Abstract
Background and Objectives
Breast cancer (BC) is the most common cancer in women. It imposes a huge disease burden and a significant impact on health‐related quality of life (HRQoL). Our study focused on HRQoL of patients with BC in Latin America and the Caribbean (LAC). We conducted a systematic review to identify relevant articles published between 2008 and August 2018. We conducted several meta‐analyses and subgroup analyses by country, disease stage, and instrument used (Prospective Register Of Systematic Reviews registration number: CRD42018106835).
Results
From 2,265 initial references, we finally included 75 articles (8,806 participants) that assessed HRQoL. The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 and B23 modules (34 studies; 8 countries; 4,866 participants) were the most used instruments, followed by the Short Form 36‐item, the abbreviated version of the World Health Organization Quality of Life instrument, and the Functional Assessment of Cancer Therapy – Breast instrument. Only four studies reported specific HRQoL data of patients with metastatic disease. Half the studies were rated as having moderate quality (38/75), and 38% (29/75) as high quality. We identified substantial heterogeneity. As expected, the meta‐analyses revealed that patients with metastatic disease reported lower HRQoL values and high symptom burden compared with patients at earlier stages. Similar results can be observed when we compared patients with early breast cancer in active treatment phases versus those in follow‐up.
Conclusion
This study provides a synthesis of breast cancer HRQoL reported in LAC and exposes existing evidence gaps. Patients with BC in active treatment or with metastatic disease had worse HRQoL compared with survivors during the follow‐up period.
Implications for Practice
This systematic review provides an exhaustive synthesis of breast cancer health‐related quality of life in women in the Latin American and Caribbean region. Patients with breast cancer in active treatment or with metastatic disease had worse health‐related quality of life compared with survivors during the different follow‐up periods. This study also shows important evidence and methods gaps that can help inform future research.
Keywords: Quality of life, Breast cancer, Cancer, Latin America, Caribbean, Systematic review, Meta‐analysis
Short abstract
This review reports the health‐related quality of life for patients with breast cancer living in Latin American countries and explores relationships with disease stage and treatment in real‐world settings.
Introduction
Breast cancer (BC) is the most frequent tumor and the leading cause of death among women worldwide [1]. Developing countries are more affected by BC, representing roughly half of the incidence and 60% of deaths. BC is the most frequent cancer and cause of specific death among women living in Latin America and the Caribbean (LAC) region, with 200,000 new cases and more than 52,000 deaths per year [1].
The diagnosis of BC causes a great physical, psychological, and economic impact on the patients and their families and on their surrounding social networks. It entails a modification of the natural course of personal life and family dynamics. The news of the diagnosis imposes a significant impact on health‐related quality of life (HRQoL). The actual symptoms, the potential changes perceived regarding life expectancy, and the menace of potential adverse events from treatment significantly affect its different domains.
Quality of life is a subjective, multidimensional, and dynamic concept that includes physical, emotional, social, and functional well‐being. According to the World Health Organization, it refers to the person's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns [2]. But defining HRQoL is more challenging, and multiple definitions can be found in the literature [3]. HRQoL is a health‐focused QoL concept that embraces aspects of health—both physical and mental—that influence QoL (e.g., “those aspects of self‐perceived wellbeing that are related to or affected by the presence of disease or treatment” [3, 4]).
Many generic and disease‐specific instruments have been developed and validated to measure HRQoL. Reference values for each instrument are necessary for assessing individual patients’ data and comparing them with general or specific populations. Several studies have been performed to assess HRQoL in patients with BC and survivors living in LAC, but there is no study that has synthesized this body of evidence. Disparities in access to diagnosis as well as treatment of BC, and different sociocultural contexts across LAC countries, make it difficult to extrapolate reference values from other regions of the world or the use of normative data obtained in individual countries.
Our objective was to characterize the HRQoL in patients with BC living in LAC and to explore relationships with disease stage and treatment in real‐world settings. We additionally aimed to provide a detailed analysis of the specific instruments used and their results. In order to accomplish this, we conducted a systematic review and meta‐analysis. This study is part of a larger project that also intended to depict the costs of medical care, the loss of labor productivity of patients with BC, and the out‐of‐pocket expenditures of patients and their families in LAC. This study is being published separately.
Materials and Methods
We followed the Meta‐Analysis of Observational Studies in Epidemiology guidelines and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement for reporting systematic reviews and meta‐analyses [5, 6]. The protocol was registered on the International Centre for Reviews and Dissemination Prospective Register Of Systematic Reviews under the registration number CRD42018106835.
We performed a systematic search of published and unpublished data on the main international and regional databases: PubMed, LILACS, EMBASE, the CEA registry, CRD, EconLit, and PsycINFO. We also searched conference proceedings of international oncology societies’ meetings. No language restriction was applied. The search strategy used is presented in supplemental online Table 1. The systematic search was performed on August 28, 2018.
Studies were included only if they reported results on HRQoL through a validated generic or specific HRQoL questionnaire in a sample of at least 20 patients (conventional cutoff) living in any LAC country. Exclusion criteria were the use of nonvalidated instruments to measure HRQoL, or a publication date prior to 2008. The integrated yield of searches was fed into the Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia) for independent screening by pairs of reviewers, out of a total of eight, and those studies potentially eligible were then selected for full text appraisal. For every study that met inclusion criteria, new pairs of reviewers independently extracted all relevant information and assessed their inherent risk of bias in a prepiloted Microsoft Excel (Microsoft Corporation, Redmond, WA) online spreadsheet for data extraction. Discrepancies were resolved by consensus of the whole team. To assess the risk of bias of the studies reporting HRQoL, we implemented the Mols et al. [7] quality assessment tool with modifications agreed upon by our team (supplemental online Table 2). Extracted data were synthesized using both descriptive and meta‐analytic approaches by outcome measures.
HRQoL domains and scores were summarized using means, standard deviations, and 95% confidence intervals (95% CIs). Whenever missing, the 95% CIs for the mean were computed with the following formula: 95%CI = Media +/− [Zα/2*(SD/√sample)] [8].
When the median and interquartile range were reported, we derived the mean and SD based on the methodology of Hozo et al. [9]. In those studies not reporting SD, we proceeded as follows: we initially considered the data from each of the studies of the same clinical group reporting a mean and SD in each score or domain. Then, for each study, the relative value of the SD was estimated in relation to the mean (relative SD study of i [RSDi]) = Mean i / SDi). The weighted average of this coefficient was then used to estimate the SD of the study or the studies that did not report it (imputed SD of study x = Mean x* weighted average of RSDs) [8]. When pooling data from different instruments, we initially mapped conceptually equivalent domains among instruments. Then, in order to be able to pool these results in the analysis, we proceeded to transform all individual domain scores to a common 0–100 scale.
Regarding statistical analyses, we first pooled the results from all individual studies using the random‐effects model of meta‐analysis described by DerSimonian and Laird [10]. Heterogeneity for each outcome among studies was assessed using the I‐squared statistics. Results were considered heterogeneous if the I‐squared statistic was >30% (30%–60%: moderate heterogeneity; >60%: substantial heterogeneity). Then we conducted the following subgroup analyses, prespecified in the study protocol, in order to assess heterogeneity of results: by questionnaire used, by stage of disease and being on treatment, and by country of origin of patients. To characterize the HRQoL of patients with BC throughout the various stages of the disease and treatment, three groups were defined: (a) those with early breast cancer (stage I, II, or III) who were receiving active treatment; (b) those with early breast cancer who had finished the treatment phase; and (c) patients with metastatic breast cancer (stage IV). Within the active treatment group, we included those studies that reported HRQoL of women who had undergone recent surgery (less than 6 months after surgery) or were receiving adjuvant treatment with chemotherapy or radiotherapy. Those studies with patients under hormonal treatment, or simply on follow‐up, were included in the other group. If studies included more than 75% of participants in any of the subgroups considered, and no information was reported by the authors regarding subgroup‐specific HRQoL, we assigned the results to the corresponding subgroup. Studies were excluded from the subgroup analysis when it was not possible to attribute the patients precisely to any of the prespecified subgroups owing to lack of information.
Results
A total of 2,233 articles were retrieved from the initial search in different databases, and another 32 were found in the gray literature. After removal of 32 duplicate records, we screened 2,233 based on title and abstract. Of these, 1,848 were excluded because it was evident from the title or abstract that they were not relevant to the review. Three hundred eighty‐five articles remained and were assessed for eligibility based on the full text. Finally, 267 studies were discarded because they did not meet inclusion criteria (93 wrong outcomes; 32 duplicated; 21 wrong study design; 18 no breast cancer–specific data; 15 wrong setting; 8 no country‐specific data; 2 wrong patient population; and finally 78 because of other reasons like non‐LAC country or publication date prior to 2008). Considering published and unpublished studies after screening and selection using the predefined inclusion and exclusion criteria, we finally included 75 studies encompassing 8,806 patients that assessed HRQoL. No additional studies were found from references cited in the papers included. The study flowchart is shown in Figure 1. The studies included and their characteristics are summarized in Table 1.
