Abstract
Breast cancer (BC) is the leading cause of cancer death in women and the second-most common cancer. An estimated 281 550 new cases of invasive BC will be diagnosed in women in the United States, and about 43 600 will die during 2021. Continual research has shed light on all disease areas, including tumor classification and biomarkers for diagnosis/prognosis. As research investigations evolve, new classes of drugs are emerging with potential benefits in BC treatment that are covered in this manuscript. The initial sections present updated classification and terminology used for diagnosis and prognosis, which leads to the following topics, discussing the past and present treatments available for BC. Our review will generate interest in exploring the complexity of the cell cycle and its association with cancer biology as part of the plethora of target factors toward developing newer drugs and effective therapeutic management of BC.
Keywords: Breast cancer, anti-cancer therapy, clinical trial, hormone receptor, anti-cancer drug resistance, FDA
Introduction
Breast cancer (BC) is the leading cause of cancer death in women and the second-most common cancer overall, with 2 million new cases diagnosed in 2018 and more than 600 000 deaths attributed to the disease.1
In the United States, an estimated 281 550 new cases of invasive BC will be diagnosed in women, and about 43 600 will die during 2021.2 This scenario is prompting continual research into all areas of the disease—including tumor classification and proposed therapeutic interventions.
Historically, BC was classified into histological groups, with the 2 most common subtypes being invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC), respectively.3 This classification has since become of secondary importance to molecular approaches.
Molecular classifications of BC have greater utility for prognosis and guiding therapeutic strategies. The implementation of staining for hormone receptor (HR) status, specifically estrogen receptor (ER) and progesterone receptor (PR), has led to a semistandard method to select patients for endocrine therapies involving selective ER modulators (SERM), aromatase inhibitors (AIs), and anthracycline and taxane-based chemotherapy.4 The other commonly tested protein in BC biopsies is the human epidermal growth factor receptor 2 (HER2),5 which, among other considerations is used to select for therapies including monoclonal antibodies (mAbs),6 antibody-drug conjugates (ADCs),7 tyrosine kinase inhibitors (TKI), poly ADP-ribose polymerase (PARP) inhibitors, and cyclin-dependent kinase (CDK) 4/6 inhibitors.8 Further information is discussed along with the text within the respective topics.
The latest drugs/classes approved by the US Food and Drug Administration (FDA) exhibiting positive results are ADCs,9 PARP inhibitors,10,11 and programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) inhibitors for selected patients.12 Recently the gamma delta (γδ) T lymphocytes have come to light as a potential BC immunotherapy due to their unique biology and established role in cancer immunosurveillance.13,14 Histone deacetylase inhibitors (HDACi) are another promising class,15 but not currently approved. An ongoing phase-III trial with the HDACi tucidinostat combined with exemestane has evaluated progression-free survival (PFS) data. It is expected to have mature overall survival (OS) data in early 2021.16
This review covers relevant past and current treatments available for BC and biomarkers as targets that show promising results for future treatments. We first present the classification and terminology for diagnosis and prognosis, which leads to the next topics, which describe the drugs’ mechanism, main clinical indications, and adverse effects.
Classification and Terminology for Diagnosis and Prognosis
According to the World Health Organization (WHO), based on histomorphology and growth patterns alone, there are 21 histological types of BC that differ in risk factors, presentation, response to treatment, and outcomes.17 A component that is always included in a pathology report and has been a cornerstone in the determination of BC prognosis is histological classification and grade.18 The first major division is in situ versus invasive carcinoma. Invasive carcinoma is then broken down into multiple subtypes, including the most common infiltrating ductal carcinoma and invasive lobular.19 Determination of HR positivity through immunohistochemistry (IHC) is used in conjunction with histology as a starting point for determining therapeutic management. Tumors can then be classified molecularly for added prognostic value and therapeutic guidance.20
Biomarkers for molecular subtyping of BC
Up to 10 different subgroups of molecular BC have been proposed, though 5 main groups have substantial clinical relevance: (1) Luminal A: HR positive (HR+) (ER+ and/or PR+) and HER2 negative (HER2−); (2) Luminal B: HR+, and either HER2 positive (HER2+) or HER2−; (3) HER2-enriched BC: HR negative (HR−) and HER2+; (4) Triple-negative breast cancer (TNBC)/basal-like: HR− and HER2−; (5) Normal-like BC, which like luminal A, is HR+, HER2−, but its prognosis is slightly worse than luminal A.21-24
HR+ means that greater than 1% of tumor nuclei express ER and/or PR, as determined by IHC.25 Two hypotheses explain the estrogen and the ER roles in BC. The first suggests that ER binding stimulates mammary cell proliferation, increasing cell division and DNA synthesis, thereby increasing the risk for replication errors and accumulation of mutations in processes of DNA repair, cell proliferation, and apoptosis.26 The other hypothesis states that the metabolism of estrogen causes the formation of genotoxic by-products that directly damage DNA, causing mutations. HR+ cancer has the advantage of having a high response rate to hormonal therapy, including SERMs and AIs.26
Immunohistochemical staining can reveal HR+ cancer; however, a significant number of women present with resistance or develop resistance during endocrine-based therapies.27 Estrogen receptor status and mutation, as well as the crosstalk between ER, HER2 signaling pathways, and growth factors, are common contributors to endocrine resistance.28,29 Other mechanisms that explain these drug resistances include estrogen-independent growth, hypersensitivity to low estrogen concentrations, cyclin D1 overexpression, constitutive nuclear factor kappa B (NFκ-B) activation, upregulation of growth-factor-signaling pathways, and downregulation of ER-alpha expression.27 The identification of resistance mechanisms leverages fruitful research on biomarkers of clinically significant and the development of new drug classes and targeted therapy.
Approximately 20 to 30% of patients with BC demonstrate overexpression of HER2,30 a member of the ERBB family of receptor tyrosine kinases (RTK), which is involved in critical cellular functions, including cell growth and survival.31 Human epidermal growth factor receptor 2 is an oncoprotein connected to the ERBB signaling pathways, mediating cell-cell interactions in organogenesis and adulthood. ERBB2, the gene that encodes HER2, may be amplified, leading to the overexpression of HER2 on the surface of BC cells known as HER2+. This overexpression of HER2 causes overactivation of the ERBB2 signaling pathway.32
The American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) updated the guideline recommendations for HER2 testing in BC and advocated the improvement of the accuracy of HER2 testing by IHC or in situ hybridization (ISH).33 Human epidermal growth factor receptor 2+ criteria were defined as HER2 protein overexpression (IHC, microscopic field of vision > 10% of adjacent homogeneous tumor tissue cell region with complete and intense circumferential membrane staining) or gene amplification, ISH, average HER2 copy number ⩾6.0 signals/cell or average HER2 copy number ⩾4.0 signals/cell and HER2/chromosome enumeration probe 17 (CEP17) ratio ⩾2.0. If indeterminate results appear, a reflex test using an alternative assay (IHC or ISH) is required. If the test results do not conform to other histological tests, they should be repeated. The test results from laboratories should be highly consistent with the validated HER2 test, and the test should be carried out in the laboratories certified by CAP or other authorized institutions.34
The prognostic implication of HER2 status has also been discussed in the settings of ductal carcinoma in situ (DCIS).35 Ductal carcinoma in situ represents 20 to 25% of all BC detected by population-based BC screening programs.35 Although it is not invasive, DCIS has a higher proportion of HER2 amplification than the invasive disease, 34% and 13%, respectively.35,36 The frequency of HER2 positivity in DCIS was found comparable to invasive BC, and HER2+ and DCIS were associated with poor prognosis features.37,38
Guided by selected biomarkers, these molecular subtypes are relevant for prognosis and planning therapeutic strategies. Patients with Luminal A BC have low levels of a biomarker that participates in controlling the rate at which cancer cells grow (Ki-67) and have the best prognosis. Compared with luminal A, the normal-like BC has low levels of Ki-67. Luminal B has high levels of Ki-67 and usually grows slightly faster than luminal A, and the patients’ prognosis is slightly worse than luminal A. Human epidermal growth factor receptor 2-enriched cancers tend to grow faster than luminal BC (A and B) and can have a worse prognosis.24,35,38
Nowadays, decision-making is based on numerous factors, including tumor morphology and grade classification, tumor size, presence of lymph node metastases, and expression of ER, PR, and HER2 (a review on this topic was published by Fragomeni et al).39
The molecular subtype, defined by PAM50, which considers the expression profiles of 50 different genes, was proposed to add prognostic and predictive information gene signature to classify invasive BC into the 5 intrinsic subtypes (luminal A, luminal B, HER2-enriched, basal-like, and normal-like).40 However, specific properties for the PAM50 subtypes reflect changes in the microenvironment instead of specific molecular changes in epithelial cells, highlighting the importance of the tumor microenvironment for the progression and disease outcome.41 Prat et al42 proposed a surrogate immunohistochemical-based definition of luminal A tumors as HR+/HER2−/Ki-67 less than 14%, and PR+ more than 20%. PAM50 and determination of luminal A or luminal B are further used for the determination of prognosis. Patients with luminal A BC carry a higher 5-year OS rate of nearly 96% compared to a rate of about 86% in luminal B43 in premenopausal women treated with tamoxifen versus tamoxifen followed by anastrozole, respectively. These molecular subtypes can also be used to assess recurrence rates, with luminal A having a 93.9% distant recurrence-free survival as opposed to 82.2% in luminal B.44
When considering the expression of HER2, cancers that are HER2+ have a better prognosis if they are HR+ as well. A study looking at routine clinical care of molecular subtypes of BC found that HR+ and HER2+ cancers had a 5-year OS of 92.5% while HER2+ tumors that were HR− had an OS of 85.6%.45 This is an improvement from 20 years ago due to the development of anti-HER2 therapies, such as trastuzumab,46 pertuzumab,47 lapatinib,48 neratinib,49 and ado-trastuzumab emtansine, also known as trastuzumab emtansine (T-DM1).50 However, resistance and adverse events (AEs) are frequently observed during HER2-directed therapy and are obstacles to the continuous administration of these agents.51 Therefore, it is crucial to improve anti-HER2 strategies for patients who are intolerant of standard therapies, as well as determine the mechanisms of resistance (for a review, see Chen et al52).
