Abstract
The cervical sympathetic chain (CSC) innervates post-ganglionic sympathetic neurons within the ipsilateral superior cervical ganglion (SCG) of all mammalian species studied to date. The post-ganglionic neurons within the SCG project to a wide variety of structures, including the brain (parenchyma and cerebral arteries), upper airway (e.g., nasopharynx and tongue) and submandibular glands. The SCG also sends post-ganglionic fibers to the carotid body (e.g., chemosensitive glomus cells and microcirculation), however, the function of these connections are not established in the mouse. In addition, nothing is known about the functional importance of the CSC-SCG complex (including input to the carotid body) in the mouse. The objective of this study was to determine the effects of bilateral transection of the CSC on the ventilatory responses [e.g., increases in frequency of breathing (Freq), tidal volume (TV) and minute ventilation (MV)] that occur during and following exposure to a hypoxic gas challenge (10% O2 and 90% N2) in freely-moving sham-operated (SHAM) adult male C57BL6 mice, and in mice in which both CSC were transected (CSCX). Resting ventilatory parameters (19 directly recorded or calculated parameters) were similar in the SHAM and CSCX mice. There were numerous important differences in the responses of CSCX and SHAM mice to the hypoxic challenge. For example, the increases in Freq (and associated decreases in inspiratory and expiratory times, end expiratory pause, and relaxation time), and the increases in MV, expiratory drive, and expiratory flow at 50% exhaled TV (EF50) occurred more quickly in the CSCX mice than in the SHAM mice, although the overall responses were similar in both groups. Moreover, the initial and total increases in peak inspiratory flow were higher in the CSCX mice. Additionally, the overall increases in TV during the latter half of the hypoxic challenge were greater in the CSCX mice. The ventilatory responses that occurred upon return to room-air were essentially similar in the SHAM and CSCX mice. Overall, this novel data suggest that the CSC may normally provide inhibitory input to peripheral (e.g., carotid bodies) and central (e.g., brainstem) structures that are involved in the ventilatory responses to hypoxic gas challenge in C57BL6 mice.
Keywords: cervical sympathetic chain transection, superior cervical ganglion, hypoxic gas challenge, ventilatory parameters, C57BL6 mice
Introduction
Pre-ganglionic sympathetic neurons emanating from the thoracic spinal cord (T1–T4) course in the left and right cervical sympathetic chains (CSC) and terminate on the cell bodies of post-ganglionic sympathetic neurons in the ipsilateral superior cervical ganglia (SCG) (Rando et al., 1981; Tang et al., 1995a,b,c; Llewellyn-Smith et al., 1998). The majority of post-ganglionic cells leave the SCG via the internal and external carotid nerves (Bowers and Zigmond, 1979; Buller and Bolter, 1997; Asamoto, 2004; Savastano et al., 2010), with the ganglioglomerular nerve (GGN) branching from the external carotid nerve to innervate glomus cells, chemoafferent nerve terminals, and vasculature within the carotid body (Biscoe and Purves, 1967; Zapata et al., 1969; Bowers and Zigmond, 1979; McDonald and Mitchell, 1981; McDonald, 1983; Verna et al., 1984; Torrealba and Claps, 1988; Ichikawa, 2002; Asamoto, 2004; Savastano et al., 2010), and baroafferent nerve terminals within the carotid sinus (Floyd and Neil, 1952; Rees, 1967; Bolter and Ledsome, 1976; Felder et al., 1983; Buller and Bolter, 1993).
The projections of the SCG to a series of inter-related structures, such as the carotid body and carotid sinus, upper airway and tongue (Flett and Bell, 1991; Kummer et al., 1992; O’Halloran et al., 1996, 1998; Hisa et al., 1999; Wang and Chiou, 2004; Oh et al., 2006), and nuclei within the hypothalamus and brainstem, including the nucleus tractus solitarius (nTS) (Cardinali et al., 1981a,b, 1982; Gallardo et al., 1984; Saavedra, 1985; Wiberg and Widenfalk, 1993; Westerhaus and Loewy, 1999; Esquifino et al., 2004; Hughes-Davis et al., 2005; Mathew, 2007), provide evidence that the SCG is a vital integrative structure regulating cardiorespiratory function. Previous studies have shown that electrical stimulation of the CSC decreases arterial blood pressure and upper airway resistance in rats (O’Halloran et al., 1996, 1998). Moreover, there is substantial (and conflicting) evidence as to the ability of sympathetic innervation to the carotid body to influence resting activity of glomus cells and chemoafferents within the carotid sinus nerve (CSN), and modulate changes in activity during hypoxic gas challenge (HXC) (Prabhakar, 1994). For instance, CSC and GGN activity increases during hypoxic challenge, suggesting the release of neurotransmitters, such as norepinephrine, dopamine and neuropeptide Y (Lahiri et al., 1986; Matsumoto et al., 1986, 1987, Yokoyama et al., 2015), and conversely activation of the GGN decreases the hypoxic response of chemosensors within the cat carotid body (McQueen et al., 1989). A variety of responses have also been reported upon application of neurotransmitters released by CSC and GGN nerve terminals (e.g., norepinephrine, dopamine and neuropeptide Y) to in vivo and in vitro carotid body preparations, including (1) a biphasic pattern consisting of initial brief bursts in CSN activity and then long-lasting inhibition (Bisgard et al., 1979), (2) a biphasic pattern consisting of initial brief decreases in CSN activity and then long-lasting excitation (Matsumoto et al., 1981), (3) indirect excitation of carotid body glomus cells via constriction of arteriolar blood flow in the carotid body (Potter and McCloskey, 1987; Yokoyama et al., 2015), (4) direct activation of glomus cells and/or chemosensory afferents (Matsumoto et al., 1980; Lahiri et al., 1981; Milsom and Sadig, 1983; Heinert et al., 1995; Pang et al., 1999), and (5) direct inhibitory action of glomus cells and/or chemosensory afferents (Zapata et al., 1969; Zapata, 1975; Llados and Zapata, 1978; Mills et al., 1978; Folgering et al., 1982; Kou et al., 1991; Pizarro et al., 1992; Bisgard et al., 1993; Prabhakar et al., 1993; Ryan et al., 1995; Almaraz et al., 1997; Overholt and Prabhakar, 1999).
Wild-type and genetically-engineered mice are widely used to understand the mechanisms by which hypoxic challenges elicit carotid body-dependent and -independent ventilatory responses (He et al., 2000, 2002, 2003; Kline and Prabhakar, 2000; Pérez-García et al., 2004; Kline et al., 2005; Pichard et al., 2015; Gao et al., 2017; Wang et al., 2017; Ortega-Sáenz et al., 2018; Peng et al., 2018; Prabhakar et al., 2018). The morphology, neurophysiology and neuropharmacology of the mouse CSC-SCG has received considerable investigation over the years (Black et al., 1972; Yokota and Yamauchi, 1974; Banks and Walter, 1975; Inoue, 1975; Lewis and Burton, 1977; Forehand, 1985; Kidd and Heath, 1988; Gibbins, 1991; Kasa et al., 1991; Little and Heath, 1994; Jobling and Gibbins, 1999; El-Fadaly and Kummer, 2003; David et al., 2010; Cadaveira-Mosquera et al., 2012; Pashai et al., 2012; Alberola-Die et al., 2013; Liu and Bean, 2014; Martinez-Pinna et al., 2018; Mitsuoka et al., 2018; Feldman-Goriachnik and Hanani, 2019; Simeone et al., 2019; Rivas-Ramírez et al., 2020). Mouse tissues that receive post-ganglionic projections from the SCG have also been heavily investigated (Krieger et al., 1976; García et al., 1988; Kawaja and Crutcher, 1997; Maklad et al., 2001; Pankevich et al., 2003a,b; Ivanusic et al., 2013; Karlsen et al., 2013; Lindborg et al., 2018; Ziegler et al., 2018; Teshima et al., 2019). Nonetheless, there is no direct evidence that the CSC-SCG complex innervates the carotid bodies of mice, and this is likely on the basis of the presence of sympathetic (i.e., tyrosine-hydroxylase-positive) nerve terminals and adrenergic receptors in these structures (Prieto-Lloret et al., 2007; Roux et al., 2008; Kåhlin et al., 2010; Chai et al., 2011).
Patients with chronic T1–T4 spinal cord injury present with cardiorespiratory disturbances consistent with diminished activity of the CSC-SCG complex (DiMarco et al., 2009; Heutink et al., 2014; Sankari et al., 2014, 2019; Berlowitz et al., 2016; Hachmann et al., 2017; Shin et al., 2019) including, enhanced peripheral (carotid body) chemoreflex sensitivity, which is a primary cause of sleep-disordered breathing in these subjects (Tester et al., 2014; Bascom et al., 2016). In contrast, studies in rats have found that mid-thoracic spinal cord injury is associated with enhanced cardiac sympathetic activity and cardiac sympathetic hyperinnervation that increases the susceptibility to life-threatening arrhythmias (Rodenbaugh et al., 2003; Collins et al., 2006; Lujan and DiCarlo, 2007; Lujan et al., 2009, 2010, 2012, 2014). Studies in rats have also confirmed that hypoactivity of the CSC greatly enhances the likelihood of stroke in hypertensive models (Sadoshima et al., 1981; Sadoshima and Heistad, 1983; Werber and Heistad, 1984).
The effects of bilateral transection of the CSC (CSCX) or bilateral removal of the SCG (SCGX) have been investigated on a variety of physiological functions/variables in the mouse (Krieger et al., 1976; García et al., 1988; Kawaja and Crutcher, 1997; Pankevich et al., 2003a,b; Karlsen et al., 2013; Lindborg et al., 2018; Ziegler et al., 2018). However, to our knowledge, no studies, in any species, have determined the roles of the CSC-SCG complex on ventilatory functions and responses to hypoxic challenges in vivo. The aim of this study was to determine the effects of sham-operated (SHAM) and bilateral CSCX (performed 4 days before testing) on resting ventilatory parameters in freely-moving adult male C57BL6 mice, and on their ventilatory responses to HXC using whole-body plethysmography as described previously (Palmer et al., 2013a,b; Gaston et al., 2014; Getsy et al., 2014; Palmer et al., 2015).
The C57BL6 mouse has proven to be an invaluable murine model in which to investigate the physiological systems involved in ventilatory control processes (Tankersley et al., 1994, 2000), and this strain is used widely to generate genetic knock-out mutants to investigate the molecular mechanisms underlying the responses of mice to hypoxic and/or hypercapnic gas challenges (Kline and Prabhakar, 2000, Kline et al., 2005; Palmer et al., 2013a). The ventilatory responses that will be described in the SHAM mice (during and following the HXC) were consistent with previous studies from our laboratory (Palmer et al., 2013a,b, 2015; Gaston et al., 2014; Getsy et al., 2014). Our Getsy et al. (2014) manuscript provides a detailed set of analyses of the ventilatory responses that occur in C57BL6, Swiss Webster and B6AF1 mice before, during, and after a brief HXC. We found that (1) the HVR in C57BL6 mice consists of an initial increase in frequency of breathing (Freq) followed by substantial decline (roll-off) toward pre-HXC values, whereas the Freq responses in Swiss Webster and B6AF1 mice were robust with minimal roll-off, and (2) the post-HXC (i.e., return to room-air) responses consisted of a rapid and sustained rise in Freq in C57BL6 mice, a sustained rise in the B6AF1 mice, which is known as a form of short-term potentiation (STP) (Powell et al., 1998), and a gradual return to pre-hypoxic challenge levels in the Swiss-Webster mice.
The ways and mechanisms by which activation of sympathetic nerves affects carotid body function under normoxic and hypoxic conditions have received considerable attention (Overholt and Prabhakar, 1999). For instance, compelling evidence shows that sympathetic nerve terminals innervate glomus cells and the microvasculature within the carotid bodies (Vázquez-Nin et al., 1978; McDonald, 1983; Verna et al., 1984), and that norepinephrine is the major neurotransmitter released by these terminals (Almaraz et al., 1997). The roles of norepinephrine and dopamine and α1-, α2-, and β-adrenoceptors and dopamine receptors have also been extensively studied. It is apparent that activation of sympathetic nerves can induce a multiplicity of effects within the carotid body. First, activation of the sympathetic nerves indirectly activate glomus cells by constricting arterioles (by activation α1-adrenoceptors and dopamine receptors) in the carotid body, effectively resulting in a hypoxic environment for glomus cells (Llados and Zapata, 1978; Majcherczyk et al., 1980; Matsumoto et al., 1981; Yokoyama et al., 2015). In addition, co-release of neuropeptide Y from sympathetic nerve terminals also reduces blood flow within the carotid bodies (Potter and McCloskey, 1987). In addition, activation of β-adrenoceptors and dopamine receptors on glomus cells directly activates these cells (Eldridge and Gill-Kumar, 1980; Lahiri et al., 1981; Gonsalves et al., 1984). On the other hand, intra-carotid artery infusions of norepinephrine depress resting chemoreceptor activity and also attenuate hypoxic excitation of the carotid body, effects mediated by α2-adrenoceptors (Kou et al., 1991; Pizarro et al., 1992; Prabhakar et al., 1993; Almaraz et al., 1997). Moreover, endogenous norepinephrine indirectly inhibits carotid body chemoafferent activity (Overholt and Prabhakar, 1999) via inhibition of glomus cell activity (presumably the release of excitatory neurotransmitters) by decreasing the magnitude and rate of macroscopic Ca2+ current influx (Overholt and Prabhakar, 1999). As such, it is likely to question how the absence of functional sympathetic input to the carotid bodies and other structures controlling ventilatory processes, would affect baseline parameters and the responses to HXC.
As mentioned, it has been established that HXC increases neural activity in the CSC and GGN (Lahiri et al., 1986; Matsumoto et al., 1986, 1987). As such, it is proposed that HXC activates a carotid sinus (chemoafferent) nerve-brainstem-descending spinal cord pathway that increases pre-ganglionic sympathetic nerve activity, which in turn activates post-ganglionic neurons in the SCG, including those projecting to the carotid body via the GGN. It should be noted that there is compelling evidence that SCG cells are not directly responsive to hypoxia (Rigual et al., 1999; Nunes et al., 2012; Buckler and Turner, 2013; Gao et al., 2019; Bernardini et al., 2020), although there is equally compelling evidence that SCG cells [and in particular small intensely fluorescent (SIF) cells] are hypoxia-sensitive (Hanson et al., 1986; Brokaw and Hansen, 1987; Dinger et al., 1993; Strosznajder, 1997; Nunes et al., 2010, 2012).
One principal finding of this study is that transection of the CSC is not equivalent to bilateral removal of the SCG (see Conclusion), suggesting multiple effects of hypoxia on neural signaling within the CSC-SCG pathway.
Materials and Methods
Permissions
All studies were carried out in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committee of Case Western Reserve University.
