Abstract
Background
Cervical cancer (CC) is the most common female cancer in many countries of sub‐Saharan Africa (SSA). We assessed treatment guideline adherence and its association with overall survival (OS).
Methods
Our observational study covered nine population‐based cancer registries in eight countries: Benin, Ethiopia, Ivory Coast, Kenya, Mali, Mozambique, Uganda, and Zimbabwe. Random samples of 44–125 patients diagnosed from 2010 to 2016 were selected in each. Cancer‐directed therapy (CDT) was evaluated for degree of adherence to National Comprehensive Cancer Network (U.S.) Guidelines.
Results
Of 632 patients, 15.8% received CDT with curative potential: 5.2% guideline‐adherent, 2.4% with minor deviations, and 8.2% with major deviations. CDT was not documented or was without curative potential in 22%; 15.7% were diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage IV disease. Adherence was not assessed in 46.9% (no stage or follow‐up documented, 11.9%, or records not traced, 35.1%). The largest share of guideline‐adherent CDT was observed in Nairobi (49%) and the smallest in Maputo (4%). In patients with FIGO stage I–III disease (n = 190), minor and major guideline deviations were associated with impaired OS (hazard rate ratio [HRR], 1.73; 95% confidence interval [CI], 0.36–8.37; HRR, 1.97; CI, 0.59–6.56, respectively). CDT without curative potential (HRR, 3.88; CI, 1.19–12.71) and no CDT (HRR, 9.43; CI, 3.03–29.33) showed substantially worse survival.
Conclusion
We found that only one in six patients with cervical cancer in SSA received CDT with curative potential. At least one‐fifth and possibly up to two‐thirds of women never accessed CDT, despite curable disease, resulting in impaired OS. Investments into more radiotherapy, chemotherapy, and surgical training could change the fatal outcomes of many patients.
Implications for Practice
Despite evidence‐based interventions including guideline‐adherent treatment for cervical cancer (CC), there is huge disparity in survival across the globe. This comprehensive multinational population‐based registry study aimed to assess the status quo of presentation, treatment guideline adherence, and survival in eight countries. Patients across sub‐Saharan Africa present in late stages, and treatment guideline adherence is remarkably low. Both factors were associated with unfavorable survival. This report warns about the inability of most women with cervical cancer in sub‐Saharan Africa to access timely and high‐quality diagnostic and treatment services, serving as guidance to institutions and policy makers. With regard to clinical practice, there might be cancer‐directed treatment options that, although not fully guideline adherent, have relevant survival benefit. Others should perhaps not be chosen even under resource‐constrained circumstances.
Keywords: Cervical cancer, Sub‐Saharan Africa, Population‐based, Access to care, Radiotherapy, Survival
Short abstract
With a multinational collection of registry data and multimodal evaluation of degree of therapy guideline adherence, this study adds population‐based evidence on the status of cervical cancer care and outcomes in the setting of sub‐Saharan Africa.
Introduction
Cervical cancer (CC) shows large differences in outcome globally depending on stage at presentation to the health system and access to high‐quality care. Both may vary depending on individual patient factors and local or country‐specific availability of diagnostic and treatment services. Assessing of treatment guideline adherence at the patient level and linking this to outcome is an established approach [1, 2]. This is a multinational, population‐based study of the pattern and degree of adherence to guidelines of care, and its association with outcome, in patients with CC in sub‐Saharan Africa (SSA).
The burden of CC is currently decreasing in high‐income countries. For example, age‐standardized annual incidence of CC in the U.S. fell to 7.4 in 100,000 in 2010–2014 from more than 40 in 100,000 in 1947–1948 largely because of wide dissemination of screening during this period [3]. In contrast, in SSA—without comprehensive screening—age‐standardized incidence rates range from 26.8 in Central Africa to 43.1 in 100,000 in Southern Africa, with Zimbabwe even reporting 62.3 in 100,000 in 2018. Of the estimated 570,000 CC diagnoses and 311,000 cervical cancer deaths in the world in 2018, 112,000 (20%) of new diagnoses and 76,000 (24%) of the deaths occur in SSA [4], despite SSA accounting for only 9.4% of women older than 20 years worldwide [5].
Population‐based data on stage at diagnosis are limited in SSA, and those that are available report a substantial proportion of cervical cancer cases diagnosed at late stages. For example, 30% of patients in Uganda presented with International Federation of Gynecology and Obstetrics (FIGO) stage III–IV disease, and 58% of patients in Zimbabwe presented with regional and metastatic disease [6, 7]. With a higher proportion of staged patients, but more selective by nature, recent hospital cohorts yield comparable stage patterns, for example, 81% with stage IIb–IV in a center in Addis Ababa, Ethiopia [8].
Similarly, population‐based survival data for CC are limited, but a recently published large survey reports age‐standardized relative survival (ASRS) of 69.8%, 44.5%, and 33.1% at 1, 3, and 5 years [9]. Additionally, there are premillennium cohorts that report 49% 5‐year ASRS in Uganda and 45% 3‐year ASRS in Zimbabwe [6, 7].
The situation of CC care in SSA from a health care infrastructure point of view can be gauged first from the gaps between calculated need and actual availability of radiotherapy services [10] and, secondly, from Global Surgery 2030's estimate that 93% of SSA's population does not have access to safe, timely, and affordable surgery [11]. In addition, although access to chemotherapy is increasing, it is still limited, and its safe administration is a major concern where there is a shortage of oncology personnel [12].
The consequences of these shortfalls in SSA health care systems have so far rarely been examined at an individual level. No previous study has described the pattern of CC care and guideline adherence using a population‐based approach, nor has there been a longitudinal examination of the degree to which guideline adherence is linked to survival of patients with CC in SSA. This led to our main research questions: Firstly, what is the quality of CC therapy in SSA in terms of degree of guideline adherence? Secondly, to what extent is overall survival associated with therapy guideline adherence when adjusted for patient characteristics and stage?
