Abstract
BACKGROUND
The role of uric acid (UA) in survival of patients with hypertrophic obstructive cardiomyopathy (HOCM) has not been fully evaluated. This study aimed to determine whether UA could be an independent risk factor of cardiac death in patients with HOCM.
METHODS
A total of 317 patients with HOCM, who were receiving conservative treatment in Fuwai Hospital from October 2009 to December 2014, all of them completed UA evaluations, were analyzed. Patients were divided into three groups according to the UA levels: Tertile 1 (≤ 318 μmol/L, n = 106), Tertile 2 (319 to 397 μmol/L, n = 105), and Tertile 3 (≥ 398 μmol/L, n = 106).
RESULTS
During a median follow-up of 45 months, 29 cardiac deaths (9.1%) occurred, including 6 sudden cardiac deaths and 23 heart failure-related deaths. Cardiac death in Tertile 3 (n = 16, 55.2%) was significantly higher than in Tertile 1 (n = 6, 20.7%) and Tertile 2 (n = 7, 24.1%). In univariate model, UA level (continuous value) showed predictive value of cardiac death [hazard ratio (HR) = 1.006, 95% CI: 1.003−1.009,P = 0.009]. Univariate Cox survival analysis had shown a significant higher property of cardiac death in patients of Tertile 3 when compared with those of Tertile 1, but cardiac death in patients of Tertile 2 did not show significant prognositic value compared with those of Tertile 1 (HR = 3.927, 95% CI: 0.666−23.162,P = 0.131). UA was found to be an independent risk factor (HR = 1.005, 95% CI: 1.001−1.009,P = 0.009) of cardiac death in the multivariate regression analysis after the adjustment for age, body mass index, atrial fibrillation, hemoglobin, creatinine, high-sensitivity C-reactive protein, interventricular septum/left ventricular posterior wall ratio, left ventricular outflow tract and left ventricular ejection fraction.
CONCLUSIONS
UA concentration was found to be independently associated with cardiac death in HOCM patients receiving conservative treatment. Randomized trials of UA-lowering agents for HOCM patients are warranted.
Hypertrophic cardiomyopathy is one of the most common genetic cardiovascular diseases with a diverse clinical symptoms.[1] Two third of these patients have combined with left ventricular outflow tract (LVOT) obstruction,[2] which is defined as hypertrophic obstructive cardiomyopathy (HOCM). It has been well established as a contributor to deteriorated cardiac function,[3,4] and associated with poor clinical outcomes.[5] Main invasive treatments for HOCM patients include septal myectomy and alcohol septal ablation. Septal myectomy was once the only invasive strategy before the clinical application of alcohol septal ablation, bringing severe trauma and mental stress to the patients. Compared with septal myectomy, alcohol septal ablation could be performed with less trauma and lower periprocedural mortality, but higher rates of pacemaker implantations and re-intervention.[6] Considering the risk and difficulty of these procedures, both of these LOVT reduction operations are suggested to be done in experienced centers by skilled operators. Thus, both septal myectomy and septal alcohol septal ablation are treated as tough challenges, not like other widespread cardiac operations such as percutaneous coronary intervention and coronary artery bypass graft.[1] In addition, the economic burden caused by surgery may be unacceptable for some patients. In this scenarios, a conservative treatment and approach may still remain the most widely accepted treatment for HOCM patients in the real world.
Several clinical indicators have been used for risk stratification in patients with HOCM, such as family history of sudden death, degree of left ventricular wall thickness, LVOT obstruction, atrial fibrillation (AF), and congestive symptoms. However, the clinical outcomes of HOCM are still not fully evaluated. Some biochemical indices may serve as tools of risk stratification.
Uric acid (UA) is the final metabolite of purine, and has potential effects on increased oxidative stress. Experimental and human studies have demonstrated the role of UA as a pro-oxidant inducing endothelial dysfunction.[7] Epidemiological studies have found an association between increased serum UA levels and elevated cardiovascular event rate. Recent retrospective research found that elevated serum UA levels are independently associated with poor long-term survival and increased risk of cardiovascular hospitalization in patients with chronic heart failure.[8] Previous studies about UA and hypertrophic cardiomyopathy did not excluded patients who received invasive treatments.[9] The purpose of this study was to determine the usefulness of UA in the risk stratification of HOCM patients receiving conservative treatments.
