Abstract
Patients with brain tumors are at high risk for thromboembolic complications and frequently require anticoagulation. Direct oral anticoagulants (DOACs) are a less burdensome treatment for cancer‐associated thrombosis with safety and efficacy comparable to those of low molecular weight heparin (LMWH); however, there are few data to support the use of DOACs in patients with brain tumors. The purpose of this study was to better understand the safety profile of anticoagulants in patients with primary and metastatic brain tumors, with particular interest in the safety and efficacy of DOACs. Our hypothesis was that DOACs are as safe and effective as LWMH in this population. This study was conducted through a single‐center retrospective chart review of 125 patients with primary and metastatic brain tumors on anticoagulation. Our primary outcomes were major bleeding and intracranial hemorrhage (ICH), with secondary outcomes of minor bleeding and recurrent thrombosis. The rate of major bleeding was 26% in the LMWH group versus 9.6% in the DOAC group (p = .03). The rate of ICH was 15% in the LMWH group versus 5.8% in the DOAC group (p = .09). The severity of ICH in both groups was low with median Common Terminology Criteria for Adverse Events version 5 scores of 2 in the LMWH group and 3 in the DOAC group. The rates of minor bleeding and recurrent thrombosis were low in both groups. Our conclusion is that DOAC use in patients with brain tumors is not associated with increased rates of major bleeding compared with LMWH and is a safe and effective option.
Implications for Practice
Patients with brain tumors are at high risk for venous thromboembolism and frequently require anticoagulation. Direct oral anticoagulants (DOACs) are less burdensome than low molecular weight heparin (LMWH) for treatment of thromboembolism, but there is concern in the community over increased risk of bleeding. This study provides much‐needed objective evidence that there are fewer major bleeding events in patients with brain tumors on DOACs compared to LMWH with similar efficacy. As the paradigm of anticoagulation in patients with cancer shifts from LWMH toward DOACs, this work is particularly meaningful as it suggests DOACs are safe and effective for patients with brain tumors.
Keywords: Venous thromboembolism, Anticoagulation, Brain tumors, Intracranial hemorrhage, Thrombosis and hemostasis
Short abstract
Direct oral anticoagulants (DOACs) are a less burdensome treatment for cancer‐associated thrombosis and have comparable safety and efficacy to low molecular weight heparin; however, there are little data to support the use of DOACs in patients with brain tumors. This article reports on the safety profile of anticoagulants in patients with primary and metastatic brain tumors, focusing on the safety and efficacy of DOACs.
Introduction
Patients with brain tumors are at high risk for thromboembolic complications and frequently require anticoagulation. Recent studies have shown that between 13% and 30% of patients diagnosed with glioblastoma multiforme will develop venous thromboembolism (VTE) over the course of their illness [1, 2]. Low molecular weight heparins (LMWHs) have become favored over warfarin in patients with cancer and VTE as they have demonstrated better efficacy in preventing recurrent VTE and no significant difference in bleeding risk [3]. However, daily subcutaneous injections make this anticoagulant uncomfortable and burdensome for many patients. Direct oral anticoagulants (DOACs) have emerged as a less burdensome treatment for cancer‐associated VTE with safety and efficacy comparable to those of LMWH [4, 5]. Although DOACs are being used more frequently in patients with cancer, there remains little data to support their use in patients with primary and metastatic brain tumors. Current guidelines recommend either LMWH, rivaroxaban, or edoxaban for at least 6 months for the treatment of VTE in patients with cancer. These guidelines make the same recommendation for patients with primary or metastatic central nervous system malignancies, although they note that there is uncertainty regarding which agent is most beneficial [6, 7]. Previous trials of anticoagulant use in patients with cancer have included few patients with brain tumors [3, 4, 5, 8], and there are currently no randomized controlled trials evaluating anticoagulants in this patient population. This has led to a high level of concern in the medical community regarding the use of DOACs in patients with brain tumors for fear of major bleeding and intracranial hemorrhage. Additionally, recent studies have shown that these events are more common in patients with brain tumors receiving therapeutic anticoagulation and lead to high morbidity and mortality [9, 10, 11]. Our objective was to further assess the safety and efficacy of anticoagulant treatments in patients with primary and metastatic brain tumors, with particular interest in rates of major bleeding and intracranial hemorrhage in patients on DOACs compared with those on LMWH.
