Abstract
Background
The modified Glasgow prognostic score (mGPS), a clinical tool that incorporates albumin and C‐reactive protein, has proven useful in the prognostication of multiple cancers. Several immune checkpoint inhibitors (ICIs) have been approved for the treatment of metastatic urothelial cell carcinoma (mUC), but a prognostic biomarker is needed. We investigated the impact of mGPS on survival outcomes in patients with mUC receiving ICIs.
Materials and Methods
We retrospectively reviewed patients with mUC treated with ICIs (programmed cell death protein 1 or programmed cell death ligand 1 inhibitors) at Winship Cancer Institute from 2015 to 2018. Overall survival (OS) and progression‐free survival (PFS) were measured from the start date of ICI until death or clinical or radiographic progression, respectively. mGPS was defined as a summary score with one point given for C‐reactive protein >10 mg/L and/or albumin <3.5 g/dL. Univariate (UVA) and multivariate (MVA) analyses were carried out using Cox proportional hazard model. These outcomes were also assessed by Kaplan‐Meier analysis.
Results
A total of 53 patients were included with a median follow‐up 27.1 months. The median age was 70 years, with 84.9% male and 20.8% Black. Baseline mGPS was 0 in 43.4%, 1 in 28.3% and 2 in 28.3%. Increased mGPS at the time of ICI initiation was associated with poorer OS and PFS in UVA, MVA, and Kaplan‐Meier analyses.
Conclusion
The mGPS may be a useful prognostic tool in patients with mUC when treatment with ICI is under consideration. These results warrant a larger study for validation.
Implications for Practice
The ideal prognostic tool for use in a busy clinical practice is easy‐to‐use, cost‐effective, and capable of accurately predicting clinical outcomes. There is currently no universally accepted risk score in metastatic urothelial cell carcinoma (mUC), particularly in the immunotherapy era. The modified Glasgow prognostic score (mGPS) incorporates albumin and C‐reactive protein and may reflect underlying chronic inflammation, a known risk factor for resistance to immune checkpoint inhibitors (ICIs). This study found that baseline mGPS is associated with survival outcomes in patients with mUC treated with ICIs and may help clinicians to prognosticate for their patients beginning immunotherapy.
Keywords: Immunotherapy, Checkpoint inhibitors, Urothelial cell carcinoma, Inflammation, Glasgow prognostic score
Short abstract
Several immune checkpoint inhibitors have been approved for the treatment of metastatic urothelial cell carcinoma, but a prognostic biomarker is needed. This article reports on the effect of the Glasgow prognostic score on survival outcomes in patients with metastatic urothelial cell carcinoma receiving immune checkpoint inhibitor therapy.
Introduction
Bladder cancer is the ninth most common cancer worldwide [1]. It is more common in well‐developed countries, and in 2020, it is estimated that there will be 81,400 new cases and 17,980 deaths resulting from bladder cancer in the U.S. alone [2]. Although the majority of cases are localized at diagnosis, the 5% who present with distant metastases have a disappointing 5‐year survival rate of 5.5% [3]. Urothelial carcinoma (UC) accounts for approximately 90% of bladder cancers.
Platinum‐based combination chemotherapy has been the cornerstone of treatment for patients with incurable metastatic disease. However, duration of response tends to be short, and many patients are ineligible to receive cisplatin because of impaired renal function or poor performance status. The advent of immune checkpoint inhibitors (ICIs) altered the landscape of management of metastatic disease given a relatively favorable toxicity profile and potential for long‐term, durable tumor responses [4]. Pembrolizumab and atezolizumab are currently approved in the first line for those whose tumors express programmed cell death ligand 1 (PD‐L1) or in patients ineligible for platinum‐containing chemotherapy. Other approved agents in the second line include nivolumab, avelumab, and durvalumab [5]. Unfortunately, response rates to second‐line ICIs ranging from 13%–21% demonstrate that the majority of patients will experience disease progression despite immunotherapy [6, 7]. Thus, a risk stratification tool capable of predicting clinical outcomes at baseline in patients with UC treated with ICIs would be of significant clinical utility.
Systemic inflammation alone and in combination with other clinical factors has been shown to predict poorer response to ICI across genitourinary cancers including UC [8, 9, 10, 11, 12, 13, 14]. C‐reactive protein (CRP) is an acute phase reactant that increases in response to proinflammatory cytokines such as interleukin (IL)‐6 and IL‐1β and has negative prognostic value across multiple malignancies and stages [15, 16]. Serum albumin is negatively affected by both systemic inflammation and malnutrition, and hypoalbuminemia is known to portend poorer prognoses in cancer [17]. The Glasgow prognostic score (GPS) was initially developed by Forrest et al. for prognostication in patients with advanced non‐small cell lung cancer and incorporates both CRP and albumin as a composite surrogate marker of systemic inflammation [18]. The modified GPS (mGPS) assigns 1 point for albumin <3.5 g/dL and 1 point for CRP ≥10 mg/L and produces scores of low (0 point), intermediate (1 point), and high risk (2 points). The difference between the mGPS and the original score is that solitary hypoalbuminemia in the absence of elevated CRP is not awarded a point; this heavier weighting of the inflammatory component of the score was found to better correlate with survival outcomes across tumor types [19].