METHODS
Study Population
A total of 965 adult patients with HOCM (age > 16 years) were enrolled and evaluated in Fuwai Hospital (National Center for Cardiovascular Diseases, Beijing, China) from October 2009 to December 2014. Among them, 502 patients had taken ventricular septum myectomy, 138 patients had taken alcohol septal ablation and eight patients were without UA evaluations. The remaining 317 patients had complete information of UA concentration, clinical characters and medical history (without any other heart or systemic diseases that induced cardiac hypertrophic changes, such as uncontrolled hypertension of home blood pressure monitoring > 140/90 mmHg, congenital heart disease). The diagnosis of HOCM was based on the following criteria [10]: (1) wall thickness ≥ 15 mm in one or more left ventricular myocardial segments, as measured by any imaging technique (echocardiography, cardiac magnetic resonance imaging, or computed tomography); or (2) wall thickness 13 to 14 mm with family history, noncardiac symptoms and signs, electrocardiogram abnormalities, laboratory tests, and multimodality cardiac imaging; and (3) patients with dynamic LVOT obstruction with a LVOT gradient ≥ 30 mmHg at rest or during physiological provocation such as valsalva maneuver, standing, and exercise. Significant dynamic LVOT obstruction was documented with two-dimensional and Doppler echocardiography. In cases that echocardiography was insufficient, invasive hemodynamic catheterization with provocation would be used.
The study was in accordance with the ethical guidelines of the Declaration of Helsinki, and China’s regulations and guidelines on good clinical practice. The study was also approved by the Ethics Committee of Fuwai Hospital (No.2011-349) and informed consent was obtained from all participants before starting the study.
UA Measurement
Blood samples were obtained at enrollment. Plasma UA concentration was analyzed with a Technicon SMA 12/60 Analyzer (Technicon Instruments, Tarrytown, NY, USA) using a colorimetric phosphotungstic acid procedure according to the methodology of Crowley. All specimen measurements were processed in the laboratory of Fuwai Hospital.
Follow-up and Clinical Outcome
Follow-up data were collected from record of outpatient clinic visit, phone calls or medical record in readmission. The primary endpoint was general cardiac death. Cardiac death was defined as death due to heart failure, sudden cardiac death, cardiogenic shock, or cardiac caused multiorgan failure. The unwitnessed death and death of unknown causes were also classified as cardiac death. Patients who were lost during the follow-up were censored at the last known contact date.
Statistical Analysis
Statistical analysis was performed with the SPSS 26.0 (SPSS Inc., IBM, Armonk, NY, USA). Patients were divided into three groups according to the UA levels: Tertile 1 (≤ 318 μmol/L, n = 106), Tertile 2 (319 to 397 μmol/L, n = 105), and Tertile 3 (≥ 398 μmol/L, n = 106). Descriptive statistics were used to summarize baseline characteristics. Normality of all variables was tested by one sample Kolmogorov-Smirnov normality test. Continuous variables were presented as mean ± SD or median (interquartile range, analyzed by Kruskal–Wallis test). Categorical variables were presented as frequency and percentage. The difference of continuous variables was tested by Student’s t-test. The difference of categorical variables was tested by Pearson’s chi-squared test and Fisher’s exact probability test. Univariate and multivariate Cox proportional hazards model were performed to estimate hazard ratio (HR) and 95% confidence intervals (CIs). The covariables in multivariate analysis were mainly selected for the following reasons: the variables that has significance in univariable analysis; the variables which are known to be related to cardiovascular events and thus may act as potential confounders. Estimate of survival between each group were analyzed with Kaplan-Meier method and log-rank test. All statistical tests were two-sided, and P-value < 0.05 was defined as statistically significant.
RESULTS
Baseline Clinical Characteristics
Table 1 shows the baseline clinical characteristics of the study population according to the UA levels. Patients in Tertile 1 had the lowest percentage of male (P < 0.001). Significant differences were found in hemoglobin ( P < 0.001), creatinine ( P < 0.001), high-sensitivity C-reactive protein (hs-CRP, P = 0.031), low-density lipoprotein cholesterol (P < 0.001), and total cholesterol ( P < 0.001) among the three groups.
