Abstract
This review aimed to provide a summary on the traditional uses, phytochemistry, and pharmacological activities in the cardiovascular system and cardiotoxicity of Melissa officinalis (MO), with the special emphasis on the protective mechanisms in different cardiovascular pathologies. MO is a perennial aromatic herb commonly known as lemon balm, honey balm, or bee balm, which belongs to Lamiaceae family. Active components are mainly located in the leaves or essential oil and include volatile compounds, terpenoid (monoterpenes, sesquiterpenes, triterpenes), and polyphenolic compounds [rosmarinic acid (RA), caffeic acid, protocatechuic acid, quercitrin, rhamnocitrin, luteolin]. For centuries, MO has been traditionally used as a remedy for memory, cognition, anxiety, depression, and heart palpitations. Up until now, several beneficial cardiovascular effects of MO, in the form of extracts (aqueous, alcoholic, and hydroalcoholic), essential oil, and isolated compounds, have been confirmed in preclinical animal studies, such as antiarrhythmogenic, negative chronotropic and dromotropic, hypotensive, vasorelaxant, and infarct size–reducing effects. Nonetheless, MO effects on heart palpitations are the only ones confirmed in human subjects. The main mechanisms proposed for the cardiovascular effects of this plant are antioxidant free radical–scavenging properties of MO polyphenols, amelioration of oxidative stress, anti-inflammatory effects, activation of M2 and antagonism of β1 receptors in the heart, blockage of voltage-dependent Ca2+ channels, stimulation of endothelial nitric oxide synthesis, prevention of fibrotic changes, etc. Additionally, the main active ingredient of MO-RA, per se, has shown substantial cardiovascular effects. Because of the vastness of encouraging data from animal studies, this plant, as well as the main ingredient RA, should be considered and investigated further as a tool for cardioprotection and adjuvant therapy in patients suffering from cardiovascular diseases.
Keywords: Melissa officinalis, lemon balm, cardioprotection, myocardium, polyphenols
Introduction
During the history, for centuries people have been widely using medicinal plants as remedies for various cardiovascular diseases (CVDs) such as congestive heart failure, hypertension, angina pectoris, atherosclerosis, cerebral insufficiency, venous insufficiency, and arrhythmia (Mashour et al., 1998). Nowadays, the number one cause of death worldwide belongs to CVDs, taking approximately 17.9 million lives each year (31%), and despite the wide spectrum of synthetic cardiovascular drugs, the prevalence of CVDs is still growing (World Health Organization, 2021). Because of this ongoing health, social, and economic problem, science has turned to investigation of alternative medicine and natural products that are marked as traditionally efficient and safe in the treatment of CVDs (Mashour et al., 1998; Li et al., 2015; World Health Organization, 2021). Popularity of natural products in the science field has revived interest in traditional remedies as new cardiovascular drugs.
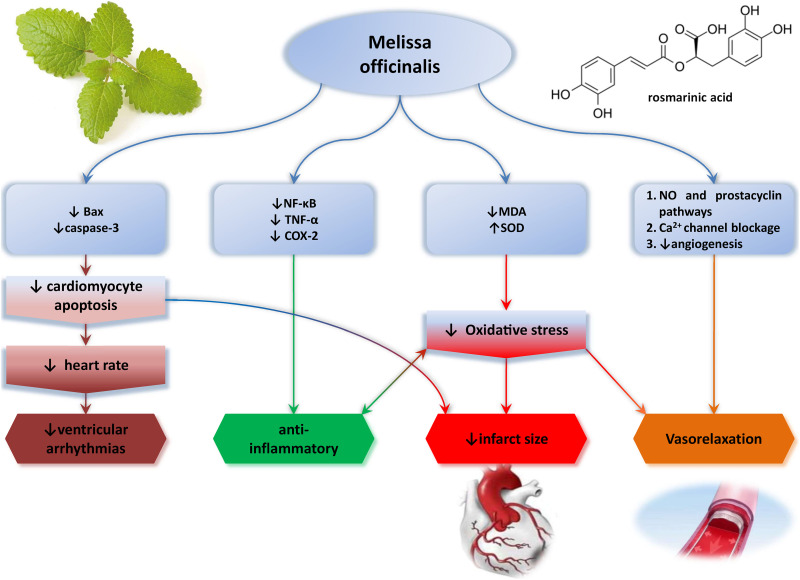
Despite the fact that the usage of Melissa officinalis (MO) in some cardiac pathologies was reported, up until now, there is a restricted knowledge regarding its cardioprotective potential and mechanisms involved. Thus, this review article aimed to summarize the current knowledge about MO effects on cardiovascular system and point out its possible usage as adjuvant therapy in CVD (Figure 1).
FIGURE 1.
Cardioprotection induced by Melissa officinalis.
Taxonomy
Kingdom: Plantae—Plants; subkingdom: Tracheobionta—vascular plants; superdivision: Spermatophyta—seed plants; division: Magnoliophyta—flowering plants; class: Magnoliopsida—dicotyledons; subclass: Asteridae; order: Lamiales; family: Lamiaceae—mint family; genus: Melissa L.—balm P; species: Melissa officinalis L.
Botany
Melissa officinalis (Greek word “Melissa”—honeybee) is a perennial aromatic herb commonly known as lemon balm, honey balm, or bee balm. It belongs to Lamiaceae (mint) family of plants and is also a member of genus Melissa L. together with Melissa axillaris, Melissa flava, and Melissa yunnanensis. MO has square-shaped stem or quadrangular stem, which grows up to 0.5–1.5 m in height, characteristically for Lamiaceae members. The leaves are in decussate pairs, green ovate to cordate, and are used as herbal drug, because of the best active component content (Canadanović-Brunet et al., 2008; Abdel-Naime et al., 2016; Shakeri et al., 2016; Abdel-Naime et al., 2020). Also, the leaves possess lemon-like scent and taste due to the presence of volatile compounds (monoterpenes and sesquiterpenes) (Aharizad et al., 2012). During the summer, white/pink pale flowers are formed, whereas hairy root system includes several lateral roots (Canadanović-Brunet et al., 2008; Abdel-Naime et al., 2016, 2020; Shakeri et al., 2016). It is native to southern Europe and central and western Asia, especially the Mediterranean, but also in gardens and naturalized in many parts of the United States (Eastern, Midwestern, and Pacific Northwest states (Abdel-Naime et al., 2016, 2020).
Active Compounds
MO is considered to be a great source of a wide range of active chemical compounds present in leaves or essential oil, including different terpene and polyphenolic compounds.
A vast number of studies investigated the MO essential oil content and claim that the major compounds of MO essential oil are volatile compounds such as monoterpenes and sesquiterpenes citrals (geranial and neral, which give citrus-like aroma), geraniol, citronellal, thymol, and β-caryophyllene in different proportions. The presence of additional components and their percentage vary, depending on the method of obtaining the essential oil, distillation conditions, region’s climate, plant species, and maturity stage of MO (Aharizad et al., 2012; Ehsani et al., 2017). Analysis of two essential oils obtained by hydrodistillation method from MO grown in Iran and Turkey discovered more than 20 different components and very similar content of the major components: citronellal (37.33% vs. 36.62–43.78%), thymol (11.96% vs. 0.40–11.94%), citral (10.10% vs. 10.10–17.43%), and β-caryophyllene (7.27% vs. 5.91–7.27%) (Cosge et al., 2009; Ehsani et al., 2017). On the other hand, MO essential oil obtained from Algeria is composed predominantly of oxygenated monoterpenes [neral (30.2%), geranial (44.2%) and citronellal (6.3%)], whereas the sesquiterpene fraction was lower: α-copaene (1.8%) and β-caryophyllene (1.3%) and oxygenated sesquiterpene caryophyllene oxide (1.3%) (Abdellatif et al., 2014). The essential oil of MO grown in Balkan, Serbia, was reported to be composed of mostly citrals (geranial + neral, 39.9%), citronellal (13.7%), limonene (2.2%), geraniol (3.4%), β-caryophyllene (4.6%), β-caryophyllene oxide (1.7%), and germacrene D (2.4%) (Mimica-Dukic et al., 2004). The variability in essential oil composition obtained from 15 different MO subspecies grown under Central European climate conditions was recently investigated, with the aim to compare the content between two harvests in June or August. It seems that leaves collected in June are richer in oil compared to the ones collected in August, whereas the oil content [dominantly citrals (geranial, neral) and citronellal] does not significantly differ between these two cuts (Chizzola et al., 2018). Plant age may also influence essential oil content, according to the recent investigation. Namely, high fluctuations in the content exist when comparing the first and the second year of growth, in the favor of richer chemical composition of the 2 year-old plant (Nurzyńska-Wierdak et al., 2014).
Phytochemical examinations of the MO leaves have pointed out the presence of the following main compounds: previously mentioned monoterpenes, sesquiterpenes (Mimica-Dukic et al., 2004; Cosge et al., 2009; Aharizad et al., 2012; Abdellatif et al., 2014; Nurzyńska-Wierdak et al., 2014; Ehsani et al., 2017; Chizzola et al., 2018), triterpenes, and phenolic compounds such as polyphenols, phenolic acids [rosmarinic acid (RA), caffeic acid, protocatechuic acid] (Ibragic et al., 2014), flavonoids (quercitrin, rhamnocitrin, luteolin) (Mencherini et al., 2007), and tannins. Rich phenolic content of lemon balm is mostly responsible for its therapeutic effects, due to well-known antioxidant potential of these compounds, so it is important to optimize the extraction conditions to achieve optimal phenolic content and therefore the treatment goals. Phenolic content also varies, depending on the plant origin. For example, hot water extracts of lemon balm leaves of Bosnian origin have higher content of rosmarinic, gallic, and chlorogenic acid compared to Turkish one, whereas the content of cinnamic acid was higher in Turkish origin lemon balm (Ibragic et al., 2014). The main triterpenoid compounds isolated from lemon balm are ursolic and oleanolic acid (Ibragic et al., 2014; Tantry et al., 2014), but additional two sulfated ursane-type triterpenes, one sulfated oleanane-type triterpene, and one oleanane triterpene were confirmed in the hydroalcoholic extract of the lemon balm leaves (Tantry et al., 2014). Triterpenes with sulfate groups in their structure, attached to the carbohydrate chain, are known to possess higher biological activity, relative to the one attached to the aglycone group (Park et al., 2014). Characteristically for Lamiaceae family, flavonoid compounds are mostly concentrated in the aerial parts of MO. Several flavonoid compounds have been isolated from lemon balm, divided into four groups by their chemical structure: flavones (luteolin, apigenin, and their derivatives), flavanones (hesperidin, hesperetin, naringin, naringenin), flavanols (catechin, epicatechin, rutin), and flavonols (isoquercitrin, rhamnocitrin) (Patora and Klimek, 2002; Mencherini et al., 2007; Dastmalchi et al., 2009; Pereira et al., 2014; Tantry et al., 2014; Shakeri et al., 2016). The main constituents are presented in Table 1.
