Abstract
BACKGROUND
M1 polarization of macrophages is an important pathological process in myocardial ischemia reperfusion injury, which is the major obstacle for the treatment of acute myocardial infarction. Currently, the strategies and mechanisms of inhibiting M1 polarization are poorly explored. This study aims to investigate the role of soluble death receptor 5-Fc (sDR5-Fc) in regulating M1 polarization of macrophages under extreme conditions and explore the mechanisms from the aspect of glycolysis.
METHODS
Extreme conditions were induced in RAW264.7 cells. Real-time quantitative polymerase chain reaction and western blot were used to detect the expression of mRNA and proteins, respectively. Cell counting kit-8 was used to investigate the proliferation activity of cells. Expression levels of inflammatory cytokines were determined by enzyme-linked immunosorbent assay.
RESULTS
We found that sDR5-Fc rescues the proliferation of macrophages under extreme conditions, including nutrition deficiency, excessive peroxide, and ultraviolet irradiation. In addition, administration of sDR5-Fc inhibits the M1 polarization of macrophages induced by lipopolysaccharide (LPS) and interferon-gamma (IFN-γ), as the expression of M1 polarization markers CD86, CXC motif chemokine ligand 10, matrix metalloproteinase 9, and tumor necrosis factor-α, as well as the secretion of inflammatory factors interleukin (IL)-1β and IL-6, were significantly decreased. By further investigation of the mechanisms, the results showed that sDR5-Fc can recover the LPS and IFN-γ induced pH reduction, lactic acid elevation, and increased expression of hexokinase 2 and glucose transporter 1, which were markers of glycolysis in macrophages.
CONCLUSIONS
sDR5-Fc inhibits the M1 polarization of macrophages by blocking the glycolysis, which provides a new direction for the development of strategies in the treatment of myocardial ischemia reperfusion injury.
Acute myocardial infarction (AMI) is one of the leading causes of death worldwide. More than ten million people around the world die of cardiovascular diseases every year, and about seven million people die of AMI.[1] At present, the treatment of AMI mainly depends on drugs, coronary artery bypass surgery and interventional treatment, which significantly enhances the perfusion of myocardial tissues and improves the prognosis of patients. However, reperfusion can lead to injury of myocardial cells, even irreversible damages, which is known as myocardial ischemia reperfusion injury (MIRI).[2] MIRI is a major obstacle in the treatment of cardiovascular diseases, which can cause damage of oxidative stress to myocardial cells, lead to changes in myocardial ultrastructure, abnormal energy metabolism, cardiac function and electrophysiological disorders, or even sudden death of patients due to malignant arrhythmia.[3,4] Although the adverse effect of MIRI has been recognized for a long time, few applicable methods have been developed for promotion in clinical practice.[3,5] Thus, it is in urgent need to develop appropriate strategies to alleviate the MIRI.
Macrophages play important roles in the physiology of myocardial cells, which promote the conduction of electrical signals between cardiomyocytes, thereby enhancing the function of myocardial contraction and blood pumping.[6,7] According to the differences in phenotypes and secreted cytokines, macrophages can be divided into two types of mutually convertible polarization: classical activated type (M1-type) and alternative activated type (M2-type).[8] The M1 polarization of macrophages leads to arrhythmia and atrial fibrillation by secreting inflammatory factors,[9] such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and chemokines.[10] Inhibition of M1 polarization has been proved to be effective to improve the MIRI-induced cardiac dysfunction.[11,12] However, the underlying mechanisms of M1 polarization of macrophages are not fully understood at present. Glycolysis is an essential pathway of glucose metabolism for cells under the extreme conditions such as oxygen deficiency, which is the main source of energy in M1-type macrophages. During glycolysis, a large number of reactive oxygen species and inflammatory cytokines were produced.[13] Interestingly, inhibition of glycolysis can promote the recovery of energy metabolism in macrophages from glycolysis to oxidative phosphorylation pathway under normal oxidative state, further blocking the M1 polarization, thereby improving the conditions of atherosclerosis, myocardial infarction, and heart failure.[13–15] Currently, there are rare studies exploring the possibility of manipulating the glycolysis in the M1 polarization of macrophages.
Death receptor 5 (DR5) is a group of markers on cell surface discovered in recent years, which transmits apoptosis signals to the cells through a series of pathways including apoptosis.[16] Studies have shown that elevated DR5 expression played an important role in MIRI process, in which it can induce the transformation of macrophages to the pro-inflammatory direction and exert inflammatory effects.[17,18] Soluble death receptor 5-Fc (sDR5-Fc) fusion protein is an extracellular region structure of DR5, which can suppress DR5 and block a series of subsequent signal transduction processes by competitively combining the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) with DR5 on the cell membrane surface, thus protecting cells from being induced apoptosis.[19] Previous studies have shown that blocking the interaction between endogenous TRAIL and DR5 through sDR5-Fc can effectively reduce the death of ischemic neurons.[20] In this study, we investigated the effect of sDR5-Fc on the polarization of macrophages and the mechanisms from the aspect of glycolysis. We hope to provide new directions for the development of treatment strategies to alleviate MIRI in AMI.