Triple-negative BC is considered the most aggressive form of BC and the worst prognostically among the 4 main molecular subtypes.9,53 Homologous recombination repair (HRR) pathway deficiency results in chromosomal instability, which characterizes TNBC,54 germline BRCA1/2 mutations, and BRCA gene promoter methylation, and genetic mutations of the HRR pathway are considered the common causes for the deficiency in the HRR pathway.55 Atchley et al56 reported more than 80% of BC involving BRCA mutations being triple negative.57 Studies have shown that TNBC has a higher risk of recurrence and worse prognosis after recurrence than HR+ cancers (median survival 12-18 months vs 50-60 months).58 Although targeted therapies are becoming part of first-line treatments for several cancers, sequential chemotherapy remains the standard of care for TNBC due to the lack of receptor expression for targeting.58 The OS for TNBC is lower than all non-TNBC, regardless of the staging of the disease.59
Testing for ER, PR, and HER2 status has been standard in the BC evaluation for some time, though other biomarkers are also emerging in research data.60 The predictive value of different biomarkers is under investigations, such as estrogen-receptor1 (ESR1, a gene that encodes the ER), CDK4, mitogen-activated protein kinase kinase kinase 1 (MAP3K1).61,62
Considering advanced breast cancer (ABC), PIK3CA mutations have a strong predictive value for treatment with α-selective and β-sparing PI3K inhibitors, and its use has recently entered clinical practice.61-63
Pharmacological BC Therapies and Predictive Biomarkers
Chemotherapy
Chemotherapy is the typical treatment of most molecular types of BC. According to Herr et al,64 nodal positive patients with luminal A BC have a limited benefit of adjuvant chemotherapy. Adjunct chemotherapy is an approach with estrogen-targeted therapy (SERM or AI) for luminal B breast tumors or in combination with trastuzumab in HER2+ BC.65,66 In metastatic breast cancer (MBC), systemic chemotherapy is the first-line approach in HER2+ and TNBC and a possible addition in ER+ MBC.67
Chemotherapy is focused on taxane and anthracycline agents (Figure 3). A Cochrane review reported that a taxane-containing chemotherapy regimen has significantly improved disease-free survival (DFS) and OS when used as an adjuvant in early BC.68 The current adjuvant regimen for early invasive breast carcinoma comprises doxorubicin or (epirubicin) and cyclophosphamide followed by paclitaxel in a dose-dense schedule of docetaxel, doxorubicin, and cyclophosphamide.69,70 These chemotherapeutic drugs are equally effective and differ only in their toxicities.69 Either of these regimens can be used as a monotherapy or in combination with endocrine therapy in HR+ BC. In ABC or MBC, chemotherapy is routinely used in a sequential method. This has been shown to have no difference in OS but a significant improvement in the quality of life.71
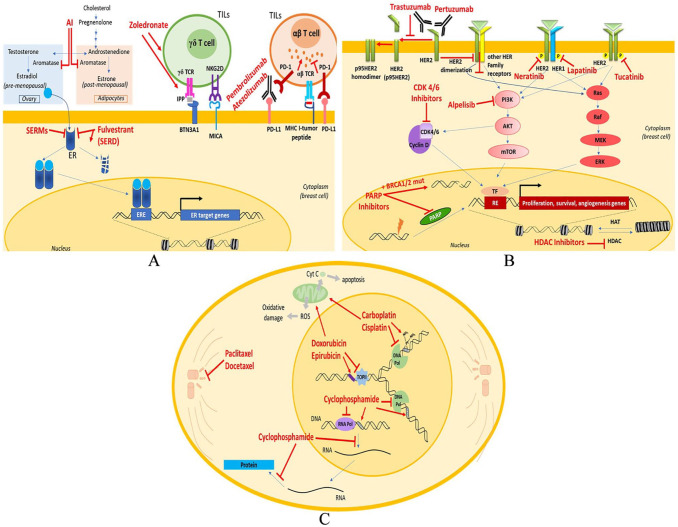
Figure 3.
Schematic diagram depicting sites of action of prominent drugs affecting principal pathways associated with breast cancer: (A) aromatase is the enzyme that catalyzes the conversion of androgens to estrogens. AIs inhibit the enzyme, therefore reducing the levels of estrogen which is needed for ER+ cancer cells growth. SERMs (ER antagonism) act as estrogen receptor antagonists in breast tissue. SERD (fulvestrant) is an ER antagonist leading to degradation and downregulation of ER. Zoledronate is a direct γδ T cell stimulator and also leads to accumulation of IPP in cancer cells leading to stimulation and activation of γδ T cell via γδ TCR recognition of phosphoantigens presented by butyrophilin 3A1 (BTN3A1) on the BC target cells and/or interaction of MICA with NKG2D. The immune response of γδ T cells can be via stimulatory and regulatory effects on other components of the immune system (secretion of cytokines or direct antigen presentation) and via direct cytotoxicity (through perforin-granzymes). Monoclonal antibodies: (B) PARP inhibitors lead to PARP inhibition and PARP trapping at sites of DNA damage. PARP acts as damage recognition repair protein for initiation of base excision repair of DNA SSB. PARP inhibition causes inability to repair and accumulation of SSB, leading to DSBs, which in cells with BRCA1/2 mutation cannot be fixed and accumulate ultimately triggering cell death. CDK4/6 inhibitors prevent DNA replication by arresting progression from the G1 to the S phase of the cell cycle. mAbs inhibit activation of the signaling pathway of HER involved in promoting cell growth and opposing apoptosis. Pertuzumab inhibits HER2 dimerization; trastuzumab prevents cleavage of the extracellular domain of the HER2 receptor, which leads to the formation of a truncated form of HER2 (p95HER2). p95HER2, which contains tyrosine kinase activity, can form constitutively active stable homodimers. PI3K inhibitor (alpelisib) selectively inhibits the p110α catalytic subunit of PI3K interrupting both AKT-dependent and AKT-independent PI3K signaling pathways. HDAC inhibitors block the enzyme, resulting in hyperacetylation of histones, relaxation of the chromatin, and allowing for higher transcription of the DNA. Tyrosine kinase inhibitors: tucatinib, lapatinib, neratinib are homologous of the adenosine triphosphate (ATP) that act by competing for the ATP-binding domain of protein kinases preventing phosphorylation and subsequent activation of the signal transduction pathways, leading to apoptosis and decreasing cellular proliferation. Moreover, TKIs target other kinase receptors due to the homology that they share with the EGFR family in the catalytic domain. (C) Anthracyclines: doxorubicin and epirubicin inhibit cancer through multiple pathways: to intercalate within DNA base pairs, causing breakage of DNA strands and inhibition of both DNA and RNA synthesis. Doxorubicin inhibits the enzyme topoisomerase II (TopII), causing DNA damage and induction of apoptosis—also cause ROS-mediated oxidative damage to DNA, further limiting DNA synthesis. Alkylating agents: cyclophosphamide (a nitrogen mustard compound), carboplatin and cisplatin (platinum-containing compounds) develop cytotoxic effects mainly due to substitution of alkyl groups for hydrogen atoms on DNA, resulting in the formation of cross-links within the DNA chain and thereby resulting in misreading of the DNA code and the inhibition of DNA, RNA, and protein synthesis and the triggering apoptosis in rapidly proliferating tumor cells. Taxanes: paclitaxel and docetaxel stabilize microtubules act mainly by binding to beta-tubulin, enhancing its proliferation and stabilizing its conformation. Doing so inhibits the proper assembly of microtubules into the mitotic spindle, arresting the cell cycling during G2/M. conferring enhanced survival.
AI indicates aromatase inhibitor; AKT, serine/threonine-protein kinase 1 (also known as PKB); ATP, adenosine triphosphate; BC, breast cancer; BRCA, breast cancer gene; CDK, cyclin-dependent kinase; DSB, double-strand breaks; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERE, estrogen-responsive element; ERK, extracellular signal-regulated kinase; HAT, histone acetyl transferase; HDAC, histone deacetylase; HER2, human epidermal growth factor receptor 2; IPP, isopentenyl pyrophosphate; MHC, major histocompatibility complex; MICA, MHC Class I polypeptide-related sequence type A; mTOR, mechanistic target Of rapamycin; PARP, poly ADP-ribose polymerase; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; RE, responsive element; ROS, reactive oxygen species; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator; SSB, single-strand break; TF, transcription factor; TIL, tumor-infiltrating lymphocytes; TKI, tyrosine kinase inhibitor; γδ TCR, gamma delta T cell receptor.
Platinum containing agents, particularly carboplatin, are also used in neoadjuvant treatment in HER2+ BC. Carboplatin has been shown to be effective when used in a regimen with trastuzumab and a taxane for HER2+ early stage of BC. Carboplatin, trastuzumab, and docetaxel used in the neoadjuvant setting were just as efficacious as doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab.72 The main difference in these 2 regimens is that the carboplatin regimen has fewer acute toxic events and lower levels of cardiotoxicity. Additional information about mAbs is further presented.
Treatment for TNBC with a BRCA 1/2 germline mutation can be enhanced by adding a platinum-containing chemotherapeutic agent (Figure 3), such as carboplatin, whether used as adjuvant or neoadjuvant therapy. The addition of a platinum agent to the treatment results in double the objective response rate (68% in carboplatin vs 33% in docetaxel).73
Platinum-containing neoadjuvant chemotherapy was tested in TNBC to improve long-term outcomes. Despite resulting in varied results, it may be considered an option in TNBC patients.74,75 Although the use of a platinum-containing chemotherapy agent can be expanded to all women with TNBC, it does not appear to have the same positive effects in patients without a BRCA 1/2 mutation or a mutation in another HRR gene when compared to those patients who do have a mutation.64
Although chemotherapy remains the standard treatment of BC, particularly in early stages76 and TNBC, the absolute benefits may be small and not worth the added risk of toxicity among women with a low baseline risk of recurrence. Likewise, chemotherapy is neither feasible nor likely to change OS77 for patients with significant comorbidities78 or advanced age. In this sense, chemotherapy has been supplemented by other drug classes, especially mAbs.
Hormone-based therapies
Selective ER modulators
Around 78% of breast tumors are ER+, which can block estrogen’s action in the breast, a suitable therapy for most tumors.79 One of the original pharmacological agents used to treat BC is tamoxifen, followed by toremifene (Figure 1).
Figure 1.
Timeline of drug approvals by the FDA (1974-2020) and the respective main clinical indications to treat breast cancers.
ABC indicates advanced breast cancer; AI, aromatase inhibitor; BC, breast cancer; BRCA, breast cancer gene; DCIS, ductal carcinoma in situ; EBC, early breast cancer; ER, estrogen receptor; FDA, Food and Drug Administration; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MBC, metastatic breast cancer; PD-L1, programed cell death ligand 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; TNBC, triple-negative breast cancer.
SERMs act as partial ER agonists in some tissues while acting as ER antagonists in other tissues (Figure 3). The standard endocrine therapy for premenopausal women is tamoxifen. On the other hand, raloxifene and toremifene are SERMs of clinical significance in treating BC in postmenopausal women, acting as antagonists in breast tissue.80,81 Their pharmacological differences are seen in other tissues. In all ER+ cancers, tamoxifen therapy is indicated as an adjuvant with a standard of treatment lasting at least 5 years. Regardless of menopausal status or age, the initial standard adjuvant therapy with tamoxifen for 5 years presented a reduction of BC recurrence and mortality by 30% and 33% after 15 years, respectively.82 The ATLAS and aTTom trials compared 10 years of adjuvant tamoxifen to 5 years in women of any menopausal status or age. Both trials showed that 10 years of adjuvant tamoxifen had an absolute reduction of recurrence by 3.7% and mortality by 2.8% at 10 years compared to 5 years of treatment.83,84 The SOFT and TEXT trials built upon tamoxifen and exemestane’s therapeutic outcomes, showing that the addition of ovarian suppression to both resulted in significant increases in 8-year DFS and OS. The addition of ovarian suppression was also associated with increased osteoporosis, thrombosis, embolism, and musculoskeletal symptoms. Due to the increased adverse effects, the clinical usage of ovarian suppression is assessed in a case-by-case manner and is usually reserved for those considered to be at higher risk.85,86 Adverse effects of tamoxifen and raloxifene result from their effects as agonists in tissues other than breasts, such as bones (raloxifene) and uterus (tamoxifen).87-89 The clinically significant risks include increased rates of thromboembolic disease, cataracts, endometrial cancer, and stroke.81 Despite potentially severe AEs, use of tamoxifen and raloxifene results in a decrease in all-cause mortality due to their reduction in BC mortality.81,82
Five-year treatment with tamoxifen decreased BCs’ risk in the next 20 years from 12.3 to 7.8%. The risk of invasive BC within 20 years, specifically, was reduced from 8.3% with placebo to 4.9% with tamoxifen.90 These results indicated that the number needed to treat (NNT) for 5 years of tamoxifen treatment was 22 to prevent one BC over the next 20 years. The most significant factor found to decrease tamoxifen’s benefit in BC prevention was the concurrent use of menopausal hormone therapy while taking tamoxifen.90 Tamoxifen is also used for the prevention of BC in patients who are at increased risk, such as women above 60, or with a history of lobular carcinoma in situ, or a 5-year risk of BC of at least 1.66%.91
Initial results from the MORE trial showed that raloxifene reduced invasive BC risk by 76%. Since that time, the STAR trial updates have reigned in these numbers, comparing 5 years of tamoxifen versus raloxifene. The trial showed that after a median follow-up of 81 months, postmenopausal women treated with raloxifene resulted in a higher rate of invasive BC, about 24% higher than at the same time in the tamoxifen group.91
Despite a more significant BC risk reduction, tamoxifen comes with an increased risk of endometrial cancer, cataract, and thromboembolic events.91
Ultimately, studies have been inconclusive on the effects of prophylactic use of tamoxifen and raloxifene on both BC-specific survival and OS, leading to a minimal use of these agents in chemoprevention in clinical practice.90,92
Aromatase inhibitors
Anastrozole, exemestane, and letrozole are AIs. They inhibit the enzyme aromatase that converts androgens into estrogens in target cells (Figure 3). AIs were developed after tamoxifen and are becoming an essential part of the therapeutic plan for many ER+ cancers (Figure 1). AIs are used in pre or postmenopausal women. However, treatment efficacy is influenced by different variables. Resistance to AI therapy is common, occurring in more than 20% of patients with early-stage disease, and is inevitable in patients with metastatic disease. The development and maintenance of AI resistance involve mechanisms dependent on interactions with cell types within the tumor microenvironment, such as fibroblasts, immune cells, adipose cells, and mesenchymal stem cells (for a review, see Ma et al93). Obesity is the most substantial variable embodying probable endocrine resistance78,94 in premenopausal patients, and it is associated with an increased risk of BC recurrence.95
Along with SERMs, AIs are used as a long-term treatment to decrease mortality and recurrence long after diagnosis and primary therapeutic interventions. Postmenopausal women treated for 5 years with AIs presented a decrease in BC recurrence by 3.6% over 10 years and a mortality reduction by 2.1% over 10 years when compared to tamoxifen.96 Extension of treatment to 10 years in postmenopausal women further increased DFS by 4% compared to the standard 5-year treatment.97 The aforementioned SOFT and TEXT trials also looked at exemestane. They found that the addition of ovarian suppression to exemestane improved DFS and OS,85,86 which is of particular importance when using AIs in premenopausal women. AIs block peripheral conversion of androgens to estrogens, and in premenopausal women, most of their estrogen is formed in the ovaries; therefore, when using AIs in this population, it is necessary to use ovarian suppression to increase the efficacy.98 Like tamoxifen, exemestane and anastrozole reduced the risk of BC after menopause by up to 65%.99 Despite the results showing the efficacy of exemestane and anastrozole in chemoprevention of BC, they are rarely used for this purpose in clinical practice. Their low utilization in clinical practice may be due to lack of awareness on the part of primary-care physicians of the clinical trials showing the efficacy of exemestane and anastrozole as methods of chemoprevention for BC and concerns over adverse effects, including loss of bone mineral density and intensification of menopausal symptoms, mainly hot flashes and fatigue.100 Since 2013, AIs, particularly letrozole, have gained increased clinical significance in combination with other agents, including CDK 4/6 inhibitors, PI3K inhibitors, and HDAC inhibitors.15,61,101,102 Details about these agents will be discussed in the next sections.