Figure 1.
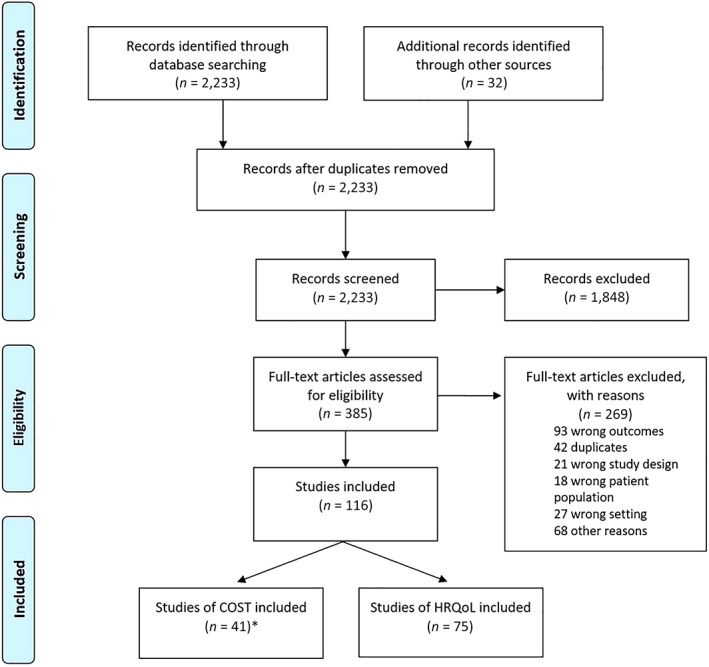
Study flowchart. Abbreviation: COST, costs of medical care; HRQoL, health‐related quality of life.
Table 1.
Characteristics of the included health‐related quality of life studies by instrument
| Author | Year | Country | Instrument | Participants | Mean age (yr) | Cancer stage included | Patients in active treatment | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| Gercovich [11] | 2012 | Argentina | EORTC QLQ‐C30 | 72 | 56.0 | All a | Yes | Low |
| Nicolussi [12] | 2011 | Brazil | EORTC QLQ‐C30 | 35 | 52.7 | All | Yes | Moderate |
| Garabeli [13] | 2012 | Brazil | EORTC QLQ‐C30 | 202 | 53.8 | I–II and III | No | Moderate |
| Moros [14] | 2010 | Chile | EORTC QLQ‐C30 | 22 | 49.0 | I–II and III | Yes | High |
| Finck [15] | 2018 | Colombia | EORTC QLQ‐C30 | 95 | 55.7 | Not specified | Not specified | Moderate |
| Villalta Cordova [16] | 2016 | Ecuador | EORTC QLQ‐C30 | 40 | 50.4 | I–II and III | Not specified | Moderate |
| Doubova [17] | 2018 | Mexico | EORTC QLQ‐C30 | 136 | 53.6 | Not specified | Not specified | Low |
| Hernández Moreno [18] | 2014 | Mexico | EORTC QLQ‐C30 | 50 | 48.7 | Not specified | Not specified | Low |
| Barber [19] | 2018 | Argentina | EORTC QLQ‐C30 and B23 | 171 | 51.0 | I–II and III | No | Moderate |
| Bevacqua [20] | 2016 | Argentina | EORTC QLQ‐C30 and B23 | 100 | 61.8 | I–II and III | No | Moderate |
| Costa [21] | 2017 | Brazil | EORTC QLQ‐C30 and B23 | 400 | Not specified | All | Yes | Moderate |
| Paiva [22] | 2016 | Brazil | EORTC QLQ‐C30 and B23 | 153 | 51.9 | I–II and III | No | Moderate |
| Castellar [23] | 2014 | Brazil | EORTC QLQ‐C30 and B23 | 75 | 54.4 | I–II and III | Not specified | Moderate |
| De Aguiar [24] | 2014 | Brazil | EORTC QLQ‐C30 and B23 | 544 | 59.1 | I–II and III | No | Moderate |
| Evangelista [25] | 2012 | Brazil | EORTC QLQ‐C30 and B23 | 354 | 51.7 | I–II and III | No | High |
| Ferreira [26] | 2011 | Brazil | EORTC QLQ‐C30 and B23 | 195 | 58.2 | I–II and III | No | High |
| Garcia [27] | 2017 | Brazil | EORTC QLQ‐C30 and B23 | 48 | 46.0 | I–II and III | No | Low |
| Michels [28] | 2013 | Brazil | EORTC QLQ‐C30 and B23 | 100 | 56.5 | I–II and III | Not specified | Low |
| Silva [29] | 2013 | Brazil | EORTC QLQ‐C30 and B23 | 28 | 52.0 | I–II and III | Yes | High |
| Lanza [30] | 2015 | Brazil | EORTC QLQ‐C30 and B23 | 57 | 62.9 | Not specified | No | Moderate |
| Dell'Antonio [31] | 2017 | Brazil | EORTC QLQ‐C30 and B23 | 87 | 55.5 | Not specified | Yes | Low |
| Gozzo [32] | 2013 | Brazil | EORTC QLQ‐C30 and B23 | 48 | 48.4 | Not specified | Yes | Moderate |
| Lobo [33] | 2013 | Brazil | EORTC QLQ‐C30 and B23 | 145 | 52.0 | Not specified | Yes | High |
| Velloso [34] | 2011 | Brazil | EORTC QLQ‐C30 and B23 | 45 | 58.9 | Not specified | No | High |
| Alfano [35] | 2014 | Brazil | EORTC QLQ‐C30 and B23 | 126 | 51.4 | Only metastatic disease | Yes | High |
| Irarrázaval [36] | 2016 | Chile | EORTC QLQ‐C30 and B23 | 91 | 60.0 | I–II and III | No | Moderate |
| Ardila Rojas [37] | 2017 | Colombia | EORTC QLQ‐C30 and B23 | 362 | 55.7 | Not specified | No | Moderate |
| Cortes‐Flores [38] | 2014 | Mexico | EORTC QLQ‐C30 and B23 | 139 | 49.7 | All a | No | Moderate |
| Enríquez Reyna [39] | 2018 | Mexico | EORTC QLQ‐C30 and B23 | 95 | 55.0 | All a | Yes | Moderate |
| Sat‐Muñoz [40] | 2011 | Mexico | EORTC QLQ‐C30 and B23 | 314 | 52.2 | All | Yes | Moderate |
| Gomez‐Rico [41] | 2009 | Mexico | EORTC QLQ‐C30 and B23 | 102 | 51.8 | I and II | Yes | Moderate |
| Cerezo [42] | 2012 | Mexico | EORTC QLQ‐C30 and B23 | 234 | 59.6 | I–II and III | Yes | High |
| Recalde [43] | 2012 | Paraguay | EORTC QLQ‐C30 and B23 | 125 | 55.0 | All a | Yes | Moderate |
| Soto‐Cáceres Cabanillas [44] | 2013 | Peru | EORTC QLQ‐C30 and B23 | 76 | Not specified | I–II and III | Not specified | Moderate |
| Sanchez‐Pedraza [45] | 2012 | Colombia | FACT‐B | 198 | 54.2 | All | Yes | Moderate |
| Bezerra [46] | 2013 | Brazil | FACT‐B | 197 | 53.0 | I–II and III | Not specified | High |
| Baigorri [47] | 2015 | Argentina | FACT‐B | 156 | 60.7 | I–II and III | No | Moderate |
| Fernandez‐Suarez [48] | 2010 | Mexico | FACT‐B | 142 | 55.0 | All | Not specified | |
| Pinto e Silva [49] | 2008 | Brazil | FACT‐B | 89 | 55.5 | I and II | Yes | High |
| Oliveira [50] | 2010 | Brazil | FACT‐B | 55 | 52.7 | Not specified a | Not specified | Moderate |
| Recchia [51] | 2017 | Brazil | FACT‐B | 30 | 51.2 | I–II and III | No | High |
| Verde [52] | 2009 | Brazil | FACT‐B | 25 | 46.0 | I and II | Yes | High |
| Perroud [53] | 2016 | Argentina | FACT‐B | 20 | 57.0 | Only metastatic disease | Yes | High |
| Hundelhausen [54] | 2015 | Colombia | SF‐36 | 50 | 55.0 | Not specified a | No | Moderate |
| Reich [55] | 2011 | Uruguay | SF‐36 | 116 | 50.8 | All a | No | High |
| Lostaunau [56] | 2013 | Peru | SF‐36 | 53 | 48.1 | I–II and III | Yes | High |
| Palacios Benzaquen [57] | 2014 | Peru | SF‐36 | 100 | 56.2 | Not specified a | No | High |
| Soares [58] | 2013 | Brazil | SF‐36 | 70 | 55.4 | All a | No | High |
| Rancatti [59] | 2013 | Argentina | SF‐36 | 221 | 52.0 | I and II | No | High |
| Tiezzi [60] | 2017 | Brazil | SF‐36 | 112 | 49.4 | I–II and III | No | High |
| Manganiello [61] | 2011 | Brazil | SF‐36 | 100 | 48.5 | I–II and III | No | Moderate |
| Veiga [62] | 2010 | Brazil | SF‐36 | 96 | 51.7 | I–II and III | No | Moderate |
| Veiga [63] | 2010 | Brazil | SF‐36 | 87 | 49.9 | I–II and III | No | Moderate |
| Freitas‐Silva [64] | 2010 | Brazil | SF‐36 | 70 | 49.2 | I–II and III | No | High |
| Simeao [65] | 2013 | Brazil | SF‐36 | 50 | 57.2 | I–II and III | No | Moderate |
