Abstract
Purpose
Partial nephrectomy is associated with a 1%–2% risk of renal iatrogenic vascular lesion (IVL) that are commonly treated with selective angioembolization (SAE). The theoretical advantage of SAE is preservation of renal parenchyma by targeting only the bleeding portion of the kidney. Our study aims to assess the long-term effect of SAE on renal function, especially that this intervention requires potentially nephrotoxic contrast load injection.
Materials and Methods
A retrospective review of patients undergoing partial nephrectomy between 2002 and 2018 was performed, and patients who developed IVL were identified. A 1:4 matched case-control analysis was performed. Paired t-test and χ2 test were used for continuous and categorical variables, respectively. Multivariable logistic and Cox proportional hazards regression analyses were used to identify risk factors and confounders for SAE and postoperative renal function.
Results
Eighteen patients found to have an IVL after partial nephrectomy were matched with 72 control patients. IVL's were more common in patients after minimally invasive partial nephrectomy (89% vs. 70%, p=0.008) and in those with higher RENAL nephrometry scores (8.8±2.0 vs. 6.5±1.8, p<0.001). On multivariable analysis, lower RENAL scores proved to decrease the odds of requiring postoperative SAE. No significant difference in renal function outcomes was seen at 24 months of follow-up after surgery.
Conclusions
SAE for the management of IVL following partial nephrectomy is a safe and efficient procedure with no significant impact on short or long-term renal function. Less complex renal tumors with lower RENAL scores are less likely to require postoperative SAE.
Keywords: Embolization, Kidney function tests, Nephrectomy, Postoperative period
INTRODUCTION
The incidence of renal cell carcinoma is approximately 60,000 cases per year [1] with partial nephrectomy (PN) being the most common surgical approach (52%) for the treatment of T1a renal tumors (<4 cm), followed by radical nephrectomy (27%), active surveillance (20%), and ablative interventions [2]. PN, when applicable, offers decreased risk of chronic kidney disease by preserving functional nephrons, as well as decreased risk of cardiovascular morbidity, while maintaining comparable oncologic control [3,4].
Whether through an open or minimally invasive approach, one of the most frequent complications following PN is an iatrogenic vascular lesion (IVL) leading to post-operative bleeding. IVLs include both renal artery pseudoaneurysms and arteriovenous fistulae (AVF), both of which may lead to life threatening hemorrhage [5,6,7]. A standardized approach to their management is not specifically defined, but rather clinician dependent. While observation of IVLs is possible, given the propensity of such lesions to progress to life threatening hemorrhage, most physicians prefer to intervene.
The most commonly utilized treatment for IVLs is selective percutaneous angioembolization (SAE), as reoperation for bleeding after PN often results in loss of the kidney. The theoretical advantage of SAE is to preserve renal function by maintaining normal blood flow to the uninvolved portion of the kidney [8]. In addition, this technique requires intravenous injection of iodinated contrast for visualization of the IVL or an actively bleeding vessel. To note, iodinated contrast can compromise renal function through several mechanisms like hypo-perfusion, direct cytotoxic effects on endothelial and tubular epithelial cells, and constriction of descending vasa recta through reduction of Nitric Oxide levels [9,10,11,12]. This study aims to assess the short and the two-year effect of SAE on renal function.
MATERIALS AND METHODS
After Institutional Review Board of the University of Chicago approval (approval number: 18-1435), we performed a retrospective review of a prospectively maintained database of patients undergoing partial nephrectomy between January 2002 and December 2018. Patients with post-operative bleeding at computed tomographic angiogram (CTA) and/or conventional angiography requiring SAE, were identified and matched to a control group. Informed consent was waived by the study protocol due to the lack of patient contact. Presence of IVL was defined by the detection of a renal pseudo-aneurysm or AVF. Each case was matched to four controls based on age, sex, preoperative comorbidities, tumor number, location and size, nephrometry score, and renal function. Demographics, perioperative data, postoperative outcomes (POD) at 90 days, 12 and 24 months of follow-up were collected and compared between groups. Glomerular filtration rate (GFR) was estimated using the chronic kidney disease epidemiology collaboration equation [13]. Exclusion criteria include immunosuppression, preoperative estimated glomerular filtration rate (eGFR) less than 15 mL/min, uninephric patients (including kidney transplant recipients) and those with bilateral cystic kidney disease.
