Abstract
Purpose
Phosphodiesterase type 5 (PDE5) inhibitors are effective treatments for erectile dysfunction, and several recent studies have reported positive effects of PDE5 inhibitors on semen parameters as well. However, the data are still controversial. We investigated the effect of PDE5 inhibitors on sperm function by analyzing sperm motility and acrosome reaction.
Materials and Methods
This study included young healthy men who underwent fertility evaluation; 32 cases were finally included. Men were excluded if they used a PDE5 inhibitor within 2 weeks or if they had insufficient semen volume (≤2 mL), leukocytospermia, or a genitourinary infection. Changes in sperm motility and acrosome reaction were determined after in vitro exposure to the maximal semen concentration of oral intake of sildenafil (100 mg) or tadalafil (20 mg).
Results
Mean age of the participants was 35.4±4.9 years, mean sperm concentration was 68.7±32.4 ×106/mL, and mean sperm motility was 50.38%±8.41%. All three groups (control, sildenafil, tadalafil) experienced trends of decreased average sperm motility over time, but these changes were not significant. There were no significant differences between the three groups in the acrosome reaction after 120 minutes of drug exposure, either. The maximal semen concentration of oral intake of sildenafil (100 mg) or tadalafil (20 mg) did not substantially affect sperm motility or acrosome reaction.
Conclusions
Our results suggest that on-demand use of a PDE5 inhibitor is safe and useful for the male partner of an infertile couple; however, further studies are warranted for daily PDE5 inhibitor use.
Keywords: Erectile dysfunction, Infertility, Phosphodiesterase type 5 inhibitor, Spermatozoa
INTRODUCTION
Phosphodiesterase type 5 (PDE5) inhibitors, such as sildenafil and tadalafil, are effective treatments for male erectile dysfunction. Infertility is a major social issue in many industrialized countries and is associated with various types of stress that can lead to diminished sexual performance of the male partner [1]. Because of the easy accessibility of PDE5 inhibitors and the high incidence of male sexual problems in couples with fertility issues, males attempting to achieve pregnancy are increasingly using PDE5 inhibitors. Therefore, many studies have investigated the potential effects of PDE5 inhibitors on male fertility and semen parameters [2,3]. Several recent studies reported that PDE5 inhibitors improve semen parameters, especially sperm motility [4,5,6,7]. However, data on this issue are conflicting, and evidence of the underlying mechanism is incomplete [8]. Moreover, one study on sildenafil reported that this drug might adversely affect male fertility by inducing abnormal sperm acrosome reaction [9]. On the other hand, there is recent evidence for the presence of additional isoforms of PDEs in sperm cells and the male reproductive system [10]. Earlier researches indicated the presence of PDE1, PDE3, and PDE4 in human sperm cells [11,12]. A recent study reported that PDE11, the most recently discovered member of this family, is also highly expressed in the testis, prostate gland, and developing sperm cells, suggesting that the PDEs might have diverse physiologic roles in the male reproductive tract [13]. Another study reported that although sildenafil is a selective inhibitor of PDE5, it may also inhibit other PDE subtypes [14]. Therefore, a thorough assessment regarding the effects of PDE5 inhibitors on sperm cell function and male fertility is clinically important because both on-demand and daily usage of PDE5 inhibitors are popular these days. The objective of this study was to investigate the effects of PDE5 inhibitors on sperm cell function by analyzing sperm motility and acrosome reaction in an effort to provide guidance on use of these drugs by the male partners of infertile couples seeking pregnancy.
MATERIALS AND METHODS
1. Study subjects and sample selection
This study included healthy male participants who visited a single center for fertility evaluation from January 2018 to July 2018 (Table 1). The study was approved by Institutional Review Board of the CHA Gangnam Medical Center (approval number: GCI-18-37).
Table 1. Clinical characteristics of the patients and initial semen parameters (n=32).
| Patient characteristic | Value |
|---|---|
| Age (y) | 35.4±4.9 |
| Serum FSH (mIU/mL) | 4.4±1.8 |
| Serum testosterone (ng/mL) | 4.3±1.3 |
| Rt. testis volume (mL) | 17.6±1.9 |
| Lt. testis volume (mL) | 17.5±1.9 |
| Semen pH | 7.6±0.2 |
| Semen volume (mL) | 3.3±0.9 |
| Sperm concentration (×106/mL) | 68.7±32.4 |
| Sperm total motility (%) | 50.38±8.41 |
| Sperm progressive motility (%) | 47.09±9.31 |
| Grade A sperm motility (%) | 15.6±9.8 |
| Sperm vitality (%) | 71.6±7.2 |
| Strict sperm morphology (%) | 2.9±1.4 |
Values are presented as mean±standard deviation.
