Abstract
Background
Paclitaxel treatment produces significant peripheral neuropathy, but the time course of neuropathy development and outcomes are unclear. Dose reduction is the only strategy to prevent neurotoxicity, however, the impact of dose‐reduction on neuropathy outcomes remains unknown. This study aimed to prospectively evaluated neuropathy development from weekly paclitaxel treatment and evaluate the impact of dose‐reduction on post‐treatment neuropathy outcomes.
Patients and Methods
Breast cancer patients receiving paclitaxel (80mg/m2) weekly for 12‐weeks were prospectively assessed using patient reported (Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity; FACTGOG‐Ntx), clinical (Total Neuropathy Score clinical version; TNSc) and neurophysiological measures up to 12‐months post completion. The impact of dose‐reduction on post‐treatment (3.6 ± 0.1 months) clinical and patient reported outcomes was evaluated in 105 weekly paclitaxel‐treated patients.
Results
Significant neuropathy was present by 6‐weeks across patient‐reported, clinical, and objective neurophysiological assessments, increasing in prevalence and severity over the treatment course. Limited recovery occurred, with significant neuropathy being maintained up to 12 months (p < .05). Patients who received dose reduction had worse patient reported (FACT‐GOG‐Ntx: 40.2 ± .1.4) and clinical neuropathy outcomes (TNSc: 4.3 ± 0.4) compared to those who received the full dose (FACT‐GOG‐Ntx: 45.9 ± 0.9; TNSc: 3.3 ± 0.3, p < .05). Patients who ceased treatment early demonstrated the worse deficits (TNSc: 5.0 ± 0.6; FACT‐GOG‐Ntx: 37.3 ± 2.7) compared to those who received the complete dose (TNSc: 3.5 ± 0.3; FACT‐GOG‐Ntx: 45.3 ± 0.9, p < .05).
Conclusion
Weekly paclitaxel produces symptomatic and objective neuropathy early in the treatment course which can persist. Dose reduction does not necessarily lead to more favorable neuropathy outcomes, with individual risk factors likely important in addition to cumulative dose.
Implications for Practice
Weekly paclitaxel schedules are extensively used in breast cancer. Patients may develop symptomatic and objective neuropathy early in the treatment course, with these individuals requiring closer monitoring. Furthermore, neuropathy is a long‐term sequela that may impact quality of life and require appropriate supportive services. Results suggest that dose reduction does not necessarily lead to better neuropathy outcomes. Understanding schedule‐specific toxicity and risk factors for neuropathy will be critical to determining individualized treatment strategies and improving quality of life in breast cancer survivors.
Keywords: Paclitaxel, Chemotherapy, Neuropathy, Dose reduction, Treatment, Neurotoxicity
Short abstract
This article evaluates the development of peripheral neuropathy related to paclitaxel treatment and the effect of dose reduction on post‐treatment neuropathy outcomes.
Introduction
Chemotherapy‐induced peripheral neuropathy (CIPN) is a common and debilitating side effect of neurotoxic cancer treatment. Estimated to occur in up to 80% of paclitaxel‐treated patients with breast cancer [1, 2], neuropathy symptoms can interfere with function, increasing the risk of falls [3] and reducing quality of life [1, 4, 5]. However, despite a high incidence, patient impact and outcomes remain poorly understood. Many prior studies of CIPN have lacked comprehensive quantitative CIPN assessment measures, sufficient follow‐up time, and treatment homogeneity [6], limiting their ability to provide clinically useful prognostic information.
Paclitaxel‐induced CIPN has a major impact on treatment tolerability, resulting in dose reduction and early cessation [4, 7]. Approximately 25% of patients with breast cancer receive reductions to their adjuvant paclitaxel treatment because of CIPN [8], which may affect clinical and survival outcomes. Dose reduction is the only current strategy to mitigate CIPN and relies on accurate identification of CIPN symptoms. However, CIPN can be challenging for clinicians to identify, evaluate, and manage. As there is no consensus on a gold standard CIPN assessment tool [9, 10, 11] and no defined objective thresholds or pharmacokinetic parameters to identify risk of CIPN [12, 13], clinicians depend on clinical experience to provide the maximum effective dose while ensuring patient quality of life. However, accurately identifying patients at risk of severe neuropathy remains a challenge, and neuropathy outcomes for those who receive dose reduction remain ill defined.
Weekly paclitaxel administration has become the most commonly used regime for adjuvant treatment of early breast cancer, demonstrating superiority over other schedules in both disease‐free progression and overall survival [2]. However, the incidence and severity of paclitaxel‐induced CIPN has been linked to increased cumulative dose and the frequency of exposure, with those receiving weekly administration demonstrating greater neuropathy than other schedules [2, 14, 15]. Given that the 5‐year survival rate for patients with early breast cancer is greater than 90% [16], the impact of CIPN on function and quality of life is a significant consideration for this population. A comprehensive understanding of the clinical manifestations and outcomes of weekly paclitaxel‐induced neuropathy may provide clinicians with resources to better inform treatment decisions and counsel patients [17]. Accordingly, this study prospectively evaluated neuropathy development and deficits in patients with breast cancer during weekly paclitaxel treatment. Furthermore, we evaluated the impact of dose reduction on posttreatment clinical and patient‐reported neuropathy outcomes.