MATERIALS AND METHODS
Materials
sDR5-Fc was presented by En-Yun SHEN, Zhongke Amshenn Pharmaceutical Co. Ltd (Shenzhen, China). Lipopolysaccharide (LPS) was purchased from Sigma (St. Louis, MO, USA). Recombinant murine interferon-gamma (IFN-γ) and TRAIL were purchased from Peprotech (Rocky Hill, NJ, USA). Anti-CD86-FITC antibody was from BD Pharmingen (San Diego, CA, USA). Anti-inducible nitric oxide synthase (iNOS) antibody, anti-peroxisome proliferator-activated receptor gamma (PPARγ) antibody, anti-hexokinase 2 (HK2) antibody, anti-glucose transporter 1 (GLUT1) antibody, were purchased from Abcam (Cambridge, MA, USA). Anti-arginase-1 (Arg-1) antibody was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Anti-β-actin antibody was purchased from Rayantibody (Beijing, China).
Cell Culture
Murine macrophage cell line RAW264.7 cells, as described previously,[21] were obtained from Institute of Basic Medical Science, Peking Union Medical College (Beijing, China), maintained in high glucose Dulbecco’s modified eagle medium (DMEM, Corning Inc., Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Grand Island, NY, USA) and 1 × penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in a 5% CO2 humidified incubator (Thermo Scientific, Waltham, MA, USA) at 37 °C.
Establishment of Extreme Conditions
RAW264.7 was inoculated into 96-well plates (Corning Inc., Corning, NY, USA) at 1 × 105 cells/mL, 100 μL/well. After 24 h, the culture medium of some cells was changed to 1% serum medium; for some cells, H2O2 with a final concentration of 500 μM was added into the culture medium; some cells were irradiated under 40 w ultraviolet environment (Thermo Scientific, Waltham, MA, USA) for 6 min. Twenty-four hours after these treatments were completed, sDR5-Fc with a concentration of 0, 4, 20, 100, 500 μg/mL were added to the cells under various extreme conditions, respectively. After 24 h of incubation, the viability of RAW264.7 cells was measured by cell counting kit-8 (CCK-8) assay.
CCK-8 Assay
Murine macrophage cell line RAW264.7 was cultured in 96-well plate at 37 °C for indicated time respectively, then 10 μL of CCK-8 reagent (Meilunbio, Dalian, China) was added to each well, and after 3 h of incubation at 37 °C, the absorbance value of optical density (OD) at 450 nm was measured by a Microplate Reader (Molecular Devices, Silicon Valley, CA, USA). Cell survival rate (%) = OD test well/OD control well × 100%. All samples were repeated for three times.
Flow Cytometry Analysis
The exponentially growing RAW264.7 cells were seeded in 6-well plates, after treatment, cells were collected, counted and stained with the anti-CD86-FITC antibody (BD Pharmingen, Cat. No.561962, 5 μg/mL) in dark for 30 min, then the stained cells were detected with the BD Accuri C6 flow cytometer (BD, Franklin Lakes, NJ, USA). All conditions were repeated for triplicate.
Real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from 1 μg RNA using a FastQuant cDNA First-Strand Synthesis Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR Green PCR Master Mix (Tiangen, Beijing, China). A Roche Light-Cycler 96 system (Roche, Basel, Switzerland) was used for PCR amplification and the 2−ΔΔCT method was used to assess the relative mRNA expression level. The primer sequences are shown in Table 1.
Table 1. The primer sequence used in the experiment.
