Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation intervention investigated for the treatment of depression. Clinical results have been heterogeneous, partly due to the variability of electric field (EF) strength in the brain owing to interindividual differences in head anatomy. Therefore, we investigated whether EF strength was correlated with behavioral changes in 16 depressed patients using simulated electric fields in real patient data from a controlled clinical trial. We hypothesized that EF strength in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC), brain regions implicated in depression pathophysiology, would be associated with changes in depression, mood and anxiety scores. SimNIBS were used to simulate individual electric fields based on the MRI structural T1-weighted brain scans of depressed subjects. Linear regression models showed, at the end of the acute treatment phase, that simulated EF strength was inversely associated with negative affect in the bilateral ACC (left: β = − 160.463, CI [− 291.541, − 29.385], p = 0.021; right: β = − 189.194, CI [− 289.479, − 88.910], p = 0.001) and DLPFC (left: β = − 93.210, CI [− 154.960, − 31.461], p = 0.006; right: β = − 82.564, CI [− 142.867, − 22.262], p = 0.011) and with depression scores in the left ACC (β = − 156.91, CI [− 298.51, − 15.30], p = 0.033). No association between positive affect or anxiety scores, and simulated EF strength in the investigated brain regions was found. To conclude, our findings show preliminary evidence that EF strength simulations might be associated with further behavioral changes in depressed patients, unveiling a potential mechanism of action for tDCS. Further studies should investigate whether individualization of EF strength in key brain regions impact clinical response.
Keywords: Transcranial direct current stimulation, Electric field modeling, tDCS modeling, Major depressive disorder, Depression, SimNIBS, General linear models
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation intervention that applies direct electric currents to modulate cortical excitability according to the parameters of stimulation [1]. The technique has been used in several neuropsychiatric conditions, most notably in major depressive disorder (MDD) [2, 3], in which electrodes are usually positioned over the dorsolateral prefrontal cortex (DLPFC), which tends to be hypoactive in depression [4, 5].
The clinical efficacy of tDCS for treatment of MDD has been investigated in several randomized clinical trials [6–8]. Although meta-analyses have shown active tDCS to be superior to sham for response, remission and depression improvement [3, 9], clinical results have been moderate and heterogeneous [3, 7, 10]. Recently, it has been hypothesized [11] that variations in the electrical current on the targeted brain region might account for such heterogeneity [12–15]. Although invasive estimation of such currents is not feasible in most cases, recent technical developments permit, using brain imaging, the simulation of the strength and spatial distribution of the electrical current injected into the brain, as well as to assess individual differences in strength and distribution of such currents owing to one’s head and brain anatomy [16]. Neurophysiological studies have shown that tDCS-induced electric fields (EFs) in the brain of healthy volunteers correlate with changes in gamma-aminobutyric acid (GABA), measured by magnetic resonance spectroscopy [17], and with the resting motor threshold, measured by transcranial magnetic stimulation [18]. Nonetheless, individual EFs have not been systematically investigated as a predictor of tDCS clinical effects.
Given these initial results, we hypothesized that tDCS-induced EF strength (measured in Vm−1 and representing the intensity of the electric field distributed over a given anatomical region) in brain regions of interest (ROIs) would be associated with behavioral changes in depressed patients within a controlled clinical trial previously performed by our group [7]. The selected ROIs were the DLPFC and the anterior cingulate cortex (ACC) bilaterally, since these regions are structurally implicated in MDD [19–21] and are targeted by brain stimulation interventions [22, 23]. For instance, a recent meta-analysis quantifying structural brain changes associated with MDD showed that gray matter reduction in the ACC to be one of the most robust findings between studies [19]. Additionally, clinical improvement has been associated with increase in gray matter volume of the DLPFC [24], and connectivity between these two regions predicted antidepressant response to rTMS [25]. Our primary outcome variable was changes in the Hamilton depression rating scale (HDRS-17) [26]. As secondary outcomes, we explored changes in affective scores (indexed by the Positive and Negative Affect Scale (PANAS) [27]) and in trait or state anxiety (measured by the State–Trait Anxiety Inventory (STAI) [28]), as previous studies have shown that prefrontal tDCS modulates affective and anxiety processing [29–31].
Methods
Study design
Our Escitalopram versus Electrical Current Therapy for Treating Depression Clinical Study (ELECT-TDCS) trial, a non-inferiority triple-arm study, randomized patients into three groups: sham-tDCS/placebo-pill (placebo group), sham-tDCS/escitalopram (escitalopram group) and active-tDCS/placebo-pill (tDCS group) [32]. The original study compared 22, 2-mA, 30-min tDCS sessions (1 × 1 tDCS-CT, SoterixMedical, New York, NY) applied in a 10-week period, with 15 sessions applied consecutively once a day (expect for weekends), and 7 more sessions applied once per week until the study endpoint at week 10, to a first-line antidepressant treatment (escitalopram 20 mg/day), and found it to be superior to placebo and not non-inferior to escitalopram [7]. Our study was registered in ClinicalTrials.gov (NCT01894815).
Participants
We recruited patients aged 18–75 years who were diagnosed with major depressive disorder during an acute depressive episode per DSM-5 criteria (Diagnostic and Statistical Manual of Mental Disorders, 5th edition). The main inclusion criteria were: (1) ≥ 17 points on HDRS-17; (2) baseline low risk of suicide; (3) at least 8 years of schooling; (4) and adherence to study protocol. Exclusion criteria were other neuropsychiatric disorders (except for anxiety disorders as a comorbidity), pregnancy, specific contraindications to tDCS (e.g., cranial plates), current or previous escitalopram use, and previous or concomitant participation in other tDCS trials. Patients under antidepressant drug therapy underwent drug washout. Benzodiazepines were allowed up to 20 mg/day diazepam-equivalent.
