Abstract
Introduction
Cabozantinib is an oral tyrosine kinase inhibitor that is approved for the treatment of metastatic renal cell carcinoma (mRCC). Cabozantinib is a weak base that exhibits a pH‐dependent solubility profile in vitro which raises concerns about its bioavailability in patients treated with proton pump inhibitors (PPIs). The purpose of this study was to investigate whether PPI use has an impact on the efficacy, safety, and residual concentration (Ctrough) of cabozantinib in patients with mRCC.
Materials and Methods
This is a retrospective review of a prospectively collected electronic database of patients with mRCC who received cabozantinib at Gustave Roussy between February 2014 and December 2018. The Kaplan‐Meier method was used for survival analysis and the Cox proportional‐hazard model for uni‐ and multivariate analysis. In parallel, we conducted a pharmacokinetic study of cabozantinib in a distinct cohort of 50 mRCC patients, in which cabozantinib Ctrough was assayed using a validated tandem mass spectrometry–liquid chromatography method.
Results
We identified 99 patients treated with cabozantinib, including 43 patients being PPI users. With a median follow‐up of 30.3 months, PPI users showed similar progression‐free survival and overall survival outcomes compared with PPI nonusers. Similarly, the incidence of adverse events was not significantly different between the PPI users and nonusers, although PPI users required dose reductions more often. In the independent pharmacokinetic cohort, of whom 21 received PPI concomitantly, Ctrough was similar between the two groups.
Conclusion
In line with the pharmacologic data, the concomitant use of PPI does not significantly impact the efficacy or safety of cabozantinib in patients with mRCC.
Implications for Practice
Drug interactions, especially between targeted therapies and proton pump inhibitors (PPI), were shown to potentially impact the outcomes of cancer patients. Cabozantinib, a current therapeutic standard in metastatic renal cell carcinoma (mRCC), exhibits a pH‐dependent solubility profile, which raises concerns about its bioavailability in patients treated with proton pump inhibitors (PPI). At the present time, there is no evidence regarding the effect of PPIs on cabozantinib's efficacy and safety in patients with mRCC. This study found that the concomitant use of PPI during cabozantinib treatment in mRCC patients does not appear to impact the residual concentration, efficacy, and safety of cabozantinib in a real‐life context.
Keywords: Cabozantinib, Pharmacology, Proton pump inhibitors, Renal cell carcinoma, Overall survival, Toxicity
Short abstract
This article reports on the effect of proton pump inhibitors on the pharmacology, efficacy and safety outcomes of patients with metastatic renal cell carcinoma treated with cabozantinib.
Introduction
Approximately 400,000 cases of renal cell carcinoma are diagnosed worldwide every year, with nearly a third having advanced‐stage or metastatic disease at the time of diagnosis [1, 2] The majority of patients diagnosed with clear‐cell metastatic renal cell carcinoma (mRCC) will be treated with vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) and/or immune checkpoint inhibitors in the metastatic setting [3]. These treatment options have yielded substantial response rates and have significantly improved the survival of patients with mRCC [4, 5]. Cabozantinib is an oral VEGFR‐TKI that inhibits the activity of c‐MET, vascular endothelial growth factor receptor, AXL, and other tyrosine kinases. It is approved in patients with mRCC that had progressed after VEGFR‐targeted therapy based on the METEOR trial (NCT01865747), which showed progression‐free survival (PFS; hazard ratio [HR], 0.51; 95% confidence interval [CI], 0.41–0.62) and overall survival (OS; HR, 0.66; 95% CI, 0.53–0.83) benefit in comparison with everolimus [6]. Cabozantinib was later approved in the first‐line treatment of patients with intermediate/poor‐risk mRCC based on the CABOSUN trial (NCT01835158), which showed a longer PFS for cabozantinib in comparison to sunitinib (HR, 0.48; 65% CI, 0.31–0.74) but was not powered to show an OS benefit (HR, 0.80; 95% CI, 0.53–1.21) [7].
Drug interactions, especially with proton pump inhibitors (PPIs), were shown to potentially impact the outcomes of patients treated with TKIs for solid tumors [7, 8, 9]. For instance, a retrospective cohort of patients with non‐small cell lung cancer treated with the anti‐EGFR TKI (erlotinib) showed that OS was negatively impacted by administration of acid‐suppressor drugs. Moreover, a detrimental impact of gastric acid suppression in terms of both OS and PFS was demonstrated in patients with soft tissue sarcoma treated with pazopanib. In mRCC, a single retrospective chart review study demonstrated a lower PFS and OS in patients treated with both sunitinib and a PPI.
