Abstract
The overall goal of this research is to design novel amphiphilic biodegradable systems based on polyanhydrides for the stabilization and sustained release of peptides and proteins. Accordingly, copolymers of the anhydrides, 1,6-bis(p-carboxyphenoxy)hexane (CPH) and 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG), which are monomer-containing oligomeric ethylene glycol moieties, have been synthesized. Microspheres of different CPTEG:CPH compositions have been fabricated by two non-aqueous methods: solid/oil/oil double emulsion and cryogenic atomization. The ability of this amphiphilic polymeric system to stabilize model proteins (i.e., lysozyme and ovalbumin) was investigated. The structure of both the encapsulated as well as the released protein was monitored using gel electrophoresis, circular dichroism, and fluorescence spectroscopy. It was found that the CPTEG:CPH system preserves the structural hierarchy of the encapsulated proteins. Activity studies of the released protein indicate the CPTEG:CPH system retains the biological activity of the released protein. These results are promising for future in vivo studies, which involve the design of novel biodegradable polyanhydride carriers for the stabilization and sustained release of therapeutic peptides and proteins.
Keywords: Polyanhydrides, Degradation products, Microspheres, Lysozyme, Ovalbumin
1. Introduction
A recent study revealed that over a quarter of all new drugs approved are biopharmaceuticals with an annual global market surpassing $30 billion [1]. This group includes peptides and proteins intended for therapeutic treatment of a wide range of diseases, including reproductive disorders, blood-related diseases, hepatitis B and C, and cancer [2]. Nevertheless, the efficient delivery of these fragile molecules, which exhibit both physical and chemical instability leading to short in vivo half lives, remains a challenge.
The ideal protein carrier must protect the protein from the physiological environment and provide sustained release kinetics ranging from days to months depending on the application. Biodegradable polymeric microspheres have been used successfully in protein delivery [3]. Some of the characteristics of biodegradable carriers that can be manipulated to maintain protein stability include: water swelling, hydrophobicity, and chemical nature of degradation products [4].
Proteins must be stabilized during device preparation, storage, and administration. Previous research has demonstrated limitations in these stages that lead to protein inactivation. During device fabrication, the common methods employed for encapsulation expose the protein drug to aqueous/organic interfaces, which are known to be problematic for protein stability [5,6]. Protein migrates towards the aqueous dispersing phase and as much as 40% of protein loaded has been shown to be lost during fabrication [3,7]. New attempts to improve protein stability and maximize loading during microsphere fabrication circumvent this problem by using non-aqueous methods, such as solid/oil/oil (S/O/O) double emulsion and cryogenic atomization (CA) [8–11]. Besides promoting protein stability, these techniques increased the encapsulation efficiency, with efficiencies as high as 85% in S/O/O and ~100% in CA, as we reported recently [10].
During protein administration, the chemistry of the polymeric carrier must be carefully chosen so as to provide a gentle environment that will maintain the activity of the protein drug. Among biodegradable polymers, bulk-erodible polyesters (e.g., poly(lactic-co-glycolic acid) or PLGA) have been extensively investigated [12–18]. Deleterious processes occurring during protein release from these polymers have been reported including an acidic microenvironment and strong hydrophobic interactions [4,19,20]. As PLGA degrades, the water content increases, characteristic of bulk erodible systems, and the local acidic environment produced by accumulation of degradation products are significant sources for irreversible physical and chemical inactivation of polypeptides and proteins.
Another class of biodegradable polymers investigated for protein delivery is polyanhydrides, which differ from polyesters in their erosion mechanism. Polyanhydrides exhibit surface erosion, which may prevent covalent aggregation by reducing water penetration into the device [21–24]. However, these materials are hydrophobic and strong hydrophobic interactions between the polymer and the protein may lead to non-covalent aggregation.
A promising alternative for the polymers discussed above is the use of amphiphilic carriers for protein stabilization [25–28]. Correspondingly, we have designed a novel amphiphilic polyanhydride system based on copolymers of the anhydride monomers, 1,6-bis(p-carboxyphenoxy)hexane (CPH) and 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) (Fig. 1), which contains oligomeric ethylene glycol [29,30]. The incorporation of oligomeric ethylene glycol into the backbone of an aromatic polyanhydride creates the necessary hydrophilicity to create the amphiphilic environment needed for protein stabilization. Moreover, it is due to this amphiphilicity that the erosion mechanism of the CPTEG:CPH system can be tuned from bulk to surface by increasing the CPH content of the copolymer.
Fig. 1.
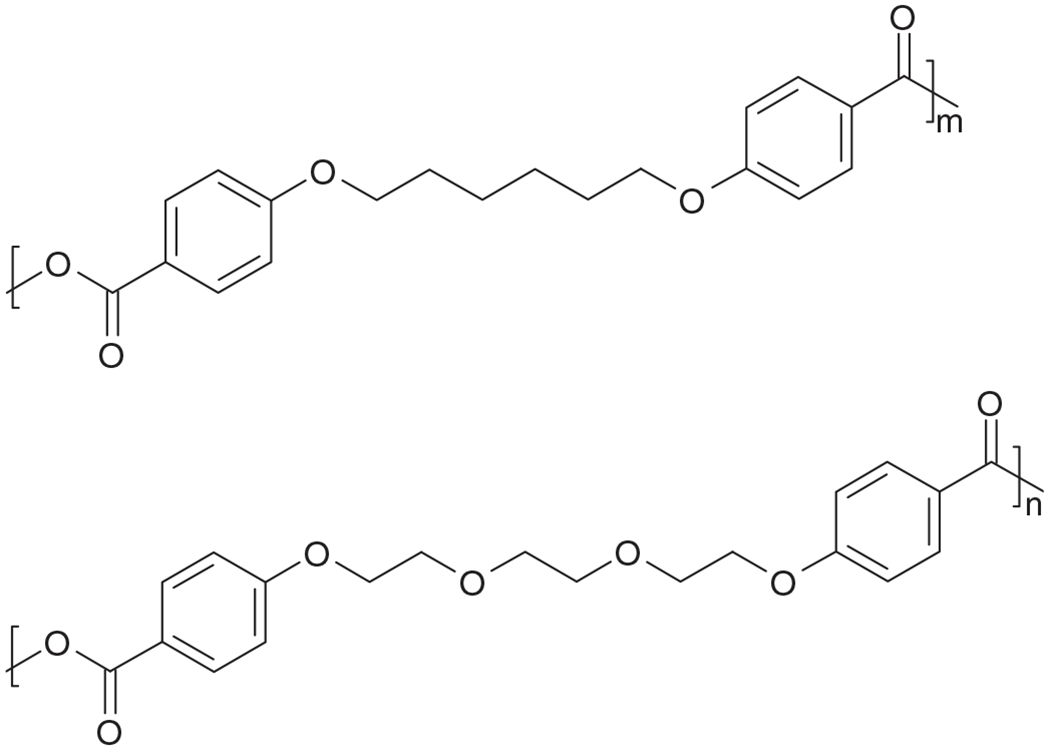
Chemical structures of poly(CPH) (top) and poly(CPTEG) (bottom).
This paper focuses on protein stabilization and sustained release from microspheres based on the CPTEG:CPH system. The proteins chosen for this study are hen egg white lysozyme (Lys) and ovalbumin (Ova). Lys is an acid-stabilized protein of 129 amino acids (14 kDa) that has been well studied, and the value of using it as a model protein for release relies on its similar size to therapeutic cytokines such as interferons and interleukins [17]. On the other hand, Ova has been well studied as a model antigen and is composed of 385 residues (48 kDa). It has a molten globular or intermediate unstable state in acidic surroundings, which is deleterious for its activity [31]. Two non-aqueous methods, S/O/O and CA, were used to fabricate the microspheres.