In this ancillary study, all participants received active tDCS according to the protocol described above. As they were part of a placebo-controlled study, these patients also received placebo pill, as they were not aware to which study group they were assigned to.
Magnetic resonance imaging
All images were acquired in 3-T MR system (Achieva, Philips Healthcare, Netherlands). Volumetric images were based on T1-weighted sequences using a 3D FFE pulse sequence with the following parameters: FOV 240 × 240 × 180 mm3, spatial resolution 1 × 1 × 1 mm3, TR 7 ms, TE 3.2 ms, FA 8°, 180 sagittal slices. MR acquisitions were performed up to 8 days before baseline and were performed at the Department of Radiology (Hospital das Clínicas da Universidade de São Paulo, São Paulo) during the weekends.
tDCS modeling
SimNIBS (v3.1, Danish Research Centre for Magnetic Resonance, Copenhagen, Denmark) [33] was used for tDCS modeling. It is a free and open-source software package for the simulation of tDCS-induced electric field in the individual brain. It allows for realistic calculations using the finite element method (FEM), and integrates free software for neuroimaging, computer graphics and FEM calculations into one coherent pipeline. TDCS modeling was done using T1-weighted anatomical images of each subject to reconstruct a high-resolution head model of that individual using the SimNIBS pipeline. We manually verified each segmentation to check for possible errors in the established boundaries between tissues, and no subjects were excluded in the process. The estimated EF distribution in one’s brain is obtained by placing simulated electrodes on the head model and setting simulated electric current intensity according to the stimulation protocol used in the clinical trial.
Parameters of the tDCS modeling were set according to the ELECT-TDCS protocol [32]: current intensity was set to 2 mA and electrode sizes of 5 × 5 cm. For electrode positioning, we simulated the F5 and F6 areas, according to the EEG 10–20 system, for the anode and cathode, respectively, targeting the left and right DLPFC. The DLPFC has a complex cortical structure that is highly variable between individuals and, despite its widespread use as a target, there remains a lack of consensus for how this region should be best localised. Although in the original study we used the Omni-Lateral Electrode (OLE) system, the F5-F6 positioning was employed since it can be directly implemented in SimNIBS and considering that the simulated EF strength in the brain for both montages is similar [5].
Electric field values
According to our hypotheses, a ROI-based approach was used to define the DLPFC and ACC, brain areas in which simulated EF strength was evaluated. To define the DLPFC, we used the Sallet et al. atlas [34], which provides a parcellation of the dorsal frontal cortex based on functional and tractography data in a cross-species comparison of both humans and primates, and divides it into 10 subregions (clusters) also identified by their corresponding Brodmann areas (BAs). This approach was used in a previous study by our group correlating structural DLPFC changes with tDCS antidepressant response [15]. This atlas was chosen as it allows to identify ROIs in the proximity of the DLPFC area, while incorporating anatomical and functional data. The Sallet et al. atlas also incorporates motor and premotor areas, but they were not included in our analysis as they were not part of our hypotheses. We defined the DLPFC by Sallet et al. clusters 3, 4, 5, 6, 7, 8 and 10, which correspond to Brodmann Areas (BAs) 8, 9, 10 and 46. For the ACC ROI, we used the parcellation of the Brainnetome atlas [35], a whole-brain, multimodal parcellation atlas based on structural magnetic resonance imaging (MRI), diffusion tensor imaging and resting-state fMRI connectivity.
As significant effects in these hypothesis-driven regions were observed for HDRS-17 and PANAS, and as performed in our previous study [15], we analyzed subregions of the DLPFC and the ACC in an exploratory manner so as to identify subregions driving these effects. In the DLPFC, we investigated 7 clusters according to Sallet et al.: cluster 3 (corresponds to BA 9), cluster 4 (BA 10), cluster 5 (BA 9/46D), cluster 6 (BA 9/46V), cluster 7 (BA 46), cluster 8 (BA 8A) and cluster 10 (BA 8B). In the ACC, we further explored the subgenual ACC (sgACC) and pregenual ACC (pgACC) using the “A32sg” and “A32p” ROIs from the Brainnetome atlas, because of their particular roles in predicting antidepressant response specifically in the rTMS literature [36–38].
Statistical analysis
We used Python 3.7.0 [39], Spyder 3.3.6 and the StatsModels library [40] to perform a regression analysis to explore in which brain regions simulated EF strength was associated with depression improvement. Statistical results were considered significant under a p threshold of 0.05.
EF strength was obtained as the average EF strength within the ROI (Emean), calculated by summing the simulated EF in each voxel and dividing it by the number of voxels. We used linear regression models, adjusted for gender and age, with changes in the HDRS-17, STAI, and PANAS scales as dependent variables and Emean at the ROIs as independent variables. We evaluated whether simulated EF would be correlated with changes immediately after the end of the acute treatment phase (i.e., 15 sessions) and at study endpoint (i.e., week 10). These two time frames were also used in main and ancillary analyses of ELECT-TDCS [7, 15, 41, 42] and reflect timepoints in which acute and long-lasting tDCS effects are usually observed [3]. These analyses were not corrected for multiple comparisons since they were hypothesis driven. Also, five patients did not complete the study, and models for study endpoint include only trial completers.
Additionally, we investigated the subregions of the DLPFC and ACC in an exploratory manner using the same models, which produced another 9 models for each hemisphere per outcome. For this exploratory analysis, the correction for multiple comparisons was done using the Bonferroni correction for each outcome individually, in both hemispheres. For each outcome variable, a total number of 18 tests were performed (9 subregions—7 in the DLPFC and 2 in the ACC—in two hemispheres); therefore, the correction was performed using a threshold α = 0.05/18.
