Abstract
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is revising ICH E6 Good Clinical Practice (GCP). The Clinical Trials Transformation Initiative (CTTI) initiated a project to identify and provide ICH with stakeholders’ priority areas and suggestions for revising ICH E6 GCP. We conducted a global online survey to identify areas of ICH E6 GCP that are and are not in need of revision. A total of 327 stakeholders completed the survey. Stakeholders represent many research roles and types of organizations, are employed in 39 countries, and conduct research in 153 countries. The ICH E6 GCP principles mentioned most often (range, 25%–29%) in need of revision were implementing systems that assure quality, providing medical care by qualified physicians/dentists, protecting confidentiality and privacy, obtaining informed consent, and documenting and storing information. The Investigator section (n = 244, 75%) and Sponsor section (n = 242, 74%) of ICH E6 GCP were identified as needing the most revision and the Investigator Brochure section (n = 166, 51%) as needing the least revision. The topic most frequently mentioned as needing revision is Monitoring (n = 146; 45%) in the Sponsor section. Although none of the principles or topics in ICH E6 GCP were identified as needing revision by the majority of stakeholders, a meaningful percentage of stakeholders identified areas that they believe need revision. These findings, which represent the views of a wide variety of stakeholders, may be useful to ICH for identifying where specifically to focus their revision efforts. CTTI provided the final report to ICH with the project findings for their consideration.
Keywords: ICH E6 GCP, Clinical trials, Stakeholders, Good clinical practice, Ethics, Regulatory
1. Introduction
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is currently revising ICH E6 Good Clinical Practice (GCP) [1]. The mission of ICH is to achieve worldwide harmonisation for developing safe, effective, and high quality medicines [2], and they have published numerous clinical guidelines to facilitate this mission, such as the ICH E6 GCP Guideline [1]. The ICH E6 GCP guideline serves as an “international ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects” (ICH, 2016, page 1). Guideline compliance provides assurance to the public, according to ICH, that trial participants’ rights, safety and well-being are protected, and data collected are credible. ICH emphasizes that the E6 GCP guideline is intended for clinical trials that are conducted for regulatory submission [1]. ICH is revising their guidelines so that E6 GCP addresses diverse trial types and data sources and facilitates the use of technologies in trials [3,4].
The Clinical Trials Transformation Initiative (CTTI) independently initiated a multi-method project to identify areas in ICH E6 GCP that are of greatest need for revision and to describe stakeholder experiences with implementing ICH E6 GCP, including suggested ways to revise the guidance. CTTI is a public-private partnership cofounded by Duke University and the US Food and Drug Administration that seeks to develop and drive adoption of practices that will increase the quality and efficiency of clinical trials. We aimed to provide an opportunity for a diverse group of individuals who use ICH E6 GCP worldwide to share their views on how the guideline should be revised, addressing a criticism of the ICH guideline development process: lack of broad stakeholder engagement [5]. Our project consisted of 3 phases: (1) a global online survey, (2) qualitative, in-depth telephone interviews, and (3) an open comment platform [6].
The primary purpose of the survey was to identify areas of ICH E6 GCP that stakeholders believe are and are not in need of revision, thus highlighting the areas where revisions are needed the most. The follow-up qualitative interviews focused on gathering stakeholders’ experiences in implementing ICH E6 GCP as well as their suggestions for how the guideline should be revised. The open comment platform provided stakeholders an opportunity to provide line-by-line edits to the guideline. The full reports of the qualitative interviews and open comments can be found on the CTTI webpage [6]. A summarized version of the qualitative interviews will be published elsewhere. Here we describe the survey findings.
2. Methods
2.1. Study design
We conducted an on-line descriptive survey with stakeholders of ICH E6 GCP.
2.2. Recruitment
Organizations that have robust global professional research networks forwarded the survey invitation to their network members so we could reach a wide variety of stakeholders worldwide. These organizations were identified by CTTI leadership, internet searches, and the CTTI advisory group for this project, which included representatives from regulatory agencies, pharmaceutical companies, ethics review boards, contract research organizations, patient groups, clinical trials professional societies, academic institutions, and community-based health care organizations. Participating organizations forwarded a recruitment email to their members that included a link to the online survey and a request that recipients forward the recruitment email to others who might be interested in completing the survey. CTTI also posted the survey link via Twitter and LinkedIn. The initial response to the survey was limited in areas outside of North America and Europe; we therefore conducted a second wave of recruitment focusing on stakeholders who were part of research networks in ICH member countries, specifically Brazil, China, Republic of Korea, Japan, and Singapore, as well as research networks that conduct research in Africa. Data were collected from August 15 to September 20, 2019.