2. Materials and methods
2.1. Materials
The chemicals needed for monomer synthesis 4-p-hydroxybenzoic acid, 1,6-dibromohexane, 1-methyl-2-pyrrolidinone, and tri-ethylene glycol were purchased from Sigma Aldrich (St. Louis, MO); 4-p-fluorobenzonitrile was obtained from Apollo Scientific (Chesire, UK); potassium carbonate, dimethyl formamide, toluene, sulfuric acid, acetic acid, and acetonitrile were purchased from Fisher Scientific (Fairlawn, NJ). The chemicals needed for the polymerization, acetic anhydride, methylene chloride, and petroleum ether, were purchased from Fisher Scientific. Chicken egg white Ova, hen egg white Lys, monoclonal anti-chicken egg albumin (clone Ova-14), rabbit anti-chicken egg albumin, alkaline phosphatase conjugated goat anti-rabbit IgG, fetal calf serum, Coommasie R-250, Sigma 104 phosphatase substrate, p-nitrophenyl phosphatase liquid substrate system, and XTT in vitro toxicology assay kit were purchased from Sigma Aldrich. Ready precast gels (15% acrylamide) and protein molecular weight standards were purchased from Bio-Rad Laboratories (Hercules, CA). The enzymatic activity of Lys was determined with the EnzCheck® Lysozyme assay kit from Invitrogen (Carlsbad, CA). Bicinchoninic acid (BCA) protein assay kit and Slide-A-Lyzer dialysis cassettes (10,000 MW cut-off membrane) were obtained from Pierce Biotechnology Inc. (Rockford, IL).
2.2. Polymer synthesis and characterization
The CPH and CPTEG diacids were synthesized as previously described [29,32]. Subsequently, as we reported recently, CPTEG:CPH copolymers were synthesized by melt polycondensation of CPH and CPTEG diacids [29]. The purity and degree of polymerization of the polymers was analyzed using 1H NMR spectra obtained from a Varian VXR-300 MHz NMR spectrometer.
2.3. Protein incubation in monomer solutions
Saturated solutions of CPTEG and CPH diacids in deionized water and phosphate buffer (0.1 M, pH 7.4) were placed in the incubator for 2 days (37 °C, 100 rpm) and, subsequently, were filtered (0.22 μM). Lyophilized protein (final concentration 250 μg/mL) was added to the respective solutions (CPTEG, 50/50 CPTEG/CPH, CPH, phosphate buffer) and incubated for 1 week (37 °C, 100 rpm) prior to structural analysis of the protein. These studies were performed in triplicate and the protein structural analysis described below was done as recently described [33].
2.3.1. Primary structure
The amino acid sequence of each protein before and after incubation was studied with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. After centrifuging samples (10,000 rpm, 4 °C, 10 min), 10 μL of each sample was mixed with 10 μL of the reducing buffer (SDS (1% w/v), Tris–HCl (pH 6.8, 0.06 mM), glycerol (3mM), bromophenol blue (0.01% w/v), and β-mercaptoethanol (0.05% v/v)). The 20 μL solution was heated for 10 min at 96 °C, cooled to room temperature, and loaded into 15% acrylamide precast gel and run for 60 min at 130 V. Exactly 5 μL of prestained low-range protein standards were used for reference. After running the gels, these were stained with Coommasie Blue for 30 min and destained overnight.
2.3.2. Secondary structure
Far UV circular dichroism (CD) (190–250 nm) was used to monitor the protein secondary structure as recently described [33].
2.3.3. Tertiary structure
Fluorescence spectra characteristic of protein residues were used to monitor changes in tertiary structure after incubation in monomer solutions. The emission spectrum (300–500 nm) at an excitation wavelength of 280 nm was analyzed as reported [33].
2.3.4. Ovalbumin antigenicity
An enzyme-linked immunosorbent assay (ELISA) was used to study the antigenicity of Ova prior to and after incubation with monomer solution and the protocol followed is described elsewhere [33]. The epitope availability of incubated samples was obtained by normalizing with protein solutions prior to incubation with the CPTEG:CPH monomers and the antigenicity was reported as relative epitope availability. The assay was also performed for protein released from microspheres (see below).
2.3.5. Lysozyme enzymatic activity
The enzymatic activity of Lys after incubation with degradation products was evaluated with the EnzChek® Lysozyme Assay Kit. Lys hydrolyzes linkages of various cell walls of microorganisms. This assay measures the activity in Micrococcus lysodeikticus cell walls, which are previously labeled with fluorescein. The experimental protocol was followed as described by the manufacturer. Triplicates of each sample were averaged. Assays were also performed on samples after release from CPTEG:CPH microspheres (see below). The results were normalized by the activity measured initially (i.e., prior to incubation and release) and reported as fractional Lys activity.
2.4. Microsphere fabrication
2.4.1. Solid/oil/oil (S/O/O)
Prior to protein encapsulation, the protein was lyophilized as described before [10]. The S/O/O method was modified from previous reports [8–10]. Briefly, lyophilized protein (2–3 mg) was suspended in a solution of 100 mg of 20:80 CPTEG:CPH copolymer dissolved in 2 mL of methylene chloride to produce the first emulsion. The suspension was obtained by homogenizing the solution at 20,000 rpm for 3 min using a Tissue-Tearor™. The second emulsion was produced after adding a solution of Dow Corning oil 550 (3mL) saturated with methylene chloride (4mL). The mixture was then poured into 200 mL of heptane on ice bath and stirred for 2h at 300 rpm. Microspheres were collected by filtration and dried under vacuum overnight.
2.4.2. Cryogenic atomization (CA)
As we obtained higher encapsulation efficiencies with CA in previous experiments with unmodified polyanhydrides [10], we fabricated microspheres of both 20:80 and 10:90 CPTEG:CPH with this method. The procedure was modified from previously reported studies [10,11]. Lyophilized protein (2–3 mg) was suspended in a polymer solution of methylene chloride (7 mL for 20:80 CPTEG:CPH and 4mL for 10:90 CPTEG:CPH) at 10,000 rpm for 1 min using a Tissue-Tearor™. The solutions were placed on ice bath before pumping. The solution was then pumped with a syringe pump through an 8700–1200MS ultrasonic atomizing nozzle (Sono Tek Corporation, Milton, NY) into 200 mL of frozen ethanol overlaid with ~100mL of liquid nitrogen. The parameters used for these experiments were: 3mL/min and 1.5W for 20:80 CPTEG:CPH and 1.5mL/min and 2.5W for 10:90 CPTEG:CPH. This procedure was performed at 4 °C in order to maintain the temperature below the glass transition temperature of the polymer during pumping. After atomization, the resulting polymer/protein solution was stored at −80 °C for 3 days to allow the methylene chloride to be extracted. After the first 24 h, 200 mL of cold ethanol was added to the solution to prevent aggregation of microspheres. The solution was stirred at 300 rpm for 30 min on ice bath and placed in the freezer (−80 °C) for the remaining 2 days. The microspheres were then collected by filtration and dried under vacuum overnight.
The microsphere morphology was characterized by SEM. The particle size distribution was obtained from SEM images (250–500 ×) using a soft imaging system software (analySIS®, Soft Imaging System Corp, Lakewood, CO). An average of 250 particles per image was analyzed.