| Gene | Primer sequence |
| CXCL10: CXC motif chemokine ligand 10; DR5: death receptor 5; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GLUT1: glucose transporter 1; HK2: hexokinase 2; IL-1β: interleukin-1β; MMP9: matrix metalloproteinases 9; PPARγ: peroxisome proliferator-activated receptor gamma; TNF-α: tumor necrosis factor-α. | |
| CXCL10 | Forward: 5’-CCAAGTGCTGCCGTCATTTTC-3’
Reverse: 5’-GGCTCGCAGGGATGATTTCAA-3’ |
| MMP9 | Forward: 5’-ACGTGCAGCTACTGCATGTGA-3’
Reverse: 5’-AGAAGGAACACGTTGTCAGCG-3’ |
| PPARγ | Forward: 5’-ACGTGCAGCTACTGCATGTGA-3’
Reverse: 5’-AGAAGGAACACGTTGTCAGCG-3’ |
| IL-1β | Forward: 5’-GTCACAAGAAACCATGGCACAT-3’
Reverse: 5’-GCCCATCAGAGGCAAGGA-3’ |
| TNF-α | Forward: 5’-CAGGCGGTGCCTATGTCTC-3’
Reverse: 5’-CGATCACCCCGAAGTTCAGTAG-3’ |
| HK2 | Forward: 5’-TGATCGCCTGCTTATTCACGG-3’
Reverse: 5’-AACCGCCTAGAAATCTCCAGA-3’ |
| GLUT1 | Forward: 5’- CAGTTCGGCTATAACACTGGTG-3’
Reverse: 5’-GCCCCCGACAGAGAAGATG-3’ |
| DR5 | Forward: 5’-CGCTGCACCAGGTGTGATT-3’
Reverse: 5’-GTGCCTTCTTCGCACTGACA-3’ |
| GAPDH | Forward: 5’-AGCAGTCCCGTACACTGGCAAAC-3’
Reverse: 5’-TCTCCTGTAAATGTAGTGGTGTCT-3’ |
Western Blot Analysis
Total proteins from cell lysate were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, separated and electro-blotted onto a nitrocellulose membrane (Pierce, Rockford, IL, USA). After blocking with 5% non-fat milk, membranes were probed using various primary antibodies (Anti-iNOS antibody, Abcam, Cat. No.ab178945, 1:2000; anti-Arg-1 antibody, Thermo Fisher, Cat. No.711765, 1:1000; anti-PPARγ antibody, Abcam, Cat. No.ab45036, 1:2000; anti-HK2 antibody, Abcam, Cat. No.ab209847, 1:1000; anti-GLUT1 antibody, Abcam, Cat. No.ab115730, 1:5000; anti-β-actin antibody, Rayantibody, Cat. No.RM2001, 1:2000) at 4 °C overnight, followed with incubation for 1 h with 1:5000 diluted horseradish peroxidase conjugated secondary antibodies (Beyotime Biotechnology, Shanghai, China) at room temperature. The antigen-antibody complexes were detected by the electrogenerated chemiluminescence detection reagent (Meilunbio, Dalian, China). Relative protein expression levels were visualized using Image J software (National Institutes of Health, Bethesda, MD, USA).
Inflammatory Cytokine Detection
Levels of IL-1β and TNF-α were measured using enzyme-linked immunosorbent assay kits (Abcam, Cambridge, MA, USA) according to the protocols. Absorbance was read at 450 nm by Microplate Reader (Molecular Devices, Silicon Valley, CA, USA).
Statistical Analysis