With its multinational collection of registry data and multimodal evaluation of degree of therapy guideline adherence, the present study adds population‐based evidence on status of CC care and outcomes in a SSA setting.
Materials and Methods
Study Design
This is a multinational retrospective population‐based study, drawing patients from nine population‐based cancer registries: Abidjan (Ivory Coast), Addis Ababa (Ethiopia), Bamako (Mali), Bulawayo (Zimbabwe), Cotonou (Benin), Eldoret (Kenya), Kampala (Uganda), Maputo (Mozambique), and Nairobi (Kenya). These registries cover populations between 800,000 (Cotonou) and four million (Abidjan) inhabitants. All are members of the African Cancer Registry Network (AFCRN), which since 2013 has coordinated sub‐Saharan population‐based cancer registries as the International Agency for Research on Cancer's regional hub [13].
Sources of Data and Study Population
After excluding cases registered based on a death certificate only, random samples of patients diagnosed with invasive cancers of the cervix (International Classification of Diseases‐10 C53.x) between January 1, 2010, and June 30, 2016, were drawn within the sampling frame of the database of the African Cancer Registry Network. In Addis Ababa, we included all cases diagnosed from January to March 2012 and 2014. A sample size of 700 produces a two‐sided 95% confidence interval with a width equal to 0.075 when the sample proportion of patients with adequate care is 0.500. We drew a simple random sample of 45 to 125 patients per registry (mean n = 75) to amount to 700 patients. For logistic reasons, it was impossible to include all patients diagnosed in that period. Follow‐up was open for 7 years until December 31, 2017 (Fig. 1).
Figure 1.
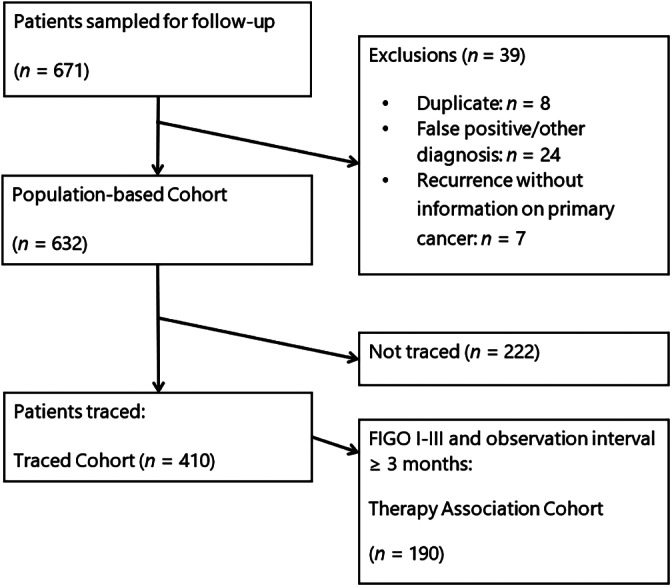
Trial flow diagram. Patients with hospital files found or successful telephone contact were considered to be traced.Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Data collection was integrated into registration work, based on the AFCRN Standard Procedure Manual Version 2 [14]. The databases of the participating registries include basic demographic and tumor characteristics (including basic staging) and, infrequently, basic initial treatment data. Clinical records of registered cases were traced via the source(s) recorded in the registry, information on date of diagnosis and stage was verified or updated, and any duplicates were excluded (Fig. 1). The registry records were updated with information on diagnostic procedures, treatment received, and patients’ vital status. However, if this information could not be found in clinical records, we attempted to contact the patient or their relatives through all phone numbers available in the records and hospital information systems to ascertain treatment details and survival status. This also enabled us to inquire about within‐country and international referral undocumented in the records. Cases for which a health record or additional information was found after this active follow‐up are subsequently referred to as “traced cases” and “traced cohort.”
Stage at diagnosis was obtained from physicians’ clinical assessments in the records in line with FIGO's 2009 classification [15]; T1–T3 with radiologically or pathologically positive pelvic nodes were grouped as FIGO stage III. In some cases, clinical FIGO stage was amended by additional information from imaging or pathology findings in line with the abovementioned AFCRN Manual. Performance status at diagnosis as Eastern Cooperative Oncology Group (ECOG) score was collected. Four detailed aspects of cancer‐directed therapy (CDT) were recorded: surgery, external beam radiation therapy (EBRT), brachytherapy, and chemotherapy. When details such as hysterectomy or radiotherapy dose were not further specified but the record reported “complete,” we assumed the treatment was performed with adherence to guidelines as a necessary simplification.
Therapy Evaluation
U.S. National Comprehensive Cancer Network (NCCN) CC Guidelines 1.2010 (actually prepared for the high‐income setting) reflected the optimum standard of CC care at the beginning of our study period [16]. These were in widespread use in low‐ and middle‐income countries and parts of SSA and were therefore chosen as a point of reference [17, 18]. Physicians also used locally adapted guidelines, other guidelines, or adjusted treatment according to specific patient characteristics and resource limitations. Because of the retrospective nature of the study using real‐world data, these factors were not captured in our analytical database. Still, we aimed to use NCCN Guidelines as standard to give an overall picture on access to care rather than a posteriori judging the individual treatment decisions. We compiled a scheme for evaluating degree of adherence (Table 1). Guideline adherence was assessed for cases known to be FIGO stage I–III. Each stage‐dependent category includes key procedures and modalities required to reach a certain degree of adherence. Note that not all possible treatment variations were depicted, and possible overtreatment was not the focus of the study. “Guideline‐adherent” was the minimum sufficient therapy recommended. Courses of chemotherapy alone, EBRT <45 Gy, and surgical intervention without removal of the tumor were defined as “CDT without curative potential.”
Table 1.