TABLE 1.
Major chemical constituents of M. officinalis essential oil and leaves.
| M. officinalis | Essential oil | Leaf |
| Terpenes -Monoterpenes | Citronellal (Aharizad et al., 2012; Cosge et al., 2009; Ehsani et al., 2017) Thymol (Cosge et al., 2009; Aharizad et al., 2012; Ehsani et al., 2017) Citral (neral and geranial) (Cosge et al., 2009; Aharizad et al., 2012; Abdellatif et al., 2014; Ehsani et al., 2017) | |
| -Sesquiterpenes | β-Caryophyllene (Cosge et al., 2009; Aharizad et al., 2012; Abdellatif et al., 2014; Ehsani et al., 2017) Caryophyllene oxide (Mimica-Dukic et al., 2004; Abdellatif et al., 2014) Limonene (Mimica-Dukic et al., 2004) Germacrene D (Mimica-Dukic et al., 2004) | |
| -Triterpenes | Ursolic acid (Ibragic et al., 2014; Tantry et al., 2014) Oleanolic acid (Ibragic et al., 2014; Tantry et al., 2014) 3β,16β,23-Trihydroxy-13,28-epoxyurs-11- ene-3-O-β-D-glucopyranoside (Mencherini et al., 2007) 3,23-Disulfate ester of 3β,19α,23- trihydroxyurs-12-en-28-oic acid (Mencherini et al., 2007) 3,23-Disulfate ester of 2α,3β,19α,23- tetrahydroxyurs-12-en-28-oic acid (Mencherini et al., 2007) 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oic acid 28-O-β-D-glucopyranoside (Mencherini et al., 2007) 3,23-Disulfate ester of 3β,23,29- trihydroxyolean-12-en-28-oic acid (Mencherini et al., 2007) 3,23-Disulfate ester of 2α,3β,23,29- tetrahydroxyolean-12-en-28-oic acid (Mencherini et al., 2007) 3,23-Disulfate ester of 2α,3β-23,29- tetrahydroxyolean-12-ene-28-oic acid, 28-Oβ-D-glucopyranoside (Tantry et al., 2014) 23-Monosulfate ester of 2α,23-dihydroxyurs12-ene-28-oic acid, 3-O-β-D- glucopyranoside (Tantry et al., 2014) | |
| Polyhenolics -Phenolic acids | Rosmarinic acid (Ibragic et al., 2014) Caffeic acid (Ibragic et al., 2014) Protocatechuic acid (Ibragic et al., 2014) Cinnamic acid (Ibragic et al., 2014) Chlorogenic acid (Ibragic et al., 2014) Gallic acid (Ibragic et al., 2014) Ferulic acid (Arceusz et al., 2015) Ellagic acid (Arceusz et al., 2015) p-Coumarinic acid (Arceusz et al., 2015) Salvianolic acid (Barros et al., 2013) | |
| Flavonoids -Flavones | Luteolin (Mencherini et al., 2007) Apigenin (Mencherini et al., 2007) | |
| -Flavanones | Hesperidin (Pereira et al., 2014; Shakeri et al., 2016) Hesperetin (Pereira et al., 2014; Shakeri et al., 2016) Naringin (Pereira et al., 2014; Shakeri et al., 2016) Naringenin (Pereira et al., 2014; Shakeri et al., 2016) | |
| -Flavanols | Catechin (Patora and Klimek, 2002; Shakeri et al., 2016) Epicatechin (Patora and Klimek, 2002; Shakeri et al., 2016) Routin (Patora and Klimek, 2002; Shakeri et al., 2016) | |
| -Flavonols | Quercetin (Arceusz et al., 2015) Myricetin (Arceusz et al., 2015) Quercitrin (Patora and Klimek, 2002; Mencherini et al., 2007; Shakeri et al., 2016) Rhamnocitrin (Patora and Klimek, 2002; Mencherini et al., 2007; Shakeri et al., 2016) Isoquercitrin (Patora and Klimek, 2002; Mencherini et al., 2007; Shakeri et al., 2016) |
Traditional Use of MO
Lemon balm has been extensively used in traditional medicine for different medical purposes due to its history dating back more than 2,000 years ago (Zarei et al., 2015). The father of pharmacology Dioscorides was the first who mentioned this herb in his Pharmacopeia of medicinal plants, De Materia Medica. Since then, the healing properties of MO have been noted in many other medical books. In addition to its large use in traditional medicine, MO is also used in the food industry as well as in aromatherapy due to its fresh smell. It is noteworthy that only aerial plant parts are traditionally used, whereas its roots attract less attention (Shakeri et al., 2016). Several studies pointed out different effects of lemon balm such as sedative and mild hypnotic (Nour Eddine et al., 2005; Chen et al., 2006; Rasmussen, 2011), hypoglycemic, hepatoprotective, antibacterial, anti-inflammatory, antioxidant, antiviral, antispasmodic, and neuroprotective (Zarei et al., 2015; Moacǎ et al., 2018). Nevertheless, there is evidence that indicates the cytotoxic effects of MO extract (MOE) on breast (Zarei et al., 2015) and colon carcinoma (Weidner et al., 2015). Because of the ability to induce apoptosis and inhibit colon cancer cell proliferation, it has been proven that RA from MOE is responsible for its antimigratory effect on colon cancer cells (Encalada et al., 2011).
Since it has been well known that hypertriglyceridemia is one of the most important factors for CVD development, a strong effort has gone into prevention strategies including a phytochemical diet. Additionally, studies showed that daily drinking of MO tea may ameliorate metabolic parameters through reduction of serum lipid concentration and lipid peroxidation. Although the lipid-lowering mechanism of lemon balm remains unclear, it is proposed that the presence of quercetin is responsible for this pharmacological effect (Jun et al., 2012). In addition, Bolkent et al. (2005) reported that besides the reduction of total cholesterol (TC), MOE also possesses the ability to increase glutathione (GSH) levels in the liver tissue. However, the hypolipidemic effects of MOE are likely related to its powerful antioxidant properties due to lemon balm containing phenolic compounds, as the most important antioxidant agents (Zarei et al., 2015). It is assumed that the presence of RA, a derivate of phenylpropanoid, is associated with its antioxidant properties that are up to 10 times stronger than the antioxidant action of vitamin C (Shakeri et al., 2016). Furthermore, lemon balm extract acts as a potential scavenger of both synthetic and natural free radicals during their early or later stages of formation (Pereira et al., 2009). Compounds of MO possess the ability to bind to acetylcholine, as well as inhibit enzyme acetylcholinesterase, which in turn leads to improved cognitive function such as memory (Rostami et al., 2010). Finally, the researchers stand out that MOE prevents diseases that are related to oxidative stress including diabetes, CVDs, or neurodegenerative disorders such as Parkinson and Alzheimer diseases (Pereira et al., 2009). Daily intake of MO at low doses improves glucose tolerance and adjusted gene expression that is involved in hepatic gluconeogenesis. All together, these mechanisms contribute to the hypoglycemic properties of this herb (Chung et al., 2010). When it comes to anxiolytic effects of MOE, the results of several studies implicate that lower doses of aqueous extract have anxiolytic properties; whereas applied in higher doses, it has a sedative effect (Zarei et al., 2015). In some European countries, this herb is traditionally used for relaxation, especially when a disturbance occurs in the first stage of sleep. Also, it is found that MOE can reduce agitation, as well as muscle tone in patients with Alzheimer disease (Soulimani et al., 1991). Traditionally, use of lemon balm in mentally ill patients results in significant improvement in irritability, insomnia, headaches, and heart disease (Ulbricht et al., 2005). All of these beneficial effects can be attributed to the presence of significant amounts of rosmarinic, oleanolic, ursolic acid, and triterpenoids in the herb, assuming that all of these active principles can inhibit γ-aminobutyric acid (GABA) transport activity and increase the level of this neurotransmitter in the brain (Awad et al., 2007; Ibarra et al., 2010).
The use of herbs to relieve pain has a long history in the world of medicine. The fact that the essential oil of lemon balm has anti-inflammatory properties supports the traditional use of this herb in the treatment of numerous diseases associated with inflammation or pain (Bounihi et al., 2013). Literature data indicate that anti-inflammatory and antinociceptive effects of MO are contributed to RA, as well as to flavonoids and terpenoids present in the extract. Although acute analgesic property of MOE does not differ from morphine or aspirin, its chronic analgesic effect is less effective. On the other hand, active principles in the MO inhibit the monoamine oxidase enzyme and thus prevent degradation of catecholamines, but also inhibit cyclooxygenase enzyme and decrease prostaglandins and inflammatory cytokine production as in response to inflammation stimuli. In this way, the traditional use of MO is absolutely justified in the treatment of inflammatory diseases (Zarei et al., 2015). There is also evidence regarding the antiviral effects of MOE at a very early stage of the infection with respect to herpes simplex virus type 1 (HSV-1) (Miraj et al., 2017). Based on the results of an in vitro study, it is suggested that MO possesses a high virucidal effect against HSV-1 even at a very low concentration. It is assumed that RA contributed to this antiviral effect (Astani et al., 2012). Lemon balm oil affects virus before adsorption, but it is not effective after virus penetration into the host cell. Finally, taking into account that lemon balm essential oil is able to penetrate the skin, thanks to its lipophilic nature, it is worth noting that MO oil should be suitable for topical treatment of herpetic infection (Wolbling and Leonhardt, 1994).
