Abstract
An update on the African swine fever (ASF) situation in the 10 affected Member States (MS) in the EU and in two neighbouring countries from the 1 September 2019 until the 31 August 2020 is provided. The dynamics of the proportions of PCR‐ and ELISA‐positive samples since the first ASF detection in the country were provided and seasonal patterns were investigated. The impact of the ASF epidemic on the annual numbers of hunted wild boar in each affected MS was investigated. To evaluate differences in the extent of spread of ASF in the wild boar populations, the number of notifications that could be classified as secondary cases to a single source was calculated for each affected MS and compared for the earliest and latest year of the epidemic in the country. To evaluate possible risk factors for the occurrence of ASFV in wild boar or domestic pigs, a literature review was performed. Risk factors for the occurrence of ASF in wild boar in Romanian hunting grounds in 2019 were identified with a generalised linear model. The probability to find at least one PCR‐confirmed ASF case in wild boar in a hunting ground in Romania was driven by environmental factors, wild boar abundance and the density of backyard pigs in the hunting ground area, while hunting‐related variables were not retained in the final model. Finally, measures implemented in white zones (ASF‐free zones that are geographically adjacent to an area where ASF is present in wild boar) to prevent further spread of ASF were analysed with a spatially, explicit stochastic individual‐based model. To be effective, the wild boar population in the white zone would need to be drastically reduced before ASF arrives at the zone and it must be wide enough. To achieve the necessary pre‐emptive culling targets of wild boar in the white zone, at the start of the establishment, the white zone should be placed sufficiently far from the affected area, considering the speed of the natural spread of the disease. This spread is faster in denser wild boar populations. After a focal ASF introduction, the white zone is always close to the infection hence pre‐emptive culling measures in the white zone must be completed in short term, i.e. in a few months.
Keywords: African swine fever, epidemiology, risk factor, seasonality, wild boar, domestic pigs, management, prevention, white zones
Summary
The European Commission requested EFSA to provide an updated analysis of the epidemiological situation of ASF in the Member States (MS) of the European Union (EU) affected by African swine fever virus (ASFV) Genotype II.
Term of reference 1 (TOR 1) of the mandate requested to analyse the epidemiological data on ASF from MS and non‐EU countries affected by ASFV Genotype II, including an analysis of the temporal and spatial distribution of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention had to be paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
A narrative update was provided on the ASF situation in each of the 10 affected MS (Belgium, Bulgaria, Estonia, Greece, Hungary, Latvia, Lithuania, Poland, Romania and Slovakia) during the reporting period (from 1 September 2019 until 31 August 2020) and in two neighbouring countries (Serbia and Russia). As the incursion of ASF in Germany occurred in September 2020, the update on the ASF epidemic in Germany will be provided in the next epidemiological report (from 1 September 2020 until 31 August 2021).
All phases of the ASF epidemic were represented in the affected MS during the reporting period. In Bulgaria, Hungary, Poland, Romania and Slovakia, the epidemic has expanded further. In Latvia and Lithuania, the epidemic seems to be stagnating. In Estonia, the epidemic is fading out, and in Belgium and Greece, the infection has been successfully controlled. The combination of control measures implemented in Belgium, including tools such as fencing, night shooting, trapping and carcass removal of wild boar, with intensities adapted to the epidemiological situation in the specific wild boar management areas, was shown to be effective to eradicate ASF after a focal introduction in the country.
Greece is the only MS, where only the domestic pig sector has been involved in the epidemic during this reporting period, whereas Belgium, Estonia and Hungary had only wild boar populations affected. All other MS affected during this reporting period had outbreaks and cases in domestic pigs and wild boar, respectively.
In Serbia, the ASF outbreaks in domestic pigs have been contained successfully. In wild boar, the infection has expanded slowly in the south‐eastern region of the country. In Russia, ASF was present in wild boar and domestic pigs from the outmost western to eastern part of the country. Control measures in Russia focused mainly on attempts to reduce wild boar population and to eliminate backyard farms.
The poor level of biosecurity in backyard farms has been identified as the predominant reason for introduction of ASFV in most of the affected pig holdings also during this reporting period, as reported by Bulgaria, Lithuania, Poland, Romania and Slovakia, based on their epidemiological investigations during the outbreaks. However, quantitative evidence is not available.
During this reporting period, human‐mediated spread, demonstrated by the sudden detection of distant cases of ASF in wild boar populations, which cannot be explained by natural spread, was suspected in Estonia after detecting a positive case in the north‐western part of the country after 18 months without any PCR‐positive case. The spread of ASF into the wild boar populations at the Western side of the Danube in Hungary and Serbia was also assumed to be human‐mediated.
To provide an insight into temporal trends, time profiles were provided, showing the evolution of the proportions of positive samples since the first detection. Based on data submitted to EFSA's data collection framework from the beginning of 2016 up to the end of this reporting period, a persisting decreasing trend in proportions of PCR‐positive carcasses was observed indicating fade out of the virus in some MS, whereas in other MS, it remained high, indicating continuing spread. In addition, there has been no general increase in the proportion of seropositive samples in wild boar.
Possible patterns of seasonality were investigated. There is a clear seasonality in the proportions of PCR‐positive samples from wild boar found dead, although the patterns are slightly different in the different MS. Overall, there is a decline in summer and an increase in winter in the proportion of PCR‐positive samples from wild boar found dead. There is a clear peak observed in the proportions of PCR‐positive samples from domestic pigs between May and September in Lithuania, Poland and Romania. The reason for the ASF seasonality and the different patterns observed in domestic pigs and wild boar require further investigation.
The possible impact of the ASF epidemic on the wild boar population in each affected MS was investigated by looking at the evolution of the annual number of wild boar hunted in the last two decades. The annual number of wild boar, that were hunted in the Baltic States has declined rapidly since the introduction of ASF, ranging from 30,000 to 50,000 wild boar in 2014 to between 5,000 and 15,000 wild boar in 2019. In the other affected MS, an increasing trend of the number of hunted wild boar was observed in the last two decades, up to 2019. An obvious decline after the ASF introduction was not observed in these countries, either because the epidemic lasted only a relatively short time, or it affected only a limited part of the country's wild boar population and data were aggregated on a country level. The hunting was perhaps also intensified in the ASF‐free areas of the affected MS, and this increases temporarily the hunting bag of the affected country.
In addition, to evaluate the extent of spread of ASF in the wild boar populations in each affected MS, the number of notifications that could be classified as secondary to a single source was calculated. Furthermore, to understand the evolution of the epidemic, i.e. whether it was in an expanding phase or in decline, the average number of notifications classified as secondary to a single source during the beginning of the epidemic was compared with that of the reporting period in each country. In most MS, this was lower than in the first year after introduction, indicating a reduced extent of spread. In Bulgaria, Poland and Slovakia, however, the average number of notifications that could be classified as secondary to a single source case clearly increased in the year before the last notification, indicating an increased extent of spread.
Term of reference 2 (TOR 2) requested a review of the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures. This assessment should aim to identify risk factors involved in the occurrence of ASF in domestic pig farms in Romania.
First, a narrative literature review identified field studies and studies based on modelling surveillance data that quantitatively evaluated possible risk factors for the occurrence of ASFV in wild boar or domestic pigs in Europe.
Field evidence found in literature regarding the exact introduction routes of ASF in domestic pig holdings is still scarce. Four studies identified wild boar observed in the vicinity of the domestic pig farms as a risk factor, but the definitive route of ASFV introduction into the farms was not identified in any of them. Wild boar density has been identified to be a risk factor for the occurrence of ASF in backyard farms in a study carried out in Romania. The proximity of growing crops near the backyard farms attractive to wild boar or the provision of fresh forage to pigs has been also identified to be a significant risk factor for the occurrence of ASF in backyard farms in Romania. The vicinity of domestic pig outbreaks in less than 2 km has proven to be a significant risk factor for the occurrence of ASF in backyard farms and commercial farms in Romania. Several risk factors have been identified for the occurrence of ASF in domestic pigs in Sardinia, such as a higher density of backyard farms and pigs, a higher road density and density of outdoor farms per administrative level. Increased wild boar density has been identified to be a risk factor for ASF case detection in wild boar in Estonia. Several environmental parameters have shown to have an impact on the probability of detecting positive wild boar cases in Poland, such as the percentage of young forest cover or meadows.
Then, possible risk factors for the occurrence of ASF in domestic pig farms and wild boar in Romania were assessed, using two different methodologies depending on the data availability. On the hunting ground level, a generalised linear model (GLM) was used to evaluate potential risk factors for the occurrence of ASF in the wild boar populations in 2019. The probability to find at least one PCR‐confirmed ASF case in wild boar in a hunting ground in Romania was mainly driven by environmental factors, wild boar abundance and the density of backyard pigs in the hunting ground area. The number of hunting days and the use of dogs during hunting were not identified as risk factors for occurrence of ASF in wild boar. We observed that wild boar abundance is correlated with the number of feeders per hunting ground, suggesting that reducing wild boar feeding could be helpful in wild boar population control, although causality cannot be inferred from the results. This field deserves more research once sufficiently detailed data on the possible covariates (environmental data, hunting modalities and related to pig production) of several years become available.
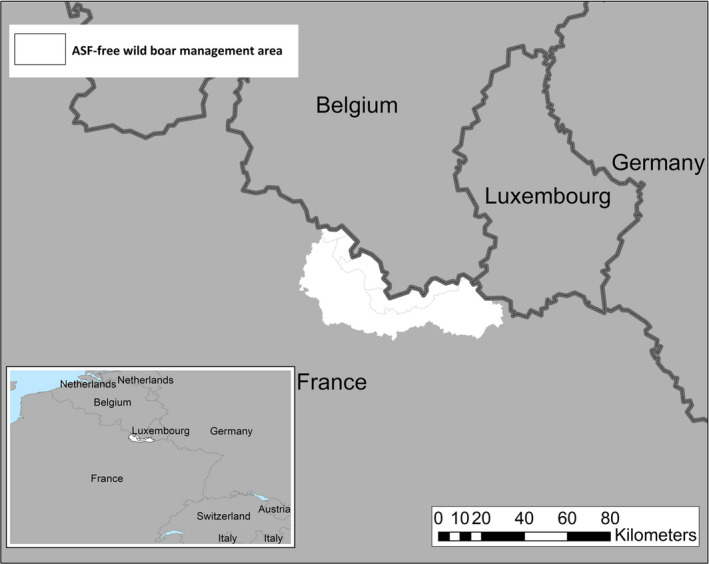
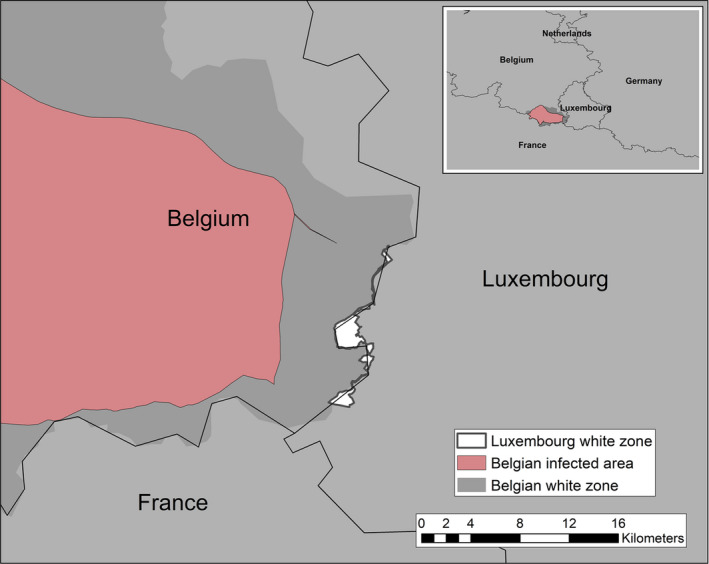
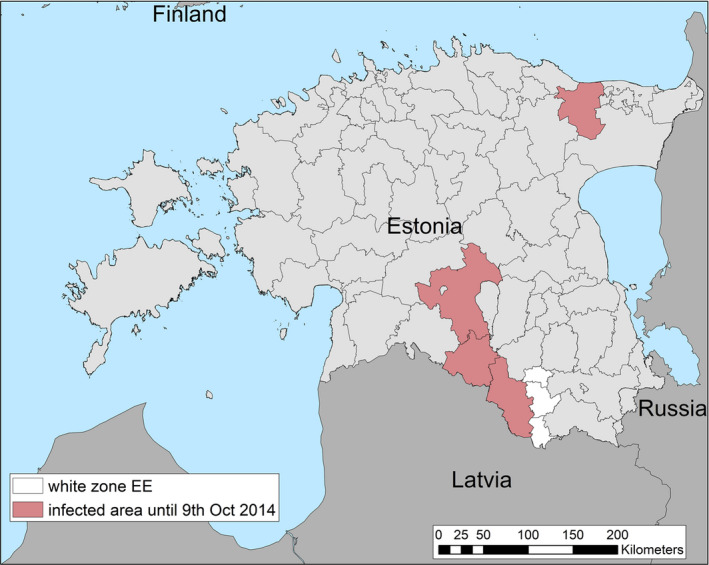
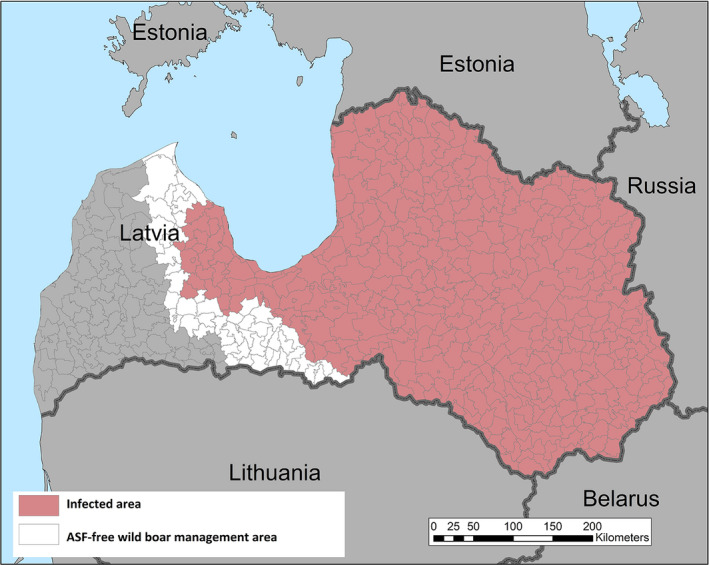
Term of reference 3 (TOR 3) requested to analyse the data and information on the geographical areas called ‘white zones’ (zones blanches) applied by free MS (France and Luxembourg at the border with Belgium) for preventing the spread of the disease in wild boar; the objective was to assess the effectiveness of the measures and to review scientific literature addressing these measures and assess the robustness and effectiveness of the boundaries used for the determination/demarcation of these areas. In this report, white zones were defined as ASF‐free (negative) management areas that are geographically adjacent to an area, where ASF is circulating in wild boar (ASF positive area), where measures are implemented to prevent further spread of ASF, in case it would be introduced. Whether these zones were adjacent to administrative borders was not of importance to evaluate the effectiveness of the measures.
To evaluate the historical effectiveness of measures applied in four different white zones scenes in Estonia, Latvia, Czechia and France, data were collected on the size, the time of establishment and the implementation of the measures applied in the selected white zones, including information about the fences used as demarcation and the numbers of shot animals and carcasses found. The empiric outcome of the measures in the four scenarios, i.e. whether the measures implemented in the particular white zones were successful to stop the spread of ASF, were analysed with a spatially explicit stochastic individual‐based model.
The failure rate of white zones that solely used standard (e.g. in Estonia in 2014) or intensified hunting (e.g. in Latvia in 2016) as a measure to stop the spread of ASF was very high (94% in Latvia a 100% in Estonia).
The failure rate of white zones that implemented fencing AND drastic, concentrated depopulation measures as measures to stop the spread of ASF (e.g. in France in 2018) was low (from 20% to 30%).
The greater the initial wild boar density in a region without ASF was the faster ASF spread forward in the wild boar population and, hence, the shorter was the time the infection needed to enter the white zone.
The success of the control measures in Czechia was most likely due to the silent culling (efforts to cull the maximum of a defined (or fenced) wild boar population with minimal disturbance, for instance, by trapping, sharp shooting or using silencers) of wild boar in an early stage of the epidemic (i.e. 2.5 months after initial detection) and not due to the measures applied in the low‐risk + intensive hunting area. In the model runs with ‘induced’ ASF infection spreading beyond the fenced part (‘highest‐risk’) into Czech white zone, a failure rate of the measures to stop the spread of ASF between 80% and 90% was observed.
Silent culling of wild boar can be initiated a soon as the risk area, established by intensive carcass searching, is reliably fenced.
To be effective, fast and intensive culling measures would need to be readily implemented in the white zone before ASF arrives, and the white zone should be wide enough. The trade‐off is that the implementation of culling measures requires time and wider white zones increased resources to be achievable.
To allow sufficient time to achieve the necessary pre‐emptive culling targets of wild boar in the white zone, it should be sufficiently far from the outermost ASF case in wild boar, taking into account the natural speed of the spread of the disease, which increases with wild boar density.
As carcass removal is a measure to eliminate ASFV sources from an infected area, this is not a pre‐emptive measure. Nonetheless, carcass detection and testing in the white zone will add to early detection and control of ASF after possible incursion in the white zone.
Several recommendations were provided, based on these model outputs. Tangible, absolute population reduction targets, in terms of numbers wild boar per km2 in the white zone after a certain management period, should be specified for the white zone implementation. The distance of the border of the white zone to non‐free area needs to consider the speed of the natural spread of the disease in wild boar. This average speed could range from 2.9 to 11.7 km per year depending on the wild boar density (EFSA, 2020). The white zone should have a minimum width to prevent ASF passing through by short infection chains as wild boar‐free white zones are unlikely to be achieved. The white zone in a focal ASF introduction context needs a reliable fence protection towards the white zone or very intensive measures that allow fast and drastic depopulation of the white zone.
1. Introduction
1.1. Background
African swine fever (ASF) is an infectious lethal disease affecting domestic pigs and wild boar. It can be transmitted via direct animal contact or via dissemination of contaminated food or equipment. This disease has serious economic implications for the pig meat and related sectors, including indirect costs related to trade restrictions. There is no vaccine or cure despite active ongoing research. The persistence of the disease in wild boar and the limited number of control measures available represent a challenge for the whole EU agricultural sector, in particular the pig farming industry.
From the beginning of 2014 up to now, Genotype II of ASFV has been notified in Belgium, Bulgaria, the Czech Republic, Estonia, Germany,1 Greece, Hungary, Latvia, Lithuania, Poland, Romania and Slovakia, causing very serious concerns. The disease has also been reported in Belarus, Moldova, Serbia, Russia and Ukraine, what creates a constant risk for all the Member States that share a border with these third countries. Czechia was recognised as officially ASF‐free in March 2019.
There is knowledge, legislation, technical and financial tools in the EU to properly face ASF. EU legislation primarily targets domestic pig and, when needed, lays down specific aspects related to wild boar. The main pieces of the EU legislation relevant for ASF are:
Council Directive 2002/60/EC2 of 27 June 2002 laying down specific provisions for the control of ASF and amending Directive 92/119/EEC as regards Teschen disease and ASF: it mainly covers prevention and control measures to be applied where ASF is suspected or confirmed either in holdings or in wild boar to control and eradicate the disease.
Commission Implementing Decision 2014/709/EU3 of 9 October 2014 concerning animal health control measures relating to ASF in certain Member States and repealing Implementing Decision 2014/178/EU: it provides the animal health control measures relating to ASF in certain Member States by setting up a regionalisation mechanism in the EU. These measures involve mainly pigs, pig products and wild boar products. A map summarising the current regionalisation applied is available online.4
Council Directive No 82/894/EEC5 of 21 December 1982 on the notification of animal diseases within the Community which has the obligation for Member States to notify the Commission of the confirmation of any outbreak or infection of ASF in pigs or wild boar.
In addition, a strategic approach to the management of ASF for the EU has been developed based on earlier scientific recommendations by EFSA. This strategy is constantly evolving based on new science available and on new experiences gained. The ASF Strategic approach is aimed to the EU countries affected by the disease and to EU countries free from the disease with a risk of introduction.
Some EU free countries, neighbouring infected or restricted areas, are at higher risk of getting ASF infection via natural spread of the disease through wild boar. On the basis of previous EFSA reports and on the basis of expert's recommendations geographical areas called white zones (zones blanches), were put in place to enable early detection (through active search of carcasses) and effectively reduce the wild boar population.
The Commission is in need of an updated epidemiological analysis based on the data collected from the Member States affected by ASFV Genotype II. This analysis should take into account the previous EFSA opinions and technical reports on ASF.
The use of the EFSA Data Collection Framework is encouraged given it promotes the harmonisation of data collection. Any data that is available from neighbouring non‐EU countries should be used as well.
1.2. Terms of Reference as provided by the requestor
TOR 1: Analyse the epidemiological data on ASF from Member States and non‐EU countries affected by ASFV Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention should be paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
TOR 2: Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures. Risk factors involved in the occurrence of ASF in domestic pig farms in Romania should be identified.
TOR 3: Analyse the data and information on the geographical areas called white zones (zones blanches) applied by free Member States (in particular France and Luxembourg at the border with Belgium) for preventing the spread of the disease in wild boar. Assess the effectiveness of the measures and review scientific literature addressing these measures. Review and assess the robustness and effectiveness of the boundaries used for the determination/demarcation of these areas.
1.3. Interpretation of the Terms of Reference
TOR 1: Analyse the epidemiological data on ASF from Member States and non‐EU countries affected by ASFV Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention should be paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
A narrative update is provided on the ASF situation in each of the 10 affected MS (Belgium, Bulgaria, Estonia, Greece, Hungary, Latvia, Lithuania, Poland, Romania and Slovakia) during the reporting period (from 1 September 2019 until 31 August 2020) and in two neighbouring countries (Serbia and Russia). As the incursion of ASF in Germany occurred in September 2020, the update on the ASF epidemic in Germany will be provided in the next epidemiological report (from 1 September 2020 until 31 August 2021).
To provide an insight into temporal trends, time‐profiles were provided showing the evolution of the proportions of positive samples since the first detection, and possible patterns of seasonality were investigated. The possible impact of the ASF epidemic on the wild boar population in each affected MS was investigated by looking at the evolution of the annual number of wild boar hunted in the last 2 decades. In addition, to evaluate the extent of spread of ASF in the wild boar populations in each affected MS, the number of potential secondary cases that could be attributed to a single source were calculated (means of bootstraps calculated with a network analysis). Furthermore, to better understand the evolution of the epidemic, i.e. whether it was in an expanding phase or in decline, the number of potential secondary cases during the beginning of the epidemic was compared with that of the reporting period in each country.
TOR 2: Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures. Risk factors involved in the occurrence of ASF in domestic pig farms in Romania should be identified.
First, a narrative literature review identified field studies and studies based on modelling surveillance data that quantitatively evaluated possible risk factors for the occurrence of ASFV in wild boar or domestic pigs in Europe. Then, possible risk factors for the occurrence of ASF in domestic pig farms and wild boar in Romania were assessed, using two different methodologies depending on the data availability. On a NUTs 3 spatial resolution a Besag York Mollié model was used to evaluate potential risk factors for the occurrence of ASF either in wild boar or in domestic pigs between 2017 and 2019. Furthermore, on the hunting ground level, a generalised linear model was used to evaluate potential risk factors for the occurrence of ASF in the wild boar populations in 2019.
TOR 3: Analyse the data and information on the geographical areas called white zones (zones blanches) applied by free Member States (in particular France and Luxembourg at the border with Belgium) for preventing the spread of the disease in wild boar. Assess the effectiveness of the measures and review scientific literature addressing these measures. Review and assess the robustness and effectiveness of the boundaries used for the determination/demarcation of these areas.
During the kick off meeting of this mandate it was agreed that ASF‐free (negative) management areas (equivalently called ‘white zones’) are zones that are geographically adjacent to an area where ASF is circulating in wild boar (ASF positive area), where measures are implemented to prevent further spread of ASF, in case it would be introduced.
To evaluate the effectiveness of the measures applied in the white zones, data were collected about the size, the time of establishment and implementation of the measures in the selected white zones, the fences used as demarcation and the numbers of shot animals and carcasses found. Per MS that provided sufficient input, the quantitative data were summarised together with a map representing the geographical situation at the time of establishment of the white zone. The empiric outcome of the scenarios, i.e. whether the measures implemented in the white zone was successful in halting the spread, were analysed with a spatially explicit stochastic individual‐based model.
2. Data
2.1. ASF outbreak reports and surveillance data
2.1.1. ASF notification data extracted from the Animal Disease Notification System Database
Data on ASF cases and outbreaks in wild boar and domestic pigs, respectively, notified between 1 January 2014 and 31 August 2020, were extracted from the ADNS database. Table 1 displays the notifications in the period from 1 September 2019 to 31 August 2020, i.e. the update of the disease since the last report of 2019 (EFSA, 2020) compared to the total number of outbreaks and cases reported from the first incursion of ASFV in the EU, on 24 January 2014 to 31 August 2020.
Table 1.
Number of African swine fever virus genotype II outbreaks in domestic pigs and cases in wild boar notified to the Animal Disease Notification System up to 31 August 2020
| Country* | Date of first incursion in the country (DFI) | Number of outbreaksa domestic pigs in period | Number of cases b in wild boar in period | ||
|---|---|---|---|---|---|
| DFI‐31 Aug 2020 | 1 Sep 2019–31 Aug 2020 | DFI‐31 Aug 2020 | 1 Sep 2019–31 Aug 2020 | ||
| EU | |||||
| LITHUANIA | 24/1/2014 (WB) | 141 | 7 | 3,826 | 244 |
| POLAND | 17/2/2014 (WB) | 338 | 80 | 8,619 | 3,621 |
| LATVIA | 26/6/2014 (DP & WB)** | 67 | 3 | 3,936 | 310 |
| ESTONIA | 8/9/2014 (WB) | 27 | 0 | 2,803 | 59 |
| CZECHIA | 26/6/2017 (WB) | 0 | 0 | 230 | 0 |
| ROMANIA | 31/7/2017 (DP) | 3,469 | 1,045 | 1,437 | 810 |
| HUNGARY | 21/4/2018 (WB) | 0 | 0 | 4,990 | 3,934 |
| BULGARIA | 31/8/2018 (DP) | 63 | 27 | 535 | 476 |
| SLOVAKIA | 24/7/2019 (DP) | 22 | 11 | 170 | 164 |
| BELGIUM | 13/9/2018 (WB) | 0 | 0 | 647 | 5 |
| GREECE | 5/2/2020 (DP) | 1 | 1 | 0 | 0 |
| Neighbouring EU | |||||
| UKRAINE | 7/1/2017 (DP) | 285 | 22 | 93 | 5 |
| SERBIA | 31/7/2019 (DP) | 30 | 13 | 41 | 41 |
| Total | 4,827 | 1,202 | 27,292 | 9,634 | |
DP: domestic pigs; WB: wild boar; DFI: date of first incursion.
An outbreak of ASF in domestic pigs refers to one or more confirmed cases detected in a pig holding.
Both sero‐ and virus‐positive wild boar are included among ‘cases’.
Only countries where ASFV genotype II outbreaks or cases have been reported to the ADNS until 31 August 2020 are listed in the table. Countries where ASF was introduced after the reporting period (i.e. Germany) are not listed.
The first case in wild boar and outbreak in domestic pig were detected at the same day.
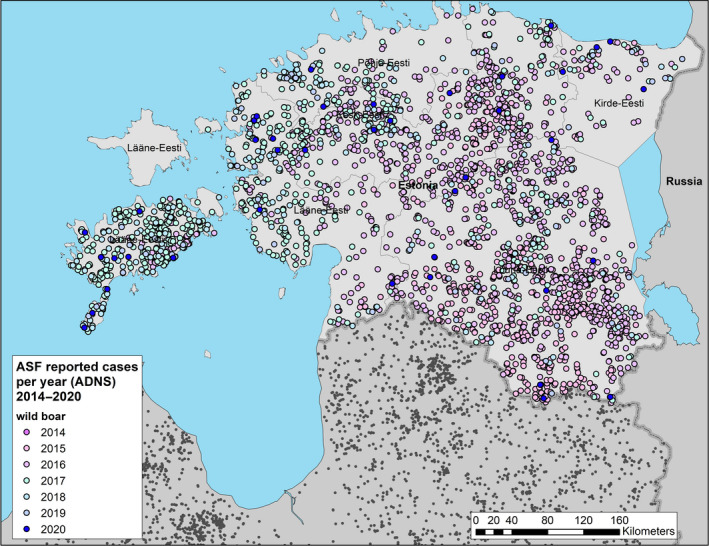
Figure 1 displays the total number of cases in wild boar and outbreaks in domestic pig farms, respectively, reported to the ADNS in the EU MS from 1 January 2014 to 31 August 2020, per calendar year. The data that were extracted from the ADNS were used for the creation of the maps with outbreaks and cases (Sections 4.1.1 and 4.1.2) and the secondary cases network (Section 4.1.6).
Figure 1.

