Abstract
We have developed and implemented an undergraduate microbiology course in which students isolate, characterize, and perform whole genome assembly and analysis of Salmonella enterica from stream sediments and poultry litter. In the development of the course and over three semesters, successive teams of undergraduate students collected field samples and performed enrichment and isolation techniques specific for the detection of S. enterica. Eighty-eight strains were confirmed using standard microbiological methods and PCR of the invA gene. The isolates’ genomes were Illumina-sequenced by the Center for Food Safety and Applied Nutrition at the FDA and the Virginia state Division of Consolidated Laboratory Services as part of the GenomeTrakr program. Students used GalaxyTrakr and other web- and non-web-based platforms and tools to perform quality control on raw and assembled sequence data, assemble, and annotate genomes, identify antimicrobial resistance and virulence genes, putative plasmids, and other mobile genetic elements. Strains with putative plasmid-borne antimicrobial resistance genes were further sequenced by students in our research lab using the Oxford Nanopore MinIONTM platform. Strains of Salmonella that were isolated include human infectious serotypes such as Typhimurium and Infantis. Over 31 of the isolates possessed antibiotic resistance genes, some of which were located on large, multidrug resistance plasmids. Plasmid pHJ-38, identified in a Typhimurium isolate, is an apparently self-transmissible 183 kb IncA/C2 plasmid that possesses multiple antimicrobial resistance and heavy-metal resistance genes. Plasmid pFHS-02, identified in an Infantis isolate, is an apparently self-transmissible 303 kb IncF1B plasmid that also possesses numerous heavy-metal and antimicrobial resistance genes. Using direct and indirect measures to assess student outcomes, results indicate that course participation contributed to cognitive gains in relevant content knowledge and research skills such as field sampling, molecular techniques, and computational analysis. Furthermore, participants self-reported a deeper interest in scientific research and careers as well as psychosocial outcomes (e.g., sense of belonging and self-efficacy) commonly associated with student success and persistence in STEM. Overall, this course provided a powerful combination of field, wet lab, and computational biology experiences for students, while also providing data potentially useful in pathogen surveillance, epidemiological tracking, and for the further study of environmental reservoirs of S. enterica.
Keywords: Salmonella, course-based undergraduate research, pathogen reservoirs, bioinformatics, pathogen surveillance, environmental pathogens, public health microbiology, genomic epidemiology
Introduction
Bacterial genomic epidemiology – the use of genomics-based methods to aid in the epidemiological investigation of communicable diseases – has become an important new tool in the hands of public health laboratories tasked with tracking pathogen outbreaks (Deng et al., 2016; Armstrong et al., 2019). Most outbreaks have been studied retrospectively due to the costs and time involved in analyzing pathogens using pulsed-field gel electrophoresis. However, the introduction of massively parallel sequencing technologies – along with the application of bioinformatics algorithms for assembly, typing, annotation, and phylogenetic analysis – have begun to enable the real-time tracking of outbreaks for infection control and prevention. Whole genome sequencing (WGS) can help public health scientists better understand the origins and dynamics of the outbreak itself (Tang et al., 2017; Armstrong et al., 2019), while providing important information about outbreak strains – such as their serotype, antibiotic susceptibility, potential virulence factors, and toxins – in a single, in silico assay (Nadon et al., 2017).
Non-typhoidal Salmonella enterica (NTS) are the leading cause of foodborne illness in the United States, and one of the main causes of gastrointestinal disease globally. Worldwide, there are 1.3 billion reported cases of gastroenteritis, 16 million cases of typhoid fever, and 3 million deaths annually attributed to all S. enterica infections (Bhunia, 2018), with ca. 535,000 attributed to NTS in 2017 (Parisi et al., 2020). NTS are spread via the fecal-oral route and transmitted via contaminated foods (e.g., poultry, beef, dairy, and eggs), water, and direct animal contact (Silva et al., 2014; Bhunia, 2018). Non-typhoidal S. enterica typically causes self-limiting gastroenteritis; however, it can cause invasive disease under certain circumstances (Crump et al., 2015). Effective real-time tracking of new outbreaks requires a large database of Salmonella from clinical, food, animal, and environmental sources. As of this writing, over 286,000 draft and complete genomes of Salmonella have been sequenced. However, to date only ca. 18,608 are identified as having been isolated from environmental sources such as freshwaters and soils1. Irrigation waters are potential sources of Salmonella outbreaks in foods (Bell et al., 2015; Liu et al., 2018), and recreational waters may also act as sources for infections in both humans and animals (Levantesi et al., 2012). Unlike other enteric bacteria like E. coli, S. enterica has high survival rates in aquatic systems and soils, can persist in poultry houses for over a year, and is generally more resistant to environmental fluctuations. It has been postulated that its long-term survival in such secondary habitats facilitates passage to its next host (Winfield and Groisman, 2003). In order to rapidly source human infections in the event of an outbreak, potential environmental reservoirs as well as clinical sources of S. enterica need to be monitored.
In keeping with national reform calls in science, technology, engineering, and mathematics (STEM) education to engage all undergraduates in the authentic practice of scientific discovery (National Research Council (US) Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century, 2003; American Association for the Advancement of Science, 2011; National Academies of Sciences, Engineering, and Medicine, 2017) we designed a semester-long course-based undergraduate research experience (CURE) in which upper-division undergraduate students are immersed in the isolation, characterization, and genomic analysis of S. enterica isolated from the local environment. As students gain access to more advanced research practices as part of their normal laboratory coursework, a CURE such as this offers students an equitable and inclusive pathway to participate in authentic research (Bangera and Brownell, 2014) and develop skills and knowledge for future careers in the field. This CURE also exposes students to the public health applications of working with foodborne pathogens as well as provides fundamental genomics training applicable to genomic epidemiology (e.g., variant tracking of SARS-COV-2). Students may, as is also described herein, elect to carry out more advanced investigations on their isolates and their genomes. In this paper, we intend to show not only the methods and tools that might be useful for faculty considering implementing a CURE in pathogen genomics, but also a model for how data derived from the isolates and their genomes can be used to address real-world needs and applications in public health genomic epidemiology and in the comparative genomics of foodborne pathogens.
Materials and Methods
Course Background and Implementation
The bulk of this research was implemented in a one-semester upper-division laboratory course at James Madison University. The prerequisite for the course is a general microbiology course, both laboratory and lecture. The course consists of two 2 hour-long lab periods per week, as well as regular lab activities outside of the formal lab periods. The course size during this study ranged from 12 to 24 students per semester and has one or two student teaching assistants who have taken the course. Consistent with Auchincloss et al.’s (2014) framework for CURE design, the course employed a collaborative, iterative, discovery-based approach intended to meaningfully engage students in authentic scientific research comparable to that of the community of practice. The course is divided up into a wet lab module, Module 1, and a computational module, Module 2. In Module 1 students work in teams to collect samples, from stream sediments and from poultry litter; then they enrich, purify, identify, and characterize S. enterica from these samples. In Module 2 the teams assess read quality, assemble, serotype, and annotate their isolates’ genomes, identify mobile genetic elements, resistance, and virulence genes, etc.
Either module can be implemented independently or they can be employed sequentially over the course of a semester as is done in this course. Details concerning course implementation, including lesson plans for each module, recommended time lines, assessments, etc. are available in Jurgensen et al. (in press). Complete and detailed wet lab and computational protocols, designed and formatted for use in the course, are freely available on the course Open Science Framework (OSF) page2. For Module 1, in addition to stream and manure sources of Salmonella as described here, S. enterica can be isolated from captive or wild reptiles (Marin et al., 2020), amphibians (Ribas and Poonlaphdecha, 2017) and rodents (Meerburg and Kijlstra, 2007; Swanson et al., 2007). Most of the protocols and methods can be modified for use with E. coli as well, which can be readily isolated from many urban and rural surface waters. All the work can be done in a typical college microbiology laboratory, albeit following Biosafety Level 2 protocols (see safety documents on OSF2). No specialized equipment beyond that found in a typical teaching microbiology laboratory is required, other than perhaps a thermal cycler. Essentially all the work described was carried out by undergraduates each semester over the span of three iterations of the one-semester course, except the nanopore sequencing, phylogenetic analyses, and the advanced aspects of plasmid identification and annotation – which were done by undergraduates and an M.S. student in the research lab – and the Illumina sequencing, which was done by the Virginia D.C.L.S.
Environmental Sample Collection
Stream sediment was collected from seven sites on four streams near James Madison University in the Shenandoah Valley of Virginia. Water temperature, salinity, and conductivity were collected using a SondeTM probe (YSI Incorporated, OH, United States). Metadata was recorded using the mobile application Epicollect53. Stream sediment was collected by inverting a sterile 50 mL Falcon® tube and inserting it straight down into the sediment with a gloved hand while avoiding plant matter and gravel. Each tube was filled approximately 3/4 full with sediment and water. Sediment samples were stored at 4°C during transport and in the lab until processing. Poultry litter was acquired from a chicken farm in northern Rockingham County, Virginia, housing approximately 150,000 birds. The farmer was provided with a clean plastic container for filling with litter. Litter was stored at room temperature (20–25°C) until processing.
