Abstract
Introduction
The identification of patients on antiretroviral therapy (ART) with virologic failure (VF) and the response in the public health sector remains a significant challenge. We previously reported improvement in routine viral load (VL) monitoring after ART commencement through a health system strengthening, nurse-led “VL champion” program as part of a multidisciplinary team in three public-sector clinics in Durban, South Africa. Here, we report on the impact of the “VL champion” model adapted to identify, support, and coordinate the management of individuals with VF on first-line ART in a setting with limited electronic based record capacity.
Methods
We evaluated the VL champion model using a controlled before-after study design. A paper based tool, “High VL Register”, was piloted under the supervision of the VL champion to improve data management, monitoring of counseling support, and enacting clinical decisions. We abstracted chart and electronic data (TIER.net) for eligible individuals with VF in the year before and after implementation of the program, and compared outcomes for individuals in these periods. Our primary outcome was the successful completion of the VF pathway, defined as a repeat VL<1,000 copies/mL or a change to second-line ART within 6 months of VF. In a secondary analysis, we assessed the completion of each step in the pathway.
Results
We identified 60 and 56 individuals in the pre-intervention and post-intervention periods respectively with VF who met inclusion criteria. Socio-demographic and clinical characteristics were similar between periods. Repeat VL testing was completed in 61.7 % and 57.8% individuals in these two groups respectively. We found no difference in the proportion achieving our primary outcome in the pre- and post-intervention periods, 11/60 (18%, 95% CI 9–28%) and 15/56 (27%, 95% CI 15–38%), respectively (P=0.28). In multivariable logistic regression models adjusted for potential confounding factors, individuals in the post-intervention period had a non-significant doubling of the odds of achieving the primary outcome (AOR 2.07, 95% CI 0.75–5.72). However, there was no difference in the rates of completion of each step along the first-line VF cascade of care.
Discussion
This enhanced intervention to improve VF in the public sector using a paper-based data management system failed to achieve significant improvements in first-line VF management over standard of care. In addition ,interventions to better address patient centered factors that contribute to VF, we believe that there are substantial limitations and staffing requirements to utilize a paper-based tool. This emphasizes the need to further expand and upgrade the electronic medical record system with capabilities for prompting staff regarding patients with missed visits and critical laboratory results demonstrating VF.
Introduction
The identification and management of virological failure (VF) among people living with HIV (PLWH) is a cornerstone of successful HIV care. Fortunately viral load (VL) monitoring of individuals on therapy, recommended by the World Health Organization,[1] has expanded vastly in the sub-Saharan African region.[2] Despite this expansion, there remain significant weakness in identification of patients experiencing VF failure and delays in appropriate switching to second-line ART.[3]
Prompt response to individuals failing therapy decreases the risk of mortality, opportunistic infections, drug resistance, and HIV transmission.[4–6] Furthermore, management of VF is complex, particularly for over-burdened public-sector clinics. Most guidelines recommend that individuals with VF undergo a complex series of clinical encounters. These include multiple visits for adherence support interventions, repeat VL testing and, visits to consider regimen changes to second-line antiretroviral therapy (ART). Notably, regimen changes to second-line ART often requires approval from a senior clinical staff member. Moreover, individuals with VF represent an inherently complex patient population and often experience additional barriers to care.[7] These complexities lead to low re-suppression rates and poor outcomes among persons with VF.[8–13]
Consequently, interventions to improve monitoring and management of individuals with VF are needed. We previously reported on the design of a health system strengthening nurse-led “Viral load (VL) champion” program as part of a multidisciplinary team in three public-sector clinics in Durban, South Africa. The program achieved significant improvement in VL testing rates after ART initiation [14] but did not assess the management of patients after VF. Beginning in 2017, we piloted a paper based tool, the “High VL Register”, as part VL champion model to strengthen the response to VF management. We now report results of a controlled before-after study design of the pilot program to assess whether it improved process and clinical outcomes for PLWH with VF.
Methods
Study setting
The VL champion pilot program was implemented at three public-sector HIV clinics in Durban, South Africa: Clairwood Hospital, Wentworth Hospital, and King Dinizulu Hospital. Prior to the intervention in 2017, monitoring of patients with VF was expected to be conducted in accordance with the South African Department of Health guidelines.[7] However, no specific clinic staff were delegated the identification or monitoring of patients with VF, which generally occurred on an ad hoc basis. A paper-based tool, the “High VL Register” (Appendix 1), was used in the VL champion model as part of a multidisciplinary team within a standard operating procedure sharing (SOP) to monitor patient visits and assist in management of the VF cascade of care.