2.3. Eligibility
Stakeholders were eligible to complete the survey if they (1) self-reported that they are involved in research in a professional capacity, (2) have access to a computer and a reliable internet connection, and (3) read English; we offered the survey in English only, the official language of ICH.
2.4. Data collection
We purposefully created the survey to be short and targeted, keeping questions broad and focused on identifying priority areas for revision. We asked stakeholders to (1) review a list of the ICH E6 GCP principles and sections/topics and indicate whether they believe that the area is or is not in need of revision, or if they have no comments, and (2) answer demographic questions. Stakeholders reflected on the ICH E6 GCP R2 addendum [1].
2.5. Data analysis
We used descriptive statistics to summarize the data and describe the survey findings.
2.6. Ethics
The project was reviewed and determined exempt from research oversight by the Duke University Health System Institutional Review Board.
3. Results
3.1. Study population
Of the 737 stakeholders who initiated the survey, 327 responded to all questions and were included in the final sample. The most common type of research conducted by stakeholders (n=289; 88%) was phase I, II, or III clinical research on medicinal products (drugs, vaccines, and biologicals) (Table 1). Stakeholders were from 39 countries representing every region of the world, although most stakeholders’ places of employment were in Europe and Central Asia (n = 193; 60%), primarily European countries, and North America (n = 98; 31%) (eAppendix 1, Table 1). Stakeholders conducted research in 153 countries worldwide (eAppendix 1, Table 2), were affiliated with a wide range of organizational types (eAppendix 1, Table 3), and represented many research roles (eAppendix 1, Table 4); 79% (n = 259) had 10 or more years of experience in research (eAppendix 1, Table 5), nearly all (n = 304; 93%) received training on ICH E6 GCP, and most (n = 258; 79%) regularly relied on ICH E6 GCP in their research role (eAppendix 1, Table 6).
Table 1.
Type of research stakeholders conduct (current and past).
| Type of Research | No. (%)a |
|---|---|
| Phase I, II, or III clinical research on medicinal products (drugs, vaccines, and biologicals) | 289 (88.4) |
| Observational clinical research | 186 (56.9) |
| Phase IV: Post-marketing/post-approval clinical research on medicinal products | 182 (55.7) |
| Epidemiological research | 112 (34.3) |
| Other clinical research not on medicinal products | 74 (22.6) |
| Diagnostic studies | 73 (22.3) |
| Other clinical research on medicinal products | 68 (20.8) |
| Social science and behavioral research | 46 (14.1) |
Stakeholders selected all that applied.
3.2. Stakeholder recommendations for revising the principles of ICH E6 GCP
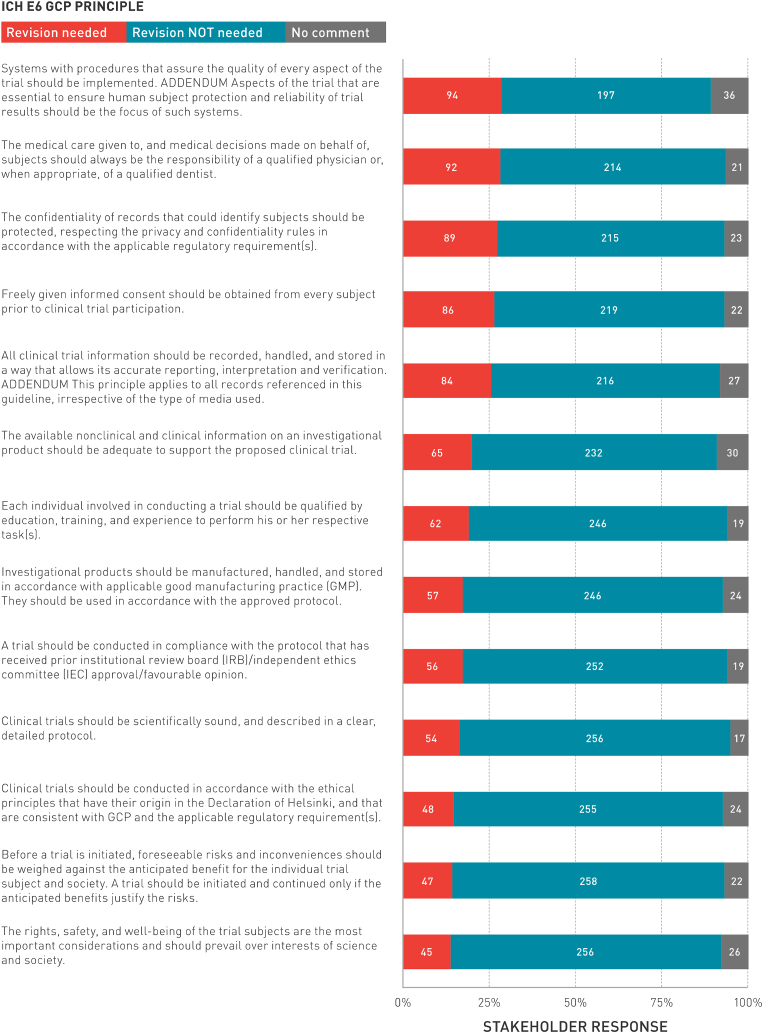
Fig. 1 presents stakeholder's recommendations for revising the ICH E6 GCP principles, ranked in order of need. The 5 principles most commonly identified by stakeholders in need of revision are:
-
1.