Materials and Methods
Design and Oversight
This study was a single‐center retrospective chart review of patients with primary and metastatic brain tumors on full‐dose anticoagulation. The study protocol was approved as exempt, as defined by federal regulation 45 CFR §46, by the University of Pittsburgh Institutional Review Board before data collection occurred. The primary investigator was responsible for data acquisition and statistical analysis. Both authors were responsible for writing and editing the manuscript and submission for publication.
Study Population
Potential cases for inclusion in this study were identified by querying records at the Hillman Cancer Institute Center of Neuro‐Oncology for patients diagnosed with primary and metastatic brain tumors on treatment dose anticoagulation between the dates of August 1, 2015, and August 8, 2018. All patients with recent imaging demonstrating active primary or metastatic brain tumors on treatment dose anticoagulation for deep vein thrombosis (DVT)/pulmonary embolism (PE), atrial fibrillation, or hypercoagulable states were included in the study pool. DOAC cases included patients on rivaroxaban, apixaban, or dabigatran, whereas LMWH cases included patients on enoxaparin or dalteparin.
Data Acquisition
The electronic medical record was used to systematically review patients and record age, sex, tumor histology, oncologic treatment, imaging, indication for anticoagulation, bleeding complications, recurrent thrombosis, and comorbid conditions.
Outcomes
The primary outcomes of this study were the rates of major bleeding and intracranial hemorrhage in the patient population. Major bleeding was defined according to the International Society on Thrombosis and Hemostasis as overt bleeding that was associated with a decrease in hemoglobin level of 2 g per deciliter or more, led to transfusion of two or more units of packed red cells, occurred in a critical site, or contributed to death [12]. Intracranial hemorrhage was determined by review of documented radiology report on computed tomography or magnetic resonance imaging as well as neuro‐oncologist documented interpretation. The severity of intracranial hemorrhage was graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5 criteria ranging from 1 (asymptomatic imaging finding) to 5 (death) [13]. Important secondary outcomes in this study included minor bleeding, defined as any documented bleeding not meeting criteria for major bleeding, and recurrent thrombosis, defined as extension of prior clot on Doppler report or evidence of new or worsening burden of clot on imaging while on anticoagulation.
Statistical Analysis
Our hypothesis was that DOACs are as safe and effective as LMWH in patients with primary and metastatic brain tumors. Using medcalc software, two‐tailed Fisher's exact test was used to compare primary and secondary outcomes between DOAC and LWMH groups and derive p values.
Subgroup Analysis
In the patient population that developed the primary outcome of major bleeding while on anticoagulation, key comorbidities that were recorded included treatment of hypertension at the time of event, ongoing bevacizumab therapy at time of event, thrombocytopenia (platelet count less than 150) at time of event, and traumatic injury preceding the event.
Results
Characteristics of the Patients
From review of charts at Hillman Cancer Center, between August 8, 2015, and August 8, 2018, there were a total of 125 patients with active primary or metastatic brain tumors on full‐dose anticoagulation whose data were abstracted for the study. The baseline characteristics were well balanced (Table 1). The mean age of the population was 63 years, and the majority (57%) were male. The majority of patients in this study had a primary diagnosis of glioblastoma multiforme (50%), whereas the most common metastatic brain tumor primary was lung (8.8%), reflecting a typical patient population in a tertiary neuro‐oncology referral center. The study population had a fairly even split of patients on LMWH (45%) and DOACs (42%). Among the 52 patients in the study on DOACs, 40 were on rivaroxaban, 11 were on apixaban, and 1 patient was on dabigatran. There were relatively few patients in the study on warfarin (13%) who were not included in statistical analysis. Anticoagulation by tumor type is shown in Table 2 and further demonstrates the balance in the population. The major indication for anticoagulation in this population was DVT/PE (86%) with 53 patients having DVT (42%) and 54 (43%) having PE.
Table 1.