Cabozantinib is a weak base that exhibits a pH‐dependent solubility profile in vitro that raises concerns about its bioavailability in patients treated with PPIs [10]. The concomitant prescription of cabozantinib and PPIs is not rare, as 12%–27% of patients receiving cabozantinib develop dyspepsia as an adverse event of the drug itself [7, 12, 13]. A phase I study performed on healthy volunteers has previously shown that mean plasma peak concentration (Cmax) and overall exposure (area under the curve [AUC] 0–t) did not differ with the addition of omeprazole after a single dose of cabozantinib [14]. As a result, the label of cabozantinib does not warn against the concomitant use of acid‐suppressive medication such as PPIs. However, the available data do not report on the clinical implications of the concomitant use of continuous administered cabozantinib and PPIs. For instance, the two pivotal trials of cabozantinib in mRCC, METEOR (NCT01865747) and CABOSUN (NCT01835158), avoided the concomitant use of PPIs and cabozantinib, although PPI usage was allowed at least 2 hours (preferably 4 hours) after taking cabozantinib and at least 14 hours before the next dose of cabozantinib, if possible [6, 7]. The CANTATA trial (NCT03428217), in which cabozantinib is administered with the glutaminase inhibitor telaglenostat or placebo, in contrast, excluded patients who required continued PPI use after randomization. In this article, we investigate the impact of PPIs on the efficacy and safety outcomes of patients with mRCC treated with cabozantinib.
Materials and Methods
Study Design and Outcomes
The mRCC cohort of patients treated at Gustave Roussy prospectively included all adult patients with biopsy‐proven mRCC starting February 2014. All adult patients who received cabozantinib at any point during treatment for mRCC were selected from this cohort. These patients are followed‐up regularly in consultation every 2 weeks during the first month, then every 4 weeks subsequently with clinical examination and a blood test, and every 12 weeks with an imaging assessment. Details concerning patient characteristics, pathology, prognostic factors based on International mRCC Database Consortium (IMDC) risk groups, previous therapeutic strategies including the history of primary tumor resection, and lines of treatment were collected. The use of PPIs was also collected according to the specific agent used and duration. Patients who received PPIs for at least 3 weeks during the treatment with cabozantinib were considered as PPI users; otherwise, patients were considered PPI nonusers. All the patients included in the pharmacokinetics cohort were evaluated for therapeutic adherence at the time blood draw was performed. Therapeutic adherence was defined by the number of days of cabozantinib intake reported by the patient divided by 28. For the evaluation of response, computed tomography imaging was locally reviewed by the same radiologist (A.B.Z.) according to RECIST version 1.1. Cabozantinib‐related adverse events were defined and evaluated according to the Common Terminology Criteria for Adverse Events, version 5.0. Patients with missing concomitant medication information and those receiving a cabozantinib‐based combination were excluded from the analysis.
Pharmacokinetics Analysis
We conducted a separate study in an independent cohort of patients treated with cabozantinib for mRCC and enrolled in a routine monitoring pharmacokinetic (PK) study (INDS MR 5612140520). All the patients had already received cabozantinib and a PPI concomitant for at least 30 days at the time of PK analysis. Plasma samples for PK assay were obtained at least 7 hours after the last cabozantinib dose and were analyzed through a validated tandem liquid chromatography–mass spectrometry method. Residual (Ctrough) was estimated by using a standard pharmacological equation (Cmin = Cmeas × 0.5 dosing interval − 24/t1/2, where Cmin is the estimated residual concentration of cabozantinib and Cmeas is the concentration of cabozantinib measured with liquid chromatography–mass spectrometry), starting from measured cabozantinib concentration.
Statistical Analysis
Descriptive statistics were used to describe patient characteristics, including pathology and lines of treatment and overall response rates (ORR). The χ2 test , Student t test or Mann‐Withney U test was used to assess the difference between the groups as appropriate. Parametric or nonparametric distribution of continuous variables was assessed per Shapiro‐Wilk test. The median PFS and OS were estimated using the Kaplan‐Meier method. PFS was defined from the date of the cabozantinib initiation to the date of progression or death, and OS was defined from the date of cabozantinib initiation to the date of death. Patients who did not progress and were alive at the time of analysis were censored at the time they were last seen for PFS and OS analyses, respectively. Comparisons according to the patient demographics and disease characteristics were performed with the use of a log‐rank test with a two‐sided α level. HRs and associated 95% CIs were calculated with the use of a Cox proportional‐hazard model for uni‐ and multivariate analysis. All statistical analyses were performed using IBM SPSS Statistics version 20.
Results
Patient and Disease Characteristics
A total of 103 patients were identified from the electronic records as having received cabozantinib therapy for mRCC. Four patients were excluded from this analysis, as they received cabozantinib‐based combinations. Of the 99 eligible patients, median age was 61 years, and most patients were male (67 patients; 67.7%) and had clear cell histology (75 patients; 75.8%). With regard to IMDC risk groups, most patients had an intermediate‐risk disease (61 patients; 61.6%), whereas the rest had a poor‐risk score (19 patients; 19.2%) or a good‐risk score (19 patients, 19.2%). The majority of patients received cabozantinib as a third or further line treatment (68 patients; 68.7%).
Forty‐three patients (43.4%) were considered PPI users. The most widely used PPI was omeprazole at 40 mg daily dose (23 patients; 53.5%), followed by esomeprazole at 40 mg (13 patients; 29.3%), pantoprazole at 20 mg (3 patients; 6.9%), lansoprazole at 30 mg (3 patients; 6.9%), and rabeprazole (1 patient; 2.2%). No other acid suppressor agents such as histamine 2 receptor antagonists were administered. The majority of PPI users (60.5%) received PPIs for a preexisting medical condition (such as gastro‐esophageal reflux disease, hiatal herniation, and symptomatic dyspepsia), whereas a minority (17 patients, 39.5%) were prescribed PPIs to manage cabozantinib‐related adverse events (such as nausea, vomiting, or both). The baseline characteristics were generally well balanced between the concomitant PPI users (56 patients; 56.6%) and PPI nonusers (43 patients; 43.4%, Table 1).