Cardiac Effects (Arrhythmia, Ischemia–Reperfusion Injury, Myocardial Infarction)
Preclinical Studies
Because of the long known antiarrhythmic effect of lemon balm and its traditional use for heart palpitations, a wide spectrum of in vitro, in vivo, and ex vivo animal studies were conducted in order to elucidate the mechanisms of this therapeutic property (Table 2).
TABLE 2.
Cardiovascular effects of M. officinalis.
| Study | Effect | Model | Material | M. officinalis formulation | Dosage | Mechanism |
| Gazola et al. (2004) | ↓Cardiac rate | Langendoff technique ex vivo isolated rat heart | Rattus norwegicus albinus | Aqueous extract | 0.038, 0.38, 3.8, and 38 mg acutely heart perfusion (0.5 mL) | Activation of cardiac M2 receptors Blockage of Ca2+ channels |
| Joukar et al. (2014) | ↓Ventricular arrhythmias Blood pressure | LAD model of in vivo myocardial I/R injury | Wistar albino rats | Aqueous extract | 50, 100, 200, and 400 mg/kg intraperitoneally | Activation of cardiac M2 receptors Antioxidant effect of phenolics present in MOE |
| Joukar and Asadipour (2015) | Prolonged QTc JT and TpTe interval | ECG | Wistar albino rats | Aqueous extract | 50, 100, and 200 mg/kg | Blockage of K+ channels and slowing ventricular conductivity |
| Joukar et al. (2016) | ↓Blood pressure ↓Heart rate by lower doses of MOE | Isoproterenol-induced myocardial infarction | Wistar albino rats | Aqueous extract | 50, 100, and 200 mg/kg per os 7 days | ↓Oxidative stress ↓MDA |
| Sedighi et al. (2019) | ↓Infarct size ↓Ventricular arrhythmias ↓Oxidative stress | LAD In vivo regional I/R injury | Sprague–Dawley rats | Ethanolic extract | 25, 50, and 100 mg/kg per os 14 days | ↓MDA, ↑SOD Free radical–scavenging of phenolic compounds |
| Hamza et al. (2016) | Dose-dependent amelioration of doxorubicin-induced cardiotoxicity | Doxorubicin-induced cardiotoxicity | Wistar albino rats | Ethanolic extract Macerate | 250, 500, and 750 mg/kg per os 10 days | ↓Oxidative stress (↓MDA, ↓TOC, ↓protein carbonyl, ↑SOD) antioxidant capacity of MOE anti-inflammatory (↓NF-κB, TNF-α, and COX-2 gene expression) antiapoptotic effect (↓Bax, ↓caspase-3) |
| Akhondali et al. (2015) | ↓Heart rate ↓Incidence of ventricular arrhythmias | CaCl2-induced arrhythmia | Sprague–Dawley rats | Ethanolic extract Macerate | 100 and 200 mg/kg Gastric gavage 14 days | Antioxidant effect of phenolics present in MOE |
| Safaeian et al. (2016) | Cytoprotection Only via doses 100–500 μg/mL | H2O2-induced toxicity in vascular cells | In vitro HUVECs | Hydroalcoholic extract Macerate | 25, 50, 100, 250, and 500 μg/mL | Antioxidant effects of rosmarinic acid and other phenolics present in MOE |
| Ersoy et al. (2008) | Dose-dependent vasorelaxant effect Of the extract and isolated rosmarinic acid | Rat isolated thoracic aorta | Wistar albino rats | Aqueous extract Infuse | 0.001–1 mg/mL dilution in Krebs–Henseleit solution | NO pathways and prostacyclin pathways involvement |
| Alijaniha et al. (2015) | ↓Incidence of heart palpitation episodes | Benign heart palpitation | Healthy human volunteers (n = 71) | Lyophilized aqueous extract | 500 mg per os 2 × a day, for 14 days | — |
One of the initial researches regarding this subject was conducted in the isolated rat hearts by using Langendorff technique. It has been revealed that acute application of aqueous MOE in the isolated rat heart had negative chronotropic effect, without the alteration of contractile force. Multiple mechanisms triggered by plant’s alkaloids may be considered responsible for these effects, such as stimulation of muscarinic receptors in the heart and/or by voltage-dependent calcium channel voltage blockage inducing bradycardia (Gazola et al., 2004). However, Joukar et al. did not confirm this effect, possibly due to different model of myocardial ischemia–reperfusion injury (I/R) injury, in vivo [ligation of left descending coronary artery (LAD)] vs. previously used ex vivo isolated rat heart model. They reported a mild antiarrhythmic effect of aqueous MOE compared to standard antiarrhythmic drug amiodarone, with multimodal mechanism of action. MOE application–induced slower heart electrical conduction through partial PR and QTc prolongation in electrocardiogram (ECG), decreased susceptibility to reperfusion–associated ventricular arrhythmias (fibrillation), and an improvement in blood pressure especially when given in a dose of 200 mg/kg intraperitoneally. All of these changes were of a less extent relative to amiodarone and attributed to both antioxidant effects of phenolic compounds in lemon balm and stimulation of muscarinic receptors, acetylcholine K+ channels, which may be connected with slower conduction in the heart and prolonged PR interval (Joukar et al., 2014). Lemon balm also contains flavonoids, phenolic acids, terpenes, RA, and caffeic acid, all of which can have antioxidant effects. One year later, the same research group evaluated the effect of 8-day consumption of water MOE in three different doses (50, 100, and 200 mg/kg) on ECG and electrophysiology of the rat heart. They reported that MOE consumption is associated with prolonged QRS, QTc, JT, and TpTe interval in a dose-dependent way with no alterations of RR interval, PR interval, amplitudes of ECG waves (P duration, P amplitude, Q amplitude, R amplitude, S amplitude, T amplitude, and ST height), heart rate, and blood pressure. QRS prolongation implies the possible antiarrhythmic effect of MOE by slowing ventricular conductivity, similarly to class I antiarrhythmics. On the other hand, QTc and JT prolongation induced by MOE is also characteristic for class III antiarrhythmics via blockage K+ channels (Joukar and Asadipour, 2015). Nonetheless, both of these alterations could potentially turn into proarrhythmogenic if MOE is applied in higher doses, subsequently inducing early after-depolarizations that might increase the risk of ventricular extrasystoles, reentry phenomenon, and torsade de pointes arrhythmia (Gupta et al., 2007). Cardiac effects of MOE were also evaluated in a well-established model of isoproterenol-induced myocardial infarction, emphasizing the importance of MOE dosage. They emphasized that lower doses of MOE (50 and 100 mg/kg) provide better cardioprotection by reducing the arterial pressure and heart rate and also by improving redox state via a decline in malondialdehyde (MDA) levels. More importantly, this study added new facts regarding the safety of MOE, as high dose of MOE (200 mg/kg) led to intensification isoproterenol-induced cardiac injury under myocardial ischemia conditions, as a consequence from increasing of cardiac contractility, increasing of myocardial oxygen demand, and hence more risk of cardiac injury (Joukar et al., 2016).
Novel study pointed out potent cardioprotective effect of ethanolic MOE against cardiac I/R and subsequent arrhythmias in in vivo rat model of regional heart ischemia (LAD model) (Sedighi et al., 2019). Namely, 2-week oral application of this extract led to infarct size reduction, decrease of ventricular tachycardia, and ventricular ectopic beats episodes, stabilizing the ST segment changes and QTc shortening, and increased the R and T wave amplitudes and the heart rate during ischemia. Antiarrhythmic effects were dose-dependent, 100 mg/kg being the most effective dose of MOE. Cinnamic acid, as a major phenolic compound of this extract, was considered to be responsible for these effects via amelioration of oxidative stress. Rich phenolic content of lemon balm may induce cardioprotection in several mechanisms as reducing agents, free radical scavenging, and potential chelation of pro-oxidant metals (Miraj et al., 2017; Sedighi et al., 2019). Consistent results regarding antiarrhythmic effects of ethanolic MOE were also confirmed in CaCl2-induced arrhythmia model in Sprague–Dawley rats. Two-week consumption of MOE decreased the heart rate and the incidence of ventricular tachycardia, ventricular fibrillation, and ventricular premature beats, especially in a higher dose of 200 mg/kg (Akhondali et al., 2015). These ECG changes were linked with antioxidant effects of polyphenolics and vasorelaxant effect of monoterpene citral from MOE (Wolbling and Leonhardt, 1994; Gazola et al., 2004; Gupta et al., 2007; Astani et al., 2012; Devi et al., 2012; Joukar et al., 2014, 2016; Akhondali et al., 2015; Joukar and Asadipour, 2015; Miraj et al., 2017; Sedighi et al., 2019). Taking the fact that the methanolic extracts of MO were previously reported to induce vasorelaxation through blockage of the Ca2+ channels and that these channels play an important role in pacemaker currents in atrioventricular node, this may be the mechanism of MOE’s heart rate–slowing property (Devi et al., 2012). Capability of ethanolic MOE to alleviate the most frequent cytotoxic drug–induced cardiotoxicity and doxorubicin was thoroughly investigated by Hamza et al. (2016) Namely, they proved dose-dependent ability of MOE to preserve cardiac function and morphology in well-established doxorubicin-induced cardiotoxicity rat model via oxidative stress modulation and amelioration of both inflammation and apoptosis in rat heart. Surprisingly, optimal cardioprotection was induced by MOE in a dose of 750 mg/kg (Hamza et al., 2016), suggesting great therapeutic index of MOE regarding safety and efficacy, as most previous studies that investigated the cardiovascular effects of MOE used lower doses such as 50, 100, and 200 mg/kg (Joukar et al., 2014, 2016; Sedighi et al., 2019). It is widely known that anthracycline antibiotic doxorubicin, commonly used for various malignancies, causes deleterious effects on the myocardium, leading to cardiomyopathy and/or subsequent heart failure, thus limiting its successful usage in oncology patients. In recent years, various studies unraveled the mechanisms of this cardiotoxicity including reactive oxygen species (ROS)–induced oxidative damage, interfering with inflammation and apoptosis of cardiomyocytes (Zhao and Zhang, 2017; Renu et al., 2018; Songbo et al., 2019). Ethanolic MOE in the dose of 750 mg/kg abolished all of these harmful effects of doxorubicin via amelioration of oxidative stress through increase in antioxidant capacity [superoxide dismutase (SOD)] and lipid peroxidation decrease (MDA reduction). Additionally, MOE abrogated inflammation by downregulating the expression of nuclear factor κ light-chain enhancer of activated B cells (NF-κB), tumor necrosis factor α (TNF-α), and cyclooxygenase 2 (COX-2) genes and showed antiapoptotic activity through decrease in Bax and caspase-3 expression (Hamza et al., 2016). What is more, MOE prevented the leakage of cardiac enzymes [creatine kinase myocardial band (CK-MB), troponin I, and troponin T] and preserved the morphology of the myocardium histopathologically when administered as adjuvant therapy with doxorubicin. Again, all of these beneficial effects of MOE extract are most likely induced by synergistic interactions of phenolic compounds and other triterpene acids of MOE.