Animals
C57BL6 male mice were purchased from Jackson Laboratory (Bar Harbor, ME, United States). Mice were delivered pathogen free, and housed under specific-pathogen free conditions with a 12 h light-dark cycle. All procedures were performed in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals1 and were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Cervical Sympathetic Chain Transection (CSCX)
Adult mice (12 weeks) were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg, Ketaset, Zoetis, Parsippany, NJ, 100 mg/ml solvent) and xylazine (10 mg/kg, Akorn Animal Health, Lake Forest, IL, United States, 20 mg/ml solvent), and placed on a surgical station allowing body temperature to be maintained at 37°C via a heating pad (SurgiSuite, Kent Scientific Corporation, Torrington, CT, United States). The adequacy of anesthesia was regularly checked by nociceptive stimulus (e.g., a toe pinch). The SCG-CSC was identified behind the carotid artery bifurcation (Figure 1), and the CSC was cut using micro-scissors approximately 1 mm from the point where the CSC enters the SCG. In SHAM mice, the SCG-CSC was identified but not cut. The mice were allowed 4 days to recover from surgery. This time-point for recovery was chosen based on evidence that catecholamine levels in the carotid bodies, identified by tyrosine hydroxylase positive nerve terminals, are markedly reduced 3–4 days after removal of the ipsilateral SCG (Mir et al., 1982; González-Guerrero et al., 1993; Ichikawa and Helke, 1993; Ichikawa, 2002). All mice were monitored for pain and distress every day following surgery. Mice were given an injection of the non-steroidal anti-inflammatory drug, carprofen (2 mg/kg, IP), 24 and 48 h post-surgery to reduce any pain or inflammation at the incision site. None of the mice showed any signs of pain or inflammation from the surgeries and began moving about the cages and eating and drinking approximately 1 h after surgery. Mice were weighed daily to ensure proper weight gain. We have determined that these injections of carprofen do not affect resting ventilation or the response to HXC on day 4 post-surgery (data not shown).
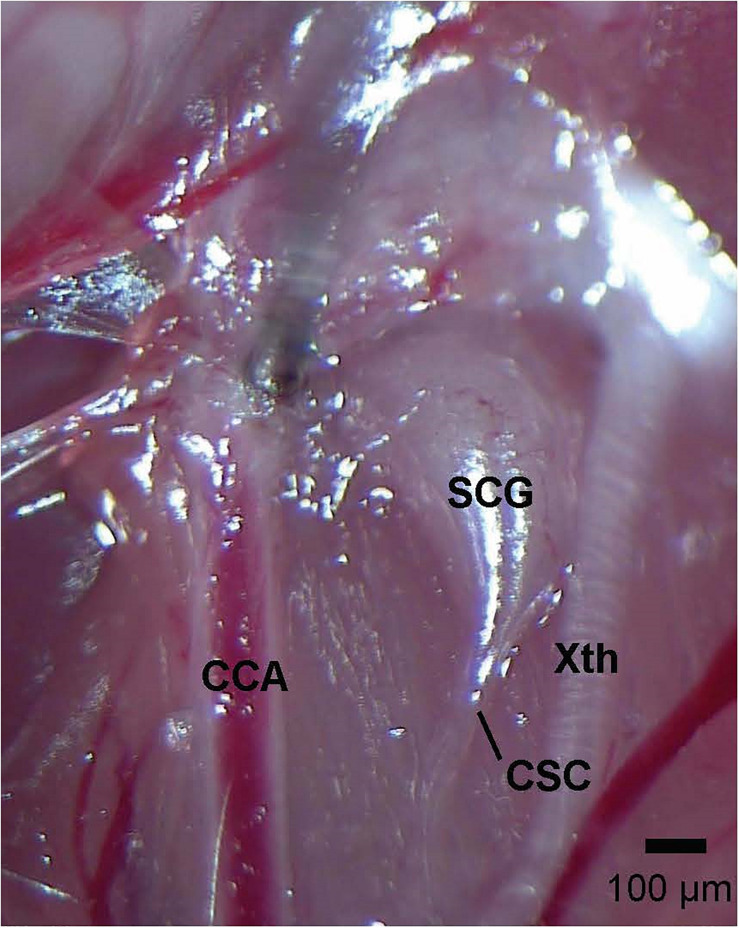
FIGURE 1.
Photograph of the left cervical sympathetic chain (CSC) and left superior cervical ganglion (SCG) of an adult C57BL6 mouse. The vagus nerve (Xth) and common carotid artery (CCA) are also shown. The scale bar is 100 μm.
Whole-Body Plethysmography
On the day of study, mice were placed in individual whole-body plethysmographs (Buxco® Small Animal Whole Body Plethysmography, DSI a division of Harvard Biosciences, Inc., St. Paul, MN, United States) to continuously record ventilatory parameters in the freely-moving state, as described in detail previously (Palmer et al., 2013a,b, 2015; Gaston et al., 2014; Getsy et al., 2014). The whole body plethysmography experiments were done by investigators (P.M.G, G.A.C, or Y-H.H) who were blinded to which surgery the mouse had undergone. The protocols were designed to ensure that the studies were done in a controlled fashion. More specifically, sub-groups of SHAM (n = 2) and CSCX (n = 2) mice were studied on the same day. The studies therefore took a total of 6 days over a 3 weeks period to study 11 SHAM and 12 CSCX mice. Because of the experimental paradigm, the collected data are likely to accurately define the effects of CSCX on resting ventilatory parameters and the response to HXC. The array of ventilatory parameters were chosen to provide an in-depth analysis of the differences in resting breathing patterns and responses to the hypoxic gas challenge in SHAM and CSCX mice (Solberg et al., 2006; Moore et al., 2012; Palmer et al., 2013a,b, 2015; Gaston et al., 2014; Getsy et al., 2014; Villiere et al., 2017). Directly recorded parameters were (a) Freq, (b) tidal volume, (TV), (c) inspiratory time (Ti, duration of inspiration) and expiratory time (Te, duration of expiration), (d) end inspiratory pause (EIP, pause between end of inspiration and start of expiration) and end expiratory pause (EEP, pause between end of expiration and start of inspiration), (e) expiratory flow at 50% expired TV (EF50), (f) peak inspiratory flow (PIF) and peak expiratory flow (PEF), (g) relaxation time (decay of respiration to 36% of maximum PIF), (h) relative rate of achieving PEF (Rpef), and (i) rejection index (RI, % of non-eupneic breaths such as apneas per epoch). Calculated parameters were (a) minute ventilation (MV, Freq × TV), (b) Ti/Te and Ti/(Ti + Te), (c) PIF/PEF, (d) inspiratory drive (TV/Ti) and expiratory drive (TV/Te), (e) rejection index corrected for respiratory frequency (RI/Freq), and (f) the number of apneic pauses per epoch (Te/RT)-1. With respect to the rejection index, the plethysmography system included a rejection algorithm that was set to reject breaths that did not reflect normal TV breathing, and as such rejected (a) abnormal breaths (abnormal balance of inspiratory and expiratory volumes), apneas, sighs, post-sighs, sniffs, and waveforms that most likely arose from activities such as grooming the face, hands, and rear (Getsy et al., 2014). Minimum TV was set at 0.05 ml, minimum Ti was set at 0.04 s, maximum Te was set at 0.5 s, and the ratio of inspiratory volume/expiratory volume (i.e., volume balance) was set to a range of 90–110%. Values were rejected when they fell outside the above criteria and when (a) Ti was greater than 2 times Te, (b) PIF could not be distinguished from PEF, and (c) EF50, Rpef, or RT could not be computed (Getsy et al., 2014). All directly recorded parameters (i.e., Freq, TV, MV, Ti, Te, EIP, EEP, PIF, PEF, Rpef, relaxation time and rejection index) were extracted from the raw waveforms using proprietary Biosystem XA and FinePointe software (Data Sciences International, St. Paul, MN, United States) as described previously for mice (Palmer et al., 2013a,b; Gaston et al., 2014; Getsy et al., 2014) and as detailed in the Data Sciences International/Buxco website reference to parameters provided by FinePointe Software using whole-body plethysmography2. Data was extracted as individual data points (e.g., a Freq value for a particular 15 s epoch) and placed in excel spreadsheets.
Protocols for Hypoxic Gas Challenge
SHAM and CSCX mice were placed in the plethysmography chambers and allowed approximately 60 min to settle to allow for resting parameters to reach stable levels before the freely-moving mice were exposed to a 5 min HXC (10% O2 and 90% N2) and then re-exposed to room-air for 15 min.
Statistics
A data point before (15 min), during (5 min) and after (15 min) HXC was collected every 15 s. To determine the total responses (cumulative % changes from pre-HXC values) during HXC and return to room-air for each mouse, we summed the values recorded before and during the challenge and those upon return to room-air. Regarding the pre-HXC (baseline) phase, the breaths for each 15 s epoch were averaged over the last 5 min of the entire 15 min baseline recording period resulting in 20 values for each mouse that was averaged to give the resting value for each mouse. The mean and SEM for the 11 SHAM and 12 CSCX mice was then derived from the individual values. Similarly, twenty 15 s epoch values were derived during the 5 min hypoxic challenge and sixty 15 s epoch values were derived for the post-hypoxia (room-air) phase. Again, the mean and SEM for the 11 SHAM and 12 CSCX mice was derived from the individual values for the HXC and room-air phases under examination. We then determined the cumulative response for each mouse by the formulas, (a) total HXC response = (sum of the 20 values during HXC) − (mean of the pre-HXC values × 20), and (b) total room-air response = (sum of the 60 values during room-air phase) − (mean of the pre-HXC values × 60). We then determined the mean and SEM of the group data. We also calculated the total responses during the HXC 0–105 sec epoch and 106–300 sec epoch, in addition to the entire HXC 5 min (0–300 sec epoch). All data are presented as mean ± SEM. All data were analyzed by one-way or two-way ANOVA followed by Student’s modified t-test with Bonferroni corrections for multiple comparisons between means (Palmer et al., 2013a,b).
Results
Resting Parameters
A summary of the mouse descriptors and resting ventilatory parameters is provided in Table 1. There were 11 mice in the SHAM group and 12 mice in the CSCX group. The ages and body weights of the two groups were similar to one another (P > 0.05, for both comparisons). Accordingly, no corrections for body weights were applied to the ventilatory data pertaining to volumes (e.g., TV and peak inspiratory and expiratory flows). There were no between-group differences for any recorded or calculated ventilatory parameter (P > 0.05, for all comparisons). In the following Figures 3–10, the left panel of each row will show the actual values recorded before, during the 5 min hypoxic (10% O2 and 90% N2) challenge, and upon to room-air. The middle panels of each row will show the arithmetic change recorded over the first 60 s of hypoxic exposure. The right panels of each row will show the total response (% change from pre) during three epochs of the 5 min (300 s) hypoxic challenge, namely 0–105, 106–300, and 0–300 s. These epochs were those which best represented the dramatic differences between SHAM and SCGX mice (see Conclusion).
TABLE 1.
Baseline parameters in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX).
| Parameter | SHAM | CSCX |
| Number of mice | 11 | 12 |
| Age, days | 101 ± 1 | 102 ± 1 |
| Body weight, grams | 27.2 ± 0.5 | 27.5 ± 0.8 |
| Frequency (breaths/min) | 196 ± 5 | 188 ± 6 |
| Tidal volume (TV, ml) | 0.149 ± 0.007 | 0.157 ± 0.014 |
| Minute ventilation (ml/min) | 28.9 ± 1.1 | 28.6 ± 1.8 |
| Inspiratory time (Ti, sec) | 0.112 ± 0.003 | 0.114 ± 0.003 |
| Expiratory time (Te, sec) | 0.207 ± 0.006 | 0.220 ± 0.009 |
| End inspiratory pause (EIP, msec) | 2.90 ± 0.08 | 2.85 ± 0.07 |
| End expiratory pause (EEP, msec) | 34.5 ± 3.8 | 32.7 ± 6.1 |
| Ti/Te | 0.551 ± 0.016 | 0.526 ± 0.015 |
| Ti/(Ti + Te) | 0.353 ± 0.007 | 0.343 ± 0.006 |
| Peak inspiratory flow (PIF, ml/sec) | 2.38 ± 0.09 | 2.44 ± 0.16 |
| Peak expiratory flow (PEF, ml/sec) | 1.51 ± 0.06 | 1.42 ± 0.09 |
| PIF/PEF | 1.59 ± 0.05 | 1.74 ± 0.07 |
| Expiratory flow at 50% exhaled TV (EF50, ml/sec) | 0.072 ± 0.004 | 0.071 ± 0.003 |
| Relaxation time (RT, sec) | 0.100 ± 0.003 | 0.103 ± 0.004 |
| Rate of achieving PEF (Rpef) | 0.138 ± 0.13 | 0.166 ± 0.019 |
| Inspiratory drive (TV/Ti, ml/sec) | 1.36 ± 0.06 | 1.38 ± 0.09 |
| Expiratory drive (TV/Te, ml/sec) | 0.72 ± 0.03 | 0.70 ± 0.04 |
| Rejection index (RI, %) | 15.4 ± 1.9 | 15.2 ± 1.9 |
| (Rejection index/frequency) × 100 | 7.9 ± 0.9 | 8.3 ± 1.2 |
| Apneic pauses (Te/RT)-1 | 1.07 ± 0.03 | 1.15 ± 0.05 |
The data are presented as mean ± SEM. There were no between-group differences for any parameter (P > 0.05, for all comparisons).
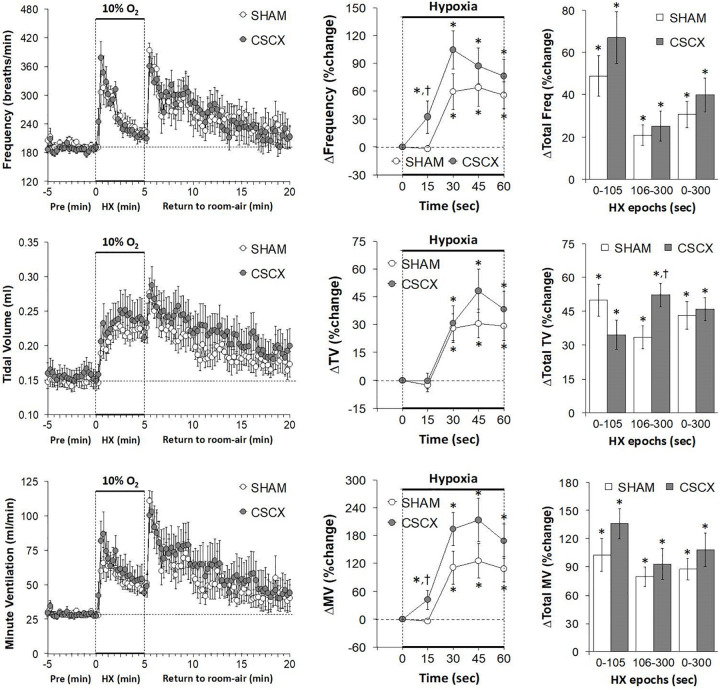
FIGURE 3.