2.5. Protein release
Microspheres (15 mg) were placed in 1 mL of phosphate buffer (0.1 m, pH 7.4) and incubated at 37 °C and 100 rpm. Sodium azide (0.01% w/w) was added to the buffer to prevent microbial contamination [34]. At different time intervals, aliquots of 750 μL of supernatant were collected and replaced with fresh buffer. The aliquots were stored at 4 °C and were centrifuged (10,000 rpm for 10 min) prior to BCA analysis. Protein concentration was measured using the BCA analysis from the absorbance at 570 nm. At least two replicates of samples in each experiment were evaluated. The experiment was repeated twice, the values were averaged, and the standard deviation was determined.
2.5.1. Total protein encapsulated
After 1 month of release, the remaining microspheres were analyzed for residual protein content. Using a recently reported procedure [10], the microspheres were suspended in 1 mL of 17 mM of sodium hydroxide and sonicated (Sonics & Materials Inc., Newton, CT). The sample was withdrawn with a syringe, and the vials were washed twice with 1 mL of the same solvent to ensure no residual protein was lost. The 3 mL solution was transferred to a dialysis cassette (Slide-A-Lyzer® 10,000 MWCO, Pierce Biotechnology Inc.) using the same syringe. The cassettes were placed in 600 mL of 17 mM sodium hydroxide and incubated at 100 rpm and 40 °C for 1 week in order to accelerate the degradation of the polymer. This temperature was chosen as it is below the denaturation temperature of Lys (81 °C) and Ova (71 °C) [35]. After the incubation period, the protein was quantified by BCA analysis. The total protein encapsulated in the microspheres was determined by adding the protein released and the amount remaining in the microspheres. The cumulative release was normalized by this total amount and reported as fractional protein released.
3. Results
3.1. Protein stability in the presence of degradation products
3.1.1. Primary structure
The primary structure of Ova and Lys was analyzed with SDS-PAGE (Fig. 2). Samples prior to and after incubation were loaded into the gels and were compared to molecular weight standards. Non-lyophilized protein was also loaded to ensure that lyophilization process did not alter the primary structure of native protein. From Fig. 2, it can be seen that no structural change occurred in the proteins during lyophilization or incubation as no aggregation or hydrolysis was perceived in the gels. Saturated concentrations of both CPTEG and CPH diacids were not detrimental to the model proteins used in this study, and characteristic bands of Ova at 48 kDa and Lys at 14 kDa were unchanged after 1 week of incubation.
Fig. 2.
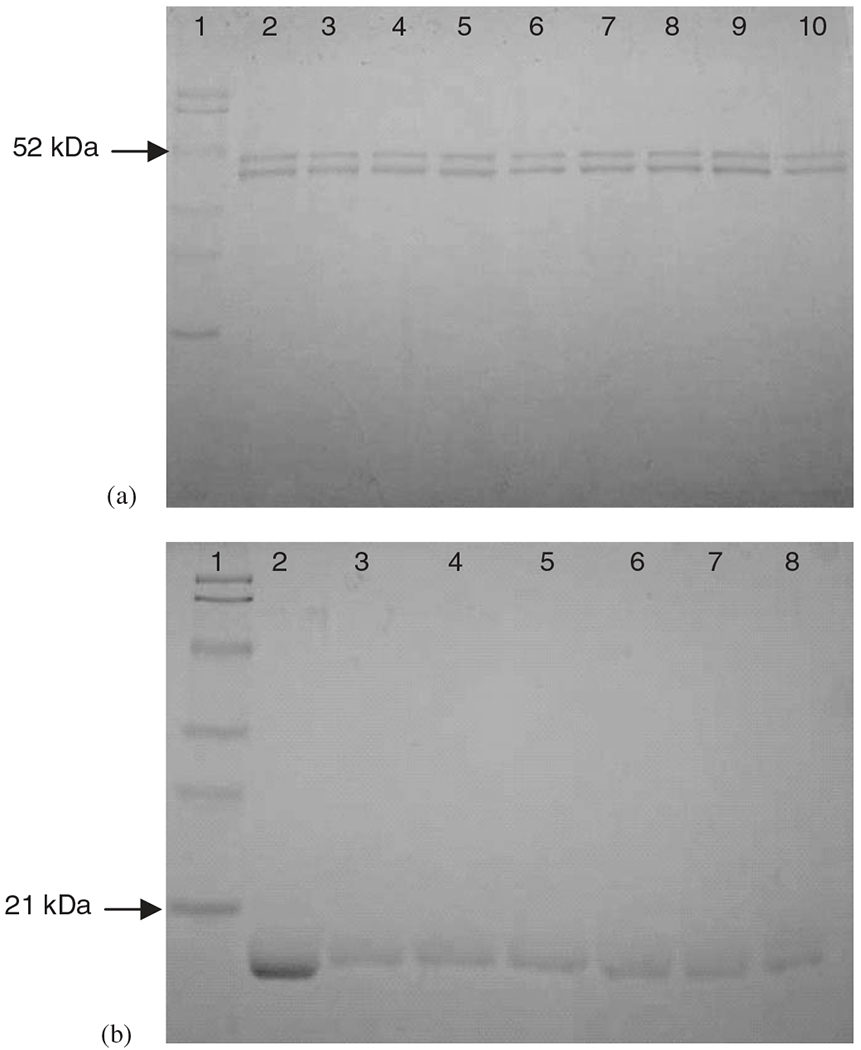
SDS-PAGE of Ova (a) and Lys (b). Lane 1: MW standard ladder; lane 2: non-lyophilized protein; lanes 3, 4: protein in CPTEG solution (day 0, 7); lanes 5, 6: protein in 50/50 CPTEG/CPH solution (day 0, 7); lanes 7, 8: protein in CPH solution (day 0, 7); lanes 9, 10 in gel (a) Ova in phosphate buffer (day 0, 7).
3.1.2. Secondary structure
CD was used to monitor the secondary structure of Lys and Ova during the incubation studies to estimate the type of secondary structure (α-helix vs. β sheet vs. coil) present in the proteins [36]. The CD spectra of Ova (Fig. 3) incubated in CPTEG and CPH diacid saturated solutions were identical at 0 and 7 days, showing two minima (208 and 222 nm) that are signatures of α-helices and α-helices + β-sheets. The secondary structure of Lys was also preserved and the characteristic minima were present in the spectra (data not shown).
Fig. 3.