FSH, follicle-stimulating hormone; Rt., right; Lt., left.
Semen samples were obtained from participants who agreed to participate and signed the informed consent documents. All semen samples were obtained by masturbation into a wide-mouthed plastic container after at least 3 days of sexual abstinence. Samples were allowed to liquefy for at least 20 minutes at 37℃ before analysis. Immediately after liquefaction, diagnostic semen analysis was performed according to the fifth edition of the World Health Organization (WHO) manual [15]. Sperm concentration and motility were assessed by using a Makler counting chamber, and sperm vitality was evaluated by using eosin-nigrosin staining. Sperm morphology was evaluated by using Papanicolaou staining according to strict criteria.
Thirty-two young healthy men who met the eligibility criteria were finally included. Target sample size was calculated by using the G-power program (version 3.1.9.4) based on the prior studies [9,16]. We included only healthy men who had normal sperm concentration and motility for this study; sperm morphology was not considered because of prior reported variations among laboratories. Men were excluded if they had used a PDE5 inhibitor within 2 weeks or if they had insufficient semen volume (≤2 mL), leukocytospermia, or a genitourinary infection. In addition, serum reproductive hormones (follicle-stimulating hormone [FSH] and testosterone) were measured by using an electrochemiluminescence immunoassay analyzer (Roche Cobas E 601; Hitachi, Tokyo, Japan), and testis volume was measured by an experienced andrologist using an orchidometer.
Sperm motility and acrosome reaction were evaluated with fresh ejaculated semen samples in vitro. After initial diagnostic semen analysis, the residual semen sample was divided into three equal volumes (0.5 mL each), and each volume was transferred into a 5-mL polystyrene round-bottom tube (Falcon; Corning Science, Tamaulipas, Mexico). One sample was a control, one was added to a 1 μg/mL sildenafil solution (Cerilliant, Round Rock, TX, USA), and the other was added to a 0.1 μg/mL tadalafil solution (Cerilliant). The final concentration was equivalent to the maximal semen concentration after single oral intake of sildenafil (100 mg) or tadalafil (20 mg), which were determined according to the manufacturer's information guide (http://labeling.pfizer.com/ShowLabeling.aspx?id=652#section-11.3, https://uspl.lilly.com/cialis/cialis.html#section-11.3).
2. Assessment of sperm motility by computer-assisted semen analysis
Sperm motility was measured by using computer-assisted semen analysis (CASA; Sperm Class Analyzer; version evolution 6.0.0.1; Microptic, Barcelona, Spain) according to the WHO classification. An external quality-control service (SCA Maintenance; Microptic, Barcelona, Spain) was performed for this study. Sperm motility was classified into 4 groups. (grade A: fast progressive, grade B: slow progressive, grade C: nonprogressive, grade D: immotile). The CASA settings were according to the manufacturer's instructions. Each reported measurement was an average of five different captured fields. Total motility (A+B+C), progressive motility (A+B), and grade A sperm after in vitro exposure to a PDE5 inhibitor for 0, 30, 60, and 120 minutes were measured.
3. Evaluation of sperm acrosome reaction by fluorescein isothiocyanate (FITC)-peanut agglutinin (PNA) staining
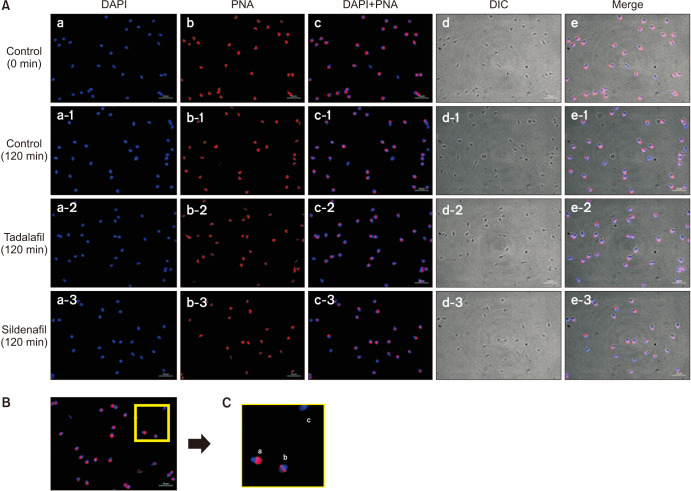
Semen samples were washed twice using Dulbecco's phosphate-buffered saline (GE Lifesciences, Salt Lake City, UT, USA) and then centrifuged for 5 minutes at 1,500 rpm. The suspended pellets were smeared on a slide coat (Histobond; Paul Marienfeld GmbH & Co., Lauda-Königshofen, Germany), and the prepared slides were then stained with peanut agglutinin (1:200; lectin PNA Alexa Fluor 568 conjugate; Invitrogen, Carlsbad, CA, USA) for 15 minutes. Then, the slides were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:1,000; Invitrogen) and allowed to react for 10 minutes in the dark at room temperature. Acrosome reaction was evaluated by using a fluorescence microscope (AXIO Imager A2; Zeiss, Göttingen, Germany) and classified as previously described: (1) acrosome intact (whole acrosome fluorescence labeling); (2) partially acrosome reacted (patchy acrosome fluorescence labeling); and (3) acrosome reacted (no fluorescence or equatorial segment only labeling) (Fig. 1) [9]. At least 200 sperm cells were analyzed per slide.