Table 1. Baseline characteristics of the study population stratified by UA levels.
| Variables | UA ≤ 318 μmol/L
(n = 106) |
319 μmol/L ≤ UA ≤ 397 μmol/L
(n = 105) |
UA ≥ 398 μmol/L
(n = 106) |
Total
(n = 317) |
P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). Categorical variables were analyzed by Pearson’s chi-squared test and Fisher’s exact probability test. Normal variables were analyzed by Student’s t-test, non-normal variables were analyzed by Mann-Whitney U test. ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; IVS: interventricular septum; NT-ProBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; UA: uric acid. | |||||
| Demographics | |||||
| Male | 44 (41.5%) | 71 (67.6%) | 72 (67.9%) | 187 (59.0%) | < 0.001 |
| Age, yrs | 55.64 ± 15.16 | 54.85 ± 11.68 | 54.94 ± 14.39 | 55.14 ± 13.79 | 0.903 |
| Body mass index, kg/m2 | 25.52 ± 11.90 | 26.60 ± 4.72 | 25.66 ± 4.09 | 25.92 ± 7.83 | 0.604 |
| Hypertension | 42 (39.6%) | 52 (49.5%) | 52 (49.1%) | 146 (46.1%) | 0.265 |
| Diabetes mellitus | 5 (4.7%) | 9 (8.6%) | 11 (10.5%) | 25 (7.9%) | 0.287 |
| Hyperlipidemia | 36 (34.0%) | 44 (41.9%) | 47 (44.8%) | 47 (40.2%) | 0.253 |
| Coronary heart disease | 26 (24.5%) | 33 (31.4%) | 27 (25.5%) | 86 (27.1%) | 0.474 |
| Smoking | 42 (39.6%) | 51 (48.6%) | 53 (50.0%) | 146 (46.1%) | 0.260 |
| Alcohol consumption | 25 (23.6%) | 35 (33.3%) | 36 (33.9%) | 96 (30.3%) | 0.183 |
| Atrial fibrillation | 15 (14.2%) | 18 (17.1%) | 22 (20.8%) | 55 (17.4%) | 0.446 |
| NYHA III or IV | 11 (10.3%) | 10 (9.5%) | 18 (16.9%) | 39 (12.3%) | 0.195 |
| Devices | |||||
| Implantable cardiac defibrillator/Pace maker | 5 (4.7%) | 8 (7.6%) | 13 (12.3%) | 26 (8.2%) | 0.130 |
| Medication | |||||
| Calcium channel blockers | 24 (22.6%) | 28 (26.7%) | 32 (30.2%) | 84 (26.5%) | 0.460 |
| β-blocker | 59 (55.7%) | 73 (69.5%) | 64 (60.4%) | 196 (61.8%) | 0.109 |
| ACEI/ARB | 11 (10.4%) | 13 (12.4%) | 22 (20.8%) | 46 (14.5%) | 0.075 |
| Statin | 19 (17.9%) | 23 (21.9%) | 29 (27.4%) | 71 (22.4%) | 0.255 |
| Diuretics | 9 (8.4%) | 7 (6.6%) | 11 (10.3%) | 27 (8.5%) | 0.236 |
| Laboratory test | |||||
| Hemoglobin, g/L | 125.98 ± 17.65 | 137.59 ± 16.24 | 136.17 ± 20.67 | 133.25 ± 18.95 | < 0.001 |
