Abstract
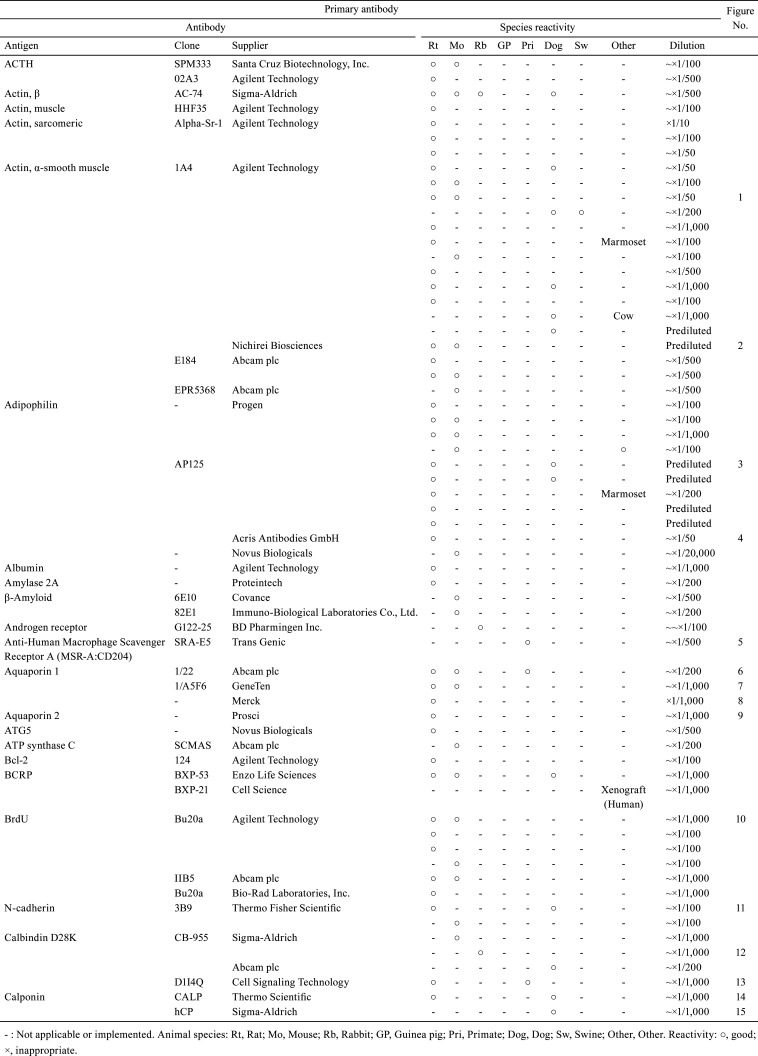
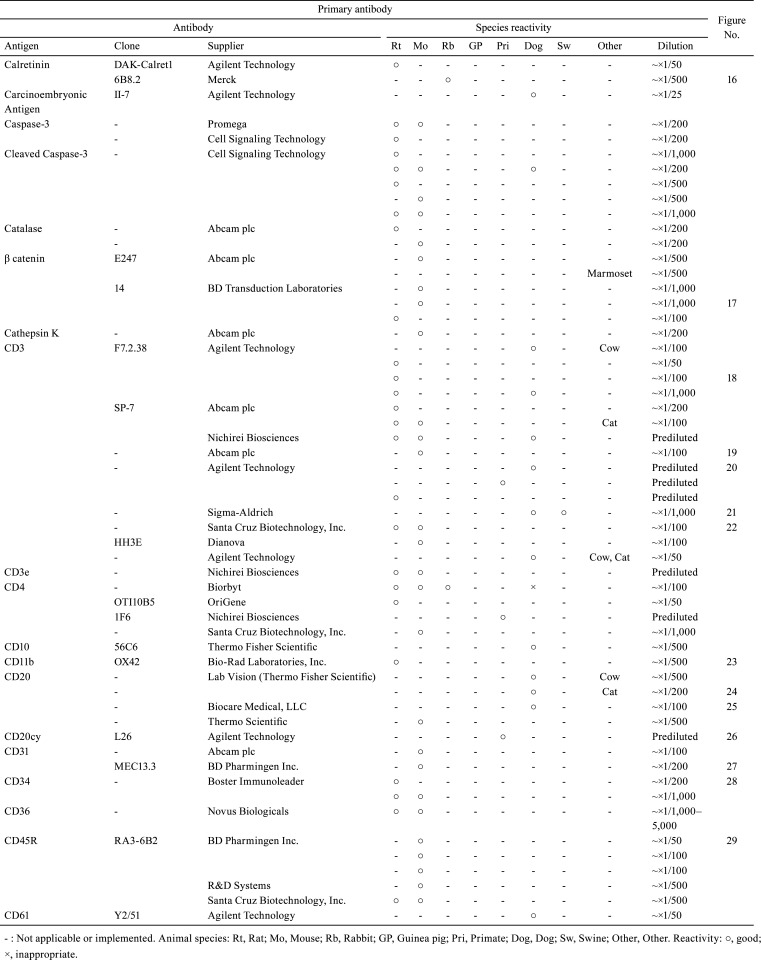
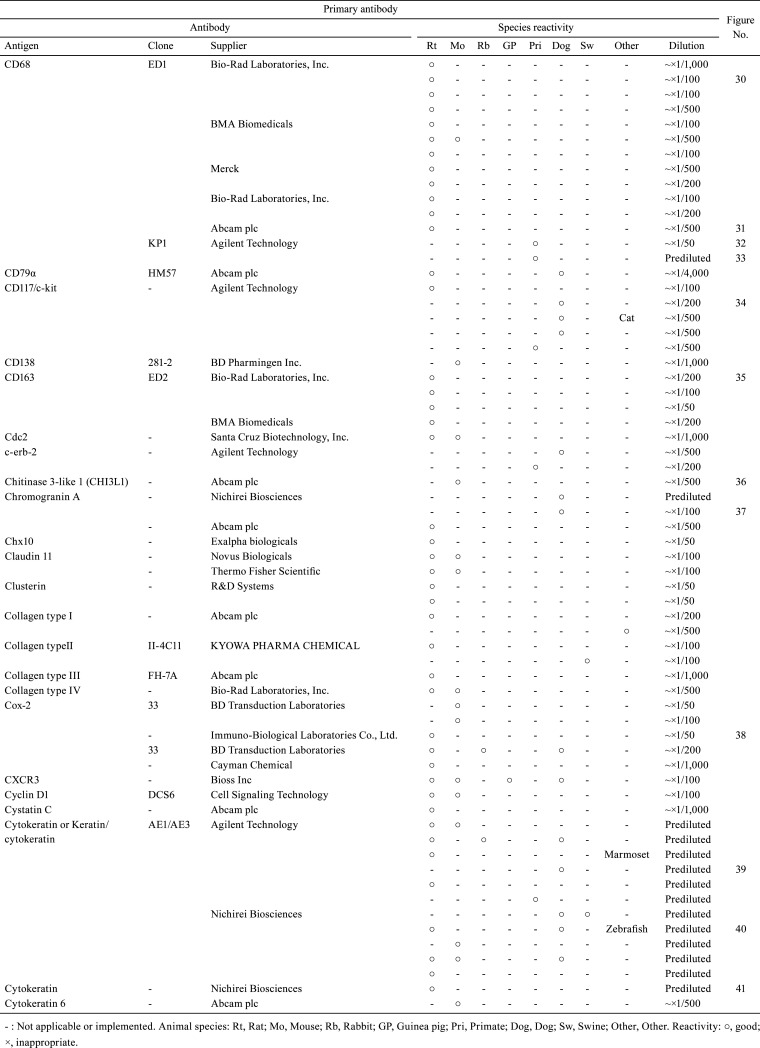
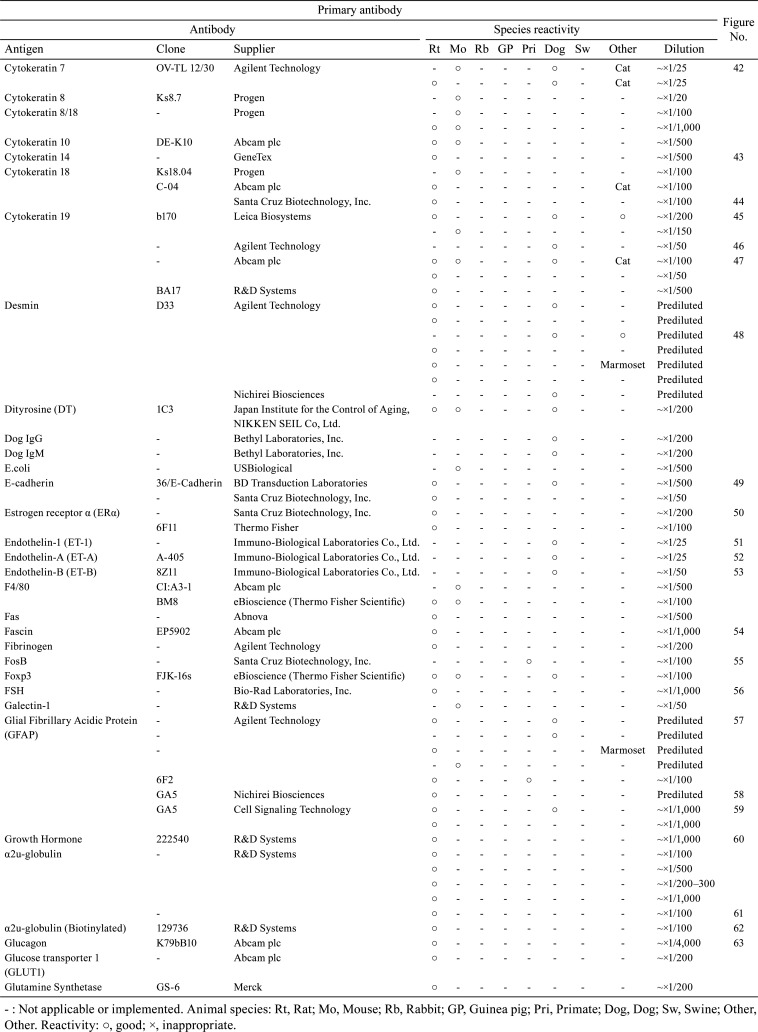
With the aim of sharing information about the technical aspects of immunohistochemistry (IHC) and facilitating the selection of suitable antibodies for histopathological examination, this technical report describes the results of a questionnaire distributed during the period of 2018 to 2019 among members of the Conference on Experimental Animal Histopathology. Additionally, it describes the immunological properties and supplier details (clone, supplier, catalog number, species reactivity, etc.) as well as the IHC staining conditions (fixing solution, fixing time, embedding, antigen retrieval method, antibody dilution, incubation time, incubation temperature, positive control tissue, blocking condition, secondary antibody information, etc.) for a total of 509 primary antibodies (comprising 220 different types). These survey results were an update on the contents reported by CEAH in 2017.
Keywords: antibody, immunohistochemistry, toxicological pathology, experimental animal
Immunohistochemistry (IHC) is a biochemical method that involves the utilization of an antibody-based strategy for identifying a specific antigen, in order to understand the distribution as well as localization of biomarkers and differentially expressed proteins in different regions of a biological tissue1. IHC is widely used for diagnostic interpretation and understanding of pathogenesis, whereby it has become a routine tool for toxicological pathology. In 2017 (survey period: 2014 to 2015), the IHC database summarized various IHC staining conditions, as reported by the Conference on Experimental Animal Histopathology (CEAH), which includes 89 research institutes—such as pharmaceutical companies, chemical companies, universities, public research institutes, and contract research organizations—involved in experimental animal pathological research in Japan and Korea2. Since the IHC database is of particular significance to pathologists, it is important to provide the latest information on IHC and to revise the discontinued antibodies or changes in supplier name. Therefore, a questionnaire about IHC was distributed during the period of 2018 to 2019 among members of the CEAH, and the database was updated based on its results. In addition, blocking condition information has been added to this updated database.
A total of 509 primary antibodies (comprising 220 different types) were available from 62 research institutes, according to the responses to the questionnaire. With the aim of sharing information about the technical aspects of IHC and facilitating the selection of suitable antibodies for histopathological examination, the present technical report describes the IHC questionnaire results. Moreover, the IHC histological photographs of some primary antibodies have been provided in the figures to clarify the antigen localization and staining conditions in the respective tissues. The immunological properties and supplier details of primary antibodies (clone, supplier, catalog number, species reactivity, etc.), as well as IHC staining conditions (fixing solution, fixing time, embedding, antigen retrieval method, antibody dilution, incubation time, incubation temperature, positive control tissue, blocking condition, secondary antibody information, etc.) are presented in Table 1, 2, 3, 4, 5, 6, 7, 8, 9. and Supplementary Table 1–12: online only, while Fig. Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24, Fig. 25, Fig. 26, Fig. 27, Fig. 28, Fig. 29, Fig. 30, Fig. 31, Fig. 32, Fig. 33, Fig. 34, Fig. 35, Fig. 36, Fig. 37, Fig. 38, Fig. 39, Fig. 40, Fig. 41, Fig. 42, Fig. 43, Fig. 44, Fig. 45, Fig. 46, Fig. 47, Fig. 48, Fig. 49, Fig. 50, Fig. 51, Fig. 52, Fig. 53, Fig. 54, Fig. 55, Fig. 56, Fig. 57, Fig. 58, Fig. 59, Fig. 60, Fig. 61, Fig. 62, Fig. 63, Fig. 64, Fig. 65, Fig. 66, Fig. 67, Fig. 68, Fig. 69, Fig. 70, Fig. 71, Fig. 72, Fig. 73, Fig. 74, Fig. 75, Fig. 76, Fig. 77, Fig. 78, Fig. 79, Fig. 80, Fig. 81, Fig. 82, Fig. 83, Fig. 84, Fig. 85, Fig. 86, Fig. 87, Fig. 88, Fig. 89, Fig. 90, Fig. 91, Fig. 92, Fig. 93, Fig. 94, Fig. 95, Fig. 96, Fig. 97, Fig. 98, Fig. 99, Fig. 100, Fig. 101, Fig. 102, Fig. 103, Fig. 104, Fig. 105, Fig. 106, Fig. 107, Fig. 108, Fig. 109, Fig. 110, Fig. 111, Fig. 112, Fig. 113, Fig. 114, Fig. 115, Fig. 116, Fig. 117, Fig. 118, Fig. 119, Fig. 120, Fig. 121, Fig. 122, Fig. 123, Fig. 124, Fig. 125, Fig. 126, Fig. 127, Fig. 128, Fig. 129, Fig. 130, Fig. 131, Fig. 132, Fig. 133, Fig. 134, Fig. 135, Fig. 136 depict IHC histological photographs.
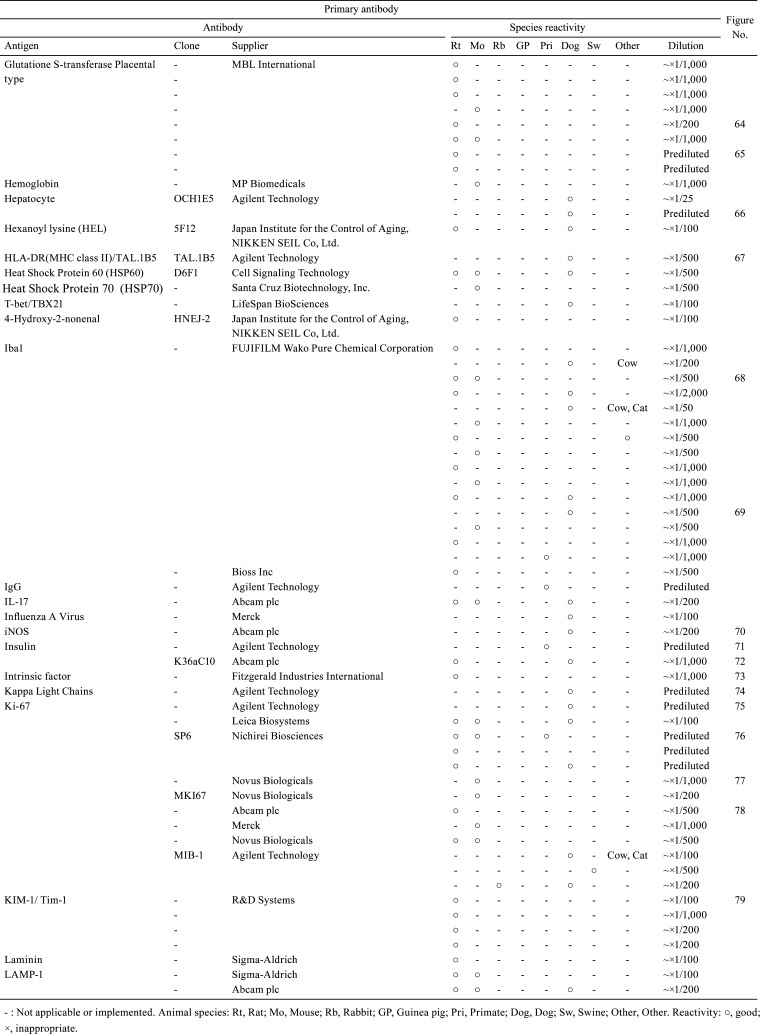
Table 1. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
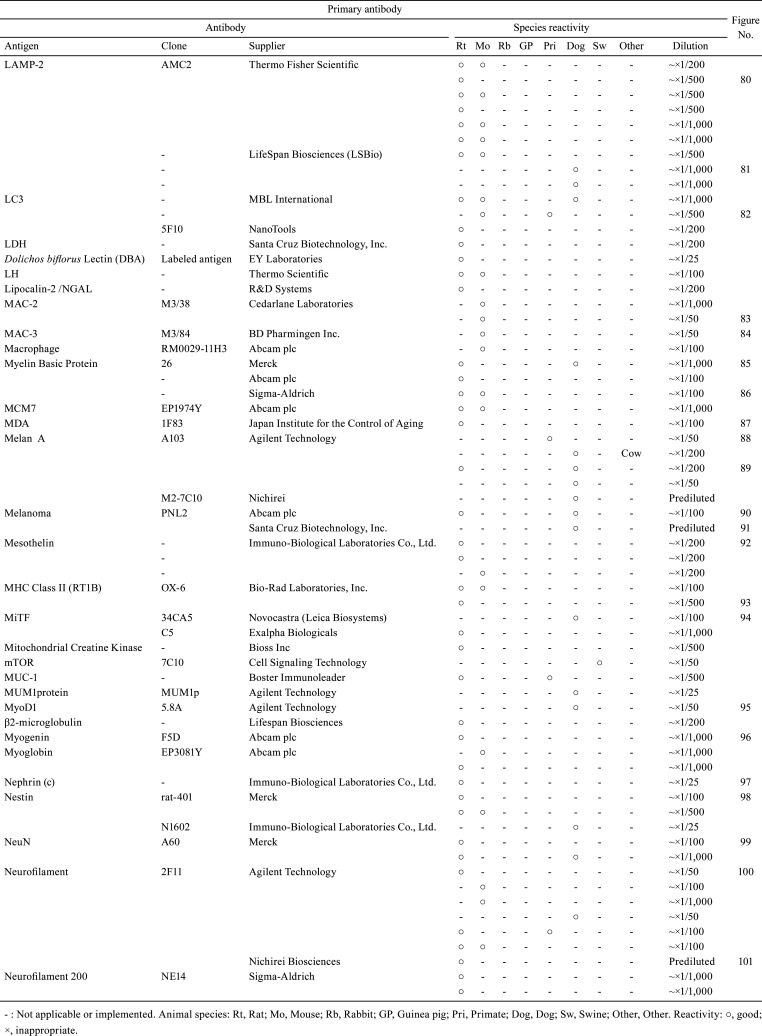
Table 2. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
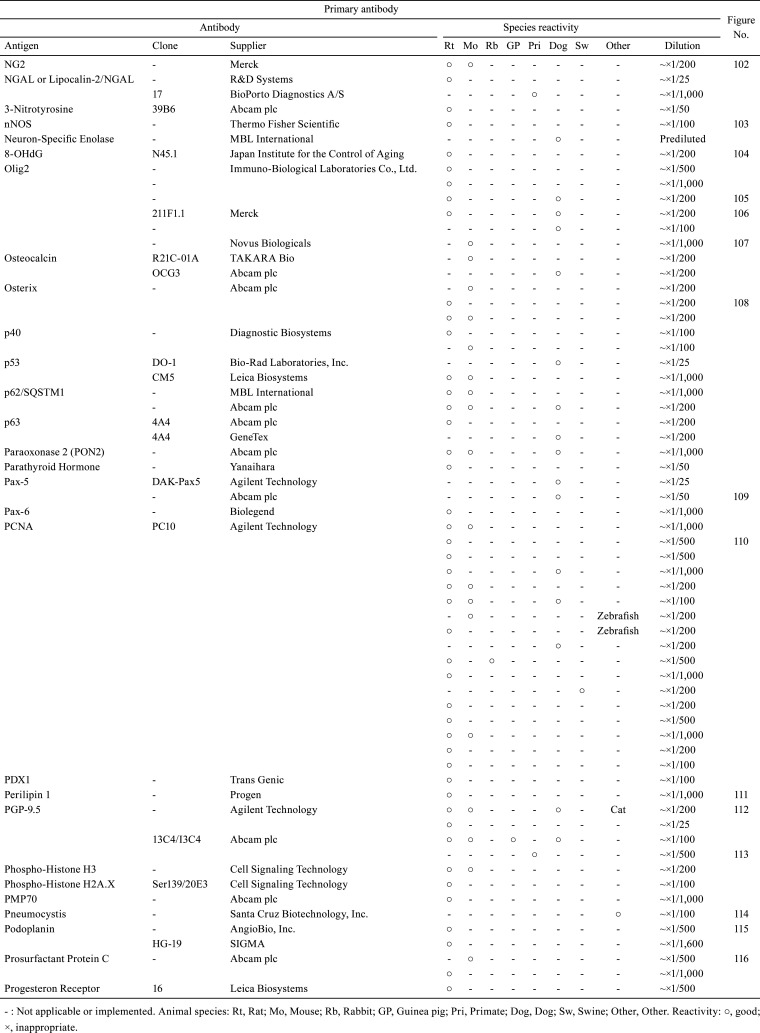
Table 3. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
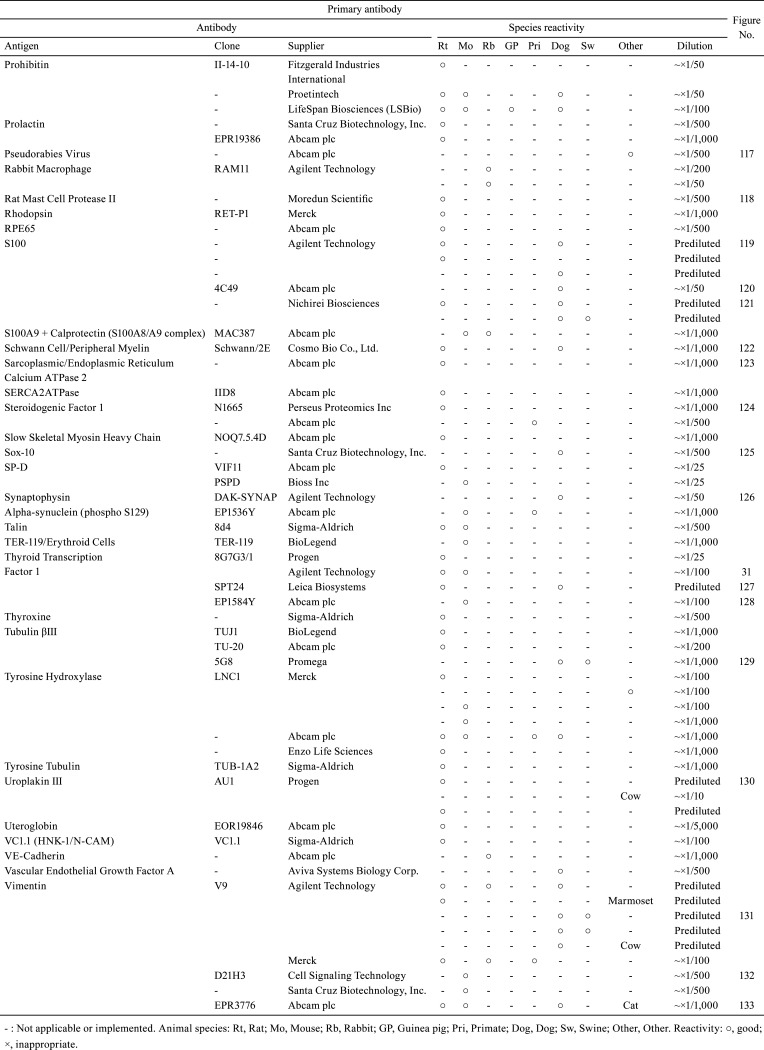
Table 4. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Table 5. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Table 6. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Table 7. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Table 8. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Table 9. Immunological Properties of Primary Antibodies and Their Respective IHC Staining Conditions.
