Abstract
A whole virus, inactivated, Porcine Circovirus 2b (PCV2b) vaccine was submitted to a quantal assay of potency, as explained in detail in our companion paper [1]. To this purpose, twenty, 45-day old piglets, checked for maternally-derived antibody (MDA), were allocated to four groups of 5 animals each; these were vaccinated with 800/266/88/0 nanograms, respectively, of an inactivated PCV2b strain, consisting of two distinct virion populations. Twenty-six days later, all the pigs were challenged intranasally with the homologous PCV2b strain. In the presence of a clear dose-dependent protection in terms of viremia, no such effect was observed in terms of weight gain after challenge. The 800 and 266-ng payloads were associated with neutralizing antibody titers above the MDA levels in oral fluids. Higher levels of viremia in control and 88-ng groups [1] coincided with a higher Natural Killer activity of tracheobronchial lymph node cells from PCV2-infected pigs. The PCV2 ORF2-specific ELISPOT assay for IFN-g– secreting cells showed very few (2–4) ORF2-specific cells/105 peripheral blood mononuclear cells beyond the basal levels under our experimental conditions (non-significant differences among groups). Also, no significant differences were observed in the degree of lymphoid tissue hyperplasia among the different groups.
Keywords: Pig, Porcine Circovirus 2, Vaccine, Neutralizing antibody, Weight gain, Cell-mediated immunity
Specifications Table
| Subject | Veterinary Science and Veterinary Medicine |
| Specific subject area | The paper deals with some parameters of the immune response of swine to an inactivated PCV2 vaccine, in the framework of a quantal potency assay. |
| Type of data | Table Figure |
| How data were acquired | The Natural Killer (NK) cells activity was measured using an ImmunoSpot S6 Ultimate reader and the HUMAN NK-TVA™ KIT (code: #NK-TVA-5), both provided by Cellular Technology Limited, CTL, Cleveland, OH, USA. The kit was used according to the manufacturer's directions, using Cell Counting software, NK TVA application. The ELISPOT assays were carried out on Multiscreen Filter plates (MAIPS4510, Millipore, MA) and read on an ImmunoSpot S6 Ultimate reader (CTL, Cleveland, OH, USA), using Immunospot software. Neutralizing antibody (NA) in group oral fluid (OF) samples was investigated in assays on PK-15 cells in 96-well, tissue culture microtiter plates, using a Nikon Eclipse TE 2000-S fluorescence microscope and selecting filter B2-A for FITC conjugates. Sucrose gradient analysis (SGA) of PCV2 was carried out using a tube piercer (Teledyne Isco, Lincoln, NE, USA) and a flow cell (Uvicord SII, GE Healthcare, Little Chalfont, UK) connected to a UV recorder. |
| Data format | Raw Analyzed |
| Parameters for data collection | NK and ELISPOT assays were carried out with at least 90% vitality of effector cells. The same parameter was adopted for K-562 target cells in the NK assay. Fluorescence-based NA assays on OF samples were carried out with 100–300 Focus Forming Units (FFUs) in the control wells. |
| Description of data collection | PBMC were obtained from PCV2-vaccinated and/or infected pigs and stored in liquid nitrogen. Later on, cells were thawed and employed in ELISPOT assays. Tracheobronchial lymph node cells were collected from PCV2-infected, slaughtered pigs and stored in liquid nitrogen. Later on they were employed in NK assays on K-562 target cells. OF were collected by a cotton rope with a frayed end and stored at −20 °C. Later on they were used in NA assays on PK-15 cells in 96-well microtiter plates. Pelletted PCV2 was resuspended, clarified and applied onto continuous 10 - 25% sucrose gradients. Gradients were centrifuged and submitted then to UV analysis. Fixed tissues were processed for histopathologic examination. These were embedded in paraffin wax, sectioned at 4 μm thickness and stained with hematoxylin and eosin. |
| Data source location | Institution: Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia-Romagna City: Brescia Country: Italy Latitude: 45°31′32″52 N Longitude: 10°13′41″88 E |
| Data accessibility | With the article |
| Related research article | Guarneri F, Tresoldi ET, Sarli G, Boniotti MB, Lelli D, Barbieri I, Bacci B, D'Annunzio G, Amadori M. Protective immunity in swine induced by Porcine Circovirus 2b inactivated vaccines with different antigen payload. Vet Microbiol. 2021 Jan; 252:108,887. doi: 10.1016/j.vetmic.2020.108887. Epub 2020 Oct 13. PMID: 33,276,254. |
Value of the Data
-
•
This paper provides new data about the mucosal antibody response and cell-mediated immunity of PCV2-vaccinated and infected pigs.