Total numbers of cases in wild boar and outbreaks in pigs per year reported to Animal Disease Notification System from 1/1/2014 to 31/8/2020, per year
2.1.2. Sample‐based ASF surveillance data submitted to EFSA's Data Collection Framework
The data on samples from wild boar from the Laboratory Information Management System (LIMS) of the national laboratories of the affected MS were collected in the EFSA's Data Collection Framework (DCF) (EFSA, 2017). The data reported to the DCF by the different MS contained the information on samples tested for ASFV between January 2014 and 31 August 2020.
Samples were tested for ASFV using polymerase chain reaction (PCR) (testing for virus genome) and AB‐enzyme‐linked immunosorbent assay (ELISA) confirmed by immunoblotting (IB) or immune‐peroxidase (IPT) (tests for antibodies). It should be noted that positive Ab ELISA test results were not systematically confirmed with confirmatory tests (IPT or WB) in all the MS, and therefore, only the ELISA tests results were used when reporting on the serology results in this report. In addition, the ELISA test has not been validated for testing samples taken from carcass fluids from wild boar and the results related to wild boar found dead should be interpreted with caution.
The analysis in Sections 4.1.3, 4.1.4 and 4.2 has been performed based on the test results submitted to the DCF (Table 2).
Table 2.
Numbers of ELISA and PCR tests performed on different samples taken from wild boar, since the first occurrence in the countries, that were submitted to EFSA's DCF from 2014 until 31/8/2020 (from all tested samples in the affected areas since the first ASF)
| Country | Found dead | Hunted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | ELISA* | PCR | ELISA* | |||||||||
| Tot | Nr. pos | % pos | Tot | Nr. pos | % pos | Tot | Nr. pos | % pos | Tot | Nr. pos | % pos | |
| BE | 1,274 | 800 | 62.8 | ND | ND | ND | 3,500 | 33 | 0.9 | ND | ND | ND |
| CZ | 385 | 233 | 60.5 | 9 | 0 | 0 | 643 | 18 | 2.8 | 559 | 17 | 3 |
| EE | 2,263 | 1,858 | 82.1 | 205 | 25 | 12.2 | 35,307 | 1,164 | 3.3 | 35,176 | 1322 | 3.8 |
| HU | 9,533 | 6,182 | 64.8 | ND | ND | ND | 86,406 | 1,134 | 1.3 | ND | ND | ND |
| LV | 2,580 | 2,083 | 80.7 | 279 | 62 | 22.2 | 49,101 | 1,228 | 2.5 | 48,148 | 2761 | 5.7 |
| LT | 7,426 | 4,407 | 59.3 | 1235 | 2 | 0.2 | 35,521 | 764 | 2.2 | 28,270 | 852 | 3 |
| PL | 1,9703 | 1,5474 | 78.5 | 716 | 63 | 8.8 | 73,217 | 1,089 | 1.5 | 62,749 | 768 | 1.2 |
| RO** | 3,093 | 2,183 | 70.6 | 662 | 64 | 9.7 | 62,254 | 740 | 1.2 | 47,303 | 211 | 0.4 |
| SK | 259 | 221 | 85.3 | ND | ND | ND | 559 | 65 | 11.6 | ND | ND | ND |
| BG | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
ND: no data submitted to DCF.
All these samples were tested by ELISA for antibodies, but some of them have been confirmed by IPT test; however, these results were not shown.
Data from RO are until 31/12/2019.
2.2. Wild boar population data
Wild boar data (sources of hunting bag data aggregated on country level are provided in Table A.1 in Appendix A) over the last two decennia were retrieved from the national hunting association websites and used to visually display the trends in wild boar population density (Section 4.1.5).
Table A.1.
Data sources of wild boar hunting data in ASF affected Member states
In addition, detailed data on Romanian wild boar populations based on estimates from the national hunters’ organisations of the Romanian wild boar population size in 2019 were provided by the Ministry of Waters and Forests. The data were provided with sufficient detail per hunting ground, including the hunting efforts (i.e. number of dogs, baiting places, number of hunters as well as a monthly wild boar hunted per hunting) to carry out the risk factor analysis (Section 4.2). Only data from 2019 were available with this spatial resolution.
2.3. Domestic pig population data in Romania
Information about the pig holdings types from 2019 was provided by the National Sanitary Veterinary and Food Safety Authority of Romania. This data set al.so contained the number of pigs per holding and the number of holdings in the different Romanian municipalities. Only data from 2019 were available with this spatial resolution.
According to Order no. 16/2010 of the National Sanitary Veterinary and Food Safety Authority modified by the Order no. 112/2010, the type of pig farms in the provided data set were defined as following:
Non‐commercial pig farm: Holding used for domestic purposes, with animals registered in the National System of identification and registration of animals (S.N.I.I.A) held by the persons who are not registered at the Trade Registry Office.
Commercial type A pig farm: Pig farm registered in the S.N.I.I.A. and registered by the Sanitary Veterinary and Food Safety Directorates in the counties. These farms are complying with the provisions of the specific biosecurity norms, and fulfil the condition from the annex 50, held by authorised persons, individual companies, family businesses or legal persons organised according to the law, registered and authorised at the Trade Registry Office.
Commercial pig farm: Pig farm registered in the S.N.I.I.A., which fulfils the provisions of the specific biosecurity norms and is authorised by the Sanitary Veterinary and Food Safety Directorates in the counties; owned by authorised persons, individual companies, family enterprises or legal persons organised according to the law; registered and authorised by the Trade Register Office.
2.4. Aggregation of data on potential risk factors in Romania spatial unit and assessment of possible collinearity
In a first step, the values of the possible risk factor were aggregated per spatial unit (NUTS3 and hunting grounds of Romania), and the average values for the specific areas were calculated. For instance, the total number of wild boar hunted in a specific hunting ground in 2019 was divided by the surface of the hunting ground to find the average number of wild boar hunted in that year per km2 in that area. Subsequently, all the aggregated values were standardised by dividing them by the maximum value of the same potential risk factor for all the spatial units.
Thereafter, to avoid multicollinearity, the potential risk factors were assessed using the variance inflation factor (VIF) (Imdad et al., 2016, 2019) and only those potential risk factors for which the VIF value was below 5 were retained to be further used in the model building process. The results are listed in Table 3 for all potential risk factors available on NUTS 3 level and Hunting ground level, and the heat map in Figure 2 visualises the pairwise correlation between the variables; red indicating positive correlation and blue indicating a negative relationship.
Table 3.
Potential risk factors based on the available data used in the analysis
| Acronyms | Description | Explanation | VIF nuts 3 | VIF hunting ground | Source |
|---|---|---|---|---|---|
| Potential risk factors related to wild boar habitat | |||||
| Habitat | Percentage of area with suitable habitat for wild boar | Habitat quality could drive wild boar density | 26.36 | 9 | ENETWILD consortium (2020) |
| BIOCMEAN | BIOCMEAN is a measure of wild boar habitat suitability | Habitat quality could drive wild boar density | 6.96 | NA | ENETWILD consortium (2019) |
| Waterbodies | Percentage of waterbodies in the area | Wild boar could aggregate at near water bodies. | 3.45 | 1.4 | https://www.esa-landcover-cci.org/?q=node/158 |
| CropRain | Percentage of the area covered by rain‐fed crops | The land cover could have an impact on wild boar behaviour, e.g. some crops attract wild boar and would facilitate aggregation and impact on transmission rates | 1.54 | 4.6 | https://www.esa-landcover-cci.org/?q=node/158 |
| Herbaceous | Percentage of the area that is covered by herbaceous land cover | 6.60 | 4.8 | https://www.esa-landcover-cci.org/?q=node/158 | |
| TreeShrub | Percentage of the area that is covered by trees and shrubs | 1.96 | 1.6 | https://www.esa-landcover-cci.org/?q=node/158 | |
| Growth | Length of vegetation growing period | 2 | http://www.appsolutelydigital.com/DataPrimer/part154.html) | ||
| Bare | Bare areas | 1.69 | 1.4 | https://www.esa-landcover-cci.org/?q=node/158 | |
| Altitude | Average altitude | 29.86 | 9.4 | https://lta.cr.usgs.gov/SRTM1Arc | |
| Sun | Average yearly sun radiation | Climatic conditions could have an effect both on the survival of the virus in the environment and on the wild boar habitat. It could also have an impact on vector distribution, which potentially could play a role in the transmission of ASFV | 2 | https://worldclim.org/version2 | |
| Snow | Average yearly snow depth | 12.59 | 5.8 | Hall and Riggs (2015) | |
| Prec | Average Precipitation | 3.18 | NA | https://worldclim.org/version2 | |
| Tmean | Average yearly mean temperature | 2869.3 | https://worldclim.org/version2 | ||
| Tmin | Average yearly minimum temperature | 5.67 | 728.1 | https://worldclim.org/version2 | |
| Tmax | Average yearly minimum temperature | 898.8 | https://worldclim.org/version2 | ||
| Potential risk factors related to hunting activity and wild boar management | |||||
| WBDNS | Wild boar density =number of wild boar hunted in the area divided by the surface of the area | Wild boar density could have an effect on the transmission rate | 1.84 | 3.8 | Ministry of Waters and Forests, Romania |
| Hunters | Density of hunters/km2 | Different hunting modalities or targets have an influence on wild boar density and behaviour and could influence the probability of ASF transmission | NA | 1.3 | Ministry of Waters and Forests, Romania |
| Dog | Density of hunting dogs/km2 | NA | 1.4 | Ministry of Waters and Forests, Romania | |
| Feeders | Density of feeders/km2 | NA | 1.8 | Ministry of Waters and Forests, Romania | |
| Feed | Density of feeding/baiting places/km2 | NA | 1 | Ministry of Waters and Forests, Romania | |
| Days | Number of hunting days per hunting ground | NA | 1.3 | Ministry of Waters and Forests, Romania | |
| PigletsSow | Average numbers of piglets observed per sow | NA | 1.8 | Ministry of Waters and Forests, Romania | |
| Females | Numbers of females shot | NA | 4.7 | Ministry of Waters and Forests, Romania | |
| Potential risk factors related to the pig farming system | |||||
| BYFarmDNS | Density of backyard farms/km2 | Different biosecurity levels are assumed to predominate in different farm types which could influence the occurrence of the disease | 2.07 | 1.9 | Veterinary Services Romania |
| BYPigDNS | Density of pigs from backyard farms/km2 | 2.13 | 1.5 | Veterinary Services Romania | |
| TypeAFarmDNS | Density of Type A farms/km2 | 1.99 | 1.2 | Veterinary Services Romania | |
| TypeAPigSDNS | Density of pigs from type A farms/km2 | 2.26 | NA | Veterinary Services Romania | |
| COMFarmDNS | Density of commercial farms/km2 | 2.51 | 1.3 | Veterinary Services Romania | |
| COMPigSDNS | Density of pigs from Commercial farms/km2 | 2.44 | NA | Veterinary Services Romania | |
| Potential anthropogenic risk factors | |||||
| HFP | Human footprint index | A higher human activity in an area could influence the occurrence of the disease | 4.63 | 3.1 | Venter et al. (2018) |
| Urban | Percentage of the surface occupied by urbanised areas | 11.59 | 2.3 | https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2020.EN-1871 | |
NA: Data not available or not calculated on this spatial level; VIF: Variance Inflation Factor. Data in red: VIF > 5: excluded from analysis due to collinearity.
Figure 2.