Results
Out of the original sample, only 68 patients received MRI at baseline. The most important reasons for the absence collection of MRI were (1) the delayed start of the MRI collection that initiated only after 30% of the sample had already been recruited, (2) patient refusal, and (3) lack of MRI slots available. Other reasons included MRI contraindications and technical reasons. Moreover, MRI scans of 15 patients were excluded after an initial quality check (absence of T1 anatomical sequences, abnormal anatomical findings, and poor quality due to head motion). The remaining 53 scans were divided into three groups: active tDCS (16 patients), escitalopram (16 patients) and placebo (21 patients). Here, we performed simulations in the 16 patients who had undergone tDCS. Their characteristics are shown in Table 1, and the individual simulated EF strength distribution in Fig. 1. It can be visually depicted that such distribution is notably different between participants (Table 2).
Table 1.
Patient group characteristics
| tDCS | |
|---|---|
| Gender (male/female) | 7/9 |
| Age (mean ± SD), years | 42.8 ± 10.9 |
| HDRSa | |
| Baseline | 21.6 ± 3.9 |
| Week 3 | 14.3 ± 6.1 |
| Week 10 | 13.8 ± 10.1 |
| PA | |
| Baseline | 17.1 ± 6.3 |
| Week 3 | 21.5 ± 9.1 |
| Week 10 | 25.0 ± 11.4 |
| NA | |
| Baseline | 29.6 ± 9.3 |
| Week 3 | 26.4 ± 11.4 |
| Week 10 | 21.8 ± 10.1 |
| STAI—state | |
| Baseline | 54.6 ± 10.1 |
| Week 3 | 51.6 ± 13.4 |
| Week 10 | 48.3 ± 16.4 |
| STAI—trait | |
| Baseline | 65.6 ± 6.3 |
| Week 3 | 60.6 ± 11.5 |
| Week 10 | 54.3 ± 17.7 |
| Emean (V/m) | |
| DFLP—left | 0.317 ± 0.055 |
| DLFPC—right | 0.332 ± 0.059 |
| ACC—left | 0.153 ± 0.030 |
| ACC—right | 0.145 ± 0.030 |
Distribution of characteristics, clinical outcomes, and mean electric field strength of the four main analyzed regions. Values are displayed as mean ± standard deviation
HDRS Hamilton depression rating scale, PA positive affect, NA negative affect, STAI state–trait anxiety inventory, Emean Mean electric field strength inside ROI, DLPFC dorsolateral prefrontal cortex, ACC anterior cingulate cortex
Scores on the 17-item Hamilton depression rating scale (0–52, the higher the more severely depressed)
Fig. 1.
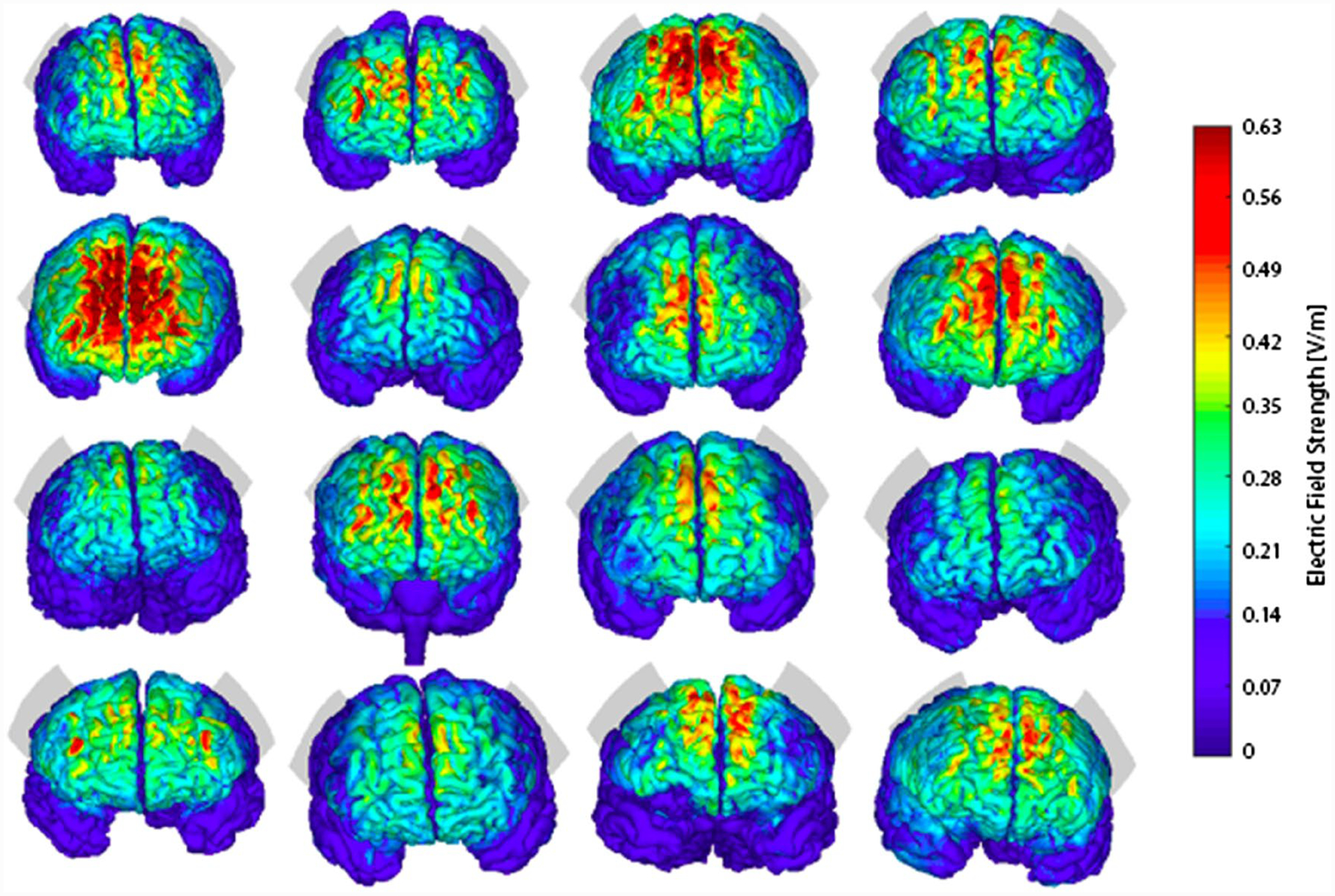
Individual tDCS-induced simulated brain electrical field strength distribution. Illustrates the location of the stimulation electrodes (EEG-based F5-F6 location) and the distribution of the electric field on all 16 subjects of the study. Peak electric field strength (measured in V/m) occurs in intermediate regions between stimulation electrodes and is notably different between participants
Table 2.