Clinical Studies
Although clinical data regarding cardiovascular benefits of MO and its different formulations are limited, there are some encouraging results from novel clinical trials (Table 2). Namely, double-blind, randomized, placebo-controlled trial of efficacy and safety was conducted in order to investigate and justify traditional use of MO for heart palpitations. It was found that 2 weeks of consumption lyophilized aqueous MOE (500 mg; two times per day) reduced heart palpitation episodes by 36% compared to 4.2% reduction in the placebo group. Additionally, no side effects were noted in the subjects who finished the study (n = 55) (Alijaniha et al., 2015). Other available clinical trial results were focused on the influence of MO on cardiometabolic parameters, such as lipid status, glycemia, and inflammatory status as factors promoting cardiovascular risk in diabetic patients (Asadi et al., 2018, 2019). Both trials came to similar results that MO application improves lipid ratios (Alijaniha et al., 2015), high-density lipoprotein (HDL), triglycerides (TGs), high-sensitivity C-reactive protein (hs-CRP) levels, systolic blood pressure (Asadi et al., 2019; Nayebi et al., 2019), and diastolic blood pressure (Ibragic et al., 2014), thus preventing cardiovascular risk in this patient population. It is noteworthy that these beneficial effects were achieved by a higher dose of MO (700 mg, 2 × a day, per os) and longer (3 months) usage (Asadi et al., 2018, 2019) than for heart palpitations (Zhao and Zhang, 2017). Nonetheless, no dose-dependent adverse reactions were reported (Asadi et al., 2018, 2019). Beneficial effects of hot water MOE were highlighted by Yui et al. (2017), in the aspect of the reductions in brachial-ankle pulse wave velocity, which reflects arterial stiffness in healthy adult subjects.
Vascular Effects of MO
Vasorelaxant effects of aqueous MOE on isolated thoracic aortic rings were first revealed and reported by Ersoy et al. (2008) They proved that aqueous MOE induces endothelium-dependent vasorelaxation, and that possible mechanism of this effect involves mainly nitric oxide (NO) pathway, but also EDHF (endothelium-derived hyperpolarizing factor) and prostacyclin pathways. It may be assumed that this vasorelaxation may be induced by monoterpene component citral and mediated by aortic Ca2+ channel blockage also, besides NO pathway involvement. The reason for this is that it has been shown that methanolic MOE exhibits vasorelaxation by the Ca2+ channel blockage (Devi et al., 2012). Additionally, as RA was shown to be the most abundant component of aqueous MOE, it has been tested on isolated rat thoracic aorta. In that way, dose-dependent vasorelaxant effect of RA was proven (Ersoy et al., 2008).
Antioxidant and cytoprotective effects of hydroalcoholic MOE were also confirmed at the cellular level in a model of H2O2-induced oxidative stress in HUVECs (human umbilical vein endothelial cells) vascular cells, almost a decade later. It was found that hydroalcoholic MOE protects the vascular cells against H2O2-induced toxicity only when applied at higher concentrations (100–500 μg/mL) as evidenced by FRAP assay, FOX1 method, and MTT assay. Importantly, MOE was investigated for an eventual toxicity on HUVECs, and it was proven that MOE did not change cell viability 24 h after the exposure, except in the case of the highest concentration of MOE (1,000 μg/mL) (Yui et al., 2017). These cytoprotective effects of MOE are linked with the main component of MOE-RA, which provides vascular cytoprotective effects via several mechanisms: antioxidant (superoxide scavenging, inhibition of low-density lipoprotein (LDL) oxidation in human aortic endothelial cells) and antiangiogenetic (reduction in the expression of H2O2-dependent endothelial growth factor and release of interleukin 8 (IL-8) from endothelial cells and inhibition of the proliferation, migration, adhesion, and tube formation of HUVECs) (Pearson et al., 1997; Huang and Zheng, 2006; Safaeian et al., 2016).
Effect of MO On Inflammatory Heart Diseases
Therapeutic effects of MO in inflammatory heart disease have not been investigated to date. Nonetheless, over a decade ago, pioneer evidence regarding the influence of water MOE on immune response in mice was reported (Drozd and Anuszewska, 2003). Additionally, several phenolic components present in MOE were shown to be beneficial in animal models of myocarditis, such as flavonoids quercetin, luteolin, and apigenin as they modulate immune response, decrease inflammation, and subsequently may suppress cardiac tissues remodeling, which occurs in dilative cardiomyopathy characteristic for chronic phase of myocarditis (Milenković et al., 2010; Zhang et al., 2016; Wu et al., 2020). Namely, chronic 3-week quercetin supplementation showed dose-dependent protective effect in experimental autoimmune myocarditis rat model through suppression of proinflammatory cytokines TNF-α and IL-17 and up-regulation of IL-10 (Milenković et al., 2010). Apigenin treatment ameliorated experimental autoimmune myocarditis in BALB/c mice by modulating TH1/TH2 balance through downregulation of TH1 cytokines (TNF-α, IL-2, and interferon γ) and up-regulation of TH2 cytokines (IL-4, IL-10) (Zhang et al., 2016). On the other hand, luteolin was proven to inhibit coxsackievirus B3 (CVB3) replication via depressing the phosphorylation of p38 MAPK and JNK MAPK and inhibiting NF-κB nuclear translocation and further attenuation of the inflammatory cytokine expression in CVB3-infected cells. Thus, luteolin may be beneficial in CVB3-induced myocarditis by inhibiting heart inflammation (Wu et al., 2020). Having this in mind, MO represents a great phenolic mixture with high potential as an adjuvant therapy for inflammatory heart disease, which should be clarified in future studies.
Cardiometabolic Effects of MO
Cardiometabolic diseases have become the leading cause of death in the modern era worldwide. The meaning of this term has been expanded, and nowadays, it refers to CVD, diabetes mellitus, and chronic renal failure (de Waard et al., 2019). Etiology is mostly explained by unhealthy sedentary lifestyle, lack of physical activity, inadequate diet, and smoking. A wide spectrum of risk factors that may lead to progression of cardiometabolic disease, including insulin resistance; increased TC, TGs, and LDL; and decreased HDL. In that sense, the plant extract supplementation may be of great importance as a prevention strategy for cardiometabolic diseases, as polyphenol-rich plants are recognized as safe and supplements with great benefit on the health, especially on lipid status and glycemia (Cicero et al., 2017). Bolkent et al. (2005) were among the first to provide evidence about hypolipidemic properties of MOE. Namely, aqueous MOE when applied in a dose of 2 g/kg induced significant decrease in serum cholesterol and total lipids in high-fat diet model of hyperlipidemia in rats (Bolkent et al., 2005; Heshmati et al., 2020). Essential oil of MO (12.5 μg/d per os) was also shown to possess hypolipidemic properties via reduction of plasma TG in apolipoprotein E (ApoE) transgenic mice (Jun et al., 2012). Possible explanation for these hypolipidemic effects may lay in synergistic action of MOE active compounds, especially rosmarinic, ursolic, and oleanolic acids on serum lipids (Yuliang et al., 2015; Chen et al., 2017; Guo et al., 2020). Exact mechanisms are still not completely clear, but it is assumed that decreased expression of sterol regulatory element-binding protein-1c and its associated genes lead to decreased hepatic synthesis of fatty acids (Jun et al., 2012).
The usage of MO in cardiometabolic disease has been thoroughly explored in meta-analyses by Heshmati et al. (2020), and it seems that MO application most likely lowers TC and SBP, according to several studies results. Because of its strong antioxidant activity, MOE may be useful in treating or preventing dyslipidemia and possibly subsequent atherosclerotic process. This hypothesis was confirmed in several experimental studies and clinical trials (Bolkent et al., 2005; Jun et al., 2012; Weidner et al., 2014; Jandaghi et al., 2016; Asadi et al., 2018; Javid et al., 2018; Heshmati et al., 2020). In order to achieve these effects in human, attention should be given to optimization of MOE dosage. LDL-lowering effects of MO were reported when applied in a dose of 1,000 mg three times daily per os for 2 months in hyperlipidemic patients (Jun et al., 2012). Similarly, improvement of lipid status via Apo A-I, Apo B/Apo A-I, and lipid ratios was proven in type 2 diabetic patients treated with hydroalcoholic MOE 700 mg two times daily for 12 weeks (Asadi et al., 2018), whereas in patients with stable angina pectoris, higher doses (3 g/d) are needed to provide improvement in lipid profile (TC, LDL, HDL, TG), MDA, hs-CRP, and paraoxonase 1. Taking these aforementioned facts, the usage of MO may be a safe and useful way to improve metabolic markers and reduce the risk of cardiovascular consequences.
Safety of MO and Possible Drug Interactions
The traditional usage of MO is generally considered to be well tolerated and safe, as it is assigned to the Food and Drug Administration GRAS (Generally Recognized as Safe) list in the United States. Up until now, no serious adverse effects have been reported following lemon balm usage. Because of insufficient information about lemon balm usage in sensitive populations such as pediatrics or during pregnancy or lactation, theoretically, in these cases it may be marked as possibly unsafe. Per os usage of MO has been reported to be well tolerated, with no adverse events when taken at less than 8 weeks. To date, sporadic minor side effects have been reported such as electroencephalographic changes in doses of 1,200 mg, reduced alertness with caution to driving in dose of 900 mg, possible increase of intraocular pressure, thyroid hormone inhibition, palpitations, headache, diarrhea, and vomiting (Ulbricht et al., 2005). Increased appetite was also reported as a side effect in a clinical trial investigating MO effects on heart palpitations (Alijaniha et al., 2015). On the other hand, topical administration of MO preparations might cause local redness, burning sensation, or irritation. It is considered that MO has no toxic effects on the liver, as proven in cell cultures (Carocho et al., 2015). Toxic effects of MO essential oil on the stomach, duodenum, liver, and kidneys have been described when applied in a dose of 1 mg/kg per os. The calculated LD50 (median lethal dose) for BALB/c mice is 2.57 g/kg, and this is thought to be moderately toxic for human usage (Stojanović et al., 2019). These findings are contrary to the previous study by Bounihi et al. (2014), which reported absence of toxicity after 2 g/kg MO essential oil of MO.
