Abstract
Background
IDH‐mutant anaplastic astrocytomas (AAs) are chemosensitive tumors for which the best choice of adjuvant chemotherapy between procarbazine, lomustine, and vincristine (PCV) or temozolomide (TMZ) after radiotherapy (RT) remains unclear.
Methods
In a large cohort of patients with histologically proven 2016 World Health Organization classification AA with IDH1/2 mutations included in the French national POLA cohort (n = 355), the primary objective was to compare progression‐free survival (PFS) between the two treatment regimens (n = 311). Secondary endpoints were overall survival (OS), progression type, pseudoprogression rate, and toxicity.
Results
The 4‐year PFS in the RT + PCV arm was 70.8% versus 53.5% in the RT + TMZ arm, with a hazard ratio (HR) of 0.58 (95% confidence interval [CI], 0.38–0.87; p = .0074) in univariable analysis and 0.63 (95% CI, 0.41–0.97; p = .0348) in multivariable analysis. The 4‐year OS in the RT + PCV arm was 84.3% versus 76.6% in the RT + TMZ arm, with an HR of 0.57 (95% CI, 0.30–1.05; p = .0675) in univariable analysis. Toxicity was significantly higher in the RT + PCV arm with more grade ≥3 toxicity (46.7% vs. 8.6%, p < .0001).
Conclusion
RT + PCV significantly improved PFS compared with RT + TMZ for IDH‐mutant AA. However, RT + TMZ was better tolerated.
Implications for Practice
In the absence of fully conducted randomized trials comparing procarbazine, lomustine, and vincristine (PCV) with temozolomide (TMZ) in adjuvant treatment after radiotherapy (RT) for the management of IDH‐mutant anaplastic astrocytoma (AA) and a similar level of evidence, these two chemotherapies are both equally recommended in international guidelines. This study in a national cohort of IDH‐mutant AA defined according the 2016 World Health Organization (WHO) classification shows for the first time that the RT + PCV regimen significantly improves progression‐free survival in comparison with the RT + TMZ regimen. Even if at the time of analysis the difference in overall survival was not significant, this result provides new evidence for the debate about the chemotherapy regimen to prescribe in adjuvant treatment to RT for WHO 2016 IDH‐mutant AA.
Keywords: Anaplastic astrocytoma; Temozolomide; Procarbazine, lomustine, and vincristine; IDH mutation
Short abstract
Different subgroups of anaplastic gliomas have been established. This study compared two chemotherapies in combination with radiotherapy to determine best management of IDH‐mutant anaplastic astrocytomas.
Introduction
In 2016, the new World Health Organization (WHO) classification for brain tumors was published, based on histological and biomolecular features [1]. Although the prognosis of anaplastic gliomas was very heterogenous in the previous classification [2], Cairncross et al. showed in the RTOG 9402 study that three different subgroups could be established by integrating mutations of isocitrate dehydrogenase (IDH) and the co‐deletion of chromosomal arm 1p and 19q (1p/19q co‐deletion) [3]: IDH‐mutant and 1p19q co‐deleted gliomas corresponding to anaplastic oligodendrogliomas with a median overall survival (OS) with radiotherapy (RT) alone of 6.8 years; IDH nonmutant and 1p19q non–co‐deleted gliomas corresponding to poor prognosis IDH wild‐type anaplastic astrocytoma (AA) with an average OS of 1.3 years; and IDH‐mutant and 1p19q non–co‐deleted gliomas corresponding to AA of moderate prognosis with a median OS of 3.3 years after RT only.
The benefit of adding chemotherapy to RT is now confirmed in these tumors. The RTOG 9402 study and the EORTC 26951 study, two large, randomized trials originating in the 1990s, showed a benefit of polychemotherapy by procarbazine, lomustine (also known as CCNU), and vincristine (PCV) in adjuvant or neoadjuvant treatment after RT [4, 5]. The role of adjuvant temozolomide (TMZ), well known since 2005 for its action on glioblastomas [6], was recently specified in the CATNON trial, with an improved OS compared with RT alone in this non–co‐deleted cohort [7].
Given these similar levels of evidence, the international guidelines recommend equally PCV or TMZ in adjuvant after RT for the management of IDH‐mutant AA [8]. In the absence of fully conducted randomized trials comparing PCV with TMZ in this setting, practices vary from one center to another.
The aim of this multicenter retrospective analysis was to compare these two chemotherapies in association with RT in order to provide stronger evidence for the management of IDH‐mutant AA, as defined in the WHO 2016 classification.
Materials and Methods
Study Population
In this multicenter retrospective noninterventional study, we included, in a first phase, patients treated in three centers in France (Institut Universitaire du Cancer de Toulouse; Hôpital de la Pitié Salpêtrière Paris; and Hôpital Neurologique Pierre Wertheimer, Centre Hospitalier Universitaire de Lyon) between October 2008 and March 2019. Inclusion criteria were age ≥ 18 years, with histologically proven AA with IDH1/2 mutations and non–co‐deleted 1p19q, according to the WHO 2016 classification for brain tumors. Most of these patients were also included in the French national POLA cohort.
