Abstract
Background
Leukocyte activation by anti-neutrophil cytoplasmic antibody (ANCA) and the subsequent leukocyte–endothelium interaction play a key role in the development of endothelial damage in ANCA-associated vasculitis (AAV). In contrast to that of leukocyte activation, the exact role of the leukocyte–endothelium interaction via integrin remains unclear. Here, we performed microarray and validation analyses to explore association between the expression levels of lymphocyte function-associated antigen-1 (LFA-1) and the clinical characteristics of patients with AAV.
Methods
We performed gene set enrichment analysis (GSEA) to identify the functional gene sets differentially expressed between patients with AAV and other types of vasculitis and the healthy controls (HCs). Flow cytometry was performed to validate the GSEA results. Treatment-naïve patients were monitored until 24 weeks of treatment. To examine the role of LFA-1 in the neutrophil–endothelium interaction, we performed a leukocyte adhesion and transmigration assay using peripheral blood and human umbilical vein endothelial cells (HUVECs).
Results
GSEA revealed that the molecular pathways involving integrin-related genes were significantly upregulated in patients with AAV compared to that in patients with other types of vasculitis and the HCs. Flow cytometry revealed that the percentage of neutrophils expressing LFA-1 was significantly higher in patients with AAV than in those with large-vessel vasculitis or polyarteritis nodosa and the HCs. LFA-1 levels in the neutrophils were higher in patients with MPO-ANCA-positive expression than in those with a positive PR3-ANCA expression and correlated with the peripheral eosinophil count, serum rheumatoid factor titre, serum C-reactive protein levels, and the vasculitis activity score of systemic and chest components. After 24 weeks of treatment, including prednisolone, cyclophosphamide, rituximab, azathioprine, methotrexate, and/or tacrolimus, neutrophil LFA-1 expression remained high in the non-responder patients, but decreased in the responder patients. The in vitro assay showed that leukocyte migration toward HUVECs was dependent on the interaction between LFA-1 and intercellular adhesion molecule-1 (ICAM1); the migration of leukocytes was inhibited by blocking the adhesion of LFA-1 to ICAM1.
Conclusions
The expression of LFA-1 in neutrophils is increased in patients with AAV. Neutrophil LFA-1 levels correlate with the clinical features of AAV. Inhibiting the adhesion of LFA-1 and ICAM1 impedes the neutrophil–endothelium interaction and may have a therapeutic role in AAV.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13075-021-02510-1.
Keywords: ANCA-associated vasculitis, Gene expression, β2-integrin, Lymphocyte function-associated antigen-1
Background
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is an autoimmune disease that affects small- to medium-sized blood vessels and includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [1–3]. Neutrophils in the peripheral blood are elevated and play a central role in the pathogenesis of AAV [4–7]. Although the phenotypes and functional changes in the endothelial cells of AAV patients are not fully understood, an elevated proportion of circulating endothelial cells is associated with disease activity in AAV [8–10]. While vasculitis is associated with abnormalities in circulating immune cells and vascular endothelial cells [11–13], the exact role of the neutrophil–endothelium interaction in AAV remains unclear.
Activated leukocytes in the peripheral blood are known to display altered levels of surface molecules. For example, CD11b levels in neutrophils and monocytes are elevated, while CD62L levels in monocytes are decreased in patients with GPA [14–18]. ANCA can induce neutrophil activation via interaction with the αM (CD11b) and β2 (CD18) subunits of integrin Mac-1 [14, 15]. Granules from activated neutrophils and neutrophil extracellular traps stimulate endothelial reactive species and induce endothelial damage [19].
The leukocyte–endothelium interaction is essential for governing the movement of leukocytes toward the site of inflammation and regulating leukocyte recruitment. Lymphocyte function-associated antigen-1 (LFA-1) is an αL (CD11a) and β2 (CD18) integrin subunit, which interacts with intercellular adhesion molecule-1/2 (ICAM1/2) on endothelial cells [20–22]. We previously reported that LFA-1 is upregulated in patients with systemic lupus erythematosus with vasculitis [23]. However, the role of LFA-1 in AAV is not well understood.
AAV is primarily managed with cyclophosphamide- or rituximab-based treatments; however, close to 50% of patients experience disease relapses [24–26], and a therapeutic drug that can target neutrophil activation has not yet been developed. Considering the fact that the neutrophil–endothelium interaction is an essential process [27, 28], integrin-mediated neutrophil–endothelium adhesion may act as a promising therapeutic target in AAV.
In this study, we performed a microarray analysis and validation to identify the key molecules involved in integrin-mediated cell adhesion occurring in AAV. Subsequently, we identified LFA-1 and explored its role in AAV.
Patients and methods
Patients and healthy controls
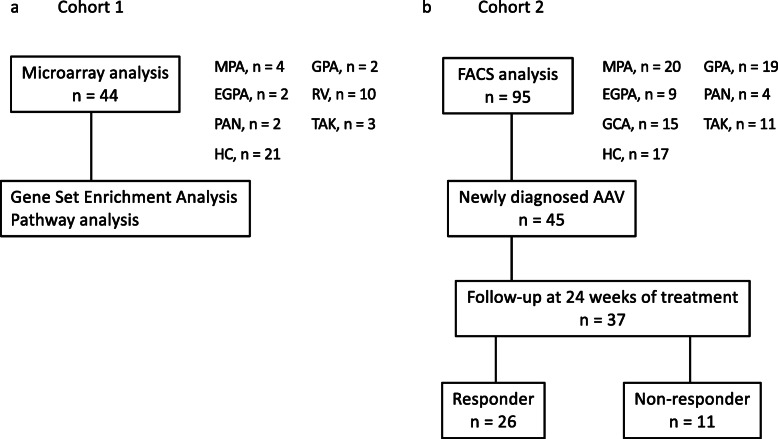
To explore the key molecule associated with the clinical characteristics of patients with AAV, we first conducted a microarray analysis and analysed the data using the Gene Set Enrichment Analysis (GSEA) and a pathway analysis (Fig. 1a). Following this, a fluorescence-activated cell sorting (FACS) analysis was performed to validate the microarray results (Fig. 1b). Whole blood samples were collected from patients at the Keio University Hospital, between April 2008 and January 2021. Patients with MPA (n = 4), GPA (n = 2), EGPA (n = 2), rheumatoid vasculitis (RV; n = 10) [29], polyarteritis nodosa (PAN; n = 2) [30], and Takayasu arteritis (TAK; n = 3) [31], who met the respective international classification criteria, and 21 healthy controls (HCs) were enrolled in the microarray analysis. Additionally, active patients with AAV (total number = 48; MPA, n = 20; GPA, n = 19; EGPA, n = 9), LVV (total number = 26; TAK, n = 11; giant cell arteritis [32], n = 15), PAN (n = 4), and HCs (n = 17) were enrolled in the validation analysis. Patients with GPA were further compared between generalised (n = 15) and localised (n = 4) forms, as previously categorised [33]. We confirmed that the HCs did not report a history of any autoimmune disease, severe allergic disorder, malignancy, or infection.
