Abstract
Background
Many systemic therapies for advanced hepatocellular carcinoma (HCC) may cause hypothyroidism; however, in these patients, hypothyroidism prevalence before therapy and its prognostic impact remain unclear.
Materials and Methods
We previously established a prospective cohort of patients who received sorafenib as first‐line therapy for advanced HCC. No patients had been clinically diagnosed with hypothyroidism before or during sorafenib treatment. We retrospectively determined the levels of thyrotropin and free thyroxine before initiation of systemic therapy. Hypothyroidism was defined as thyrotropin level higher than the upper limit of the normal range. Among patients with hypothyroidism, free thyroxine level less than the lower normal range was defined as overt hypothyroidism, and free thyroxine level within the normal range was defined as subclinical hypothyroidism.
Results
In total, 79 patients were enrolled; of them, 16 (20%) had hypothyroidism (overt hypothyroidism, 10; subclinical hypothyroidism, 6). Patients with hypothyroidism, compared with those without hypothyroidism, were more likely to be older than 65 years (56% vs. 29%, p = .037), have a serum α‐fetoprotein level of >400 ng/mL (81% vs. 52%, p = .037), and have a significantly poorer overall survival (OS; median, 5.5 vs. 11.6 months, p = .043). After adjusting for other potential prognostic factors, hypothyroidism remained an independent predictor for poorer OS (hazard ratio, 2.53, p = .018). Patients with overt hypothyroidism and subclinical hypothyroidism exhibited similarly poor OS (p = .768).
Conclusion
Underdiagnosis of hypothyroidism in patients with advanced HCC was common. Hypothyroidism, whether overt or subclinical, is associated with poor prognosis of advanced HCC.
Implications for Practice
The results of this study showed the underdiagnosis of hypothyroidism in patients with advanced hepatocellular carcinoma (HCC) and its influence on prognosis. These findings implied the importance of thyroid function check before initiation of systemic therapy for patients with advanced HCC.
Keywords: Hepatocellular carcinoma, Hypothyroidism, Prognosis, Survival
Short abstract
Systemic therapies for advanced hepatocellular carcinoma (HCC) can cause hypothyroidism. This study examined the prevalence and prognostic impact of hypothyroidism in patients with HCC before receiving any systemic therapy.
Introduction
Targeted therapy and immunotherapy are the main treatment options for advanced hepatocellular carcinoma (HCC). Many approved targeted therapies for advanced HCC, especially small molecule multikinase inhibitors such as lenvatinib [1, 2], cabozantinib [3], and regorafenib [4], can cause hypothyroidism. The condition can also be a manifestation of immune‐related adverse events in patients receiving immunotherapy with checkpoint inhibitors [5, 6]. In total, 5%–10% of patients who receive PD1 blockade therapy could experience hypothyroidism [5].
In a recent randomized study on pembrolizumab as second‐line treatment for advanced HCC, hypothyroidism occurred in approximately 5% of patients, whether they received pembrolizumab or a placebo [7]. Hyperthyroidism developed in 3% of patients who received pembrolizumab, but no patients who received a placebo experienced hyperthyroidism. Thus, hypothyroidism may still occur even in patients with advanced HCC who are not currently under active therapy.
Many factors could contribute to the increase in hypothyroidism incidence in patients with advanced HCC. Interferon therapy, previously used regularly in patients with chronic hepatitis C, can cause hypothyroidism [8, 9, 10, 11, 12, 13]. Transarterial chemoembolization (TACE), a standard treatment for intermediate‐stage HCC, can also cause thyroid function disorders, although temporarily in most patients [14]. Despite these findings, studies have reported that 10%–20% of patients with chronic hepatitis C before receiving interferon therapy or patients with liver cirrhosis exhibit thyroid disorders [15, 16, 17, 18].
There is a possibility of increased occurrence of hypothyroidism and identifying it as an adverse event in patients with advanced HCC. In the current study, we examined the prevalence and prognostic impact of hypothyroidism in patients with advanced HCC before they received any systemic therapy.
Materials and Methods
Study Population
The study cohort comprised patients with advanced HCC receiving sorafenib as first‐line therapy at the National Taiwan University Hospital (NTUH), Taipei, Taiwan. The participants provided consent to undergo pretreatment peripheral blood collection. All clinicopathological variables were prospectively collected from patient chart records. This biomarker study was approved by the Research Ethical Committee of NTUH.