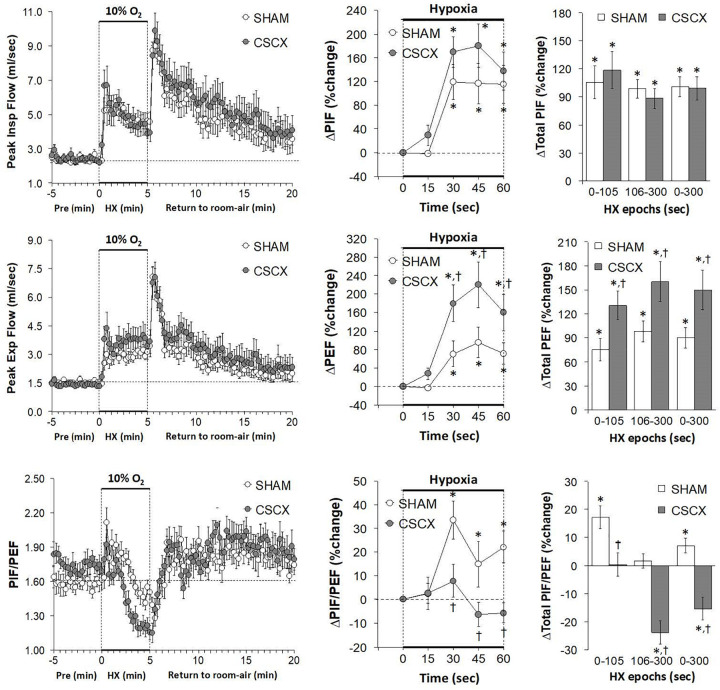
(Left) Frequency of breathing, tidal volume and minute ventilation values before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
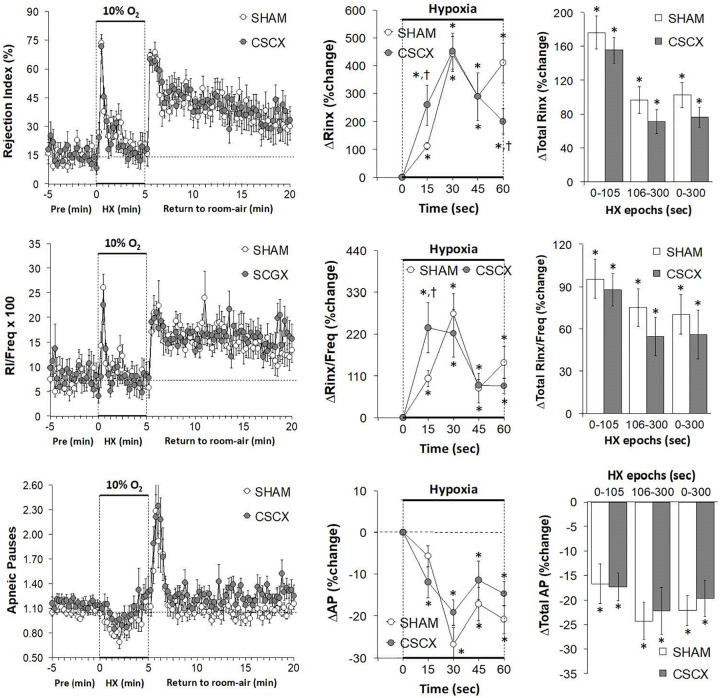
FIGURE 10.
(Left) Rejection Index (RI, Rinx) values, RI/frequency ratios and Apneic Pauses (AP) before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – Frequency of Breathing, Tidal Volume and Minute Ventilation
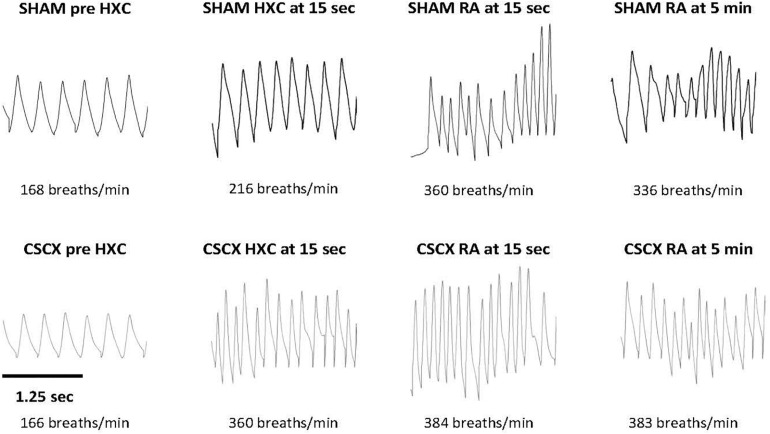
Examples of respiratory waveforms during various stages of the experiment in a SHAM mouse and a CSCX mouse are shown in Figure 2. A time bar (1.25 s) is shown in the bottom left of the figure. Resting Freq (pre-HXC values) was similar in both groups of mice. The initial HXC response (HXC at 15 s) was far greater in the CSCX mouse (166–360 breaths/min = 194 breaths/min, +119%) than the SHAM mouse (168–216 breaths/min = 48 breaths/min, +29%). The roll-off values at the end of the 5 min HXC were similar between the two groups (data not shown) as were the increases in Freq immediately upon return to room-air (RA at 15 s, +131 and +114% for the SHAM and CSCX mouse, respectively) and at 5 min (RA at 5 min, +131 and +100% for the SHAM and CSCX mouse, respectively).
FIGURE 2.
Example traces of respiratory waveforms during various stages of the experiment in a sham-operated (SHAM) mouse and in a mouse with bilateral cervical sympathetic chain transection (CSCX). A time bar (1.25 s) is shown in the bottom left of the figure. HXC, hypoxic gas challenge. RA, room-air.
The changes in Freq, TV, and MV in SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 3. As seen in the top row of panels, exposure to HXC in SHAM mice elicited a typical initial increase in Freq that was subject to pronounced roll-off (left panel). The responses in CSCX mice were essentially similar compared to SHAM except that the rise in Freq was significantly higher at the 15 s time-point (middle panel). The total increases in Freq in the three designated epochs (0–105, 106–300, and 0–300 s) were similar in the SHAM and CSCX mice (right panel). The return to room-air elicited the expected dramatic increase in Freq in SHAM mice and CSCX mice (left panel), and the total room-air responses were similar in both groups (Table 2). As seen in the middle row of panels, exposure to the HXC elicited immediate and sustained increases in TV that were similar in most aspects in the SHAM and CSCX mice except that the total increase recorded during the 106–300 s epoch were higher in CSCX than SHAM mice (right panel). The return to room-air elicited the expected small initial increase in TV followed by gradual decline toward baseline in SHAM and CSCX mice (left panel). The total room-air responses were similar in both groups (Table 2). As seen in the bottom row of panels, exposure to HXC in the SHAM mice elicited a typical initial increase in MV that was subject to a pronounced roll-off (left panel). The MV responses in CSCX mice were similar except that the rise in MV was significantly higher at the 15 s time-point (middle panel). The total increases in MV in the three designated epochs were similar in the SHAM and CSCX mice. The return to room-air elicited the expected dramatic increase in MV in SHAM and CSCX mice (left panel). The total room-air responses were similar in both groups (Table 2).
TABLE 2.
Total changes that occurred during the 15 min return to room-air.
| Parameter | SHAM | CSCX |
| Number of mice | 11 | 12 |
| Frequency (%) | +33.0 ± 11.2 | +39.4 ± 13.2 |
| Tidal volume (TV, %) | +33.3 ± 7.1 | +39.8 ± 7.4 |
| Minute ventilation (%) | +87.1 ± 22.1 | +105.3 ± 25.8 |
| Inspiratory time (Ti, %) | −26.5 ± 5.5 | −28.3 ± 5.0 |
| Expiratory tme (Te, %) | +3.2 ± 7.9 | +1.2 ± 8.2 |
| +Te,% | +17.2 ± 3.4 (6) | +18.4 ± 3.1 (8) |
| −Te, % | −26.6 ± 4.0 (5) | −33.3 ± 5.1 (4) |
| Ti/Te, % | −25.3 ± 2.3 | −25.2 ± 3.5 |
| Ti/(Ti + Te), % | −18.9 ± 1.6 | −19.0 ± 2.7 |
| End inspiratory pause (EIP, %) | −12.9 ± 2.0 | −10.7 ± 2.0 |
| End expiratory pause (EEP, %) | +181 ± 29 | +298 ± 61 |
| Peak inspiratory flow (PIF, %) | +106 ± 19 | +122 ± 21 |
| Peak expiratory flow (PEF, %) | +92 ± 20 | +128 ± 28 |
| PIF/PEF, % | +13.5 ± 3.4 | +6.9 ± 4.8 |
| Expiratory flow at 50% exhaled TV (EF50, %) | +89 ± 22 | +114 ± 30 |
| Relaxation time (RT, %) | −0.3 ± 6.4 | −4.1 ± 7.3 |
| +RT,% | +16.6 ± 3.0 (5) | +24.6 ± 7.9 (7) |
| −RT, % | −14.3 ± 3.7 (6) | −16.6 ± 3.0 (5) |
| Rate of achieving PEF (Rpef, %) | +27.1 ± 13.1 | +21.9 ± 3.6 |
| +Rpef,% | +61 ± 7 (6) | +21.9 ± 3.6* (5) |
| −Rpef, % | −14 ± 5 (5) | −23.6 ± 2.5 (7) |
| Inspiratory drive (TV/Ti, %) | +114 ± 21 | +133 ± 24 |
| Expiratory drive (TV/Te, %) | +59 ± 19 | +78 ± 25 |
| Rejection index (RI, %) | +227 ± 50 | +200 ± 33 |
| (Rejection index/frequency) × 100 (%) | +123 ± 27 | +132 ± 28 |
| Apneic pauses (Te/RT)-1 (%) | +8.2 ± 7.0 | +13.8 ± 5.4 |
| +(Te/RT)-1,% | +26.7 ± 6.5 (5) | +19.8 ± 3.3 (10) |
| −(Te/RT)-1, % | −7.2 ± 2.7 (6) | −16.5 ± 5.8 (2) |
The data are presented as mean ± SEM. *P < 0.05, CSCX versus SHAM. The values in parentheses equal the number of mice in the sub-groups for Te, RT, Rpef and (Te/RT)-1.
Hypoxic Challenges – Inspiratory Time and Expiratory Time
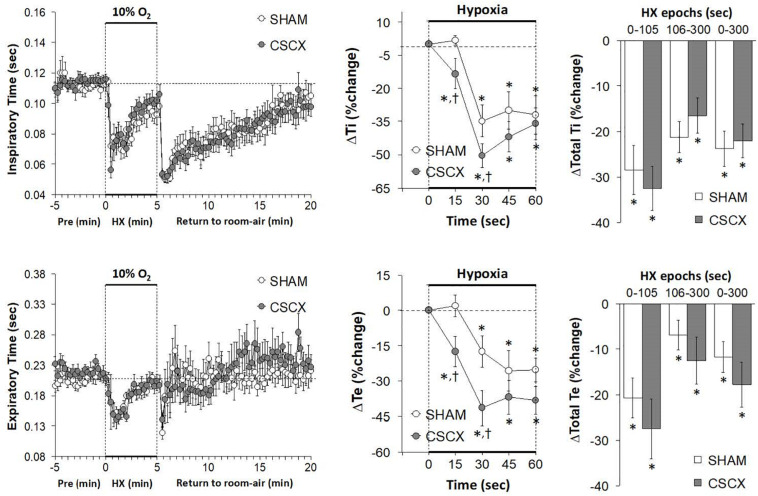
The changes in Ti and Te values in SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 4. Exposure to HXC in SHAM mice elicited initial decreases in Ti and Te that were subject to roll-off (left panels). The decreases in Ti and Te occurred more rapidly in CSCX mice (middle panels), but the overall (total) responses for Ti and Te were similar in both groups (right panels). Return to room-air elicited a rapid transient decrease in Te, but a rapid and sustained decrease in Ti (left panels). The actual (left panels) and total room-air responses (Table 2) were similar in SHAM and CSCX mice. Additionally, the total Te responses upon return to room-air fell into two groups, those in which total Te fell and those in which Te rose (Table 2).
FIGURE 4.
(Left) Inspiratory time (Ti) and expiratory time (Te) values before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – Inspiratory Time/Expiratory Time and Inspiratory Quotient
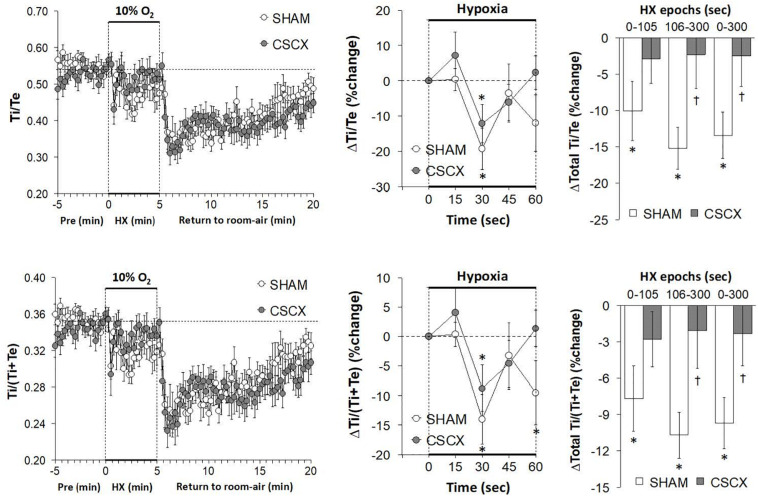
The changes in Ti/Te values and inspiratory quotients [Ti/(Ti + Te)] in SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 5. The resulting changes in Ti and Te during HXC (Figure 4) translated into minor changes in Ti/Te values and inspiratory quotients in SHAM mice (left and middle panels) that were nevertheless significantly smaller in the CSCX mice (right panels). As seen in the left panels, the return to room-air resulted in substantial decreases in Ti/Te values and inspiratory quotients that gradually returned toward baseline values in both groups. As seen in Table 2, the changes in total Ti/Te and Ti/(Ti + Te) values upon return to room-air were similar in the SHAM and CSCX mice.
FIGURE 5.
(Left) Inspiratory time/expiratory time (Ti/Te) and inspiratory time/(inspiratory time + expiratory time) ratios [Ti/(Ti + Te)] before, during and after a 5 min hypoxic (HX, 10% O2 and 90 %N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – End Inspiratory Pause, and End Expiratory Pause
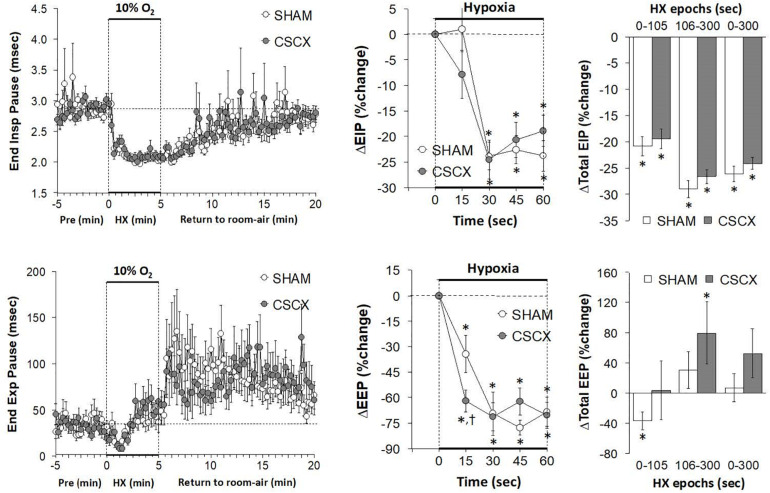
The changes in EIP and EEP in SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 6. The hypoxic challenge elicited a prompt decrease in EIP in SHAM and CSCX mice (top left panel). The initial (top middle) and total responses (top right panel) were similar in SHAM and CSCX mice. The HXC elicited a fall in EEP of about 2 min in duration in SHAM and CSCX mice (bottom left panel). Then EEP quickly returned to baseline values in the SHAM mice during the remainder of the hypoxic challenge, but went above baseline in the CSCX mice. The initial decrease in EEP during the HXC occurred faster in the CSCX mice (bottom middle panel) and the total changes in EEP (bottom right panel) reflected the biphasic changes described above. Return to room-air resulted in a gradual recovery of EIP toward baseline values, but substantial and variable increases in EEP (left panels) for both groups. As seen in Table 2, the total EIP and EEP responses upon return to room-air were similar in the SHAM and CSCX mice.