Partial nephrectomy was performed via either open or minimally invasive approach. Patients with suspected postoperative bleeding who underwent CTA received 120 mL of Iohexol (Omnipaque 350 mg/mL), a water-soluble radiographic contrast agent, intravenously. If a source of bleeding was identified, patients subsequently underwent conventional angiogram and SAE. Another larger subset of patients, however, proceeded directly to conventional angiogram with immediate SAE if confirmed positive. Procedural technique for SAE entailed obtaining percutaneous access via the common femoral artery. Once a catheter was placed into the ipsilateral renal artery, selective angiogram was performed, the IVL was identified, and embolization was performed. Embolization using coils, Gelfoam, and/or particles was performed depending on the size of the renal branch, level of catheterization, and/or extent of the IVL. Technical success was defined as angiographic arrest of bleeding and/or IVL resolution.
Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Build 1.0.0.1461 64-bit edition (released 2020; IBM Co., Armonk, NY, USA). A 1:4 case-control pairing was performed to match cases and controls based on the aforementioned preoperative variables. Continuous data were reported as mean±standard deviation. Postoperative success rates were calculated as the proportion of events among the number of patients available for follow-up, and 95% confidence intervals were then calculated for a single proportion. F- and D'Agostino-Pearson tests were performed to reject equal variance and normal distribution, respectively. Paired sample t-test, after logarithmic transformation, or Welch-test were used to compare continuous variables from independent samples. χ2 test was used to analyze data collected on non-continuous variables. Fisher's exact test was utilized for categorical variables with fewer than ten events. Multivariable logistic and Cox proportional hazards regression analyses were used to identify risk factors and confounders for SAE and postoperative renal function. A p-value <0.05 was considered statistically significant.
RESULTS
A total of 685 nephron sparing surgeries were performed during the study period: 598 (87%) minimally invasive (MIPN) and 87 (13%) open (OPN). Postoperative bleeding was suspected in 23 (3.8%) patients. Among those, 4 (17.3%) patients underwent CTA followed by angiography and SAE. Nineteen (82.7%) patients proceeded directly to percutaneous conventional angiography. A total of 18 patients (2.6%) were found to have an IVL or arterial contrast extravasation that required intervention. These cases were matched with 72 control patients. No significant differences in age, sex, race, body mass index, smoking status, and Charlson comorbidity index were observed (Table 1). The impact of ischemia time on long term renal function was standardized by matching both groups as well. No significant differences were seen in preexisting comorbidities, such as diabetes mellitus and hypertension. Mean mass diameter was comparable among groups (4.4±2.0 cm vs. 3.5±1.6 cm, p=0.081), but patients with IVL had significantly higher RENAL scores (8.8±2.0 vs. 6.5±1.8, p<0.001) (Table 2). The median size of IVL was 1 cm (interquartile range 0.75–1). Regarding surgical technique, a higher proportion of cases had undergone MIPN compared to controls (89% vs. 70%, p=0.008) (Table 2). Hemostatic agents were less often used in patients who ultimately bled and required SAE compared to the control group (56% vs. 89%, p<0.001) (Table 2). On multivariable logistic regression analysis, only lower RENAL scores proved to be protective as they decrease the odds of requiring postoperative SAE (OR 1.72, p<0.001) (Table 3). Surgical technique, the number of resected masses, and the volume of intraoperative blood loss did not influence the need for SAE (p>0.05).
Table 1. Demographic and perioperative data of study and control groups.
| Variable | SAE | Control | p-value | |
|---|---|---|---|---|
| Age (y) | 59.1±13.6 | 62.8±12 | 0.7 | |
| Sex | Male | 13 (72.2) | 46 (63.9) | 0.6 |
| Female | 5 (27.8) | 26 (36.1) | ||
| Race | Caucasian | 13 (72.2) | 49 (68.1) | 0.3 |
| African American | 4 (22.2) | 20 (27.8) | ||
| Asian | 1 (5.6) | 0 (0.0) | ||
| Other/unknown | 0 (0.0) | 3 (4.1) | ||
| Body mass index (kg/m2) | 30.6±8.7 | 30.4±8.5 | 0.9 | |
| Preoperative eGFR (mL/min) | Average | 73.9±19.9 | 70.1±25.5 | 0.2 |
| >90 | 4 (22.2) | 14 (19.4) | 0.1 | |
| 60–89 | 11 (61.1) | 27 (37.5) | ||
| 30–59 | 2 (11.1) | 29 (40.3) | ||
| 15–29 | 1 (5.6) | 2 (2.8) | ||
| <15 | 0 (0.0) | 0 (0.0) | ||
| Charlson comorbidity index | Average | 4.4±2.6 | 5.0±2.0 | 0.4 |
| ≥4 | 10 (55.6) | 49 (68.1) | 0.3 | |
| Patients with DM | 4 (22.2) | 20 (27.7) | 0.8 | |
| Patients with HTN | 12 (66.7) | 49 (68.1) | 0.6 | |
| Smoking | No | 11 (61.1) | 36 (50.0) | 0.5 |
| Current | 1 (5.6) | 12 (16.7) | ||
| Former | 5 (27.8) | 22 (30.6) | ||
| Unknown | 1 (5.6) | 2 (2.7) | ||
| CL kidney present | 17 (94.4) | 68 (94.4) | 0.9 | |
| Any anticoagulation | 9 (50.0) | 25 (34.7) | 0.2 | |
Values are presented as mean±standard deviation or number (%).