Table 1.
Demographic and disease characteristics of the patients at study inclusion
| Characteristic | PPI users, n = 43 (43.4%) | PPI nonusers, n = 56 (56.6%) | p value |
|---|---|---|---|
| Age, median (range), yr | 62 (30–78) | 58 (22–78) | .377 |
| Male gender, n (%) | 26 (60.5) | 41 (73.2) | .179 |
| Histology, n (%) | .105 | ||
| Clear cell RCC | 36 (83.7) | 39 (69.6) | |
| Nonclear cell RCC, n (%) | 7 (16.3) | 17 (30.4) | |
| IMDC risk groups, n (%) | .507 | ||
| Good risk | 6 (13.9) | 13 (23.2) | |
| Intermediate risk | 28 (65.1) | 33 (58.9) | |
| Poor risk | 9(20.9) | 10 (17.8) | |
| Previous nephrectomy, n (%) | 35 (81.4) | 47 (83.9) | .740 |
| Cabozantinib line of treatment, n (%) | .130 | ||
| First and second line | 10 (23.3) | 21 (37.5) | |
| Third line and beyond | 33 (76.7) | 35 (62.5) |
Abbreviations: IMDC, International Metastatic RCC Database Consortium; PPI, proton pump users; RCC, renal cell carcinoma.
Effect of PPI Use on Efficacy Outcomes
With a median follow‐up of 30.3 months from cabozantinib initiation, 82 events of treatment failure, 92 events of progression or death, and 67 deaths had occurred. The median TTF was 12.7 months (95% CI, 9.5–16.0), the median PFS was 9.4 months (95% CI, 8.6–10.2), and the median OS was 20.1 months (95% CI, 16.4–23.8). Overall, the results were similar when the efficacy outcomes were assessed according to PPI use (for PFS and OS, Figs. 1, 2; Table 2). The ORR and DCR as assessed by blinded, independent central radiologic review were 33.4% and 95.2% in the PPI users group and 38.2% and 89.1% in the PPI nonusers group, respectively.
Figure 1.
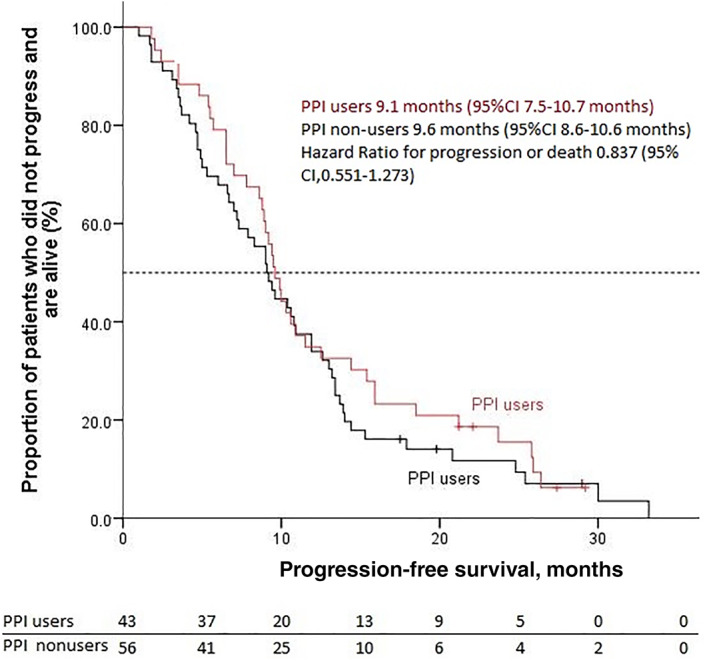
Kaplan‐Meier estimates of progression‐free survival. Abbreviations: CI, confidence interval; PPI, proton pump inhibitor.
Figure 2.
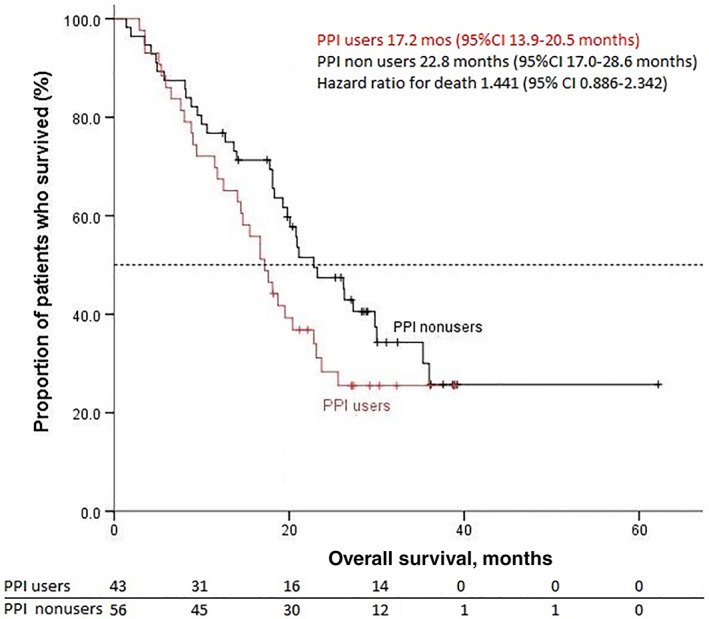
Kaplan‐Meier estimates of overall survival. Abbreviations: CI, confidence interval; PPI, proton pump inhibitor.