In France, since 2008, a dedicated program has been set up for more homogeneous management of de novo adult high‐grade glioma with an oligodendroglial component, called the Prise en charge des oligodendrogliomes anaplasiques (POLA) network. One of the aims of the program was to provide a pathological centralized review of the cases and centralized molecular analysis. Patients prospectively included into the POLA cohort provided their written consent for clinical data collection and genetic analysis according to national and POLA network policies.
Thereafter, in a second phase, we widened our cohort to add patients, with the same inclusion criteria, from the whole French nationwide POLA cohort.
Data Collection
Comprehensive data were collected from the POLA database. For the patients in Toulouse, Paris, and Lyon, an exhaustive review of medical records and imaging data was performed on site to collect precise data on treatment progress, toxicity, and progression.
Treatments
All patients received planning computed tomography, and planning volumes were delineated after magnetic resonance imaging (MRI) registration. The treatment techniques included three‐dimensional conformal or intensity‐modulated radiotherapy. TMZ chemotherapy was given either according to the Stupp protocol, that is, concomitantly with RT at 75 mg/m2 daily, 7 days per week from the first day of RT until the last day of RT and after a 1‐month break in maintenance, for six cycles according to the standard 5‐day schedule every 28 days at the dose of 150 mg/m2 for the first cycle and 200 mg/m2 for the next cycles in the absence of hematologic toxicity, or either only in adjuvant without concomitance, at the standard 5‐day schedule every 28 days from 6 to 12 cycles. Chemotherapy by PCV was given according to the standard schedule for six cycles. PCV could be given before or after radiotherapy but never concomitantly. Each cycle consisted of lomustine 110 mg/m2 orally on day 1 (with a maximum dose of 200 mg), procarbazine 60 mg/m2 orally on days 8 to 21 (with a maximum dose of 100 mg), and vincristine 1.4 mg/m2 intravenous on days 8 and 29 (with a maximum dose of 2 mg).
Endpoints
Our primary endpoint was progression‐free survival (PFS). PFS was defined according response assessment in neuro‐oncology criteria combining imaging and clinical features. It was defined as any increase of contrast‐enhancing lesions more than 25% or significant increase in T2‐weighted fluid‐attenuated inversion recovery (T2‐FLAIR) nonenhancing lesions or any new lesion or clinical deterioration (not attributable to other nontumor causes and not caused by steroid decrease). Neuroimaging evaluation was not centrally reviewed. The secondary endpoints were OS, progression type, pseudoprogression rate, and toxicity. Pseudoprogression was radiologically defined as an increase of contrast‐enhancing lesion that may occur from the first few weeks to 6 months after treatment and subsequently subsides on follow‐up MRI without change in therapy.
Toxicity was graded using the Common Terminology Criteria for Adverse Events version 4.0.
Molecular biomarkers—IDH mutations, 1p19q co‐deletion status, and CDKN2A homozygous deletion status—were collected from POLA database where all cases were centrally reviewed [9].
Statistical Analysis
Data were summarized by the median, minimum, and maximum for quantitative variables and by frequency and percentage for qualitative variables. In univariable analysis, categorical variables were compared using the chi‐square and Fisher tests, and continuous variables were compared using the Kruskal‐Wallis test. All survival times were calculated from the initiation of the treatment and estimated by the Kaplan‐Meier method with 95% confidence intervals (CIs) using the following first‐event definitions: progression or death from any cause for PFS and death from any cause for OS. Patients who did not experience the event of interest were censored at their last follow‐up. Univariable and multivariable analysis were performed using the log‐rank test and Cox proportional hazards model; hazard ratios (HRs) were estimated with 95% CIs. Tests were two‐sided, and values of p < .05 were considered significant. Statistical analyses were performed using STATA 13 software.
This study was approved by the National Commission for Data Protection and Liberties (CNIL‐France) and the institutional review boards (Comité de Protection des Personnes).
Results
Analysis of the Paris, Lyon, and Toulouse Centers
Patients and Treatment Characteristics
First, we included 139 patients from Paris, Lyon, and Toulouse treated according to the international recommendations by combined RT and chemotherapy. Out of these 139 patients, 72 received temozolomide, either concurrent and adjuvant or adjuvant only (RT + TMZ arm), and 67 received PCV (RT + PCV arm). Patients’ clinical and demographic characteristics are summarized in supplemental online Table 1. The characteristics of the two arms were equally distributed except for age, with significantly younger patients in the RT + PCV arm (median 37 vs. 39 years, p = .0139). The median RT dose was 60 Gy (range, 45–60). The RT dose was 60 Gy for 59.9% of patients, 59.4 Gy for 31.4%. Only one patient received a dose below 50 Gy (45 Gy). The median number of PCV cycles in the RT + PCV arm was six (range, one to six). The median number of temozolomide cycles in the RT + TMZ arm was 6 (range, 1–24).
Table 1.