Therapy evaluation scheme for patients with known FIGO stage
| Therapy; FIGO stage | Guideline adherent (FIGO stage I–III applicable only) | Minor deviation (FIGO stage I–III applicable only) | Major deviation (FIGO stage I–III applicable only) | CDT without curative potential (FIGO stage I–III applicable only) | No CDT detected, FU <3 months (FIGO stage I–III applicable only) | No CDT detected, FU ≥3 months (FIGO stage I–III applicable only) |
|---|---|---|---|---|---|---|
| Curative primary surgery | ||||||
| IA1 | Excision with free margins, e.g., through conization, simple hysterectomy | — | Any cancer‐directed surgery with possible tumor destruction, e.g., laser vaporization or cryotherapy | — | No CDT identified, but patient dead/lost to FU <3 months after diagnosis | No CDT identified in patients with FU ≥3 months |
| IA2–IIA | (IA2: Modified) Radical hysterectomy + pelvic LAE | (IA2: Modified) Radical hysterectomy | Any less radical procedure for removal of tumor, e.g., simple hysterectomy | Any surgery with remaining parts of cervix/primary tumor | No CDT identified, but patient dead/lost to FU <3 months after diagnosis | No CDT identified in patients with FU ≥3 months |
| IIB | — | Radical hysterectomy + pelvic LAE | Radical hysterectomy | Any less radical surgery than radical hysterectomy, | No CDT identified, but patient dead/lost to FU <3 months after diagnosis | No CDT identified in patients with FU ≥3 months |
| Curative primary radiotherapy | ||||||
| IB–III | EBRT ≥45 Gy + concurrent chemotherapy ≥2 cycles + brachytherapy ≥16.6 Gy | EBRT ≥45 Gy + brachytherapy ≥16.6 Gy | EBRT ≥45 Gy (with or without chemotherapy | EBRT <45 Gy or missing | No CDT identified, but patient dead/lost to FU <3 months after diagnosis | No CDT identified in patients with FU ≥3 months |
| T1–3 N1 | EBRT ≥45 Gy + concurrent chemotherapy ≥2 cycles + brachytherapy ≥16.6 Gy if primary is not resected | EBRT ≥45 Gy + brachytherapy ≥16.6 if primary is not resected | EBRT ≥45 Gy (with or without chemotherapy) | EBRT <45 Gy or missing | No CDT identified, but patient dead/lost to FU <3 months after diagnosis | No CDT identified in patients with FU ≥3 months |
| Obligatory palliative care: IVA–IVB | Individual approaches with or without CDT, labeled “FIGO stage IV, any approach” | |||||
Therapy was considered for evaluation if documented within 2 years and not indicated for relapse. References and considerations on which this scheme is based apart from National Comprehensive Cancer Network Guidelines version 1.2010 can be found in supplemental online Table 1.
Abbreviations: CDT, cancer‐directed therapy; EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; FU, follow‐up, observation after date of incidence; LAE, lymphadenectomy; N1, radiologically or pathologically involved pelvic lymph nodes.
Outcome
Outcome, in terms of date and vital status (alive/dead) at the last known contact, as recorded by the cancer registries, was verified and/or updated from the clinical records. When no information could be found, contact by telephone with the patient or next of kin was attempted. The precise cause of death, as certified by a medical practitioner, could rarely be determined.
Statistical Methods
Overall survival (OS) was estimated using the Kaplan‐Meier method, and differences according to prognostic factors were assessed with the log rank test. ASRS was calculated for the traced cohort. Relative survival was determined using SAS macro “periodh” [19]. Because of the small number of patients per registry per year and because differences in baseline mortality of the age groups studied between the countries were small (see supplemental online Table 2) [20], only a single life table was created: World Health Organization life tables from the eight countries for the year 2013 as the median year of diagnosis of all patients were retrieved and the average calculated [20]. For age standardization the direct method and International Cancer Survival Standard 2 with its “broad age groups” were employed [21]. We assume that the small sample of cases (632) is representative of cervix cancer cases in sub‐Saharan Africa and that the missing cases (35% of patients who cannot be traced; 2% of patients whose files that miss staging information) were missing at random. Extrapolation of therapy evaluation results for SSA was done by using simple multiplication with rounding to 1,000 and assuming representativeness and missing information at random.
To assess the association between treatment guideline adherence and survival, Cox multiple regression was employed for the therapy association cohort (follow‐up ≥3 months, FIGO stage ≤III). The inclusion criteria were chosen to reduce survivorship bias. The assumption of proportionality of hazards was checked graphically and found to be satisfactory.
Ethics
The study protocol was approved by the AFCRN review committee (02.03.2016) and Halle University Review Board (votum no. 2019‐009). The study group used anonymized secondary data, which were collected under existing regulations and national laws in the respective registries. Funding sources had no role in study design, collection, analysis, or interpretation of the data.
Results
The median age at diagnosis in our population‐based cohort was 50 years. The most common stage was FIGO III, and the most common histology was squamous cell carcinoma (Table 2).
Table 2.