Table 2.
Efficacy outcomes of cabozantinib in patients with mRCC according to PPI use
| Efficacy outcomes | PPI users, n = 43 (43.4%) | PPI nonusers, n = 56 (56.6%) | p value |
|---|---|---|---|
| Overall response rate, n (%) | 14 (33.4) | 21 (38.2) | .674 |
| Disease control rate, n (%) | 40 (95.2) | 49 (89.1) | .464 |
| Median PFS, (95% CI) mo | 9.6 (95% CI, 8.6–10.6) | 9.1 (95% CI, 7.5–10.7) | .402 |
| HR (95% CI) | 0.837 (0.551–1.273) | 0.837 (0.551–1.273) | |
| Median OS, (95% CI) mo | 17.2 (95% CI, 13.9–20.5) | 22.8 (95% CI, 17.0–28.6) | .138 |
| HR (95% CI) | 1.441 (0.886–2.342) | 1.441 (0.886–2.342) |
Abbreviations: CI, confidence interval; HR, hazard ratio; mRCC, metastatic renal cell carcinoma; OS, overall survival, PFS, progression‐free survival; PPI, proton pump inhibitor; TTF, time‐to‐treatment failure.
In univariate analysis, a longer PFS was associated with a good and intermediate‐risk IMDC score at cabozantinib start (HR, 0.346; 95% CI, 0.164–0.729; p = .005 and HR, 0.425; 95% CI, 0.231–0.785; p = .006), whereas longer OS was correlated to good IMDC score at treatment start (HR, 0.153; 95% CI, 0.054–0.432, p < .001). In the multivariate analysis, a longer PFS was associated with good and intermediate‐risk IMDC score (HR, 0.345; 95% CI, 0.162–0.734, p = .006 and HR, 0.48; 95% CI, 0.253–0.91, p = .025, respectively). A longer OS was associated with a good‐risk IMDC score (HR, 0.144; 95% CI, 0.048–0.428; p < .001); a trend for a longer OS was observed in patients with an intermediate‐risk IMDC score and those who have undergone a previous nephrectomy (HR, 0.529; 95% CI, 0.279–1.003; p = .051 and HR, 0.492; 95% CI, 0.293–1.015; p = .055, respectively). PPI use was not associated with survival in either the univariate or in the multivariate analysis.
Effect of PPI Use on Safety Outcomes
Adverse events attributed to cabozantinib included diarrhea in 61.6% (grade 3–4, 1%), nausea in 43.4% (grade 3–4, 2%), loss of appetite in 36.3% (grade 3–4, 2%), dyspepsia in 21.2% (grade 3–4, not reported), mucositis in 37.4% (grade 3–4, 1%), vomiting in 20.2% (grade 3–4%, 3%), increased liver function tests in 44.4% (grade 3–4, not reported), and weight loss in 57,8% (grade 3, 10 %). Overall, the incidence of the reported adverse events was similar between the PPI users and nonusers. PPI users required dose reductions more often (83.7% vs. 64.3%; p = .041), but the treatment discontinuation did not differ between the two groups (72.1% vs. 73.3%, p = .587; Table 3).
Table 3.
Adverse events and reasons for treatment discontinuation
| Adverse events | PPI users, n = 43 (43.4%) | PPI nonusers, n = 56 (56.6%) | p value | ||
|---|---|---|---|---|---|
| Grade 1–2, n (%) | Grade 3–4, n (%) | Grade 1–2, n (%) | Grade 3–4, n (%) | ||
| Diarrhea | 27 (64.3) | 1 (2.4) | 33 (58.9) | 0 (0) | .401 |
| Nausea | 15 (34.9) | 0 (0) | 26 (46.4) | 2 (3.4) | |
| Loss of appetite | 19 (45.2) | 0 (0) | 15 (27.3) | 2 (3.6) | .106 |
| Dyspepsia | 10 (23.8) | 0 (0) | 11 (2.0) | 0 (0) | .652 |
| Mucositis | 15 (35.7) | 1 (2.4) | 21 (38.2) | 0 (0) | .510 |
| Vomiting | 11 (26.2) | 0 (0) | 8 (14.5) | 3 (5.5) | .131 |
| Increased liver function tests | 19 (46.3) | 0 (0) | 25 (45.5) | 0 (0) | .931 |
| Hyponatremia | 11 (26.8) | 0 (0) | 17 (3.9) | 0 (0) | .664 |
| Weight loss a | 21 (53.8) | 4 (10.2) | 24 (47) | 5 (9.8) | .488 |
| Cabozantinib dose reduction | 36 (83.7) | 36 (83.7) | 36 (64.3) | 36 (64.3) | .031 |
| Reasons for treatment discontinuation | .587 | ||||
| Progression | 20 (46.5) | 20 (46.5) | 31 (55.4) | 31 (55.4) | |
| Adverse events | 11 (25.6) | 11 (25.6) | 10 (17.9) | 10 (17.9) | |
Data at every visit unavailable for 9 patients: 5 PPI users and 4 PPI nonusers
Abbreviation: PPI, proton pump inhibitor.