Fig. 1. (A) DAPI- and PNA-labeled sperm images and merged images of DAPI and PNA staining (×1,000). (B) All sperm were stained with DAPI for the nucleus and PNA for the acrosome. (C) Stained sperms were divided into the following groups according to the degree of PNA staining: (a) intact acrosome, (b) partially reacted acrosome, and (c) reacted acrosome.
4. Statistical analyses
Statistical analysis was performed with R software version 3.6.2. Data were expressed as means±standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare the three groups. A repeated-measures ANOVA was used to evaluate changes in sperm motility according to time of drug exposure. A p-value less than 0.05 was considered significant.
RESULTS
Fifty-six patients underwent initial screening tests, and 32 of them met the eligibility criteria and were finally enrolled (Table 1). Mean age was 35.4±4.9 years. Mean sperm concentration was 68.7±32.4 ×106/mL and mean sperm motility was 50.38%±8.41%. Mean serum FSH level was 4.4±1.8 mIU/mL and mean serum testosterone level was 4.3±1.3 ng/mL. Mean right testicular volume was 17.6±1.9 mL and mean left testicular volume was 17.5±1.9 mL.
1. Effect of PDE5 inhibitors on sperm motility
We measured sperm motility at 0, 30, 60, and 120 minutes after in vitro exposure to a PDE5 inhibitor. Initially (time=0 minute), the progressive motility (A+B) was 47.09%±9.31%, and the grade A motility was 15.60%±9.82%. There were trends of decreased average motility in all groups over time, but these differences were not significant. Grade A motility at 120 minutes was 12.97%±9.29% in the control group, 14.82%±10.93% in the tadalafil group, and 14.57%±8.96% in the sildenafil group (p=0.950) (Table 2). The decline in sperm motility appeared smaller in groups exposed to the PDE5 inhibitor than in the control group, but a repeated-measures ANOVA indicated no significant differences (Fig. 2).
Table 2. Changes in total sperm motility, progressive motility, and grade A sperm motility.
| Variable | 0 min | 30 min | 60 min | 120 min | |
|---|---|---|---|---|---|
| Total sperm motility (%) | Control | 50.38±8.41 | 47.00±9.75 | 46.22±9.42 | 44.16±10.18 |
| Tadalafil | 50.38±8.41 | 48.22±9.23 | 46.97±8.98 | 45.56±9.56 | |
| Sildenafil | 50.38±8.41 | 48.69±9.09 | 47.81±8.59 | 46.38±8.82 | |
| p-value | 0.778 | 0.643 | 0.759 | ||
| Progressive motility (%) | Control | 47.09±9.31 | 43.91±10.43 | 44.31±12.42 | 41.06±10.9 |
| Tadalafil | 47.09±9.31 | 45.38±9.61 | 44.06±9.18 | 42.59±10.31 | |
| Sildenafil | 47.09±9.31 | 45.53±9.83 | 44.50±9.11 | 42.72±10.04 | |
| p-value | 0.986 | 0.779 | 0.772 | ||
| Grade A sperm motility (%) | Control | 15.60±9.82 | 13.83±9.11 | 13.93±10.61 | 12.97±9.29 |
| Tadalafil | 15.60±9.82 | 13.10±9.23 | 15.61±10.98 | 14.82±10.93 | |
| Sildenafil | 15.60±9.82 | 13.58±9.27 | 14.66±10.09 | 14.57±8.96 | |
| p-value | 0.815 | 0.714 | 0.950 |
Values are presented as mean±standard deviation.