The IBM SPSS 19.0 (IBM Corporation, Somers, NY, USA) was used for data analysis in this study. All the data were tested for normality before further processing. Data with normal distribution were expressed as mean ± SD, and one-way analysis of variance (ANOVA) was used for comparisons among multiple groups and least significant difference (LSD) method was used for post-hoc pairwise test. Data that did not conform to the normal distribution were compared with Kruskal-Wallis test, and Nemenyi method was used for the pairwise comparisons. A two-sided P-value < 0.05 was considered statistically significant.
RESULTS
sDR5-Fc Recovers the Proliferation Ability of Macrophages Under Extreme Conditions
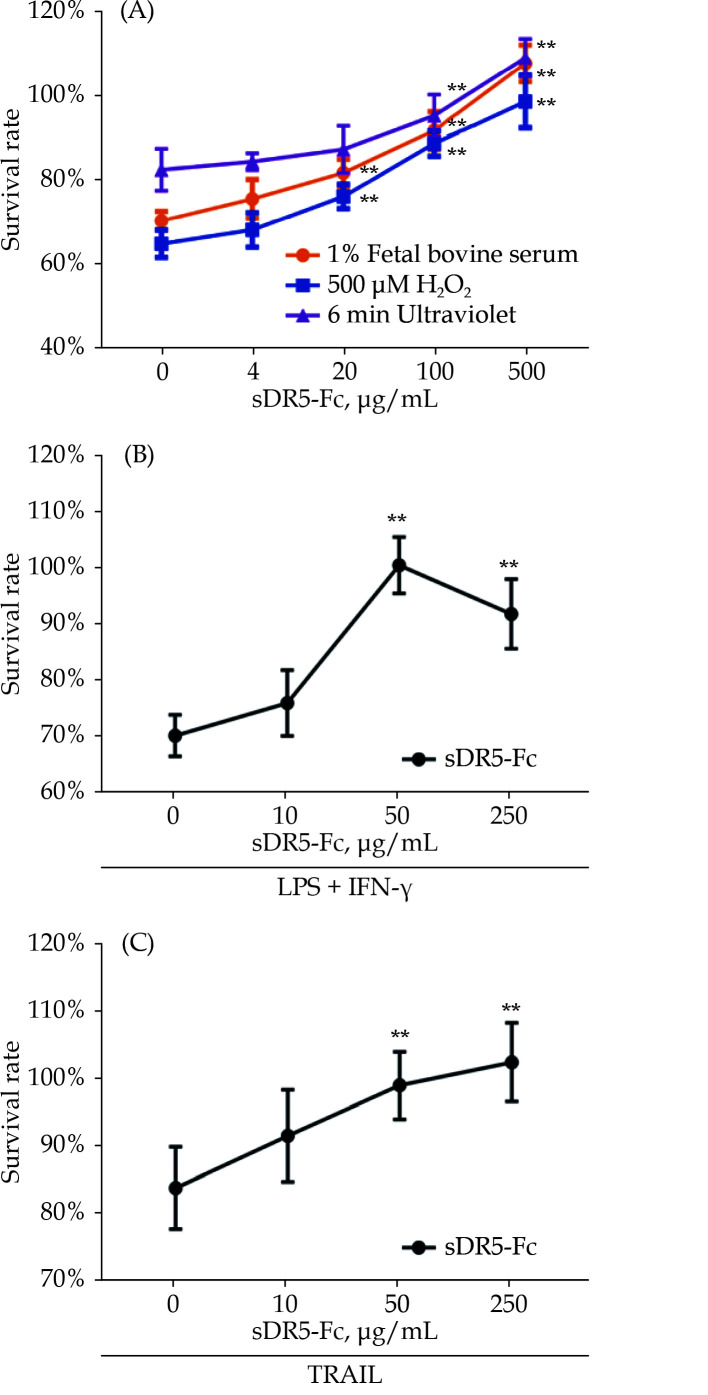
In order to investigate the effects of sDR5-Fc on the proliferation of macrophages under extreme conditions, we applied the nutrition deficiency, excessive hyperoxide and ultraviolet exposure to the macrophage cell line RAW264.7. With the increase of sDR5-Fc concentrations, the vitality of macrophages was significantly recovered with a dose-dependent manner (Figure 1A). Meanwhile, RAW264.7 cells were treated with LPS and IFN-γ to induce M1 polarization, which were characterized with low viability. Interestingly, sDR5-Fc significantly improved the survival rates of M1 macrophages (Figure 1B). The binding of TRAIL with DR5 was regarded as the trigger of cell apoptosis. We further analyzed the effect of sDR5-Fc on TRAIL-induced polarization of macrophages. As shown in Figure 1, the apoptosis process was significantly alleviated by sDR5-Fc (Figure 1C).
Figure 1.
sDR5-Fc recovered the proliferation of macrophage under extreme conditions in a dose-dependent manner.
All samples were exposed in different conditions for 24 h firstly and then treated with different concentrations of sDR5-Fc. (A): RAW264.7 cells were cultured in 1% fetal bovine serum (orange), 500 μM H2O2 (purple) or ultraviolet exposure (blue) separately; (B): RAW264.7 cells were induced with LPS and IFN-γ; and (C): RAW264.7 cells were supplemented with TRAIL. Data are expressed as mean ± SD from at least three representative experiments. **P < 0.01, compared with the group of sDR5-Fc with indicated concentration of 0 μg/mL. IFN-γ: interferon-gamma; LPS: lipopolysaccharide; sDR5-Fc: soluble death receptor 5-Fc; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand.
sDR5-Fc Inhibits the M1 Polarization and Normal Function of Macrophages
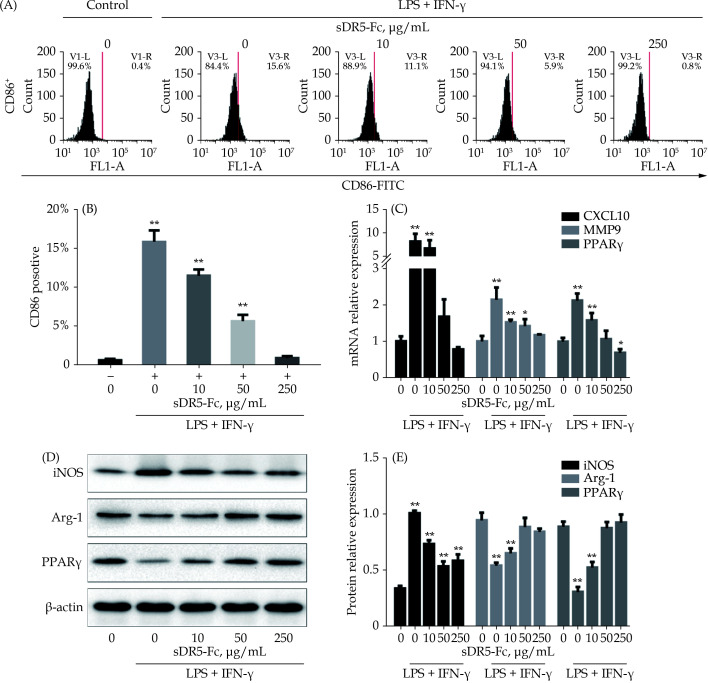
To confirm the effect of sDR5-Fc on inhibition of M1 polarization of macrophage, we stimulated the RAW264.7 cells with LPS and IFN-γ for 24 h. The expression levels of CD86, markers of M1 polarization, were significantly increased (Figure 2A & 2B), suggesting successful induction of M1 polarization. Administration of sDR5-Fc dose-dependently recovered the LPS and IFN-γ-induced M1 polarization, as indicated by the decreased expression of CD86 (Figure 2A & 2B).