SAE, selective angioembolization; eGFR, estimated glomerular filtration rate; DM, diabetes mellitus; HTN, hypertension; CL, contralateral kidney.
Table 2. Tumor and procedure-specific data of study and control groups.
| Variable | SAE | Control | p-value | |
|---|---|---|---|---|
| Mass diameter (cm) | 4.4±2 | 3.5±1.6 | 0.081 | |
| Masses removed | 1.4±1 | 1.1±0.2 | 0.220 | |
| Radius (cm) | <4 | 8 (44.4) | 49 (68) | 0.211 |
| 4–7 | 9 (50) | 21 (29.2) | ||
| >7 | 1 (5.6) | 2 (2.8) | ||
| Total RENAL points | 8.8±2 | 6.5±1.8 | <0.001 | |
| Surgical approach | Open surgery | 2 (11.1) | 22 (30.5) | 0.008 |
| Laparoscopic surgery | 4 (22.2) | 30 (41.7) | ||
| Robotic surgery | 12 (66.7) | 20 (27.8) | ||
| EBL (mL) | 110±79 | 199±185 | 0.075 | |
| OR time (min) | 190±21 | 219±58 | 0.065 | |
| Ischemia time (min) | Warm | 27.7±6.6 | 28.4±11 | 0.745 |
| Cold | 21.5±4.9 | 21.7±8.4 | 0.835 | |
| Hemostatic agent | Yes | 10 (56) | 64 (89) | <0.001 |
| No | 8 (44) | 8 (11) | ||
Values are presented as mean±standard deviation or number (%).
SAE, selective angioembolization; EBL, estimated blood loss; OR, operating room.
Table 3. Multivariable logistic regression analysis showing the odds of postoperative SAE based on RENAL score, EBL, number of mass removed, surgical approach, and ischemia time.
| Variable | RR | 95% | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| RENAL score | 1.72 | 1.28 | 2.29 | <0.001 |
| EBL (mL) | 0.99 | 0.98 | 1.00 | 0.122 |
| Masses removed (n) | 2.04 | 0.58 | 7.14 | 0.235 |
| Surgical technique (LS vs. OS) | 2.76 | 0.39 | 19.47 | 0.333 |
| Surgical technique (RS vs. OS) | 2.27 | 0.34 | 14.89 | 0.425 |
| Ischemia time (min) | 0.99 | 0.92 | 1.08 | 0.915 |
SAE, selective angioembolization; OR, odds ratio; CI, confidence interval; EBL, estimated blood loss; OS, open surgery; LS, laparoscopic surgery; RS, robotic surgery.
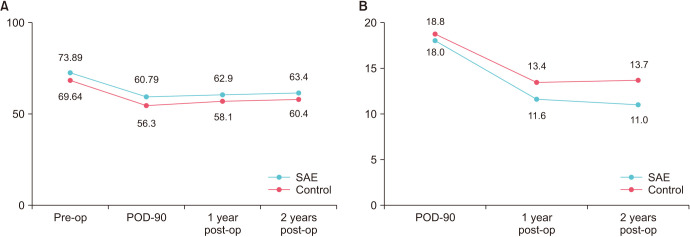
Mean preoperative GFR was comparable among groups (73.9 mL/min [19.9] vs. 70.1 mL/min [25.5], p>0.05). No significant differences in renal function were seen among the cases and controls at POD 90, 12, and 24 months after surgery (p>0.05) (Fig. 1). Cox proportional hazards regression analysis revealed that SAE, surgical technique, and minor differences in baseline preoperative eGFR do not significantly impact renal function after nephron sparing surgery (p>0.05) (Table 4).