Fig. 1.
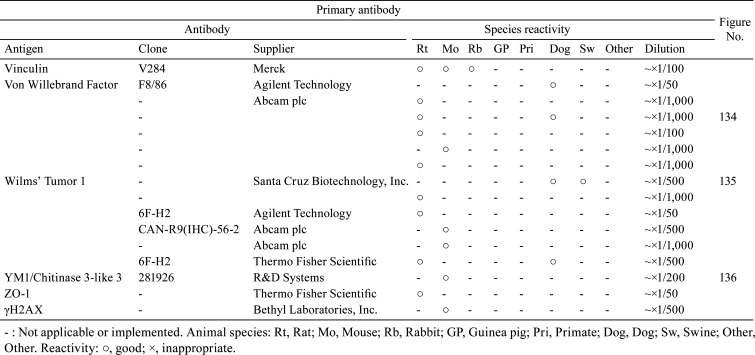
Actin, α-smooth muscle /1A4 /Agilent Technology /M085129-2, Mammary gland /Rat.
Fig. 2.
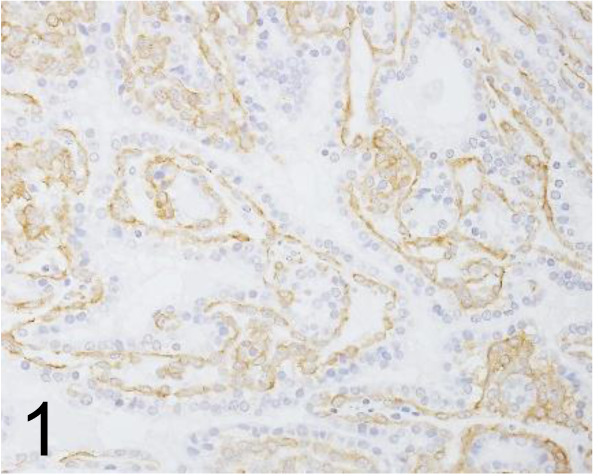
Actin, α-smooth muscle /1A4 /Nichirei Biosciences /412021, Kidney /Mouse.
Fig. 3.
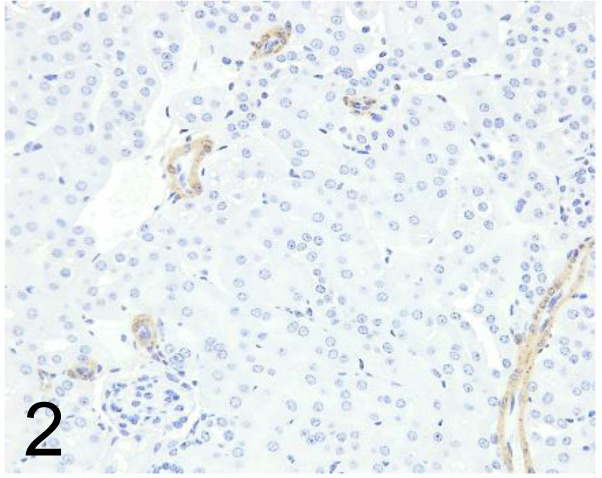
Adipophilin /AP125 /Progen /651102, Testis /Rat.
Fig. 4.
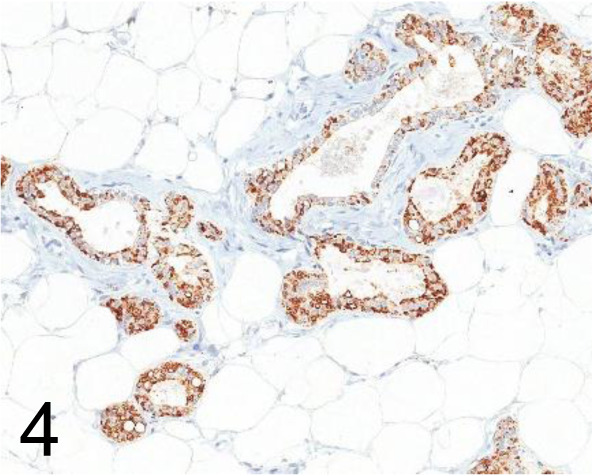
Adipophilin /AP125 /Acris Antibodies GmbH /BM5051, Mammary gland /Rat.
Fig. 5.
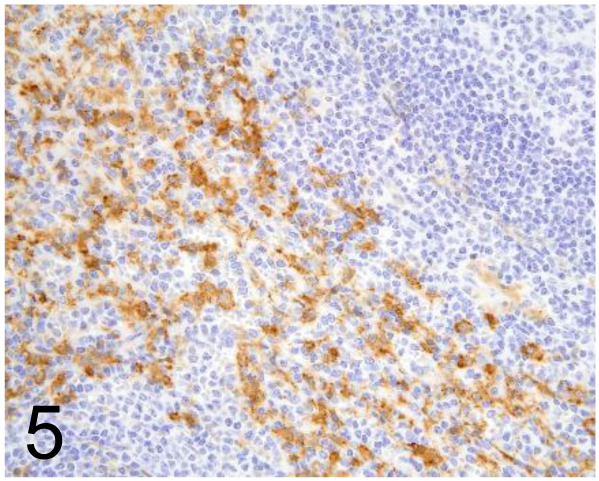
Anti-Human Macrophage Scavenger Receptor A (MSR-A: CD204) /SRA-E5 /Trans Genic Inc /KT022, Spleen /Primate.
Fig. 6.
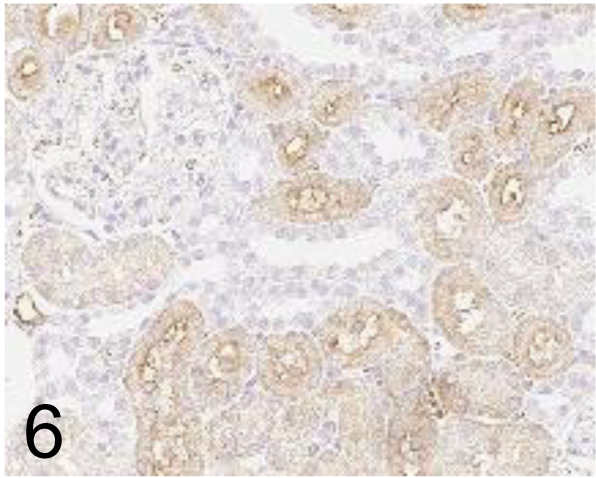
Aquaporin 1 /1/22 /Abcam plc /ab9566, Kidney /Rat.
Fig. 7.
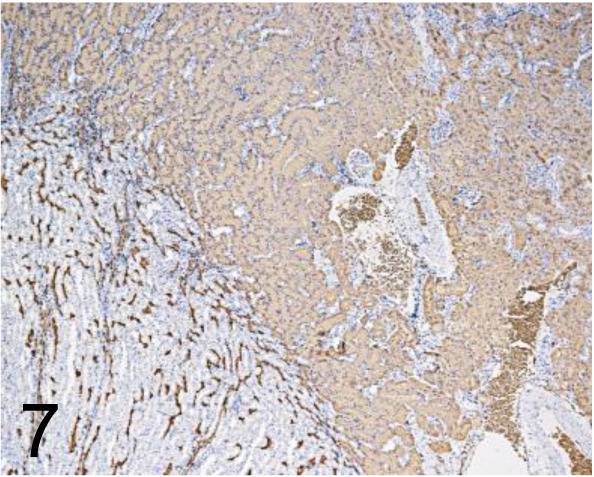
Aquaporin 1 /1/A5F6 /GeneTex /GTX11023, Kidney /Rat.
Fig. 8.
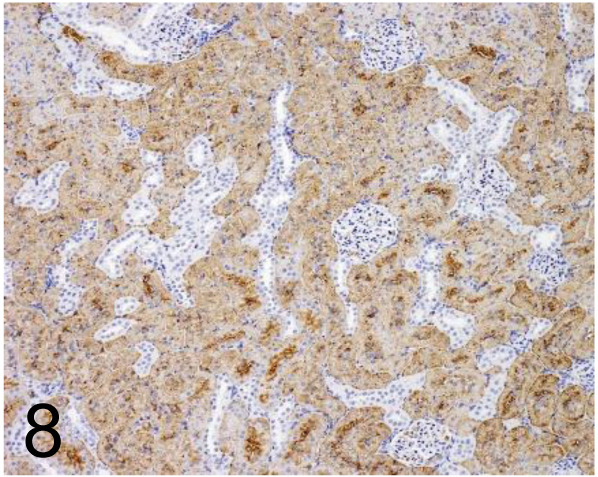
Aquaporin 1 /- /Merck /AB2219, Kidney /Rat.
Fig. 9.
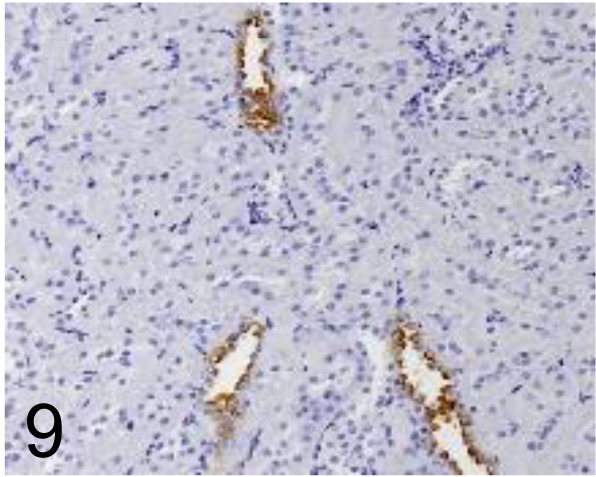
Aquaporin 2 /- /Prosci /50-225, Kidney /Rat.
Fig. 10.
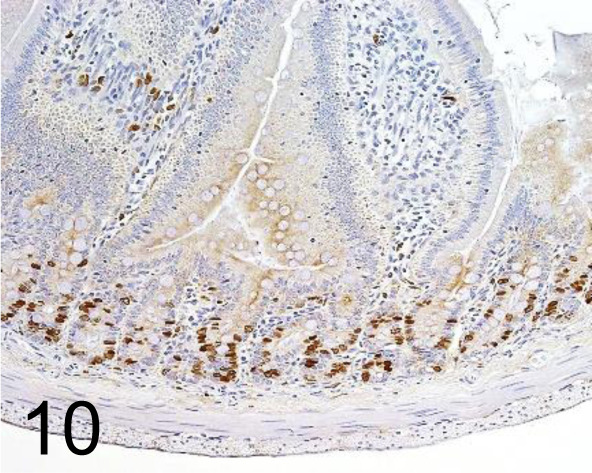
BrdU /Bu20a /Agilent Technology /M074401-8, Small Intestine /Rat.
Fig. 11.
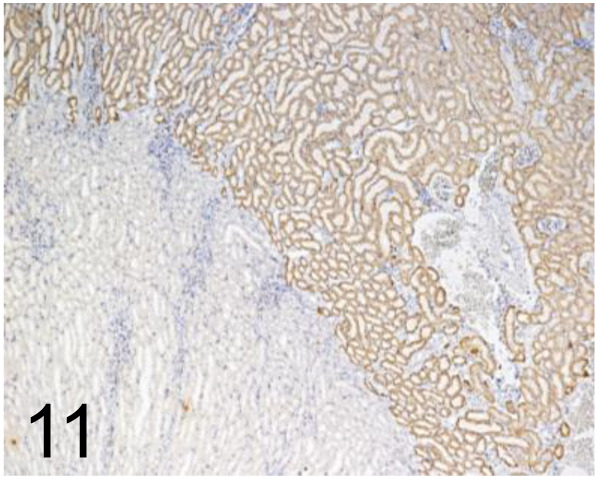
N-cadherin /3B9 /Thermo Fisher Scientific /33-3900, Kidney /Rat.
Fig. 12.
Calbindin D28K /CB-955 /Sigma-Aldrich /C9848, Eye /Rabbit.
Fig. 13.
Calbindin D28K /D1I4Q /Cell Signaling Technology / #13176, Kidney /Rat.
Fig. 14.
Calponin /CALP /Thermo Scientific /MA5-11620, Mammary gland /Dog.
Fig. 15.
Calponin /hCP /Sigma-Aldrich /C2687, Complex carcinoma, eyelid /Dog.
Fig. 16.
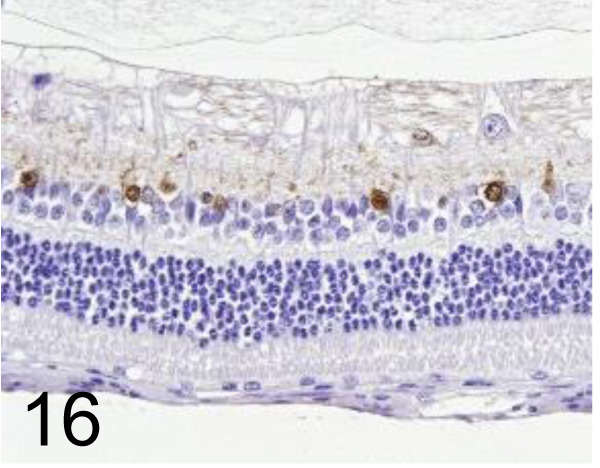
Calretinin /6B8.2 /Merck /MAB1568, Eye /Rabbit.
Fig. 17.
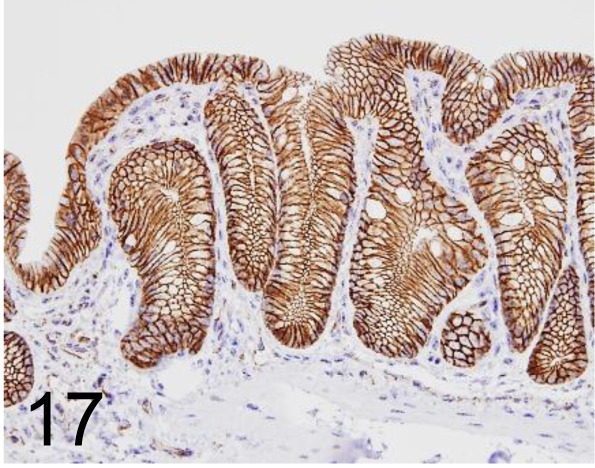
β catenin /14 /BD Transduction Laboratories /610153, Colon /Mouse.
Fig. 18.
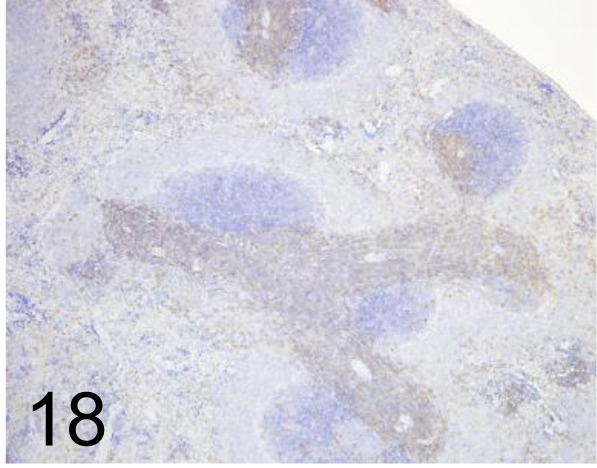
CD3 /F7.2.38 /Agilent Technology /M725401-2, Spleen /Rat.