Baseline characteristics
| Characteristic | n = 125, n (%) |
|---|---|
| Age, mean, years | 63 |
| Gender: male, % | 57 |
| Tumor type | |
| GBM | 63 (50) |
| Glioma | 15 (12) |
| Meningioma | 7 (5.6) |
| CNS lymphoma | 19 (15) |
| Lung | 11 (8.8) |
| Breast | 5 (4.0) |
| Renal | 2 (1.6) |
| Ovary | 1 (0.8) |
| Uterine | 1 (0.8) |
| Melanoma | 1 (0.8) |
| Anticoagulant | |
| Warfarin | 16 (13) |
| DOAC | 52 (42) |
| LMWH | 57 (45) |
| Indication | |
| DVT/PE | 107 (86) |
| Atrial fibrillation | 12 (9.6) |
| CVST | 4 (3.2) |
| Genetic thrombophilia | 1 (0.8) |
| Mechanical valve | 1 (0.8) |
Abbreviations: CNS, central nervous system; CVST, cerebral venous sinus thrombosis; DOAC, direct oral anticoagulant; DVT/PE, deep vein thrombosis/pulmonary embolism; GBM, glioblastoma multiforme; LMWH, low molecular weight heparin.
Table 2.
Anticoagulation by tumor type
| Tumor type | Total, n | DOAC, n | LMWH, n | Warfarin, n |
|---|---|---|---|---|
| Primary brain tumors | ||||
| GBM | 63 | 22 | 33 | 8 |
| Glioma | 15 | 7 | 5 | 3 |
| Meningioma | 7 | 5 | 1 | 1 |
| CNS lymphoma | 19 | 10 | 8 | 1 |
| Metastatic tumors | ||||
| Lung | 11 | 7 | 4 | 0 |
| Breast | 5 | 0 | 4 | 1 |
| Renal | 2 | 0 | 1 | 1 |
| Ovary | 1 | 0 | 0 | 1 |
| Uterine | 1 | 1 | 0 | 0 |
| Melanoma | 1 | 0 | 1 | 0 |
| Total | 125 | 52 | 57 | 16 |
Abbreviations: CNS, central nervous system; DOAC, direct oral anticoagulant; GBM, glioblastoma multiforme; LMWH, low molecular weight heparin.
Primary Outcome
There were 22 major bleeding events in this population, with 14 of those major bleeding cases attributed to intracranial hemorrhage (ICH; Tables 3 and 4). In the entire study population, major bleeding events occurred in 5 of 52 patients (9.6%) in the DOAC group compared with 15 of 57 patients (26%) in the LWMH group with a significant p value of .03. The rate of intracranial hemorrhage was lower in the DOAC group at 3 of 52 patients (5.8%) compared with the LWMH group at 9 of 57 patients (16%), although the p value was insignificant at .09. The severity of ICH in both groups was low with median CTCAE version 5 scores of 2 and 3 in the LMWH and DOAC groups, respectively (Fig. 1). When evaluating only the patients in the study with primary brain tumors, the rates of major bleeding remained lower in the DOAC group at 4 of 44 patients (9.1%) compared with the LMWH group at 13 of 47 patients (28%) with a significant p value of .02. The rate of ICH also remained lower in the DOAC group at 2 of 44 patients (4.5%) than in the LWMH group at 7 of 47 patients (15%), although the p value remained insignificant at .10. There were few primary events in the metastatic tumor population and no significant differences noted between DOAC and LWMH groups. There was one fatal intracranial hemorrhage in the entire patient population that occurred in a patient with glioma on LMWH for DVT/PE.
Table 3.
