Abstract
Lessons Learned
Treatment for patients with metastatic colorectal cancer (mCRC) typically involves multiple lines of therapy with eventual development of treatment resistance.
In this single‐arm, phase II study involving heavily pretreated patients, the combination of sorafenib and capecitabine yielded a clinically meaningful progression‐free survival of 6.2 months with an acceptable toxicity profile.
This oral doublet therapy is worthy of continued investigation for clinical use in patients with mCRC.
Background
Capecitabine (Cape) is an oral prodrug of the antimetabolite 5‐fluorouracil. Sorafenib (Sor) inhibits multiple signaling pathways involved in angiogenesis and tumor proliferation. SorCape has been previously studied in metastatic breast cancer.
Methods
This single‐arm, phase II study was designed to evaluate the activity of SorCape in refractory metastatic colorectal cancer (mCRC). Patients received Sor (200 mg p.o. b.i.d. max daily) and Cape (1,000 mg/m2 p.o. b.i.d. on days 1–14) on a 21‐day treatment cycle. Primary endpoint was progression‐free survival (PFS) with preplanned comparison with historical controls.
Results
Forty‐two patients were treated for a median number of 3.5 cycles (range 1–39). Median PFS was 6.2 (95% confidence interval [CI], 4.3–7.9) months, and overall survival (OS) was 8.8 (95% CI, 4.3–12.2) months. One patient (2.4%) had partial response (PR), and 22 patients (52.4%) had stable disease (SD) for a clinical benefit rate of 54.8% (95% CI, 38.7%–70.2%). Hand‐foot syndrome was the most common adverse event seen in 36 patients (85.7%) and was grade ≥ 3 in 16 patients (38.1%). One patient (2.4%) had a grade 4 sepsis, and one patient (2.4%) died while on treatment.
Conclusion
SorCape in this heavily pretreated population yielded a reasonable PFS with manageable but notable toxicity. The combination should be investigated further.
Keywords: Sorafenib, Capecitabine, Colorectal cancer, Metastatic cancer, Oral therapy
Discussion
The prognosis of mCRC after previous exposure to effective therapies remains poor with limited treatment options resulting in successively shorter PFS intervals for those with relapsed and refractory disease. In this study, the median number of prior lines of therapy before study entry was three (range, two to seven). Thus, the vast majority of patients in this trial had exhausted most effective forms of treatment before entering this trial. In such a late setting, best supportive care alone offers a median PFS of 1.8 months and OS of 4.6 months, highlighting the natural history of the disease in this situation. This single‐arm, phase II study of SorCape demonstrated a median PFS of 6.2 months, 3‐month PFS of 83.3%, and median OS of 8.8 months in a heavily pretreated patient population. These results compare favorably with anticipated historical PFS and OS outcomes of 3 months and 6–7 months, respectively, with oral monotherapy salvage treatment options (Fig. 1; Table 1).
Figure 1.
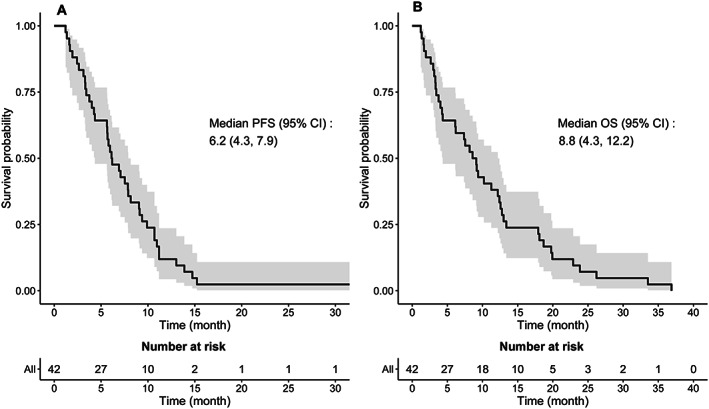
Kaplan‐Meier curves of median PFS shown on the left (A) and median OS shown on the right (B). Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival.
Table 1.
