Abstract
Background
The purpose of periodontal treatments is to reduce inflammation, restore gingival health and clinical attachment level gain by controlling microbial plaque formation and other etiological factors. One of the drugs that has been tested in many areas and shown good anti-inflammatory properties is erythropoietin (EPO). We evaluated the effect of this drug on the improvement of periodontitis after the phase I treatment.
Methods
This study was conducted on 30 patients with stage III periodontitis who had at least two bilateral teeth with CAL of ≥ 5 mm and PPD ≥ 6 mm at ≥ 2 non‐adjacent teeth and bleeding on probing. After oral hygiene instruction and scaling and root planning (SRP), EPO gel containing a solution of 4000 units was applied deeply in the test group and placebo gel was deeply administered in the control pockets (5 times, every other day). The clinical parameters of the plaque index (PI), gingival index (GI), clinical attachment level (CAL), probing depth (PD) and bleeding index (BI) were measured at baseline and after three months of follow up. The P-value was set at 0.05.
Results
All clinical variables improved after treatment in both groups. The BI and GI scores (which reflects the degree of gingival inflammation) showed statistically more reduction in test group. The CAL decreased from 5.1 ± 4.1 to 3.40 ± 2.71 mm; and 5.67 ± 4.32 to 4.33 ± 3.19 mm in test and control group, respectively (P < 0.00). After the treatment, there was a significant greater reduction in CAL and also PD values in test group (P < 0.01).
Conclusion
Local application of EPO gel in adjunct to SRP can improve clinical inflammation and CAL gain in periodontitis.
Trial registration: This study was registered at 2017-11-06 in IRCT. All procedures performed in this study were approved with ID number of IR.TUMS.DENTISTRY.REC.1396.3139 in Tehran University of medical science.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-021-01607-y.
Keywords: Non-surgical periodontal treatment, Erythropoietin (EPO), Periodontitis
Background
Periodontal disease is one of the most common infectious diseases associated with dental plaque accumulation. Periodontitis may lead to tooth loss and disability and it negatively affects chewing function and aesthetics [1]. Therefore accurate diagnosis and treatment of this disease is essential and based on reduction or elimination of local factors, risk management and elimination of the harmful effects of disease [2]. Periodontal deterioration occurs as a result of both direct (due to microbial invasion) and indirect tissue destruction (due to host defense response). The diffusion of bacterial products (antigens, lipopolysaccharide) across the junctional epithelium stimulate the host immune-inflammatory response in the tissues that is the major factor in the periodontal tissue degradation [3–5].
Some studies introduced effectiveness of adjunctive pharmacotherapies in the management of periodontitis which particularly targets the host immune response and a wide variety of drugs are under research for evaluating their ability to modulate destructive components of the host response [6–8].
Local drug delivery system, is one of such commonly used practices [9]. Local administration of anti-infective agents (e.g. Doxycycline), directly into the periodontal pocket, has the potential to provide greater concentrations of drug into the infected area and to reduce possible systemic side effects [9, 10].
Erythropoietin (EPO) is a hormone produced by kidneys with modifying effects on the host immune system. Contrary to the past speculation that EPO was only effective in the development of erythropoiesis, today, EPO is recognized for its regenerative properties in acute and chronic tissue damage [11, 12]. EPO attenuates both primary and secondary injury, as well as facilitates healing and restoration of function by local down-regulation of inflammatory processes triggered by injury [11]. In a study by Strunk et al. [13], investigators observed that IL-2, -6, -8, c-interferon (IFN-c) and TNF-α stimulated by lipopolysaccharide (LPS) could be significantly inhibited by rh-EPO. So, a beneficial role for administering exogenous EPO could be to rescue cells at risk of apoptosis within the penumbra by signaling through its already expressed receptor [14, 15]. In addition, EPO plays a role in invoking stem cells [16] and in improving blood supply to surrounding tissues as a protective molecule for vascular endothelial cells, which undergo apoptosis in hypoxic conditions [17]. By increasing the proliferation of stem cells, including dermal stem cells, EPO is effective in skin restorations [18–20]. In a study on mice with heat damage, treatment with EPO was associated with improved and accelerated wound healing by increasing vascular proliferation, extracellular matrix maturation, angiogenesis and vascular density [20]. Recent studies have shown the effects of EPO on the recovery of damaged nerves by increasing vascular proliferation [21]. Also, topical application of EPO improves palatal wound healing during the third and fourth weeks after free gingival graft procedures [22]. Although systemic administration of EPO have been reported to result in improved recovery of neuronal structures following ischemic neuronal damage [23, 24], this administration route was mainly studied in animal models. Human clinical studies are ongoing to confirm the safety of systemic application of EPO.