Selective ER degraders
The prevalence of ER+ BC, combined with the risk of recurrence after treatment with both AIs and SERMs, led to the development of the third class of drugs that acts through ER known as SERDs. This class currently contains an orphan drug, fulvestrant, a 17-β-estradiol analog,103 which acts as a selective ER antagonist that also speeds the ER degradation and downregulation.104 Due to its mechanism of action (Figure 3), fulvestrant is useful for treating HR+ cancer and is mainly reserved for local ABC or MBC.
Fulvestrant is currently approved only for use in postmenopausal women. In head-to-head trials, fulvestrant resulted in an increased OS and PFS over anastrozole.105,106 Fulvestrant monotherapy is approved for postmenopausal women with HR+ MBC following antiendocrine therapy, or those with HR+, HER2− ABC not previously treated with endocrine therapy (Figure 1).107 However, fulvestrant is most often used as an adjuvant instead of monotherapy.108,109 In combination with palbociclib, fulvestrant resulted in an absolute prolongation of OS of 6.9 months among patients with HR+, HER2− ABC who had disease progression after previous endocrine therapy.106
The most common adverse effects for fulvestrant are arthralgia, hot flush, fatigue, nausea, and back pain.106 Although infrequent, cardiac failure, and arrhythmias are the most severe adverse effects leading patients to discontinue the therapy.110,111
Targeted therapy and immunotherapy
Cyclin-dependent kinases 4/6 inhibitors
A combination of acquired and new resistance to hormonal based treatments has led to the search for newer agents to aid in treating HR+ tumors. Food and Drug Administration-approved CDK 4/6 inhibitors include palbociclib, abemaciclib, and ribociclib.62 CDK4/6 interact with the protein cyclin D1 (CCND1) to allow for progression through the G1 checkpoint and into the S phase of the cell cycle. CDK4/6 inhibition prevents DNA replication by arresting progression from the G1 to S phase (Figure 3).112
Mainly due to ABC, current guidelines include CDK4/6 inhibitor combined with endocrine therapy for the treatment of premenopausal/postmenopausal women with HR+/HER2− (Figure 1).113 Palbociclib combined with letrozole was shown to increase PFS by 45% when compared to letrozole alone in HR+, HER2− BC101 and significantly improved OS by 48% versus letrozole alone.114
Abemaciclib and ribociclib presented similar results as palbociclib in recent clinical trials. The MONARCH 2 trial compared the use of abemaciclib + fulvestrant versus placebo + fulvestrant in HR+, HER2− BC. Abemaciclib resulted in significant increase in both OS and PFS when compared to placebo.115 The MONALEESA-7 trial, ribociclib + endocrine therapy (AI or tamoxifen + goserelin) was compared to placebo + endocrine therapy in HR+, HER2− BC. The results showed the addition of ribociclib significantly increased OS.116
Selected biomarkers are predictive of these pharmacological agents’ efficacy. High CDK4 expression was associated with resistance to letrozole, indicated by a shorter PFS than low CDK4 expression; the addition of palbociclib mitigated this.62 Higher expression levels of fibroblast growth factor receptor-2 (FGFR2), and ERBB2 RTK 3 were associated with longer PFS when treated with letrozole plus palbociclib.62 Finally, low expression of CCNE1 was associated with greater PFS when treated with letrozole.117 Despite these findings, testing of these biomarkers is not used routinely in clinical practice.118,119
Common AEs associated with all CDK4/6 inhibitors are neutropenia and leukopenia. Patients often report fatigue, nausea, and arthralgia. Serious AEs occurred at a rate of 19.6% with the use of palbociclib and 12.6% with letrozole alone.27 Of note, the rate of neutropenia with abemaciclib was much lower than with palbociclib or ribociclib.62,101,102
Phosphoionositide-3 kinase inhibitors
Phosphoionositide-3 kinase is a critical enzyme for many cellular functions, including proliferation, apoptosis, and nutrient response.120 Approximately 40% of HR+, HER2− BC patients presented with mutations in the PIK3CA gene, leading to constitutive activation of the alpha subunit (p110α) of PI3K.121-123
The novel PI3K inhibitors (Figure 3) used to treat BC are alpelisib, taselisib, and copansilib.61,124-126 Of the 3, the only currently FDA approved is alpelisib in combination with fulvestrant for postmenopausal women or men, who have HR+, HER2−, PIK3CA mutated, ABC or MBC (Figure 1).127-132 Alpelisib works as an α-specific PI3K inhibitor, by selectively inhibiting p110α, leading to interruption of PI3K signaling pathways.128-132 The SOLAR-1 trial alpelisib + fulvestrant resulted in a PFS of 11 months versus 5.7 months in the placebo group. This effect was only seen in patients with PIK3CA mutated cancer, though, with the effect disappearing in those without the mutation.124 Taselisib and copansilib, have shown promise, particularly in combination with other anticancer drugs, with all showing increased PFS in clinical trials involving HER2−, HR+ advanced, or MBC.125,126
Currently, the clinical benefit of PI3K inhibitors is only achieved in patients with PIK3CA mutations. Recently it was shown that double mutations might increase sensitivity even more than a single mutation. When comparing the response to a PI3K inhibitor taselisib, patients with multiple PIK3CA mutations had an overall response rate (ORR) of 30.2% compared to 8.7% in placebo. Patients with a single mutation only had an ORR of 18.1% compared to 10% in placebo, which was not a statistically significant difference.133 The presence of MAP3K1 mutation simultaneously with PIK3CA mutation is associated with the enhanced clinical benefit of PI3K inhibitors.61 Like other potential biomarkers mentioned, this offers an avenue for further studies to determine the clinical impact of testing for inactivating MAP3K1 mutations in combination with activating PIK3CA mutations.61
AEs associated with PI3K inhibitors are determined by the isoform they affect. Alpelisib is more specific to the α isoform of PI3K, and therefore, the most common adverse effects are hyperglycemia, diarrhea, and nausea. The most common grade 3 or above AEs witnessed with alpelisib + fulvestrant treatment is hyperglycemia, with rash, and diarrhea following.124
Many of these agents have been abandoned in development due to toxicity issues or lack of efficacy, such as buparlisib and pictilisib that have both had trials terminated.134,135
Tyrosine kinase inhibitors
Receptor tyrosine kinases are a family of tyrosine protein kinases. Receptor tyrosine kinases are transmembrane proteins with binding sites at their extracellular domains for polypeptide hormones and growth factors as ligands. Receptors of growth factors are members of the RTK family, such as the epidermal growth factor helping the regulation of cell growth and differentiation. The proto-oncogene HER2 encodes epidermal growth factor receptor (EGFR) with tyrosine kinase activity.33,136
HER2/neu is a transmembrane tyrosine kinase receptor that forms part of this ErbB family signaling network. Abnormal signaling by this network is present in BC.137 The EGFR/HER1, a different ErbB receptor, is expressed or highly expressed in many tumors. Although this remains controversial,138,139 overexpression of EGFR is linked to a more aggressive breast tumor phenotype, involving the increased potential for invasiveness and metastasis.140-142
TKIs play an essential role in the modulation of growth factor signaling. The mechanism of TKI is depicted in Figure 3. The TKIs targeting BC treatment are lapatinib, neratinib, tucatinib, pyrotinib, and afatanib. Among them, only lapatinib, tucatinib, and neratinib are approved by the FDA to treat BC (Figures 1 and 2).
Figure 2.
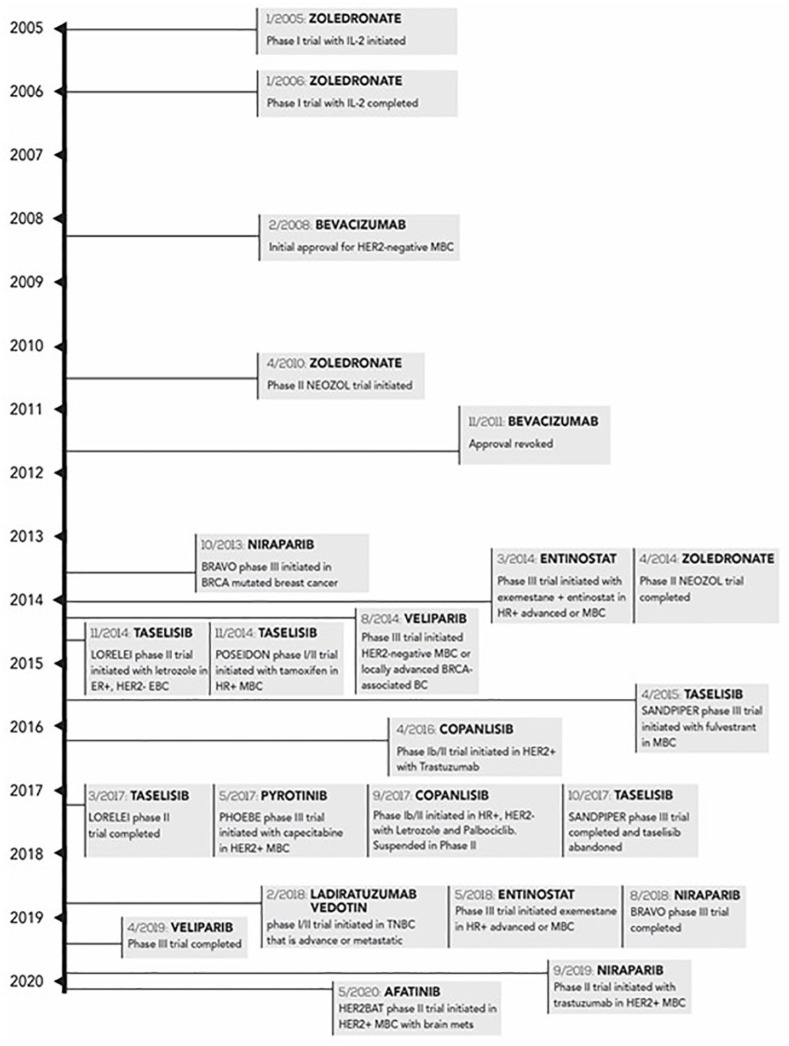
Timeline (2007-2020) of drugs under clinical trial toward the treatment of breast cancers and those that had their trials not completed.