Isolation of Salmonella enterica From Stream Sediment/Poultry Litter
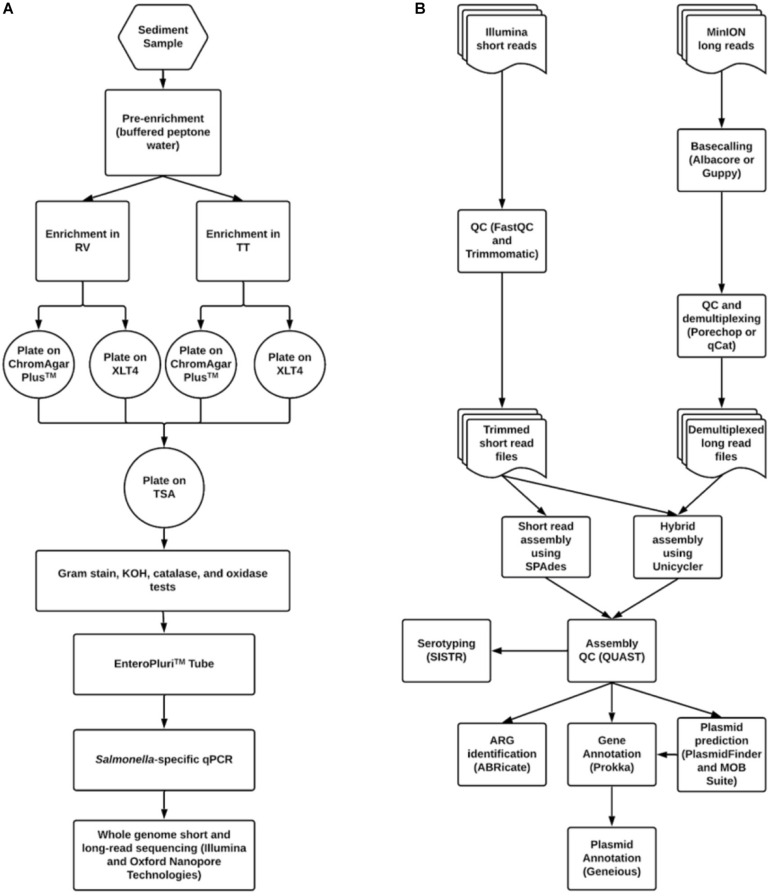
An outline of the methods used to isolate and identify S. enterica in sediment and poultry samples is shown in Figure 1A. The procedure was based loosely on the United States FDA Bacteriological Analytical Manual Salmonella isolation protocol (Andrews et al., 2014). Both sediment and litter were processed in the same manner. Pre-enrichment began within 24 h of sample collection. Fifty grams of sediment or litter were transferred to sterile 250 mL Erlenmeyer flasks in duplicate. One hundred milliliters of buffered peptone water (10 g peptone, 5 g NaCl, 7 g Na2HPO4, 3 g KH2PO4 per liter) were added to each flask and mixed by swirling. Pre-enrichments were incubated with shaking at 35°C for 16–22 h. After incubation, 1 mL of supernatant was added to screw-cap tubes containing 10 mL of either Tetrathionate (TT) or Rappaport-Vasilliadis (RV) broth. TT was made in one liter batches (5 g polypeptone, 1 g bile salts, 10 g CaCO3, 30 g Na2S2O3 per liter) with additional 20 mL iodine-potassium iodide (5 g KI, 6 g iodine resublimated) added. RV was also made in one liter batches consisting of 100 mL magnesium chloride solution (400 g MgCl2 6H2O per liter) and 10 mL malachite green oxalate solution (0.4 g malachite green oxalate per liter) to one liter of broth base (5 g tryptone, 8 g NaCl, 1.6 g KH2PO4 per liter). RV was autoclaved prior to the addition of pre-enrichment. All enrichments were shaken at 42°C for 5 days.
FIGURE 1.
Workflows for (A) Salmonella isolation and (B) bioinformatic data processing and analysis.
One hundred microliter aliquots from each enrichment were spread-plated onto Xylose Lysine Tergitol-4 (XLT4) agar (Becton Dickinson, Franklin, NJ, United States) and CHROMagarTM Salmonella agar (DRG International Inc., Springfield, NJ). Plates were incubated at 35°C for 16–22 h. Putative Salmonella colonies on each medium were identified based on morphology and then streaked onto the complementary agar. If Salmonella-like morphology was seen on both media, then colonies were streaked onto tryptic soy agar (TSA) (Becton Dickinson, Franklin, NJ, United States) for purification.
Identification of S. enterica
Gram-negative, oxidase-negative, catalase- and KOH-positive isolates were grown in an EnteroPluriTM tube (Becton-Dickinson, Franklin Lakes, NJ, United States). The EnteroPluriTM tube allows for the simultaneous inoculation of multiple media types and the execution of 15 separate biological reactions. Isolates identified as Salmonella were then subjected to an endpoint PCR using primers targeting the Salmonella-specific gene invA (Malorny et al., 2003) for confirmation. Briefly, a small number of cells were acquired by touching an inoculating needle to a colony. The cells were added to 5 μL of ddH2O in a 0.2 mL PCR tube and lysed in a thermocycler at 95°C for 5 minutes. A master mix was made consisting of 12.5 μL of 2X AmpliTaq Gold® (ThermoFisher Scientific, Waltham, MA, United States) (0.625 U AmpliTaq Gold DNA polymerase, 30 mM Tris/HCl, pH 8.05, 100 mM KCl, 400 μM each dNTP, 5 mM MgCl2), one μL of both invA 139 primer (5′-GTGAAATTATCGCCACGTTCGGGCAA-3′) and invA 141 primer (5′-TCATCGCACCGTCAAAGGAACC-3′) at 10 μM concentrations, and 5 μL of ddH2O. Twenty microliters of master mix were added to the lysed cell mixture and run according to the program of Malorny et al. (2003): 95°C for one minute followed by 36 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 30 s, then a final extension at 72°C for four minutes. For agarose gel electrophoresis, eight microliters of PCR product were added to 2 μL of 5X loading dye prior to loading. The gel was run at 5 V/cm for ca. 120 min and stained with 0.5% GelRed (Biotium Inc., Fremont, CA, United States) for 20-30 min followed by de-staining with ddH2O for 5 min. Bands were visualized using a UV transilluminator. A band size of 285 bp was expected for an invA positive result. For long term storage, one mL of culture was combined with one mL of sterile glycerol in a 2 mL cryogenic freezer tube and stored at −80°C. Strain names were derived from the initials of student teams that isolated them.
Genomic DNA Extraction for Oxford Nanopore DNA Sequencing
Cells were grown in tryptic soy broth for 16–20 h. The QiagenTM DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, United States) was used following the manufacturer’s instructions for Gram-negative bacteria with some modifications: cell density was not assayed prior to extraction, during the incubation step with proteinase K the length of incubation was kept to a maximum of 1 h, and after elution with ddH2O, the DNA was left to dissolve for 24 h at 4°C.
Genomic DNA Sequencing
Short read sequence data were generated by the United States FDA Center for Food Safety and Applied Nutrition, as well as the Virginia State Department of Consolidated Laboratory Services. Both carried out sequencing on an Illumina® MiSeq sequencer using either a 300 cycle (2 × 151) or 500 cycle kit (2 × 251). Raw sequence data were uploaded to Illumina BaseSpace, GalaxyTrakr, and to NCBI’s Sequence Read Archive (SRA).
For nanopore sequencing, DNA quality and concentrations were assessed using a Synergy H1 Multi-Mode Reader (BioTek Instruments, VT, United States) and a Qubit 2.0 fluorometer, respectively. An OD260/280 of 1.8–2.0 was used as the quality cutoff. DNA concentration was determined using the Qubit dsDNA broad range kit. DNA samples were concentrated for samples where when necessary using Microcon® centrifugal filters (Merck Millipore Ltd., MA, United States) according to the manufacturer’s instructions.
Prior to nanopore sequencing, a flow cell QC was performed according to the manufacturer’s instructions. Library preparation was done using the rapid barcoding kit (SQK-RBK004) according to the manufacturer’s instructions (version RNK_9054_v2_revA_23Jan2018; Oxford Nanopore Technologies, Oxford, United Kingdom). Sequencing on the ONT MinION proceeded for up to 48 h using a FLO-MIN106 flowcell (R9.4.1 pore type).
Sequence Data Quality Control and Analysis
Sequence data were processed according to the pipeline shown in Figure 1B. Bioinformatic processing and analyses were done in GalaxyTrakr (Gangiredla et al., 2021) or using the command line interface on a computer with an Ubuntu 16.04 LTS operating system. Short read data were quality checked using FastQC4 version 0.72 or 0.69. Low-quality data was removed using Trimmomatic version 0.36.4 or 0.36.3 (Bolger et al., 2014). Trimmomatic operations consisted of sliding window trimming using a window of four bases with an average quality cutoff of 20, then an overall average quality trimming with a cutoff of 27, and finally a minimum length trimming with a cutoff of 70% the maximum read length (i.e., for data with read length of 251, reads below 75 bp were removed). Trimmed reads were again run through FastQC. Assembly using short read data was carried out using SPAdes version 3.11.1 with default options and specified k-mer values of 21, 33, 55, 77, 99, and 127 (Bankevich et al., 2012).
For long read data, basecalling was performed using either Albacore version 2.2.7 or Guppy 3.0.3 (Oxford Nanopore, Oxford, United Kingdom). Adapter removal and demultiplexing was performed using either Porechop5 version 0.2.3 or Qcat6 version 1.0.6. These data were used in conjunction with short read data to carry out a hybrid assembly using Unicycler version 0.4.1.1 (Wick et al., 2017). Assembly quality was assessed using QUAST version 4.6.3 (Gurevich et al., 2013). Assembly quality thresholds used were: N50 > 200,000, number of contigs <200, sequence length ca. 4.4 to 5 Mbp.
Serotyping of Salmonella Isolates
Serotyping was done in silico using Seqsero2 version 2.0 and SISTR version 1.0.2 on GalaxyTrakr. Additionally, serotyping using SMART PCR (Leader et al., 2009) was carried out on isolates HJ-01 to HJ-26. Trimmed short reads (as FASTQ files) were used as inputs to Seqsero2. Assembled genomes (either short read only or hybrid assemblies, as FASTA files) were used as inputs to SISTR. Agreement between the two in silico tools and, if necessary, SMART PCR were used to determine the consensus serotype of a given isolate.
Antimicrobial Resistance Genotyping
Antimicrobial resistance genes (ARGs) were identified using ABRicate7 versions 0.7.0, 0.8.0, 0.8.7, and ResFinder version 3.2 (Zankari et al., 2012). ABRicate was used through GalaxyTrakr and the command line. Default settings were used on both platforms with two exceptions: a minimum identity cutoff of 80% was specified and the database used was NCBI. ResFinder was run through the Center for Genomic Epidemiology website8. For ResFinder, the “acquired ARGs” option was used and final assemblies were submitted.
Phylogenetic Analysis
Eighty-eight S. enterica isolates were used to generate a phylogenetic tree in Enterobase (Zhou et al., 2020). A neighbor-joining tree was generated using the algorithm RapidNJ in Enterobase from Enterobase’s cgMLST scheme, a set of alleles for 3,002 loci that make up S. enterica’s core genome. GrapeTree (Zhou et al., 2018) and TreeGraph2 (Stöver and Müller, 2010) were used to visualize the tree. The tree was rooted using the genome of S. enterica subspecies salamae strain 1315 K. Phandango (Hadfield et al., 2018) was used to visualize source and serotype metadata mapped onto the tree.
In silico Identification and Annotation of Plasmids
MOB_Suite version 1.4.8 (Robertson and Nash, 2018) and PlasmidFinder’s most recent version (Carattoli et al., 2014) were used to identify potential plasmids from short read-only assemblies. PlasmidFinder was used through the Center for Genomic Epidemiology website. The Enterobacteriaceae database was employed, with an identity cutoff of 90% and a minimum coverage cutoff of 80%. For MOB_Suite, the mob_recon command’s basic options, which require only an input FASTA file and an output directory location, were used, along with the mob_typer command for plasmid typing. MOB_Suite is now available on GalaxyTrakr9.