Fig. 19.
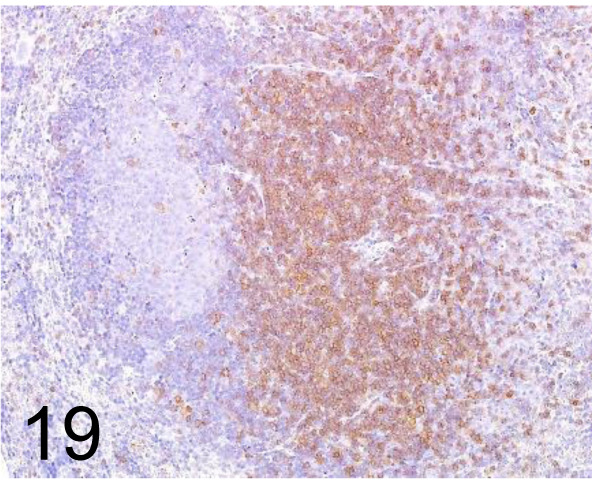
CD3 /- /Abcam plc /ab5690, Lymph node /Mouse.
Fig. 20.
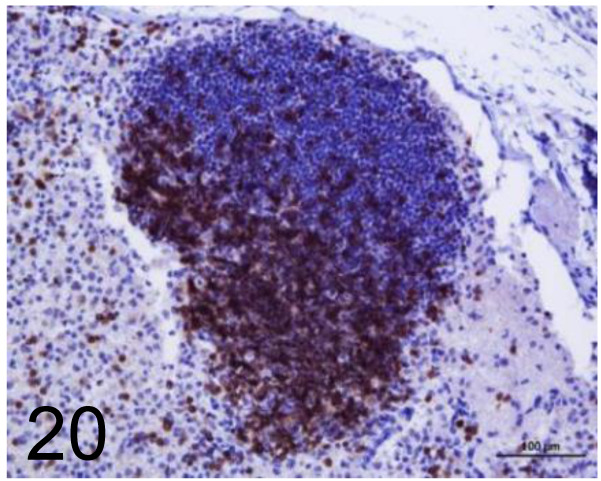
CD3 /- /Agilent Technology /IS50330-2J, Lymph node /Dog.
Fig. 21.
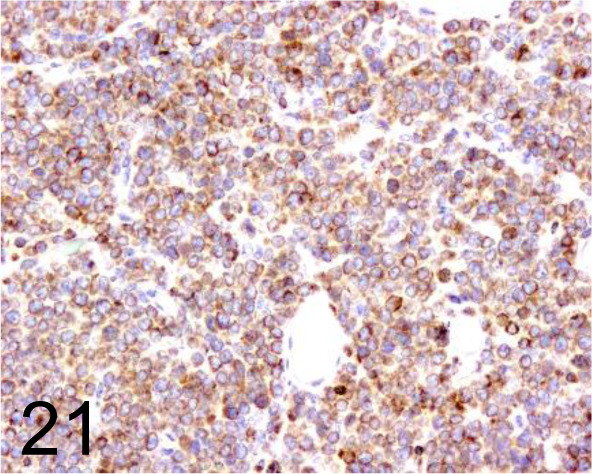
CD3 /- /Sigma-Aldrich /C7930, T cell lymphoma /Dog.
Fig. 22.
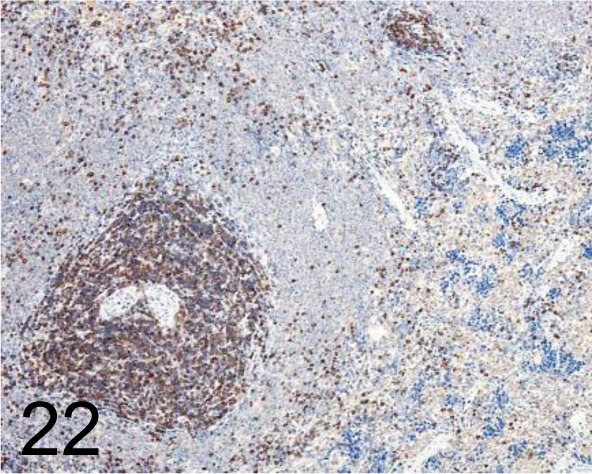
CD3 /Santa Cruz Biotechnology, Inc. /sc-1127, Spleen /Rat.
Fig. 23.
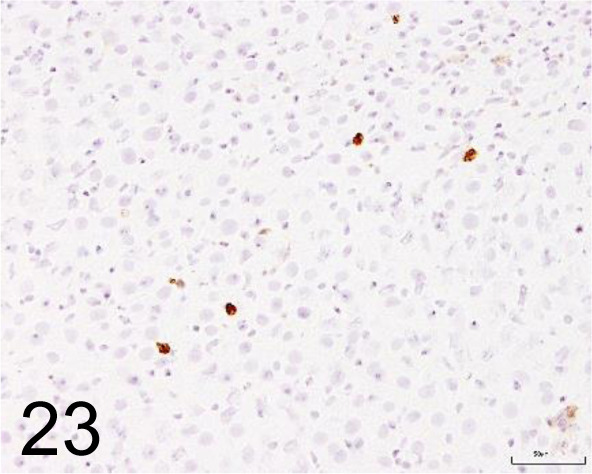
CD11b /OX42 /Bio-Rad Laboratories, Inc. /MCA74G, Liver /Rat.
Fig. 24.
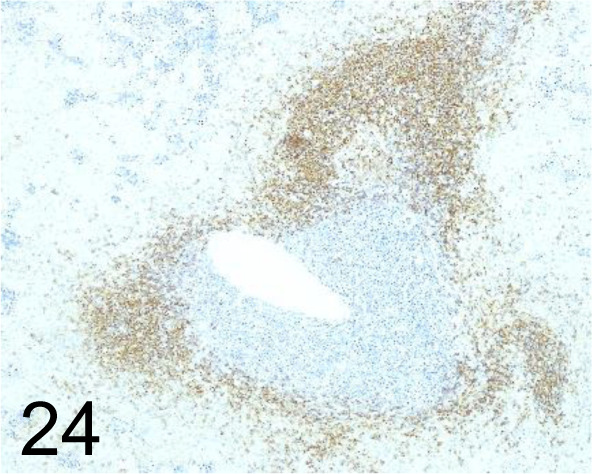
CD20 /- /Lab Vision™ (Thermo Fisher Scientific)/RB-9013, Spleen /Dog.
Fig. 25.
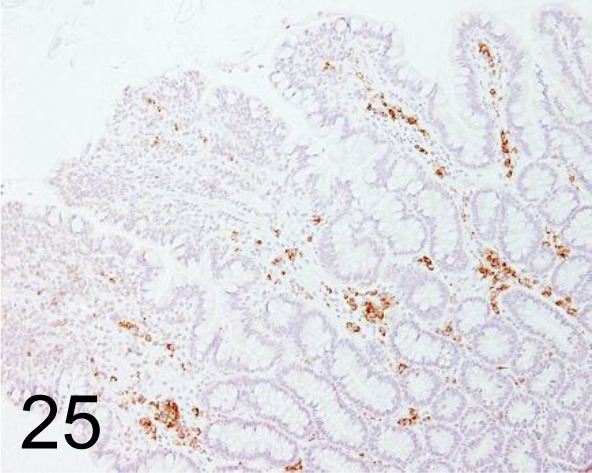
CD20 /- /Biocare Medical, LLC /ACR 3004 A, Intestine /Dog.
Fig. 26.
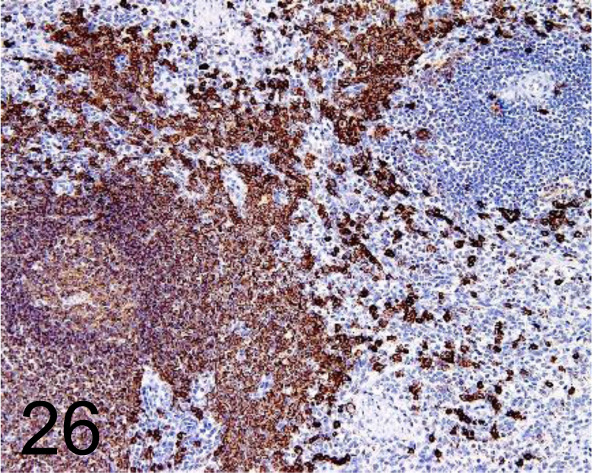
CD20cy /L26 /Agilent Technology /IS60430-2J, Spleen /Primate.
Fig. 27.
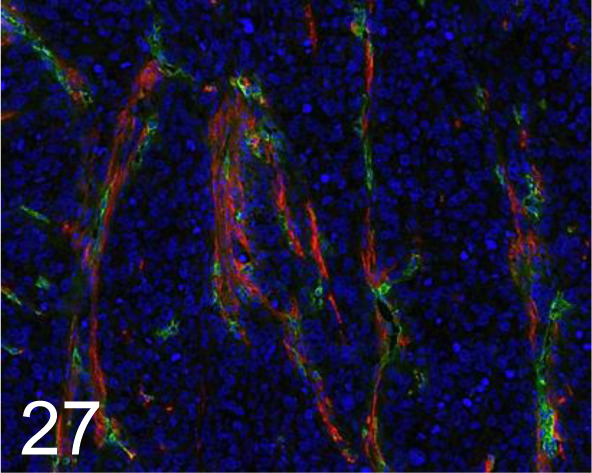
Green, CD31 /MEC13.3 /BD Pharmingen Inc. /550274, Tumor /Mouse.
Fig. 28.
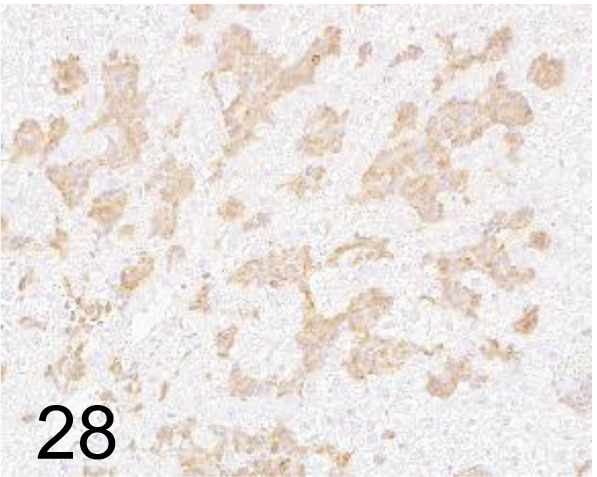
CD34 /- /Boster Immunoleader /PA1334, Hemangiosarcoma, liver /Rat.
Fig. 29.
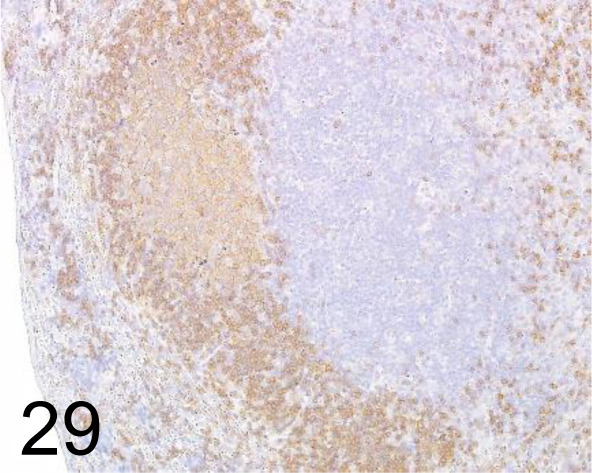
CD45R /RA3-6B2 /BD Pharmingen Inc. /550280, Lymph node /Mouse.
Fig. 30.
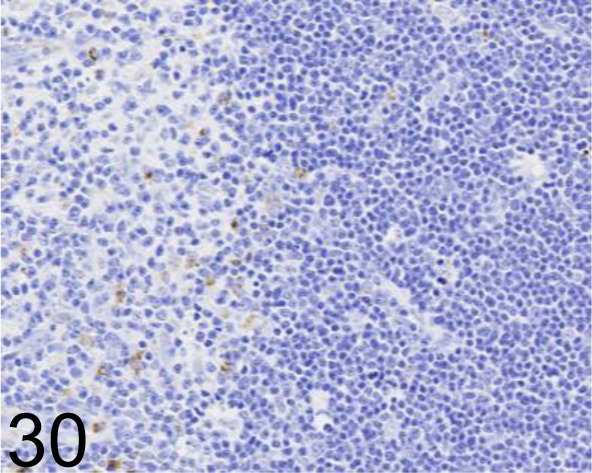
CD68 /ED-1 /Bio-Rad Laboratories, Inc. /MCA341GA, Thymus /Rat.
Fig. 31.
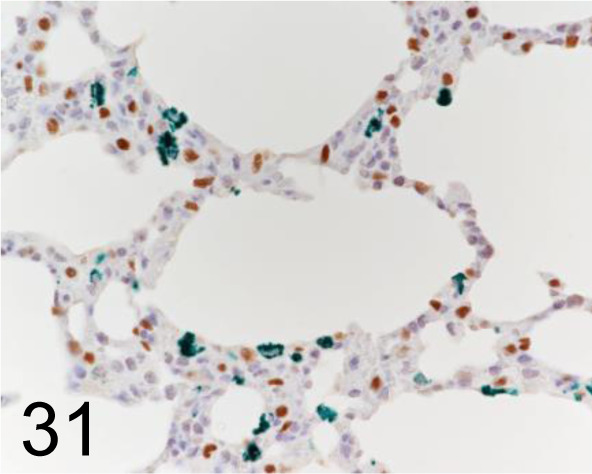
Green, CD68 /ED-1 /Abcam plc /ab31630, Lung /Rat. Brown, Thyroid Transcription Factor 1 /8G7G3/1 / Agilent Technology /M357501-2, Lung /Rat.
Fig. 32.
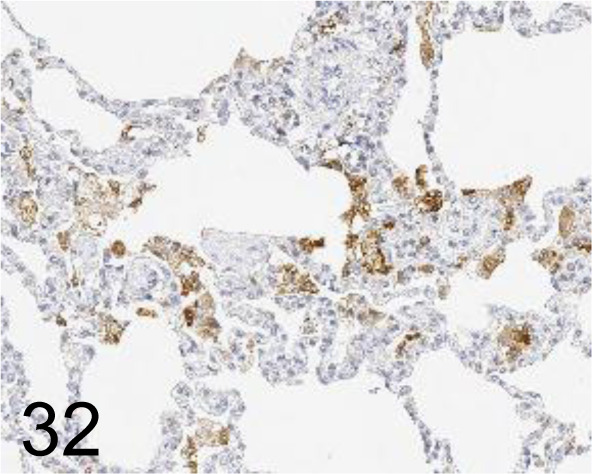
CD68 /KP1 /Agilent Technology /M081401-2, Lung /Monkey.
Fig. 33.
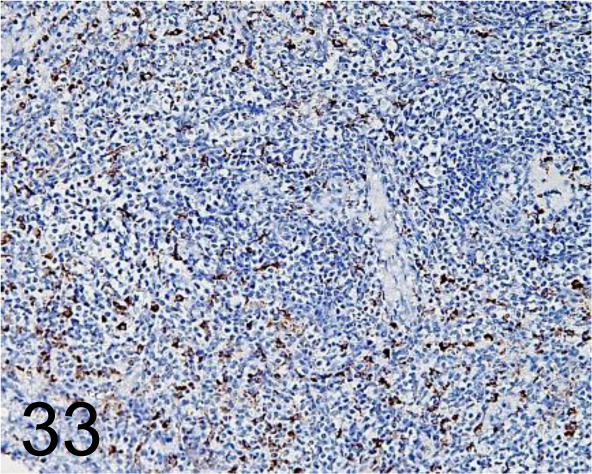
CD68 /KP1 /Agilent Technology /IS61330-2, Spleen /Primate.
Fig. 34.
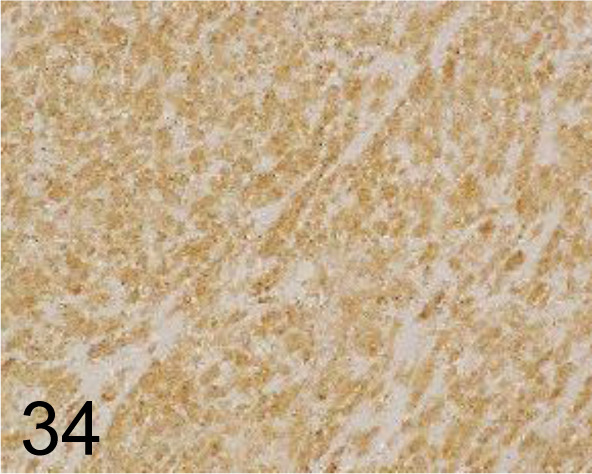
CD117/c-kit /- /Agilent Technology /A450229-2, GIST /Dog.
Fig. 35.
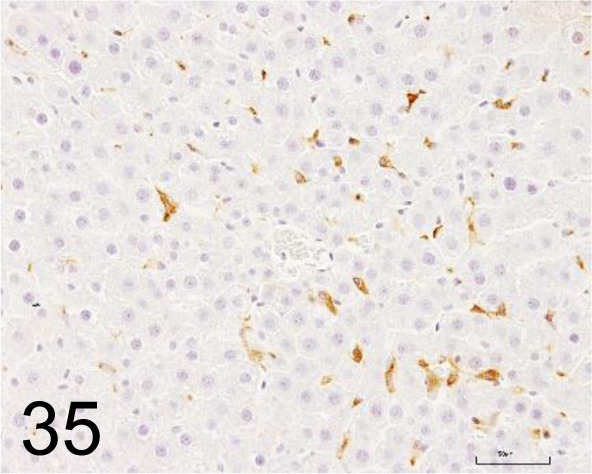
CD163 /ED2 /Bio-Rad Laboratories, Inc. /MCA342R, Liver /Rat.
Fig. 36.
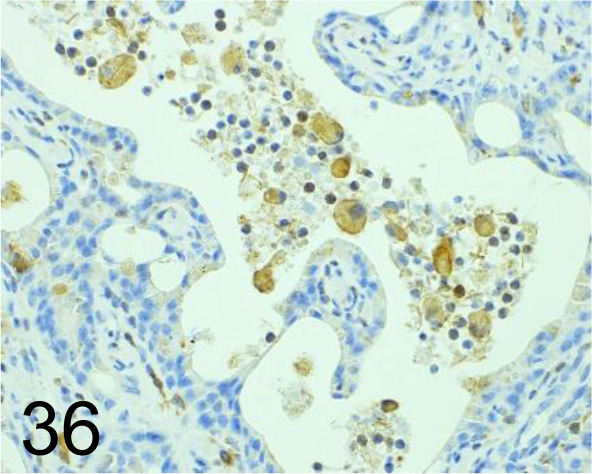
CHI3L1 /- /Abcam plc /ab77528, Liver /Mouse.
Fig. 37.
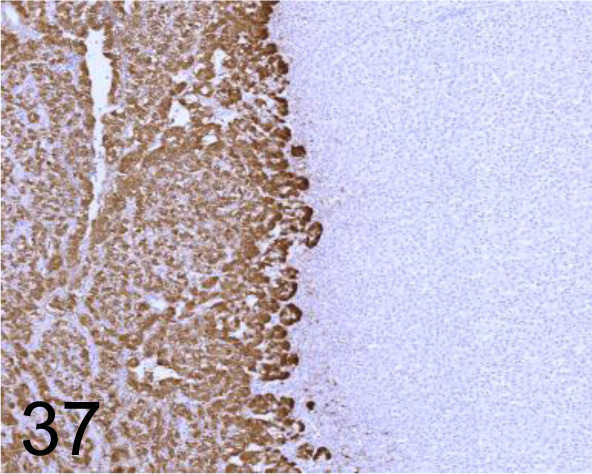
Chromogranin A /- /Nichirei Biosciences /412751, Adrenal gland /Dog.