Primary and secondary outcomes
| Outcomes | Warfarin, n (%) | DOAC, n (%) | LMWH, n (%) | p value |
|---|---|---|---|---|
| Primary + metastatic brain tumors | (n = 16) | (n = 52) | (n = 57) | |
| Major bleeding | 2 (12.5) | 5 (9.6) | 15 (26) | .03 |
| ICH | 2 (12.5) | 3 (5.8) | 9 (16) | .09 |
| Minor bleeding | 1 (6.2) | 10 (19) | 12 (21) | .79 |
| Recurrent thrombosis | 0 | 1 (1.9) | 3 (5.3) | .35 |
| Primary brain tumors | (n = 13) | (n = 44) | (n = 47) | |
| Major bleeding | 1 (7.7) | 4 (9.1) | 13 (28) | .02 |
| ICH | 1 (7.7) | 2 (4.5) | 7 (15) | .10 |
| Minor bleeding | 1 (7.7) | 8 (18) | 11 (23) | .56 |
| Recurrent thrombosis | 0 | 1 (2.3) | 2 (4.2) | .61 |
| Metastatic tumors | (n = 3) | (n = 8) | (n = 10) | |
| Major bleeding | 1 (33) | 1 (12) | 2 (20) | .65 |
| ICH | 1 (33) | 1 (12) | 2 (20) | .65 |
| Minor bleeding | 0 | 2 (25) | 0 | .10 |
| Recurrent thrombosis | 0 | 0 | 1 (10) | .37 |
Abbreviations: DOAC, direct oral anticoagulant; LMWH, low molecular weight heparin; ICH, intracranial hemorrhage.
Table 4.
Bleeding events
| Bleeding event | n |
|---|---|
| Major bleed (n = 22) | |
| Intracranial hemorrhage | 14 |
| Muscle hematoma | 4 |
| Gastrointestinal bleed | 2 |
| Retroperitoneal hematoma | 1 |
| Vaginal bleed | 1 |
| Minor bleed (n = 23) | |
| Gastrointestinal bleed | 9 |
| Epistaxis | 5 |
| Muscle hematoma | 4 |
| Hematuria | 3 |
| Hemoptysis | 2 |
Figure 1.
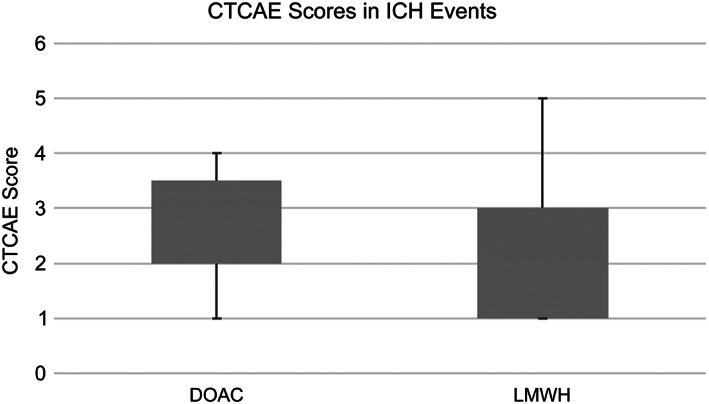
CTCAE scores in ICH events. Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; DOAC, direct oral anticoagulant; ICH, intracranial hemorrhage; VTE, venous thromboembolism.
Secondary Outcomes
There was no significant difference in minor bleeding (10/52 [19%] in the DOAC group vs. 11/57 [19%] in the LWMH group; p = .79) or recurrent thrombosis (1/52 [1.9%] in the DOAC group vs. 3/57 [5.3%] in the LWMH group; p = .35) in the patient population. This trend held true when the population was further divided into primary tumors and metastatic tumors. Further data expressing event rate by each tumor type can be found in Table 5.
Table 5.
Primary and secondary outcomes by tumor type
| Tumor type | ICH | Median CTCAE | Major bleed | Minor bleed | Recurrent thrombosis |
|---|---|---|---|---|---|
| Primary brain tumors | |||||
| GBM | 5 | 3 | 10 | 14 | 1 |
| Glioma | 4 | 1 | 5 | 2 | 0 |
| Meningioma | 0 | N/A | 1 | 1 | 0 |
| CNS lymphoma | 1 | 1 | 2 | 3 | 1 |
| Metastatic tumors | |||||
| Lung | 1 | 3 | 1 | 3 | 0 |
| Breast | 1 | 1 | 1 | 0 | 1 |
| Renal | 0 | N/A | 0 | 0 | 0 |
| Ovary | 1 | 1 | 1 | 0 | 0 |
| Uterine | 0 | N/A | 0 | 0 | 0 |
| Melanoma | 1 | 3 | 1 | 0 | 0 |
Abbreviations: CNS, central nervous system; CTCAE, Common Terminology Criteria for Adverse Events; GBM, glioblastoma multiforme; ICH, intracranial hemorrhage; N/A, not applicable.