Intervention description
In January 2017, we implemented a health systems strengthening program to manage VF at each of the three clinics. The program included the following elements:
Assignment of a nurse as the “VL champion” at each clinic to supervise the staff responsible for the monitoring all patients with a detectable VL;
Development of a standard operating procedure (SOP) for management of VF by clinic staff. Training on the SOP was provided to a) a lay counsellor or nurse assigned to adherence counselling, b) a nurse and/or doctor assigned to manage the VF clinic, c) an administrative clerk for records handling, and d) a data clerk to ensure same day data entry. In brief, VL results were reviewed daily by the VL champion and filed or entered in the patient charts. Patient charts with a high VL result were identified with a sticker and filed separately from the remaining clinic charts by the administrative clerk. Upon the return to the clinic, those marked with a high VL sticker were identified by the administrative clerk and referred directly to the counsellor, who would perform adherence counseling and then refer to the high VL physician or nurse. The lay counsellor also managed the completion of the high VL register (Supplemental Appendix) and was expected to call patients within a week who missed a clinic appointment at any point of the follow up period.
Study Population and Data Sources
All adult patients on first line ART (zidovudine or tenofovir, lamivudine and efavirenz) with VL > 1 000 copies/ml were screened for eligibility from the following data sources: TIER.net,[15] patient clinical charts or the weekly clinic electronic dashboard of the National Health Laboratory Services (NHLS). We excluded pregnant patients and those actively participating in other research studies in the clinc. Data was abstracted at three intervention clinics during December 2015 – December 2017. For each participant meeting criteria, sociodemographic data, laboratory data (CD4 count and HIV-1 RNA VL), dates of clinic visits and enhanced adherence counseling (EAC) events, and dates of regimen changes were obtained.
Study design and statistical methods
We evaluated the VL champion intervention using a controlled before-after observational design. Primary exposure of interest was the outcomes in the cascade in the two periods, the pre-intervention (December 2015 – December 2016) and post-intervention with the VL champion model (January 2017 – December 2017). Individuals were allocated by their first clinic visit after a VL > 1 000 copies/mL during 2016 as being in the pre-intervention period, and those with their first visit after a VL > 1 000 copies/mL during 2017 as being in the post-intervention period. Those with a VL > 1 000 copies/mL and no additional visits were included in the analysis and categorized by the year of their high VL.
The primary outcome was appropriate response completed to the repeat VL; specifically a VL load < 1 000 copies/mL or change to a protease inhibitor (PI) based regimen after a repeat VL > 1 000 copies/mL within six months of VF. Secondary outcomes included completion of at least one EAC session and completion of a repeat VL within six months.
Fisher’s exact and chi-squared statistical techniques were used to compare descriptive indices of individuals in the pre- and post-intervention periods. Crude rates of outcomes by intervention period were estimated and graphically depicted the “second-line cascade of care” to describe the proportion of people with first-line VF who successfully completed > 1 EAC, a repeat VL, and had appropriate response completed. Finally, we fit logistic regression models to identify correlates of our primary outcome with and without confounders, including age, sex, CD4 count, ART duration, ART regimen, and clinic of attendance. All data analyses were completed with Stata Version 15.1 (StataCorp, College Station, TX, USA).
Ethical considerations
The study protocol was approved by the KwaZulu-Natal Provincial Health Research Committee and the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BFC 377/16).
Results
A total of 116 individuals were identified as eligible for the assessment on first-line ART with a VL > 1 000 copies/mL. During the observation period 60 individuals (including 3 with no additional visit) were eligible in the pre-intervention period and 56 (including 4 with no additional visit) in the post-intervention period (Table 1). Participants in the two periods were similar in terms of sociodemographic and clinical factors. The primary outcome, confirmation of a repeat VL < 1 000 copies/mL or a change to second-line ART within 6 months, was similar in the pre- and post-intervention groups, respectively (11/60 [18%, 95%CI 9–28%] and 15/56 [27%, 95%CI 15–38%], P=0.28. In multivariable logistic regression models adjusted for potential confounding factors, individuals in the post-intervention period had a non-significant doubling of the odds of achieving the primary outcome (AOR 2.07, 0.75, 5.72, Table 2).
Table 1. Cohort characteristics.