Annotation of plasmids was done using the commercial platform Geneious PrimeTM 2019 (Biomatters Ltd., San Diego, CA, United States). Bandage (Wick et al., 2015) was used to visualize assemblies and to identify potential plasmids from hybrid assembly graphs generated by Unicycler. Plasmid sequences were downloaded as FASTA files from Bandage graphs and submitted to Prokka version 0.13.0 (Seemann, 2014) for automatic annotation using the default parameters: Locus tag prefix PROKKA, Locus tag counter increment = 1, GFF version = 3, no forced GenBank/ENA/DDJB compliance, minimum contig size = 200, kingdom = Bacteria, genetic code = 11, similarity e-value cut-off = 0.000001. Not used: “gene” feature for “CDS” feature, genus-specific BLAST database, improve gene prediction for highly fragmented genomes, fast mode, searching for ncRNAs, rRNA search with Barnap, and tRNA search with Aragorn. In addition, identification of unidentified coding sequences was attempted using BLASTx. Parameters used for BLASTx were: max target sequences = 100, expect threshold = 10, word size = 6, and maximum number of matches in a query = 0. The BLOSUM62 matrix was used for scoring. GFF3 files from Prokka were uploaded to Geneious for plasmid mapping and editing.
Results
Course Development
Due to the large amount of poultry and cattle farming in the central Shenandoah Valley of Virginia, we hypothesized that Salmonella could be isolated from area streams that drain these farms. If this proved to be true, we intended to develop an elective upper-level research course in which undergraduates would learn to isolate and identify Salmonella and to assemble and annotate their genomes. We hoped thereby also to initiate a long-term study on Salmonella in streams in our area and to develop the methods necessary for other colleges and universities to replicate the course or a portion thereof. Our focus was particularly on the sediments of streams, as these have been shown to harbor a more stable population of introduced bacteria, particularly Enterobacteriaceae, than does the water column itself (Hendricks, 1971; Burton et al., 1987; Pachepsky and Shelton, 2011). Initial attempts to isolate Salmonella from sediments in various agriculturally impacted streams were unsuccessful. However, after lengthening the time of enrichment from 24 h to 5 days (Figure 1A) we were able to routinely isolate S. enterica from all of our tested stream sites.
We wished to use WGS of these isolates both to understand the population-level dynamics of these Salmonella and to train students in some basic bioinformatics methods in microbial genomics. A bioinformatics workflow was developed and used for the QC, assembly, and annotation of Salmonella genomes (Figure 1B). This workflow was carried out primarily using GalaxyTrakr, an FDA Galaxy instance developed particularly for the use of public health laboratories for analyzing the genomes of foodborne pathogens (Gangiredla et al., 2021).
These isolation and computational protocols were piloted in a new course, BIO346 Bacterial Discovery, beginning in the spring of 2018. Over three separate semesters, CURE students (n = 52) isolated and characterized 15 S. enterica strains. Three of these students went on to isolate an additional 34 strains in the Herrick research lab. These combined with the 39 strains isolated during the methods development for the course resulted in a total of 88 strains isolated from October 2016 through September 2018 (Table 1). Of these, 83 strains were isolated from the sediment of seven sites on four streams and five strains from a broiler poultry house, all in the Shenandoah River watershed Rockingham County, Virginia.
TABLE 1.
Salmonella enterica strains isolated from stream sediment and poultry litter in the Shenandoah Valley of Virginia from October 2016 through September 2018.
| Isolate | SRR number | Collection date | Sourcea | Source type | Serotype | MLSTb | cgMLSTc |
| HJ-01 | SRR5886281 | 2016/10/02 | MC | Sediment | Give | 654 | 97788 |
| HJ-02 | SRR5886286 | 2016/10/02 | MC | Sediment | Give | 654 | 80760 |
| HJ-03 | SRR5886299 | 2016/10/02 | PR | Sediment | Uganda | 684 | 80845 |
| HJ-04 | SRR5886298 | 2016/10/02 | PR | Sediment | Uganda | 684 | 70491 |
| HJ-05 | SRR5886283 | 2016/10/16 | CC11 | Sediment | Litchfield | 214 | 80915 |
| HJ-06 | SRR5886290 | 2016/10/16 | CC11 | Sediment | Schwarzengrund | 96 | 80849 |
| HJ-07 | SRR5886279 | 2016/10/16 | PR | Sediment | Muenster | 321 | 80796 |
| HJ-08 | SRR5886351 | 2016/10/16 | PR | Sediment | Muenster | 321 | 80796 |
| HJ-09 | SRR5886350 | 2016/10/16 | CC704 | Sediment | Mbandaka | 413 | 80794 |
| HJ-10 | SRR5884063 | 2016/12/05 | MC | Sediment | Anatum | 64 | 80537 |
| HJ-11 | SRR5884068 | 2016/12/05 | CC704 | Sediment | Schwarzengrund | 96 | 80539 |
| HJ-12 | SRR5884053 | 2016/12/05 | CC704 | Sediment | Senftenberg | 14 | 80536 |
| HJ-13 | SRR5884069 | 2017/01/15 | CC11 | Sediment | Hadar | 33 | 80533 |
| HJ-14 | SRR5884070 | 2017/01/15 | CC11 | Sediment | Hadar | 33 | 80527 |
| HJ-15 | SRR5884058 | 2017/01/15 | CC11 | Sediment | Hadar | 33 | 80538 |
| HJ-16 | SRR5884066 | 2017/02/01 | L | Litter | Cerro | 367 | 80542 |
| HJ-17 | SRR5884067 | 2017/02/01 | L | Litter | Typhimurium | 19 | 80529 |
| HJ-18 | SRR5884056 | 2017/02/01 | L | Litter | Typhimurium | 19 | 80535 |
| HJ-19 | SRR5884062 | 2017/02/01 | L | Litter | Typhimurium | 19 | 80530 |
| HJ-20 | SRR5884079 | 2017/02/01 | L | Litter | Typhimurium | 19 | 80534 |
| HJ-21 | SRR5884080, SRR13268785d | 2017/02/05 | CC11 | Sediment | Typhimurium | 19 | 80528 |
| HJ-22 | SRR5884077 | 2017/02/26 | CC704 | Sediment | Muenchen | 112 | 80532 |
| HJ-23 | SRR5884081 | 2017/02/26 | CC704 | Sediment | Muenchen | 112 | 80526 |
| HJ-24 | SRR6832866, SRR13268782d | 2017/09/10 | PR | Sediment | Montevideo | 138 | 111799 |
| HJ-25 | SRR6832877 | 2017/09/10 | PR | Sediment | Montevideo | 138 | 169196 |
| HJ-26 | SRR6832873 | 2017/09/10 | MC | Sediment | Senftenberg | 14 | 111800 |
| HJ-27 | SRR6366729 | 2017/10/22 | CC704 | Sediment | Cerro | 367 | 101373 |
| HJ-28 | SRR6367403 | 2017/10/22 | CC704 | Sediment | Cerro | 367 | 101373 |
| HJ-29 | SRR6369106, SRR13268783d | 2017/10/22 | CC704 | Sediment | Anatum | 64 | 11895 |
| HJ-30 | SRR6367413 | 2017/10/22 | CCP | Sediment | Braenderup | 22 | 4601 |
| HJ-31 | SRR6367404 | 2017/10/22 | CCP | Sediment | Braenderup | 22 | 101420 |
| HJ-32 | SRR6367414 | 2017/10/22 | CCP | Sediment | Braenderup | 22 | 4601 |
| HJ-33 | SRR6367467 | 2017/10/22 | MC | Sediment | Montevideo | 138 | 101411 |
| HJ-34 | SRR6832876 | 2018/01/21 | CCP | Sediment | Braenderup | 22 | 113399 |
| HJ-35 | SRR6832896 | 2018/01/21 | CCP | Sediment | Braenderup | 22 | 4601 |
| HJ-36 | SRR6832925 | 2018/01/21 | CCP | Sediment | Typhimurium | 19 | 113353 |
| HJ-37 | SRR6832911 | 2018/01/21 | CCP | Sediment | Typhimurium | 19 | 111797 |
| HJ-38 | SRR6832904, SRR13268784d | 2018/01/21 | CCP | Sediment | Typhimurium | 19 | 111798 |
| HJ-39 | SRR6832916 | 2018/01/21 | CCP | Sediment | Typhimurium | 19 | 111391 |