FIGURE 6.
(Left) End inspiratory pause (EIP) and end expiratory pause (EEP) values before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – Inspiratory Drive and Expiratory Drive
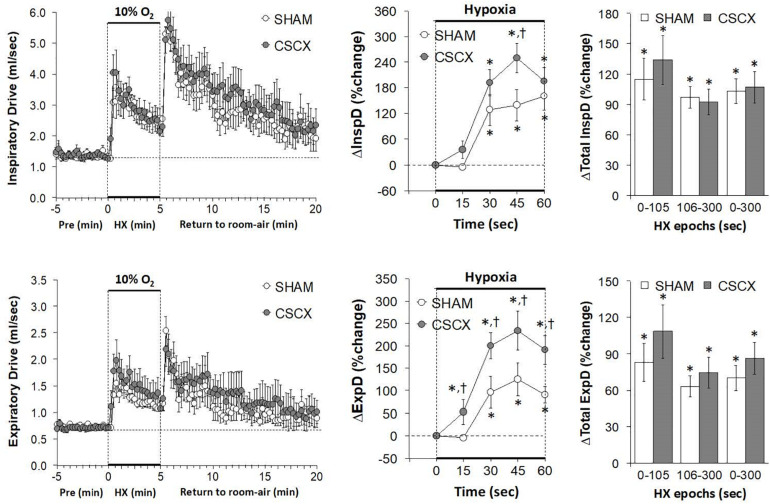
The changes in inspiratory drive (TV/Ti) and expiratory drive (TV/Te) in the SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 7. The HXC elicited prompt and sustained increases in both inspiratory and expiratory drives (left panels) that occurred more rapidly in CSCX mice (middle panels) although the total responses were similar in both groups (right panels). Return to room-air elicited initial increase in both inspiratory and expiratory drives in SHAM and CSCX mice that gradually returned toward baseline (left panels). The total room-air changes were similar in both groups (Table 2).
FIGURE 7.
(Left) Inspiratory Drive (TV/Ti) and Expiratory Drive (TV/Te) before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – Peak Inspiratory and Expiratory Flows
The changes in PIF, PEF, and PIF/PEF ratios in the SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are shown in Figure 8. The HXC elicited prompt and sustained increases in PIF and PEF in SHAM and CSCX mice, with the PIF responses being of greater magnitude during the first half of the hypoxic challenge resulting in higher PIF/PEF ratios (left panels). The initial PIF responses during the HXC in the CSCX mice were similar to those in the SHAM mice, whereas the initial PEF responses were higher in CSCX mice, such that the expected increase in initial PIF/PEF ratios seen in SHAM mice did not occur in CSCX mice (middle panels). Total PIF responses to the HXC were similar in SHAM and CSCX mice, whereas the increases in PEF were higher in the CSCX mice compared to SHAM during each designated epoch (right panels). PIF/PEF ratios over the 106–300 and 0–300 s epochs were markedly lower in CSCX mice than in SHAM mice (bottom right panel). The return to room-air elicited initial increases in PIF and PEF in SHAM and CSCX mice, and the changes resulted in sustained increases in PIF/PEF ratios (left panels). Total PIF, PEF and PIF/PEF responses upon return to room-air were similar in both groups (Table 2).
FIGURE 8.
(Left) Peak Inspiratory flow (PIF) and Peak Expiratory Flow (PEF) values and PIF/PEF ratios before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – EF50, Rpef, and Relaxation Time
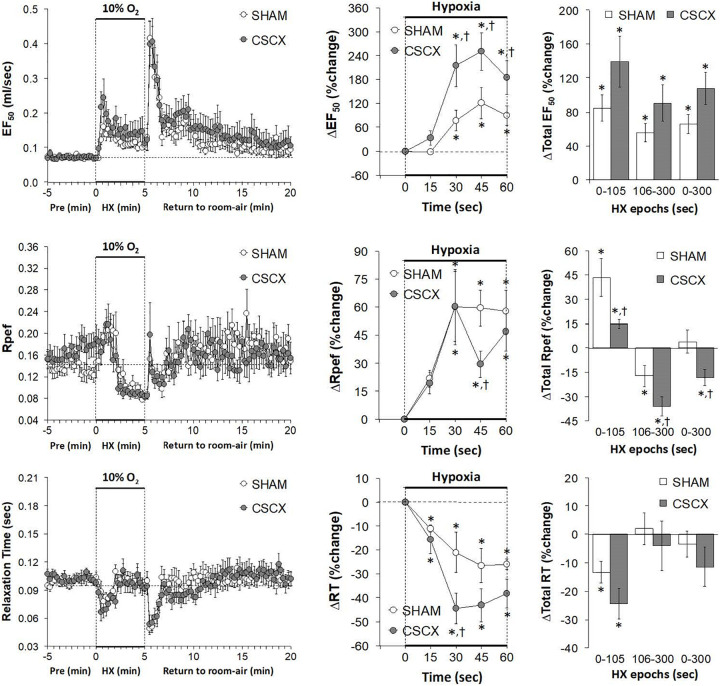
The changes in EF50, Rpef, and RT values in the SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 9. HXC elicited a robust increase in EF50 that occurred more rapidly in the CSCX mice, although the total responses were similar in the SHAM and CSCX mice (top row: left, middle, and right panels). HXC-induced initial brief increases in Rpef in SHAM and CSCX mice that were followed by sustained decreases (middle row: left panel). These Rpef responses occurred more rapidly in the SHAM mice than CSCX mice at the 45 sec time-point (middle row: middle panel), and the responses during the 0–105 sec epoch were significantly smaller in the CSCX mice compared to SHAM, and significantly more reduced from baseline during the 106–300 sec epoch and overall 0–300 sec epoch (middle row: right panel). HXC elicited brief reductions in RT that occurred more rapidly in the CSCX mice than SHAM mice although the overall responses were similar in both groups (bottom row: left, middle, and right panels). The return to room-air elicited prompt increases in EF50 and Rpef that were associated with prompt decreases in RT (left panels). The overall room-air changes in EF50 were similar in both groups. The room-air changes in RT in the SHAM and CSCX mice fell into two categories of mice with roughly equal numbers, those in which RT was elevated and those in which RT was decreased. These two categories of changes were equivalent in the SHAM and CSCX mice. The changes in Rpef upon return to room-air also fell into two categories, roughly of equal numbers of mice, those in which Rpef was elevated and those in which Rpef was decreased. The values for those in which Rpef rose were significantly smaller in the CSCX mice than the SHAM mice (Table 2).
FIGURE 9.
(Left) EF50, Rpef and Relaxation Time values before, during and after a 5 min hypoxic (HX, 10% O2, 90% N2) gas challenge in sham-operated (SHAM) mice and in mice with bilateral transection of the cervical sympathetic chain (CSCX). (Middle) Responses (expressed as % of pre-values) during the first minute of HX challenge in SHAM and CSCX mice. (Right) Total responses (sum of all % changes from pre) during the first 105 s, between 106–300 s and 0–300 s. Data are expressed as mean ± SEM. *P < 0.05, significant response. †P < 0.05, CSCX versus SHAM. There were 11 mice in the SHAM group and 12 mice in the CSCX group.
Hypoxic Challenges – Rejection Index, Rejection Index/Frequency of Breathing, Apneic Pauses
The changes in RI, RI/Freq and Apneic Pause values in the SHAM and CSCX mice in response to the 5 min HXC and upon return to room-air are summarized in Figure 10. The HXC elicited immediate, but short-lived increases in RI and RI/Freq values that were not accompanied by increases in the numbers of apneic pauses, which on the contrary, showed relatively transient decreases in the earlier stage of the HXC (left panels). The increases in RI and RI/Freq occurred more rapidly in the CSCX mice, but the overall changes were similar in the SHAM and CSCX mice (top and middle rows: middle and right panels). The changes in apneic pauses during the HXC were similar in the SHAM and CSCX mice (bottom row). The return to room-air was associated with rapid and substantial increases in RI, RI/Freq and apneic pauses (left panel) that were similar in magnitude in the SHAM and CSCX mice (Table 2).
Discussion
Due to the multiplicity and often opposing effects of sympathetic nerves/catecholamines in the carotid bodies (see Introduction), the question as to how the absence of functional sympathetic input to the carotid bodies and other structures controlling ventilatory processes would affect baseline parameters and the responses to HXC was not easy to predict. The major finding that many of the ventilatory responses (e.g., increases Freq, PEF and expiratory drive) during HXC occurred faster in CSCX mice suggests that activation of SCG sympathetic input to the carotid bodies of these mice normally blunts the initiation of these responses. Accordingly, it appears that loss of sympathetic input/catecholamine-induced suppression of carotid body activity (Kou et al., 1991; Pizarro et al., 1992; Prabhakar et al., 1993; Almaraz et al., 1997; Overholt and Prabhakar, 1999) out ways the loss of mechanisms which promote hypoxic responses including vasoconstriction in arterioles associated with glomus cells (Llados and Zapata, 1978; Eldridge and Gill-Kumar, 1980; Majcherczyk et al., 1980; Lahiri et al., 1981; Matsumoto et al., 1981; Gonsalves et al., 1984; Potter and McCloskey, 1987; Yokoyama et al., 2015). Deeper mechanistic insights would certainly come from studies designed to evaluate blood flow responses in the mouse carotid body and cerebral circulation in SHAM and CSCX mice during HXC and how systemic hemodynamic responses in these mice may influence the expression of the ventilatory responses.
Ventilatory Responses During Hypoxic Gas Challenge
This study demonstrates that HXC in adult male C57BL6 mice elicits a complex array of ventilatory responses over and above what have been reported previously. In response to the HXC, the SHAM C57BL6 mice displayed an increase in Freq that was subject to roll-off and which was accompanied with decreases in Ti, Te, EEP, Rpef and RT that were also subject to roll-off. In contrast, the decrease in EIP was sustained throughout HXC. HXC was also associated with robust and sustained increases in TV, MV, PIF, PEF, EF50, and inspiratory and expiratory drives. The question arises as to why some ventilatory parameters in C57BL6 mice are subject to roll-off whereas others are not. First, it should be noted that the C57BL6 mouse is widely considered to be a “normal” healthy model to study the physiology of cardiorespiratory systems, and is used to generate genetically-engineered mice to study the mechanisms involved in cardiorespiratory-thermoregulatory control processes, including responses to HXCs (Tankersley et al., 2002; Campen et al., 2004, 2005; Palmer et al., 2013a,b; Tewari et al., 2013; Gaston et al., 2014). Despite considerable normal physiology, the C57BL6 mouse is of major interest to sleep-apnea researchers because it displays disordered breathing (irregular breathing patterns, including apneas and sighs) and cardiovascular disturbances during sleep and wakefulness, and shows disordered breathing upon return to room-air after exposure to HXC (Han and Strohl, 2000; Han et al., 2001, 2002; Tagaito et al., 2001; Yamauchi et al., 2008a,b,c, 2010; Getsy et al., 2014). The genetic (Tankersley et al., 1994, 2000, 2002; Tankersley, 2001, 2003; Han et al., 2001, 2002; Tagaito et al., 2001; Yamauchi et al., 2008b) and neurochemical processes (Tankersley et al., 2002; Price et al., 2003; Groeben et al., 2005; Yamauchi et al., 2008a,c, 2010; Moore et al., 2012, 2014), underlying the breathing patterns of C57BL6 mice, and the responses to HXC are well studied. The potential role of structural differences in the carotid bodies has also been studied (Yamaguchi et al., 2003, 2006; Chai et al., 2011). Nonetheless, evidence that the breathing patterns of C57BL6 mice and their responses to HXC have a strong genetic component has not posed an explanation as to why some ventilatory components, such as ventilatory timing (e.g., Freq and EEP) and mechanics (e.g., Rpef and RT) are subject to roll-off whereas other timing (e.g., EIP) and mechanics (e.g., PIF, PEF, and EF50) are not. Regardless of the explanation, understanding the importance of each of these ventilatory responses to HXC will help us better understand the processes by which breathing disorders occur in disease states and point to therapeutic strategies.
Resting ventilatory parameters (19 directly recorded or calculated variables) were similar in the SHAM and CSCX mice. This would suggest that (presumed) loss of input from the SCG to structures controlling breathing, such as the carotid bodies, upper airway and brainstem structures (see Introduction), do not obviously effect ventilatory timing or mechanics or the quality of breathing (e.g., occurrence of non-eupneic breathing events, such as apneic pauses) in C57BL6 mice. Again, the caveat is that these studies were performed only 4 days after CSCX, and it would seem possible that changes in baseline ventilatory performance would occur at longer post-CSCX time-points. Nevertheless, there were numerous important differences in the responses of CSCX and SHAM mice to the HXC. For example, the increases in Freq (and associated decreases in Ti, Te, EEP, and RT) and the increases in MV, inspiratory and expiratory drives, PEF and EF50 occurred more quickly in CSCX mice than in SHAM mice. Although both PIF and PEF increased during HXC in the CSCX mice, the PIF/PEF ratio fell because the rise in PEF exceeded that of the rise in PIF, and the PIF/PEF ratio fell more dramatically, and to a greater extent, in the CSCX mice because of the exaggerated rise in PEF in the CSCX mice. The overall (total) responses in the SHAM and CSCX mice to the HXC were similar to one another with some important exceptions. Specifically, the overall increases in TV during the latter half of the hypoxic challenge appeared greater in the CSCX mice, and as mentioned above, the PIF/PEF ratio fell and to a greater extent in the CSCX mice. Moreover, the total decreases in Ti/Te and respiratory quotient [Ti/(Ti + Te)] observed in the SHAM mice were absent in CSCX mice. These findings clearly demonstrate that CSC-SCG input to respiratory control structures influence the ventilatory responses to HXC in C57BL6 mice.
Ventilatory Responses Upon Return to Room-Air
Following exposure to HXC, the return to room-air resulted in respiratory patterns that can be classified as short-term potentiation, in which ventilation remains elevated (Powell et al., 1998; Getsy et al., 2014) or post-hypoxic frequency decline, in which breathing frequency falls below baseline (Dick and Coles, 2000). Our C57BL6 mice displayed robust short-term potentiation upon return to room-air, accompanied by a substantial prolonged phase of disordered breathing (e.g., elevated rejection index). The mechanisms responsible for post-HXC disordered breathing have received considerable investigation, and at present, evidence is in favor of disturbances in central signaling (Wilkinson et al., 1997; Strohl, 2003) including, the pons area of the brainstem (Coles and Dick, 1996; Dick and Coles, 2000) rather than processes within the carotid bodies (Vizek et al., 1987; Brown et al., 1993), even though it is evident that carotid body chemoafferents play a vital role in the expression of disordered breathing such as, sleep apnea (Smith et al., 2003). The post-HXC responses were similar in our SHAM and CSCX mice, suggesting that diminished input to the SCG and (presumably decreased activity of SCG cells) do not have an obvious impact on the post-HXC responses, including the disordered breathing. The one exception was that total Rpef responses (positive rather than negative responders) (Table 2) after return to room-air were smaller in the CSCX mice than in the SHAM mice. This suggests that CSC-SCG activity is normally a positive factor in achieving maximal Rpef upon recovery from a HXC challenge in C57BL6 mice. The major differences with respect to the initial (i.e., first 60 sec of the HXC) changes in Freq, MV, EEP, rejection index (Rinx), and Rinx/Freq did occur during the first 15 s although other differences between the SHAM and CSCX groups occurred at 30 s for relaxation time and inspiratory drive; 45 s for Rpef; 15 and 30 s for Ti and Te; 30, 45, and 60 s for PEF, PEF/PIF and EF50; and 15, 30, 45, and 60 s for expiratory drive.