Patient characteristics of the population‐based cohort (n = 632)
| Characteristics | n (%) |
|---|---|
| Age group (median: 50 years; IQR: 40–58 years; range 16–99 years) | |
| <40 years | 143 (23) |
| 40–59 years | 335 (53) |
| ≥60 years | 154 (24) |
| Registry | |
| Abidjan, Ivory Coast | 67 (11) |
| Addis Ababa, Ethiopia | 92 (15) |
| Bamako, Mali | 59 (9) |
| Bulawayo, Zimbabwe | 55 (9) |
| Cotonou, Benin | 37 (6) |
| Eldoret, Kenya | 82 (13) |
| Kampala, Uganda | 60 (9) |
| Maputo, Mozambique | 122 (19) |
| Nairobi County, Kenya | 59 (9) |
| HIV status | |
| Negative | 78 (12) |
| Positive | 82 (13) |
| Unknown | 250 (40) |
| Not traced | 222 (35) |
| ECOG performance | |
| ECOG 0–1 | 88 (14) |
| ECOG 2 | 61 (10) |
| ECOG 3–4 | 25 (4) |
| Unknown | 236 (37) |
| Not traced | 222 (35) |
| FIGO stage | |
| I | 49 (8) |
| II | 91 (14) |
| III (incl. T1–T3, pelvic N1) | 123 (19) |
| IV | 99 (16) |
| Unknown | 48 (8) |
| Not traced | 222 (35) |
| Histology | |
| Squamous cell carcinoma | 443 (70) |
| Adenocarcinoma | 40 (6) |
| Other | 4 (1) |
| Carcinoma | 41 (6) |
| Neoplasm, malignant | 104 (16) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range.
For the population‐based cohort (n = 632) in general, we found that about one‐eighth of patients had received some form of external beam radiotherapy (EBRT) and one‐eighth some form of surgery. Information additional to that recorded by the cancer registries could not be found for 35% of the patients. Of the patients we could trace (n = 410), more than half (or 31% of the total cases) lacked essential information for therapy evaluation. Guideline adherence of care varied according to FIGO stage group (supplemental online Table 3).
Quality and delay of radiotherapy were assessed. Only one‐fifth of the traced cohort (n = 410) received primary EBRT. In detail, there were 73 nonsurgical patients, and of these 60 (82%) were staged FIGO I–III in need of curative EBRT with concurrent chemotherapy and subsequent brachytherapy [16]; of these latter 60 patients in need, 8 (13%) were documented as certainly incomplete. Furthermore, only 8 (13%) of 60 patients had brachytherapy as part of their treatment, and only 22 (37%) of 60 patients received concurrent chemotherapy. A median delay of 14 weeks (range, 1–73 weeks) between diagnosis and the start of EBRT was noted in 45 patients whose files had exact EBRT dates.
Radiation was also incomplete for 10 patients with node‐positive disease who had received operations. Only three of them had documented EBRT after surgery, whereas four of the remaining seven patients with node‐positive disease were observed for ≥12 months without EBRT.
Chemotherapy as the only CDT was seen in 66 (16%) of patients in the traced cohort, of whom there were 42 (64%) patients with FIGO stage I–III. Eighteen (43%) of these 42 patients were observed for more than 12 months without further CDT being documented.
Statements on guideline adherence and quality of care were possible for two‐thirds of traced patients. Evaluation was impossible for one‐third of traced patients because of lack of information on stage, early death, and observation less than 3 months. When we evaluated the degree of guideline adherence among the whole population‐based cohort, the proportion of patients with known optimal guideline‐adherent therapy came down to a total of only 5%; an additional 11% received therapy with curative potential showing minor or major deviations (Fig. 2). The proportions of guideline‐adherent therapy were higher among patients with early stages compared with late‐stage presentation (see supplemental online Table 3 and supplemental online Fig. 1). A total of 19% of patients certainly received therapy without curative potential or no therapy at all. In the worst‐case scenario, that is, no further CDTs in the untraceable patients, this would mean that only 16% received any CDT with curative potential, whereas 67% of patients were receiving CDT without curative potential or no therapy at all. Additionally, 17% of patients were known FIGO stage IV in need of palliative care (Fig. 2).
Figure 2.
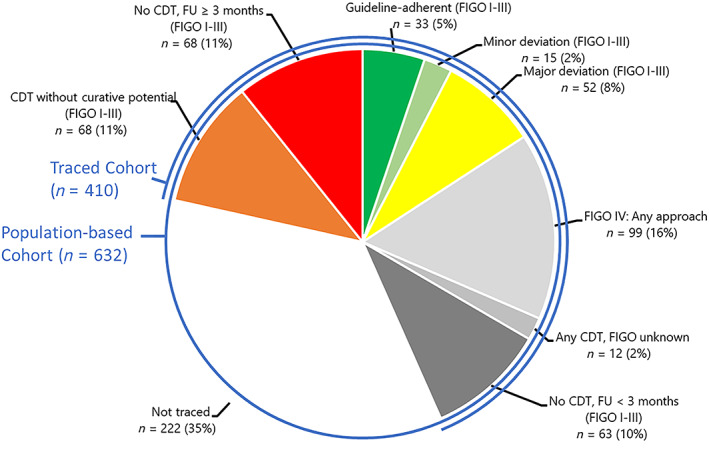
Therapy evaluation in the population‐based cohort (n = 632). Evaluations refer to the therapy evaluation scheme in Table 1. Colors depict the degree of adherence: green indicates optimal, light green minor deviation, yellow major deviation, orange CDT without curative potential, and red no CDT. Light gray indicates patients with FIGO stage IV, middle and darker gray indicates missing stage or observation time, and no color indicates untraced patients. Patients with hospital files found or successful telephone contact were considered to be traced.Abbreviations: CDT, cancer‐directed therapy; FIGO, International Federation of Gynecology and Obstetrics; FU, follow‐up (time of observation since diagnosis).
We found large disparities in care within the populations of the different countries. Populations from centers with radiotherapy available (Addis Ababa, Kampala, and Nairobi) had higher proportions of patients with guideline‐adherent therapy or minor and major deviations compared with those centers without radiotherapy facilities (Fig. 3).
Figure 3.
