Abstract
Objectives:
In Turkey, the genus Moltkia Lehm. is represented by two species, namely Moltkia aurea Boiss. and M. coerulea (Willd.) Lehm., which are used as both food and for medicinal purposes. This study aimed to evaluate the antidiabetic and antioxidant potential and phytochemical profiles of leaf, flower, and root extracts of Moltkia species.
Materials and Methods:
α-Glucosidase and α-amylase inhibitory activities, antioxidant effects, and total phenol and flavonoid contents of Moltkia extracts were evaluated. High-performance liquid chromatography was performed for identifying and quantifying phenolic compounds, which are responsible for various activities of these extracts.
Results:
Among the investigated phenolic compounds, caffeic and rosmarinic acids and rutin were determined and quantified in methanol extracts. Rutin was the major compound in the flower extract of M. aurea. Rutin and rosmarinic acid were the major compounds in the leaf extract of M. aurea. The flowers, leaves and roots of M. coerulea were also rich in rosmarinic acid. The antioxidant and antidiabetic potential of these extracts may be attributable to their rutin and rosmarinic acid content.
Conclusion:
Moltkia species can be used as natural sources of antioxidants. Notably, M. aurea extracts can be used for the development of herbal products with antidiabetic potential.
Keywords: Moltkia, Boraginaceae, antidiabetic, antioxidant, HPLC, phenolic compounds
INTRODUCTION
The Boraginaceae family is distributed in tropical, subtropical, and temperate areas of the world and comprises approximately 2500 species.1 Moltkia, Heliotropium, Cordia, Arnebia, Echium, and Onosma are some of the important genera of Boraginaceae. Most members of this family are medicinally important plants that contain secondary metabolites, including flavonoids, terpenoids, alkaloids, fatty acids, glycosides, and phytosterols, and various proteins.2,3
Moltkia comprises five species, all of which grow in the Eastern part of the Mediterranean region. Moltkia coerulea (Willd.) Lehm. is endemic to Anatolia, Lebanon, and the Crimea; M. aurea Boiss. is endemic to Anatolia.4 Moltkia species of Turkey, known as “emzik cicegi, sancı out, sormuk, sarı kesen”, are traditionally used for various health problems, such as kidney disorders, diarrhea, and abdominal pain.5,6 In Sivas, leaves of M. coerulea are consumed as food; in Nigde, the sweet flowers of M. coerulea are eaten by children.7 Studies on Moltkia species of Turkey have revealed that these plants possess antioxidant, antibacterial, and cytotoxic activities.3,5
Inhibition of α-glucosidase in the bowel slows oligosaccharide degradation, thereby reducing the glucose level in the circulatory system. α-amylase degrades long-chain carbohydrates. Inhibition of such enzymes has been an important approach to decrease blood glucose levels and diabetes-related complications. Oxidative stress is an important determinant of diabetes-related complications, and the overproduction of free radicals is related to hyperglycemia.8 Although studies comparing anatomical and morphological features of M. aurea and M. coerulea growing in Turkey1 are available, only a few studies have examined the biological activity and phytochemistry of both species together.5,9 Therefore, we investigated the antidiabetic and antioxidant potential of flowers, leaves, and roots of Turkish Moltkia species and compared their phytochemical profiles.
MATERIALS AND METHODS
Plant material
Plants from Moltkia species were collected in the flowering stage from Eryaman-Ankara, Turkey in May, 2015. Plants were collected by N. Orhan and Ç. Orhan and identified by Assoc. Prof. Dr. N. Orhan. Voucher specimens GUEF 3239 and GUEF 3240 (M. aurea) and GUEF 3241 and GUEF 3242 (M. coerulea) were deposited at Gazi University’s Faculty of Pharmacy.
Extract preparation
Water extract: Dried and ground plant parts (leaf, flower, and root) were extracted with 50 mL hot water (4% w/v) on a heating-magnetic stirrer for 6 h and filtered. The residues were treated with 50 mL water using the same procedure. Filtered aqueous extracts were combined and freeze-dried.
Ethyl acetate and methanol (MeOH) extracts: Dried and powdered plant parts (leaf, flower, and root) were treated with 200 mL MeOH and ethyl acetate (2.5% w/v) on a shaker for 18 h at 25°C and filtered. This procedure was repeated two times; extracts were pooled and solvents were removed using a rotary evaporator. Extract yields are presented in Table 1.