Response rates
| Response | Assessment (n = 42) | % patientsa (95% CI) |
|---|---|---|
| Overall response rate | 1 | 2.4 (0.01–0.13) |
| Clinical benefit rate | 23 | 54.8 (0.39–0.70) |
| Best response | ||
| Complete response | 0 | 0 |
| Partial response | 1 | 2.4 |
| Stable disease | 22 | 52.4 |
| Progressive disease | 14 | 33.3 |
| Could not be evaluatedb | 5 | 11.9 |
Percentages may not total 100 because of rounding.
Response not evaluable due to patients prematurely stopping treatment or not receiving second scan.
Abbreviation: CI, confidence interval.
The adverse events (AEs) attributed to this doublet therapy were not surprising given the well‐established side effect profile of each individual agent and were overall manageable. As expected, the most common AE was hand‐foot syndrome, which is one of the overlapping toxicities with both agents. Although it was noted to be present in the majority of patients (n = 36; 85.7%), it was grade 3 or greater in only 16 patients (38.1%). Other common side effects included fatigue and gastrointestinal side effects including nausea, anorexia, diarrhea, and vomiting. Most of these anticipated side effects were mild and easily mitigated with supportive care medications (i.e., antidiarrhea and antinausea medications).
The endpoint of PFS was chosen as we did not anticipate a robust response rate with the SorCape combination, but rather clinical benefit in the form of disease control. We used a historical PFS as a comparative analysis, which is admittedly harder to interpret without a contemporaneously randomized control arm using best supportive care and is confounded by potential patient selection bias.
Additional correlative analyses in larger studies using this combination therapy are required to identify subsets of patients or molecular profiles that could support a predictive biomarker.
Trial Information
| Disease | Colorectal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | More than two prior regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints |
Overall survival Overall response rate Toxicity PFS rate at 3 months OS rate at 3 months Clinical benefit rate |
| Additional Details of Endpoints or Study Design | |
| The primary endpoint of the study was PFS defined as the time from the treatment to disease progression or death from any cause, whichever came first. Patients who did not progress or were lost to follow‐up were censored at the day of their last objective tumor assessment. OS was defined as the time from the date of treatment start to the date of death from any cause. If the patient was alive at the end of the follow‐up period or was lost to follow‐up, OS was censored on the last date the patient was known to be alive. SD was defined by RECIST version 1.1 measurements as a component of best overall response. It was calculated from the start of treatment time until the criteria for progression were met, taking as reference the smallest measurements recorded since the treatment started. Upon treatment discontinuation, subjects were contacted every 8 weeks to assess survival status. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Capecitabine | |
| Generic/Working Name | Capecitabine |
| Trade Name | Xeloda |
| Company Name | Roche |
| Drug Type | Fluoropyrimidine with antineoplastic activity |
| Drug Class | Antimetabolite |
| Dose | 1,000 milligrams (mg) per squared meter (m2) |
| Route | Oral (p.o.) |
| Schedule of Administration | Capecitabine was administered at 1,000 mg/m2 by mouth twice daily. The dose of capecitabine was calculated with a maximum body surface area (BSA) of 2.0 m2 for patient safety. Capecitabine dose was adjusted if BSA changed >5% from baseline. |
| Sorafenib | |
| Generic/Working Name | Sorafenib |
| Trade Name | Nexavar |
| Company Name | Bayer and Onyx Pharmaceutics |
| Drug Type | Small molecule |
| Drug Class | Tyrosine kinase inhibitor |
| Dose | 200 milligrams |
| Route | Oral (p.o.) |
| Schedule of Administration | Sorafenib was administered at 200 mg by mouth twice daily. Dose was increased to 400 mg by mouth each morning and 200 mg by mouth each evening (dose level + 1) for cycle 2, and then to 400 mg by mouth twice daily (dose level + 2) for cycle 3 and onward, assuming no attributable AEs during the prior cycle. |
Patient Characteristics
| Number of Patients, Male | 33 |
| Number of Patients, Female | 9 |
| Stage | Stage IV: 42 (100%) |
| Age | Median (range): 56.6 (36–78) years |
| Number of Prior Systemic Therapies | Median (range): 3 (2–7) |
| Performance Status: ECOG |
0 — 19 1 — 19 2 — 3 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes |
BRAF mutated — 0 BRAF wild type — 12 (31%) KRAS mutated — 21 (50%) KRAS wild type — 18 (42.9%) Microsatellite instability (MSI) high — 0 MSI low — 1 (2.4%) MSI stable — 12 (28.6%) |
| Other | See Table 2 and Figure 2. |
Table 2.