Implementing systems that assure quality (n = 94; 29%)
-
2.
Providing medical care by a qualified physician or dentist (n = 92; 28%)
-
3.
Protecting the confidentiality of participant records and privacy (n = 89; 27%)
-
4.
Obtaining informed consent (n = 86; 26%)
-
5.
Documenting and storing clinical trial information to ensure accurate reporting, interpretation and verification (n = 84; 25%)
Fig. 1.
Stakeholder recommendations for revising the ICH E6 GCP principles.
The 5 most common principles stakeholders believed did not need revision are:
-
1.
Weighing risks and benefits (n = 258; 79%)
-
2.
Considering the rights, safety, and well-being of the trial subjects over interests of science and society (n = 256; 78%)
-
3.
Implementing scientifically sound clinical trials with a clear, detailed protocol (n = 256; 78%)
-
4.
Conducting clinical trials in accordance with the ethical principles and GCP (n = 255; 78%)
-
5.
Obtaining approval from an independent ethics committee approval (n = 252; 77%)
3.3. Stakeholder recommendations for revising the content of ICH E6 GCP
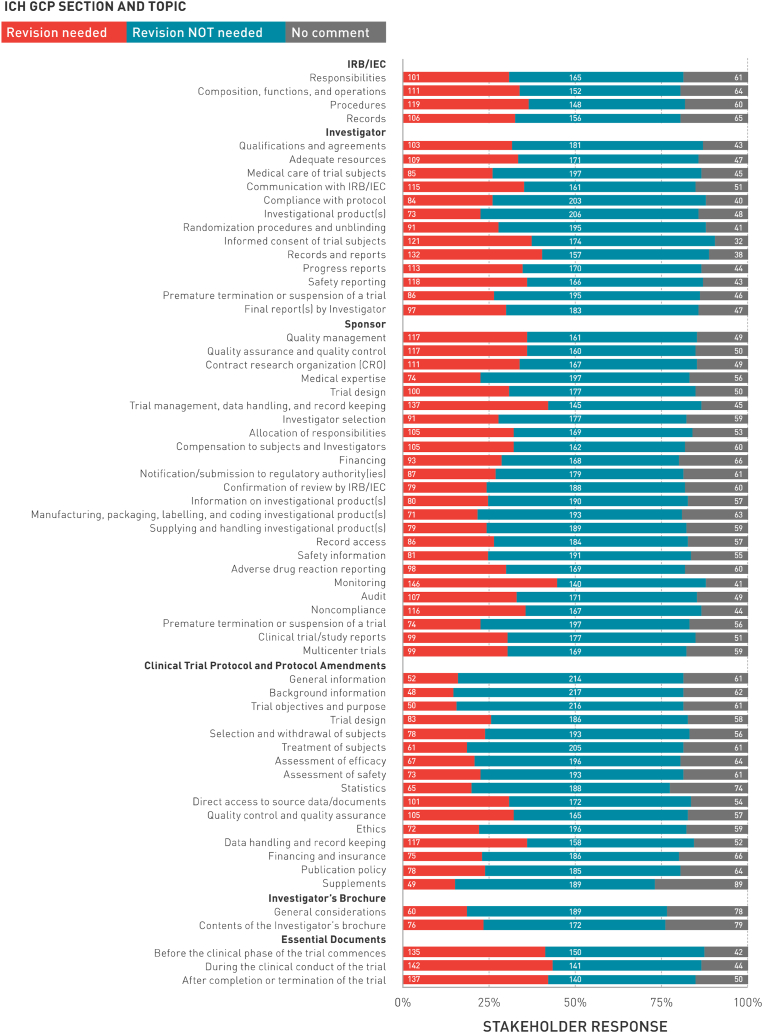
The sections in most need of revision, based on the number of stakeholders indicating that at least one topic in that section should be revised, are the Investigator (n = 244, 75%) and Sponsor (n = 242, 74%) sections. Fig. 2 presents stakeholder's recommendations for revising the ICH E6 GCP topics, by section. The top 5 topics reported in need of revision are:
-
1.