Fig. 2. Evaluation of sperm motility with time. The decline in sperm motility appeared smaller in groups that received the phosphodiesterase type 5 (PDE5) inhibitor than in the control group, but a repeatedmeasures ANOVA indicated no significant differences (p=0.312).

2. Effects of PDE5 inhibitors on acrosome reaction
Sperm acrosome status was evaluated at 0 and 120 minutes using FITC-FNA staining. The acrosome reaction status did not differ significantly between groups after 120 minutes of exposure to the maximal semen concentration of sildenafil and tadalafil solution (Fig. 3).
Fig. 3. Comparison of sperm acrosome reaction status. Sperm acrosome reaction status after 2 hours of exposure to the maximal semen concentration of sildenafil and tadalafil solution did not differ significantly between the groups.

DISCUSSION
We found that in vitro exposure to the maximal semen concentration of single oral intake of sildenafil (100 mg) or tadalafil (20 mg) up to 120 minutes had no significant effect on sperm motility or acrosome reaction. These results differ from recent studies that reported that these drugs improve semen parameters [4,5,6,7]. Our data suggest that a PDE5 inhibitor could be a safe and useful drug for the male partner of infertile couples seeking pregnancy. Diminished sexual function is common in male partners of infertile couples, and use of PDE5 inhibitors by men of reproductive age is common as well [17]. Infertile couples reasonably and commonly ask about the potential risks of using any drug, including PDE5 inhibitors, while attempting to conceive. Since the initial introduction of sildenafil in 1998, early studies have mainly focused on the safety of PDE5 inhibitors, and most studies indicated no definite adverse effects on semen parameters or sperm function [18,19,20]. However, the detailed effects of PDE5 inhibitors on sperm cells and the fertilization process have not been studied thoroughly, partly because most studies focused on sexual satisfaction in elderly men with erectile dysfunction. Our findings are consistent with initial studies regarding the influence of PDE5 inhibitors on the semen parameters and their safety. In addition, our data showed that the extent of motility decrease tended to be smaller in the PDE5 inhibitor exposed groups than in the control group, which suggests that PDE5 inhibitors may prevent the deterioration of sperm motility (Fig. 2).
Various in vivo and in vitro studies have reported improvements in semen parameters and sperm function after treatment with a PDE5 inhibitor. One study suggested that in vitro exposure to a sildenafil citrate solution has a concentration-related stimulatory effect on ejaculated sperm motility [4]. However, other in vitro studies have reported conflicting effects of PDE5 inhibitors on sperm motility and acrosome reaction [9,16]. Several in vivo studies also showed that oral administration of a PDE5 inhibitor improves sperm motility [5,6]. A recent meta-analysis concluded that an oral PDE5 inhibitor can increase sperm motility and improve sperm morphology in infertile men [21]. Those authors further suggested that chronic administration of a PDE5 inhibitor may yield better effects than on-demand use. Researchers have suggested that the effect of PDE5 inhibitors on sperm motility could be explained by alterations in the cGMP, cAMP-protein kinase A pathway or a calcium signaling pathway [8,10]. However, the underlying mechansim of sperm motility change has not been sufficiently elucidated. One study suggested that PDE5 inhibitors can reduce ejaculation-associated stress, resulting in an ejaculation with higher sexual satisfaction and therefore an increased number of high-quality spermatozoa [22].
Appropriate sperm acrosome reaction is another important element for normal fertilization that occurs in the sperm head as it approaches a female ovum. If acrosome reaction occurs at an abnormal rate, fertilization capacity may be compromised. A previous study suggested that PDE5 inhibitors might reduce male fertility by causing abnormal premature sperm acrosome reaction [9]. These authors found that sildenafil caused significant increase in the proportion of acrosome-reacted sperm, and suggested that this drug could adversely affect male fertility. However, another study reported no significant difference in the premature acrosome reaction rate after on-demand administration of sildenafil or tadalafil [16]. Our study showed that the maximal semen concentration of oral intake of sildenafil (100 mg) or tadalafil (20 mg) did not negatively affect the sperm acrosome reaction, thus ensuring the safety of this drug for males of infertile couples seeking pregnancy.