The GPS and mGPS has been found to be predictive of survival outcomes in nonmuscle‐invasive bladder cancer, muscle‐invasive disease after radical cystectomy, and early stage upper tract UC [20, 21, 22, 23]. However, the utility of the mGPS has not been previously investigated in advanced, nonoperable UC. In addition, because chronic inflammation affecting the tumor microenvironment has been posited as a factor in the development of resistance to ICI, the mGPS may be a particularly useful prognostic biomarker in cases of metastatic UC treated with immunotherapy [24]. We hypothesized that mGPS at baseline in patients with metastatic disease treated with immunotherapy was associated with survival outcomes.
Materials and Methods
We performed a retrospective analysis of 53 patients. Inclusion criteria were biopsy‐proven UC with spread to at least one metastatic site (including pelvic or retroperitoneal lymph nodes), prior treatment with a checkpoint inhibitor (either an anti–programmed cell death protein 1 [PD‐1] or anti–PD‐L1 monoclonal antibody) in any line of treatment at the Winship Cancer Institute of Emory University between the years of 2015 and 2018 and baseline CRP value available for analysis. Patients were identified through a drug administration pharmacy database. Demographic and clinical data including age, sex, race, smoking status, Eastern Cooperative Oncology Group (ECOG) performance score, body mass index (BMI), baseline laboratory data within 2 weeks of immunotherapy initiation, and treatment course and response were collected from the electronic medical record. Pertinent laboratory values collected included baseline albumin and CRP.
The primary outcome measures assessed were progression‐free survival (PFS), defined as the time in months from ICI initiation to clinical or radiographic progression or death, whichever occurred first, and the overall survival (OS), defined as the time of months from initiation until death. Radiographic progression was defined using RECIST version 1.1. Univariate (UVA) and multivariate (MVA) analyses were carried out using Cox proportional hazard model for OS and PFS with backward variable elimination procedure under a removal criterion of p > .1. The strategy was to avoid overparameterizing the model given the small sample size. The hazard ratio (HR) and its 95% confidence interval (CI) will be reported. The overall significance level was set at p < .05. The covariates to be adjusted include age, race, sex, smoking status, baseline BMI, prior lines of therapy, and number of metastatic sites. The relationship between mGPS and survival outcomes (OS and PFS) was assessed using Kaplan‐Meier analysis. Statistical analysis was conducted using SAS Version 9.4 and SAS macros developed by the Biostatistics Shared Resource at Winship Cancer Institute [25].
Results
Baseline Demographic and Disease Characteristics
We identified 53 patients who met the inclusion criteria (Table 1). Within this cohort, 84.9% were male, the median age was 70, and 77.4% were white. The large majority (88.7%) of patients had an ECOG score of 0 or 1. Over half of the patients (54.7%) were receiving immunotherapy in the first or second‐line setting and 47.2% had received prior cisplatin and gemcitabine doublet chemotherapy. The ICI administered was atezolizumab in 73.5%, pembrolizumab in 20.8%, and nivolumab in 5.7%. At baseline, 43.4, 28.3 and 28.3% of the cohort had an mGPS of 0, 1, and 2, respectively.
Table 1.
Patient characteristics
| Characteristic | n = 53, n (% or range) |
|---|---|
| Age (median) | 70 (32–86) |
| Male gender | 45 (84.9) |
| Race | |
| White | 41 (77.4) |
| Black | 11 (20.8) |
| Asian | 1 (1.8) |
| Ever smoker | 28 (52.8) |
| ECOG performance score | |
| 0–1 | 47 (88.7) |
| 2–3 | 6 (11.3) |
| Sites of metastases | |
| Lymph node | 39 (73.6) |
| Bone | 14 (26.4) |
| Lung | 17 (32.1) |
| Brain | 1 (1.9) |
| Liver | 11 (20.8) |
| No. of prior therapies | |
| 0–1 | 29 (54.7) |
| 2 | 11 (20.8) |
| 3–6 | 13 (24.5) |
| Prior gemcitabine/cisplatin | 25 (47.2) |
| Prior BCG | 13 (24.5) |
| Therapy type | |
| Atezolizumab | 39 (73.5) |
| Pembrolizumab | 11 (20.8) |
| Nivolumab | 3 (5.7) |
| BMI (median) | 25.3 (15.8–49.5) |
| Baseline lab values | |
| Albumin (median) | 3.7 (2.3–4.4) |
| CRP (median) | 14.6 (0.48–244.6) |
| Baseline mGPS | |
| 0 | 23 (43.4) |
| 1 | 15 (28.3) |
| 2 | 15 (28.3) |
Abbreviations: BCG, Bacillus Calmette Guerin; BMI, body mass index; CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; mGPS, modified Glasgow prognostic score.
Baseline mGPS and Survival Outcomes in Kaplan‐Meier Analysis
Median follow‐up for the entire cohort was 27.1 months. The median OS of the entire cohort was 11.4 months (CI, 6.6–21.9), with 45.9% of patients surviving at 12 months. The median PFS was 4.3 months (CI, 3.2–7.0), with 27.9% of patients without progression at 12 months.