BC, breast cancer; BRCA, breast cancer gene; EBC, early breast cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HER2BAT, a study of HER2+ breast cancer patients with active brain metastases treated with afatinib & T-DM1 vs. T-DM1 alone; HR, hormone receptor; IL-2, interleukin-2; LORELEI, study of neoadjuvant letrozole + taselisib versus letrozole + placebo in post-menopausal women with breast cancer; BRAVO, trial of niraparib versus physician’s choice in HER2 negative, germline BRCA mutation-positive breast cancer patients; MBC, metastatic breast cancer; PHOEBE, pyrotinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2+ metastatic breast cancer; POSEIDON, safety, efficacy and circulating tumor DNA response of the beta isoform-sparing PI3K inhibitor taselisib (GDC-0032) combined with tamoxifen in hormone receptor-positive metastatic breast cancer patients; SANDPIPER, a study of taselisib + fulvestrant versus placebo + fulvestrant in participants with advanced or metastatic breast cancer who have disease recurrence or progression during or after aromatase inhibitor therapy; TNBC, triple-negative breast cancer.
Lapatinib (a prodrug metabolized by CYP3A4), combined with capecitabine, is used to treat patients with HER2-overexpressing MBC who received an anthracycline, a taxane, and trastuzumab as prior therapy. Among the 3 TKIs approved, lapatinib is the only intracellular blocker acting on both HER2 (ErbB-2) and HER1 (ErbB-1), acting as a dual reversible inhibitor for receptors, thus blocking the downstream MAPK/Erk1/2 and PI3K/AKT pathways.143,144
The most common adverse effects during therapy with lapatinib plus capecitabine were gastrointestinal disorders (diarrhea, nausea, and vomiting) or dermatologic, such as palmar-plantar erythrodysesthesia and rash.145
Neratinib is an irreversible TKI of HER1, HER2, HER4, approved in combination with capecitabine for adult patients with advanced or metastatic HER2+ BC who have received 2 or more prior anti-HER2-based regimens in the metastatic setting.146 Compared with lapatinib, neratinib has a more valid and consistent inhibitory effect in feasible resistance pathways.49 Neratinib was shown to significantly improve the 2-year invasive DFS after trastuzumab-based adjuvant therapy in HER2+ BC. The most common adverse effects among patients using neratinib include diarrhea, and less frequently, neutropenia and dehydration were reported. These adverse effects were reversible and manageable with dose reduction, pause interruption, and proper supportive care.147
Tucatinib is approved in combination with trastuzumab and capecitabine for adult patients with advanced unresectable or metastatic HER2+ BC, including patients with brain metastases who have received one or more prior anti-HER2-based regimens in the metastatic setting.148 Tucatinib increased PFS at 1 year to 33.1% compared to 12.3% in placebo and led to an increased OS at 2 years to 44.9% from 26.6% in placebo.149 Tucatinib’s common adverse effects are diarrhea, palmar-plantar erythrodysesthesia, nausea, fatigue, and vomiting.146
Pyrotinib and Afatinib are both TKIs that have shown promise in studies, with pyrotinib being approved in China.150,151 Neither have been approved by the FDA, but they remain in numerous phases III and II trials (Figure 2).
ADP-ribose polymerase inhibitors
Cancer cells with deleterious mutations in BRCA1/2 are deficient in the repair mechanism for DNA double-strand breaks (DSB), leaving these tumors highly dependent on the repair pathway for single-strand DNA breaks (SSB).10,152 DNA-SSB results directly from oxidative damage.152 Poly ADP-ribose polymerase comprise a family of enzymes that modify targeted proteins by catalyzing the transfer of poly(ADP-ribose) (PAR) to these target proteins. Poly ADP-ribose polymerase acts as a damage recognition repair protein of an SSB and triggers the repair of SSBs in the DNA by base excision repair (BER).153 Poly ADP-ribose polymerase detects SSBs, binds to DNA, and undergoes auto-poly ADP-ribosylation, which acts as a signal for the recruitment of hundreds of downstream proteins that regulate DNA repair and eventually repair these SSBs.154,155 Nevertheless, if these SSBs are not repaired, they finally progress to DBSs, which are cytotoxic. Poly ADP-ribose polymerase inhibition (Figure 3) is thought to decrease PAR levels, one of the earliest cellular responses to genotoxic stress.156 In other words, targeted therapy with PARP inhibitors leads to SSBs accumulation and, consequently, DSB formation fostering DSB repair.154-157
In addition to catalytic inhibition, PARP inhibitors induce PARP trapping at sites of DNA damage. The capacity to trap PARP–DNA complexes varies among PARP inhibitors and is not correlated with PARP catalytic inhibition.157-159 The synthetic lethality approach combines a PARP inhibitor and a BRCA mutation in a condition where a deficiency in one gene does not lead to cell death. Still, a combination of 2 or more deficiencies do.158,160 Poly ADP-ribose polymerase inhibitors target PARP enzymes, mainly PARP1 and PARP2.161
Regarding PARP inhibitor treatment of TNBC, TP53, ATM, PALB2, and RAD51C might be prognostic biomarkers or predictive indicators for treatment response and could also provide targets for novel treatment strategies.157
To date, there are 4 PARP inhibitors approved by the FDA: olaparib, rucaparib, niraparib, and talazoparib.162-164 Olaparib and talazoparib are the PARP inhibitors currently FDA approved as targeted therapies for treating MBC caused by a BRCA mutation (Figure 1).
Olaparib has been approved for the treatment of patients with advanced ovarian cancer as of 2014. The OlympiAD phase III trial then evaluated the efficacy and safety of olaparib in patients with metastatic HER2− BC that could be either HR+ or HR−.165 The results indicated olaparib increased PFS by 2.8 months and response rate by 31.1% compared to placebo.166-169
Talazoparib (Figure 1) is a potent PARP inhibitor. It provides 100 times greater catalytic inhibition and PARP-trapping potential than other PARP inhibitors, indicating the trapping PARP on DNA may be more effective in inducing cancer-cell death than enzymatic inhibition alone.170After the phase III EMBRACA trial, talazoparib showed superior PFS by 3 months and increased ORR by 35.4% compared to standard chemotherapy.10,171 The most common adverse effects observed with PARP inhibitors are anemia, fatigue, nausea, vomiting, and neutropenia.
Histone deacetylase inhibitors
Resistance to antiestrogens in ER+ tumors can either present initially or develop during treatment. One potential mechanism of antiestrogen resistance is through the alteration of genome sequences, resulting in gene silencing.172 This has created an additional avenue for dealing with resistance beyond targeting new pathways, instead by altering the epigenetics of tumor cells. Acetylation of histones increases the transcriptional activity of DNA, and, in the face of antiestrogen resistance, HDAC has become a target for pharmacological intervention.173,174 Histone deacetylase inhibitors block the enzyme, resulting in hyperacetylation of histones, relaxation of the chromatin, allowing for higher transcription of the DNA (Figure 3).
Preclinical studies determined that HDAC inhibitors increased transcription of the ER gene, restoring ER+ status and AI responsiveness to cell lines that were previously resistant.175,176 Currently, no HDAC inhibitors are approved by the FDA to treat BC, though entinostat is presently in 2 phase III trials (Figure 2).177
The most common adverse effects triggered by entinostat use are fatigue, nausea, neutropenia, and peripheral edema. The most frequently observed grade 3 or higher AEs were neutropenia and fatigue.15
Monoclonal antibodies
Breast cancer with HER2 overamplification (HER2+) was historically more challenging to treat than the corresponding HER2−, resulting in a worse OS and prognosis.178 This was before the development of mAbs, which have since improved the prognosis of HER2+ cancers, though they remain worse prognostically.179,180 Overamplification of HER2 over-activates signaling pathways, causing proliferation, motility, and survivability of the tumor cells, which makes difficult the treatment. Human epidermal growth factor receptor 2 tyrosine kinase pathway targets ER and promotes human BC cells to grow in a hormone-independent way.181
This scenario has been changed by introducing mAb, trastuzumab, and pertuzumab, targeting the HER2 receptor as a new strategy to treat HER2+ disease.182
Trastuzumab binds to extracellular domains of HER2183 activating antibody-dependent cellular cytotoxicity, blocks HER2 signaling (mostly PI3K/Akt pathway), and finally interrupts HER2 angiogenesis.184-187 Trastuzumab also blocks the proteolytic cleavage of the extracellular domain of HER2, therefore preventing the formation of a truncated, membrane-bound, constitutively active form of HER2 (known as p95HER2) (Figure 3).184-187 Pertuzumab binds extracellular domains of HER2 and inhibits the dimerization of HER2188 with other proteins of the HER family and therefore inhibits activation of the signaling pathway involved in promoting cell growth and opposing apoptosis (Figure 3).189
A third mAb, bevacizumab, is an anti-vascular endothelial growth factor (VEGF) targeting HER2− MBC. It is approved in Europe190 and Australia191 to treat BC. In the United States and Canada, bevacizumab is approved against other cancers, but not BC.192 Clinical trials had previously suggested that bevacizumab combined with taxanes seemed to be a highly effective first-line treatment for MBC.193,194 According to the FDA, based on 4 clinical trials (Figure 2), this mAb did not improve survival enough to outweigh the risks of increased blood pressure, internal bleeding, chest pain, and pulmonary embolism.191
Many patients with HER2+ tumors, especially those with MBC, demonstrate primary de novo or intrinsic resistance to trastuzumab as a monotherapy195-197 Truncation of the HER2 molecule itself198 represents the most prominent mechanism of resistance of HER2+ BC against trastuzumab.199 In the same way, most patients who initially respond to trastuzumab demonstrate disease progression within 1 year of treatment initiation. On the other hand, trastuzumab improves prognosis when used in combinations. It delays the disease progression and increases the OS when used as an adjuvant to traditional chemotherapeutic regimens with doxorubicin, cyclophosphamide, and paclitaxel.66,200,201 Trastuzumab administered concurrently with paclitaxel after doxorubicin, and cyclophosphamide is the most frequently used adjuvant schedule to treat HER2+ BC.200 The joint analysis of North Central Cancer Treatment Group NCCTG N9831 (combination chemotherapy with or without trastuzumab in treating women with HER2 overexpressing BC) and the National Surgical Adjuvant Breast and Bowel Project NSABP B-31 (doxorubicin and cyclophosphamide plus paclitaxel with or without trastuzumab in treating women with node-positive BC that overexpresses HER2) reported that adding trastuzumab to paclitaxel after doxorubicin and cyclophosphamide in early-stage HER2+ BC leads to a substantial reduction in cancer recurrence.200 Trastuzumab concurrently with paclitaxel increased 5 years DFS rates to 84.5%, and paclitaxel plus sequential trastuzumab increased 5 years DFS rates 80.1%.201
The NeoSphere trial showed the efficacy of mAb agents extends into the neoadjuvant setting as well, indicating that the combination of trastuzumab and pertuzumab with docetaxel had a 16.8% improvement.202 The same group203 previously reported a sustained benefit in event-free survival (EFS) from trastuzumab-containing neoadjuvant therapy followed by adjuvant trastuzumab in patients with locally advanced or inflammatory BC and provided a positive insight into the association between complete pathological remission and long-term outcomes in HER2+ disease.203
The addition of pertuzumab to the standard treatment of chemotherapy in combination with trastuzumab increased in 3 years DFS from 93.2 to 94.1% in patients with node-positive or high-risk node-negative HER2+ BC.204