Despite the recognized properties of anti-inflammatory, anti-apoptotic, and angiogenic properties of EPO, and the role of the host response in pathogenesis of periodontal diseases, there are no studies on the use of this hormone in periodontal treatments. So, we decided to evaluate the effects of local application of EPO on the clinical outcomes of non-surgical treatment of periodontitis.
Methods
Study group and research design
This triple blinded randomized clinical trial conducted at the Periodontology Department of School of Dentistry at Tehran University of medical sciences. Thirty patients with stage III periodontitis were selected from the patients referred to the Periodontics department. All procedures performed in this study were approved on 06/11/2017 with ID number of IRCT2017091636203N1 in Tehran University of medical sciences. Procedures were in accordance with the ethical standards from the last update of Helsinki Declaration [25].
Patients who entered the study were over 18 years old, did not undergo scaling and root planing during the past 6 months and had CAL of ≥ 5 mm and PPD ≥ 6 mm with bleeding on probing around ≥ 2 non‐adjacent teeth [26].
Exclusion criteria: systemic diseases (e.g., uncontrolled diabetes mellitus, rheumatoid arthritis, osteoporosis), medications known to alter the immune response (e.g., chronic nonsteroidal anti-inflammatory drugs, glucocorticoids, bisphosphonates, calcitonin, methotrexate and antibiotics) affecting periodontal inflammation and bone turnover, paraphysiological conditions (e.g., pregnancy and breast feeding), and surgical periodontal therapy within the last year.
Patient selection
The randomization was conducted using computer-generated sequencing. The sequence was placed in a sealed opaque envelope including the patient number and the treatment code. Patients were randomly assigned groups immediately after scaling and root planing. The practitioner (S.Y.), the examiner (H.A.) and the patient were blinded to group assignment. Informed consent was obtained from all subjects. According to amounts of α = 0.05, X2 = 9.3, X1 = 7.3 and β = 0.2, sample size set at n = 15 (standard deviation was considered 1.6 and 2.1 respectively).
Clinical interventions
At the screening visit, the plaque index (PI) [27], gingivitis index (GI) [28], bleeding index (BI) [29], probing depth (PD), and clinical attachment level (CAL) were measured by an examiner (H.A) other than the practitioner who was blinded to the treatment assignment. All clinical parameters were measured on experimental teeth. The examiner was calibrated by “examiner alignment and assessment” protocol before measurements [30]. Intra-examiner reproducibility was assessed at the start of the study. All clinical measurements were performed twice for 5 patients (10 sites) with 24 h interval. The intra-examiner agreement was > 0.85. Indices were measured using a calibrated and standardized UNC-15 periodontal probe.
One day after the screening visit, oral hygiene instruction based on “targeted hygiene” was provided. Then, patients underwent full mouth supra- and subgingival scaling and root planning (SRP). The medications were applied immediately following the completion of the initial SRP, then repeated every other day for a total of 5 sessions. EPO is available as an ampule containing a solution of 4000 units [22]. One cc of EPO solution was mixed with 1 cc of carboxymethylcellulose gel to form an EPO gel at a concentration of 2000 units per cc. The resulting mixture was entirely (whole of 2 cc) applied in the test pockets (PPD ≥ 5 mm) using a 28-gauge needle. To ensure that EPO reached the desired depth, the tip of the canula was derived to the depth of the pocket. On the control sites, a placebo mixture of distilled water and carboxymethylcellulose gel, with the same volume of the test gel, was applied.