Fig. 1.
Study design and analytical strategy. a Microarray analysis and b fluorescence-activated cell sorting (FACS) analysis for validation. AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; RV, rheumatoid vasculitis; PAN, polyarteritis nodosa; TAK, Takayasu arteritis; HC, healthy control
This study was approved by the Institutional Review Board of Keio University School of Medicine (#20140335) and was conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all participating individuals.
Clinical assessment
Clinical information was obtained from patient records. Disease activity in vasculitis patients was recorded using the Birmingham Vasculitis Activity Score (BVAS) 2003, along with clinical signs [34]. Evidence of organ involvement (mucous membranes/eyes, ear, nose, throat, chest, kidney, and nervous system) and laboratory data, including the erythrocyte sedimentation rate, white blood cell count, haemoglobin level, platelet count, C-reactive protein (CRP) level, IgG level, ANCA positivity, ANCA titre, and rheumatoid factor (RF) levels, were assessed. Remission was defined as the absence of clinical signs in the disease activity, as indicated by a BVAS of 0 maintained for at least 2 months [26]. Patients who were treatment-naïve at the time of inclusion received treatment, including prednisolone, cyclophosphamide, rituximab, azathioprine, methotrexate, and/or tacrolimus, during the 24 weeks of follow-up evaluations. These patients were categorised into responder and non-responder groups according to whether they were in remission at 24 weeks of treatment.
RNA extraction
Blood samples were collected in the PAXgene blood RNA tubes (PreAnalytiX, Hombrechtikon, Switzerland). Total RNA was extracted using the PAXgene blood RNA kit (PreAnalytiX) according to the manufacturer’s instructions. Total RNA quantity and quality were determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), respectively. All RNA samples met the following criteria: RNA integrity > 7 and optical density at 260/280 nm > 1.6.
Microarray experiment
Cyanine 3-labelled complementary RNAs (cRNAs) were synthesised using the QuickAmp Labelling Kit (Agilent Technologies). The cRNAs were hybridised to the whole human genome at 65 °C for 17 h using Microarray 8 × 60 K v 2.0 (Agilent Technologies). After washing, the microarrays were scanned using an Agilent DNA microarray scanner (Agilent Technologies). The intensity value for each scanned feature was extracted using the Agilent Feature Extraction software (Agilent Technologies).
GSEA and pathway analysis
We performed GSEA with v 2.1.0 (http://www.broadinstitute.org/gsea/index.jsp) using GeneSpring GX v 11.0.2 (Agilent technologies) to identify the functional gene sets that were differentially expressed among patients with AAV, disease controls, and HCs. Fold change (FC) was calculated to determine whether a set of genes showed statistically significant, concordant differences between two biological states. A two-sided unpaired Welch’s t test was performed for each pair of comparison groups, and adjusted P values were calculated using the Benjamini and Hochberg correction. Statistically significant changes in proteins or metabolites were selected using an adjusted P value < 0.05 and absolute FC > 1.3. Pathway analysis was performed using MAPPFinder (GenMAPP, www.genmapp.org).
FACS analysis
We investigated the surface expression of the α- and β-integrin subunits on neutrophils, monocytes, and lymphocytes using FACS analysis. FACS analysis of the cells present in 20 μL of heparinised blood samples was performed. For gating purposes, the neutrophil population was verified by staining with an anti-CD16b-PE antibody (Clone CLB-gran11.5; BD Biosciences, San Jose, CA, USA). Monocytes and lymphocytes were gated using forward-and side-scatter. The antibodies used to probe surface molecules included anti-CD11a/CD18-Fluor488 (Clone M24; BioLegend, San Diego, CA, USA), anti-CD11a-PE-Cy7 (Clone HI111; BioLegend), anti-CD11b-FITC (Clone M1/70; BioLegend), anti-CD11c-VioBlue (Clone 4.9; BioLegend), and anti-CD18-APC (Clone TS1/18; BioLegend). Isotype-matched control IgGs for the corresponding antibodies were used as negative controls.
The fluorescence-activated cell sorting (FACS) analysis was conducted on an MACS Quant Analyser (Miltenyi Biotec, Auburn, CA, USA) using FlowJo v 10.1. (Tree Star, Ashland, OR, USA). Details of the gating strategy are provided in Supplementary Fig. 1. We collected clinical data and peripheral blood samples from patients with AAV until 24 weeks of treatment.
In vitro leukocyte adhesion and transmigration assay
To examine the function of LFA-1 in the leukocyte–endothelium interaction, we performed a leukocyte adhesion and transmigration assay using human umbilical vein endothelial cells (HUVECs) and peripheral leukocytes from HCs, as previously described [35]. HUVECs were grown in EGM™-2 (Lonza, Basel, Switzerland) to 70–80% confluence and then treated with 10 ng/mL tumour necrosis factor (TNF)-α (PeproTech, Rocky Hill, NJ, USA) for 24 h to induce ICAM expression. Peripheral blood was used after haemolysis using HetaSep (Veritas, Tokyo, Japan). Subsequently, the Leuko Tracker solution (Cell Biolabs, San Diego, CA, USA) and 100 ng/mL of lipopolysaccharide (LPS)-pre-treated peripheral blood (1 × 105 cells/well) were loaded into the upper chamber of Transwell inserts (3.0 μm pore size, 24-well plates; Corning, NY, USA). Recombinant interleukin (IL)-8 (10 ng/mL, PeproTech) and N-formyl-met-leu-phe (fMLP) (100 nM, Sigma-Aldrich, St Louis, MO, USA) were added to the lower compartment of each well as chemoattractants. Transmigrated cells were collected after co-incubation of leukocytes and HUVECs for 2 h and quantified by measuring the fluorescence intensity of Leuko Tracker solution-labelled leukocytes in the culture medium. The leukocyte transmigration assay was also conducted after treatment with 10 μg/mL of neutralising anti-LFA-1 antibody (Clone hu1124; Novus Biologicals, Littleton, CO, USA), anti-ICAM1 antibody (Clone 1A29; Thermo Fisher Scientific), and isotype-matched control IgG. The experiments were replicated in six healthy subjects.