The addition of carboplatin in a dose-equivalent, taxane (paclitaxel)-containing regimen plus trastuzumab had a significant advantage with respect to PFS and ORR over paclitaxel plus trastuzumab in women with HER-2-overexpressing MBC.205
Tryphaena conducted a randomized study to assess the cardiotoxicity of the addition of pertuzumab plus trastuzumab with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2+ early BC. The results demonstrated that the addition of pertuzumab does increase rates of cardiotoxicity, but the overall incidence was low.206
The cardiac safety profile of a neoadjuvant doxorubicin-based regimen followed by paclitaxel, trastuzumab, and pertuzumab with completion of 1 year of adjuvant trastuzumab-based therapy was confirmed by Yu et al.207
The most significant AEs related to mAb agents are cardiac. The risk of a primary and overall risk of cardiac events is slightly higher with the concurrent use of pertuzumab and trastuzumab than trastuzumab alone (0.7% pertuzumab vs 0.3% with trastuzumab alone).204 Apart from cardiac toxicity, the most common severe adverse effects associated with those mAb are diarrhea, neutropenia, and anemia.204
Antibody-drug conjugates
Monoclonal antibodies are also components of a subclass of drugs used to treat BC, the ADCs. They are composed of a targeted mAb linked to an antineoplastic agent.208 Currently, this class contains the following drugs approved by the FDA for BC treatment: trastuzumab emtansine (T-DM1)209 and trastuzumab-deruxtecan29,210 and sacituzumab govitecan-hziy.209 Trastuzumab emtansine is composed of trastuzumab linked to a fungal derivative that is a microtubule inhibitor.7 Trastuzumab emtansine is indicated for HER2+ MBC in both men and women following results of the EMILIA phase III trial showing an increased PFS with T-DM1 when compared to lapatinib plus capecitabine.50 The most frequent AEs seen with T-DM1 administration was thrombocytopenia, followed by elevated transaminases.50
The efficacy of T-DM1 for treating advanced HER2+ BC was further tested in the MARIANNE trial, which compared T-DM1 use to taxane plus trastuzumab. The findings showed that the PFS with T-DM1 was equivalent to taxane plus trastuzumab, but T-DM1 appeared more tolerable due to a lower proportion of grade 3+ AEs.211 The TH3RESA trial looked at T-DM1 versus physician’s choice treatment in patients with HER2+ ABC, which had progressed after treatment with 2 or more HER2-directed regimens. Trastuzumab emtansine demonstrated an absolute increase in OS of 6.9 months compared to physician’s choice treatment.212
The KATHERINE trial expanded the indications of T-DM1 by comparing adjuvant usage of T-DM1 to trastuzumab in women with HER2-positive early BC who had undergone neoadjuvant chemotherapy + trastuzumab yet still had invasive breast disease. The results showed an increase in DFS from 77% in the control group to 88.3% with T-DM1. This has led to the expansion of the clinical indications for T-DM1 and the development of a new standard of care.213
Three-year results from KRISTINE214,215 data showed a higher risk of an EFS associated with adjuvant T-DM1 plus pertuzumab and neoadjuvant, a similar risk of an invasive DFS, and less quality-of-life impairment in the neoadjuvant phase compared with neoadjuvant docetaxel, carboplatin, trastuzumab plus pertuzumab followed by adjuvant trastuzumab plus pertuzumab. Furthermore, relative to docetaxel, carboplatin, trastuzumab plus pertuzumab followed by adjuvant trastuzumab plus pertuzumab, the conventional systemic chemotherapy-sparing regimen of T-DM1 plus pertuzumab was associated with fewer serious AEs, grade 3 or greater AEs, and more AEs leading to treatment discontinuation. Overall, the observed worse EFS and similar invasive DFS associated with T-DM1 plus pertuzumab indicate the importance of selecting patients for conventional chemotherapy-sparing neoadjuvant regimen.214,215 Data from KAITLIN may further outline the clinical utility of adjuvant T-DM1 plus pertuzumab in patients with HER2-positive early BC.216
Trastuzumab-deruxtecan is composed of the mAb linked to a topoisomerase I inhibitor, similar to irinotecan.217 It is indicated in patients with HER2+ MBC that has previously been treated with T-DM1 following the results of the DESTINY trial showing an increase in response rate and OS.210 There are other DESTINY breast trials testing the efficacy of trastuzumab-deruxtecan in different populations or compared to other regimens for treating HER2-positive BC.218-220 Frequent AEs include decreased neutrophil count, anemia, and nausea. However, the most worrisome AE is the development of interstitial lung disease.210
ADCs have shown efficacy in treating HER2-negative cancers, with one being approved for treatment in April 2020: sacituzumab govitecan-hziy, an ADC composed of SN-38 an active metabolite of the topoisomerase I inhibitor irinotecan coupled to an anti-Trop-2 mAb. A trial in patients with metastatic TNBC showed an ORR of 33.3%, with a duration of response of 7.7 months.221 Other ADCs are on the horizon, one of which is ladiratuzumab vedotin, which is a microtubule disrupting agent conjugated to a mAb against the zinc transporter LIV-1, which was originally identified as an estrogen-induced gene in the cell line ZR-75-1 of BC.222 The presence of LIV-1 mRNA is associated with an ER + status and has been correlated with lymph node involvement of BC, suggesting a role for LIV-1 in metastasis.223
There are currently multiple phase I and II studies evaluating the safety and efficacy of ladiratuzumab vedotin in different BC populations.224,225
Bone modifying drugs
Denosumab, a human mAb that acts by neutralizing the receptor activator of nuclear factor κB ligand (RANKL)226 and zoledronate are targeted therapies classified as bone modifying drugs. Zoledronate acts by binding to and accumulating in the bone, inhibiting bone resorption by osteoclasts. Denosumab, on the other hand, binds to the RANK receptor on osteoclasts and promotes osteoclast differentiation and activity without accumulating in the bone. They are indicated for the prevention of skeletal-related events in patients with BC227,228 with bone metastases and the treatment of osteoporosis.229-232
The ASCO recommends adjuvant bisphosphonates to reduce bone recurrence and improve survival in postmenopausal patients with non-MBC.233,234 According to the ASCO, the absolute benefit is more significant in patients who are at higher risks of recurrence, and almost all trials were conducted in patients who also received systemic therapy. Most studies evaluated zoledronic acid or clodronate, and data are extremely limited for other bisphosphonates. While denosumab was found to reduce fractures, long-term survival data are still required.231
St. Gallen consensus (2019)234 considered the use of bisphosphonates in premenopausal patients on ovarian suppression with either tamoxifen or AIs. For postmenopausal patients, the panel strongly supported the use of bisphosphonates to improve DFS. And the panelists were clear in stating that denosumab twice a year should not be used as a substitute for bisphosphonate as suggested by the ABCSG-18 Trial,235 despite a recent publication on the positive impact on DFS.236
Immune checkpoint inhibitors and gamma-delta T cell stimulators
The microenvironment of BC tumors is composed of fibroblasts, leukocyte lineage cells (including lymphocytes, macrophages and myeloid-derived stromal cells), and the extracellular matrix, all of which play an important role in BC development and progression.237 Triple-negative breast cancer and HER2+ show the highest immunogenicity among the BC molecular subtypes.238,239 We further discuss 2 emerging immunotherapies, ICI of the PD-L1/PD-1 receptor and gamma-delta (γδ) T cells.
Immune checkpoint inhibitors (ICI). Some BC cells express the immune checkpoint regulator PD-L1 to suppress anti-tumor immune responses, leading to a pro-tumor microenvironment. PD-L1 inhibitors relieve the anti-tumor immune responses’ suppression, allowing the immune system to attack and kill the cancer cells.240
Programed death ligand 1, as a ligand of the PD-1 receptor, is an active biomarker that interacts with PD-1 receptor to suppress the immune system’s response to cancer. Prgramed death 1 is a receptor that triggers inhibitory actions; it is quickly induced on naïve T cells to counteract T cell activation. PD-1 activation regulates T cell activation, tolerance, and exhaustion, and effector T cell responses.241,242 Programed death ligand 1 expression can predict a favorable response to antibodies designed to unfetter anti-tumor activity.243 This means that PD-L1 and its receptor PD-1 represent crucial therapeutic targets to treat cancer as immune checkpoints regulating host immunity.244
There are 2 monoclonal antibodies targeting PD-1 (nivolumab, pembrolizumab) and 3 targeting PD-L1 (atezolizumab, avelumab, and durvalumab) approved by the FDA. These drugs have been approved with different indications as monotherapy or as combination therapy with radiation, chemotherapeutics, or other ICI.241 In this review, we approach pembrolizumab and atezolizumab, which are approved to treat BC.
Based on the presence or absence of T cells and the expression of PD-L1 by cancer cells, a tumor can be categorized into 4 groups: (1) PD-L1 positive, T cell positive; (2) PD-L1 negative, T cell positive; (3) PD-L1 positive, T cell negative; and (4) PD-L1 negative, T cell negative.245-247 Tumor responses with anti–PD1/L1 antibodies are not mediated by the antibody per se, but by tumor antigen-specific T cells that had been previously blocked by the PD1-PD-L1 interaction248,249
Many kinds of cancers are currently identified by IHC, which can detect the PD-L1 protein expression enhancing the response to anti-PD-L1-blockade. However, this method is not absolute regarding the use of PD-L1 as a predictor of responsiveness to the different anti-PD-L1 therapies.250 The facts that some patients who test positive for PD-L1 may not respond to the treatment, and that some patients who test negative may still respond are potential caveats to predict therapeutic response through PD-L1251,252 (reviewed by Ribas and Hu‑Lieskovan250). Reasons for the concerns on the limitations of the predictive value of PD-L1 include the differences in the sensitivity/sensibility of the commercial assays available or artifacts related to IHC and tissue sampling. In addition, PD-L1 expression in the tumor microenvironment is highly complex, being upregulated by aberrant genetic alterations and is highly regulated at the transcriptional, posttr6anscriptional, and protein levels. Thus, PD-L1 IHC seems to be insufficient to fully understand the relevance of PD-L1 levels in the whole body and their dynamics to improve therapeutic outcomes.251 Davis and Patel253 evaluated PD-L1 as a predictive biomarker based on all ICI approved by the FDA from 2011 to 2019. They reported that PD-L1 was predictive in only 28.9% of cases and was either not predictive (53.3%) or not tested (17.8%) in the remaining cases.253 A broad review on this topic has been published by Nimmagadda241 and Cottrell and Taube.243
Nonetheless, fortunately, numerous IHC assays have been approved254-256 to detect PD-L1 expression levels for specific drugs; each ICI has its own IHC assay specific to a distinct anti-PD-L1 antibody clone and a particular staining platform with specific tumor cell or immune cell thresholds.243,257
Although the different FDA-approved tests may detect the same biomarker, these tests’ performance characteristics differ, meaning the tests may detect different patient populations, as they are based on the data generated in the course of the corresponding therapeutic trial. Thus, a test is uniquely paired with a specific drug, and FDA approval of this drug-device pair was shown to be safe and effective for the stated intended use, including the specific indication.258,259
The availability of multiple PD-L1 assays and the appropriate choice for immune checkpoint therapy has become increasingly significant in TNBC. Higher expression of PD-L1 is observed in TNBC, more than in other molecular subtypes of BC.260 Moreover, in TNBC, PD-L1 is expressed mainly in tumor-infiltrating immune cells259,261 making PD-L1 inhibition attractive immunotherapy for the treatment of TNBC.