Abbreviations: IQR – Interquartile range
| Characteristic | Pre-Intervention Period (n=60) | Post-Intervention-Period (n=56) | P-value |
|---|---|---|---|
| Age (median, IQR) | 36 (23–41) | 35 (30 – 39) | 0.18 |
| Female (n, %) | 35 (58%) | 25 (45%) | 0.14 |
| ART duration, years (median, IQR) | 1.3 (0.5–3.2) | 1.5 (0.5–3.3) | 0.74 |
| Viral load at failure, copies/mL (median, IQR) | 19,443 (4,751–82,910) | 16,963 (5,621–144,977) | 0.64 |
| Baseline CD4 count, cells/μl (median, IQR) | 130 (38–223) | 114 (28–193) | 0.41 |
Table 2.
Logistic regression model for correlates of a successful outcome after virologic failure, defined as a repeat viral load < 1 000 copies/mL or a change to second-line therapy.
| Univariable Models | Multivariable Model | |||
|---|---|---|---|---|
| Odd’s Ratio (95%CI) | P-value | Adjusted Odd’s Ratio (95%CI) | P-value | |
| Age (each 10 years) | 0.99 (0.91, 1.06) | 0.76 | 1.35 (0.78, 2.35) | 0.29 |
| Female sex | 1.81 (0.86, 3.81) | 0.12 | 3.59 (1.21, 10.6) | 0.02 |
| Baseline viral load (log 10 copies/mL) | 0.99 (0.62, 1.59) | 0.97 | 0.89 (0.48, 1.65) | 0.70 |
| Pre-post intervention | 1.63 (0.67, 3.94) | 0.28 | 2.07 (0.75, 5.72) | 0.16 |
We found similar trends across the first-line failure cascade of care (Fig 1). In the pre- and post-intervention group, second VL done was 37/60 (61.7%) and 29/56 (51.8%) respectively .Of these with a second VL result < 1 000 copies/ml, only 8.3% and 10.7% respectively were managed as per guidelines by continuation of first line ART. The proportion that were changed to second line ART with VL > 1 000 copies/ml were 10.1% and 16.1% respectively within 6 months of detection of VF
Figure 1. Second-line cascade of care before and after implementation of a nurse-led viral load monitoring and management program.
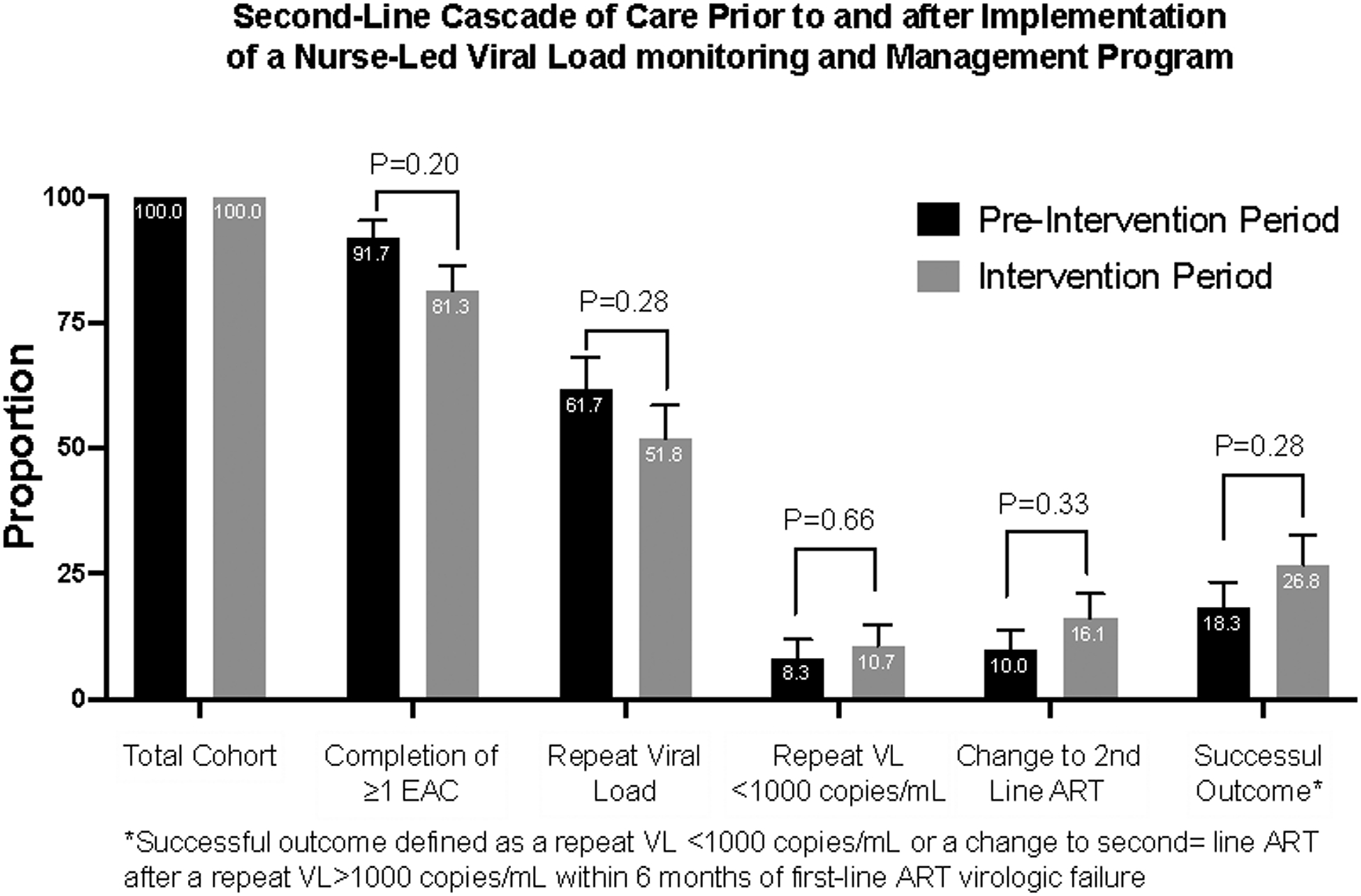
Successful outcome defined as a repeat viral load < 1 000 copies/mL or a change to second-line antiretroviral therapy after a repeat viral load > 1 000 copies/mL within six months of first-line antiretroviral therapy virologic failure. ART: antiretroviral therapy; EAC: enhanced adherence counselling; VL: viral load
Discussion