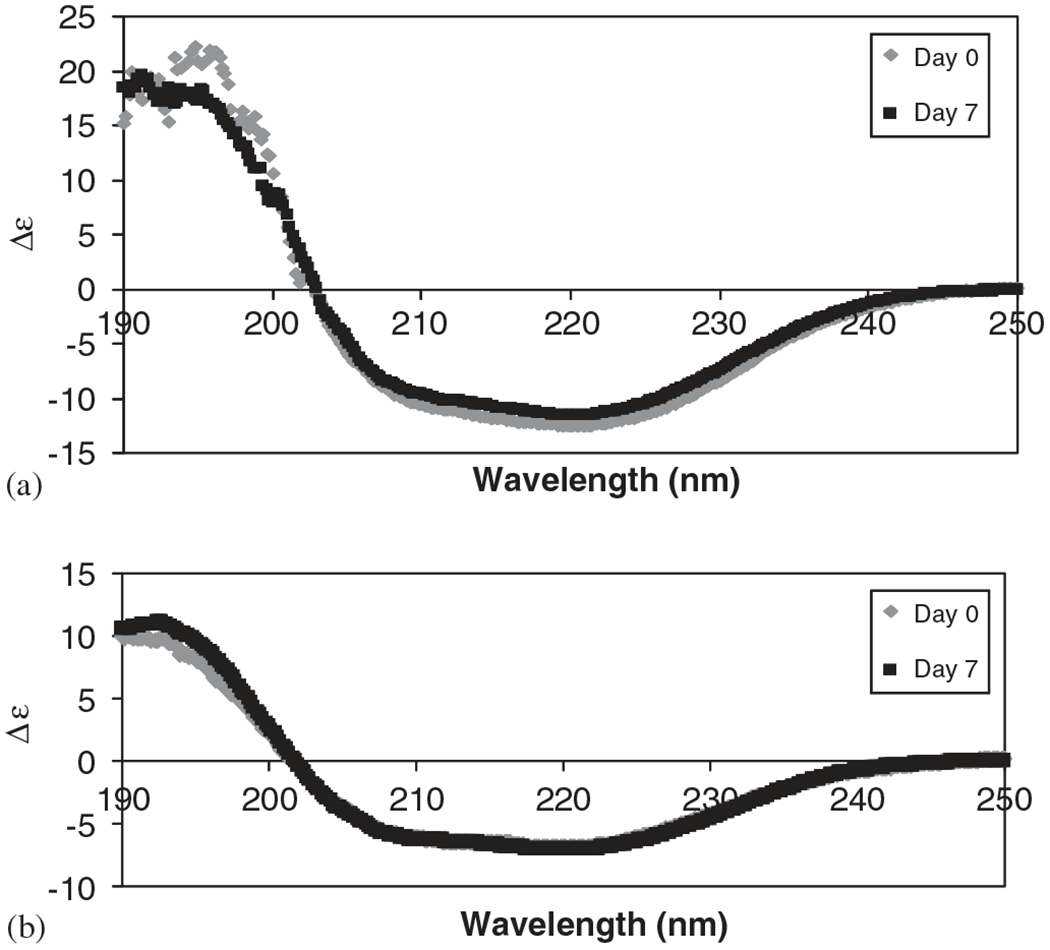
CD spectra of Ova incubated in (a) CPTEG saturated solution and (b) CPH saturated solution.
3.1.3. Tertiary structure
Proteins are usually biologically active only when folded in their native conformations, so understanding their 3D structures is key to understanding how they function [37]. The most common exception to two-state folding transitions is the occurrence of a stable, partially folded state, known as the molten globule [37]. The formation of a molten globule state may retain the secondary structure, as it is almost as compact as the fully folded protein, but the tertiary structure is disrupted/unfolded [38]. It has been reported that this molten globular state can be produced in Ova as a result of harsh environments [39]. On the other hand, Lys does not have a molten globule structure and unfolds globally by guanidine hydrochloride in the two-state type [37,40,41]. With this in mind, the fluorescence spectra were analyzed. Fig. 4 shows that in the fluorescence spectra of Ova incubated in CPTEG and CPH saturated solution, the maximum wavelength at days 0 and 7 was in the 336–340 nm range, and suggested that a molten globule state was not formed during the incubation period. Similarly, fluorescence spectra of Lys contained maximum wavelengths in the 343–345 nm range (data not shown). The emission spectrum at the wavelength range of 330–345 nm is characteristic of the tryptophan residues [42]. No loss of tertiary structure was detected, and it can be concluded that conformational stability of the two model proteins (Ova and Lys) was preserved in the presence of CPTEG and CPH monomers.
Fig. 4.
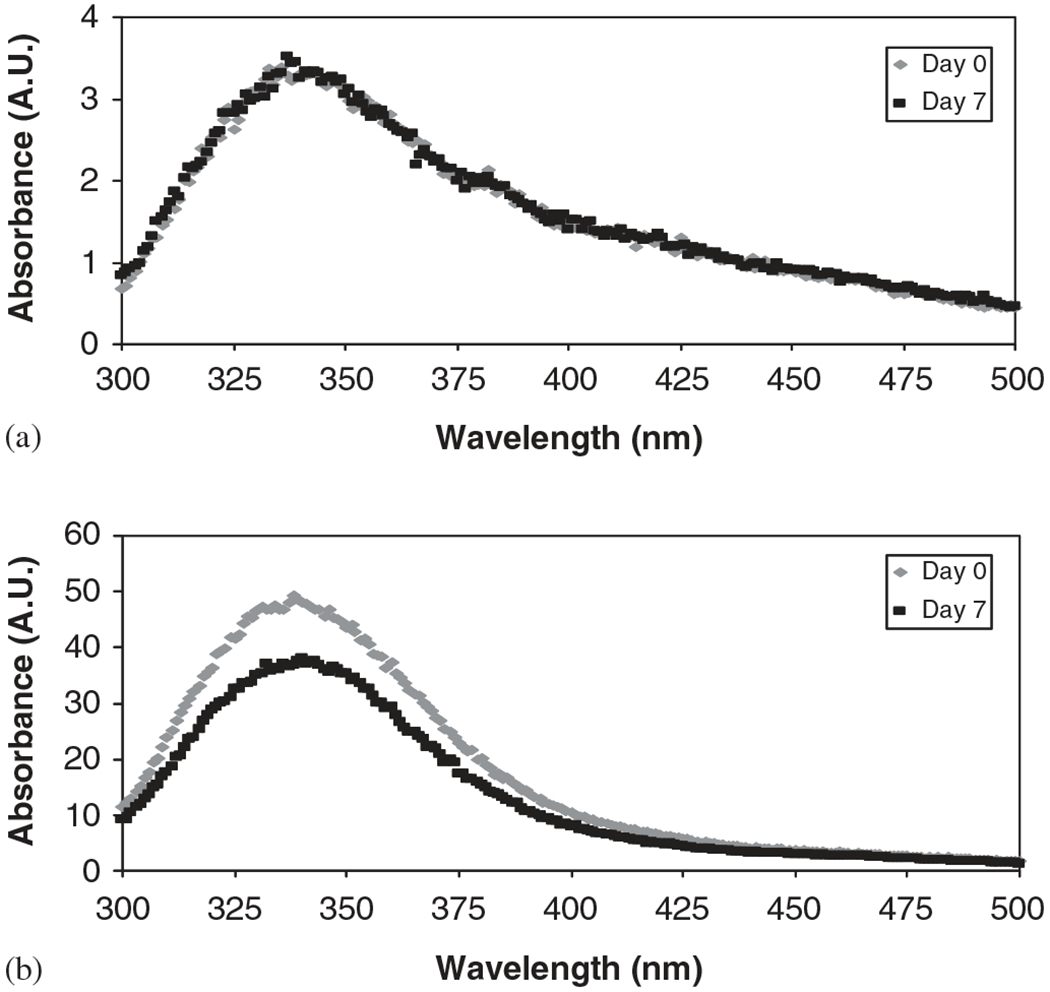
Fluorescence spectra of Ova incubated in (a) CPTEG saturated solution and (b) CPH saturated solution.
3.1.4. Protein activity
The activity after incubation of the model proteins with CPTEG:CPH degradation products was assessed by measuring the antigenicity of Ova and the enzymatic activity of Lys. The results of the ELISA performed in the Ova samples (Fig. 5a) demonstrate that neither CPTEG nor CPH diacid solutions caused a statistically significant change in the antigenicity of Ova after 7 days of incubation. More perturbations were caused when the protein was incubated in phosphate buffer alone, where an increase of ~50% after incubation suggests that protein exposed more epitopes to be recognized and quantified by the assay. The enzymatic activity of Lys was also measured (Fig. 5b), and as expected, it was maintained during the incubation period, with less than 10% loss in all the solutions analyzed. All these results support our hypothesis that the novel amphiphilic CPTEG:CPH system provides a conducive environment for protein stability.
Fig. 5.