Sample Preparation and Definition Of Hypothyroidism
Blood samples were carefully layered onto Histopaque‐1077 (Sigma, St. Louis, MO, USA) and then centrifuged at 400 × g (relative centrifugal force) for 30 minutes at 4°C. The plasma was separated and stored at −80°C. Thyrotropin and free thyroxine levels were determined using the quantitative chemiluminescent immunoassay at the central laboratory of NTUH. The normal range of thyrotropin and free thyroxine levels followed the NTUH standards (0.35–4.94 μIU/mL and 0.7–1.48 ng/dL, respectively). Hypothyroidism was defined when the thyrotropin level was higher than the upper limit of the normal range (ULN) or when the free thyroxine level was lower than the lower limit of the normal range (LLN). The patients with thyrotropin levels higher than ULN were considered to have overt and subclinical hypothyroidism when their free thyroxine levels were lower than the LLN and were within the normal range, respectively. The patients with thyroxine levels lower than LLN but normal or decreased thyrotropin levels were considered to have central hypothyroidism.
Statistical Methods
To examine the association between hypothyroidism and patient characteristics, we used the χ2 or Fishers exact test, when appropriate, for nominal variables and the independent t test for continuous variables. The Kaplan‐Meier method was used to estimate survival. To compare survival between the groups, the log‐rank test was used in univariate analysis and a Cox proportional hazards model was used in multivariate analysis. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). A two‐sided p value of ≤.05 was considered statistically significant.
Results
Patient Characteristics
In total, 79 patients (women, 15 [19%]; men, 64 [81%]) were included in the study. No patient had been clinically diagnosed as having thyroid dysfunction before the sample collection or during sorafenib treatment. Basic patient characteristics are listed in Table 1. The mean patient age was 60.7 years, and 27 (34%) patients were older than 65 years. Regarding hepatitis etiology, 65% and 24% of patients had chronic hepatis B and chronic hepatitis C, respectively. One patient with chronic hepatitis C had received interferon‐α treatment approximately 10 years before. No other patients had received either interferon‐α or ribavirin treatment previously. Most patients had Barcelona Clinic Liver Cancer C disease (92%), Child‐Pugh A liver reserve (94%), and Eastern Cooperative Oncology Group performance status of 0 or 1 (96%). Most (80%) patients had received prior HCC therapy, and 63% had received TACE.
Table 1.
Patient characteristics and comparisons between patients with and without hypothyroidism
| Characteristics | All | Hypothyroidism | ||
|---|---|---|---|---|
| Yes | No | p | ||
| Total, n (%) | 79 (100) | 16 (100) | 63 (100) | |
| Mean age (SD), years | 60.7 (10.6) | 67.5 (9.2) | 58.9 (10.3) | .003 |
| Age >65 y | 27 (34) | 9 (56) | 18 (29) | .037 |
| Gender, n (%) | .490 | |||
| Female | 15 (19) | 4 (25) | 11 (17) | |
| Male | 64 (81) | 12 (75) | 52 (83) | |
| Hepatitis virus, n (%) | ||||
| HBsAg positive | 51 (65) | 8 (50) | 43 (68) | .173 |
| Anti‐HCV positive | 19 (24) | 5 (31) | 14 (22) | .516 |
| Extrahepatic metastasis, n (%) | 49 (62) | 9 (56) | 40 (63) | .594 |
| Macrovascular invasion, n (%) | 52 (66) | 12 (75) | 40 (63) | .386 |
| AFP >400 ng/mL, n (%) | 46 (58) | 13 (81) | 33 (52) | .037 |
| Child‐Pugh status, n (%) | 1.000 | |||
| A | 74 (94) | 15 (94) | 59 (94) | |
| B | 5 (6) | 1 (6) | 4 (6) | |
| BCLC stage, n (%) | 1.000 | |||
| B | 6 (8) | 1 (6) | 5 (8) | |
| C | 73 (92) | 15 (94) | 58 (92) | |
| ECOG PS, n (%) | .637 | |||
| 0 | 36 (46) | 7 (44) | 29 (46) | |
| 1 | 40 (51) | 9 (56) | 31 (49) | |
| 2 | 3 (4) | 0 (0) | 3 (5) | |
| Prior HCC treatment, n (%) | 63 (80) | 13 (81) | 50 (79) | 1.000 |
| Prior TACE, n (%) | 50 (63) | 10 (63) | 40 (63) | .941 |
| Mean thyrotropin (SD) | 4.8 (10.9) | 16.3 (20.9) | 1.9 (1.1) | |
| Mean free thyroxine (SD) | 0.90 (0.21) | 0.68 (0.23) | 0.95 (0.16) | |
All data were presented as n (%) unless otherwise indicated.