Atezolizumab, a mAb against PD-L1, has shown mixed results in clinical trials. The Impassion-031 trial studied atezolizumab combined with nab-paclitaxel (a paclitaxel protein-bound) followed by doxorubicin and cyclophosphamide as a neoadjuvant treatment for patients with early-stage TNBC eligible for surgery. The trial showed a polymerase chain reaction (pCR) of 58% in the atezolizumab group and 41% in the placebo group.262 Another trial, Impassion-130 looked at atezolizumab with nab-paclitaxel as a first-line treatment in untreated metastatic TNBC. Findings from this trial showed an increased PFS to 7.2 months with atezolizumab when compared to 5.5 months with placebo. Furthermore, Impassion130 showed that this increase in PFS was even greater in those patients who had PD-L1 positive tumors (7.5 months vs 5.0 months).263 Final results from this trial showed an improvement in median PFS of 2.2 months with atezolizumab plus nab-paclitaxel for patients with tumors that express PD-L1 on immune cells that cover 1% or more of the tumor area (PD-L1 immune cell-positive patients)264 while maintaining patient’s health-related quality of life.265 The results of these trials led to the approval of atezolizumab for early and metastatic TNBC with nab-paclitaxel. More recently, the Impassion-131 trial had less optimistic results when looking at atezolizumab in combination with paclitaxel for metastatic TNBC. The trial revealed that atezolizumab did not significantly improve PFS and that OS results at interim favored placebo over atezolizumab.266 This led to the FDA releasing a statement that further studies are needed to confirm the efficacy of atezolizumab in treating BC, and that continued approval of atezolizumab with nab-paclitaxel could be contingent on these additional studies.266
Pembrolizumab, a mAb anti-PD-L1, was evaluated in 2 different phase III clinical trials to be used in conjunction with chemotherapy in the treatment of TNBC.267,268 Keynote-522, a phase III trial, evaluated pembrolizumab plus chemotherapy (paclitaxel/carboplatin followed by cyclophosphamide) compared with placebo plus chemotherapy as neoadjuvant therapy followed by adjuvant pembrolizumab or placebo in patients with early-stage TNBC.267 This phase III trial showed a significantly higher percentage of patients with a pCR among those who received pembrolizumab plus neoadjuvant chemotherapy than those who received placebo plus chemotherapy regardless of PD-L1 status.267 Pembrolizumab was also evaluated combined with chemotherapy (nab-paclitaxel; paclitaxel; or gemcitabine/carboplatin) as a first-line treatment for patients with locally recurrent inoperable or metastatic TNBC in the Keynote-355 phase III trial.268 This study showed a statistically significant and clinically meaningful improvement in PFS when pembrolizumab was added to 3 different chemo regiments in TNBC patients whose tumors expressed PD-L1 (Combined Positive Score ⩾ 10).268 Such results lead the FDA, in November 2020, to grant an accelerated approval of pembrolizumab in combination with chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin) for the treatment of patients with locally recurrent unresectable or TNBC whose tumors express PD-L1 (CPS ⩾ 10).12
Gamma-delta T (γδ T) cell stimulators: The γδ T cells are a subset of T lymphocytes characterized by T cell receptors (TCR) composed of γ and δ chains which can recognize a great variety of antigens including peptides, unprocessed proteins, sulfatides, and phospholipids without a requirement for major histocompatibility complex (MHC) antigen presentation.13,14,269,270 Moreover, γδ T cells also express natural killer receptors such as NKG2D.13,14 Different subtypes of γδ T cells display spectrums of phenotypic and functional characteristics ranging from innate to adaptive like features.271,272
γδ T cells are involved in the lymphoid stress-surveillance response by recognizing antigens that are upregulated in stressed and transformed cells but not expressed in healthy tissues, giving it an advantage as the first line of defense against transformed cells.13,14,272,273 There are 2 major subtypes of γδ T lymphocytes in humans, the Vδ1+ T cells, which are present in skin and intestine and the Vδ2+ T cells which are present in the peripheral blood.13,14,274 Vδ1+ T cells represent the predominant γδ T cell subtype that has been found in healthy breast tissue272 as well as tumor-infiltrating lymphocytes (TILs) for many cancers14,275,276 and have also been implicated to be involved in BC.13,274,277,278 A recent clinical study demonstrated PFS and OS correlated with Vδ1+ T cells representation for TNBC patients.273 A subset of Vδ2+ T cells expressing Vγ9Vδ2+ TCRs is abundant in human peripheral blood, making it optimal for isolation and use in clinical trials. Vγ9Vδ2+ T cells display antitumor immunity in response to phosphoantigens produced by cellular pathogens and overexpressed by cancers. Aminobisphosphonates (N-bis), such as zoledronate (zoledronic acid), also a phosphoantigen, is a promising drug in the treatment of cancer by targeting the mevalonate pathway (Figure 3).13,14,279 As discussed above, zoledronate is the most potent and efficacious clinically approved N-bis, which are prescribed for osteoporosis.280,281 and is also under clinical trial for BC as a γδ T cell stimulator (Figure 2), which is the newest potential class of drugs to treat ABC and MBC.274
Conclusion
Resistance to prior standard treatments is an important driving force for the discovery and development of new BC drugs and/or new strategies applying the existing drugs. Combined therapies comprising different classes of drugs is an approach successfully used as reasonable options to establish the best prognosis providing patients with the opportunity to obtain the best benefit while minimizing or abolishing recurrence, resistance, and toxic effects. Besides that, researches on cellular pathways have led to studies of new agents targeting or inhibiting tumorigeneses.
This rationale yielded the researches on biomarkers of clinical significance such as CDK4/6 expression and PIK3CA mutations. PIK3 inhibitors are the newest BC treatments approved by the FDA.
The forefront current drugs under clinical trials are mAb, ADC, HDAC inhibitors, PARP inhibitors, and ultimately the γδ T cell stimulators, but their role is unclear and still under investigation.
As research continues, new classes of drugs are emerging with increased benefits in BC treatment. The complexity of the cell cycle and its association with cancer biology provides a plethora of factors toward the development of more efficient agents as well as biomarkers with high predictive values.
Acknowledgments
To Michigan State University (MSU): College of Osteopathic Medicine, Department of Biochemistry and Molecular Biology and Department of Pharmacology and Toxicology. The authors are grateful to Dr Karen Liby of MSU, for updating information about breast cancer classification and treatment. They also thank Dr John Wang of MSU for his comments and suggestions during the preparation of the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support for the publication fees of this article from MSU-COM.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: J.S., R.P.R., and C.R. contributed equally. C.R. wrote the bulk of the manuscript and edited the final version. C.R. and R.P.R. conceived the project. C.R. coordinated manuscript production. C.R., J.S., and R.P.R. participated in literature searching. C.R., J.S., and R.P.R. wrote, edited, and approved the final version of the manuscript.
ORCID iD: Carolina Restini  https://orcid.org/0000-0001-5158-485X
https://orcid.org/0000-0001-5158-485X
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Updated January 12, 2021. Accessed January 15, 2021.
- 3. Li C, Uribe D, Daling J. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046-1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kittaneh M, Montero AJ, Gluck S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013;5:61-70. doi: 10.4137/BIC.S9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533-1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134-1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 7. Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280-9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 8. Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance [published online ahead of print March 19, 2019]. Ther Adv Med Oncol. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao S, Zuo WJ, Shao ZM, Jiang YZ. Molecular subtypes and precision treatment of triple-negative breast cancer. Ann Transl Med. 2020;8:499. doi: 10.21037/atm.2020.03.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Litton JK, Rugo HS, Hurvitz SA, et al. Talazoparib in patients with advanced breast cancer and germline BRCA mutation. N Engl J Med. 2018;379:753-763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomized, open-label, phase 3 trial. Lancet Oncol. 2017;18:732-742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pembrolizumab is now also approved by the FDA for metastatic PDL1+ (CPS>10) TNBC. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple. Accessed January 13, 2021.
- 13. Morrow ES, Roseweir A, Edwards J. The role of gamma delta T lymphocytes in breast cancer: a review. Transl Res. 2019;203:88-96. doi: 10.1016/j.trsl.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 14. Nussbaumer O, Koslowski M. The emerging role of γδ T cells in cancer immunotherapy. Immuno-Oncol Technol. 2019;1:3-10. doi: 10.1016/j.iotech.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31:2128-2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang Z, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:806-815. doi: 10.1016/S1470-2045(19)30164-0. [DOI] [PubMed] [Google Scholar]
- 17. Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel). 2013;8:149-154. doi: 10.1159/000350774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:23-31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421-1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 20. Mueller C, Haymond A, Davis JB, Williams A, Espina V. Protein biomarkers for subtyping breast cancer and implications for future research. Expert Rev Proteomics. 2018;15:131-152. doi: 10.1080/14789450.2018.1421071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumors reveals novel subgroups. Nature. 2012;486:346-352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747-752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 23. Yersal O, Barutca S. Biological subtypes of breast cancer—prognostic and therapeutic implications. World J Clin Oncol. 2014;5:412-424. doi: 10.5306/wjco.v5.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molecular subtypes of breast cancer. https://www.breastcancer.org/symptoms/types/molecular-subtypes. Accessed October 20, 2020.
- 25. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346-1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 26. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561-570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szostakowska M, Trębińska-Stryjewska A, Grzybowska EA, Fabisiewicz A. Resistance to endocrine therapy in breast cancer: molecular mechanisms and future goals. Breast Cancer Res Treat. 2019;173:489-497. doi: 10.1007/s10549-018-5023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217-233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7:1511-1519. doi: 10.4155/fmc.15.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eroglu Z, Tagawa T, Somlo G. Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer. Oncologist. 2014;19:135-150. doi: 10.1634/theoncologist.2013-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerbin CS. Activation of ERBB receptors. Nat Educ. 2010;3:35. https://www.nature.com/scitable/topicpage/activation-of-erbb-receptors-14457210/. Accessed June 20, 2020. [Google Scholar]
- 32. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 33. Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54:34-44. doi: 10.4132/jptm.2019.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xuhong J-C, Qi X-W, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019;9:2103-2119. [PMC free article] [PubMed] [Google Scholar]
- 35. van Seijen M, Lips EH, Thompson AM, et al. PRECISION team. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer. 2019;121:285-292. doi: 10.1038/s41416-019-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Latta EK, Tjan S, Parkes RK, O’Malley FP. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002;15:1318-1325. doi: 10.1097/01.MP.0000038462.62634.B1. [DOI] [PubMed] [Google Scholar]
- 37. Borgquist S, Zhou W, Jirström K, et al. The prognostic role of HER2 expression in ductal breast carcinoma in situ (DCIS); a population-based cohort study. BMC Cancer. 2015;15:468. doi: 10.1186/s12885-015-1479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Purdie CA, Baker L, Ashfield A, et al. Increased mortality in HER2 positive, oestrogen receptor positive invasive breast cancer: a population-based study. Br J Cancer. 2010;103:475-481. doi: 10.1038/sj.bjc.6605799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27:95-120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gil Del Alcazar CR, Huh SJ, Ekram MB, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7:1098-1115. doi: 10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lesurf R, Aure MR, Mørk HH, et al. Molecular features of subtype-specific progression from ductal carcinoma in situ to invasive breast cancer. Cell Rep. 2016;16:1166-1179. doi: 10.1016/j.celrep.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 42. Prat A, Cheang MCU, Martín M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol. 2013;31:203-209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18:4465-4472. doi: 10.1158/1078-0432.CCR-12-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339-345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 45. Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16:734. doi: 10.1186/s12885-016-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92-98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 49. Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301-1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 50. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783-1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415-2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 52. Chen Q, Ouyang D, Anwar M, et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with HER2-positive metastatic breast cancer: a real-world, multicentre analysis. Front Oncol. 2020;10:811. doi: 10.3389/fonc.2020.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Criscitiello C, Azim HA, Jr, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23:vi13-vi18. [DOI] [PubMed] [Google Scholar]
- 54. Konstantinopoulos PA, Ceccaldi R, Shapiro G, II, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137-1154. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura M. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep. 2020;10:1-8. doi: 10.1038/s41598-020-59671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282-4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press). 2016;8:93-107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Székely B, Silber ALM, Pusztai L. New therapeutic strategies for triple-negative breast cancer. Oncology (Williston Park). 2017;31:130-137. http://www.ncbi.nlm.nih.gov/pubmed/28205193. [PubMed] [Google Scholar]
- 59. Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279-287. doi: 10.1007/s10549-016-4059-6. [DOI] [PubMed] [Google Scholar]
- 60. The Cancer Genome Atlas program. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga. Accessed June 20, 2020.