Heat map displaying the pairwise correlation between potential risk factors, where blue and red shades indicate negative and positive pairwise correlations
3. Methodologies
3.1. Descriptive epidemiology – TOR 1
3.1.1. Update the ASF situation in individual affected Member States
A narrative overview of the epidemic during the reporting period in the different affected MS was provided by the working group member from each MS, focusing on the 1) evolution of ASF epidemic in this reporting period in the EU; 2) specific prevention and control measures in each MS; 3) the most likely sources of introduction in domestic pig holdings, if relevant and 4) the probable human mediated ASF spread in wild boar population (jumps), if relevant.
3.1.2. Time‐profile of proportions of positive samples tested with Ab ELISA or PCR in wild boar hunted and found dead
The proportion of positive samples reported through the DCF (either tested by PCR or Ab ELISA) were calculated as the number of positive animals divided by the total number of tested animals (either hunted or found dead) per month, in the affected MS. As there was no consistent reporting of results of the IB or IPT confirmatory tests, the results of the ELISA tests were taken as results for the serology results. Local regression or local fitting (LOESS) smoothing (Cleveland and Devlin, 1988) was used to estimate the average profiles describing the global trends of the PCR‐ or Ab ELISA‐positive samples. As the plots extended below 0 or above 1, generalised linear mixed models restricted cubic splines were fitted. Confidence bands are also presented to show uncertainties in the estimation of the smoothing curves.
The time profiles were provided per country displaying the proportion of positive samples from only the affected areas where at least one positive case has been found, from the first positive detection in that area onwards. Data were available on NUTS 3, LAU 1 or LAU 2 level from year 2016 onwards. The affected regions only contributed to the estimation of proportion of positive samples in the months after the first infection was found in that country.
3.1.3. Seasonality of proportions of positive samples in wild boar hunted and found dead
A visual inspection was done to compare the number of cases in wild boar and the number of outbreaks in domestic pigs notified to the ADNS by season in the Baltic countries combined with Poland as well as in Romania.
Subsequently, the seasonal patterns of the numbers of cases reported through EFSA's DCF were analysed. Therefore, the data were aligned according geographical location (sampling region), the sampling date and the final test result (for this analysis, a sample was considered an ASF case in a wild boar if it tested PCR positive). ELISA positive results were not considered given that the results do not reflect incidence. Each LAU 2 region was included from the date on which the first positive sample was reported for that LAU 2 region, e.g. starting date. Previous negative reports for that region were excluded from the analysis. A local regression or local fitting (LOESS) smoothing (Cleveland and Devlin, 1988) was used to estimate the average profiles describing the global trends of the PCR‐ or Ab ELISA‐positive samples. Confidence bands (CI 95%) are also presented to show uncertainties in the estimation of the smoothing curves.
3.1.4. Evolution of yearly wild boar density in affected Member States
The total numbers of harvested wild boar per year per country were aggregated and represented for the last 20 years to visualise possible trends in population density. Two graphs were provided, one for the three Baltic States, and one for the other MS in the EU that have been affected by ASFV genotype II in the last decennia.
3.1.5. Secondary cases network
To build the Directed Acyclic Graph (DAG), representing the network connecting the nodes and the directed edges representing the potential parent‐child relation between nodes in the network) representing the ASF epidemic based on the case‐report data submitted to the ADNS, the location and confirmation date were used. The wild boar cases reported were sorted by confirmation date. Starting from the first reported case (considered to be the source), the distance to each subsequent case that occurs in a window of 60 days were calculated (using great circle distance), in accordance with Barongo et al. (2015), only those that were in a band of 1 km to the closest outbreak to the potential source were considered to be linked to the source outbreak. This figure was considered to be in line with a median velocity of spread calculated for Belgium, Czechia, Estonia, Hungary, Latvia, Lithuania and Poland between 2.9 and 11.7 km/year (EFSA, 2020). Once a source was identified, no other source could be linked to the recipient node. A schematic representation of the procedure followed is presented in Figure 3.
Figure 3.
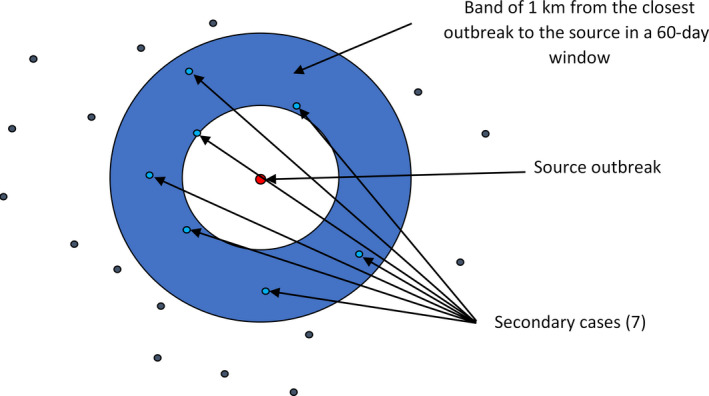
Schematic representation to build the DAG network and calculate the number of nodes connected to each source of infections
Then once the DAG was built, the number of edges coming out from all sources of infections was calculated and this information was used to build a frequency table of notifications that could be classified as secondary to the source cases and bootstrapping (a total of 100,000 bootstraps) was used to quantify uncertainty around the mean of secondary cases obtained from the DAG build.
3.2. Risk factor analysis – TOR 2
3.2.1. Besag York Mollié model to analyse risk factors for the occurrence of African swine fever in domestic pigs and wild boar on NUTS3 level
The Besag York Mollié (BYM) model is a lognormal Poisson model, which includes both an intrinsic conditional autoregression for spatial smoothing and an ordinary random‐effects component for non‐spatial heterogeneity. Details about the models used can be found in EFSA (2017) and in the Zenodo repository (Varewyck et al., 2017).
Section 2.4 describes how the data were aggregated and checked for collinearity. Then the model was fitted with the remaining potential risk factors (Table 3) aggregated per county, to evaluate their effect on the probability of occurrence of ASF in: 1) domestic pigs and 2) in wild boar in the different counties (NUTS 3) in Romania.
Using a backward elimination procedure, the potential risk factors were removed one by one, if their significance level was p > 0.05, given their lack of significant contribution to model.
After the analysis was performed on a NUTS3 level, the potential risk factors for the occurrence of ASF in wild boar were assessed on the hunting ground level. Only covariate data related to pig farms for×were provided with enough spatial detail and the BYM analysis could not be performed as it considers a random effect component for the non‐spatial heterogeneity, but only one single measurement per spatial unit was available making the data at hand unsuited for such a modelling approach (not allowing estimation of the random effect variance with a single observation per spatial unit). Therefore, a Generalised Linear Model was used (see Section 3.3.2) on the hunting ground level.
3.2.2. Generalised linear model
The data reported by Romania through the DCF is used to build a binary indicator per hunting ground, which takes the value 0, if no PCR positive for ASF was reported for that hunting ground, and 1, if at least one PCR positive was reported in the year 2019 for that hunting ground. For each hunting ground then, the hunting activity indicators were reported (such as number of hunters, dogs, feeders, hunted, females hunted, etc.), information regarding domestic pig farms per hunting ground (number of pigs, number of farms, etc.), environmental information (temperature, snow depth, hours sun, suitability scores, etc.) as well as information regarding human footprint index in the area. A logistic regression model was used to explore the effect of the covariates and a backward selection procedure was applied to eliminate covariates in the model that were not significantly (p > 0.05) associated with the presence of at least one ASF PCR‐positive result in a hunting ground area. The proportion of ASF PCR‐positive results in each hunting ground is presented in a choropleth map.
3.3. Review wild boar management for controlling the spread of ASF, in the areas called white zones (zones blanche), and the robustness and effectiveness of the boundaries used for the determination/demarcation of this areas
In a white zone, measures are undertaken to preventively impact the wild boar population before ASF may enter from the adjacent positive area (or not). These measures entail the preparation of the white zone to act as buffer towards even more distant ASF‐free areas yet without management. The intended functionality of the white zone inherently foresees that ASF might enter, but the infection is expected not to leave off the wild boar population in the once demarcated white zone. In other words, a white zone, and ASF‐free management or negative area still remains in function, even if no longer ‘white’, ‘ASF‐free’ or ‘negative’ – the importance is whether eventually the infection chain ceases inside the demarcated area. Nonetheless, in practice white zones usually will be extended (precautionary), once ASF enters the original extension.
These principles are basic to the methodology described in the following sections and the assessment of the capability of white zone measures to control the spread of ASF.
3.3.1. Field evidence
The first step of this assessment included the collection of field evidence of measures applied to white zones in different areas of the EU. Evidence was collected for different periods (as early as 2014, or 5–6 years later when more experience in controlling the disease was obtained), different wild boar population structures (low‐ to high‐density habitat affected) and alternative control situations (advancing epidemic front vs. focal introduction) recorded in the affected MS. The associated data were collected from the MS to: a) have quantitative input about the intensity of the applied measures and b) access the spatial details of the white zones established. The collected data included the size, the time of establishment and the timing of the implementation of the measures in the selected white zones, a description of the fences used as demarcation and the numbers of shot animals and carcasses found. Additionally, the planned outcomes of measures in white zones (targets) were collected. Per MS that provided sufficient input, the quantitative data were tabulated together with a map representing the geographical situation at the time of establishment of the white zone. The empiric outcome of the scenes, i.e. whether the particular white zone was successful in halting the spread of ASF, can be read from details in Section 4.3.2.
3.3.2. Spatial explicit stochastic model
Next, the detailed situation in each MS was implemented in a spatially‐explicit, stochastic individual‐based model. The model is developed to simulate spread and control of ASF in wild boar in structured landscapes of wild boar habitat. The tool was used in support of previous EFSA outputs relating to ASF in wild boar, in particular to assess the capacity to manage ASF spread in alternative scenarios (i.e. large‐scale front, EFSA AHAW Panel, 2015, 2017, or focal introduction, EFSA, 2018). The disease component of the model was updated with knowledge on ASF infection and epidemiology as reviewed in EFSA AHAW Panel, 2021.
The model uses habitat maps to represent population distribution and dynamics. These maps determine population growth and local density variations. Per Member State, the model population is parameterised with the data as described under Section 3.3.1. The structure of the model habitat is based on Pittiglio et al. (2018). The maximum abundance or density is calibrated to estimations of the MS for the particular region (see Section 3.3.1). Finally, the data provided by the MS regarding hunting record and carcasses found in and around the white zone were used to validate or adjust the population numbers emerging from the model habitats. The purpose was to understand the reproducibility of the observation and the possible resulting calibration.
On the geographic landscape, the historic spread of ASF according to ADNS is reconstructed until the white zone has to be established. From there on, ASF spread is independently simulated, and control efforts applied to the white zone including fencing, ASF related excess hunting, depopulation activities and carcass search/removal. The purpose is to investigate each white zone under the epidemiological situation where it was established, and where one possible outcome was already known from field.
Model output is aggregated to inform about:
the likelihood of the observed outcome in a particular white zone (post hoc),
the probability of successful control over time of the applied measures and
potential amendments to the previous suggestions on measures in ASF‐free management zones (EFSA, 2015, 2017, 2018).
Dynamic visualisations of simulation output are available in Lange et al. (2021).
4. Assessment
4.1. Descriptive epidemiology – TOR 1
4.1.1. Update of the ASF situation
During the 12‐months period in question for this report, a first outbreak of ASF was reported from one additional EU‐MS (Greece). At the end of the period, i.e. 31 August 2020, 10 EU MSs (BE, BG, EE, EL, HU, LV, LT, PL, RO and SK) were thus affected by ASF. In addition, ASF was present in all the non‐member countries on the eastern border of the EU, except Turkey, as well as Serbia (Figures 4 and 5).
Figure 4.
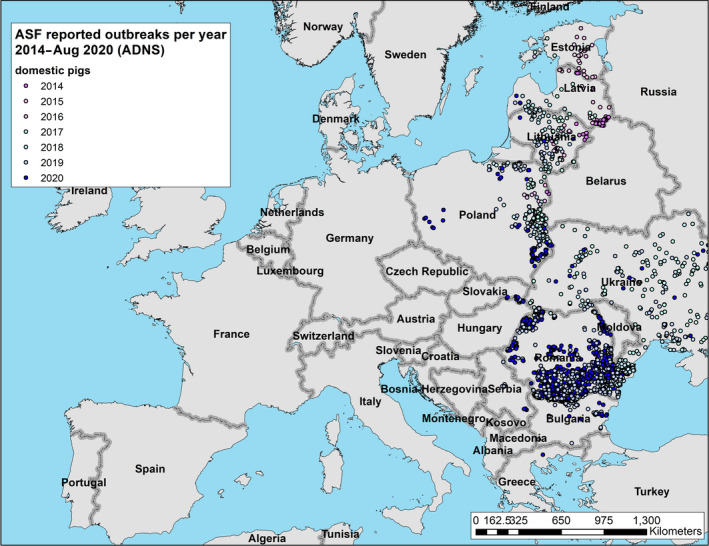
Reported ASF genotype II outbreaks in domestic pigs since the first introduction in the EU, Ukraine and Serbia until 31 August 2020
Figure 5.
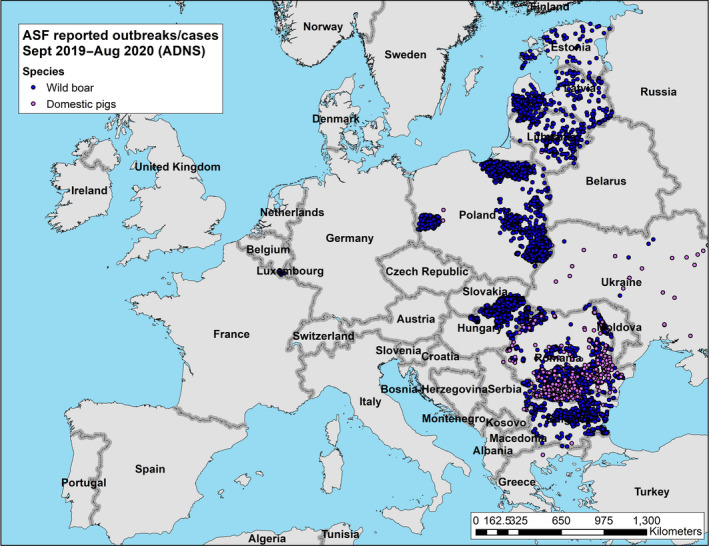
Reported ASF genotype II cases and outbreaks in pigs and wild boar during the reporting period (1 September until 31 August 2020) in the EU, Ukraine and Serbia
Within the EU, all phases of the ASF epidemic were represented during the reporting period, including non‐affected areas (i.e. most MS), affected areas in which the situation was still evolving following e.g. geographic expansion of affected areas and/or increasing numbers of reported cases or outbreaks (e.g. PL, RO, SK and HU), affected areas where prevalence had reached a plateau (areas of PL), areas of reducing prevalence (e.g. LT, LV, EE) and areas in which control measures implemented had managed to stop active virus circulation (BE and EL).
4.1.2. Update the ASF situation in affected Member States and neighbouring countries
4.1.2.1. Belgium
4.1.2.1.1. Evolution of ASF epidemic in this reporting period, Belgium
On 13 September 2018, the presence of ASF was confirmed in Belgium for the first time since 1985. The two‐first positive cases, one adult found‐dead and one young wild boar sanitary‐shot, were detected in the Bois de Buzenol (province of Luxembourg, south‐east of Wallonia). The cases were found about 12 and 17 km, respectively, from the borders of France and the Grand Duchy of Luxembourg. Within the first 11 months (from mid‐September 2018 to mid‐August 2019), 827 ASFV‐positive wild boar were detected in the south‐eastern area of Belgium (Figure 6).
Figure 6.
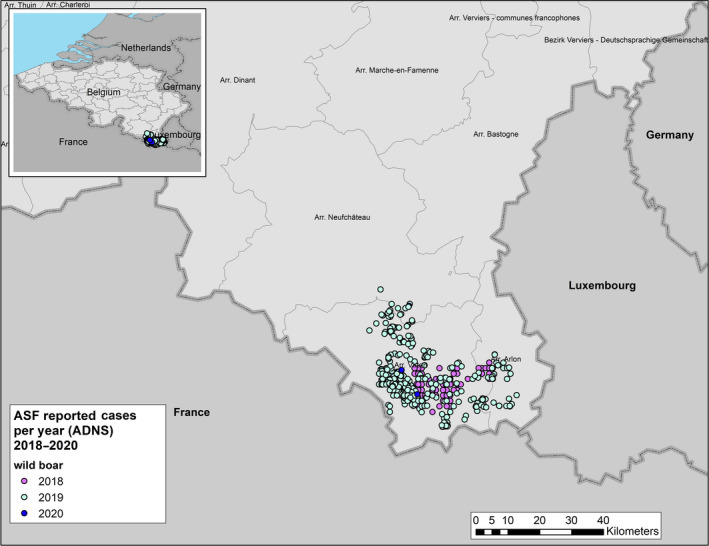
Reported ASF cases in wild boar since the first introduction in Belgium up to 31 August 2020
4.1.2.1.2. Specific prevention and control measures (besides those laid down in the EU legislation and the Strategic approach to the management of ASF for the EU)
Preventive measures and surveillance in domestic pigs
About 2 years after the emergence of ASF in wild boar, there were still no cases among domestic pigs. The self‐declaration of « Free status of ASF for domestic and wild pigs kept in captivity » submitted by Belgium to the OIE was approved in April 2019.7 The obligations related to registration of pig holdings, infrastructure and hygiene are controlled by the agents of the Federal Agency for the Safety of the Food Chain (FASFC). Measures carried out by (FASFC), including enhanced passive surveillance in all pig holdings, strict biosecurity measures and prohibition of assembly of pigs, were maintained during the second year of the crisis. Moreover, an active surveillance on pig farms was introduced in 2020. Although surveillance covers pig farms throughout Belgium, it specifically targets ‘farms at risk for virus introduction’, i.e. farms located in the province of Luxembourg (in or near the ASF‐affected area), farms keeping free‐range pigs and farms that market breeding and rearing pigs.
Enhanced passive surveillance: Persons in charge of any pig operation are expected to immediately call the farm veterinarian, if signs of disease are simultaneously observed in several animals or if multiple mortalities are noted. The veterinary surgeon is then expected to examine all pigs in the herd within 24 hours and, even if no evidence of ASF is detected, must comply with the FASFC's instructions for increased vigilance. According to the latter, 3 blood samples and/or a fresh carcass have to be sent to the first‐line laboratory for differential diagnosis before initiating any treatment. From September 13, 2018 to October 11, 2020, 20,843 pigs from 2,778 holdings were sampled, and all samples were PCR‐negative. Further, when new pigs are introduced into a holding, they must undergo quarantine for 4 weeks before being introduced into the herd.
Implementation of active surveillance: During the year 2020, blood samples were taken from 5,804 pigs from 341 holdings. The corresponding 5,804 serological and 3,773 virological analyses were performed, and all were found negative (by PCR tests).
Control of biosecurity measures in pig holdings: in addition to the adaptations of the Belgian legislation reinforcing biosecurity in the domestic swine sector, the usual controls of registration, infrastructure and hygiene obligations by FASFC officials were extended to biosecurity measures. This was made feasible by the hiring of additional veterinary inspectors. Further, from 2021 onwards, the farmers are expected to contact their veterinary surgeons once a year for evaluating the biosecurity measures implemented in the corresponding farms. This evaluation will be mandatorily and carried out with the use of a new, dedicated computer app (BioCheck), which is made available by and automatically transfers the results to the FASFC.
Control measures in wild boar
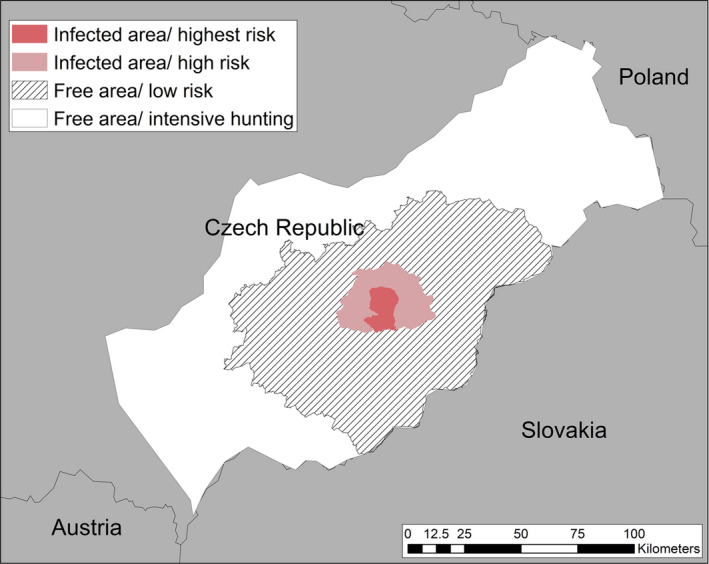
Zoning: regulated zones were created in October 2018 according to the European legislation (Figure 7). According to the specific measures prescribed by the European legislation, the areas were adapted four times, as new positive cases were detected. In January 2019, an extension was created to the West (close to France), in February and March 2019, to the North and North West and again to the North‐West in January 2020, when a PCR‐positive wild boar bone was detected outside the infected area. In May 2020, the infected area was reduced as no new cases had been detected for more than 12 months. Each adaptation has been the subject of a Decision of the EU Commission. In August 2020, Part II and I combined covered 1,106 km2, with Part II extending over 572 km2, of which forests accounted for 302 km2.
Figure 7.
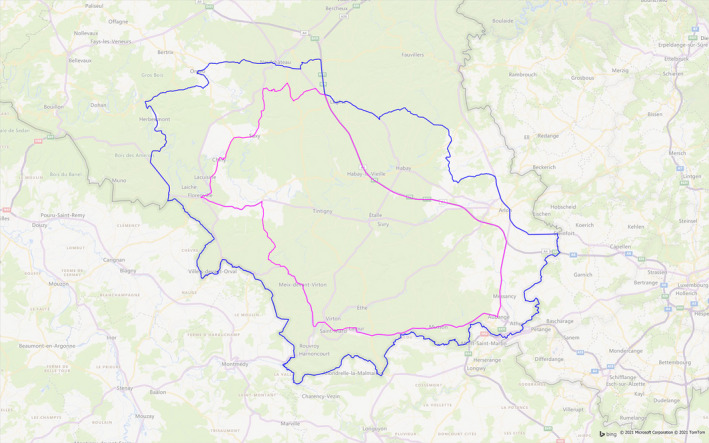
Regulated zones in Belgium, May 2020. Blue line: border of Part I, surrounding zone II and in which no cases of ASF has been recorded; Pink line: border of Part II in which ASF has only been detected in wild boar
Restrictions: In October 2018, restriction measures were enforced, aimed at limiting the spread of ASFV in the affected area. The objectives were to avoid disturbing wild boar, avoid passive dissemination of the virus and avoid physical risks for average citizens during depopulation operations. The restrictions consisted of a strict ban on feeding and hunting wild boar and on free circulation in the forests for walking, hiking and professional forestry activities. In April 2019, some safe areas were released for walking/hiking activities during the summer. In October 2019, the infected area was totally closed again to allow intensification of depopulation activities. In January 2020, light forestry activities (handheld equipment only) were authorised, provided that strict biosecurity measures were implemented. In May 2020, heavy professional activities were authorised, again provided strict disinfection of equipment was implemented and access of the forests to tourists was reopened during daylight hours (depopulation activities were maintained during the nights). Finally, in August 2020, the authorities issued some new authorisations to take firewood outside the forest and to prepare the coming wild cervids hunting season, provided strict disinfection procedures were implemented.
Enhanced passive surveillance: Systematic searches for dead wild boar with immediate carcass removal and ground disinfection were and are still organised over the two zones since the beginning of the outbreak. In Part I, intense active search was expected, throughout the crisis, to provide early detection of ASFV positive cases outside the infected zone and/or detect possible sealing failures of the network of fences. Throughout this reporting period, each found‐dead wild boar was packed according to strict biosecurity procedures and was transported to the principal collection centre by professionals of the Civil Protection and by the administration. Twenty‐five workers were hired in September 2019 to reinforce the carcasses’ search‐packing‐removal‐testing activities. Overall, since the beginning of the outbreak, more than 56,000 hours of active search were organised by the regional authorities. During the reporting period (September 2019–August 2020, 18,000 h of active search, C. Malengreaux, personal communication), 171 wild boar were found dead in Part II, 96% of which were in skeletonised condition (165/171). Of these, 74% (122/165) could no longer be analysed because of advanced decay (no DNA retrieved). Of the 43 analysable bones, 37 were found ASFV‐negative and 6 were found positive. The six positive bones had been found in the forest between October 2019 and March 2020. In addition, 71 animals were found dead in Part I, of which 72% were found in the skeletonised condition. Among them, 59% were no longer analysable for the same reasons. All analysable animals from Part I were found to be ASFV‐negative.
Fencing: A network of concentric fences was built on the border and within the aforementioned areas (about 300 km in total).
Between November 2018 and September 2019:
Belgian fences were connected to those built in France and Grand‐Duchy of Luxemburg wherever pertinent. The goal was/is twofold: (i) slowing down the centrifugal geo‐diffusion of the disease, and (ii) creating tight corridors in which depopulation can be carried out without taking the risk of causing the movement of animals over long distances. Fences were built progressively around the infected zone first, then in front of the non‐infected zone to have at least a fence in advance on the virus spread.
Between September 2019 and August 2020:
The fence network was completed where gaps remained. First, the Libramont‐Bouillon junction (28 km) was built in November 2019 (it is a junction in the north‐west of Part II, along the N89 road between Recogne and Bertrix, approximately 15 km from the limit of Part II). This fence was built far outside of the disease propagating front to anticipate a possible centrifugal spread of the virus and also to hinder the centripetal immigration of wild boar into the area where intense depopulation activities were being undertaken. Then, a junction between the Belgian network of fences and that of the Grand‐Duchy of Luxemburg at the level of Athus (0.6 km, end of November 2019) was built.
Depopulation: Many depopulation methods were used (trapping, night shooting, single hunting on baiting points, driven hunts with/without dogs) with specific restrictions according to the area. All these measures were carried out under the supervision of the regional authorities with the objective to drastically depopulate the different areas. The depopulation‐associated decisions were rendered mandatory by issuing specific regional legislations. Culling of wild boar within the infected zone was carried out by the public authorities, whereas hunters were enrolled with the administration for depopulating the periphery. Compensations (50 € or 100 € per wild boar, depending on the area) were provided for participating hunters. The latter were enrolled, provided they had received specific training on biosecurity procedures, including for packaging and transportation of culled wild boar to the collection/diagnostic centres.
Among different methods, the night shots proved essential for implementing a targeted depopulation strategy without disturbing wild boar populations. They were exclusively carried out by the public authorities since January 2019. Traps also proved an efficient ancillary method. Up to 170 traps have been installed by the public authorities between January and June 2019. They were operated with the collaboration of hunters for baiting and culling.
The rationale underlying the depopulation activities relied on a regular adaptation to the epidemiologic situation. During the epidemic phase (rapid spread of the disease with recurrent extensions of the infected area, from September 2018 to April 2019), it was strictly forbidden for the public to hunt in the infected area and the public authorities progressively intensified night shots and trapping for depopulation purposes in the infected area. Besides, in the surrounding still ASFV‐free areas, i.e. Part I and outside Part I, hunters were invited to organise driven hunts with or without dogs, according to the presence of fences or not. During the residual (post‐epidemic) phase (typically when detection of ASFV‐positive cases became sporadic, at constant found‐dead searching power, from May 2019 onwards), night shots and trapping for depopulation purposes were intensified in the infected area. In the infected area (572 km2), the objective was to eliminate as much as possible « residual » wild boar. According to the network of camera traps installed over the entire zone, the estimated living population was 50–150 wild boar over the infected area in July 2020 (A. Licoppe, personal communication). Between September 2019 and August 2020, 148 wild boar were culled over the infected zone (111 shot at night, 15 trapped/culled and 22 culled by public authorities). All proved ASFV‐negative. In the surrounding area (534 km2), 1087 were culled (325 night shots, 273 trapped and 489 culled), all of them proven ASFV‐negative. As the population is dynamic, the Belgian depopulation strategy will be maintained over the whole area at least until the end of 2021.
Testing: The three carcass collection centres (one main and two secondary) set up at the beginning of the crisis remained fully functional during the period September 2019 to August 2020. A total of 1504 wild boar carcasses were analysed for the presence of ASFV with a strategy identical to that implemented during the preceding phase: 100% of found‐dead (zones II and I) animals as well as 100% (Part II) and 20–30% (Part I) of the night‐shot/trapped/culled animals. During the reporting period, 171 carcasses were removed from the infected area (Part II). The majority of these (96%) were reduced to a pack of bones. Six of them were proven weakly ASFV‐positive by PCR, they were all detected in the infected area between October 2019 and March 2020. For these six weakly ASFV‐positive (Ct > 34) bones found in 2020, based on the date of discovery, climatic conditions and macroscopic appearance, the post‐mortem interval was estimated at > 6 months, according to Samsuwan and colleagues (2018). Further, the ASFV EURL (INIA, Madrid, Spain) was unable to isolate infectious virus from these samples. Taken together, the testing results show that the last fresh ASFV‐positive wild boar we detected in the infected area dates from 13 August 2019.
Since 1 October 2020, Belgium has declared itself free of ASF, and this was approved by OIE in December 2020 (OIE, 2020). Nevertheless, the surveillance is maintained to continue to reduce the density of wild boar populations throughout Wallonia.
4.1.2.1.3. Probable human‐mediated ASF spread in wild boar population
When ASFV entered in Belgium, the nearest wild boar case was about 1,000 km far from the place of introduction, and therefore, this introduction is considered to be human‐mediated. Up to now, however, the cause of the long‐distance jump of ASFV into the Belgian wild boar population could not be proven.
Key points
No outbreaks have been reported in domestic pigs in Belgium
The reporting period September 2019–August 2020 is in the residual (post epidemic) phase. The last fresh ASFV positive case in wild boar was reported in August 2019. Since then, six PCR‐positive bones (the last in March 2020) were detected by active search of carcasses, all in the infected area.
Since the beginning of the outbreak: 833 ASFV‐positive cases were detected in the infected area (EU Part II) of ~ 572 km2.
Preventive measures in pig holdings and control strategies in wild boar populations have proved effective to avoid introduction of the disease into pig farms and to contain the virus in wild boar in a controlled area.
Control strategies in wild boar are a combination of tools (including fencing, night shooting and traps) adapted to the epidemiological situation and to the specific zone they are implemented in.
Active search and removal of carcasses and depopulation are maintained in the regulated zones.
The authorities are keeping up the pressure to eradicate the disease.
Free status from the first of October 2020.
4.1.2.2. Bulgaria
4.1.2.2.1. Evolution of ASF epidemic in this reporting period in Bulgaria
Figure 8.

ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Bulgaria up to 31 August 2020
Figure 9.
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Bulgaria up to 31 August 2020
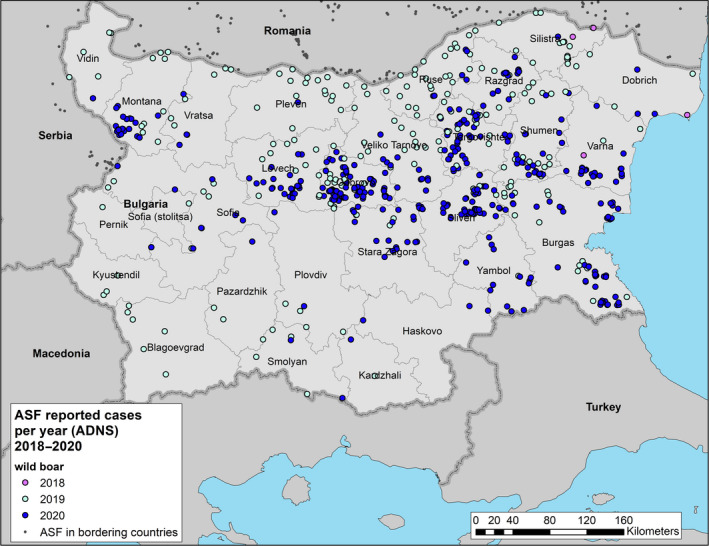
During this reporting period, ASF was detected both in domestic pigs and in the wild boar population in Bulgaria.
Twenty‐seven outbreaks in domestic pigs and 534 cases in wild boar were notified by the Competent Authority. Descriptive data on the outbreaks per holding category, wild boar cases and animals affected are provided in Table 4.
Table 4.
Descriptive data on the ASF outbreaks per holding category in Bulgaria from the first of September 2019 until the 31 August 2020
| Category | Outbreaks/cases, n | Affected pigs/WB |
|---|---|---|
| Industrial farms | 3 | 67,624 |
| Family farms | 1 | 29 |
| Backyards | 3 | 12 |
| East Balkan pigs | 20 | 1,809 |
| Wild boar | 534 | 1,649 |
There were four regions in Bulgaria where domestic pigs were affected by ASF during the reporting period. The majority of the outbreaks were located in the central and north‐eastern parts of the country – two industrial farms and the East Balkan pig's farms were affected in the Shumen and Varna regions, one family farm and a backyard farm in the Gabrovo and Sliven regions, respectively.
The outbreaks in East Balkan pigs were confirmed throughout the reporting period, whereas the outbreaks in the other pig categories were confirmed in the beginning of 2020. No outbreaks were confirmed in the months from May to July. The low density of backyard pigs resulting from the preventive measures taken in July 2019 (depopulation of the backyard farms and ban for repopulation till September 2020) and amendment of the national legislation (see below) have contributed to the epidemiological situation with ASF in the country.
Almost the same situation is observed in the wild boar population, namely the spread of ASF cases towards the central and eastern part of the country. ASF cases in wild boar have been confirmed in 26 administrative regions, out of 28 regions. In total, 1,649 positive wild boar were confirmed as PCR positive. Out of 41,756 hunted wild boar, 630 were positive, while 1,019 positive cases were in wild boar found dead.
The highest percentage of PCR‐positive wild boar found dead was reported in the age category between 2 and 6 years (86%), followed by the category from 1 to 2 years (75%), whereas in shot positive wild boar, a higher percentage of positive pigs was observed in the age group from 1 to 2 years (24%). Out of the PCR‐positive hunted wild boar, 1.35% were animals with atypical behaviour, in the age category up to 2 years. From the wild boar testing positive, 48% were females and 39% were males and the sex of the rest (13%) was not known.
4.1.2.2.2. Specific prevention and control measures (besides those laid down in the EU legislation and the Strategic approach to the management of ASF for the EU)
Measures were implemented in line with in with Directive 2002/60/EC. In addition, in the beginning of 2020, the national legislation was amended, resulting in easier registration for backyard farms, strengthened biosecurity requirements for pig farms and improved cooperation with local institutions and new, improved compensation rules in case of animal disease outbreaks.
A multi‐institutional plan for control and prevention of ASF was adopted in January 2020 by the Council of Ministers, which laid down the roles, responsibilities, commitments of all governmental institutions regarding the implementation of the prevention, control and eradication measures for ASF.
Additionally, a massive training and awareness campaign was carried out in the reporting period, targeting farmers and hunters, which focused on pig holding registration, biosecurity measures in pig holdings and during hunting.
4.1.2.2.3. Most likely routes of introduction in domestic pig holdings
The most likely routes of introduction into the pig holdings are listed below and are based on the outcomes of the epidemiological investigation carried out for each outbreak.
Domestic pig farms, which were not applying strong biosecurity measures, were detected during the checks by the Veterinary Officials. Some of the farms were surrounded by forest or crop land areas attractive for wild boar, or areas where several dead wild boar were found and later tested PCR‐positive for ASF. Additionally, the likely pathway of virus spread for one of the affected farms was due to its commercial links with a previous confirmed outbreak farm. The backyard farms that reported positive for ASF did not have any or sufficient biosecurity measures in place.
East Balkan Pigs (EBP) farms are allowed by the national law to be kept in three regions of the country, resulting in a higher density of these farms in those areas. This type of pig breeding is paired with very low levels of biosecurity. However, there is a ban for free range and outdoor rearing of pigs in the ASF affected areas, which is unfortunately not complied with by some pig owners. The spread in those areas where EBP are reared was therefore just a matter of time. At the time being, more than 50% of the EBP farms have been affected by ASF.
Thus, the main conclusion about the possible source of ASF in the affected farms is that the lack of strong biosecurity in place, the high concentration of EBP farms and high levels of environmental contamination with ASFV (as almost 80% of the country is affected by ASF in WB) are the most likely causes contributing to ASF spread.
4.1.2.3. Estonia
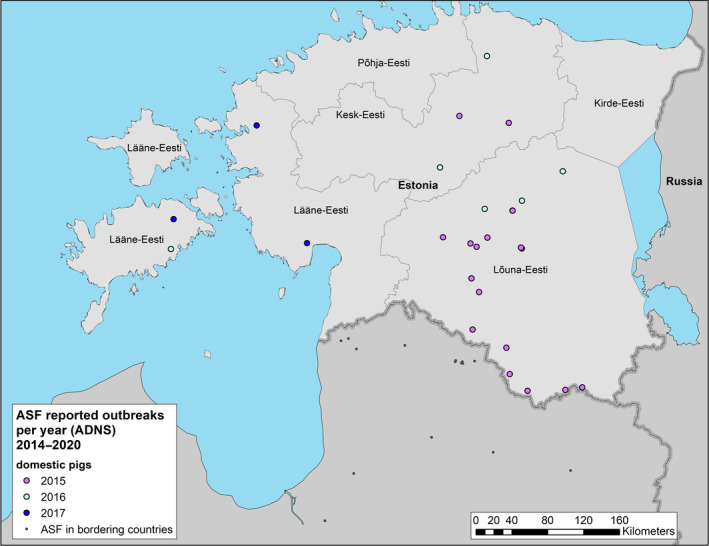
4.1.2.3.1. Evolution of ASF epidemic in this reporting period in Estonia
Figure 10.
ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Estonia up to 31 August 2020
Figure 11.
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Estonia up to 31 August 2020
During the reporting period (1 September 2019–31 August 2020), the decrease in numbers of detected cases as well as the prevalence of ASF PCR‐ and/or antibody‐positive wild boar continued. No outbreaks were observed in domestic pig herds. Until the re‐emergence of PCR‐positive cases among wild boar in August 2020, the last clusters of PCR‐positive wild boar were observed in late 2018–early 2019 in the west most part of the country and on the eastern border with Russia (EFSA, 2020), the latter being likely epidemiologically linked to the ASF situation over the border, where ASFV circulation was registered from September to November 2018 (Shulz et al., 2020). Between 6 February 2019 and 28 August 2020 (for more than 18 months), only cases of seropositive wild boar were sporadically detected in Estonia. During the year 2019, seropositive animals were detected in all the 14 previously affected counties. However, there were eight counties, where all the detected seropositive animals were older than 1 year. The proportion of seropositive wild boar in the affected counties in total was 2.1%. On the county level, the prevalence ranged from 0.5% (Valgamaa) to 8.2% (Läänemaa), whereas on the mainland of Läänemaa County, where virus spread among wild boar was observed the latest, the average seroprevalence was 17.4%.
From January until August 2020, all detected seropositive wild boar were in the age class older than one year. In July, however, two PCR‐negative and seropositive piglets were hunted on the island of Saaremaa, both reported to be younger than 6 months (M. Kristian, personal communication), possibly indicating that these animals may have had maternal antibodies. Although it cannot be excluded that these animals were infected, this is not supported by the fact that no virus has been detected consecutively in wild boar hunted or found dead in this area or Saaremaa island in general. The proportion of seropositive animals in total in affected counties had decreased to 1.3% by the end of September 2020. On the county level, the prevalence of seropositive findings among hunted wild boar ranged from 0 (two counties in the South‐East of the country) to 5.0% (Läänemaa County in the west). The two counties in the South‐East of Estonia, Valgamaa and Põlvamaa, free of PCR and antibody positive findings during 2020, had their last seropositive wild boar detected in January and July 2019, respectively.
In late August 2020, a new cluster of PCR‐positive wild boar was detected in one hunting ground located in Raplamaa County in the western part of the country. In this hunting ground, 13 (11 found dead 2 hunted) PCR‐positive wild boar were detected, all located within a radius of approximately 3 km. In October 2020, three seropositive but PCR‐negative wild boar younger than 1 year old were hunted in the same area, likely the surviving piglets of the infected group (M. Kristian, personal communication). The last previous PCR case in county of Raplamaa was detected in February 2018 in the same municipality, ~ 13 km from the case detected on 25 August 2020. The time span between these two findings is 30 months. During this period in the Rapla County, 16 seropositive wild boar were detected (after February 2018 – 8, 2019 – 5, before August 2020 – 3), all but one older than one year. The only seropositive piglet in the age group of younger than one year was hunted in August 2019 (~ 9 km away from the location of the last PCR‐positive detection), which means the animal must have been the offspring of the same year and had to be younger than 6 months and therefore could have had maternal antibodies.
The number of carcasses reported during the period from January 2018 to the end of August 2020 in Rapla County has been on average 2 per 1000 km2 (including wild boar killed in traffic accidents). However, the reporting has not been well distributed over the territory of the county. In 2019, all carcasses were reported from one municipality (Märjamaa). No carcasses were reported, neither from the municipality nor from the hunting ground affected by the present outbreak.
The origin of the outbreak is not known. The probability that virus circulation in the local wild boar population has remained undetected over 30‐month period is considered low. Despite the lack of passive surveillance in the presently affected municipality, there has been ongoing active surveillance and seropositive animals have been detected. The proportion of seropositive animals among hunted wild boar in the Rapla County has been declining over the whole period. This has not been previously observed in areas where the virus has been circulating. Furthermore, the seropositive animals have been (with one exception) adults, indicating lack of recent infections.
The closest PCR‐positive cases in wild boar or outbreaks in domestic pigs detected during the period February 2019 to August 2020 were in the south western part of Latvia and European part of Russia, both more than 300 km away from the Rapla County. This makes it unrealistic to assume any role of migrating wild boar to be a cause of this outbreak.
The probability that the virus has survived in the environment over a 30‐month period should be considered unlikely (see EFSA, 2021). The only plausible way for the virus to survive this long is if it has been frozen. Thus, the new release of the virus by a human could be considered as a plausible cause of this outbreak. The third, but highly hypothetical option, would be the release of the virus from a long‐term infectious animal. To date, however, there is no evidence that such shedding of virus for longer than 60–70 days exist (EFSA, 2021).
4.1.2.3.2. Specific prevention and control measures in Estonia
No new measures during the reporting period.
4.1.2.3.3. Most likely routes of introduction in domestic pig holdings
No domestic pig outbreaks during the reporting period.
4.1.2.3.4. Probable human mediated ASF spread in wild boar population
A cluster of virus‐positive wild boar was detected in Raplamaa County, in the North‐Western part of the country in late August 2020 after 18 months from the last detection of the PCR‐positive wild boar in Estonia. One of the possible reasons of the re‐emergency of the virus in the area is reintroduction by humans. No direct or circumstantial evidence to support the hypothesis could be revealed in epidemiological investigation.
4.1.2.4. Greece
4.1.2.4.1. Evolution of ASF epidemic in this reporting period in Greece
Figure 12.

ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Greece up to 31 August 2020
The first outbreak of ASF in Greece occurred in a small non‐commercial farm of 32 pigs (in Nikoklia village of Serres Regional Unit in the Region of Central Macedonia in Greece). The suspicion of the outbreak was raised on 3.2.2020 and the presence of the virus was confirmed in the laboratory on 5.2.2020. The location of the outbreak was in the village Nikoklia in the Vissaltia Municipality, Serres Regional Unit in the Region of Central Macedonia.
The epidemiological investigation revealed that the holding included two epidemiological subunits: the first unit contained 4 sows, 1 boar, 13 piglets and 11 fattening pigs and the second unit was located in an olive grove, surrounded by electric fence, where a number of fattening pigs were transferred according to the farmer.
4.1.2.4.2. Specific prevention and control measures in Greece
The measures were implemented in line with in with Directive 2002/60/EC.
4.1.2.4.3. Most likely routes of introduction in domestic pig holdings
Taking into account the incubation period reported by OIE (2019), and the moment of clinical signs and death observed on the farm, the assumed period of introduction of ASFV on the farm was during the period between 20.12.2019 and 9.1.2020.
According to the epidemiological investigation conducted by the official veterinarians in the Serres Regional Unit, it is considered likely that the pigs were exposed to food leftovers thrown in the olive grove where fattening pigs were allowed to graze since the 5 January 2020, by people working in a greenhouse located close to the farm. These workers visit regularly their country of origin which is ASF‐affected, and maybe they transported contaminated pig meat/pig meat products to Serres following Christmas holidays and leftovers were thrown in the olive grove, accessible to the pigs.
Thus, based also on the available surveillance data until now in the extended surveillance/protection zones and the fact that no new cases have been detected, it seems that this was an outbreak restricted only in a small farm due to human translocation of the virus. Transmission through wild boar is considered less likely but cannot be excluded taking into account the limited available surveillance data in wild boar. According to recent data, population of this game species is estimated to be 10484 animals in Serres Regional Unit, while reported hunting bag is 2428 wild boars.
4.1.2.5. Hungary
4.1.2.5.1. Evolution of ASF epidemic in this reporting period in Hungary
Figure 13.

ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Hungary up to 31 August 2020
In Hungary, the ASF virus is only present in wild boar. Until September 2019, the following counties were affected: Heves, Szabolcs‐Szatmár‐Bereg, Nógrád, Borsod‐Abaúj‐Zemplén, Hajdú‐Bihar and Jász‐Nagykun‐Szolnok.
On 28 September 2019, the virus was found in dead wild boar in the Pest County, which previously was considered as low‐risk area. The cases were found in a fenced wild boar garden. The garden is located under the Hungarian law in a forest with public access. Visitors are allowed to enter inside the fenced boar garden, and Hungarian as well as foreign tourists thus visit the area. We assumed that the virus entered the area via contaminated food waste.
On 9 December 2019, ASF was confirmed in dead wild boar in the high‐risk area of Békés County. The most likely source of the infection was the natural spread of infected wild boar from Romania.
On 15 February 2020, ASF was confirmed in the Komárom‐Esztergom County. The most likely source of the infection was the natural spread of infected wild boar from the Pest County.
Up to the end of this reporting period, domestic pigs have not been affected in Hungary.
4.1.2.5.2. Specific prevention and control measures in Hungary
At the end of December 2019, the entire free area was declared as medium‐risk area and there is no low‐risk area in the country since then.
On 6 March 2020, the Eradication Plan was modified. The main modifications were the following:
The Game Management Units in the infected zones with secondary cases automatically should become part of the strictly/highly restricted area (SRA).
In the SRA only individual diagnostic shooting can be allowed.
In the SRA dogs can only be used at small game group hunting.
Fenced wild boar must be eliminated in the infected area within 6 months
Wild boar population reduction with sampling ordered by the veterinary authority should be performed at a level 150% of hunting year 2019–2020 for all age groups. If this target is not met, external help can be used for diagnostic shooting
Special equipment (thermal camera, silencer) can be approved by the leader of the National Disease Control Centre for dedicated persons
Home slaughter is to be notified in the SRA and infected area
Before using grains as feed for pigs, these must be stored minimum 90 days in place inaccessible to wild boar.
A new definition for small scale non‐commercial holdings has been established: a small‐scale non‐commercial holding is a holding where no breeding pigs (sow or boar) are reared on the holding and the pigs are fattened only for own consumption and the pigs and the products thereof do not leave the holding.
4.1.2.5.3. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
There is no direct proof of human‐mediated spread, although it was assumed that the virus entered the area in the Pest County via contaminated food waste as the place of the finding was in the low risk area, about 72 km from the nearest wild boar case in Heves County and these were the first cases on the west side of the Danube.
4.1.2.6. Lithuania
4.1.2.6.1. Evolution of ASF epidemic in this reporting period in Lithuania
Figure 14.

ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Lithuania up to 31 August 2020
Figure 15.
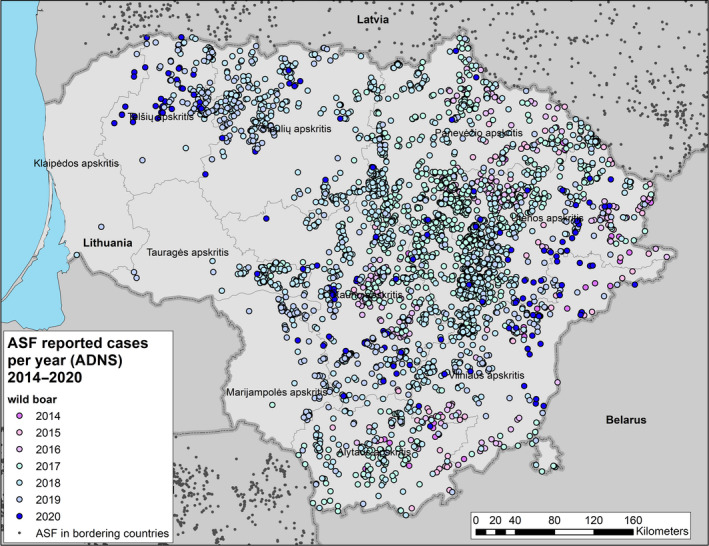
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Lithuania up to 31 August 2020
In the period between 1 September 2019 and 31 August 2020, 306 affected wild boar have been reported: 116 were found dead and 190 were hunted. The State Food and Veterinary Service of Lithuania carries out inspections in all hunting grounds. Presumably, the improved biosecurity during hunting is crucial and it helps to contain the disease in limited areas. The wild boar population has substantially decreased in Lithuania (Figure 49).
Figure 49.
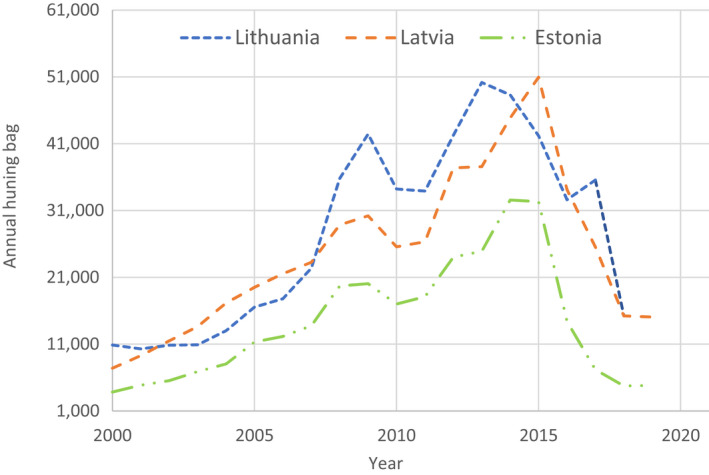
Annual number of hunted wild boar in the ASF‐affected the Baltic States in the last two decades
From 1 September 2019 to 31 August 2020, the presence of ASF was confirmed in three farms:
one in a commercial farm with 8,555 pigs.
two in backyard farms with 8 pigs.
A substantial decrease in ASF outbreaks in domestic pigs was observed during this reporting period, compared to the previous period, which was probably due to:
the lower virus pressure from the environment, based on lower numbers of cases in wild boar compared to the previous period.
improvement of biosecurity.
the reduction of the number of backyard farms (from 14,000 in 2017 to 7,700 in 2020)
4.1.2.6.2. Specific prevention and control measures in Lithuania
The surveillance in Lithuania is performed in line with the National Surveillance Plan and the Strategic Approach to the Management of ASF for the EU:
All backyard farms are inspected at least once per year. A special expert group performs biosecurity inspections in commercial farms at least twice a year.
Regular training courses are organised for pig keepers and hunters. The training courses cover theoretical and practical topics with the demonstration of implementation of major biosecurity principals in pig farms. Official inspections of biosecurity requirements are regularly performed on hunting grounds.
The completion of the Trade Control and Expert System (TRACES) of the European Commission with trade data of the movements of pigs from one place to another was started, which helps to ensure the traceability more effectively.
4.1.2.6.3. Most likely routes of introduction in domestic pig holdings
The investigations carried out on outbreak farms lead to the conclusion that the reasons of the outbreaks in pig farms were related to the particularities of activities carried out on those farms, the biosecurity infringements, and the increased risks for the introduction of ASF from environment. An intensive spread of ASF in the wild boar population was recorded within a radius of 10 km around the outbreaks in non‐commercial farms in 2020 for several months.
The epidemiological investigations resulted in the identification of the following most likely sources of infection:
Not changing of clothes and footwear when entering the pig facilities.
Owners of backyard farms visiting forests.
Not fenced pig housing facilities.
Storing other farm materials in pig housing facilities.
During epidemiological investigations it was determined that the human factor was the most likely reason of virus introduction (builders, workers who do not oblige with biosecurity rules).
4.1.2.6.4. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
No direct evidence of human mediated spread in wild boar could be provided.
4.1.2.7. Latvia
4.1.2.7.1. Evolution of ASF epidemic in this reporting period in Latvia
Figure 16.
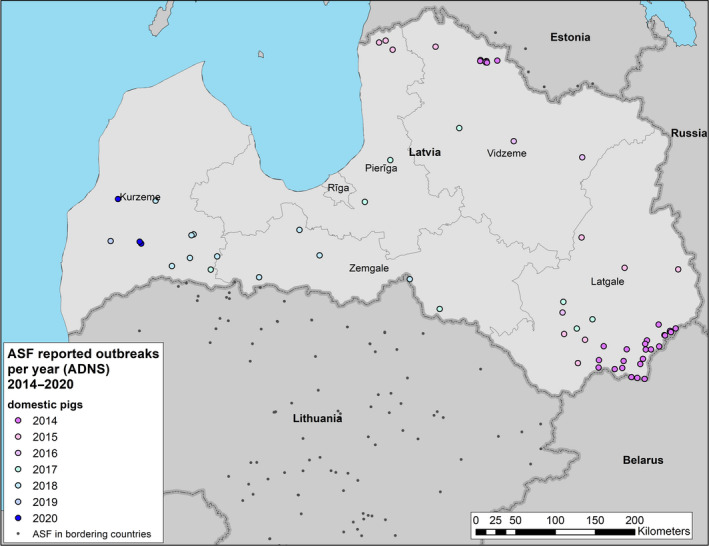
ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Latvia up to 31 August 2020
Figure 17.
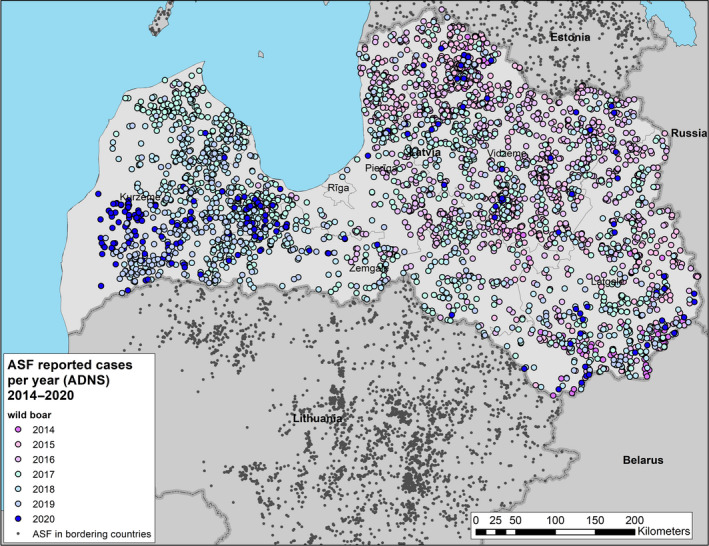
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Latvia up to 31 August 2020
In the reporting period, 14,974 hunted wild boar and 134 found dead wild boar (including four road kills) were tested for the presence of ASFV. In total, 416 ASF wild boar cases (336 hunted and 80 found dead) have been confirmed. Only 35% of all ASF cases were confirmed as PCR positive, 98% of which were located in the southwestern part of Latvia forming a cluster near the border with Lithuania. This cluster is a frontline of the current epidemic wave that is still moving very slowly towards the Baltic Sea. Only three ASFV (PCR)‐positive cases were confirmed in the eastern part of Latvia, where the disease was introduced in 2014.
Out of 336 cases in hunted wild boar, 270 (80%) only had seropositive results. All PCR‐positive cases (n = 66) in hunted wild boar were found in the western part of Latvia.
During this period, 3,246 domestic pigs have been tested in the frame of enhanced passive surveillance, i.e. weekly testing of dead pigs. In July 2020, three ASF outbreaks in pig farms were confirmed. Two out of three ASF outbreaks were detected by weekly testing of dead pigs. All three ASF affected farms were located in the south‐west of Latvia, where most of the ASF virus cases in wild boar are clustered.
4.1.2.7.2. Specific prevention and control measures in Latvia
In order to improve passive surveillance system in wild boar, since July 2020 incentives are paid for the notification of found dead wild boar.
4.1.2.7.3. Most likely routes of introduction in domestic pig holdings
In three ASF outbreaks, detected in July 2020, the most probable source of infection was indirect contact with infected wild boar/contaminated environment.
4.1.2.7.4. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
Not observed in Latvia since 2016.
4.1.2.7.5. Poland
4.1.2.7.6. Evolution of ASF epidemic in this reporting period in Poland
Figure 18.

ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Poland up to 31 August 2020
Figure 19.
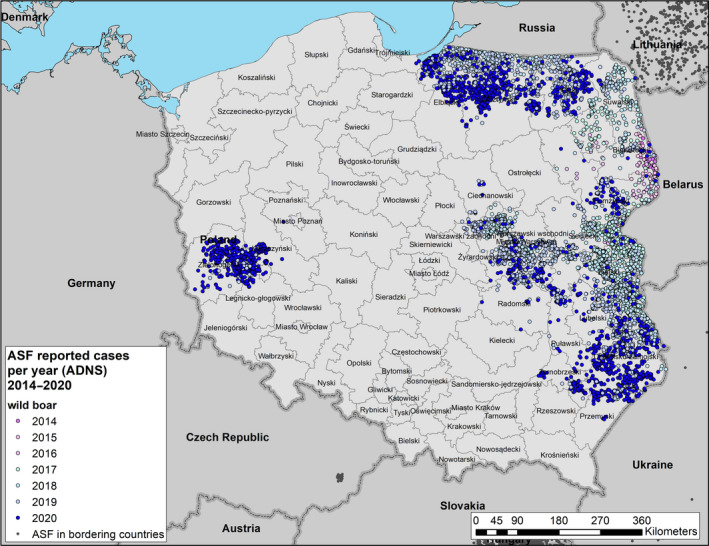
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Poland up to 31 August 2020
In November 2019, the first case of ASF was identified in western Poland, in the Lubuskie voivodeship. The virus was confirmed in a carcass of a wild boar killed in a traffic accident. After the first confirmation of ASF in western Poland, the Polish Veterinary Inspectorate intensified an active and passive surveillance in several neighbouring counties. The result of these activities was the confirmation of ASF in 878 wild boar, killed or shot by the end of February 2020 in three voivodeships: Lubuskie, Wielkopolskie and Dolnośląskie. Until the end of August 2020, in total 4,361 wild boar found dead and 755 hunted wild boar have been confirmed positive for ASFV. The highest number of ASF cases has been found in the Warmińsko‐Mazurskie, Lubuskie and Lubelskie voivodeships with 1924, 1334 and 859 positive wild boar, respectively. From the wild boar hunting bag data (data from individual forest districts dated March 2019), the wild boar abundance is estimated to range from 0.27 to 0.52 wild boar per km2 in Western Poland. The prevalence of ASF in wild boar found dead in western Poland, where the ASF epidemic in wild boar occurred, was at the level of approximately 80–100%. Figure 21 shows the newly affected area in western Poland with the location of confirmed ASF cases.
Figure 21.
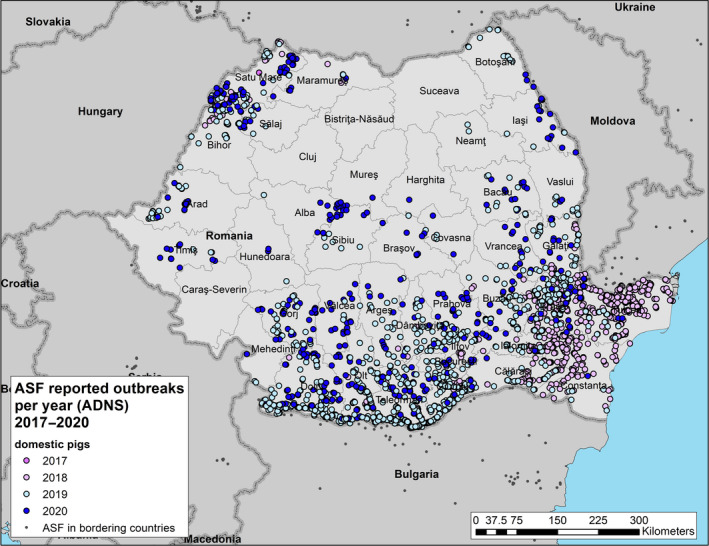
ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Romania up to 31 August 2020
Figure 20.

ASF‐affected area in western Poland and the confirmed cases (red dots). The areas of forests have been indicated in green. The region marked with red indicates the estimated buffer zone (10 km) from the confirmed ASF cases
The current epidemiological situation in the domestic pig sector resulted in identification of in total 48 ASF outbreaks in domestic pigs which have been confirmed in 2019 and 94 outbreaks confirmed in 2020.
4.1.2.7.7. Specific prevention and control measures in Poland
At the beginning of the ASF epidemic in western Poland, fenced areas were established around 10 km of the recently confirmed ASF cases. The fence is 1.5 m tall with the mesh size of 10 cm. The bottom part of the mesh (50 cm) is folded back and immobilised in the ground. Additionally, stations with odour repellents have been installed. In the enhanced surveillance, the ad‐hoc task forces composed of shooters (police, army, border guards and state fire brigades) have been involved to search for carcasses. These tasks aimed to identify wild boar carcass in the most effective way.
4.1.2.7.8. Most likely routes of introduction in domestic pig holdings
The most likely source of ASF introduction to pig farms during the reported period were ASFV‐contaminated crops, represented by hay, straw or grain harvested from the area where ASF spreads in the wild boar population. The contaminated agriculture equipment including harvesters or tractors used at the ASF‐affected territory could also be a source of ASF in domestic pig farms.
4.1.2.7.9. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
The distance of the newly affected region in western Poland to the nearest ASF case in central Poland was over 300 km. The potential spill over of ASF cases in western Poland was likely caused by human‐mediated spread. However, the true route of spread is impossible to determine. The study on genetic identity of Polish ASFV isolates showed the similarity between isolates identified in southern part of Mazovieckie voivodeship and isolates detected in western Poland (Mazur‐Panasiuk et al. 2020).
4.1.2.8. Romania
4.1.2.8.1. Evolution of ASF epidemic in this reporting period in Romania
Figure 22.

ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Romania up to 31 August 2020
The first half of this reporting period
In fall 2019, the number of ASF outbreaks in domestic pigs decreased, similar to the seasonal pattern observed in 2018. Measures have been taken to demonstrate that there is no ASFV circulating in the restricted areas and in areas where the outbreaks were resolved the restrictions have been lifted. Regarding the evolution of ASF in wild boar, an increase in the number of confirmations during the hunting season could be observed.
The second half of this reporting period
The number of outbreaks reported during the first three months of 2020 was considerably higher than during the same period in 2019. On the other hand, the peak in July and August was flatter than in the preceding years. This reduction of outbreaks in 2020 could be due to the restrictions imposed during the state of emergency because of the Covid‐19 pandemic, which demonstrates that human‐mediated spread is one of the main drivers for the spread of the disease.
4.1.2.8.2. Specific prevention and control measures in Romania
The main measures taken by the Veterinary Services that have led to a decrease in the number of outbreaks are:
-
–
the organisation of traffic controls by teams composed of representatives of the Sanitary Veterinary and Food Safety Directorates, the County Police Inspectorates and the County Inspectorates of Gendarmes to stop illegal movements of pigs and pork/products;
-
–
advising pig farmers by private veterinarians on the biosecurity measures they must comply with and the obligation to report any disease/death of the animals on the holding;
-
–
training of veterinarians within the Sanitary Veterinary and Food Safety Directorates, and private veterinarians regarding the clinical picture and measures to be applied in case of ASF outbreaks;
-
–
updating and approving the plan of measures for the prevention and control of ASF in domestic pigs and wild boar in each county's Local Centers for Disease Control.
4.1.2.8.3. Most likely routes of introduction in domestic pig holdings
The epidemiological investigations carried out at the outbreak farms demonstrated that the most likely causes of new outbreaks of ASF are the following:
-
–
the purchase of pigs without sanitary‐veterinary documents attesting their health status;
-
–
illegal movement of pigs outside the protection and surveillance zones established around the outbreaks (minimum 10 km), in accordance with the requirements of Directive 2002/60/EC;
-
–
uncontrolled movements of pigs carried out by illegal traders of live animals;
-
–
slaughtering sick pigs without the supervision of the official veterinarian and preservation of the products derived from them, taking into account that scientific data have shown that the virus persists in frozen/preserved products for years;
-
–
illegal use of untreated food waste in pig feed;
-
–
non‐compliance with biosecurity measures, especially in backyard farms, but also in some commercial holdings.
4.1.2.8.4. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
Although evidence of specific cases of human‐mediated spread of ASF in the wild boar population cannot be provided, the continued spread of ASF in wild boar populations in Romania is likely linked to the following aspects:
-
–
failure to reduce the wild boar density according to prescribed hunting quotas,
-
–
non‐performance of passive surveillance, i.e. sufficient wild boar carcasses are not collected to test them for ASF.
4.1.2.9. Russia
4.1.2.9.1. Evolution of ASF epidemic in this reporting period in Russia
During this reporting period, ASF occurred in the Russian Federation from the most western Kaliningrad oblast to the Russian Far East. The virus has persisted as local epidemics in domestic pig populations and wildlife in several previously affected regions in the central and southern European part of the country (in some of them since 2008) and in the regions bordering with Kazakhstan, Mongolia and China.
Figure 23.
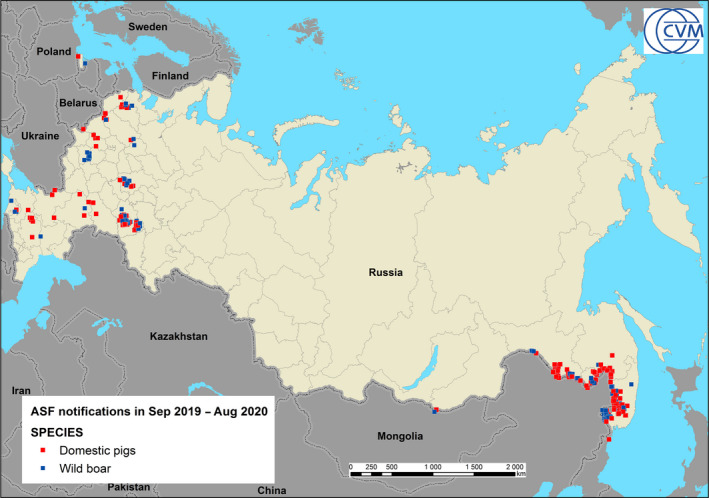
ASF notifications in domestic pigs and wild boar from September 2019 to August 2020 in Russia up to 31 August 2020
In the past 2 years, many outbreaks have been reported in the most eastern oblasts of Russia, mainly bordering the Chinese border.
Figure 24.
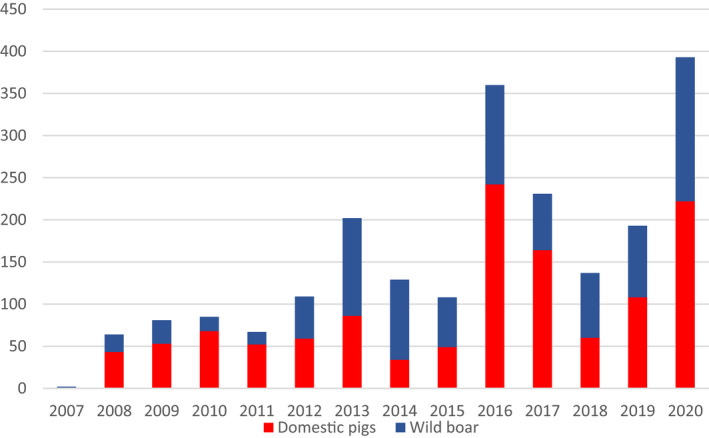
Number of ASF outbreaks in domestic pigs and wild boar between 2007 and 2020 in Russia
4.1.2.9.2. Specific prevention and control measures in Russia
No specific prevention and control measures have been implemented since 2011 to control the spread of ASF in wild boar populations. The main measures are aimed at reducing the number of low biosecurity pig farms (backyards and small holdings) and the wild boar density. Most low biosecurity small farms were gradually eliminated. At the same time, the production of pork is growing due to increasing number of industrial farm types.
To prevent the spread of ASF in wildlife, the Russian authorities recommended to maintain wild boar density less than 2.5–5 wild boar per km2 for both affected regions and regions at risk.
4.1.2.9.3. Most likely routes of introduction in domestic pig holdings
Backyards, low biosecurity pig farms and free‐ranging farms play a leading role in the spread of ASFV (Figure 25). This was supported by a number of statistical models (Korennoy et al., 2014; Vergne et al., 2014; 2017) where the density of pigs kept in the low biosecurity sector was found to be strongly associated with the number of ASF outbreak reports.
Figure 25.
Distribution of ASF affected pig holdings by size (A) and holding types (B)
4.1.2.9.4. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
Since the first introduction of ASFV in Russia, ASF epidemics are characterised by distant jumps of the infection most likely mediated by humans, followed by local epidemics in susceptible populations.
4.1.2.10. Slovakia
4.1.2.10.1. Evolution of ASF epidemic in this reporting period in Slovakia
Figure 26.
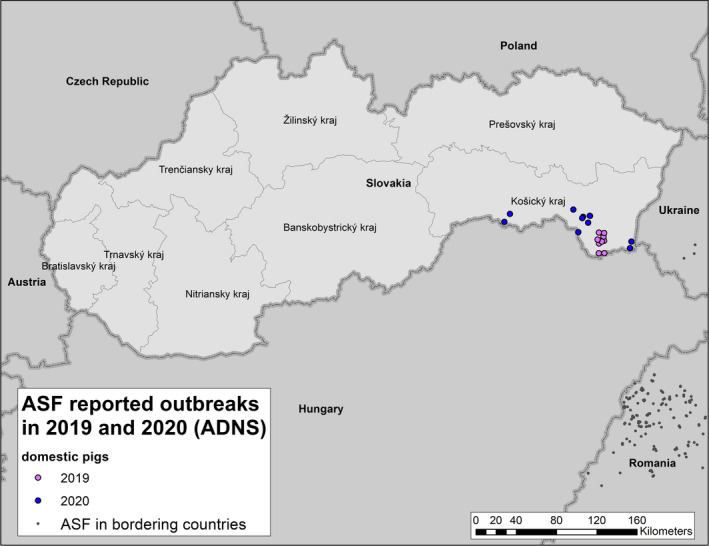
ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Slovakia up to 31 August 2020
Figure 27.
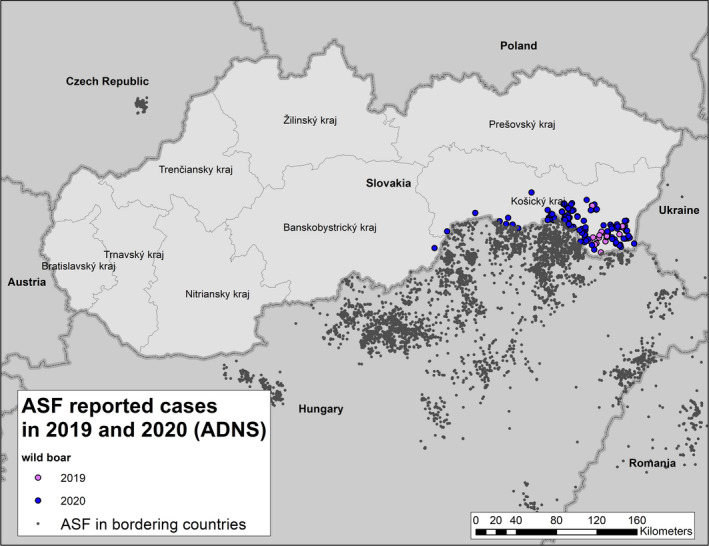
ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Slovakia up to 31 August 2020
In this reporting period, ASF in wild boar has spread in the south east part of Slovakia and 32,087 hunted wild boar and 1,409 dead wild boar were tested for the presence of ASFV using PCR. The percentage of PCR‐positive hunted wild boar was 0.19% (61 positive samples) and 14.76% of dead wild boar (208 positive samples). Serology was done by IPT test on 32,076 hunted wild boar (25 positive) and 738 wild boar found dead (9 positive). All ASF cases in wild boar are from six district only. The districts with the largest numbers of positive animals are Trebišov (123), Košice‐okolie (109) and Michalovce (33). There were two districts with just one ASFV positive animal, and one district with just two positive animals. The disease was moving along the border with Hungary and Ukraine.
There was no ASF outbreak in domestic pigs from 19.8.2019 until 10.7.2020, when a new outbreak in a backyard holding was confirmed. It was a small backyard holding 3 km from the Hungarian border and 1.5 km from the Ukrainian border. In total, we recorded 14 new outbreaks in domestic pigs, 12 in backyards holdings and two in commercial farms (together 601 pigs). All outbreaks are from two districts: Trebišov and Košice‐okolie.
Outdoor keeping of pigs is not allowed in the whole territory of Slovakia.
In Slovakia, active and passive surveillance system has been in place for several years before the first case in both wild boar and in domestic pigs was confirmed.
From 1 January 2020, caused by the development and spread of ASFV in Poland closer to the Slovakian border, a new buffer zone near the Polish border has been defined to have an overview of the situation in the northern part of country. For the time being, all results of laboratory investigation originated from this region were negative.
4.1.2.10.2. Specific prevention and control measures in Slovakia
In the ASF‐free areas, intensive wild boar hunting has been ordered and the hunters are motivated to reduce the wild boar population through financial incentives. Financial support is provided for:
hunting of adult female wild boar and female yearlings, after submitting of genital organs.
hunted virologically positive wild boar regardless of age and weight and
found dead virologically positive wild boar.
According to the hunters’ database, the total hunting bag for 2020 was 60,697 wild boar in Slovakia. The hunting quota are considered to be minima and there are no administrative limitations to exceed the number of hunted wild boar above those quota.
Moreover, the recommendation to stop pig breeding in infected areas and/or areas with high risk (non‐commercial holdings only) has been issued by the chief veterinary officer. District Veterinary and Food Administration may decide to order temporary ban of breeding of pigs in backyard farms in the municipalities of villages corresponding the hunting ground, where positive WB have been confirmed and where the owner is not able to fulfil the biosecurity requirements preventing introduction of the virus into the holding.
4.1.2.10.3. Most likely routes of introduction in domestic pig holdings
Following the epidemiological investigations, most outbreak farms were non‐commercial farms with poor level of biosecurity. Introduction through direct transmission has not been reported. However, introduction of disease into the holding was likely via fomites.
4.1.2.10.4. Probable human mediated ASF spread in wild boar population (jumps‐when evidence)
No direct evidence of human‐mediated spread in wild boar could be provided.
4.1.2.11. Serbia
4.1.2.11.1. Evolution of ASF epidemic in this reporting period in Serbia
Figure 28.
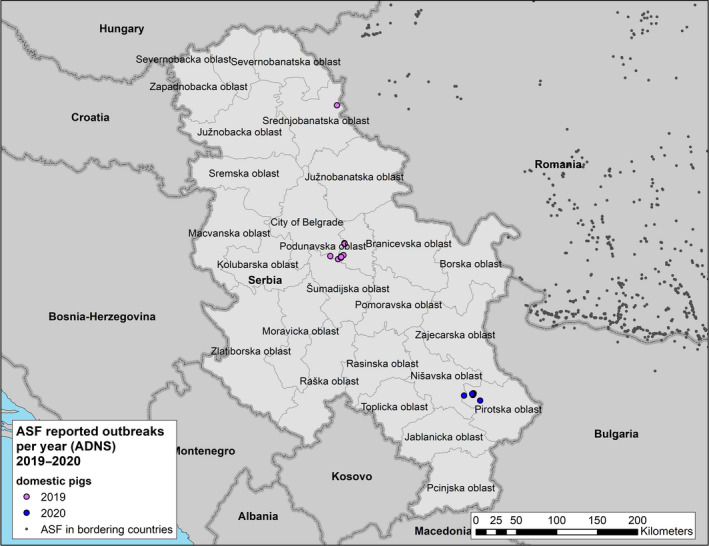
ASF outbreaks reported to the Animal Disease Notification System in domestic pigs since the first introduction in Serbia up to 31 August 2020
Figure 29.

ASF cases reported to the Animal Disease Notification System in wild boar since the first introduction in Serbia up to 31 August 2020
Despite the measures in the initially identified risk‐zones near the bordering regions to Hungary, Romania, Bulgaria, ASF was introduced in Serbia on 31 July 2019 in the in the central part of the country in the Podunavska Oblast and the City of Belgrade. Thereafter, in total 37 outbreaks occurred in domestic pigs until the 31 August 2020. The last outbreak occurred in the Srednjebanatski district, close to the Romanian border. After applying appropriate measures and no further evidence of the presence of ASF during the overall surveillance activities, the outbreaks were reported as resolved to OIE in December 2020. In total, 634 pigs were destroyed in Serbia (including pre‐emptive culling in holdings with low biosecurity) in 47 holdings. Large commercial farms have not been affected.
The first appearance of ASF in found dead wild boar was confirmed on the 3 January 2020 in the Pirotska district, near the Bulgarian border and the next day in the Borska oblast. So far, the presence of the virus has been confirmed in three oblasts (Borska, Zaječarska and Pirotska). The last case of ASF in wild boar for the reporting period was officially confirmed in a dead wild boar in August in the Pirotska Oblast, in the same area where the first one occurred. In total, 70 wild boar were found PCR positive within the reporting period.
4.1.2.11.2. Specific prevention and control measures in Serbia
Every year, the surveillance programme in the domestic pig sector is updated according to the epidemiological situation in Serbia, including intense active and passive surveillance activities. From 16 September to 1 December 2019, in total 347,535 questionnaires have been carried out on domestic pig farms and 16,296 samples were taken from pigs to be tested for ASF. In the first semester of 2020, a total of 2377 samples were tested (PCR) without positive findings until the end of the reporting period.
In 2019, a representative number (95% CI) of wild boar had to be examined for ASF and Classical Swine Fever (CSF), especially in the period of intensive hunting (November–March). All dead wild boar or wild boar shot during ‘sanitary hunting’ (shooting because of the suspicion of possible disease) had to be reported and tested for ASF and CSF. Legal hunting entities, i.e. hunting clubs, were obliged to report hunting not later than 48 hours prior the hunting started, to organise veterinary inspection and sampling at the time of hunting. The monitoring and surveillance plan was further extended to a wider territory in Serbia, considered as high‐risk areas, in 10 districts bordering to Hungary, Romania and Bulgaria.
In 2020, all wild boar found during passive surveillance had to be tested by PCR and additional serological testing in affected areas had to be carried out in parallel. To ensure continuity of surveillance on ASF during the year, after the completion of intensive hunting, all wild boar that were hunted during the off‐season period had to be examined.
In hunting areas affected by ASF, all shot wild boar had to be examined during the duration of the measures by PCR and if necessary serological testing, for at least 12‐month period, except during the hunting ban.
As a result of surveillance and diagnostics in wild boar, there were 1865 shot or found dead animals tested in the first semester of 2020, without positive findings outside the affected area.
The most important measures implemented in the affected areas:
Hunting grounds:
Placing all hunting grounds under official supervision by the competent, authorised, official veterinarian‐veterinary inspector;
Temporary ban of hunting, except sanitary shooting and shooting for diagnostic purposes under the control and by persons from the hunting and guarding service under the supervision of the veterinary and hunting inspector and keeping prescribed records, for a period of at least 30 days. After the 30 days of hunting prohibition have passed, intensive hunting of wild pigs was carried out in order to reduce the population density, in accordance with the epidemiological situation and risk analysis, under the control of the competent veterinary and hunting authorities;
Control of the trading of meat, products and by‐products originating from wild boar, which may be a possible source and way of spreading the infection;
Continuous active surveillance in order to control the health status of wild boar and enhanced passive surveillance in order to find the carcasses or sick wild boar;
Prohibition of entering the hunting ground to all persons except officially authorised veterinarians and persons authorised by hunting entities.
Domestic pig holdings:
Pigs on the holding should be isolated from feral pigs and must not have access to any material that may have come in contact with feral pigs/wild boar.
Prohibition of entry and exit of domestic pigs to and from the holding without the approval of the veterinary inspector, taking into account the epidemiological situation;
Clinical examination and diagnostic testing on the holding of all dead pigs or pigs showing clinical signs of ASF;
Preventive killing or slaughtering of domestic pigs in all low‐level biosecurity holdings based on a risk analysis and a ban on repopulation of pigs on these holdings.
Establishment of disinfection barriers using appropriate disinfectants at the entrances and exits of buildings and farms and, if necessary, disinfection of premises;
The implementation of appropriate hygiene and biosecurity measures by persons coming into contact with feral pigs/wild boar to reduce the risk of the spread of ASFV;
Prohibition of the introduction into the holding of organs or tissues of wild boar, whether shot or found dead, as well as accessories or equipment which could be contaminated with the ASFV;
Control of the movement of pigs, pig meat and products and by‐products from pigs, feed, semen, ova or embryos of pigs from holdings in the infected area;
Temporary prohibition of organising fairs, exhibitions, livestock markets and other gathering of pigs.
Measures implemented in additional intensive hunting area (buffer zone)
Hunting grounds:
Intensive shooting of wild boar in order to reduce their numbers in the hunting area, in accordance with the epidemiological situation and risk analysis, under the control of the competent veterinary and hunting inspector, in accordance with regulations and acts in the field of hunting and veterinary matters;
Prohibition of bringing pork into the hunting grounds, not intended for human consumption, which does not originate from the approved selections and is not in the original packaging.
Domestic pig holdings:
Prohibition on outdoor keeping and releasing domestic pigs on the open area;
Installation of barriers with cleaners and disinfectants at the entrance to the farm. Vehicles are forbidden from entering and cleaning equipment that has not been cleaned, washed and disinfected in an economically efficient manner;
Prohibition of unauthorised access to the pig farms, proper storage of equipment, food and material;
Prohibition on the bringing of pork, offal, by‐products and by‐products originated from wild boar or domestic pigs killed or slaughtered on other holdings.
4.1.2.11.3. Most likely routes of introduction into domestic pig holdings
Taking into account the fact that ASF was circulating in the wild boar population in the Pirotska and Borska oblasts, as well as the extensive way of keeping pigs in the area with low levels of biosecurity, it was expected that the disease would be transmitted to domestic pigs in backyard farms.
Although wild boar hunting was banned in the infected area, to avoid further possible spread by hunters, and direct contact between pigs and wild boar could not be excluded, considering the location of the outbreak in the vicinity of wild boar carcasses, human mediated spread seems to be the most likely source of introduction.
Preventive depopulation of domestic pigs in holding in the infected hunting ground in Pirotska and Borska oblasts slowed the spread of the disease, but it could not prevent the virus to be continuously present in the environment. The consequent occurrence of ASF in domestic pigs was subsequently confirmed in the surrounding area with a higher density of small farms, but the disease did not spread to commercial farms or outside the affected hunting grounds.
4.1.2.11.4. Probable human‐mediated ASF spread in wild boar population
Based on the fact that ASF in wild boar was initially confirmed in the Borska and Pirotska oblasts, two locations without epidemiological connection, in the same period, the possible sources of introduction of ASF in those areas should be analysed separately.
When it comes to the occurrence of ASF in wild boar in Borska oblast, although the location of the first positive case in the municipality of Kladovo was near the border with Romania, as the closest affected area, the width of the Danube represents a significant physical barrier that is hard to ignore in assessing the possible spread of diseases by the natural way. In addition, the common habits of the local population and the natural connection of people on both sides of the border, highly increase the probability of human involvement in the introduction of the virus.
However, for the appearance of ASF in the Pirotska oblast, there were no indications that indicate a role of humans in the spread of the disease, although this option can never be ruled out. The hunting area where the virus was first confirmed in a wild boar carcass is considered as a natural habitat of wild boar (part of Stara Planina mountain) and geographically connected with an area of the same characteristics in the border zone to Bulgaria. Furthermore, a natural migration routes are accelerated by the appearance of large fires that occurred in the border area in late autumn 2019 due to unusually high temperatures and strong wind which seems to be most likely as predisposing factor for the rapid movement of the wild boar population. This theory is supported by the fact that some carcasses were found on bare areas outside the forest vegetation which is a rather unnatural phenomenon.
In general, the problem of ASF control in the wild boar population in Serbia is reflected in lack of professional hunters and people employed or engaged in supervising and carcass searching activities in hunting grounds. In the large hunting area, the possibility of finding carcasses is low and so is early detection of the disease. For this reason, in certain parts of the hunting grounds in the infected zone, restrictive measures of prohibiting group/driven hunting are still in force in order to reduce the human contact with the virus. However, this also limits the access to important epidemiological parameters to assess the epidemiological situation in real time.
4.1.3. Time profile of proportions of positive samples tested with Ab ELISA or PCR in wild boar hunted and found dead
Figures 30A–37A show the observed proportions of positive samples of wild boar found dead, tested by PCR and by Ab ELISA. Only samples tested since 2016 are shown. Figures 30B37B show the same proportions, but only from the hunted wild boar. As for the previous reporting period, in the affected areas, the proportion of wild boar found dead testing positive with PCR has always been much higher than the proportions testing positive to Ab ELISA. Further, no general increase in the proportion of seropositives over time can be observed.
Figure 30.

Proportion of ASFV‐positive samples (only by PCR) over the tested samples from wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Belgium (1 January 2016–31 August 2020)
Figure 37.

Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Romania (1 January 2016–31 December 2019)
Similarly, Figure 38 shows the proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all domestic pigs in the ASF‐affected areas of Lithuania (A), Poland (B) and Romania (C) for the period from 1 January 2016 to 31 August 2020, which is investigated further in Section 4.1.4.
4.1.3.1. Wild boar
Figure 31.

Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Czechia (1 January 2016–31 August 2020)
Figure 32.

Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Estonia (1 January 2016–31 August 2020)
Figure 33.

Proportion of ASFV‐positive samples (only by PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Hungary (1 January 2016–31 August 2020)
Figure 34.

Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Lithuania (1 January 2016–31 August 2020)
Figure 35.
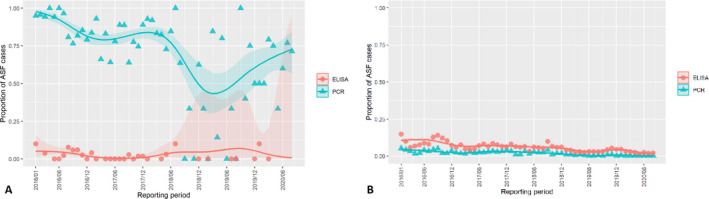
Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Latvia (1 January 2016–31 August 2020)
Figure 36.
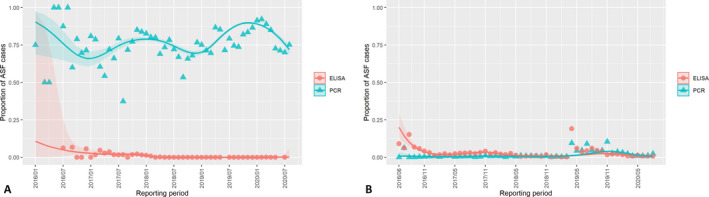
Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all wild boar found dead (A) and from hunted wild boar (B) in the ASF‐affected areas of Poland (1 January 2016–31 August 2020)
4.1.3.2. Domestic pigs
Figure 38.

Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all domestic pigs in the ASF‐affected areas of Lithuania (1 January 2016–31 August 2020) (A), Poland (1 January 2016–31 August 2020) (B) and Romania (1 January 2016–31 December 2019) (c) (a)
4.1.4. Seasonality of African swine fever outbreaks and cases
4.1.4.1. Seasonality of proportions of tested samples testing PCR‐positive
Figures 40–46 show the proportions of PCR‐positive wild boar found dead or hunted in the affected MS. In hunted animals, the proportions of PCR‐positive samples remain low throughout the year without visible seasonal patterns, with the exception of Czechia that seems to have an increase in proportion of PCR‐positive samples in winter. The latter is probably due to the short period that wild boar were infected in Czechia, and therefore, the Czech data are less suitable to observe seasonality.
Figure 40.
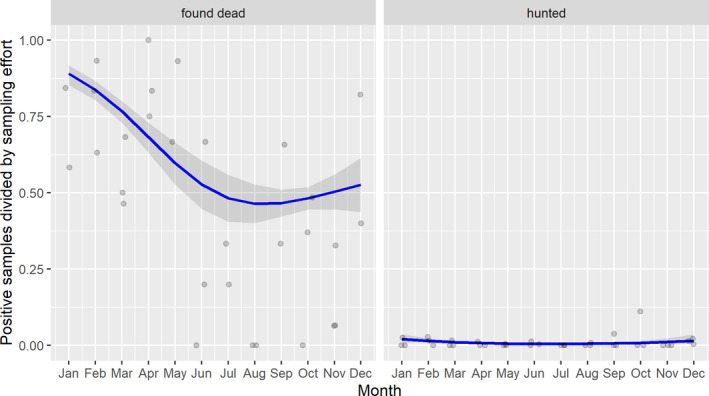
Proportion of wild boar testing positive to ASF (PCR) in Belgium by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 46.
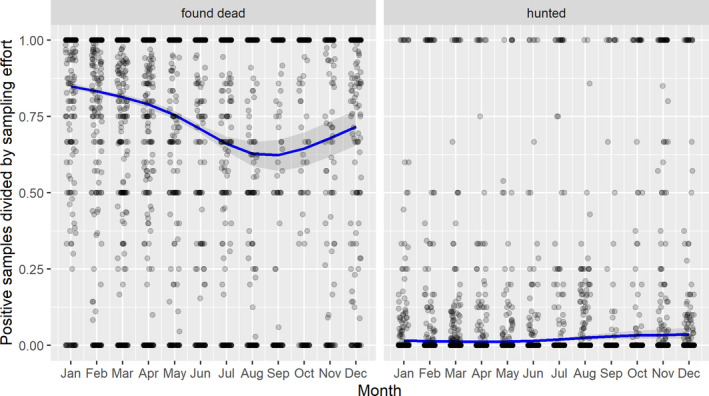
Proportion of wild boar testing positive to ASF (PCR) in Poland by calendar month, date and region for animals found dead (left) or hunted (right)
On the other hand, there is a clear seasonality in the proportions of PCR‐positive samples taken from wild boar found dead, although the patterns are slightly different for the different MS. This pattern is not synchronised with the seasonal pattern observed in samples taken from domestic pigs, displayed in Figure 39, where a clear peak of proportions PCR‐positive samples between May and September is observed in Lithuania, Poland and Romania, whereas in wild boar found dead, the proportions clearly decline during the same period, to then increase during the colder months of the year. The cause of the decline of positive proportions of wild boar found dead in summer is not known. It could be due to the shorter survival time of the virus in carcasses, or a higher baseline mortality in summer of piglets. Also, the exact cause of the summer peak in the incidence of outbreaks in domestic pig holdings is not known. It could be related to a peak of activities that are intrinsic to the pig husbandry system or other human related activities that are more abundant in summer. Also, mechanic transmission by vectors would potentially lead to a summer incidence peak of ASF outbreaks (see Section 4.2.1 on the outcomes of the literature review for risk factors for the occurrence of ASF in domestic pigs and wild boar).
Figure 39.
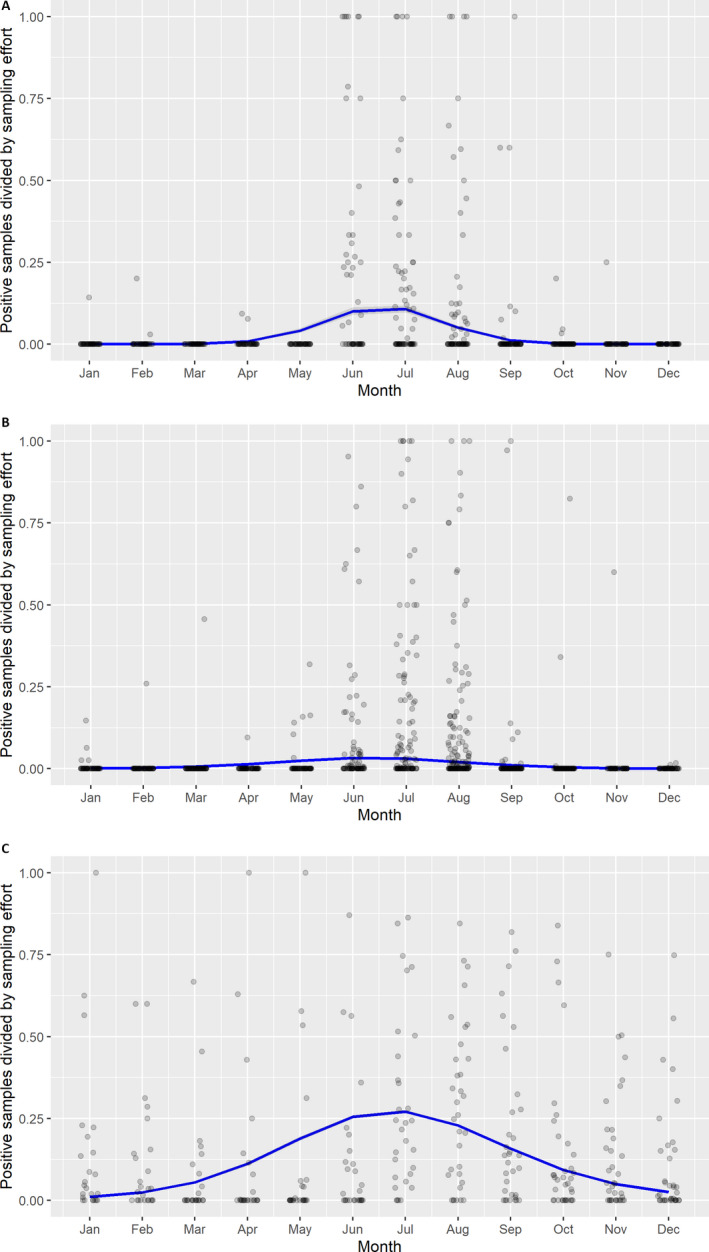
Proportion of domestic pigs testing positive to ASF (PCR) in Lithuania (A), Poland (B) and Romania (C) by calendar month, date and region
Figure 41.
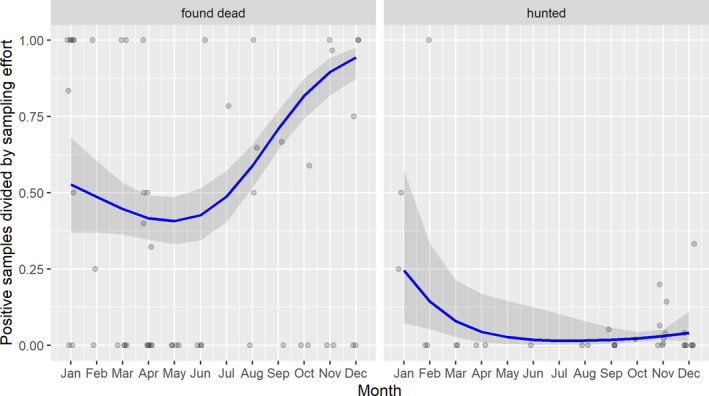
Proportion of wild boar testing positive to ASF (PCR) in Czechia by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 42.
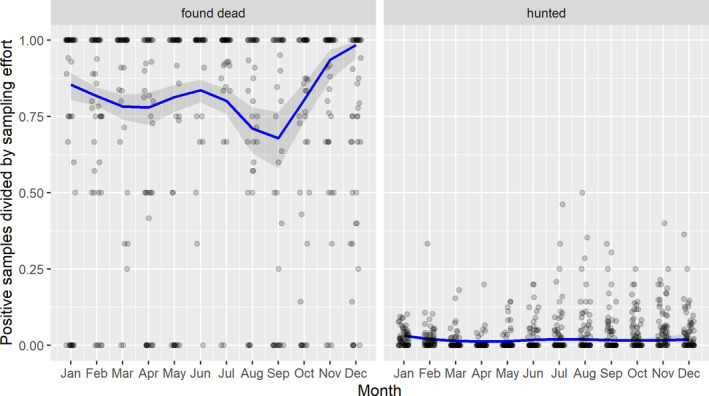
Proportion of wild boar testing positive to ASF (PCR) in Estonia by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 43.
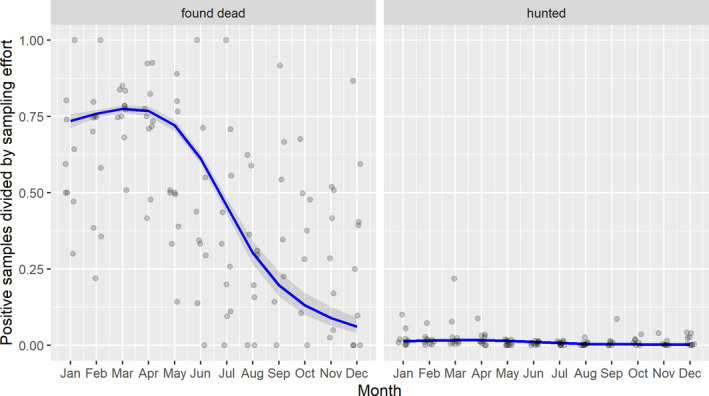
Proportion of wild boar testing positive to ASF (PCR) in Hungary by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 44.
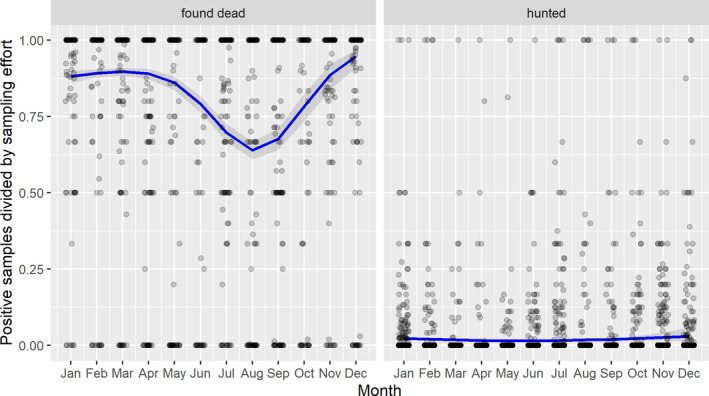
Proportion of wild boar testing positive to ASF (PCR) in Lithuania by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 45.
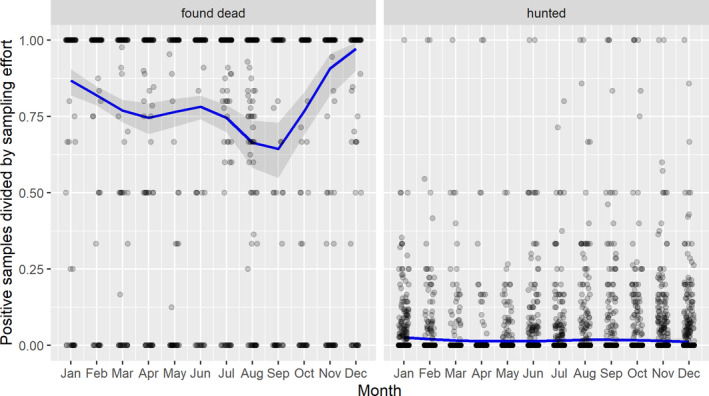
Proportion of wild boar testing positive to ASF (PCR) in Latvia by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 47.