Treatment‐related adverse events by treatment in the population of the three centers of Paris, Lyon, and Toulouse
| Adverse event | RT + temozolomide (n = 72), n (%) | RT + PCV (n = 67), n (%) | Total (n = 139), n (%) | p value |
|---|---|---|---|---|
| Dose decrease due to toxicity | 13 (23.2) | 54 (87.1) | 67 (56.8) | <.0001 |
| Missing | 16 | 5 | 21 | |
| Treatment discontinuation due to toxicity | 22 (39.5) | 47 (75.8) | 69 (58.5) | <.0001 |
| Missing | 16 | 5 | 21 | |
| All toxicities | ||||
| All grades | 37 (63.8) | 60 (100) | 97 (82.2) | <.0001 |
| Grade ≥3 | 5 (8.6) | 28 (46.7) | 33 (28.0) | <.0001 |
| Missing | 14 | 7 | 21 | |
| Hepatotoxicity | ||||
| All grades | 5 (8.9) | 19 (33.9) | 24 (21.4) | .0013 |
| Grade ≥3 | 0 (0.0) | 11 (19.6) | 11 (9.8) | .0005 |
| Missing | 16 | 11 | 27 | |
| Hematotoxicity | ||||
| All grades | 21 (36.2) | 52 (86.7) | 73 (61.9) | <.0001 |
| Grade ≥3 | 3 (5.2) | 16 (26.7) | 19 (16.1) | .0015 |
| Missing | 14 | 7 | 21 | |
| Nausea/vomiting | ||||
| All grades | 11 | 8 | 19 | |
| Grade ≥3 | 0 | 0 | 0 | |
| Neuropathy | ||||
| All grades | 0 | 16 | 16 | |
| Grade ≥3 | 0 | 2 | 2 | |
| Asthenia | ||||
| All grades | 8 | 5 | 13 | |
| Grade ≥3 | 1 | 0 | 1 | |
| Anaphylaxy | ||||
| All grades | 3 | 9 | 12 | |
| Grade ≥3 | 0 | 1 | 1 | |
| Constipation | ||||
| All grades | 0 | 4 | 4 | |
| Grade ≥3 | 0 | 0 | 0 | |
| Pain | ||||
| All grades | 0 | 5 | 5 | |
| Grade ≥3 | 0 | 0 | 0 |
Abbreviations: PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Outcomes
The median follow‐up was 44.6 months (range, 38.6–54.4). At the time of the analysis, 52 patients (37.4%) had disease progression, 40 in the RT + TMZ arm and 12 in the RT + PCV arm. The 4‐year PFS was 46.0% in the RT + TMZ arm versus 80.7% in the RT + PCV arm, with an HR of 0.36 (95% CI, 0.18–0.68; p = .0012). At the time of the analysis, 25 patients (18%) were dead, 19 in the RT + TMZ arm versus 6 in the RT + PCV arm. The 4‐year OS was 78.9% in the RT + TMZ arm versus 90.5% in the RT + PCV arm, with an HR of 0.66 (95% CI, 0.25–1.74; p = .4005; Fig. 1).
Figure 1.
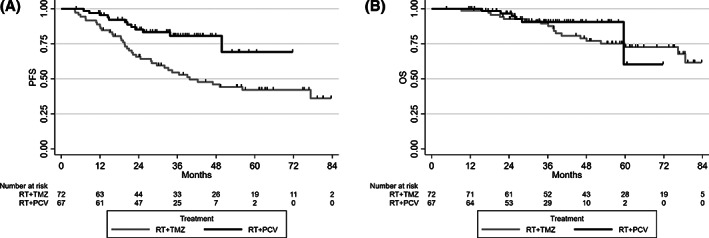
Progression‐free survival (A) and overall survival (B) by treatment in the first analysis of the three centers of Paris, Lyon, and Toulouse. Abbreviations: OS, overall survival; PCV, procarbazine, lomustine, and vincristine; PFS, progression‐free survival; RT, radiotherapy; TMZ, temozolomide.
A pseudoprogression analysis could be performed on 124 patients. This subacute treatment‐related reaction that mimics tumor progression was evidenced in 22 patients (17.7%): 5 (7.8%) in the RT + TMZ arm versus 17 (28.3%) in the RT + PCV arm.
MRI at progression could be analyzed in 49 of 52 patients with disease progression. Among them, 38 (77.6%) corresponded to an emergence/extension of local T1‐contrast enhancement, 9 (18.4%) to a distant T2‐FLAIR extension, and 2 (4.1%) to the appearance of distant T1‐contrast enhancement. These progressions occurred in 91.8% of the cases in the planning target volume treated with high dose.
Toxicity
Treatment‐related adverse events occurred in 63.8% of patients in RT + TMZ arm and 100% in the RT + PCV arm. Dose decrease or treatment discontinuation because of treatment‐related adverse events occurred less in the RT + TMZ arm. Grade ≥3 adverse events occurred in 8.6% of patients in the RT + TMZ arm and in 46.7% in the RT + PCV arm. The most common adverse events were hepatotoxicity (8.9% vs. 33.9%) and hematotoxicity (36.2% vs. 86.7%). Details are summarized in Table 1.