Patients were asked not to use any chemotherapeutic mouthwashes or other antibacterial or anti-inflammatory products. Patients were evaluated every two weeks for prophylaxis and oral hygiene reinforcement up to 3 months to prevent supra and sub- gingival plaque formation, and to keep the PI to 40% (based on the Sillness and Loe plaque index). Clinical indices were evaluated and measured by the same examiner at this time. In order to evaluate the need for future periodontal surgery, number of residual pockets with PPD ≥ 5 mm with bleeding upon probing was recorded.
Statistical analysis
This study reports on the (3 month) change in clinical parameters between SRP + EPO and SRP only groups. We considered the BI and GI as primary outcomes and CAL, PD and PI as secondary outcomes. To determine the difference between the data before and after the treatment, as well as the differences in test and control groups data, the Wilcoxon Rank-Sum Test was used and the P-value below 0.05 was considered significant. The data were analyzed using SPSS software (Version 22.0, Chicago, IL, USA).
Results
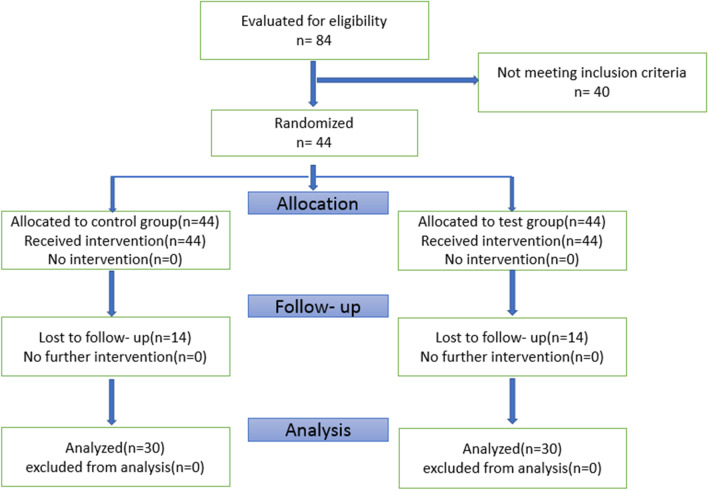
In this study, a total of 84 patients were examined, of which 44 were eligible to enter the study. Fourteen patients refused to continue their collaboration for various reasons, and finally, the study was conducted on 30 patients, 15 patients in each group (16 females and 14 males; age 32–68) (Table 1). In general, 60 teeth (30 in the experimental and 30 in the control groups) were included. None of the patients reported an allergic reaction to the drug or any other side effects. Consort flowchart is shown in Fig. 1.
Table 1.
Baseline demographic data
| Parameter | Test group (n = 15) | Control group (n = 15) |
|---|---|---|
| Age | 32–68 | 32–68 |
| Male/female | 6/9 | 8/7 |
| Teeth | 30 | 30 |
Fig. 1.
Consort flow chart
At baseline, there were no significant difference in the scores of all of the clinical parameters between test and control groups (P > 0.05) (Table 2).
Table 2.