| NT-ProBNP, pg/mL | 1216.09 (668.50−2469.30)* | 1098.80 (612.50−1679.80)* | 1369.55 (633.35−2744.57)* | 1181.80 (635.45−2261.95)* | 0.890 |
| Creatinine, μmol/L | 65.30 (56.28−77.66)* | 73.05 (64.54−81.70)* | 82.36 (69.62−94.90)* | 73.05 (62.48−84.19)* | < 0.001 |
| High-sensitivity C-reactive protein, mg/L | 1.70 (0.60−8.27)* | 1.38 (0.77−2.63)* | 1.90 (1.12−5.14)* | 1.66 (0.78−3.95)* | 0.031 |
| Total cholesterol, mmol/L | 4.01 (3.54−4.65)* | 4.58 (3.81−5.32)* | 4.36 (3.84−5.15)* | 4.28 (3.73−5.07)* | < 0.001 |
| High-density lipoprotein cholesterol, mmol/L | 1.07 (0.84−1.34)* | 1.07 (0.90−1.26)* | 1.00 (0.83−1.17)* | 1.06 (0.86−1.25)* | 0.091 |
| Low-density lipoprotein cholesterol, mmol/L | 2.40 ± 0.71 | 2.84 ± 0.85 | 2.79 ± 0.95 | 2.68 ± 0.85 | < 0.001 |
| Echocardiography | |||||
| Left ventricular outflow tract gradient, mmHg | 58.90 (36.00−87.35)* | 58.80 (38.50−89.50)* | 57.00 (38.85−80.25)* | 57.80 (37.50−84.80)* | 0.823 |
| Left ventricular ejection fraction | 70.00 (64.55−75.00)* | 69.00 (65.00−74.00)* | 68.00 (63.60−72.82)* | 70.00 (65.00−74.90)* | 0.127 |
| Left ventricular end-diastolic diameter, mm | 41.00 (37.00−45.00)* | 42.00 (39.00−46.00)* | 42.00 (39.00−47.25)* | 42.00 (39.00−46.00)* | 0.193 |
| IVS/Left ventricular posterior wall ratio | 1.50 (1.20−1.90)* | 1.59 (1.30−2.00)* | 1.53 (1.33−2.06)* | 1.54 (1.27−2.00)* | 0.883 |
| Ventricular septum thickness, mm | 18.00 (16.00−23.00)* | 19.00 (16.00−23.00)* | 19.00 (16.00−23.00)* | 19.00 (16.00−23.00)* | 0.987 |
| IVS > 30 mm | 9 (8.4%) | 7 (6.6%) | 8 (7.5%) | 24 (7.5%) | 0.528 |
Prognostic Value of the UA Level in HOCM and Survival Analysis
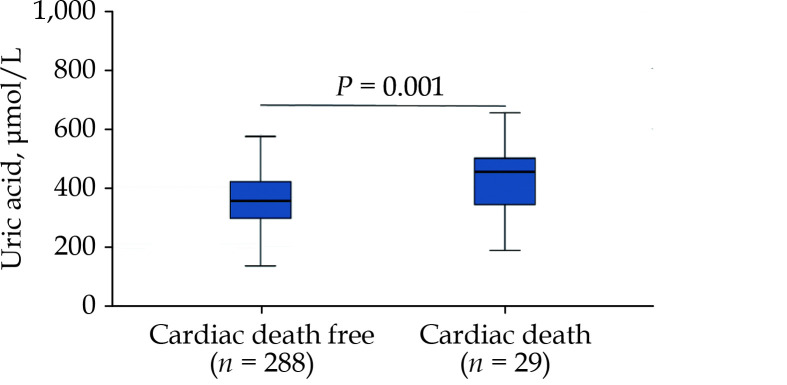
During a median follow-up of 45 months, 29 cardiac deaths (9.1%) occurred, including 6 sudden cardiac deaths and 23 heart failure-related deaths. Cardiac death in Tertile 3 (n = 16, 55.2%) was significantly higher than in Tertile 1 (n = 6, 20.7%) and Tertile 2 (n = 7, 24.1%). Compared to patients with cardiac death free, patients with cardiac death had substantially higher UA concentration (P = 0.001) (Figure 1).
Figure 1.
Comparison of serum uric acid concentration in patients with cardiac death free and cardiac death.