Fig. 38.
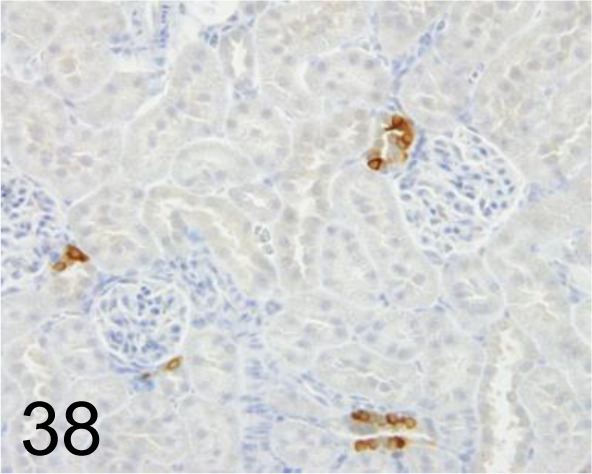
Cox-2 /- /Immuno-Biological Laboratories Co., Ltd. /18955, Kidney /Rat.
Fig. 39.
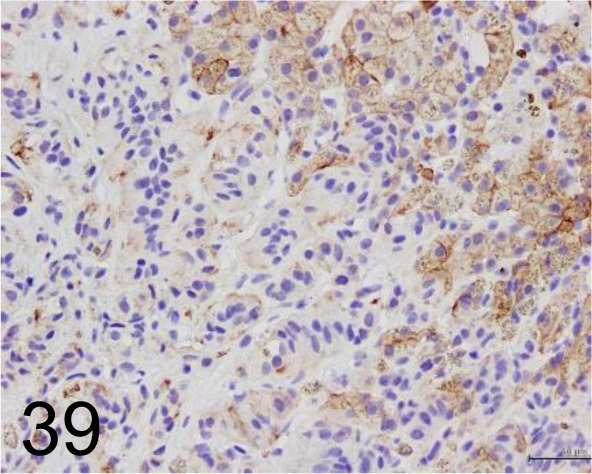
Cytokeratin /AE1/AE3 /Agilent Technology /IR05361-2J, Hepatic carcinoid /Dog.
Fig. 40.
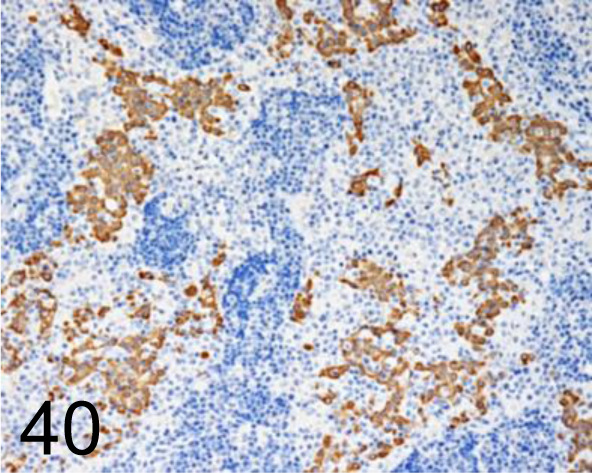
Keratin /cytokeratin /AE1/AE3 /Nichirei Biosciences /412811, Mesothelial cell in thoracic mass /Dog.
Fig. 41.
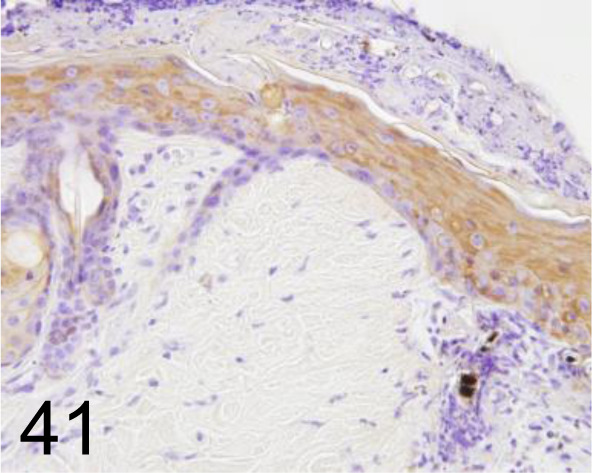
Keratin /cytokeratin /- /Nichirei Biosciences /422061, Skin /Rat.
Fig. 42.
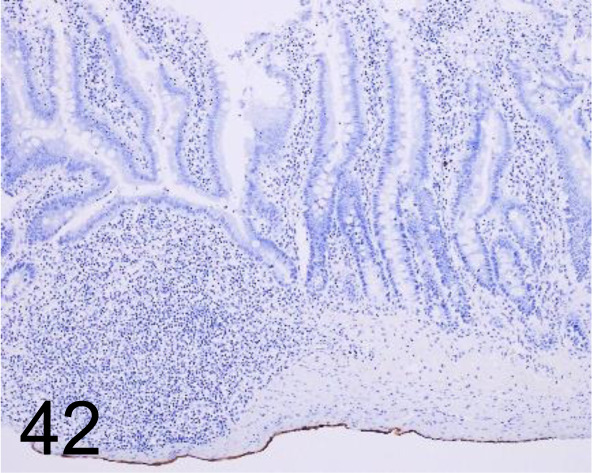
Cytokeratin 7 /OV-TL 12/30 /Agilent Technology /M701801-2, Ileum /Rat.
Fig. 43.
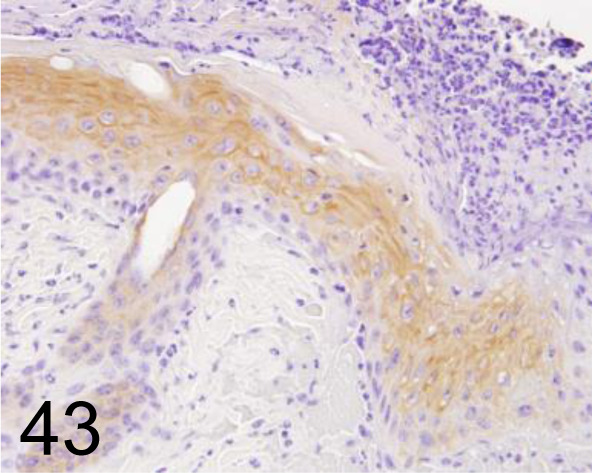
Cytokeratin 14 /- /GeneTex /GTX104124, Skin /Rat.
Fig. 44.
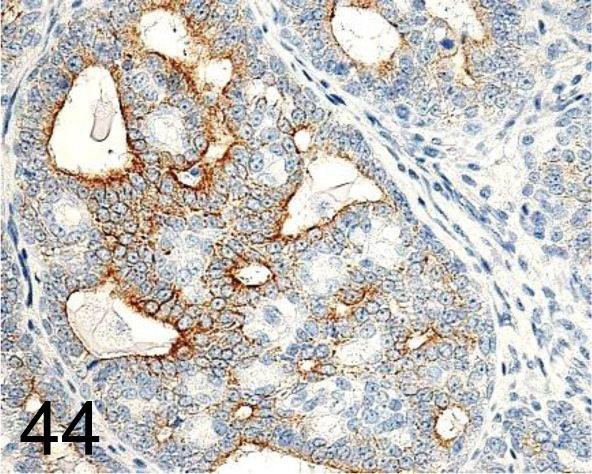
Cytokeratin 18 /C-04 /Santa Cruz Biotechnology, Inc. /SC-51582, Mammary gland /Rat.
Fig. 45.
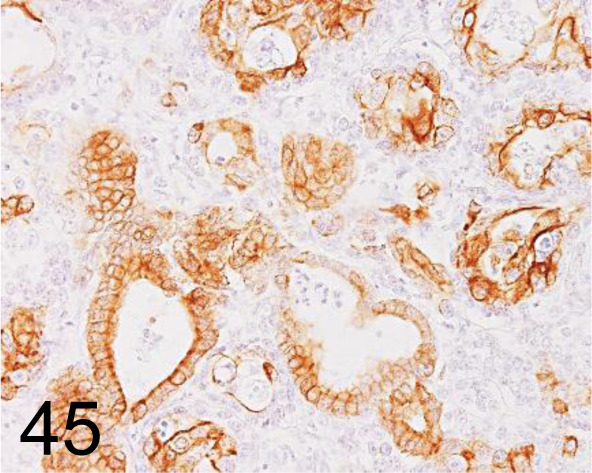
Cytokeratin 19 /b170 /Leica Biosystems /NCL-CK19, Bile duct carcinoma /Dog.
Fig. 46.
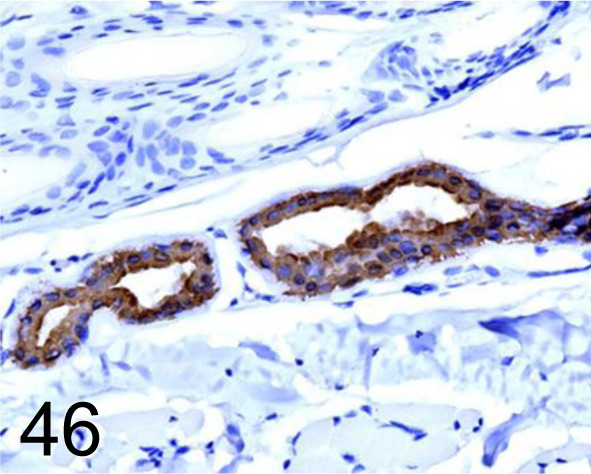
Cytokeratin 19 /- /Agilent Technology /M088801-2, Skin /Dog.
Fig. 47.
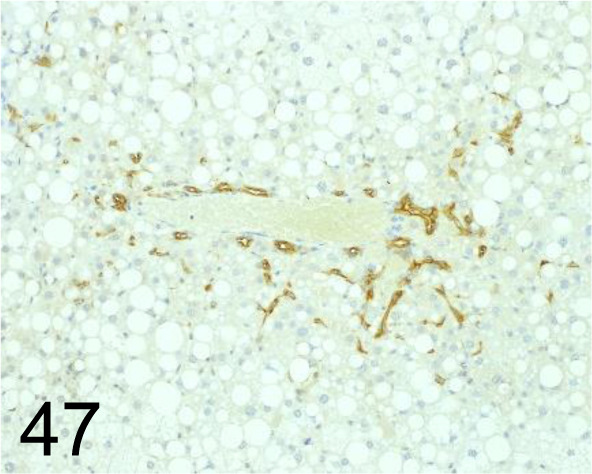
Cytokeratin 19 /- /Abcam plc /ab15463, Liver /Mouse.
Fig. 48.
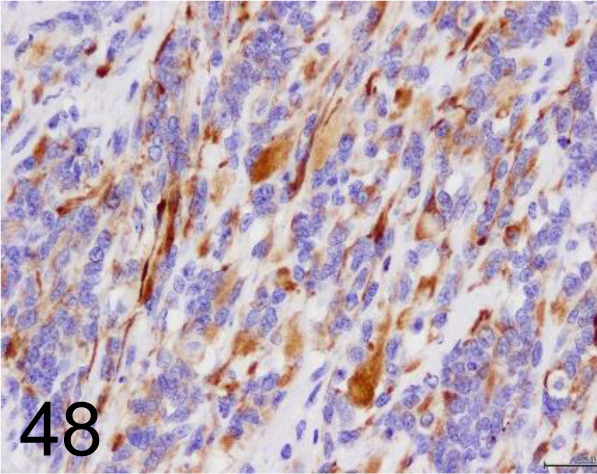
Desmin /D33 /Agilent Technology /IR60661-2J, Rhabdomyosarcoma /Cow.
Fig. 49.
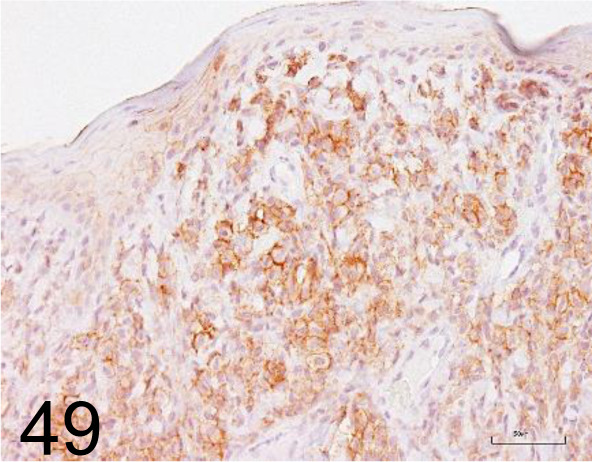
E-cadherin /36/E-Cadherin /BD Transduction Laboratories /610181, Cutaneous histiocytoma /Dog.
Fig. 50.
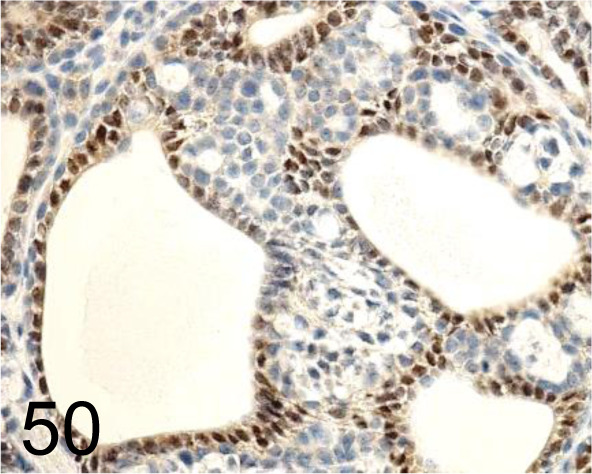
Estrogen receptor α (ERα) /Santa Cruz Biotechnology, Inc. /SC-543, Mammary gland /Rat.
Fig. 51.
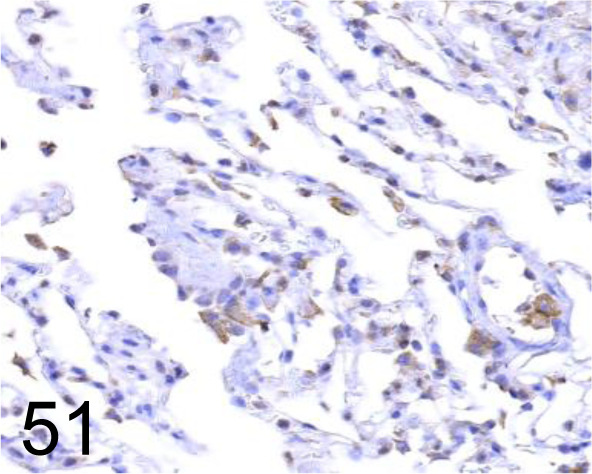
Endothelin-1 (ET-1) /- /Immuno-Biological Laboratories Co., Ltd. /18201, Lung /Dog.
Fig. 52.
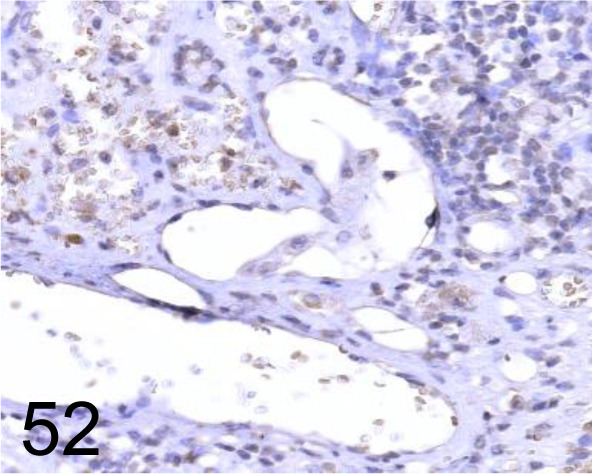
Endothelin-A(ET-A) /A-405 /Immuno-Biological Laboratories Co., Ltd. /16201, Liver /Dog.
Fig. 53.
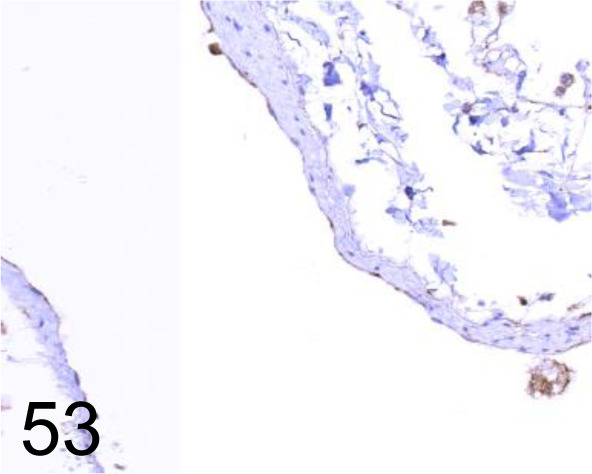
Endothelin-B (ET-B) /8Z11 /Immuno-Biological Laboratories Co., Ltd. /10253, Lung /Dog.
Fig. 54.
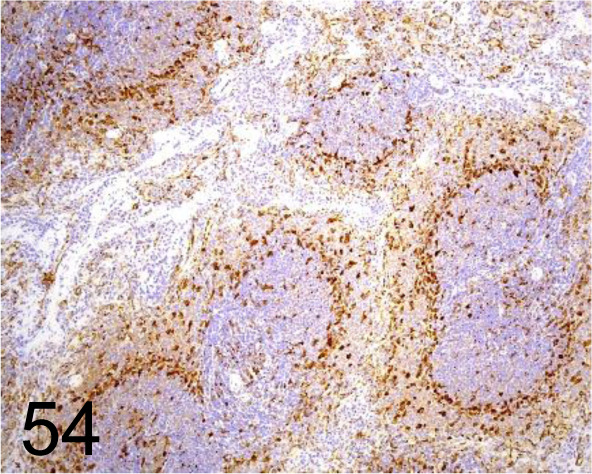
Fascin /EP5902 /Abcam plc /ab126772, Spleen /Rat.
Fig. 55.
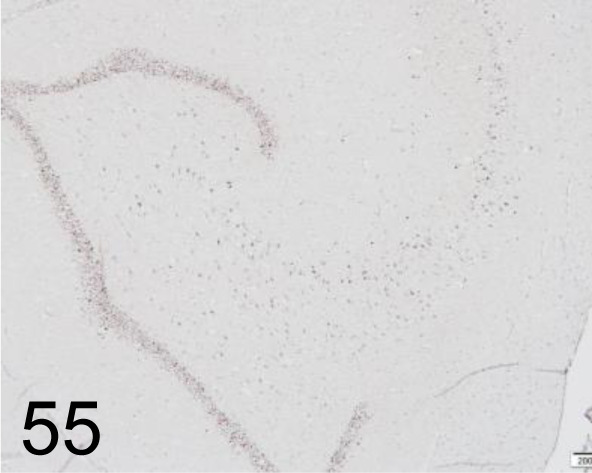
FosB /- /Santa Cruz Biotechnology, Inc. /sc-7203, Brain /Primate.
Fig. 56.
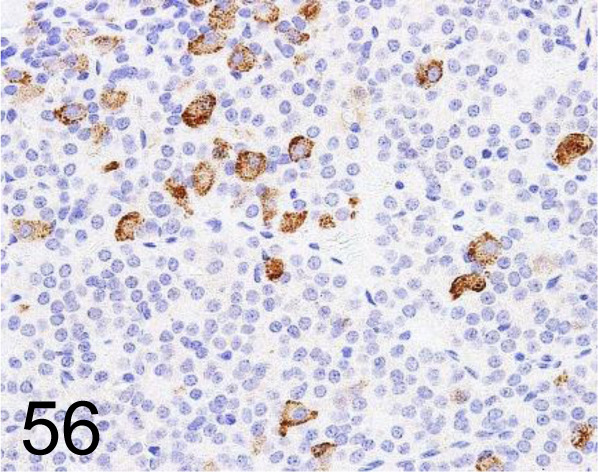
FSH /- /Bio-Rad Laboratories, Inc. /4561-6959, Pituitary gland /Rat.