It is familiar that patients tend to use herbal products in the purpose of healing and curing considering it completely safe regardless of pharmacotherapy they are assigned to. As MO is most commonly used as a mild sedative because of its calming effects, to date, the only described interactions of MO refer to synergism with sedatives and barbiturates and inhibition of SSRIs (selective serotonin reuptake inhibitors) (Posadzki et al., 2013). MO exerts its therapeutic properties via inhibition of acetylcholinesterase activity and GABA transaminase, so it potentiates the effects of drugs acting in the same way. Thus, the usage of MO OTC preparations should be careful and monitored or advised by a pharmacist in patients using these drugs. This is because there is a possibility that MO potentiates the depressing effect on the central nervous system. Theoretically, MO may also interact with thyroid hormone therapy in the conditions of hypothyroidism, because of its ability to inhibit the thyroid hormone receptors (Ulbricht et al., 2005). Identification of MO interactions with cardiovascular drugs is of great significance as few years ago, a descriptive study employing cardiopathy patients on anticoagulation therapy, well known to be prone to dangerous drug and/or herb interactions, reported that high percentage of these patients self-used herbal medications, with MO being on the third place by frequency (Leite et al., 2016). There is limited amount of data regarding the possible interactions of MO or its components with cardiovascular drugs. Interestingly, it was recently reported that the most abundant component of MO, RA, may weakly inhibit the two isoforms of cytochrome P450 oxidase, CYP2C19, and CYP2E1 and moderately competitively inhibit UGT1A1, UGT1A6, and UGT2B7 (Kim et al., 2019). This fact may be important in predicting possible pharmacokinetic interactions with cardiovascular drugs metabolized by the mentioned isoforms such as carvedilol, simvastatin (UGT1A1 substrates) (Marques and Ikediobi, 2010), dabigatran etexilate, simvastatin, and fluvastatin (UGT2B7 substrates) (Drug Bank, 2021). Of course, these claims remain theoretical, as no preclinical or clinical studies evaluated these interactions. Another component of MO, flavonoid quercetin, was proven to interact with digoxin in terms of raising its bioavailability, which may result in the potentially harmful effect of this drug, well known to possess narrow therapeutic index (Wang et al., 2004).
Data regarding the aspects of MO and its active compounds in different formulations such as adverse effects of the extracts and potential interactions or synergistic effects, especially in polyherbal preparations, with standard cardiovascular medications, are still limited, thus calling for future studies especially in humans.
Therapeutic Potential of RA
RA [Rosmarinus officinalis (RO)], as an isolated compound, was mentioned for the first time by Scarpati and Oriente in 1958 and carries the name in accordance with the plant from which it was first isolated, RO. It is a water-soluble ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid and is prevalent in a wide range of plants. Additionally, it is a predominant phenol of several medicinal plants that belong to the Lamiaceae family, such as rosemary, sage, basil, mint, and lemon scent plants-emon balm (MO) (Prasannarong et al., 2019; Ghasemzadeh Rahbardar and Hosseinzadeh, 2020). It has been reported that the presence of RO in medicinal plants has generated healthy and beneficial effects such as antioxidant, astringent, antimicrobial, anti-inflammatory, antiviral, antiangiogenic, antirheumatic, antiallergic, antidiabetic, antidepressant, and antitumor (Ferreira et al., 2013). However, reports on its activity in the cardiovascular system are scarce but showed that RO possesses cardioprotective effects.
RO was shown to reduce blood pressure in ways that could be related to inhibition and/or modulation of angiotensin-converting enzyme or promotion of NO production and downregulation of endothelin-1 production (Li et al., 2008; Karthik et al., 2011). The mechanisms involved in the endothelium-dependent vasodilating effect of RO remain unknown. Nevertheless, this effect can be explained by the fact that RO is a polyphenolic compound that has a vasodilating activity, through activation of NO pathway, EDHF, and prostacyclin (Fernandes et al., 2005; Ersoy et al., 2008). There is evidence claiming that RO can improve both cardiovascular and metabolic problems in hypertensive conditions, which is confirmed by the results of a study that evaluated the acute and chronic effects of RO in hypertensive rats. The acute and chronic treatment with RO reduced blood pressure and fasting plasma glucose levels, while the acute RA treatment increased skeletal muscle glucose transport activity along with extracellular signal–regulated kinase activity (Prasannarong et al., 2019). Cardioprotective effects of the treatment with RO were also shown in a model of doxorubicin-induced cardiotoxicity and pressure overload–induced cardiac dysfunction in mice. Possible explanation of these effects may be reflected in the fact that RA alleviates cardiomyocyte apoptosis via cardiac fibroblast (Zhang et al., 2018, 2019). Preconditioning with RO could strongly prevent cardiac hypertrophy, lipid peroxidation, weakness in myocardial contractility and relaxation, and stretch-induced arrhythmias after acute myocardial infarction induced by supramaximal administration of isoproterenol in rats. The cardioprotective effect of RO, which was reflected through the reduction of cardiac hypertrophy, may be related to the inhibition of the isoproterenol-induced cardiac hyperactivity, resulting in inhibition of oxidative stress and lipid peroxidation (Javidanpour et al., 2018). In addition, 2 week application of RO exerted the beneficial effects on heart electrophysiology of rats with myocardial infarction. Namely, RO induced QRS complex voltage increment and dramatic lowering of ST elevation, especially via the highest dose applied (30 mg/kg) compared to non-treated animals with myocardial infarction. These results suggest the protective effect of RO on plasma membrane. RO pretreatment diminishes almost all harmful heart alterations in isoproterenol-induced myocardial infarction. This refers to antioxidants boosting antioxidant activity and up-regulation of gene expression of RyR2 and SERCA2, which are involved in Ca2+ homeostasis and possibly through RA antiadrenergic effect (Javidanpour et al., 2017). Considering these facts, application of RO isolated from MO may also be a useful tool for cardioprotection. Nonetheless, the synergistic action of all compounds present in MOE may provide even better effects.
Cardioprotective Mechanisms of Other MO Active Compounds
There are a vast number of studies claiming that triterpene compounds of MO ursolic and oleanolic acid are per se cardioprotective. For example, ursolic acid exerts cardioprotection in different ways. First, it was shown that ursolic acid possesses negative inotropic and dromotropic effect, thus inducing antiarrhythmic effect in rats (Seo et al., 2018). Additionally, it was shown that pretreatment with ursolic acid alleviates myocardial I/R, isoproterenol induces myocardial infarction (Radhiga et al., 2012), and doxorubicin induced cardiotoxicity (Mu et al., 2019) via several mechanisms: antiapoptotic effect on cardiomyocytes, anti-inflammatory effect, antioxidant effect through suppression of ROS formation, and enhancement of antioxidant protection [SOD, catalase (CAT), GSH] (Wang et al., 2018). There is also evidence that oleanolic acid could improve the release of NO from the endothelium and induce vasorelaxation, which is mediated by phosphoinositide-3-kinase–dependent phosphorylation of Akt-Ser473 followed by phosphorylation of eNOS-Ser1177 (Rodriguez-Rodriguez et al., 2008).
Besides thoroughly described phenolic acid most abundant in MO-RA, additional phenolic acids such as gallic acid, caffeic acid, ferulic acid, etc., may also contribute to cardioprotection induced by MO through several mechanisms. Gallic acid applied in a dose of 20 mg/kg was proven to reduce cardiac remodeling and hypertrophy via activation of autophagy, which subsequently causes the degradation of epidermal growth factor receptor, gp130, and CaNA and finally leads to the inactivation of their downstream pathways (ERK1/2, AKT, JAK2, STAT3, and NFATc1) (Yan et al., 2019). Gallic acid (13.1 mg/kg) was also reported to improve cardiac dysfunction and fibrosis in a model of pressure overload–induced heart failure via decreased expression of collagen type I and connective tissue growth factor (Jin et al., 2018). Ferulic acid application (20 and 40 mg/kg) was shown to protect the heart against doxorubicin-induced cardiotoxicity as proven by the decrease of natriuretic peptides atrial natriuretic peptide and brain natriuretic peptide, cardiac enzymes CK-MB and lactate dehydrogenase, decrease of proinflammatory cytokines IL-1β and IL-6, and increase of antioxidant GSH in the myocardium. It is suggested that ferulic acid acts as an antihypertensive because of the existing evidence about its ability to decrease systolic blood pressure, left ventricular diastolic stiffness, antioxidant protection enzymes SOD and CAT boosting, amelioration of kidney damage caused by hypertension, and vasorelaxant effect on isolated thoracic aorta (Alam et al., 2013).
Flavonoid components such as quercetin, rutin, and hesperetin are widely investigated as cardioprotective agents, with its potency and efficacy varying on applied doses or plant they are isolated from. As they are present in different extracts of MO, they could also be responsible for cardiovascular effects of this plant, together with the others. To date, numerous beneficial effects of quercetin on cardiovascular system have been described including antihypertensive, antiatherosclerotic, anti-ischemic, anti-inflammatory, and antioxidant effects. Dozens of mechanisms are proven to be involved in these effects. First, increased bioavailability of NO and decreased NADPH oxidase and eNOS activity lead to vasodilation and angiotensin-converting enzyme inhibition, all of them enabling better blood pressure regulation. Second, decreased oxidative stress, through augmentation of antioxidant protection enzymes CAT, SOD, and GSH; decreased lipid peroxidation; and decreased oxidation of LDL providing antiatherosclerotic action. Third, antiapoptotic effect and decrease of proinflammatory markers make quercetin a great candidate for cardiovascular drug (Patel et al., 2018).