Statistical analysis
Continuous data are expressed as median and interquartile range (IQR), and categorical data are expressed as numbers and percentages. The Mann-Whitney U test was used to examine the differences between two groups, and the chi-squared test was used for nominal variables. Wilcoxon’s signed-rank test was used to compare paired samples. The spearman’s rank correlation coefficient was used for correlation analysis. Statistical significance was set at P < 0.05. All analyses were performed using the R statistics package (v 3.6.1; The R Foundation for Statistical Computing, Vienna, Austria), SPSS Statistics v 26.0 (IBM Corp., Armonk, NY, USA), and GraphPad Prism v 8.0 (GraphPad, La Jola, CA, USA).
Results
Identification of characteristic molecular profile for patients with AAV
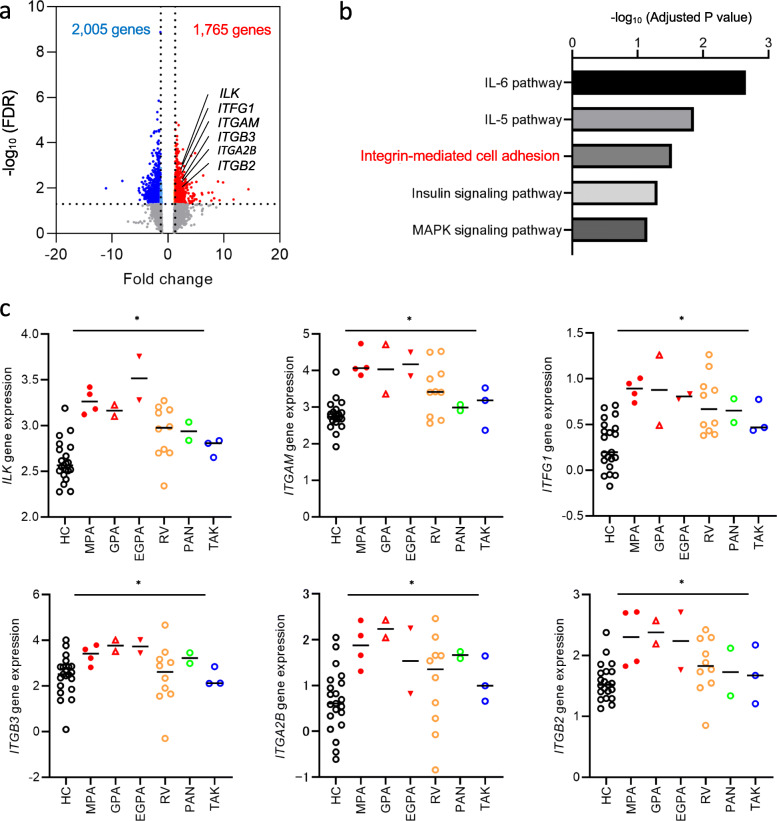
Baseline characteristics of the patients are shown in Table 1 and Supplementary Table 1. Among the 24 patients enrolled for transcriptome analysis, six were treatment-naïve and the remaining 18 were undergoing treatment (Supplementary Table 1). We used GSEA to identify the molecular biological features of each type of vasculitis. Table 2 shows the pathways that were upregulated and downregulated in AAV, RV, PAN, and TAK, compared to those in the HCs, using the permute P value < 0.05. At a significance threshold of the FDR adjusted P value < 0.05, and fold change > 1.3, we identified 3770 differentially expressed genes between patients with AAV and the HCs (Fig. 2a). Among the 1765 upregulated genes, the pathways related to IL-6 (adjusted P value = 0.0022), IL-5 (adjusted P value = 0.014), integrin-mediated cell adhesion (adjusted P value = 0.030), and insulin signalling (adjusted P value = 0.049) were significantly upregulated in patients with AAV compared to those in the HCs; the MAPK signalling pathway tended to be upregulated in patients with AAV (adjusted P value = 0.071) (Fig. 2b). As the leukocyte–endothelium interaction plays an important role in endothelial damage, we focused on integrin families. Regarding individual genes, the expression levels of genes associated with integrin-mediated cell adhesion (ILK, ITGAM, ITFG1, ITGB3, ITGA2B, and ITGB2) were higher in patients with AAV than in the disease controls and HCs (Fig. 2c).
Table 1.
Baseline characteristics of patients, assessed using FACS analysis
| Variable | AAV | LVV | PAN | HC |
|---|---|---|---|---|
| n = 48 | n = 26 | n = 4 | n = 17 | |
| Baseline characteristics | ||||
| Age, years | 70 (57–80) | 67 (47–72) | 64 (48–72) | 43 (32–56) |
| Male, n (%) | 15 (31) | 14 (54) | 1 (25) | 5 (29) |
| Race, Japanese, n (%) | 48 (100) | 26 (100) | 4 (100) | 17 (100) |
| MPA/GPA/EGPA, n | 20/19/9 | – | – | – |
| GCA/TAK, n | – | 15/11 | – | – |
| Newly diagnosed/major relapse, n | 45/3 | 24/2 | 4/0 | – |
| BVAS | 12 (8–18) | 3 (3–6) | 15 (12–19) | – |
| Organ involvement | ||||
| Systemic, n (%) | 34 (71) | 23 (88) | 2 (50) | – |
| Skin, n (%) | 5 (10) | 1 (4) | 3 (75) | – |
| Mucous membranes/eyes, n (%) | 5 (10) | 0 (0) | 0 (0) | – |
| Ear, nose, throat, n (%) | 22 (46) | 0 (0) | 0 (0) | – |
| Chest, n (%) | 28 (58) | 0 (0) | 1 (25) | – |
| Vascular, n (%) | 3 (6) | 9 (35) | 1 (25) | – |
| Renal, n (%) | 17 (35) | 3 (12) | 1 (25) | – |
| Nervous system, n (%) | 20 (42) | 0 (0) | 3 (75) | – |
| Laboratory test | ||||
| ESR, mm/h | 87 (40–121) | 109 (59–122) | 71 (33–112) | – |
| White blood cells, 103 cells/μL | 9.5 (7.0–13) | 9.5 (7.0–14) | 7.7 (6.6–9.1) | – |
| Neutrophils, 103 cells/μL | 6.2 (4.4–8.4) | 5.4 (4.6–7.0) | 6.1 (2.6–8.4) | – |
| Lymphocytes, 103 cells/μL | 1.5 (1.0–1.8) | 1.4 (1.1–1.8) | 1.1 (0.8–1.6) | – |
| Monocytes, cells/μL | 414 (308–623) | 460 (343–565) | 444 (235–737) | – |
| Eosinophils, cells/μL | 223 (116–814) | 119 (67–192) | 109 (7–755) | – |
| Haemoglobin, g/dL | 11 (9.6–13) | 11 (10–12) | 12 (11–13) | – |
| Platelets, 104 cells/μL | 34 (25–41) | 34 (29–42) | 28 (18–36) | – |
| CRP, mg/dL | 3.9 (0.7–8) | 4.8 (2.4–6.5) | 0.8 (0.3–5.2) | – |
| IgG, mg/dL | 1648 (1120 − 1905) | 1437 (1140–1690) | 1325 (1124–2223) | – |
| MPO-ANCA-/PR3-ANCA-positive/ANCA-negative, n | 34/8/6 | 1/0/25 | 0/0/4 | – |
| MPO-ANCA titre, U/mL | 41 (13–171), n = 34 | 28, n = 1 | – | – |
| PR3-ANCA titre, U/mL | 14 (10–39), n = 8 | – | – | – |
| Rheumatoid factor positive, n (%) | 37 (79) | 2 (8) | 1 (25) | – |
| Rheumatoid factor, IU/mL | 65 (32–141) | 29 (16–42) | 41, n = 1 | – |
AAV ANCA-associated vasculitis, MPA microscopic polyangiitis, GPA granulomatosis with polyangiitis, EGPA eosinophilic granulomatosis with polyangiitis, LVV large-vessel vasculitis, GCA giant cell arteritis, TAK Takayasu arteritis, PAN polyarteritis nodosa, BVAS Birmingham vasculitis activity score, ESR erythrocyte sedimentation rate, CRP C-reactive protein, MPO myeloperoxidase, PR3 proteinase 3
Table 2.