Fig. 57.
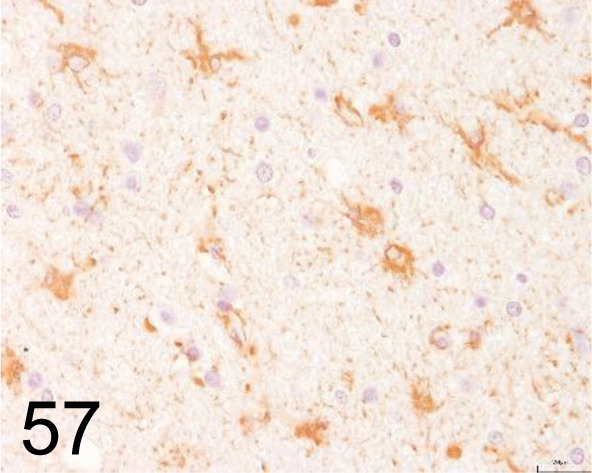
Glial Fibrillary Acidic Protein (GFAP) /- /Agilent Technology /IR52461-2J, Brain /Dog.
Fig. 58.
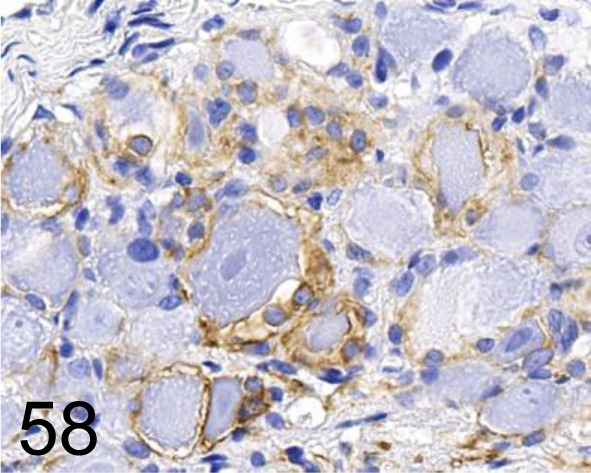
Glial Fibrillary Acidic Protein (GFAP) /GA5 /Nichirei Biosciences /422261, Dorsal root ganglion /Rat.
Fig. 59.
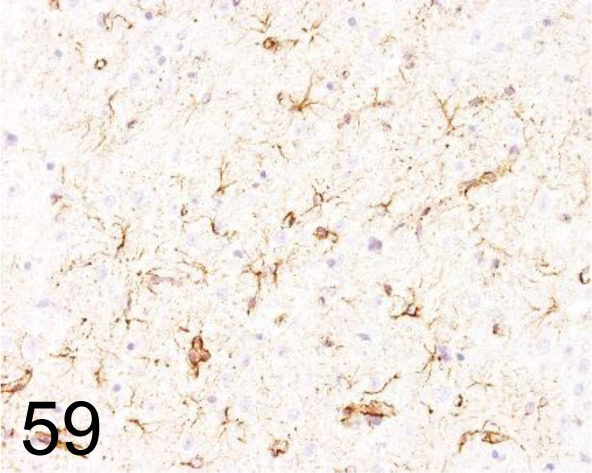
Glial Fibrillary Acidic Protein (GFAP) /GA5 /Cell Signaling Technology /3670, Brain /Rat.
Fig. 60.
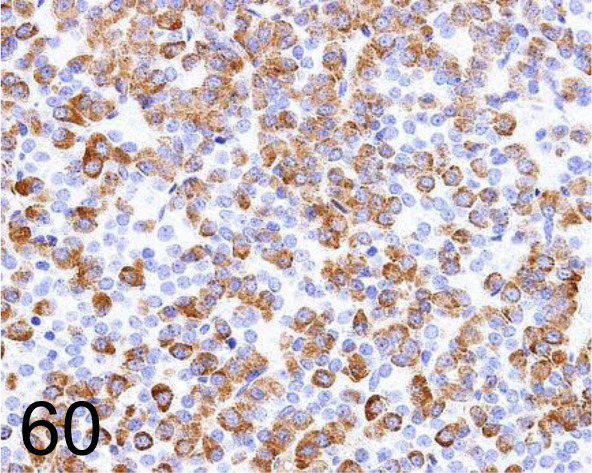
Growth Hormone /222540 /R&D Systems /MAB1566, Pituitary gland /Rat.
Fig. 61.
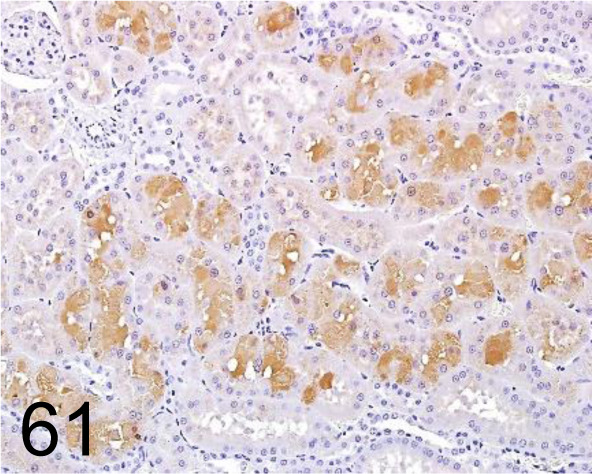
α2u-globulin /- /R&D Systems /AF 586, Kidney /Rat.
Fig. 62.
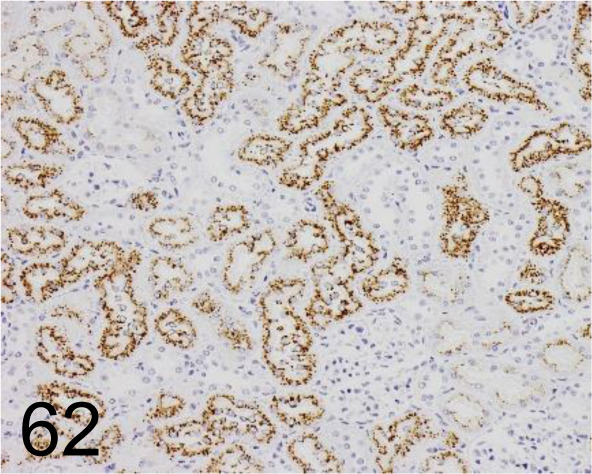
α2u-globulin (Biotinylated) /129736 /R&D Systems /BAM586, Rat /Kidney.
Fig. 63.
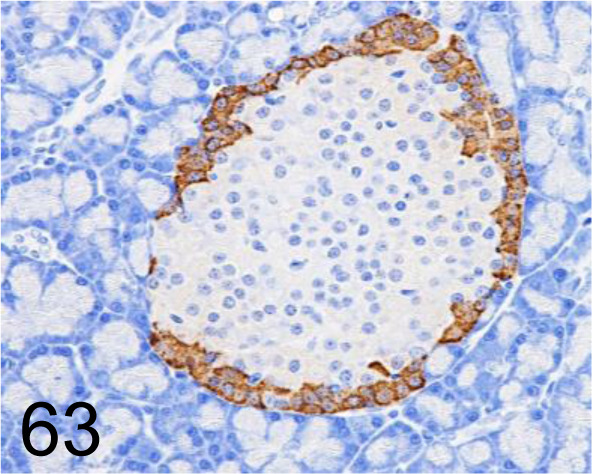
Glucagon /K79bB10 /Abcam plc /ab10988, Pancreas /Rat.
Fig. 64.
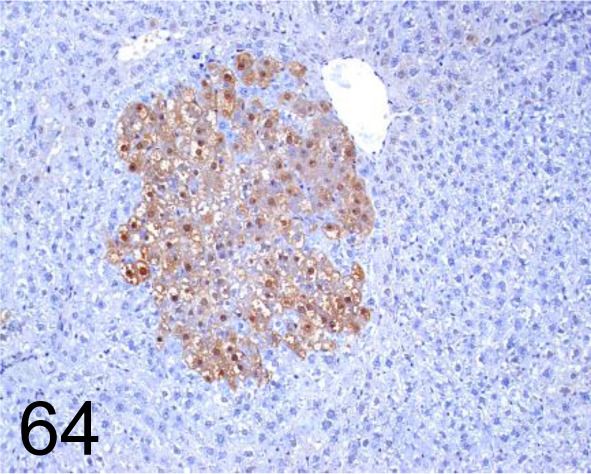
Glutathione S-transferase Placental type /- /MBL International /311, Liver /Rat.
Fig. 65.
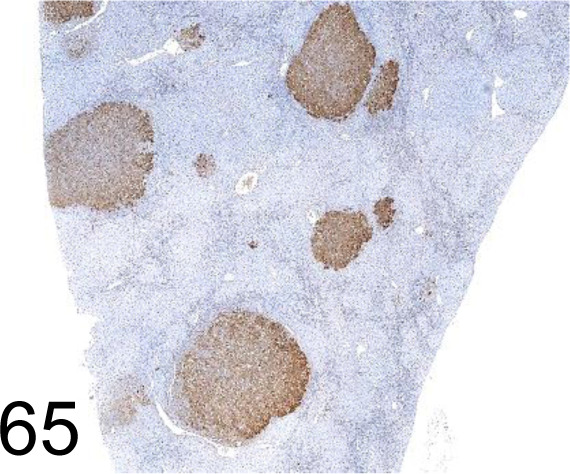
Glutathione S-transferase Placental type /- /MBL International /311-H, Liver /Rat.
Fig. 66.
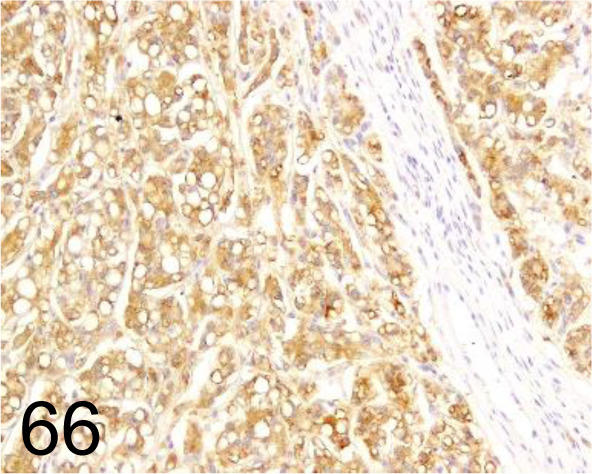
Hepatocyte /OCH1E5 /Agilent Technology /M715801-2, Hepatocellular carcinoma /Dog.
Fig. 67.
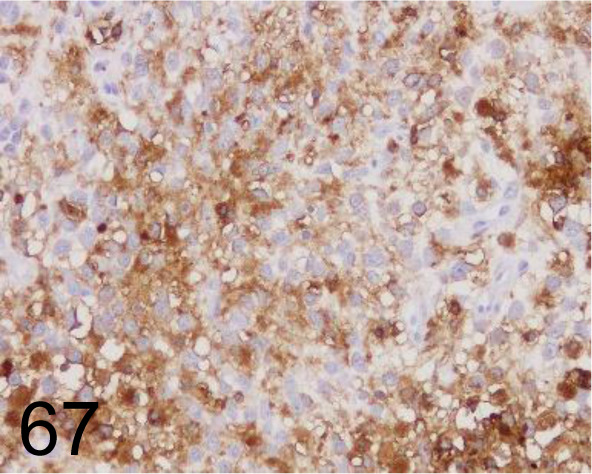
HLA-DR (MHC class II)/TAL.1B5 /TAL.1B5 /Agilent Technology /M074601-2, Histiocytic sarcoma /Dog.
Fig. 68.
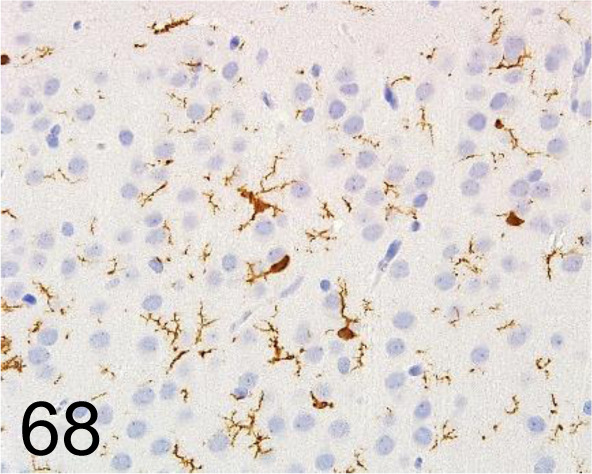
Iba 1 /- /FUJIFILM Wako Pure Chemical Corporation /019-19741, Brain /Rat.
Fig. 69.
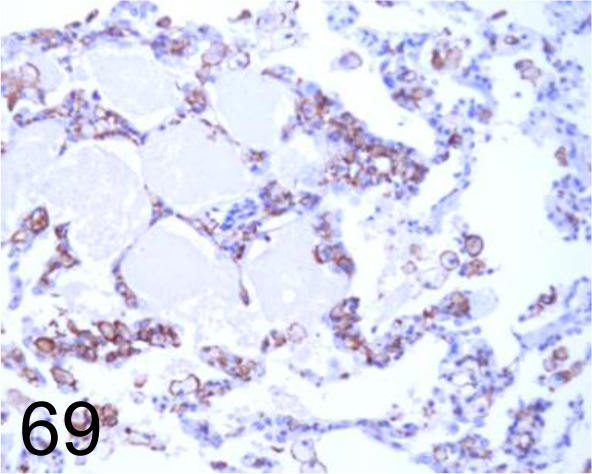
Iba 1 /- /FUJIFILM Wako Pure Chemical Corporation /019-19741, Lung /Dog.
Fig. 70.
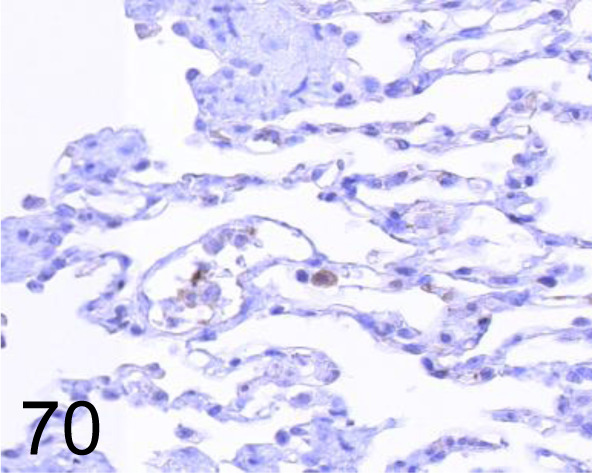
iNOS /- /Abcam plc /AB3523, Lung /Dog.
Fig. 71.
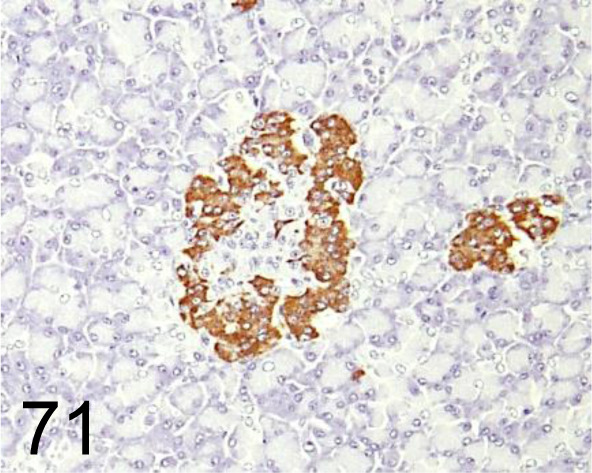
Insulin /- /Agilent Technology /IR00261-2J, Pancreas /Primate.
Fig. 72.
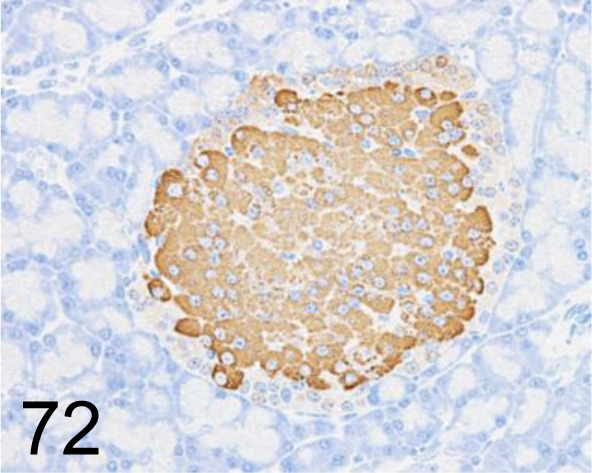
Insulin /K36aC10 /Abcam plc /ab6995, Pancreas /Rat.
Fig. 73.
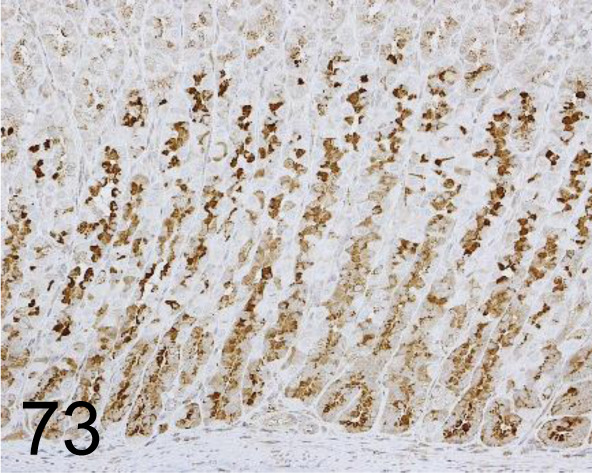
Intrinsic factor /- /Fitzgerald Industries International /20-IR51, Stomach /Rat.
Fig. 74.
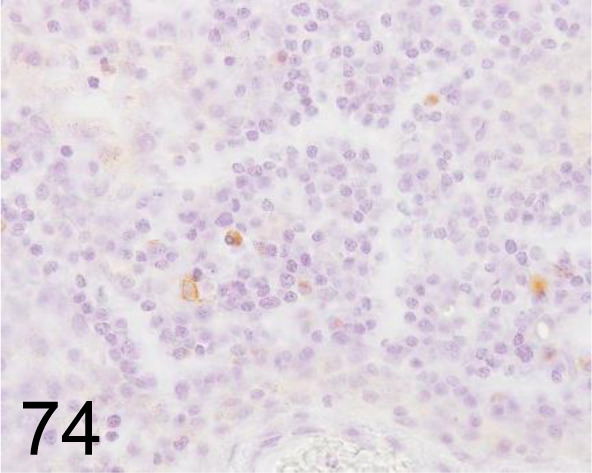
Kappa Light Chains /- /Agilent Technology /IR50661-2J, Lymph node /Dog.
Fig. 75.
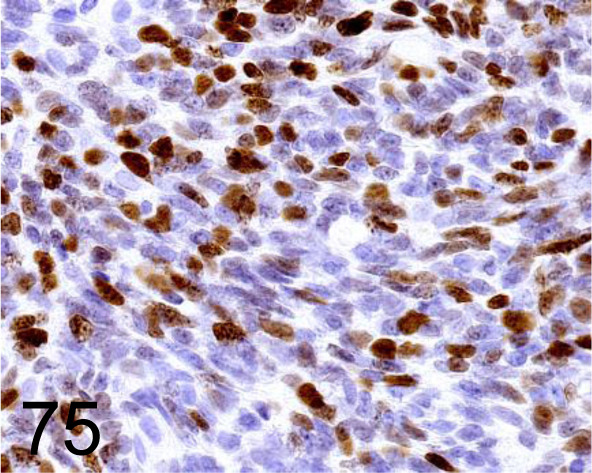
Ki-67 /- /Agilent Technology /IR62661-2, Odontogenic tumor /Dog.