Linear models for the DLPFC
| Linear models for DLPFC | Left hemisphere | Right hemisphere | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p | Beta | 95% CI | p | |
| HDRS (week 3) | − 48.25 | [− 131.74, 35.25] | 0.232 | − 45.34 | [− 123.32, 32.64] | 0.229 |
| HDRS (week 10) | 24.23 | [− 174.97, 223.42] | 0.782 | 19.40 | [− 169.51, 208.31] | 0.815 |
| PA (week 3) | 42.50 | [− 33.92, 118.91] | 0.249 | 41.22 | [− 29.86, 112.30] | 0.230 |
| PA (week 10) | − 43.92 | [− 185.99, 98.15] | 0.489 | − 45.02 | [− 178.65, 88.60] | 0.452 |
| NA (week 3) | − 93.21 | [− 154.96, − 31.46] | 0.006 | − 82.56 | [− 142.87, − 22.62] | 0.011 |
| NA (week 10) | 17.51 | [− 133.60, 168.63] | 0.792 | 23.52 | [− 118.78, 165.82] | 0.708 |
| STAI—state (week 3) | − 12.21 | [− 112.61, 88.19] | 0.795 | 2.73 | [− 91.36, 96.83] | 0.951 |
| STAI—state (week 10) | 43.78 | [− 177.29, 264.86] | 0.654 | 73.82 | [− 128.24, 275.88] | 0.416 |
| STAI—trait (week 3) | − 3.72 | [− 117.92, 110.48] | 0.945 | − 6.73 | [− 113.40, 99.94] | 0.893 |
| STAI—trait (week 10) | 30.84 | [− 208.47, 270.14] | 0.769 | 49.3 | [− 174.47, 273.07] | 0.618 |
Results of the linear models obtained for the dorsolateral prefrontal cortex in both brain hemispheres. Table values marked in bold indicate regions in which the correlation was found to be significant. Beta is the linear coefficient of the relation between mean electric field strength and negative affect change. 95% CI is the confidence interval for the linear coefficient with a 95% confidence level. p is the p-value of the linear model and was not corrected for multiple comparisons since these analyses were hypothesis driven
HDRS Hamilton depression rating scale, PA positive affect, NA negative affect, STAI state–trait anxiety inventory, DLPFC dorsolateral prefrontal cortex
Changes in depression scores
For the acute treatment phase, HDRS-17 change was significantly correlated with Emean in the left ACC (β = − 156.91, CI [− 298.51, − 15.30], p = 0.033) (Table 3). No other significant correlation was found for this time frame or at study endpoint (Tables 2 and 3).
Table 3.
Linear models for the ACC
| Linear models for ACC | Left | Right | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p | Beta | 95% CI | p | |
| HDRS (week 3) | − 156.91 | [− 298.51, − 15.30] | 0.033 | − 110.46 | [− 257.21, 36.29] | 0.127 |
| HDRS (week 10) | − 55.89 | [− 398.49, 286.72] | 0.711 | 14.24 | [− 338.41, 366.89] | 0.927 |
| PA (week 3) | 101.91 | [− 41.73, 245.54] | 0.148 | 86.84 | [− 50.67, 224.34] | 0.194 |
| PA (week 10) | − 1.61 | [− 256.31, 253.08] | 0.988 | − 57.70 | [− 312.12, 196.71] | 0.608 |
| NA (week 3) | − 160.46 | [− 291.54, − 29.38] | 0.021 | − 189.19 | [− 289.48, − 88.91] | 0.001 |
| NA (week 10) | − 1.18 | [263.70, 261.35] | 0.992 | 30.73 | [− 235.43, 296.88] | 0.792 |
| STAI—state (week 3) | − 30.98 | [− 225.64, 163.69] | 0.735 | − 74.34 | [− 252.33, 103.66] | 0.381 |
| STAI—state (week 10) | 10.11 | [− 377.74, 397.95] | 0.953 | 41.68 | [− 351.86, 435.33] | 0.809 |
| STAI—trait (week 3) | − 54.18 | [− 273.47, 165.11] | 0.600 | − 34.63 | [− 242.26, 172.99] | 0.723 |
| STAI—trait (week 10) | − 63.78 | [− 476.09, 348.55] | 0.725 | − 4.53 | [− 428.75, 419.69] | 0.980 |
Results of the linear models obtained for the anterior cingulate cortex in both brain hemispheres. Table values marked in bold indicate regions in which the correlation was found to be significant. Beta is the linear coefficient of the relation between mean electric field strength and negative affect change. 95% CI is the confidence interval for the linear coefficient with a 95% confidence level. p is the p-value of the linear model and was not corrected for multiple comparisons since these analyses were hypothesis driven
HDRS Hamilton depression rating scale, PA positive affect, NA negative affect, STAI state–trait anxiety inventory, ACC anterior cingulate cortex
Changes in positive and negative affect
For the acute treatment phase, negative affect reduction was associated to Emean in the left DLPFC (β = − 93.21, CI [− 154.96, − 31.46], p = 0.006) (Table 2), right DLPFC (β = − 82.56, CI [− 142.87, − 22.62], p = 0.011) (Table 2), left ACC (β = − 160.46, CI [− 291.54, − 29.38], p = 0.021) (Table 3), right ACC (β = − 189.19, CI [− 289.48, − 88.91], p = 0.001) (Table 3) (Fig. 2). No significant correlation was found for the 10-week period (Tables 2 and 3).