| Tolentino [66] | 2010 | Brazil | SF‐36 | 22 | 48.8 | I–II and III | No | High |
| Trejo‐Ochoa [67] | 2013 | Mexico | SF‐36 | 74 | 48.4 | I–II and III a | Yes | Moderate |
| Fontes [68] | 2017 | Brazil | SF‐36 | 135 | 48.5 | Not specified a | No | High |
| Medina [69] | 2010 | Mexico | SF‐36 | 125 | 54.0 | Not specified a | Not specified | Moderate |
| Mendes [70] | 2014 | Brazil | SF‐36 | 49 | 53.9 | Not specified a | No | Moderate |
| Oliveira [71] | 2014 | Brazil | SF‐36 + WHOQOL‐Bref + FACT‐B | 106 | 49.2 | All a | No | High |
| Aguirre‐Loaiza [72] | 2016 | Colombia | SF‐36 + FACT‐B | 39 | 56.2 | All | Not specified | Moderate |
| Binotto [73] | 2016 | Brazil | WHOQOL‐Bref | 272 | 58.5 | All a | Yes | Moderate |
| Canario [74] | 2016 | Brazil | WHOQOL‐Bref | 215 | 52.7 | All a | Yes | Moderate |
| Kluthcovsky [75] | 2015 | Brazil | WHOQOL‐Bref | 202 | 54.5 | I–II and III | No | High |
| Rabin [76] | 2008 | Brazil | WHOQOL‐Bref | 73 | 47.9 | I–II and III | No | High |
| Porciúncula Frenzel [77] | 2013 | Brazil | WHOQOL‐Bref | 70 | 55.6 | I–II and III | Yes | Moderate |
| Seidel [78] | 2017 | Brazil | WHOQOL‐Bref | 58 | 51.6 | Not specified a | Not specified | Moderate |
| Araújo Neto [79] | 2017 | Brazil | WHOQOL‐Bref | 50 | 54.0 | Not specified | No | Moderate |
| Gomes [80] | 2015 | Brazil | WHOQOL‐Bref | 37 | 56.1 | Not specified | No | Moderate |
| Elias [81] | 2015 | Brazil | WHOQOL‐Bref | 26 | 52.5 | I–II and III | Not specified | Low |
| Santos [82] | 2010 | Brazil | WHOQOL‐Bref | 25 | 50.4 | All a | Yes | Moderate |
| Zapata [83] | 2010 | Colombia | WHOQOL‐Bref | 220 | 53.5 | Not specified | No | High |
| Pineda‐Higuita [84] | 2017 | Colombia | WHOQOL‐Bref | 82 | 57.8 | Not specified | Not specified | Moderate |
| Álviz Amador [85] | 2016 | Colombia | WHOQOL‐Bref | 23 | 50.2 | Not specified | Not specified | Moderate |
These studies did not specify stage of disease but, according to study protocol or distribution of included patients, were assumed to represent patients in stages I, II, and III.
Abbreviations: EORTC QLQ, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; FACT‐B, Functional Assessment of Cancer Therapy – Breast; SF‐36, Short Form 36‐item; WHOQOL‐Bref, abbreviated version of the World Health Organization Quality of Life.
The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C30 and B23 modules were the most used instruments. Most of the studies were from Brazil. Their sample size varied between 20 and 544 subjects. We found important heterogeneity in the age of participants, their means ranging from 42 to 62.7 years. The distribution of patients by countries and instrument to measure HRQoL is shown in Table 2.
Table 2.
Distribution of patients broken down by countries and instruments
| Country | Patients, n (%) | EORTC QLQ‐C30 and B23 (55.3%) | FACT‐B (10.4%) | SF‐36 (17.8%) | WHOQOL‐Bref (16.6%) |
|---|---|---|---|---|---|
| Argentina |
740 (8) |
343 | 176 | 221 | — |
| Brazil |
4,963 (56) |
2,642 | 396 | 791 | 1,134 |
| Colombia |
1,069 (12) |
457 | 198 | 89 | 325 |
| Mexico |
1,411 (16) |
1,070 | 142 | 199 | — |
| Other |
623 (7) |
354 | — | 269 | — |
| Total |
8,806 (100) |
4,866 | 912 | 1,569 | 1,459 |
Abbreviations: —, No studies found; EORTC QLQ, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; FACT‐B, Functional Assessment of Cancer Therapy – Breast; SF‐36, Short Form 36‐item; WHOQOL‐Bref, abbreviated version of the World Health Organization Quality of Life.
Most of the studies included patients in early (stage I–II) and locally advanced (III) stages (36 studies; n = 4,288 patients). Some studies included all stages of the disease, but more than 75% were in stage I, II, or III (10 studies, n = 1,235). Regarding metastatic disease, 5.7% of studies (18 studies; n = 505) included patients in this stage, but only in 4 studies (four countries; n = 315) were results reported for this specific group. Two of them included only patients in this stage (n = 147).
We found no studies reporting HRQoL individual data according to any of the common BC molecular subtypes (i.e., luminal A; luminal B; triple‐negative/basal‐like or human epidermal growth receptor 2 [HER2]‐enriched). Of the 75 studies included, 50% were published before 2014 (38 studies; n = 4,275). The I2 statistic in the meta‐analyses of HRQoL was higher than 75% in all countries and virtually with all instruments, and particularly high with the EORTC C30 and B23 (information available on demand), denoting substantial heterogeneity.
Risk of Bias Assessment
Half of the studies were rated as having moderate quality (38/75), and 38% (29/75) were considered high quality. The mean quality score was 7.36. However, most studies had a high risk of selection bias. Also, most studies (65%; 49/75) did not describe the overall response rates (or when stated, they were lower than 75%) or the way in which sample patients were drawn (77%; 58/75). In those that did, methodological shortcomings consisted mainly of the lack of information on characteristics of nonresponders. Many studies included did report on all questionnaire domains (23%, 17/75) or even on position or dispersion of statistical parameters (20%; 15/75).
Pooled Estimates of HRQoL
In Tables 3 and 4, we show the results of the meta‐analyses by HRQoL instruments and disease stage. Through random‐effects models, we estimated that patients with stage IV disease had worse HRQoL than earlier stages. As expected, we found very high levels of heterogeneity among instruments, and domains, even when stratifying by relevant subgroups. Thus, we consider that the confidence intervals of the pooled estimates are more meaningful than the central estimate.
Table 3.
