Short abstract
This letter to the editor outlines the use of stereotactic body radiotherapy for metastatic renal cell carcinoma in the U.S., identifying clinical predictors of utilization using the National Cancer Database.
Radiotherapy (RT) may be used to treat symptomatic sites of metastatic renal cell carcinoma (mRCC) or where oligometastasis or oligoprogression is present. mRCC is historically considered intrinsically resistant to conventional fractionated RT: however, modern techniques such as stereotactic body radiotherapy (SBRT) demonstrate excellent locoregional control rates reaching 90% and in certain disease settings may improve patient survival [1, 2]. Information on the community utilization of SBRT is limited; this study sought to evaluate temporal trends in the use of SBRT in the U.S. and identify clinical predictors of utilization using the National Cancer Database.
Among 65,345 patients diagnosed with mRCC between 2004 and 2016, 1,919 patients (2.9%) underwent SBRT, 15,871 (24.3%) received conventional RT, and 47,555 (72.8%) did not receive RT. The proportion of patients undergoing SBRT increased from 1.4% in 2004 to 3.7% in 2016, whereas conventional RT utilization decreased over the same period (26.6% in 2004, 24.1% in 2016) (Figure 1). The Cochran‐Armitage test for trend showed a significant increase in utilization of SBRT both in the overall cohort (slope = 0.0023, p < .001) and among patients receiving either conventional RT or SBRT (slope = 0.00839, p < .001). The most common site of treatment for patients undergoing SBRT was brain/neck (75.1%), followed by spine (14.5%), whereas for patients undergoing conventional RT, the most frequent sites of treatment were spine (35.6%) and other bony sites (24.5%). At multivariable logistic regression analysis, factors associated with a higher odds of receiving SBRT included a later year of diagnosis (2007–2009: odds ratio [OR], 1.50; 95% confidence interval [CI], 1.26–1.79; 2010–2012: OR, 2.00; 95% CI, 1.69–2.35; 2013–2016: OR, 2.42; 95% CI, 2.08–2.83; all p < .001) and higher educational status (intermediate‐high: OR, 1.26; 95% CI, 1.06–1.49; p = .007; high: OR, 1.44; 95% CI, 1.18–1.74; p < .001). Age ≥70 years (OR, 0.71; 95% CI, 0.61–0.84), female gender (OR, 0.80; 95% CI, 0.72–0.88), Black race (OR, 0.53; 95% CI, 0.43–0.65), and Medicaid insurance (OR, 0.79; 95% CI, 0.66–0.94) or no insurance (OR, 0.63; 95% CI, 0.49–0.81) were independent predictors of lower likelihood of receipt of SBRT.
Figure 1.
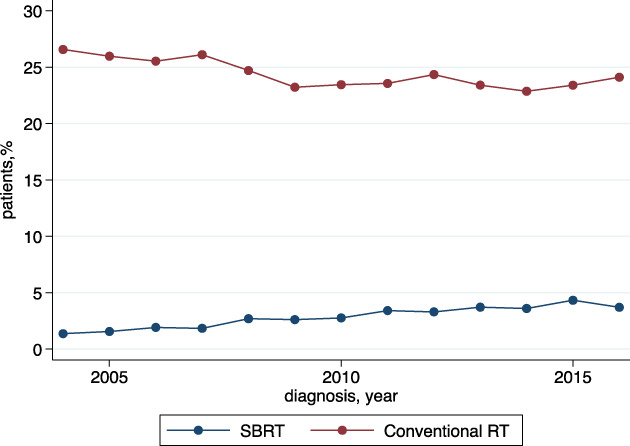
Trend in utilization of stereotactic body radiotherapy and conventional radiotherapy; percentage of patients with metastatic disease. Abbreviations: RT, radiotherapy; SBRT, stereotactic body radiotherapy.
