, von Gontard A, et al. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004;171(6 Pt 2):2545–2561. doi: 10.1097/01.ju.0000111504.85822.b2. [DOI] [PubMed] [Google Scholar]
- 15.Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006;176:314–324. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- 16.Kessler TM, Bachmann LM, Minder C, Löhrer D, Umbehr M, Schünemann HJ, et al. Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach. PLoS One. 2011;6:e16718. doi: 10.1371/journal.pone.0016718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, et al. Effect of (R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther. 2007;321:642–647. doi: 10.1124/jpet.106.115840. [DOI] [PubMed] [Google Scholar]
- 18.Lee SD, Chung JM, Kang DI, Ryu DS, Cho WY, Park S. Efficacy and tolerability of solifenacin 5 mg fixed dose in Korean children with newly diagnosed idiopathic overactive bladder: a multicenter prospective study. J Korean Med Sci. 2017;32:329–334. doi: 10.3346/jkms.2017.32.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho WY, Kim SC, Kim SO, Park S, Lee SD, Chung JM, et al. Can recording only the day-time voided volumes predict bladder capacity? Investig Clin Urol. 2018;59:194–199. doi: 10.4111/icu.2018.59.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fryer S, Nicoara C, Dobson E, Griffiths M, McAndrew HF, Kenny SE, et al. Effectiveness and tolerability of mirabegron in children with overactive bladder: a retrospective pilot study. J Pediatr Surg. 2020;55:316–318. doi: 10.1016/j.jpedsurg.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 21.Kopp Kallner H, Elmér C, Altman D. Urodynamics as a prognosticator of mirabegron treatment outcomes. Gynecol Obstet Invest. 2019;84:472–476. doi: 10.1159/000496606. [DOI] [PubMed] [Google Scholar]
- 22.Zinner N, Noe L, Rasouliyan L, Marshall T, Seifeldin R. Impact of solifenacin on resource utilization, work productivity and health utility in overactive bladder patients switching from tolterodine ER. Curr Med Res Opin. 2008;24:1583–1591. doi: 10.1185/03007990802081766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The difference in bladder capacity between before and after medication according to drug useda
Subjective treatment outcomes reported by patients and/or parents
Difference in baseline aBCR according to nocturnal enuresis