Demographics and baseline characteristics (n = 42)
| Characteristic | n (%) |
|---|---|
| Age group | |
| 18 to <50 | 8 (19) |
| 50 to <65 | 22 (52.4) |
| ≥65 | 12 (28.6) |
| Gender | |
| Male | 33 (78.6) |
| Female | 9 (21.4) |
| Race | |
| White | 31 (73.8) |
| Black | 11 (26.2) |
| Tumor grade | |
| Unknown | 14 (33.3) |
| Well differentiated or G1 | 3 (7.1) |
| Moderately differentiated or G2 | 13 (31) |
| Poorly differentiated or undifferentiated or G3 | 4 (9.5) |
| Grade cannot be assessed or Gx | 8 (19) |
| BRAF | |
| Wild type | 13 (31) |
| Mutated | 0 |
| Unknown | 29 (69) |
| KRAS | |
| Wild type | 18 (42.9) |
| Mutated | 21 (50) |
| Unknown | 3 (7.1) |
| MSI | |
| Low | 1 (2.4) |
| Stable | 12 (28.6) |
| High | 0 |
| Unknown | 29 (69) |
| Number of lines of therapy prior to enrollment | |
| Median number of lines of therapy (min–max) | 3 (2–7) |
| <3 | 7 (16.7) |
| ≥3 | 35 (83.3) |
| Prior treatment with bevacizumab | |
| Yes | 34 (81) |
| No | 8 (19) |
| Prior treatment with capecitabine | |
| Yes | 17 (40.5) |
| No | 24 (57.1) |
| Unknown | 1 (2.4) |
| Prior treatment with cetuximab or panitumumab | |
| Yes | 18 (42.9) |
| No | 24 (57.1) |
Abbreviations: BRAF, B‐Raf; G1, grade 1; G2, grade 2; G3, grade 3; Gx, grade not assessed; KRAS, K‐ras; MSI, microsatellite instability.
Figure 2.
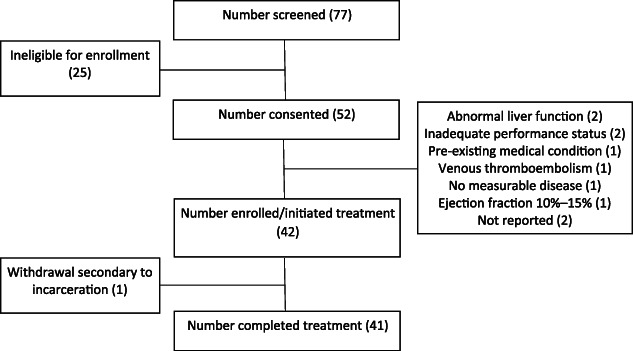
CONSORT diagram of enrollment. All patients who met enrollment eligibility criteria were included in the analysis. Patients who received at least one dose of a study drug were included in the safety analysis.
Primary Assessment Method
| Title | Response assessment |
| Number of Patients Screened | 77 |
| Number of Patients Enrolled | 42 |
| Number of Patients Evaluable for Toxicity | 42 |
| Number of Patients Evaluated for Efficacy | 42 |
| Evaluation Method | RECIST version 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 2.4 (1%) |
| Response Assessment SD | n = 52.4 (22%) |
| Response Assessment PD | n = 33.3 (14%) |
| Response Assessment OTHER | n = 11.9 (5%) |
| (Median) Duration Assessments PFS | 6.2 months, CI: 4.3–7.9 |
| (Median) Duration Assessments OS | 8.8 months, CI: 4.3–12.2 |
| Outcome Notes | |
| Patients received a median of 3.5 cycles (range, 1–39 cycles) of SorCape. The median PFS was 6.2 months (95% CI, 4.3–7.9; Fig. 1A), which was longer when compared with historical controls (3 months; p = .0004). The median OS for this study cohort was 8.8 months (95% CI, 4.3–12.2; Fig. 1B). The 3‐month PFS rate was 83.3% (95% CI, 68.2%–91.7%), 6‐month PFS was 52.4% (95% CI, 36.4%–66.1%), 3‐month OS was also 83.3% (95% CI, 68.2%–91.7%), and 6‐month OS was 64.3% (95% CI, 47.9%–76.7%). One patient had PR (2.4%), and 22 patients had SD (52.4%) per RECIST version 1.1, achieving an overall response rate of 2.4% (95% CI, 0.1%–12.6%) and clinical benefit rate of 54.8% (95% CI, 38.7%–70.2%). | |
Adverse Events
Table 3.