Figure 2.
sDR5-Fc inhibited the polarization of macrophages to M1 type in a dose-dependent manner.
All samples were simulated with LPS + IFN-γ for 24 h firstly and then treated with different concentrations of sDR5-Fc. (A & B): The ratio of M1 macrophages cells marked with CD86 were analyzed and counted using flow cytometry; (C): the relative expression of mRNA of CXCL10, MMP9 and PPARγ in different groups; and (D & E): the expression of proteins of iNOS, Arg-1 and PPARγ in different groups. Data are expressed as mean ± SD from at least three representative experiments. *P < 0.05, **P < 0.01, compared with the group of sDR5-Fc and LPS + IFN-γ with indicated concentration of 0 μg/mL. Arg-1: arginase-1; CXCL10: CXC motif chemokine ligand 10; IFN-γ: interferon-gamma; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; MMP9: matrix metalloproteinases 9; PPARγ: peroxisome proliferator-activated receptor gamma; sDR5-Fc: soluble death receptor 5-Fc.
In addition, we further tested other markers of M1 polarization of macrophage including CXC motif chemokine ligand 10 (CXCL10), matrix metalloproteinases 9 (MMP9), PPARγ, iNOS, and Arg-1. The results showed that sDR5-Fc significantly decreased the mRNA expression of CXCL10, MMP9, PPARγ (Figure 2C) and the protein expression of iNOS, Arg-1 and PPARγ (Figure 2D & 2E).
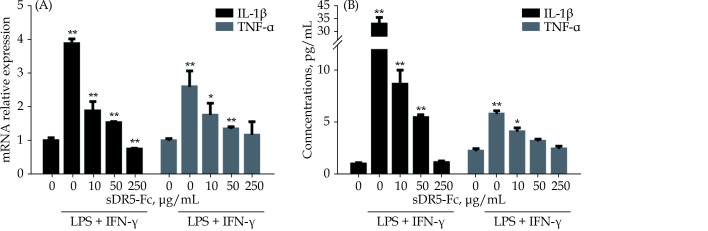
sDR5-Fc Suppresses the Secretion of Inflammatory Factors of M1 Polarization of Macrophages
One of the key steps of M1 macrophages to exert harmful effect is to release a series of inflammatory factors such as IL-1β and TNF-α. We further investigated whether sDR5-Fc played a role in regulating the release of the inflammatory factors in M1 polarization of macrophages. Interestingly, the results demonstrated that sDR5-Fc dramatically inhibited the expression of IL-1β, TNF-α, mRNA and proteins (Figure 3A & 3B).
Figure 3.
sDR5-Fc suppressed the release of IL-1β and TNF-α in M1 macrophages.
All samples were simulated with LPS + IFN-γ for 24 h firstly and then treated with different concentrations of sDR5-Fc. With the growing of sDR5-Fc, IL-1β and TNF-α were suppressed in mRNA (A) and protein level (B). Data are expressed as mean ± SD from at least three representative experiments. *P < 0.05, **P < 0.01, compared with the group of sDR5-Fc and LPS + IFN-γ with indicated concentration of 0 μg/mL. IFN-γ: interferon-gamma; IL-1β: interleukin-1β; LPS: lipopolysaccharide; sDR5-Fc: soluble death receptor 5-Fc; TNF-α: tumor necrosis factor-α.
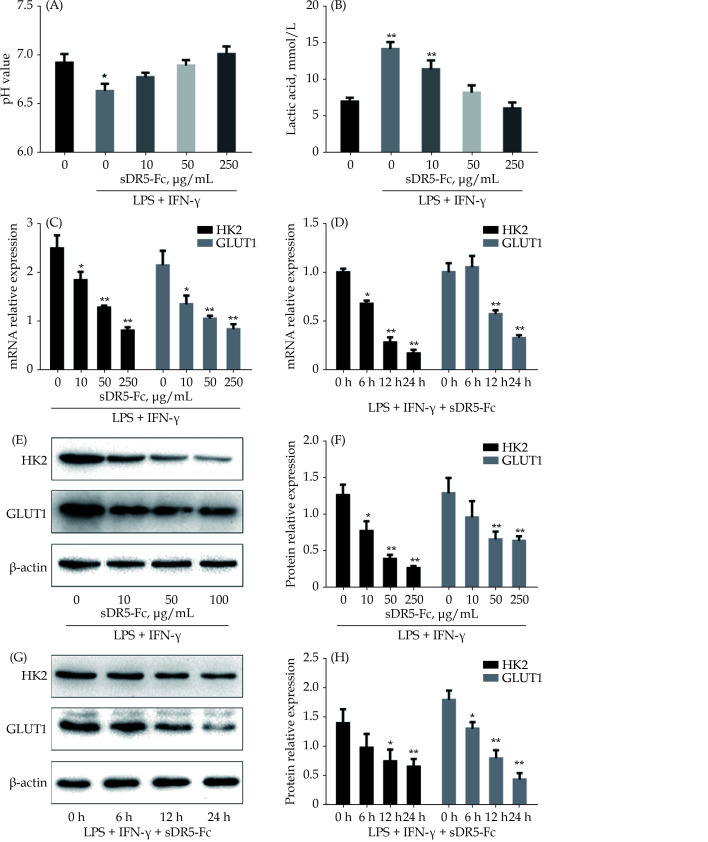
sDR5-Fc Regulates Glycolysis in the M1 Macrophages
As mentioned above, glycolysis is essential for the M1 polarization of macrophages, which is involved in the MIRI, we finally explored the effect of sDR5-Fc on the glycolysis in the M1 macrophages. LPS and IFN-γ-induced M1 polarization of macrophage led to significant decrease of pH and elevation of lactic acid (Figure 4A & 4B). Administration of sDR5-Fc significantly rescued the effects of LPS and IFN-γ on macrophages (Figure 4A & 4B). HK2 and GLUT1 are the key factors in glycolysis. Consistently, sDR5-Fc dose-dependently and time-dependently inhibited the expression of mRNA of HK2 and GLUT1 (Figure 4C & 4D), as well as the protein expression of HK2 and GLUT1 (Figure 4E–4H).