-
•
Companies involved in PCV2 vaccine production, the scientific community at large and agencies in charge of vaccine control could benefit from the described parameters of immune response.
-
•
The described parameters of immune response could be reused for a proper definition of reliable correlates of protection induced by PCV2 vaccines.
-
•
The definition in vitro of reliable correlates of protection after PCV2 vaccination will be conducive to a substantial reduction of animal experiments in the framework of a general 3Rs approach (Replacement, Reduction and Refinement).
1. Data Description
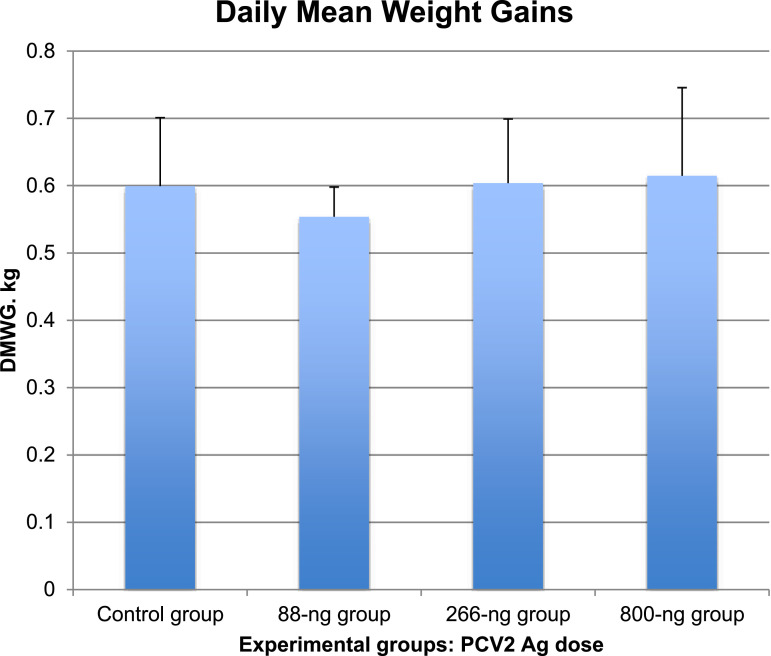
Fig. 1. Weight gain of PCV2-vaccinated and infected pigs. Daily Mean Weight Gains (DMWG) in terms of kg were reckoned in each experimental group. This is shown in a histogram with error bars corresponding to 1 standard deviation. No significant difference among groups was evidenced by one-way ANOVA (P 0.773). The file is based upon the weights of pigs measured 8 days before vaccination and immediately after sacrifice, which took place on three different days, i.e. at Days Post Infection (DPI) 51±2. This enabled us to reckon DMWG in each group.
Fig. 1.
Pigs were individually weighed 8 days before vaccination and immediately after sacrifice. Daily mean weight gains (DMWG) and standard deviations are shown for each group. The observed differences were not significant (One–way ANOVA, P 0.773).
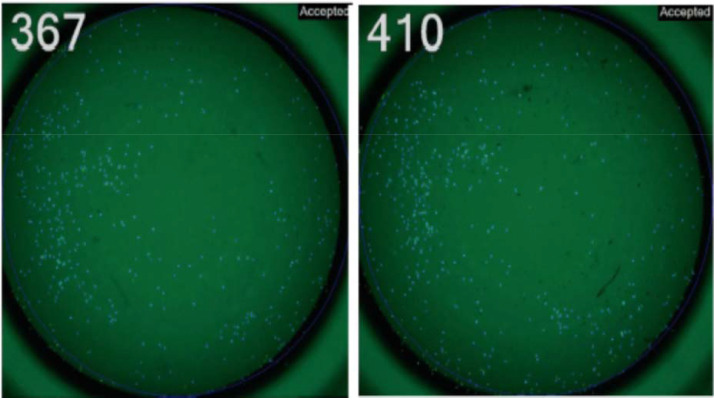
Fig. 2. Layout of NK assay on K-562 cells. Effector cells were employed in a 4-h NK assay on fluorescence-labeled K-562 cells (human chronic myelogenous leukemia, Biobanking of Veterinary Resources, BVR, Brescia, Italy, code BS TCL 33), using an ImmunoSpot S6 Ultimate reader and the HUMAN NK-TVA™ KIT (Cellular Technology Limited, CTL, Cleveland, OH), according to the manufacturer's directions. In this example, numbers refer to viable K-562 cells detected in 96-well, tissue culture plates [2]. Right picture: labelled cells + medium (control). Left picture: labelled + effector cells. The file refers to 5000 viable K-562 target cells reacted with 125,000 effector cells (E:T ratio = 25: 1).