Analysis of Extended National POLA Cohort
Patients and Treatment Characteristics
In this next phase, the cohort was extended to add the whole national POLA database with the same eligibility criteria. We included a total of 355 patients: 311 patients treated by combined RT and chemotherapy and 44 by monotherapy only (chemotherapy or radiotherapy). Out of these 311 patients, 122 received chemotherapy by PCV (RT + PCV arm) and 189 by TMZ (RT + TMZ arm). In the RT + TMZ arm, 165 patients were treated with both concurrent and adjuvant TMZ and 24 with adjuvant TMZ only.
The clinical and demographic characteristics of the 311 patients treated by the association of RT and chemotherapy were equally distributed in the two arms except for age, with significantly younger patients in the RT + PCV arm (median 36.5 vs. 40 years, p = .0085; Table 2). In our cohort of IDH‐mutant AA treated with the association of RT and chemotherapy, only six patients (three in the RT + TMZ arm and three in the RT + PCV arm) had a CDKN2A homozygous deletion, which is known to be a highly adverse prognostic factor [9].
Table 2.
Patient's clinical and demographic characteristics in the national POLA cohort
| Characteristic | RT + temozolomide (n = 189), n (%) | RT + PCV (n = 122), n (%) | Total (n = 311), n (%) | p value |
|---|---|---|---|---|
| Median age (range), year | 40 (19–75) | 36.5 (19–68) | 38 (19–75) | .0085 |
| Sex | ||||
| Male | 110 (58.2) | 65 (53.7) | 175 (56.5) | .4375 |
| Female | 79 (41.8) | 56 (46.3) | 135 (43.5) | |
| Missing | 0 | 1 | 1 | |
| Performance status | ||||
| 0 | 91 (63.6) | 70 (66.4) | 161 (64.4) | .0550 |
| 1 | 37 (25.9) | 34 (29.5) | 71 (28.4) | |
| 2 | 15 (10.5) | 3 (2.8) | 18 (7.2) | |
| Missing | 46 | 15 | 61 | |
| Initial surgery | ||||
| Biopsy | 32 (17.0) | 23 (19.0) | 55 (17.8) | .6558 |
| Resection | 156 (83.0) | 98 (81.0) | 254 (82.2) | |
| Missing | 1 | 1 | 2 | |
| Localization | ||||
| Frontal | 140 (77.3) | 73 (68.2) | 213 (74.0) | .0882 |
| Temporal | 61 (33.7) | 50 (46.7) | 111 (38.5) | .0282 |
| Parietal | 31 (17.1) | 18 (16.8) | 49 (17.0) | .9470 |
| Occipital | 10 (5.5) | 8 (7.5) | 18 (6.3) | .5085 |
| Missing | 8 | 15 | 23 | |
| Side | .2195 | |||
| Right | 90 (49.2) | 46 (39.0) | 136 (45.2) | |
| Left | 82 (44.8) | 64 (54.2) | 146 (48.5) | |
| Median | 11 (6.0) | 8 (6.8) | 19 (6.3) | |
| Missing | 6 | 4 | 10 | |
| IDH mutation type | ||||
| IDH1 | 188 (99.5) | 119 (97.5) | 307 (98.7) | |
| IDH2 | 1 (0.5) | 3 (2.5) | 4 (1.3) | |
| Mib‐1 (range) | 12 (1–80) | 10 (2–30) | 10 (1–80) | .2342 |
| ≤10% | 81 (48.2) | 65 (57.0) | 146 (51.8) | .1465 |
| >10% | 87 (51.8) | 49 (43.0) | 136 (48.2) | |
| Missing | 21 | 8 | 29 |
Abbreviation: PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Outcomes
The median follow‐up was 48.2 months (range, 45.1–53.2) for the 311 patients treated by RT and chemotherapy. At the time of analysis, 121 patients (38.9%) had disease progression, 89 (47%) in the RT + TMZ arm and 32 (26.2%) in the RT + PCV arm. The 4‐year PFS in the RT + TMZ arm was 53.5% versus 70.8% in the RT + PCV arm. PFS was significantly longer in the RT + PCV arm (HR, 0.58; 95% CI, 0.38–0.87; p = .0074). At the time of the analysis, 65 patients (20.9%) had died, 51 (26.9%) in the RT + TMZ arm and 14 (11.4%) in the RT + PCV arm. The 4‐year OS in the RT + TMZ arm was 76.6% versus 84.3% in the RT + PCV arm, with an HR in favor of RT + PCV (HR, 0.57; 95% CI, 0.30–1.05; p = .0675; Fig. 2).
Figure 2.
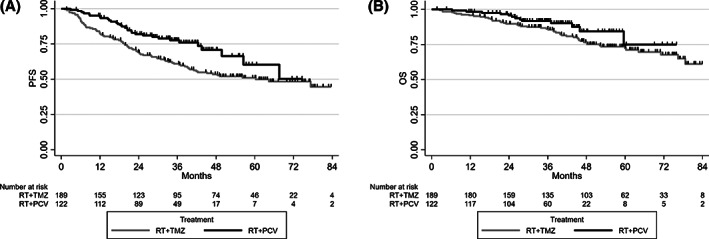
Progression‐free survival (A) and overall survival (B) by treatment in the second analysis of the national POLA cohort. Abbreviations: OS, overall survival; PCV, procarbazine, lomustine, and vincristine; PFS, progression‐free survival; RT, radiotherapy; TMZ, temozolomide.
