Abstract
Objectives:
Cutaneous leishmania sis (CL) is considered as one of the most critical infections worldwide, in which the protozoa of the genus Leishmania infects a person. Today, the common and selective drugs for the treatment of CL are antimonial compounds present some limitations to their usage. The objective of this study is to investigate the cytotoxic and antileishmanial effects of various extracts of Capparis spinosa L. on the in vitro model.
Materials and Methods:
The primary phytochemical analysis of the C. spinosa extracts was performed to assess the presence of tannins, alkaloids, saponins, flavonoids, terpenoids, and glycosides. Furthermore, the in vitro cytotoxic and antileishmanial effects of C. spinosa extracts on Leishmania tropica promastigote were evaluated. Additionally, these effects on the J774-A1 macrophage cells by colorimetric cell viability 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide assay were also assessed.
Results:
In this study, the findings of primary phytochemical screening of the C. spinosa extracts demonstrated the existence of flavonoids, tannins, terpenoids, glycosides, and alkaloids in this plant. Importantly, the findings indicated that the aqueous and methanolic extracts of C. spinosa exhibit a high potency to inhibit the growth of L. tropica promastigotes with inhibitory concentration 50 values of aqueous and methanolic extracts being 28.5 and 44.6 μg/mL, respectively. Based on the obtained results, C. spinosa extracts did not display a considerable cytotoxicity on the J774-A1 macrophage cells.
Conclusion:
The obtained findings exhibited remarkable antileishmanial effects of C. spinosa extracts on L. tropica, thereby indicating the ability of C. spinosa as a herbal product to be developed as a new antileishmanial drug. Nevertheless, supplementary investigations will be obligatory to achieve these findings, especially in human subjects.
Keywords: Herbal medicines, in vitro, Leishmania tropica, macrophage, promastigote
INTRODUCTION
In present times, cutaneous leishmaniasis (CL) is one of the main parasitic infections worldwide, in which human beings are infected by the Leishmania protozoan parasites. The most important characteristics of this disease are chronic and prolonged ulcers that leave scars even after recovery.1 Every year, about 1.5 million people become infected with this disease; hence, it can be considered as a main health and economic challenge.2 Previous studies have demonstrated that in Iran, the common types of CL are anthroponotic CL (Leishmania tropica) and zoonotic CL (L. major).3
Today, the common and selective chemotherapies for CL treatment are antimonial compounds such as meglumine antimoniate and sodium stibogluconate; however, recent studies have suggested some restrictions about the use of these drugs such as excessive side effects and parasitic resistance to these agents.4,5 Therefore, it is highly believed that the discovery of a new drug with same efficacy to the current agents and even higher than them along with lower toxicity can be a priority for researchers.
From centuries ago, the use of natural compounds has been considered for the treatment of several diseases such as infectious ones.6,7 Capparis spinosa L. from the family of Capparidaceae, which is called “Kabar” in Persian, widely grows in the various parts of the world, especially in Iran. Previous studies have shown that various parts of this plant represent some biological and medicinal effects such as antimicrobial, antioxidant, and anticancer activities.8 Therefore, the objective of this study is to investigate the in vitro cytotoxic and the leishmanicidal activities of extracts of C. spinosa.
MATERIALS AND METHODS
Parasite strain
Here, we obtained the L. tropica (MHOM/IR/2002/Mash2) strain from the Leishmaniasis Research Center (Kerman, Iran). The promastigotes were cultured in the NNN medium, and then subcultured in RPMI-1640, complemented with penicillin (200 IU/mL), streptomycin (100 µg/mL), and 15% heat-inactivated fetal calf serum.
Collection of plant materials
We collected the aerial parts of C. spinosa from the mountains of Lorestan Province, Iran. The materials were recognized by a botanist, and a voucher specimen was deposited at the herbarium of Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran.
Preparation of extracts
After chopping the fruits into smaller portions and drying them in shade, the fruits were powdered. Afterward, the powdered materials were extracted using the technique of percolation with methanol and water for 3 days at 21°C. The obtained extracts were allowed to pass through a filter paper to remove the excess particles. Finally, by means of a rotary evaporator (Heidolph, Germany), the extracts were vacuum concentrated at 50°C and kept at -20°C until testing.9,10,11
Phytochemical analysis
The primary phytochemical analysis of the both C. spinosa extracts was conducted to assess the presence of tannins, alkaloids, flavonoids, saponins, terpenoids, and glycosides via following reagents and chemicals:12 Alkaloids with Mayer and Dragendorff’s reagents, flavonoids by using Mg and HCl, tannin with 1% gelatin and 10% NaCl solutions, terpenoids with chloroform, and concentrated sulfuric acid, glycosides with FeCl2 and H2SO4, and saponin with the ability of producing suds.