Nonetheless, there is no evidence about the cardiovascular effects of these pure compounds isolated concretely from MO. As the beneficial effects of the mentioned compounds highly depend on dose, frequency, administration route, and treatment duration, it remains a question whether application of themselves would exert the same effect when isolated from MO. It is the most probable that the MO-induced cardioprotection is mediated through various mechanisms of these compounds and its synergistic action.
Conclusion
Although there is promising preclinical evidence regarding cardiovascular benefits of MOEs and essential oil application, there is an urgent need for clarification of the exact mechanisms of cardioprotection, safety, and pharmacokinetics of MO on cellular level, so the translation of these results into clinical practice could be possible. Additionally, optimization of dosage in human remains a challenge as reported cardiac benefits in animals are achieved with different doses (50–500 mg/kg), whereas improvement of cardiometabolic markers is achieved with much higher doses, which should be questioned in clinical practice. Given the fact that insufficiently is known regarding the possible interaction of MO formulations with frequently used cardiovascular drugs or other medicinal plants, future studies should be focused on these problems. Additionally, the usage of MO was investigated in various models of CVD; nonetheless, in inflammatory heart disease, it has not been investigated so far. Future studies should also focus on revealing the effects of MO in this pathology also, as there is evidence about its anti-inflammatory effects. This review provided detailed information regarding ethnomedical, preclinical, and possible clinical usage of MO in various cardiovascular entities.
Author Contributions
IM and ND substantially contributed to the conception and the design of the manuscript. ND, MA, MR, and JJ wrote sections of the manuscript. IS, TNT, JJ, AS, and MT selected extracted relevant manuscript of this manuscript. VJ, SB, and IM reviewed and approved the final version. All authors had full access to data and critically revised and approved the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- CAT
catalase
- CK-MB
creatine kinase myocardial band
- COX-2
cyclooxygenase 2
- CVB3
coxsackievirus B3
- CVDs
cardiovascular diseases
- CYP
cytochrome P450 oxidase
- ECG
electrocardiogram
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- FOX1
ferrous oxidation–xylenol orange
- FRAP
ferric reducing ability of plasma
- GABA
transaminase–γ-aminobutyric acid transaminase
- GRAS
generally recognized as safe
- GSH
glutathione
- H2O2
hydrogen peroxide
- HUVECs
human umbilical vein endothelial cells
- IL-10
interleukin 10
- IL-17
interleukin 17
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-8
interleukin 8
- JNK MAPK
c-JunN-terminal kinases mitogen-activated protein kinase
- LAD
left descending coronary artery
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- MO
Melissa officinalis
- MOE
Melissa officinalis extract
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor κ light-chain enhancer of activated B cells
- NO
nitric oxide
- OTC
over the counter
- p38 MAPK
p38 mitogen-activated protein kinase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SSRIs
Selective serotonin re − uptake inhibitors
- TNF-α
tumor necrosis factor α
- UGT
uridine 5’-diphospho-glucuronosyltransferase.
Footnotes
Funding. The authors would like to express gratitude to the Faculty of Medical Sciences, University of Kragujevac for Grant No. JP 26/20.
References
- Abdellatif F., Boudjella H., Zitouni A., Hassani A. (2014). Chemical composition and antimicrobial activity of the essential oil from leaves of Algerian Melissa officinalis L. EXCLI J. 13 772–781. [PMC free article] [PubMed] [Google Scholar]
- Abdel-Naime W. A., Fahim J. R., Fouad M. A., Kamel M. S. (2016). Botanical studies of the leaf of Melissa officinalis L., Family: labiatae, cultivated in Egypt. J. Pharm. Phytochem. 5 98–104. [Google Scholar]
- Abdel-Naime W. A., Fahim J. R., Fouad M. A., Kamel M. S. (2020). Botanical studies on the stem and root of Melissa officinalis L. (lemon balm). J. Adv. Biomed. Pharm. Sci. 3 184–189. 10.21608/jabps.2020.29774.1085 [DOI] [Google Scholar]
- Aharizad S., Rahimi M. H., Moghadam M., Mohebalipour N. (2012). Study of genetic diversity in lemon balm (Melissa officinalis l.) populations based on morphological traits and essential oils content. Ann. Biol. Res. 3 5748–5753. [Google Scholar]
- Akhondali Z., Dianat M., Radan M. (2015). Negative chronotropic and antidysrhythmic effects of hydroalcoholic extract of lemon balm (Melissa officinalis L.) on CaCl2-induced arrhythmias in rats. Electron. Physician. 7 971–976. 10.14661/2015.971-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. A., Sernia C., Brown L. (2013). Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 61 240–249. 10.1097/FJC.0b013e31827cb600 [DOI] [PubMed] [Google Scholar]
- Alijaniha F., Naseri M., Afsharypuor S., Fallahi F., Noorbala A., Mosaddegh M., et al. (2015). Heart palpitation relief with Melissa officinalis leaf extract: double blind, randomized, placebo controlled trial of efficacy and safety. J. Ethnopharmacol. 164 378–384. 10.1016/j.jep.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Arceusz A., Wesolowski M., Ulewicz-Magulska B. (2015). Flavonoids and phenolic acids in methanolic extracts, infusions and tinctures from commercial samples of lemon balm. Nat. Prod. Commun. 10 977–981. 10.1177/1934578X1501000645 [DOI] [PubMed] [Google Scholar]
- Asadi A., Shidfar F., Safari M., Hosseini A. F., Fallah Huseini H., Heidari I., et al. (2019). Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A randomized, double-blind, clinical trial. Phytother. Res. 33 651–659. 10.1002/ptr.6254 [DOI] [PubMed] [Google Scholar]
- Asadi A., Shidfar F., Safari M., Malek M., Hosseini A. F., Rezazadeh S., et al. (2018). Safety and efficacy of Melissa officinalis (lemon balm) on ApoA-I, Apo B, lipid ratio and ICAM-1 in type 2 diabetes patients: A randomized, double-blin ded clinical trial. Complement. Ther. Med. 40 83–88. 10.1016/j.ctim.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Astani A., Reichling J., Schnitzler P. (2012). Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy 58 70–77. 10.1159/000335590 [DOI] [PubMed] [Google Scholar]
- Awad R., Levac D., Cybulska P., Merali Z., Trudeau V. L., Arnason J. T. (2007). Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 85 933–942. 10.1139/Y07-083 [DOI] [PubMed] [Google Scholar]
- Barros L., Dueñas M., Dias M. I., Sousa M. J., Santos-Buelga C., Ferreira I. C. (2013). Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 136 1–8. 10.1016/j.foodchem.2012.07.107 [DOI] [PubMed] [Google Scholar]
- Bolkent S., Yanardag R., Karabulut-Bulan O., Yesilyaprak B. (2005). Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: a morphological and biochemical study. Ethnopharmacol 14 391–398. 10.1016/j.jep.2005.02.038 [DOI] [PubMed] [Google Scholar]
- Bounihi A., Alnamer R., Hajjaj G., Cherrah Y., Zellou A. (2014). Investigation of essential oil of Melissa officinalis for acute and sub-chronic oral toxicity. Int. J. Pharm. 4 40–46. [Google Scholar]
- Bounihi A., Hajjaj G., Alnamer R., Cherrah Y., Zellou A. (2013). In vivo potential anti-inflammatory activity of Melissa officinalis L. essential oil. Adv. Pharmacol. Sci. 2013:101759. 10.1155/2013/101759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadanović-Brunet J., Cetković G., Djilas S., Tumbas V., Bogdanović G., Mandić A., et al. (2008). Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L. extracts. J. Med. Food 11 133–143. 10.1089/jmf.2007.580 [DOI] [PubMed] [Google Scholar]
- Carocho M., Barros L., Calhelha R. C., Ćirić A., Soković M., Santos-Buelga C., et al. (2015). Melissa officinalis L. decoctions as functional beverages: a bioactive approach and chemical characterization. Food Funct. 6 2240–2248. 10.1039/c5fo00309a [DOI] [PubMed] [Google Scholar]
- Chen S., Wen X., Zhang W., Wang C., Liu J., Liu C. (2017). Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 31 1085–1096. 10.1096/fj.201601022R [DOI] [PubMed] [Google Scholar]
- Chen X. K., Yang Q., Smith G., Krewski D., Walker M., Wen S. W. (2006). Environmental lead level and pregnancy-induced hypertension. Environ. Res. 100 424–430. 10.1016/j.envres.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Chizzola R., Lohwasser U., Franz C. (2018). Biodiversity within Melissa officinalis: variability of bioactive compounds in a cultivated collection. Molecules 23:294. 10.3390/molecules23020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. J., Cho S. Y., Bhuiyan M. J., Kim K. H., Lee S. J. (2010). Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 104 180–188. 10.1017/S0007114510001765 [DOI] [PubMed] [Google Scholar]
- Cicero A. F. G., Fogacci F., Colletti A. (2017). Food and plant bioactives for reducing cardiometabolic disease risk: an evidence based approach. Food Funct. 8 2076–2088. 10.1039/c7fo00178a [DOI] [PubMed] [Google Scholar]
- Cosge B., Ipek A., Gurbuz B. G. C. (2009). /MS analysis of herbage essential oil from lemon balms (Melissa officinalis L) grown in Turkey. J. Appl. Bio. Sci. 3 149–152. [Google Scholar]
- Dastmalchi K., Ollilainen V., Lackman P., Boije af Gennäs G., Dorman H. J., Järvinen P. P., et al. (2009). Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem. 17 867–871. 10.1016/j.bmc.2008.11.034 [DOI] [PubMed] [Google Scholar]