Recent basic researches on the PDE family have indicated the existence of more isoforms of PDE in human sperm cells and in male reproductive organs [11,12,13]. These findings could bear important clinical implications, because daily usage of PDE5 inhibitors is also popular these days. With daily usage, the PDE5 inhibitor would be present in the plasma and other parts of the male reproductive tract, such as the epididymis, during the whole spermatogenesis period. Sperm cells are reportedly more vulnerable to damage by reactive oxygen species during the prolonged period when they pass through the epididymis [23]. Previously, two pharmaceutical industry-sponsored studies reported that daily intake of PDE5 inhibitors for 6 to 9 months had no detrimental effects on semen characteristics or the serum levels of reproductive hormones [3,24]. However, these studies did not evaluate sperm function, such as acrosome reaction. Other researchers also expressed concerns about the potential effects of long-term exposure to PDE inhibitors on other PDE family members, spermatogenesis and the male reproductive system [13]. But in the case of on-demand usage of PDE5 inhibitors, the exposure of sperm cells or the male reproductive system to PDE5 inhibitors would be short, thereby minimizing the likelihood of adverse effects.
A few limitations of our study, including the relatively small number of cases, must be addressed. First, we evaluated the effect of PDE5 inhibitors with regard to sperm motility and acrosome reaction. Evaluation of more functional parameters would be needed to further demonstrate a potential effect. With the limited volume of the semen sample from each individual, we chose to study two important functional parameters of the sperm cell for fertilization (sperm motility and acrosome reaction). In addition, we used the maximal semen concentration of oral intake of sildenafil (100 mg) or tadalafil (20 mg), for which prior studies have reported conflicting results [4,9,16]. We think that potential technical issues and publication bias might be in part accountable for the conflicting results from prior studies. Second, the CASA system is well known to bear several inherent limitations, such as the inability to discriminate sperm cells from non-sperm cells and seminal debris. To minimize these errors, we excluded patients with leukocytospermia or genital infection and two experienced reproductive biologists rechecked the CASA data manually.
Appropriate counseling would be helpful for infertile couples seeking pregnancy when the male partner has erectile dysfunction. Further basic research on this topic is also warranted.
CONCLUSIONS
Our study showed that the maximal semen concentration of oral intake of sildenafil (100 mg) or tadalafil (20 mg) did not substantially affect sperm motility or sperm acrosome reaction. These results suggest that on-demand use of a PDE5 inhibitor is safe and useful for the male partner of an infertile couple seeking pregnancy. However, exercising prudence might be recommended when using a PDE5 inhibitor on a daily basis.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B508379413). The sponsor had no role in the design and conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Seung-Hun Song and Dong Suk Kim.
- Data acquisition: Mi Hee Oh, Jung Won Baek, Suye Sung, and Jin Hee Eum.
- Statistical analysis: Dong Hyuk Shin and Young Sun Her.
- Data analysis and interpretation: Seung-Hun Song.
- Drafting of the manuscript: Seung-Hun Song.
- Critical revision of the manuscript: Seung-Hun Song.
- Obtaining funding: Seung-Hun Song.
- Administrative, technical, or material support: Yohan Heo.
- Supervision: Dong Suk Kim.
- Approval of the final manuscript: Seung-Hun Song.
References
- 1.O'Brien JH, Lazarou S, Deane L, Jarvi K, Zini A. Erectile dysfunction and andropause symptoms in infertile men. J Urol. 2005;174:1932–1934. doi: 10.1097/01.ju.0000177453.14334.a2. discussion 1934. [DOI] [PubMed] [Google Scholar]
- 2.Corvasce A, Albino G, Leonetti T, Buonomo AF, Marucco EC. Once-a-day Tadalafil administration improves the spermogram parameters in fertile patients. Arch Ital Urol Androl. 2015;87:210–213. doi: 10.4081/aiua.2015.3.210. [DOI] [PubMed] [Google Scholar]
- 3.Jarvi K, Dula E, Drehobl M, Pryor J, Shapiro J, Seger M. Daily vardenafil for 6 months has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels. J Urol. 2008;179:1060–1065. doi: 10.1016/j.juro.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 4.Mostafa T. In vitro sildenafil citrate use as a sperm motility stimulant. Fertil Steril. 2007;88:994–996. doi: 10.1016/j.fertnstert.2006.11.182. [DOI] [PubMed] [Google Scholar]