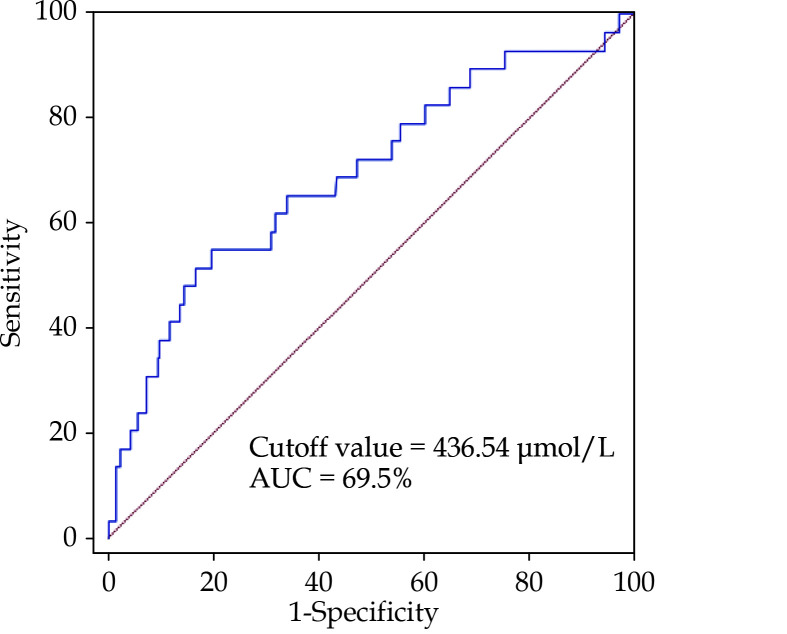
A receiver operating characteristic curve was performed to determine the cutoff value of UA for cardiac death. The cutoff value was 436.54 μmol/L, with an area under the curve of 69.5% (Figure 2). Indexes, which were found significant difference in baseline clinical characteristic (Table 1) and risk factors may affect survival of HOCM, are put into univariate Cox regression analysis for cardiac death. The results are shown in Table 2: age (HR = 1.079, 95% CI: 1.046−1.114,P < 0.001), body mass index (HR = 0.885, 95% CI: 0.794−0.986, P = 0.027), AF (HR = 0.293, 95% CI: 0.115−0.498,P < 0.001), interventricular septum/left ventricular posterior wall ratio (HR = 0.305, 95% CI: 0.113−0.826, P = 0.02), LVOT (HR = 0.980, 95% CI: 0.967−0.994,P = 0.004), left ventricular ejection fraction (HR = 0.980, 95% CI: 0.936−0.974,P < 0.001), hemoglobin (HR = 0.964, 95% CI: 0.947−0.981, P < 0.001), creatinine (HR = 1.019, 95% CI: 1.012−1.027, P < 0.001) and hs-CRP (HR = 1.136, 95% CI: 1.070−1.207, P < 0.001) were found to be associated with cardiac death in the univariate Cox analysis.
Figure 2.
Receiver operating characteristic curve of uric acid for cardiac death.
AUC: area under the curve.
Table 2. Univariate Cox analysis for cardiac death.
| Variables | HR (95% CI) | P-value |
| *Refers to Model 1 which consider UA as continuous value in the Cox survival analysis. **Refers to Model 2 which consider UA as categorical value in the Cox survival analysis. CI: confidence interval; HR: hazard ratio; UA: uric acid. | ||
| Male | 0.595 (0.286−1.237) | 0.165 |
| Age | 1.079 (1.046−1.114) | < 0.001 |
| Body mass index | 0.885 (0.794−0.986) | 0.027 |
| Atrial fibrillation | 0.293 (0.115−0.498) | < 0.001 |
| Hemoglobin | 0.964 (0.947−0.981) | < 0.001 |
| Creatinine | 1.019 (1.012−1.027) | < 0.001 |
| High-sensitivity C-reactive protein | 1.136 (1.070−1.207) | < 0.001 |
| Total cholesterol | 0.722 (0.486−1.071) | 0.106 |
| Low-density lipoprotein cholesterol | 0.860 (0.553−1.338) | 0.505 |
| Ventricular septum thickness | 0.951 (0.883−1.023) | 0.176 |
| Interventricular septum/Left ventricular posterior wall ratio | 0.305 (0.113−0.826) | 0.02 |
| Left ventricular outflow tract at rest, mmHg | 0.980 (0.967−0.994) | 0.004 |
| Left ventricular ejection fraction | 0.980 (0.936−0.974) | < 0.001 |
| UA*, μmol/L | 1.006 (1.003−1.009) | < 0.001 |
| UA levels** | ||
| Tertile 1 (≤ 318 μmol/L) | Reference | |
| Tertile 2 (319 to 397 μmol/L) | 1.315 (0.417−4.146) | 0.64 |