| FHS-01 | SRR6832910 | 2018/01/23 | CC704 | Sediment | Montevideo | 138 | 166542 |
| FHS-02 | SRR6832913, SRR13268786d | 2018/01/23 | CC704 | Sediment | Infantis | 32 | 111896 |
| FHS-04 | SRR6832912 | 2018/01/23 | CC704 | Sediment | Montevideo | 138 | 152383 |
| DG-01 | SRR8360264 | 2018/05/20 | PR | Sediment | Muenchen | 112 | 131452 |
| DG-02 | SRR8360271 | 2018/05/20 | PR | Sediment | Montevideo | 138 | 131598 |
| DG-03 | SRR8104581 | 2018/05/20 | PR | Sediment | Montevideo | 138 | 131599 |
| DG-04 | SRR8104579 | 2018/05/20 | CC11 | Sediment | Hadar | 33 | 131627 |
| DG-05 | SRR8104582 | 2018/05/20 | CC11 | Sediment | Hadar | 33 | 131627 |
| DG-06 | SRR7504390 | 2018/05/20 | CC11 | Sediment | Hadar | 33 | 131627 |
| DG-07 | SRR7506701 | 2018/05/20 | CC11 | Sediment | Hadar | 33 | 136579 |
| DG-08 | SRR7506695 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 136587 |
| DG-09 | SRR7506699 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 131813 |
| DG-10 | SRR8360260 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 153131 |
| DG-11 | SRR7504359 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 153155 |
| DG-12 | SRR7506710 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 153158 |
| DG-13 | SRR7499244 | 2018/05/20 | CC704 | Sediment | Typhimurium | 19 | 153152 |
| DG-14 | SRR7499253 | 2018/05/20 | MC | Sediment | Meleagridis | 463 | 135814 |
| DG-15 | SRR7499245 | 2018/05/20 | MC | Sediment | Meleagridis | 463 | 135813 |
| DG-16 | SRR7499272 | 2018/05/20 | MC | Sediment | Cerro | 367 | 135849 |
| DG-17 | SRR7499280 | 2018/05/20 | MC | Sediment | Meleagridis | 463 | 135804 |
| DG-18 | SRR7499278 | 2018/05/20 | MC | Sediment | Meleagridis | 463 | 135809 |
| DG-19 | SRR7889322 | 2018/05/20 | CC11 | Sediment | Schwarzengrund | 96 | 144617 |
| DG-20 | SRR7878396 | 2018/05/20 | CC704 | Sediment | Alachua | 1298 | 144057 |
| DG-21 | SRR7889352 | 2018/05/20 | CCP | Sediment | Mbandaka | 413 | 144638 |
| DG-22 | SRR7878395 | 2018/05/20 | BR | Sediment | Mbandaka | 413 | 144059 |
| AP-01 | SRR8179982 | 2018/09/03 | CC11 | Sediment | Bareilly | 2553 | 153210 |
| AP-02 | SRR8179943 | 2018/09/03 | CC11 | Sediment | Bareilly | 2553 | 153210 |
| AP-03 | SRR8179958 | 2018/09/03 | CC11 | Sediment | Bareilly | 2553 | 153210 |
| AP-04 | SRR8179911 | 2018/09/03 | CC11 | Sediment | Bareilly | 2553 | 153210 |
| BES-01 | SRR8179966 | 2018/09/03 | CC704 | Sediment | Reading | 412 | 153206 |
| BES-02 | SRR8179907 | 2018/09/03 | CC11 | Sediment | Reading | 412 | 153208 |
| PPL-01 | SRR8179980 | 2018/09/03 | CC11 | Sediment | Braenderup | 22 | 4601 |
| PPL-02 | SRR8179892 | 2018/09/03 | CC11 | Sediment | Braenderup | 22 | 4601 |
| WEK-01 | SRR8179901 | 2018/09/03 | CC704 | Sediment | Montevideo | 138 | 153130 |
| WEK-02 | SRR8179936 | 2018/09/03 | CC11 | Sediment | Mbandaka | 413 | 153196 |
| WEK-03 | SRR8179927 | 2018/09/03 | CC11 | Sediment | Braenderup | 22 | 4601 |
| WEK-04 | SRR8179981 | 2018/09/03 | CC11 | Sediment | Mbandaka | 413 | 153188 |
| WMD-01 | SRR8570270 | 2018/09/30 | PR | Sediment | Muenchen | 112 | 164635 |
| WMD-02 | SRR8570265 | 2018/09/30 | CC11 | Sediment | Give | 654 | 169281 |
| WMD-03 | SRR8570271 | 2018/09/30 | MC | Sediment | Montevideo | 138 | 164641 |
| WMD-04 | SRR8570267 | 2018/09/30 | MC | Sediment | Montevideo | 138 | 164640 |
| WMD-05 | SRR8570264 | 2018/09/30 | CCA | Sediment | Muenster | 321 | 164639 |
| WMD-07 | SRR8573695 | 2018/09/30 | PR | Sediment | Montevideo | 138 | 164637 |
| WMD-08 | SRR8570269 | 2018/09/30 | CCA | Sediment | Montevideo | 138 | 164636 |
| WMD-09 | SRR8570273 | 2018/09/30 | MC | Sediment | Montevideo | 138 | 164643 |
| WMD-10 | SRR8570263 | 2018/09/30 | CCA | Sediment | Cerro | 367 | 164638 |
| WMD-11 | SRR8570268 | 2018/09/30 | MC | Sediment | Typhimurium | 19 | 164642 |
| WMD-12 | SRR8570266 | 2018/09/30 | BR | Sediment | Give | 654 | 164644 |
| WMD-13 | SRR8570272 | 2018/09/30 | CCA | Sediment | Cerro | 367 | 164645 |
aPR: Pleasant Run, CC11: Cook’s Creek Rt. 11, CC704: Cook’s Creek Rt. 704, CCP: Cook’s Creek Park, CCA: Cook’s Creek Arboretum, MC: Muddy Creek, BR: Black’s Run, and L: poultry litter.
bMLST: 7-gene multilocus sequence type.
ccgMLST: core-genome multilocus sequence type, Enterobase scheme (Zhou et al., 2018).
dOxford Nanopore fastq SRR numbers.
Distribution of Sero- and Genotypes
Serotypes were determined by consensus of both SeqSero2 and SISTR and, for strains HJ1 to HJ26, using SMART PCR (Leader et al., 2009). SeqSero2 was unable to serotype strains HJ-02, HJ-04, HJ-13, and HJ-20; however, SMART PCR agreed with SISTR in its serotype determination for these isolates. The 88 isolates were distributed in 19 serotypes, of which the largest number (n = 16) were Typhimurium. The serotypes exactly aligned with the seven-gene multilocus sequence type (MLST), i.e., each serotype corresponded with exactly one MLST. It was found that not only were different serotypes isolated from within the same source, but the same serotype was isolated from different sources as well (Figure 2). Also, while certain serotypes were confined to a single source (e.g., Hadar, Bareilly, and Meleagridis), others such as Typhimurium, Montevideo, and Cerro were isolated from a number of different sources.
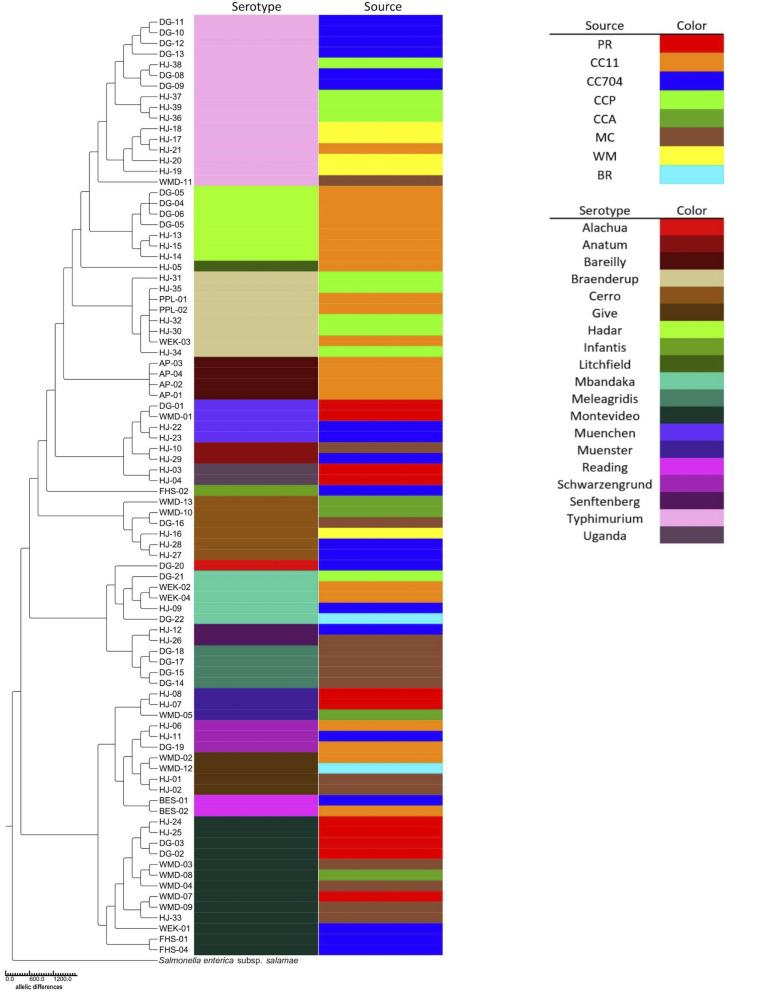
FIGURE 2.
Neighbor-joining cgMLST phylogenetic tree of 88 S. enterica isolates generated using the Enterobase GrapeTree RapidNJ algorithm. Branch lengths correspond to the number of allelic differences between isolates. The tree was rooted using S. enterica subspecies salamae strain 1315K. Isolate source and serotype are indicated by colored bars.
Among the 88 isolates, 75 distinct core-genome multilocus sequence types (cgMLST) were found (Table 1). Thirteen were apparently duplicates, having the same cgMLST as at least one other strain isolated from the same source on the same date. However, six serotype Braenderup strains with the same cgMLST (#4601; strains HJ-30, -32, and-35, PPL-01 and -02, and WEK-03) were isolated from different areas of Cooks Creek over the course of three samplings in 1 year. They were isolated in October 2017 (HJ-30 and HJ-32) and January 2018 (HJ-35) from the upstream CCP site, and in September 2018 the same sequence type was isolated three times (PPL-01, PPL-2, and WEK-03) downstream at the CC11 sampling site (Table 1).
Antibiotic Resistance and Plasmid Characterization
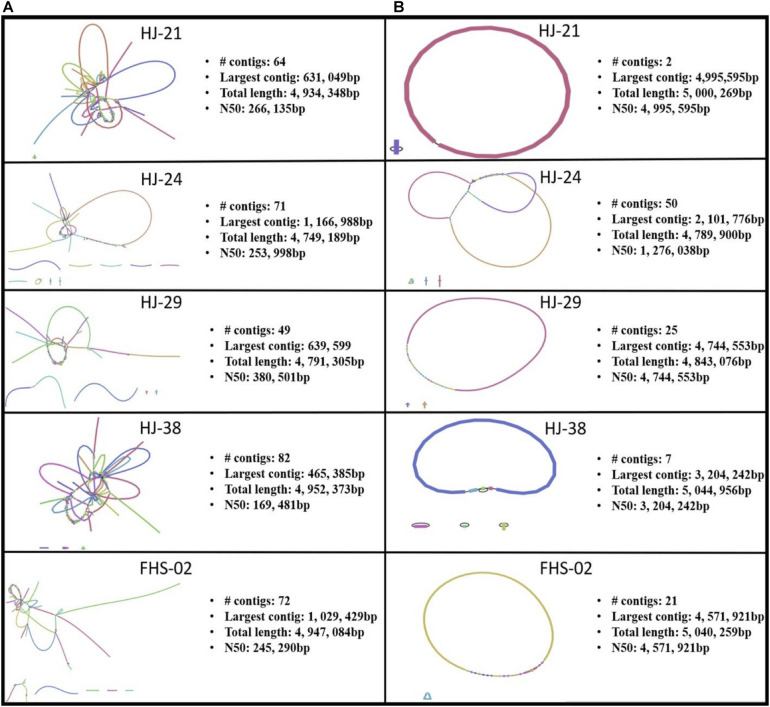
Of the 88 isolates collected, 31 were found to contain one or more ARGs (data not shown). Of the 31 isolates with ARGs, 26 were predicted using MOB_Suite and/or PlasmidFinder to house one or more plasmids (data not shown). However, both these tools use assemblies based on short read data as inputs. Short read sequencing data, though highly accurate, result in fragmented, discontiguous assemblies (Figure 3A). A hybrid, whole genome assembly approach, incorporating both short and long read data, allowed for the resolving of complete or nearly complete genomes (Figure 3B).