In univariable analysis, by adding adjuvant PCV, variables associated with improved PFS were surgical resection versus biopsy (HR, 0.45; 95% CI, 0.30–0.68; p < .0001), Mib‐1 ≤ 10% (HR, 0.64; 95%, 0.43–0.93; p = .0201), and performance status (PS) <2 (HR, 0.37; 95% CI, 0.20–0.70; p = .0015). There was no association between PFS and location in the brain, subventricular zone contact, or age. Surgical resection, Mib‐1 ≤ 10%, and PS <2 were also associated with better OS: HRs were 0.57 (95% CI, 0.32–0.99; p = .0420), 0.39 (95% CI, 0.22–0.67; p = .0005), and 0.26 (95% CI, 0.11–0.58; p = .004), respectively (supplemental online Tables 2 and 3).
Table 3.
Multivariable analysis of progression‐free survival and overall survival in the national POLA cohort
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR 95% CI | p value | HR 95% CI | p value | |
| Treatment | .0348 | .2156 | ||
| RT + temozolomide | 1.00 | 1.00 | ||
| RT + PCV | 0.63 (0.41–0.97) | 0.67 (0.36–1.26) | ||
| Initial surgery | .0010 | .0511 | ||
| Biopsy | 1.00 | 1.00 | ||
| Resection | 0.47 (0.30–0.74) | 0.54 (0.29–1.00) | ||
| Mib‐1 | .0135 | .0004 | ||
| >10% | 1.00 | 1.00 | ||
| ≤10% | 0.61 (0.41–0.90) | 0.35 (0.20–0.62) | ||
| Age | 1.01 (0.99–1.03) | .2828 | 1.02 (1.00–1.04) | .0522 |
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PCV, procarbazine, lomustine, and vincristine; PFS, progression‐free survival; RT, radiotherapy.
Bold indicates p values of statistical significance.
In multivariable analysis adjusted on initial surgery (resection vs. biopsy), Mib‐1, and age, the RT + PCV arm remained associated with improved PFS compared with the RT + TMZ arm with an HR of 0.63 (95% CI, 0.41–0.97; p = .0348), but this benefit was still not found for OS (HR, 0.67; 95% CI, 0.36–1.26; p = .2156). Lastly, although Mib‐1 ≤ 10% remained associated with improved PFS and OS in multivariable analysis, initial surgery remained significant in PFS (HR, 0.47; 95% CI, 0.30–0.74; p = .001) but no longer in OS (HR, 0.54; 95% CI, 0.29–1.00; p = .051; Table 3).
In the RT + TMZ arm, the comparison between concurrent + adjuvant TMZ versus adjuvant TMZ showed no difference in PFS or in OS: HRs were 1.27 (95% CI, 0.64–2.54) and 1.47 (95% CI, 0.53–4.08; Fig. 3).
Figure 3.
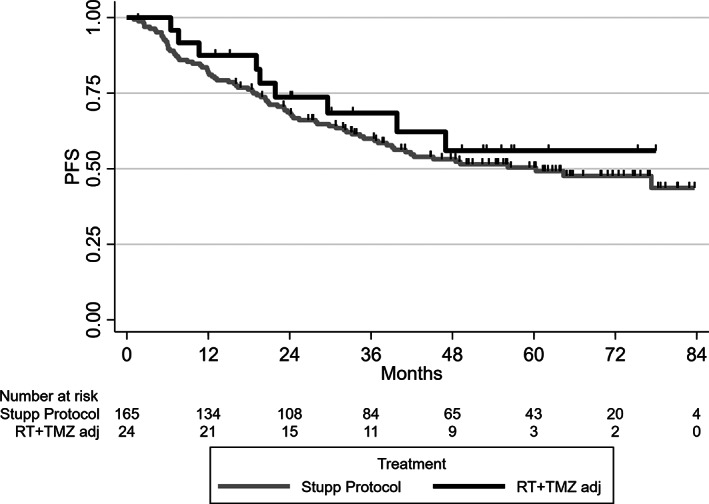
Progression‐free survival in the RT + TMZ arm between concurrent and adjuvant TMZ (Stupp Protocol) and adjuvant TMZ only in the national POLA cohort.Abbreviations: PFS, progression‐free survival; RT, radiotherapy; TMZ adj, adjuvant temozolomide.
Discussion
To our knowledge, this is the first study to compare directly the two chemotherapies recommended in the treatment of IDH‐mutant AA defined according to the new WHO classification of brain tumors [8]. We show a significant improvement in PFS with PCV compared with TMZ in this subgroup of patient.