Effect of PPI Use on Cabozantinib Ctrough
Fifty patients treated with cabozantinib were enrolled in the pharmacokinetics study, of whom 21 (42%) received concomitant PPIs for at least 30 days. Therapeutic adherence in this patients’ cohort, expressed as proportion of days covered, was 93.5%: 93.2% for PPI users and 94.5% for PPI nonusers. Seven out of 21 patients (33.3%) received PPIs to manage cabozantinib‐related adverse events, whereas the rest received PPIs for other medical conditions. Sixteen patients from this cohort were also included in the retrospective survival analysis. The characteristics of the patients did not differ between PPI users and nonusers (Table 4). The most widely used PPI was omeprazole at 40 mg (10 patients; 47.6%), followed by lansoprazole at 30 mg (5 patients; 23.8%), esomeprazole at 40 mg (4 patients; 19.1%), and pantoprazole at 20 mg (2 patients; 9.5%). No patient in this cohort was treated with other acid‐suppressive agents (e.g., histamine 2 receptor antagonists). A total of 82 plasma samples were considered as evaluable for this analysis: 32 obtained from PPI users and 50 from PPI nonusers. There was no significant difference between the two groups of patients (see supplemental online Fig. 1).
Table 4.
Demographic and disease characteristics of the patients included in the independent pharmacokinetics cohort*
| Characteristic | PPI users, n = 21 (42%) | PPI nonusers, n = 29 (58%) | p value |
|---|---|---|---|
| Age, median (range), yr | 58 (40–79) | 57 (22–73) | .748 |
| Male gender, n (%) | 17 (80.9) | 23 (79.3) | .354 |
| Histology, n (%) | |||
| Clear cell RCC | 17 (80.9) | 21 (72.4) | .068 |
| Nonclear cell RCC | 4 (19.1) | 8 (27.6) | |
| Cabozantinib line of treatment, n (%) | .130 | ||
| First and second line | 5 (23.3) | 8 (27.6) | |
| Third line and beyond | 16 (76.7) | 21 (72.4) | |
| Median duration of treatment, wk | 26.7 | 39.6 | .794 |
| Cabozantinib dose intensity b /blood draw, n (%), mg per day | .117 | ||
| 20 | 1 (3.1) | 4 (8) | |
| 28.57 | 7 (21.9) | 4 (8) | |
| 30 | 0 | 1 (2) | |
| 35.71 | 0 | 1 (2) | |
| 40 | 17 (53 ) | 14 (28) | |
| 42.85 | 3 (9.4) | 8 (16) | |
| 50 | 0 | 1 (2) | |
| 60 | 3 (9.4) | 13 (26) |
16 patients are included in both the clinical study set and the pharmacokinetic cohort.
Cumulative cabozantinib dose received in the previous 4 weeks divided by 28.
Abbreviations: IMDC, International Metastatic RCC Database Consortium; PPI, proton pump users; RCC, renal cell carcinoma.
Discussion
We hypothesized that the efficacy outcomes of cabozantinib in patients with mRCC could be lower in PPI users because the solubility of cabozantinib is decreased in a higher gastric pH. From a chemical point of view, cabozantinib can be present either in an ionized or a nonionized form, depending on the gastric pH and the acid‐base dissociation constant of the drug [15]. Ionized forms normally dissolve easier than nonionized forms, thus leading to a wider absorption; however, the concomitant use of PPI increases the gastric pH, and thus it may potentially decrease the drug absorption [14]. The results of this analysis showed that PPI use does not negatively impact the efficacy and safety of cabozantinib in patients with mRCC.
The available evidence reporting on the effect of concomitant PPIs with other TKIs in mRCC remains inconclusive. A retrospective study including 90 patients with mRCC treated with pazopanib, of whom 66 concomitantly received an acid‐suppressing drug, showed similar efficacy outcomes between PPI users and nonusers (median PFS 9.0 vs 11.0 months [p = .85] and median OS 28.0 vs 30.1 months [p = .92]) [16]. Another retrospective study including 231 patients with mRCC treated with sunitinib, of whom 45 were PPI users, showed a shorter survival among PPI users (median PFS 5.9 vs 4.7 months [p = .04] and median OS 15.6 vs. 10.2 months [p = .02]) [8]. A large retrospective study of 2,188 patients with mRCC treated with sunitinib, axitinib, or sorafenib, of whom 120 patients were PPI users, showed similar efficacy between PPI users and nonusers (median PFS 5.5 vs. 8.0 months [p = .902] and median OS 21.1 vs. 21.3 [p = .754]) [17]. In a large SEER‐Medicare retrospective study performed on 12,538 patients with cancer aged more than 65 years receiving concomitantly a PPI and a TKI for various oncological indications (such as erlotinib for non‐small cell lung cancer, dasatinib and nilotinib for chronic myeloid leukemia, and sorafenib and sunitinib for mRCC), the concomitant use of PPIs and TKIs was reported in 22.7%. This study showed a higher risk of death at 90 days and 1 year for PPI users (HR, 1.16; 95% CI, 1.05–1.28 and HR, 1.10; 95% CI, 1.04–1.17, respectively). However, when the analysis was restricted to 847 patients receiving sunitinib for mRCC, no difference in 90‐day and 1‐year survival was demonstrated (HR, 0.99; 95% CI, 0.66–1.49 and HR, 0.98; 95% CI, 0.77–1.25 respectively) [18]. Table 5 gives an overview of the published data reporting on the efficacy outcomes of tyrosine kinase inhibitors among patients with mRCC with and without concomitant PPI use.