The median survival of the cohort differed significantly based on stratification by mGPS at treatment initiation as assessed by Kaplan‐Meier analysis (Fig. 1). The median OS of the patients with mGPS of 0, 1, and 2 was 21.9 months (CI, 11.4–not evaluable [NE]), 10.2 months (CI, 2.2–NE) and 4.6 months (CI, 1.0–10.1), respectively (p = .0025; Fig. 1A). The median PFS of the patients with mGPS of 0, 1, and 2 was 8.3 months (CI, 3.8–NE), 4.2 (CI, 1.4–8.6), and 2.6 (CI, 0.4–4.6), respectively (p = .0029; Fig. 1B).
Figure 1.
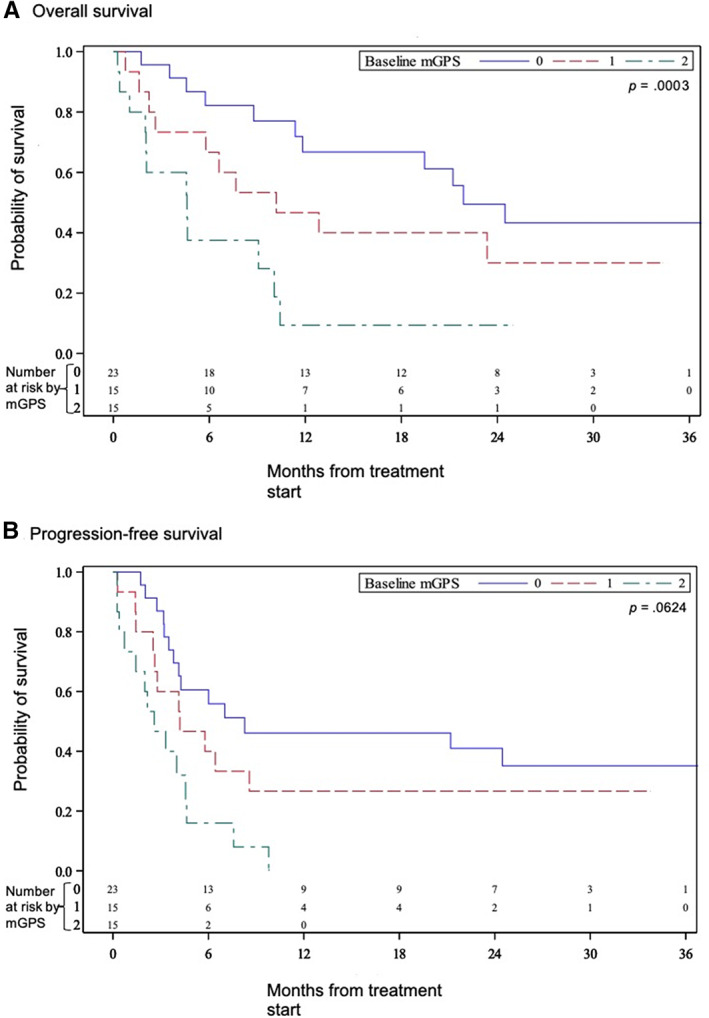
Kaplan‐Meier estimates of (A) overall survival and (B) progression‐free survival in patients with metastatic urothelial carcinoma treated with immunotherapy stratified by baseline modified Glasgow prognostic score.Abbreviation: mGPS, modified Glasgow prognostic score.
Baseline mGPS and Survival Outcomes in Univariate and Multivariate Analyses
The association of multiple variables including mGPS with survival outcomes including PFS and OS were assessed in both univariate and multivariate analyses. In UVA (Table 2), an mGPS at treatment start of 2 was significantly associated with worse PFS (HR, 3.54; CI, 1.64–7.65; p = .001) and OS (HR, 4.08; CI, 1.75–9.54, p = .001). An mGPS of 1 at baseline was also associated with shorter PFS (HR, 1.57; CI, 0.71–3.47, p = .265) and OS (HR, 1.72; CI, 0.73–4.06; p = .214), although the association was not statistically significant.
Table 2.