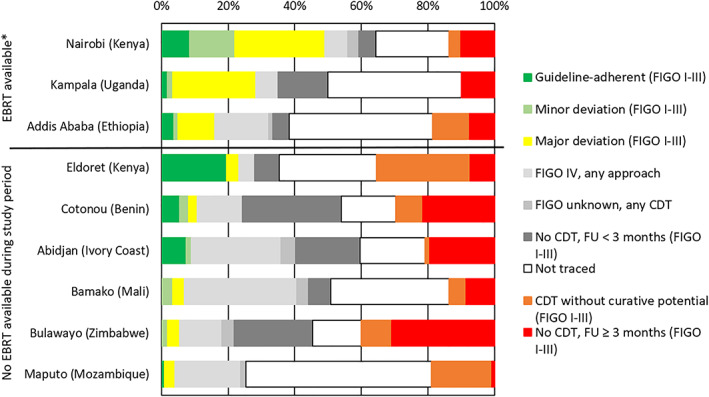
Therapy evaluation in the population‐based cohort (n = 632) stratified by registry. Evaluations refer to the therapy evaluation scheme in Table 1. Colors depict the degree of adherence: green indicates optimal, light green minor deviation, yellow major deviation, orange CDT without curative potential, and red no CDT. Light gray indicates patients with FIGO stage IV, middle and darker gray indicates missing stage or observation time, and white indicates the proportion of untraced patients. *, Principal EBRT availability at the study site did not exclude overstrain or temporary breakdown of machines. EBRT in Bulawayo was nonfunctional during the whole study period.Abbreviations: CDT, cancer‐directed therapy; EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; FU, follow‐up (time of observation since diagnosis).
Data come from eight countries only, but to highlight the possible broader implications of our findings, we extrapolated the findings of our cohort to all 112,000 estimated newly diagnosed cervical cancer cases each year in SSA [4]. This translated to 9,000 (8%) patients with FIGO stage I–III who received guideline‐adherent care, 4,000 (4%) with FIGO stage I–III who received minor deviations and 15,000 (13%) major deviations, 19,000 (17%) with FIGO stage I–III who received CDT without curative potential, 19,000 (17%) more patients with FIGO stage I–III who did not receive any CDT though observed beyond 3 months, 18,000 (16%) patients with FIGO stage I–III who died or got lost to follow‐up within 3 months of diagnosis and had no CDT documented, and 28,000 (25%) patients who were diagnosed with FIGO stage IV and, hopefully, were subject to individualized care. Patients in the inconclusive categories “Not traced” (n = 222) and “Any CDT, FIGO unknown” (n = 12) were omitted at this point.
OS in the traced cohort (n = 410) at 1, 2, and 3 years was 74% (95% confidence interval [CI], 69.3%–78.7%), 51.3% (95% CI, 45%–57.6%), and 41.3% (95% CI, 34.6%–48%), respectively (Fig. 4). A total of 22 patients died within the first month (median at 7 days) after formal diagnosis.
Figure 4.
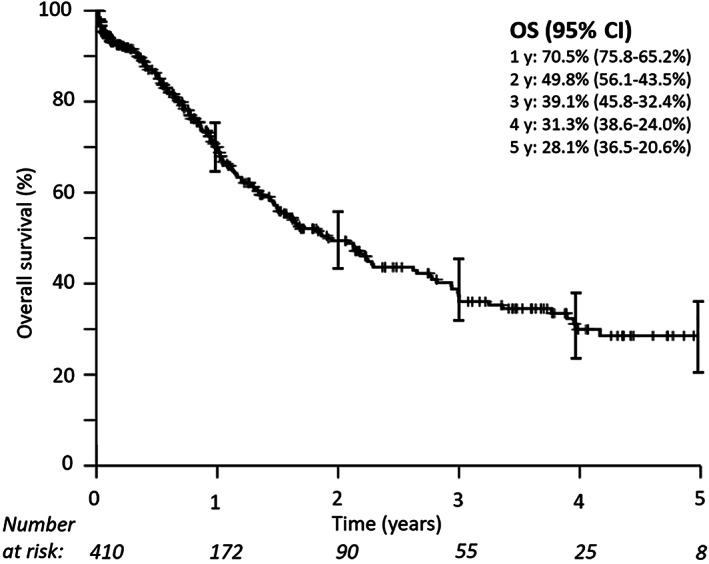
Overall survival in the traced cohort (n = 410). Median overall survival was 23 months. Patients with hospital files found or successful telephone contact were considered to be traced.Abbreviations: CI, confidence interval; OS, overall survival.
One‐, 3‐, and 5‐year ASRSs were 75.6% (95% CI, 70.9%–80.3%), 42.4% (95% CI, 35.5%–49.7%), and 28.7% (95% CI, 19.9%–37.5%). OS differed between FIGO stages I and II versus stages III and IV (p < .001). Three‐year OS was similar for women with FIGO stage I and II cancer (60.8% and 58.2%) but considerably lower for women with FIGO stage III and IV cancer (27.8% and 17.8%) (supplemental online Fig. 2).
Multiple Cox regression analysis was done with adjustment for FIGO stage, age group, HIV status, and ECOG performance status among patients with known stage and more than 3 months’ observation time. Lack of CDT was the variable most strongly associated with negative effect on survival. CDT without curative potential (hazard rate ratio [HRR], 3.88; 95% CI, 1.19–12.71) and no CDT (HRR, 9.43; 95% CI, 3.03–29.33) were associated with worse survival. Minor (HRR, 1.73; 95% CI, 0.37–7.37) and major deviations (HRR, 1.97; 95% CI, 0.59–6.56) were associated with somewhat worse survival. FIGO stage III (HRR, 2.21; 95% CI, 1.01–4.48) and HIV positivity (HRR, 2.00; 95% CI, 1.01–3.96) status were also associated with worse survival (Fig. 5).
Figure 5.