| Tertile 3 (≥ 398 μmol/L) | 3.158 (1.165−8.562) | 0.024 |
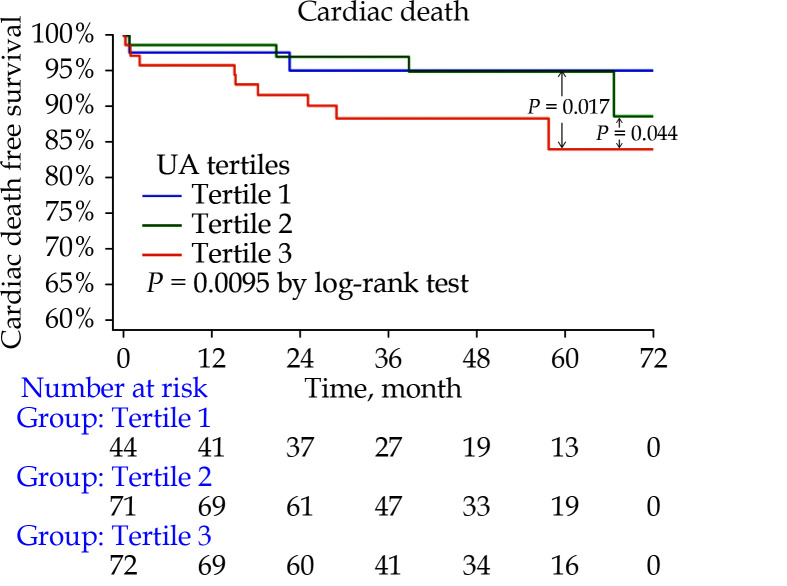
Two multivariate models were constructed, taking UA as either a continuous variable (Model 1) or a categorical variable (Model 2; Tertiles 1−3) (Table 3). In Model 1, UA concentration was significantly associated with cardiac death (HR = 1.005, 95% CI: 1.001−1.009,P = 0.009; Table 3). In Model 2, the group with highest UA concentration (Tertile 3) showed a significant rising trend of cardiac death when compared with the Tertile 1 (HR = 4.732, 95% CI: 1.082−20.69,P = 0.004; Table 3). Tertile 2 did not show significant difference when compared with Tertile 1 (HR = 3.927, 95% CI: 0.666−23.162,P = 0.131; Table 3). Body mass index, AF, interventricular septum/left ventricular posterior wall ratio, LVOT, hemoglobin and hs-CRP were also found independently correlated with cardiac death in both Model 1 and Model 2. In the Kaplan-Meier survival analysis, significant difference was found between three groups (P = 0.0095). Significant differences were also found in both Tertile 3 versus Tertile 1 (P = 0.017) and Tertile 3 versus Tertile 2 (P = 0.044) (Figure 3).
Table 3. Multivariate Cox analysis for cardiac death.
| Variables | HR (95% CI) | P-value |
| *Refers to Model 1 which consider UA as continuous value in the multivariate Cox survival analysis. **Refers to Model 2 which consider UA as categorical value in the multivariate Cox survival analysis. CI: confidence interval; HR: hazard ratio; UA: uric acid. | ||
| Age | 1.030 (0.995−1.066) | 0.109 |
| Body mass index | 0.884 (0.767−1.020) | 0.090 |
| Atrial fibrillation | 0.270 (0.088−0.823) | 0.021 |
| Hemoglobin | 0.965 (0.937−0.994) | 0.018 |
| Creatinine | 0.684 (0.144−3.243) | 0.632 |
| High-sensitivity C-reactive protein | 1.120 (1.015−1.237) | 0.025 |
| Interventricular septum/Left ventricular posterior wall ratio | 0.412 (0.119−1.437) | 0.164 |
| Left ventricular outflow tract at rest, mmHg | 0.978 (0.960−0.997) | 0.095 |
| Left ventricular ejection fraction | 0.980 (0.945−1.028) | 0.509 |
| UA*, μmol/L | 1.005 (1.001−1.009) | 0.009 |
| UA levels** | ||
| Tertile 1 (≤ 318 μmol/L) | Reference | |
| Tertile 2 (319 to 397 μmol/L) | 3.927 (0.666−23.162) | 0.131 |
| Tertile 3 (≥ 398 μmol/L) | 4.732 (1.082−20.691) | 0.039 |
Figure 3.
Kaplan-Meier curves comparing the probability of cardiac death stratified by uric acid concentration (Tertile 1: ≤ 318 μmol/L, Tertile 2: 319 to 397 μmol/L, Tertile 3: ≥ 398 μmol/L).