Study Limitations
A limitation of this study is that it only provides a snap-shot of the temporal changes in ventilatory responses to HXC after bilateral CSCX. Therefore, it is imperative to study what the patterns of ventilatory responses to HXC would be at longer time-points post-CSCX. We chose to test the mice 4 days after CSCX surgery in order to compare with our findings related to the effects of bilateral removal of the SCG on HXC (see Conclusion). The 4-day recovery period was chosen for our other study as it would be the earliest time in which SCGX would result in virtually complete loss of sympathetic terminals within the carotid bodies (Mir et al., 1982; González-Guerrero et al., 1993; Ichikawa and Helke, 1993; Ichikawa, 2002). We have yet to establish whether this loss is true in mice, and also whether there is significant loss of sympathetic terminals in other structures, such as the brainstem. This presence/absence of intact terminals is vital since it is established that hypoxia elicits action potential/extracellular Ca2+-independent release of catecholamines from sympathetic nerve terminals (Schömig et al., 1987; Chahine et al., 1994; Kurz et al., 1995, 1996; Du et al., 1997). This evidence is especially relevant to our study since CSCX would presumably diminish activity of post-ganglionic neurons within the SCG without causing the degeneration of these post-ganglionic nerve terminals. Moreover, substantial data has shown that spinal cord damage, which markedly reduces pre-ganglionic outflow, does not necessarily eliminate all post-ganglionic sympathetic nerve activity (Meckler and Weaver, 1985; Qu et al., 1988; Stein and Weaver, 1988; McLachlan, 2007). Nonetheless, to our knowledge this has not been studied for T1–T4 spinal cord-CSC-SCG pathways. It would seem reasonable to suggest that since we transected the left and right CSC and therefore the pre-ganglionic fibers in these nerves, we could expect that (1) the activity of a post-ganglionic fibers emanating from the SCG including those to the carotid bodies would be markedly if not totally diminished, and (2) HXC would not be able to elicit centrally-mediated changes in SCG neuronal activity. However, it certainly remains possible that HXC directly activates post-ganglionic neurons denervated of pre-ganglionic input and/or more likely, direct release of neurotransmitters from sympathetic nerve terminals themselves as demonstrated in the heart and saphenous veins (Dart and Riemersma, 1991; Kamath et al., 1993; Chahine et al., 1994; Santos et al., 1996), if vesicular release mechanisms are intact.
Conclusion
Our data show that under baseline (normoxic) environmental conditions, the potential loss of active sympathetic input to the carotid bodies (via CSCX-induced quiescence of post-ganglionic projections to the carotid body) does not have a noticeable effect on ventilatory parameters, which suggests that resting activity (e.g., neurotransmitter release) of carotid body glomus cells is not altered in a way that would lead to activation of carotid body chemoafferents and therefore enhancement of breathing. In contrast, our data shows that CSCX does alter the initial hypoxic ventilatory response and therefore suggests that diminished SCG input to the carotid bodies (or other targets, such as those in the brainstem) does influence the ability of glomus cells and/or other neuronal structures to respond to HXC. Planned studies involving bilateral transection of ganglioglomerular nerves (that project only to the carotid bodies and carotid sinus) will help to establish the relevant neuronal pathways/mechanisms. Overall, this novel data suggest that the CSC may normally provide inhibitory input to peripheral (e.g., carotid bodies) and central (e.g., brainstem) structures that are involved in the ventilatory responses to HXC in C57BL6 mice. Moreover, the results of our CSCX study lend support to the concept that a loss of CSC-SCG activity may be involved in the etiology of ventilatory disorders, such as sleep-disordered breathing. With respect to mechanistic insights provided by our data, it would be reasonable to assume that post-ganglionic sympathetic nerves innervating the ipsilateral carotid bodies (and other targets such as those within the brain) would be quiescent following CSCX as a result of the loss of pre-ganglionic input to the SCG. Whether the presumed diminution of sympathetic activity alters the expression of functional proteins (i.e., tyrosine hydroxylase, catecholamine-containing vesicles, and fusion proteins mediating vesicular exocytosis) within the sympathetic nerve terminals themselves and/or the target tissues, such as glomus cells in the carotid bodies needs to be addressed in future experiments. This is especially important since these post-transection adaptations in protein expression, while not being noticeable at rest (i.e., under normoxia) may have a direct effect on the ability of glomus cells (for example) to respond to the hypoxic challenge and/or secrete neurotransmitters. We have data that demonstrates that removal of the SCG has dramatically augmented effects on HXC compared to CSCX (unpublished observations). This raises important questions as to whether HXC may directly alter the activity of SCG neurons independently of the CSC input. One key question pertains to which of the SCG projections normally driven by the CSC are responsible for mediating the neuromodulatory effects of the CSC on the processes that drive ventilatory responses to HXC. As detailed in the Introduction section, the direct projections of the SCG to structures that control ventilation are extensive and include projections to the carotid bodies via the GGN. In order to better determine which of the post-ganglionic projections of the SCG regulate the ventilatory responses to HXC, we are currently planning to perform studies in mice in which the major post-ganglionic branches of the SCG, namely the internal carotid nerves, external carotid nerves and GGN are transected. We intend to perform these studies 4, 14, and 30 days post-transection in C57BL6 mice and in other strains, such as Swiss-Webster and A/J mice (Getsy et al., 2014) to determine temporal and genetic aspects of the role of the CSC-SCG complex in the control of ventilation and the responses to HXC.
The data in this study demonstrates that the primary effect of CSCX appears to be changes in the immediate responsiveness to the HXC. For example, the increases in Freq (and associated decreases in Ti, Te, and EEP), MV, expiratory drive, and rejection index occurred more rapidly in CSCX mice than SHAM mice (changes at 15 sec were significant in CSCX mice but not SHAM mice and between-group differences for expiratory drive were maintained at 15, 30, 45, and 60 s). In contrast, between-group differences in the responses of relaxation time, Rpef, PIF, PEF, PEF/PIF, EF50, and inspiratory drive, were evident at 30, 45, or 60 s with between-group differences for PEF, PEF/PIF, and EF50 being evident at 30, 45, and 60 s. Additionally, it is important to remember for the interpretation of the effects of CSCX that it was evident that the initial increases in TV and PIF during HXC were similar in SHAM and CSCX mice. Taken together, it is apparent that the loss of post-ganglionic SCG input to structures, such as the carotid body and brainstem, has a strong impact on ventilatory performance in C57BL6 mice. The findings that the decreases in Ti and Te were larger in the CSCX mice than the SHAM mice suggests that SCG input to neural structures regulating ventilatory timing events equally affect inspiratory and expiratory control processes. However, with respect to flow parameters it was evident that (a) the increases in TV were similar in SHAM and CSCX mice, (b) the responses of PIF and inspiratory drive in CSCX mice were minimally different from SHAM mice, whereas the changes in PEF, EF50, PEF/PIF and expiratory drive were substantially different between the groups. As such, CSCX appears to have much more of an influence on PEF than PIF. Whether this pattern of effects is due primarily to altered carotid body function must await more definitive studies in which, for example, the effects of bilateral GGN transection are investigated.
The data in the present manuscript and in that of our recent study which investigated the effects of bilateral SCGX (unpublished observations) provides the beginning of understanding how the loss of pre-ganglionic and/or post-ganglionic fibers in the CSC-SCG complex affect resting ventilatory parameters and the responses to HXC. On-going studies will extend our investigations by (1) determining how expression of proteins in sympathetic nerve terminals (e.g., tyrosine hydroxylase, fusion proteins, and vesicular stores of norepinephrine) and carotid body glomus cells (e.g., tyrosine hydroxylase, voltage-gated Na+, K+, and Ca2+-channels) change after CSCX, and (2) differentiating the effects of sympathetic input to various areas by performing ventilatory studies in mice with (a) transection of the left and right internal carotid nerves (a major post-ganglionic SCG trunk), (b) transection of the left and right external carotid nerves (the other major post-ganglionic SCG trunk), and (c) transection of the left and right ganglioglomerular nerves (a branch of the external carotid nerve), which projects only to the carotid bodies and carotid sinus.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Case Western Reserve University Institution’s Animal Care and Use Committee.
Author Contributions
PG, GC, Y-HH, and SL conceived and designed the study. PG performed the mouse surgeries. PG and GC performed the plethysmography studies. PG and SL analyzed the data and prepared the figures. All authors contributed to writing the manuscript, and revised, read, and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Dr. James N. Bates (Department of Anesthesia, University of Iowa) for his critical comments about the manuscript and helping with the clinical perspectives of the study.
Abbreviations
- CSC
cervical sympathetic chain
- CSCX
cervical sympathetic chain transection
- SCG
superior cervical ganglion
- SCGX
superior cervical ganglionectomy
- HXC
hypoxic gas challenge
- Freq
frequency of breathing
- TV
tidal volume
- MV
minute ventilation
- Ti
inspiratory time
- Te
expiratory time
- EIP
end inspiratory pause
- EEP
end expiratory pause
- PIF
peak inspiratory flow
- PEF
peak expiratory flow
- RT
relaxation time
- EF50
expiratory flow at 50% expired total volume
- Rpef
rate of achieving peak expiratory flow.
Funding. This study was funded by an NIH-SPARC award to SL (10T20D023860; Functional Mapping of the afferent and Efferent Projections of the Superior Cervical Ganglion Interactome).
References
- Alberola-Die A., Reboreda A., Lamas J. A., Morales A. (2013). Lidocaine effects on acetylcholine-elicited currents from mouse superior cervical ganglion neurons. Neurosci. Res. 75 198–203. 10.1016/j.neures.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Almaraz L., Pérez-García M. T., Gómez-Nino A., González C. (1997). Mechanisms of alpha2-adrenoceptor-mediated inhibition in rabbit carotid body. Am. J. Physiol. 272 C628–C637. 10.1152/ajpcell.1997.272.2.C628 [DOI] [PubMed] [Google Scholar]
- Asamoto K. (2004). Neural circuit of the cervical sympathetic nervous system with special reference to input and output of the cervical sympathetic ganglia: relationship between spinal cord and cervical sympathetic ganglia and that between cervical sympathetic ganglia and their target organs. Kaibogaku Zasshi. 79 5–14. [PubMed] [Google Scholar]
- Banks B. E., Walter S. J. (1975). Proceedings: the effects of axotomy and Nerve Growth Factor on the neuronal population of the superior cervical ganglion of the mouse. J. Physiol. 249 61–62. [PubMed] [Google Scholar]
- Bascom A. T., Sankari A., Badr M. S. (2016). Spinal cord injury is associated with enhanced peripheral chemoreflex sensitivity. Physiol. Rep. 4:e12948. 10.14814/phy2.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlowitz D. J., Wadsworth B., Ross J. (2016). Respiratory problems and management in people with spinal cord injury. Breathe (Sheff). 12 328–340. 10.1183/20734735.012616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini A., Wolf A., Brockmeier U., Riffkin H., Metzen E., Acker-Palmer A., et al. (2020). Carotid body type I cells engage flavoprotein and Pin1 for oxygen sensing. Am. J. Physiol. Cell Physiol. 318 C719–C731. 10.1152/ajpcell.00320.2019 [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. (1967). Observations on carotid body chemoreceptor activity and cervical sympathetic discharge in the cat. J. Physiol. 190 413–424. 10.1113/jphysiol.1967.sp008218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard G., Warner M., Pizarro J., Niu W., Mitchell G. (1993). Noradrenergic inhibition of the goat carotid body. Adv. Exp. Med. Biol. 337 259–263. 10.1007/978-1-4615-2966-8_36 [DOI] [PubMed] [Google Scholar]
- Bisgard G. E., Mitchell R. A., Herbert D. A. (1979). Effects of dopamine, norepinephrine and 5-hydroxytryptamine on the carotid body of the dog. Respir. Physiol. 37 61–80. 10.1016/0034-5687(79)90092-6 [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. (1972). The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J. Physiol. 221 149–159. 10.1113/jphysiol.1972.sp009745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolter C. P., Ledsome J. R. (1976). Effect of cervical sympathetic nerve stimulation on canine carotid sinus reflex. Am. J. Physiol. 230 1026–1030. 10.1152/ajplegacy.1976.230.4.1026 [DOI] [PubMed] [Google Scholar]
- Bowers C. W., Zigmond R. E. (1979). Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J. Comp. Neurol. 185 381–391. 10.1002/cne.901850211 [DOI] [PubMed] [Google Scholar]
- Brokaw J. J., Hansen J. T. (1987). Evidence that dopamine regulates norepinephrine synthesis in the rat superior cervical ganglion during hypoxic stress. J. Auton. Nerv. Syst. 18 185–193. 10.1016/0165-1838(87)90117-2 [DOI] [PubMed] [Google Scholar]
- Brown D. R., Forster H. V., Greene A. S., Lowry T. F. (1993). Breathing periodicity in intact and carotid body-denervated ponies during normoxia and chronic hypoxia. J. Appl. Physiol. (1985). 74 1073–1082. 10.1152/jappl.1993.74.3.1073 [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Turner P. J. (2013). Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol. 591 3549–3563. 10.1113/jphysiol.2013.257741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller K. M., Bolter C. P. (1993). The localization of sympathetic and vagal neurones innervating the carotid sinus in the rabbit. J. Auton. Nerv. Syst. 44 225–231. 10.1016/0165-1838(93)90035-s [DOI] [PubMed] [Google Scholar]