Clinical parameters at baseline and 3 months post treatment
| Baseline | Post-treatment | P-value (Between- group comparison) | |||
|---|---|---|---|---|---|
| Test | Control | Test | Control | ||
| Plaque index (PI) | 2.53 ± 0.89αμ | 2.71 ± 0.46αμ | 1.1 ± 0.79α | 1.1 ± 0.68α | 0.705 |
| Gingival Index (GI) | 2.6 ± 0.72μ | 2.75 ± 0.75μ | 0.98 ± 0.58β | 1.33 ± 0.07β | 0.002 |
| Bleeding Index (BI) | 2.71 ± 0.65μ | 2.53 ± 0.89μ | 0.84 ± 0.48β | 1.3 ± 0.79β | 0.000 |
| Probing depth (mm) | 3.72 ± 0.72μ | 3.94 ± 0.78μ | 1.95 ± 0.76β | 2.55 ± 0.86β | 0.004 |
| CAL (mm) | 5.1 ± 4.1μ | 5.67 ± 4.32μ | 3.40 ± 2.71β | 4.33 ± 3.19β | 0.002 |
μNo statistically significant difference between groups in baseline measurements (p > 0.05)
αPI was not significantly different between groups at any time points
βGI, BI, PD and CAL were significantly different between groups after 3 months
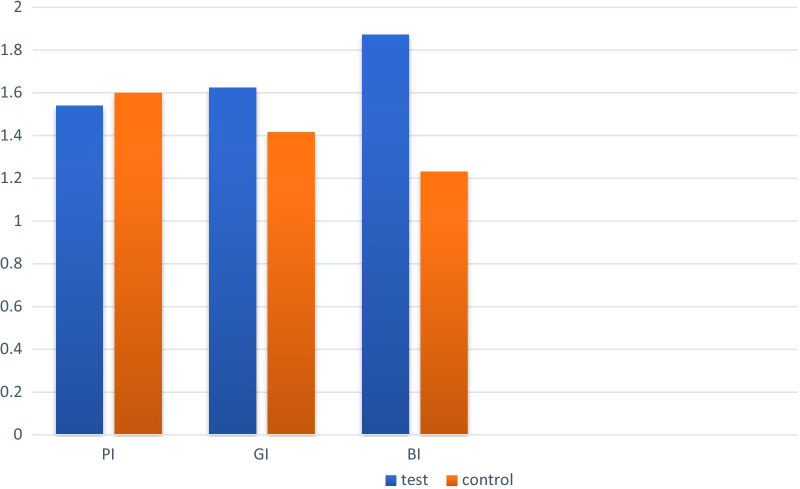
After 3 months, the GI scores decreased to 0.98 ± 0.58 and 1.33 ± 0.07 in test and control groups respectively. When compared between groups, the difference between two groups was significant (P-value = 0.002). At 3 months recall visit, BI scores decreased significantly in both groups (0.84 0.48 in test and 1.3 0.89 in control group). Comparison between groups revealed a significant difference between groups (P-value = 0.000). PI decreased in both groups without significant difference between groups (P-value = 0.705) (Table2).
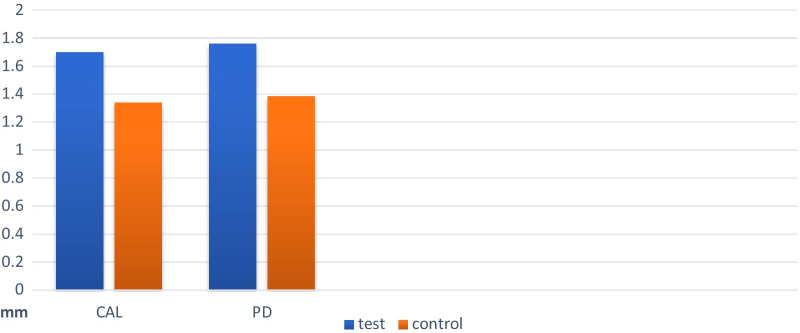
In the post-treatment examinations, the PD values showed significant decrease within both study groups. When the comparison carried out between groups, there was a significantly greater decrease in PD in the test group (P-value = 0.004). There was a significant improvement in CAL values after treatment in both groups, this improvement was statistically greater in the EPO treated group (P-value = 0.002) (Table2).
Comparison of periodontal parameters at baseline and 3 months post treatment in test and control groups are presented in Figs. 2 and 3.
Fig. 2.
The amount of changes in periodontal parameters (CAL, PD) in each group during 3 months
Fig. 3.
The amount of changes in clinical periodontal parameters (PI, BI, GI) in each group during 3 months follow up
There was no significant difference in need for periodontal surgery. In all, 48% of EPO treated and 52% of control sites required surgical interventions (remaining pockets > 5 mm with BOP).
Discussion
This study was aimed to investigate the efficacy of a topical erythropoietin for treatment of Stage III grade B/C periodontitis. After 3 months, the BI and GI scores (which reflecting the degree of gingival inflammation) decreased significantly in both groups. There was a statistically significant greater reduction in EPO treated group in comparison to patient received SRP alone.