FIGURE 3.
Bandage visualizations of five Salmonella enterica plasmid assemblies using (A) short read sequence data only, and (B) hybrid assemblies using short and long read data.
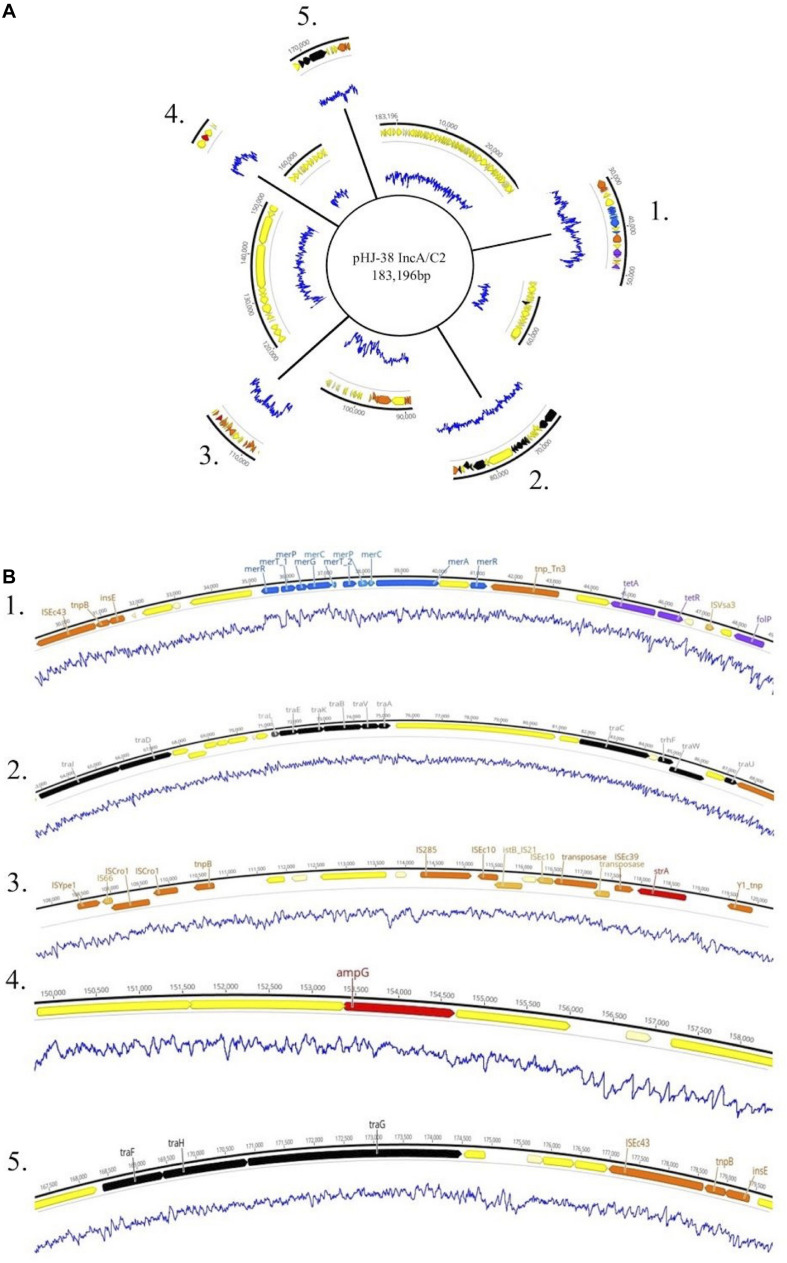
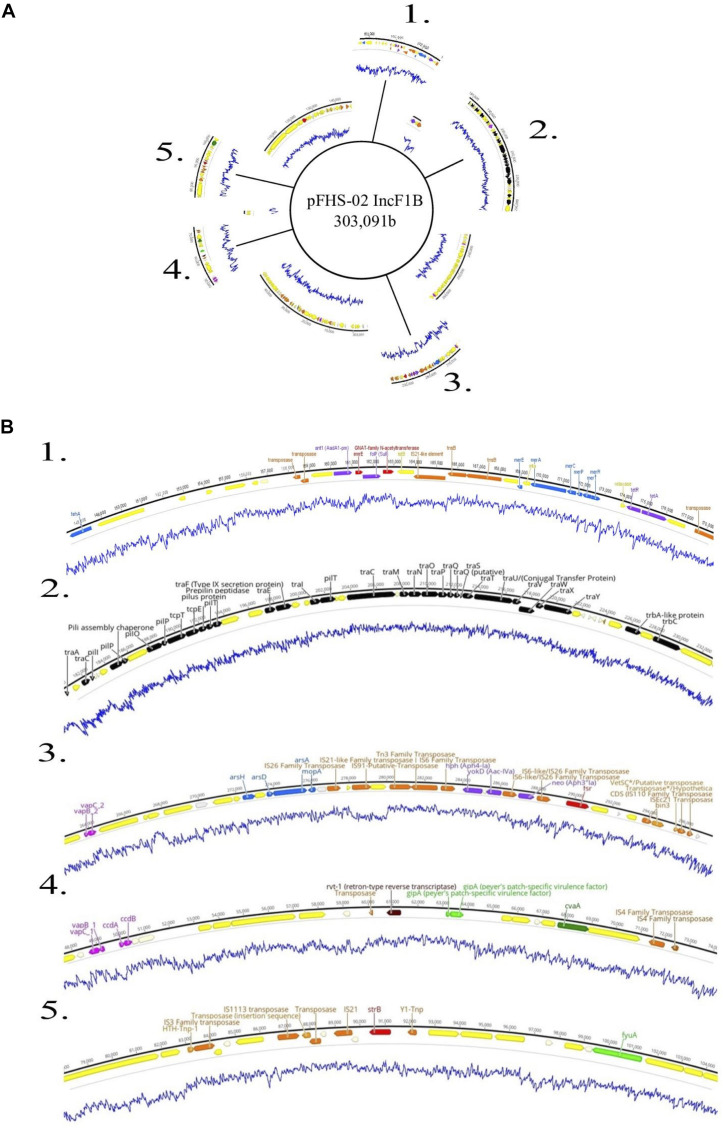
Plasmids identified in isolates that contained one or more ARGs were annotated using Prokka (Seemann, 2014). Two of these plasmids are shown in Figures 4, 5. Plasmid pHJ-38 was an IncA/C2 plasmid found to have multiple ARGs as predicted by ABRicate and ResFinder (Figure 4). Other notable features identified were multiple heavy metal resistance genes and tra genes. Tra genes are essential for plasmid conjugation between potential host bacteria (Zatyka and Thomas, 1998; Thomas, 2000) and suggest this is a self-transmissible plasmid. Similarly, in the IncF1B plasmid pFHS-02, multiple antibiotic and heavy metal resistance genes were identified (Figure 5). Along with tra genes, pil genes were also present in both plasmids. These genes encode a different pilus than those typically encoded by tra genes, one usually associated with conjugation in liquid environments (Bradley, 1984; Zatyka and Thomas, 1998). Plasmid pFHS-02 was also found to contain multiple toxin-antitoxin (or “plasmid addiction”) systems. These systems exist to ensure a plasmid’s successful replication during host cell division (Hayes, 2003).
FIGURE 4.
(A) Genetic map of the 184 kb IncA/C2 plasmid pHJ-38. Excised regions 1–5 of the plasmid contain mobility associated genes and genetic elements, antimicrobial and metal resistance genes, virulence genes, and other regions of interest. (B) Annotated gene regions containing heavy metal resistance genes (blue); virulence factors (light green); colicin genes (dark green); toxin-antitoxin gene cassettes (pink); genes associated with transposons, IS elements, or integrons (orange); and conjugation genes (black) are shown. Antimicrobial resistance genes identified by both Prokka and Abricate (purple) or by Prokka alone (red) are also shown. Yellow and cream-colored gene regions indicate hypothetical proteins. Blue lines indicate percent GC content.
FIGURE 5.
(A) Genetic map of the 303 kb IncF1B plasmid pFHS-02. Regions 1–5 of the plasmid containing mobility associated genes and genetic elements, antimicrobial and metal resistance genes, virulence genes, and other regions of interest are shown excised. (B) Annotated gene regions; genes and labels are colored as indicated in Figure 4. Blue lines indicate percent GC content.
Assessment of Educational Outcomes
To determine the impact of the CURE on the development of participants’ content knowledge, skills, and attitudes in the domain, direct measures of classroom performance (see Jurgensen et al., in press for an extended discussion of the employed assessments) were partnered with indirect self-report data collected via in-class surveys near the beginning and end of the term. During the first 2 weeks of class, students were asked to complete a short set of closed-response items regarding their academic and demographic background with a limited number of open-ended questions focused on their course expectations. At the end of the term, students responded to Likert scale questions drawn from multiple validated instruments (Hurtado and Carter, 1997; Smith et al., 2013; Corwin et al., 2015b; Hanauer et al., 2017; Maltese et al., 2017) combined with closed-response and open-response questions designed for this study to capture data pertaining to changes in academic/career interests and course design. Survey data were collected online and aggregately analyzed from students in three consecutive semesters of the course (fall 2018, spring 2019, fall 2019) with approval by the university’s Institutional Review Board (IRB). Student participants (n = 50) were all biology majors with 74% self-identifying as female, 72% white, 30% first-generation students, 8% had participated in an apprenticeship-like undergraduate research experience, and 26% were working >15 h per week “to make ends meet,” which suggests the course offered an opportunity to engage students who may not be able to volunteer as a traditional, mentored undergraduate researcher due to financial considerations (Bangera and Brownell, 2014). Here, given the focus of this special issue, we report on a subset of post-survey items used to address questions as to how participation in the research course influenced students’ academic/career interest and persistence in science, while a description of conferred cognitive outcomes and perceptions of the course is presented in Jurgensen et al. (in press).