Antileishmanial effects of C. spinosa extracts
To determine the antileishmanial effects of C. spinosa extracts, we used the colorimetric cell viability 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method as explained by some researchers.13,14,15 After adjusting the promastigotes from the logarithmic growth phase to 106 cells per each mL, 0.1 mL of suspension of promastigotes was put in a 96-well plate. In the next step, promastigotes were treated with the various concentrations of each plant extract (0-200 µg/mL) at 25°C±1°C for 3 days. After finishing the exposure time, 0.01 ml of MTT solution (5 mg/mL) was poured into wells and again incubated at 25°C for 4 hours. Next, to solve the formazan crystals and subsequently generate the purple color, 0.1 mL of isopropanol was added into wells. At the end, an ELISA reader (BioTek-ELX800) was used and the absorbance level of wells was determined at 490 nm. The complete medium containing promastigote and no extract was considered as a positive control, whereas a complete medium with no parasite and extract considered as a negative control (blank).
Cytotoxic effects
To assess the cytotoxic effects, the J774-A1 cells cultured at Dulbecco’s modified eagle’s medium were adjusted at 5x105 cell per mL. Then, they were treated in 96-well plates with different concentrations of each extract (0-5.000 µg/mL) at 37°C in 5% CO2 for 48 hours. Finally, the cytotoxic effects of extracts were measured by the colorimetric MTT assay as mentioned above.15,16,17
Statistical analysis
We performed experiments in triplicates. The collected data were analyzed by SPSS software version 22.0. Moreover, [cytotoxic concentration for 50% (CC50)of macrophages] and inhibitory concentration 50 (IC50) (50% ICs for promastigotes) were measured by the linear regression method. Furthermore, the selectivity index (SI) was measured as the equation of CC50 for J774-A1/IC50 for promastigotes to assess the toxicity and activity of C. spinosa extracts. Additionally, One-Way ANOVA test was applied to assess the variations among the test and control groups. Furthermore, p<0.05 was considered to be statistically significant for this study.
RESULTS
Phytochemical analysis
In this study, the findings referred to the primary phytochemical screening of the C. spinosa methanolic and aqueous extracts demonstrated the presence of tannins, flavonoids, terpenoids, glycosides, and alkaloids in this plant.
Antileishmanial effects of C. spinosa extracts
Figure 1 shows the antileishmanial effects of different extracts of C. spinosa on L. tropica promastigote. The obtained findings showed that different extracts of C. spinosa, mostly methanolic extract, displayed effective antileishmanial effects on L. tropica promastigote in a dose-dependent manner (p<0.05). The obtained IC50 values of aqueous and methanolic extracts on L. tropica promastigote were 28.5 and 44.6 µg/mL, respectively. Meglumine antimoniate also as control drug revealed effective antileishmanial effects with the IC50 value of 35.7 µg/mL on L. tropica promastigotes.
Figure 1.
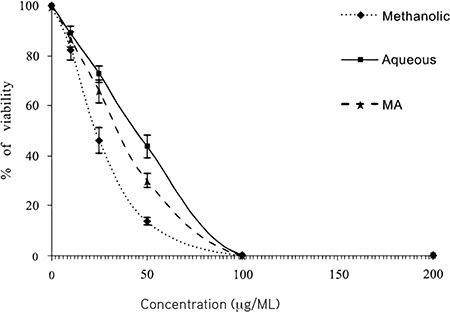
Antileishmanial effects of various extracts of Capparis spinosa on the viability rate of Leishmania tropica promastigote. Data are expressed as mean ± SD (n=3)
SD: Standard deviation
Cytotoxic activity
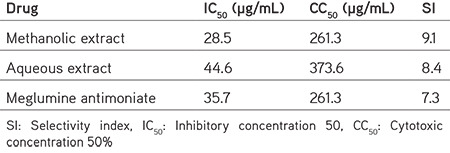
Based on the obtained results, C. spinosa extracts did not display considerable cytotoxicity on the J774-A1 macrophage cells. As shown in Table 1, the CC50 values of aqueous and methanolic extracts of C. spinosa on J774-A1 macrophage cells were 261.3 and 373.6 µg/mL, respectively. Table 1 presents the SI values of different extracts of C. spinosa.
Table 1. CC50 values of various extracts of Capparis spinosa on the J774-A1 macrophage cells as well as their IC50 and selectivity index values on Leishmania tropica promastigotes.