- de Waard A. M., Hollander M., Korevaar J. C., Nielen M. M. J., Carlsson A. C., Lionis C., et al. (2019). SPIMEU Project Group. Selective prevention of cardiometabolic diseases: activities and attitudes of general practitioners across Europe. Eur. J. Public Health 29 88–93. 10.1093/eurpub/cky112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi R. C., Sim S. M., Ismail R. (2012). Effect of cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid. Based Complement. Altern. Med. 2012:539475. 10.1155/2012/539475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd J., Anuszewska E. (2003). The effect of the Melissa officinalis extract on immune response in mice. Acta Pol. Pharm. 60 467–470. [PubMed] [Google Scholar]
- Drug Bank. (2021). UGT2B7 Substrates. Available online at: https://go.drugbank.com/categories/DBCAT004610 (accessed March 9, 2021). [Google Scholar]
- Ehsani A., Alizadeh O., Hashemi M., Afshari A., Aminzare M. (2017). Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum. 8 223–229. [PMC free article] [PubMed] [Google Scholar]
- Encalada M. A., Hoyos K. M., Rehecho S., Berasategi I., de Ciriano M. G., Ansorena D., et al. (2011). Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum. Nutr. 66 328–334. 10.1007/s11130-011-0256-y [DOI] [PubMed] [Google Scholar]
- Ersoy S., Orhan I., Turan N. N., Sahan G., Ark M., Tosun F. (2008). Endothelium-dependent induction of vasorelaxation by Melissa officinalis L. ssp. officinalis in rat isolated thoracic aorta. Phytomedicine 15 1087–1092. 10.1016/j.phymed.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Fernandes L., Fortes Z. B., Casarini D. E., Nigro D., Tostes R. C., Santos R. A., et al. (2005). Role of PGI2 and effects of ACE inhibition on the bradykinin potentiation by angiotensin-(1-7) in resistance vessels of SHR. Regul. Pept. 127 183–189. 10.1016/j.regpep.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Ferreira L. G., Celotto A. C., Capellini V. K., Albuquerque A. A., Nadai T. R., Carvalho M. T., et al. (2013). Is rosmarinic acid underestimated as an experimental cardiovascular drug? Acta Cir. Bras. 28 83–87. 10.1590/s0102-86502013001300016 [DOI] [PubMed] [Google Scholar]
- Gazola R., Machado D., Ruggiero C., Singi G., Macedo Alexandre M. (2004). Lippia alba, Melissa officinalis and Cymbopogon citratus: effects of the aqueous extracts on the isolated hearts of rats. Pharmacol. Res. 50 477–480. 10.1016/j.phrs.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh Rahbardar M., Hosseinzadeh H. (2020). Effects of rosmarinic acid on nervous system disorders: an updated review. Naunyn Schmiedebergs Arch. Pharmacol. 393 1779–1795. 10.1007/s00210-020-01935-w [DOI] [PubMed] [Google Scholar]
- Guo C., Shangguan Y., Zhang M., Ruan Y., Xue G., Ma J., et al. (2020). Rosmarinic acid alleviates ethanol-induced lipid accumulation by repressing fatty acid biosynthesis. Food Funct. 11 2094–2106. 10.1039/c9fo02357g [DOI] [PubMed] [Google Scholar]
- Gupta A., Lawrence A. T., Krishnan K., Kavinsky C. J., Trohman R. G. (2007). Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am. Heart J. 153 891–899. 10.1016/j.ahj.2007.01.040 [DOI] [PubMed] [Google Scholar]
- Hamza A. A., Ahmed M. M., Elwey H. M., Amin A. (2016). Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anticancer activity on MCF-7 cells. PLoS One 11:e0167049. 10.1371/journal.pone.0167049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati J., Morvaridzadeh M., Sepidarkish M., Fazelian S., Rahimlou M., Omidi A., et al. (2020). Effects of Melissa officinalis (Lemon Balm) on cardio-metabolic outcomes: a systematic review and meta-analysis. Phytother. Res. 34 3113–3123. 10.1002/ptr.6744 [DOI] [PubMed] [Google Scholar]
- Huang S. S., Zheng R. L. (2006). Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 239 271–280. 10.1016/j.canlet.2005.08.025 [DOI] [PubMed] [Google Scholar]
- Ibarra A., Feuillere N., Roller M., Lesburgere E., Beracochea D. (2010). Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine 17 397–403. 10.1016/j.phymed.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Ibragic S., Salihovic M., Tahirovic I., Toromanovic J. (2014). Quantification of some phenolic acids in the leaves of Melissa officinalis L. from Turkey and Bosnia. Bull. Chem. Tech. Bosnia Herzegovina 42 47–50. [Google Scholar]
- Jandaghi P., Noroozi M., Ardalani H., Alipour M. (2016). Lemon balm: a promising herbal therapy for patients with borderline hyperlipidemia-A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 26 136–140. 10.1016/j.ctim.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Javid A. Z., Haybar H., Dehghan P., Haghighizadeh M. H., Mohaghegh S. M., Ravanbakhsh M., et al. (2018). The effects of Melissa officinalis (lemon balm) in chronic stable angina on serum biomarkers of oxidative stress, inflammation and lipid profile. Asia Pac. J. Clin. Nutr. 27 785–791. 10.6133/apjcn.022018.01 [DOI] [PubMed] [Google Scholar]
- Javidanpour S., Dianat M., Badavi M., Mard S. A. (2017). The cardioprotective effect of rosmarinic acid on acute myocardial infarction and genes involved in Ca2+ homeostasis. Free Radic. Res. 51 911–923. 10.1080/10715762.2017.1390227 [DOI] [PubMed] [Google Scholar]
- Javidanpour S., Dianat M., Badavi M., Mard S. A. (2018). The inhibitory effect of rosmarinic acid on overexpression of NCX1 and stretch- induced arrhythmias after acute myocardial infarction in rats. Biomed. Pharmacother. 102 884–893. 10.1016/j.biopha.2018.03.103 [DOI] [PubMed] [Google Scholar]
- Jin L., Sun S., Ryu Y., Piao Z. H., Liu B., Choi S. Y., et al. (2018). Gallic acid improves cardiac dysfunction and fibrosis in pressure overload-induced heart failure. Sci. Rep. 8:9302. 10.1038/s41598-018-27599-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukar S., Asadipour H. (2015). Evaluation of Melissa officinalis (Lemon Balm) effects on heart electrical system. Res. Cardiovasc. Med. 4:e27013. 10.5812/cardiovascmed.4(2)2015.27013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukar S., Asadipour H., Sheibani M., Najafipour H., Dabiri S. (2016). The effects of Melissa officinalis (lemon balm) pretreatment on the resistance of the heart to myocardial injury. Pharm. Biol. 54 1005–1013. 10.3109/13880209.2015.1091845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukar S., Zarisfi Z., Sepehri G., Bashiri A. (2014). Efficacy of Melissa officinalis in suppressing ventricular arrhythmias following ischemia-reperfusion of the heart: a comparison with amiodarone. Med. Princ. Pract. 23 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H. J., Lee J. H., Jia Y., Hoang M. H., Byun H., Kim K. H., et al. (2012). Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c-dependent fatty acid synthesis. J. Nutr. 142 432–440. 10.3945/jn.111.152538 [DOI] [PubMed] [Google Scholar]
- Karthik D., Viswanathan P., Anuradha C. V. J. (2011). Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. Cardiovasc. Pharmacol. 58 514–521. 10.1097/FJC.0b013e31822c265d [DOI] [PubMed] [Google Scholar]
- Kim S. B., Kim K. S., Kim D. D., Yoon I. S. (2019). Metabolic interactions of rosmarinic acid with human cytochrome P450 monooxygenases and uridine diphosphate glucuronosyltransferases. Biomed. Pharmacother. 110 111–117. 10.1016/j.biopha.2018.11.040 [DOI] [PubMed] [Google Scholar]
- Leite P. M., Castilho R. O., Ribeiro A. L., Martins M. A. (2016). Consumption of medicinal plants by patients with heart diseases at a pharmacist-managed anticoagulation clinic in Brazil. Int. J. Clin. Pharm. 38 223–227. 10.1007/s11096-016-0270-0 [DOI] [PubMed] [Google Scholar]
- Li L., Zhou X., Li N., Sun M., Lv J., Xu Z. (2015). Herbal drugs against cardiovascular disease: traditional medicine and modern development. Drug Discov. Today. 20 1074–1086. 10.1016/j.drudis.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Li Q. L., Li B. G., Zhang Y., Gao X. P., Li C. Q., Zhang G. L. (2008). Three angiotensin-converting enzyme inhibitors from Rabdosia coetsa. Phytomedicine 15 386–388. 10.1016/j.phymed.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Marques S. C., Ikediobi O. N. (2010). The clinical application of UGT1A1 pharmacogenetic testing: gene-environment interactions. Hum. Genomics 4 238–249. 10.1186/1479-7364-4-4-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour N. H., Lin I. G., Frishman W. H. (1998). Herbal medicine for the treatment of 703 cardiovascular disease clinical considerations. Arch. Intern. Med. 158 2225–2234. [DOI] [PubMed] [Google Scholar]
- Mencherini T., Picerno P., Scesa C., Aquino R. (2007). Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. J. Nat. Prod. 70 1889–1894. 10.1021/np070351s [DOI] [PubMed] [Google Scholar]
- Milenković M., Arsenović-Ranin N., Stojić-Vukanić Z., Bufan B., Vuěićević D., Janěić I. (2010). Quercetin ameliorates experimental autoimmune myocarditis in rats. J. Pharm. Pharm. Sci. 13 311–319. 10.18433/j3vs3s [DOI] [PubMed] [Google Scholar]
- Mimica-Dukic N., Bozin B., Sokovic M., Simin N. (2004). Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 52 2485–2489. 10.1021/jf030698a [DOI] [PubMed] [Google Scholar]
- Miraj S., Rafieian-Kopaei, Kiani S. (2017). Melissa officinalis L: a review study with an antioxidant prospective. J. Evid. Based Complement. Altern. Med. 22 385–394. 10.1177/2156587216663433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moacǎ E. A., Farcaş C., Ghiţu A., Coricovac D., Popovici R., Cǎrǎba-Meiţǎ N. L., et al. (2018). A comparative study of melissa officinalis leaves and stems ethanolic extracts in terms of antioxidant, cytotoxic, and antiproliferative potential. Evid. Based Complement Alternat Med. 2018 7860456. 10.1155/2018/7860456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu H., Liu H., Zhang J., Huang J., Zhu C., Lu Y., et al. (2019). Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through eNOS activation and inhibition of eNOS uncoupling. J. Cell Mol. Med. 23 2174–2183. 10.1111/jcmm.14130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayebi N., Esteghamati A., Meysamie A., Khalili N., Kamalinejad M., Emtiazy M., et al. (2019). The effects of a Melissa officinalis L. based product on metabolic parameters in patients with type 2 diabetes mellitus: a randomized double-blinded controlled clinical trial. J. Complement. Integr. Med. 16:20180088. 10.1515/jcim-2018-0088 [DOI] [PubMed] [Google Scholar]