Fig. 2.
NK fluorescent assay.
NK fluorescent assay on K-562 cells. Effector cells are incubated with fluorescence-labeled K-562 target cells. Following NK-mediated lysis, target cells lose their fluorescent signal. The direct visualization of remaining viable target cells determines the percentage of cytotoxicity at each effector to target (E:T) ratio. Numbers refer to viable K-562 cells detected in 96-well, tissue culture plates. Right picture: labelled cells + medium (control). Left picture: labelled + effector cells.
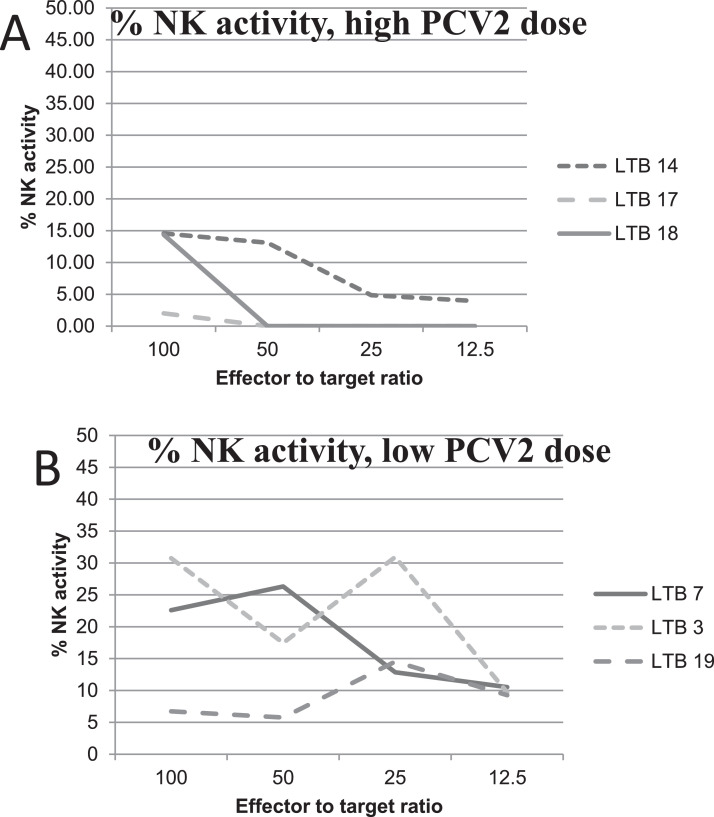
Fig. 3. NK cells activity of tracheobronchial lymph node cells from PCV2-vaccinated and infected pigs. Tracheobronchial lymph node cells of PCV2-infected pigs were employed in a 4-h NK assay on K-562 cells The assay was performed using an ImmunoSpot S6 Ultimate reader and the HUMAN NK-TVA™ KIT (Cellular Technology Limited, CTL, Cleveland, OH, USA, code #NK-TVA-5), according to the manufacturer's directions (see Fig. 2). Panel A: NK cells activity of 3 pigs of the 800-ng group. Panel B: NK cells activity of 3 pigs from the 266-ng, 88-ng and control group, respectively. Tracheobronchial lymph node cells showed NK cells activity at different E:T ratios. The file depicts a greater NK cells activity of tracheobronchial cells from pigs vaccinated with low or no Ag dose, compared to the same effector cells from full-dose vaccinated pigs.
Fig. 3.
Natural killer activity of tracheobronchial lymph node cells from PCV-2 vaccinated and infected pigs.
Tracheobronchial lymph node cells of PCV2-infected pigs were employed in a 4-h NK assay on K-562 cells. Panel A: NK activity of 3 pigs of the 800-ng group. Panel B: NK activity of 3 pigs from the 266-ng, 88-ng and control group, respectively.
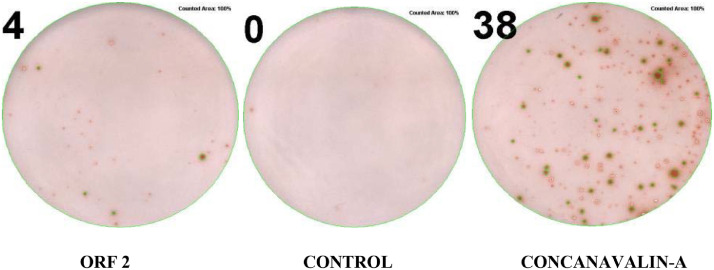
Fig. 4. ELISPOT Assay for cytokine-secreting cells. The picture shows IFN-γ secreting cells in PBMC of PCV2-infected pigs exposed to ORF2 protein of PCV2b, medium (negative control) and Concanavalin-A (10 μg/mL, positive control), respectively, in Multiscreen Filter plates. In the whole study, very few (2–4) ORF2-specific cells/2 × 105 PBMC beyond the basal levels were revealed under our experimental conditions (non-significant differences).