Most common adverse reactions with sorafenib plus capecitabine
| Adverse event | CTCAE toxicity grade | |
|---|---|---|
| All grades, n (%) | Grade ≥3, n (%) | |
| Palmar‐plantar erythrodysesthesia syndrome | 36 (85.7) | 16 (38.1) |
| Fatigue | 26 (61.9) | 5 (11.9) |
| Nausea | 17 (40.5) | 1 (2.4) |
| Anorexia | 17 (40.5) | 0 |
| Diarrhea | 16 (38.1) | 1 (2.4) |
| Vomiting | 11 (26.2) | 1 (2.4) |
| Hypertension | 9 (21.4) | 6 (14.3) |
| Abdominal pain | 9 (21.4) | 4 (9.5) |
| Oral mucositis | 9 (21.4) | 2 (4.8) |
| Weight loss | 9 (21.4) | 0 |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events version 4.0.
Table 4.
All adverse reactions grouped by organ system
| Adverse events | CTCAE toxicity grade | |||
|---|---|---|---|---|
| Grade 1, a n (%) | Grade 2, a n (%) | Grade 3, a n (%) | Total/any grade, b n (%) | |
| Constitutional | ||||
| Fatigue | 19 (45.2) | 6 (14.3) | 5 (11.9) | 26 (61.9) |
| Fever | 1 (2.4) | 1 (2.4) | 1 (2.4) | |
| Flu like symptoms | 2 (4.8) | 2 (4.8) | 4 (9.5) | |
| Malaise | 2 (4.8) | 2 (4.8) | ||
| Pain | 2 (4.8) | 2(4.8) | 3(7.1) | 5 (11.9) |
| Dehydration | 1(2.4) | 3 (7.1) | 2(4.8) | 4(9.5) |
| Weight loss | 7 (16.7 | 2 (4.8) | 9 (21.4) | |
| Hematological | ||||
| Anemia | 2 (4.8) | 2 (4.8) | ||
| Thrombocytopenia | 1 (2.4) | 1 (2.4) | ||
| Gastrointestinal | ||||
| Nausea | 10 (23.8) | 7 (16.7) | 1 (2.4) | 17 (40.5) |
| Vomiting | 6 (14.3) | 5(11.9) | 1(2.4) | 11 (26.2) |
| Diarrhea | 12 (28.6) | 4 (9.5) | 1 (2.4) | 16 (38.1) |
| Dyspepsia | 1 (2.4) | 1 (2.4) | 2 (4.8) | |
| Gastritis | 2 (4.8) | 2 (4.8) | ||
| Enterocolitis | 1 (2.4) | 1 (2.4) | ||
| Flatulence/bloating | 2 (4.8) | 2 (4.8) | ||
| Anorexia | 13 (31) | 5 (11.9) | 17 (40.5) | |
| Ascites | 1 (2.4) | 1 (2.4) | ||
| Hepatobiliary side effect NOS | 1 (2.4) | 1 (2.4) | ||
| Gastrointestinal side effect NOS | 1 (2.4) | 1 (2.4) | 2 (4.8) | |
| Cardiovascular: Hypertension | 4 (9.5) | 6 (14.3) | 9(21.4) | |
| Respiratory: Hoarseness | 1 (2.4) | 1 (2.4) | 2 (4.8) | |
| Skin and mucosa | ||||
| Palmar‐plantar erythrodysesthesia syndrome | 33 (78.6) | 28 (66.7) | 16 (38.1) | 36 (85.7) |
| Photosensitivity | 3 (7.1) | 3 (7.1) | ||
| Pruritis | 1 (2.4) | 1 (2.4) | ||
| Maculopapular rash | 2 (4.8) | 1 (2.4) | 3 (7.1) | |
| Skin and subcutaneous tissue side effect NOS | 1 (2.4) | 1 (2.4) | 2 (4.8) | |
| Mucosal bleeding (epistaxis) | 1 (2.4) | 1 (2.4) | ||
| Oral mucositis | 4 (9.5) | 4 (9.5) | 2 (4.8) | 9 (21.4) |
| Musculoskeletal | ||||
| Arthralgia | 1 (2.4) | 1 (2.4) | ||
| Musculoskeletal side effect NOS | 2 (4.8) | 2(4.8) | 2 (4.8) | |
| Neurological | ||||
| Dysgeusia | 1 (2.4) | 1 (2.4) | ||
| Peripheral sensory neuropathy | 1 (2.4) | 1 (2.4) | 1 (2.4) | 3 (7.1) |
| Vertigo | 1 (2.4) | |||
| Infectious disease | ||||
| Pelvic infection | 1 (2.4) | 1 (2.4) | ||
| Splenic infection | 1 (2.4) | 1 (2.4) | ||
In each column, n represents a unique patient with the listed adverse event.