Univariate analysis of explored covariates with survival outcomes
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR (CI) | p value | HR (CI) | p value | |
| Baseline mGPS | ||||
| 2 (n = 15) | 3.54 (1.64–7.65) | .001 a | 4.08 (1.75–9.54) | .001 a |
| 1 (n = 15) | 1.57 (0.71–3.47) | .265 | 1.72 (0.73–4.06) | .214 |
| 0 (n = 23) | ref | NA | ref | NA |
| Race | ||||
| White or Asian (n = 42) | 0.95 (0.44–2.07) | .897 | 1.33 (0.55–3.22) | .527 |
| Black (n = 11) | ref | NA | ref | NA |
| Sex | ||||
| Female (n = 8) | 0.61 (0.24–1.57) | .310 | 0.37 (0.11–1.22) | .103 |
| Male (n = 45) | ref | NA | ref | NA |
| Smoker | ||||
| No (n = 25) | 0.83 (0.44–1.56) | .560 | 0.82 (0.41–1.63) | .576 |
| Yes (n = 28) | ref | NA | ref | NA |
| ECOG PS | ||||
| 2–3 (n = 6) | 2.34 (0.91–6.03) | .079 | 2.30 (0.80–6.60) | .121 |
| 0–1 (n = 47) | ref | NA | ref | NA |
| Prior lines of therapy | ||||
| 0–1 (n = 29) | 0.94 (0.44–1.99) | .869 | 1.34 (0.56–3.20) | .511 |
| 2 (n = 11) | 0.70 (0.27–1.86) | .476 | 1.24 (0.44–3.55) | .683 |
| 3–6 (n = 13) | ref | NA | ref | NA |
| Number of metastatic sites | ||||
| 2–5 (n = 30) | 2.14 (1.08–4.22) | .029 a | 2.08 (1.01–4.29) | .046 a |
| 0–1 (n = 23) | ref | NA | ref | NA |
| Sites of metastases | ||||
| Lymph node (n = 39) | 1.36 (0.64–2.88) | .420 | 1.07 (0.50–2.31) | .860 |
| Bone (n = 14) | 2.63 (1.31–5.26) | .006 a | 2.81 (1.35–5.87) | .006 a |
| Liver (n = 11) | 1.45 (0.66–3.16) | .354 | 1.68 (0.73–3.88) | .226 |
| Lung (n = 17) | 1.04 (0.53–2.03) | .905 | 0.90 (0.43–1.90) | .791 |
| Sites of metastases | ||||
| <25 (n = 21) | 0.99 (0.52–1.88) | .977 | 1.21 (0.60–2.41) | .596 |
| ≥25 (n = 32) | ref | NA | ref | NA |
| Baseline CRP | ||||
| >11.4 mg/L (n = 30) | 1.94 (1.01–3.73) | .047 a | 2.14 (1.05–4.39) | .037 a |
| ≤11.4 mg/L (n = 23) | ref | NA | ref | NA |
| Baseline albumin | ||||
| <3.8 g/dL (n = 28) | 3.55 (1.80–7.01) | <.001 a | 4.89 (2.25–10.59) | <.001 a |
| ≥3.8 g/dL (n = 25) | ref | NA | ref | NA |
Abbreviations: BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; mGPS, modified Glasgow prognostic score; NA, not applicable; OS, overall survival; PFS, progression‐free survival; ref, reference group.
Statistical significance at α < 0.05.
In MVA (Table 3), baseline mGPS of 2 was again significantly associated with worse PFS (HR 3.91; CI, 1.74–8.82; p < .001) and OS (HR 6.37; CI, 2.46–16.48; p < .001). A score of 1 was associated with shorter PFS (HR 2.00; CI, 0.88–4.52; p = .098), although this association was not statistically significant; alternatively, its association with OS (HR 2.42; CI, 1.01–5.81; p = .048) was found to be significant. Other factors in the MVA associated with worse survival outcomes were ECOG performance (PS) score of 2 or 3 (PFS: HR 4.34; CI, 1.42–13.24; p = .010; OS: HR 5.60; CI, 1.50–20.94; p = .010) and the presence of bone metastases (PFS: HR 2.31; CI, 1.11–4.80; p = .025; OS: HR 2.99; CI, 1.35–6.62; p = .007). Female sex was associated with improved survival outcomes (PFS: HR 0.41; CI, 0.15–1.10; p = .077; OS: HR, 0.19; CI, 0.05–0.64; p = .008).
Table 3.
Multivariate analysis of explored covariates with survival outcomes
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR (CI) | p value | HR (CI) | p value | |
| Baseline mGPS | ||||
| 2 (n = 15) | 3.91 (1.74–8.82) | <.001 a | 6.37 (2.46–16.48) | <.001 a |
| 1 (n = 15) | 2.00 (0.80–84.52) | .098 | 2.42 (1.01–5.81) | .048 a |
| 0 (n = 23) | ref | NA | ref | NA |
| Sex | ||||
| Female (n = 8) | 0.41 (0.15–1.10) | .077 | 0.19 (0.05–0.64) | .008 a |
| Male (n = 45) | ref | NA | ref | NA |
| ECOG PS | ||||
| 2–3 (n = 6) | 4.23 (1.42–13.24) | .010 a | 5.60 (1.50–20.94) | .010 a |
| 0–1 (n = 47) | ref | NA | ref | NA |
| Bone metastases | ||||
| Yes (n = 14) | 2.31 (1.11–4.80) | .025 a | 2.99 (1.35–6.62) | .007 a |
| No (n = 39) | ref | NA | ref | NA |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; mGPS, modified Glasgow prognostic score; NA, not applicable; OS, overall survival; PFS, progression‐free survival; ref, reference group.
Multivariate analysis controlled for age, sex, baseline BMI, prior lines of therapy, race, smoking status, and number of metastatic sites.
Statistical significance at α < 0.10.
Discussion
In the management of metastatic UC, treatment with ICIs provides hope for long‐term disease control with a generally well tolerated side effect profile. Unfortunately, the majority of patients will not respond to ICIs, and with the risk of grade 3 or 4 immune‐related side effects as high as 10%–15%, a method of predicting response to immunotherapy is clinically useful and currently lacking [26]. In our study, we found the mGPS to be a cost‐effective, accessible tool capable of prognosticating in this cohort of patients with metastatic UC treated with ICI in any line of therapy.