Materials and Methods
Patients and Study Design
Patients with breast cancer who were prescribed monotherapy with 80 mg/m2 paclitaxel weekly for 12 weeks undertook comprehensive clinical and neurophysiological assessments. Patients were included if they had no evidence of preexisting polyneuropathy or any prior neurotoxic chemotherapy treatment. During the course of the study, no patients received additional neurotoxic chemotherapy such as platinum agents or additional lines of taxane‐based therapy. However, patients were able to receive non‐neurotoxic cancer treatment such as trastuzumab. Written informed consent was obtained in accordance with the Declaration of Helsinki, with studies approved by the Sydney Local Health District Human Research Ethics Committee and South Eastern Sydney Local Health District Human Research Ethics Committee.
Eighty‐three patients were prospectively recruited prior to cycle 1 of paclitaxel for a longitudinal analysis of the natural progression and recovery of paclitaxel‐induced peripheral neuropathy. Patients were assessed at baseline (week 0), midtreatment (week 6), final treatment (week 12), and post‐treatment at 3, 6, and 12 months, with follow‐up assessments only conducted in patients who did not recommence neurotoxic treatment. In addition, 23 patients who had completed weekly paclitaxel cancer treatment underwent a one‐off cross‐sectional assessment postpaclitaxel completion (Fig 1; Table 1). Cross‐sectional evaluations were undertaken between 3 to 6 months postcompletion of paclitaxel treatment. Cross‐sectional patients were comparable to the prospectively recruited cohort in terms of cumulative dose, age, and neuropathy severity based on patient report and clinical examination (supplemental online Table 1). To analyze the impact of dose reduction, cross‐sectionally assessed patients were analyzed together with data from prospectively assessed patients at the 3‐ or 6‐month postpaclitaxel completion time point, for a total of 105 paclitaxel‐treated patients with breast cancer.
Figure 1.
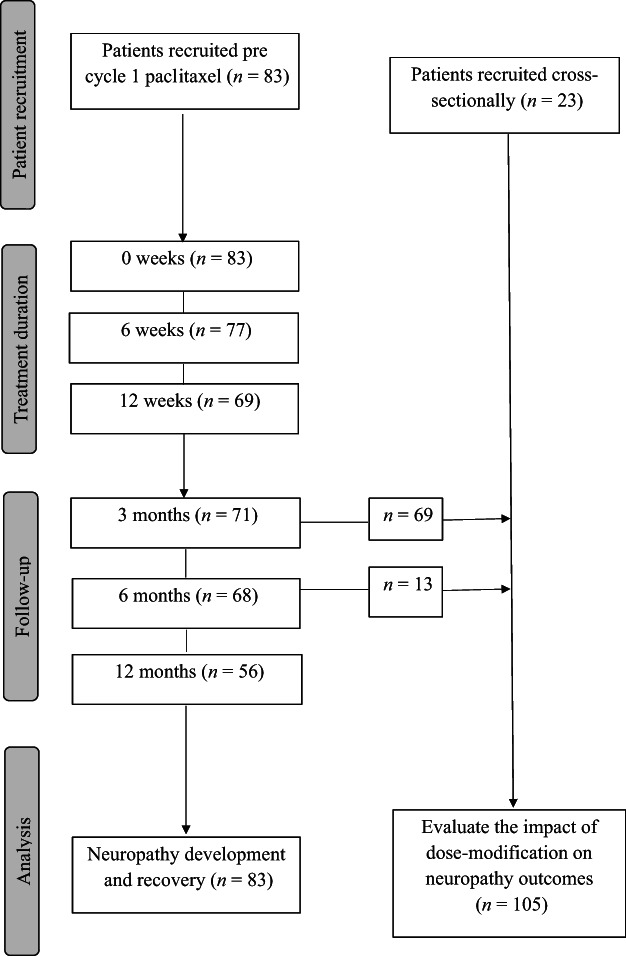
Consort diagram.
Table 1.
Patient characteristics
| Demographic data | Values |
|---|---|
| Age, yr | |
| Mean ± SE (range) | 52.7 ± 1.2 (28–76) |
| BMI, kg/m2 | |
| Mean ± SE (range) | 27.8 ± .64 (18.6–51.4) |
| Diabetes (n, %) | 7 (6.7) |
| Prescribed CIPN treatment, n (%) a | 9 (8.6) |
| Breast cancer stage, n (%) | |
| I | 6 (5.7) |
| II | 47 (44.8) |
| III | 37 (35.2) |
| IV | 7 (6.7) |
| Unknown | 8 (7.6) |
| Receptor status, n (%) | |
| ER positive | 71 (67.6) |
| PR positive | 58 (55.2) |
| HER2 positive | 27 (25.7) |
| Unknown | 3 (2.85) |
| Time since taxane treatment, mo | |
| Mean ± SE (range) | 3.57 ± .16 (2–8) |
| Cumulative paclitaxel dose, mean ± SE (range), mg/m2 | |
| Prospective cohort (n = 83) | 861.8 ± 15.9 (474–960) |
| Whole cohort (n = 105) | 848.9 ± 14.8 (400–960) |
| No dose modification (n = 53) | 960.0 ± 0 |
| Any dose modification (n = 52) | 736.0 ± 20.0 (400–940) |
| Early cessation (6–9 cycles) (n = 19) | 582.1 ± 29.9 (400–720) |
| Late cessation (10–11 cycles) (n = 25) | 817.8 ± 12.6 (660–880) |
| No treatment discontinuation (n = 61) | 944.7 ± 5.6 (760–960) |
| Reason for dose modification, n (%) | |
| Neuropathy | 38 (36.2) |
| Other b | 14 (13.3) |
Patient taking medication to treat CIPN at time of assessment including pregabalin and gabapentin.