Fig. 4.
ELISPOT assay for cytokine-secreting cells.
The picture shows IFN-γ secreting cells in PBMC of PCV2-infected pigs exposed to ORF2 protein of PCV2b, medium (negative control) and Concanavalin-A (10 μg/mL, positive control), respectively, in Multiscreen Filter plates.
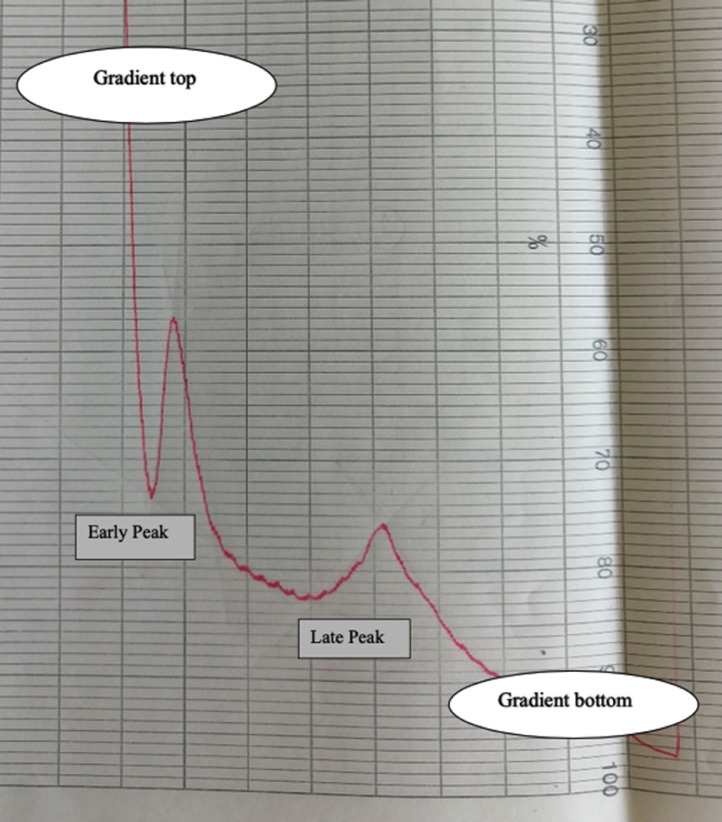
Fig. 5. SGA of inactivated PCV2. PCV2 was 20-fold concentrated and pelleted. The virus was resuspended overnight in PBS at 4 °C and clarified (10,000 rpm, 10′, 4°). Next, PCV2 was layered on the top of linear, 10–25% sucrose gradients and analyzed as previously described [3]. Two UV peaks could be discriminated: both were unambiguously identified as PCV2 by means of PCR. The “late” peak corresponds to PCV2 57S particles [4]. The “early” peak corresponds to lighter, presumably Defective Interfering PCV2 particles. Vaccine antigen payload was calculated as a sum of the two virus populations as previously described [3].
Fig. 5.
Sucrose gradient analysis of inactivated PCV2.