Multiple other scoring systems have been investigated in the metastatic UC space. Perhaps the first model published was by Geller et al. (1991), who found that baseline normal alkaline phosphatase, normal hemoglobin, high Karnofsky prognostic score (KPS), and age greater than 60 predicted longer survival in a population of patients with advanced UC receiving chemotherapy [27]. Bajorin et al. in 1999 incorporated KPS less than 80% and visceral metastases as the two risk factors capable of independently predicting outcomes in patients with metastatic UC on chemotherapy; Lin et al. (2007) found the same in addition to alkaline phosphatase [28, 29]. A nomogram devised by Galsky et al. included visceral metastases, ECOG PS, leukocyte count, site of primary tumor, and presence of lymph node metastases and was internally and externally validated as prognostic for patients with metastatic UC treated with cisplatin‐based chemotherapy [30]. Bellmunt et al. [31] prospectively evaluated potential prognostic markers in patients with platinum‐refractory advanced UC and found that when given a point for an ECOG PS more than 0, hemoglobin less than 10 g/dL, and/or the presence liver metastases, patients with a score of 0, 1, 2, and 3 had a median overall survival of 14.2, 7.3, 3.8, and 1.7 months (p < .001), respectively, when validated in an external cohort [32]. Building on this work, Sonpavde et al. (2013) found that time from prior chemotherapy (TFPC) as a fourth variable enhanced the prognostic capabilities of the other three factors in this second‐line setting [32]. These models are based largely on disease extent and functional status and were validated in cohorts treated with chemotherapy, as was the standard of care at all lines of therapy at the time.
The role of inflammation in the development and progression of cancer is well reviewed and widely accepted [33, 34, 35]. Su et al. created a novel prognostic score for patients with metastatic UC treated with chemotherapy based on laboratory values that increase in the setting of systemic inflammation: platelet count and neutrophil‐lymphocyte ratio (NLR) [36]. Owari et al. used multivariate modeling to create a score capable of predicting cancer‐specific survival in those with bone metastases across multiple genitourinary malignancies which incorporated type of cancer (prostate, renal cell or urothelial carcinoma), ECOG performance status, the presence of visceral metastases, elevated GPS, and elevated NLR [37]. Matsumoto et al. similarly combined ECOG PS >0, CRP ≥1 mg/dL, and poor response to prior chemotherapy as a cumulative negative prognostic score in a cohort of patients with metastatic UC receiving second‐line chemotherapy [38]. Sonpavde et al. (2016) ultimately improved the concordance statistic of their model incorporating ECOG PS, hemoglobin, liver metastasis, and TFPC from 0.616 to 0.646 by adding low albumin, a known marker of inflammation, as a fifth and final factor in their prognostic classification of patients receiving chemotherapy in the salvage setting [39]. While investigating the factors most predictive of clinical outcomes in patients with metastatic UC treated with ICI, our own group developed the Emory Risk Scoring System that associates increased baseline platelet‐lymphocyte ratio, low baseline albumin, metastasis to the liver, and higher ECOG PS with decreased survival [11]. Associating elevated levels of systemic inflammation through a variety of biomarker proxies with prognosis is a path often traveled, as evidenced above. In addition, incorporating other patient‐related factors such as ECOG status as these models did is consistent with our finding of association between higher ECOG PS and worse survival outcomes in MVA. However, the ideal prognostic tool for use in a busy clinical practice, in addition to being accurate in outcome prediction, is easily remembered, used without calculation, cost‐effective, and derived from blood biomarkers measured routinely. The mGPS not only meets these requirements but may be particularly relevant in the context of patients receiving immunotherapy.
ICIs block inhibitory signaling intended to dampen T‐cell receptor signaling by interacting with either cytotoxic T‐lymphocyte‐associated protein 4 or the PD‐1/PD‐L1 circuit, thus leading to a more robust antitumoral immune response. There are multiple mechanisms by which tumors have or develop resistance to ICI including low tumor immunogenicity, patient‐specific genetic variations leading to impaired antigen presentation and activation of the adaptive immune response and inhibitory aspects of the tumor microenvironment (TME) [24, 40, 41, 42, 43]. The TME represents the complex interplay between cancer, immune, vascular, and stromal cells with the surrounding extracellular matrix and the cytokine milieu in the vicinity. The effect of ICI can be blunted by the presence of immunosuppressive cells and cytokines in the TME. A recent investigation into biomarkers predictive of response to pembrolizumab across 20 different tumor types showed that those with a high T‐cell–inflamed gene expression profile, an mRNA‐based measure indicative of a T‐cell–inflamed microenvironment with increased interferon gamma signaling and cytotoxic tumor‐infiltrating lymphocytes, were more likely to respond to pembrolizumab [44, 45] Chronic, cancer‐related inflammation can lead to an increase in cytokines including interleukin‐6 (IL‐6) and transforming growth factor beta (TGF‐β), both of which have been cited as mediators of T‐cell exhaustion [46]. In a cohort of patients with urothelial carcinoma unresponsive to atezolizumab, increased TGF‐β signaling was noted and correlated with exclusion of CD8+ T cells from the tumor parenchyma; rather, the cytotoxic cells were confined to peritumoral stroma [47]. Alternatively, IL‐1β and IL‐6 are two of the proinflammatory mediators known to upregulate myeloid‐derived suppressor cells (MDSCs), an immunosuppressive cell in the TME known to inhibit the function of CD4+ and CD8+ T cells and promote tumor angiogenesis and metastasis [48]. MDSCs were found to be enriched in the peripheral blood of patients with metastatic melanoma that did not respond to ipilimumab [49]. Through these multifactorial mechanisms, chronic inflammation promotes tumorigenesis broadly but also specifically in the setting of ICI by leading to T‐cell exhaustion and dysfunction.