Meta‐analysis of health‐related quality of life by EORTC QLQ‐C30 and B23 domains broken down by stage of the disease and treatment
| Domains | Global | Stages I, II, and III | Stage IV | |
|---|---|---|---|---|
| Active treatment | Follow‐up | |||
| EORTC QLQ‐C30 | ||||
| No. of studies (no. of patients) | 34 (4,866) | 14 (1,534) | 12 (2,419) | 3 (295) |
| Global health status | 70.5 (68.2–72.7) | 67.1 (64.6–69.5) | 73.3 (69.5–77.2) | 64.9 (56.4–73.3) |
| Physical functioning | 74.5 (70.6–78.4) | 75.7 (71.9–79.6) | 81 (77.8–84.6) | 64.8 (60.4–69.2) |
| Role functioning | 67.2 (60.4–74.1) | 60.2 (52.8–67.6) | 81.8 (76.4–87.1) | 56.3 (38.7–73.8) |
| Emotional functioning | 63.2 (60.5–65.9) | 65.2 (62.5–68) | 65.5 (61.2–69.9) | 57.4 (52.7–62.1) |
| Cognitive functioning | 74.2 (69–79.4) | 81.5 (76.9–86.1) | 72.5 (67.6–77.4) | 73.8 (64.6–83) |
| Social functioning | 76.3 (69.7–82.9) | 76.1 (67.5–84.8) | 86.7 (85.1–88.3) | 82.1 (79.2–84.9) |
| Fatigue | 31.4 (25.1–37.6) | 27.3 (23.7–30.8) | 21 (18–24.1) | 34.7 (31.1–38.4) |
| Nausea and vomiting | 22.5 (17–28) | 29.1 (25.2–33) | 20.4 (16.7–24.1) | 29.4 (21 – 37.8) |
| Pain | 31.2 (27.8–34.6) | 14.7 (10.3–19.1) | 6.6 (4.6–8.5) | 22.5 (13.8–31.3) |
| Dyspnea | 21.6 (18–25.3) | 7.5 (6.1–8.9) | 11 (9.7–12.3) | 29.4 (21–37.9) |
| Insomnia | 34.5 (28.6–40.3) | 28.4 (25.5–31.3) | 28.7 (25.7–31.7) | 34.3 (30.7–38) |
| Appetite loss | 25.3 (21.3–29.3) | 14.2 (12.9–15.5) | 10.2 (8.2–12.3) | 30 (21.5–38.5) |
| Constipation | 27.6 (21.6–33.7) | 14.3 (11.7–16.9) | 17.6 (14.2–20.9) | 29.8 (19.9–39.6) |
| Diarrhea | 21.6 (15.9–23.6) | 6.8 (5.1–8.4) | 5.3 (4.4–6.2) | 18.7 (10.5–26.9) |
| Financial difficulties | 37.2 (29.3–45) | 32.6 (27–38.2) | 18.8 (16.4–21.1) | 36.8 (27.8–45.8) |
| EORTC QLQ‐B23 | ||||
| No. of studies (no. of patients) | 25 (4,342) | 11 (1,435) | 12 (2,032) | 3 (295) |
| Body image | 72.3 (59.6–85) | 70.3 (54.4–86.2) | 75.9 (62.7–89.3) | 82.4 (73.8–91.1) |
| Sexual enjoyment | 45.7 (38–53.4) | 41.3 (31.9–50.7) | 48.4 (35.8–61.1) | 43.7 (21.6–65.8) |
| Sexual functioning | 45.2 (31.9–58.5) | 18 (16.6–19.4) | 59.9 (45.0–74.8) | 49.2 (20.8–77.7) |
| Future perspective | 50.6 (40.5–60.8) | 50.2 (37.5–62.9) | 49.5 (35.1–63.9) | 18.4 (13.1–23.7) |
| Arm symptoms | 30.5 (26.4–34.6) | 30.3 (26.0–34.6) | 20.1 (17.6–22.5) | 22.3 (20.6–23.8) |
| Breast symptoms | 31.7 (24.7–38.7) | 33.5 (15.7–51.4) | 16.9 (14.5–19.4) | 16.8 (15.5–18.1) |
| Systemic therapy side effects | 33.9 (28.1–39.6) | 36.2 (29.1–43.4) | 21.5 (18.6–24.5) | 25.5 (23.5–27.6) |
| Upset by hair loss | 44.7 (34.5–54.9) | 49.1 (34.2–63.9) | 33.6 (22.4–64.8) | 29.3 (20.9–37.7) |
Data are shown as mean (95% confidence interval).
Abbreviation: EORTC QLQ, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire.
Table 4.
Meta‐analysis of HRQoL by SF‐36, FACT‐B, and the WHOQOL‐Bref instrument broken down by stage of the disease and treatment
| Domains | Global | Stages I, II, and III | Stage IV | |
|---|---|---|---|---|
| Active treatment | Follow‐up | |||
| SF‐36 | ||||
| No. of studies (no. of patients) | 19 (1,675) | 2 (127) | 15 (1,384) | 1 (4) |
| Physical functioning | 67 (62.8–71.2) | 77.8 (56.7–98.9) | 71.3 (67–75.5) | 20.8 (8.3–33.3) |
| Role physical | 48.4 (34.9–61.9) | 51.4 (39.3–63.5) | 51.9 (35.3–68.6) | 12.5 (0–37) |
| Bodily pain | 59.7 (54.2–65.3) | 76.3 (67.4–85.2) | 60.1 (53.6–66.7) | 38.6 (3.5–73.7) |
| General health | 67.6 (64.9–70.3) | 68.2 (64–72.5) | 69.1 (66–72.2) | 38.7 (11.5–65.9) |
| Vitality | 63.8 (61.2–66.4) | 72.3 (67.9–76.8) | 64.1 (61.1–67.1) | 39.5 (6.7–72.3) |
| Social functioning | 72.7 (67.3–78.0) | 79.7 (67.1–92.2) | 78.3 (74.6–82) | 45 (40.6–49.4) |
| Role emotional | 61.8 (53.5–70.2) | 64.3 (58.9–69.8) | 67.5 (56.3–78.8) | 20.8 (0–45.3) |
| Mental health | 68.3 (66.2–70.3) | 69.7 (62.4–77) | 69.3 (66.9–71.6) | 45 (14.3–75.7) |
| FACT‐B | ||||
| No. of studies (no. of patients) | 11 (1,057) | 6 (529) | 2 (186) | 2 (24) |
| Physical well‐being | 20.8 (19.4–22.2) | 21.2 (19.8–22.6) | 17.5 (15.3–19.7) | 14.9 (6.5–23.4) |
|
Social/family well‐being |
19.4 (17.7–21.2) | 18.3 (15.6–20.9) | 21.8 (18.6–24.9) | 13.9 (2.1–25.8) |
|
Emotional well‐being |
17.2 (14.9–19.5) | 17.5 (16.3–18.7) | 11.8 (8–15.6) | 10.2 (8.1–12.4) |
|
Functional well‐being |
18.9 (17.2–20.6) | 17.9 (17.1–18.8) | 21 (17–24.9) | 12.7 (9.8–15.6) |
|
Breast cancer subscale |
23.1 (20.6–25.5) | 23.6 (22.3–24.8) | 16.1 (14–18.2) | 23.7 (13.1–34.3) |
| WHOQOL‐Bref a | ||||
| No. of studies (no. of patients) | 13 (1,436) | 4 (607) | 5 (333) | — |
| Global HRQoL | 67.3 (59.8–74.8) a | 69 (58.9–79.1) | NR | NR |
| Satisfaction with health | 67.9 (57.8–77.9) b | NR | NR | NR |
| Physical health | 59.8 (57.0–62.7) | 61.4 (56.8–65.9) | 59.2 (54.2–64.1) | NR |
| Psychological | 65.4 (61.4–69.3) | 63.8 (56.4–71.2) | 67.6 (62.6–72.6) | NR |
| Social relationships | 66.7 (60.9–72.4) | 68.9 (59.5–78.4) | 70.8 (65.4–76.2) | NR |
| Environment | 63.7 (61.2–66.4) | 62.2 (58.2–66.3) | 61.5 (56.7–66.3) | NR |
Data are shown as mean (95% confidence interval).
Only six studies (n = 713) report this domain.
Only two studies (n = 95) report this domain.
Abbreviations: —, no equivalent domain was identified; FACT‐B, Functional Assessment of Cancer Therapy – Breast; HRQoL, health‐related quality of life; NR, not reported by any of the included studies; SF‐36, Short Form 36‐item; WHOQOL‐Bref, abbreviated version of the World Health Organization Quality of Life.
HRQoL with EORTC QLQ C30 and B23 Subscale Questionnaires
A total of 34 articles (eight countries; n = 4,866) reported HRQoL with EORTC QLQ‐C30. Total sample sizes ranged from 22 to 544 patients, and the average age of participants from 42 to 62.7 years. Fifty‐three percent of studies were rated as moderate quality (18/34), and 32% (11/34) as high quality. The mean quality score was 6.52. The pooled scores for global health status, symptom, and functional scales, broken down by stage of the disease and treatment, are shown in Table 3.
Mean scores of global health status and functional scales domains (except for the social functioning score) of patients with stage IV disease were lower than those of patients with stage I, II, or III, regardless of the treatment phase. Similar results were observed for all symptom scales.
In general, comparisons between patients with early BC show that mean scores are higher in most scales in the follow‐up group versus those in active treatment. More pronounced differences were observed in the global health status, physical, role, and social functioning domains.
Twenty‐five articles (seven countries; n = 4,342) reported HRQoL with the EORTC QLQ‐B23 module (Table 3). Patients with metastatic disease reported lower HRQoL and high symptom burden compared with patients with earlier stages. Similar results can be observed when comparing patients with active treatment versus those in follow‐up.
HRQoL with the SF‐36 Health Survey
A total of 19 articles (six countries; n = 1,675) reported HRQoL with the Short Form 36‐item (SF‐36) in patients with BC living in LAC. Total sample sizes of the studies ranged from 22 to 221 patients, and the average age of participants ranged from 48.1 to 57.2 years. Fifty‐two percent of studies were rated as moderate quality (10/19), and 48% (9/19) attained scores above 75% of the maximum score. The maximum attainable score ranged from 5 to 10 points with a mean quality score of 7.42.