Abbreviations: AFP, α‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; ECOG PS, Eastern Cooperative Oncology Group performance status; HBsAg, hepatitis B virus surface antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; TACE, transarterial chemoembolization.
Hypothyroidism and Its Association with Demographics
No patient had received prior systemic therapy, including targeted therapy or immunotherapy, for advanced HCC during peripheral blood sample collection. Of the 79 patients, 16 (20%) were identified as having hypothyroidism (overt hypothyroidism, 10; subclinical hypothyroidism, 6; central hypothyroidism, 0). Patients with hypothyroidism, compared with those without hypothyroidism, were more likely to be older than 65 years (56% vs. 29%, p = .037) and have a serum α‐fetoprotein (AFP) level of >400 ng/mL (81% vs. 52%, p = .037) (Table 1). One‐third of patients aged >65 years had hypothyroidism. Chronic hepatitis B and C, performance status, prior HCC treatment, or prior TACE was not found to be associated with hypothyroidism. The patient who had received prior interferon treatment for chronic hepatitis C was identified as not having hypothyroidism.
Hypothyroidism and Prognosis
For the entire study cohort, the median overall survival (OS) was 9.8 (95% confidence interval, 5.7–13.8) months. Patients with hypothyroidism, compared with patients without hypothyroidism, exhibited significantly poorer OS (median, 5.5 vs. 11.6 months, p = .043; Fig. 1A). After adjusting for age, sex, hepatitis etiology, liver reserve, tumor extent, performance status, AFP level, and prior HCC treatment, hypothyroidism remained an independent predictor for poorer OS (hazard ratio, 2.53; 95% confidence interval 1.177–5.439; p = .018; Table 2). Patients with overt hypothyroidism and subclinical hypothyroidism exhibited similarly poor OS (median, 5.5 vs. 4.6 months, p = .768; Fig. 1B).
Figure 1.
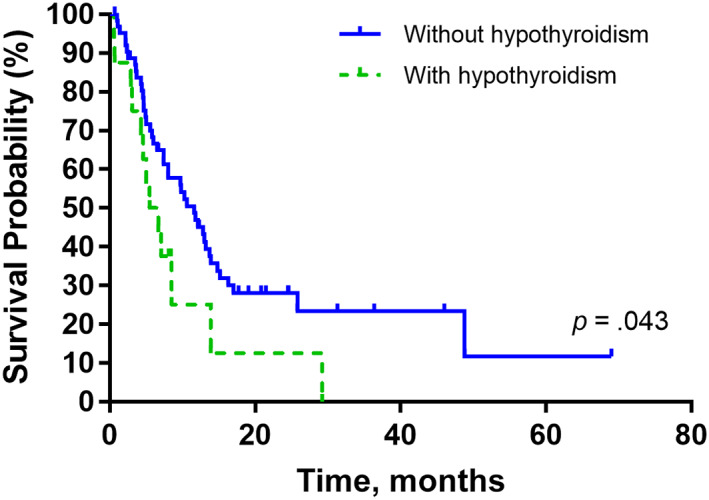
Kaplan‐Meier curves for the overall survival of (A) patients with and without hypothyroidism and (B) patients with overt and subclinical hypothyroidism. The p values were conducted using the log‐rank test.
Table 2.
Multivariate analysis using a Cox proportional hazards model for overall survival predictors
| Variables | P value | Hazard ratio (95% CI) |
|---|---|---|
| Hypothyroidism | .018 | 2.53 (1.177–5.439) |
| Sex | .583 | 0.814 (0.391–1.696) |
| Age >65 years | .204 | 0.641 (0.323–1.272) |
| HBsAg positive | .353 | 1.424 (0.676–3.002) |
| Anti‐HCV positive | .577 | 0.79 (0.345–1.808) |
| Macrovascular invasion | .035 | 2.066 (1.053–4.052) |
| AFP >400 ng/mL | .138 | 1.61 (0.858–3.022) |
| Extrahepatic spread | .040 | 1.977 (1.033–3.784) |
| Child‐Pugh B (vs. A) | .262 | 1.991 (0.598–6.625) |
| ECOG PS 0 (vs. 1 or 2) | .245 | 0.696 (0.378–1.281) |
| Prior HCC treatment | .006 | 0.388 (0.197–0.764) |
Abbreviations: AFP, α‐fetoprotein; ECOG PS, Eastern Cooperative Oncology Group performance status; HBsAg, hepatitis B virus surface antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Discussion
In this study, 20% of the patients with advanced HCC who had not received prior systemic therapy were identified as having hypothyroidism. The high prevalence was not attributable to either interferon therapy or previous TACE. Hypothyroidism, whether overt or subclinical, was associated with poor prognosis even after adjusting for other potential prognostic factors. This finding is in line with a previous study on patients with unresectable HCC, which demonstrated that increased thyrotropin levels were associated with more advanced disease and decreased OS [19].