Proportion of wild boar testing positive to ASF (PCR) in Romania by calendar month, date and region for animals found dead (left) or hunted (right)
Figure 48.
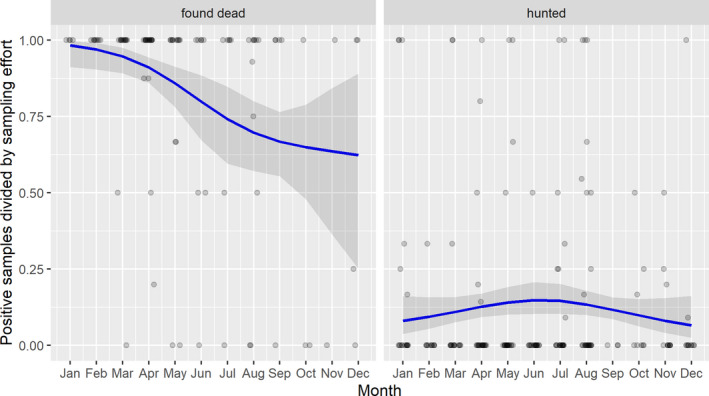
Proportion of wild boar testing positive to ASF (PCR) in Slovakia by calendar month, for animals found dead (left) or hunted (right)
4.1.5. Annual wild boar hunting harvest in ASF‐affected countries
Figure 49 shows the evolution of the annual number of wild boar that were hunted in the Baltic States in the last two decades. The number of wild boar that were hunted in the Baltic States declined rapidly since the introduction of ASF, ranging from 30,000 to 50,000 wild boar in 2014 to between 5,000 and 15,000 wild boar in 2019.
Figure 50 shows the evolution of the number of wild boar that were hunted in the all the other affected countries in the last 2 decennia (up to 2019). Contrary to the decline observed in the Baltic States, the clear decline in the wild boar populations could not be observed in the other affected countries when data are aggregated on a country level. This was either because the epidemic lasted relatively a short time in some countries (e.g. BE and CZ), or it affected only a limited part of the country's wild boar population (e.g. Hu, PL or SI). The hunting was perhaps also intensified in the ASF‐free areas of the affected MS, and this increased temporarily the hunting bag of the affected country.
Figure 50.
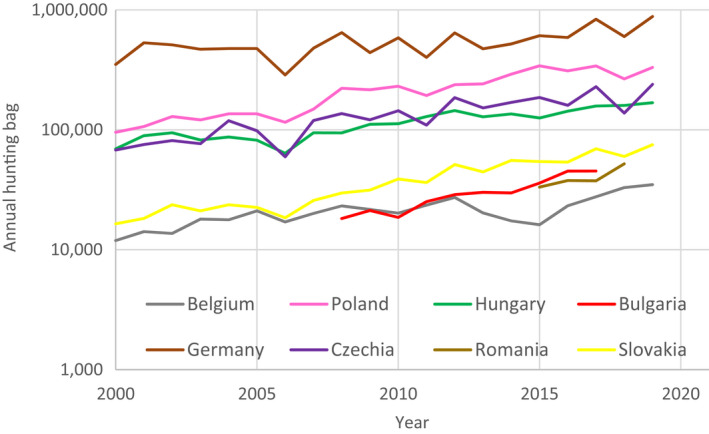
Annual number of hunted wild boar in several EU Member States affected by ASF in the last decades
4.1.6. Secondary cases network
Several studies have estimated the reproduction number (R0) in different countries affected with ASFV genotype II. In Russia, the R0 was estimated to be 1.58 (95%CI 1.13–3.77) (Iglesias et al. 2016), in Czechia 1.95 (Marcon et al., 2019), and in Belgium 1.65 (Marcon et al., 2019). In Sardinia, the R0 estimates in different subregions for ASFV genotype I were estimated to be minimum 1.12 (95% CI: 1.10–1.15) and maximum 1.17 (95% CI: 1.01–1.33) (Loi et al., 2020). The reproduction numbers estimated in these studies were calculated for a particular period in the epidemic and do not completely allow comparing the initial and later stages of epidemic.
The purpose of this investigation was to evaluate if there was a development in the numbers of notifications that could be classified as secondary cases to a single‐source case and to compare this for the beginning of the epidemic and the ongoing reporting period. Although this average number of notifications that could be classified as secondary cases (means of bootstraps calculated with a network analysis) is not to be confused with the actual reproduction number, it can be considered as a proxy for the extent of spread in the evaluated time period, and it therefore allows comparison between periods in the epidemic. This can be useful to help understanding the evolution of epidemic, i.e. if it is still in the expanding phase, or if it is rather fading out.
Except for Belgium and Czechia, probably due to the smaller sample size due to the limited spread and subsequent eradication of ASF the after focal introduction, the average number of notifications classified as potential secondary cases calculated for a single source (ASF case in wild boar) were of similar order of magnitude. They are provided in Table 5.
Table 5.
Average number of notifications classified as potential secondary cases per source case in wild boar in the affected EU Member States
| Country | Date first notification | Average number of potential secondary cases in the year after the first notification (95%CI) | Date last notification in epidemic (in the reporting period or in the country) | Average number of potential secondary cases in the year before the last notification (date last case in epidemic, or in the reporting period) |
|---|---|---|---|---|
| Latvia | 26/6/2014 | 2.01 (1.84–2.17) | 31/8/2020 | 1.78 (1.63–1.93) |
| Lithuania | 24/1/2014 | 2.00 (1.65–2.42) | 31/8/2020 | 1.83 (1.69–1.98) |
| Belgium | 24/1/2014 | 4.41 (4.12–4.72) | 28/10/2019 | 3.11 (2.75–3.48) |
| Ukraine | 5/2/2017 | 1.72 (1.38–2.10) | 4/2/2020 | ND |
| Hungary | 21/4/2018 | 3.15 (2.94–3.40) | 31/8/2020 | 3.08 (2.97–3.17) |
| Czechia | 26/6/2017 | 6.33 (5.69–6.95) | 19/4/2018 | 1.93 (1.46–2.46) |
| Estonia | 8/9/2014 | 3.5 (3.09–3.96) | 28/8/2020 | 1.63 (1.33–1.97) |
| Bulgaria | 23/10/2018 | 1.73 (1.48–2.01) | 31/8/2020 | 1.87 (1.75–1.99) |
| Romania | 29/5/2018 | 2.14 (1.98–2.29) | 31/8/2020 | 2.00 (1.89–2.10) |
| Poland | 29/5/2018 | 1.65 (1.30–2.04) | 31/8/2020 | 2.45 (3.39–2.52) |
| Slovakia | 8/8/2019 | 2.00 (1.5–2.5) | 31/8/2020 | 2.36 (2.08–2.65) |
ND: no sufficient data available for network analysis; 95%CI: 95% CI.
As can be seen in Table 5, in seven out of 10 MS the average number of notifications classified as potential secondary cases declined when comparing the first year after introduction with the number estimated in the year before the last notification of a ASF case in wild boar in this reporting period. In Bulgaria, Poland and Slovakia; however, the average number of notifications classified as potential secondary cases per source case has increased in the year before the last notification suggesting an increased extent of spread.
Figures 51–52 display the frequencies of notifications classified as potential secondary cases, caused by a single source case, obtained by bootstrapping in Estonia and Bulgaria.
Figure 51.
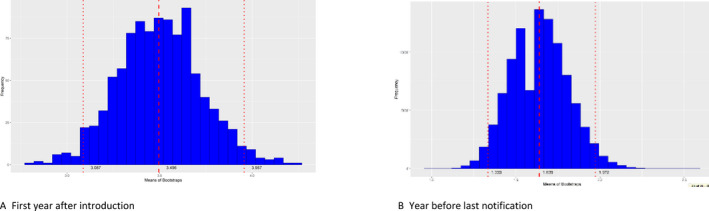
Frequencies of potential secondary cases caused by a single source case in Estonia, obtained by bootstrapping
Figure 52.
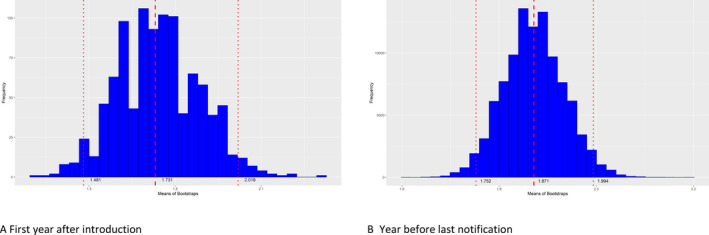
The frequencies of potential secondary cases caused by a single source case in Bulgaria, obtained by bootstrapping
4.2. Risk factor analysis –TOR 2
4.2.1. Update from narrative literature review
Since the introduction of ASF in Georgia in 2007, the ASF epidemic has insidiously and progressively extended across the European continent, eventually reaching the EU in 2014. Whilst in the Baltic countries the virus has mainly been detected in wild boar and only sporadically in domestic pigs, in Romania and Poland the domestic pig population has been largely affected (ADNS). The increased number of ASF outbreaks in Romanian domestic pig holdings provided an opportunity to investigate risk factors for the pig sector, but field evidence is still scarce, especially with regards to specific introduction routes in the holdings. Conversely, analyses of wild boar surveillance data from Baltic countries and Poland have contributed to a better understanding of the disease dynamics in the wild host.
The scope of this narrative literature review was research focused on risk factor analysis for the occurrence of ASF in Europe, from 2014 onwards. Research results were grouped into: (1) field studies (with varying objective and degrees of robustness depending on study design and sample size) and (2) studies based on modelling surveillance data (with different degrees of data granularity).
4.2.1.1. Evidence from field studies
With regard to risk factors in the domestic pig sector, wild boar vicinity has been implicated as a risk factor in four field studies, but the definitive route of viral introduction has not been identified in any instance (Nurmoja et al., 2018; Zani et al., 2019; Boklund et al., 2020; Lamberga et al., 2020). In Estonia, wild boar presence was detected within 15 km radius in 88% of the 26 outbreaks that occurred in commercial and backyard pig farms. The results suggested that indirect transmission due to insufficient biosecurity was the most likely source (Nurmoja et al., 2018). Similarly, results from outbreak investigations in two large commercial farms in Latvia could not identify the definitive cause of viral introduction, although both farms were located in areas with wild boar presence, with one wild boar case being detected only 3 km away from the farm (Lamberga et al., 2020). Wild boar cases were detected shortly after the first outbreak in a Bulgarian backyard farm, and contaminated material was the most likely source of viral introduction in the holding. Nonetheless, the specific routes of introduction remained speculative (Zani et al., 2019). In Latvia, it was suggested that feeding backyard pigs with contaminated fodder was directly related to the outbreaks in those units, although this has not been proven and swill feeding as a source could not be excluded (Olsevskis et al., 2016).
A field study carried out in Romania succeeded in identifying additional management variables related to ASF incursion, with wild boar presence in the vicinity being one of the risk factors for backyard farms (Boklund et al., 2020). A matched case–control study involving 655 domestic farms studied potential risk factors in backyard and commercial holdings (Boklund et al., 2020). For commercial farms, the only risk factor identified as relevant was the proximity to an ASF outbreak in a nearby domestic pig farm; but the lack of identification of additional risk factors could result from a low number of commercial farms enrolled in the study. Information on biosecurity levels was not available for every holding, and therefore, inferences on the impact of this variable were also limited. For backyard farms, a larger herd size, a higher number of outbreaks in domestic holdings in the vicinity of the farm (2 km radius) and abundance of wild boar in the surroundings were identified as risk factors for ASF. The risk of ASF was also higher in backyard farms surrounded by crops that were attractive to wild boar, and if the forage used on farm to feed the pigs was grown in ASF affected areas (Boklund et al., 2020). Additionally, a greater number of visits from professionals during the high‐risk period increased the likelihood of an ASF outbreak in a backyard farm. The same risk factor (i.e. entrance of visitors on the farm) had been previously identified as the most likely cause for ASF secondary outbreaks in a study conducted in 32 Latvian holdings (Olsevskis et al., 2016; Bellini et al., 2021). These data confirm that thorough biosecurity in pig holdings remains essential.
Recently, field studies have also focused on the specific role of infected carcasses on ASF spread and persistence in the environment. A study in Lithuania investigated wild boar behaviour towards domestic pig carcasses intentionally disposed in the forest (Masiulis et al., 2019). The authors observed that contacts occurred only rarely and that wild boars were more interested in the soil underneath and in the vicinity of the carcass than on the carcass itself (Masiulis et al., 2019). To investigate viral persistence of ASFV in soil and buried wild boar carcasses another study estimated viral presence in carcasses unburied at different times; with all carcasses buried for at least one summer period (Zani et al., 2019). Whilst ASFV genome could be detected in seventeen out of twenty burial sites, ASFV could not be isolated in any of the instances, including in the soil. These results suggested that buried carcasses were unlikely to be involved in long‐term survival of ASFV in the environment (Zani et al., 2019). These data appear to be in agreement with an experimental study revealing that no ASFV infectiousness could be detected in carcasses stored at room temperature after one week (Fischer et al., 2020). ASFV isolates were, however, detected in muscle, spleen and bones for several months in carcasses that were stored at lower temperatures (4°C and –20°C) (Fischer et al., 2020). A separate study tested the hypothesis that other scavenger species (such as birds, raccoons, marten and fox) could be involved in ASF spread; it was concluded that the role of these species was likely to be minor or non‐existent and that they could even contribute to reducing viral persistence by metabolising infected carcasses (Probst et al., 2020).
4.2.1.2. Evidence from modelling surveillance data
Domestic pigs
Other studies have looked at the probability of ASF occurrence in the domestic pig sector at the geographical unit level (rather than farm level), and its relationship with geographical, human‐related factors, as well as general pig management factors. A study in Sardinia, Italy, an ASF endemic territory, indicated that the likelihood of ASF presence in a ‘commune’ (i.e. the smallest administrative unit) was shown to increase with a larger number of backyard farms, higher road density, higher mean altitude, a larger number of outdoor fattening farms and a larger number of pigs per administrative level area (Martínez‐López et al., 2015). The same study showed that, in contrast, the presence of farms with at least one annual census was a protective factor, suggesting that unsupervised farms without veterinarian authority visits were a risk for ASF occurrence. Indeed, the illegal trade of pigs and pig products was demonstrated to be a likely factor in the maintenance of ASF on the island (Mur et al., 2016) and the involvement of wild boar in disease spread is probably limited (Bellini et al., 2016; Loi et al., 2020), with the local, free‐ranging, illegal pig population representing the most likely true reservoir (Loi et al., 2020). Socio‐economic factors were also demonstrated to play an important role on ASF occurrence in Sardinia (Loi et al., 2019), with the risk of an ASF outbreak increasing four‐fold in economically and materially deprived areas of the island (Cappai et al., 2018). It is still unclear, however, how much of these results can be extrapolated to other areas of Europe given the special characteristics of pig production in Sardinia and the important role of free‐range pigs in maintaining the disease in the area.
Wild boar populations
An analysis of ASF test results of wild boar found dead or hunted allowed to explore individual (age), population‐level (density) and ecological (season) factors potentially related to the probability of detection of an ASF case in a wild host. Wild boar surveillance data from Estonia and Latvia indicated that the probability of detecting a wild boar testing positive for ASF (ASFV genome or serology) was significantly higher in younger animals (Nurmoja et al., 2018, Schulz et al., 2019). This contrasted, however, with results from an experimental study, in which no age‐dependent susceptibility could be observed (Pietschmann et al., 2015). At the population level, a higher wild boar density was correlated with a higher probability of a case detection (Nurmoja et al., 2018; EFSA, 2019).
Environmental parameters can also influence the probability of detecting ASF cases in an area, due to their influence on the easiness of finding the carcasses. The probability of finding a positive wild boar was greater in winter (at least 4.5 times higher) rather than in summer, possibly due to forest coverage that hampered carcass identification in Poland (Frant et al., 2021). A field study in the Czech Republic concluded that carcasses were more likely to be found in younger forests (less than 40 years of age); on meadows, infected wild boar carcasses were more often found in a higher herb layer compared to non‐infected individuals. Infected carcases were more frequently found in locations distant from roads and forest edges (Cukor et al., 2020). Such information can help refining passive surveillance efforts.
4.2.2. Risk factors for the occurrence of ASF in the different counties of Romania analysed with BYM model
Domestic pigs
Figure 53 displays the probability to obtain a PCR‐positive test result in samples taken from wild boar shot or found dead in each of the different countries of Romania, from 2017 to 2019 as generated by the BYM model.
Figure 53.
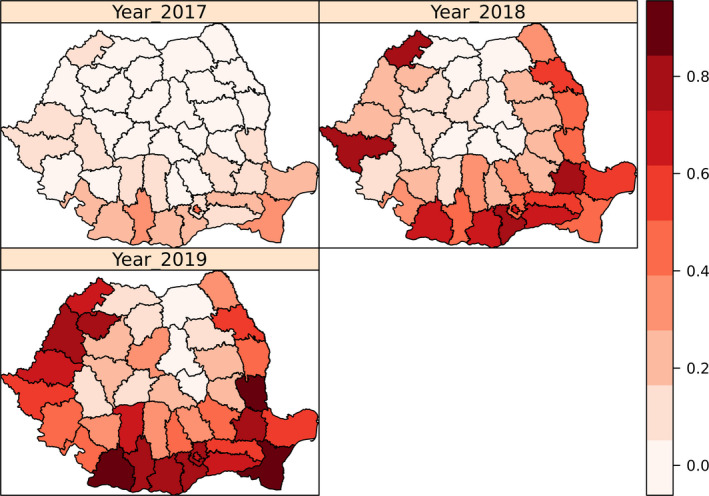
Average probability to get a PCR‐positive test result in samples from domestic pig in the different countries of Romania, from 2017 to 2019
Only the Human Footprint Index was kept as a significant covariate after stepwise elimination of non‐significant parameters on NUTS 3 level (Table 6). In addition, as can be observed in Figure 53 as well, it was clearly more likely to observe a positive result in 2018 and 2019 compared to 2017. The human footprint index is a measure for the cumulative human pressure on the environment and it is measured using eight variables including built‐up environments, population density, electric power infrastructure, crop lands, pasture lands, roads, railways, and navigable waterways. As the human‐mediated transmission, especially in backyard farms, has been reported to play an important role in the spread of the disease in the domestic pig sector (EFSA, 2020). To obtain more meaningful results, it would be necessary to collect systematically more detailed information on the potential covariates, such as detailed georeferenced pig population data. In addition, pig surveillance data should be either georeferenced, or linked to pig farms identification, so they could be georeferenced indirectly.
Table 6.
Outcomes of Bayesian Hierarchical model after stepwise elimination of non‐significant variables
| Odds ratio | 0.025 quantile | 0.975 quantile | |
|---|---|---|---|
| Effect year 2018 | 89.12 | 2.60 | 6.92 |
| Effect year 2019 | 1236.45 | 4.93 | 9.86 |
| Human footprint index | 4.3−11 | –38.16 | –11.36 |
Wild boar
Stepwise elimination of non‐significant parameters on NUTS 3 level did not retain any of the analysed potential risk factors as significant covariate for the occurrence of ASF in the wild boar populations, when using the BYM model. As for the domestic pigs, the analysis on a spatial level as large as NUTs 3 was probably not detailed enough to come up with any meaningful results.
4.2.3. Risk factors for the occurrence of ASF in wild boar the different hunting grounds of Romania, analysis with Generalised Linear model
Figure 54 shows the proportion of ASF PCR positive reported per hunting ground (HG) region in 2019. It should be highlighted that for the majority of HG region the proportion of PCR‐positive findings is zero and only around the borders with other countries are the proportions larger than 0.4, while in the centre of the country the proportions reported are below 0.4, and mostly zero.
Figure 54.
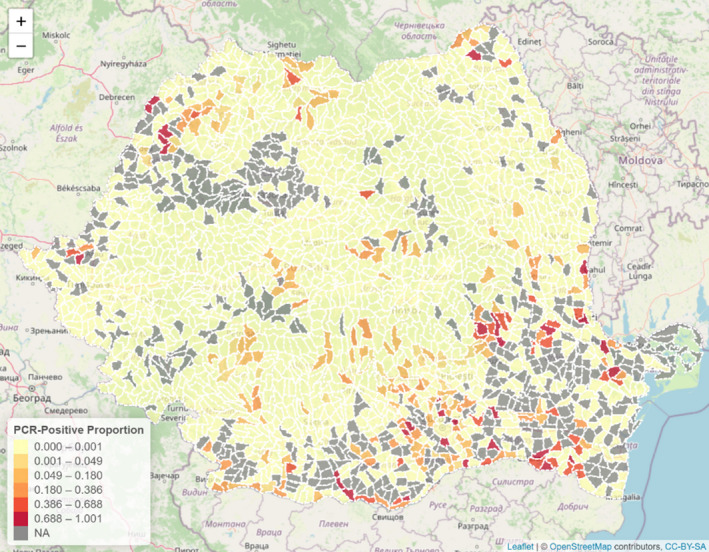
Proportion of ASF PCR positive reported per hunting ground (HG) region in 2019 in Romania
A generalised linear model was used to estimate the probability to observe ASF PCR‐positive results in a hunting ground (Table 7) based on the set of based on a set of potential covariates. Covariates with a Variance Inflation Factor (VIF) > 5 were excluded from analysis due to collinearity (Section 2.4). The final model obtained when using backward selection procedure is presented below.
Table 7.
Results of Generalised Linear Model to estimate the probability to observe a PCR‐positive results in Romanian hunting grounds based on analysis a set of potential covariates
| Risk factor | Abbreviation | Odds ratio | 0.025 quantile | 0.975 quantile | p Wald test |
|---|---|---|---|---|---|
| Wild boar density | WBDNS | 8.10 | 1.37 | 47.93 | 0.021 |
| Density of pigs from backyard farms/km2 | BYPigDNS | 61,613.37 | 0.86 | 4,401,748,956.56 | 0.053 |
| Length of vegetation growing period | Growth | 0.0025 | 0 | 0.05 | < 0.001 |
| Percentage of the area that is covered by trees and shrubs | TreeShrub | 11.07 | 1.79 | 68.47 | 0.01 |
| Percentage of the surface occupied by urbanised areas | Urban | 313.88 | 8.25 | 11,942.64 | 0.00 |
After discarding many variables due to multicollinearity issues, the generalised linear model found that the probability to find at least one PCR‐confirmed ASF case in wild boar in a hunting ground was mainly influenced by environmental factors, wild boar abundance (WBDNS), and the density of backyard pigs in the hunting ground (BYPigsDNS). Environmental variables influencing ASF presence/absence in wild boar included those driving wild boar distribution or carcass detectability (negative vegetation growth, tree/shrub cover) and the human footprint index of the area (urban cover).
Variables included in the model correlate with other relevant risk factors. Wild boar abundance, estimated based on the hunting bag per km2 (WBDNS), correlates with the number of feeders. The number of backyard pigs correlates with the number of backyard farms.
The probability to find at least one PCR‐confirmed ASF case in wild boar in a hunting ground is mainly driven by environmental factors, wild boar abundance, and backyard pigs. Hunting‐related variables such as the number of hunters, hunting days, and dogs, were not selected by the model. It was observed that wild boar abundance is correlated with the number of feeders per hunting ground, possibly suggesting that reducing wild boar feeding could be helpful in wild boar population control. The possible interference of wild boar feeding with ASF control, due to the higher underlying host density and longer breeding season associated with supplementary feeding, had previously been suggested by models (O'Neill et al. 2020). This field deserves more research once sufficiently detailed data on the possible covariates (environmental data, hunting modalities and related to pig production) of several years become available.
4.3. Evaluation of measures applied in ASF free areas adjacent to affected wild boar areas
The effectiveness of control measures applied in situations with ongoing epidemic spread of ASF in wild boar has repeatedly been investigated by EFSA, using spatially explicit modelling. The particular interest was on the efficacy of measures foreseen to protect the region adjacent to (EFSA, 2015, 2017) or surrounding (EFSA, 2018) the area affected by circulating ASF infection. With these models, the effectiveness was reported also for the intensity of depopulation measures (removal of animals through culling or trapping) and removal of carcasses in areas adjacent to the affected areas (the ‘white zones’). Moreover, adequate spatial dimensions of white zones in different epidemiological settings have been made available (Lange, 2015; Thulke and Lange, 2017; Lange et al., 2018). The following section provides the data available of historic white zones in different MS together with the measures applied and possible population reduction targets. Thereafter, the model results provide an evaluation of the particular white zones using multiple stochastic repetitions and sensitivity investigations regarding uncertain quantitative parameterisation of the WZ measures.
4.3.1. Description of White Zones
Table 8 summarises a description of the measures implemented in four white zones scenes, that will form the basis of the evaluation carried out by the stochastic model. The results will be provided in Section 4.3.2.
Table 8.
Description of white zones and the control measures implemented
| White zone | Fence | Depopulation measures | ||||
|---|---|---|---|---|---|---|
| France | Description fence | Description measures | Start date | End date | No. wild boar killed | No. wild boar killed/km2 |
|
Figure 55. White zone in France
|
|
Culling:
|
19/10/2018 | At least up to 31/8/2020 (end of reporting period) | 963 | 3.21 |
|
Carcass removal | 19/10/2018 | At least up to 31/8/2020 (end of reporting period) | NA | NA | |
| Luxembourg | Description fence | Description measures | Start date | End date | No. wild boar killed | No. wild boar killed/km2 |
|
Figure 56. White zone in Luxembourg
|
Figure 57. Fence bordering white zone in Luxembourg
|
Culling:
|
May 2019 | At least up to 31/8/2020 (end of reporting period) | 55 | 18.3 |
| Carcass removal | May 2019 | At least up to 31/8/2020 (end of reporting period) | 3 | 1 | ||
| Estonia | Fence | Depopulation measures | Start date | End date | No. wild boar killed | No. wild boar killed/km2 |
|
Figure 58. White zone in Estonia
|
None | Hunting
|
9/10/2014 | 31/08/2015 | 136 | 0.1 |
Carcass removal
|
9/10/2014 | 31/08/2015 | 0 | 0 | ||
| Latvia | Fence | Depopulation measures | Start date | End date | Total killed | Total/km2 |
|
Figure 59. Latvian white zone
|
None | Hunting
|
12.08.2016 | 23.10.2016 | 26 | 0.1 |
Carcass removal
|
12.08.2016 | 23.10.2016 | 10 | 0 | ||
| Czechia | Fence | Depopulation measures | Start date measure | End date measure | No. wild boar killed | No. wild boar killed/km2 |
|
Figure 60. White zone in Czechia
|
Figure 61. Fence bordering white zone in Czechia
|
Culling
|
11/9/2017 | 31.07.2018 | 757 | 4.7 |
Depopulation
|
16/10/2017 | 28/2/2018 | 157 | 1.0 | ||
Carcass removal
|
Sep. 2017 | 31.07.2018 | 420 | 2.6 | ||
|
None | Hunting
|
21.7.2017 | 31.7.2018 | 2,601 | 3.0 |
Carcass removal
|
31.7.2018 | 119 | 0.1 | |||
|
None | Hunting
|
21.7.2017 | 31.7.2018 | 17,992 | 3.1 |
Carcass removal
|
31.7.2018 | 526 | 0.1 | |||
NA: not applicable
4.3.2. Evaluation of efficacy of measures applied in white zones (model outcomes)
4.3.2.1. Simulation outcome per individual white zones
The field data provided by the MS in Table 8 facilitated the model‐based analysis of four different layouts for white zones (WZ). The first two WZ scenes are in the context of WZ implemented in front of an epidemic expansion on a large scale and the next two WZ scenes are in the context of control measures applied on a small scale, after a focal introduction of ASF.
The first WZ scene is derived from Estonia and is based on field data collected very early in the European ASF genotype II epidemic (2014). The second scene is derived from Latvia and takes place in a more advanced stage of the epidemic, when control measures are already well established (2016). Third WZ scene refers to the management after the focal introduction of ASF in the Zlin region of Czech Republic, which was the first control of ASF in the EU after the focal introduction in 2017. The fourth scene addresses the French ‘Zone Blanche’, which was also origin of the concept, being a preventatively managed zone in response to the focal introduction across the border in Belgium in 2018. The results are presented per WZ scene with the following details (a more comprehensive collection of simulation output can be consulted at Lange et al. (2021)):
Model implementation of the scene.
Summary box of data input and simulation results for the most similar configuration of population and measures.
Spatial heat map for different scenarios highlighting the effect of the WZ.
Possible insights from the model outcomes for the specific WZ scene, which can be useful also in other contexts when designing a WZ.
4.3.2.1.1. White zone scene of Estonia (WZ‐EE)
Figure 62 shows the details of the simulation landscape in WZ‐EE. The squared pixels represent wild boar group habitat patches of different quality, according to the wild boar distribution model of Pittiglio et al. (2018). The simulation of the spread of the ASF infection started from the west (infected area = grey cells) and ran through the population in eastward direction, equivalent with the direction of the movement of the infections according to historic notifications to the Animal Disease Notification System (ADNS) in that specific area in Estonia. The simulation also included identified human‐mediated translocation events (yellow dots) until the 9th of October 2014. On that date, the WZ was established (red shaded habitat cells) towards the still ASF‐free area to the east (blue shaded cells). After this date human‐mediated spread events were excluded from the simulations.
Figure 62.