Neutropenia or thrombocytopenia (n = 3), fluid retention (n = 2), patient travel (n = 2), abnormal liver function (n = 1), arthralgias (n = 1), cardiovascular concerns (n = 1), diarrhea (n = 1), fever (n = 1) and unspecified (n = 2).
Abbreviations: BMI, body mass index; CIPN, chemotherapy‐induced peripheral neuropathy; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Dosing Information
Demographic and paclitaxel dose data were collected from medical records. Dose reductions or cessations were recorded with the reason for the modification. Patients were classified as having “no dose reduction,” “reduction due to peripheral neuropathy,” or “reduction due to other causes.” Patient who discontinued treatment prior to receiving all 12 prescribed cycles were classified as ceasing early (ceasing between cycle 6–9) or late (ceasing between cycle 10–11) in the treatment course.
Assessment of Neurotoxicity
Neuropathy burden was assessed via the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (FACT‐GOG‐Ntx; range, 0–52), a validated patient‐reported outcome [18, 19]. Lower scores were associated with greater symptomatic burden, with a 10% reduction in scores from baseline being considered clinically significant [20]. The Total Neuropathy Score clinical version (TNSc; Johns Hopkins University), a validated composite measure of patient symptom report and neurological assessment, was used to clinically assess neuropathy (supplemental online Methods) [21, 22], with higher scores indicating greater neuropathy severity (range, 0–24) [21, 22]. Nerve conduction studies (NCSs) were undertaken as previously described [23], recording compound sensory nerve action potential amplitudes from the sural nerve. Specific tests of distal sensation were performed using von Frey monofilaments in the upper limb and two‐point discrimination in the lower limb (supplemental online Methods).
Statistical Analysis
Generalized estimating equations [24] were used to evaluate the progression of neuropathy over time. Results are presented as predicted mean estimate of change from baseline (week 0). An exchangeable correlation structure was used to account for repeated measures. Baseline sural NCSs were added as a covariate to account for the variation in starting values. Two‐tailed Mann‐Whitney U tests were conducted to confirm that those with missing data at 12 months post‐treatment did not differ significantly in dose, age, or neuropathy severity (supplemental online Table 2). To evaluate the impact of dose reduction, nonparametric data were analyzed using two‐tailed Mann‐Whitney U tests to investigate the differences between patients with respect to dosing. Chi‐square tests of independence were used to examine relationships with symptom severity. Statistical analysis was performed using SPSS (Version 17; IBM, Armonk, NY), with statistical significance set at p ≤ .05 and results presented as mean with SE, unless otherwise specified.
Results
Development and Recovery Profile of Weekly Paclitaxel‐Induced Peripheral Neuropathy
The natural history of neuropathy development and recovery was documented using comprehensive, multimodal assessment tools. Weekly paclitaxel‐treated patients (n = 83, Table 1) underwent baseline assessment (week 0), with further assessments carried out after 6 ± 0.1 and 12 ± 0.2 weeks of treatment (Fig. 1). Follow‐up assessments were conducted at 3 ± 0.2, 6 ± 0.1, and 12 ± 0.2 months postcompletion of paclitaxel treatment.
Development of Neuropathy
Based on scores from the FACT‐GOG‐Ntx, 54.5% (n = 42) of patients reported numbness and tingling in the hands by week 6 of treatment, with 13% (n = 10) reporting “quite a bit” or “very much.” Similarly, 58.4% (n = 45) reported symptoms in the feet, with 13% (n = 10) reporting greater severity (Fig 2A). Interestingly, some patients were already reporting functional deficits, such as difficulty feeling the shape of small objects (14.3%, n = 11) and problems with walking (11.7%, n = 9; Fig. 2B).
Figure 2.
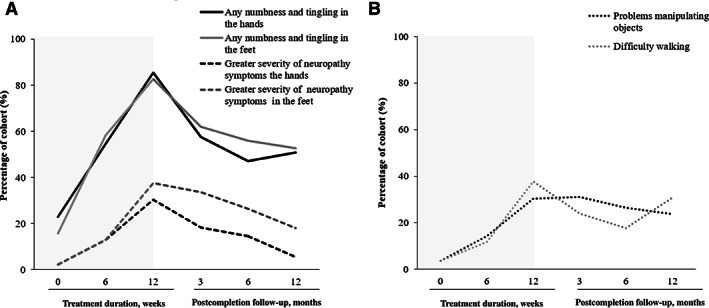
(A): The percentage of patients reporting any (solid) numbness and tingling in the hands and feet and those reporting greater severity of symptoms in the hands and feet (dash). (B): The percentage of patients reporting functional impairments in the upper limb (difficulty manipulating objects) and lower limb (problems walking) during paclitaxel treatment (0–12 weeks) and postcompletion (3–12 months)
Concurrently, overall patient‐reported neuropathy burden increased, with a decline in FACT‐GOG‐Ntx scores (lower scores indicating worse neuropathy) of 4.1 points (Table 2) by week 6. Objective evidence of neuropathy also developed by week 6, with a mean increase in total neuropathy scores consistent with mild neuropathy (1.9 points, p < .05; Fig 3A) [25]. Similarly, a significant reduction in sural amplitude (−3.0μV, p < .05; Table 2) was also evident by 6 weeks, suggestive of early axonal dysfunction.
Table 2.