DISCUSSION
Since long ago, herbal medicines have been recognized as one of the main therapeutic agents worldwide. In recent years, the therapeutic and preventive use of medicinal plants has attracted increased attention because of low post-consumption complications and the various biological properties.18,19,20
So far, researchers have demonstrated the antileishmanial effects of a broad spectrum of medicinal herbs, such as black cumin, garlic, savory, pistacia, berberis, myrtle, periwinkle, black beans, and others, on CL.21 Although previous investigations have reported a number of pharmacological benefits of C. spinosa such as antioxidant, anticancer, and antibacterial activities, there is no documented study regarding the antiparasitic effects of this plant. Thus, we decided to investigate the in vitro antileishmanicidal and cytotoxic activities of C. spinosa extracts. The results revealed that different extracts of C. spinosa, mostly methanolic extract, displayed effective antileishmanial effects on L. tropica promastigote in a dose-dependent manner (p<0.05). The obtained IC50 values for methanolic and aqueous extracts on L. tropica promastigote were 28.5 and 44.6 µg/mL, respectively.
In this study, the results of the primary phytochemical analysis of the C. spinosa extracts indicated the existence of tannins, flavonoids, terpenoids, glycosides, and alkaloids in this plant. Previous studies on phytochemical analysis C. spinosa have proven that this plant contains high amounts of bioactive components, such as alkaloids, flavonoids, steroids, terpenoids, and tocopherols.8 Moreover, a study conducted by Tlili et al.22 on the phytochemical analysis of C. spinosa showed that aerial parts of this plants are rich in quaternary ammonium compounds, alkaloids, phenolic compounds, and glycosides, such as glucosinolates, further indicating various pharmacological properties useful in modern medicine.
Regarding the antileishmanial effects of polyphenolic compounds, Antwi et al.23 demonstrated that rosmarinic acid (as a phenolic compound) exerted antileishmanial effect through iron chelation that results in the morphological changes and cell cycle arrest against the promastigote and intracellular amastigote forms of L. donovani. Monzote et al.24 demonstrated the potent antileishmanial activity of ten phenolic compounds including cinnamic acid, coumaric acid isomers, gallic acid, sinapic acid, gentisic acid, morin, rutin extrasynthese, and ellagic acid, vanillic acid against intracellular amastigotes as well as experimental CL in BALB/c mice infected with L. amazonensis.
Regarding antileishmanial activity of alkaloids, Delorenzi et al.25 showed that indole alkaloid coronaridine have shown considerable antileishmanial effects, which led to the growth of promastigote and amastigote forms. Through change in their mitochondrial functions. Tasdemir et al.26 also demonstrated that some flavonoid compounds exert potent antileishmanial and antitrypanosomal effects against Trypanosoma brucei rhodesiense, Trypanosoma cruzi, and L. donovani in vitro and in vivo.
Arruda et al.27 demonstrated that nerolidol as a sesquiterpene (terpenoids) prevented the growth of L. amazonensis, L. braziliensis, L. chagasi promastigotes, and L. amazonensis amastigotes with IC50 values of 85, 74, 75, and 67 µM, respectively; whereas a reduction of lesion sizes was observed in L. amazonensis-infected BALB/c mice treated with nerolidol. Considering the mechanisms of the antimicrobial action of polyphenolic compounds, some studies have shown that antimicrobial mechanisms of polyphenolic compounds are associated with their lipophilia as well as their effects on protein synthesis.28,29,30,31 Previously, Puupponen-Pimiä et al.32 have shown that polyphenolic compounds, through their disruptive action on the external membrane, can inhibit the growth of bacteria.32,33,34 Therefore, although the accurate antileishmanial mechanisms of C. spinosa is unclear, we can suggest that antiparasitic effects of this plant is referred to the existence of polyphenolic compounds in it. Here, we found that C. spinosa extracts did not display considerable cytotoxicity on the J774-A1 macrophage cells; moreover, the SI values above ten of methanolic and aqueous extracts of C. spinosa revealed their immunity against the macrophages and specificity to the parasite, according to Weninger et al.35,36
CONCLUSION
The obtained findings exhibited remarkable antileishmanial effects of C. spinosa extracts on L. tropica, thereby indicating the ability of C. spinosa as a natural ingredient to create a new antileishmanial drug. Nevertheless, supplementary investigations will be obligatory to achieve these findings, especially in human subjects.
Footnotes
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of the paper.