Periodontal treatment primarily is intended to reduce the bacterial bioburden, by the mechanical removal of plaque and calculus (either by a nonsurgical, or surgical approach). Research into the pathogenesis of periodontal disease, particularly the role of the host response, has introduced opportunities for adjunctive pharmacotherapies in the management of periodontitis [2]. A variety of topical and systemically administered medications have been evaluated for their ability to modulate destructive components of the host response (e.g., Host Modulatory Therapies, HMTs) [31–33]. Although there is no study on the effectiveness of EPO on periodontal treatment, the positive effects of EPO on epithelial regeneration and healing process and its anti- inflammatory characteristic have been demonstrated [11, 18, 34]. Erythropoietin also exerts both cytoprotective and proangiogenic effects [12, 35, 36]. The osteogenic and angiogenic potential of erythropoietin are of particular interest for orthopedics [37]. Some studies showed that EPO improved bone healing and could serve as a therapeutic means enhancing bone regeneration [38, 39]. So, it was assumed that subgingival administration of EPO could be beneficial for its anti-inflammatory and regenerative properties in non- surgical periodontal therapies.
In 2004, Galeano and colleagues examined the effect of EPO on the extent of wound healing in diabetic rats on days 3, 6 and 12 after injury. They found that EPO had a significant effect on the level of angiogenesis, parallel with increased secretion of vascular endothelial growth factor (VEGF). VEGF is effective in both increasing the proliferation as well as in reducing the apoptosis of endothelial cells [40].
In a study by Fayaz-zadeh and colleagues in 2012, the effect of EPO and fibroblast growth factor (FGF2) on preventing the development of necrosis in skin flaps was investigated. Based on this study, both EPO treated and FGF2-treated groups had significantly less necrosis than control group, although this decrease was higher in FGF2 group [41]. They surveyed the magnitude of angiogenesis in all three groups (normal saline, EPO group, FGF-2 group) using a 400-magnification microscope and found no significant difference in the number of visible vessels in the three groups. This may means that less necrosis in the experimental groups is due to the improved microcirculation and increased number of small capillaries that are not visible with their assessment tool. Fayaz-zadeh and colleagues used EPO during different therapeutic periods. They observed that although high dose and frequent use of EPO did not make a significant difference in experimental group, the short-term (day 10) use with optimum dosage (100 U/kg/day) shows the best results [41].
In a systematic review, Hamed and colleagues studied the effect of EPO on wound healing of diabetic and non-diabetic animals and humans. The results showed that EPO was beneficial in the healing process of all these groups due to the effect of EPO on various stages of wound healing including the proliferative stage (the effect on fibroblastic proliferation and collagen synthesis) and the inflammatory stage (reduction of inflammatory cytokines and intrinsic immune cells). Bader and colleagues in 2011 studied the effect of EPO on the healing of foot skin lesions in humans. The result showed that EPO had a significant effect on the amount of epithelialization. In the EPO-treated group, epithelization was completed on the seventh post-treatment day while in the control group, the lesions were still red and exudating [19]. Our data showed that CAL and PD decreased in both groups after treatment, but the decrease in the experimental group was higher and there was a statistically significant difference between experimental and control group 3 months post treatment.
The cytoprotective effects of EPO (increased proliferation of fibroblasts and synthesis of collagen, increased number of macrophages, stimulated angiogenesis and reduced inflammatory response) and the expression of EPO receptors on the basal cells of the oral mucosa, could be assumed as the potential underlying mechanisms for greater reduction in BI and GI after non-surgical periodontal therapy [18].
To date, conventional treatments have succeeded in controlling periodontal inflammation but they had limited potentials toward regeneration of tissues destroyed during active periodontal disease [42].
Several studies have suggested that EPO can trigger bone formation from mesenchymal stem cells (mScs) [43, 44]. Kim et al., reported that EPO can regulate differentiation of both osteoblasts and osteoclasts through mechanistic target of rapamycin kinase (mtor) signaling. In this process, EPO improved bone formation of mScs [44]. In the study by Hamed, investigators reported that EPO promotes healing and stimulate the synthesis of collagen [45]. Accordingly, the increased clinical attachment gain could be attributed to the effects of EPO on bone formation and epithelial attachment.