Seventeen studies (six countries; n = 1,475) that measured HRQoL in early (I and II) and locally advanced (III) stages, and only one study from Colombia (n = 4) that disaggregated data of patients with metastatic disease, were included in subgroup meta‐analysis. Given these assumptions, the pooled mean reported in Table 4 is assumed as representative of the population in stages I, II, and III, whereas the HRQoL of metastatic disease IV is only represented by four patients. Comparison of limited versus metastatic disease groups shows significantly worse HRQoL scores in patients with advanced BC. However, the small number of patients included in this last group limits the significance of the observed difference. A very similar mean score was observed between patients in early breast cancer, with the exception of bodily pain and vitality domains.
HRQoL with the FACT‐B Instrument
Of the 75 studies included in the systematic review, HRQoL was assessed by the Functional Assessment of Cancer Therapy – Breast (FACT‐B) instrument in 11 studies (four countries; n = 1,057). Most studies were performed in Brazil (n = 6). Sample sizes of the studies ranged from 20 to 198 patients, and the average age of participants ranged from 40 to 60.7 years. Sixty‐two percent of the studies (6/11) attained scores above 75% of the maximum score, whereas the rest were rated as moderate quality. The maximum attainable score ranged from 5 to 9 points with a mean quality score of 7.36 (Table 4).
In the meta‐analysis of the FACT‐B questionnaire, we found significant impairment in the HRQoL of patients with stage IV disease. Meaningful lower values in physical and emotional domains’ estimates were observed in patients in the subsequent cross‐sectional surveys through the natural disease evolution of these patients.
HRQoL with the WHOQOL‐Bref Instrument
Of the 75 studies included in the systematic review, HRQoL was assessed by the abbreviated version of the World Health Organization Quality of Life (WHOQOL‐Bref) instrument in 14 studies (two countries; n = 1,459). All the studies were carried out in two countries, and most of them were from Brazil (n = 11, n = 1,134). Sample sizes ranged from 23 to 273 patients, and the average age of participants from 47.9 to 57.8 years. Only 28% of the studies (4/14) were judged as high quality, whereas the rest were rated as moderate and only one as low quality. The score ranged from 5 to 9 points with a mean quality score of 6.85. The overall mean for domains in all populations ranged between 59.8 and 63.7 (Table 4). No studies have individually reported HRQoL of women with metastatic disease stages with the WHOQOL‐Bref instrument. So, these results are mostly representative of the Brazilian population with stage I, II, or III disease because most of the sample came from this country and the distribution of patient subgroup could not be determined in the Colombian studies.
Supplemental online Table 3A–3D shows the comparative HRQoL measured by the different instruments (SF‐36, QLQ‐C30, QLQ‐B23, and FACT‐B) by country and disease stage. It can be shown, for example, that for SF‐36, most studies were performed in mild to moderate disease, and only one Colombian paper includes women with stage IV disease. The physical functioning domain seemed to have a higher average for Argentina, as did the social functioning and emotional role ones. This same pattern was observed in most countries, albeit at different levels; notably, Colombian women scored lower among most countries. Additional details are shown in the abovementioned supplement.
Meta‐Analysis of Conceptually Common Domains Across the Different HRQoL Instruments
In a further analysis, we reviewed the grammatical and conceptual structure of the multiple instruments to identify those conceptually equivalent domains with the objective of carrying out a pooled analysis. The specified analysis yielded seven conceptually similar domains. Table 5 shows the instruments included and the domains identified as conceptually equivalent.
Table 5.
Conceptually equivalent domains of the instruments
| EORTC QLQ‐C30 | SF‐36 | FACT‐B | WHOQOL‐Bref |
|---|---|---|---|
| Global health status | General health | — | Global HRQoL |
| Physical functioning | Physical functioning | Functional well‐being | Physical health |
| Role functioning | Role physical | — | — |
| Emotional functioning | Role emotional | Emotional well‐being | Psychological |
| Social functioning | Social functioning | Social/family well‐being | Social relationships |
| Pain | Bodily pain | — | — |
Abbreviations: —, No equivalent domain was identified; EORTC QLQ, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; FACT‐B, Functional Assessment of Cancer Therapy – Breast; HRQoL, health‐related quality of life; SF‐36, Short Form 36‐item; WHOQOL‐Bref, abbreviated version of the World Health Organization Quality of Life.
The reported HRQoL scores of all domains were converted to a positive unified scale of 0–100, in which 0 is the worst possible score in each particular domain of each particular instrument and 100 the best possible score. In a similar manner to previous analyses, we performed a global analysis, as well as another one according to the stage of the disease. Table 6 shows the overall results and the subgroup analyses performed by stage and treatment.
Table 6.
Results of the joint analysis of the conceptually equivalent domains according to stage and phase of treatment
| Domains | Global | Stages I, II, and III | Stage IV | ||
|---|---|---|---|---|---|
| Active treatment | Follow‐up | ||||
| Global | No. of studies (no. of patients) | 59 (7,529) | 18 (2,089) | 26 (3,782) | 3 (295) |
| Mean (95% CI) | 67.7 (62.6–72.7) | 67.7 (65.1–70.3) | 69.4 (51.1–79.6) | 64.9 (56.3–73.4) | |
| Physical | No. of studies (no. of patients) | 69 (8,843) | 22 (2,436) | 28 (3,659) | 5 (319) |
| Mean (95% CI) | 69.9 (67.6–72.2) | 70.8 (65.5–75.2) | 74.9 (72.4–77.3) | 64.9 (56.3–73.4) | |
| Emotional | No. of studies (no. of patients) | 68 (8,615) | 22 (2,416) | 29 (4,192) | 5 (319) |
| Mean (95% CI) | 65.4 (62.4–68.5) | 65.8 (60.7–71) | 66.7 (61.9–71.5) | 54.9 (49.3–60.7) | |
| Social | No. of studies (no. of patients) | 68 (8,441) | 24 (2,552) | 30 (4,358) | 5 (319) |
| Mean (95% CI) | 72.8 (69.2–76.3) | 71.0 (64.7–77.3) | 80.9 (78.3–83.5) | 74.1 (64.6–83.6) | |
| Role | No. of studies (no. of patients) | 47 (6,672) | 13 (1,362) | 25 (3,697) | 4 (299) |
| Mean (95% CI) | 60.8 (55.8–65.8) | 54.3 (45.7–62.9) | 65.1 (56.2 – 74.0) | 49.7 (32.7–66.8) | |
| Pain | No. of studies (no. of patients) | 42 (5,782) | 5 (872) | 11 (2,216) | 4 (177) |
| Mean (95% CI) | 66.9 (65.3–68.6) | 72.9 (69–76.8) | 67.2 (63.5–70.8) | 47.9 (31.7–64.2) | |
Abbreviations: CI, confidence interval.
In these pooled analyses of common domains, more patients were included with gains in statistical precision. We found, as compared with patients in follow‐up and no active treatment, lower values in all domains of HRQoL of patients in active treatment (I, II, and III) and metastatic disease.
Discussion
The present study provides the most exhaustive analysis and synthesis to date of a heterogeneous body of evidence of breast cancer HRQoL in LAC.
We found 75 studies reporting HRQoL. Brazil was the most represented country. The EORTC QLQ‐C30 questionnaire and the B23 module (disease‐specific instruments) were the most commonly used tools. Most of them measured HRQoL in early (I and II) and locally advanced (III) stages. Other frequently used instruments were generic instruments, such as the SF‐36 and WHOQOL. We did not find studies that assessed HRQoL for other subgroups of interest, such as women with HER2‐positive breast cancer. Using the random‐effects model, we estimated that patients with a diagnosis of BC in active treatment or with metastatic disease had worse HRQoL in follow‐up compared with survivors.
Several studies tried to characterize HRQoL in patients with breast cancer in whole countries or regions. A systematic review with meta‐analyses in patients with cancer from the Eastern Mediterranean region included 36 studies from 12 countries totaling 8,347 patients from 2008 to 2018 [86]. The most frequent instrument was the EORTC QLQ‐C30 (20 studies; n = 6,043). The mean score of the global HRQoL ranged between 31.1 and 75.6. Based on the results of the random‐effects method, the mean overall was 60.5. Comparisons show that the mean score of global HRQoL domains of this study are lower, indicating better QoL in LAC. The comparison of values in other domains shows very similar results.
A systematic review about HRQoL in women with breast cancer was performed in Spain, searching from 1993 to 2009 [88]. They identified 25 studies encompassing 2,236 women. In descending order of frequency, the questionnaires we used were the EORTC, FACT‐B, Fonts’ quality of life questionnaire, SF‐12, Functional living index questionnaire, Rotterdam Symptom Checklist, and Quality of life Questionnaire. Most studies examined HRQoL according to the type of treatment. Few differences were detected by type of chemotherapy, with the single exception of worse results among younger women treated with radiotherapy. In the short term, better results were reported for all HRQoL components by women undergoing conservative rather than radical surgery. The presence of lymphedema was associated with worse HRQoL. Psychosocial disorder and level of depression and anxiety, regardless of treatment or disease stage, worsened HRQoL.