- 5.Rago R, Salacone P, Caponecchia L, Marcucci I, Fiori C, Sebastianelli A. Effect of vardenafil on semen parameters in infertile men: a pilot study evaluating short-term treatment. J Endocrinol Invest. 2012;35:897–900. doi: 10.3275/8368. [DOI] [PubMed] [Google Scholar]
- 6.Pomara G, Morelli G, Canale D, Turchi P, Caglieresi C, Moschini C, et al. Alterations in sperm motility after acute oral administration of sildenafil or tadalafil in young, infertile men. Fertil Steril. 2007;88:860–865. doi: 10.1016/j.fertnstert.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: a randomized trial. BJU Int. 2010;106:1181–1185. doi: 10.1111/j.1464-410X.2010.09243.x. [DOI] [PubMed] [Google Scholar]
- 8.Mostafa T. Oral phosphodiesterase-5 inhibitors and sperm functions. Int J Impot Res. 2008;20:530–536. doi: 10.1038/ijir.2008.29. [DOI] [PubMed] [Google Scholar]
- 9.Glenn DR, McVicar CM, McClure N, Lewis SE. Sildenafil citrate improves sperm motility but causes a premature acrosome reaction in vitro. Fertil Steril. 2007;87:1064–1070. doi: 10.1016/j.fertnstert.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Dimitriadis F, Giannakis D, Pardalidis N, Zikopoulos K, Paraskevaidis E, Giotitsas N, et al. Effects of phosphodiesterase-5 inhibitors on sperm parameters and fertilizing capacity. Asian J Androl. 2008;10:115–133. doi: 10.1111/j.1745-7262.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- 11.Fisch JD, Behr B, Conti M. Enhancement of motility and acrosome reaction in human spermatozoa: differential activation by type-specific phosphodiesterase inhibitors. Hum Reprod. 1998;13:1248–1254. doi: 10.1093/humrep/13.5.1248. [DOI] [PubMed] [Google Scholar]
- 12.Lefièvre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod. 2002;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- 13.Wayman C, Phillips S, Lunny C, Webb T, Fawcett L, Baxendale R, et al. Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. Int J Impot Res. 2005;17:216–223. doi: 10.1038/sj.ijir.3901307. [DOI] [PubMed] [Google Scholar]
- 14.Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10:69–73. doi: 10.1038/sj.ijir.3900354. discussion 73–4. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 16.Yang Y, Ma Y, Yang H, Jin Y, Hu K, Wang HX, et al. Effect of acute tadalafil on sperm motility and acrosome reaction: in vitro and in vivo studies. Andrologia. 2014;46:417–422. doi: 10.1111/and.12097. [DOI] [PubMed] [Google Scholar]
- 17.Jannini EA, Lombardo F, Salacone P, Gandini L, Lenzi A. Treatment of sexual dysfunctions secondary to male infertility with sildenafil citrate. Fertil Steril. 2004;81:705–707. doi: 10.1016/j.fertnstert.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Purvis K, Muirhead GJ, Harness JA. The effects of sildenafil on human sperm function in healthy volunteers. Br J Clin Pharmacol. 2002;53(Suppl 1):53S–60S. doi: 10.1046/j.0306-5251.2001.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger M, Sikka SC, Bivalacqua TJ, Lamb DJ, Hellstrom WJ. The effect of sildenafil on human sperm motion and function from normal and infertile men. Int J Impot Res. 2000;12:229–234. doi: 10.1038/sj.ijir.3900551. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom WJ, Overstreet JW, Yu A, Saikali K, Shen W, Beasley CM, Jr, et al. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J Urol. 2003;170:887–891. doi: 10.1097/01.ju.0000081053.97792.da. [DOI] [PubMed] [Google Scholar]
- 21.Tan P, Liu L, Wei S, Tang Z, Yang L, Wei Q. The effect of oral phosphodiesterase-5 inhibitors on sperm parameters: a meta-analysis and systematic review. Urology. 2017;105:54–61. doi: 10.1016/j.urology.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Sofikitis NV, Miyagawa I. Endocrinological, biophysical, and biochemical parameters of semen collected via masturbation versus sexual intercourse. J Androl. 1993;14:366–373. [PubMed] [Google Scholar]
- 23.Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, Azzari C, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21:109–122. doi: 10.2119/molmed.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellstrom WJ, Gittelman M, Jarow J, Steidle C, McMurray J, Talley D, et al. An evaluation of semen characteristics in men 45 years of age or older after daily dosing with tadalafil 20mg: results of a multicenter, randomized, double-blind, placebo-controlled, 9-month study. Eur Urol. 2008;53:1058–1065. doi: 10.1016/j.eururo.2007.09.046. [DOI] [PubMed] [Google Scholar]