In this column, n represents the total number of patients with the listed adverse event.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; NOS, not otherwise specified.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Colorectal cancer (CRC) remains the third leading cause of cancer‐related death for both men and women in the U.S. [1]. Systemic therapies, including fluoropyrimidines, platinum derivatives (oxaliplatin), topoisomerase II inhibitors (irinotecan), vascular endothelial growth factor (VEGF) inhibitors (e.g., bevacizumab, aflibercept, regorafenib), and epidermal growth factor receptor (EGFR) inhibitors (e.g., cetuximab and panitumumab), have improved survival in metastatic CRC (mCRC) [2]. Capecitabine is a prodrug of the antimetabolite 5‐fluorouracil (5‐FU) that undergoes a series of enzymatic steps in its conversion to 5‐FU and has been approved for treatment of colon, breast, and gastric cancer [3]. Sorafenib (bi‐aryl urea, BAY 43‐9006) is a potent inhibitor of Raf‐1, which is a member of the RAF/MEK/ERK signaling pathway. Additionally, it demonstrates activity against several receptor tyrosine kinases that are involved in angiogenesis and tumor progression [4]. It is well established that both the RAF/MEK/ERK pathway and angiogenesis are clinically actionable biologic targets in mCRC. The safety and efficacy of sorafenib as a single agent [5, 6, 7, 8] and in combination with other agents [9, 10] have been demonstrated in a series of studies conducted in patients with solid tumors.
This single‐arm, phase II study of sorafenib plus capecitabine (SorCape) demonstrated a median progression‐free survival (PFS) of 6.2 months, 3‐month PFS of 83.3%, and median overall survival (OS) of 8.8 months in a patient population of heavily pretreated patients. These results compare favorably with the anticipated PFS and OS outcomes of 3 months and 6–7 months, respectively, with oral monotherapy salvage treatment options and are far better than best supportive care (BSC) alone [11, 12, 13, 14].
Despite major advances in therapeutic options, the prognosis of mCRC after previous exposure to effective therapies remains poor. The use of biologic therapies has expanded the treatment algorithm for mCRC. Bevacizumab, for example, is a potent VEGF inhibitor and has been shown to have a modest improvement in response rate, PFS, and OS in mCRC when used in combination with cytotoxic chemotherapy [15, 16, 17]. Cetuximab, a chimerized IgG1 antibody, and panitumumab, a fully humanized IgG2 antibody, target EGFR and block receptor dimerization, tyrosine kinase phosphorylation, and downstream signal transduction. However, the clinical benefit of these EGFR targeting agents is restricted only to a subset of patients with KRAS wild type mCRC [18]. In our present study, the majority of patients had received prior bevacizumab (81%), and 18 patients (42.9%) were KRAS wild type, supporting a similar 43% having received a prior EGFR inhibitor therapy. The median number of prior lines of therapy before study entry was three (range, two to seven). Thus, the vast majority of patients in this trial had exhausted most effective forms of treatment before entering this trial. In a salvage setting, BSC alone offers a median PFS of 1.8 months and a median OS of 4.6 months [18], highlighting the natural history of the disease in this situation.