Fig. 1. (A) Change in estimated GFR as a function of time in the SAE vs. control group. (B) Percentage change in eGFR over time in the SAE vs. control group. GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate; SAE, selective angioembolization; POD, postoperative day; Pre-op, pre-operative; Post-op, post-operative.
Table 4. Cox proportional hazards regression analysis investigating the effect of several variables (SAE, baseline eGFR, and surgical technique) on renal function over time.
| Variable | RR | 95% | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| SAE | 2.05 | 0.95 | 4.55 | 0.072 |
| Baseline eGFR | 0.99 | 0.98 | 1.01 | 0.440 |
| Surgical technique (LS vs. OS) | 1.09 | 0.47 | 2.51 | 0.815 |
| Surgical technique (RS vs. OS) | 2.32 | 0.93 | 5.77 | 0.072 |
SAE, selective angioembolization; eGFR, estimated glomerular filtration rate; RR, risk ratio; OR, odds ratio; CI, confidence interval; OS, open surgery; LS, laparoscopic surgery; RS, robotic surgery.
DISCUSSION
Nephron sparing surgery (NSS) has emerged as the gold standard for cT1 kidney tumors due to the renal function preservation and subsequent advantages in cardiovascular survival. An unwanted complication of NSS/PN is the development of IVL, which have been reported to be around 1%–2% after open or minimally invasive nephron sparing surgery [14]. Such lesions can potentially be detrimental if they bleed and their treatment could compromise renal function. Over a 16-year period, 2.6% of patients at our institution had significant bleeding events following partial nephrectomy and were ultimately diagnosed with an IVL on imaging. Clinical presentation is dependent on IVL size and/or the severity of bleeding. Though some remain asymptomatic, most patients present within two weeks of surgery with gross hematuria, flank tenderness, hypotension, anemia, or clot retention [5,14,15].
The first reported IVL was a pseudoaneurysm reported in 1973 by Rezvani et al. [16]. Since then, numerous reports have been published about IVLs occurring after PN, with frequencies ranging from 0%–1% for OPN and 1%–2% for MIPN [14,17]. These lesions have become more common due to the increased adoption of PN for T1 cortical tumors, especially the surge in utility of minimally invasive surgery in the last decade. Therefore, it is imperative to understand IVLs and the effect of SAE on kidney function.
Although a small subset of IVLs following partial nephrectomy may resolve with observation alone, the majority require intervention given their instability and propensity for significant hemorrhage [5,6,7]. Some clinicians are concerned with worsening renal function following percutaneous SAE, the mainstay treatment of IVLs [18,19]. In our series, no significant differences in serum creatinine and eGFR were seen acutely or at 12- and 24-months post-operative. These findings suggest that SAE may not significantly compromise renal function compared to surgery alone. In patients presenting with signs and symptoms of post-operative bleeding, this data supports the safety of proceeding with early SAE. There have been some published reports on the influence of SAE on kidney function. A similarly designed matched cohort previously published has shown that SAE is associated with a decline in kidney function [20]. The difference could be attributed to the interventional radiologist experience, amount of coils placed, and amount of contrast used. On the other hand, several studies have found no difference in GFR before and after SAE similar to our findings [21,22]. However, we are the first to show that there has been no renal impairment after a 2-year period of follow-up.
Many factors may contribute to the likelihood of a patient developing an IVL following partial nephrectomy. The RENAL nephrometry score is currently used to stratify renal masses by complexity to assist in surgical decision-making by taking into account mass size and location [23]. In our study population, those who ultimately required SAE for IVLs had higher RENAL nephrometry scores, suggesting an increased likelihood of IVL with increased mass complexity. A potential explanation for this finding is the proximity of these kidney tumors to larger intrarenal vessels with increase in mass size and proximity to the kidney hilum. The impact of RENAL on the incidence on postoperative IVL and the need for SAE has shown contradictory evidence in the literature, whereby some studies show no correlation [20,21,22], while others show a strong association [24].
Additionally, surgical approach may impact the likelihood of developing a post-operative IVL. Most studies report higher incidence of IVL following MIPN compared to OPN [14,17]. Some postulate this may be related to the use of larger needles in MIPN causing greater trauma, looser parenchymal approximation, and/or obscuring small vascular lesions by the pneumoperitoneum [17,25]. Multivariable logistic regression analysis in our series showed that there was no difference in the incidence of IVL's among both surgical approaches. This could be due to increased experience with MIPN in our center whereby surgical technique in resecting the tumor and nephrorrhaphy mimic the safety of OPN in terms of small vessel injury. Though regression analysis showed a possible protective effect of hemostatic agents on SAE risk (OR 0.19, p=0.022), this cannot be generalized to a wider and more heterogeneous population. This investigation was specifically designed to focus on patients with postoperative bleeding and its impact on renal function, reason why a selection bias was reported among the study limitations.
Our findings concur with those of Collins et al. [26], in that SAE does not adversely affect GFR. In addition, our study shows that the hypothetical additive nephrotoxic effect of SAE after PN did not, in fact, alter kidney function for up to two years of follow-up. Iatrogenic infarction of nephrons after blocking the feeding arterioles causes parenchymal death; however, with the preservation of the contralateral kidney we found that SAE can be safely performed.
Limitations of our study include its retrospective nature, small sample size, and relatively short follow-up interval. Also, since the patients selected were from a referral center, there could be a referral bias towards complex kidney masses, which may have increased the incidence of IVL. A 1:4 matched control analysis is not optimal due to selection bias; however, the rarity of IVL precludes the conduction of an impactful powered prospective study.
CONCLUSIONS
The use of SAE for the management of IVL following partial nephrectomy is a safe and efficient procedure with no significant impact on short or long-term renal function. Less complex renal tumors are indeed safer to resect as they decrease the need for postoperative SAE. Further larger-scale, randomized, prospective studies are necessary to validate our findings.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Logan Galansky and Ragheed Saoud.
- Data acquisition: Ragheed Saoud, Logan Galansky, and Joshua Aizen.
- Statistical analysis: Ciro Andolfi.
- Data analysis and interpretation: Nassib Abou Heidar and Ciro Andolfi.
- Drafting of the manuscript: Nassib Abou Heidar, Ragheed Saoud, Joshua Aizen, and Logan Galansky.
- Critical revision of the manuscript: Nassib Abou Heidar, Osmanuddin Ahmed, and Arieh L. Shalhav.
- Administrative, technical, or material support: Arieh L. Shalhav.
- Supervision: Arieh L. Shalhav.
- Approval of the final manuscript: Arieh L. Shalhav, Osmanuddin Ahmed, Ragheed Saoud, Ciro Andolfi, Nassib Abou Heidar, and Joshua Aizen.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hwang EC, Yu HS, Kwon DD. Small renal masses: surgery or surveillance. Korean J Urol. 2013;54:283–288. doi: 10.4111/kju.2013.54.5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capitanio U, Terrone C, Antonelli A, Minervini A, Volpe A, Furlan M, et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol. 2015;67:683–689. doi: 10.1016/j.eururo.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. 2012;62:1097–1117. doi: 10.1016/j.eururo.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Hyams ES, Pierorazio P, Proteek O, Sukumar S, Wagner AA, Mechaber JL, et al. Iatrogenic vascular lesions after minimally invasive partial nephrectomy: a multi-institutional study of clinical and renal functional outcomes. Urology. 2011;78:820–826. doi: 10.1016/j.urology.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 6.Guiu B, Kermarrec I, Ladoire S, Cormier L, Ricolfi F. Transcatheter embolization of a renal artery pseudoaneurysm after open partial nephrectomy. Am J Surg. 2011;202:e25–e26. doi: 10.1016/j.amjsurg.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Kessaris DN, Bellman GC, Pardalidis NP, Smith AG. Management of hemorrhage after percutaneous renal surgery. J Urol. 1995;153(3 Pt 1):604–608. doi: 10.1097/00005392-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Richstone L, Reggio E, Ost MC, Seideman C, Fossett LK, Okeke Z, et al. First Prize (tie): hemorrhage following percutaneous renal surgery: characterization of angiographic findings. J Endourol. 2008;22:1129–1135. doi: 10.1089/end.2008.0061. [DOI] [PubMed] [Google Scholar]
- 9.Zorn KC, Starks CL, Gofrit ON, Orvieto MA, Shalhav AL. Embolization of renal-artery pseudoaneurysm after laparoscopic partial nephrectomy for angiomyolipoma: case report and literature review. J Endourol. 2007;21:763–768. doi: 10.1089/end.2006.0332. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro EY, Hakimi AA, Hyams ES, Cynamon J, Stifelman M, Ghavamian R. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. Urology. 2009;74:819–823. doi: 10.1016/j.urology.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Shigeta M, Mita K, Shoji K, Marukawa K, Toyota N, Usui T. Renal artery pseudoaneurysm occurring after laparoscopic partial nephrectomy. Urol Int. 2008;80:332–334. doi: 10.1159/000127353. [DOI] [PubMed] [Google Scholar]
- 12.Andreucci M, Faga T, Serra R, De Sarro G, Michael A. Update on the renal toxicity of iodinated contrast drugs used in clinical medicine. Drug Healthc Patient Saf. 2017;9:25–37. doi: 10.2147/DHPS.S122207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Nyirenda T, Yates J, Munver R. Incidence of renal artery pseudoaneurysm following open and minimally invasive partial nephrectomy: a systematic review and comparative analysis. J Urol. 2013;189:1643–1648. doi: 10.1016/j.juro.2012.11.170. [DOI] [PubMed] [Google Scholar]
- 15.Netsch C, Brüning R, Bach T, Gross AJ. Management of renal artery pseudoaneurysm after partial nephrectomy. World J Urol. 2010;28:519–524. doi: 10.1007/s00345-010-0572-0. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani A, Ward JN, Lavengood RW., Jr Intrarenal aneurysm following partial nephrectomy. Urology. 1973;2:286–288. doi: 10.1016/0090-4295(73)90466-4. [DOI] [PubMed] [Google Scholar]
- 17.Ghoneim TP, Thornton RH, Solomon SB, Adamy A, Favaretto RL, Russo P. Selective arterial embolization for pseudoaneurysms and arteriovenous fistula of renal artery branches following partial nephrectomy. J Urol. 2011;185:2061–2065. doi: 10.1016/j.juro.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Baumann C, Westphalen K, Fuchs H, Oesterwitz H, Hierholzer J. Interventional management of renal bleeding after partial nephrectomy. Cardiovasc Intervent Radiol. 2007;30:828–832. doi: 10.1007/s00270-007-9033-6. [DOI] [PubMed] [Google Scholar]
- 19.Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377–383. doi: 10.1111/j.1464-410X.2005.05304.x. [DOI] [PubMed] [Google Scholar]
- 20.Walach MT, Rathmann N, Porubsky S, Pfalzgraf D, Diehl SJ, Ritter M, et al. Influence of symptomatic pseudoaneurysms on postoperative renal function after partial nephrectomy: results of a matched pair analysis. Int Urol Nephrol. 2019;51:33–40. doi: 10.1007/s11255-018-2024-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Yang M, Wu P, Li T, Ning X, Peng S, et al. Renal arterial pseudoaneurysm and renal arteriovenous fistula following partial nephrectomy. Urol Int. 2018;100:368–374. doi: 10.1159/000443700. [DOI] [PubMed] [Google Scholar]
- 22.Chavali JSS, Bertolo R, Kara O, Garisto J, Mouracade P, Nelson RJ, et al. Renal arterial pseudoaneurysm after partial nephrectomy: literature review and single-center analysis of predictive factors and renal functional outcomes. J Laparoendosc Adv Surg Tech A. 2019;29:45–50. doi: 10.1089/lap.2018.0364. [DOI] [PubMed] [Google Scholar]
- 23.Canter D, Kutikov A, Manley B, Egleston B, Simhan J, Smaldone M, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 2011;78:1089–1094. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N, Patel A, Ensor J, Ahrar K, Ahrar J, Tam A, et al. Multiple renal artery pseudoaneurysms in patients undergoing renal artery embolization following partial nephrectomy: correlation with RENAL nephrometry scores. Cardiovasc Intervent Radiol. 2017;40:202–209. doi: 10.1007/s00270-016-1473-4. [DOI] [PubMed] [Google Scholar]
- 25.Singh D, Gill IS. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. J Urol. 2005;174:2256–2259. doi: 10.1097/01.ju.0000181827.49239.8e. [DOI] [PubMed] [Google Scholar]
- 26.Collins CS, Eggert CH, Stanson AJ, Garovic VD. Long-term follow-up of renal function and blood pressure after selective renal arterial embolization. Perspect Vasc Surg Endovasc Ther. 2010;22:254–260. doi: 10.1177/1531003510395605. [DOI] [PubMed] [Google Scholar]