Results of gene set enrichment analysis
| MAPP name | Number changed | Number measured | Number on MAPP | Permute P | Adjusted P | |
|---|---|---|---|---|---|---|
| AAV > HC | IL-6 pathway | 26 | 89 | 100 | < 0.001 | 0.0022 |
| IL-5 pathway | 25 | 63 | 69 | < 0.001 | 0.014 | |
| Integrin-mediated cell adhesion | 24 | 79 | 99 | < 0.001 | 0.030 | |
| Insulin signalling pathway | 38 | 128 | 159 | < 0.001 | 0.049 | |
| MAPK signalling pathway | 36 | 129 | 162 | 0.001 | 0.071 | |
| EGFR1 pathway | 38 | 148 | 177 | < 0.001 | 0.093 | |
| Focal adhesion | 34 | 132 | 187 | < 0.001 | 0.13 | |
| MAPK cascade | 10 | 22 | 29 | 0.001 | 0.057 | |
| B cell receptor pathway | 36 | 146 | 158 | 0.001 | 0.23 | |
| Regulation of actin cytoskeleton | 25 | 95 | 146 | 0.005 | 0.33 | |
| IL-3 pathway | 24 | 93 | 101 | 0.008 | 0.39 | |
| Pentose phosphate pathway | 4 | 7 | 7 | 0.011 | 0.31 | |
| Glycogen metabolism | 10 | 31 | 36 | 0.011 | 0.59 | |
| Proteasome degradation | 16 | 59 | 61 | 0.014 | 0.62 | |
| Cytochrome P450 | 9 | 28 | 71 | 0.016 | 0.65 | |
| IL-1 pathway | 11 | 35 | 38 | 0.017 | 0.57 | |
| Eicosanoid synthesis | 6 | 16 | 19 | 0.030 | 0.67 | |
| AAV < HC | Ribosomal proteins | 27 | 86 | 88 | < 0.001 | < 0.001 |
| Homologous recombination | 4 | 12 | 13 | 0.012 | 0.002 | |
| Inflammatory response pathway | 5 | 21 | 33 | 0.029 | 0.071 | |
| Synthesis and degradation of ketone bodies | 2 | 4 | 5 | 0.035 | 0.076 | |
| RV > HC | Proteasome degradation | 9 | 59 | 61 | < 0.001 | 0.020 |
| Prostaglandin synthesis regulation | 4 | 20 | 31 | 0.003 | 0.099 | |
| Apoptosis | 6 | 76 | 82 | 0.019 | 0.80 | |
| Nucleotide GPCRs | 2 | 10 | 105 | 0.037 | 0.54 | |
| Electron transport chain | 6 | 87 | 105 | 0.042 | 0.93 | |
| RV < HC | Inflammatory response pathway | 2 | 21 | 33 | 0.041 | 0.84 |
| PAN > HC | Oxidative stress | 3 | 18 | 28 | 0.010 | 0.31 |
| Nucleotide GPCRs | 2 | 10 | 11 | 0.016 | 0.44 | |
| Statin pathway | 2 | 12 | 20 | 0.038 | 0.64 | |
| Eicosanoid synthesis | 2 | 16 | 19 | 0.048 | 0.85 | |
| PAN < HC | Wnt signalling and pluripotency | 4 | 64 | 97 | 0.015 | 0.83 |
| TAK > HC | Apoptosis | 4 | 76 | 82 | 0.008 | 0.63 |
| Hypertrophy model | 2 | 14 | 20 | 0.014 | 0.23 | |
| GPCRDB class A Rhodopsin-like | 4 | 115 | 262 | 0.049 | 0.96 | |
| TAK < HC | S1P signalling | 2 | 20 | 25 | 0.038 | 0.67 |
MAPP microarray pathway profiler, AAV ANCA-associated vasculitis, RV rheumatoid vasculitis, PAN polyarteritis nodosa, TAK Takayasu arteritis, HC healthy controls
Fig. 2.
Upregulation of the molecules involved in integrin-mediated cell adhesion in AAV. a Volcano plot of 3770 differentially expressed genes between patients with AAV and the healthy controls (HCs) (fold change > 1.3, adjusted P < 0.05). b Top five pathways that were differentially regulated between patients with AAV and HCs. c Z-score variance of representative genes within each integrin family among patients with each type of vasculitis and the HCs, are shown. *P < 0.05 using the Kruskal-Wallis test. MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic polyangiitis; RV, rheumatoid vasculitis; PAN, polyarteritis nodosa; TAK, Takayasu arteritis; ILK, integrin-linked kinase; ITGAM, integrin alpha M; ITFG1, integrin alpha FG-GAP repeat containing 1; ITGB3, integrin beta 3; ITGA2B, integrin alpha 2b; ITGB2, integrin beta 2
Neutrophil LFA-1 upregulation in patients with AAV
Given that α- and β-integrins are preferentially expressed in human neutrophils, monocytes, and lymphocytes [20–22], we examined their surface expression using FACS analysis to confirm our GSEA results.