Subgroup Analysis
In pooling data from the 22 cases of major bleeding (regardless of anticoagulation type), it was determined that treatment for hypertension was present in 11 of 22 (50%) and active treatment with bevacizumab was occurring in 11 of 22 (50%) of cases at time of diagnosis of major bleed.
Discussion
Patients with primary and metastatic brain tumors have significantly increased risk of venous thromboembolism, which leads to substantial morbidity and mortality [1, 2, 9, 10, 11]. However, anticoagulation in these patients has proven difficult, as many providers have concerns over precipitating bleeding complications, particularly intracranial hemorrhage. Prior studies have established that LMWH is superior to warfarin for cancer‐associated thromboembolism [3]; however, there is a growing body of evidence that DOACs are safe and effective for this indication. Two randomized control trials have shown that DOACs have noninferior efficacy to LWMH for malignancy‐associated VTE but also showed that DOACs are associated with an increased rate of major bleeding [4, 5]. Additionally, these trials included very few patients with brain tumors.
Our single‐center retrospective study of patients with primary and metastatic brain tumors on full‐dose anticoagulation showed that there was not an increased rate of major bleeding or intracranial hemorrhage in the DOAC group compared with the LWMH group. In fact, our data show that there were significantly fewer major bleeding events in the DOAC group compared with the LMWH group and a trend toward fewer intracranial hemorrhage events. This was true both in our composite analysis of all patients in the study and in the primary brain tumor population. There were few primary outcome events in the metastatic tumor group, which limited ability to generalize based on anticoagulation type. Our data show a higher rate of non‐ICH major bleeding events in the LMWH group, which may be a result of provider bias to treat more critically ill patients with LMWH. This is supported by a recent retrospective study that evaluated patterns of anticoagulation prescribing in patients with cancer and found that providers tend to avoid DOACs in patients with advanced disease [14]. The data in our study appear to contradict previously mentioned recent trials that show an increased major bleeding rate with patients with cancer on DOACs [4, 8]. However, these studies found that the increased major bleeding rates occurred in patients with gastrointestinal and gynecologic malignancies, of which there are very few in our patient population. We also found that there was no significant difference in minor bleeding or recurrent thrombosis in patients on DOACs versus LMWH in the population. The overall rate of intracranial hemorrhage in our study was higher than some previously reported studies that cite a rate of 2% [15, 16] but was in line with more recent studies showing around 14.7% severe ICH in patients with brain tumors on therapeutic anticoagulation [11, 17]. Additionally, most ICH events in this study were of low severity and required no aggressive intervention. Our results are consistent with a recent retrospective cohort study by Carney et al., which found a decreased rate of ICH events in patients with brain tumors on DOACs versus LMWH [18]. Our research adds to this study by also evaluating rates of major bleeding and adding secondary outcomes including minor bleeding and recurrent thrombosis. Of note, the patients in this study were not included in the recent CARAVAGGIO trial comparing the safety of apixaban with LWMH in patients with cancer. The results of our study lend further support to current guidelines that recommend the choice of either LMWH, rivaroxaban, or edoxaban for at least 6 months for the treatment of VTE in select patients with cancer, including patients with primary and metastatic brain tumors [6, 7].
The majority of the primary outcome events in our study occurred in patients with primary brain tumors, which is unsurprising as these patients comprised 80% of the study population. There were few primary outcome events in patients with metastatic tumors known to cause hemorrhage (melanoma, renal cancer); however, there were very few cases in the study population. The majority of the primary outcome events were attributed to intracranial hemorrhage and to muscle wall hematomas. Most of the minor bleeding events consisted of gastrointestinal bleeding and epistaxis, which is also unsurprising given that these are very common side effects of full‐dose anticoagulation [19]. When evaluating the patients who suffered a primary outcome in the population, treatment with bevacizumab and medical management of hypertension were common, which is also expected given their respective contribution to hemorrhagic complications [20, 21].