Figure 4.
sDR5-Fc suppressed the glycolysis of M1 macrophages.
All samples were simulated with LPS + IFN-γ for 24 h firstly and then treated with different concentrations of sDR5-Fc. With the growing concentration of sDR5-Fc, pH of culture medium was rising to neutral (A) while the concentrations of lactic acid were reduced (B). sDR5-Fc decreased the mRNA expression of HK2 and GLUT1 in the M1 macrophages with a concentration-dependent manner (C) and time-dependent manner (D). Also, sDR5-Fc decreased the protein expression of HK2 and GLUT1 in the M1 macrophages with a concentration-dependent manner (E & F) and time-dependent manner (G & H). Data are expressed as mean ± SD from at least three representative experiments. *P < 0.05, **P < 0.01, compared with the group of sDR5-Fc and LPS + IFN-γ with indicated concentration of 0 μg/mL. GLUT1: glucose transporter 1; HK2: hexokinase 2; IFN-γ: interferon-gamma; LPS: lipopolysaccharide; sDR5-Fc: soluble death receptor 5-Fc.
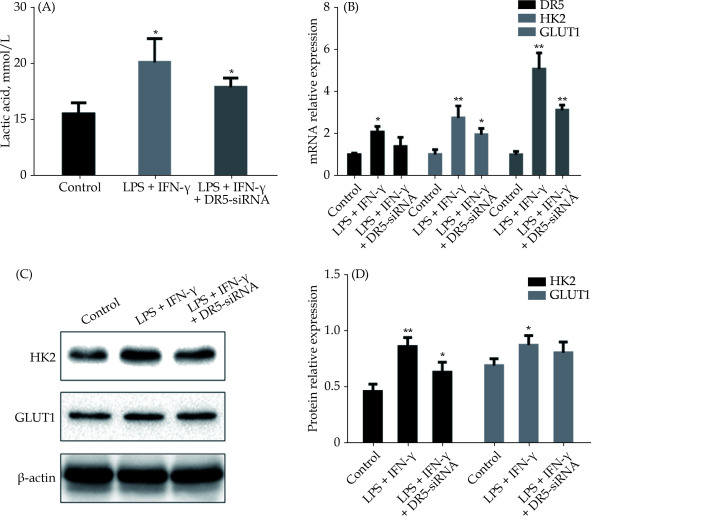
To further confirm the essential role of DR5 in the glycolysis in M1 macrophages, we applied small interfering RNA technology to knock-down the DR5. Interestingly, knock-down of DR5 recovered the increased pH (Figure 5A) and the mRNA expression of HK2 and GLUT1 (Figure 5B). Also, the levels of HK2 and GLUT1 proteins were decreased after DR5 knock-down in M1 polarization of macrophages (Figure 5C & 5D).
Figure 5.
Knock-down of DR5 inhibited the glycolysis of M1 macrophages.
(A): DR5-siRNA significantly reduced the elevated pH induced by LPS + IFN-γ; (B): DR5-siRNA reduced the elevated expression of mRNA of HK2 and GLUT1 induced by LPS + IFN-γ; and (C & D): DR5-siRNA reduced the elevated expression of HK2 and GLUT1 proteins in M1 macrophages. Data are expressed as mean ± SD from at least three representative experiments. *P < 0.05, **P < 0.01, compared with the control group. DR5: death receptor 5; GLUT1: glucose transporter 1; HK2: hexokinase 2; IFN-γ: interferon-gamma; LPS: lipopolysaccharide; sDR5-Fc: soluble death receptor 5-Fc; siRNA: small interfering RNA.