- Nour Eddine D., Miloud S., Abdelkader A. (2005). Effect of lead exposure on dopaminergic transmission in the rat brain. Toxicology 207 363–368. 10.1016/j.tox.2004.10.016 [DOI] [PubMed] [Google Scholar]
- Nurzyńska-Wierdak R., Bogucka-Kocka A., Szymczak G. (2014). Volatile constituents of Melissa officinalis leaves determined by plant age. Nat. Prod. Commun. 9 703–706. [PubMed] [Google Scholar]
- Park J. I., Bae H. R., Kim C. G., Stonik V. A., Kwak J. Y. (2014). Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem 2:77. 10.3389/fchem.2014.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. V., Mistry B. M., Shinde S. K., Syed R., Singh V., Shin H. S. (2018). Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 155 889–904. 10.1016/j.ejmech.2018.06.053 [DOI] [PubMed] [Google Scholar]
- Patora J., Klimek B. (2002). Flavonoids from lemon balm (Melissa officinalis L. Lamiaceae). Acta Pol. Pharm. 59 139–143. [PubMed] [Google Scholar]
- Pearson D. A., Frankel E. N., Aeschbach R., German J. B. (1997). Inhibition of endothelial cell-mediated oxidation of low-density lipoprotein by rosemary and plant phenolics. J. Agric. Food Chem. 45 578–582. [Google Scholar]
- Pereira R. P., Boligon A. A., Appel A. S., Faschinetto R., Speroni Ceroc C., Tanus-Santos J., et al. (2014). Chemical composition, antioxidant andanticholinesterase activity of Melissa officinalis. Ind. Crops Prod. 53 34–45. 10.1016/j.indcrop.2013.12.007 [DOI] [Google Scholar]
- Pereira R. P., Fachinetto R., de Souza Prestes A., Puntel R. L., Santos da Silva G. N., Heinzmann B. M., et al. (2009). Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res. 34 973–983. 10.1007/s11064-008-9861-z [DOI] [PubMed] [Google Scholar]
- Posadzki P., Watson L., Ernst E. (2013). Herb-drug interactions: an overview of systematic reviews. Br. J. Clin. Pharmacol. 75 603–618. 10.1111/j.1365-2125.2012.04350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasannarong M., Saengsirisuwan V., Surapongchai J., Buniam J., Chukijrungroat N., Rattanavichit Y. (2019). Rosmarinic acid improves hypertension and skeletal muscle glucose transport in angiotensin II-treated rats. BMC Complement. Altern. Med. 19:165. 10.1186/s12906-019-2579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhiga T., Rajamanickam C., Sundaresan A., Ezhumalai M., Pugalendi K. V. (2012). Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie 94 1135–1142. 10.1016/j.biochi.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Rasmussen P. (2011). Lemon balm-Melissa officinalis; also known as lemon balm, bee balm, garden balm, Melissa, melissengeist. J. Prim. Health Care 3 165–166. [PubMed] [Google Scholar]
- Renu K., V G A P B Tp, Arunachalam S. (2018). Molecular mechanism of doxorubicin-induced cardiomyopathy - An update. Eur. J. Pharmacol. 818 241–253. 10.1016/j.ejphar.2017.10.043 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez R., Stankevicius E., Herrera M. D., Ostergaard L., Andersen M. R., Ruiz-Gutierrez V., et al. (2008). Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br. J. Pharmacol. 155 535–546. 10.1038/bjp.2008.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami S., Momeni Z., Behnam R., Ghayour N. (2010). Comparison of antioxidant effect of Melissa officinalis leaf and vitamin C in lead acetate induced learning deficits in rat [In Persian]. Sci. Res. J. Shahed Univ. 17 47–54. [Google Scholar]
- Safaeian L., Sajjadi S. E., Javanmard S. H., Montazeri H., Samani F. (2016). Protective effect of Melissa officinalis extract against H2O2-induced oxidative stress in human vascular endothelial cells. Res. Pharm. Sci. 11 383–389. 10.4103/1735-5362.192488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedighi M., Faghihi M., Rafieian-Kopaei M., Rasoulian B., Nazari A. (2019). Cardioprotective effect of ethanolic leaf extract of melissa officinalis L against regional ischemia-induced arrhythmia and heart injury after five days of reperfusion in rats. Iran J. Pharm. Res. 18 1530–1542. 10.22037/ijpr.2019.1100761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D. Y., Lee S. R., Heo J. W., No M. H., Rhee B. D., Ko K. S., et al. (2018). Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 22 235–248. 10.4196/kjpp.2018.22.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri A., Sahebkar A., Javadi B. (2016). Melissa officinalis L. - A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 188 204–228. 10.1016/j.jep.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Songbo M., Lang H., Xinyong C., Bin X., Ping Z., Liang S. (2019). Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 307 41–48. 10.1016/j.toxlet.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Soulimani R., Fleurentin J., Mortier F., Misslin R., Derrieu G., Pelt J. M. (1991). Neurotropic action of the hydroalcoholic extract of Melissa officinalis in the mouse. Planta Med. 57 105–109. 10.1055/s-2006-960042 [DOI] [PubMed] [Google Scholar]
- Stojanović N. M., Randjelović P. J., Mladenović M. Z., Ilić I. R., Petrović V., Stojiljković N., et al. (2019). Toxic essential oils, part VI: acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 133 110794. 10.1016/j.fct.2019.110794 [DOI] [PubMed] [Google Scholar]
- Tantry M. A., Bhat G. A., Idris A., Dar J. A., Al Omar S. Y., Masoodi K. Z., et al. (2014). Sulfated triterpenes from Lemon balm. Helv. Chim. Acta 97 1497–1506. 10.1002/hlca.201400001 [DOI] [Google Scholar]
- Ulbricht C., Brendler T., Gruenwald J., Kligler B., Keifer D., Abrams T. R., et al. (2005). Lemon balm (Melissa officinalis L.): an evidence-based systematic review by the Natural Standard Research Collaboration. J. Herb. Pharmacother. 5 71–114. [PubMed] [Google Scholar]
- Wang X. T., Gong Y., Zhou B., Yang J. J., Cheng Y., Zhao J. G., et al. (2018). Ursolic acid ameliorates oxidative stress, inflammation and fibrosis in diabetic cardiomyopathy rats. Biomed. Pharmacother. 97 1461–1467. 10.1016/j.biopha.2017.11.032 [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Chao P. D., Hsiu S. L., Wen K. C., Hou Y. C. (2004). Lethal quercetin-digoxin interaction in pigs. Life Sci. 74 1191–1197. 10.1016/j.lfs.2003.06.044 [DOI] [PubMed] [Google Scholar]
- Weidner C., Rousseau M., Plauth A., Wowro S. J., Fischer C., Abdel-Aziz H., et al. (2015). Melissa officinalis extract induces apoptosis and inhibits proliferation in colon cancer cells through formation of reactive oxygen species. Phytomedicine 22 262–270. 10.1016/j.phymed.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Weidner C., Wowro S. J., Freiwald A., Kodelja V., Abdel-Aziz H., Kelber O., et al. (2014). Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol. Nutr. Food Res. 58 903–907. 10.1002/mnfr.201300477 [DOI] [PubMed] [Google Scholar]
- Wolbling R. H., Leonhardt K. (1994). Local therapy of herpes simplex with dried extract from Melissa officinalis. Phytomedicine 1 25–31. 10.1016/S0944-7113(11)80019-X [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021). Cardiovascular Disease. Available online at: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed January 10, 2021) [Google Scholar]
- Wu S., Wang H. Q., Guo T. T., Li Y. H. (2020). Luteolin inhibits CVB3 replication through inhibiting inflammation. J. Asian Nat. Prod. Res. 22 762–773. 10.1080/10286020.2019.1642329 [DOI] [PubMed] [Google Scholar]
- Yan X., Zhang Y. L., Zhang L., Zou L. X., Chen C., Liu Y., et al. (2019). Gallic acid suppresses cardiac hypertrophic remodeling and heart failure. Mol. Nutr. Food Res. 63:e1800807. 10.1002/mnfr.201800807 [DOI] [PubMed] [Google Scholar]
- Yui S., Fujiwara S., Harada K., Motoike-Hamura M., Sakai M., Matsubara S., et al. (2017). Beneficial effects of lemon balm leaf extract on in vitro glycation of proteins, arterial stiffness, and skin elasticity in healthy adults. J. Nutr. Sci. Vitaminol. (Tokyo) 63 59–68. 10.3177/jnsv.63.59 [DOI] [PubMed] [Google Scholar]
- Yuliang W., Zejian W., Hanlin S., Ming Y., Kexuan T. (2015). The hypolipidemic effect of artesunate and ursolic acid in rats. Pak. J. Pharm. Sci. 28 871–874. [PubMed] [Google Scholar]
- Zarei A., Changizi-Ashtiyani S., Taheri S., Hosseini N. (2015). A brief overview of the effects of Melissa officinalis L. extract on the function of various body organs. Zahedan J. Res. Med. Sci. 15 29–34. 10.17795/zjrms1007 [DOI] [Google Scholar]
- Zhang S., Liu X., Sun C., Yang J., Wang L., Liu J., et al. (2016). Apigenin attenuates experimental autoimmune myocarditis by modulating Th1/Th2 Cytokine balance in mice. Inflammation 39 678–686. 10.1007/s10753-015-0294-y [DOI] [PubMed] [Google Scholar]
- Zhang X., Ma Z. G., Yuan Y. P., Xu S. C., Wei W. Y., Song P., et al. (2018). Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell Death Dis. 9:102. 10.1038/s41419-017-0123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu J. X., Ma Z. G., Wu H. M., Xu S. C., Song P., et al. (2019). Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 15 556–567. 10.7150/ijbs.29907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang B. (2017). Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 7:44735. 10.1038/srep44735 [DOI] [PMC free article] [PubMed] [Google Scholar]