Measures
Multiple data points were collected to gain an understanding of how the experience influenced students’ academic and career interests. First, using an approach comparable to Maltese and Harsh (2015), students were asked at the onset and end of term to report their intentions (e.g., STEM graduate school, professional school, non-STEM career, and unsure) upon graduation. Exiting students were also prompted if their future intentions had shifted over the term, and if so, they were asked to qualitatively describe whether the course had influenced their plans. Additionally, we also asked students to rate their level of interest in research and science, in general, after the class on a five-point scale. Then, students completed eight parallel items using five-point Likert-type scales (1 = far less interested, 5 = far more interested) created for this project that assessed their interest in pursuing future coursework and research opportunities in specific course topics (i.e., microbiology, molecular biology, bioinformatics, genomics, and microbial ecology) via the stem prompt, “After this class, I am [interest level] in future [coursework or research opportunities] in [topic].” Finally, we used the Persistence in the Sciences survey (PITS) and measures of sense of belonging to assess conferred psychosocial outcomes to research course participants that are relevant to STEM persistence (Chemers et al., 2011; Estrada et al., 2011). The PITS instrument (Hanauer et al., 2016) is designed to measure psychological variables related to student persistence (e.g., project-ownership, self-efficacy, science identity), and has been previously used to assess the effectiveness of CURE educational designs (Hanauer et al., 2017; Corwin et al., 2018; Cooper et al., 2019; Zelaya et al., 2020). Each item is scored on a scale ranging from one to four or five (depending on variable), and then averaged to form a composite variable score. In addition, we also used items modified from Hurtado and Carter (1997) and Smith et al. (2013) to measure sense of belonging assessed using five-point Likert-type scales (1 = strongly disagree, 5 = highly agree).
Findings
Students entered the course largely with the intentions of attending graduate school in a STEM field (42%) or professional school (42%) after degree completion, with fewer intending to seek immediate employment in STEM fields (12%) or who were unsure of their respective plans (4%). Fifty-six percent of participants reported their academic or career plans changed to some degree during the term, with 36% of all students specifically identifying that the course influenced their future intentions. Most often, similar to interest shifts observed in mentored undergraduate research experience (Maltese and Harsh, 2015), students indicated that the experience refined or triggered their interest through exposure to fields of study and careers related to genomic epidemiology. For instance, two students stated in open-ended questions that “[The course] opened the door and exposed me to genomics and bioinformatics in which I had never thought about in my future career” and “It significantly influenced my future plans and [sic] considering to attend a graduate school specifically [to study] foodborne pathogens in relation to the environment so that I possibly could work for the CDC or FDA in the future.” A notable proportion of students upon exiting the course indicated that the experience enhanced their interest, to some degree, in coursework in bioinformatics (40%), microbial ecology (33%), molecular biology (24%), and genomics (22%). While the balance of respondents largely reported no change in their prior interests, a small subset (<10%) indicated decreased interests in these topical areas resultant of class participation. A modest shift in interest in microbiology coursework was also noted (8% increase, 92% no change), which likely reflects the preexisting interests of students that opted into such an upper-level course. Results also showed that 77% percent of students identified that course participation increased their overall interest in pursuing future research opportunities. More specifically, a fair proportion of students indicated that the experience enhanced their interests, to some degree, in research in bioinformatics (42% of respondents), molecular biology (34%), genomics (31%), and microbial ecology (24%) research with a smaller shift for microbiology (16%). On the other hand, approximately 20% of students indicated that they became less interested in research over the term in the respective areas of microbial ecology, bioinformatics, and genomics. Together, these results suggest that the research course refined student interest by the opportunity to test the proverbial waters of different fields through authentic practice, which may guide their later academic and career intentions in a means comparable to that of a traditional undergraduate research experience (Hunter et al., 2007; Maltese and Harsh, 2015).
In addition to observing shifts in academic and/or career interests, we sought to assess the impact of course participation on psychosocial aspects often correlated with academic success and STEM persistence (Perez et al., 2014; Trujillo and Tanner, 2014). Most students (80, 72, and 76%, respectively) reported on the post-survey that participation in the course directly contributed to a greater sense of belonging to the department, campus community, and scientific community. Students reported high ratings on PITS items measuring their science-identity (M = 4.52, SD = 0.46), self-efficacy (M = 4.32, SD = 0.77), cognitive project ownership (M = 4.22, SD = 0.59), and networking (M = 4.10, SD = 0.71) as related to course participation. These ratings are comparable to or higher than those reported in previously published studies on biologically focused CUREs (Corwin et al., 2015a; Hanauer et al., 2017; Cooper et al., 2019; Zelaya et al., 2020) that use the same instruments (Hanauer and Dolan, 2014; Hanauer et al., 2016) to assess student outcomes. The overall pattern of early findings suggests the research course contributes to psychosocial outcomes that influence STEM persistence, though additional data is needed to allow comparisons to be drawn between groups to assess the impact of participation of the experience on all students.
Discussion
Animal husbandry plays a prominent role in the economy of the Shenandoah Valley of Virginia, with an estimated 159 million chickens, 16 million turkeys, and over a half million cattle raised in 2012 in four counties alone. These were estimated to produce over 400 tons and 1.28 billion gallons of manure in that year10. We hypothesized that agricultural runoff, particularly from poultry and cattle, would result in detectable enteric pathogens such as Salmonella in these streams and rivers. In the approximately 15 months before beginning this course, and then over three semesters of the course itself, the sediments of seven sites on four streams and one poultry house were sampled and a total of 88 distinct S. enterica strains were isolated. Standard microbiological techniques along with the Enteropluri tube and PCR of the invA gene were used to verify the isolates’ identity (Figure 1A). Students typically characterized their isolates further by examining phenotypic antibiotic resistance (using Kirby-Bauer or SensititreTM MIC panels) and by isolating native plasmids (Heringa et al., 2007). After short read Illumina sequencing, students in the course used the FDA’s GalaxyTrakr web platform for sequence trimming and filtering, assembly of draft genomes, sequence and genome assembly quality control, annotation of assemblies, and in silico serotyping. GalaxyTrakr is an instance of Galaxy11 that was developed by the FDA as a bioinformatics platform for use by United States public health laboratories. However, it is particularly advantageous for educational use, as the computational tools included are only those typically used for studying microbial genomes in general, particularly those of pathogens (Gangiredla et al., 2021). After they assembled, annotated, and serotyped their isolate genomes, students were then asked to pursue specific questions and hypotheses related to genes and gene functional categories of interest in their isolates, including those relating to antibiotic resistance, virulence, phages, plasmids, transposons, etc.
The 88 strains of S. enterica represented 19 unique serotypes. In the CDC’s report on the top 10 serotypes responsible for human infections in the United States12, five of the ten – Typhimurium, Infantis, Muenchen, Montevideo, and Braenderup – were repeatedly isolated in this study. Interestingly, a notable serotype that was not isolated was Enteritidis. This was surprising because Enteritidis is one of the most common serotypes associated with poultry13, yet no samples near or even directly from a poultry farm yielded this serotype.
Enterobase was used to identify 75 distinct core genome multilocus sequence types (cgMLSTs) among the 88 isolates (Table 1 and Figure 2). The cgMLST implemented in Enterobase is a highly discriminatory typing scheme. It reflects a so-called “soft-core genome” which in Salmonella consists of 3,002 genes found in ≥98%, intact in ≥94% and of “unexceptional diversity” in over 3,000 Salmonella genomes (Alikhan et al., 2018). A single base-pair difference in any of these genes would result in a different cgMLST. Six Braenderup isolates of cgMLST 4601 were isolated at different times and from different portions of Cooks Creek, with three isolates found upstream and three isolated 9–11 months later from an area ca. 7.5 km downstream. Since all belonged to the same core genome sequence type, there was no detected divergence between the isolates (Figure 2), suggesting that they may have come from a common source. It is possible that members of the same population of Salmonella from the upstream site moved down to the downstream site, or that there were independent introductions of the same type, perhaps from poultry litter spread on fields within the watershed.
Five strains were further sequenced using the Oxford Nanopore MinIONTM and hybrid-assembled to yield complete or near-complete circular assemblies. Two of these plasmids, pHJ38 and pFHS-02, were annotated and found to have multiple ARGs and two types of pilus genes potentially facilitating conjugation under multiple environmental conditions (Figures 3, 4). Plasmid pFHS-02 was particularly notable as it was a very large (303 kb) megaplasmid containing 11 predicted ARGs, 11 predicted heavy metal (mercury, arsenic, tellurite, and molybdenum) resistance genes, four predicted toxin-antitoxin systems, multiple transposons and IS elements, and a phage-associated virulence gene (gipA) associated with Peyer’s patch colonization and macrophage survival (Stanley et al., 2000; Vazeille et al., 2016). We have previously isolated numerous large, self-transmissible multidrug-resistant plasmids from many of these streams (Herrick et al., 2014).
We present here a model laboratory course design for introducing upper level microbiology undergraduates to real-world public health and pathogen surveillance methods and applications, as well as to laboratory research techniques and computational biology methods. At our university, this elective one-semester course is offered to students who have taken a course in general microbiology, including laboratory. Most of these students are majors in biology, typically with a concentration in microbiology, although some are allied health students majoring in health science, nursing, etc.
The course is divided into separate modules – one on the wet lab methods used for Salmonella isolation, isolation, and identification, and the other covering bioinformatics techniques. Linking the two modules is the gratis sequencing provided, in our case, by the United States FDA and our state public health laboratory, the Virginia DCLS. Foodborne and other related pathogens are of interest to many regional, state, and national public health laboratories for genomic epidemiological surveillance and these agencies are often willing, even eager, to sequence these at no cost. Although Module 1, focusing on the isolation of Salmonella, requires knowledge and skills in general microbiology laboratory techniques, the computational methods of Module 2 could conceivably be utilized by students with only a background in general college biology concepts. Module 2 in particular can be employed as a standalone research experience. Students could download the raw reads of Salmonella or other pathogens that are readily available from the NCBI Sequence Read Archive and work with them using the Bioinformatics Lab Guides available for this course2.