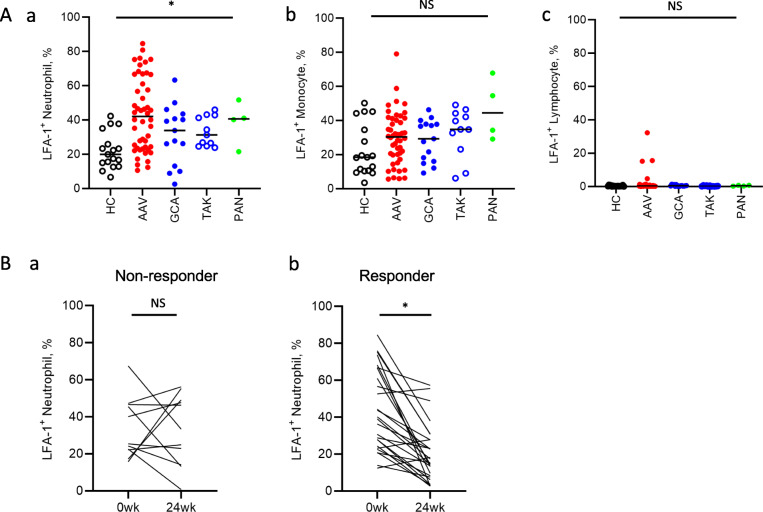
In concordance with our GSEA results, the percentage of neutrophils expressing LFA-1 was higher in the neutrophils of patients with AAV than in those of the disease controls and HCs (HC vs. AAV vs. GCA vs. TAK vs. PAN; 44% vs. 34% vs. 31% vs. 41% vs. 20%, P = 0.0006) (Fig. 3A-a). In contrast, LFA-1 expression in the monocytes and lymphocytes was not significantly different between patients with AAV and LVV and the HCs (Fig. 3A-b and -c). Furthermore, CD11c expression in the monocytes was lower in patients with AAV than in the HCs (52% vs. 73%, P = 0.0007) (Supplementary Fig. 2C). In contrast, there was no significant difference in the expression of other integrins, including CD11a (Supplementary Fig. 2A), CD11b (Supplementary Fig. 2B), and CD18 (Supplementary Fig. 2D), between patients with AAV and the HCs.
Fig. 3.
Upregulation of LFA-1 expression in the neutrophils of patients with AAV. (A) Expression of LFA-1 in (a) neutrophils, (b) monocytes, and (c) lymphocytes. (B) Temporal change in LFA-1 expression in the neutrophils of (a) non-responder and (b) responder AAV patients. *P < 0.05 using the (A) Kruskal-Wallis test or (B) Wilcoxon’s signed-rank test. LFA-1, lymphocyte function-associated antigen-1; AAV, ANCA-associated vasculitis; HC, healthy control; MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; GCA, giant cell arteritis; TAK, Takayasu arteritis; wk., week; NS, not significant
Temporal changes in LFA-1 expression in the responder and non-responder groups
Of the 45 treatment-naïve patients, 37 were monitored for at least 24 weeks following the procedure. These 37 patients were categorised as responders (n = 26) and non-responders (n = 11) based on the criteria described in the ‘Patients and methods’ section. LFA-1 expression in the neutrophils of non-responder patients was comparable at the onset and at 24 weeks of treatment (25% vs. 33%, P = 0.90) (Fig. 3B-a), but decreased in the responder patients (40% vs. 16%, P < 0.0001) during follow-up (Fig. 3B-b). The expression levels of neutrophil LFA-1 at baseline (40% vs. 25%, P = 0.11) and at 24 weeks of treatment (16% vs. 33%, P < 0.0001) were not significantly different between the responder and non-responder groups. Treatments received by the responder and non-responder patients from week 0 to week 24 are shown in Supplementary Table 2.
Correlation between LFA-1 expression in neutrophils and clinical features
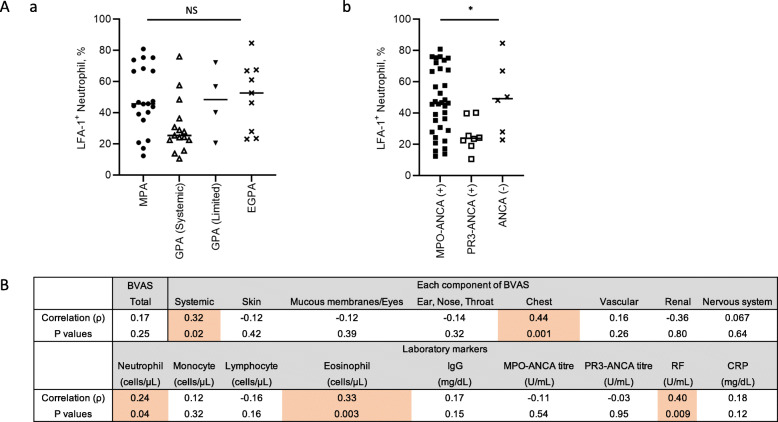
We compared the levels of LFA-1 with clinical phenotypes, which revealed no differences among patients with MPA, generalised GPA, localised GPA, and EGPA (Fig. 4A-a). The Kruskal-Wallis test and post-hoc test revealed that the levels of LFA-1 in neutrophils were comparable between patients with MPO-ANCA-positive and ANCA-negative expression, but were higher than those in patients with PR3-ANCA-positive expression (46% vs. 48% vs. 24%, P = 0.023) (Fig. 4A-b). We also examined the relationship between neutrophil LFA-1 expression levels and disease activity based on the BVAS and laboratory markers. Correlation analysis was conducted to determine the Spearman’s rank correlation coefficient between LFA-1 expression in neutrophils and disease activity (Fig. 4B). Correlation analysis showed that the BVAS scores in systemic (ρ = 0.32, P = 0.028) and chest (ρ = 0.48, P = 0.001) components, excluding total BVAS and BVAS scores in other components, were significantly correlated with the expression of LFA-1 in neutrophils. Moreover, LFA-1 expression in the neutrophils was significantly correlated with eosinophil count (ρ = 0.29, P = 0.048), serum RF level (ρ = 0.39, P = 0.018), and serum CRP level (ρ = 0.30, P = 0.038) in patients with AAV. These data indicate that elevated LFA-1 expression in neutrophils may be associated with systemic disease activity in AAV.
Fig. 4.