DISCUSSION
In this study, we found that sDR5-Fc, a competitive agent of DR5, can rescue the survival rate of macrophages under extreme conditions. Furthermore, sDR5-Fc inhibits the M1 polarization of macrophages and the secretion of inflammatory factors. These effects may be mediated by the effect of sDR5-Fc on inhibition of glycolysis in the M1 macrophages, owing to a series of markers including pH, lactic acid, expression of HK2 and GLUT1 were recovered by the sDR5-Fc in M1 polarization of macrophages. These results indicated that sDR5-Fc may be a promising agent to inhibit cell death caused by various factors, which has the potential application in clinical practice for the cell death-related diseases. MIRI can cause outbreak of inflammatory responses and cascade amplification reactions by reactive oxygen species and other harmful free radicals.[22] Although the serious consequences of MIRI have been recognized clinically, and many strategies have been developed and used for reducing MIRI, most of the methods have not been widely applied in clinical applications.[3,5] Therefore, developing clinical drugs, studying the damage mechanism and treatment strategies of MIRI, and reducing the serious consequences caused by MIRI are of great clinical significance.
Macrophages are the major components of innate immunity, and can be divided into M1 and M2 subtypes due to the high capacity of plasticity.[23] More and more evidence showed that M1 polarization deteriorated the tissue injury in liver[23] and kidney[24] repair. As for the cardiac muscles, reducing the M1 macrophages or increasing the M2 macrophages can significantly alleviate the damage of cardiac function induced by MIRI.[11,12,25] Outbreak of main factors during MIRI such as free radicals, mitochondrial damage, energy metabolism disorder and autophagy can promote to the M1 polarization of macrophages, release of reactive oxygen species and a series of relevant factors, including IL-1β, IL-6, IL-12, IL-23, IL-27, TNF-α, CXCL3, CXCL9, CXCL10, CXCL11, MMP1, MMP2, MMP7, MMP9, and MMP12.[26–28] Also, nicotinamide adenine dinucleotide phosphate oxidase and iNOS were produced during these processes.[10] Inflammatory damage and even inflammatory storms caused by this series of inflammatory factors are the main factors leading to death. Therefore, M1-type macrophages play a key role in MIRI, and inhibiting the over-polarization of macrophages into M1-type can be an effective method to repair MIRI. Here, the sDR5-Fc has been proved to be effective in blocking the M1 polarization of macrophages, suggesting a promising strategy for the treatment of MIRI.
DR5 is a family of surface receptors discovered in recent years. By binding with its ligand, TRAIL, it can transduce signals to cells and induce apoptosis.[29] Studies have shown that the production of TRAIL and the increase of DR5 expression were commonly observed during the processes of MIRI, suggesting that inhibition of DR5 may be effective to reduce the MIRI. Also, DR5 can mediate the signal transduction in macrophages to promote the M1 polarization, which were pro-inflammatory effects.[17,18,30] Previous studies confirmed the elevated expression of TRAIL and DR5 in mice with cerebral ischemia-reperfusion.[20] Therefore, it is reasonable to speculate that interfering with DR5 by the competitive agent sDR5-Fc can rescue the survival of macrophages in MIRI. Indeed, our results showed that sDR5-Fc dose-dependently recovered the LPS and IFN-γ-induced M1 polarization. More importantly, sDR5-Fc significantly inhibited the effects of IL-1β and TNF-α in M1 macrophages, further confirming its role in down-regulating the inflammatory responses by inhibiting the M1 polarization. However, the mechanisms underlying sDR5-Fc mediated M1 polarization inhibition are unknown. Since the pro-inflammatory responses involve a large number of complicated signal pathways and MIRI is closely related to the energy metabolism, we focused on the regulatory role of sDR5-Fc in the glycolysis.
Metabolic reprogramming plays an important role in the control of immune responses, especially the innate responses.[31] When there is lack of oxygen, the lactate was produced from pyruvate during the process of glycolysis. Phosphatidylinositol-3-kinase/Akt pathway promotes, but adenosine monophosphate-activated protein kinase pathway blocks, the cells from a metabolic state under normal oxygen to deficiency of oxygen,[32] in which inflammatory factors such as IL-10 were involved.[33] Thus, glycolysis may be the key factor determining the polarization direction of macrophages. Here, we found that sDR5-Fc can significantly recover the decreased pH and elevated lactic levels in LPS and IFN-γ-induced M1 macrophages. These results enriched our understanding of the relationship between M1 polarization and glycolysis. But the complex signaling pathways in this process are not clarified, which should be the focus of research in the future.