Table 5.
Overview of the published data reporting on the efficacy outcomes of tyrosine kinase inhibitors among mRCC patients with and without concomitant proton pump inhibitor use
| Study | TKI | Number of patients | Outcomes (nonusers vs users) | Comment |
|---|---|---|---|---|
| Ha et al. (2015) [8] | Sunitinib | 231, 45 PPI users (19.4%) | mPFS 5.9 vs 4.7 months (p = .04); mOS 15.6 vs 10.2 months (p = .02) | |
| Lalani et al. (2017) [17] | Sunitinib, axitinib, sorafenib | 2,188, 120 PPI users (5.5%) | mPFS 8 vs 5.5 months (p = 0 .902); mOS 21.3 vs 21.1 months (p = .754) | Patients enrolled in clinical trials |
| McAllister et al. (2018) [16] | Pazopanib | 90, 63 PPI users (70%), 66 PPI + H2 antagonist users (73.3%) | mPFS 9.0 vs 11.0 months (p = .85); mOS 28.0 vs 30.1 months (p = .92) | Survival analysis performed by grouping all acid suppressing drug users |
| Sharma et al. (2019) [18] | Sunitinib | 847 mRCC; 22.7% (whole study population including NSCLC, CML, pancreatic cancer and HCC) | HR for death at 90 days 0.99, (95% CI, 0.66–1.49); HR = 0.98, (95% CI, 0.77–1.25) | Medicare retrospective study performed on several diseases. Data for mRCC extrapolated |
Abbreviations: CI, confidence interval; CML, chronic myeloid leukemia; HCC, hepatocellular carcinoma; HR, hazard radio; mOS, median overall survival; mPFS, median progression‐free survival; mRCC, metastatic renal cell carcinoma; NSCLC, non‐small cell lung cancer
Our analysis did not show a significant difference in the median PFS or OS between PPI users and nonusers. This is consistent with the available cabozantinib pharmacokinetics data that did not demonstrate a reduction in the AUC of cabozantinib in healthy volunteers treated with esomeprazole [14]. No relevant differences were noted in terms of safety between the two groups although a higher proportion of PPI users required dose reduction of cabozantinib, which did not seem to impact the clinical efficacy. A plausible explanation for our observation is the prescription of cabozantinib in the morning and the PPI in the evening, which may diminish the impact of the PPI on the absorption of cabozantinib. The effect of PPI may last longer than 12 hours, but the absorption of cabozantinib might not have been affected in a clinically significant manner that would influence the patient outcomes [19].
In our cohort, the median Ctrough of cabozantinib did not differ between PPI users and nonusers. Our pharmacokinetic results are consistent with those deriving from the phase I pharmacokinetic study of cabozantinib, which did not demonstrate a relevant difference in terms of Cmax and AUC 0–infinity in healthy volunteers treated with a single oral dose of cabozantinib before and after the use of PPI [13].
The rate of PPI use in the clinical trial of TKIs in patients with mRCC compares differently to those in the real‐world setting [17]. The results of this study are relevant to the daily clinical practice because the prevalence of PPI use (43.4%) parallels that of patients in the clinical setting, as almost 20% of patients take PPIs at baseline and 21.2% (our series) of patients develop dyspepsia attributed to cabozantinib prescription [20]. As such, it is more plausible that our cohort represents the patients encountered in daily practice having more competing comorbidities and requiring polymedications [21].
To our knowledge, this study is the first to specifically report on the impact of PPI use on the outcomes of cabozantinib in patients with mRCC. The data set is prospectively collected, the imaging assessment is blindly and centrally reviewed, and the details about the specific PPI and schedule are available. More broadly, our study results are in line with previous clinical experiences of concomitant PPI and TKI use in mRCC patients. The clinical efficacy and safety outcomes of our patients are comparable with those of patients enrolled in the pivotal clinical trials and the largest retrospective series currently published [6, 7, 22, 23]. Nevertheless, solid conclusions are limited by the relatively small sample size, the retrospective nature of the study, and the lack of standardized indications for PPIs, which are prescribed at the discretion of the treating oncologist. Furthermore, therapeutic adherence was assessed prospectively for the patients included in the pharmacokinetics cohort and through patient self‐reporting, a therapeutic adherence tool that may be biased by patients’ over‐reporting of drug adherence [24]. However, the self‐reported adherence rates to cabozantinib were comparable between the PPI users and nonusers; therefore the impact of the therapeutic adherence in this cohort can be considered negligible. Taking into consideration the pharmacologic data and the safe use of PPIs with other TKIs may suggest that PPIs do not impact the efficacy or safety of cabozantinib. The current development of cabozantinib as a drug is centered on combination regimens with either immune‐checkpoint inhibitors or CB 839, a glutaminase inhibitor. It is noteworthy that our results cannot be extrapolated to cabozantinib‐based combinations with immune checkpoint inhibitors (nivolumab in the CheckMate 9ER [NCT03141177], nivolumab plus ipilimumab in COSMIC‐313 [NCT03937219], and atezolizumab in CONTACT 03 [NCT03170960]) [24, 25, 26, 27]. Thus, PPIs may influence the efficacy of immune checkpoint inhibitors by modifying the gut microbiota [28, 29]. Conversely, PPI use is not allowed for patients enrolled in the phase II trial CANTATA in which cabozantinib is administered with placebo or CB 839.