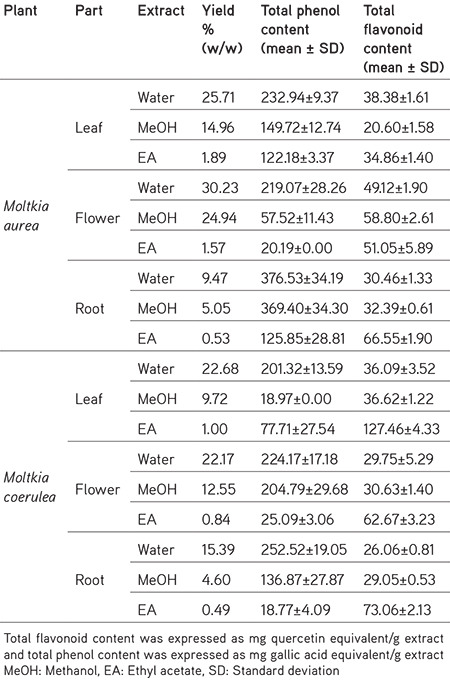
Table 1. Yield percentages and total phenol and flavonoid contents in Moltkia extracts.
Enzyme inhibitory activity
α-Amylase inhibitory activity
α-Amylase (porcine pancreatic, EC 3.2.1.1, Sigma) was dissolved in distilled water and added to plant extracts. Incubation was performed at 37°C for 3 min. Then, potato starch solution (0.5% w/v) was added to the mix and incubated at 37°C for 5 min. Subsequently, 3,5-dinitrosalicylic acid solution was added to the mix and incubated in an 85°C heater. Finally, distilled water was added, and the tubes were allowed to cool. Absorbance values were recorded at 540 nm. Acarbose was run as the reference.10 Absorbance (A) due to maltose formation was estimated according to the formula: AControl or sample =ATest - ABlank.
The quantity of maltose formation was assessed by using the maltose standard calibration curve (0-0.1% w/v) and the gained net absorbance value. Inhibition ratio was estimated as:
Inhibition % = [(Maltose Control − Maltose Sample) / Maltose Control)] × 100.
α-Glucosidase inhibitory activity
α-Glucosidase inhibitory potential of Moltkia samples was evaluated using the assay of Lam et al.11 α-Glucosidase (Sigma Co., St. Louis, USA) obtained from Bacillus stearothermophilus was dissolved in phosphate buffer (0.5 M) at pH 6.5. Extracts and enzyme solution were suspended in hydroalcohol (80%) and preincubated at 37°C. Then, 20 mM p-nitrophenyl-α-d-glucopyranoside (NPG, Sigma) was added as the substrate. Samples were incubated for nearly 30 min at 37°C. The difference in absorbance at 405 nm, due to the the hydrolysis of NPG by the enzyme, was quantified. The α-glucosidase inhibitor acarbose (Bayer Group, Turkey) was chosen as a reference. The inhibition ratio (%) was estimated using the following equation:
Inhibition % =[(AControl - ASample) / AControl] × 100
Antioxidant activity
Radical [1.1-diphenyl-2-picrylhydrazy (DPPH)] scavenging activity
A total of 40 µL DPPH solution was vortexed with 160 µL of the extract and incubated in the dark. Then, absorbance was measured at 520 nm.12 All calculations were performed using the Softmax PRO 4.3.2.LS software. Butylated hydroxytoluene was used as a reference at 0.1, 0.3, and 1 mg/mL.
Superoxide anion scavenging activity
Superoxide radicals were generated in a nonenzymatic system. The reaction mixture, including 25-2000 µg/mL of the test fraction in 70°C ethanol, 1 mL 468 mmol/L β-NADH, 1 mL 60 mmol/L phenazine methosulphate (PMS), and 1 mL 150 mmol/L nitro blue tetrazolium chloride in phosphate buffer (0.1 mol/L, pH 7.4), was incubated. Absorbance was recorded at 560 nm against blank samples, which were free of PMS. Activity was estimated as scavenging activity (%) =[(AControl - ASample) / AControl] × 100, where AControl is the absorbance of the control and ASample is the absorbance of the extract.13,14 Quercetin was chosen as the reference at 0.1, 0.3, and 1 mg/mL.
Ferric-reducing antioxidant power
The extract or ascorbic acid was incubated with 0.2 mol/L phosphate buffer (pH 6.6) and K3Fe(CN)6. Trichloroacetic acid was added to the mixture, vortexed, and centrifuged. Water and ferric chloride were added to the supernatant and absorbance was measured at 700 nm.15 Ascorbic acid was used as reference.