Considering the high prevalence of hypothyroidism among patients with advanced HCC and that many approved therapies for advanced HCC can cause hypothyroidism [1, 2, 3, 4, 5, 6], routine examination of thyroid function in patients with advanced HCC before initiation of systemic therapy should be considered. In clinical practice, hypothyroidism can lead to symptoms, such as severe fatigue and edema, that prevent patients from receiving adequate cancer treatment. Recognizing hypothyroidism as a treatment‐related adverse event might lead to incorrect discontinuation of systemic therapy.
The mechanism underlying the association between hypothyroidism and poor prognosis of advanced HCC could be multifactorial. Overexpression of thyroid hormone receptors decreased HCC cell migration [20], and thyroid receptor expression was decreased in HCC cells [21, 22, 23]. Thyroid hormone was shown to inhibit HCC progression in animal model through various pathways [24]. Although we did not find any associations between hypothyroidism and known clinical prognostic markers, hypothyroidism could represent an unknown marker of poor HCC disease status. Above all, such speculations require further study for confirmation.
Our finding that hypothyroidism was associated with poor prognosis in patients with advanced HCC is not a universal phenomenon among patients with cancer. Hypothyroidism was reported to correlate with favorable prognosis in patients with metastatic brain cancer [25]. Combining the above potential mechanisms of how thyroid hormone may inhibit HCC progression, hypothyroidism may have a specific influence of patients with HCC. Whether levothyroxine supplementation can reverse the poor prognosis should be explored in future study.
Although levothyroxine supplementation is easy and convenient for patients with overt hypothyroidism, guidelines do not recommend routine levothyroxine supplementation in asymptomatic patients with subclinical hypothyroidism [26, 27]. A recent study on patients with subclinical hypothyroidism and acute myocardial infarction failed to demonstrate the benefit of levothyroxine supplement in improving the left ventricular ejection function [28]. Thus, levothyroxine should not be routinely given to patients with advanced HCC and subclinical hypothyroidism unless future studies prove otherwise.
Our study might have overestimated hypothyroidism prevalence. The finding of hypothyroidism may be temporary, and we did not have the chance to examine the thyrotropin level on another occasion. Nevertheless, as long as the thyrotropin level was high, patients exhibited poor survival regardless of the free thyroxine level. Studies have also reported a hypothyroidism prevalence of 10%–20% in patients with chronic hepatitis C or liver cirrhosis [15, 16, 17, 18].
Conclusion
The underdiagnosis of hypothyroidism was noted in patients with advanced HCC. Hypothyroidism, whether overt or subclinical hypothyroidism, is associated with poor prognosis of advanced HCC.
Author Contributions
Conception/design: Yu‐Yun Shao, Chih‐Hung Hsu
Provision of study material or patients: Yu‐Yun Shao, Ann‐Lii Cheng, Chih‐Hung Hsu
Collection and/or assembly of data: Yu‐Yun Shao
Data analysis and interpretation: Yu‐Yun Shao, Chih‐Hung Hsu
Manuscript writing: Yu‐Yun Shao, Ann‐Lii Cheng, Chih‐Hung Hsu
Final approval of manuscript: Yu‐Yun Shao, Ann‐Lii Cheng, Chih‐Hung Hsu
Disclosures
The authors indicated no financial relationships
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 102–2314‐B‐002–120, MOST‐103–2314‐B‐002–181‐MY2, MOST‐103–2314‐B‐002–092, MOST 104–2314‐B‐002 ‐073, MOST‐105–2314‐B‐002–194, and MOST‐108–2314‐B‐002–072‐MY3), Ministry of Health and Welfare, Taiwan (MOHW109‐TDU‐B‐211–114002), National Taiwan University Hospital (NTUH 105S2954 and NTUH 108‐S4150), and Good Liver Foundation, Taiwan
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Ann‐Lii Cheng, Email: alcheng@ntu.edu.tw.
Chih‐Hung Hsu, Email: chihhunghsu@ntu.edu.tw.