Conclusion
Approximately 43% of patients with mRCC used PPIs during cabozantinib treatment. The concomitant use of PPIs did not significantly impact the efficacy and safety of cabozantinib in patients with mRCC. The pharmacologic cohort did not identify significant differences associated with PPI use. Thus, clinicians may consider allowing patients to remain on concomitant PPIs for clinically appropriate indications.
Author Contributions
Conception/design: Elie Rassy, Luigi Cerbone, Bernard Escudier, Laurence Albiges
Provision of study material or patients: Ronan Flippot, Emeline Colomba, Angelo Paci, Bernard Escudier, Laurence Abiges
Collection and/or assembly of data: Elie Rassy, Luigi Cerbone, Axelle Benchimoll Zouari, Carolina Alves Costa Silva, Annalisa Guida, David Combarel
Data analysis and interpretation: Elie Rassy, Luigi Cerbone, Edouard Auclin, Arthur Geraud, Ronan Flippot, Bernard Escudier, Laurence Albiges
Manuscript writing: Elie Rassy, Luigi Cerbone, Edouard Auclin, Axelle Benchimoll‐Zouari, Ronan Flippot, Carolina Alves Costa Silva, Emeline Colomba, Arthur Geraud, Annalisa Guida, Olivier Mir, David Combarel, Angelo Paci, Bernard Escudier, Laurence Albiges
Final approval of manuscript: Elie Rassy, Luigi Cerbone, Edouard Auclin, Axelle Benchimoll‐Zouari, Ronan Flippot, Carolina Alves Costa Silva, Emeline Colomba, Arthur Geraud, Annalisa Guida, Olivier Mir, David Combarel, Angelo Paci, Bernard Escudier, Laurence Albiges
Disclosures
Edouard Auclin: Mundipharma (H), Sanofi‐Genzymes (ET); Emeline Colomba: Bristol‐Myers Squibb, Ipsen (C/A), Bristol‐Myers Squibb Brazil, Pfizer (Other); Ronan Flippot: Bristol‐Myers Squibb (H); Arthur Geraud: Abbvie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen‐X Bvba, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, AstraZeneca Ab, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Bioalliance Pharma, Biontech Ag, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol‐Myers Squibb, Bristol‐Myers Squibb International Corporation, Ca, Celgene Corporation, Cephalon, Chugai Pharmaceutical Co., Clovis Oncology, Cullinan‐Apollo, Daiichi Sankyo, Debiopharm S.A., Eisai, Eisai Limited, Eli Lilly & Co, Exelixis, Forma Tharapeutics, Gamamabs, Genentech, Gilead Sciences, GlaxoSmithKline, Glenmark Pharmaceuticals, H3 Biomedicine, Hoffmann La Roche Ag, Incyte Corporation, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev., Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Kgaa, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Nerviano Medical Sciences, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncomed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Ose Pharma, Pfizer, Pharma Mar, Philogen S.P.A., Pierre Fabre Medicament, Plexxikon, Rigontec Gmbh, Roche, Sanofi Aventis, Sierra Oncology, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Tioma Therapeutics, Wyeth Pharmaceuticals France, Xencor, Y's Therapeutics (RF [Institution]); Olivier Mir: Amplitude Surgical, Ipsen, Transgene (O/I), Roche (H), Janssen, Eli Lilly & Co, Lundbeck, Pfizer, Roche (C/A), Pfizer, Roche (Other); Bernard Escudier: Bristol‐Myers Squibb, EUSA Pharma, Ipsen, Novartis, Oncorena, Pfizer, Roche/Genentech (H), AVEO, Bristol‐Myers Squibb, EUSA Pharma, Ipsen, Novartis, Pfizer, Roche/Genentech (C/A), Bristol‐Myers Squibb France (RF [Institution]) Bristol‐Myers Squibb, Ipsen, MSD, Pfizer, Roche/Genentech (Other); Laurence Albiges: Pfizer, Novartis, Bristol Myer Squibb, Ipsen, Roche, Merck Sharpe & Dohme, Astra Zeneca, Merck, Amgen, Astellas, Exelixis, Corvus Pharmaceuticals, Peloton Therapeutics (C/A [Institutional]). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 2013;23:38–45. [DOI] [PubMed] [Google Scholar]
- 3. Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma: Ther Adv Med Oncol 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med. 2017;376:354–66. [DOI] [PubMed] [Google Scholar]
- 5. de Velasco G, Bex A, Albiges L et al. Sequencing and combination of systemic therapy in metastatic renal cell carcinoma. Eur Urol Oncol 2019;2:505–514. [DOI] [PubMed] [Google Scholar]
- 6. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 7. Choueiri TK, Hessel C, Halabi S et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression‐free survival by independent review and overall survival update. Eur J Cancer 2018;94:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ha VH, Ngo M, Chu MP et al. Does gastric acid suppression affect sunitinib efficacy in patients with advanced or metastatic renal cell cancer? J Oncol Pharm Pract 2015;21:194–200. [DOI] [PubMed] [Google Scholar]
- 9. Chu MP, Ghosh S, Chambers CR et al. Gastric acid suppression is associated with decreased erlotinib efficacy in non‐small‐cell lung cancer. Clin Lung Cancer 2015;16:33–39. [DOI] [PubMed] [Google Scholar]
- 10. Mir O, Touati N, Lia M et al. Impact of concomitant administration of gastric acid‐suppressive agents and pazopanib on outcomes in soft‐tissue sarcoma patients treated within the EORTC 62043/62072 trials. Clin Cancer Res 2019;25:1479–1485. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration . Center for Drug Evaluation and Research clinical pharmacology and biophamaceutics reviews(s) for cabozantinib [COMETRIQ]. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000ClinPharmR.pdf. 2012. Accessed January 26, 2021.