Mean estimates of the change in neuropathy outcomes since the beginning of paclitaxel treatment (week 0)
| FACT‐GOG‐NTX‐13 | TNSc | CSAP sural amplitude, μV | Two‐point discrimination, mm | Von Frey, mN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | 95% CI | Mean ± SE | 95% CI | Mean ± SE | 95% CI | Mean ± SE | 95% CI | Mean ± SE | 95% CI | |
| Wk 0 (n = 83) | 49.4 | (48.7 to 50.1) | 1.2 ± .2 a | (.91 to 1.5) | 18.7 | (16.4 to 20.9) | 8.4 | (7.8 to 9.1) | .37 | (.29 to .45) |
| Wk 6 (n = 77) | −4.1 ± .6 a | (−5.2 to −2.8) | 1.9 ± .2 a | (1.4 to 2.3) | −3.0 ± .8 a | (−4.7 to −1.4) | 0.3 ± .4 | (‐0.5 to 1.0) | .26 ± .16 a | (.14 to .38) |
| Wk 12 (n = 69) | −9.7 ± .9 a | (‐11.5 to −7.9) | 3.6 ± .3 a | (2.9 to 4.2) | −5.7 ± 1.0 a | (−7.7 to −3.8) | 1.6 ± .5 a | (0.7 to 2.5) | .60 ± .21 a | (.18 to 1.0) |
| 3 mo post‐treatment (n = 71) | −5.0 ± .8 a | (‐6.5 to −3.5) | 2.4 ± .3 a | (1.8 to 2.9) | −4.9 ± .9 a | (‐6.7 to −3.1) | 2.2 ± .5 a | (1.3 to 3.1) | .31 ± .14 a | (.04 to .58) |
| 6 mo post‐treatment (n = 68) | −4.8 ± .8 a | (‐6.4 to −3.2) | 1.9 ± .3 a | (1.4 to 2.6) | −3.9 ± 1.1 a | (‐6.1 to −1.8) | 1.7 ± .5 a | (0.8 to 2.5) | .33 ± .21 | (−.08 to .74) |
| 12 mo post‐treatment (n = 55) | −4.8 ± .8 a | (−6.4 to −3.2) | 1.8 ± .2 a | (1.1 to 2.5) | −2.9 ± 1.2 a | (−5.2 to −0.5) | 2.2 ± .5 a | (1.3 to 3.1) | .37 ± .36 | (−.34 to 1.1) |
Mean estimates of change from the predicted mean at week 0, presented with SE and 95% confidence intervals. FACT‐GOG‐NTX‐13: range, 0–52 points. Lower scores associated with greater symptomatic burden. TNSc: range, 0–24 points. Higher scores indicate greater neuropathy severity of neuropathy. CSAP sural amplitude: measured from the sural nerve. Baseline sural amplitude was added a covariate to improve the fit of the model. Two‐point discrimination: larger distance (mm) indicate greater sensory deficit. Von Frey monofilament: Higher force (mN) indicates greater sensory deficit.
Indicates significant the change from week 0 (p < .05). Predicted means for other measured are presented in appendix 1.
Abbreviations: CI, confidence interval; CSAP, compound sensory nerve action potential; FACT‐GOG‐NTX‐13, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity questionnaire; TNSc, Total Neuropathy Score clinical version.
Figure 3.

(A): Predicted means and 95% confidence intervals for the TNSc (higher scores indicate greater neuopathy severity) and patient‐reported neuropathy burden (FACT/GOG‐Ntx score; lower scores indicate greater neuropathy burden). Assessments collected during treatment (weeks) and at postcompletion (months). (B): Predicted means and 95% confidence intervals for von Frey monofilaments (mN) preformed in the upper limb and two‐point discrimination (mm) preformed in the lower limb. Great values indicating greater deficits. Assessments collected during treatment (weeks) and at postcompletion (months). Abbreviations: FACT/GOG‐Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity; TNSc, Total Neuropathy Score clinical version.
Consistent with patient reports of neuropathy symptoms in the hands, distal sensation in the upper limbs had worsened significantly from baseline (week 0), with an increase in mechanical detection threshold of .26 mN (p < .05; Table 2). However, there were no significant changes observed in lower limb sensation (two‐point discrimination) from baseline after 6 weeks of paclitaxel (Fig. 3B; Table 2).
After completing 12 weeks of treatment, 85.5% (n = 59) of patients reported numbness and tingling in their hands, with 82.6% (n = 57) reporting symptoms in their feet. Correspondingly, more patients reported severe symptoms (hands: 30.4%, n = 21; feet 37.7%, n = 26; Fig. 2A) and functional impairment (difficulty manipulating objects 30.4%, n = 21; problems with walking 37.7%, n = 26; Fig. 2B).
Overall patient‐reported symptom burden also peaked in severity at the end of 12 weeks of paclitaxel, with the average change from baseline representing a clinically significant deficit (−9.7 points, p < .05; Table 2) [20]. Consistent with patient‐reported symptom burden, objective neuropathy scores also increased maximally from baseline (3.6 points, p < .05; Fig. 3A), as did sensory deficits in the upper limbs (.60 mN, p < .05; Fig. 3B). Lower limb sensation also demonstrated significant deficits compared with baseline with increased detection threshold (1.6 mm, p < .05; Fig. 3B). Similarly, sensory nerve amplitudes demonstrated the greatest decline from baseline (−5.7 μV, p < .05; Table 2).