References
- 1. Tada T, Kumada T, Hiraoka A et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol Res 2020;50:75–83. [DOI] [PubMed] [Google Scholar]
- 2. Koizumi Y, Hirooka M, Hiraoka A et al. Lenvatinib‐induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr J 2019;66:787–792. [DOI] [PubMed] [Google Scholar]
- 3. Di Nunno V, Frega G, Gatto L et al. Hypothyroidism in patients with hepatocellular carcinoma receiving cabozantinib: An unassessed issue. Future Oncol 2019;15:563–565. [DOI] [PubMed] [Google Scholar]
- 4. Bruix J, Tak WY, Gasbarrini A et al. Regorafenib as second‐line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open‐label, phase II safety study. Eur J Cancer 2013;49:3412–3419. [DOI] [PubMed] [Google Scholar]
- 5. Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 6. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finn RS, Ryoo BY, Merle P et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: A randomized, double‐blind, phase III trial. J Clin Oncol 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 8. Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology 1997;26:112S–121S. [DOI] [PubMed] [Google Scholar]
- 9. Deutsch M, Dourakis S, Manesis EK et al. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology 1997;26:206–210. [DOI] [PubMed] [Google Scholar]
- 10. Okanoue T, Sakamoto S, Itoh Y et al. Side effects of high‐dose interferon therapy for chronic hepatitis C. J Hepatol 1996;25:283–291. [DOI] [PubMed] [Google Scholar]
- 11. Picciotto A, Varagona G, Cianciosi P et al. Thyroid function and interferon treatment in chronic hepatitis C. Gut 1993;34:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcellin P, Pouteau M, Renard P et al. Sustained hypothyroidism induced by recombinant alpha interferon in patients with chronic hepatitis C. Gut 1992;33:855–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lisker‐Melman M, Di Bisceglie AM, Usala SJ et al. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology 1992;102:2155–2160. [DOI] [PubMed] [Google Scholar]
- 14. Flohr F, Harder J, Seufert J et al. Hypothyroidism in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Hepatology 2008;47:2144. [DOI] [PubMed] [Google Scholar]
- 15. Antonelli A, Ferri C, Pampana A et al. Thyroid disorders in chronic hepatitis C. Am J Med 2004;117:10–13. [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez‐Torres M, Ríos‐Bedoya CF, Ortiz‐Lasanta G et al. Thyroid dysfunction (TD) among chronic hepatitis C patients with mild and severe hepatic fibrosis. Ann Hepatol 2008;7:72–77. [PubMed] [Google Scholar]
- 17. Batool N, Elahi S, Saleem N et al. Thyroid dysfunction in non‐interferon treated hepatitis c patients residing in hepatitis endemic area. Biomed Res Int 2017;2017:2390812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar KV, Pawah AK, Manrai M. Occult endocrine dysfunction in patients with cirrhosis of liver. J Family Med Prim Care 2016;5:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinter M, Haupt L, Hucke F et al. The impact of thyroid hormones on patients with hepatocellular carcinoma. PLoS One 2017;12:e0181878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chi HC, Liao CH, Huang YH et al. Thyroid hormone receptor inhibits hepatoma cell migration through transcriptional activation of Dickkopf 4. Biochem Biophys Res Commun 2013;439:60–65. [DOI] [PubMed] [Google Scholar]
- 21. Frau C, Loi R, Petrelli A et al. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology 2015;61:249–259. [DOI] [PubMed] [Google Scholar]
- 22. Martínez‐Iglesias O, Garcia‐Silva S, Tenbaum SP et al. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res 2009;69:501–509. [DOI] [PubMed] [Google Scholar]
- 23. Liao CH, Yeh CT, Huang YH et al. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology 2012;55:910–920. [DOI] [PubMed] [Google Scholar]
- 24. Kowalik MA, Puliga E, Cabras L et al. Thyroid hormone inhibits hepatocellular carcinoma progression via induction of differentiation and metabolic reprogramming. J Hepatol 2020;72:1159–1169. [DOI] [PubMed] [Google Scholar]
- 25. Berghoff AS, Wippel C, Starzer AM et al. Hypothyroidism correlates with favourable survival prognosis in patients with brain metastatic cancer. Eur J Cancer 2020;135:150–158. [DOI] [PubMed] [Google Scholar]
- 26. Bekkering GE, Agoritsas T, Lytvyn L et al. Thyroid hormones treatment for subclinical hypothyroidism: A clinical practice guideline. BMJ 2019;365:l2006. [DOI] [PubMed] [Google Scholar]
- 27. Garber JR, Cobin RH, Gharib H et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012;22:1200–1235. [DOI] [PubMed] [Google Scholar]
- 28. Jabbar A, Ingoe L, Junejo S et al. Effect of levothyroxine on left ventricular ejection fraction in patients with subclinical hypothyroidism and acute myocardial infarction: A randomized clinical trial. JAMA 2020;324:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]