Correlation between LFA-1 expression in neutrophils and disease activity. (A) Expression of LFA-1 in neutrophils among patients with (a) different disease phenotype and (b) ANCA status. (B) Correlation analysis was conducted to determine the Spearman’s correlation coefficient between LFA-1 expression in neutrophils and disease activity. LFA-1, lymphocyte function-associated antigen-1; BVAS, Birmingham Vasculitis Activity Score; RF, rheumatoid factor; CRP, C-reactive protein
Effects of LFA-1 and ICAM1 on the leukocyte–endothelium interaction
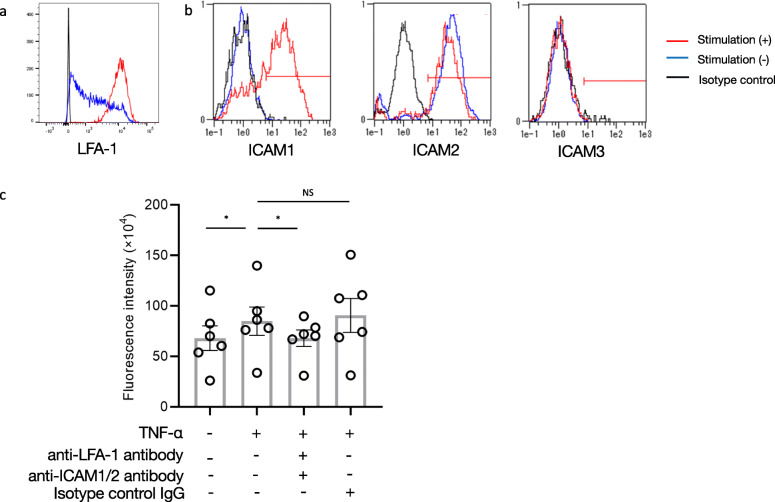
We performed leukocyte adhesion and transmigration assays to study LFA-1 function in the leukocyte–endothelium interaction. To avoid neutrophil activation during cell isolation, leukocytes were obtained from whole blood samples. LFA-1 was upregulated in the leukocytes after treatment with LPS (Fig. 5a), and levels of ICAM1, but not ICAM2/3, were upregulated in the HUVECs after treatment with TNF-α (Fig. 5b). We co-incubated LPS-stimulated leukocytes and TNF-α-pre-treated HUVECs to examine leukocyte migration via the fluorescence intensity of Leuko Tracker solution-labelled leukocytes. We observed upregulation of LPS-pre-treated leukocyte migration toward TNF-α-pre-treated HUVECs on a Transwell plate (65 × 104 vs. 82 × 104, P = 0.031), suggesting that the leukocyte–endothelium interaction was dependent on the expression of LFA-1 in the leukocytes and that of ICAM1 in the HUVECs (Fig. 5c). Notably, pre-incubation of leukocytes with the anti-LFA-1 and anti-ICAM1 neutralising antibodies significantly suppressed the interaction between leukocytes and HUVECs (82 × 104 vs. 71 × 104, P = 0.031), whereas incubation with the isotype-matched control did not significantly affect the result (82 × 104 vs. 91 × 104, P = 0.44).
Fig. 5.
Effect of LFA-1 on leukocyte–endothelium transmigration. Representative findings from flow cytometry analysis evaluating a LFA-1 expression in leukocytes and b ICAM1–3 expression in HUVECs in the presence and absence of LPS and TNF-α stimulation, respectively. c The extent of cell transmigration, as measured by the fluorescence intensity of Leuko Tracker solution-labelled leukocytes from six healthy subjects. Leukocyte migration in the presence of anti-LFA-1 antibody, anti-ICAM1 antibody, and isotype-matched control IgG was monitored. *P < 0.05, using Wilcoxon’s signed-rank test. LFA-1, lymphocyte function-associated antigen-1; ICAM, intercellular adhesion molecule-1; HUVEC, human umbilical vein endothelial cell; LPS, lipopolysaccharide; TNF, tumour necrosis factor
Discussion
We performed a microarray analysis and GSEA to compare the molecular biological features of patients with AAV, the disease controls, and the HCs. Based on these analyses, we found that proteins of the integrin family were specifically upregulated in AAV. FACS analysis revealed that LFA-1 expression in neutrophils was significantly elevated in patients with AAV compared to that in patients with LVV and in the HCs. LFA-1 levels in the neutrophils of patients with AAV were significantly associated with systemic inflammatory markers such as eosinophil count, RF level, CRP level, and BVAS score of systemic and chest components. Interestingly, LFA-1 expression in neutrophils was higher in MPO-ANCA-positive patients with AAV than in those positive for PR3-ANCA. Considering that MPO-ANCA-positive patients with AAV are at a higher risk of death [36], mechanistic biomarkers would be helpful.
The neutrophil–endothelium interaction mainly occurs due to the binding of LFA-1 to its receptor ICAM1/2 on the endothelial cells [20–22]. As neutrophil–endothelium adhesion is an essential process in neutrophil-mediated endothelial damage, upregulation of LFA-1 in neutrophils during AAV can serve as a convenient biomarker. Although the regulatory mechanism of LFA-1 expression in AAV has not yet been sufficiently explained, cytokines, such as GM-CSF and TNF-α whose levels are elevated in patients with AAV [37], can induce LFA-1 upregulation [38].
β2-integrins are necessary for leukocytic inflammation. JAK2-V617F knock-in mice (JAK2+/VF), which possess a JAK2 activating mutation, reportedly showed increased β2-integrin activity in neutrophils [39]. A previous report showed that JAK2+/VF mice had enhanced neutrophil–endothelium adhesion that led to pathologic thrombosis, which was inhibited by anti-β2 integrin neutralising antibodies [39]. Abnormal functioning of integrins in leukocytes, particularly neutrophils, may induce abnormal leukocyte–endothelium interactions, contributing to such pathological events.
Integrins have recently been identified as therapeutic targets for various inflammatory diseases [40]. For example, vedolizumab, a humanised monoclonal antibody that specifically recognises the α4 β7 heterodimer, is effective in the treatment of ulcerative colitis [41] and Crohn’s disease [42]. Furthermore, natalizumab, which targets the α4-integrin subunit, is effective for the treatment of multiple sclerosis [43] and Crohn’s disease [44]. Additionally, efalizumab, which targets LFA-1, is effective for the treatment of moderate-to-severe psoriasis [45]. However, once approved globally, it was discontinued due to an adverse event of progressive multifocal leukoencephalopathy reported in several cases [46]. In our study, LFA-1 expression in the neutrophils was upregulated in patients with AAV and was associated with systemic inflammation via the neutrophil–endothelium interaction. Thus, we believe that bispecific or multi-specific antibodies bridging LFA-1 and neutrophil surface molecules can recognise LFA-1 in neutrophils. Treatment with emicizumab, a bispecific antibody and a drug that specifically targets neutrophil LFA-1, showed a positive effect in patients with haemophilia A [47] and could be a safe and effective therapeutic alternative for AAV.