Fig. 2.
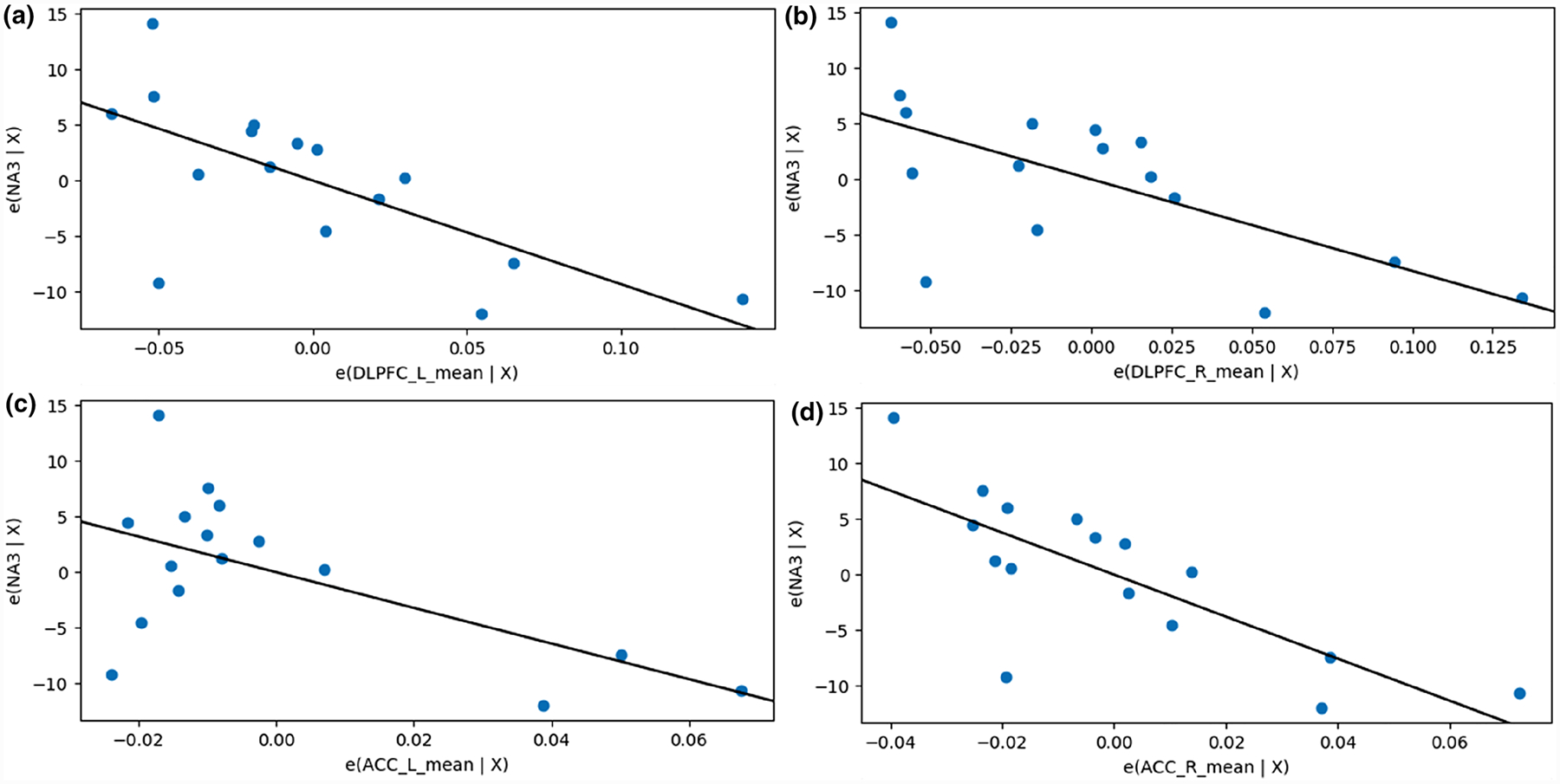
Association between negative affect and EF strength for brain regions of interest. Partial regression plots of the mean electric field strength (measured in V/m) in hypothesis-defined regions of interest in relation to change in negative affect after acute treatment phase. A higher mean field value corresponds to a more pronounced decrease of negative affect as measured by the Positive and Negative Affect Scale (PANAS). a, b, c and d show the partial regression plots of the left dorsolateral prefrontal cortex, right dorsolateral prefrontal cortex, left anterior cingulate cortex and right anterior cingulate cortex, respectively. DLPFC dorsolateral prefrontal cortex, ACC anterior cingulate cortex, NA negative affect
No significant correlations were found between change in positive affect and EF strength (Table 2 and 3).
STAI improvement
No significant correlations were found between trait and state anxiety changes and simulated EF strength for any of the explored clusters in any time frame (Table 2 and 3).
Exploratory analysis in subregions of DLPFC and ACC
Post-acute treatment phase changes in negative affect were significantly associated with Emean after Bonferroni correction in right BA 9/46D (β = − 83.87, CI [− 130.02, − 37.71], p = 0.002, pcorr = 0.034), right pgACC (β = − 159.92, CI [− 245.51, − 74.32], p = 0.002, pcorr = 0.028), and left BA 9 (β = − 49.38, CI [− 74.22, − 24.53], p = 0.001, pcorr = 0.018). No other significant associations were observed for other outcomes, regions, or timeframes (data not shown).
Discussion
In this study, we investigated the association between individual tDCS-induced simulated EF strength, using state-of-the-art computational simulation modeling approaches, and behavioral outcomes in depressed patients, based on our ELECT-TDCS trial. To the best of our knowledge, this is the first study correlating simulated EF strength with clinical outcomes in a depressed sample submitted to tDCS treatment.