- Buller K. M., Bolter C. P. (1997). Carotid bifurcation pressure modulation of spontaneous activity in external and internal carotid nerves can occur in the superior cervical ganglion. J. Auton. Nerv. Syst. 67 24–30. 10.1016/s0165-1838(97)00088-x [DOI] [PubMed] [Google Scholar]
- Cadaveira-Mosquera A., Pérez M., Reboreda A., Rivas-Ramírez P., Fernández-Fernández D., Lamas J. A. (2012). Expression of K2P channels in sensory and motor neurons of the autonomic nervous system. J. Mol. Neurosci. 48 86–96. 10.1007/s12031-012-9780-y [DOI] [PubMed] [Google Scholar]
- Campen M. J., Tagaito Y., Jenkins T. P., Balbir A., O’Donnell C. P. (2005). Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J. Appl. Physiol. (1985) 99 807–813. 10.1152/japplphysiol.00039.2005 [DOI] [PubMed] [Google Scholar]
- Campen M. J., Tagaito Y., Li J., Balbir A., Tankersley C. G., Smith P., et al. (2004). Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol. Genomics 20 15–20. 10.1152/physiolgenomics.00197.2003 [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Pisarev M. A., Barontini M., Juvenal G. J., Boado R. J., Vacas M. I. (1982). Efferent neuroendocrine pathways of sympathetic superior cervical ganglia. Early depression of the pituitary-thyroid axis after ganglionectomy. Neuroendocrinology 35 248–254. 10.1159/000123390 [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Vacas M. I., Gejman P. V. (1981a). The sympathetic superior cervical ganglia as peripheral neuroendocrine centers. J. Neural. Transm. 52 1–21. 10.1007/BF01253092 [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Vacas M. I., Luchelli de Fortis A., Stefano F. J. (1981b). Superior cervical ganglionectomy depresses norepinephrine uptake, increases the density of alpha-adrenoceptor sites, and induces supersensitivity to adrenergic drugs in rat medial basal hypothalamus. Neuroendocrinology 33 199–206. 10.1159/000123229 [DOI] [PubMed] [Google Scholar]
- Chahine R., Nadeau R., Lamontagne D., Yamaguchi N., de Champlain J. (1994). Norepinephrine and dihydroxyphenylglycol effluxes from sympathetic nerve endings during hypoxia and reoxygenation in the isolated rat heart. Can. J. Physiol. Pharmacol. 72 595–601. 10.1139/y94-085 [DOI] [PubMed] [Google Scholar]
- Chai S., Gillombardo C. B., Donovan L., Strohl K. P. (2011). Morphological differences of the carotid body among C57/BL6 (B6), A/J, and CSS B6A1 mouse strains. Respir. Physiol. Neurobiol. 177 265–272. 10.1016/j.resp.2011.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles S. K., Dick T. E. (1996). Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J. Physiol. 497 79–94. 10.1113/jphysiol.1996.sp021751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins H. L., Rodenbaugh D. W., DiCarlo S. E. (2006). Spinal cord injury alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. Prog. Brain Res. 152 275–288. 10.1016/S0079-6123(05)52018-1 [DOI] [PubMed] [Google Scholar]
- Dart A. M., Riemersma R. A. (1991). Noradrenaline release from the rat heart during anoxia: effects of changes in extracellular sodium concentration and inhibition of sodium uptake mechanisms. Clin. Exp. Pharmacol. Physiol. 18 43–46. 10.1111/j.1440-1681.1991.tb01375.x [DOI] [PubMed] [Google Scholar]
- David R., Ciuraszkiewicz A., Simeone X., Orr-Urtreger A., Papke R. L., McIntosh J. M., et al. (2010). Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur. J. Neurosci. 31 978–993. 10.1111/j.1460-9568.2010.07133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick T. E., Coles S. K. (2000). Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir. Physiol. 121 87–100. 10.1016/s0034-5687(00)00121-3 [DOI] [PubMed] [Google Scholar]
- DiMarco A. F., Kowalski K. E., Geertman R. T., Hromyak D. R., Frost F. S., Creasey G. H., et al. (2009). Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-Sponsored clinical trial. Part II: clinical outcomes. Arch. Phys. Med. Rehabil. 90 726–732. 10.1016/j.apmr.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinger B., Wang Z. Z., Chen J., Wang W. J., Hanson G., Stensaas L. J., et al. (1993). Immunocytochemical and neurochemical aspects of sympathetic ganglion chemosensitivity. Adv. Exp. Med. Biol. 337 25–30. 10.1007/978-1-4615-2966-8_4 [DOI] [PubMed] [Google Scholar]
- Du X. J., Bobik A., Esler M. D., Dart A. M. (1997). Effects of intracellular Ca2+ chelating on noradrenaline release in normoxic and anoxic hearts. Clin. Exp. Pharmacol. Physiol. 24 819–823. 10.1111/j.1440-1681.1997.tb02697.x [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P. (1980). Mechanisms of hyperpnea induced by isoproterenol. Respir. Physiol. 40 349–363. 10.1016/0034-5687(80)90034-1 [DOI] [PubMed] [Google Scholar]
- El-Fadaly A. B., Kummer W. (2003). The spatial relationship between type I glomus cells and arteriolar myocytes in the mouse carotid body. Ann. Anat. 185 507–515. 10.1016/S0940-9602(03)80114-0 [DOI] [PubMed] [Google Scholar]
- Esquifino A. I., Alvarez M. P., Cano P., Jiménez V., Duvilanski B. (2004). Superior cervical ganglionectomy differentially modifies median eminence and anterior and mediobasal hypothalamic GABA content in male rats: effects of hyperprolactinemia. Exp. Brain Res. 157 296–302. 10.1007/s00221-004-1843-z [DOI] [PubMed] [Google Scholar]
- Felder R. B., Heesch C. M., Thames M. D. (1983). Reflex modulation of carotid sinus baroreceptor activity in the dog. Am. J. Physiol. 244 H437–H443. 10.1152/ajpheart.1983.244.3.H437 [DOI] [PubMed] [Google Scholar]
- Feldman-Goriachnik R., Hanani M. (2019). The effects of sympathetic nerve damage on satellite glial cells in the mouse superior cervical ganglion. Auton. Neurosci. 221:102584. 10.1016/j.autneu.2019.102584 [DOI] [PubMed] [Google Scholar]
- Flett D. L., Bell C. (1991). Topography of functional subpopulations of neurons in the superior cervical ganglion of the rat. J. Anat. 177 55–66. [PMC free article] [PubMed] [Google Scholar]
- Floyd W. F., Neil E. (1952). The influence of the sympathetic innervation of the carotid bifurcation on chemoceptor and baroceptor activity in the cat. Arch. Int. Pharmacodyn. Ther. 91 230–239. [PubMed] [Google Scholar]
- Folgering H., Ponte J., Sadig T. (1982). Adrenergic mechanisms and chemoreception in the carotid body of the cat and rabbit. J. Physiol. 325 1–21. 10.1113/jphysiol.1982.sp014131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forehand C. J. (1985). Density of somatic innervation on mammalian autonomic ganglion cells is inversely related to dendritic complexity and preganglionic convergence. J. Neurosci. 5 3403–3408. 10.1523/JNEUROSCI.05-12-03403.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo E., Chiocchio S. R., Tramezzani J. H. (1984). Sympathetic innervation of the median eminence. Brain Res. 290 333–335. 10.1016/0006-8993(84)90951-x [DOI] [PubMed] [Google Scholar]
- Gao L., Bonilla-Henao V., García-Flores P., Arias-Mayenco I., Ortega-Sáenz P., López-Barneo J. (2017). Gene expression analyses reveal metabolic specifications in acute O2-sensing chemoreceptor cells. J. Physiol. 595 6091–6120. 10.1113/JP274684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Ortega-Sáenz P., López-Barneo J. (2019). Acute oxygen sensing-Role of metabolic specifications in peripheral chemoreceptor cells. Respir. Physiol. Neurobiol. 265 100–111. 10.1016/j.resp.2018.08.007 [DOI] [PubMed] [Google Scholar]
- García J. B., Romeo H. E., Basabe J. C., Cardinali D. P. (1988). Effect of superior cervical ganglionectomy on insulin release by murine pancreas slices. J. Auton. Nerv. Syst. 22 159–165. 10.1016/0165-1838(88)90089-6 [DOI] [PubMed] [Google Scholar]
- Gaston B., May W. J., Sullivan S., Yemen S., Marozkina N. V., Palmer L. A., et al. (2014). Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice. J. Appl. Physiol. (1985). 116 1290–1299. 10.1152/japplphysiol.01050.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy P. M., Davis J., Coffee G. A., May W. J., Palmer L. A., Strohl K. P., et al. (2014). Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice. Respir. Physiol. Neurobiol. 204 147–159. 10.1016/j.resp.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins I. L. (1991). Vasomotor, pilomotor and secretomotor neurons distinguished by size and neuropeptide content in superior cervical ganglia of mice. J. Auton. Nerv. Syst. 34 171–183. 10.1016/0165-1838(91)90083-f [DOI] [PubMed] [Google Scholar]
- Gonsalves S. F., Smith E. J., Nolan W. F., Dutton R. E. (1984). beta-Adrenoceptor blockade spares chemoreceptor responsiveness to hypoxia. Brain Res. 324 349–353. 10.1016/0006-8993(84)90047-7 [DOI] [PubMed] [Google Scholar]
- González-Guerrero P. R., Rigual R., González C. (1993). Opioid peptides in the rabbit carotid body: identification and evidence for co-utilization and interactions with dopamine. J. Neurochem. 60 1762–1768. 10.1111/j.1471-4159.1993.tb13401.x [DOI] [PubMed] [Google Scholar]
- Groeben H., Meier S., Tankersley C. G., Mitzner W., Brown R. H. (2005). Heritable and pharmacological influences on pauses and apneas in inbred mice during anesthesia and emergence. Exp. Lung Res. 31 839–853. 10.1080/01902140600586458 [DOI] [PubMed] [Google Scholar]
- Hachmann J. T., Grahn P. J., Calvert J. S., Drubach D. I., Lee K. H., Lavrov I. A. (2017). Electrical neuromodulation of the respiratory system after spinal cord injury. Mayo Clin. Proc. 92 1401–1414. 10.1016/j.mayocp.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Han F., Strohl K. P. (2000). Inheritance of ventilatory behavior in rodent models. Respir. Physiol. 121 247–256. 10.1016/s0034-5687(00)00132-8 [DOI] [PubMed] [Google Scholar]
- Han F., Subramanian S., Dick T. E., Dreshaj I. A., Strohl K. P. (2001). Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J. Appl. Physiol. (1985) 91 1962–1970. 10.1152/jappl.2001.91.5.1962 [DOI] [PubMed] [Google Scholar]
- Han F., Subramanian S., Price E. R., Nadeau J., Strohl K. P. (2002). Periodic breathing in the mouse. J. Appl. Physiol. (1985) 92 1133–1140. 10.1152/japplphysiol.00785.2001 [DOI] [PubMed] [Google Scholar]
- Hanson G., Jones L., Fidone S. (1986). Effects of hypoxia on neuropeptide levels in the rabbit superior cervical ganglion. J. Neurobiol. 17 51–54. 10.1002/neu.480170106 [DOI] [PubMed] [Google Scholar]
- He L., Chen J., Dinger B., Fidone S. (2000). Characteristics of carotid body chemosensitivity in the mouse. Baseline studies for future experiments with knockout animals. Adv. Exp. Med. Biol. 475 697–704. 10.1007/0-306-46825-5_69 [DOI] [PubMed] [Google Scholar]
- He L., Chen J., Dinger B., Sanders K., Sundar K., Hoidal J., et al. (2002). Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am. J. Physiol. Cell Physiol. 282 C27–C33. 10.1152/ajpcell.2002.282.1.C27 [DOI] [PubMed] [Google Scholar]
- He L., Chen J., Dinger B., Sanders K., Sundar K., Hoidal J., et al. (2003). Carotid body chemoreceptor activity in mice deficient in selected subunits of NADPH oxidase. Adv. Exp. Med. Biol. 536 41–46. 10.1007/978-1-4419-9280-2_5 [DOI] [PubMed] [Google Scholar]
- Heinert G., Paterson D. J., Bisgard G. E., Xia N., Painter R., Nye P. C. (1995). The excitation of carotid body chemoreceptors of the cat by potassium and noradrenaline. Adv. Exp. Med. Biol. 393 323–330. 10.1007/978-1-4615-1933-1_61 [DOI] [PubMed] [Google Scholar]
- Heutink M., Post M. W., Luthart P., Schuitemaker M., Slangen S., Sweers J., et al. (2014). Long-term outcomes of a multidisciplinary cognitive behavioural programme for coping with chronic neuropathic spinal cord injury pain. J. Rehabil. Med. 46 540–545. 10.2340/16501977-1798 [DOI] [PubMed] [Google Scholar]
- Hisa Y., Koike S., Tadaki N., Bamba H., Shogaki K., Uno T. (1999). Neurotransmitters and neuromodulators involved in laryngeal innervation. Ann. Otol. Rhinol. Laryngol. Suppl. 178 3–14. 10.1177/00034894991080s702 [DOI] [PubMed] [Google Scholar]
- Hughes-Davis E. J., Cogen J. P., Jakowec M. W., Cheng H. W., Grenningloh G., Meshul C. K., et al. (2005). Differential regulation of the growth-associated proteins GAP-43 and superior cervical ganglion 10 in response to lesions of the cortex and substantia nigra in the adult rat. Neuroscience 135 1231–1239. 10.1016/j.neuroscience.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Ichikawa H. (2002). Innervation of the carotid body: immunohistochemical, denervation, and retrograde tracing studies. Microsc. Res. Tech. 59 188–195. 10.1002/jemt.10193 [DOI] [PubMed] [Google Scholar]
- Ichikawa H., Helke C. J. (1993). Distribution, origin and plasticity of galanin-immunoreactivity in the rat carotid body. Neuroscience 52 757–767. 10.1016/0306-4522(93)90424-e [DOI] [PubMed] [Google Scholar]
- Inoue K. (1975). Electron microscopic analysis of the cervical vagus nerve in mice; its fiber constituent and relationship with the superior cervical ganglion. Kaibogaku Zasshi 50 1–14. [PubMed] [Google Scholar]
- Ivanusic J. J., Wood R. J., Brock J. A. (2013). Sensory and sympathetic innervation of the mouse and guinea pig corneal epithelium. J. Comp. Neurol. 521 877–893. 10.1002/cne.23207 [DOI] [PubMed] [Google Scholar]
- Jobling P., Gibbins I. L. (1999). Electrophysiological and morphological diversity of mouse sympathetic neurons. J. Neurophysiol. 82 2747–2764. 10.1152/jn.1999.82.5.2747 [DOI] [PubMed] [Google Scholar]
- Kåhlin J., Eriksson L. I., Ebberyd A., Fagerlund M. J. (2010). Presence of nicotinic, purinergic and dopaminergic receptors and the TASK-1 K+-channel in the mouse carotid body. Respir. Physiol. Neurobiol. 172 122–128. 10.1016/j.resp.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Kamath G. S., Rorie D. K., Tyce G. M. (1993). Altered release and metabolism of norepinephrine in superfused canine saphenous veins in the presence of halothane and hypoxia. Anesthesiology. 78 553–561. 10.1097/00000542-199303000-00019 [DOI] [PubMed] [Google Scholar]
- Karlsen A. S., Rath M. F., Rohde K., Toft T., Møller M. (2013). Developmental and diurnal expression of the synaptosomal-associated protein 25 (Snap25) in the rat pineal gland. Neurochem. Res. 38 1219–1228. 10.1007/s11064-012-0918-7 [DOI] [PubMed] [Google Scholar]
- Kasa P., Dobo E., Wolff J. R. (1991). Are there cholinergic through-fibers in the superior cervical ganglion of the mouse? Histochemistry. 96 261–263. 10.1007/BF00271545 [DOI] [PubMed] [Google Scholar]
- Kawaja M. D., Crutcher K. A. (1997). Sympathetic axons invade the brains of mice overexpressing nerve growth factor. J. Comp. Neurol. 383 60–72. [DOI] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W. (1988). Double myelination of axons in the sympathetic nervous system of the mouse. I. Ultrastructural features and distribution. J. Neurocytol. 17 245–261. 10.1007/BF01674211 [DOI] [PubMed] [Google Scholar]