Fig. 76.
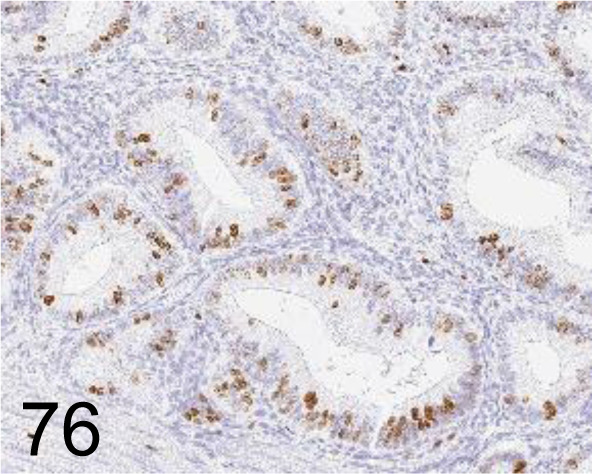
Ki-67 /SP6 /Nichirei Biosciences /418071, Uterus /Monkey.
Fig. 77.
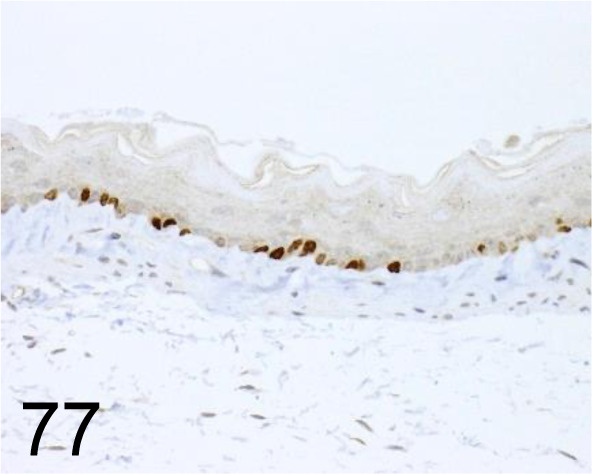
Ki-67 /- /Novus Biologicals /NB110-89717SSP, Esophagus /Mouse.
Fig. 78.
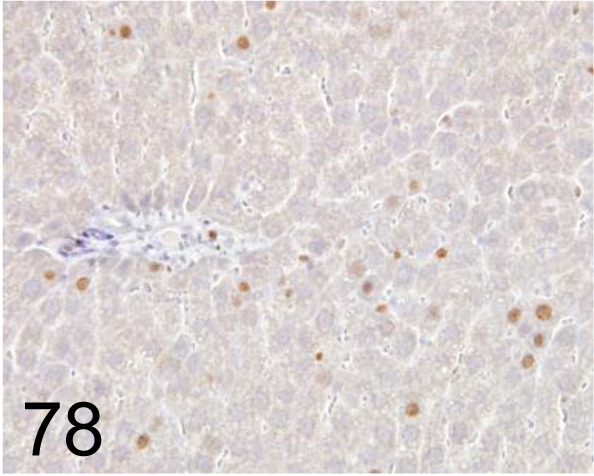
Ki-67 /- /Abcam plc /AB15580, Liver /Rat.
Fig. 79.
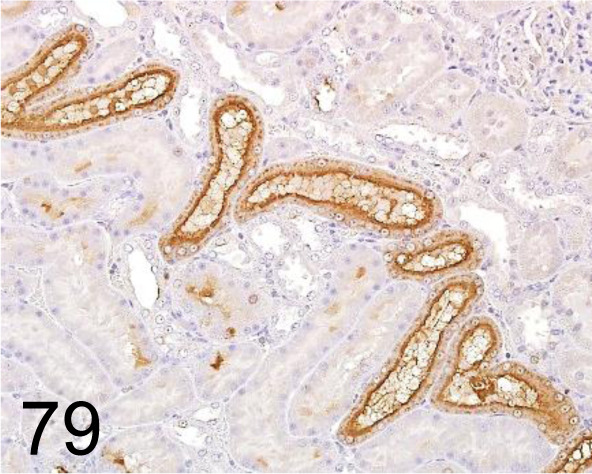
KIM-1/ Tim-1 /- /R&D Systems /AF3689, Kidney /Rat.
Fig. 80.
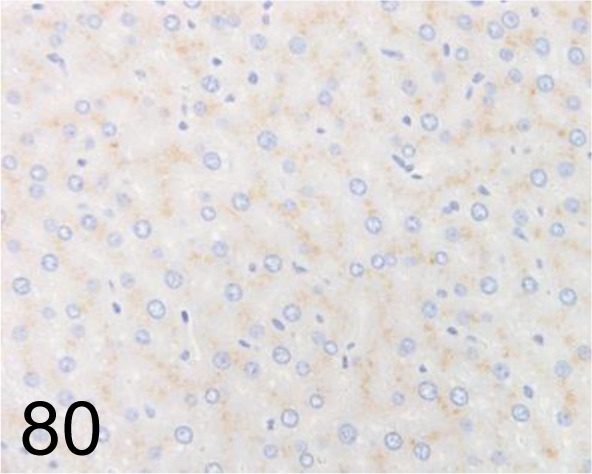
LAMP-2 /AMC2 /Thermo Fisher Scientific /51-2200, Liver /Rat.
Fig. 81.
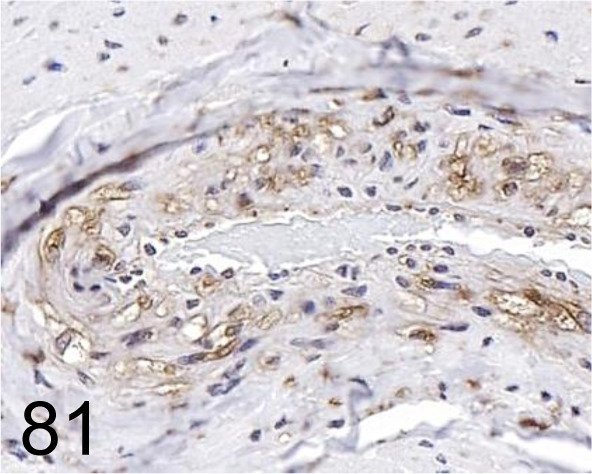
LAMP-2 /- /LifeSpan BioSciences (LSBio) /LS-B3144-50, Coronary artery /Dog.
Fig. 82.
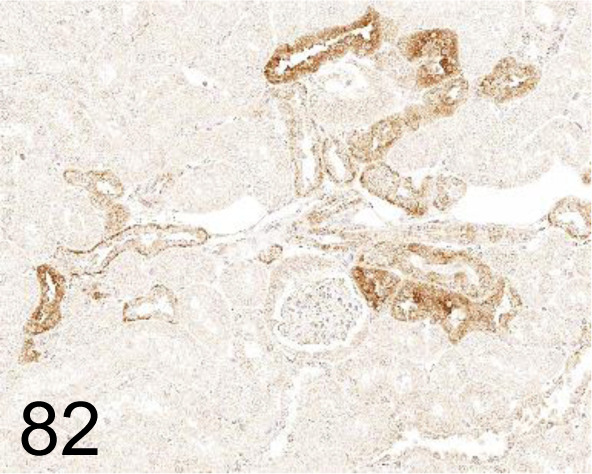
LC3 /- /MBL International /PM036, Kidney /Monkey.
Fig. 83.
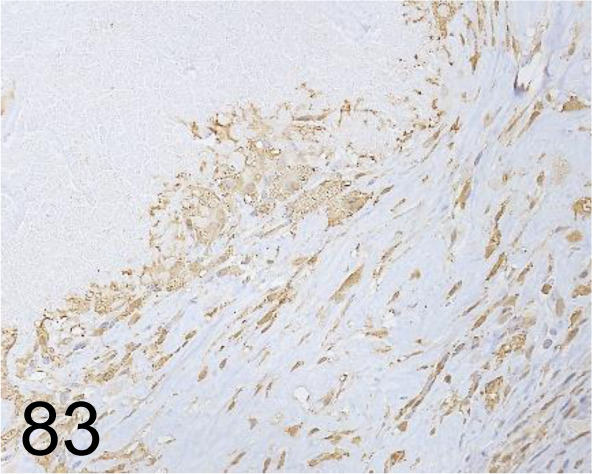
MAC-2 /M3/38 /Cedarlane Laboratories /CL8942AP, Skin /Mouse.
Fig. 84.
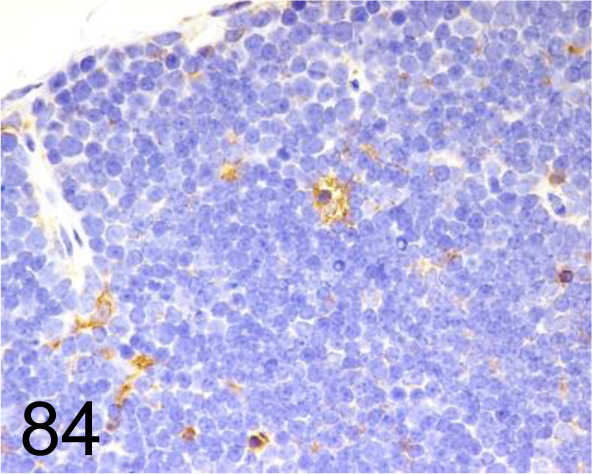
MAC-3 /M3/84 /BD Pharmingen Inc. /550292, Thymus /Mouse.
Fig. 85.
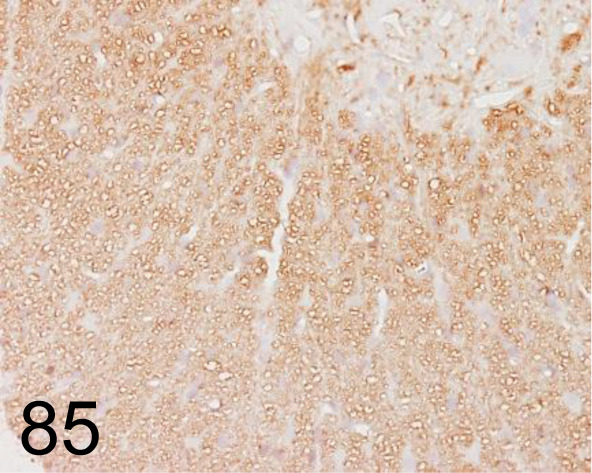
Myelin Basic Protein /26 /Merck /MAB384, Spinal cord /Rat.
Fig. 86.
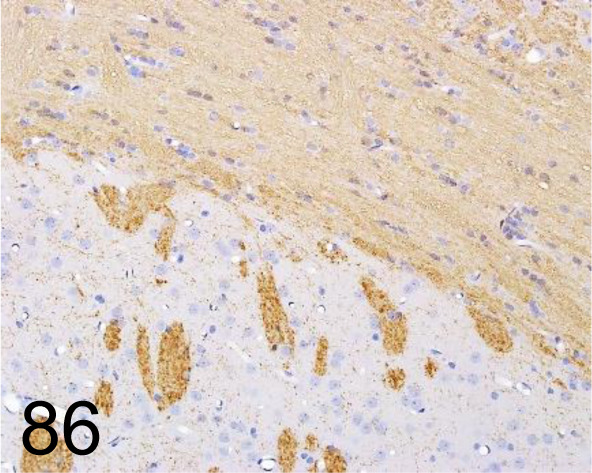
Myelin Basic Protein /- /Sigma-Aldrich /M3821, Brain /Rat.
Fig. 87.
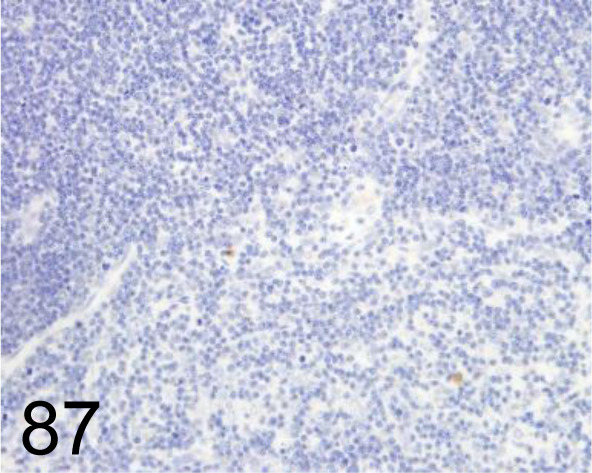
MDA /1F83 /Japan Institute for the Control of Aging /MMD-030n, Thymus /Rat.
Fig. 88.
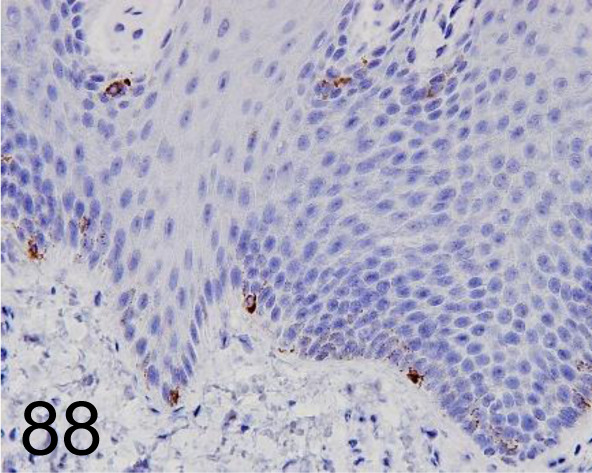
Melan A /A103 /Agilent Technology /M719601-2, Skin /Primate.
Fig. 89.
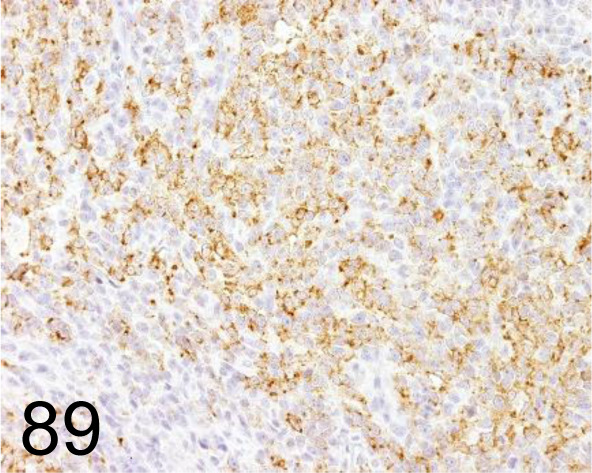
Melan A /A103 /Agilent Technology /M719629-2, Malignant melanoma /Dog.
Fig. 90.
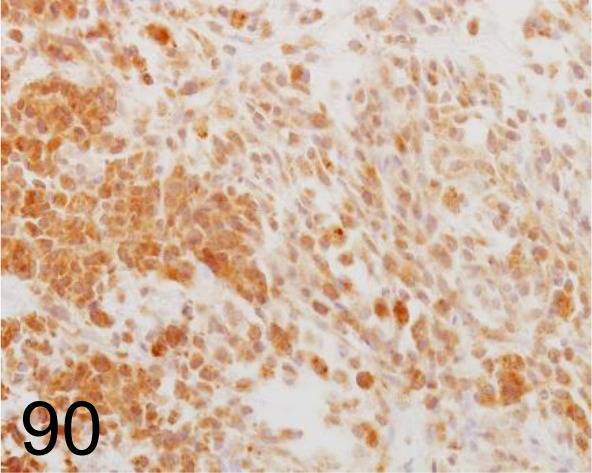
Melanoma /PNL2 /Abcam plc /ab12502, Malignant melanoma /Dog.
Fig. 91.
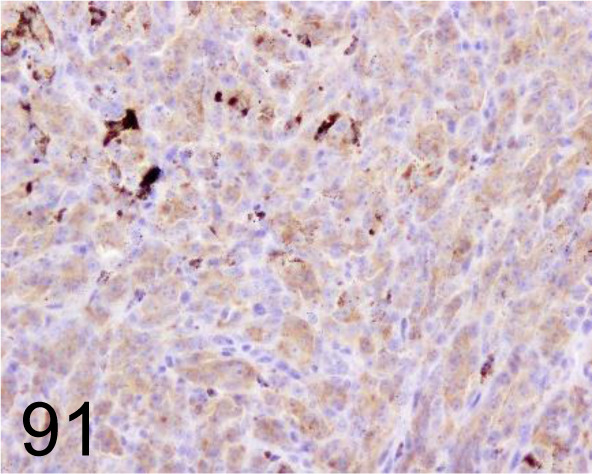
Melanoma /PLN.2 /Santa Cruz Biotechnology, Inc. /SC-59306, Melanoma /Dog.
Fig. 92.
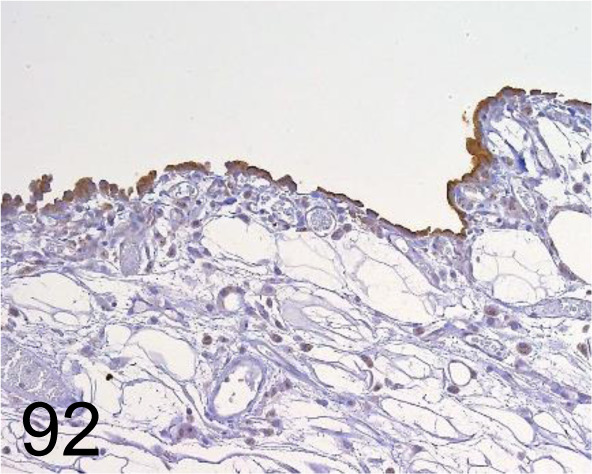
Mesothelin /- /Immuno-Biological Laboratories Co., Ltd. /28001, Mesothelial cell /Rat.
Fig. 93.
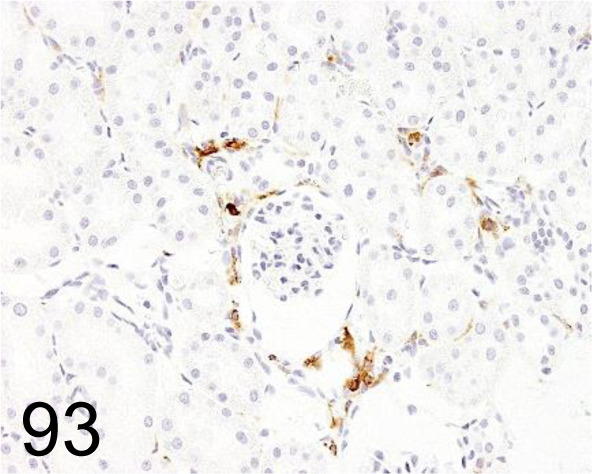
MHC Class II (RT1B) /OX-6 /Bio-Rad Laboratories, Inc. /MCA46GA, Kidney /Rat.
Fig. 94.
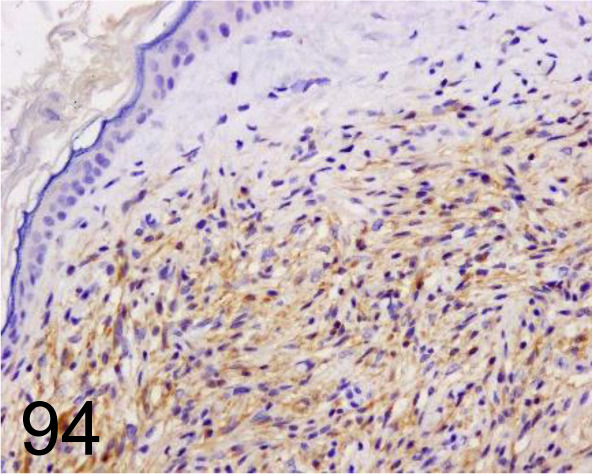
MiTF /34CA5 /Novocastra (Leica Biosystems) /MITF-L, Peripheral nerve sheath tumor /Dog.
Fig. 95.