The VL champion model was developed to address barriers to improve routine VL testing and monitoring. We previously reported successful outcomes in improving VL completion rates utilizing the VL champion model after ART initiation from 62% to > 90% at one year and maintaining high VL suppression rates.[14] We now report the results of adapting the VL champion model to use a paper-based “High VL Register” to manage the small but significant numbers of individuals with VF. There was no significant difference in the primary outcome for the confirmation of a repeat VL < 1 000 copies/mL or a change to second-line ART within 6 months of detection of VF in the pre- and post-intervention groups. In both groups, poor outcomes were reported in all stages of the VF cascade of care (number of EAC sessions, second VL and subsequent visit after repeat VL).
This study reinforces prior work demonstrating extremely poor outcomes after first-line ART failure in sub-Saharan Africa.[9] Potentially more concerning than suboptimal VL monitoring is the startlingly low response to detectable VL we observed in this cohort in the pre intervention and post intervention period. Our findings are similar to a Mozambican study which also reported a poor cascade of care in health system response to VF.[11] In that study, only 35% of those with detected VF had an appropriate repeat test in which 62% had persistent VF. Only a third of those with persistent VF appropriately started second‐line ART. An analysis from rural KwaZulu-Natal using electronic health records also reported very poor management of VF.[10] Only 34% of patients had a VL documented after 12 months starting on ART, and only 18% of these had the recommended repeat VL conducted. In total, and similar to our study, only 20% of individuals were confirmed to have virology re-suppression or change to second-line therapy after VF, and those that did change therapy did so a median of one year after VF. These results show a consistently poor response to treatment failure in the region. Additional measures are needed to better identify, monitor, and ensure – for this high-risk population – more effective clinical management.