- Kline D. D., Buniel M. C., Glazebrook P., Peng Y. J., Ramirez-Navarro A., Prabhakar N. R., et al. (2005). Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. J. Neurosci. 25 3389–3399. 10.1523/JNEUROSCI.4556-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D. D., Prabhakar N. R. (2000). Peripheral chemosensitivity in mutant mice deficient in nitric oxide synthase. Adv. Exp. Med. Biol. 475 571–579. 10.1007/0-306-46825-5_55 [DOI] [PubMed] [Google Scholar]
- Kou Y. R., Ernsberger P., Cragg P. A., Cherniack N. S., Prabhakar N. R. (1991). Role of alpha 2-adrenergic receptors in the carotid body response to isocapnic hypoxia. Respir. Physiol. 83 353–364. 10.1016/0034-5687(91)90054-m [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Hauser H., Liotta A., Zelenetz A. (1976). Circadian periodicity of epidermal growth factor and its abolition by superior cervical ganglionectomy. Endocrinology. 99 1589–1596. 10.1210/endo-99-6-1589 [DOI] [PubMed] [Google Scholar]
- Kummer W., Fischer A., Kurkowski R., Heym C. (1992). The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49 715–737. 10.1016/0306-4522(92)90239-x [DOI] [PubMed] [Google Scholar]
- Kurz T., Richardt G., Hagl S., Seyfarth M., Schömig A. (1995). Two different mechanisms of noradrenaline release during normoxia and simulated ischemia in human cardiac tissue. J. Mol. Cell Cardiol. 27 1161–1172. 10.1016/0022-2828(95)90052-7 [DOI] [PubMed] [Google Scholar]
- Kurz T., Richardt G., Seyfarth M., Schömig A. (1996). Nonexocytotic noradrenaline release induced by pharmacological agents or anoxia in human cardiac tissue. Naunyn Schmiedebergs Arch. Pharmacol. 354 7–16. 10.1007/BF00168700 [DOI] [PubMed] [Google Scholar]
- Lahiri S., Matsumoto S., Mokashi A. (1986). Responses of ganglioglomerular nerve activity to respiratory stimuli in the cat. J. Appl. Physiol. (1985) 60 391–397. 10.1152/jappl.1986.60.2.391 [DOI] [PubMed] [Google Scholar]
- Lahiri S., Pokorski M., Davies R. O. (1981). Augmentation of carotid body chemoreceptor responses by isoproterenol in the cat. Respir. Physiol. 44 351–364. 10.1016/0034-5687(81)90029-3 [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Burton P. R. (1977). Ultrastructural studies of the superior cervical trunk of the mouse: distribution, cytochemistry and stability of fibrous elements in preganglionic fibers. J. Comp. Neurol. 171 605–618. 10.1002/cne.901710411 [DOI] [PubMed] [Google Scholar]
- Lindborg J. A., Niemi J. P., Howarth M. A., Liu K. W., Moore C. Z., Mahajan D., et al. (2018). Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J. Neuroinflammation. 15:192. 10.1186/s12974-018-1222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little G. J., Heath J. W. (1994). Morphometric analysis of axons myelinated during adult life in the mouse superior cervical ganglion. J. Anat. 184 387–398. [PMC free article] [PubMed] [Google Scholar]
- Liu P. W., Bean B. P. (2014). Kv2 channel regulation of action potential repolarization and firing patterns in superior cervical ganglion neurons and hippocampal CA1 pyramidal neurons. J. Neurosci. 34 4991–5002. 10.1523/JNEUROSCI.1925-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llados F., Zapata P. (1978). Effects of adrenoceptor stimulating and blocking agents on carotid body chemosensory inhibition. J. Physiol. 274 501–509. 10.1113/jphysiol.1978.sp012163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith I. J., Arnolda L. F., Pilowsky P. M., Chalmers J. P., Minson J. B. (1998). GABA- and glutamate-immunoreactive synapses on sympathetic preganglionic neurons projecting to the superior cervical ganglion. J. Auton. Nerv. Syst. 71 96–110. 10.1016/s0165-1838(98)00069-1 [DOI] [PubMed] [Google Scholar]
- Lujan H. L., Chen Y., Dicarlo S. E. (2009). Paraplegia increased cardiac NGF content, sympathetic tonus, and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am. J. Physiol. Heart Circ. Physiol. 296 H1364–H1372. 10.1152/ajpheart.01286.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan H. L., DiCarlo S. E. (2007). T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am. J. Physiol. Heart Circ. Physiol. 293 H3333–H3339. 10.1152/ajpheart.01019.2007 [DOI] [PubMed] [Google Scholar]
- Lujan H. L., Janbaih H., DiCarlo S. E. (2012). Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J. Appl. Physiol. (1985) 113 1332–1341. 10.1152/japplphysiol.00522.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan H. L., Janbaih H., DiCarlo S. E. (2014). Structural remodeling of the heart and its premotor cardioinhibitory vagal neurons following T(5) spinal cord transection. J. Appl. Physiol. (1985) 116 1148–1155. 10.1152/japplphysiol.01285.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan H. L., Palani G., DiCarlo S. E. (2010). Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299 R985–R995. 10.1152/ajpregu.00329.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majcherczyk S., Coleridge J. C., Coleridge H. M., Kaufman M. P., Baker D. G. (1980). Carotid sinus nerve efferents: properties and physiological significance. Fed. Proc. 39 2662–2667. [PubMed] [Google Scholar]
- Maklad A., Quinn T., Fritzsch B. (2001). Intracranial distribution of the sympathetic system in mice: DiI tracing and immunocytochemical labeling. Anat. Rec. 263 99–111. 10.1002/ar.1083 [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna J., Soriano S., Tudurí E., Nadal A., de Castro F. (2018). A calcium-dependent chloride current increases repetitive firing in mouse sympathetic neurons. Front. Physiol. 9:508. 10.3389/fphys.2018.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew T. C. (2007). Scanning electron microscopic observations on the third ventricular floor of the rat following cervical sympathectomy. Folia Morphol. (Warsz) 66 94–99. [PubMed] [Google Scholar]
- Matsumoto S., Ibi A., Nagao T., Nakajima T. (1981). Effects of carotid body chemoreceptor stimulation by norepinephrine, epinephrine and tyramine on ventilation in the rabbit. Arch. Int. Pharmacodyn. Ther. 252 152–161. [PubMed] [Google Scholar]
- Matsumoto S., Mokashi A., Lahiri S. (1986). Influence of ganglioglomerular nerve on carotid chemoreceptor activity in the cat. J. Auton. Nerv. Syst. 15 7–20. 10.1016/0165-1838(86)90075-5 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Mokashi A., Lahiri S. (1987). Ganglioglomerular nerves respond to moderate hypoxia independent of peripheral chemoreceptors in the cat. J. Auton. Nerv. Syst. 19 219–228. 10.1016/0165-1838(87)90068-3 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Nagao T., Ibi A., Nakajima T. (1980). Effects of carotid body chemoreceptor stimulation by dopamine on ventilation. Arch. Int. Pharmacodyn. Ther. 245 145–155. 10.1007/978-3-642-66755-8_21 [DOI] [PubMed] [Google Scholar]
- McDonald D. M. (1983). Morphology of the rat carotid sinus nerve. I. Course, connections, dimensions and ultrastructure. J. Neurocytol. 12 345–372. 10.1007/BF01159380 [DOI] [PubMed] [Google Scholar]
- McDonald D. M., Mitchell R. A. (1981). The neural pathway involved in “efferent inhibition” of chemoreceptors in the cat carotid body. J. Comp. Neurol. 201 457–476. 10.1002/cne.902010310 [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. (2007). Diversity of sympathetic vasoconstrictor pathways and their plasticity after spinal cord injury. Clin. Auton. Res 17 6–12. 10.1007/s10286-006-0394-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. S., Evrard Y., Gordon B. H., Campbell D. B. (1989). Ganglioglomerular nerves influence responsiveness of cat carotid body chemoreceptors to almitrine. J. Auton. Nerv. Syst. 27 57–66. 10.1016/0165-1838(89)90129-x [DOI] [PubMed] [Google Scholar]
- Meckler R. L., Weaver L. C. (1985). Splenic, renal, and cardiac nerves have unequal dependence upon tonic supraspinal inputs. Brain Res. 338 123–135. 10.1016/0006-8993(85)90254-9 [DOI] [PubMed] [Google Scholar]
- Mills E., Smith P. G., Slotkin T. A., Breese G. (1978). Role of carotid body catecholamines in chemoreceptor function. Neuroscience. 3 1137–1146. 10.1016/0306-4522(78)90134-3 [DOI] [PubMed] [Google Scholar]
- Milsom W. K., Sadig T. (1983). Interaction between norepinephrine and hypoxia on carotid body chemoreception in rabbits. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55 1893–1898. 10.1152/jappl.1983.55.6.1893 [DOI] [PubMed] [Google Scholar]
- Mir A. K., Al-Neamy K., Pallot D. J., Nahorski S. R. (1982). Catecholamines in the carotid body of several mammalian species: effects of surgical and chemical sympathectomy. Brain Res. 252 335–342. 10.1016/0006-8993(82)90401-2 [DOI] [PubMed] [Google Scholar]
- Mitsuoka K., Miwa Y., Kikutani T., Sato I. (2018). Localization of CGRP and VEGF mRNAs in the mouse superior cervical ganglion during pre- and postnatal development. Eur. J. Histochem. 62:2976. 10.4081/ejh.2018.2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Akladious A., Hu Y., Azzam S., Feng P., Strohl K. P. (2014). Effects of orexin 2 receptor activation on apnea in the C57BL/6J mouse. Respir. Physiol. Neurobiol. 200 118–125. 10.1016/j.resp.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Chai S., Gillombardo C. B., Carlo A., Donovan L. M., Netzer N., et al. (2012). Two weeks of buspirone protects against posthypoxic ventilatory pauses in the C57BL/6J mouse strain. Respir. Physiol. Neurobiol. 183 35–40. 10.1016/j.resp.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Nunes A. R., Batuca J. R., Monteiro E. C. (2010). Acute hypoxia modifies cAMP levels induced by inhibitors of phosphodiesterase-4 in rat carotid bodies, carotid arteries and superior cervical ganglia. Br. J. Pharmacol. 159 353–361. 10.1111/j.1476-5381.2009.00534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A. R., Sample V., Xiang Y. K., Monteiro E. C., Gauda E., Zhang J. (2012). Effect of oxygen on phosphodiesterases (PDE) 3 and 4 isoforms and PKA activity in the superior cervical ganglia. Adv. Exp. Med. Biol. 758 287–294. 10.1007/978-94-007-4584-1_39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. J., Mazzone S. B., Canning B. J., Weinreich D. (2006). Reflex regulation of airway sympathetic nerves in guinea-pigs. J. Physiol. 573 549–564. 10.1113/jphysiol.2005.104661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran K. D., Curran A. K., Bradford A. (1996). The effect of sympathetic nerve stimulation on ventilation and upper airway resistance in the anaesthetized rat. Adv. Exp. Med. Biol. 410 443–447. 10.1007/978-1-4615-5891-0_68 [DOI] [PubMed] [Google Scholar]
- O’Halloran K. D., Curran A. K., Bradford A. (1998). Influence of cervical sympathetic nerves on ventilation and upper airway resistance in the rat. Eur. Respir. J. 12 177–184. 10.1183/09031936.98.12010177 [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P., Caballero C., Gao L., López-Barneo J. (2018). Testing acute oxygen sensing in genetically modified mice: plethysmography and amperometry. Methods Mol. Biol. 1742 139–153. 10.1007/978-1-4939-7665-2_13 [DOI] [PubMed] [Google Scholar]
- Overholt J. L., Prabhakar N. R. (1999). Norepinephrine inhibits a toxin resistant Ca2+ current in carotid body glomus cells: evidence for a direct G protein mechanism. J. Neurophysiol. 81 225–233. 10.1152/jn.1999.81.1.225 [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Kimberly deRonde, Brown-Steinke K., Gunter S., Jyothikumar V., Forbes M. S., et al. (2015). Hypoxia-induced changes in protein s-nitrosylation in female mouse brainstem. Am. J. Respir. Cell Mol. Biol. 52 37–45. 10.1165/rcmb.2013-0359OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., May W. J., deRonde K., Brown-Steinke K., Bates J. N., Gaston B., et al. (2013a). Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir. Physiol. Neurobiol. 185 571–581. 10.1016/j.resp.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., May W. J., deRonde K., Brown-Steinke K., Gaston B., Lewis S. J. (2013b). Hypoxia-induced ventilatory responses in conscious mice: gender differences in ventilatory roll-off and facilitation. Respir. Physiol. Neurobiol. 185 497–505. 10.1016/j.resp.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L., Miao Z. H., Dong L., Wang Y. L. (1999). [Hypoxia-induced increase in nerve activity of rabbit carotid body mediated by noradrenaline]. Sheng li xue bao [Acta physiologica Sinica] 51 407–412. [PubMed] [Google Scholar]
- Pankevich D., Baum M. J., Cherry J. A. (2003b). Removal of the superior cervical ganglia fails to block Fos induction in the accessory olfactory system of male mice after exposure to female odors. Neurosci. Lett. 345 13–16. 10.1016/s0304-3940(03)00471-3 [DOI] [PubMed] [Google Scholar]
- Pankevich D. E., Deedy E. M., Cherry J. A., Baum M. J. (2003a). Interactive effects of testosterone and superior cervical ganglionectomy on attraction thresholds to volatile urinary odors in gonadectomized mice. Behav. Brain Res. 144 157–165. 10.1016/s0166-4328(03)00073-1 [DOI] [PubMed] [Google Scholar]
- Pashai P., Kostuk E. W., Pilchard L. E., Shirahata M. (2012). ATP release from the carotid bodies of DBA/2J and A/J inbred mouse strains. Adv. Exp. Med. Biol. 758 279–285. 10.1007/978-94-007-4584-1_38 [DOI] [PubMed] [Google Scholar]
- Peng Y. J., Zhang X., Nanduri J., Prabhakar N. R. (2018). Therapeutic targeting of the carotid body for treating sleep apnea in a pre-clinical mouse model. Adv. Exp. Med. Biol. 1071 109–114. 10.1007/978-3-319-91137-3_14 [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Colinas O., Miguel-Velado E., Moreno-Domínguez A., López-López J. R. (2004). Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J. Physiol. 557 457–471. 10.1113/jphysiol.2004.062281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard L. E., Crainiceanu C. M., Pashai P., Kostuk E. W., Fujioka A., Shirahata M. (2015). Role of BK channels in murine carotid body neural responses in vivo. Adv. Exp. Med. Biol. 860 325–333. 10.1007/978-3-319-18440-1_37 [DOI] [PubMed] [Google Scholar]
- Pizarro J., Warner M. M., Ryan M., Mitchell G. S., Bisgard G. E. (1992). Intracarotid norepinephrine infusions inhibit ventilation in goats. Respir. Physiol. 90 299–310. 10.1016/0034-5687(92)90110-i [DOI] [PubMed] [Google Scholar]
- Potter E. K., McCloskey D. I. (1987). Excitation of carotid body chemoreceptors by neuropeptide-Y. Respir. Physiol. 67 357–365. 10.1016/0034-5687(87)90065-x [DOI] [PubMed] [Google Scholar]