Metal-chelating capacity
Extracts were incubated with FeCl2 (2 mM). The reaction was initiated after the addition of 0.2 mL of ferrozine (5 mM). After a while, absorbance was recorded at 562 nm.16 Ethylenediaminetetraacetic acid was used as a reference and FeCl2 and ferrozine as controls. Inhibition ratio of ferrozine-Fe+2 complex generation was estimated using the following formula: Metal-chelating activity (%) = [(AControl-ASample) / AControl] × 100.
Total antioxidant capacity
Molybdate reagent, water, and extracts were mixed, vortexed, incubated for 90 min at 95°C in test-tubes. Then, tubes were cooled and absorbance was recorded at 695 nm. Outcomes were shown as ascorbic acid equivalent in the extract (mg/g).17
Phytochemical content
Estimation of total phenol content
Extracts were mixed and incubated with the Folin-Ciocalteu reagent. Then, sodium carbonate solution was added to the mixture and immediately vortexed. Absorbance was measured at 735 nm after 30 min in the dark. Total phenol content of extracts in mg of gallic acid equivalent (GAE)18 was calculated using the following equation:
Absorbance=1.6342 × (conc.) + 1.417, r2 = 0.9986.
Determination of total flavonoid content
Extracts (1 mg/mL) were suspended in 80% ethanol. Sodium acetate, aluminum chloride solution, ethyl alcohol, and water were mixed and incubated with extracts for 30 min. Absorbance was measured at 415 nm. Outcomes in mg quercetin equivalent (QE)/g extract19 were calculated using the following equation: Absorbance=1.8934 × (conc.) - 0.025, and r2 value was 0.9996.
Qualitative and quantitative analyses of phenolic compounds using reverse phase-high-performance liquid chromatography (RP-HPLC)-photo diode array (PDA)
HPLC analyses were performed as described20,21 using the HP Agilent 1260 series LC system and ACE column (5 µm, 250 mm × 4.6 mm) at 30°C. The flow rate of the gradient elution was 0.8 mL/min. The mobile phase was a mixture of water (solution A), MeOH (solution B), and acetonitrile (solution C), each containing 0.1% trifluoroacetic acid. Caffeic and rosmarinic acids were analyzed at 330 nm and rutin at 360 nm by external standardization.
Statistical analysis
Experiments were performed in triplicate and mean values were obtained. All values are given as the mean ± standard deviation. Computations were performed using GraphPad InStat and Microsoft Excel.
RESULTS
Results of the total phenol content assay revealed that lyophilized water and MeOH extracts of all investigated parts of both Moltkia species were rich in total phenolics. Water and MeOH extracts of M. aurea roots had the highest total phenolic content (376.53±34.19 and 369.40±34.30 mg GAE/g extract, respectively). The highest amount of total flavonoid was recorded in the ethyl acetate extract of M. coerulea leaves (127.46±4.33 mg QE/g extract). In addition, amount of total flavonoid in the MeOH extract of M. aurea flowers was high (58.80±2.61 mg QE/g extract), which was consistent with results from HPLC analysis, indicating high rutin content. Extract yields and obtained results for total phenol and flavonoid content are presented in Table 1.
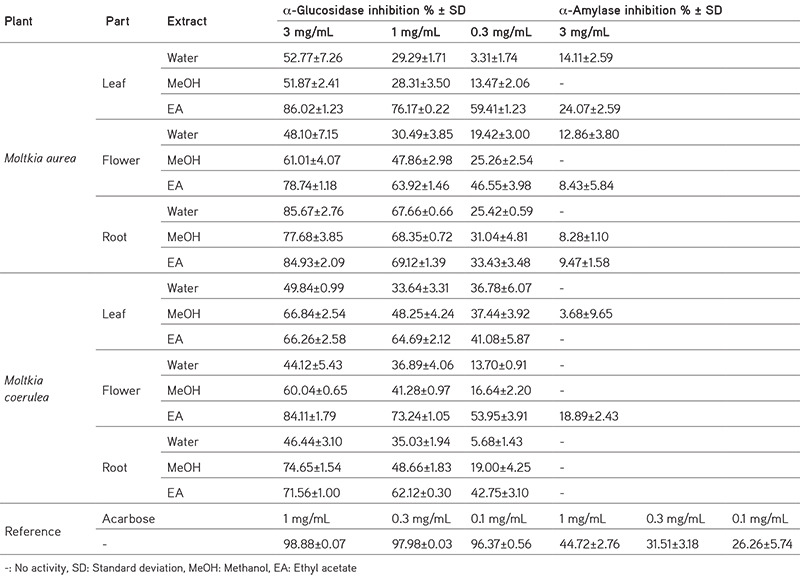
One approach for managing diabetes includes inhibiting α-amylase and α-glucosidase activity. In this study, Moltkia species displayed mild enzyme inhibitory activity compared to the reference acarbose (Table 2). Ethyl acetate and water extracts of M. aurea leaves inhibited α-amylase (24.07%±2.59% and 14.11%±2.59%, respectively) together with ethyl acetate extracts of M. coerulea flowers (18.89%±2.43%) at 3 mg/mL. MeOH and ethyl acetate extracts inhibited α-glucosidase activity more strongly than water extracts (Table 2).