Sponsor: Monitoring (n = 146; 45%)
-
2.
Essential Documents: During the Clinical Conduct of the Trial (n = 142; 43%)
-
3.
Essential Documents: After Completion or Termination of the Trial (n = 137; 42%)
-
4.
Sponsor: Trial Management, Data Handling, and Record Keeping (n = 137; 42%)
-
5.
Essential Documents: Before the Clinical Phase of the Trial Commences (n = 135; 41%)
Fig. 2.
Stakeholder recommendations for revising the ICH E6 GCP topics by section.
The section in least need of revision, based on the number of stakeholders indicating that no topic in that section should be revised, is the section on the Investigator Brochure (n = 166, 51%). The top 5 topics that stakeholders reported not needing revision are:
-
1.
Clinical Trial Protocol and Protocol Amendments: Background Information (n = 217; 66%)
-
2.
Clinical Trial Protocol and Protocol Amendments: Trial Objectives and Purpose (n = 216; 66%)
-
3.
Clinical Trial Protocol and Protocol Amendments: General Information (n = 214; 65%)
-
4.
Investigator: Investigational Product(s) (n = 206; 63%)
-
5.
Clinical Trial Protocol and Protocol Amendments: Treatment of Subjects (n = 205; 63%)
4. Discussion
We aimed to identify aspects of ICH E6 GCP that stakeholders believed needed to be revised—and not revised—based on their experiences in implementing the guideline. Overall, none of the principles or topics in ICH E6 GCP were identified as needing revision by the majority of stakeholders. However, a meaningful percentage of stakeholders identified areas that they believe need revision. Of the 6 sections of ICH E6 GCP, the Sponsor and Investigator sections were identified as needing the most revision, although topics from other sections were also identified as needing revision. Of less need for revision was the Investigator Brochure section, although stakeholders identified topics from other sections that were also in less need of revision.
A strength of the research is that we describe the views of a wide variety of stakeholders who represent multiple countries, a diversity of research roles, and numerous types of organizations. However, even with significant effort, we had limited stakeholder involvement in areas outside of North America and Europe. Offering the survey in English only, the official language of ICH, may have been a barrier. In addition, as with all descriptive research, the findings presented here represent the views of the individuals who participated; a different group of individuals could have yielded different findings. Lastly, to facilitate ease of completion, we solicited stakeholders’ feedback within the current familiar organizational structure of ICH E6 GCP; a different format may have yielded different findings.
CTTI provided the final report [6] to ICH for their consideration as they were revising ICH E6 GCP, although CTTI has no agreement with ICH that they will use the findings in their planned revision. We hope these data will be useful to ICH to determine where specifically to focus their revision efforts.
Funding
Funding for this work was made possible, in part, by the US Food and Drug Administration through cooperative agreement U18FD005292 and grant R18FD005292. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government. Partial funding was also provided by pooled membership fees from the Clinical Trials Transformation Initiative's (CTTI) member organizations.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
We thank all stakeholders for sharing their recommendations with us. The authors would also like to recognize Adora Nsonwu, BA, Duke University, for her assistance in implementing the research and preparing the manuscript; Damon M. Seils, MA, Duke University, for editorial assistance, and Kerry Stenke, for assistance with the figures. All staff who contributed to implementation of the research or manuscript preparation were compensated as part of their salaries. The authors would also like to thank the project's CTTI advisory group for their contributions to this effort.
eAppendix 1. Detailed Stakeholder Demographic Information
Table 1.