Salmonella enterica is one of the leading causes of foodborne illness in the world (Majowicz et al., 2010). It is also one of the most-sequenced organisms on earth2, due primarily to the massive efforts of agencies like the United States Food and Drug Administration and individual United States state public health laboratories. These and other national and regional agencies are interested in tracking the occurrence and spread of S. enterica and other pathogenic bacteria (Allard et al., 2018) and therefore are often willing to sequence, at no cost, the whole genomes of isolated strains, especially those isolated from less-sampled sources. However, public health agencies’ interest in the huge number of Salmonella, E. coli, Clostridioides, Vibrio, and other pathogens being sequenced worldwide rarely extends beyond cataloging their genomes for possible future epidemiological use. There is therefore a vast repository of essentially unanalyzed raw sequencing reads that have never been analyzed beyond a simple automated assembly and annotation, let alone examined for important accessory and other genetic elements such as plasmids, phages, transposons, ARGs, CRISPR regions, or virulence genes. This opens up an opportunity for students to work on authentic and important problems not only in genomic epidemiology and pathogen surveillance but also in mobile gene transfer, antibiotic resistance, the evolution of virulence, microbial ecology, etc. Students can potentially use and analyze either (1) strains they isolate themselves, or (2) the archived pathogen genomes in NCBI and other genome repositories. Depending on their needs, instructors could deploy a course such as this as a whole or as separate modules, one focusing on the wet lab isolation, identification, and characterization of Salmonella and the other on the genomics of Salmonella or other pathogens. Although Salmonella is a Biosafety II level (BSL 2) pathogen, it is relatively safe to work with in a classroom setting. It is not uncommonly cultivated for use in upper-division and even general microbiology laboratory courses (Ponder and Sumner, 2009; Marvasi et al., 2015). We have recently published details on safely setting up and using either module or the course in its entirety (Jurgensen et al., in press). We have also established an OSF page containing protocols, bioinformatics guides, safety documents, posters, etc. related to the course (see text footnote 1).
Over three-quarters of the students who took the course indicated they developed increased interest in research. Over 40% were interested in pursuing further study or research in bioinformatics and genomics and more than a third said that it influenced their future academic or career plans, particularly in relation to pathogen genomics and genomic epidemiology. Students in the course also benefited from their interaction with our research lab, where we are seeking to understand more specifically the ecology of the Enterobacteriaceae in secondary habitats such as streams, as well as their evolution via horizontal gene transfer. This course serves as a “feeder” of both data and interested students to more advanced research projects. Graduate and advanced undergraduate students from our research lab also serve as mentors to students in the course, especially with the more advanced aspects of their projects. Data generated from the course has been presented in a regional symposium14 and used in thesis projects (Jurgensen, 2018; Greenman, 2019).
This course provided a unique opportunity for microbiology students to gain valuable skills in pathogen isolation and identification, and in the basics of WGS and microbial genomics. Students were also introduced to the applications of these methods in public health microbiology, genomic epidemiology, pathogen surveillance, and in genome research in general. Course-based research experiences such as this can provide many of the benefits of traditional mentor-guided, open-ended and authentic research to students who might not otherwise have such an opportunity. They can also provide opportunities for students who have the interest to pursue further and deeper research questions on their isolates and their genomes, questions that are directly applicable to genomic epidemiology and to understanding the genomics and ecology of foodborne pathogens in the environment.
Data Availability Statement
Illumina raw sequencing reads were deposited in the NCBI Sequence Read Archive (SRA) under BioProject PRJNA186035 for isolates HJ-01 through HJ-23 and BioProject PRJNA219491 for all others. Nanopore sequences for isolates HJ-21, HJ-24, HJ-29, HJ-38, and FHS-02 have been deposited in the SRA under BioProject PRJNA605356. SRR identifiers can be found in Table 1.
Ethics Statement
Procedures involved in the collection and analysis of student data to assess the efficacy and impact of the research course, including the informed consent process and confidentiality parameters, were reviewed and approved by the James Madison University’s Institutional Review Board (IRB No. 18-0508).
Author Contributions
JBH conceived the project. NAG, SKJ, CPH, CJK, and JBH designed the experiments. SKJ, CPH, CJK, RED, WMM, and JBH developed the methods. SKJ, RED, WMM, and JBH developed the instructional aspects of the Bacterial Discovery course. NAG, SKJ, CPH, CJK, RED, WMM, DE, BAE, SMH, TNK, HGL, DJL, and BW performed the experiments and conducted bioinformatics data analysis (undergraduates DE, BAE, TNK, HGL, DJL, and BW carried these out during the course itself). JAH gathered, compiled, and analyzed student assessment data. NAG, JBH, and JAH wrote the manuscript. SKJ, CJK, and RED edited it. The figures and table were generated by NAG. JSK, CPH, RED, WMM, DE, BAE, SMH, TNK, HGL, DJL, and BW were undergraduates at the time this work was done, NAG and CJK were graduate students, and JAH and JBH were on the faculty at James Madison University. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge Dr. Lauren Turner and the Virginia Department of Consolidated Laboratory Services and Drs. Marc Allard and Rebecca Bell of the United States Food and Drug Administration Center for Food Safety and Applied Nutrition for DNA sequencing support. Kevin Libuit also provided useful bioinformatics advice. Dr. Jonathan Frye and Lari Hiott provided technical support in serotyping and in antibiotic resistance phenotyping. The authors are especially grateful for the ongoing support of the James Madison University Department of Biology and College of Science and Mathematics.
Funding. The development and implementation of this study and course were supported by Madison Trust grant #MT23. Support was also provided by the Virginia 4VA program and the JMU Department of Biology.
References
- Alikhan N.-F., Zhou Z., Sergeant M. J., Achtman M. (2018). A genomic overview of the population structure of Salmonella. PLoS Genet. 14:e1007261. 10.1371/journal.pgen.1007261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard M. W., Bell R., Ferreira C. M., Gonzalez-Escalona N., Hoffmann M., Muruvanda T., et al. (2018). Genomics of foodborne pathogens for microbial food safety. Curr. Opin. Biotechnol. 49 224–229. [DOI] [PubMed] [Google Scholar]
- American Association for the Advancement of Science (2011). Vision and Change in Undergraduate Biology Education: a Call to Action. Washington, DC: American Association for the Advancement of Science. [Google Scholar]
- Andrews W. H., Jacobson A., Hammack T. (2014). Bacteriological Analytical Manual: Salmonella. Silver Spring: U.S. Food and Drug Administration. [Google Scholar]
- Armstrong G. L., MacCannell D. R., Taylor J., Carleton H. A., Neuhaus E. B., Bradbury R. S., et al. (2019). Pathogen genomics in public health. N. Engl. J. Med. 381 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss L. C., Laursen S. L., Branchaw J. L., Eagan K., Graham M., Hanauer D. I., et al. (2014). Assessment of course-based undergraduate research experiences: a meeting report. CBE Life Sci. Educ. 13 29–40. 10.1187/cbe.14-01-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangera G., Brownell S. E. (2014). Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci. Educ. 13 602–606. 10.1187/cbe.14-06-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Zheng J., Burrows E., Allard S., Wang C. Y., Keys C. E., et al. (2015). Ecological prevalence, genetic diversity, and epidemiological aspects of Salmonella isolated from tomato agricultural regions of the Virginia Eastern Shore. Front. Microbiol. 6:415. 10.3389/fmicb.2015.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K. (2018). “Salmonella enterica,” in Foodborne Microbial Pathogens: Mechanisms and Pathogenesis, ed. Bhunia A. K. (New York, NY: Springer; ), 271–287. [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. (1984). Characteristics and function of thick and thin conjugative pili determined by transfer-derepressed plasmids of incompatibility groups I1. I2, I5, B, K and Z. J. Gen. Microbiol. 130 1489–1502. 10.1099/00221287-130-6-1489 [DOI] [PubMed] [Google Scholar]
- Burton G. A., Jr., Gunnison D., Lanza G. R. (1987). Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 53 633–638. 10.1128/aem.53.4.633-638.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A., Zankari E., Garciá-Fernández A., Larsen M. V., Lund O., Villa L., et al. (2014). In silico detection and typing of plasmids using Plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58 3895–3903. 10.1128/aac.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemers M. M., Zurbriggen E. L., Syed M., Goza B. K., Bearman S. (2011). The role of efficacy and identity in science career commitment among underrepresented minority students: efficacy and identity in science career commitment. J. Soc. Issues 67 469–491. 10.1111/j.1540-4560.2011.01710.x [DOI] [Google Scholar]
- Cooper K. M., Blattman J. N., Hendrix T., Brownell S. E. (2019). The impact of broadly relevant novel discoveries on student project ownership in a traditional lab course turned cure. CBE Life Sci. Educ. 18:ar57. 10.1187/cbe.19-06-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. A., Graham M. J., Dolan E. L. (2015a). Modeling course-based undergraduate research experiences: an agenda for future research and evaluation. CBE Life Sci. Educ. 14:es1. 10.1187/cbe.14-10-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. A., Runyon C., Robinson A., Dolan E. L. (2015b). The laboratory course assessment survey: a tool to measure three dimensions of research-course design. CBE Life Sci. Educ. 14:ar37. 10.1187/cbe.15-03-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. A., Runyon C. R., Ghanem E., Sandy M., Clark G., Palmer G. C., et al. (2018). Effects of discovery, iteration, and collaboration in laboratory courses on undergraduates’ research career intentions fully mediated by student ownership. CBE Life Sci. Educ. 17:ar20. 10.1187/cbe.17-07-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump J. A., Sjölund-Karlsson M., Gordon M. A., Parry C. M. (2015). Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 28 901–937. 10.1128/cmr.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., den Bakker H. C., Hendriksen R. S. (2016). Genomic epidemiology: whole-genome-sequencing–powered surveillance and outbreak investigation of foodborne bacterial pathogens. Annu. Rev. Food Sci. Technol. 7 353–374. 10.1146/annurev-food-041715-033259 [DOI] [PubMed] [Google Scholar]