Table 2. Enzyme inhibitory effects of Moltkia extracts.
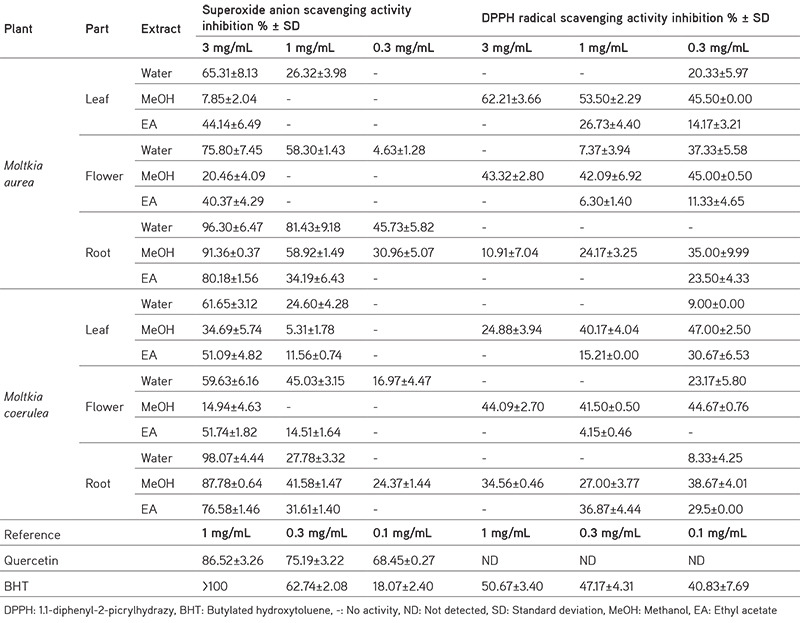
Plant extracts have excellent antioxidant activity.22 To evaluate the antioxidant potential of Moltkia species, various in vitro tests were performed. Analysis of total antioxidant capacity revealed that ethyl acetate extracts displayed significant antioxidant potential compared with extracts prepared using other solvents. Water, and especially MeOH, extracts exhibited significant ferric-reducing power compared to ethyl acetate extracts. All water extracts exhibited significant metal-chelating activity (Table 3). Although the superoxide-scavenging activity of water, MeOH, and ethyl acetate extracts of roots of both species were found to be promising, MeOH extracts of all samples scavenged the DPPH free radical significantly (Table 4).
Table 3. Metal-chelating activity, ferric-reducing power, and total antioxidant capacity of Moltkia extracts.