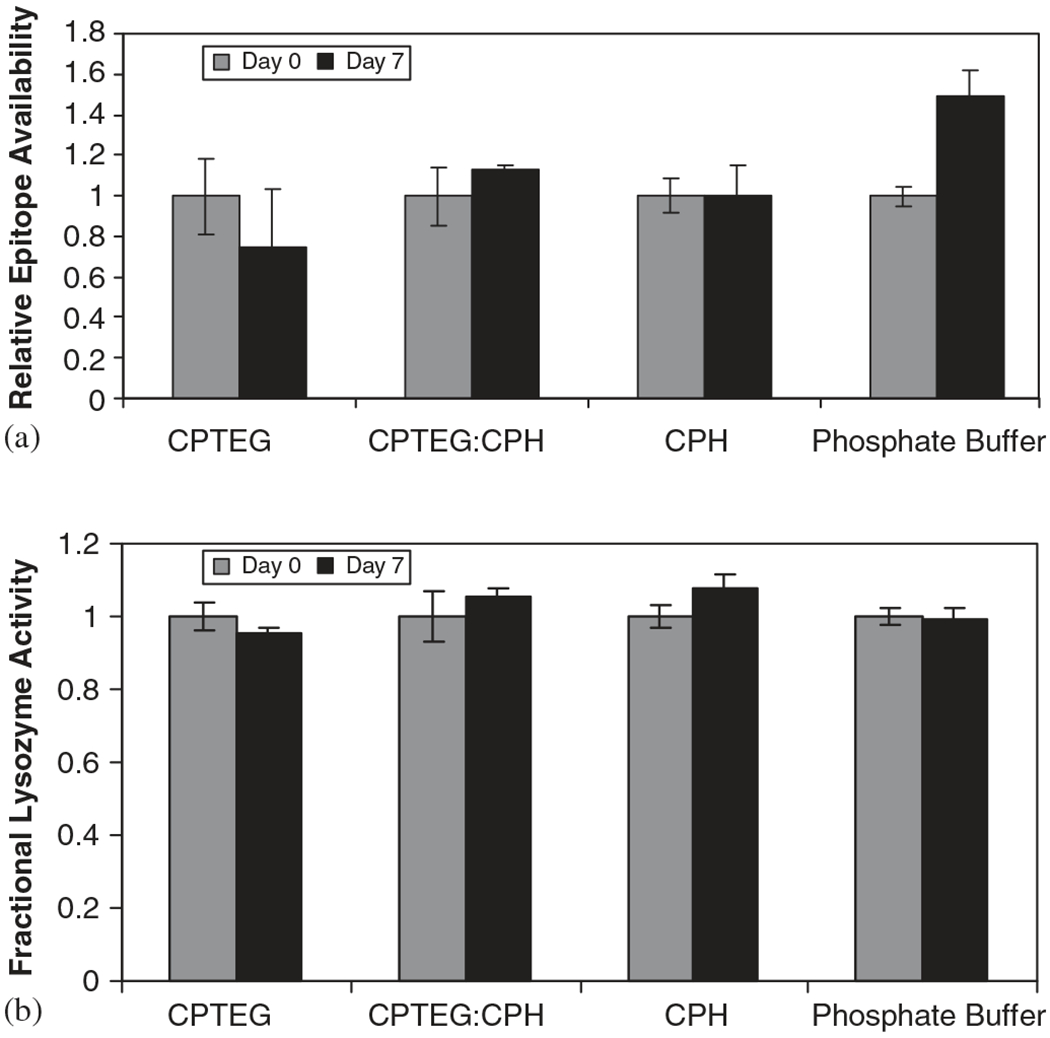
Protein activity after incubation with CPTEG:CPH degradation products. (a) Antigenicity of Ova and (b) enzymatic activity of Lys. Error bars indicate standard deviation of triplicate samples.
3.2. Microsphere fabrication
It is desirable to minimize drug particle size during encapsulation in order to minimize the burst effect [43]. As the size of the protein particles and stability of the dispersion are directly relevant for microsphere performance, the proteins (Ova, Lys) were lyophilized prior to their encapsulation into CPTEG:CPH microspheres.
Microspheres fabricated by S/O/O had a relatively smooth surface of the microspheres prior to and following protein encapsulation (images not shown). The typical size distribution of these microspheres was in the range of 4–60 μM, with the majority of the population in the 10–15 μM range, which is adequate for injectable formulations.
The second method studied was CA, which besides avoiding the deleterious effect of the water/organic interface, maximizes protein encapsulation [10]. Microspheres of 10:90 and 20:80 CPTEG:CPH were successfully fabricated and images of 10:90 CPTEG:CPH microspheres and particle size distribution are shown in Fig. 6. Similar microsphere surface structure was obtained for the 20:80 CPTEG:CPH copolymers, indicating that the slight change in hydrophobicity did not have any effect on the microsphere formation (images not shown). The difference in surface roughness when compared to S/O/O microspheres is due to the difference in solvent extraction rates during the fabrication process. The particle size from cryogenic microspheres resulted in unimodal distributions with diameters in the range of 2–16 μM.
Fig. 6.
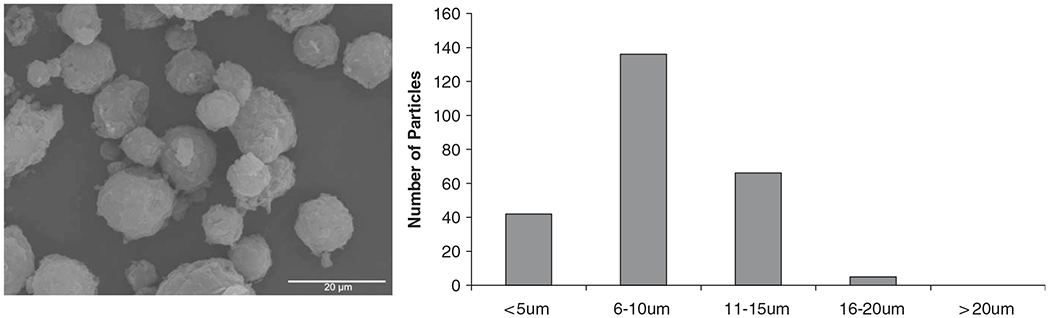
SEM image and particle size distribution of Ova-loaded 10:90 CPTEG:CPH microspheres fabricated by cryogenic atomization. Scale bar represents 20 μM.
3.3. Protein release
The release of Ova and Lys from CPTEG:CPH microspheres is shown in Fig. 7. The cumulative protein release was normalized with respect to the total protein encapsulated, which was determined as described before. On average, ~100% encapsulation efficiencies were observed for both proteins and fabrication methods studied, which is in agreement with previous work [10]. A sustained protein release and relatively low initial burst were achieved with all the CPTEG:CPH formulations, which is characteristic of amphiphilic systems where protein is more uniformly distributed. In addition, the initial bursts of both CA and S/O/O microspheres were comparable to recently published studies with polyanhydride microspheres composed of 20% CPH [10].
Fig. 7.