Geographic Location of Stakeholders' Employment (n = 321*)
| Region/Country | No. (%) |
|---|---|
| East Asia and Pacific | 21 (6.5) |
| Australia | 16 (5.0) |
| China | 1 (0.3) |
| Japan | 4 (1.2) |
| Europe and Central Asia | 193 (60.1) |
| Austria | 3 (0.9) |
| Belgium | 6 (1.9) |
| Bulgaria | 1 (0.3) |
| Croatia | 1 (0.3) |
| Czechia (Czech Republic) | 4 (1.2) |
| Denmark | 5 (1.6) |
| Estonia | 2 (0.6) |
| Finland | 4 (1.2) |
| France | 7 (2.2) |
| Germany | 56 (17.4) |
| Greece | 3 (0.9) |
| Hungary | 2 (0.6) |
| Ireland | 10 (3.1) |
| Italy | 9 (2.8) |
| Netherlands | 7 (2.2) |
| Norway | 4 (1.2) |
| Poland | 1 (0.3) |
| Portugal | 5 (1.6) |
| Romania | 3 (0.9) |
| Serbia | 2 (0.6) |
| Slovakia | 3 (0.9) |
| Slovenia | 3 (0.9) |
| Spain | 9 (2.8) |
| Sweden | 4 (1.2) |
| Switzerland | 11 (3.4) |
| Turkey | 1 (0.3) |
| United Kingdom | 27 (8.4) |
| Latin America and Caribbean | 3 (0.9) |
| Argentina | 1 (0.3) |
| Colombia | 2 (0.6) |
| Middle East and North Africa | 4 (1.2) |
| Iraq | 1 (0.3) |
| Israel | 2 (0.6) |
| Lebanon | 1 (0.3) |
| North America | 98 (30.5) |
| Canada | 48 (15.0) |
| United States of America | 50 (15.6) |
| South Asia | 1 (0.3) |
| Sri Lanka | 1 (0.3) |
| Sub-Saharan Africa | 1 (0.3) |
| Tanzania | 1 (0.3) |
*Data are missing from 6 stakeholders.
Table 2.
Geographic Location of Stakeholders' Researcha
| Country | No. (%) |
|---|---|
| East Asia and Pacific | 97 (29.7) |
| Australia | 72 (22.0) |
| Cambodia | 3 (0.9) |
| China | 40 (12.2) |
| Indonesia | 14 (4.3) |
| Japan | 41 (12.5) |
| Laos | 1 (0.3) |
| Malaysia | 18 (5.5) |
| Maldives | 1 (0.3) |
| Marshall Islands | 1 (0.3) |
| Mongolia | 1 (0.3) |
| Myanmar | 3 (0.9) |
| New Zealand | 47 (14.4) |
| Papua New Guinea | 1 (0.3) |
| Philippines | 16 (4.9) |
| Singapore | 27 (8.3) |
| South Korea | 35 (10.7) |
| Taiwan | 30 (9.2) |
| Thailand | 27 (8.3) |
| Vietnam | 12 (3.7) |
| Europe and Central Asia | 230 (70.3) |
| Albania | 4 (1.2) |
| Andorra | 3 (0.9) |
| Armenia | 2 (0.6) |
| Austria | 69 (21.1) |
| Azerbaijan | 3 (0.9) |
| Belarus | 12 (3.7) |
| Belgium | 78 (23.9) |
| Bosnia and Herzegovina | 6 (1.8) |
| Bulgaria | 30 (9.2) |
| Croatia | 26 (8.0) |
| Cyprus | 8 (2.4) |
| Czechia (Czech Republic) | 55 (16.8) |
| Denmark | 71 (21.7) |
| Estonia | 27 (8.3) |
| Finland | 50 (15.3) |
| France | 89 (27.2) |
| Georgia | 8 (2.4) |
| Germany | 134 (41.0) |
| Greece | 39 (11.9) |
| Greenland | 2 (0.6) |
| Hungary | 51 (15.6) |
| Iceland | 14 (4.3) |
| Ireland | 73 (22.3) |
| Italy | 93 (28.4) |
| Kazakhstan | 3 (0.9) |
| Kosovo | 2 (0.6) |
| Kyrgyzstan | 3 (0.9) |
| Latvia | 21 (6.4) |
| Liechtenstein | 3 (0.9) |
| Lithuania | 25 (7.6) |
| Luxembourg | 11 (3.4) |
| Malta | 4 (1.2) |
| Moldova | 9 (2.8) |
| Monaco | 3 (0.9) |
| Montenegro | 1 (0.3) |
| Netherlands | 80 (24.5) |