Reduced gingival inflammation secondary to mechanical control of microbial biofilm and irritants promotes establishment of long junctional epithelium and consequently, a new periodontal attachment [46]. In addition, decreased penetration of periodontal probe may be a result of reduced tissue inflammation [47]. In a 12 weeks experimental study by Omlor etal, authors concluded that systemic or locally administration of EPO, as an additional treatment option, was beneficial to increase bone healing in rabbit long- bone defects. In addition administration of EPO significantly increased blood vessel formation after either local of systemic single-dose EPO treatment [48]. Therefore, the higher reduction in PD may be a result of reduced inflammatory response, gingival recession and bone regeneration in EPO treated sites.
Several studies showed the beneficial effect of administration of sub anti- microbial dose Doxycycline (SDD) [49, 50] and host modulatory drugs [51] on periodontal parameters improvement which could reduce the need for surgical interventions. In our study, the difference in the percentage of patients requiring surgery (PD > 5 mm) between the experimental and the control group was not statistically significant at the end of the study, which implicates further studies designed to investigate different doses of EPO within shorter intervals on larger amount of patients [41].
Locally delivered, controlled-release antimicrobials have been designed to maintain high and clinically relevant concentrations of drug in the GCF for extended periods [10]. Administration of controlled-release EPO may have more desirable effects. These systems can maximize the clinical benefit for patients. In addition, with controlled-release systems, the medication is applied in fewer visits with longer intervals. So, the main limitation of this study is that EPO was not formulated in a controlled-release gel. In order to improve the drug effectiveness, the EPO gel was applied 5 times every other day for 10 days.
Topical erythropoietin may increase the plasma erythropoietin concentration that can cause hematological changes and result in systemic side effects [48]. However, in our study blood samples were not collected to determine the plasma erythropoietin levels and blood cell counts before and after the topical administration of the drug. So, we are unable to conclude that this treatment modality is systemically safe.
Conclusion
Considering the limitations of this study, the results showed that topical administration of EPO has no beneficial effect on the reduction of microbial plaque. However, when used as an adjunct to non-surgical periodontal therapy, it provides a promising result in treatment of patients with moderate to severe chronic periodontitis. Since in this study only three-month follow ups were conducted, we suggest further studies with longer follow up periods. Moreover, we suggest further studies on larger number of subjects and with various doses of EPO to determine the optimal dose of the drug.
Supplementary Information
Additional file 1. Ethics-informed consent.
Acknowledgements
None.
Abbreviations
- SRP
Scaling and root planning
- EPO
Erythropoietin
- PI
Plaque index
- GI
Gingival index
- CAL
Clinical attachment level
- PD
Probing depth
- BI
Bleeding index
- HMTs
Host modulatory therapies
- VEGF
Vascular endothelial growth factor
- FGF2
Fibroblast growth factor
- mScs
From mesenchymal stem cells
- mtor
Mechanistic target of rapamycin kinase
- SDD
Sub anti- microbial dose doxycycline
Authors' contributions
Conceptualization: HA, SY, Methodology: SA, Data Analysis: SA, SY, Investigation: NK, HA, Writing—review and editing: NK, HA. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials are available with corresponding author.
Declarations
Ethics approval
All procedures performed in this study were approved with ID number of IRCT2017091636203N1in Tehran University of medical sciences and were in accordance with the ethical standards from the last update of Helsinki Declaration. Informed consent obtained from all the participants.
Informed consent
Uploaded as Additional file 1.