Compared with the EORTC‐C30 reference values of 2008 for all stages of breast cancer, the mean scores of our study are slightly lower in most scales, indicating worse HRQoL in LAC [89]. However, the global health/QoL mean score in LAC was better than normative scores (70.5 vs. 61.8). Comparison of global health/QoL mean score between active treatment and follow‐up stage subsets with reference values shows the same difference (67.1 and 73.3 vs. 61.8). One possible explanation for the differences observed is that reference values are based on pretreatment HRQoL data and patients who are off treatment were not included.
Our study is the first systematic review, to our knowledge, that focuses on BC in LAC. Some limitations might undermine our findings. Even though we had a very sensitive search strategy for all countries, that incorporated traditional databases, gray literature, reference identification, and experts, we found studies in only 9 of the 46 LAC countries. Also, study quality was heterogeneous: participation and response rates for patient groups were not described in a significant percentage of the studies, and selection bias was present in most of studies, which may hamper internal validity and, ultimately, generalizability. Lastly, most studies did not report key clinical variables that could influence HRQoL, such as average time since diagnosis, last treatment performed, or the exact moment in which HRQoL was measured in the patients with BC. HRQoL is a dynamic multidimensional measurement. All the aforementioned factors could partly explain the very high heterogeneity found in the main results, thus calling for caution in the interpretation of pooled results. This substantial level of heterogeneity is commonly found in epidemiological systematic reviews including different countries, and a meaningful way to address it is by bringing more attention to the range of uncertainty around them (i.e., 95% confidence intervals).
Comparison of limited versus diffuse disease, or early stages in follow‐up versus active treatment, showed significantly worse HRQoL scores in the last groups with an important limitation when performing this analysis. Of note, there was only a handful of studies (in four countries; total n = 315) that reported detailed data from patients with metastatic disease. In a large proportion of included studies, the precise stage of the disease was not determined, or women belonged to a mix of stages, which prevented knowing specific HRQoL data without access and reanalysis of the primary data. It is reasonable to expect changes throughout the continuum of care of the disease. Owing to the short time frame of the questionnaires (ranging from 1 to 4 weeks), the inability to temporarily relate the measured quality of life to an exact moment of the continuum of care is another significant limitation of the studies included. Another aspect that could not be well informed by our results relates to the longitudinal changes in HRQoL in these patients along the natural history of breast cancer. There were no longitudinal studies assessing this aspect, and our analysis mainly focuses on cross‐sectional analysis and comparisons of predefined patient populations. All these limitations of external validity were also generally found in the aforementioned systematic reviews that assessed HRQoL in BC and thus must be interpreted with caution.
Our study did not focus on the comparative validity of the different instruments in different populations and contexts, so prospective researchers do not have a straightforward solution on which instrument to include in a study. Their choice should be based partly on the instrument dissemination and validity evidence in their setting; their main research interest (i.e., choose a specific instrument such as the EORTC QLQ‐C30 or a generic one that can help compare the HRQoL impact in this population of patients with other health conditions); or specific domains of interest (instruments usually share selected dimensions but also have domains that are not present in other potential instruments; see Table 5 for clarification of this aspect).
Our results also expose important evidence gaps. Additional research is needed to better report HRQoL in the future in clearly defined patient subgroups to determine HRQoL of women with metastatic disease or in other subgroups such as those that present overexpression of the HER2/neu gene. Economic evaluations are an increasingly important component in making decisions about the inclusion of treatments and resource allocation in benefits packages, and HRQoL is a key component. The reference values provided from our study can be used to derive quality‐adjusted life years and improve health decision making in Latin America.
Conclusion
This study provides an exhaustive analysis and synthesis of a body of evidence of BC HRQoL in LAC and gives reference values averaged across nine countries. The research published was mainly from Brazil, followed by Mexico and Colombia. We summarized existing evidence and its inherent uncertainty by countries and patient subgroups, also exposing the existence of evidence gaps. Estimates should be interpreted with caution owing to important heterogeneity and selection bias. Our results also signal research priorities in LAC, such as better reporting, and conducting future HRQoL studies, studying it in women with metastatic disease or different relevant subgroups such as those that present overexpression of the HER2/neu gene.
Author Contributions
Conception/design: Lucas Gonzalez, Ariel Bardach, Alfredo Palacios, Agustin Ciapponi, Andres Pichón‐Riviere, Federico Augustovski
Provision of study material or patients: Lucas Gonzalez, Ariel Bardach, Alfredo Palacios, Agustin Ciapponi, Andres Pichón‐Riviere, Federico Augustovski
Collection and/or assembly of data: Lucas Gonzalez, Ariel Bardach, Claudia Peckaitis, Agustin Ciapponi, Andres Pichón‐Riviere, Federico Augustovski
Data analysis and interpretation: Lucas Gonzalez, Ariel Bardach, Federico Augustovski
Manuscript writing: Lucas Gonzalez, Ariel Bardach, Federico Augustovski
Final approval of manuscript: Lucas Gonzalez, Ariel Bardach, Alfredo Palacios, Claudia Peckaitis, Agustin Ciapponi, Andres Pichón‐Riviere, Federico Augustovski
Disclosures
Claudia Peckaitis: Janssen‐Cilag (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information
Acknowledgments
We thank Daniel Comandé, librarian at Institute for Clinical Effectiveness and Health Policy, for his help with the electronic searches.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. International Agency for Research on Cancer, GLOBOCAN 2018 accessed via Global Cancer Observatory . Available from https://gco.iarc.fr/today/online‐analysis‐table?v=2020&mode=cancer&mode_population=regions&population=900&populations=915_916_931&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1#. Accessed September 2018
- 2. WHO | WHOQOL: Measuring Quality of Life . World Health Organization 2014. Available from https://www.who.int/tools/whoqol#:~:text=WHO%20defines%20Quality%20of%20Life,%2C%20expectations%2C%20standards%20and%20concerns.
- 3. Karimi M, Brazier J. Health, health‐related quality of life, and quality of life: What is the difference? Pharmacoeconomics 2016;34:645–649. [DOI] [PubMed] [Google Scholar]
- 4. Ebrahim S. Clinical and public health perspectives and applications of health‐related quality of life measurement. Soc Sci Med 1995;41:1383–1394. [DOI] [PubMed] [Google Scholar]
- 5. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: A proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mols F, Vingerhoets AJJM, Coebergh JW et al. Quality of life among long‐term breast cancer survivors: A systematic review. Eur J Cancer 2005;41:2613–2619. [DOI] [PubMed] [Google Scholar]
- 8. Cochrane Handbook for Systematic Reviews of Interventions. 2019.