References
- 1.World Health Organization. Control of the Leishmaniasis. Vol. 949. Geneva, Switzerland: WHO. 2010. [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immun Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi F, Sharifi I, Zarean M, Parizi MH, Aflatoonian M, Harandi MF, Zahmatkesh R, Mashayekhi M, Kermanizadeh A. Spatial distribution and molecular identification of Leishmania species from endemic foci of South-eastern Iran. Iran J Parasitol. 2012;7:45–52. [PMC free article] [PubMed] [Google Scholar]
- 4.Santos DO, Coutinho CE, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC. Leishmaniasis treatment-a challenge that remains: a review. Parasitol Res. 2008;103:1–10. doi: 10.1007/s00436-008-0943-2. [DOI] [PubMed] [Google Scholar]
- 5.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:11–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasoulian B, Kheirandish F. Herbal medicines: from traditional medicine to modern experimental approaches. Herb Med J. 2017;2:1–2. [Google Scholar]
- 7.Saedi Dezaki E, Mahmoudvand H, Sharififar F, Fallahi S, Monzote L, Ezatkhah F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm Biol. 2016;54:752–758. doi: 10.3109/13880209.2015.1079223. [DOI] [PubMed] [Google Scholar]
- 8.Nabavi SF, Maggi F, Daglia M, Habtemariam S, Rastrelli L, Nabavi SM. Pharmacological Effects of Capparis spinosa L. Phytother Res. 2016;30:1733–1744. doi: 10.1002/ptr.5684. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoudvand H, Asadi A, Harandi MF, Sharififar F, Jahanbakhsh S, Dezaki ES. In vitro lethal effects of various extracts of Nigella sativa seed on hydatid cyst protoscoleces. Iran J Basic Med Sci. 2014;17:1001–1006. [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoudvand H, Sharififar F, Rahmat MS, Tavakoli R, Dezaki ES, Jahanbakhsh S, Sharifi I. Evaluation of antileishmanial activity and cytotoxicity of the extracts of Berberis vulgaris and Nigella sativa against Leishmania tropica. J Vector Borne Dis. 2014;51:294–299. [PubMed] [Google Scholar]
- 11.Mahmoudvand H, Saedi Dezaki E, Sharififar F, Ezatpour B, Jahanbakhsh S, Fasihi Harandi M. Protoscolecidal effect of Berberis vulgaris root extract and its main compound, berberine in cystic echinococcosis. Iran J Parasitol. 2014;9:503–510. [PMC free article] [PubMed] [Google Scholar]
- 12.Trease GE, Evans WC. Pharmacognosy. 15th ed. Saunders Publishers, London. 2002:42–44. [Google Scholar]
- 13.Mahmoudvand H, Kheirandish F, Mirbadie SR, Kayedi MH, Rezaei Riabi T, Ghasemi AA, Bamorovat M, Sharifi I. The potential use of methotrexate in the treatment of cutaneous leishmaniasis: in vitro assays against sensitive and meglumine antimoniate-resistant strains of Leishmania tropica. Iran J Parasitol. 2017;12:339–347. [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoudvand H, Shakibaie M, Tavakoli R, Jahanbakhsh S, Sharifi I. In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucan-time-resistant Leishmania tropica. Iranian J Parasitol. 2014;9:452–460. [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoudvand H, Saedi Dezaki E, Ezatpour B, Sharifi I, Kheirandish F, Rashidipour M. In vitro and in vivo antileishmanial activities of Pistacia vera essential oil. Planta Med. 2016;82:279–284. doi: 10.1055/s-0035-1558209. [DOI] [PubMed] [Google Scholar]
- 16.Kheirandish F, Delfan B, Mahmoudvand H, Moradi N, Ezatpour B, Ebrahimzadeh F, Rashidipour M. Antileishmanial, antioxidant, and cytotoxic activities of Quercus infectoria Olivier extract. Biomed Pharmacother. 2016;82:208–215. doi: 10.1016/j.biopha.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki ES. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. Korean J Parasitol. 2015;53:21–27. doi: 10.3347/kjp.2015.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoudvand H, Mirbadie SR, Sadooghian S, Harandi MF, Jahanbakhsh S, Saedi Dezaki E. Chemical composition and scolicidal activity of Zataria multiflora Boiss essential oil. J Essential Oil Res. 2017;29:42–47. [Google Scholar]