Our findings showed that simulated EF strength in the left ACC was correlated with changes in HDRS-17. This is relevant since ACC acts as a bridge between attentional and emotional processing. In fact, alterations in its structure and function have been implicated in the pathophysiology of MDD. Depressed patients show decreased gray matter volume in the ACC [43], and, although our previous study did not find a significant correlation between baseline gray matter volume in the ACC and tDCS treatment response [15], it has been suggested that increases in ACC volume after repetitive transcranial magnetic stimulation (rTMS) and electroconvulsive therapy (ECT), other brain stimulation techniques, are correlated with its antidepressant effect [44, 45]. Additionally, connectivity between the DLPFC and ACC is altered in MDD [46], and changes in DLPFC-ACC connectivity are associated with rTMS efficacy [36, 47]. Therefore, our finding suggests that the ACC is implicated in prefrontal tDCS antidepressant effects. Future studies should investigate whether tDCS induces volumetric or connectivity changes in the ACC of depressed patients, and whether such changes are correlated with the EF strength in this brain region.
We also found that simulated EF strength in the bilateral ACC and DLPFC was inversely associated with negative affect, i.e., greater score reductions were associated with larger EF strengths in these regions. Since both brain regions are involved in implementing emotion regulation strategies and modulating activity in other emotion-encoding brain regions [48–52], it is possible that the applied EF contributed to increasing the functional coupling within this neural circuitry, facilitating emotional regulation of affect. In fact, this finding might be associated with the direct effects of tDCS over the PFC that regulates negative affect [53]. In previous studies using ELECT-TDCS data, we found that negative affect was the most important predictor associated with tDCS antidepressant response [54] and that the DLPFC volume predicted antidepressant response [15]. Moreover, several studies showed that prefrontal tDCS can enhance affective processing of emotionally loaded tasks [55–57]. Thus, our study confirms and expands previous evidence suggesting that changes in negative affect are implicated in tDCS antidepressant mechanisms of action. Further studies are necessary to explore this hypothesis and determine the specific mechanisms by which this is accomplished.
We found no correlation between trait or state anxiety and EF strength over the DLPFC and ACC. Although some studies suggested that tDCS can downregulate anxiety [31, 58], negative findings have been also reported. For instance, recent trials showed modest or null effects of prefrontal tDCS in ameliorating anxiety symptoms [59, 60]. In this context, other tDCS protocols that could be more effective in improving anxiety symptoms should be investigated [61]. In addition, tDCS effects on anxiety might be more effective when down-regulating stress-induced tasks [62, 63].
All the observed effects occurred immediately after the acute treatment phase (3 weeks of trial onset), but not at study endpoint (week 10). Interestingly, most studies have observed that tDCS effects are delayed, i.e., only differentiate from placebo after the acute treatment phase [7, 64, 65]. It is possible that other, non-specific factors (e.g., placebo effects, natural history of disease, regression to the mean) occurring between weeks 3 and 10 mitigated a possible association between simulated EF strength and our behavioral outcomes. Conversely, another possible explanation is that missing data from patients who did not complete the trial decreased the power of our analyses.
Our study has several limitations worth notice. First, our sample size is small as only a subsample of patients from the original study had MRI data collected. Therefore, some analyses might have been underpowered and our results should be primarily interpreted as hypothesis driven for future studies. Our limited sample size highlights the urgent need for larger tDCS depression studies performing baseline MRI measurements for replication of our findings. In addition, as 40 models were performed, at least 2 false positive findings might have emerged just by chance. Second, we are using simulated electric fields in reconstructed models of patient’s heads. Although validated and considered state of the art [66, 67], they nonetheless represent an approximation of the “real” current distribution in the brain, which cannot be measured in a non-invasive manner. Third, the electrode positioning for the simulations on the models does not follow the exact correct location of the electrodes on the montage of the clinical trial (OLE system, which uses a 10 cm distance between electrodes), because of technical difficulties positioning virtual electrodes over simulated models’ scalps using a 10 cm distance on irregular surfaces with distinct curvatures. Instead, the F5-F6 montage used in this study’s simulations favors uniformity in the electrode positioning between subjects. Finally, the model is static, i.e., it does not incorporate fluctuations in blood flow and changes in tissue conductivity that likely occur when tDCS is applied [68].
Whether further studies confirm that EF strength of certain brain regions correlates with clinical response, it would be possible to tailor individual tDCS montages and parameters to increase EF strength in such areas, theoretically improving clinical outcomes. This would represent an advancement towards individualizing tDCS parameters [69] whose parameters have been hitherto mostly fixed, not considering one’s brain and skull anatomy.
Conclusion
We have investigated the association between simulated EF strength in brain regions implicated in depression pathophysiology and changes in behavioral outcomes in 16 depressed patients. We found that simulated EF strength presented a large variation in individual brains, even under the same parameters of stimulation. According to our hypotheses, associations were observed between simulated EF strength in the DLPFC and ACC and negative affect and depression scores. Nonetheless, the sample size was small and multiple tests were performed. Therefore, our findings should be regarded as exploratory. Notwithstanding, they show that EF strength might be associated with behavioral changes in clinical samples, suggesting a potential mechanism of action of tDCS antidepressant effects and fomenting further studies exploring whether tDCS interventions could be tailored to maximize EF strength in key brain regions to enhance clinical outcomes.
Funding
ELECT-TDCS funding: São Paulo Research State Foundation (FAPESP): grants FAPESP 2012/20911-5 and 2018/21722-8.
Footnotes
Conflict of interest On behalf of all authors, the corresponding author states that there are no conflicts of interest to disclose.