There was a drop in numbers of EAC sessions and second VL done in the post intervention period. If adherence counseling was not performed with sufficient expertise or if staff implementation of EAC was not consistent, the remainder of the intervention could be less effective as reported in other studies.[16–18] The results of our intervention demonstrate that addressing health system factors alone might not be sufficient to improve VF management. We hypothesize that a failure to demonstrate improved outcomes was the result of multiple factors. Attention was directed predominantly towards standard operating procedures (SOP), training, and support for laboratory testing and results reporting. The patient-specific factors, that may need to be addressed are transportation costs, work/clinical care tradeoffs, stigma and mental health issues, which have all been shown to affect outcomes in this patient population.[19] A differentiated care model, which adds focus and resources to patients with VF, might serve to improve on-treatment HIV care in the public sector.[20,21]
Both groups had similar rates of repeated VL testing, yet the number that returned for follow up management was very low. Although the team contacted missing patients, additional measures for those who could not be found were limited by funds and staffing time. There is no inherent capacity in paper-based tools to prompt clinic staff to follow-up missed visits. This system requires manual entry for reconciliation of laboratory results, visit schedules, and clinical reporting. Additionally, the available electronic data repositories used in KwaZulu-Natal are not updated in real-time and are often not available to nursing staff. We believe there should be a priority to upgrade the electronic medical record system with the capacity to prompt staff to track patients and universalize access to up-to-date electronic data repositories. Efforts to advance online medical record systems across South Africa, as successfully implemented in the Western Cape, may meaningfully improve patient flow and clinical care.[22]
Clearly, additional novel strategies are needed to more promptly identify and switch patients who qualify for second-line ART, specifically patients with advanced HIV, the group with the worst outcomes after treatment failure.[23,24] In addition to improving online medical record systems, a greater emphasis on task‐shifting to allow nurses to change patients to second‐line with physician approval is worthy of further investigation.[25] Another strategy currently under investigation is the incorporation of resistance testing into the management of VF which might differentiate those with failure into adherence and resistance‐based causes and could encourage clinicians to respond earlier.[26] The roll-out of dolutegravir-based ART as first-line therapy in the region, a regimen with a higher barrier to resistance and better tolerability, is also likely to change the relative importance of the workflow components for monitoring VF. It will perceivably make poor adherence rather than ART resistance the primary determinant of treatment failure.[27] This might further highlight the importance of adherence counseling in the management of VF and require further optimization of the current VF monitoring and switch guidelines.[28]
Our study had limitations. The first was the small sample size, which may not have allowed sufficient power to detect a modest improvement in the outcome of interest. Second, as an observational study with a historical control group, there may have been unmeasured changes in patient characteristics or management that accrued over time that also had an impact on our outcomes. Third, ours was an observational study that followed very soon after the establishment of a new model of care; there may not have been sufficient time for full uptake of the new model to affect patient care outcomes. Last, the clinics under investigation were mainly urban, and to make it generalizable, it will be essential to include rural clinics with different human resource profiles.
In summary, this study lays the basis upon which more comprehensive, evidence-based, clinical operational strategies are sorely needed to improve VF management based upon implementation science research. These programs must address both health system and individual barriers to care and consider the complex mechanisms of detection, monitoring, and response to VF. Until this standard of practice is available and programmatic guidelines reflect their results, high rates of losses from care, delays in appropriate switch to second-line ART, and poor outcomes among people with VF in the region will remain unacceptably common, and threaten the success and durability of global HIV treatment programs. The VL Champion model improves VL completion rates after ART commencement and may be expanded as a national public health intervention, the model for improving VF requires further implementation research.
Supplementary Material
Acknowledgements:
We are thankful for the substantial efforts and contributions from the patients who participated in this program, the nurse and ancillary staff at the clinics, and our partners at the Department of Health for their support of this work. This project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development (USAID) under the Cooperative agreement number AID-674-A-12-00019. The contents are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. A PEPFAR funded non-governmental organization provided on-site data management support in addition to training and mentorship for enhanced adherence counseling and project instruments.