- 19.Mahmoudvand H, Mahmoudvand H, Oliaee RT, Kareshk AT, Mirbadie SR, Aflatoonian MR. In vitro protoscolicidal effects of cinnamomum zeylanicum essential oil and its toxicity in mice. Pharmacogn Mag. 2017;13:S652–S657. doi: 10.4103/pm.pm_280_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudvand H, Mirbadie SR, Ghasemi Kia M, Badparva E, Shamsadini Lori S, Fasihi Harandi M. Efficacy of pistacia khinjuk fruits on viability of hydatid cyst protoscoleces and its acute toxicity in mice model. Iran J Parasitol. 2016;11:383–388. [PMC free article] [PubMed] [Google Scholar]
- 21.Bahmani B, Saki K, Ezatpour B, Shahsavari S, Eftekhari Z, Jeldori M, Kopaei MR, Sepahvand R. Leishmaniosis phytotherapy: review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pacific J Trop Biomed. 2015;5:695–701. [Google Scholar]
- 22.Tlili N, Elfalleh W, Saadaoui E, Khaldi A, Triki S, Nasri N. The caper (Capparis L.): ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia. 2011;82:93–101. doi: 10.1016/j.fitote.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Antwi CA, Amisigo CM, Adjimani JP, Gwira TM. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLoS Negl Trop Dis. 2019;13:e0007206. doi: 10.1371/journal.pntd.0007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monzote L, Perera Córdova WH, García1 M, Piñón A, Setzer WN. In-vitro and In-vivo Activities of Phenolic Compounds Against Cutaneous Leishmaniasis. Rec Nat Prod. 2016;10:269–276. [Google Scholar]
- 25.Delorenzi JC, Attias M, Gattass CR, Andrade M, Rezende C, da Cunha Pinto A, Henriques AT, Bou-Habib DC, Saraiva1 EMB. Antileishmanial Activity of an Indole Alkaloid from Peschiera australis. Antimicrob Agents Chemother. 2001;45:1349–1354. doi: 10.1128/AAC.45.5.1349-1354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, Rüedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arruda DC, D’Alexandri FL, Katzin AM, Uliana SR. Antileishmanial activity of the terpene nerolidol. Antimicrob Agents Chemother. 2005;49:1679–1687. doi: 10.1128/AAC.49.5.1679-1687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucchini JJ, Corre J, Cremieux A. Antibacterial activity of phenolic compounds and aromatic alcohols. Res Microbiol. 1990;141:499–510. doi: 10.1016/0923-2508(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 29.Tavakoli Kareshk A, Keyhani A, Mahmoudvand H, Tavakoli Oliaei R, Asadi A, Andishmand M, Azzizian H, Babaei Z, Zia-Ali N. Efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice model. Iran J Parasitol. 2015;10:625–631. [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoudvand H, Tavakoli Oliaei R, Mirbadie SR, Kheirandish F, Tavakoli Kareshk A, Ezatpour B, Mahmoudvand H. Efficacy and safety of bunium persicum (Boiss) to inactivate protoscoleces during hydatid cyst operations. Surg Infect (Larchmt). 2016;17:713–719. doi: 10.1089/sur.2016.010. [DOI] [PubMed] [Google Scholar]
- 31.Niazi M, Saki M, Sepahvand M, Jahanbakhsh S, Khatami M, Beyranvand M. In vitro and ex vivo scolicidal effects of Olea europaea L. to inactivate the protoscolecs during hydatid cyst surgery. Ann Med Surg (Lond). 2019;42:7–10. doi: 10.1016/j.amsu.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A, Oksman-Caldentey KM. Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 33.Kar N, Ghosh S, Kumari L, Chakraborty S, Bera T. Search for new antileishmanial chemotherapeutics. Inter J Pharm Pharm Sci. 2018;10:46–52. [Google Scholar]
- 34.Kaur R, Kaur J, Kaur S, Joshi J. Evaluation of the antileishmanial efficacy of medicinal plant Chenopodium album Linn. against experimental visceral leishmaniasis. Inter J Pharm Pharm Sci. 2016;7:227–231. [Google Scholar]
- 35.Weninger B, Robledo S, Arango GJ, Deharo E, Arango R, Munoz V, Callapa J, Lobstein A, Anton R. Antiprotozoal activities of Colombian plants. J Ethnopharmacol. 2001;78:193–200. doi: 10.1016/s0378-8741(01)00346-4. [DOI] [PubMed] [Google Scholar]
- 36.Ezatpour B, Saedi Dezaki E, Mahmoudvand H, Azadpour M, Ezzatkhah F. In vitro and in vivo antileishmanial effects of Pistacia khinjuk against Leishmania tropica and Leishmania major. Evid Based Complement Alternat Med. 2015;2015:149707. doi: 10.1155/2015/149707. [DOI] [PMC free article] [PubMed] [Google Scholar]