- 9. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 11. Gercovich D, Lopez PL, Bortolato D et al. Rol del distrés Psicológico en la relación entre la percepción de enfermedad y calidad de vida en pacientes con cáncer de mama. Psicooncologia 2012;9:403–414. [Google Scholar]
- 12. Nicolussi, AC , & Sawada, NO . (2011). Qualidade de vida de pacientes com câncer de mama em terapia adjuvante. Revista Gaúcha de Enfermagem, 32(4), 759‐766. [DOI] [PubMed] [Google Scholar]
- 13. Kluthcovsky ACGC, Urbanetz AA, de Carvalho DS et al. Fatigue after treatment in breast cancer survivors: Prevalence, determinants and impact on health‐related quality of life. Support Care Cancer 2012;20:1901–1909. [DOI] [PubMed] [Google Scholar]
- 14. Teresa Moros M, Ruidiaz M, Caballero A et al. Ejercicio físico en mujeres con cáncer de mama. Rev Med Chil 2010;138:715–722. [DOI] [PubMed] [Google Scholar]
- 15. Finck C, Barradas S, Zenger M et al. Quality of life in breast cancer patients: Associations with optimism and social support. Int J Clin Health Psychol 2017;18:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Córdova FXV. Calidad de vida de pacientes mastectomizadas que reciben tratamiento adyuvante en Solca núcleo de Loja, período 2015. Universidad Nacional de Loja; 2016. [Google Scholar]
- 17. Doubova SV, Casales‐Hernández MG, Perez‐Cuevas R. Supportive care needs and association with quality of life of Mexican adults with solid cancers. Cancer Nurs 2018;41:E1–E12. [DOI] [PubMed] [Google Scholar]
- 18. Hernández Moreno F, Landero Hernández R. Aspectos psicosociales relacionados con la calidad de vida en mujeres con cáncer de mama. Summa psicol UST 2014;11(1):99–104. [Google Scholar]
- 19. Barber MJ, Berdinelli D, Varela EB et al. Impacto del diagnóstico y tratamiento del cáncer de mama en la calidad de vida de las pacientes. Rev Argentina Mastología 2018;36:57–90. [Google Scholar]
- 20. Ana Laura UB. Calidad De Vida En Pacientes Con Cáncer De Mama. Rev Argentina Mastología 2016;36:92–113. [Google Scholar]
- 21. Costa WA, Monteiro MN, Queiroz JF et al. Pain and quality of life in breast cancer patients. Clinics (Sao Paulo) 2017;72:758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paiva CE, Rezende FF, Paiva BS et al. Associations of body mass index and physical activity with sexual dysfunction in breast cancer survivors. Arch Sex Behav 2016;45:2057–2068. [DOI] [PubMed] [Google Scholar]
- 23. Castellar JI, Fernandes CA, Tosta CE. Beneficial effects of pranic meditation on the mental health and quality of life of breast cancer survivors. Integr Cancer Ther 2014;13:341–350. [DOI] [PubMed] [Google Scholar]
- 24. De Aguiar SS, Bergmann A, Mattos IE. Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res 2014;23:627–637. [DOI] [PubMed] [Google Scholar]
- 25. Evangelista Lopes A. (2012) Verificar a associação entre o nível de atividade física e qualidade de vida em mulheres com cãncer de mama tratadas com intuito de cura. Doctoral thesis. Antonio Prudente Fundation. Aviable from http://www.luzimarteixeira.com.br/wp-content/uploads/2010/04/Alexandre.pdf
- 26. Ferreira DB. Qualidade de vida em pacientes em tratamento de câncer de mama ‐ associação com rede social, apoio social e atividade física. 2011. ix,104 f. Dissertação (Saúde e Meio Ambiente) ‐ Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro, 2011. Aviable from https://www.arca.fiocruz.br/handle/icict/24626
- 27. Garcia SN, Coelho RCFP, dos Santos PND et al. Changes in social function and body image in women diagnosed with breast cancer undergoing chemotherapy. Acta Sci Health Sci 2017;39:57–64. [Google Scholar]
- 28. Michels FAS, Latorre MRDO, Maciel MDS. Validity, reliability and understanding of the EORTC‐C30 and EORTC‐BR23, quality of life questionnaires specific for breast cancer. Rev Bras Epidemiol 2013;16:352–363. [DOI] [PubMed] [Google Scholar]
- 29. Dantas SM, Tirolli RettM, Carvalho Rabelo MA, et al. Qualidade de Vida e Movimento do Ombro no Pós‐Operatório de Câncer de Mama: um Enfoque da Fisioterapia. Rev. Bras. Cancerol 2013;59(3):419‐426. [Google Scholar]
- 30. Lanza M, Bergmann A, Ferreira MG et al. Quality of life and volume reduction in women with secondary lymphoedema related to breast cancer. Int J Breast Cancer 2015;2015:586827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dell'Antonio Pereira L, Brandao‐Souza C, Amaral Musso MA et al. Quality of life of women with pre‐and post‐operative breast cancer. Investig y Educ en Enferm 2017;35:109–119. [DOI] [PubMed] [Google Scholar]
- 32. Gozzo Tde O, Moyses AM, da Silva PR et al. Nausea, vomiting and quality of life in women with breast cancer receiving chemotherapy [in Portuguese]. Rev Gauch Enferm 2013;34:110–116. [DOI] [PubMed] [Google Scholar]
- 33. Aguilar Lobo S. Qualidade de vida de mulheres com cãncer de mama em quimioterapia. Universidad Federal de Ceará; 2013. [Google Scholar]
- 34. Velloso FSB, Barra AA, Dias RC. Functional performance of upper limb and quality of life after sentinel lymph node biopsy of breast cancer. Rev Bras Fisioter 2011;15:146–153. [DOI] [PubMed] [Google Scholar]
- 35. Alfano ACC, Paiva CE, Rugno FC et al. Biologically based therapies are commonly self‐prescribed by Brazilian women for the treatment of advanced breast cancer or its symptoms. Support Care Cancer 2014;22:1303–1311. [DOI] [PubMed] [Google Scholar]
- 36. Irarrázaval ME, Kleinman P, Silva RF et al. Quality of life in Chilean breast cancer survivors. Rev Med Chil 2016;144:1567–1576. [DOI] [PubMed] [Google Scholar]
- 37. Ardila Rojas E. Descripción de la calidad de vida en mujeres sobrevivientes al cáncer de seno (Estudio Cavicsen). Universidad de Cartagena; 2017. [Google Scholar]
- 38. Cortés‐Flores AO, Morgan‐Villela G, Zuloaga‐Fernández del Valle CJ et al. Quality of life among women treated for breast cancer: A survey of three procedures in Mexico. Aesthetic Plast Surg 2014;38:887–895. [DOI] [PubMed] [Google Scholar]
- 39. Enriquez Reyna MC, Vargas Flores MLA. Factores personales que afectan la calidad de vida de mujeres con cancer de mama del noreste de Mexico: Personal factors that affect quality of life of women with breast cancer from the northeast of Mexico. Hisp Health Care Int 2018;16:70–75. [DOI] [PubMed] [Google Scholar]
- 40. Sat‐Muñoz D, Contreras‐Hernández I, Balderas‐Peña LMA et al. Quality of life in Mexican women with breast cancer in different clinical stages and its association with socio‐demographic features, comorbidity states and care process characteristics in the Mexican Institute of Social Security [in Spanish]. Value Health 2011;14(5 suppl 1):S133–S136. [DOI] [PubMed] [Google Scholar]
- 41. Gomez‐Rico JA, Altagracia‐Martinez M, Kravzov‐Jinich J, et al. Breast cancer quality of life evaluation in Mexican women at La Raza Hospital, Mexico City: A preliminary approach. Clinicoecon Outcomes Res 2009;1:1–6. [PMC free article] [PubMed] [Google Scholar]
- 42. Cerezo O, Oñate‐Ocaña LF, Arrieta‐Joffe P et al. Validation of the Mexican‐Spanish version of the EORTC QLQ‐C30 and BR23 questionnaires to assess health‐related quality of life in Mexican women with breast cancer. Eur J Cancer Care (Engl). 2012;21:684–691. [DOI] [PubMed] [Google Scholar]
- 43. Recalde MT, Samudio M. Calidad de vida en pacientes con cáncer de mama en tratamiento oncológico ambulatorio en el Instituto de Previsión Social en el año 2010. Mem. Inst. Investig. Cienc. Salud 2012;10(2),13‐29. [Google Scholar]
- 44. Cabanillas RSC, Soto Caceres V. Level of quality of life perception in patients with and without breast cancer radical surgery at the National Hospital Almanzor Aguinaga. Chiclayo. Rev cuerpo méd HNAAA 2013;6(1):25‐29. [Google Scholar]
- 45. Sánchez‐Pedraza R, Matamoros FAS, Lopez‐Daza DF. Colombian validation of the FACT‐B scale for measuring breast cancer patients’ quality of life. Rev Colomb Obs Ginecol 2012;63:196–206. [Google Scholar]
- 46. Bezerra KB, Silva DS, Chein MB et al. Quality of life of women treated for breast cancer in a city of the northeast of Brazil [in Portuguese]. Cien Saude Colet 2013;18:1933–1941. [DOI] [PubMed] [Google Scholar]
- 47. Baigorri TE, Terrer TM, Figueroa NLD et al. Estudio longitudinal del crecimiento postraumÁtico y la calidad de vida en mujeres supervivientes de cÁncer de mama. Psicooncologia 2015;12:303–314. [Google Scholar]
- 48. Fernández‐Suárez HG, Blum‐Grynberg B, Aguilar‐Villalobos EJ et al. Validation of an instrument to measure life quality in breast cancer patients [in Spanish]. Rev Med Inst Mex Seguro Soc 2010;48:133–138. [PubMed] [Google Scholar]
- 49. Pinto e Silva MP, Sarian LO, Morais SS et al. Implications of a postoperative rehabilitation program on quality of life in women with primary breast cancer treated with sentinel lymph node biopsy or complete axillary lymph node dissection. Ann Surg Oncol 2008;15:3342–3349. [DOI] [PubMed] [Google Scholar]