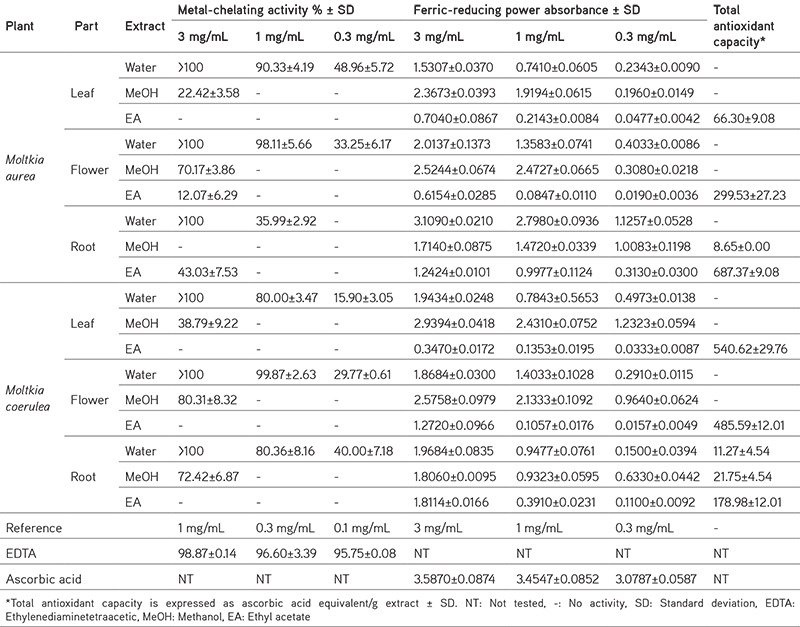
Table 4. Superoxide anion and DPPH radical scavenging activities of Moltkia extracts.
HPLC analyses revealed that among the investigated phenolics (Rt chlorogenic acid: 8.9 min, Rt caffeic acid: 12.3 min, Rt ferulic acid: 20.25 min, Rt rutin: 23.1 min, Rt rosmarinic acid: 30.6 min, Rt quercetin: 36.5 min, Rt luteolin: 37.3 min, and Rt apigenin: 40.3 min) only caffeic acid, rutin, and rosmarinic acid were detected and quantified in methanolic extracts of flowers, leaves, and roots (Figures 1, 2, 3, 4, 5, 6). Chromatograms of standard compounds are shown in Figures 7, 8. Ultraviolet spectra of quantified compounds, which were overlaid with those of standard compounds, are shown in Figures 9, 10, 11.
Figure 1.
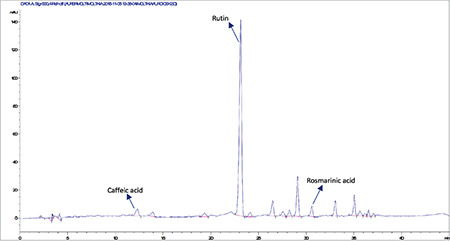
High-performance liquid chromatography analysis of the methanolic extract of Moltkia aurea flowers
Figure 2.
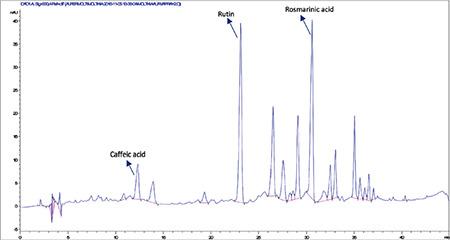
High-performance liquid chromatography analysis of the methanolic extract of Moltkia aurea leaves
Figure 3.
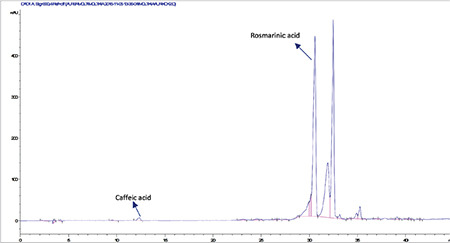
High-performance liquid chromatography analysis of the methanolic extract of Moltkia aurea roots
Figure 4.
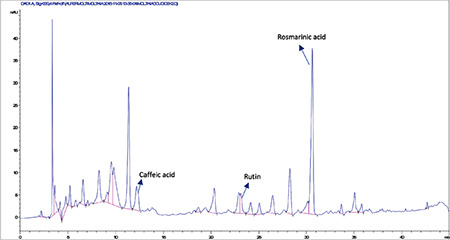
High-performance liquid chromatography analysis of the methanolic extract of Moltkia coerulea flowers
Figure 5.
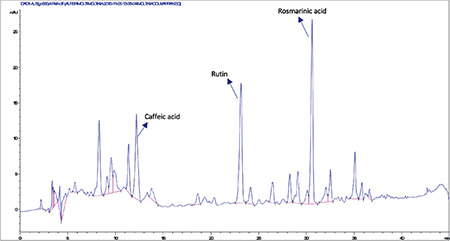
High-performance liquid chromatography analysis of the methanolic extract of Moltkia coerulea leaves
Figure 6.
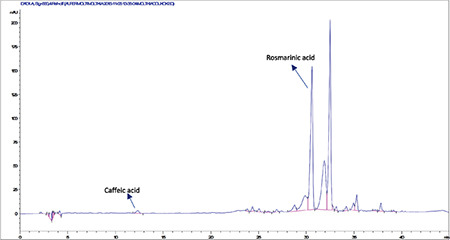
High-performance liquid chromatography analysis of the methanolic extract of Moltkia coerulea roots
Figure 7.