Recovery Profile
Following completion of weekly paclitaxel treatment, there was some evidence of symptomatic recovery, with a reduction in the proportion of patients reporting neuropathy symptoms at 3 months postcompletion, compared with week 12. However, 57.7% (n = 41) of patients reported residual neuropathy in the hands and 62% (n = 44) in the feet, with more patients reporting greater severity of symptoms in the feet (33.8%, n = 24) compared with the hands (18.3%, n = 13; Fig. 2A). Functional difficulties were reported by 31% (n = 22) of patients in the hands and by 23.9% (n = 17) with walking (Fig. 2B).
Six months after paclitaxel completion, approximately half the cohort still reported neuropathy symptoms (hands: 47.1%, n = 32; feet: 55.9%, n = 38), which were maintained up to 12 months postcompletion (hands: 50.9%, n = 28; feet: 52.7%, n = 29; Fig. 2A). Severe symptoms in the feet were still reported by 26.5% (n = 18) of patients at 6 months and 18.1% (n = 10) at 12 months. However, fewer patients reported severe neuropathy symptoms in the hands (6 months: 14.7%, n = 10; 12 months: 5.5%, n = 3), suggesting greater symptomatic improvement in the upper limbs (Fig. 2A)
Correspondingly, deficits in lower limb sensation (two‐point discrimination) increased maximally from baseline at 3 months (2.2 mm, p < .05) and were maintained up to 12 months postcompletion (2.2 mm, p < .05; Fig. 3B). However, by 6 months postcompletion, changes in upper limb sensation (von Frey monofilaments) were no longer significantly different from baseline (6 months: 0.33 mN; 95% confidence interval [CI], −.08 to.74 mN; 12 months: 0.37 mN; 95% CI, −.34 to 1.1 nM, N.S.; Table 2), suggesting variations in the pattern of neuropathy recovery between upper and lower limbs (Fig. 3).
Compared with the end of treatment (12 weeks), some improvement was seen in overall patient‐reported neuropathy burden; however, deficits remained clinically significant from baseline (FACT‐GOG‐Ntx: −5.0 points, p < .05; Table 2) and did not improve over the 12 month follow‐up period (6 and 12 months: −4.8 points, p < .05, Fig 3A).
Similarly, despite some improvement, TNSc scores remained significantly elevated from baseline at the 3‐month (2.4 points, p < .05), 6‐month (1.9 points, p < .05), and 12‐month follow‐up assessments (1.8 points, p < .05; Table 2). Correspondingly, sural nerve conduction studies showed continued deficits from week 0 up to 12 months postcompletion (−2.9 μV, p < .05; Table 2), suggesting incomplete recovery of objective and symptomatic neuropathy by 12 months posttreatment.
Dose Reduction and Neuropathy Outcomes
To evaluate the effect of dose reduction on neuropathy outcomes, 105 patients with breast cancer (Table 1) were assessed cross‐sectionally 3.6 ± 0.1 months postpaclitaxel treatment.
In total, 49.5% (n = 52) of these patients experienced paclitaxel dose reduction, with neuropathy accounting for 73.1% of these reductions (n = 38; Table 1). In total, 36.2% of the cohort required dose reduction for neuropathy (n = 38). The dose reduction group received lower cumulative paclitaxel dose (736 ± 20 mg/m2 vs. 960 mg/m2, p < .01), with those discontinuing treatment early (n = 19) in the course receiving the lowest dose (582.1 ± 29.9 mg/m2; Table 1).
At follow‐up, residual neuropathy was reported in 77% (n = 40) of patients who had received any dose reduction and 70% (n = 37) who received the full dose, with those receiving dose reduction more likely to report “quite a bit” or “very much” numbness and tingling in the hands or feet (X2(1, n = 105) = 4.29, p < .05).
Those receiving dose reduction demonstrated significantly greater deficits, based on clinical grading (TNSc: 4.3 ± 0.4), compared with those who received the full dose (TNSc: 3.3 ± 0.3, Mann‐Whitney U = 1,063, p < .05). Concurrently, patient‐reported symptom burden was significantly worse for patients who received a reduced dose (FACT‐GOG‐Ntx dose reduction: 40.2 ± .1.4; full dose: 45.9 ± 0.9, Mann‐Whitney U = 876, p < .05). However, there were no significant differences in specific tests of distal sensation in the upper or lower limbs (N.S.).
Treatment was discontinued for 18% (n = 19) of patients early in the course (6–9 cycles), with the majority of these ceasing because of neuropathy (79%, n = 15). Patients requiring early cessation had significantly worse neuropathy based on clinical grading (TNSc: 5.0 ± 0.6, Mann‐Whitney U = 388.5, p < .05; Fig 4A) and reported greater overall neuropathy burden (FACT‐GOG‐Ntx: 37.3 ± 2.7, Mann‐Whitney U = 341, p < .05; Fig. 4B) compared with those who received all cycles regardless of dose reduction (n = 61, TNSc: 3.5 ± 0.3; FACT‐GOG‐Ntx: 45.3 ± 0.9). However, these differences in patient report and clinical examination were not evident for those who ceased paclitaxel late in the treatment course (10–11 cycles, n = 25), with outcomes being comparable at follow‐up (TNSc: 3.5 ± 0.6, Mann‐Whitney U = 743, N.S; FACT‐GOG‐Ntx: 42.2 ± 1.9, Mann‐Whitney U = 622.5; N.S) to those who did not discontinue treatment (n = 61; Fig. 4A, B). Similarly, those discontinuing early demonstrated significantly worse distal sensation in the lower limbs (two‐point discrimination: 12.3 ± 0.9 mm) compared with those who did not cease treatment (9.76 ± 0.6 mm, Mann‐Whitney U = 262, p < .05), but upper limb sensation did not differ between groups.