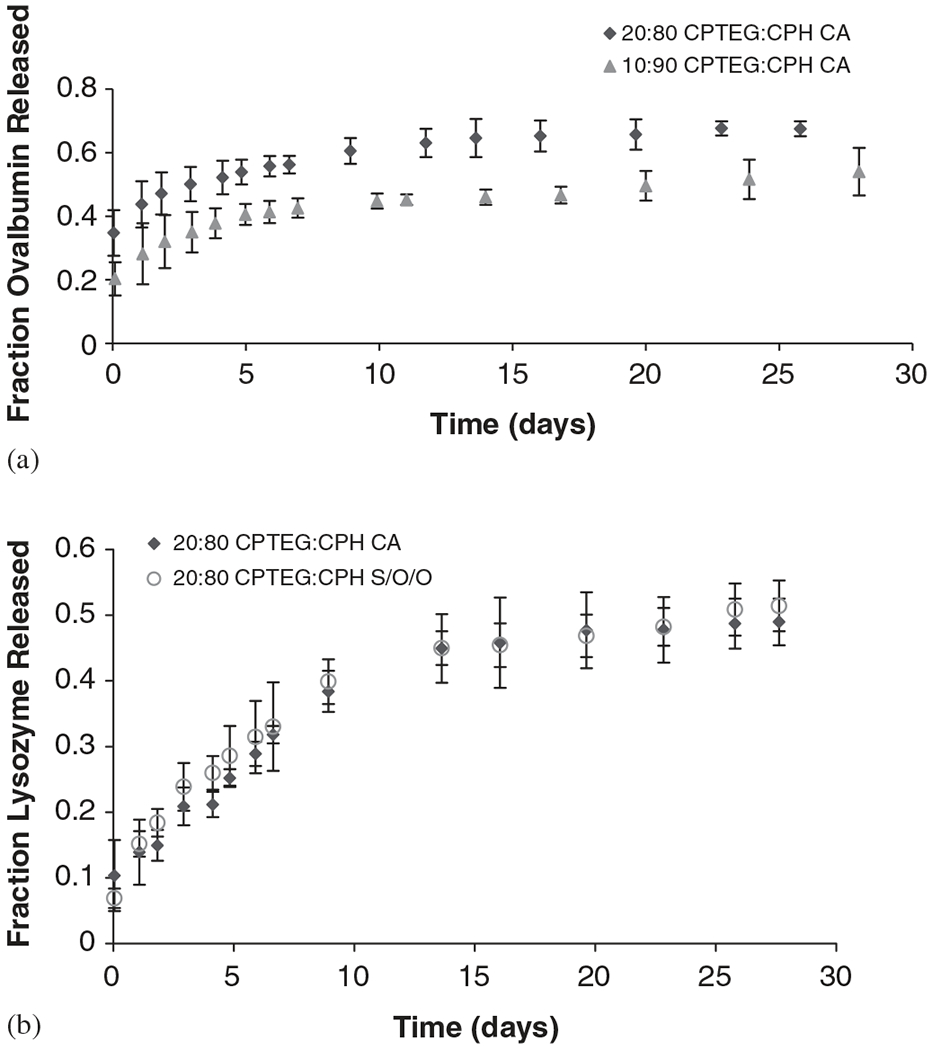
Protein released from CPTEG:CPH microspheres. (a) Ova released from 20:80 and 10:90 CA microspheres, (b) Lys released from 20:80 CA and S/O/O microspheres. Error bars represent standard deviation of triplicate samples.
Ova was released from both 20:80 and 10:90 CPTEG:CPH microspheres fabricated by CA to study the effect of copolymer composition on protein release (Fig. 7a). The polymer hydrophobicity had the expected effect on protein release, as protein released faster from 20:80 CPTEG:CPH (67% ) than from 10:90 CPTEG:CPH microspheres (54%) in 1 month.
The effect of microsphere fabrication method on protein release was analyzed from Lys release from 20:80 CPTEG:CPH microspheres fabricated by CA and S/O/O (Fig. 7b). As can be seen, there is excellent agreement between the two fabrication methods. During 1 month of release, 49% and 51% of the total Lys encapsulated were released from CA and S/O/O microspheres, respectively. It is important to note that bursts of less than 10% were observed in these microspheres, suggesting that Lys was homogenously distributed. These results, when compared to the Ova studies described above, demonstrate that protein characteristics influence their distribution and hence the subsequent release. Ova encapsulated in the same polymer formulation (i.e., 20:80 CPTEG:CPH CA microspheres) produced an initial burst of 35%, compared to the 10% obtained with Lys.
3.3.1. Protein activity after release
The activities of Ova and Lys were analyzed after release from the CPTEG:CPH microspheres similar to the methods used to analyze the proteins incubated in the presence of the degradation products. The antigenicity of Ova released from the CA microsphere formulations (i.e., 20:80 and 10:90 CPTEG:CPH) shown in Fig. 8a indicates that the released Ova did not lose significant antigenicity after being released from the two copolymer compositions. Similarly, the activity of Lys was essentially maintained in both 20:80 CPTEG:CPH CA and S/O/O microspheres formulations (Fig. 8b). No significant differences were found in the enzymatic activity of the protein when released from the CA microspheres. On the contrary, Lys activity released from the S/O/O formulation was statistically different (p<0.05). Possible causes for this difference among the microspheres are the processes involved during fabrication, where different solvents (i.e., methylene chloride, ethanol in CA, methylene chloride, silicon oil, heptane in S/O/O) were involved. The methylene chloride extraction rate in each method was different. In S/O/O, the protein-loaded microsphere formulations were subjected to more stress and organic phases than in CA. These studies suggest that minimizing protein instability during processing and maximizing protein encapsulation are desirable characteristics of the CA method, which make it suitable for protein delivery applications.
Fig. 8.
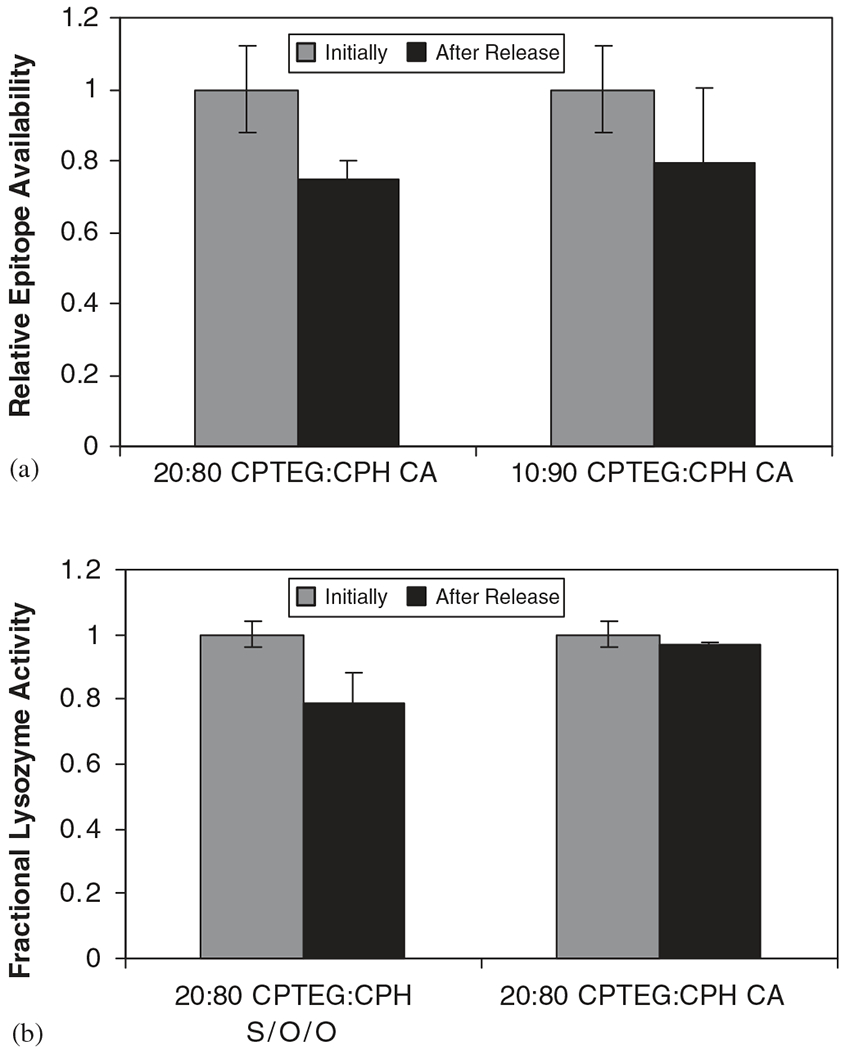
Protein activity after release from CPTEG:CPH microspheres. (a) Antigenicity of Ova after release from 20:80 and 10:90 CA microspheres, (b) enzymatic activity of Lys after release from 20:80 S/O/O and CA microspheres. Error bars indicate standard deviation of triplicate samples. * represents p-value<0.05 as determined by statistical test.