Consent to publish
All the authors declare that have Consent to publish this article in the “BMC oral health” journal.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albandar JM. Underestimation of periodontitis in NHANES surveys. J Periodontol. 2011;82(3):337–341. doi: 10.1902/jop.2011.100638. [DOI] [PubMed] [Google Scholar]
- 2.Preshaw PM, Seymour RA, Heasman PA. Current concepts in periodontal pathogenesis. Dent Update. 2004;31(10):570–578. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 3.Kantarci A, Van Dyke TE. Lipoxin signaling in neutrophils and their role in periodontal disease. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):289–299. doi: 10.1016/j.plefa.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol. 1997;14(1):112–143. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 5.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol. 1997;14(1):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 6.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 7.Higgs G, Vane J. Inhibition of cyclo-oxygenase and lipoxygenase. Br Med Bull. 1983;39(3):265–270. doi: 10.1093/oxfordjournals.bmb.a071831. [DOI] [PubMed] [Google Scholar]
- 8.Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71(4):521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 9.Wesley SJ. Microbial evaluation of a single subgingival irrigation with chlorhexidine and benzydamine in advanced periodontitis. World. 2014;5(1):37–41. [Google Scholar]
- 10.Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza's clinical periodontology. Amsterdam: Elsevier Health Sciences; 2011. [Google Scholar]
- 11.Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264(5):405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 12.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78(3):183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 13.Strunk T, Härtel C, Temming P, Matzke N, Zimmer J, Schultz C. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;97(1):16–20. doi: 10.1111/j.1651-2227.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 14.Sakanaka M, Wen T-C, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci. 1998;95(8):4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci. 2002;99(16):10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhanam AVR, d’Uscio LV, Peterson TE, Katusic ZS. Activation of endothelial nitric oxide synthase is critical for erythropoietin-induced mobilization of progenitor cells. Peptides. 2008;29(8):1451–1455. doi: 10.1016/j.peptides.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson T, Katusic ZS. EPO tecting the endothelium. Br J Pharmacol. 2007;150(7):823–825. doi: 10.1038/sj.bjp.0707162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014;22(1):23–33. doi: 10.1111/wrr.12135. [DOI] [PubMed] [Google Scholar]
- 19.Bader A, Lorenz K, Richter A, Scheffler K, Kern L, Ebert S, et al. Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation Res. 2011;14(1):57–66. doi: 10.1089/rej.2010.1050. [DOI] [PubMed] [Google Scholar]
- 20.Giri P, Ebert S, Braumann U-D, Kremer M, Giri S, Machens H-G, et al. Skin regeneration in deep second-degree scald injuries either by infusion pumping or topical application of recombinant human erythropoietin gel. Drug Des Dev Ther. 2015;9:2565. doi: 10.2147/DDDT.S79425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geary MB, Li H, Zingman A, Ketz J, Zuscik M, De Mesy Bentley KL, et al. Erythropoietin accelerates functional recovery after moderate sciatic nerve crush injury. Muscle Nerve. 2017;56(1):143–151. doi: 10.1002/mus.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaghobee S, Rouzmeh N, Aslroosta H, Mahmoodi S, Khorsand A, Kharrazifard MJ. Effect of topical erythropoietin (EPO) on palatal wound healing subsequent to free gingival grafting (FGG). Brazilian Oral Research. 2018;32. [DOI] [PubMed]
- 23.Brines M. From the cover: erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci. 97(19). [DOI] [PMC free article] [PubMed]
- 24.de Lucas CA, Bond WS, Rex TS. Safety and angiogenic effects of systemic gene delivery of a modified erythropoietin. Gene Ther. 2015;22(5):365–373. doi: 10.1038/gt.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson RV, Boyd KM, Webb DJ. The revision of the declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89:S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 27.Silness J, Löe H. Periodontal disease in pregnancy II Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22(1):121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 28.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. 1959.