References
- 1.Brunoni AR, Nitsche MA, Bolognini N et al. (2012) Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5:175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moffa AH, Brunoni AR, Nikolin S, Loo CK (2018) Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am 41:447–463 [DOI] [PubMed] [Google Scholar]
- 3.Moffa AH, Martin D, Alonzo A, et al. (2019) Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: an individual patient data meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry pp 109836. [DOI] [PubMed] [Google Scholar]
- 4.Grimm S, Beck J, Schuepbach D et al. (2008) Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatr 63:369–376 [DOI] [PubMed] [Google Scholar]
- 5.Seibt O, Brunoni AR, Huang Y, Bikson M (2015) The pursuit of DLPFC: non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS). Brain Stimul 8:590–602 [DOI] [PubMed] [Google Scholar]
- 6.Brunoni AR, Valiengo L, Baccaro A et al. (2013) The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatr 70:383–391 [DOI] [PubMed] [Google Scholar]
- 7.Brunoni AR, Moffa AH, Sampaio-Junior B et al. (2017) Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med 376:2523–2533 [DOI] [PubMed] [Google Scholar]
- 8.Sampaio-Junior B, Tortella G, Borrione L et al. (2018) Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatr 75:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunoni AR, Moffa AH, Fregni F et al. (2016) Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatr 208:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loo CK, Husain MM, McDonald WM et al. (2018) International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul 11:125–133 [DOI] [PubMed] [Google Scholar]
- 11.Lisanby SH (2017) Noninvasive brain stimulation for depression—the devil is in the dosing. N Engl J Med 376:2593–2594 [DOI] [PubMed] [Google Scholar]
- 12.Lang N, Nitsche MA, Dileone M et al. (2011) Transcranial direct current stimulation effects on I-wave activity in humans. J Neurophysiol 105:2802–2810 [DOI] [PubMed] [Google Scholar]
- 13.Fresnoza S, Paulus W, Nitsche MA, Kuo M-F (2014) Nonlinear dose-dependent impact of D1 receptor activation on motor cortex plasticity in humans. J Neurosci 34:2744–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta A, Truong D, Minhas P et al. (2012) Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatr 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulubas L, Padberg F, Bueno PV et al. (2019) Antidepressant effects of tDCS are associated with prefrontal gray matter volumes at baseline: evidence from the ELECT-TDCS trial. Brain Stimul 12:1197–1204 [DOI] [PubMed] [Google Scholar]
- 16.Opitz A, Paulus W, Will S et al. (2015) Determinants of the electric field during transcranial direct current stimulation. Neuroimage 109:140–150 [DOI] [PubMed] [Google Scholar]
- 17.Antonenko D, Thielscher A, Saturnino GB et al. (2019) Towards precise brain stimulation: is electric field simulation related to neuromodulation? Brain Stimul 12:1159–1168 [DOI] [PubMed] [Google Scholar]
- 18.Mikkonen M, Laakso I, Sumiya M et al. (2018) TMS motor thresholds correlate with TDCS electric field strengths in hand motor area. Front Neurosci 12:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bora E, Fornito A, Pantelis C, Yücel M (2012) Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 138:9–18 [DOI] [PubMed] [Google Scholar]
- 20.Lai C-H (2013) Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatr Res 211:37–46 [DOI] [PubMed] [Google Scholar]
- 21.Liao Y, Huang X, Wu Q et al. (2013) Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatr Neurosci 38:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunoni AR, Sampaio-Junior B, Moffa AH et al. (2019) Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatr 41:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeken C, Brem A-K, Arns M et al. (2019) Repetitive transcranial magnetic stimulation treatment for depressive disorders. Curr Opin Psychiatr 32:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith R, Chen K, Baxter L et al. (2013) Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J Affect Disord 146:414–419 [DOI] [PubMed] [Google Scholar]
- 25.Weigand A, Horn A, Caballero R et al. (2018) Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatr 84:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton M (1980) Rating depressive patients. J Clin Psychiatr 41:21–24 [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070 [DOI] [PubMed] [Google Scholar]
- 28.Spielberger CD, Gorsuch RL (1983) State-trait anxiety inventory for adults: manual, instrument, and scoring guide. Mind Garden Incorporated, Menlo Park [Google Scholar]
- 29.Brunoni AR, Zanao T, Vanderhasselt MA, et al. (2013) Enhancement of affective processing induced by bi-frontal transcranial direct current stimulation in patients with major depression. Neuromodulation [Epub–ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Moreno ML, Vanderhasselt M-A, Carvalho AF et al. (2015) Effects of acute transcranial direct current stimulation in hot and cold working memory tasks in healthy and depressed subjects. Neurosci Lett 591:126–131 [DOI] [PubMed] [Google Scholar]
- 31.Ironside M, O’Shea J, Cowen PJ, Harmer CJ (2016) Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biol Psychiatr 79:823–830 [DOI] [PubMed] [Google Scholar]
- 32.Brunoni AR, Sampaio-Junior B, Moffa AH et al. (2015) The escitalopram versus electric current therapy for treating depression clinical study (ELECT-TDCS): rationale and study design of a non-inferiority, triple-arm, placebo-controlled clinical trial. Sao Paulo Med J 133:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thielscher A, Antunes A, Saturnino GB (2015) Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? In: 2015 37th annual international conference of the IEEE engineering in medicine and biology society (EMBC). pp 222–225 [DOI] [PubMed] [Google Scholar]
- 34.Sallet J, Mars RB, Noonan MP et al. (2013) The organization of dorsal frontal cortex in humans and macaques. J Neurosci 33:12255–12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan L, Li H, Zhuo J et al. (2016) The Human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 26:3508–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox MD, Buckner RL, White MP et al. (2012) Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatr 72:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C-T, Wang S-J, Hirvonen J et al. (2010) Antidepressant mechanism of add-on repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J Affect Disord 127:219–229 [DOI] [PubMed] [Google Scholar]