4. Discussion
Loss of the folded structure of proteins can be readily followed by observing changes in absorption spectra, CD, fluorescence spectra or in the dimensions of the protein, which generally increase upon denaturation. The interactions are so dependent upon each other that disruption of a very limited number of interactions tends to disrupt all of them [37]. Any structure present in unfolded proteins is local, however, and the global co-operative interactions characteristic of the native state are absent. Thus, it is important to verify that the native structure of the protein is preserved at all structural levels (i.e., primary, secondary, and tertiary structures). The stability of proteins in the presence of degradation products provides invaluable information by mimicking the microenvironment inside the polymeric device during release. After ensuring that no perturbations take place at the structural level, it is equally important to check if the protein maintained its activity. The results obtained in these studies indicate that the two model proteins studied maintained both their structure and activity in the presence of the CPTEG:CPH monomers.
When designing delivery systems for protein drugs, all the processes involved, from fabrication to delivery, which can alter the stability and hinder the therapeutic drug efficacy, must be considered. This paper demonstrates that during the three stages of device fabrication using amphiphilic polyanhydrides, the model proteins Ova and Lys were efficiently stabilized. The stability of the proteins in saturated concentrations of degradation products of CPTEG:CPH was unaltered at all the structural sublevels. In Table 1, the pH of the CPTEG and CPH diacid solutions used in these studies is compared to that of the degradation products of polyesters. It is interesting to note that an acidic microenvironment is produced by the ester monomers even below their saturation concentration. On the other hand, saturated solutions of CPTEG and CPH diacids have pH of 6.5 and 5.5, respectively. Thus, even at saturated monomer concentrations, the microenvironments of the CPTEG:CPH eroding device show little decrease in pH. Not surprisingly, the protein released from CPTEG:CPH microspheres was stable and the activity was essentially unaltered.
Table 1.
Effect of the dissolution of polymer degradation products on pH.
| Monomer Solution | Concentration (mm) | Type of monomer | pH |
|---|---|---|---|
| CPTEG | 9 (saturated) | Anhydride | 6.5 |
| CPHa | 1 (saturated) | Anhydride | 5.5 |
| LAa | 5 | Ester | 3.5 |
| GAa | 5 | Ester | 3.6 |
Data was obtained from Ref. [32].
The model proteins (Ova and Lys) differed in their chemical structure and function, and therefore their mechanisms of instability were dissimilar. The less acidic microenvironments (i.e., CPTEH:CPH) improved Ova stability than at acidic pH, where stronger complexation can result in protein aggregation [31,44]. On the other hand, precipitation of albumin is expected when the pH of the aqueous environment approaches the isoelectric point (~4.8). Under these conditions most proteins expose hydrophobic domains which are inherently attractive, a process that will likely occur in degrading environment of polyesters, but not in the CPTEG:CPH system [44]. In contrast, Lys is a monomeric globular protein that is acid stabilized [45], and severe methods such as disulfide scrambling are needed for its partial denaturation [40]. It is not surprising that the acidic environment of the polyester degradation products provide a stable environment for this protein [33].
5. Conclusions
This study demonstrates that the amphiphilic CPTEG:CPH system is a promising protein carrier. Our studies showed that the amphiphilic environment does not alter protein structure and provides a sustained release profile from microspheres. These results are promising for future in vivo studies, which involve the design of novel biodegradable polyanhydride carriers suitable for the stabilization and sustained release of different therapeutic peptides and proteins.
Acknowledgements
The authors thank Dr. Marit Nilsen-Hamilton for the use of the fluorescence spectrometer, and Dr. Tanya Prozorov, Sikander Hakim, Mary Byron, and Martin Gran for helping with the analysis and experiments. They also thank the Whitaker Foundation and the ISU Institute for Combinatorial Discovery for financial support. Maria Torres acknowledges support of the NSF-Iowa AGEP Fellowship.
References
- [1].Walsh G Biopharmaceutical benchmarks—2003. Nat Biotechnol 2003;21(8):865–70. [DOI] [PubMed] [Google Scholar]
- [2].Walsh G Pharmaceutical biotechnology products approved within the European Union. Eur J Pharm Biopharm 2003;55(1):3–10. [DOI] [PubMed] [Google Scholar]
- [3].Bilati U, Allemann E, Doelker E. Protein drugs entrapped within micro- & nanoparticles: an overview of therapeutic challenges & scientific issues. Drug Deliv Technol 2005;5(8):40–7. [Google Scholar]
- [4].Schwendeman SP, Costantino HR, Gupta RK, Langer R. Peptide, protein, and vaccine delivery from implantable polymeric systems. In: Park K, editor. Controlled drug delivery: challenges and strategies. Washington, DC: ACS; 1997. p. 229–67. [Google Scholar]
- [5].Perez C, Griebenow K. Effect of salts on lysozyme stability at the water–oil interface and upon encapsulation in poly(lactic-co-glycolic) acid microspheres. Biotechnol Bioeng 2003;82(7):825–32. [DOI] [PubMed] [Google Scholar]
- [6].Castellanos IJ, Crespo R, Griebenow K. Poly(ethylene glycol) as stabilizer and emulsifying agent: a novel stabilization approach preventing aggregation and inactivation of proteins upon encapsulation in bioerodible polyester microspheres. J Control Rel 2003;88:135–45. [DOI] [PubMed] [Google Scholar]
- [7].Van de Weert M, Hoechstetter J, Hennink WE, Crommelin DJA. The effect of a water/organic solvent interface on the structural stability of lysozyme. J Control Rel 2000;68:351–9. [DOI] [PubMed] [Google Scholar]
- [8].Carrasquillo KG, Stanley AM, Aponte-Carro JC, De Jesus P, Costantino HR, Bosques CJ, et al. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J Control Rel 2001;76(3):199–208. [DOI] [PubMed] [Google Scholar]
- [9].Carrasquillo KG, Carro JC, Alejandro A, Toro DD, Griebenow K. Reduction of structural perturbations in bovine serum albumin by non-aqueous microencapsulation. J Pharm Pharmacol 2001;53(1): 115–20. [DOI] [PubMed] [Google Scholar]
- [10].Determan A, Graham J, Pfeiffer K, Narasimhan B. The role of microsphere fabrication methods on the stability and release kinetics of ovalbumin encapsulated in polyanhydride microspheres. J Microencapsul 2006; in press. [DOI] [PubMed] [Google Scholar]
- [11].Lam XM, Duenas ET, Daugherty AL, Levin N, Cleland JL. Sustained release of recombinant insulin-like growth factor-I for treatment of diabetes. J Control Rel 2000;67:281–92. [DOI] [PubMed] [Google Scholar]