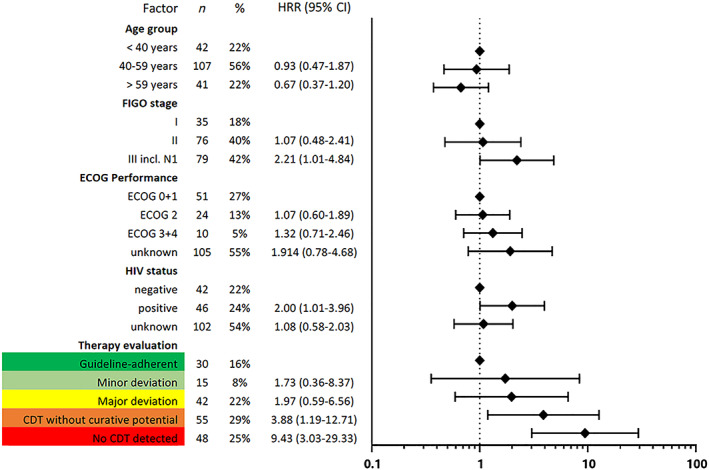
Results of multiple Cox regression for risk of early death in the therapy association cohort (n = 190) are shown: through inclusion criteria (FIGO stages I–III and follow‐up ≥3 months), bias was reduced. Therapy evaluation refers to Table 1.Abbreviations: CDT, cancer‐directed therapy; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HRR, hazard rate ratio.
To facilitate quantitative comparison with a 2005–2011 Australian cohort [22], we additionally analyzed a subcohort including only patients with FIGO stage I and II (n = 111). In this subcohort, adherence to guidelines was associated with a substantially better survival (HRR, 0.30; CI, 0.11–0.86).
Discussion
The most alarming finding in our population‐based, cross‐sectional assessment of NCCN Guidelines–recommended receipt of therapy in eight SSA countries was that for two‐thirds of patients with CC, no documented CDT could be found despite thorough investigations, and in the worst‐case scenario, these patients did not receive any CDT at all. Additionally, of the 37% patients with valid treatment evaluation, only half received CDT with curative potential. By country, the proportion of patients receiving CDT with curative potential varied from 4% in Maputo (Mozambique) to 49% in Nairobi (Kenya). But also, within countries we saw huge inequality. Our study was performed mainly in capital cities (exceptions: Eldoret and Bulawayo, both still major centers). All have tertiary referral oncology centers, which, however, were only partly equipped with radiotherapy facilities, and patients within population‐based registry areas lived close to those centers. According to international recommendations, all centers had far too few radiotherapy facilities [23]. In this respect, we found that cancer centers in registry areas with EBRT available managed to provide CDT with curative potential to only 15%–49% of patients (Addis Ababa, Nairobi, and Kampala), whereas only 10% of patients in countries without radiotherapy facilities received CDT with curative potential—except Eldoret (Kenya) with 23%, where we know that a screening program is in place [24]. In general, economic, epidemiologic, and radiotherapy indicators confirm differences between the countries in our scope but also the backlog relative to Australia and the U.S., which we used for comparisons elsewhere in this report (supplemental online Table 4).
Excluding subjects with missing information, our estimated findings imply that only 28,000 of 112,000 annual patients with CC in SSA received CDT with curative potential [4]; 38,000 up to 56,000 received CDT without curative potential or no CDT. Approximately 28,000 patients presented in FIGO stage IV needing palliative care. These projections are optimistic because they assume that results in large city situations are generalizable to the whole population, including rural settings where access to therapy is likely to be worse.
In general, care of patients with CC requires specialized multimodal therapy with radiotherapeutic and surgical options. This applies to an even greater extent to patients with FIGO stage ≥II (86.5% of patients with staging information available). Given the patient pathways and observed treatment patterns, we assume that certain factors may have greatly reduced the proportion of patients receiving guideline‐adherent care. The identified problems include a lack of specialized facilities and personnel for diagnosis [25], surgery [11], interrupted provision of chemotherapy drugs [12], and both individual poverty and lack of health insurance. The well‐known and still widespread lack of EBRT and brachytherapy services has great impact and is also seen in our cohort [10]. Only 13% of patients with known FIGO stages I–III received primary EBRT and brachytherapy. This is comparable to findings from a population‐based Ugandan cohort of 261 patients described 20 years ago (1995–1997): only 25% of patients with FIGO stages I–IV received primary EBRT and brachytherapy [6]. In contrast, in the Surveillance, Epidemiology, and End Results (SEER) program areas of the U.S., 59%–83% of patients with FIGO stages IB2–IVA received adequate radiotherapy in 1988–2009 [26]. Similarly, in Australia, treatment for patients with FIGO stages I–IVa was guideline adherent for more than half (54.1%) of the patients in 2005–2011 [22]. Our most important result of 16% strict guideline adherence among 190 patients (in the therapy association cohort; Fig. 5) is by far the lowest rate reported in the literature to this date.
This low adherence was associated with poor outcome. Analysis of survival showed 1‐, 3‐, and 5‐year‐ASRSs of 75.6%, 42.4%, and 28.7%. This survival is similar to Ugandan (81.4% and 49%) and Zimbabwean (66% and 44.9%) 1995–1997 population‐based 1‐ and 3‐year ASRS estimates, although the reference population for standardization slightly different [6, 7]. In contrast, the U.S. SEER estimate of 67.1% 5‐year ASRS for the 2007–2013 period [27], taken as example of CC survival in a high‐income country, is much higher. As expected, patients with FIGO stages I and II had considerably better outcome probabilities than those with FIGO stages III and IV. This should encourage education of health care workers to be able to recognize and interpret symptoms of CC and refer patients earlier.
