Abstract
Pyoderma gangrenosum manifests as an ulceration of the skin often associated with several systemic diseases. The diagnosis is usually made by exclusion criteria with suggestions made by clinical findings and histological features. It can occur any site but more common in the legs. Advances in translational medicine led to the development of new forms of therapy in chronic inflammatory diseases by the oral administration of Janus kinase inhibitors. We report two cases of chronic ulceration of the skin consistent with the diagnosis of Pyoderma Gangrenosum that went into complete remission after the use of baricitinib.
Keywords: Pyoderma gangrenosum, Treatment, Baricitinib
Highlights
-
•
Advances in cytokine signalling through translational intracellular pathways led to the development of Janus kinases critical for a large family of cytokines.
-
•
This new category of drugs may have potential role for the treatment of chronic immune inflammatory disorder.
-
•
Treatment of Pyoderma Gangrenosum may be successful with the Janus kinase inhibitor Baricitinib.
1. Introduction
Pyoderma gangrenosum (PG) is an uncommon cutaneous condition with a challenging diagnosis, given its multifactorial pathogenesis [1,2]. In this research communication, we report on the first successful treatment of two PG patients with the novel JAK inhibitor, baricitinib.
1.1. Case report
A 71-year-old woman with remitting multi-resistant tender papules with a metameric disposition was initially treated as herpes zoster. She later developed a chronic ulcer on the scalp that, after investigation, was indicative of pyoderma gangrenosum (PG) or pyoderma-like. She had a previous history of pulmonary tuberculosis and PG on both legs in 2003. She was treated with cyclosporine, infliximab, clofazimine, doxycycline, and intralesional triamcinolone injections with varying results for over four years. PG was finally controlled in mid-2007. She remained asymptomatic until March 2020, when intense and sudden pain on the vertex and left parietal region developed. Multiple tender papules with peripheral erythema were present. Although no vesicles were found, a tentative diagnosis of cephalic herpes zoster was suspected. Despite treatment with valacyclovir and pregabalin, symptoms increased, and papules evolved into multiple, deep, ulcerated lesions with a central necrotic scab and undermining border. Expression yielded a significant amount of purulent secretion. Skin biopsy findings were of an ulcer with exuberant granulation tissue. Histology study was performed on the material obtained while curetting one of the large abscesses rather than by a wedge excision of the border. Although the vertex aspect on the scalp suggested an erosive pustular dermatosis of the scalp, histology was more consistent with PG. Attempts to obtain microbiological cultures were twice negative.
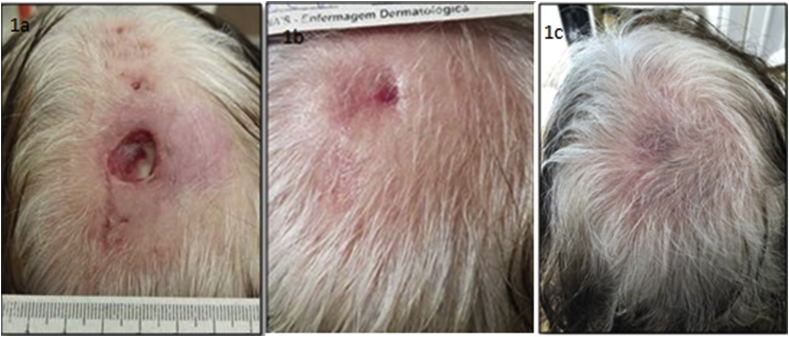
Previous comorbidities include IgA multiple myeloma in remission for the past seven years and remission of pulmonary tuberculosis treated with triple therapy 18 years ago. In the process of choosing a proper therapy, the Quantiferon test was performed, which was negative. After a short period of use of oral Dapsone, the disease progressed. Due to the previous history of tuberculosis and possible contra-indications for anti-TNF alpha therapy, she was started on baricitinib, a novel Janus kinase (JAK) inhibitor, 4 mg orally daily, taking into consideration the past attempts with more conservative approaches mentioned above. The idea of using JAK inhibitors was considered in the case of failed treatments, which proved helpful. Lesions started to improve, and by seven days, no new lesions were identified. Complete regression was achieved after five weeks, leaving a depressed scar (Fig. 1, Fig. 2).
Fig. 1.
1a, 1b and 1c: Gradual reduction on the ulcer size, and complete healing.