The RTOG 9813 study published in 2017 randomized 201 patients with AA to adjuvant TMZ versus nitrosourea. Only 44% of these tumors were IDH‐mutant, that is, slightly different from our population. Despite early closure because of insufficient accrual, this study showed comparable outcomes (median survival 3.9 years for RT + TMZ vs. 3.8 years for RT + nitrosourea), with lower toxicity in the TMZ arm [10]. The authors therefore recommended the use of TMZ in adjuvant, supporting their argument with the results of the large international CATNON trial. In 2017, a preplanned interim analysis of this study demonstrated a benefit in OS in patients with anaplastic gliomas without 1p19q co‐deletion by adding adjuvant TMZ for 12 months versus RT alone. Overall survival at 5 years was 55.9% with and 44.1% without adjuvant temozolomide [7]. The update presented by van den Bent during the 2019 American Society of Clinical Oncology (ASCO) meeting showed that adjuvant TMZ produced significant 5‐year OS benefits only in patients with IDH‐mutant tumors, which corresponds to our population study [11]. Although these two major studies emphasized the value of adjuvant TMZ in AA with IDH mutation, PCV combination chemotherapy is also well studied. In his post hoc analysis of the RTOG 9402 study, Cairncross et al. showed a significant benefit of adding PCV to RT alone in the subgroup of patients with IDH‐mutant and non–co‐deleted tumors with a median OS of 5.5 years in RT + PCV arm versus 3.3 years in RT alone arm [3]. Moreover, a post hoc analysis of the RTOG 9802, a phase III trial of high‐risk low‐grade gliomas treated with RT with and without PCV, presented at the 2019 ASCO meeting, support the benefit of adding PCV in this molecular subgroup [12]. Indeed, it showed that both IDH‐mutant subgroups (co‐deleted and non–co‐deleted) were significantly correlated with longer PFS and OS in the RT + PCV arm, whereas no difference was observed with the addition of PCV chemotherapy in the IDH wild‐type subgroup.
This recent result of the RTOG 9802 study emphasizes the importance of incorporating the new WHO 2016 subgroups for population selection, which is a major strength of our study. Indeed, critical breakthroughs in molecular biomarkers have changed our understanding of gliomas classification. In 2006, Brandes et al. had already compared adjuvant PCV and TMZ for AA in a nonrandomized prospective study and had showed no difference in OS and PFS between the two arms [13]. But, as in RTOG 9813, the diagnosis of astrocytoma was based only on the histological criteria of the WHO 2000 classification. Nowadays, molecular diagnostic criteria provide valuable prognostic and predictive insights. We hypothesized that this biomolecular selection has enabled us to show a benefit of PCV in our subgroup of anaplastic gliomas.
We also assumed that despite the increase in PFS in univariate and multivariate analysis, the absence of a significant benefit in OS in our patients could be partly the result of a chemotherapy crossover at the time of progression. Indeed, patients who received second‐line treatment at progression were treated 24% by surgery, 9% by reirradiation, and 67% by systemic treatment. Among progressing patients in the RT + TMZ arm, 25% received a second‐line chemotherapy by PCV and 18% a nitrosourea‐based monotherapy, and among progressing patients in the RT + PCV arm, 42.9% received a second‐line chemotherapy by TMZ, which corresponds to a significant crossover.
Another issue was the short follow‐up of our study. Indeed, with a median follow‐up of 48.1 months and only 20.9% death at the time of analysis, this low incidence rate could explain a lack of statistical power to show a significant difference in OS.
Although it is a large national cohort with more than 300 patients, the retrospective nature of our study remains an important limitation. Consequently, we performed multivariable analysis to prevent any selection bias inherent in retrospective studies. After adjustment on potentially confounding variables (i.e., primary surgery, Mib‐1 rate, and age), the PFS benefit with PCV still remained significant. In multivariable analysis, no adjustment was performed on performance status (because of the small number of cases with PS ≥2).
The two other variables that remained associated with improved PFS in the multivariable analysis were surgical resection and and Mib‐1 rate ≤ 10%. Many nonrandomized studies have shown that the extent of resection is a strong prognostic factor in malignant gliomas. In the light of molecular features, Kawaguchi et al. studied the impact of gross total resection in grade 3 gliomas according to IDH mutation and 1p/19q co‐deletion [14]. They showed that gross total resection compared with nontotal resection was associated with a longer OS in the group with IDH mutation and without 1p/19q co‐deletion (i.e., IDH‐mutant AA), whereas it had no impact in 1p/19q co‐deleted or in IDH wild‐type gliomas. The prognostic impact of Mib‐1 rate, also called Ki‐67, was studied in the large meta‐analysis by Chen et al. [15]. They found an inverse effect on survival with a cutoff <10%. In a more homogeneous population of IDH‐mutant anaplastic gliomas from the EORTC 26951 study, Preusser et al. also showed a better PFS and OS in patients with a low Ki‐67 index [16]. The results of these studies are in accordance with our present findings.
Regarding the role of concurrent TMZ plus adjuvant TMZ versus adjuvant alone, our study did not show any difference in PFS and OS between the two regimens. In the second interim analysis of the CATNON trial, concurrent TMZ did not increase OS in the whole cohort [11]. However, for patients with IDH mutations who also received adjuvant TMZ, it showed a trend toward a benefit in 5‐year OS by adding concurrent TMZ, with a 5‐year OS rate of 84.4% in concurrent and adjuvant TMZ arm versus 80.4% in adjuvant without concurrent TMZ arm.