In addition to the clinical outcomes noted in this study, the SorCape regimen is an entirely oral regimen, which might be favorable or preferred for some patients for whom routine travel or a central venous access device is not convenient or feasible. The adverse events attributed to this doublet therapy were not surprising given the well‐established side effect profile of each individual agent and were overall manageable. As noted, the most common adverse event was hand‐foot syndrome, which is one of the overlapping toxicities anticipated with both agents. Although it was noted to be present in the majority of patients (n = 36; 85.7%), it was only grade 3 or greater in 16 patients (38.1%). This is noteworthy because the median dose of sorafenib administered in the study was 200 mg b.i.d., less than the monotherapy dose approved by the U.S. Food and Drug Administration (FDA). Other common side effects included fatigue and gastrointestinal side effects including nausea, anorexia, diarrhea, and vomiting. Most of these anticipated side effects were mild and easily mitigated with supportive care medications (i.e., antidiarrhea and antinausea medications).
Although it provides potentially impactful clinical data, the study does have several limitations. Notably, this was a single‐arm study with a relatively modest sample size. The endpoint of PFS was chosen as we did not anticipate a robust response rate with the SorCape combination, but rather clinical benefit in the form of disease control. This led us to use a historical PFS as a comparative analysis, which is harder to interpret without a contemporaneously randomized control arm using BSC and is confounded by potential patient selection bias. There have also been additional FDA‐approved therapies since this study was completed, including TAS‐102 (tipiracil hydrochloride, an oral drug that combines two agents, trifluridine and tipiracil hydrochloride) and regorafenib, which were only starting to become clinically available during the conduct of this study. However, the clinical results from this trial could still be interpreted as comparable to, if not potentially better than, the results of those more recent interventions. Lastly, we have been unable to identify a biomarker or other clinical variable that could identify a subset of patients in whom this therapy might be more valuable (Table 5). Whole exome sequencing of tumors from patients with the longest PFS has failed to identify any candidate overlapping genes that suggest a targeted biomarker (data not shown). The combination regimen of SorCape should continue to be explored in larger studies to verify the clinical benefit seen in this trial and identify subsets of patients or molecular profiles that could support a predictive biomarker.
Table 5.
Univariate Cox analysis for progression‐free survival
| Variable | HR (95% CI) | p value |
|---|---|---|
| Gender: Male vs. female | 1.3 (0.6–2.8) | .475 |
| Race: White vs. Black | 1.2 (0.6–2.3) | .686 |
| Age group: 50 to <65 vs. 18 < 50 years | 0.8 (0.4–1.9) | .628 |
| Age group: ≥65 vs. 18 to <50 years | 0.7 (0.3–1.7) | .448 |
| Tumor grade | ||
| G2 vs. G1 | 0.3 (0.1–1) | .059 |
| G3 vs. G1 | 0.2 (0–1) | .053 |
| Gx vs. G1 | 0.4 (0.1–1.4) | .147 |
| KRAS: Mutated vs. wild type | 1.9 (1–3.8) | .058 |
| BRAF: Unknown vs. wild type | 0.8 (0.4–1.6) | .506 |
| MSI: Stable vs. low | 1 (0.1–7.5) | .966 |
| PI3K: Mutated vs. wild type | 0.8 (0.1–8.5) | .829 |
| Number of lines of therapy: ≥3 lines vs. <3 lines | 0.5 (0.2–1.2) | .145 |
| Prior treatment with bevacizumab: Yes vs. no | 1.4 (0.6–3.1) | .381 |
| Prior treated with cetuximab or panitumumab: Yes vs. no | 0.6 (0.3–1.2) | .131 |
| Prior treatment with capecitabine: Yes vs. no | 1.1 (0.6–2.1) | .788 |
| Hypertension: Yes vs. no | 0.9 (0.4–1.8) | .698 |
Abbreviations: BRAF, B‐Raf; CI, confidence interval; G1, grade 1; G2, grade 2; G3, grade 3; Gx, grade not assessed; HR, hazard ratio; KRAS, K‐ras; MSI, microsatellite instability.