- 29.Newbrun E. Indices to measure gingival bleeding. J Periodontol. 1996;67(6):555–561. doi: 10.1902/jop.1996.67.6.555. [DOI] [PubMed] [Google Scholar]
- 30.Hefti AF, Preshaw PM. Examiner alignment and assessment in clinical periodontal research. Periodontol. 2012;59(1):41–60. doi: 10.1111/j.1600-0757.2011.00436.x. [DOI] [PubMed] [Google Scholar]
- 31.Golub LM, Lee HM. Periodontal therapeutics: current host-modulation agents and future directions. Periodontol. 2020;82(1):186–204. doi: 10.1111/prd.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkhouli A. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study) J Periodontal Res. 2011;46(2):261–268. doi: 10.1111/j.1600-0765.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 33.Özden FO, Sakallioğlu EE, Demir E, Bilgici B, Tunçel ÖK, Gökosmanoğlu F, et al. Effect of bisphosphonate as an adjunct treatment for chronic periodontitis on gingival crevicuar fluid levels of nuclear factor-κB ligand (RANKL) and osteoprotegerin in postmenopausal osteoporosis. J Oral Sci. 2017;59(1):147–155. doi: 10.2334/josnusd.16-0241. [DOI] [PubMed] [Google Scholar]
- 34.Cervellini I, Sacre S, Ghezzi P, Mengozzi M. Erythropoietin does not affect TNF and IL-6 production directly. J Biol Regul Homeost Agents. 2013;27(1):189–196. [PubMed] [Google Scholar]
- 35.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141(1):14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 36.Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78(1):41–53. doi: 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- 37.Rölfing JHD, Bendtsen M, Jensen J, Stiehler M, Foldager CB, Hellfritzsch MB, et al. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J Orthop Res. 2012;30(7):1083–1088. doi: 10.1002/jor.22027. [DOI] [PubMed] [Google Scholar]
- 38.Wan L, Zhang F, He Q, Tsang WP, Lu L, Li Q, et al. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS ONE. 2014;9(7):e102010. doi: 10.1371/journal.pone.0102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klontzas E, Kenanidis I, MacFarlane J, Michail R, Potoupnis TE, Heliotis M, et al. Investigational drugs for fracture healing: preclinical & clinical data. Expert Opin Investig Drugs. 2016;25(5):585–596. doi: 10.1517/13543784.2016.1161757. [DOI] [PubMed] [Google Scholar]
- 40.Galeano M, Altavilla D, Bitto A, Minutoli L, Calò M, Cascio PL, et al. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit Care Med. 2006;34(4):1139–1146. doi: 10.1097/01.CCM.0000206468.18653.EC. [DOI] [PubMed] [Google Scholar]
- 41.Fayyazzadeh E, Ahmadi SH, Rabani S, Boroumand MA, Salavati A, Sotoudeh AM. A comparative study of recombinant human basic fibroblast growth factor (bFGF) and erythropoietin (EPO) in prevention of skin flap ischemic necrosis in rats. 2012. [PubMed]
- 42.Chen F-M, Zhang J, Zhang M, An Y, Chen F, Wu Z-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Wu F, Song Y, Duan Y, Jin Z. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 MAPK pathway. Int J Mol Med. 2018;41(2):829–835. doi: 10.3892/ijmm.2017.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113(1):220–228. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Investig Dermatol. 2010;130(1):287–294. doi: 10.1038/jid.2009.219. [DOI] [PubMed] [Google Scholar]
- 46.Pradeep A, Karvekar S, Nagpal K, Patnaik K, Guruprasad C, Kumaraswamy K. Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: a randomized, placebo-controlled clinical trial. J Periodontol. 2015;86(6):738–745. doi: 10.1902/jop.2015.140631. [DOI] [PubMed] [Google Scholar]
- 47.Garnick J, Keagle J, Searle J, King G, Thompson W. Gingival resistance to probing forces: II. The effect of inflammation and pressure on probe displacement in beagle dog gingivitis. J Periodontol. 1989;60(9):498–505. doi: 10.1902/jop.1989.60.9.498. [DOI] [PubMed] [Google Scholar]
- 48.Omlor GW, Kleinschmidt K, Gantz S, Speicher A, Guehring T, Richter W. Increased bone formation in a rabbit long-bone defect model after single local and single systemic application of erythropoietin. Acta Orthop. 2016;87(4):425–431. doi: 10.1080/17453674.2016.1198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emingil G, Gürkan A, Tervahartiala T, Hernandez M, Özgül S, Sorsa T, et al. Adjunctive effects of a sub-antimicrobial dose of doxycycline on clinical parameters and potential biomarkers of periodontal tissue catabolism. Dentistry J. 2019;7(1):9. doi: 10.3390/dj7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preshaw PM, Hefti AF, Jepsen S, Etienne D, Walker C, Bradshaw MH. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis: a review. J Clin Periodontol. 2004;31(9):697–707. doi: 10.1111/j.1600-051X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 51.Preshaw PM. Host modulation therapy with anti-inflammatory agents. Periodontol. 2018;76(1):131–149. doi: 10.1111/prd.12148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Ethics-informed consent.
Data Availability Statement
Data and materials are available with corresponding author.