The limitations of this study are its retrospective nature, the small sample size, and data abstracted from a single site. As previously mentioned, there were very few patients in the population with metastatic brain tumors with only three patients with tumor types most associated with intracranial hemorrhage (melanoma and renal cell carcinoma). In future efforts, a randomized control trial comparing LMWH with DOACs in patients with brain tumors would be the most ideal way to analyze the efficacy and safety of these agents. However, a retrospective study using data from multiple sites may be the most reasonable means by which to obtain a larger data set and further evaluate this clinical question.
Conclusion
Our study builds upon recent evidence showing that DOACs are not associated with increased major bleeding, ICH, or recurrent thrombosis compared with LMWH in patients with primary or metastatic brain tumors. Given the ease of administration of DOACs compared with LMWH and the decreased patient burden, DOACs appear to be a safe and effective choice for anticoagulation in neuro‐oncology patients.
Author Contributions
Conception/design: Andrew W. Swartz, Jan Drappatz
Provision of study material or patients: Jan Drappatz
Collection and/or assembly of data: Andrew W. Swartz, Jan Drappatz
Data analysis and interpretation: Andrew W. Swartz, Jan Drappatz
Manuscript writing: Andrew W. Swartz, Jan Drappatz
Final approval of manuscript: Andrew W. Swartz, Jan Drappatz
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We thank the Neuro‐Oncology team at Hillman Cancer Center, including Megan Mantica, M.D., Ashley Pritchard, P.A.‐C., Donna Scalercio, B.S.N., and Lauren Theis, B.S.N., who assisted in identifying patients appropriate for this study and supported the authors.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol 2006;24:1310–1318. [DOI] [PubMed] [Google Scholar]
- 2. Reidl J, Preusser M, Nazari PM et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017;129:1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AYY, Levine MN, Baker RI et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 4. Raskob GE, van Es N, Verhamme P et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
- 5. Young AM, Marshall A, Thirlwall J et al. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT‐D). J Clin Oncol 2018;36:2017–2023. [DOI] [PubMed] [Google Scholar]
- 6. Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2019;38:496–520. [DOI] [PubMed] [Google Scholar]
- 7. Farge D, Frere C, Connors JM et al.; International Initiative on Thrombosis and Cancer (ITAC) Advisory Panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–e581. [DOI] [PubMed] [Google Scholar]
- 8. Carrier M, Abou‐Nassar K, Mallick R et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 9. Sjöblom L, Hårdemark HG, Lindgren A et al. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: A Swedish multicenter study. Stroke;32:2567–2574. [DOI] [PubMed] [Google Scholar]
- 10. Chai‐Adisaksopha C, Linkins LA, Al Kindi SY et al. Outcomes of low‐molecular‐weight heparin treatment for venous thromboembolism in patients with primary and metastatic brain tumours. Thromb Haemost 2017;117:589–594. [DOI] [PubMed] [Google Scholar]
- 11. Zwicker JI, Carrier M. A meta‐analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation: Reply. J Thromb Haemost 2016;14:2082. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 13. Basch E, Reeve BB, Mitchell SA et al. Development of the national cancer institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). J Natl Cancer Inst 2014;106:dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiang E, Ahuja T, Raco V et al. Anticoagulation prescribing patterns in patients with cancer. J Thromb Thrombolysis 2018;45:89–98. [DOI] [PubMed] [Google Scholar]
- 15. Choucair AK, Silver P, Levin VA. Risk of intracranial hemorrhage in glioma patients receiving anticoagulant therapy for venous thromboembolism. J Neurosurg 1987;66:357–358. [DOI] [PubMed] [Google Scholar]
- 16. Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983;13:334–336. [DOI] [PubMed] [Google Scholar]
- 17. Mantia C, Uhlmann EJ, Puligandla M et al. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood 2017;129:3379–3385. [DOI] [PubMed] [Google Scholar]
- 18. Carney BJ, Uhlmann EJ, Puligandla M et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost 2019;17:72–76. [DOI] [PubMed] [Google Scholar]
- 19. Trautmann A, Seitz CS. The complex clinical picture of side effects to anticoagulation. Med Clin North Am 2010;94:821–834. [DOI] [PubMed] [Google Scholar]
- 20. Hemphill JC 3rd, Greenberg SM, Anderson CS et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 21. Nghiemphu P, Green RM, Pope WB et al. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol 2008;10:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]