CONCLUSIONS
In conclusion, we confirmed the role of sDR5-Fc, a competitive agent of DR5, in inhibition of M1 polarization of macrophage by regulating the glycolysis. On one hand, these results provide new insights into the mechanisms of M1 polarization and the important role of glycolysis in this process. On the other hand, our results provide new directions for developing strategies to treat the MIRI in AMI. Future studies should further confirm the effects of sDR5-Fc on alleviating the MIRI through regulation of M1 polarization and glycolysis and clarify the mechanisms underlying these processes.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of Beijing, China (No.7212027 & No.7214223), National Key Research and Development Program of China (2017YFC0908800), and the Beijing Municipal Health Commission (PXM2020_026272_000002 & PXM2020_026272_000014). All authors had no conflicts of interest to disclose.
References
- 1.Reed GW, Rossi JE, Cannon CP Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Chang G, Gao L, et al Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy. J Mol Med (Berl) 2018;96:791–806. doi: 10.1007/s00109-018-1664-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Chen B, Yang X, et al S100a8/a9 signaling causes mitochondrial dysfunction and cardiomyocyte death in response to ischemic/reperfusion injury. Circulation. 2019;140:751–764. doi: 10.1161/CIRCULATIONAHA.118.039262. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Zheng YY, Song YT, et al Pretreatment with low-dose gadolinium chloride attenuates myocardial ischemia/reperfusion injury in rats. Acta Pharmacol Sin. 2016;37:453–462. doi: 10.1038/aps.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Couto G, Liu W, Tseliou E, et al Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–3162. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski FK, Nahrendorf M Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulsmans M, Clauss S, Xiao L, et al Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FO, Gordon S The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Zhou D, Xie X, et al Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol. 2016;111:63. doi: 10.1007/s00395-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peet C, Ivetic A, Bromage DI, et al Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116:1101–1112. doi: 10.1093/cvr/cvz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue Y, Huang S, Wang L, et al M2b macrophages regulate cardiac fibroblast activation and alleviate cardiac fibrosis after reperfusion injury. Circ J. 2020;84:626–635. doi: 10.1253/circj.CJ-19-0959. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Li F, Jian Y, et al Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. Eur J Pharmacol. 2020;871:172916. doi: 10.1016/j.ejphar.2020.172916. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Bories G, Lantz C, et al Immunometabolism of phagocytes and relationships to cardiac repair. Front Cardiovasc Med. 2019;6:42. doi: 10.3389/fcvm.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao P, Zhou W, Zhang Y, et al Aminooxyacetic acid attenuates post-infarct cardiac dysfunction by balancing macrophage polarization through modulating macrophage metabolism in mice. J Cell Mol Med. 2020;24:2593–2609. doi: 10.1111/jcmm.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouton AJ, Li X, Hall ME, et al Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126:789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa K, Liu W, Zhao L, et al Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yang P, Wu Q, et al Death receptor 5-targeted depletion of interleukin-23-producing macrophages, Th17, and Th1/17 associated with defective tyrosine phosphatase in mice and patients with rheumatoid arthritis. Arthritis Rheum. 2013;65:2594–2605. doi: 10.1002/art.38057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Chen H, Gao J, et al Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27–41. doi: 10.1016/j.yjmcc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Graves JD, Kordich JJ, Huang TH, et al Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity. Cancer Cell. 2014;26:177–189. doi: 10.1016/j.ccr.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Cui M, Wang L, Liang X, et al Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol Dis. 2010;39:138–147. doi: 10.1016/j.nbd.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Falcone DJ Heparin stimulation of plasminogen activator secretion by macrophage-like cell line RAW264.7: role of the scavenger receptor. J Cell Physiol. 1989;140:219–226. doi: 10.1002/jcp.1041400205. [DOI] [PubMed] [Google Scholar]
- 22.Fondevila C, Shen XD, Tsuchihashi S, et al The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008;14:1133–1141. doi: 10.1002/lt.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun YY, Li XF, Meng XM, et al Macrophage phenotype in liver injury and repair. Scand J Immunol. 2017;85:166–174. doi: 10.1111/sji.12468. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Huen S, Nishio H, et al Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Xing H, Mao X, et al Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model. Scand J Immunol. 2015;81:31–38. doi: 10.1111/sji.12245. [DOI] [PubMed] [Google Scholar]
- 26.Chistiakov DA, Myasoedova VA, Revin VV, et al The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223:101–111. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y, Guo H, Wang L, et al Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav Immun. 2017;60:346–360. doi: 10.1016/j.bbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Le Y, Cao W, Zhou L, et al Infection of mycobacterium tuberculosis promotes both M1/M2 polarization and MMP production in cigarette smoke-exposed macrophages. Front Immunol. 2020;11:1902. doi: 10.3389/fimmu.2020.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mert U, Sanlioglu AD Intracellular localization of DR5 and related regulatory pathways as a mechanism of resistance to TRAIL in cancer. Cell Mol Life Sci. 2017;74:245–255. doi: 10.1007/s00018-016-2321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y, Chen X, Fan M, et al TRAIL facilitates cytokine expression and macrophage migration during hypoxia/reoxygenation via ER stress-dependent NF-kappaB pathway. Mol Immunol. 2017;82:123–136. doi: 10.1016/j.molimm.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Biswas SK, Mantovani A Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 33.Murray PJ Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]