- 61. Nixon MJ, Formisano L, Mayer IA, et al. PIK3CA and MAP3K1 alterations imply luminal A status and are associated with clinical benefit from pan-PI3K inhibitor buparlisib and letrozole in ER+ metastatic breast cancer. NPJ Breast Cancer. 2019;5:31. doi: 10.1038/s41523-019-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Finn RS, Liu Y, Zhu Z, et al. Biomarker analyses of response to cyclin dependent kinase 4/6 inhibition of endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26:110-121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 63. Brandão M, Caparica R, Eiger D, de Azambuja E. Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Ann Oncol. 2019;30:x27-x42. doi: 10.1093/annonc/mdz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Herr D, Wischnewsky M, Joukhadar R, et al. Does chemotherapy improve survival in patients with nodal positive luminal A breast cancer? A retrospective Multicenter Study. PLoS ONE. 2019;14:e0218434. doi: 10.1371/journal.pone.0218434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martín M, Rodríguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805-814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 66. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 67. Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23:489-502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 68. Willson ML, Burke L, Ferguson T, Ghersi D, Nowak AK, Wilcken N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2019;9:CD004421. doi: 10.1002/14651858.CD004421.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Swaim SM, Tang G, Geyer CE. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol. 2013;31:3197-3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 71. Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicine and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003;21:588-592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 72. Slamon D, Eiermann W, Robert N, Pienkowski T, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273-1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24:628-637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ Book. 2016;35:34-42. doi: 10.1200/EDBK_159135. [DOI] [PubMed] [Google Scholar]
- 75. Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497-1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 76. Peto R, Davies C, Godwin J, et al.; EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432-444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055-2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29:2653-2659. doi: 10.1200/jco.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 79. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593-602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 80. Qin T, Yuan ZY, Peng RJ. Efficacy and tolerability of toremifene and tamoxifen therapy in premenopausal patients with operable breast cancer: a retrospective analysis. Curr Oncol. 2013;20:196-204. doi: 10.3747/co.20.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65:125-134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 82. EBCTCG—Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomized trials. Lancet. 2011;378:771-784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gray RG, Rea D, Handley K, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31:5. doi: 10.1200/jco.2013.31.18_suppl.5.23169505 [DOI] [Google Scholar]
- 85. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436-446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122-137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437-1452. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gottardis MM, Ricchio ME, Styaswaroop PG, Jordan VC. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res. 1990;50:3189-3192. [PubMed] [Google Scholar]
- 89. DeMichele A, Troxel AB, Berlin JA, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008;26:4151-4159. doi: 10.1200/JCO.2007.14.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67-75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Euhus DM, Diaz J. Breast cancer prevention. Breast J. 2014;21:76-81. doi: 10.1111/tbj.12352. [DOI] [PubMed] [Google Scholar]
- 93. Ma C, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15:261-275. doi: 10.1038/nrc3920. [DOI] [PubMed] [Google Scholar]
- 94. Ioannides SJ, Barlow PL, Elwood JM, Porter D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res Treat. 2014;147:237-248. doi: 10.1007/s10549-014-3091-7. [DOI] [PubMed] [Google Scholar]
- 95. Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411-3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 96. EBCTCG—Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:341-352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 97. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209-219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pistelli M, Mora AD, Ballatore Z, Berardi R. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr Oncol. 2018;25:e168-e175. doi: 10.3747/co.25.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 100. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 102. Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow up. Breast Cancer Res Treat. 2019;174:719-729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867-3873. [PubMed] [Google Scholar]
- 104. Pike AC, Brzozowski AM, Walton J, et al. Structural insights into the mode of action of a pure antiestrogen. Structure. 2001;9:145-153. doi: 10.1016/s0969-2126(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 105. Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500mg versus anastrozole 1 mg for the first line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33:3781-3787. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomized, double-blind, phase 3 trial. Lancet. 2016;388:2997-3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 107. Mahtani RL. A role for fulvestrant monotherapy in the first-line treatment of ER+ metastatic breast cancer? Breast J. 2020;26:109-111. doi: 10.1111/tbj.13495. [DOI] [PubMed] [Google Scholar]
- 108. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209-219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 109. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926-1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 110. Ma XC, Sparano JA. Treatment Approach to Metastatic Hormone Receptor-Positive, HER2-Negative Breast Cancer: Endocrine Therapy and Targeted Agents. Waltham, MA: UpToDate. https://www.uptodate.com/contents/treatment-approach-to-metastatic-hormone-receptor-positive-her2-negative-breast-cancer-endocrine-therapy-and-targeted-agents?topicRef=83848&source=see_link. Accessed November 3, 2020. [Google Scholar]
- 111. Rusz O, Kószó R, Dobi Á, et al. Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: a case series. Onco Targets Ther. 2018;11:5459-5463. doi: 10.2147/OTT.S170736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130-146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25-35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 114. DeMichele A, Cristofanilli M, Brufsky A, et al. Overall survival for first-line palbociclib plus letrozole vs letrozole alone for HR+/HER2- metastatic breast cancer patients in U.S. real-world clinical practice. Cancer Res. 2020;80:4. doi: 10.1158/1538-7445.SABCS19-P1-19-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy- MONARCH 2. JAMA Oncol. 2020;6:116-124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307-316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 117. Sobhani N, D’Angelo A, Pittacolo M, et al. Updates on the CDK4/6 inhibitory strategy and combinations in breast cancer. Cells. 2019;8:321. doi: 10.3390/cells8040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Duffy MJ, Harbeck N, Nap M, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European group on tumor markers (EGTM). Eur J Cancer. 2017;75:284-298. doi: 10.1016/j.ejca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 119. Le Du F, Ueno NT, Gonzalez-Angulo AM. Breast cancer biomarkers: utility in clinical practice. Curr Breast Cancer Rep. 2013;5:284-292. doi: 10.1007/s12609-013-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954-2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 121. Koboldt D, Fulton R, McLellan M, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61-70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mollon L, Aguilar A, Anderson E, et al. A systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in HR+/HER2-metastatic breast cancer. Cancer Res. 2018;78:1207. doi: 10.1158/1538-7445.AM2018-1207. [DOI] [Google Scholar]
- 123. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379:2052-2062. doi: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 124. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929-1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 125. Dickler MN, Saura C, Richards DA, et al. Phase II study of taselisib (GDC-0032) in combination with fulvestrant in patients with HER2-negative, hormone receptor-positive advanced breast cancer. Clin Cancer Res. 2018;24:4380-4387. doi: 10.1158/1078-0432.CCR-18-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. FDA approves first PI3K inhibitor for breast cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-first-pi3k-inhibitor-breast-cancer. Accessed November 9, 2020. [DOI] [PubMed]
- 128. Fritsch C, Huang A, Chatenay-Rivauday C, et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther. 2014;13:1117-1129. doi: 10.1158/35-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 129. Al-Sukhun S, Lataifeh I, Al-Sukhun R. Defining the prognostic and predictive role of PIK3CA mutations: sifting through the conflicting data. Curr Breast Cancer Rep. 2016;8:73-79. doi: 10.1007/s12609-016-0215-6. [DOI] [Google Scholar]
- 130. Croessmann S, Sheehan JH, Lee KM, et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (PI3K), generate oncogene dependence, and are exquisitely sensitive to PI3Kα inhibitors. Clin Cancer Res. 2018;24:1426-1435. doi: 10.1158/1078-0432.CCR-17-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6:154-166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127-150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vasan N, Razavi P, Johnson JL, et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science. 2019;366:714-723. doi: 10.1126/science.aaw9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progression on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2017;19:87-100. doi: 10.1016/s1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 135. Vanderbilt-Ingram Cancer Center. GDC-0941 and cisplatin in treating patients with androgen receptor-negative triple negative metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT01918306. Update May 2017. Accessed January 24, 2021.
- 136. Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989;7:1120-1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- 137. Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression. Oncologist. 2002;7:31-39. doi: 10.1634/theoncologist.7-suppl_4-31. [DOI] [PubMed] [Google Scholar]
- 138. Ferrero JM, Ramaioli A, Largillier R, et al. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12:841-846. doi: 10.1023/a:1011183421477. [DOI] [PubMed] [Google Scholar]
- 139. Rampaul RS, Pinder SE, Wencyk PM, et al. Epidermal growth factor receptor status in operable invasive breast cancer: is it of any prognostic value? Clin Cancer Res. 2004;10:2578. [PubMed] [Google Scholar]
- 140. Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13:3-17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 141. Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71:67-75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 142. Lee K, Jang MH, Chung YR, et al. Prognostic significance of centromere 17 copy number gain in breast cancer depends on breast cancer subtype. Hum Pathol. 2017;61:111-120. doi: 10.1016/j.humpath.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 143. Burris HA, 3rd. Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9:10-15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 144. Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170-1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 145. Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13:1114-1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- 146. FDA approves neratinib for metastatic HER2-positive breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neratinib-metastatic-her2-positive-breast-cancer. Accessed October 31, 2020.
- 147. Chan A. Neratinib in HER-2-positive breast cancer: results to date and clinical usefulness. Ther Adv Med Oncol. 2016;8:339-350. doi: 10.1177/1758834016656494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer. Accessed October 31, 2020.
- 149. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597-609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 150. Blair HA. Pyrotinib: first global approval. Drugs. 2018;78:1751-1755. doi: 10.1007/s40265-018-0997-0. [DOI] [PubMed] [Google Scholar]
- 151. Harbeck N, Huang CS, Hurvitz S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 2016;17:357-366. doi: 10.1016/S1470-2045(15)00540-9. [DOI] [PubMed] [Google Scholar]
- 152. Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619-631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 153. Morales J, Li L, Fattah FJ, et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24:15-28. doi: 10.1615/critreveukaryotgeneexpr.2013006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lin KY, Kraus WL. PARP inhibitors for cancer therapy. Cell. 2017;169:183. doi: 10.1016/j.cell.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 155. Patel M, Nowsheen S, Maraboyina S, Xia F. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review. Cell Biosci. 2020;10:35. doi: 10.1186/s13578-020-00390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409-433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Geenen JJJ, Linn SC, Beijnen JH, Schellens JHM. PARP inhibitors in the treatment of triple-negative breast cancer. Clin Pharmacokinet. 2018;57:427-437. doi: 10.1007/s40262-017-0587-4. [DOI] [PubMed] [Google Scholar]
- 158. Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152-1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293-301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387-393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610-621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gourley C, Balmana J, Ledermann JA, et al. Moving from poly (ADPRibose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J Clin Oncol. 2019;37:2257-2269. [DOI] [PubMed] [Google Scholar]
- 163. Jiang X, Li W, Li X, Bai H, Zhang Z. Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Cancer Manag Res. 2019;11:4371-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yi M, Dong B, Qin S, Chu Q, Wu K, Luo S. Advances and perspectives of PARP inhibitors. Exp Hematol Oncol. 2019;8:29. doi: 10.1186/s40164-019-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523-533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 166. Caulfield SE, Davis CC, Byers KF. Olaparib: a novel therapy for metastatic breast cancer in patients with a BRCA1/2 mutation. J Adv Pract Oncol. 2019;10:167-174. doi: 10.6004/jadpro.2019.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. FDA approves olaparib for germline BRCA-mutated metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer. Published 2018.