Funding sources:
MJS receives research support from the National Institutes of Health (NIH R01 AI124718). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References:
- 1.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO, 2015. http://www.ncbi.nlm.nih.gov/books/NBK327115/ (accessed 5 October 2020) [PubMed] [Google Scholar]
- 2.Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV 2015;2(7):e271–278. 10.1016/S2352-3018(15)00087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy RA, Court R, Maartens G, Sunpath H. Second-Line Antiretroviral Therapy in Sub-Saharan Africa: It Is Time to Mind the Gaps. AIDS Res Hum Retroviruses 2017;33(12):1181–4. 10.1089/AID.2017.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis 2008;46(10):1589–97. 10.1086/587109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 2016;375(9):830–9. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016;16(5):565–75. 10.1016/S1473-3099(15)00536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med 2017;18(1):776. 10.4102/sajhivmed.v18i1.776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston V, Fielding KL, Charalambous S, Churchyard G, Phillips A, Grant AD. Outcomes following virological failure and predictors of switching to second-line antiretroviral therapy in a South African treatment program. J Acquir Immune Defic Syndr 2012;61(3):370–80. 10.1097/QAI.0b013e318266ee3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunpath H, Naidu KK, Pillay S, Siedner MJ. The Second Cascade: Management of Patients with High Viral Loads in Public HIV Clinics in Durban, South Africa. Amsterdam: 2018. [Google Scholar]
- 10.Iwuji CC, Shahmanesh M, Koole O, et al. Clinical outcomes after first-line HIV treatment failure in South Africa: the next cascade of care. HIV Med 2020;21(7):457–62. 10.1111/hiv.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swannet S, Decroo T, de Castro SMTL, et al. Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health 2017;9(4):206–14. 10.1093/inthealth/ihx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mgosha PC. Barriers to Switching Patients to Second-Line Antiretroviral Treatment Among Clinicians in Tanzania. 2017; 10.1016/S2352-3018(15)00087-9 [DOI]
- 13.Ssempijja V, Nakigozi G, Chang L, et al. Rates of switching to second-line antiretroviral therapy and impact of delayed switching on immunologic, virologic, and mortality outcomes among HIV-infected adults with virologic failure in Rakai, Uganda. BMC Infect Dis 2017; 17(1):582. 10.1186/s12879-017-2680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunpath H, Hatlen TJ, Naidu KK, et al. targeting the third ‘90’: introducing the viral load champion. Public Health Action 2018; 8(4):225–31. 10.5588/pha.18.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osler A, Boulez A. Three interlinked electronic registers (TIER.Net) Project, Working Paper [Internet]. 2010;Available from: http://webdav.uct.ac.za/depts/epi/publications/documents/TIER.Net%20%5BSept%202010%5D
- 16.Bango F, Ashmore J, Wilkinson L, van Cutsem G, Cleary S. Adherence clubs for long-term provision of antiretroviral therapy: cost-effectiveness and access analysis from Khayelitsha, South Africa. Trop Med Int Health 2016;21(9):1115–23. 10.1111/tmi.12736 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee JS, Barry D, Weatherford RD, Desai IK, Farmer PE. Community-Based ART Programs: Sustaining Adherence and Follow-up. Curr HIV/AIDS Rep 2016; 13(6):359–66. 10.1007/s11904-016-0335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MP, Pascoe S, Huber AN, et al. Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: Results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. Plops Med 2019;16(7):e1002874. 10.1371/journal.pmed.1002874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MP, Pascoe SJS, Huber AN, et al. Effectiveness of interventions for unstable patients on antiretroviral therapy in South Africa: results of a cluster-randomized evaluation. Trop Med Int Health 2018;23(12):1314–25. 10.1111/tmi.13152 [DOI] [PubMed] [Google Scholar]
- 20.Grimsrud A, Bygrave H, Doherty M, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016;19(1):21484. 10.7448/IAS.19.1.21484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015;528(7580):S68–76. 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Org 2008; 86(12):939–47. 10.2471/BLT.07.049908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villa G, Abdullahi A, Owusu D, et al. Determining virological suppression and resuppression by point-of-care viral load testing in a HIV care setting in sub-Saharan Africa. EClinicalMedicine 2020; 18:100231. 10.1016/j.eclinm.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroufi A, Van Cutsem G, and Cambiano V, et al. Simplifying switch to second-line antiretroviral therapy in sub Saharan Africa: predicted effect of using a single viral load to define efavirenz-based first-line failure. AIDS 2019;33(10):1635–44. 10.1097/QAD.0000000000002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowley T, Mayers P. Trends in task shifting in HIV treatment in Africa: Effectiveness, challenges and acceptability to the health professions. Afr J Prim Health Care Fam Med 2015; 7(1). 10.4102/phcfm.v7i1.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siedner MJ, Bwana MB, Moosa M-YS, et al. The REVAMP trial to evaluate HIV resistance testing in sub-Saharan Africa: a case study in clinical trial design in resource limited settings to optimize effectiveness and cost effectiveness estimates. HIV Clin Trials 2017; 18(4):149–55. 10.1080/15284336.2017.1349028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruciani M, Parisi SG. Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: A systematic review and meta-analysis. PLoS ONE 2019;14(9):e0222229. 10.1371/journal.pone.0222229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy, Adolescents, Children, Infants and Neonates [Internet]. 2019; Available from: http://bit.ly/2019-ART-Guidelines
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