- 50. Oliveira MM, de Souza GA, Miranda MdS et al. Upper limbs exercises during radiotherapy for breast cancer and quality of life [in Portugeuse]. Rev Bras Ginecol Obstet 2010;32:133–138. [DOI] [PubMed] [Google Scholar]
- 51. Recchia TL, Prim AC, Luz CM. Upper limb functionality and quality of life in women with five‐year survival after breast cancer surgery. Rev Bras Ginecol Obstet 2017;39:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verde SMML, São Pedro BMO, Mourão Netto M et al. Aversão alimentar adquirida e qualidade de vida em mulheres com neoplasia mamária. Rev Nutri 2009;22(6),795‐807. [Google Scholar]
- 53. Perroud HA, Alasino CM, Rico MJ et al. Quality of life in patients with metastatic breast cancer treated with metronomic chemotherapy. Future Oncol 2016;12:1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hundelhausen MM, Paola A, Porto J, Meza E, Perez RK, Ana G, et al. Calidad de vida en mujeres mastectomizadas en dos instituciones de Cartagena en el año 2015. (2016). Degree thesis. University of Cartagena. Faculty of Nursing Aviable from https://repositorio.unicartagena.edu.co/handle/11227/3099
- 55. Reich M, Remor E. Calidad de vida relacionada con la salud y variables psicosociales: caracterización de una muestra de mujeres uruguayas con cáncer de mama. Psicooncologia 2011;8:453–471. [Google Scholar]
- 56. Lostaunau Calero AV, Torrejón Salmón CS, Cassaretto M. Stress, Coping And Health‐Related Quality Of Life In Breast Cancer Women. Actualidades en Psicología. 2017b;31(122):75‐90. [Google Scholar]
- 57. del Palacios Benzaquen MP, del Palacios Benzaquen MP. Calidad de vida en mastectomizadas por cáncer de mama a un año de terapia adyuvante en un Hospital de Lambayeque 2008‐2010. Degree thesis. Católica Santo Toribio de Mogrovejo Univertsity‐ USAT. 2014. Aviable from http://tesis.usat.edu.pe/xmlui/handle/20.500.12423/300
- 58. Soares PBM, Carneiro JM, Rocha LA et al. The quality of life of disease‐free Brazilian breast cancer survivors. Rev Esc Enferm USP 2013;47:69–75. [DOI] [PubMed] [Google Scholar]
- 59. Rancati A, Soderini A, Dorr J et al. One‐step breast reconstruction with polyurethane‐covered implants after skin‐sparing mastectomy. J Plast Reconstr Aesthet Surg 2013;66:1671–1675. [DOI] [PubMed] [Google Scholar]
- 60. Tiezzi MF, de Andrade JM, Romao AP et al. Quality of life in women with breast cancer treated with or without chemotherapy. Cancer Nurs 2017;40:108–116. [DOI] [PubMed] [Google Scholar]
- 61. Manganiello A, Hoga LAK, Reberte LM et al. Sexuality and quality of life of breast cancer patients post mastectomy. Eur J Oncol Nurs 2011;15:167–172. [DOI] [PubMed] [Google Scholar]
- 62. Veiga, Daniela Francescato , Campos, Fabíola Soares Moreira , Ribeiro, Leda Marques , Archangelo Junior, Ivanildo , Veiga Filho, Joel , Juliano, Yara , Sabino Neto, Miguel , & Ferreira, Lydia Masako . (2010). Mastectomy versus conservative surgical treatment: the impact on the quality of life of women with breast cancer. Revista Brasileira de Saúde Materno Infantil, 10(1), 51‐57. [Google Scholar]
- 63. Veiga DF, Veiga‐Filho J, Ribeiro LM et al. Quality‐of‐life and self‐esteem outcomes after oncoplastic breast‐conserving surgery. Plast Reconstr Surg 2010;125:811–817. [DOI] [PubMed] [Google Scholar]
- 64. Freitas‐Silva R, Conde DM, de Freitas‐Junior R et al. Comparison of quality of life, satisfaction with surgery and shoulder‐arm morbidity in breast cancer survivors submitted to breast‐conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics (Sao Paulo) 2010;65:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simeao SF, Landro IC, De Conti MH et al. Quality of life of groups of women who suffer from breast cancer [in Portuguese]. Cien Saude Colet 2013;18:779–788. [DOI] [PubMed] [Google Scholar]
- 66. Tolentino GP, Battaglini CL, Araujo SS et al. Cardiorespiratory fitness and quality‐of‐life analysis posttreatment in breast cancer survivors. J Psychosoc Oncol 2010;28:381–398. [DOI] [PubMed] [Google Scholar]
- 67. Trejo‐Ochoa JL, Maffuz‐Aziz A, Said‐Lemus FM et al. Impact on quality of life after reconstructive surgery breast cancer treatment. Ginecol Obstet Mex 2013;81:510–518. [PubMed] [Google Scholar]
- 68. Fontes KP, Veiga DF, Naldoni AC et al. Physical activity, functional ability, and quality of life after breast cancer surgery. J Plast Reconstr Aesthet Surg 2019;72:394–400. [DOI] [PubMed] [Google Scholar]
- 69. Medina‐Franco H, García‐Alvarez MN, Rojas‐García P et al. Body image perception and quality of life in patients who underwent breast surgery. Am Surg 2010;76:1000–1005. [PubMed] [Google Scholar]
- 70. dos Mendes I S,de Freitas STT, de C Souza G et al. Correlation pain‐quality of life in women after being submitted to surgical treatment of breast cancer. Mundo saúde (Impr). 2014;38(2):189–196. [Google Scholar]
- 71. Oliveira IS, Costa LC, Manzoni AC et al. Assessment of the measurement properties of quality of life questionnaires in Brazilian women with breast cancer. Brazilian J Phys Ther 2014;18:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aguirre‐Loaiza HH, Nunez C, Navarro AM et al. Calidad de vida según el estadio del cáncer de seno en mujeres: Análisis desde el FACT‐B Y SF‐36. Psychologia 2017;11:109–120. [Google Scholar]
- 73. Binotto M, Daltoé T, Formolo F, et al. Physical activity and its benefits in quality of life of women with breast cancer: a cross sectional study in Caxias do Sul ‐ RS. Rev. bras. ativ. fís. saúde; 2016;21(2):154‐161. [Google Scholar]
- 74. Canario AC, Cabral PU, de Paiva LC et al. Physical activity, fatigue and quality of life in breast cancer patients. Rev Assoc Med Bras 2016;62:38–44. [DOI] [PubMed] [Google Scholar]
- 75. Kluthcovsky AC, Urbanetz AA. Fatigue and quality of life in breast cancer survivors: A comparative study. Rev Bras Ginecol Obstet 2015;37:119–126. [DOI] [PubMed] [Google Scholar]
- 76. Rabin EG, Heldt E, Hirakata VN et al. Quality of life predictors in breast cancer women. Eur J Oncol Nurs 2008;12:53–57. [DOI] [PubMed] [Google Scholar]
- 77. Porciuncula Frenzel A, Aberici Pastore C, Gonzalez MC. The influence of body composition on quality of life of patients with breast cancer. Nutr Hosp 2013;28:1475–1482. [DOI] [PubMed] [Google Scholar]
- 78. Seidel W, Bins‐Ely J, Ongaratto Barazzetti D et al. Breast reconstruction after mastectomy for breast cancer: Comparative analysis of early and delayed reconstruction. Minerva Chir 2017;72:188–199. [DOI] [PubMed] [Google Scholar]
- 79. Araújo Neto EA, Alves BCA, Gehrke FDS et al. Quality of life of post‐mastectomywomen living in a semi‐arid region of Brazil. Int J Environ Res Public Health 2017;14:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gomes NS, Soares MBO, da Silva SR. Self‐esteem and quality of life in women undergoing breast cancer surgery. REME rev. min. enferm; 2015,19(2):120‐126. [Google Scholar]
- 81. Elias AC, Ricci MD, Rodriguez LH et al. The biopsychosocial spiritual model applied to the treatment of women with breast cancer, through RIME intervention (relaxation, mental images, spirituality). Complement Ther Clin Pract 2015;21:1–6. [DOI] [PubMed] [Google Scholar]
- 82. dos Santos Júnior NC, dos Santos MA, Castro JGD, et al. Depression, anxiety and quality of life in women in treatment of breast cancer. Rev bras Mastol. 2010;20(2):81–85. [Google Scholar]
- 83. Zapata CS, Romero HG. Quality of life and associated factors in women with breast cancer in Antioquia, Colombia [in Spanish]. Rev Panam Salud Publica 2010;28:9–18. [DOI] [PubMed] [Google Scholar]
- 84. Pineda‐Higuita SE, Andrade‐Mosquera SM, Montoya‐Jaramillo YM. Factores asociados a la calidad de vida en mujeres con cáncer de mama. Medellín 2013. Rev Gerenc y Polit Salud 2017;16:85–95. [Google Scholar]
- 85. Álviz Amador A, Martínez Zambrano J, Marrugo Padilla A et al. Adherence, treatment satisfaction and quality of life in patients with breast cancer at the Hospital Universitario del Caribe. (Cartagena, Colombia). Pharm Care Espana 2016;18:251–264. [Google Scholar]
- 86. Hashemi SM, Balouchi A, Al‐Mawali A et al. Health‐related quality of life of breast cancer patients in the Eastern Mediterranean region: A systematic review and meta‐analysis. Breast Cancer Res Treat 2019;174:585–596. [DOI] [PubMed] [Google Scholar]
- 87. Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: A systematic review of the literature. J Cancer Surviv 2011;5:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Delgado‐Sanz MC, García‐Mendizábal MJ, Pollán M et al. Heath‐related quality of life in Spanish breast cancer patients: A systematic review. Health Qual Life Outcomes 2011;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scott NW, Fayers P, Aaronson NK et al. EORTC QLQ‐C30 Reference Values Manual. EORTC Quality of Life Group; 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information