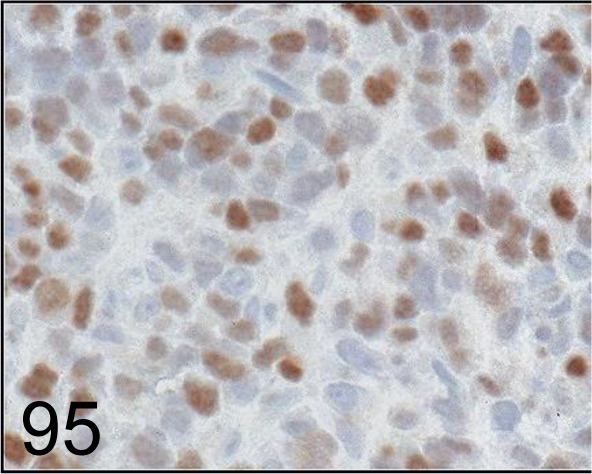
MyoD1 /5.8A /Agilent Technology /M351201-2, Rhabdomyosarcoma /Dog.
Fig. 96.
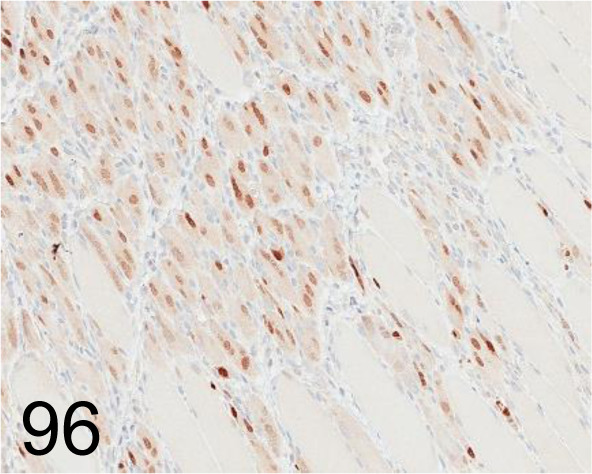
Myogenin /F5D /Abcam plc /ab1835, Myoblast /Rat.
Fig. 97.
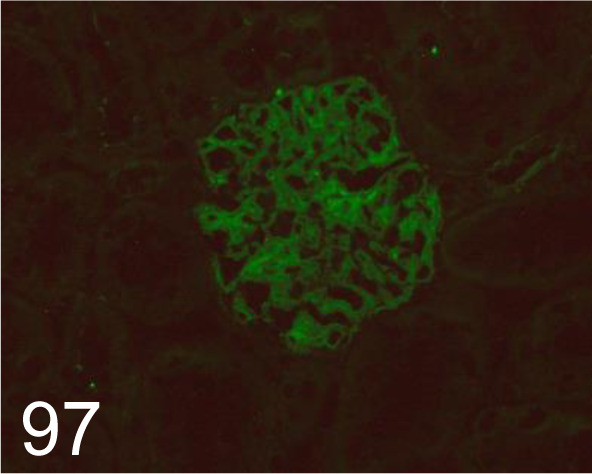
Nephrin (c)/- /Immuno-Biological Laboratories Co., Ltd. /29070, Kidney /Rat.
Fig. 98.
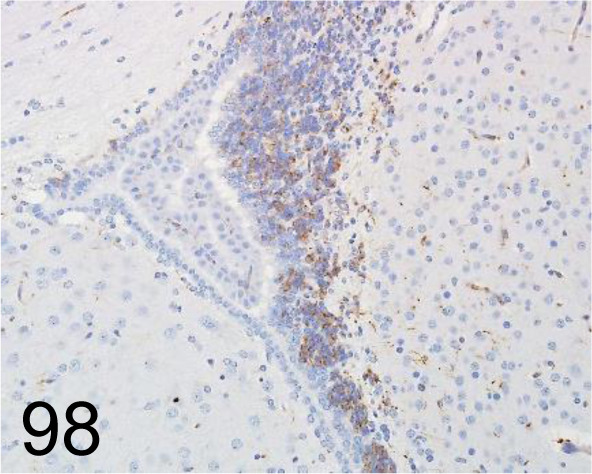
Nestin /Rat-401 /Merck /MAB353, Brain /Rat.
Fig. 99.
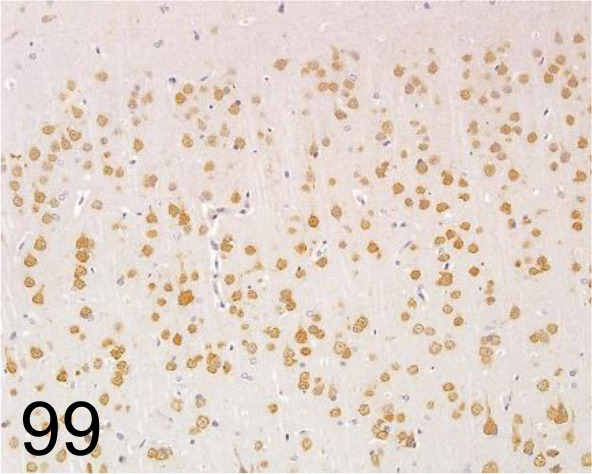
NeuN /A60 /Merck /MAB377, Brain /Rat.
Fig. 100.
Neurofilament /2F11 /Agilent Technology /M076229-2, Brain /Rat.
Fig. 101.
Neurofilament /2F11 /Nichirei Biosciences /412551, Dorsal root ganglion /Rat.
Fig. 102.
Red, NG2 /- /Merck /AB5320, Pericyte in tumor /Mouse.
Fig. 103.
nNOS /- /Thermo Fisher Scientific /61-7000, Nerve /Rat.
Fig. 104.
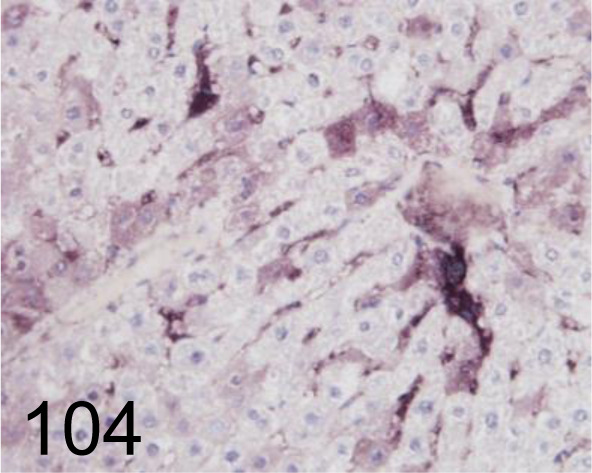
8-OHdG /N45.1 /Japan Institute for the Control of Aging /MOG-020P, Liver /Rat.
Fig. 105.
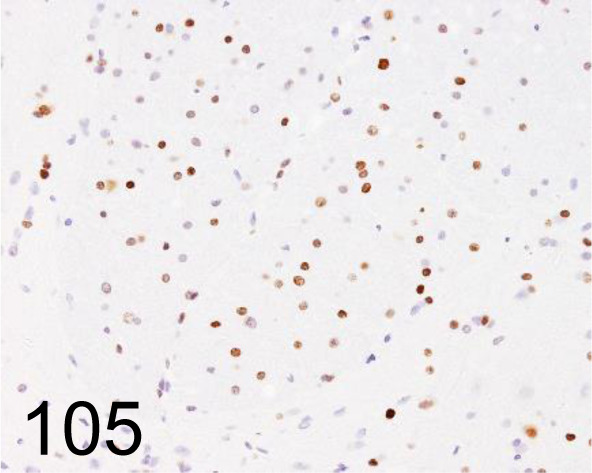
Olig2 /- /Immuno-Biological Laboratories Co., Ltd. /18953, Spinal cord /Rat.
Fig. 106.
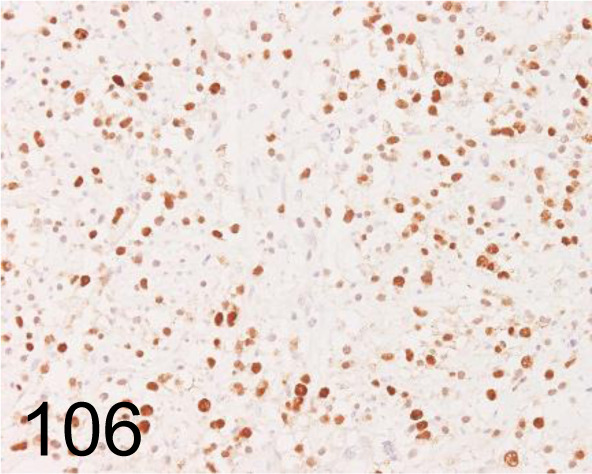
Olig2 /211F1.1 /Merck /MABN50, Oligodendroglioma /Dog.
Fig. 107.
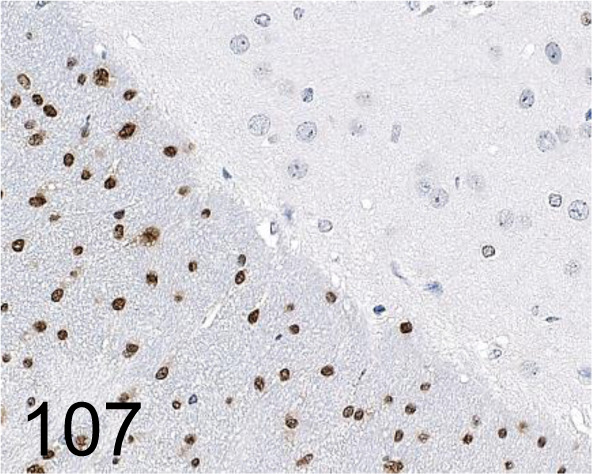
Olig2 /- /Novus Biologicals /NBP1-28667, Cerebrum /Mouse.
Fig. 108.
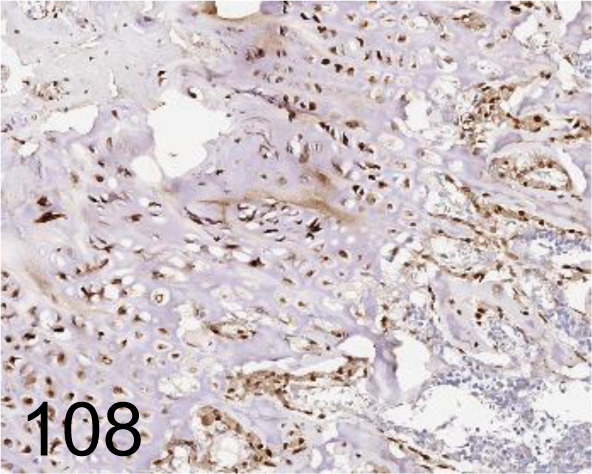
Osterix /- /Abcam plc /ab22552, Femur /Rat.
Fig. 109.
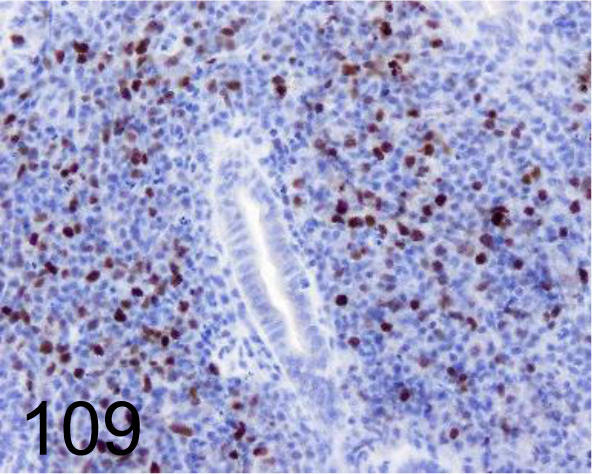
Pax-5 /- /Abcam plc /ab15164, Intestinal B cell lymphoma /Dog.
Fig. 110.
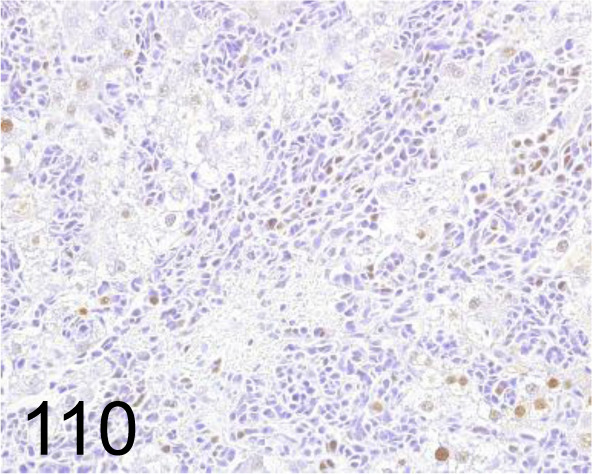
PCNA /PC10 /Agilent Technology /M087901-2, Hemangiosarcoma, liver /Rat.
Fig. 111.
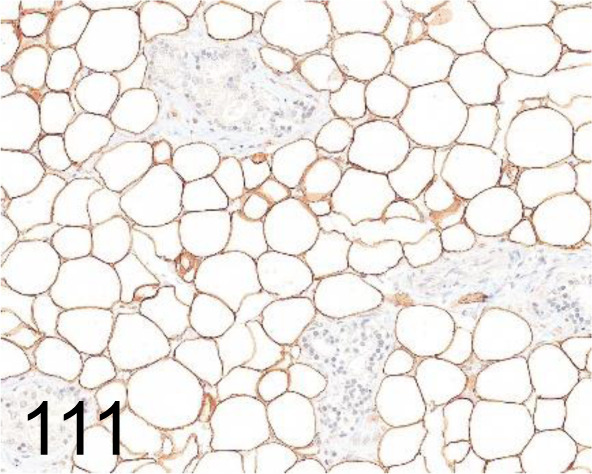
Perilipin 1 /- /Progen /GP29, Adipose tissue /Rat.
Fig. 112.
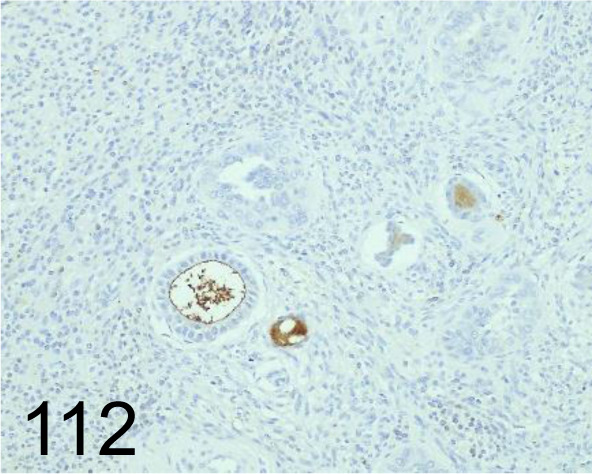
PGP-9.5 /- /Agilent Technology /Z511601-2, Ovary /Dog.
Fig. 113.
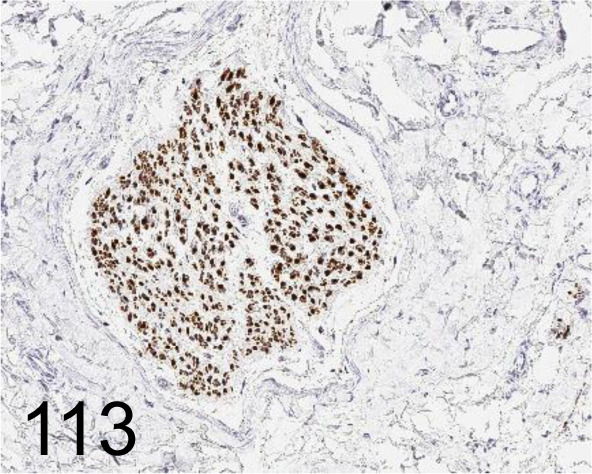
PGP-9.5 /13C4/I3C4 /Abcam plc /ab8189, Peripheral nerve /Monkey.
Fig. 114.
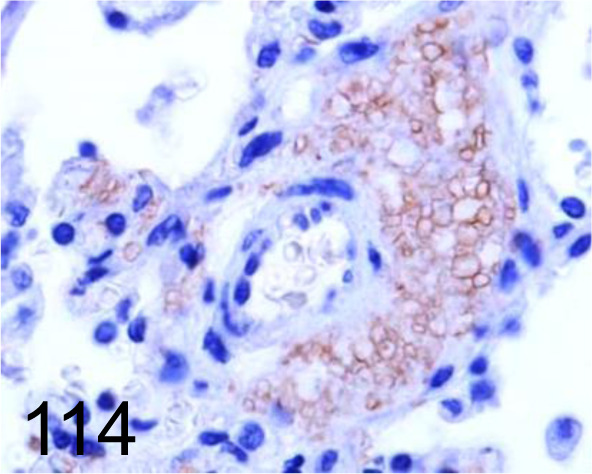
Pneumocystis /- /Santa Cruz Biotechnology, Inc. /sc-71915, Lung /Dog.
Fig. 115.
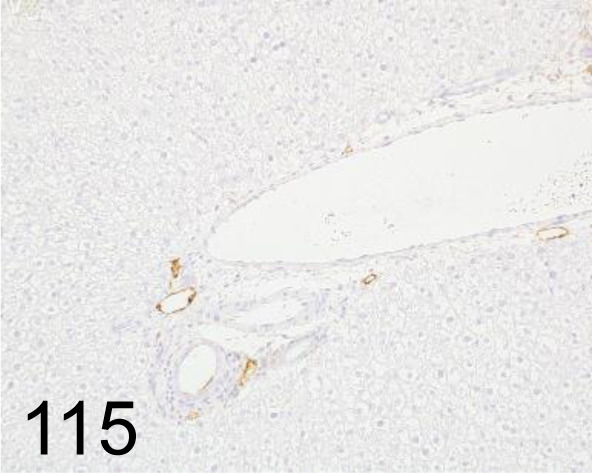
Podoplanin /- /AngioBio, Inc. /11-035, Liver /Rat.
Fig. 116.
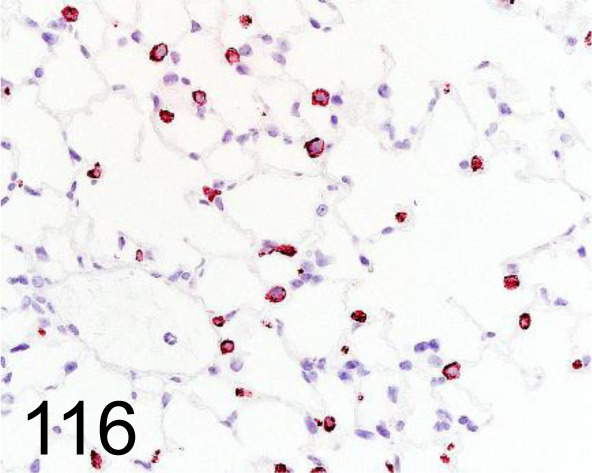
Prosurfactant Protein C /- /Abcam plc /ab90716, Lung /Mouse.
Fig. 117.
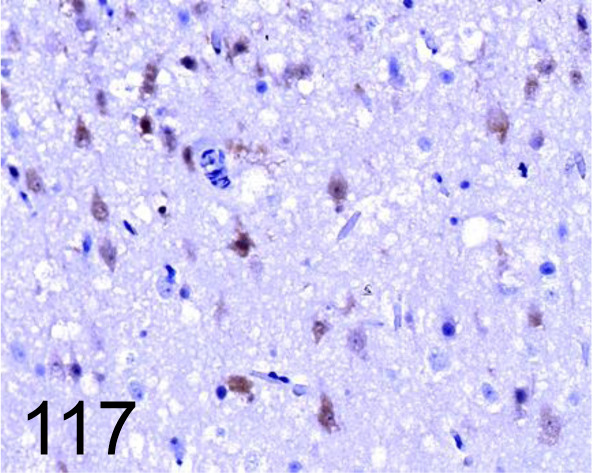
Pseudorabies Virus /- /Abcam plc /ab3534, Cerebrum /Dog.
Fig. 118.