The mGPS is a composite biomarker of inflammation based on its two components, CRP and albumin. Inflammatory stimuli from the TME may lead to increased production of CRP by hepatocytes. In fact, cytokines IL‐1β and IL‐6, which stimulate MDSCs, are also directly responsible for upregulating the transcription of CRP within hepatocytes [16, 50]. Both inflammation and malnutrition suppress albumin in patients with cancer via the catabolic action of IL‐1β, IL‐6, and tumor necrosis factor alpha [17, 51]. When applied to a cohort of patients with metastatic non‐small cell lung cancer treated with ICI, Freitas et al. found that a lower mGPS was associated with improved PFS and OS, as we did in metastatic UC [52]. The mGPS as a marker of systemic inflammation is a logical prognostic score in this treatment setting given that it reflects some of the mechanisms underlying resistance to ICI.
Last, because there is only a 13%–21% response rate to second‐line immunotherapy, a predictive biomarker capable of dichotomizing patients into responders and nonresponders to ICI across multiple treatment settings is desperately needed to assist oncologists with clinical decision making regarding ordering of therapies. One such biomarker is PD‐L1; however, there has been inconsistency in its predictive value across trials, across assays with different expression thresholds for defining positivity, and between lines of therapy. For example, in the IMvigor210 phase II study of atezolizumab in the second‐line setting after progression on platinum‐based chemotherapy, the objective response rate (ORR) was 15% in all patients, 26% in those with high expression, and as low as 8% in those with no PD‐L1 expression [53]. However, when it was studied in the first‐line setting in patients ineligible for chemotherapy, the ORR was 24% across patients with expression greater than 5% but was still 24% in all comers and 21% in those with no expression; the mOS was actually higher in patients negative for PD‐L1 compared with those who were positive [54]. Tumor mutational burden has also been explored as a predictive biomarker for response to immunotherapy based on the concept that a higher load of germline or somatic mutations equates to more neoantigens that can stimulate immunogenicity. Although TMB holds promise as a predictor of durable responses, across studies of ICIs in mUC, there were responders with low TMB and nonresponders with high TMB, illustrating the need for better definition of high versus low TMB as well as an exploration of the relevance of the heterogeneity of neoantigens in addition to quantity [55, 56]. Other potential predictive biomarkers such as the Cancer Genome Atlas gene expression signature, T‐cell receptor clonality, and the gut microbiome are also under investigation and represent progress toward a much needed and what will likely be a composite predictive biomarker to guide treatment decisions and complement prognostic biomarkers like mGPS [57].
Although our findings of mGPS as a prognostic marker in patients with metastatic UC treated with ICIs were both statistically and clinically relevant, there are also some limitations to our investigation. We retrospectively examined a small cohort of 53 patients, and although this was large enough to demonstrate a significant signal, our findings should be externally validated in a larger cohort and across multiple malignancies treated with ICI. The retrospective nature of our analysis increases the risk of selection bias, although we included all cases of metastatic UC treated with immunotherapy treated at our institution between the inclusion dates. We also looked at patients treated with a variety of ICI including pembrolizumab, atezolizumab, and nivolumab at any line of therapy. Although the majority of our patients received atezolizumab and were treated in the first or second lines of therapy, the mixed cohort introduces some variables to our analysis and a more homogeneous population should be examined in future studies. It is also worth acknowledging that in the simplicity of the mGPS also lies limitation. The half‐lives of CRP and albumin are 19 hours and roughly 21 days, respectively; although we ensured that the baseline levels were collected within 2 weeks of ICI initiation, these measurements are subject to variation, and we did not examine the cases for nonmalignant insults such as concomitant infection. Finally, the landscape of metastatic UC is ever‐changing in recent years. Although not yet approved by the U.S. Food and Drug Administration, ICIs are currently being studied in combination with nonimmunotherapy approaches such as enfortumab vedotin, a first‐in‐class antibody‐drug conjugate targeting nectin‐4 expressed on UC cells, with promising preliminary results [58]. The prognostic value of mGPS will need to be investigated and validated in cohorts with mUC treated with these combination approaches.
Conclusion
We found that higher mGPS was significantly associated with worse PFS and OS in patients with metastatic UC treated with ICIs. In addition to our retrospective analysis, there are logical reasons why mGPS as a composite biomarker of systemic inflammation might forecast response to immunotherapy in particular given the role of inflammation in increasing resistance to ICI. The goal of the prognostic score is not to preclude patients from receiving ICI but rather to inform clinical decision making and patient discussions about the expected impact of these agents. These results are thought provoking as well as clinically relevant and deserve validation in a larger, prospective investigation.