Figure 4.

(A): Total neuropathy score clinical version for patients who ceased treatment early (cycle 6–9) or late in the treatment course (cycle 10–11) compared with those who completed 12 cycles of paclitaxel. *p < .05. Higher scores indicate greater neuropathy severity. (B): Fact/GOG‐Ntx for patients who ceased treatment early (cycle 6–9) or late in the treatment course (cycle 10–11) compared with those who completed 12 cycles of paclitaxel. *p < .05. Lower scores indicate greater neuropathy severity. Abbreviation: FACT/GOG‐Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity.
Discussion
This study used comprehensive multimodal assessment to assess neuropathy development and evaluate recovery up to 1 year postcompletion in a homogenous cohort of weekly paclitaxel‐treated patients. Across patient‐reported, clinical, and objective neurophysiological assessments, significant neuropathy was already present by 6 weeks of treatment, increasing in prevalence and severity over treatment. Limited recovery occurred during follow‐up, with significant neuropathy being maintained up to 12 months, with deficits more pronounced in lower limbs. Moreover, neuropathy was a significant dose‐limiting side effect, with more than a third of patients requiring dose reductions because of neuropathy. Three months after treatment, patients who received dose reduction had worse patient‐reported and clinical neuropathy outcomes, with those ceasing treatment early demonstrating the most deficits, despite receiving the lowest cumulative dose.
CIPN Phenotype Associated with Weekly Paclitaxel Administration
Weekly paclitaxel treatment regimens form a key foundation of the treatment of early‐stage breast cancer [2, 26]. Neuropathy is a well‐recognized toxicity of weekly paclitaxel treatment, with significant grade ≥ 2 CIPN reported in 27% of patients [2]. Our study identified symptoms of CIPN of any severity in 85% of weekly paclitaxel‐treated patients, with severe symptoms in 38%. This is a similar range to other studies examining CIPN with weekly paclitaxel [14, 27, 28], although rates of CIPN vary considerably depending on the specific assessment method used.
More than half the cohort reported numbness and tingling after 6 weeks, with some patients already describing severe symptoms and functional deficits. This finding was supported by clinical examination and significant reduction in sural amplitude from baseline, suggestive of early axonal dysfunction. Neuropathy peaked at the end of treatment, in terms of incidence, severity, and functional deficits. Similar to previous reports, neuropathy severity in the hands and feet were similar during treatment [29]. However, greater symptomatic improvement was seen in the upper limbs. Lower limb predominance is consistent with length dependent neuropathy [5, 30] and may support the role of disrupted axonal transport as a potential mechanism of paclitaxel‐induced neuropathy.
A critical feature of the present study is the high proportion of patients experiencing symptoms at 12 months postpaclitaxel completion. Despite some improvement from the end of treatment, patient‐reported symptom burden remained clinically significant at 12 months postcompletion, in line with previous reports of the persistence of paclitaxel‐induced peripheral neuropathy [20, 29, 31]. However, in contrast to platinum‐induced peripheral neuropathy, there was no evidence of a “coasting” effect with neuropathy worsening after treatment completion, highlighting different pathophysiological mechanisms between agents [17].
Dose Reduction and Neuropathy Outcome
Dose reduction occurred in nearly half of patients, with reduction due to neuropathy affecting more than a third of the cohort (36.2%). Paclitaxel dose reduction is common in weekly schedules, occurring in 29% of patients in clinical trial settings [2] and in 47% of patients in community oncology practice [32]. A high proportion of dose reductions in weekly paclitaxel schedules are due to CIPN, necessitating dose reduction in 25%–32% of patients [8, 33, 34]. Importantly, although investigations into alternate preventative strategies are ongoing, dose reduction is the only current preventative strategy for CIPN recommended by the American Society of Clinical Oncology guidelines [10]. Although broadly, neuropathy prevalence and severity are associated with cumulative dose [5], there remains little evidence concerning its effect on neuropathy outcome on an individual patient basis. In the present study, patients who experienced dose reduction demonstrated worse neuropathy at follow‐up. Although patients who ceased treatment after cycle 10 had comparable outcomes to those who did not discontinue treatment, patients who ceased early (cycles 6–9) had worse neuropathy outcomes despite receiving the lowest paclitaxel dose. These findings suggest that the relationship between dose reduction and neuropathy outcomes is not straightforward and likely reflect individual neuropathy risk profiles. Patients who require early dose reductions may be more vulnerable to neuropathy, and these effects may be persistent despite dose modification.
There are number of demographic and genetic characteristics that may influence neuropathy risk [35, 36, 37]. Although many of these are known risk factors, such as older age [38], there are many aspects of neuropathy risk that remain ill defined. These findings suggest that dose reduction alone may be insufficient to ensure favorable neuropathy outcomes. It is possible that susceptible patients develop early symptoms of neuropathy and remain worse affected regardless of dose reduction. This emphasizes the need to identify risk factors that can be used to determine risk of lasting neuropathy. Interestingly, paclitaxel plasma concentration during the first treatment cycle may predict eventual dose reductions due to neuropathy [34]. This suggests that individualized dosing protocols may be able to ensure maximum benefit from chemotherapy while reducing side effects. However, future individualized treatment protocols will need to include clinical or genetic neuropathy risk information, as dose alone is insufficient to predict neuropathy risk.