In contrast to LFA-1 expression in the neutrophils, CD11c expression in the monocytes is decreased in patients with AAV. We recently reported that CD14++ and CD16+, which are intermediate monocytes, play a substantial role in the development of AAV [7]. As CD11c is a characteristic of both intermediate and classical monocytes [48, 49], CD11c expression may be involved in monocyte migration to the local site of inflammation; however, further investigation is needed to confirm this hypothesis.
Our study had several limitations. First, the number of patients enrolled in this study may not be sufficient to clarify the specificity of the changes in LFA-1 expression in AAV. Second, the significance of LFA-1 as a biomarker is not sufficient to distinguish between the disease controls and HCs because they overlap among groups. Third, we did not study the phenotype of endothelial cells or platelets in this study, although several abnormalities have been reported in the pathogenesis of AAV [50].
Conclusions
In conclusion, we demonstrated that LFA-1 upregulation may enhance endothelial damage in patients with AAV. To our best knowledge, this is the first study to identify the role of the neutrophil adhesion molecule LFA-1 in the pathogenesis of AAV. Further evidence on the mechanism of interaction between neutrophils and endothelial cells will be helpful in the identification of novel therapeutic targets for AAV treatment.
Supplementary Information
Acknowledgements
We are incredibly grateful to Ms. Yumi Ikeda, Ms. Yuko Takaishi, and Ms. Kumiko Tanaka for their technical support.
Abbreviations
- ANCA
Anti-neutrophil cytoplasmic antibody
- AAV
ANCA-associated vasculitis
- LFA-1
Lymphocyte function-associated antigen-1
- GSEA
Gene set enrichment analysis
- HC
Healthy control
- HUVEC
Human umbilical vein endothelial cell
- LVV
Large vessel vasculitis
- MPA
Microscopic polyangiitis
- GPA
Granulomatosis with polyangiitis
- EGPA
Eosinophilic granulomatosis with polyangiitis
- ICAM
Intercellular adhesion molecule
- RV
Rheumatoid vasculitis
- PAN
Polyarteritis nodosa
- TAK
Takayasu arteritis
- BVAS
Birmingham vasculitis activity score
- CRP
C-reactive protein
- RF
Rheumatoid factor
- cRNA
Complementary RNA
- FACS
Fluorescence-activated cell sorting
- TNF
Tumour necrosis factor
- LPS
Lipopolysaccharide
- IL
Interleukin
- fMLP
N-formyl-met-leu-phe
Authors’ contributions
KM, TK, KY, KS, and TT designed the study. KM, TK, and KS were involved in patient recruitment and data collection. All the authors were involved in writing the manuscript and approved the final version. The corresponding author had full access to all data produced in the study and had final responsibility for the decision to submit for publication.
Funding
This study was supported by grants from JSPS KAKENHI (Grant Number JP20K22911), the Japan Agency for Medical Research and Development, and the Keio University School of Medicine.
Availability of data and materials
All data generated and analysed in this study are disclosed.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Keio University School of Medicine (#20140335) and was conducted in compliance with the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from all participating individuals.
Competing interests
The authors have no competing interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CGM, Mccluskey RT, Sinico RA, Rees AJ, Es LAV, Waldherr RÜD, Wiik A. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, Mahr A, Segelmark M, Cohen-Tervaert JW, Scott D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66(2):222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CGM, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DGI, Specks U, Stone JH, Takahashi K, Watts RA. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. 2019;15(2):91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto K, Suzuki K, Yoshimoto K, Seki N, Tsujimoto H, Chiba K, Takeuchi T. Significant association between clinical characteristics and immuno-phenotypes in patients with ANCA-associated vasculitis. Rheumatology (Oxford) 2020;59(3):545–553. doi: 10.1093/rheumatology/kez327. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Suzuki K, Yoshimoto K, Seki N, Tsujimoto H, Chiba K, Takeuchi T. Longitudinal immune cell monitoring identified CD14++ CD16+ intermediate monocyte as a marker of relapse in patients with ANCA-associated vasculitis. Arthritis Res Ther. 2020;22(1):145. doi: 10.1186/s13075-020-02234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361(9353):206–210. doi: 10.1016/S0140-6736(03)12269-6. [DOI] [PubMed] [Google Scholar]
- 9.Woywodt A, Goldberg C, Kirsch T, de Groot K, Erdbruegger U, Haller H, Haubitz M. Circulating endothelial cells in relapse and limited granulomatous disease due to ANCA associated vasculitis. Ann Rheum Dis. 2006;65(2):164–168. doi: 10.1136/ard.2005.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneeweis C, Rafalowicz M, Feist E, Buttgereit F, Rudolph PE, Burmester GR, Egerer K. Increased levels of BLyS and sVCAM-1 in anti-neutrophil cytoplasmatic antibody (ANCA)-associated vasculitides (AAV) Clin Exp Rheumatol. 2010;28(1 Suppl 57):62–66. [PubMed] [Google Scholar]
- 11.Hu P, Su H, Xiao H, Gou SJ, Herrera CA, Alba MA, Kakoki M, Falk RJ, Jennette JC. Kinin B1 receptor is important in the pathogenesis of myeloperoxidase-specific ANCA GN. J Am Soc Nephrol. 2020;31(2):297–307. doi: 10.1681/ASN.2019010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu N, Westra J, Kallenberg CGM. Dysregulated neutrophil--endothelial interaction in antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides: implications for pathogenesis and disease intervention. Autoimmun Rev. 2011;10(9):536–543. doi: 10.1016/j.autrev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Halbwachs L, Lesavre P. Endothelium-neutrophil interactions in ANCA-associated diseases. J Am Soc Nephrol. 2012;23(9):1449–1461. doi: 10.1681/ASN.2012020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez GA, Rovere-Querini P, Sabbadini MG, Manfredi AA. Parietal and intravascular innate mechanisms of vascular inflammation. Arthritis Res Ther. 2015;17(1):16. doi: 10.1186/s13075-015-0528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H, Hu N, Wang C, Chen M, Zhao MH. Interaction between CD177 and platelet endothelial cell adhesion molecule-1 downregulates membrane-bound proteinase-3 (PR3) expression on neutrophils and attenuates neutrophil activation induced by PR3-ANCA. Arthritis Res Ther. 2018;20(1):213. doi: 10.1186/s13075-018-1710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikman A, Fagergren A, Johansson SGO, Lundahl J, Jacobson SH. Monocyte activation and relationship to anti-proteinase 3 in acute vasculitis. Nephrol Dial Transplant. 2003;18(9):1792–1799. doi: 10.1093/ndt/gfg216. [DOI] [PubMed] [Google Scholar]
