Figure 1.
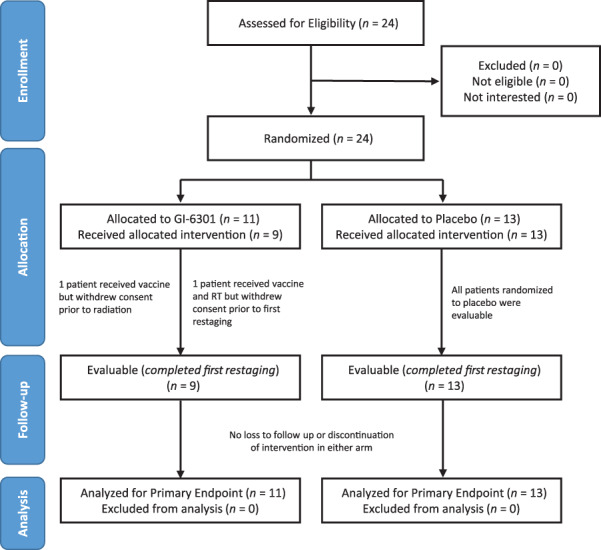
Consort flow diagram. Note that all patients who were assessed for eligibility on this protocol were enrolled and randomized. A separate National Cancer Institute screening protocol is used for the general screening of new patients, but these data are not reported. Of 24 patients who gave signed informed consent and enrolled on study, 11 were randomized to the interventional (vaccine) arm and 13 to the placebo arm. Two patients on the vaccine arm left the study before first restaging and were considered not evaluable; these two patients were included in the intention‐to‐treat analysis. Patients who received placebo were allowed to cross over to vaccine after progression, but data postcrossover are not part of the primary efficacy analysis. Abbreviation: RT, radiation therapy.