- [12].Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Del Rev 1997;28(1):5–24. [DOI] [PubMed] [Google Scholar]
- [13].Bilati U, Allemann E, Doelker E. Poly(D,L-lactide-co-glycolide) protein-loaded nanoparticles prepared by the double emulsion method—processing and formulation issues for enhanced entrapment efficiency. J Microencapsul 2005;22(2):205–14. [DOI] [PubMed] [Google Scholar]
- [14].Castellanos IJ, Carrasquillo KG, Lopez JD, Alvarez M, Griebenow K. Encapsulation of bovine serum albumin in poly(lactide-co-glycolide) microspheres by the solid-in-oil-in-water technique. J Pharm Pharmacol 2001;53(2):167–78. [DOI] [PubMed] [Google Scholar]
- [15].Ibrahim MA, Ismail A, Fetouh MI, Gopferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. J Control Rel 2005;106(3):241–52. [DOI] [PubMed] [Google Scholar]
- [16].Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev: Trends Particulate Antigen DNA Deliv Systems Vaccines 2005;57(3):391–410. [DOI] [PubMed] [Google Scholar]
- [17].Kang F, Jiang G, Hinderliter A, DeLuca PP, Singh J. Lysozyme stability in primary emulsion for PLGA microsphere preparation: effect of recovery methods and stabilizing excipients. Pharm Res 2002;19(5):629–33. [DOI] [PubMed] [Google Scholar]
- [18].O’Hagan DT, Singh M, Gupta RK. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Deliv Rev 1998;32(3):225–46. [PubMed] [Google Scholar]
- [19].Shenderova A, Burke TG, Schwendeman SP. The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm Res 1999;16(2):241–8. [DOI] [PubMed] [Google Scholar]
- [20].Wade LG. Organic chemistry. 3rd ed. New Jersey: Prentice-Hall; 1995. [Google Scholar]
- [21].Tabata Y, Gutta S, Langer R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharm Res 1993;10(4):487–96. [DOI] [PubMed] [Google Scholar]
- [22].Goepferich A, Langer R. The influence of microstructure and monomer properties on the erosion mechanism of a class of polyanhydrides. J Polym Sci, A Polym Chem 1993;31:2445–58. [Google Scholar]
- [23].Ron E, Turek T, Mathiowitz E, Chasin M, Hageman M, Langer R. Controlled release of polypeptides from polyanhydrides. Proc Natl Acad Sci USA 1993;90:4176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Determan AS, Trewyn BG, Lin VS, Nilsen-Hamilton M, Narasimhan B. Encapsulation, stabilization, and release of BSA-FITC from polyanhydride microspheres. J Control Rel 2004;100(1):97–109. [DOI] [PubMed] [Google Scholar]
- [25].Bezemer JM, Radersma R, Grijpma DW, Dijkstra PJ, Van Blitterswijk, Feijen J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers 2. Modulation of release rate. J Control Rel 2000;67:249–60. [DOI] [PubMed] [Google Scholar]
- [26].Mader K, Bittner B, Li Y, Wohlauf W, Kissel T. Monitoring microviscosity and microacidity of the albumin microenvironment inside degrading microparticles from poly(lactide-co-glycolide) (PLG) or ABA-triblock polymers containing hydrophobic poly(lactide-co-glycolide) A blocks and hydrophilic poly(ethyleneoxide) B blocks. Pharm Res 1998;15(6):787–93. [DOI] [PubMed] [Google Scholar]
- [27].Morita T, Horikiri Y, Suzuki T, Yoshino H. Applicability of various amphiphilic polymers to the modification of protein release kinetics from biodegradable reservoir-type microspheres. Eur J Pharm Biopharm 2001;51:45–53. [DOI] [PubMed] [Google Scholar]
- [28].Quaglia F, Ostacolo L, Nese G, De Rosa G, La Rotonda MI, Palumbo R, et al. Microspheres made of poly(ε-caprolactone)-based amphiphilic copolymers: potential in sustained delivery of proteins. Macromol Biosci 2005;5:945–54. [DOI] [PubMed] [Google Scholar]
- [29].Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J Biomed Mater Res 2006;76A(1):102–10. [DOI] [PubMed] [Google Scholar]
- [30].Vogel BM, Mallapragada SK. Synthesis of novel biodegradable polyanhydrides containing aromatic and glycol functionality for tailoring of hydrophilicity in controlled drug delivery devices. Biomaterials 2005;26(7):721–8. [DOI] [PubMed] [Google Scholar]
- [31].Galazka VB, Dickinson E, Ledward DA. Emulsifying properties of ovalbumin in mixtures with sulphated polysaccharides: effects of pH, ionic strength, heat and high-pressure treatment. J Sci Food Agric 2000;80:1219–29. [Google Scholar]
- [32].Conix A Poly[1,3-bis(p-carboxyphenoxy)-propane anhydride]. Macro Synth 1966:95–8. [Google Scholar]
- [33].Determan AS, Wilson JH, Kipper MJ, Wannemuehler MJ, Narasimhan B. Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials 2006;27(17):3312–20. [DOI] [PubMed] [Google Scholar]
- [34].Thomas PA, Padmaja T, Kulkarni MG. Polyanhydride blend microspheres: novel carriers for the controlled release of macromolecular drugs. J Control Rel 1997;43:273–81. [Google Scholar]
- [35].Johnson TM, Zabik ME. Gelation properties of albumen proteins, singly and in combination. Poultry Sci 1981;60:2071–83. [Google Scholar]
- [36].Klemba MW, Munson M, Regan L. De novo design of protein structure and function. In: Angeletti RH, editor. Proteins analysis and design. New York: Academic Press; 1998. p. 3139–320. [Google Scholar]
- [37].Darby NJ, Creighton TE. Protein structure. Heidelberg, Germany: IRL Press; 1993. [Google Scholar]
- [38].Bychkova VE, Ptitsyn OB. The molten globule in vitro and in vivo. Chemtracts Biochem Mol Biol 1993;4:133–63. [Google Scholar]
- [39].Koseki T, Kitabatake N, Doi E. Conformational changes in ovalbumin at acid pH. J Biochem (Tokyo) 1988;103(3):425—30. [DOI] [PubMed] [Google Scholar]
- [40].Chang JY, Li L. The unfolding mechanism and the disulfide structures of denatured lysozyme. FEBS Lett 2002:73–8. [DOI] [PubMed] [Google Scholar]
- [41].Sugai S, Kuwajima K, Nitta K. Characterization of the folding intermediates of globular proteins. In: Masahiro H, editor. Protein structural analysis, folding and design. Sendai, Japan: Japan Scientific Societies Press Elsevier; 1990. p. 53–5. [Google Scholar]
- [42].Zemser M, Friedman M, Katzhendler J, Greene LL, Minsky A, Gorinstein S. Relationship between functional properties and structure of ovalbumin. J Protein Chem 1994;13(2):261–74. [DOI] [PubMed] [Google Scholar]
- [43].Costantino HR, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo KG, et al. Protein spray-freeze drying. effect of atomization conditions on particle size and stability. Pharm Res 2000;17(11):1374–83. [DOI] [PubMed] [Google Scholar]
- [44].Coombes AGA, Breeze V, Lin W, Gray T, Parker KG, Parker T. Lactic acid-stabilised albumin for microsphere formulation and biomedical coatings. Biomaterials 2001;22:1–8. [DOI] [PubMed] [Google Scholar]
- [45].Jiang G, Woo BH, Kang F, Singh J, DeLuca PP. Assesment of protein release kinetics, stability and protein polymer interaction of lysozyme encapsulated poly(D,L-lactide-co-glycolide) microspheres. J Control Rel 2002;79:137–45. [DOI] [PubMed] [Google Scholar]