Disclosures
Krista P. Terracina: Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT01471353
- Sponsors: University of Florida Gatorade Trust Fund, UF Health Cancer Center, Bayer Healthcare Pharmaceuticals (Bayer Study #ONC‐2010‐23)
- Principal Investigator: Thomas J. George
- IRB Approved: Yes
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figures. Available at https://www.cancer.org/research/cancer‐facts‐statistics/colorectal‐cancer‐facts‐figures.html. Accessed February 2, 2021.
- 2. Martchenko K, Schmidtmann I, Thomaidis T et al. Last line therapy with sorafenib in colorectal cancer: A retrospective analysis. World J Gastroenterol 2016;22:5400–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam SW, Guchelaar HJ, Boven E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 2016;50:9–22. [DOI] [PubMed] [Google Scholar]
- 4. Wilhelm SM, Carter C, Tang L et al. BAY 43‐9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–7109. [DOI] [PubMed] [Google Scholar]
- 5. Strumberg D, Richly H, Hilger RA et al. Phase I clinical and pharmacokinetic study of the novel RAF kinase and vascular endothelial growth factor receptor inhibitor BAY 43‐9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965–972. [DOI] [PubMed] [Google Scholar]
- 6. Awada A, Hendlisz A, Gil T et al. Phase I safety and pharmacokinetics of BAY 43‐9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 2005;92:1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark JW, Eder JP, Ryan D et al. The safety and pharmacokinetics of the multi‐targeted tyrosine kinase inhibitor (including RAF kinase and VEGF kinase), BAY 43‐9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 2005;11:2005–5480. [DOI] [PubMed] [Google Scholar]
- 8. Moore M, Hirte HW, Siu L et al. Phase I study to determine the safety and pharmacokinetics of the novel RAF kinase and VEGFR inhibitor BAY 43‐9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol 2005;16:1688–1694. [DOI] [PubMed] [Google Scholar]
- 9. Siu LL, Awada A, Takimoto CH et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res 2006;12:144–151. [DOI] [PubMed] [Google Scholar]
- 10. Richly H, Henning BF, Kupsch P et al. Results of a phase I trial of sorafenib (BAY 43‐9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol 2006;17:866–873. [DOI] [PubMed] [Google Scholar]
- 11. Chen HX, Mooney M, Boron M et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: An NCI treatment referral center trial TRC‐0301. J Clin Oncol 2006;24:3354–3360. [DOI] [PubMed] [Google Scholar]
- 12. Hendlisz A, Van den Eynde M, Peeters M et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium‐90 resin microspheres radioembolization for liver‐limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687–3694. [DOI] [PubMed] [Google Scholar]
- 13. Lee JJ, Kim TM, Yu SJ et al. Single‐agent capecitabine in patients with metastatic colorectal cancer refractory to 5‐fluorouracil/leucovorin chemotherapy. Jpn J Clin Oncol 2004;34:400–404. [DOI] [PubMed] [Google Scholar]
- 14. Ardavanis AS, Ioannidis GN, Orphanos GS et al. Salvage treatment with single‐agent capecitabine in patients with heavily pretreated advanced colorectal cancer. Anticancer Res 2006;26:1669–1672. [PubMed] [Google Scholar]
- 15. Fuchs CS, Marshall J, Mitchell E et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first‐line treatment of metastatic colorectal cancer: Results from the BICC‐C study. J Clin Oncol 2007;25:4779–4786. [DOI] [PubMed] [Google Scholar]
- 16. Giantonio BJ, Catalano PJ, Meropol NJ et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group study e3200. J Clin Oncol 2007;25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 17. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 18. Van Cutsem E, Lang I, D'Haens G et al. KRAS status and efficacy in the first‐line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol 2008;26(suppl 2):2a. [Google Scholar]