Treatment‐related adverse events analysis showed a better tolerability of TMZ with 8.6% grade ≥3 toxicity in the RT + TMZ arm versus 46.7% in the RT + PCV arm. This tolerance profile in favor of TMZ was found in other studies comparing TMZ with nitrosourea‐based chemotherapy. For instance, the NOA‐04 randomized study measuring RT alone versus RT plus adjuvant chemotherapy by PCV or TMZ showed significantly higher grade ≥2 toxicity in the PCV arm than in the TMZ one (40% vs. 4%) and more treatment discontinuations because of hematologic toxicity (18% vs. 6%) [17]. Beyond toxicity, TMZ, with its 5‐day oral schedule every 28 days, is much more practical for patients than the complex PCV combination regimen. This issue must be an important consideration for some of our patients with neurocognitive disabilities because it could lead to poor compliance.
In the present study, the pseudoprogression rate was consistent with the literature [18, 19]. However, it was significantly more frequent in the RT + PCV arm than in the RT + TMZ arm (28.3% vs. 7.8%). Brandes et al. demonstrated in 2008 that pseudoprogression was an independent prognostic factor [20]. Moreover, it is known to be associated significantly with predictive biomarkers for treatment response, especially IDH mutations and MGMT promoter methylation [21]. Therefore, pseudoprogression may be assumed to reflect a good treatment response and represents perhaps an active inflammatory response against the tumor. Unsurprisingly, our most responsive treatment arm, that is, RT + PCV, was the one with the highest pseudoprogression rate.
Conclusion
Even if we did not show any significant benefit in OS, this retrospective multicenter national study showed for the first time a significantly better PFS with the RT‐PCV regimen compared with RT‐temozolomide for WHO 2016 IDH‐mutant AA. It provides new evidence for the debate about the best chemotherapy regimen for this subgroup of anaplastic gliomas defined by biomolecular markers according to the WHO 2016 classification.
Author Contributions
Conception/design: Vincent Esteyrie, Caroline Dehais, François Ducray, Elizabeth Cohen‐Jonathan Moyal
Provision of study material or patients: Vincent Esteyrie, Caroline Dehais, Catherine Carpentier, Charlotte Bronniman, Damien Pouessel, Delphine Larrieu Ciron, François Ducray, Elizabeth Cohen‐Jonathan Moyal
Collection and/or assembly of data: Vincent Esteyrie, Catherine Carpentier, Emmanuelle Uro‐Coste, Dominique Figarella‐Branger
Data analysis and interpretation: Elodie Martin
Manuscript writing: Vincent Esteyrie, Caroline Dehais, Damien Pouessel, Delphine Larrieu Ciron, François Ducray, Elizabeth Cohen‐Jonathan Moyal
Final approval of manuscript: Vincent Esteyrie, Caroline Dehais, Damien Pouessel, Delphine Larrieu Ciron, François Ducray, Elizabeth Cohen‐Jonathan Moyal
Disclosures
Damien Pouessel: AstraZeneca, Merck, Astellas, Janssen, Ipsen, Bristol‐Myers Squibb (H), AstraZeneca, Pfizer, Sanofi, Astellas, Janssen (SAB); Elizabeth Cohen‐Jonathan Moyal: Novocure (C/A), Bayer, Incyte, Astra Zeneca (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Table S1 Patient's clinical and demographic characteristics in the cohort of the three centers of Paris, Lyon and Toulouse
Table S2. Univariable analysis of progression‐free survival in the national POLA cohort
Table S3. Univariable analysis of overall survival in the national POLA cohort
Acknowledgments
The POLA network is funded by the French Institut National du Cancer. Members of the POLA network include the following: Amiens (C. Desenclos, H. Sevestre), Angers (P. Menei, A. Rousseau), Annecy (T. Cruel, S. Lopez), Besançon (M.‐I. Mihai, A. Petit), Bicêtre (C. Adam, F. Parker), Brest (R. Seizeur, I. Quintin‐Roué), Bordeaux (S. Eimer, H. Loiseau), Caen (L. Bekaert, F. Chapon), Clamart (D. Ricard), Clermont‐Ferrand (C. Godfraind, T. Khallil), Clichy (D. Cazals‐Hatem, T. Faillot), Colmar (C. Gaultier, M.C. Tortel), Cornebarrieu (I. Carpiuc, P. Richard), Créteil (W. Lahiani), Dijon (H. Aubriot‐Lorton, F. Ghiringhelli), Lille (E. Le Rhun, C.A. Maurage,), Limoges (E.M. Gueye, F. Labrousse), Lyon (D. Meyronet), Marseille (O. Chinot), Montpellier (L. Bauchet, V. Rigau), Nancy (P. Beauchesne, G. Gauchotte), Nantes (M. Campone, D. Loussouarn), Nice (D. Fontaine, F. Vandenbos‐Burel), Orléans (C. Blechet, M. Fesneau), Paris (A. Carpentier, J.Y. Delattre, K. Mokhtari, M. Polivka), Poitiers (S. Milin, M. Wager), Reims (P. Colin, M.D. Diebold), Rennes (D. Chiforeanu, E. Vauleon), Rouen (O. Langlois, A. Laquerriere), Saint‐Etienne (F. Forest, M.J. Motso‐Fotso), Saint‐Pierre de la Réunion (M. Andraud, G. Runavot), Strasbourg (B. Lhermitte, G. Noel), Suresnes (A.L. Di Stéfano, C. Villa), Tours (C. Rousselot‐Denis, I. Zemmoura), Toulon (N. Desse), and Villejuif (F. Dhermain).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 2016;131:803–820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2. Rajmohan KS, Sugur HS, Shwetha SD et al. Prognostic significance of histomolecular subgroups of adult anaplastic (WHO Grade III) gliomas: Applying the ‘integrated’ diagnosis approach. J Clin Pathol 2016;69:686–694. doi: 10.1136/jclinpath-2015-203456 [DOI] [PubMed] [Google Scholar]