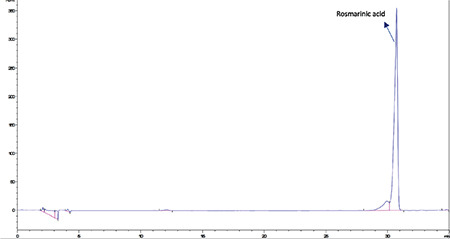
High-performance liquid chromatography analysis of the rosmarinic acid standard
Figure 8.
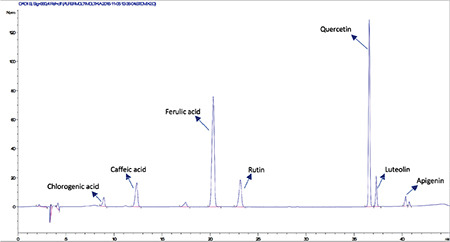
High-performance liquid chromatography analysis of a standard mixture containing chlorogenic acid (Rt: 8.9 min), caffeic acid (Rt: 12.3 min), ferulic acid (Rt: 20.25 min), rutin (Rt: 23.1 min), quercetin (Rt: 36.5 min), luteolin (Rt: 37.3 min), apigenin (Rt: 40.3 min)
Figure 9.
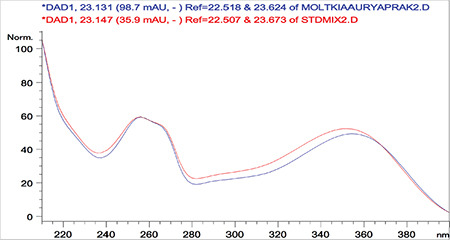
Overlaid ultraviolet spectra of standard rutin and rutin detected in the Moltkia aurea leaf extract
Figure 10.
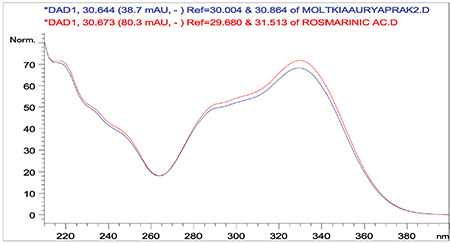
Overlaid ultraviolet spectra of standard rosmarinic acid and rosmarinic acid detected in the Moltkia aurea leaf extract
Figure 11.
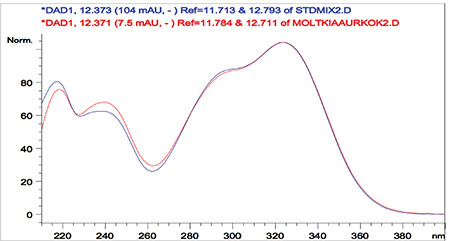
Overlaid ultraviolet spectra of standard caffeic and caffeic acid detected Moltkia aurea in the root extract
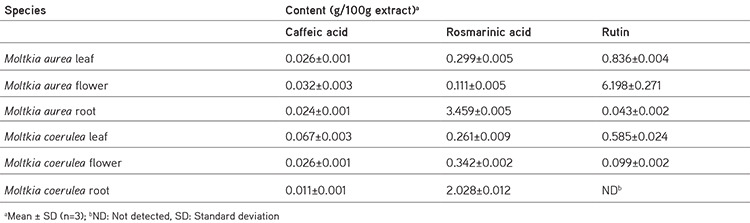
Rutin a flavonoid glycoside, occurs naturally in many fruits and vegetables and has several biological activities, such as antioxidant, antibacterial, antifungal, and antiinflammatory effects.23 As seen in the chromatograms, a significant amount of rutin was present in flower extracts of M. aurea (6.198%±0.271%; mean peak area: 3290), and MeOH extract of M. coerulea flowers contained less amount rutin (0.099%±0.002%; mean peak area: 81.23). We believe our findings reveal significant phytochemical differences between these two Moltkia taxa endemic to Turkey. Rosmarinic acid, a phenolic compound derived from hydroxycinnamic acid and mostly found in Lamiaceae and Boraginaceae, has many biological activities, including antioxidant, antimicrobial, antiinflammatory, antiproliferative, and chemopreventive effects.24 As seen from the chromatograms, root extracts of both species were rich in rosmarinic acid (3.459%±0.005% and 2.028%±0.012%, respectively). Moreover, remarkable amount of rosmarinic acid was detected in the other investigated parts (Table 5).