Using the patient group with known FIGO stages I–III and ≥3 months’ observation time, we analyzed the effect of known prognostic factors and degree of treatment completeness on outcome. In 2017, NCCN published Harmonized Guidelines specific to low‐resource regions such as SSA [28]. These guidelines contain information on standard treatment, but also alternative options when resources are not available. The impact of an implementation of these NCCN Harmonized Guidelines for SSA obviously cannot be assessed in a randomized trial. The relationship between different degrees of therapy adherence and better survival observed in our study supports these guidelines’ principles of recommending well‐considered, specific deviations from maximum care if needed. Association of therapy with survival followed a dose‐response effect, with the HRRs increasing with less guideline adherence. Treatment with minor deviations was associated with 1.7‐times increased risk of death, major deviations were associated with a doubled hazard ratio, and “CDT without curative potential” and “no CDT” were associated with detrimental fourfold and ninefold higher hazards of death, respectively, compared with guideline‐adherent treatment. As we do not expect extensive short‐term improvements in CC care in SSA, we conclude that therapy with selected minor and major deviations (Table 1) such as recommended in the NCCN Harmonized Guidelines for SSA are justifiable options.
Treatment attempts without curative potential should be avoided, such as discontinuation of radiotherapy resulting in underdosing, chemotherapy only, surgery in patients with FIGO stage >IIb, or inappropriate surgery in patients with FIGO stage ≤IIb. We found that such practices were associated with a nearly fourfold risk of early death compared with guideline‐adherent practices. It is also possible that they cause considerable morbidity as well as financial burden in patients and family members [29]. Of course, it is even less acceptable to see patients managed without any CDT in a curative situation, with risk of early death increased ninefold.
In patients with fully guideline‐adherent treatments, the risk of early death was similar in our study (HRR, 0.30; 95% CI, 0.11–0.86; n = 111) compared with an Australian subcohort with FIGO stage I and II patients (HRR, 0.22; 95% CI, 0.07–0.75; n = 106) in 2005–2011 [22].
General limitations in our study include imprecise staging, poor documentation and record keeping, and early loss to follow‐up [6, 7, 8, 9, 30]. First, to assess completeness of therapy, we included patients from the population‐based registries, among which there is no selection bias in contrast to hospital‐based studies. Second, we assume there could have been a survivorship bias, because patients with aggressive disease and early death never had a chance to receive therapy and thus could have contributed to lower survival in the group without therapy. We also anticipated immortal‐time bias for those patients receiving treatment. Therapy uptake might not have been at random but also might have been linked to factors associated with outcome. To reduce inflation of therapy effects, we only included into regression analysis patients with survival of at least 3 months after diagnosis. Consequently, the analysis started 3 months after diagnosis [31]. Third, patients without any information were a large group of 35%. We decided not to make assumptions about therapy received and to present the data as unknown. Findings on stage pattern, number of patients left untreated, 1‐ and 3‐year ASRSs, and proportion of HIV‐positive patients were similar to previous studies from Ethiopia, Kenya, and Zimbabwe and reassuring as to the representativeness of our cohort [6, 7, 8]. Seeing a total of 22 among 410 patients in the traced cohort who died within the first month (median survival 7 days) shows that late presentation and late formal diagnosis is another reason for very short survival times in our cohort. Upcoming prospective studies from population‐based cancer registries may result in more detailed information on therapy and outcome [32].
Conclusion
In this population‐based study from eight African countries, up to two‐thirds of patients with CC received treatment without curative potential or no therapy at all (worst‐case scenario assuming those without documented information were left without therapy). Lack of therapy and advanced stage were associated with very low survival rates, similar to data reported 20 years ago from Uganda and Zimbabwe. Implementation of vaccination, early detection, and screening could reduce the total of 112,000 patients with CC and reduce the estimated 28,000 patients with incurable stage IV disease in the long term. More radiotherapy facilities are urgently needed for patients presenting with curative disease. Also, specialist gynecological surgeons need to be trained to mitigate the tragic outcome of up to 75,000 women presenting with curable disease but not receiving guideline‐adherent or any treatment at all, who are thus left to suffer and die. Progress in surgical techniques managing even advanced and nodal‐positive disease without radiotherapy could be of high importance for SSA [33].
Author Contributions
Conception/design: Mirko Griesel, Eva J. Kantelhardt
Provision of study material or patients: Samukeliso Vuma, Anne Korir, Gladys C. Chesumbai, Sarah Nambooze, Cesaltina F. Lorenzoni, Marie‐Thérèse Akele‐Akpo, Amalado Ayemou, Cheick B. Traoré, Tigeneh Wondemagegnehu
Collection and/or assembly of data: Mirko Griesel, Tobias P. Seraphin, Nikolaus C.S. Mezger, Lucia Hämmerl, Jana Feuchtner, Biying Liu
Data analysis and interpretation: Mirko Griesel, Walburga Yvonne Joko‐Fru, Andreas Wienke, Christoph Thomssen, Eva J. Kantelhardt
Manuscript writing: Mirko Griesel, Mazvita Sengayi‐Muchengeti, Donald M. Parkin, Ahmedin Jemal, Eva J. Kantelhardt
Final approval of manuscript: Mirko Griesel, Tobias P. Seraphin, Nikolaus C.S. Mezger, Lucia Hämmerl, Jana Feuchtner, Walburga Yvonne Joko‐Fru, Mazvita Sengayi‐Muchengeti, Biying Liu, Samukeliso Vuma, Anne Korir, Gladys C. Chesumbai, Sarah Nambooze, Cesaltina F. Lorenzoni, Marie‐Thérèse Akele‐Akpo, Amalado Ayemou, Cheick B. Traoré, Tigeneh Wondemagegnehu, Andreas Wienke, Christoph Thomssen, Donald M. Parkin, Ahmedin Jemal, Eva J. Kantelhardt
Disclosures
Eva J. Kantelhardt: Daiichi Sankyo (other: travel support); Jana Feuchtner: Bayer Foundation (other: stipend/travel); Mirko Griesel: Friedrich Ebert Foundation (other: stipend/travel). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables
Acknowledgments
We would like to thank all registry staff involved in data collection and follow‐up. We were supported by Intramural Funding from the Research Department of the American Cancer Society (contract no. 43359) and the German Ministry for Economic and Development Cooperation (BMZ) through the ESTHER University and Hospital Partnership Initiative of German International Cooperation (GIZ) (project no. 13.2238.7‐004.41). The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Open Access funding enabled and organized by Projekt DEAL.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bach PB. Using practice guidelines to assess cancer care quality. J Clin Oncol 2005;23:9041–9043. [DOI] [PubMed] [Google Scholar]
- 2. Kruk ME, Gage AD, Arsenault C et al. High‐quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob Health 2018;6:e1196–e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devesa SS, Silverman DT. Cancer incidence and mortality trends in the United States: 1935‐74. J Natl Cancer Inst 1978;60:545–571. [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 5. United Nations, Department of Economic and Social Affairs, Population Divison (2017). World Population Prospects: The 2017 Revision, custom data acquired via website. Available at: https://population.un.org/wpp/Publications/. Accessed February 18, 2019.