| North Macedonia (Formerly Macedonia) | 3 (0.9) |
| Norway | 53 (16.2) |
| Poland | 69 (21.1) |
| Portugal | 45 (13.8) |
| Romania | 40 (12.2) |
| Russia | 48 (14.7) |
| Serbia | 18 (5.5) |
| Slovakia | 31 (9.5) |
| Slovenia | 23 (7.0) |
| Spain | 80 (24.5) |
| Sweden | 73 (22.3) |
| Switzerland | 69 (21.1) |
| Tajikistan | 1 (0.3) |
| Turkey | 29 (8.9) |
| Turkmenistan | 1 (0.3) |
| Ukraine | 32 (9.8) |
| United Kingdom | 112 (34.3) |
| Uzbekistan | 1 (0.3) |
| Vatican City | 1 (0.3) |
| Latin America and Caribbean | 59 (18.0) |
| Argentina | 39 (11.9) |
| Bahamas | 1 (0.3) |
| Barbados | 1 (0.3) |
| Belize | 1 (0.3) |
| Bolivia | 2 (0.6) |
| Brazil | 38 (11.6) |
| Chile | 27 (8.3) |
| Colombia | 21 (6.4) |
| Costa Rica | 4 (1.2) |
| Cuba | 3 (0.9) |
| Dominica | 1 (0.3) |
| Dominican Republic | 3 (0.9) |
| Ecuador | 7 (2.1) |
| El Salvador | 2 (0.6) |
| Guatemala | 5 (1.5) |
| Guyana | 1 (0.3) |
| Honduras | 1 (0.3) |
| Jamaica | 2 (0.6) |
| Mexico | 38 (11.6) |
| Nicaragua | 1 (0.3) |
| Panama | 5 (1.5) |
| Paraguay | 4 (1.2) |
| Peru | 11 (3.4) |
| Uruguay | 5 (1.5) |
| Venezuela | 10 (3.1) |
| Middle East and North Africa | 42 (12.8) |
| Algeria | 2 (0.6) |
| Bahrain | 2 (0.6) |
| Egypt | 10 (3.1) |
| Iran | 1 (0.3) |
| Iraq | 1 (0.3) |
| Israel | 30 (9.2) |
| Jordan | 4 (1.2) |
| Kuwait | 2 (0.6) |
| Lebanon | 6 (1.8) |
| Morocco | 2 (0.6) |
| Oman | 1 (0.3) |
| Qatar | 5 (1.5) |
| Saudi Arabia | 8 (2.4) |
| Syria | 1 (0.3) |
| Tunisia | 4 (1.2) |
| United Arab Emirates | 6 (1.8) |
| Yemen | 3 (0.9) |
| North America | 170 (52.0) |
| Canada | 119 (36.4) |
| United States of America | 122 (37.3) |
| South Asia | 44 (13.5) |
| Afghanistan | 1 (0.3) |
| Bangladesh | 6 (1.8) |
| India | 43 (13.1) |
| Nepal | 2 (0.6) |
| Pakistan | 6 (1.8) |
| Sri Lanka | 5 (1.5) |
| Sub-Saharan Africa | 49 (15.0) |
| Benin | 2 (0.6) |
| Botswana | 1 (0.3) |
| Burkina Faso | 1 (0.3) |
| Cameroon | 4 (1.2) |
| Central African Republic (CAR) | 1 (0.3) |
| Congo, Democratic Republic of the | 3 (0.9) |
| Cote d'Ivoire | 2 (0.6) |
| Ethiopia | 4 (1.2) |
| Gabon | 1 (0.3) |
| Gambia | 4 (1.2) |
| Ghana | 8 (2.4) |
| Guinea | 1 (0.3) |
| Kenya | 13 (4.0) |
| Liberia | 1 (0.3) |
| Malawi | 4 (1.2) |
| Mali | 1 (0.3) |
| Mozambique | 3 (0.9) |
| Namibia | 2 (0.6) |
| Nigeria | 6 (1.8) |
| Rwanda | 4 (1.2) |
| Senegal | 2 (0.6) |
| Seychelles | 1 (0.3) |
| Sierra Leone | 3 (0.9) |
| South Africa | 37 (11.3) |
| Sudan | 3 (0.9) |
| Tanzania | 9 (2.8) |
| Uganda | 10 (3.1) |
| Zambia | 5 (1.5) |
| Zimbabwe | 5 (1.5) |
aThe regional headers represent the total number and percentage of stakeholders who conduct research in one of the countries in that region. The country sub-headers represent the total number and percentage of stakeholders who conduct research in that country.
Table 3.
Stakeholders' Affiliations (n = 327)
| Organization Type | No. (%) |
|---|---|
| University/academic research center affiliated with a hospital/medical center | 132 (40.4) |
| Pharmaceutical company or biotechnology company | 61 (18.7) |
| Contract research organization (commercial/for profit) | 44 (13.5) |
| Hospital/medical center not affiliated with a university/academic research center | 21 (6.4) |
| University/academic research center not affiliated hospital/medical center | 20 (6.1) |
| Governmental organization that regulates medical products | 14 (4.3) |
| Non-governmental organization or not-for-profit organization | 12 (3.7) |
| Private research site | 8 (2.4) |
| Governmental organization that does not regulate medical products | 5 (1.5) |
| Patient advocacy group | 2 (0.6) |
| Private foundation | 2 (0.6) |
| Trade/professional organization | 1 (0.3) |
| Not affiliated with a specific organization | 1 (0.3) |
| Prefer not to respond | 4 (1.2) |
Table 4.
Stakeholders' Main Role in Research (n = 327)
| Research Role | No. (%) |
|---|---|
| Principal investigator, co-investigator, sub-investigator, site investigator | 77 (23.5) |
| Quality assurance/quality control personnel | 65 (19.9) |
| Clinical operations personnel | 57 (17.4) |
| Clinical research associate/research coordinator/study nurse | 45 (13.8) |
| Regulatory affairs personnel | 21 (6.4) |
| Data analyst | 12 (3.7) |
| Monitor | 10 (3.1) |
| Inspector | 9 (2.8) |
| Data manager | 6 (1.8) |
| Government regulator | 4 (1.2) |
| Laboratory personnel | 4 (1.2) |
| Pharmacist | 3 (0.9) |
| Ethics review/Institutional Review Board personnel | 1 (0.3) |
| Medical provider (healthcare provider who delivers medical care to study participants) | 1 (0.3) |
| Data collector | 1 (0.3) |
| Patient advocate | 1 (0.3) |
| Prefer not to respond | 10 (3.1) |
Table 5.
Length of Time Stakeholders Have Been Involved in Research (n = 327)
| Length of Time | No. (%) |
|---|---|
| Less than 1 year | 2 (0.6) |
| 1 year to less than 5 years | 24 (7.3) |
| 5 years to less than 10 years | 38 (11.6) |
| 10 years to less than 20 years | 108 (33.0) |
| 20 or more years | 151 (46.2) |
| Prefer not to respond | 4 (1.2) |
Table 6.
Stakeholders' Engagement with ICH GCP E6 (n = 327)
| Type of Engagement | No. (%) |
|---|---|
| Received training on ICH GCP | 304 (93.0) |
| How often rely on GCP to do research role | |
| Regularly | 258 (78.9) |
| Occasionally | 41 (12.5) |
| Rarely | 15 (4.6) |
| Never | 2 (0.6) |
| Prefer not to respond | 11 (3.4) |
References
- 1.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Integrated Addendum to ICHE6(r1): Guideline for Good Clinical Practice E6(R2). Step 4 version dated 9 November 2016.
- 2.International Council for harmonisation of technical Requirements for pharmaceuticals for human use (ICH) https://ich.org
- 3.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) ICH reflection on “GCP renovation”: modernization of ICH E8 and subsequent renovation of ICH E6. 2017. https://admin.ich.org/sites/default/files/2019-04/ICH_Reflection_paper_GCP_Renovation_Jan_2017_Final.pdf
- 4.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Final concept paper ICH E6(R3): guideline for Good clinical practice. 2019. https://database.ich.org/sites/default/files/E6-R3_FinalConceptPaper_2019_1117.pdf
- 5.Updated open letter to EMA & ICH in Response to ICH GCP. 26 February 2016. http://moretrials.net/the-problem
- 6.Clinical Trials Transformation Initiative Informing ICH E6 renovation. https://www.ctti-clinicaltrials.org/projects/informing-ich-e6-renovation