Author Contributions
Conception/design: Jacqueline T. Brown, Wayne B. Harris, Viraj Master, Mehmet Asim Bilen
Provision of study material or patients: Emilie Elise Hitron, Greta Anne Russler, Sarah Caulfield, Lauren Beth Yantorni, Shreyas S. Joshi, Kenneth Ogan, Wayne B. Harris, Bradley C. Carthon, Omer Kucuk, Viraj Master, Mehmet Asim Bilen
Collection and/or assembly of data: Jacqueline T. Brown, Julie M. Shabto, Dylan J. Martini, Deepak Ravindranathan
Data analysis and interpretation: Jacqueline T. Brown, Yuan Liu, Mehmet Asim Bilen
Manuscript writing: Jacqueline T. Brown, Yuan Liu, Mehmet Asim Bilen
Final approval of manuscript: Jacqueline T. Brown, Yuan Liu, Julie M. Shabto, Dylan J. Martini, Deepak Ravindranathan, Emilie Elise Hitron, Greta Anne Russler, Sarah Caulfield, Lauren Beth Yantorni, Shreyas S. Joshi, Haydn Kissick, Kenneth Ogan, Wayne B. Harris, Bradley C. Carthon, Omer Kucuk, Viraj A. Master, Mehmet Asim Bilen
Disclosures
Mehmet Bilen: Exelixis, Bayer, Bristol‐Myers Squibb, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, Sanofi (C/A), Xencor, Bayer, Bristol‐Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton Therapeutics, Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute and the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Ming Yin, Petros Grivas, Qi‐En Wang et al. Prognostic Value of DNA Damage Response Genomic Alterations in Relapsed/Advanced Urothelial Cancer. The Oncologist 2020;25:680–688.
Implications for Practice: Somatic mutations of DNA damage response (DDR) genes are frequently found in urothelial cancer and appear to play an important role in tumorigenesis, progression, treatment response, and outcomes. In a set of DDR genes, ATM alterations were associated with worse survival, while other alterations were associated with better survival in advanced urothelial cancer. The results of this study suggest a complex role of ATM in tumor progression and call for further studies to determine the underlying mechanisms and biomarker clinical utility.
References
- 1. Antoni S, Ferlay J, Soerjomataram I et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975‐2017, Bethesda, MD: National Cancer Institute. Available at https://seer.cancer.gov/csr/1975_2017/. Accessed July 14, 2020.
- 4. Postow MA, Sidlow R, Hellmann MD: Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 5. NCCN Guidelines in Oncology: Bladder Cancer,Version 3.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020.
- 6. Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powles T, Durán I, van der Heijden MS et al. Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2018;391:748–757. [DOI] [PubMed] [Google Scholar]
- 8. Martini DJ, Kline MR, Liu Y et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 2020;126:575–582. [DOI] [PubMed] [Google Scholar]
- 9. Bilen MA, Martini DJ, Liu Y et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy. The Oncologist 2020;25:e528–e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martini DJ, Liu Y, Shabto JM et al. Novel risk scoring system for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. The Oncologist 2020;25:e484–e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shabto JM, Martini DJ, Liu Y et al. Novel risk group stratification for metastatic urothelial cancer patients treated with immune checkpoint inhibitors. Cancer Med 2020;9:2752–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilen MA, Dutcher GMA, Liu Y et al. Association between pretreatment neutrophil‐to‐lymphocyte ratio and outcome of patients with metastatic renal‐cell carcinoma treated with nivolumab. Clin Genitourin Cancer 2018;16:e563–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bilen MA, Martini DJ, Liu Y et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced‐stage cancer treated with immunotherapy. Cancer 2019;125:127–134. [DOI] [PubMed] [Google Scholar]
- 14. Sekar RR, Patil D, Baum Y et al. A novel preoperative inflammatory marker prognostic score in patients with localized and metastatic renal cell carcinoma. Asian J Urol 2017;4:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health 2007;61:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito K, Kihara K. C‐reactive protein as a biomarker for urological cancers. Nat Rev Urol 2011;8:659–666. [DOI] [PubMed] [Google Scholar]
- 17. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forrest LM, McMillan DC, McArdle CS et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non‐small‐cell lung cancer. Br J Cancer 2003;89:1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Proctor MJ, Morrison DS, Talwar D et al. An inflammation‐based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br J Cancer 2011;104:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimura S, D'Andrea D, Soria F et al. Prognostic value of modified Glasgow Prognostic Score in non‐muscle‐invasive bladder cancer. Urol Oncol 2019;37:179.e19–179.e28. [DOI] [PubMed] [Google Scholar]
- 21. Ferro M, De Cobelli O, Buonerba C et al. Modified Glasgow Prognostic Score is associated with risk of recurrence in bladder cancer patients after radical cystectomy: A multicenter experience. Medicine (Baltimore) 2015;94:e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suyama T, Kanbe S, Maegawa M et al. Prognostic significance of inflammation‐based prognostic scoring in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol 2019;45:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inamoto T, Matsuyama H, Sakano S et al. The systemic inflammation‐based Glasgow Prognostic Score as a powerful prognostic factor in patients with upper tract urothelial carcinoma. Oncotarget 2017;8:113248–113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran L, Theodorescu D. Determinants of resistance to checkpoint inhibitors. Int J Mol Sci 2020;21:1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Nickleach DC, Zhang C et al. Carrying out streamlined routine data analyses with reports for observational studies: Introduction to a series of generic SAS® macros. F1000Res 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magee DE, Hird AE, Klaassen Z et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta‐analysis of randomized clinical trials. Ann Oncol 2020;31:50–60. [DOI] [PubMed] [Google Scholar]