- 6. Wabinga H, Ramanakumar AV, Banura C et al. Survival of cervix cancer patients in Kampala, Uganda: 1995‐1997. Br J Cancer 2003;89:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chokunonga E, Ramanakumar AV, Nyakabau AM et al. Survival of cervix cancer patients in Harare, Zimbabwe, 1995‐1997. Int J Cancer 2004;109:274–277. [DOI] [PubMed] [Google Scholar]
- 8. Kantelhardt EJ, Moelle U, Begoihn M et al. Cervical cancer in Ethiopia: Survival of 1,059 patients who received oncologic therapy. The Oncologist 2014;19:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sengayi‐Muchengeti M, Joko‐Fru WY, Miranda‐Filho A et al. Cervical cancer survival in sub‐Saharan Africa by age, stage at diagnosis and Human Development Index: A population‐based registry study. Int J Cancer 2020;147:3037–3048. [DOI] [PubMed] [Google Scholar]
- 10. Abdel‐Wahab M, Zubizarreta E, Polo A et al. Improving quality and access to radiation therapy: An IAEA perspective. Semin Radiat Oncol 2017;27:109–117. [DOI] [PubMed] [Google Scholar]
- 11. Meara JG, Leather AJM, Hagander L et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015;386:569–624. [DOI] [PubMed] [Google Scholar]
- 12. Wilson BE, Jacob S, Yap ML et al. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population‐based study. Lancet Oncol 2019;20:769–780. [DOI] [PubMed] [Google Scholar]
- 13. African Cancer Registry Network Web site . Available at http://afcrn.org/. Accessed December 29, 2018.
- 14. Finesse AM, Somdyala N, Chokunonga E, Parkin DM. Standard Procedure Manual for Population‐Based Cancer Registries in sub‐Saharan Africa. Version II, 2015. Available at: http://afcrn.org/resources/51-afcrndatabase/131-sop. Accessed July 31, 2017.
- 15. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet 2009;105:103–104. [DOI] [PubMed] [Google Scholar]
- 16. National Comprehensive Cancer Network . Practice Guidelines in Oncology: Cervical Cancer. Version 1.2010. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2009.
- 17. Kerr S, Jazieh AR, Kerr D. How useful are international treatment guidelines in low‐ and middle‐income countries? J Glob Oncol 2017;3:441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ismaila N, Salako O, Mutiu J et al. Oncology guidelines usage in a low‐ and middle‐income country. J Glob Oncol 2018;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brenner H, Gefeller O, Hakulinen T et al. period and periodh: Period Analysis of Survival Data, 2018. Available at: http://www.imbe.med.uni-erlangen.de/cms/software_period.html. Accessed January 2, 2019.
- 20. World Health Organization . Global Health Observatory Data Repository: Life tables by country. Available at: http://apps.who.int/gho/data/node.main.LIFECOUNTRY?lang=en. Accessed December 4, 2018.
- 21. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–2316. [DOI] [PubMed] [Google Scholar]
- 22. Chiew KL, Chong S, Duggan KJ et al. Assessing guideline adherence and patient outcomes in cervical cancer. Asia Pac J Clin Oncol 2017;13:e373–e380. [DOI] [PubMed] [Google Scholar]
- 23. Abdel‐Wahab M, Bourque JM, Pynda et al. Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol 2013:e168–e175. [DOI] [PubMed] [Google Scholar]
- 24. Were E, Nyaberi Z, Buziba N. Perceptions of risk and barriers to cervical cancer screening at Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya. Afr Health Sci 2011;11:58–64. [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson ML, Atun R, DeStigter K et al. The Lancet Commission on diagnostics: Advancing equitable access to diagnostics. Lancet 2019;393:2018–2020. [DOI] [PubMed] [Google Scholar]
- 26. Han K, Milosevic M, Fyles A et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111–119. [DOI] [PubMed] [Google Scholar]
- 27. Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975‐2014. Available at: https://seer.cancer.gov/csr/1975_2014/. Accessed February 4, 2018.
- 28. National Comprehensive Cancer Network . NCCN Harmonized Guidelines for Sub‐Saharan Africa: Cervical Cancer. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical_harmonized-africa.pdf. [Google Scholar]
- 29. Moelle U, Mathewos A, Aynalem A et al. Cervical cancer in Ethiopia: The effect of adherence to radiotherapy on survival. The Oncologist 2018;23:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allemani C, Matsuda T, Di Carlo V et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol 2008;167:492–499. [DOI] [PubMed] [Google Scholar]
- 32. Dereje N, Addissie A, Worku A et al. Extent and predictors of delays in diagnosis of cervical cancer in Addis Ababa, Ethiopia: A population‐based prospective study. J Glob Oncol 2020;6:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Höckel M, Wolf B, Schmidt K, et al. Surgical resection based on ontogenetic cancer field theory for cervical cancer: mature results from a single‐centre, prospective, observational, cohort study. Lancet Oncol 2019;20(9):1316–1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables