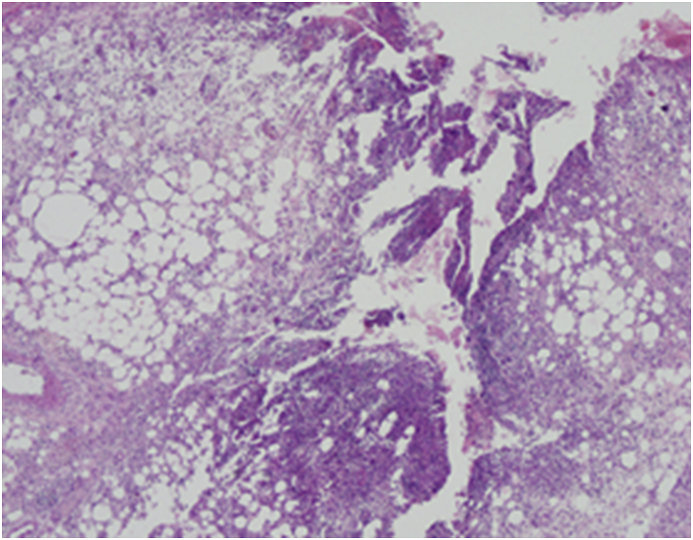
Fig. 2.
Ulcerated diffused neutrophilic inflammation involving the whole dermis and achieving the hypodermis (HE 40X tif).
Case 2 A 59-year-old female with rheumatoid arthritis since 2011, controlled with methotrexate 15 mg/week, developed in 2018 a leg skin ulcer for which PG was diagnosed. Healing was obtained with dressings and MTX maintenance.
In 2019, another PG occurred on the left leg and ankle; treatment consisted of prednisone 0.7 mg/kg/d associated with MTX 20 mg/week. PG resolved in 4 months; corticotherapy was gradually tapered, and MTX was maintained. Severe Cushing’s syndrome signs were noted, impairing quality of life.
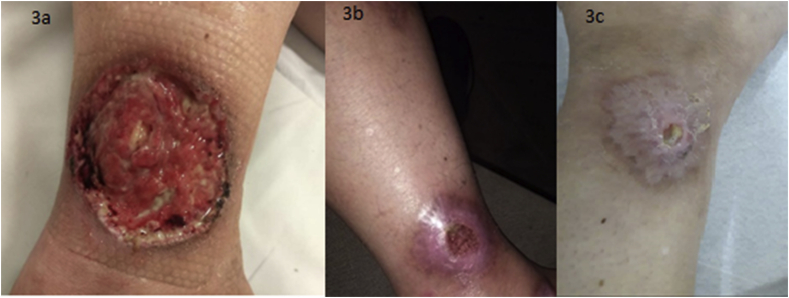
In 2020, another outbreak of PG compromised the right leg; methotrexate was suspended, and baricitinib 4 mg/day was introduced, with the lesion’s healing in 3 months presented in the photos (Fig. 3, Fig. 4).
Fig. 3.
3a, 3b and 3c: Gradual decrease and complete healing after Baricitinib.
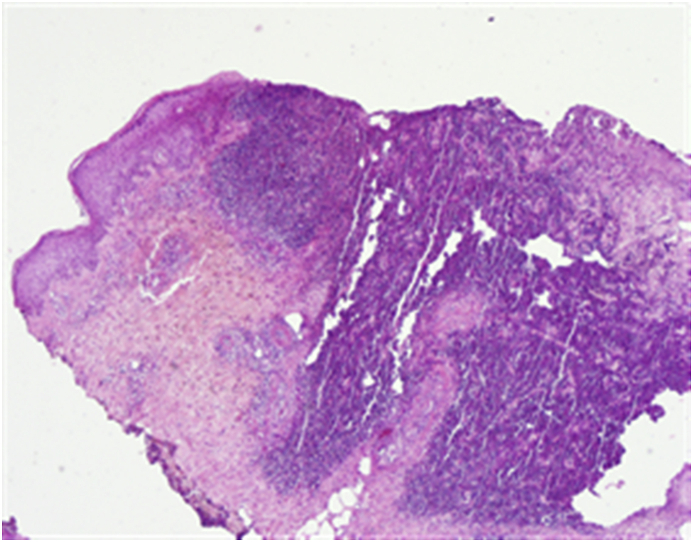
Fig. 4.
Skin biopsy pathology, inflammatory infiltrate in the derma and hypoderma with neutrophil accumulation, and small vessel necrosis, consistent with PG (HE 40X tif).
2. Discussion
PG is uncommon, challenging to treat with no gold standard therapy. The literature is mainly restricted to case reports, case series, and rare randomized clinical trials.