DISCUSSION
A number of epidemiological studies have reported a relation between serum UA levels and a wide variety of cardiovascular conditions, including hypertension, metabolic syndrome, coronary artery disease, vascular dementia, preeclampsia, and kidney disease.[11] Yet, the literature about the role of UA for HOCM is relatively scarce.[9,12] In a cohort study with 588 adult hypertrophic cardiomyopathy patients, Zhu, et al.[8] reported that elevated UA levels independently predicted adverse outcomes during the five-year follow-up. In this study, the adjusted HRs for all-cause mortality and cardiovascular death of patients in the highest Tertile of serum UA were 2.33 (95% CI: 1.11−4.89,P = 0.025) and 3.10 (95% CI: 1.37−7.04,P = 0.007) when compared with the lowest Tertial, indicating the potential clinical utility of UA in hypertrophic cardiomyopathy patients.[9] In Wang, et al.[12] study, patients were divided according to quartiles of UA concentration, and the result showed that either low or high serum UA concentrations had a higher risk of all-cause mortality and hypertrophic cardiomyopathy related mortality, which was called a U-shape relationship.[12] Certain phenomenon also occurred in some studies on coronary heart diseases.[13–16] However, none of these studies focused on HOCM patients receiving conservative treatment.
With the development of surgical procedures and techniques, the application of Morrow procedure and alcohol septal ablation are spreading in recent years. However, due to the risk and complexity, only a few centers and hospitals could perform these operations. Moreover, the surgical risk and economic burden may be unbearable for some patients, thus the conservative treatment remains the main strategy among Chinese HOCM patients. The present study was designed to determine whether UA was to be an independent risk factor of cardiac death in HOCM patients receiving conservative treatment and showed a positive result, which was consistent with Zhu, et al.[8] At the same time, the present study demonstrated the gender difference in the effect of UA on cardiac death of HOCM patients.
Another cross-sectional study from Fuwai Hospital, Zhang, et al.[17] reported that serum UA was significantly and independently associated with left ventricular mass index (LVMI) by cardiac magnetic resonance imaging in the HOCM patients. The LVMI increased progressively as the UA concentration raised in female patients, but not in male patients. This was the first study to demonstrate the gender difference in the role of UA in HOCM patients.[17] In Olivotto, et al.[18] study, higher LVMI was found in patients with greater left ventricular outflow obstruction at rest, and the increased LVMI was found markedly associated with hypertrophic cardiomyopathy related death with great sensitivity. A hypothetical was carried out that the effects of UA on increasing LVMI may play a pathogenetic role for HOCM development, especially in female patients.
The underlying mechanisms of UA in the progression of HOCM is not fully elucidated. Previous in vitro and animal studies have shown that high UA concentrations might produce an inflammatory reaction by increasing expression of inflammation cytokines in endothelial cells, such as interleukin (IL)-6, IL-8, and tumor necrosis factor-alpha (TNF-α).[19] It could also stimulate monocyte chemo-attractant protein-1 in vascular smooth muscle cells through mitogen-activated protein kinase and cyclooxygenase-2.[20] Ruggiero, et al.[21] clinical study revealed that serum UA concentration has a positive and significant association with inflammatory bio-markers, such as neutrophil count, C-reactive protein, IL-6, IL-18, and TNF-α. Recently, Zhu, et al.[22] study reported that patients with hypertrophic cardiomyopathy in the high hs-CRP group had a significant higher risk of adverse outcomes than the low hs-CRP group. In present study, levels of hs-CRP was found significantly different among three groups according to the UA levels. That’s implied that UA may have an adverse impact on HOCM by enhancing the inflammation reaction. Whether the use of urate-lowering agents has the effect of reducing inflammatory in the HOCM patients is still unknown.
LIMITATIONS
There are several mentionable limitations in the present study. Firstly, patients in this study are from a single center, which limited the generalizability of our findings. Secondly, one third of the study population (34%) had met the diagnostic criteria of hyperuricemia (> 420 μmol/L for male and > 360 μmol/L in female),[23] and might have taken urate-lowering agents. Thirdly, in the present study, data of UA concentration was taken only at the admission and the serum UA concentration at follow-up appointments was lacking, thus the change of UA concentration in the development of HOCM was unknown. In conclusion, the population of this study was relatively small, and the gender difference of UA in the prediction value to the HOCM patients may not be fully demonstrated, and some unknown risk factors may not have been adjusted in the multivariate models.