- Grey squares: ASF‐infected area;
- Red squares: white zone;
- Blue squares: ASF‐free area in front of white zone;
- Black squares: represent virtually blocked cells preventing ASF infection from bypassing the WZ to the north or south;
- Yellow dots: human‐mediated spread events before establishment of the WZ.
Box 1. Summary of model simulation of the white zone of Estonia.
1.

Figure 63 provides an overview of the impact of the WZ‐EE on the spread of the infection. The more reddish a particular wild boar habitat cell is coloured, the greater the proportion of simulations runs in which the cell contained ASF‐positive animals. Since there were no measures implemented, the expected outcome revealed no effect of the WZ (Figure 63). Note: The historic WZ‐EE was not designed for wild boar population management at that time in context of ASF.
Figure 63.
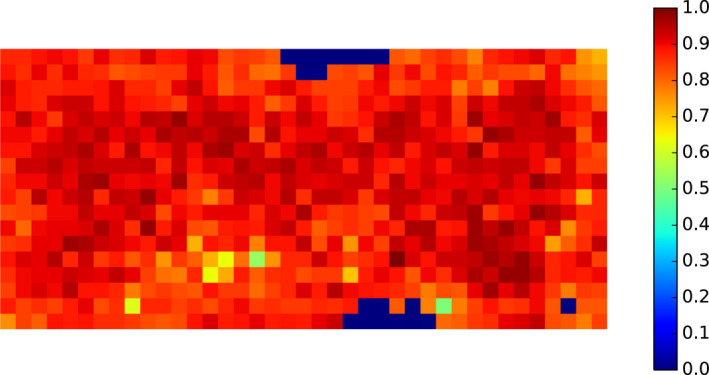
Heat map of local ASF occurrence inside and adjacent to the white zone in Estonia
The simulation seeds infection according to the notifications reported in the ADNS falling into the simulation landscape. Subsequently, ASF spread is simulated stochastically until the historic date of establishment of the WZ‐EE. After establishment of the WZ, measures are simulated in the WZ while the spread simulation is continued. Figure 64 shows the period before the simulated ASF approached the WZ‐EE (t(established); Figure 64). The greater the simulated wild boar density was in the modelled landscape, the faster the infection approached the WZ, which implies that greater population density associates with faster spread. Hence, the edge demarcating the start of the WZ should be established sufficiently far away from the outermost case detection in the ASF‐positive area and this distance should be further the higher wild boar densities are in the area.
Figure 64.
Time interval from establishment of the white zone in Estonia until the entry of ASF into the WZ. Data are shown for three different median starting densities 1.2., 1.5 and 2.3 wild boar/km2 (density scale 1.5, 2.0 and 3.0; x‐axis). Top row values represent percentage of runs in which the white zone did fail to halt the spread of ASF
4.3.2.1.2. White zone scene of Latvia (WZ‐LV)
Figure 65A shows the details of the simulation landscape for the WZ‐LV scene. The squared grid cells represent wild boar group habitat patches of increasing quality by lighter shades of grey, according to the wild boar distribution model of Pittiglio et al. (2018). The simulation of the spread of the ASF infection started from the east and ran westwards, equivalent with the direction of the historic notifications to the ADNS in that specific area in Latvia. The simulations included identified human‐mediated translocation events until the 12th of August 2016 (blue dots). On that date, the WZ was established (red area) in front of still ASF‐free area to the west and after that date human‐mediated spread events were excluded from the simulations. Further simulation of the ASF spread considered the changes to the wild boar population emerging from the applied management measures (here hunting aimed to reduce the density to 0.5 per km2 within two hunting seasons). The snapshot in Figure 65B reveals the distribution of ASF resulting from simulation at the moment of establishment of the WZ (reddish coloured cells indicate wild boar family groups that contain infectious animals). The particular location of infected wild boar is from one arbitrary simulation run and varied between repetitions due to the stochastic nature of the model.
Figure 65.
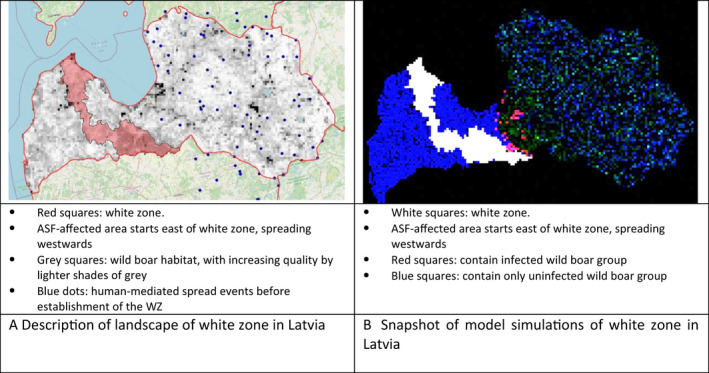
A–B. Simulation area representing the white zone in Latvia (A) and snapshot of the simulation at the moment of establishment of the white zone in Latvia (B)
Box 2. Summary of model simulation in white zone of Latvia.
1.

Figure 66 reveals the impact of the WZ‐LV on the spread of ASF in wild boar using the heat map resulting from 100 runs per three scenarios. Figure 66A on the top left displays the most optimistic scenario with a low starting wild boar density (~ 1 wild boar/km2) and a wild boar population reduction achieved within one season down to 0.5 wild boar/km2. Figure 66B on the top right displays the most pessimistic scenario with high wild boar starting density (~ 2 wild boar/km2) and only within two seasons down to only 0.75 wild boar. The larger map shows the outcome of the scenario adjusted to the provided field observations (standard scenario), i.e. with a wild boar starting density at 1.5 wild boar/km2 and a wild boar population reduction achieved within two seasons down to 0.5/km2 (Figure 66C).
Figure 66.
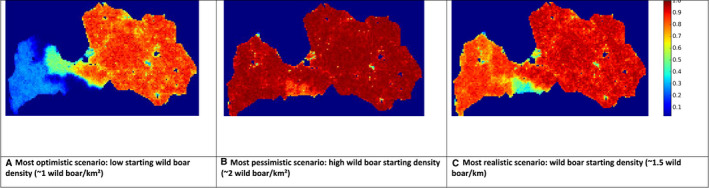
A–C. Heat map of ASF occurrence inside and adjacent to the white zone in Latvia
In particular, the optimistic scenario (Figure 66A) reveals how the pre‐emptive measures inside the WZ reduce the capacity of the infection to maintain continuous spread. However, the more wild boar inhabit the WZ, the lower is the impact of the pre‐emptive measures (Figure 66B + C). This insufficient effect relates to the narrow width in the middle of this specific WZ, which is the location through which most of the runs failed (encircled in Figure 66C).
Interestingly, the basic effect of the WZ is clearly visible in the southern part of the standard scenario (yellow to blue values, Figure 66C), while the ‘bridge’ due to thin layout in the middle section of the WZ facilitates final ASF spread into the left part of the simulation area in most runs.
To understand the role of the heterogeneous width of the WZ‐LV, the time between its establishment and the entry of ASF is of importance.
Figure 67 reports the effective time interval available to complete the pre‐emptive implementation of the planned measures. For most simulation runs, however, there were less than two years available (median 1 year, 0–2 year 90% central interval) before the entry of ASF in the WZ. Hence, the aimed reduction of wild boar density in the WZ was not reached at the moment of ASF entry. Therefore, the narrow part of the WZ layout led to failure in most runs (Figure 66).
Figure 67.

Time interval from establishment of the white zone in Latvia till entry of ASF into the white zone area. Data are shown for alternative management scenarios (x‐axis). Top row values represent percentage of runs in which the managed white zone did fail to halt the spread of ASF
The simulations addressed the population measures applied pre‐emptive to the WZ‐LV. However, when the infection would enter the WZ in the reality, also reactive control measures likewise carcass removal would apply. Therefore, in particular for the WZ‐LV, additional simulations were performed including carcass removal but only from the WZ‐LV (i.e. outside the WZ time periods and density conditions were unchanged compared to Figure 66C).
Figure 68 confirms the carcass removal as efficient measure to support the WZ in halting the spread of the infection when compared with Figure 66C. However, it also clear that high carcass detection efforts (here 20%; Figure 61A) are required to improve the situation. The more routine level of carcass detection (here 2%; Figure 61B; EFSA, 2021) cannot compensate the issues discussed as limiting the efficiency of the WZ‐LV.
Figure 68.
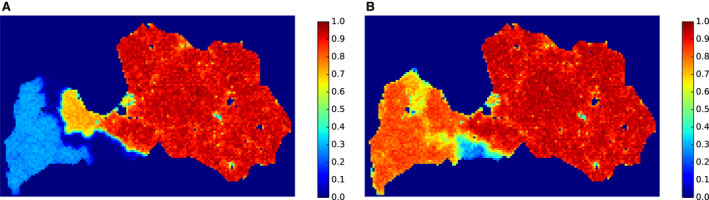
A–B. Heat map of ASF occurrence inside and adjacent to the white zone in Latvia. As Figure 66C (failure rate 94%) but simulation additionally includes carcass removal inside the WZ assuming a detection efficiency of 20% (A; failure rate 34%) and 2% (B; failure rate 90%)
4.3.2.1.3. White zone scene of Czechia (WZ‐CZ)
Figure 69 shows the details of the simulation landscape for the WZ‐CZ. The squared grid cells (Figure 69A) represent wild boar group habitat patches. Their quality increases with lighter shading, and the structure follows the wild boar distribution model of Pittiglio et al. (2018).
Figure 69.
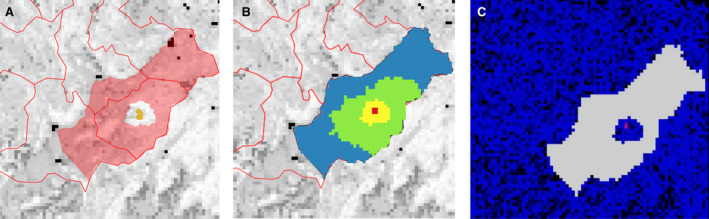
Simulation area representing the layout of the white zone in Czechia (A), the historic organisation of areas (B) and a snapshot of the simulation at the moment of establishment of the white zone in Czechia (C)
The simulated spread starts inside the fenced part (red, Figure 69B) of the core area (red + yellow areas, Figure 69B). ASF infection can stochastically cross the fence. Additionally, ASF is seeded (intentionally inserted in the model) outside the fence early in 2018 towards the south. The WZ‐CZ combines the larger areas around the core area (green+ blue areas, Figure 69B) and is treated according to Table 9 shortly after ASF detection. The core area (red + yellow areas, Figure 69B) was concentrically culled from 2.5 months after initial detection.
Table 9.
Contribution of ASF‐positive area and WZ‐CZ to the overall success derived of 600 simulation runs per density scenario
| Wild boar density factor | Numbers of runs entering WZ | % of runs entering WZ | Numbers of runs that left WZ | Number of runs that got stopped in WZ | % of runs that got stopped in WZ |
|---|---|---|---|---|---|
| 2 | 214 | 36% | 164 | 50 | 23% |
| 4 | 459 | 77% | 451 | 8 | 2% |
| 6 | 549 | 92% | 549 | 0 | 0% |
| Total | 1,222 | 68% | 1,164 | 58 | 5% |
Note: In strict sense the non‐fenced part of the core area (yellow, Figure 69B) could have been considered as white zone as well as being adjacent to the fenced ASF‐positive area and pre‐emptively treated by depopulation culling. However, the planning of this study did address the WZ‐CZ in the historic way where pre‐emptively intensified hunting was addressing the WZ‐CZ (green and blue part, Figure 69B).
Box 3. Summary of model simulation in white zone of Czechia.
1.