Although SBRT for mRCC increased significantly from 2004 to 2016 in the U.S., it was used in a minority of patients. Our findings highlight key sociodemographic differences in the receipt of SBRT. Access to cancer care is an important determinant of outcomes, and with the increasing adoption of SBRT in routine oncology practice, efforts must be made to lessen disparities such that patients who are older, female, of Black race, and with Medicaid or no insurance may receive the same access to emerging technologies such as SBRT. Less resourced individuals may derive greater benefit from shorter duration of therapy (with a reduced number of fractions), resulting in less contact time at cancer centers. The observation of a lower likelihood of being treated with SBRT at nonacademic centers highlights the importance of multidisciplinary care within institutions or integration of care between regional and major centers for patients with advanced disease.
Moving forward, as more effective front‐line therapies enter the treatment landscape, patients’ responses improve, and patterns of progression after combination immunotherapy evolve, better systemic disease control may provide increased opportunity for further integration of SBRT, with smaller and/or more limited sites of metastases. A reduction in cytoreductive nephrectomy following the pivotal CARMENA and SURTIME trials, which challenged nephrectomy as the standard of care, has created an opportunity to investigate SBRT to the primary tumor [3, 4]. The CYTOSHRINK trial is a phase II trial accruing in Canada investigating nivolumab and ipilimumab for patients with intermediate‐ and poor‐risk mRCC, randomizing patients to SBRT to the renal primary following one cycle of systemic therapy [5]. Planned for accrual within the U.S., the NRG SAMURAI trial is in development, using a similar cohort of intermediate‐ and poor‐risk patients receiving immune combination therapy (either ipilimumab‐nivolumab or PD‐1/L1 and VEGF combination), randomizing to SBRT to the primary tumor [6]. Evolving evidence of the immunomodulatory properties of radiation therapy has also renewed interest in investigating multimodality treatment strategies incorporating SBRT for patients with advanced disease [7]. Over time we predict that the utilization of SBRT will continue to increase and form an integral tool in the multidisciplinary management of renal cell carcinoma.
Disclosures
Rana R. McKay: Bayer, Pfizer, Tempus (RF), AstraZeneca, Bayer, Bristol‐Myers Squib, Calithera, Exelixis, Janssen, Novartis, Pfizer, Sanofi, Tempus (SAB), Dendreon, Vividion (C/A); Quoc‐Dien Trinh: Astellas, Bayer, Janssen, Insightec, Intuitive Surgical (H); Toni K. Choueiri: AstraZeneca, Alexion, Bayer, Bristol‐Myers Squibb/ER Squibb and Sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann‐La Roche, GlaxoSmithKline, Eli Lilly & Co., Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda (RF), AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol‐Myers Squibb/ER Squibb and Sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann‐La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up‐to‐Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates Inc. (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L‐path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly Oncology, Medscape, PrimeOncology, ESMO (H), AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol‐Myers Squibb/ER Squibb and Sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Eli Lilly & Co., Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up‐to‐Date, NCCN, Analysis Group, Pionyr, Tempest, Lilly Ventures, Infinity Pharma (C/A), Pionyr, Tempest (OI), IP related to biomarkers of immune checkpoint blockers, and circulating free methylated DNA. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com.
References
- 1. Kothari G, Foroudi F, Gill S et al. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol 2015;54:148–157. [DOI] [PubMed] [Google Scholar]
- 2. Palma DA, Olson R, Harrow S et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR‐COMET): A randomised, phase 2, open‐label trial. Lancet 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]
- 3. Méjean A, Ravaud A, Thezenas S et al. Sunitinib alone or after nephrectomy in metastatic renal‐cell carcinoma. N Engl J Med 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 4. Bex A, Mulders P, Jewett M et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME randomized clinical trial. JAMA Oncol 2019;5:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lalani AKA, Swaminath A, Pond GR et al. Phase II trial of cytoreductive stereotactic hypofractionated radiotherapy with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). J Clin Oncol 2020;38(suppl 6):TPS761a. [Google Scholar]
- 6. @DrRanaMcKay. Excited to move forward the Samurai trial of SBRT + IO to patients with advanced RCC with primary intact. December 8 , 2020. Available at https://twitter.com/drranamckay/status/1336528013735776256 Accessed December 9, 2020.
- 7. Marciscano AE, Haimovitz‐Friedman A, Lee P et al. Immunomodulatory effects of stereotactic body radiation therapy: Preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