Table 5. Content of phenolic compounds in methanol extracts of Moltkia species.
DISCUSSION
Based on our comprehensive literature survey, only a few studies on pharmacological activities and chemical profiles of related Moltkia species have been preformed. Harput et al.5 investigated the cytotoxic effect of the aerial parts of M. aurea on three cell lines and determined antioxidant activity. Moreover, dose-dependent cytotoxic activity against cancer cell lines as well as promising antioxidant activity have been reported. Additionally, some phenolic compounds were obtained from the water subextract. Erdemoglu et al.25 studied the chemical constituents of the seed oil of M. aurea.
The effect of an ointment prepared from the aerial parts of M. coerulea (collected from Iran) on wound healing was investigated by Farahpour et al.26 Chemical constituents of M. coerulea flowers collected from India included fatty acids (capric, myristic, palmitic, behenic, and undecylic acids), flavonoids (kaempferol, quercetin, nortangeretin, and rebinetin), and amino acids (ornithine, lysine, dopa, serine, glutamic acid, proline, amino-n-butyric acid, phenylalanine, and leucine).27
Zengin et al.9 investigated antioxidant, antimicrobial, antityrosinase, antiacetylcholinesterase, antibutyrylcholinesterase, and antidiabetic activities of methanolic extracts of aerial parts of M. aurea and M. coerulea in vitro. Moreover, they studied the phenolic profile of the species and performed an in vivo assay to evaluate genotoxicity. Rutin, hesperidin, and protocatechuic acid were detected as the main components of the aerial parts of M. aurea, whereas rutin, protocatechuic acid, malic acid, and hesperidin were detected as the main components of the aerial parts of M. coerulea. In this study, we evaluated antioxidant and antidiabetic activities and phenolic profiles of three parts of the species. Consistent with the results of Zengin et al.9, we found that a significant amount of rutin was present in the MeOH extract of M. aurea flowers. Moreover, rosmarinic acid was one of the most abundant compound in both species, especially in the leaf and root of M. aurea and all investigated parts of M. coerulea. The content of rosmarinic acid is the main difference between the two studies. Rutin amount was determined too high in the flower extract of M. aurea compared to that of M. coerulea. Moreover, caffeic acid should contribute to the antioxidant feature of the species.
Zengin et al.9 reported that although both species possessed antioxidant properties, M. aurea was a better antioxidant. We could not categorize the species according to their antioxidant potential. Different results were obtained with different extracts, and making correlation was difficult. Both M. aurea and M. coerulea possessed antidiabetic activity as inhibitors of carbohydrate-digesting enzymes.
CONCLUSION
M. aurae and M. coerulea exhibited potent antioxidant and mild carbohydrate-digesting enzyme inhibitory activity in in vitro assays. Both species are a significant source of rosmarinic acid, rutin, and caffeic acid, which are possibly responsible for these activities. Thus, M. aurae and M. coerulea can be used as potential natural antioxidant sources. Notably, M. aurea extracts should be studied for the development of herbal products with antidiabetic potential.
Footnotes
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of the paper.