- 12. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Numico G, Fusco V, Franco P, Roila F. Proton pump inhibitors in cancer patients: How useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol 2017;111:144–151. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen L, Holland J, Mamelok R et al. Evaluation of the effect of food and gastric pH on the single‐dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol 2015;55:1293–1302. [DOI] [PubMed] [Google Scholar]
- 15. van Leeuwen RW, van Gelder T, Mathijssen RH et al. Drug‐drug interactions with tyrosine‐kinase inhibitors: A clinical perspective. Lancet Oncol 2014;15:e315–e326. [DOI] [PubMed] [Google Scholar]
- 16. McAlister RK, Aston J, Pollack M et al. Effect of concomitant pH‐elevating medications with pazopanib on progression‐free survival and overall survival in patients with metastatic renal cell carcinoma. The Oncologist 2018;23:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lalani AKA, McKay RR, Lin X et al. Proton pump inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2017;15:724–732. [DOI] [PubMed] [Google Scholar]
- 18. Sharma M, Holmes HM, Mehta HB et al. The Concomitant use of tyrosine kinase inhibitors and proton pump inhibitors: Prevalence, predictors and impact on survival and discontinuation of therapy in older adults with cancer. Cancer 2019;125:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miner P, Katz PO, Chen Y e al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: A five‐way crossover study. Am J Gastroenterol 2003;98:2616–2620. [DOI] [PubMed] [Google Scholar]
- 20. Budha NR, Frymoyer A, Smelick GS et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: Is pH‐dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 2012;92:203–213. [DOI] [PubMed] [Google Scholar]
- 21. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008;336:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bodnar L, Kopczyńska A, Żołnierek J et al. Real‐world experience of cabozantinib as second‐ or subsequent line treatment in patients with metastatic renal cell carcinoma: Data from the polish managed access program. Clin Genitourin Cancer 2019;17:e556–e564. [DOI] [PubMed] [Google Scholar]
- 23. Procopio G, Prisciandaro M, Iacovelli R et al. Safety and efficacy of cabozantinib in metastatic renal‐cell carcinoma: Real‐world data from an Italian managed access program. Clin Genitourin Cancer 2018;16:e945–e951. [DOI] [PubMed] [Google Scholar]
- 24. Levine AM, Richardson JL, Marks G et al. Compliance with oral drug therapy in patients with hematologic malignancy. J Clin Oncol 1987;5:1469–1476 [DOI] [PubMed] [Google Scholar]
- 25. Escudier B, Porta C, Schmidinger M et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2019;30:706–720. [DOI] [PubMed] [Google Scholar]
- 26. Albiges L, Powles T, Staehler M et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune checkpoint inhibition is the new backbone in first‐line treatment of metastatic clear‐cell renal cell carcinoma. Eur Urol 2019;76:151–156. [DOI] [PubMed] [Google Scholar]
- 27. Hirsch L, Flippot R, Escudier B et al. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs 2020;80:1169–1181. [DOI] [PubMed] [Google Scholar]
- 28. Choueiri TK, Albiges L, Powles T et al. A phase III study (COSMIC‐313) of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in patients (pts) with previously untreated advanced renal cell carcinoma (aRCC) of intermediate or poor risk. J Clin Oncol 2020;38:TPS767a. [Google Scholar]
- 29. Rossi G, Pezzuto A, Sini C et al. Concomitant medications during immune checkpoint blockage in cancer patients: Novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol 2019;142:26–34. [DOI] [PubMed] [Google Scholar]
- 30. Chalabi M, Cardona A, Nagarkar DR et al. Efficacy of chemotherapy and atezolizumab in patients with non‐small‐cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020;31:525–531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information