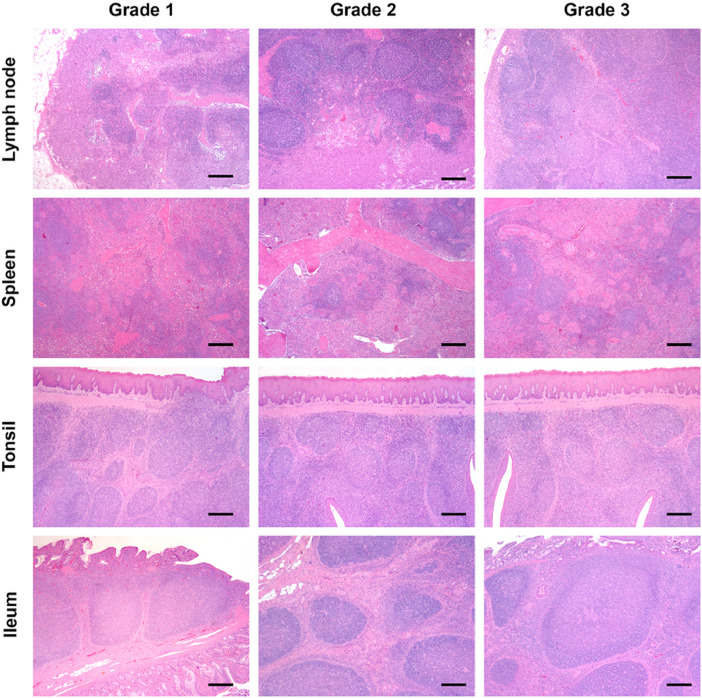
Fig. 6. Grading of lymphoid tissue hyperplasia. Grade 1. Lymph node: presence of primary follicles and well-represented medulla-like tissue; Spleen: presence of periarteriolar lymphoid sheaths without germinal centers; Tonsil: diffuse lymphoid tissue without germinal centers; Ileum: lymphoid tissue without germinal centers. Grade 2. Lymph node: presence of secondary follicles with evident germinal center in almost all the lymphoid tissue with a proportional reduction of medulla-like tissue; Spleen: enlarged periarteriolar lymphoid tissue with presence of germinal centers; Tonsil: germinal centers in no more than 50% of the lymphoid tissue; Ileum: germinal centers in no more than 50% of the lymphoid tissue. Grade 3. Lymph node: marked reactive hyperplasia and expansion of the interfollicular lymphoid tissue replacing medulla; Spleen: pericentric expansion of the periarteriolar lymphoid sheaths with presence of a high number of coalescent germinal centers; Tonsil: presence of germinal centers in all the lymphoid tissue; Ileum: presence of germinal centers in all the lymphoid tissue. All images: original magnification 50×; Haematoxylin - Eosin (H&E) stain. Bar = 500 µm.
Fig. 6.
Grading of lymphoid tissue hyperplasia.
Table 1. NA assay on group OF. Group OF were collected by a cotton rope frayed at one end at different times after PCV2 vaccination and infection. Neutralizing antibodies were investigated by immunofluorescent staining in PK-15c28 cells, as described in our previous study [5]. Titers were expressed as the dilution causing a 90% reduction of the FFUs observed in control wells. The observed differences were not significant (P 0.436 in Repeated Measures ANOVA). Higher titers were observed in the 800 and 266-ng groups at DPV 21, whereas the 1:8 titer in the control and 88-ng groups represents the background MDA level. A decrease of NA titers was also observed in group oral fluids between DPI 10 and 28 in pigs of the 800 and 266-ng groups, which coincided with a significant decrease of serum NA in the same period in the 266 and 88-ng groups [1].
Table 1.
PCV2 NA assay on group oral fluids.
| Sample number | Groups | Of sampling | Na titer |
|---|---|---|---|
| 1 | Group A, 800 ng/dose | 21 DPV | 1/16 |
| 2 | Group B, 266 ng /dose | 1/16 | |
| 3 | Group C, 88 ng/dose | 1/8 | |
| 4 | Group D, control | 1/8 | |
| 5 | Group A, 800 ng/dose | 10 DPI | 1/8 |
| 6 | Group B, 266 ng /dose | 1/8 | |
| 7 | Group C, 88 ng/dose | 1/4 | |
| 8 | Group D, control | 1/4 | |
| 9 | Group A, 800 ng/dose | 28 DPI | 1/2 |
| 10 | Group B, 266 ng /dose | 1/4 | |
| 11 | Group C, 88 ng/dose | 1/8 | |
| 12 | Group D, control | 1/16 | |
| 13 | Group A, 800 ng/dose | 35 DPI | 1/8 |
| 14 | Group B, 266 ng /dose | 1/16 | |
| 15 | Group C, 88 ng/dose | 1/8 | |
| 16 | Group D, control | 1/4 | |
Table 2. Results of the histological grading of lymphoid tissues hyperplasia and of the IHC reaction for the residual viral load in lymphoid tissues. Between the different groups, no significant differences were observed in the degree of lymphoid tissue hyperplasia. The results of IHC are reported only if different from 0. CG / M = presence of isolated giant cells and / or centrofollicular multinucleated cells. The presence of PCV2-specific antigen was measured with a 4-tier IHC scoring scale (grade 0 equal to no PCV2 antigen found in tissue, grade 1 equal to 0 to 25%, grade 2 equal to 25 to 50%, grade 3 equal to more than 50% follicles containing antigen).
Table 2.