- 27. Geller NL, Sternberg CN, Penenberg D et al. Prognostic factors for survival of patients with advanced urothelial tumors treated with methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer 1991;67:1525–1531. [DOI] [PubMed] [Google Scholar]
- 28. Bajorin DF, Dodd PM, Mazumdar M et al. Long‐term survival in metastatic transitional‐cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–3181. [DOI] [PubMed] [Google Scholar]
- 29. Lin CC, Hsu CH, Huang CY et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5‐fluorouracil‐based regimens. Urology 2007;69:479–484. [DOI] [PubMed] [Google Scholar]
- 30. Galsky MD, Moshier E, Krege S et al. Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin‐based chemotherapy. Cancer 2013;119:3012–3019. [DOI] [PubMed] [Google Scholar]
- 31. Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum‐containing regimens. J Clin Oncol 2010;28:1850–1855. [DOI] [PubMed] [Google Scholar]
- 32. Sonpavde G, Pond GR, Fougeray R et al. Time from prior chemotherapy enhances prognostic risk grouping in the second‐line setting of advanced urothelial carcinoma: A retrospective analysis of pooled, prospective phase 2 trials. Eur Urol 2013;63:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diakos CI, Charles KA, McMillan DC et al. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–e503. [DOI] [PubMed] [Google Scholar]
- 35. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Su YL, Hsieh MC, Chiang PH et al. Novel inflammation‐based prognostic score for predicting survival in patients with metastatic urothelial carcinoma. PLoS One 2017;12:e0169657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Owari T, Miyake M, Nakai Y et al. A genitourinary cancer‐specific scoring system for the prediction of survival in patients with bone metastasis: A retrospective analysis of prostate cancer, renal cell carcinoma, and urothelial carcinoma. Anticancer Res 2018;38:3097–3103. [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto R, Abe T, Ishizaki J et al. Outcome and prognostic factors in metastatic urothelial carcinoma patients receiving second‐line chemotherapy: An analysis of real‐world clinical practice data in Japan. Jpn J Clin Oncol 2018;48:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sonpavde G, Pond GR, Rosenberg JE et al. Improved 5‐factor prognostic classification of patients receiving salvage systemic therapy for advanced urothelial carcinoma. J Urol 2016;195:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fares CM, Van Allen EM, Drake CG et al. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book 2019;39:147–164. [DOI] [PubMed] [Google Scholar]
- 41. Schepisi G, Brighi N, Cursano MC et al. Inflammatory biomarkers as predictors of response to immunotherapy in urological tumors. J Oncol 2019;2019:7317964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vajaitu C, Draghici CC, Solomon I et al. The central role of inflammation associated with checkpoint inhibitor treatments. J Immunol Res 2018;2018:4625472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pitt JM, Vétizou M, Daillere R et al. Resistance mechanisms to immune‐checkpoint blockade in cancer: Tumor‐intrinsic and ‐extrinsic factors. Immunity 2016;44:1255–1269. [DOI] [PubMed] [Google Scholar]
- 44. Ott PA, Bang YJ, Piha‐Paul SA et al. T‐cell‐inflamed gene‐expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE‐028. J Clin Oncol 2019;37:318–327. [DOI] [PubMed] [Google Scholar]
- 45. Ayers M, Lunceford J, Nebozhyn M et al. IFN‐γ‐related mRNA profile predicts clinical response to PD‐1 blockade. J Clin Invest 2017;127:2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mariathasan S, Turley SJ, Nickles D et al. TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ostrand‐Rosenberg S, Sinha P. Myeloid‐derived suppressor cells: Linking inflammation and cancer. J Immunol 2009;182:4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyer C, Cagnon L, Costa‐Nunes CM et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014;63:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agrawal A, Samols D, Kushner I. Transcription factor c‐Rel enhances C‐reactive protein expression by facilitating the binding of C/EBPbeta to the promoter. Mol Immunol 2003;40:373–380. [DOI] [PubMed] [Google Scholar]
- 51. McMillan DC, Watson WS, O'Gorman P et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210–213. [DOI] [PubMed] [Google Scholar]
- 52. Freitas C, Tavares N, Jacob M et al. P1.04‐59 Modified Glasgow Prognostic Score predict survival among non‐small cell lung cancer patients treated with immune checkpoint inhibitors. J Thorac Oncol 2019;(suppl)14:S464a. [Google Scholar]
- 53. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Balar AV, Galsky MD, Rosenberg JE et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: A single‐arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: A moving target. J Immunothe Cancer 2017;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tripathi A, Plimack ER. Immunotherapy for urothelial carcinoma: Current evidence and future directions. Curr Urol Rep 2018;19:109. [DOI] [PubMed] [Google Scholar]
- 57. Zhu J, Armstrong AJ, Friedlander TW et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD‐L1, tumor mutational burden, and beyond. J Immunother Cancer 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosenberg JE, Flaig TW, Friedlander TW et al. Study EV‐103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2020;38(suppl):441a. [Google Scholar]