Figure 70 demonstrates what would have happened, if ASF would have escaped the fenced part (red, Figure 61b) of the core area (red + yellow; in Figure 69b). The more reddish a particular wild boar habitat cell is coloured, the greater the proportion of simulations runs in which the cell contained ASF‐positive animals. From the uniform, orange coloured heat map (wild boar group infected in more than 70 of simulation runs), it is clear that the measures in the WZ‐CZ would not have stopped the infection from spread in and further out the WZ.
Figure 70.
- Note to these figure: The disease incursion in Czechia's white zone is simulated to evaluate the measures applied in the zone. In the reality, there was no notified introduction of ASF in the white zone in Czechia Colour scale: proportion of simulations runs in which the square cell became infected, ranging from red (proportion of the simulation runs for which the cell became infected = 1) to dark blue (proportion of the simulation runs for which the cell became infected = 0).
Interestingly, the area around the fenced centre part of the core area (yellow in Figure 69B) – in which wild boar was culled after 2.5 months over a short period of 3 months – became involved in a much lower proportion of runs (less than 50%; green light blue scale) than the demarcated WZ‐CZ (more than 70%, orange). Although the infection was intentionally released to the south of the fence in every simulation, the ASF entry into the WZ‐CZ usually happened via the north of the core area. The simulated escapes to the south (as in the field situation) did rarely arrive at the WZ. The observed directionality of the escapes were likely due to insufficient width of the northern segment of the non‐fenced part of the core area.
Table 9 details the contribution of the different parts of the simulated management region in Czechia. Dependent on the initial density, in 36%, 77 and 92% of the simulation runs ASF actually reached the WZ (Table 9 third column). Hence, in 64%, 23 and 8% of the runs the infection was stopped already inside the ASF‐positive area. However, only 23%, 2 and 0% of those runs, which entered the WZ, did stop inside the WZ (Table 9 last column).
In the model simulations the success of the WZ‐CZ was due to the eradication of the infection in the non‐fenced and fenced core area (‘highest‐ and high‐risk part’). From the analysis of the WZ‐CZ scene it appears that it would have been useful instead to widen the non‐fenced part of the core area (yellow; in Figure 69B), in particular to the north, while subjecting it to the pre‐emptive population culling applied in reality.
In 2017 the silent culling approach was implemented in both the fenced and non‐fenced part of the core area (red and yellow area in Figure 61B) in Czechia 2.5 months after detection. The culling approach followed important preconditions to prevent spread of the wild boar and the disease in response to disturbance:
Preconditions for early depopulation in infected area:
Started only after the area where positive carcasses were found was fenced, after intensive carcass searching.
Only after the fence was established, night shooting with silencers, together with wild boar traps was implemented, but never driven hunts with dogs (‘silent culling’). Silent culling started after the epidemic peak was passed inside the fence and followed a centripetal direction towards the fenced area.
Crops were left inside the risk area to provide favourable conditions for the wild boar.
Additionally, if similar narrow dimensions as the zones that were historically implemented in the field in the Zlin region in Czech Republic would have been used in other areas with denser wild boar populations, the overall success of the approach would be unlikely.
4.3.2.1.4. White zone scene of France (WZ‐FR)
Figure 71A reflects the spatial explicit design of the simulation landscape of the WZ‐FR. In particular, the intense hashed red area (~ 300 km2) is fenced around (‘white zone’ according to the definition used in France), and the entire red hashed area (1,024 km2) is subjected to depopulations. The pink ellipse at the French–Luxembourgian border (Figure 63A) symbolises artificially placed model barriers that mimic the effect of a four‐lane highway and urbanised area (the Longwy area). Without that manipulation, the WZ would have produced mostly failure results.
Figure 71.
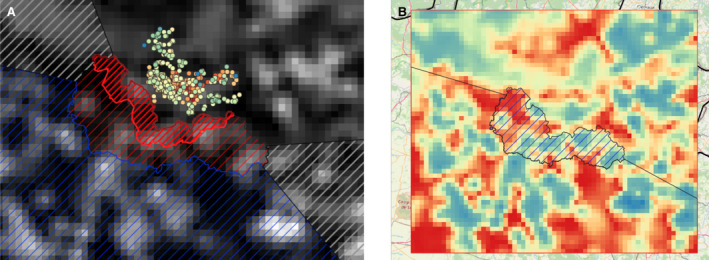
Simulation area representing the white zone in France (red hashed) and the cumulated ASF notification in BE wild boar (A) and the structure of the underlying wild boar habitat patches (B)
Box 4. Summary of model simulation in white zone of France.
1.
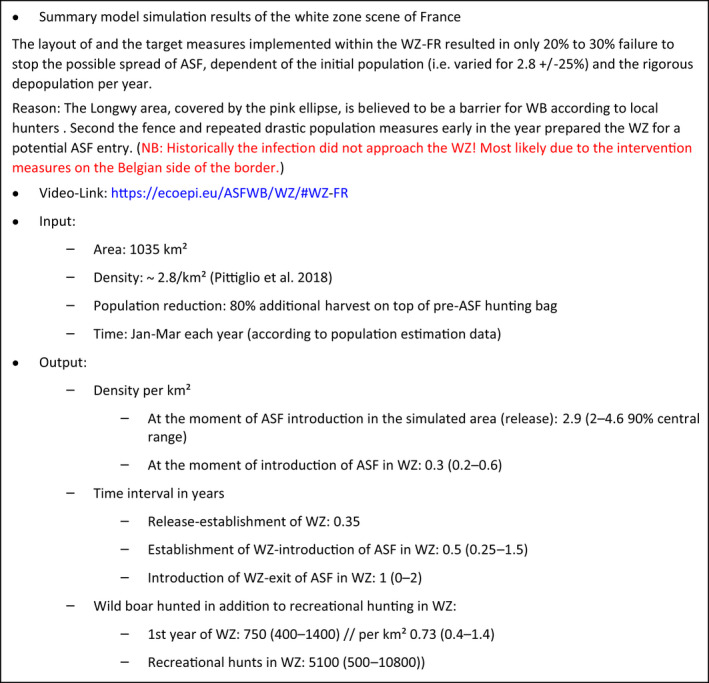
Figure 72 revealed the drastic effect of the depopulation measures on the population numbers in the WZ‐FR based on field estimates (Figure 72A) and in comparison with model output (Figure 72B). The input data of population estimate in the fenced part of the WZ in FR were used to illustrate the observed dynamics (Figure 72A). The model simulations, however, were calibrated only at start with the overall population density in the region (~ 2.8 per km2; Pittiglio et al., 2018) and a simulated 80% reduction in intense hunting sessions from January to March each year. With these two basic inputs the model adequately reproduced the population size in the fenced part of the WZ, the steep decline after the first depopulation campaign, the smooth regrowth over the reproductive season and the next dip early in 2020 (Figure 72B).
Figure 72.
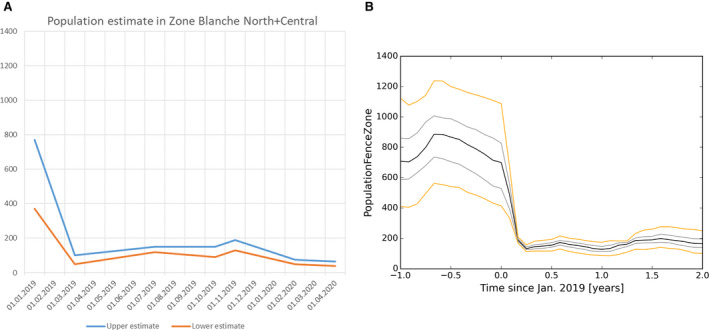
A–B. Development of the population size inside the fenced part of the WZ‐FR (intense hashed red in Figure 71) according to MS data (A) and model output (B)
Figure 73 reveals the impact of the WZ‐FR on the spread of ASF in wild boar using the heat map resulting from 100 runs per scenario. Top row shows the most plausible scenario with an initial wild boar density (~ 2.8/km2) and depopulation to target density of 0.25/km2 comparing the simulations with fence (Figure 73A) and without fence (Figure 73B). The bottom row shows the most pessimistic scenario simulated with starting density (~ 4/km2) and depopulation target only 0.75/km2 again comparing the simulations with fence (Figure 73C) and without fence (Figure 73D).
Figure 73.
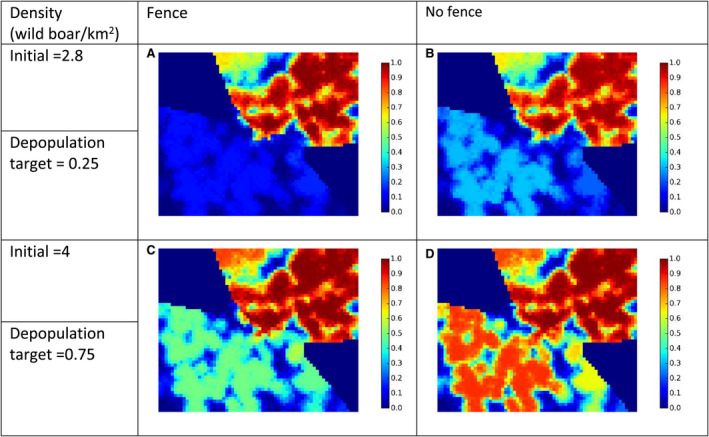
- Note to these figures: The disease incursion in the French white zone is simulated to evaluate the measures applied in the zone. In the reality, there was no introduction of ASF in France.
Both the fast and effective depopulation (top to bottom) and the fencing (left to right) did support the efficiency of the measures.
5. Conclusions
5.1. Descriptive epidemiology‐ TOR 1
5.1.1. Update the ASF situation in affected Member States and neighbouring countries
-
All phases of the ASF epidemic were represented in the MS affected by ASFV genotype II during the reporting period:
-
o
In Bulgaria, Hungary, Poland, Romania and Slovakia the epidemic has expanded further.
-
o
In Latvia and Lithuania, the epidemic seems to be stagnating.
-
o
In Estonia, the epidemic is fading out.
-
o
In Belgium and Greece, the infection has been successfully controlled.
-
o
Greece is the only MS, where only the domestic pig sector has been involved in the epidemic during this reporting period (1/9/2019–31‐8/2020), whereas Belgium, Estonia and Hungary had only wild boar populations affected. All other MS affected during this reporting period had outbreaks and cases in domestic pigs and wild boar, respectively.
In Serbia, the ASF outbreaks in domestic pigs have been contained successfully. In wild boar, the infection has expanded slowly in the south‐eastern region of the country.
In Russia, ASF was present in wild boar and domestic pigs from the outmost western to eastern part of the country. Control measures focused mainly on attempts to reduce wild boar population and to eliminate backyard farms.
The combination of control measures implemented in Belgium, including tools such as fencing, night shooting, trapping and carcass removal of wild boar, with intensities adapted to the epidemiological situation in the specific wild boar management areas, was shown to be effective to eradicate ASF after a focal introduction in the country.
The poor level of biosecurity in backyard farms has been identified as the predominant reason for introduction of ASFV in most of the affected pig holdings also during this reporting period, as reported by Bulgaria, Lithuania, Poland, Romania and Slovakia, based on their epidemiological investigations during the outbreaks. Although, quantitative evidence is not available.
During this reporting period, human‐mediated spread, demonstrated by the sudden detection of distant cases of ASF in wild boar populations that cannot be explained by natural spread, was suspected in Estonia after detecting a positive case in the north‐western part of the country after 18 months without any PCR‐positive case. The spread of ASF into the wild boar populations at the Western side of the Danube in Hungary and Serbia was also assumed to be human‐mediated.
5.1.2. Time‐profile of proportions of positive samples tested with Ab Elisa or PCR in wild boar hunted and found dead
Based on data submitted to EFSA's data collection framework from the beginning of 2016, up to the end of this reporting period, in the affected areas:
In some countries a persisting decreasing trend in proportions of PCR‐positive carcasses was observed indicating fade out of the virus (e.g. BE, CZ, EE) whereas in others it remains high indicating continuing spread.
There has been no general increase in the proportion of seropositive samples in wild boar.
5.1.3. Seasonality of African swine fever outbreaks and cases
There is a clear seasonality in the proportions of PCR‐positive samples from wild boar found dead, although the patterns are slightly different in the different MS. Overall, there is a decline in summer and an increase in winter in the proportion of PCR‐positive samples from wild boar found dead.
There is a clear peak observed in the proportions of PCR‐positive samples from domestic pigs between May and September in Lithuania, Poland and Romania.
The reason for the ASF seasonality and the different patterns observed in domestic pigs and wild boar requires further investigation.
5.1.4. Evolution yearly wild boar hunted in affected countries
The annual number of wild boar that were hunted in the Baltic States has declined rapidly since the introduction of ASF, ranging from 30,000 to 50,000 wild boar in 2014 to between 5,000 and 15,000 wild boar in 2019.
In the other affected MS, no change in the generally increasing trend in wild boar abundance has been observed.
5.1.5. Evolution of the extent of spread of the epidemic in wild boar, based on a secondary case network
In the year before the last notification of ASF compared to the first year after introduction in each MS, the average number of notifications that could be classified as secondary to a single source case declined in most MS, indicating a reduced extent of spread.
In Bulgaria, Poland and Slovakia, however, the average number of notifications that could be classified as secondary to a single source case clearly increased in the year before the last notification, indicating an increased extent of spread.
5.2. Risk factor analysis –TOR 2
5.2.1. Update from narrative literature review
5.2.1.1. Field evidence
Field evidence regarding the exact introduction routes of ASF in domestic pig holdings is still scarce.
Four studies identified wild boar observed in the vicinity of the domestic pig farms as a risk factor but the definitive route of ASFV introduction into the farms was not identified in any of them.
Wild boar density has been identified as a risk factor for the occurrence of ASF in backyard farms in a study carried out in Romania.
The proximity of growing crops attractive to wild boar near the backyard farms or the provision of fresh forage to pigs has been identified as a risk factor for the occurrence of ASF in backyard farms in Romania.
The vicinity of domestic pig outbreaks less than 2 km has identified as a risk factor for the occurrence of ASF in backyard farms and commercial farms in Romania.
5.2.1.2. Evidence from modelling surveillance data
Several risk factors have been identified for the occurrence of ASF in domestic pigs in Sardinia, such as a higher density of backyard farms and pigs, a higher road density and density of outdoor farms per administrative level.
Increased wild boar density has been identified to be a risk factor for ASF case detection in wild boar in Estonia.
Several environmental parameters have shown to have an impact on the probability of detecting positive wild boar cases, such as the percentage of young forest cover or meadows in Poland.
5.2.2. Risk factors for the occurrence of ASF in wild boar the different hunting grounds of Romania
The probability to find at least one PCR‐confirmed ASF case in wild boar in a hunting ground in Romania was mainly driven by environmental factors, wild boar abundance and the density of backyard pigs in the hunting ground area.
The number of hunting days and the use of dogs during hunting were not identified as risk factors for occurrence of ASF in wild boar.
We observed that wild boar abundance is correlated with the number of feeders per hunting ground, suggesting that reducing wild boar feeding could be helpful in wild boar population control, although causality cannot be inferred from the results.
This field deserves more research once sufficiently detailed data on the possible covariates (environmental data, hunting modalities and related to pig production) of several years become available.
5.3. Evaluation of measures applied in ASF free areas adjacent to affected wild boar areas using a stochastic model
The failure rate of white zones that solely used standard or intensified hunting as the measure to stop the spread of ASF was very high, from 94% to 100% depending on the initial wild boar density that was used in the model and the time the infection needed to reach the white zone.
The failure rate of white zones that implemented fencing AND drastic, concentrated depopulation measures as measures to stop the spread of ASF was low (from 20% to 30%) and depended on the initial wild boar density that was used in the model and time the infection needed to reach the white zone.
The success of the control measures in Czechia was most likely due to silent culling of the core area (fenced highest + high risk area) and not due to the measures applied in the low‐risk + intensive hunting area. In the model, in runs with ‘induced’ ASF infection spreading beyond the high‐risk part into Czech white zone, between 80% and 90% failure rates were observed.
Silent culling of wild boar (efforts to cull the maximum of a defined (or fenced) population with minimal disturbance, for instance, by trapping, sharp shooting or using silencers) can be initiated a soon as the risk area, established by intensive carcass searching, is reliably fenced.
The white zone would need to be very intensively hunted or even culled before ASF arrives to be effective and it should be of sufficient width. The trade‐off is that these measures require sufficient time and increased resources to be achievable.
To be successful and allow sufficient time (for instance 2 years) to achieve the necessary pre‐emptive culling targets of wild boar in the white zone, it should be sufficiently far from the outermost wild boar case, taking into account the natural speed of the spread of the disease, which varies with density.
As carcass removal is a measure to eliminate ASFV sources from an infected area, this is not a pre‐emptive measure. Nonetheless, carcass detection and testing will add to early detection and control of ASF after possible incursion in the white zone.
6. Recommendation
Tangible, absolute population reduction targets in terms of numbers wild boar per km2 in the white zone after a certain management period should be specified for the white zone implementation.
The distance at which the border of the white zone is placed to the non‐free area needs to consider the speed of the natural spread of the disease in wild boar. The speed of spread determines the time available to implement measures in the white zone. This speed did range at 2.9–11.7 km per year on average in Eastern EU MS but will be higher in densely populated areas.
The white zone should have a minimum width (i.e. several wild boar home ranges) to prevent ASF passing through by short infection chains as wild boar‐free white zones are unlikely to be achieved.
The white zone in a focal ASF introduction context needs a reliable fence protection towards the risk area or silent culling of the population. In the focal context the white zone will always be close to the risk area and it is therefore needed to perform the pre‐emptive measures in the white zone very quickly.
Before WB culling activities start after a focal ASF introduction, the infected area should be demarcated by intensive carcass search and fenced afterwards in order to prevent the dispersal of ASF.
Abbreviations
- ASF
African swine fever
- ASFV
African swine fever virus
- BYM
Besag York Mollié
- CSF
Classical Swine Fever
- DCF
Data Collection Framework
- ELISA
enzyme‐linked immunosorbent assay
- IB
immunoblotting
- IPT
immune‐peroxidase
- LIMS
Laboratory Information Management System
- S.N.I.I.A
National System of identification and registration of animals
- PCR
polymerase chain reaction
- TRACES
Trade Control and Expert System
Appendix A – Wild boar hunting data in Member States affected by African swine fever
1.
Suggested citation: European Food Safety Authority (EFSA) , Desmecht D, Gerbier G, Gortázar Schmidt C, Grigaliuniene V, Helyes G, Kantere M, Korytarova D, Linden A, Miteva A, Neghirla I, Olsevskis E, Ostojic S, Petit T, Staubach C, Thulke H‐H, Viltrop A, Richard W, Wozniakowski G, Abrahantes Cortiñas J, Broglia A, Dhollander S, Lima E, Papanikolaou A, Van der Stede Y and Ståhl K, 2021. Scientific Opinion on the epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA Journal 2021;19(5):6572, 101 pp. 10.2903/j.efsa.2021.6572
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00426
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank all European competent institutions, Member State bodies and the other organisations that provided data for this scientific output. In addition, EFSA would like to thank Alvarez Julio, Bicout Dominique, Boklund Anette, Drewe Julian, Garin‐Bastuji Bruno, Gonzales Rojas Jose, Guberti Vittorio, Loi Federica, Nielsen Søren and Pasquali Paolo for reviewing the report.
Approved: 26 March 2021
Notes
ASFV entered Germany on 10 September 2020, and was added in the above text after reception of this mandate.
References
- Barongo MB, Ståhl K, Bett B, Bishop RP, Fèvre EM and Aliro T, 2015. Estimating the basic reproductive number (R0) for African Swine Fever Virus (ASFV) transmission between pig herds in Uganda. PLoS ONE, 10. 10.1371/journal.pone.0125842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini S, Rutili D and Guberti V, 2016. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Veterinaria Scandinavica, 58. 10.1186/s130280160264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini S, Casadei G, De Lorenzi G and Tamba M, 2021. A review of risk factors of african swine fever incursion in pig farming within the European Union Scenario. Pathogens, 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund A, Dhollander S, Chesnoiu Vasile T, Abrahantes JC, Bøtner A, Gogin A, Gonzalez Villeta LC, Gortázar C, More SJ, Papanikolaou A, Roberts H, Stegeman A, Ståhl K, Thulke HH, Viltrop A, Van der Stede Y and Mortensen S, 2020. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Scientific Reports, 10, 10215. 10.1038/s41598-020-66381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai S, Rolesu A, Coccollone A, Laddomada A and Loi F, 2018. Evaluation of biological and socio‐economic factors related to persistence of African swine fever in Sardinia. Preventive Veterinary Medicine, 152, 1–11. 10.1016/j.prevetmed.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Cleveland WS and Devlin SJ, 1988. Locally weighted regression: an approach to regression analysis by local fitting. J. Amer. Statist. Assn., 83, 596–610. [Google Scholar]
- Cukor J, Linda R, Václavek P, Šatrán P, Mahlerová K, Vacek Z, Kunca T and Havránek F, 2020. Wild boar deathbed choice in relation to ASF: are there any differences between positive and negative carcasses? Prev Vet Med., 177, 104943. 10.1016/j.prevetmed.2020.104943. Epub 2020 Feb 27. PMID: 32172021. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Depner K, Gortazar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke H‐H, Viltrop A, Woźniakowski G, Cortiñas Abrahantes J, Gogin A, Verdonck F and Dhollander S, 2017. Scientific report on the epidemiological analyses of African swine fever in the Baltic States and Poland. EFSA Journal 2017;15(11):5068, 59 pp. 10.2903/j.efsa.2017.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Boklund A, Cay B, Depner K, Foldi Z, Guberti V, Masiulis M, Miteva A, More S, Olsevskis E, Satran P, Spiridon M, Stahl K, Thulke H‐H, Viltrop A, Wozniakowski G, Broglia A, Cortinas Abrahantes J, Dhollander S, Gogin A, Verdonck F, Amato L, Papanikolaou A and Gortazar C, 2018. Scientific report on the epidemiological analyse s of African swine fever in the European Union (November 2017 until November 2018). EFSA Journal 2018;16(11):5494, 106 pp. 10.2903/j.efsa.2018.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Alvarez J, Bicout D, Boklund A, Bøtner A, Depner K, More SJ, Roberts H, Stahl K, Thulke HH, Viltrop A, Antoniou SE, Cortifinas Abrahantes J, Dhollander S, Gogin A, Papanikolaou A, Vander Stede Y, Gonzalez Villeta LC and Gortazar Schmidt C, 2019. Research gap analysis on African swine fever. EFSA Journal 2019;17(8):5811. 10.2903/j.efsa.2019.5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anette B, Theodora CV, Klaus D, Daniel D, Vittorio G, Georgina H, Daniela K, Annick L, Aleksandra M, Simon M, Edvins O, Sasa O, Helen R, Mihaela S, Karl S, Hans‐Hermann, Grigaliuniene V, Arvo V, Richard W, Grzegorz W, Jose AC, Sofe D, Andrey G, Corina I, Alexandra P, Gonzalez VLC and Christian GS, 2020. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal 2020;18(1):5996, 107 pp. 10.2903/j.efsa.2020.5996 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), Berg C, Bøtner A, Browman H, De Koeijer A, Domingo M, Ducrot C, Edwards S, Fourichon C, Koenen F, More S, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke HH, Vågsholm I, Velarde A and Willeberg P, 2015. Scientific opinion on African swine fever. EFSA Journal 2015;13(7):4163, 92 pp. 10.2903/j.efsa.2015.4163 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), Nielsen SS, Alvarez J, Bicout D, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda MA, Roberts H, Pasquali P, Sihvonen L, Spoolder H, Stahl K, Winckler C, Cortinas Abrahantes J, Dhollander S, Ivanciu C, Papanikolaou A, Van der Stede Y, Blome S, Guberti V, Loi F, More SJ, Olsevskis E, Thulke H‐H and Viltrop A, 2021. Scientific Opinion on ASF Exit Strategy: providing cumulative evidence of absence of African swine fever virus circulation in wild boar populations using standard surveillance measures. EFSA Journal 2021;19(3):6419, 30 pp. 10.2903/j.efsa.2021.6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENETWILD consortium , Acevedo P, Croft S, Smith G, Antonio Blanco‐Aguiar J, Fernandez‐Lopez J, Scandura M, Apollonio M, Ferroglio E, Keuling O, Sange M, Zanet S, Brivio F, Podgorski T, Petrovic K, Soriguer R and Vicente J, 2019. ENETwild modelling of wild boar distribution and abundance: update of occurrence and hunting data models. EFSA supporting publication 2019;EN‐1674, 29 pp. 10.2903/sp.efsa.2019.EN-1674 [DOI] [Google Scholar]
- ENETWILD consortium , Acevedo P, Croft S, Smith G, Antonio Blanco‐Aguiar J, Fernandez‐Lopez J, Scandura M, Apollonio M, Ferroglio E, Keuling O, Sange M, Zanet S, Brivio F, Podgorski T, Petrovic K, Soriguer R and Vicente J, 2020. Update of occurrence and hunting yield‐based data models for wild boar at European scale: new approach to handle the bioregion effect. EFSA supporting publication 2020;17(5):EN‐1871, 28 pp. 10.2903/sp.efsa.2020.EN‐1871 [DOI] [Google Scholar]
- Fischer M, Hühr J, Blome S, Conraths FJ and Probst C, 2020. Stability of African Swine Fever Virus in Carcasses of Domestic Pigs and Wild Boar Experimentally Infected with the ASFV “Estonia 2014” Isolate. Viruses, 12, 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frant M, Wozniakowski G and Pejsak Z, 2017. African Swine Fever (ASF) and Ticks. No Risk of Tick‐mediated ASF Spread in Poland and Baltic States. Journal of Veterinary Research, 61. 10.1515/jvetres-2017-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frant M, Gal A, Bocian ?, Zi?tek‐Barszcz A, Niemczuk K and Wo?niakowski G, 2021. African Swine Fever Virus (ASFV) in Poland in 2019—Wild Boars: Searching Pattern. Agriculture. 11, 45. 10.3390/agriculture11010045 [DOI]
- Hall DK and Riggs GA, 2015. MODIS/Terra Snow Cover Monthly L3 Global 0.05Deg CMG, Version 6. [Indicate subset used]. Boulder, Colorado USA. NASA National Snow and Ice Data Center Distributed Active Archive Center. 10.5067/modis/mod10cm.006 [DOI]
- Iglesias I, Muñoz MJ, Montes F, Perez A, Gogin A, Kolbasov D and de la Torre A, 2016. Reproductive Ratio for the Local Spread of African Swine Fever in Wild Boars in the Russian Federation. Transbound. Emerg. Dis., 63, e237–e245. [DOI] [PubMed] [Google Scholar]
- Imdad MU, Aslam M and Altaf S, 2016. mctest: an R package for detection of collinearity among regressors. The R Journal, 8, 499–509. [Google Scholar]
- Imdad MU, Aslam M, Altaf S and Ahmed M, 2019. Some new diagnostics of multicollinearity in linear regression model. Sains Malaysiana, 48, 2051–2060. [Google Scholar]
- Korennoy FI, Gulenkin VM, Malone JB, Mores CN, Dudnikov SA and Stevenson MA, 2014. Spatio temporal modeling of the African swine fever epidemic in the Russian Federation, 2007–2012. Spat Spatiotemporal Epidemiol., 11, 135–41. 10.1016/j.sste.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberga K, Oļševskis E, Seržants M, Bērziņš A, Viltrop A and Depner K, 2020. African swine fever in two large commercial pig farms in LATVIA—estimation of the high risk period and virus spread within the farm. Veterinary Sciences, 7, 105. 10.3390/vetsci7030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, 2015. Alternative control strategies against ASF in wild boar populations. EFSA Supporting Publication 2015;12(7):EN‐1312, 29 pp. 10.2903/sp.efsa.2015.EN-843 [DOI] [Google Scholar]
- Lange M, Gubert V and Thulke H‐H, 2018. Understanding ASF spread and emergency control concepts in wild boar populations using individual‐based modelling and spatio‐temporal surveillance data. EFSA Supporting Publication 2018;15(11):EN‐1521, 46 pp. 10.2903/sp.efsa.2018.EN-1521 [DOI] [Google Scholar]
- Lange M, Reichold A and Thulke H‐H, 2021. Modelling wild boar management for controlling the spread of ASF in the areas called white zones (zones blanche). EFSA Supporting Publication 2021. [Google Scholar]
- Loi F, Laddomada A, Coccollone A, Marrocu E, Piseddu T and Masala G, 2019. Socio‐economic factors as indicators for various animal diseases in Sardinia. PLoS ONE, 14. 10.1371/journal.pone.0217367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi F, Cappai S, Laddomada A, Oggiano A, Franzoni G, Feliziani F, Rolesu S and Guberti V, 2020. Mathematical approach to estimating the main epidemiological parameters of African swine fever in wild boar. Vaccines (Basel), 8, 521. 10.3390/vaccines8030521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon A, Linden A, Satran P, Gervasi V, Licoppe A and Guberti V, 2019. R0 estimation for the african swine fever epidemics in wild boar of Czech Republic and Belgium. Veterinary Sciences, 7, 2. 10.3390/vetsci7010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐López B, Perez AM, Fr Feliziani, Rolesu S, Mur L and Sánchez‐Vizcaíno JM, 2015. Evaluation of the risk factors contributing to the African swine fever occurrence in Sardinia, Italy. Frontiers in Microbiology, 6, 314. 10.3389/fmicb.2015.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulis M, Bušauskas P, Jonušaitis V and Pridotkas G, 2019. Potential role of domestic pig carcasses disposed in the forest for the transmission of African swine fever. Berliner und Münchener tierärztliche Wochenschrift. Hannover: Schlütersche Verlagsgesellschaft mbH & Co. KG, 2019, Jg. 132.
- Mazur‐Panasiuk N, Walczak M, Juszkiewicz M and Woźniakowski G, 2020. The Spillover of African Swine Fever in Western Poland Revealed Its Estimated Origin on the Basis of O174L, K145R, MGF 505‐5R and IGR I73R/I329L Genomic Sequences. Viruses, 12, 1094. Published 2020 Sep 27. 10.3390/v12101094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur L, Iscaro X, Cocco M, Jurado C, Rolesu S, De Mia GM, Feliziani F, Pérez‐Sánchez R, Oleaga A and Sánchez‐Vizcaíno JM, 2016. Serological surveillance and direct field searching reaffirm the absence of ornithodoros erraticus ticks role in African swine fever cycle in Sardinia. Transbound. Emerg. Dis., 3–4. 10.1111/tbed.12485H [DOI] [PubMed] [Google Scholar]
- Nurmoja I, Mõtus K, Kristian M, Niine T, Schulz K, Depner K and Viltrop A, 2018. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Preventive Veterinary Medicine. 10.1016/j.prevetmed.2018.10.001 [DOI] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2019. African swine fever, Aetiology Epidemiology Diagnosis Prevention and Control References. ‐ Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. [Google Scholar]
- OIE (World Organisation for Animal Health), 2020. Self‐declaration of Belgium's African swine fever‐free status in all swine species. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Self-declarations/2020_12_Belgium_ASF_self-declaration_ENG.pdf
- Olsevskis E, Guberti V, Serzants M, Westergaard J, Gallardo C, Rodze I and Depner K, 2016. African swine fever virus introduction into the EU in 2014: experience of Latvia. Research in Veterinary Science, 105, 28–30. 10.1016/j.rvsc.2016.01.006 [DOI] [PubMed] [Google Scholar]
- O'Neill X, White A, Ruiz‐Fons F and Gortfiazar C, 2020. Modelling the transmission and persistence of African swinefever in wild boar in contrasting European scenarios. Scientific Reports, 10, 5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann J, Guinat C, Beer M, Pronin V, Tauscher K, Petrov Anja K and Blome S, 2015. Course and transmission characteristics of oral low‐dose infection of domestic pig and European wild boar with a Caucasian African swine fever virus isolate. Archives of Virology, 160. 10.1007/s00705-015-2430-2 [DOI] [PubMed] [Google Scholar]
- Pittiglio C, Khomenko S and Beltran‐Alcrudo D, 2018. Wild boar mapping using population‐density statistics: from polygons to high resolution raster maps. PLoS ONE. 10.1371/journal.pone.0193295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Gethmann J, Amendt J, Lutz L, Teifke JP and Conraths FJ, 2020. Estimating the postmortem interval of wild boar carcasses. Veterinary Science, 7, 6. 10.3390/vetsci7010006. PMID: 31948042; PMCID: PMC7157510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsuwan J, Somboonchokepisal T, Akaraputtiporn T, Srimuang T, Phuengsukdaeng P, Suwannarat A, Mutirangura A and Kitkumthorn N, 2018. A method for extracting DNA from hard tissues for use in forensic identification. Biomed Rep., 9, 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Staubach C, Blome S, Viltrop A, Nurmoja I, Conraths FJ and Sauter‐Louis C, 2019. Analysis of Estoniansurveillance in wild boar suggests a decline in the incidence of African swine fever. Scientific Reports, 9, 8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Staubach C, Blome S, Nurmoja I, Viltrop A, Conraths FJ, Kristian M and Sauter‐Louis C, 2020. How to demonstrate freedom from African swine fever in wild boar – Estonia as an example. Vaccines (Basel), 8, 336. 10.3390/vaccines8020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulke HH and Lange M, 2017. Simulation‐based investigation of ASF spread and control in wildlife without consideration of human non‐compliance to biosecurity. EFSA Supporting Publication 2017;12(7):EN‐843, 30 pp. 10.2903/sp.efsa.2017.EN-1312 [DOI] [Google Scholar]
- Varewyck M, Verbeke T and Cortinas Abrahantes J, 2017. Exploratory analysis for spatio‐temporal epidemiological data WEB app (spatial). Zenodo. 10.5281/zenodo.889551 [DOI] [Google Scholar]
- Venter O, Sanderson EW, Magrach A, Allan JR, Beher J, Jones KR, Possingham HP, Laurance WF, Wood P, Fekete BM, Levy MA and Watson JE, 2018. Last of the Wild Project, Version 3 (LWP‐3): 2009 Human Footprint, 2018 Release. NASA Socioeconomic Data and Applications Center (SEDAC), Palisades, NY. 10.7927/h46t0jq4. Accessed 13 February 2021. [DOI] [Google Scholar]
- Vergne T, Guinat C, Petkova P, Gogin A, Kolbasov D, Blome S, Molia S, Pinto Ferreira J, Wieland B, Nathues H and Pfeiffer DU, 2014. Attitudes and beliefs of pig farmers and wild boar hunters towards reporting of African Swine fever in Bulgaria, Germany and the Western part of the Russian Federation. Transboundary and Emerging Diseases, 63, e194–e204. 10.1111/tbed.12254 [DOI] [PubMed] [Google Scholar]
- Vergne T, Chen‐Fu C, Li S, Cappelle J, Edwards J, Martin V, Pfeiffer DU, Fusheng G and Roger FL, 2017. Pig empire under infectious threat: risk of African swine fever introduction into the People's Republic of China. Vet Rec., 181, 117. 10.1136/vr.103950 PMID: 28754737. [DOI] [PubMed] [Google Scholar]
- Zani L, Dietze K, Dimova Z, Hendrik Forth J, Denev D, Depner K and Alexandrov T, 2019. African Swine fever in a bulgarian backyard farm—a case report. Veterinary Sciences, 6, 94. 10.3390/vetsci6040094 [DOI] [PMC free article] [PubMed] [Google Scholar]