Given the use of self‐report measures, it is possible that patients who are better at communicating their symptoms are more likely to receive clinical intervention and dose reduction. Documentation of neuropathy requires both clinician‐based questioning and patient report and is more likely to occur when patients openly discuss neuropathy symptoms [39]. Although clinicians may have a better understanding of the range of severity of toxicities than patients [12], patient report is an essential component of subjective toxicities such as CIPN. Although consensus on a “gold standard” assessment is lacking [5, 40, 41], multimodal assessment of patients may assist in monitoring symptomatic patients and provide objective evidence of neurological deficits.
The current study has several strengths, including multimodal and objective assessment, prospective data collection, midtreatment evaluation and follow‐up, and a homogeneous breast cancer population receiving a uniform paclitaxel regime. However, some patients were lost to follow‐up over the course of the study. Although demographic characteristics in this group did not differ, it is possible that patients experiencing more significant neuropathy had greater motivation to remain in the study. Replication in a larger cohort would allow further comparison with those who received dose reduction for other reasons, facilitating a better understanding of at‐risk patients. However, it is imperative that investigation of CIPN uses objective and comprehensive measures rather than limited or unimodal assessment often used in larger cohort or clinical trials.
Conclusion
Weekly paclitaxel schedules are extensively used in breast cancer, and CIPN is a major consideration for both patient wellbeing and treatment decisions. Previous estimates of neuropathy have varied, reflecting differences in study design, populations, treatment schedules, and CIPN assessment methods. Schedule‐specific toxicity information is important to provide a guide for clinicians and patients regarding typical patterns of CIPN, especially as discrepancies between clinician and patient perceptions of CIPN have been highlighted [40]. Clinicians should be aware that symptomatic and objective neuropathy can develop early in the treatment course and that these patients may need closer monitoring. Furthermore, neuropathy is a long‐term sequela that may be detrimental to quality of life in cancer survivors, including the risk of deficits which may require appropriate supportive services. Importantly, results suggest that dose reduction does not necessarily lead to better neuropathy outcomes, with individual risk factors playing a role. Understanding risk factors for neuropathy will be critical to determining individualized treatment strategies and improving quality of life in breast cancer survivors.
Author Contributions
Conception/design: Hannah C. Timmins, Susanna B. Park
Provision of study material or patients: Michelle Harrison, Frances Boyle, Michael Friedlander, David Goldstein
Collection and/or assembly of data: Hannah C. Timmins, Tiffany Li, Terry Trinh
Data analysis and interpretation: Hannah C. Timmins
Manuscript writing: Hannah C. Timmins, Susanna B. Park
Final approval of manuscript: All authors.
Disclosures
Michael Friedlander: AstraZeneca, Novartis, Takeda, GlaxoSmithKline, Merck Sharp & Dohme (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting informaiton
Acknowledgments
The authors acknowledge Elizabeth Barnes for statistical advice (NHMRC Clinical Trials Centre, Sydney Medical School, University of Sydney, Australia)
This study was supported by a Cancer Institute NSW Program Grant (14/TPG/1–05) and a National Health and Medical Research Council of Australia (NHMRC) Project Grant (no. 1080521). S.B.P is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship (no. 1148595). MCK is supported by a NHMRC Practitioner Fellowship (1156093); and by ForeFront, a large collaborative research group dedicated to the study of neurodegenerative diseases and funded by the National Health and Medical Research Council of Australia Program Grant (no. 1132524).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Hershman DL, Weimer LH, Wang A et al. Association between patient reported outcomes and quantitative sensory tests for measuring long‐term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 2011;125:767–774. [DOI] [PubMed] [Google Scholar]
- 2. Sparano JA, Wang M, Martino S et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winters‐Stone KM, Horak F, Jacobs PG et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy‐induced peripheral neuropathy. J Clin Oncol 2017;35:2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowinsky EK, Chaudhry V, Cornblath DR, et al. Neurotoxicity of taxol. J Natl Cancer Inst Monogr 1993:107–115. [PubMed] [Google Scholar]
- 5. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy‐induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin 2013;63:419–437. [DOI] [PubMed] [Google Scholar]
- 6. Rivera DR, Ganz PA, Weyrich MS, et al. Chemotherapy‐associated peripheral neuropathy in patients with early‐stage breast cancer: A systematic review. J Natl Cancer Inst 2017;110:djx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez EA. Paclitaxel in breast cancer. The Oncologist 1998;3:373–389. [PubMed] [Google Scholar]
- 8. Speck RM, Sammel MD, Farrar JT, et al. Impact of chemotherapy‐induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract 2013;9:e234–e240. [DOI] [PubMed] [Google Scholar]
- 9. Smith EML, Knoerl R, Yang JJ et al. In search of a gold standard patient‐reported outcome measure for use in chemotherapy‐ induced peripheral neuropathy clinical trials. Cancer Control 2018;25:1073274818756608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy‐induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 2020;38:3325–3348. [DOI] [PubMed] [Google Scholar]
- 11. McCrary J, Goldstein D, Boyle F, et al. Optimal clinical assessment strategies for chemotherapy‐induced peripheral neuropathy (CIPN): A systematic review and Delphi survey. Support Care Cancer 2017;25:3485–3493. [DOI] [PubMed] [Google Scholar]