Histological grading of lymphoid tissues hyperplasia and of the IHC reaction.
| Group | Pig ID | Mesenteric LN | Spleen | Mediastinic LN | Superficial inguinal LN | Tonsil | Ileum |
|---|---|---|---|---|---|---|---|
| A | 15 | 3 | 1 | 2 | 1 | 3 (CG/M) | 3 (CG/M) |
| 5 | 3 | 2 | 3 | 1 | 2 (CG/M) | 2 | |
| 17 | 3 | 1 | 2 | 1 | 2 | 3 (CG/M) | |
| 18 | 3 (CG/M) | 2 | 2 (CG/M) | 2 (CG/M) | 2 (CG/M) | * | |
| 14 | 2 (CG/M) | 2 | 2 (CG/M) | 2 (CG/M) | 2 (CG/M) | 3 (CG/M) | |
| B | 10 | 3 (CG/M) | 1 | 2 | 1 | 3 | 3 |
| 13 | 3 | 1 | 3 | 2 | 3 | 3 (CG/M) | |
| 19 | 3 | 1 | 2 (CG/M) | 1 | 3 | 2 | |
| 20 | 3 | 2 | 2 (CG/M) | 2 | 2 | 2 | |
| 12 | * | 2 | 3 (CG/M) | 2 | 3 (CG/M) | * | |
| C | 11 | 3 | 2 | 1 | 2 | 3 | 3 (CG/M) |
| IHC 1 | IHC 1 | IHC 2 | |||||
| 16 | 3 (CG/M) | 2 | 2 (CG/M) | 2 | 3 (CG/M) | 2 (CG/M) | |
| IHC 1 | IHC 1 | IHC 1 | IHC 1 | ||||
| 8 | 2 | 1 | 2 | 2 | 3 (CG/M) | * | |
| 7 | 3 | 2 | 2 | 1 | 3 (CG/M) | 3 | |
| 9 | 3 | 1 | 2 | 2 | 2 (CG/M) | 2 (CG/M) | |
| D | 2 | 2 | 2 | 2 | 1 | 3 | 2 (CG/M) |
| 3 | 3 (CG/M) | 2 | 2 | 1 | 3 | 3 (CG/M) | |
| IHC 2 | IHC 1 | IHC 1 | IHC 1 | IHC 1 | |||
| 4 | 3 | 3 | 2 | 3 | 3 | 2 | |
| 1 | 3 | 2 | 3 | 2 | 3 | 3 | |
| 6 | 2 | 2 | 2 | 2 | 3 | 2 (CG/M) | |
* = section missing.
Raw data. Raw data of Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 are reported in Supplementary File 1.
2. Experimental Design, Materials and Methods
2.1. Animals, PCV2 vaccination and infection
The study was carried out on 20 Goland hybrid piglets of 3 litters, born in a farm with no evidence of Porcine Circovirus Associated Disease (PCVAD) complex. After weaning, 28-day old piglets were transferred to our isolation units and clinically inspected. Maternally-derived antibody (MDA) titers to PCV2 were measured by ELISA [1] two days later. Then, after seven further days, pigs were randomly allocated to four groups of 5 animals each with a balanced distribution of MDA titers and litters. Vaccination was carried out after a further 8 days: three groups out of four (A, B and C) were immunized intramuscularly with 800 / 266 / 88 ng, respectively, of an inactivated PCV2b strain in the commercial oil adjuvant associated to Circovac PCV2 vaccine (Ceva Santé Animale) in a final 0.5-mL volume; group D was treated instead with 0.5 ml of placebo (sterile PBS in the same oil adjuvant). Twenty-six days later, pigs were challenged intranasally with 105.3 Tissue Culture Infectious Doses 50% (TCID50) of the homologous PCV2b strain in a 2-mL volume. Blood samplings were carried out in heparinized vacuum tubes and tubes without anticoagulant at days post vaccination (DPV) 21 and DPI 7, 14, 21, 35.
Tracheobronchial lymph node cells were collected after the sacrifice of pigs, at 51±2 DPI. Group oral fluids (OF) from vaccinated and control groups were collected at DPV 21 and DPI 10, 28 and 35 by a frayed cotton rope; this was squeezed and the content was collected in 15-mL conical screw cap centrifuge tubes, and stored at −20 °C.
2.2. Weight gain of PCV2-vaccinated and infected pigs
The pigs of each experimental group were individually weighed 8 days before vaccination and immediately after sacrifice, which took place on three different days, i.e. at DPI 51±2. The initial weight was subtracted from the final one and Daily Mean Weight Gains (DMWG) in terms of kg were reckoned for each group.