- 38.Downar J, Geraci J, Salomons TV et al. (2014) Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatr 76:176–185 [DOI] [PubMed] [Google Scholar]
- 39.Rossum G (1995) Python reference manual. CWI (Centre for Mathematics and Computer Science), Amsterdam, The Netherlands. [Google Scholar]
- 40.Seabold S, Perktold J (2010) Statsmodels: econometric and statistical modeling with python of the 9th Python in science conference [Google Scholar]
- 41.Brunoni AR, Padberg F, Vieira ELM et al. (2018) Plasma biomarkers in a placebo-controlled trial comparing tDCS and escitalopram efficacy in major depression. Prog Neuropsychopharmacol Biol Psychiatr 86:211–217 [DOI] [PubMed] [Google Scholar]
- 42.Moreno ML, Goerigk SA, Bertola L et al. (2020) Cognitive changes after tDCS and escitalopram treatment in major depressive disorder: results from the placebo-controlled ELECT-TDCS trial. J Affect Disord 263:344–352 [DOI] [PubMed] [Google Scholar]
- 43.Zhang F-F, Peng W, Sweeney JA et al. (2018) Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther 24:994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan MJ, Chhetry BT, Liston C et al. (2016) Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul 9:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yrondi A, Péran P, Sauvaget A et al. (2018) Structural-functional brain changes in depressed patients during and after electroconvulsive therapy. Acta Neuropsychiatr 30:17–28 [DOI] [PubMed] [Google Scholar]
- 46.Iseger TA, van Bueren NER, Kenemans JL et al. (2020) A frontal-vagal network theory for major depressive disorder: implications for optimizing neuromodulation techniques. Brain Stimul 13:1–9 [DOI] [PubMed] [Google Scholar]
- 47.Taylor SF, Ho SS, Abagis T et al. (2018) Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J Affect Disord 232:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rive MM, van Rooijen G, Veltman DJ et al. (2013) Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 37:2529–2553 [DOI] [PubMed] [Google Scholar]
- 49.Greening SG, Osuch EA, Williamson PC, Mitchell DGV (2014) The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc Cogn Affect Neurosci 9:628–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon ML, Thiruchselvam R, Todd R, Christoff K (2017) Emotion and the prefrontal cortex: an integrative review. Psychol Bull 143:1033–1081 [DOI] [PubMed] [Google Scholar]
- 51.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE (2002) Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229 [DOI] [PubMed] [Google Scholar]
- 52.Chikazoe J, Lee DH, Kriegeskorte N, Anderson AK (2014) Population coding of affect across stimuli, modalities and individuals. Nat Neurosci 17:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heller AS, Johnstone T, Peterson MJ et al. (2013) Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry 70:1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kambeitz J, Goerigk S, Gattaz W et al. (2020) Clinical patterns differentially predict response to transcranial direct current stimulation (tDCS) and escitalopram in major depression: a machine learning analysis of the ELECT-TDCS study. J Affect Disord 265:460. [DOI] [PubMed] [Google Scholar]
- 55.Wolkenstein L, Plewnia C (2012) Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol Psychiatr. 10.1016/j.biopsych.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 56.Vanderhasselt M-A, De Raedt R, Namur V et al. (2016) Emotional reactivity to valence-loaded stimuli are related to treatment response of neurocognitive therapy. J Affect Disord 190:443–449 [DOI] [PubMed] [Google Scholar]
- 57.Sanchez-Lopez A, Vanderhasselt M-A, Allaert J et al. (2018) Neurocognitive mechanisms behind emotional attention: Inverse effects of anodal tDCS over the left and right DLPFC on gaze disengagement from emotional faces. Cogn Affect Behav Neurosci 18:485–494 [DOI] [PubMed] [Google Scholar]
- 58.Ironside M, Browning M, Ansari TL et al. (2019) Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatr 76:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Lima AL, Braga FMA, da Costa RMM et al. (2019) Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J Affect Disord 259:31–37 [DOI] [PubMed] [Google Scholar]
- 60.D’Urso G, Dell’Osso B, Rossi R et al. (2017) Clinical predictors of acute response to transcranial direct current stimulation (tDCS) in major depression. J Affect Disord 219:25–30 [DOI] [PubMed] [Google Scholar]
- 61.Sanchez A, Vanderhasselt M-A, Baeken C, De Raedt R (2016) Effects of tDCS over the right DLPFC on attentional disengagement from positive and negative faces: an eye-tracking study. Cogn Affect Behav Neurosci 16:1027–1038 [DOI] [PubMed] [Google Scholar]
- 62.Ankri YLE, Braw Y, Luboshits G, Meiron O (2020) The effects of stress and transcranial direct current stimulation (tDCS) on working memory: a randomized controlled trial. Cogn Affect Behav Neurosci. 10.3758/s13415-019-00755-7 [DOI] [PubMed] [Google Scholar]
- 63.Brunoni AR, Vanderhasselt M-A, Boggio PS et al. (2013) Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology 38:58–66 [DOI] [PubMed] [Google Scholar]
- 64.da Valiengo L, Goerigk S, Gordon PC et al. (2019) Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatr. 10.1001/jamapsychiatry.2019.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valiengo LC, Goulart AC, de Oliveira JF et al. (2016) Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatr. 10.1136/jnnp-2016-314075 [DOI] [PubMed] [Google Scholar]
- 66.Opitz A, Falchier A, Yan C-G et al. (2016) Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep 6:31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Datta A, Krause MR, Pilly PK et al. (2016) On comparing in vivo intracranial recordings in non-human primates to predictions of optimized transcranial electrical stimulation. Conf Proc IEEE Eng Med Biol Soc 2016:1774–1777 [DOI] [PubMed] [Google Scholar]
- 68.Bikson M, Brunoni AR, Charvet LE et al. (2018) Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimul 11:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borrione et al. (2020) Precision non-implantable neuromodulation therapies: a perspective for the depressed brain. Brazil J Psychiatr. 10.1590/1516-4446-2019-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]