References
- 1.Dogu S, Dinc M, Pınar NM. Anatomical and micromorphological differentiation in the genus Moltkia Lehm in Turkey. Pakistan J Bot. 2012;44:1083–1090. [Google Scholar]
- 2.Sharma RA, Singh B, Singh D, Chandrawat P. Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Boraginaceae in India. J Medic Plants Res. 2009;3:1153–1175. [Google Scholar]
- 3.Balpınar N, Ökmen G. The biological activities of Moltkia aurea Boiss., an endemic species to Turkey. Afr J Tradit Complement Altern Med. 2017;14:60–64. doi: 10.21010/ajtcam.v14i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiou O, Dimitrellos G, Georgiadis T. Moltkia petraea (Boraginaceae) in Greece. Phyton. 2000;40:57–69. [Google Scholar]
- 5.Harput US, Nagatsu A. Antioxidant and cytotoxic effects of Moltkia aurea Boiss. Rec Nat Prod. 2012;6:62–66. [Google Scholar]
- 6.Ozdemir E, Alpınar K. The wild edible plants of western Nigde Aladaglar Mountains (Central Turkey) Istanbul J Pharm. 2010-2011;41:66–74. [Google Scholar]
- 7.Ozdemir E, Alpınar K. An ethnobotanical survey of medicinal plants in western part of central Taurus Mountains: Aladaglar (Nigde-Turkey) J Ethnopharmacol. 2015;166:53–65. doi: 10.1016/j.jep.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 8.Van Quan N, Xuan TD, Tran HD, Thuy NTD, Trang LT, Huong CT, Andriana Y, Tuyen PT. Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum Bark. Molecules. 2019;24:605. doi: 10.3390/molecules24030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zengin G, Ceylan R, Katanic J, Mollica A, Aktumsek A, Boroja T, Matic S, Mihailovic V, Stanic S, Aumeerudy-Elalfi Z, Yılmaz MA, Mahomoodally MF. Combining in vitro, in vivo and in silico approaches to evaluate nutraceutical potentials and chemical fingerprints of Moltkia aurea and Moltkia coerulea. Food Chem Toxicol. 2017;107:540–553. doi: 10.1016/j.fct.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes, with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Lam SH, Chen JM, Kang CJ, Chen CH, Lee SS. α-Glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochemistry. 2008;69:1173–1178. doi: 10.1016/j.phytochem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Jung HA, Jin SE, Choi RJ, Manh HT, Kim YS, Min BS, Son YK, Ahn BR, Kim BW, Sohn HS, Choi JS. Anti tumorigenic activity of sophoraflavescenol against lewis lung carcinoma in vitro and in vivo. Arch Pharm Res. 2011;34:2087–2099. doi: 10.1007/s12272-011-1212-y. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Ooi VFC, Chang ST. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 1997;60:763–771. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 14.Adaramoye OA, Farombi EO, Adeyemi EO, Emerole GO. Comparative study on the antioxidant properties of flavonoids of Garcinia kola seeds. Pak J Med Sci. 2005;21:331–339. [Google Scholar]
- 15.Oyaizu M. Studies on products of browning reactions-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 16.Dinish TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 17.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex, specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 18.Zongo C, Savadogo A, Ouattara L, Bassole IHN, Ouattara CAT, Ouattara AS, Barro N, Koudou J, Traore AS. Polyphenols content, antioxidant and antimicrobial activities of Ampelocissus grantii (Baker) Planch. (Vitaceae): A medicinal plant from Burkina Faso. Int J Pharmacol. 2010;6:880–887. [Google Scholar]
- 19.Kosalec I, Bakmaz M, Pepeljnjak S, Vladimir-Knezevic S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharmaceut. 2004;54:65–72. [PubMed] [Google Scholar]
- 20.Gökbulut A. Validated RP-HPLC method for quantification of phenolic compounds in methanol extracts of aerial parts and roots of Thymus sipyleus and evaluation of antioxidant potential. Trop J Pharm Res. 2015;14:1871–1877. [Google Scholar]
- 21.Orhan Deliorman D, Hartevioğlu A, Orhan N, Berkkan A, Gökbulut A, Günhan Ö, Pekcan M. Subacute effects of standardized Fumaria vaillantii Lois. ethanol extract on trace element levels, biochemical and histopathological parameters in experimental liver toxicity. J Food Biochem. 2016;40:180–189. [Google Scholar]
- 22.Meot-Duros L, Le Floch G, Magne C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J Ethnopharmacol. 2008;116:258–262. doi: 10.1016/j.jep.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Tech. 2017;67:220–235. [Google Scholar]
- 24.Al-Dhabi NA, Arasu MV, Park CH, Park SU. Recent studies on rosmarinic acid and its biological and pharmacological activities. EXCLI J. 2014;13:1192–1195. [PMC free article] [PubMed] [Google Scholar]
- 25.Erdemoglu N, Kusmenoglu S, Vural M. γ-Linolenic acid content and fatty acid composition of Boraginaceae seed oils. Eur J Lipid Sci Tech. 2004;106:160–164. [Google Scholar]
- 26.Farahpour MR, Dilmaghanian A, Faridy M, Karashi E. Topical Moltkia coerulea hydroethanolic extract accelerates the repair of excision wound in a rat model. Chin J Traumatol. 2016;19:97–103. doi: 10.1016/j.cjtee.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meshkatalsadat MH, Parekh HH. Chemical investigations of the flowers of Moltkia coerulea. Planta Med. 1990;56:556–557. [Google Scholar]