- Powell F. L., Milsom W. K., Mitchell G. S. (1998). Time domains of the hypoxic ventilatory response. Respir. Physiol. 112 123–134. 10.1016/s0034-5687(98)00026-7 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R. (1994). Neurotransmitters in the carotid body. Adv. Exp. Med. Biol. 360 57–69. 10.1007/978-1-4615-2572-1_6 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Kou Y. R., Cragg P. A., Cherniack N. S. (1993). Effect of arterial chemoreceptor stimulation: role of norepinephrine in hypoxic chemotransmission. Adv. Exp. Med. Biol. 337 301–306. 10.1007/978-1-4615-2966-8_42 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Peng Y. J., Nanduri J. (2018). Recent advances in understanding the physiology of hypoxic sensing by the carotid body. F1000Res 7:F1000FacultyRev–1900. 10.12688/f1000research.16247.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E. R., Han F., Dick T. E., Strohl K. P. (2003). 7-nitroindazole and posthypoxic ventilatory behavior in the A/J and C57BL/6J mouse strains. J. Appl. Physiol. (1985) 95 1097–1104. 10.1152/japplphysiol.00166.2003 [DOI] [PubMed] [Google Scholar]
- Prieto-Lloret J., Donnelly D. F., Rico A. J., Moratalla R., González C., Rigual R. J. (2007). Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D(2) receptors. J. Appl. Physiol. (1985) 103 1269–1275. 10.1152/japplphysiol.00391.2007 [DOI] [PubMed] [Google Scholar]
- Qu L., Sherebrin R., Weaver L. C. (1988). Blockade of spinal pathways decreases pre- and postganglionic discharge differentially. Am. J. Physiol. 255 R946–R951. 10.1152/ajpregu.1988.255.6.R946 [DOI] [PubMed] [Google Scholar]
- Rando T. A., Bowers C. W., Zigmond R. E. (1981). Localization of neurons in the rat spinal cord which project to the superior cervical ganglion. J. Comp. Neurol. 196 73–83. 10.1002/cne.901960107 [DOI] [PubMed] [Google Scholar]
- Rees P. M. (1967). Observations on the fine structure and distribution of presumptive baroreceptor nerves at the carotid sinus. J. Comp. Neurol. 131 517–548. 10.1002/cne.901310409 [DOI] [PubMed] [Google Scholar]
- Rigual R., Cachero M. T., Rocher A., González C. (1999). Hypoxia inhibits the synthesis of phosphoinositides in the rabbit carotid body. Pflugers Arch. 437 839–845. 10.1007/s004240050853 [DOI] [PubMed] [Google Scholar]
- Rivas-Ramírez P., Reboreda A., Rueda-Ruzafa L., Herrera-Pérez S., Lamas J. A. (2020). PIP2 mediated inhibition of TREK potassium currents by bradykinin in mouse sympathetic neurons. Int. J. Mol. Sci. 21:389. 10.3390/ijms21020389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenbaugh D. W., Collins H. L., Nowacek D. G., DiCarlo S. E. (2003). Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am. J. Physiol. Heart. Circ. Physiol. 285 H2605–H2613. 10.1152/ajpheart.00319.2003 [DOI] [PubMed] [Google Scholar]
- Roux J. C., Dura E., Villard L. (2008). Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neurosci. Lett. 447 82–86. 10.1016/j.neulet.2008.09.045 [DOI] [PubMed] [Google Scholar]
- Ryan M. L., Hedrick M. S., Pizarro J., Bisgard G. E. (1995). Effects of carotid body sympathetic denervation on ventilatory acclimatization to hypoxia in the goat. Respir. Physiol. 99 215–224. 10.1016/0034-5687(94)00096-i [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. (1985). Central and peripheral catecholamine innervation of the rat intermediate and posterior pituitary lobes. Neuroendocrinology 40 281–284. 10.1159/000124087 [DOI] [PubMed] [Google Scholar]
- Sadoshima S., Busija D., Brody M., Heistad D. (1981). Sympathetic nerves protect against stroke in stroke-prone hypertensive rats. A preliminary report. Hypertension 3 I124–I127. 10.1161/01.hyp.3.3_pt_2.i124 [DOI] [PubMed] [Google Scholar]
- Sadoshima S., Heistad D. D. (1983). Regional cerebral blood flow during hypotension in normotensive and stroke-prone spontaneously hypertensive rats: effect of sympathetic denervation. Stroke 14 575–579. 10.1161/01.str.14.4.575 [DOI] [PubMed] [Google Scholar]
- Sankari A., Badr M. S., Martin J. L., Ayas N. T., Berlowitz D. J. (2019). Impact of spinal cord injury on sleep: current perspectives. Nat. Sci. Sleep 11 219–229. 10.2147/NSS.S197375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A., Bascom A., Oomman S., Badr M. S. (2014). Sleep disordered breathing in chronic spinal cord injury. J. Clin. Sleep Med. 10 65–72. 10.5664/jcsm.3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. S., Moreno A. J., Carvalho A. P. (1996). Relationships between ATP depletion, membrane potential, and the release of neurotransmitters in rat nerve terminals. An in vitro study under conditions that mimic anoxia, hypoglycemia, and ischemia. Stroke 27 941–950. 10.1161/01.str.27.5.941 [DOI] [PubMed] [Google Scholar]
- Savastano L. E., Castro A. E., Fitt M. R., Rath M. F., Romeo H. E., Muñoz E. M. (2010). A standardized surgical technique for rat superior cervical ganglionectomy. J. Neurosci. Methods. 192 22–33. 10.1016/j.jneumeth.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Schömig A., Fischer S., Kurz T., Richardt G., Schömig E. (1987). Nonexocytotic release of endogenous noradrenaline in the ischemic and anoxic rat heart: mechanism and metabolic requirements. Circ. Res. 60 194–205. 10.1161/01.res.60.2.194 [DOI] [PubMed] [Google Scholar]
- Shin J. C., Han E. Y., Cho K. H., Im S. H. (2019). Improvement in pulmonary function with short-term rehabilitation treatment in spinal cord injury patients. Sci. Rep. 9:17091. 10.1038/s41598-019-52526-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone X., Karch R., Ciuraszkiewicz A., Orr-Urtreger A., Lemmens-Gruber R., Scholze P., et al. (2019). The role of the nAChR subunits α5, β2, and β4 on synaptic transmission in the mouse superior cervical ganglion. Physiol. Rep. 7:e14023. 10.14814/phy2.14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Nakayama H., Dempsey J. A. (2003). The essential role of carotid body chemoreceptors in sleep apnea. Can. J. Physiol. Pharmacol. 81 774–779. 10.1139/y03-056 [DOI] [PubMed] [Google Scholar]
- Solberg L. C., Valdar W., Gauguier D., Nunez G., Taylor A., Burnett S., et al. (2006). A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm. Genome 17 129–146. 10.1007/s00335-005-0112-1 [DOI] [PubMed] [Google Scholar]
- Stein R. D., Weaver L. C. (1988). Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. J. Physiol. 396 155–172. 10.1113/jphysiol.1988.sp016956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl K. P. (2003). Periodic breathing and genetics. Respir. Physiol. Neurobiol. 135 179–185. 10.1016/s1569-9048(03)00036-3 [DOI] [PubMed] [Google Scholar]
- Strosznajder R. P. (1997). Effect of hypoxia and dopamine on arachidonic acid metabolism in superior cervical ganglion. Neurochem. Res. 22 1193–1197. 10.1023/a:1021920610766 [DOI] [PubMed] [Google Scholar]
- Tagaito Y., Polotsky V. Y., Campen M. J., Wilson J. A., Balbir A., Smith P. L., et al. (2001). A model of sleep-disordered breathing in the C57BL/6J mouse. J. Appl. Physiol. (1985) 91 2758–2766. 10.1152/jappl.2001.91.6.2758 [DOI] [PubMed] [Google Scholar]
- Tang F. R., Tan C. K., Ling E. A. (1995a). An ultrastructural study of the sympathetic preganglionic neurons that innervate the superior cervical ganglion in spontaneously hypertensive rats and Wistar-Kyoto rats. J. Hirnforsch. 36 411–420. [PubMed] [Google Scholar]
- Tang F. R., Tan C. K., Ling E. A. (1995b). A comparative study by retrograde neuronal tracing and substance P immunohistochemistry of sympathetic preganglionic neurons in spontaneously hypertensive rats and Wistar-Kyoto rats. J. Anat. 186 197–207. [PMC free article] [PubMed] [Google Scholar]
- Tang F. R., Tan C. K., Ling E. A. (1995c). A comparative study of NADPH-diaphorase in the sympathetic preganglionic neurons of the upper thoracic cord between spontaneously hypertensive rats and Wistar-Kyoto rats. Brain Res. 691 153–159. 10.1016/0006-8993(95)00658-d [DOI] [PubMed] [Google Scholar]
- Tankersley C. G. (2001). Selected contribution: variation in acute hypoxic ventilatory response is linked to mouse chromosome 9. J. Appl. Physiol. (1985) 90 1615–1622. 10.1152/jappl.2001.90.4.1615 [DOI] [PubMed] [Google Scholar]
- Tankersley C. G. (2003). Genetic aspects of breathing: on interactions between hypercapnia and hypoxia. Respir. Physiol. Neurobiol. 135 167–178. 10.1016/s1569-9048(03)00035-1 [DOI] [PubMed] [Google Scholar]
- Tankersley C. G., Elston R. C., Schnell A. H. (2000). Genetic determinants of acute hypoxic ventilation: patterns of inheritance in mice. J. Appl. Physiol. (1985) 88 2310–2318. 10.1152/jappl.2000.88.6.2310 [DOI] [PubMed] [Google Scholar]
- Tankersley C. G., Fitzgerald R. S., Kleeberger S. R. (1994). Differential control of ventilation among inbred strains of mice. Am. J. Physiol. 267 R1371–R1377. 10.1152/ajpregu.1994.267.5.R1371 [DOI] [PubMed] [Google Scholar]
- Tankersley C. G., Irizarry R., Flanders S., Rabold R. (2002). Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. J. Appl. Physiol. (1985) 92 870–877. 10.1152/japplphysiol.00904.2001 [DOI] [PubMed] [Google Scholar]
- Teshima T., Tucker A. S., Lourenço S. V. (2019). Dual sympathetic input into developing salivary glands. J. Dent. Res. 98 1122–1130. 10.1177/0022034519865222 [DOI] [PubMed] [Google Scholar]
- Tester N. J., Fuller D. D., Fromm J. S., Spiess M. R., Behrman A. L., Mateika J. H. (2014). Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med. 189 57–65. 10.1164/rccm.201305-0848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari S. G., Bugenhagen S. M., Wang Z., Schreier D. A., Carlson B. E., Chesler N. C., et al. (2013). Analysis of cardiovascular dynamics in pulmonary hypertensive C57BL6/J mice. Front. Physiol. 4:355. 10.3389/fphys.2013.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F., Claps A. (1988). The carotid sinus connections: a WGA-HRP study in the cat. Brain Res. 455 134–143. 10.1016/0006-8993(88)90122-9 [DOI] [PubMed] [Google Scholar]
- Vázquez-Nin G. H., Costero I., Echeverría O. M., Aguilar R., Barroso-Moguel R. (1978). Innervation of the carotid body. An experimental quantitative study. Acta Anat (Basel) 102 12–28. 10.1159/000145613 [DOI] [PubMed] [Google Scholar]
- Verna A., Barets A., Salat C. (1984). Distribution of sympathetic nerve endings within the rabbit carotid body: a histochemical and ultrastructural study. J. Neurocytol. 13 849–865. 10.1007/BF01148589 [DOI] [PubMed] [Google Scholar]
- Villiere S. M., Nakase K., Kollmar R., Silverman J., Sundaram K., Stewart M. (2017). Seizure-associated central apnea in a rat model: Evidence for resetting the respiratory rhythm and activation of the diving reflex. Neurobiol. Dis. 101 8–15. 10.1016/j.nbd.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizek M., Pickett C. K., Weil J. V. (1987). Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J. Appl. Physiol. (1985) 63 1658–1664. 10.1152/jappl.1987.63.4.1658 [DOI] [PubMed] [Google Scholar]
- Wang H. W., Chiou W. Y. (2004). Sympathetic innervation of the tongue in rats ORL J. Otorhinolaryngol. Relat. Spec. 66 16–20. 10.1159/000077228 [DOI] [PubMed] [Google Scholar]
- Wang J., Hogan J. O., Wang R., White C., Kim D. (2017). Role of cystathionine-γ-lyase in hypoxia-induced changes in TASK activity, intracellular [Ca2+] and ventilation in mice. Respir. Physiol. Neurobiol. 246 98–106. 10.1016/j.resp.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Werber A. H., Heistad D. D. (1984). Effects of chronic hypertension and sympathetic nerves on the cerebral microvasculature of stroke-prone spontaneously hypertensive rats. Circ. Res. 55 286–294. 10.1161/01.res.55.3.286 [DOI] [PubMed] [Google Scholar]
- Westerhaus M. J., Loewy A. D. (1999). Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J. Comp. Neurol. 414 361–378. [DOI] [PubMed] [Google Scholar]
- Wiberg M., Widenfalk B. (1993). Involvement of connections between the brainstem and the sympathetic ganglia in the pathogenesis of rheumatoid arthritis. An anatomical study in rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 27 269–276. 10.1080/02844311.1993.12005640 [DOI] [PubMed] [Google Scholar]
- Wilkinson M. H., Berger P. J., Blanch N., Brodecky V., Jones C. A. (1997). Paradoxical effect of oxygen administration on breathing stability following post-hyperventilation apnoea in lambs. J. Physiol. 504 199–209. 10.1111/j.1469-7793.1997.199bf.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Balbir A., Okumura M., Schofield B., Coram J., Tankersley C. G., et al. (2006). Genetic influence on carotid body structure in DBA/2J and A/J strains of mice. Adv. Exp. Med. Biol. 580 105–109. 10.1007/0-387-31311-7_16 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Balbir A., Schofield B., Coram J., Tankersley C. G., Fitzgerald R. S., et al. (2003). Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. J. Appl. Physiol (1985) 94 1536–1542. 10.1152/japplphysiol.00739.2002 [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Dostal J., Kimura H., Strohl K. P. (2008a). Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J. Appl. Physiol. (1985) 105 518–526. 10.1152/japplphysiol.00069.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Dostal J., Strohl K. P. (2008c). Post-hypoxic unstable breathing in the C57BL/6J mouse: effects of acetazolamide. Adv. Exp. Med. Biol. 605 75–79. 10.1007/978-0-387-73693-8_13 [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Kimura H., Strohl K. P. (2010). Mouse models of apnea: strain differences in apnea expression and its pharmacologic and genetic modification. Adv. Exp. Med. Biol. 669 303–307. 10.1007/978-1-4419-5692-7_62 [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Ocak H., Dostal J., Jacono F. J., Loparo K. A., Strohl K. P. (2008b). Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir. Physiol. Neurobiol. 162 117–125. 10.1016/j.resp.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota R., Yamauchi A. (1974). Ultrastructure of the mouse superior cervical ganglion, with particular reference to the pre- and postganglionic elements covering the soma of its principal neurons. Am. J. Anat. 140 281–297. 10.1002/aja.1001400211 [DOI] [PubMed] [Google Scholar]
- Yokoyama T., Nakamuta N., Kusakabe T., Yamamoto Y. (2015). Sympathetic regulation of vascular tone via noradrenaline and serotonin in the rat carotid body as revealed by intracellular calcium imaging. Brain Res. 1596 126–135. 10.1016/j.brainres.2014.11.037 [DOI] [PubMed] [Google Scholar]
- Zapata P. (1975). Effects of dopamine on carotid chemo- and baroreceptors in vitro. J. Physiol. 244 235–251. 10.1113/jphysiol.1975.sp010794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata P., Hess A., Bliss E. L., Eyzaguirre C. (1969). Chemical, electron microscopic and physiological observations on the role of catecholamines in the carotid body. Brain Res. 14 473–496. 10.1016/0006-8993(69)90123-1 [DOI] [PubMed] [Google Scholar]
- Ziegler K. A., Ahles A., Wille T., Kerler J., Ramanujam D., Engelhardt S. (2018). Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc. Res. 114 291–299. 10.1093/cvr/cvx227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.