PG is currently a “diagnosis of exclusion,” where attempts to improve diagnostic accuracy has been addressed in international experts’ consensus and validated diagnostic criteria for ulcerative PG [3,4]. In our cases, in which the patient lacked a major diagnostic criterion (biopsy of ulcer edge demonstrating neutrophilic infiltrate), PG diagnosis was based on the presence of several minor criteria mentioned in the consensus, namely: (a) history of papule, pustule, or vesicle ulcerating within four days of appearance; (b) peripheral erythema, undermining border, and tenderness at ulceration site; (c) multiple ulcerations, at least one on an anterior lower leg; (d) cribriform or “wrinkled paper” scar(s) at healed ulcer sites; and (e) decreased ulcer size within one month of initiating immunosuppressive medication(s). In this study, 4 of 8 minor criteria maximized discrimination, yielding sensitivity and specificity of 86% and 90%, respectively.
There is growing evidence on the efficacy of small-molecule Janus kinase inhibitors on the treatment of immune skin disorders by its effect of transduction of intracellular signals from cell surface receptors from various immune-inflammatory cytokines, Baricitinib acts as an oral anticytokine therapy by inhibiting Janus kinase activity [[5], [6], [7]]. In a recent paper Baricitinib was shown to affect other immunoinflammatory diseases resistant to steroids. The lack of response observed in our patient and the growing concept that PG is an autoimmune condition where several uncontrolled/untreated comorbidities may serve as a trigger opens the possibility that Baricitinib should be expanded to PG refractory cases where steroids or anti-cytokine therapy fails or is contraindicated [8]. Finally, one of our patients had hematological malignancy and the other rheumatoid arthritis. Both conditions have been reported to have an increased association with the development of PG in recent reports [9,10]. Patients with rheumatoid arthritis have an increase in the odds to develop PG by threefold, and patients with malignant monoclonal antibody disease are listed as one of the hematological malignancies associated with PG, raised the possibility that skin lesions PG or PG like, rheumatoid arthritis or hematological malignancies should be suspected as an associated disease.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Teagle A., Hargest R. Management of pyoderma gangrenosum. J. R. Soc. Med. 2014;107(6):228–236. doi: 10.1177/0141076814534407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marzano A.V., Damiani G., Ceccherini I., Berti E., Gattorno M., Cugno M. Autoinflammation in pyoderma gangrenosum and its syndromic form(pyoderma gangrenosum) acne and suppurative hidradenitis) Br. J. Dermatol. 2017;176:228–236. doi: 10.1111/bjd.15226. [DOI] [PubMed] [Google Scholar]
- 3.Maverakis E., Ma C., Shinkai K., Fiorentino D., Callen J.P., Wollina U., Marzano A.V., Wallach D., Kim K., Schadt C., Ormerod A., Fung M.A., Steel A., Patel F., Qin R., Craig F., Williams H.C., Powell F., Merleev A., Cheng M.Y. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018 Apr 1;154(4):461–466. doi: 10.1001/jamadermatol.2017.5980. PMID: 29450466. [DOI] [PubMed] [Google Scholar]
- 4.Alavi A., French L.E., Davis M.D., Brassard A., Kirsner R.S. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am. J. Clin. Dermatol. 2017 Jun;18(3):355–372. doi: 10.1007/s40257-017-0251-7. PMID: 28224502. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie F., Cash D., Gupta A., Cummings L.W., Ortega-Loayza A.G. Biologic and small-molecule medications in the management of pyoderma gangrenosum. J. Dermatol. Treat. 2019 May;30(3):264–276. doi: 10.1080/09546634.2018.1506083. [DOI] [PubMed] [Google Scholar]
- 6.Cinats A., Heck E., Robertson L. Janus kinase inhibitors: a review of their emerging applications in dermatology. Skin Therapy Lett. 2018 May;23(3):5–9. PMID: 29772037. [PubMed] [Google Scholar]
- 7.Scheinberg M., Maluf F.C., Wagner J. Steroid-resistant sarcoidosis treated with baricitinib. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217271. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Ahn C., Negus D., Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expet Rev. Clin. Immunol. 2018 Mar;14(3):225–233. doi: 10.1080/1744666X.2018.1438269. [DOI] [PubMed] [Google Scholar]
- 9.Montagnon C.M., Fracica E.A., Patel A.A., Camilleri M.J., Murad H., Dingli D., Wetter D.A., Tolkachjov S.N. Pyoderma Gangrenosum in hematological malignancies. J. Am. Acad. Dermatol. 2020 Jun;82(6):1346–1359. doi: 10.1016/j.jaad.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Kridrin K., Damiani G., Cohen A.D. Rheumatoid arhtirtis and pyoderma gangrenosum : a population-based case control study. Clin. Rheumatol. 2021;40:521–528. doi: 10.1007/s10067-020-05253-7. [DOI] [PubMed] [Google Scholar]