- 12. Hertz DL. Concerns regarding use of patient‐reported outcomes in biomarker studies of chemotherapy‐induced peripheral neuropathy. Pharmacogenomics J 2019;19:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salgado TM, Liu J, Reed HL, et al. Patient factors associated with discrepancies between patient‐reported and clinician‐documented peripheral neuropathy in women with breast cancer receiving paclitaxel: A pilot study. Breast 2020;51:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pace A, Nisticò C, Cuppone F, et al. Peripheral neurotoxicity of weekly paclitaxel chemotherapy: A schedule or a dose issue? Clin Breast Cancer 2007;7:550–554. [DOI] [PubMed] [Google Scholar]
- 15. Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every‐3‐weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER‐2 overexpressors and random assignment to trastuzumab or not in HER‐2 nonoverexpressors: Final results of cancer and leukemia group B protocol 9840. J Clin Oncol 2008;26:1642–1649. [DOI] [PubMed] [Google Scholar]
- 16. Australian Institute of Health and Welfare . Cancer in Australia 2019. Canberra, Austsralia: Australian Institute of Health and Welfare; 2019. [Google Scholar]
- 17. Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin‐induced neuropathy: Results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (FACT/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 2003;13:741–748. [DOI] [PubMed] [Google Scholar]
- 19. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hershman DL, Unger JM, Crew KD, et al. Two‐year trends of taxane‐induced neuropathy in women enrolled in a randomized trial of acetyl‐L‐carnitine (SWOG s0715). J Natl Cancer Inst 2018;110:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavaletti G, Jann S, Pace A, et al. Multi‐center assessment of the total neuropathy score for chemotherapy‐induced peripheral neurotoxicity. J Peripher Nerv Syst 2006;11:135–141. [DOI] [PubMed] [Google Scholar]
- 22. Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: Validation and reliability study. Neurology 1999;53:1660–1664. [DOI] [PubMed] [Google Scholar]
- 23. Timmins HC, Li T, Huynh W, et al. Electrophysiological and phenotypic profiles of taxane‐induced neuropathy. Clin Neurophysiology 2020;131:1979–1985. [DOI] [PubMed] [Google Scholar]
- 24. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 25. Park SB, Lin CSY, Krishnan AV, et al. Long‐term neuropathy after oxaliplatin treatment: Challenging the dictum of reversibility. The Oncologist 2011;16:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujii T, Le Du F, Xiao L, et al. Effectiveness of an adjuvant chemotherapy regimen for early‐stage breast cancer: A systematic review and network meta‐analysis. JAMA Oncol 2015;1:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park SB, Lin CSY, Krishnan AV, et al. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel‐induced neuropathy. Muscle Nerve 2011;43:367–374. [DOI] [PubMed] [Google Scholar]
- 28. Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy‐induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP‐HOR 02 study). Support Care Cancer 2009;17:1071–1080. [DOI] [PubMed] [Google Scholar]
- 29. Pachman DR, Qin R, Seisler D, et al. Comparison of oxaliplatin and paclitaxel‐induced neuropathy (Alliance A151505). Support Care Cancer 2016;24:5059–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Timmins HC, Li T, Kiernan MC, et al. Taxane‐induced peripheral neuropathy: Differences in patient report and objective assessment. Support Care Cancer 2020;28:4459–4466. [DOI] [PubMed] [Google Scholar]
- 31. Bandos H, Melnikow J, Rivera DR, et al. Long‐term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B‐30. J Natl Cancer Inst 2018;110:djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw 2015;13:1383–1393. [DOI] [PubMed] [Google Scholar]
- 33. Bhatnagar B, Gilmore S, Goloubeva O, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single‐center experience. Springerplus 2014;3:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hertz DL, Kidwell KM, Vangipuram K, et al. Paclitaxel plasma concentration after the first infusion predicts treatment‐limiting peripheral neuropathy. Clin Cancer Res 2018;24:3602–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kudlowitz D and Muggia F. Defining risks of taxane neuropathy: Insights from randomized clinical trials. Clin Cancer Res 2013;19:4570–4577. [DOI] [PubMed] [Google Scholar]
- 36. Robertson J, Raizer J, Hodges JS, et al. Risk factors for the development of paclitaxel‐induced neuropathy in breast cancer patients. J Peripher Nerv Sys 2018;23:129–133. [DOI] [PubMed] [Google Scholar]
- 37. Lam SW, Frederiks CN, van der Straaten T, et al. Genotypes of CYP2C8 and FGD4 and their association with peripheral neuropathy or early dose reduction in paclitaxel‐treated breast cancer patients. Br J Cancer 2016;115:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barginear M, Dueck AC, Allred JB, et al. Age and the risk of paclitaxel‐induced neuropathy in women with early‐stage breast cancer (Alliance A151411): Results from 1,881 patients from cancer and leukemia group B (CALGB) 40101. The Oncologist 2019;24:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knoerl R, Smith EML, Han A, et al. Characterizing patient‐clinician chemotherapy‐induced peripheral neuropathy assessment and management communication approaches. Patient Educ Couns 2019;102:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alberti P, Rossi E, Cornblath DR, et al. Physician‐assessed and patient‐reported outcome measures in chemotherapy‐induced sensory peripheral neurotoxicity: Two sides of the same coin. Ann Oncol 2014;25:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Argyriou AA, Park SB, Islam B, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy‐induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry 2019;90;1361–1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting informaiton