- Estrada M., Woodcock A., Hernandez P. R., Schultz P. W. (2011). Toward a model of social influence that explains minority student integration into the scientific community. J. Educ. Psychol. 103 206–222. 10.1037/a0020743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangiredla J., Rand H., Benisatto D., Payne J., Strittmatter C., Sanders J., et al. (2021). GalaxyTrakr: a distributed analysis tool for public health whole genome sequence data accessible to non-bioinformaticians. BMC Genomics 22:114. 10.1186/s12864-021-07405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman N. (2019). Assessing the prevalence of multidrug resistant Salmonella enterica from stream sediment and poultry litter in the Shenandoah Valley of Virginia. Masters Theses, 2010–2019. 594. Available online at: https://commons.lib.jmu.edu/master201019/594. [Google Scholar]
- Gurevich A., Saveliev V., Vyahhi N., Tesler G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29 1072–1075. 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield J., Croucher N. J., Goater R. J., Abudahab K., Aanensen D. M., Harris S. R. (2018). Phandango: an interactive viewer for bacterial population genomics. Bioinformatics 34 292–293. 10.1093/bioinformatics/btx610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer D. I., Dolan E. L. (2014). The project ownership survey: measuring differences in scientific inquiry experiences. CBE—Life Sci. Educ. 13 149–158. 10.1187/cbe.13-06-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer D. I., Graham M. J., Hatfull G. F. (2016). A measure of college student persistence in the sciences (PITS). CBE Life Sci. Educ. 15:ar54. 10.1187/cbe.15-09-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer D. I., Graham M. J., Sea-Phages, Betancur L., Bobrownicki A., Cresawn S. G., et al. (2017). An inclusive Research Education Community (iREC): impact of the SEA-PHAGES program on research outcomes and student learning. Proc. Natl. Acad. Sci. U S A. 114 13531–13536. 10.1073/pnas.1718188115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F. (2003). Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301 1496–1499. 10.1126/science.1088157 [DOI] [PubMed] [Google Scholar]
- Hendricks C. W. (1971). Increased recovery rate of Salmonellae from stream bottom sediments versus surface waters. Appl. Microbiol. 21 379–380. 10.1128/am.21.2.379-380.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa S. D., Monroe J. D., Herrick J. B. (2007). A simple, rapid method for extracting large plasmid DNA from bacteria. Nat Prec. 10.1038/npre.2007.1249.1 [DOI] [Google Scholar]
- Herrick J. B., Haynes R., Heringa S., Brooks J. M., Sobota L. T. (2014). Coselection for resistance to multiple late-generation human therapeutic antibiotics encoded on tetracycline resistance plasmids captured from uncultivated stream and soil bacteria. J. Appl. Microbiol. 117 380–389. 10.1111/jam.12538 [DOI] [PubMed] [Google Scholar]
- Hunter A.-B., Laursen S. L., Seymour E. (2007). Becoming a scientist: the role of undergraduate research in students’ cognitive, personal, and professional development. Sci. Educ. 91 36–74. 10.1002/sce.20173 [DOI] [Google Scholar]
- Hurtado S., Carter D. F. (1997). Effects of college transition and perceptions of the campus racial climate on latino college students’. Sense Belonging. Sociol. Educ. 70 324–345. 10.2307/2673270 [DOI] [Google Scholar]
- Jurgensen S. (2018). A cure for salmonella: engaging students in pathogen microbiology and bioinformatics. Senior Honors Projects, 2010–2019. 623. Available online at: https://commons.lib.jmu.edu/honors201019/623 [Google Scholar]
- Jurgensen S. K., Harsh J., Herrick J. B. (in press). ). A CURE for Salmonella: A Laboratory Course in Pathogen Microbiology and Genomics. CourseSource. [Google Scholar]
- Leader B. T., Frye J. G., Hu J., Fedorka-Cray P. J., Boyle D. S. (2009). High-throughput molecular determination of salmonella enterica serovars by use of multiplex PCR and capillary electrophoresis analysis. J. Clin. Microbiol. 47 1290–1299. 10.1128/jcm.02095-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levantesi C., Bonadonna L., Briancesco R., Grohmann E., Toze S., Tandoi V. (2012). Salmonella in surface and drinking water: occurrence and water-mediated transmission. Food Res. Int. 45 587–602. 10.1016/j.foodres.2011.06.037 [DOI] [Google Scholar]
- Liu H., Whitehouse C. A., Li B. (2018). Presence and persistence of Salmonella in water: the impact on microbial quality of water and food safety. Front Public Health 6:159. 10.3389/fpubh.2018.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majowicz S. E., Musto J., Scallan E., Angulo F. J., Kirk M., O’Brien S. J., et al. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50 882–889. [DOI] [PubMed] [Google Scholar]
- Malorny B., Hoorfar J., Bunge C., Helmuth R. (2003). Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl. Environ. Microbiol. 69 290–296. 10.1128/aem.69.1.290-296.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese A. V., Harsh J. A. (2015). Pathways of Entry into STEM Across K–16. Interest and the Self in K-16 Mathematics and Science Learning, Washington, DC: American Educational Research Association. [Google Scholar]
- Maltese A., Harsh J., Jung E. (2017). Evaluating undergraduate research experiences—development of a self-report tool. Educ. Sci. 7:87. 10.3390/educsci7040087 [DOI] [Google Scholar]
- Marin C., Lorenzo-Rebenaque L., Laso O., Villora-Gonzalez J., Vega S. (2020). Pet reptiles: a potential source of transmission of multidrug-resistant Salmonella. Front. Vet. Sci. 7:613718. 10.3389/fvets.2020.613718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvasi M., Choudhury M., Teplitski M. (2015). Laboratory activity to teach about the proliferation of Salmonella in vegetables. J. Microbiol. Biol. Educ. 16 230–236. 10.1128/jmbe.v16i2.948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg B. G., Kijlstra A. (2007). Role of rodents in transmission of Salmonella and Campylobacter. J. Sci. Food Agric. 87 2774–2781. [Google Scholar]
- Nadon C., Van Walle I., Gerner-Smidt P., Campos J., Chinen I., Concepcion-Acevedo J., et al. (2017). PulseNet international: vision for the implementation of whole genome sequencing (WGS) for global food-borne disease surveillance. Euro. Surveill. 22:30544. 10.2807/1560-7917.ES.2017.22.23.30544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2017). Undergraduate Research Experiences for STEM Students: Successes, Challenges, and Opportunities. Washington, D.C: National Academies Press. [Google Scholar]
- National Research Council (US) Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century (2003). BIO2010: Transforming Undergraduate Education for Future Research Biologists. Washington, D.C: National Academies Press. [PubMed] [Google Scholar]
- Pachepsky Y. A., Shelton D. R. (2011). Escherichia coli and fecal coliforms in freshwater and estuarine sediments. Crit. Rev. Environ. Sci. Technol. 41 1067–1110. 10.1080/10643380903392718 [DOI] [Google Scholar]
- Parisi A., Stanaway J. D., Sarkar K., Crump J. A. (2020). The global burden of non-typhoidal Salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Int. J. Infect. Dis. 101:341. 10.1016/j.ijid.2020.09.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez T., Cromley J. G., Kaplan A. (2014). The role of identity development, values, and costs in college STEM retention. J. Educ. Psychol. 106 315–329. 10.1037/a0034027 [DOI] [Google Scholar]
- Ponder M. A., Sumner S. (2009). Use of case studies to introduce undergraduate students to principles of food microbiology, molecular biology, and epidemiology of food-borne disease. Biochem. Mol. Biol. Educ. 37 156–163. 10.1002/bmb.20277 [DOI] [PubMed] [Google Scholar]
- Ribas A., Poonlaphdecha S. (2017). Wild-caught and farm-reared amphibians are important reservoirs of Salmonella, a study in North-East Thailand. Zoonoses Public Health 64 106–110. 10.1111/zph.12286 [DOI] [PubMed] [Google Scholar]
- Robertson J., Nash J. H. E. (2018). MOB-suite: software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microbial Genom. 4:e000206. 10.1099/mgen.0.000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Silva C., Maloy S., Calva E. (2014). One health and food-borne disease: Salmonella transmission between humans, animals, and plants. Microbiol. Spectrum 2 137–148. 10.1128/microbiolspec.oh-0020-2013 [DOI] [PubMed] [Google Scholar]
- Smith J. L., Lewis K. L., Hawthorne L., Hodges S. D. (2013). When trying hard isn’t natural: women’s belonging with and motivation for male-dominated STEM fields as a function of effort expenditure concerns. Pers. Soc. Psychol. Bull. 39 131–143. 10.1177/0146167212468332 [DOI] [PubMed] [Google Scholar]
- Stanley T. L., Ellermeier C. D., Slauch J. M. (2000). Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar typhimurium survival in Peyer’s patches. J. Bacteriol. 182 4406–4413. 10.1128/jb.182.16.4406-4413.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöver B. C., Müller K. F. (2010). TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 11:7. 10.1186/1471-2105-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S. J., Snider C., Braden C. R., Boxrud D., Wünschmann A., Rudroff J. A., et al. (2007). Multidrug-resistant Salmonella enterica serotype typhimurium associated with pet rodents. N. Engl. J. Med. 356 21–28. [DOI] [PubMed] [Google Scholar]
- Tang P., Croxen M. A., Hasan M. R., Hsiao W. W. L., Hoang L. M. (2017). Infection control in the new age of genomic epidemiology. Am. J. Infect. Control. 45 170–179. 10.1016/j.ajic.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Thomas C. M. (2000). Paradigms of plasmid organization. Mol. Microbiol. 37 485–491. 10.1046/j.1365-2958.2000.02006.x [DOI] [PubMed] [Google Scholar]
- Trujillo G., Tanner K. D. (2014). Considering the role of affect in learning: monitoring students’ self-efficacy, sense of belonging, and science identity. CBE Life Sci. Educ. 13 6–15. 10.1187/cbe.13-12-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille E., Chassaing B., Buisson A., Dubois A., de Vallée A., Billard E., et al. (2016). GipA factor supports colonization of Peyer’s patches by Crohn’s disease-associated Escherichia coli. Inflamm. Bowel Dis. 22 68–81. 10.1097/mib.0000000000000609 [DOI] [PubMed] [Google Scholar]
- Wick R. R., Judd L. M., Gorrie C. L., Holt K. E. (2017). Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13:e1005595. 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick R. R., Schultz M. B., Zobel J., Holt K. E. (2015). Bandage: interactive visualization of de novo genome assemblies. Bioinformatics 31 3350–3352. 10.1093/bioinformatics/btv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield M. D., Groisman E. A. (2003). Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69 3687–3694. 10.1128/aem.69.7.3687-3694.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatyka M., Thomas C. M. (1998). Control of genes for conjugative transfer of plasmids and other mobile elements. FEMS Microbiol. Rev. 21 291–319. 10.1111/j.1574-6976.1998.tb00355.x [DOI] [PubMed] [Google Scholar]
- Zelaya A. J., Gerardo N. M., Blumer L. S., Beck C. W. (2020). The bean beetle microbiome project: a course-based undergraduate research experience in microbiology. Front. Microbiol. 11:577621. 10.3389/fmicb.2020.577621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Alikhan N.-F., Mohamed K., Fan Y., Agama Study, Group A. S., Achatman M. (2020). The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 30 138–152. 10.1101/gr.251678.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Alikhan N.-F., Sergeant M. J., Luhmann N., Vaz C., Francisco A. P., et al. (2018). GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 28 1395–1404. 10.1101/gr.232397.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Illumina raw sequencing reads were deposited in the NCBI Sequence Read Archive (SRA) under BioProject PRJNA186035 for isolates HJ-01 through HJ-23 and BioProject PRJNA219491 for all others. Nanopore sequences for isolates HJ-21, HJ-24, HJ-29, HJ-38, and FHS-02 have been deposited in the SRA under BioProject PRJNA605356. SRR identifiers can be found in Table 1.