- 17.Wikman A, Fagergren A, Forslid J, Jacobson SH, Johansson SGO, Lundahl J. Antineutrophil cytoplasmic antibodies induce decreased CD62L expression and enhanced metabolic activity in monocytes. Scand J Immunol. 2003;57(2):179–184. doi: 10.1046/j.1365-3083.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 18.Day CJ, Hewins P, Savage CO. New developments in the pathogenesis of ANCA-associated vasculitis. Clin Exp Rheumatol. 2003;21(6 Suppl 32):S35–S48. [PubMed] [Google Scholar]
- 19.Hong Y, Elefttheliou D, Hussain AAK, Price-Kuehne FE, Savage CO, Jayne D, et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol. 2012;23(1):49–62. doi: 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem. 1996;61(4):554–561. doi: 10.1002/(SICI)1097-4644(19960616)61:4<554::AID-JCB8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 22.Kolev K, West EE, Kunz N, Chauss D, Moseman EA, Rahman J, et al. Diapedesis-induced integrin signaling via LFA-1 facilitates tissue immunity by inducing intrinsic complement C3 expression in immune cells. Immunity. 2020;52(3):513–527. doi: 10.1016/j.immuni.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi T, Amano K, Sekine H, Koide J, Abe T. Upregulated expression and function of integrin adhesive receptors in systemic lupus erythematosus patients with vasculitis. J Clin Invest. 1993;92(6):3008–3016. doi: 10.1172/JCI116924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, Höglund P, Jayne D, Luqmani R, Mahr A, Mukhtyar C, Pusey C, Rasmussen N, Stegeman C, Walsh M, Westman K, for the European Vasculitis Study Group Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70(3):488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 25.Jones RB, Tervaert JWC, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 26.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U, RAVE-ITN Research Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radford DJ, Savage CO, Nash GB. Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum. 2000;43(6):1337–1345. doi: 10.1002/1529-0131(200006)43:6<1337::AID-ANR16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Nolan SL, Kalia N, Nash GB, Kamel D, Heeringa P, Savage COS. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J Am Soc Nephrol. 2008;19(5):973–984. doi: 10.1681/ASN.2007111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott DG, Bacon PA. Intravenous cyclophosphamide plus methylprednisolone in treatment of systemic rheumatoid vasculitis. Am J Med. 1984;76(3):377–384. doi: 10.1016/0002-9343(84)90654-5. [DOI] [PubMed] [Google Scholar]
- 30.Lightfoot RW, Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33(8):1088–1093. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 31.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW Jr The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 32.Kermani TA, Warrington KJ, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Maksimowicz-McKinnon K, McAlear C, Monach PA, Seo P, Merkel PA, Ytterberg SR, Vasculitis Clinical Research Consortium Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol. 2015;42(7):1213–1217. doi: 10.3899/jrheum.141347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone JH. Limited versus severe Wegener’s granulomatosis. Arthritis Rheum. 2003;48(8):2299–2309. doi: 10.1002/art.11075. [DOI] [PubMed] [Google Scholar]
- 34.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, Specks U, Allen NB, Davis JC, Spiera RF, Calabrese LH, Wigley FM, Maiden N, Valente RM, Niles JL, Fye KH, McCune JW, St.Clair EW, Luqmani RA, for the International Network for the Study of the Systemic Vasculitides (INSSYS) A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44(4):912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Hu N, Westra J, Rutgers A, der Meer BDV, Huitema MG, Stegeman CA, et al. Decreased CXCR1 and CXCR2 expression on neutrophils in anti-neutrophil cytoplasmic autoantibody-associated vasculitides potentially increases neutrophil adhesion and impairs migration. Arthritis Res Ther. 2011;13(6):R201. doi: 10.1186/ar3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sada KE, Yamamura M, Harigai M, Fujii T, Dobashi H, Takasaki Y, Ito S, Yamada H, Wada T, Hirahashi J, Arimura Y, Makino H, the Research Committee on Intractable Vasculitides, the Ministry of Health, Labour and Welfare of Japan Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res Ther. 2014;16(2):R101. doi: 10.1186/ar4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, Fervenza FC, Fessler BJ, Hoffman GS, Iklé D, Kallenberg CGM, Krischer J, Langford CA, Mueller M, Seo P, St. Clair EW, Spiera R, Tchao N, Ytterberg SR, Johnson KJ, Merkel PA. Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Ann Rheum Dis. 2013;72(8):1342–1350. doi: 10.1136/annrheumdis-2012-201981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JG, Collinge M, Ramgolam V, Ayalon O, Fan XC, Pardi R, Bender JR. LFA-1-dependent HuR nuclear export and cytokine mRNA stabilization in T cell activation. J Immunol. 2006;176(4):2105–2113. doi: 10.4049/jimmunol.176.4.2105. [DOI] [PubMed] [Google Scholar]
- 39.Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, Philipsen L, Weinert S, Ghosh A, Saalfeld FC, Nimmagadda SC, Müller P, Braun-Dullaeus R, Mohr J, Wolleschak D, Kliche S, Amthauer H, Heidel FH, Schraven B, Isermann B, Müller AJ, Fischer T. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128(10):4359–4371. doi: 10.1172/JCI90312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Argollo M, Fiorino G, Hindryckx P, Peyrin-Biroulet L, Danese S. Novel therapeutic targets for inflammatory bowel disease. J Autoimmun. 2017;85:103–116. doi: 10.1016/j.jaut.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 42.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 43.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 44.Gordon FH, Lai CW, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121(2):268–274. doi: 10.1053/gast.2001.26260. [DOI] [PubMed] [Google Scholar]
- 45.Gordon KB, Papp KA, Hamilton TK, Walicke PA, Dummer W, Li N, Bresnahan BW, Menter A, Efalizumab Study Group Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290(23):3073–3080. doi: 10.1001/jama.290.23.3073. [DOI] [PubMed] [Google Scholar]
- 46.Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis. Br J Dermatol. 2009;161(5):987–1019. doi: 10.1111/j.1365-2133.2009.09505.x. [DOI] [PubMed] [Google Scholar]
- 47.Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, Fukazawa N, Yoneyama K, Yoshida H, Nogami K. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044–2053. doi: 10.1056/NEJMoa1511769. [DOI] [PubMed] [Google Scholar]
- 48.Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, Nguyen AT, McNamara CA, Hedrick CC. Human blood monocyte subsets: a new gating strategy defined using cell surface markers identified by mass cytometry. Arterioscler Thromb Vasc Biol. 2017;37(8):1548–1558. doi: 10.1161/ATVBAHA.117.309145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto K, Yasuoka H, Yoshimoto K, Suzuki K, Takeuchi T. Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci Rep. 2021;11:222. doi: 10.1038/s41598-020-80685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed in this study are disclosed.