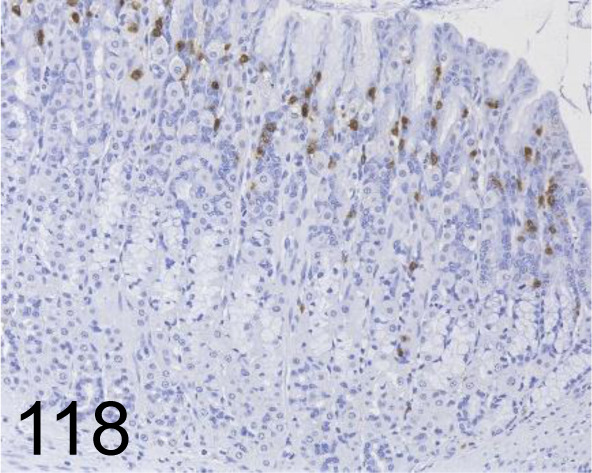
Rat Mast Cell Protease II /- /Moredun Scientific /MS-RM4, Glandular stomach /Rat.
Fig. 119.
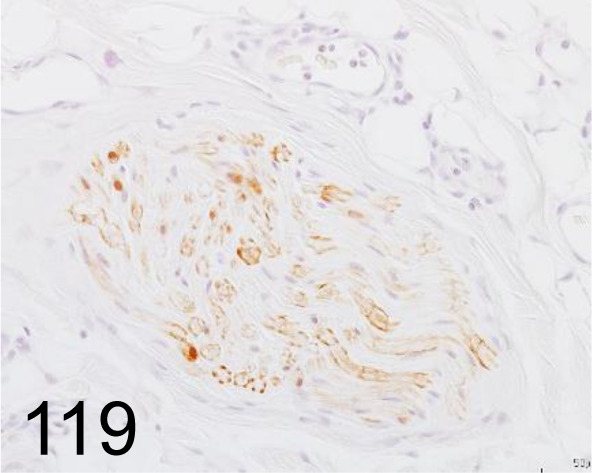
S100 /- /Agilent Technology /IR50461-2J, Skin /Dog.
Fig. 120.
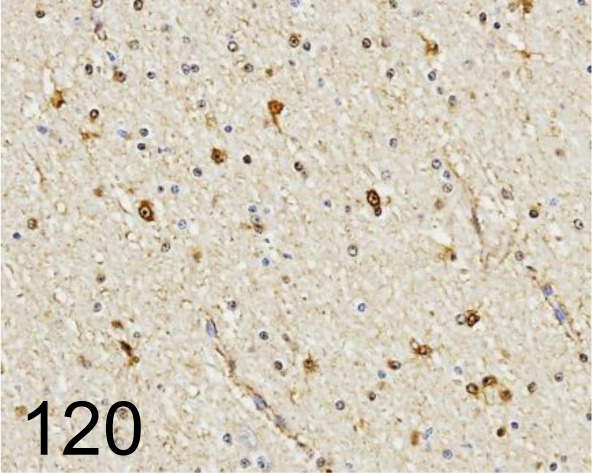
S100 /4c49 /Abcam /ab4066, Brain /Cow.
Fig. 121.
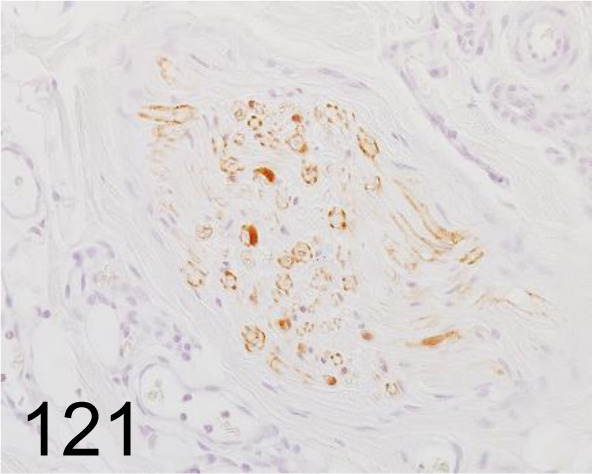
S100 /- /Nichirei Biosciences /422091, Skin /Dog.
Fig. 122.
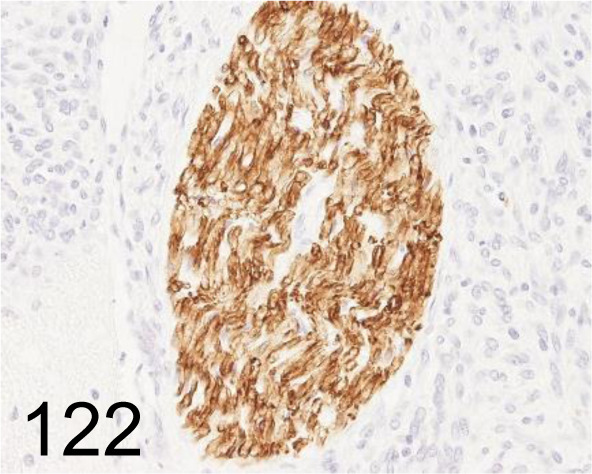
Schwann Cell/Peripheral Myelin /Schwann/2E /Cosmo Bio Co., Ltd. /GU01-M01AS-A, Nerve /Rat.
Fig. 123.
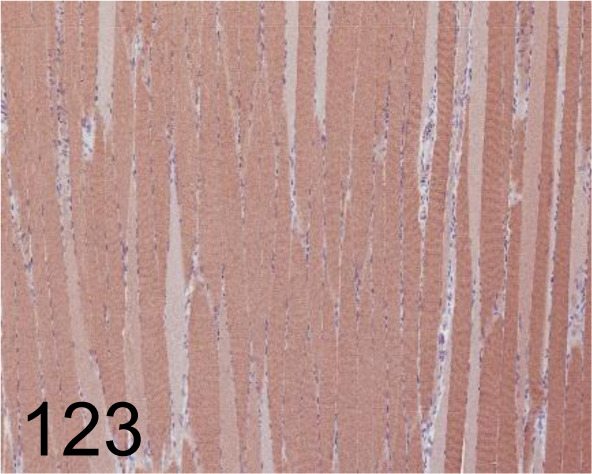
Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase 2 /- /Abcam plc /ab3625, Soleus /Rat.
Fig. 124.
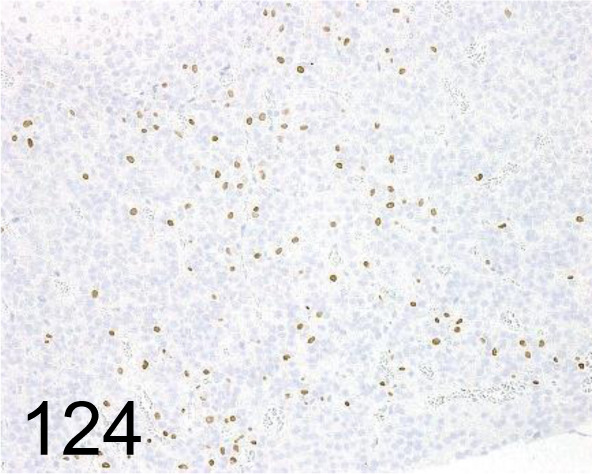
Steroidogenic Factor 1 /N1665 /Perseus Proteomics Inc. /PP-N1665-00, Pituitary gland /Rat.
Fig. 125.
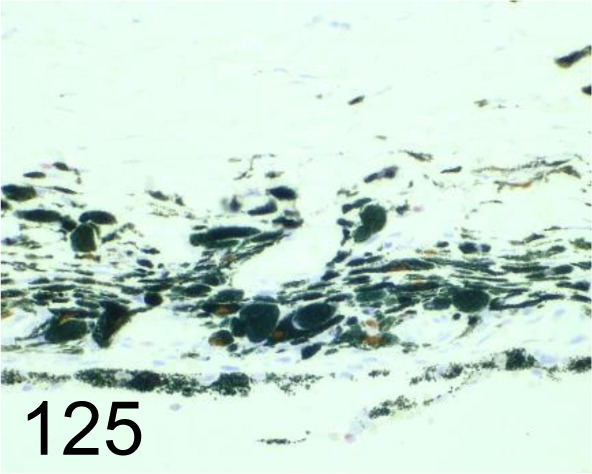
Sox-10 / Santa Cruz Biotechnology, Inc. /sc-365692, Eye /Dog.
Fig. 126.
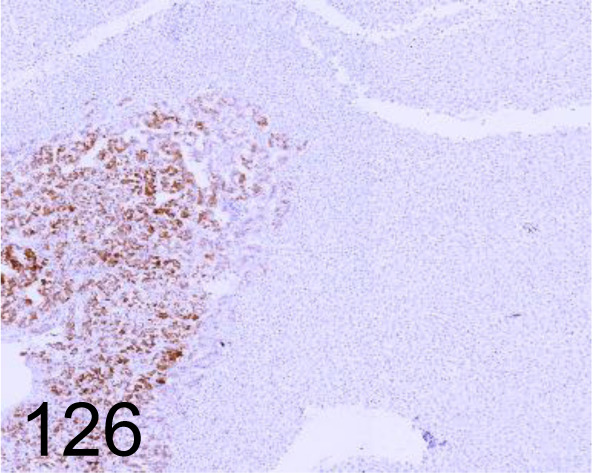
Synaptophysin /DAK-SYNAP /Agilent Technology /M731501-2, Adrenal gland /Dog.
Fig. 127.
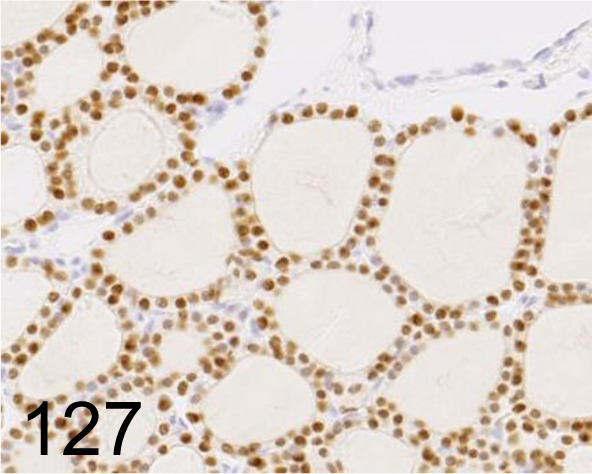
Thyroid Transcription Factor 1 /SPT24 /Leica Biosystems /PA0364, Thyroid gland /Rat.
Fig. 128.
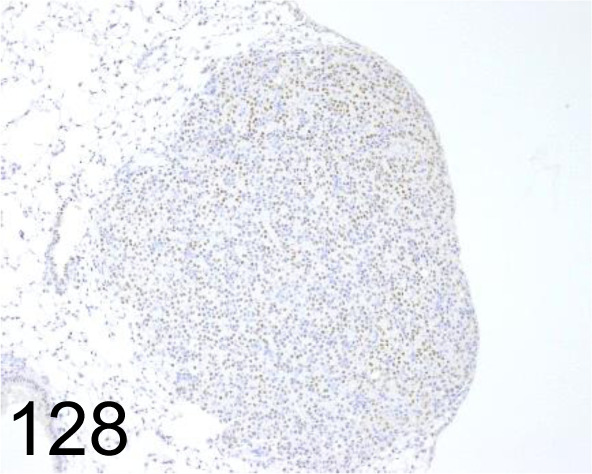
Thyroid Transcription Factor 1 /EP1584Y /Abcam plc /ab76013, Lung /Mouse.
Fig. 129.
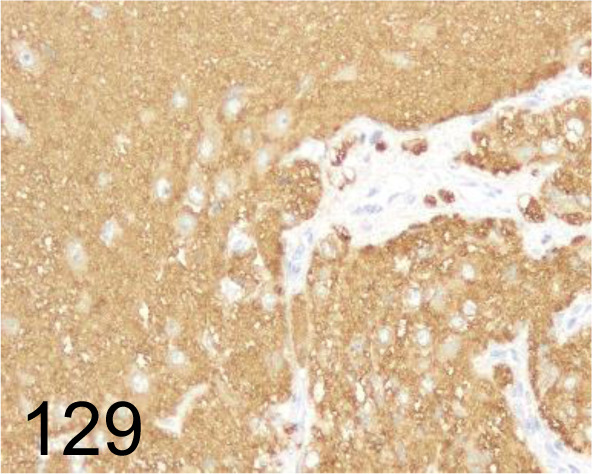
Tubulin βIII /5G8 /Promega /G7121, Brain /Dog.
Fig. 130.
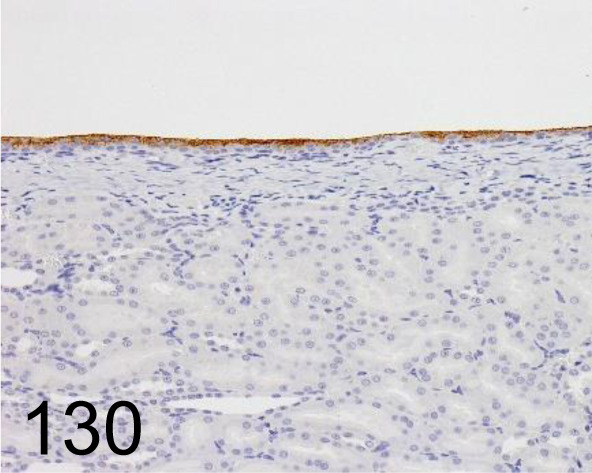
Uroplakin III /AU1 /Progen /651108, Kidney /Rat.
Fig. 131.
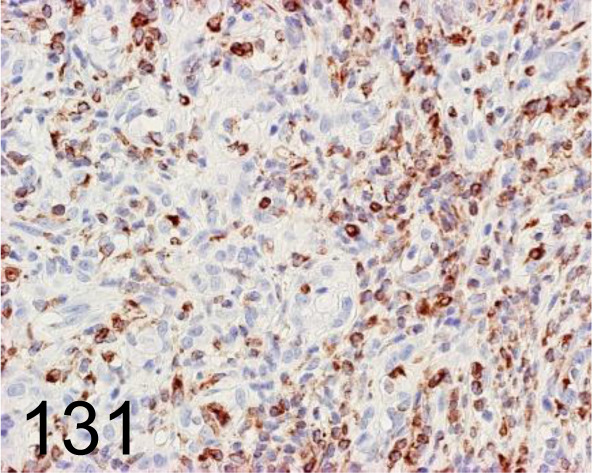
Vimentin /V9 /Agilent Technology /IR63061-2J, Complex carcinoma, eyelid /Dog.
Fig. 132.
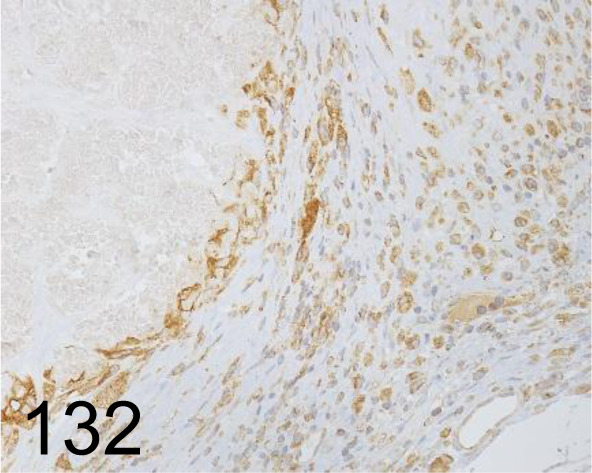
Vimentin /D21H3 /Cell Signaling Technology /5741, Skin /Mouse.
Fig. 133.
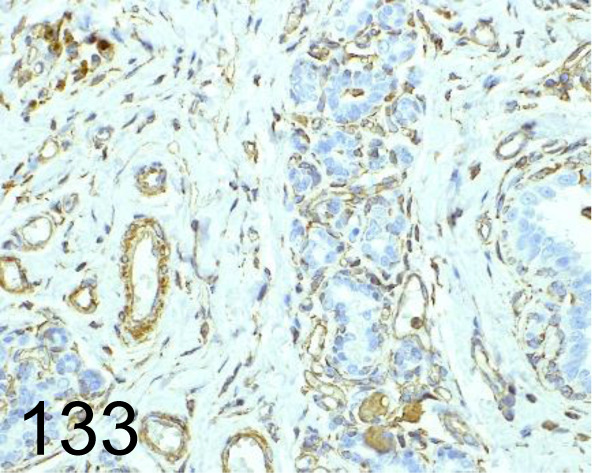
Vimentin /EPR3776 /Abcam plc /ab92547, Mammary gland /Dog.
Fig. 134.
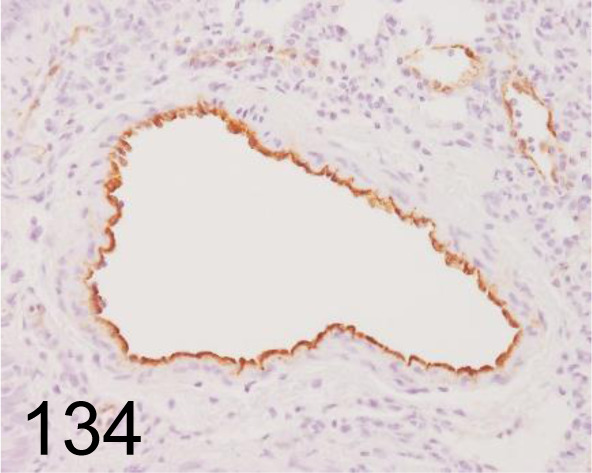
Von Willebrand Factor /- /Abcam plc /ab6994, Lung /Rat.
Fig. 135.
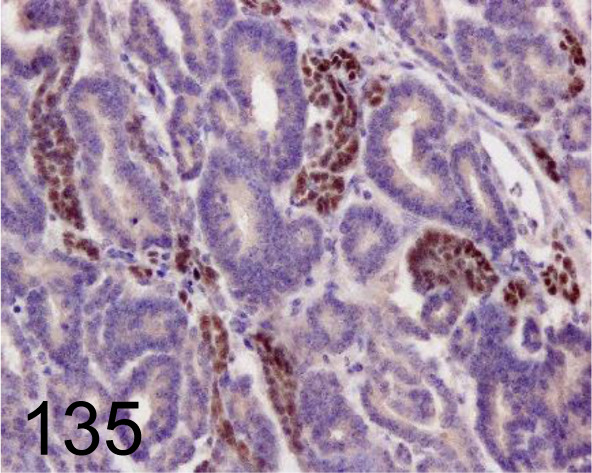
Wilms’ Tumor 1 /- /Santa Cruz Biotechnology, Inc. /SC-192, Nephroblastoma /Pig.
Fig. 136.
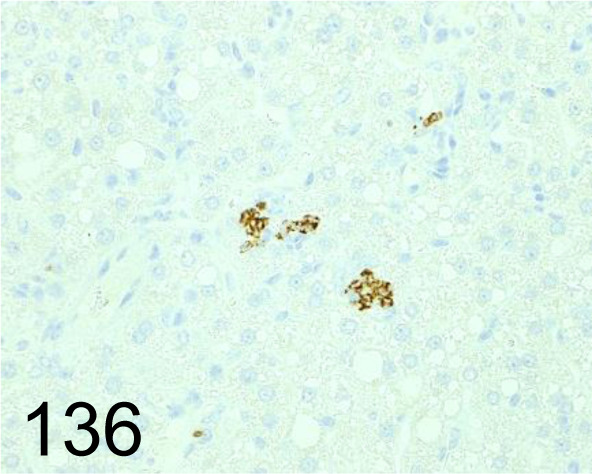
YM1/Chitinase 3-like 3 /281926 /R&D Systems /MAB2446, Liver /Mouse.
Supplementary Tables
Disclosure of Potential Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Acknowledgments
The authors would like to thank the cooperating research institutes, part of the CEAH, for generously providing the data.
References
- 1.Ramos-Vara JA, and Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol. 51: 42–87. 2014. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa S, Nagaike M, and Ozaki K. Databases for technical aspects of immunohistochemistry. J Toxicol Pathol. 30: 79–107. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.