2.3. NK assay on K-562 cells
Tracheobronchial lymph node cells of PCV2-infected pigs were stored in liquid nitrogen. They were thawed and grown overnight in RPMI 1640 + 10% heat-inactivated FCS + 20 U/mL human recombinant interleukin-2 (HIL-2 RO, Roche, cat. 10,799,068,001, Merck). Next, they were employed in a 4-h NK assay on K-562 cells (human chronic myelogenous leukemia, Biobanking of Veterinary Resources, BVR, Brescia, Italy, code BS TCL 33). The assay [2] was performed using an ImmunoSpot S6 Ultimate reader and the HUMAN NK-TVA™ KIT (Cellular Technology Limited, CTL, Cleveland, OH, USA, code #NK-TVA-5), according to the manufacturer's directions (see Fig. 2). Briefly, the kit utilizes fluorescence-labeled K-562 target cells (5000 / well). Following NK cell-mediated lysis, target cells lose their fluorescent signal. The direct visualization of remaining viable target cells determines the percentage of cytotoxicity for each effector to target (E:T) ratio.
2.4. Separation and freezing of PBMC
PBMC of pigs were separated by centrifugation of 1:2-diluted, heparinized blood on Histopaque 1.077 (Merck, code 10,771–6 × 100 mL) at 1100 g, 25 min, 20 °C. Aliquots of cells at 5 × 106 /mL were frozen at −80 °C in RPMI 1640 medium (50%), Fetal Calf Serum (FCS, 40%), Dimethyl sulfoxide (DMSO, 10%).
2.5. ELISPOT assay for IFN γ-secreting cells
Multiscreen Filter plates (MAIPS4510, Millipore, MA) were pre-coated overnight (4 °C) with anti-porcine IFN-γ mAb (P2F6 clone, ThermoScientific, MA) at 5 μg/ml in 0.1 M carbonate / bicarbonate buffer pH 9.6 . After washing twice with RPMI 1640 medium, blocking buffer (filtered RPMI 1640 + 10% heat-inactivated FCS) was added and plates were incubated at 37 °C in 5% CO2 for at least 1 h. Next, PBMC were thawed and 2 × 105 viable cells were reacted in duplicate with 2 μg/mL of recombinant, baculovirus-expressed PCV2 ORF2 or medium only (control) for 40 h at 37 °C in 5% CO2, in a final volume of 0.2 mL/well. PBMC reacted with Concanavalin-A (Sigma-Aldrich) at 10 μg/mL final served as positive control, whereas sterile medium served as a control for non-specific spots. Then, the ELISPOT assay was performed as described in a previous study [6] with minor modifications. Briefly, cells were discarded and plates were washed twice with distilled water and three times with PBS/Tween 20 0.05% under stirring conditions. 0.1 mL/well of biotinylated, anti-porcine IFN-γ mAb MP701B (ThermoFisher Scientific, Catalog # MP701) was added at 5 μg/ml in Assay Buffer, i.e. PBS-Tween 20 0.05% +0.1% Bovine Serum Albumin (BSA), 0.2 µm-filtered. Plates were incubated at room temperature (above 20 °C) over 2 h. After four, 30-s washes under stirring conditions, Pierce® Streptavidin, HRP-conjugated (ThermoFisher Scientific, catalog #21,126) was diluted 1:2000 in Assay Buffer and 0.1 mL/well was added to all wells. After 1 h at room temperature, plates were washed 6 times with PBS-Tween 20 0.05% under stirring conditions. Spots were developed by adding 0.1 mL/well of 3-amino-9-ethylcarbazole (Vector® AEC, Vector Laboratories, code SK-4200) for 5 – 7 min at room temperature. Finally, the substrate was flicked off and plates were washed 5 times with distilled water; plastic cover was removed and the back of wells was washed as well. Plates were dried for two hours in a laminar flow hood, followed by overnight storage at room temperature. Then, spots were read on an ImmunoSpot S6 Ultimate reader (CTL, Cleveland, OH, USA), using Immunospot software.
2.6. NA assay on group oral fluids
Group OF were thawed and processed to remove the bacterial load and possible toxic components. To this purpose, 0.4 ml of each OF sample was diluted with 1.6 ml of PBS supplemented with antibiotics at high concentrations (Penicillin 250 micrograms/ml, Streptomycin 250 micrograms/ml, Amphotericin B 10 micrograms/ml), and stored at 4 °C for 18 h. Next day, Nanosep® Centrifugal Devices with Omega Membrane - 10 K MWCO (Pall Corpopration, Port Washington, NY, USA) were sequentially treated with 70% ethanol and sterile distilled water. OF samples were clarified at 10,000 rpm, 10 min, 4 °C and loaded onto the centrifugal devices. Samples were centrifuged at 14,000 rpm through the membrane and the residual volume (some 50 microliters) was brought back to 400 microliters by adding PBS + antibiotics (see above). Next, neutralizing antibodies in oral fluids were investigated by immunofluorescent staining in PK-15c28 cells as described in our previous study [5]. Titers corresponded to the sample dilution causing a 90% reduction of the Focus Forming Units (FFUs) observed in control wells without OF.