CONCLUSIONS
UA concentration was found to be independently associated with cardiac death in HOCM patients receiving conservative treatment. Randomized trials of UA-lowering agents for HOCM patients are warranted.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Plan of China (2020YFC2004700), the National Natural Science Foundation of China (No.81825003 & No.91957123), the CAMS Innovation Fund for Medical Sciences (CIFMS 2016-I2M-1-009), and the Beijing Municipal Commission of Science and Technology (Z171100000417021). All authors had no conflicts of interest to disclose.
Contributor Information
Ming-Qi ZHENG, Email: mzheng2020@163.com.
Yi-Da TANG, Email: drtangyida@126.com.
References
- 1.Gersh BJ, Maron BJ, Bonow RO, et al 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Maron MS, Olivotto I, Zenovich AG, et al Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 3.Wigle ED, Sasson Z, Henderson MA, et al Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28:1–83. doi: 10.1016/0033-0620(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 4.Wigle ED, Rakowski H, Kimball BP, et al Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation. 1995;92:1680–1692. doi: 10.1161/01.CIR.92.7.1680. [DOI] [PubMed] [Google Scholar]
- 5.Maron MS, Olivotto I, Betocchi S, et al Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 6.Osman M, Kheiri B, Osman K, et al Alcohol septal ablation vs myectomy for symptomatic hypertrophic obstructive cardiomyopathy: systematic review and meta-analysis. Clin Cardiol. 2019;42:190–197. doi: 10.1002/clc.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muiesan ML, Agabiti-Rosei C, Paini A, et al Uric acid and cardiovascular disease: an update. Eur Cardiol. 2016;11:54–59. doi: 10.15420/ecr.2016:4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, Wang J, Wang Y, et al Plasma uric acid as a prognostic marker in patients with hypertrophic cardiomyopathy. Can J Cardiol. 2015;31:1252–1258. doi: 10.1016/j.cjca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Rowin EJ, Casey SA, et al Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–1928. doi: 10.1016/j.jacc.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 10.Elliott PM, Anastasakis A, Borger MA, et al 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 11.Feig DI, Kang DH, Johnson RJ Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Xu Y, Liao H, et al U-shaped association between serum uric acid concentration and mortality in hypertrophic cardiomyopathy patients. Ups J Med Sci. 2020;125:44–51. doi: 10.1080/03009734.2020.1719245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odden MC, Amadu AR, Smit E, et al Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988-1994 and 1999-2002. Am J Kidney Dis. 2014;64:550–557. doi: 10.1053/j.ajkd.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang E, Hwang SS, Kim DK, et al Sex-specific relationship of serum uric acid with all-cause mortality in adults with normal kidney function: an observational study. J Rheumatol. 2017;44:380–387. doi: 10.3899/jrheum.160792. [DOI] [PubMed] [Google Scholar]
- 15.Dahle DO, Jenssen T, Holdaas H, et al Uric acid has a J-shaped association with cardiovascular and all-cause mortality in kidney transplant recipients. Clin Transplant. 2014;28:134–140. doi: 10.1111/ctr.12290. [DOI] [PubMed] [Google Scholar]
- 16.Cho SK, Chang Y, Kim I, et al U-shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. 2018;70:1122–1132. doi: 10.1002/art.40472. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Liu R, Yuan J, et al Gender-related differences in the association between serum uric acid and left ventricular mass index in patients with obstructive hypertrophic cardiomyopathy. Biol Sex Differ. 2016;7:22. doi: 10.1186/s13293-016-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivotto I, Maron MS, Autore C, et al Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Zhen H, Gui F The role of hyperuricemia on vascular endothelium dysfunction. Biomed Rep. 2017;7:325–330. doi: 10.3892/br.2017.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanellis J, Watanabe S, Li JH, et al Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 21.Ruggiero C, Cherubini A, Ble A, et al Uric acid and inflammatory markers. Eur Heart J. 2006;27:1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Zou Y, Wang Y, et al Prognostic significance of plasma high-sensitivity C-reactive protein in patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;6:e004529. doi: 10.1161/JAHA.116.004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Alderman MH Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]