- 3. Cairncross JG, Wang M, Jenkins RB et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014;32:783–790. doi: 10.1200/JCO.2013.49.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairncross G, Wang M, Shaw E et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long‐term results of RTOG 9402. J Clin Oncol 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ, Brandes AA, Taphoorn MJB et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long‐term follow‐up of EORTC Brain Tumor Group Study 26951. J Clin Oncol 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229 [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 7. van den Bent MJ, Baumert B, Erridge SC et al. Interim results from the CATNON trial (EORTC study 26053‐22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non‐co‐deleted anaplastic glioma: A phase 3, randomised, open‐label intergroup study. Lancet 2017;390:1645–1653. doi: 10.1016/S0140-6736(17)31442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weller M, van den Bent M, Tonn JC et al. European Association for Neuro‐Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8 [DOI] [PubMed] [Google Scholar]
- 9. Appay R, Dehais C, Maurage CA et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH‐mutant gliomas. Neuro Oncol 2019;21:1519–1528. doi: 10.1093/neuonc/noz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang S, Zhang P, Cairncross JG et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: Results of NRG Oncology RTOG 9813. Neuro Oncol 2017;19:252–258. doi: 10.1093/neuonc/now236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Bent MJ, Erridge S, Vogelbaum MA et al. Second interim and first molecular analysis of the EORTC randomized phase III intergroup CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q codeletion. J Clin Oncol 2019;37(suppl 15):2000a. doi: 10.1200/JCO.2019.37.15_suppl.2000 [DOI] [Google Scholar]
- 12. Bell EH, Won M, Fleming JL et al. Updated predictive analysis of the WHO‐defined molecular subgroups of low‐grade gliomas within the high‐risk treatment arms of NRG Oncology/RTOG 9802. J Clin Oncol 2019;37(suppl 15:2002a. doi: 10.1200/jco.2019.37.15_suppl.2002 [DOI] [Google Scholar]
- 13. Brandes AA. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol 2006;8:253–260. doi: 10.1215/15228517-2006-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawaguchi T, Sonoda Y, Shibahara I et al. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co‐deletion. J Neurooncol 2016;129:505–514. doi: 10.1007/s11060-016-2201-2 [DOI] [PubMed] [Google Scholar]
- 15. Chen WJ, He DS, Tang RX et al. Ki‐67 is a valuable prognostic factor in gliomas: Evidence from a systematic review and meta‐analysis. Asian Pac J Cancer Prev 2015;16:411–420. doi: 10.7314/apjcp.2015.16.2.411 [DOI] [PubMed] [Google Scholar]
- 16. Preusser M, Hoeftberger R, Woehrer A et al. Prognostic value of Ki67 index in anaplastic oligodendroglial tumours ‐ a translational study of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Histopathology 2012;60:885–894. doi: 10.1111/j.1365-2559.2011.04134.x [DOI] [PubMed] [Google Scholar]
- 17. Wick W, Roth P, Hartmann C et al. Long‐term analysis of the NOA‐04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol 2016;18:1529–1537. doi: 10.1093/neuonc/now133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanghera P, Perry J, Sahgal A et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 2010;37:36–42. [DOI] [PubMed] [Google Scholar]
- 19. Abbasi AW, Westerlaan HE, Holtman GA et al. Incidence of tumour progression and pseudoprogression in high‐grade gliomas: A systematic review and meta‐analysis. Clin Neuroradiol 2018;28:401–411. doi: 10.1007/s00062-017-0584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Bent MJ, Dubbink HJ, Sanson M et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: A report from EORTC Brain Tumor Group Study 26951. J Clin Oncol 2009;27:5881–5886. doi: 10.1200/JCO.2009.24.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Li J, Cheng G et al. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide‐based chemoradiotherapy. Clin Neurol Neurosurg 2016;151:31–36. doi: 10.1016/j.clineuro.2016.10.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Table S1 Patient's clinical and demographic characteristics in the cohort of the three centers of Paris, Lyon and Toulouse
Table S2. Univariable analysis of progression‐free survival in the national POLA cohort
Table S3. Univariable analysis of overall survival in the national POLA cohort