2.7. Sucrose gradient analysis of PCV2
Beta-propiolactone (BPL)-inactivated PCV2 was pelleted, resuspended overnight at 4 °C in sterile PBS and clarified (10,000 rpm, 10 min). Next, 0.5-mL aliquots were loaded on top of continuous, 10–25% sucrose gradients prepared in 40 mM sodium phosphate buffer (pH 7.4). Control gradients were loaded with PBS and mock antigen (BPL-treated cryolysate of PK15c28 cells), respectively. Gradients were centrifuged at 35,000 rpm in a SW-40 rotor (Beckman Coulter) for 3 h at 10 °C. After centrifugation, tubes were inserted into a tube piercer (Teledyne Isco, Lincoln, NE, USA) and gradients were chased at 1 mL / min by means of a 50% sucrose solution in PBS for analysis in a flow cell (Uvicord SII, GE Healthcare, Little Chalfont, UK) from top to bottom. Absorbance at 254 nm was monitored in a 5-mm flow cell (Uvicord SII and Rec102 recording apparatus, Pharmacia 77 Biotech). PCV2 virion concentrations were calculated on the basis of the UV peak areas as previously described [3].
2.8. Hystology and immunohistochemistry
Tissue fragments were fixed in 10% buffered formalin (37% formaldehyde), and processed for histopathologic examination. After embedding fragments in paraffin wax, 4 μm-sections were cut and stained with hematoxylin and eosin.
Ethics Statement
This work involved animal experiments. These were performed in agreement with ARRIVE guidelines (https://arriveguidelines.org) and EU Directive 2010/63/EU for animal experiments. Accordingly, the study was carried out in compliance with the internal Ethical Committee for Animal Experimentation, after receiving a specific Project License (n. 230/2018-PR) issued by the Italian Ministry of Health.
CRediT Author Statement
Massimo Amadori: Conceptualization, Methodology, Supervision, Writing-Original draft preparation, Project administration, Funding acquisition; Flavia Guarneri: Investigation, Data Curation, Methodology, Visualization, Formal analysis; Enrico Tommaso Tresoldi: Investigation, Resources; Giuseppe Sarli: Methodology, Investigation, Project administration; Maria Beatrice Boniotti: Investigation; Davide Lelli: Investigation; Ilaria Barbieri: Investigation; Barbara Bacci: Investigation; Giulia D'Annunzio: Investigation, Data Curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
This work was supported by the Italian Ministry of Health, Grant PRC2016013, 2016. The authors want to thank Mrs. C. Mantovani and Mr. G. Savoldi (IZSLER, Brescia, Italy) for the skillful technical assistance.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.106906.
Appendix. Supplementary materials
References
- 1.Guarneri F., Tresoldi E.T., Sarli G., Boniotti M.B., Lelli D., Barbieri I., Bacci B., D'Annunzio G., Amadori M. Protective immunity in swine induced by Porcine Circovirus 2b inactivated vaccines with different antigen payload. Vet. Microbiol. 2021;252 doi: 10.1016/j.vetmic.2020.108887. Epub 2020 Oct 13. PMID: 33276254. [DOI] [PubMed] [Google Scholar]
- 2.Welter A., Sundararaman S., Li R., Zhang T., Karulin A.Y., Lehmann A. High-throughput GLP-capable target cell visualization assay for measuring cell-mediated cytotoxicity. Cells. 2018;7 doi: 10.3390/cells7050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanotti C., Amadori M. A simple method for measuring porcine circovirus 2 whole virion particles and standardizing vaccine formulation. Biologicals. 2015;43:79–83. doi: 10.1016/j.biologicals.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Allan G.M., Phenix K.V., Todd D., McNulty M.S. Some biological and physico-chemical properties of porcine circovirus, Zentralbl. Veterinarmed. B. 1994;41:17–26. doi: 10.1111/j.1439-0450.1994.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 5.Zanotti C., Martinelli N., Lelli D., Amadori M. Correlates of protection following vaccination with inactivated Porcine Circovirus 2 vaccines. Viral Immunol. 2015;28:600–608. doi: 10.1089/vim.2015.0021. [DOI] [PubMed] [Google Scholar]
- 6.Vordermeier M., Whelan A.O. ELISPOT assays to enumerate bovine IFN-gamma-secreting cells for the development of novel vaccines against bovine tuberculosis. Methods Mol. Biol. 2012;792:219–227. doi: 10.1007/978-1-61779-325-7_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.