- 168. Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579-1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]
- 169. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19:1126-1134. doi: 10.1016/S1470-2045(18)30343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Murai J, Zhang Y, Morris J, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther. 2014;349:408-416. doi: 10.1124/jpet.113.210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Exman P, Barroso-Sousa R, Tolaney SM. Evidence to date: talazoparib in the treatment of breast cancer. Onco Targets Ther. 2019;12:5177-5187. doi: 10.2147/OTT.S184971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Clarke R, Skaar TC, Bouker KB, et al. Molecular and pharmacological aspects of antiestrogen resistance. J Steroid Biochem Mol Biol. 2001;76:71-84. doi: 10.1016/s0960-0760(00)00193-x. [DOI] [PubMed] [Google Scholar]
- 173. Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73-84. doi: 10.1016/0092-8674(93)90051-Q. [DOI] [PubMed] [Google Scholar]
- 174. Richon VM, Emilani S, Verdin E, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA. 1998;95:3003-3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res. 2001;61:7025-7029. [PubMed] [Google Scholar]
- 176. Sabnis GJ, Goloubeva OG, Kazi AA, Shah P, Brodie AH. HDAC inhibitor entinostat restores responsiveness of letrozole resistant MCF-7Ca xenografts to AIs through modulation of Her-2. Mol Cancer Ther. 2013;12:2804-2816. doi: 10.1158/1535-7163.MCT-13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yeruva SLH, Zhao F, Miller KD, et al. E2112: randomized phase III trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. NPJ Breast Cancer. 2018;4:1. doi: 10.1038/s41523-017-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto oncogene in human breast and ovarian cancer. Science. 1989;244:707-712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 179. Fallahpour S, Navaneelan T, De P, Borgo A. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open. 2017;5:e734-e739. doi: 10.9778/cmajo.20170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Li Z-h, Hu P-h, Tu J-h, Yu N-s. Luminal B breast cancer: patterns of recurrence and clinical outcome. Oncotarget. 2016;7:65024-65033. doi: 10.18632/oncotarget.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Pietras RJ, Arboleda J, Reese DM, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435-2446. [PubMed] [Google Scholar]
- 182. Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Cho H, Mason K, Ramyar K, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756-760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 184. Barok M, Isola J, Pályi-Krekk Z, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065-2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 185. Molina MA, Codony-Servant J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744-4749. [PubMed] [Google Scholar]
- 186. Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumor biology: Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279-280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 187. McKeage K, Perry CM. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs. 2002;62:209-243. doi: 10.2165/00003495-200262010-00008. [DOI] [PubMed] [Google Scholar]
- 188. Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317-328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 189. Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127-137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 190. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/equidacent. Accessed January 13, 2021.
- 191. Therapeutic Goods Administration (TGA). Australian public assessment report for bevacizumab. https://www.tga.gov.au/sites/default/files/auspar-bevacizumab-200226.pdf. Update February 2020. Accessed January 13, 2021.
- 192. Breast Cancer Org. FDA removes breast cancer indication from Avastin. https://www.breastcancer.org/research-news/20111118. Accessed June 3, 2020.
- 193. Lorusso V. Bevacizumab in the treatment of HER2-negative breast cancer. Biologics. 2008;2:813-821. doi: 10.2147/btt.s3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. O’Shaughnessy JA, Brufsky AM. RiBBON 1 and RiBBON 2: phase III trials of bevacizumab with standard chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2008;8:370-373. doi: 10.3816/CBC.2008.n.045. [DOI] [PubMed] [Google Scholar]
- 195. Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737-744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 196. Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639-2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 197. Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719-726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 198. Molina MA, Sáez R, Ramsey EE, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347-353. [PubMed] [Google Scholar]
- 199. Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15:7479-7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744-3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29:4491-4497. doi: 10.1200/JCO.2011.36.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791-800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 203. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640-647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 204. von Minckwitz G, Procter M, de Azambuja E, et al.; for the APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122-131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786-2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 206. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278-2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 207. Yu AF, Singh JC, Wang R, et al. Cardiac safety of dual anti-HER2 therapy in the neoadjuvant setting for treatment of HER2-positive breast cancer. Oncologist. 2017;22:642-647. doi: 10.1634/theoncologist.2016-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chari RVJ, Martell BA, Gross JL, et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52:127-131. [PubMed] [Google Scholar]
- 209. Lambert JM, Chari RV. Ado-trastuzumab emtansine (T-DM1): an antibody drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57:6949-6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 210. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141-148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Krop IE, Kim S-B, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18:743-754. doi: 10.1016/S1470-2045(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 213. von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617-628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 214. Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37:2206-2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115-126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 216. NCT0196647. A study of trastuzumab emtansine (Kadcyla) plus pertuzumab (Perjeta) following anthracyclines in comparison with trastuzumab (Herceptin) plus pertuzumab and a taxane following anthracyclines as adjuvant therapy in participants with operable HER2-positive primary breast cancer. https://clinicaltrials.gov/ct2/show/NCT01966471. Update January 12, 2021. Accessed January 13, 2021.
- 217. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097-5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 218. AstraZeneca. DS-8201a in pre-treated HER2 breast cancer that cannot be surgically removed or has spread [DESTINY-Breast02]. NLM identifier: NCT03523585. https://clinicaltrials.gov/ct2/show/NCT03523585?term=DS8201&cond=Breast+Cancer&draw=2&rank=3. Accessed November 11, 2020. [Google Scholar]
- 219. AstraZeneca. DS-8201a versus T-DM1 for human epidermal growth factor receptor 2 (HER2)-positive, unresectable and/or metastatic breast cancer previously treated with trastuzumab and taxane [DESTINY-Breast03]. NLM identifier: NCT03529110. https://clinicaltrials.gov/ct2/show/NCT03529110?term=DS8201&cond=Breast+Cancer&draw=2&rank=1. Accessed November 11, 2020. [Google Scholar]
- 220. AstraZeneca. Trastuzumab deruxtecan (DS-8201a) versus investigator’s choice for HER2-low breast cancer that has spread or cannot be surgically removed [DESTINY-Breast04]. NLM Identifier: NCT03734029. https://clinicaltrials.gov/ct2/show/NCT03734029. Accessed November 11, 2020. [Google Scholar]
- 221. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741-751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 222. Manning DL, Daly RJ, Lord PG, Kelly KF, Green CD. Effects of oestrogen on the expression of a 4.4 kb mRNA in the ZR-75-1 human breast cancer cell line. Mol Cell Endocrinol. 1988;59:205-212. doi: 10.1016/0303-7207(88)90105-0. [DOI] [PubMed] [Google Scholar]
- 223. Manning DL, Robertson JF, Ellis IO, et al. Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur J Cancer. 1994;30A:675-678. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 224. Hoffmann-La Roche. A study evaluating the efficacy and safety of multiple immunotherapy-based treatment combinations in patients with metastatic or inoperable locally advanced triple-negative breast cancer (Morpheus-TNBC). NLM Identifier: NCT03424005. https://clinicaltrials.gov/ct2/show/NCT03424005.
- 225. Seagen. A safety study of SGN-LIV1A in breast cancer patients. NLM identifier: NCT01969643. https://clinicaltrials.gov/ct2/show/study/NCT01969643.
- 226. Yee AJ, Raje NS. Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin Interv Aging. 2012;7:331-338. doi: 10.2147/CIA.S14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132-5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 228. Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564-1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 229. von Moos R, Body JJ, Egerdie B, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer. 2013;21:3497-3507. doi: 10.1007/s00520-013-1932-2. [DOI] [PubMed] [Google Scholar]
- 230. Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082-3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 231. Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:2062-2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 232. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;148:677-692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 233. Hospices Civils de Lyon. Comparative study of neoadjuvant chemotherapy with and without zometa for management of locally advanced breast cancers (NEOZOL). NLM Identifier: NCT01367288. https://clinicaltrials.gov/ct2/show/NCT01367288?term=gamma+delta+T+cells&cond=Breast+Cancer&draw=1&rank=4. Accessed June 4, 2020. [DOI] [PubMed] [Google Scholar]
- 234. Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N.. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel). 2019;14:103-110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Gnant M, Pfeiler G, Dubsky PC, et al.; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433-443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 236. Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:339-351. doi: 10.1016/S1470-2045(18)30862-3. [DOI] [PubMed] [Google Scholar]
- 237. Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang S, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109:2796-2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast. 2017;35:142-150. doi: 10.1016/j.breast.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 239. Nagarajan D, McArdle S. Immune landscape of breast cancers. Biomedicines. 2018;6:20. doi: 10.3390/biomedicines6010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Chen DS, Mellman I. Oncology meets immunology: the cancer immunity cycle. Immunity. 2013;39:1-10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 241. Nimmagadda S. Quantifying PD-L1 expression to monitor immune checkpoint therapy: opportunities and challenges. Cancers (Basel). 2020;12:3173. doi: 10.3390/cancers12113173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813-824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 243. Cottrell TR, Taube JM. PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J. 2018;24:41-46. doi: 10.1097/PPO.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450-461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2103;19:1021-1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139-2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ock CY, Keam B, Kim S, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res. 2016;22:2261-2270. doi: 10.1158/1078-0432.CCR-15-2834. [DOI] [PubMed] [Google Scholar]
- 248. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med. 2016;213:2835-2840. doi: 10.1084/jem.20161462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 252. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 253. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. PD-L1 IHC 22C3 pharmDx. Premarket approval (PMA) database. FDA. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150013. Updated January 1, 2021. Accessed January 13, 2021. [Google Scholar]
- 255. VENTANA. PD-L1 (SP-142) assay: interpretation guide for triple-negative breast carcinoma (TNBC). productlibrary.ventana.com. (2019). Ventana Product Document Library. U.S. VERSION—Package Insert, VENTANA PD-L1 (SP142) Assay. Roche. http://productlibrary.ventana.com/ventana_portal/OpenOverlayServlet?launchIndex=1&objectId=740-48591017981US. Updated March 3, 2019. Accessed January 13, 2021.
- 256. PD-L1 IHC 28-8 PHARMDX. Premarket approval (PMA) database. FDA. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150025S003. Updated January 1, 2021. Accessed January 13, 2021. [Google Scholar]
- 257. Kerr KM. The PD-L1 immunohistochemistry biomarker: two steps forward, one step back? J Thorac Oncol. 2018;13:291-294. doi: 10.1016/j.jtho.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 258. Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051-1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Torlakovic E, Lim HJ, Adam J, et al. “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. 2020;33:4-17. doi: 10.1038/s41379-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449-5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (Impassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090-1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 263. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advance triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 264. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44-59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 265. Adams S, Diéras V, Barrios CH, et al. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann Oncol. 2020;31:582-589. doi: 10.1016/j.annonc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 266. U.S. Food and Drug Administration. FDA Alerts Healthcare Professionals & Oncology Clinical Investigators. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel. Published 2020. Accessed November 11, 2020.
- 267. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810-821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 268. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38:1000. doi: 10.1200/JCO.2020.38.15_suppl.1000. [DOI] [PubMed] [Google Scholar]
- 269. Ferreira LM. Gammadelta T cells: innately adaptive immune cells. Int Rev Immunol. 2013;32:223-248. doi: 10.3109/08830185.2013.783831. [DOI] [PubMed] [Google Scholar]
- 270. Lafont V, Sanchez F, Laprevotte E, et al. Plasticity of gamma-delta T cells: impact on the anti-tumor response. Front Immunol. 2014;5:622. doi: 10.3389/fimmu.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Davey MS, Willcox CR, Hunter S, et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9- subsets. Nat Commun. 2018;9:1760. doi: 10.1038/s41467-018-04076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467-478. doi: 10.1038/nri278. [DOI] [PubMed] [Google Scholar]
- 273. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184-196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 274. Wu Y, Kyle-Cezar F, Woolf RT, et al. An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci Transl Med. 2019;11:eaax9364. doi: 10.1126/scitranslmed.aax9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Cordova A, Toia F, La Mendola C, et al. Characterization of human γδ t lymphocytes infiltrating primary malignant melanomas. PLoS ONE. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Meraviglia S, Lo Presti E, Tosolini M, et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology. 2017;6:e1347742. doi: 10.1080/2162402X.2017.1347742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Peng G, Wang HY, Peng W, et al. Tumor-infiltrating gamma-delta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334-348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 278. Ye J, Ma C, Wang F, et al. Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer Res. 2013;73:6137-6148. doi: 10.1158/0008-5472.CAN-13-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Kabelitz D, Dechanet-Merville J. Recent advances in gamma/delta T cell biology: new ligands, new functions, and new translational perspectives. Front Immunol. 2015;6:371. doi: 10.3389/fimmu.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Devogelaer JP, Brown JP, Burckhardt P, et al. Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteoporos Int. 2007;18:1211-1218. doi: 10.1007/s00198-007-0367-3. [DOI] [PubMed] [Google Scholar]
- 281. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]