Abstract
Background and purpose
With the advent of more intensive chemotherapy regimens, neoadjuvant chemoradiotherapy (NACRT) for patients with locally advanced rectal cancer (LARC) has always been questioned due to its inevitable radiation toxicity. Hence, we conducted a meta-analysis to compare the clinical efficacy of neoadjuvant chemotherapy (NAC) and NACRT.
Materials and methods
Eligible studies were searched using PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science up to 31 July 2020, comparing the clinical efficacy of NAC versus NACRT for LARC. Short- and long-term outcomes were determined using the odds ratio (OR) with 95% confidence interval (CI).
Results
Six studies with 12,812 patients were eligible for this meta-analysis, including 677 patients in the NAC group and 12,135 patients in the NACRT group. There were no significant differences between the two groups in terms of pathological complete response rate (OR=0.62, 95%CI=0.27~1.41), N down-staging rate (OR=1.20, 95%CI=0.25~5.79), R0 resection rate (OR=1.24, 95%CI=0.78~1.98), and local relapse rate (OR=1.12, 95%CI=0.58~2.14). The pooled OR for the total response rate and T down-staging were in favor of NACRT (OR=0.41, 95%CI=0.22~0.76 versus OR=0.67 95%CI=0.52~0.87). However, the pooled OR for the sphincter preservation rate favored NAC compared with NACRT (OR=1.87, 95%CI=1.24~2.81). Moreover, NAC was found to be superior to NACRT in terms of distant metastasis (14.3% vs. 20.4%), but the difference was not significant (OR=0.84, 95%CI=0.31~2.27).
Conclusion
We concluded that NAC was superior to NACRT in terms of the sphincter preservation rate, and non-inferior to NACRT in terms of pCR, N down-staging, R0 resection, local relapse, and distant metastasis. However, the conclusion warrants further validation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-021-02251-0.
Keywords: Locally advanced rectal cancer, Neoadjuvant chemoradiotherapy, Neoadjuvant chemotherapy, Meta-analysis
Introduction
Colorectal cancer (CRC) is the third leading cancer worldwide. Approximately eight hundred and sixty thousand patients die of CRC annually [1]. Patients with rectal cancer typically have a better prognosis than those with colon cancer [1], but various kinds of neoadjuvant modalities have been tried to improve the prognosis of patients with rectal cancer, especially for those with locally advanced rectal cancer (LARC). Among the current neoadjuvant modalities, neoadjuvant chemoradiotherapy (NACRT) is preferred [2, 3].
However, the clinical value of NACRT has always been questioned. Data from the Surveillance, Epidemiology, and End Results (SEER) showed that less than 37.5% of patients received NACRT [4]. Reasons are as follows: (1) the survival benefit of NACRT has not been confirmed up to today [5–9]; (2) the current rate of pathological complete response following NACRT is 10% to 25% [10, 11], which is far from satisfactory; (3) adverse events (AEs) related to radiotherapy including radiation colitis decrease the compliance of patients [12–14]; and (4) radiotherapy is deemed to increase the difficulty of surgical dissection and the risk of postoperative complications, which often make the surgeons hesitate to applicate it.
In recent decades, intensive regimens of adjuvant chemotherapy including the FOFLOX [15] and fluorouracil and leucovorin and oxaliplatin regimens [16] have been confirmed as superior to conventional regimens. This highlights the potential use of neoadjuvant chemotherapy (NAC) alone. Matsumoto et al. [17] found that NAC could acquire a similar rate of pathological complete response (pCR) but without radiation toxicity, which was confirmed by the latter reports [18]. In addition, NAC was also found to increase the rate of sphincter preservation by Okuyama et al. [18]. Considering that the results of published studies were not consistent, we conducted a meta-analysis to compare the clinical efficacy of NAC and NACRT for LARC.
Methods
This systematic review was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [19] and Assessing the methodological quality of systematic reviews (AMSTAR) Guidelines [20].
Literature search
PubMed, MEDLINE, Embase, the Cochrane Library, and Google Scholar were used to identify the potentially eligible studies comparing the clinical efficacy of NAC and NACRT in patients with LARC, up to 31 July 2020, by two independent researchers from the same establishment. The keywords “rectal cancer,” “adenocarcinoma of rectum,” “local staging,” “locally advanced rectal cancer,” “neo-adjuvant,” “chemotherapy,” “neo-adjuvant chemotherapy,” “Concomitant Chemoradiotherapies,” “surgery,” and “Neoadjuvant Treatment” were used in all possible combinations. Studies comparing the clinical efficacy of NAC and NACRT for patient with LARC. Search strategy for MEDLINE via PubMed was depicted in Supplement 1. Searching strategies for other databases were performed correspondingly. In addition, relevant trials either ongoing or unpublished were also searched in www.clinicaltrials.gov and www.clinicaltrialsregister.eu. Any potentially eligible studies were searched manually from the included studies, reviews, letters, comments, and abstracts from meetings.
Selection criteria
Inclusion criteria are as follows: (1) patients were diagnosed as rectal adenocarcinoma by biopsy specimen; (2) patients were staged at TanyN+ M0 or T3/4Nany M0 using CT/MRI/rectal ultrasound; (3) intervention included NAC and NACRT; and (4) outcomes including rates of pCR, response, T down-staging, N down-staging, R0 resection, sphincter preservation, local relapse, distant metastasis, and complications.
Exclusion criteria are as follows: (1) patients with colorectal cancer; (2) tumors were unresectable at diagnosis; (3) single arm studies; (4) case reports, letters, and reviews; (5) follow-up information is incomplete; and (6) pCR data is unknown.
Considering short- and long-term outcomes of most of the trials were reported separately, all the publications of each trial including conference abstracts were identified.
Data extraction
All data were extracted and assessed by two independent investigators with predefined forms, which were as follows: (1) general data including title, first author, journal, publication data, and study design; (2) baseline characteristics, such as tumor stage, patient number, chemoradiotherapy regimens, surgical modality, adjuvant regimens, neoadjuvant chemoradiotherapy, and neoadjuvant chemotherapy; (3) outcomes including short- and long-term outcomes (pCR, response, T down-staging, N down-staging, R0 resection, sphincter preservation, local relapse, and distant metastasis). In the case of disagreement, a third investigator intervened for a conclusion. Considering that pCR is the most widely used surrogate indicator of preoperative neoadjuvant therapy, we took it as the primary and others as secondary outcomes in this meta-analysis.
Definition of outcomes
pCR was defined as absence of microscopic adenocarcinoma cells in the surgical specimen [21].
Local relapse was defined as evidence of tumor within the pelvic or perineal area.
Distant metastasis was defined as recurrence outside the true pelvis.
R0 resection was defined as no evidence of tumor at the surgical margin macroscopically or pathologically.
T-down staging was defined as the reduction of pathological T stage (ypT) from clinical T stage.
N-down staging was defined as the reduction of pathological N stage (ypN) from clinical N stage.
Overall survival (OS) was defined as time to death from any cause, or to end of follow-up (censored).
Disease-free survival (DFS) was defined as time to any recurrence or death, whichever occurred first, or end of follow-up (censored).
Quality assessment
The quality of each included study was determined according to the modified Newcastle-Ottawa Scale (NOS) [22], which contained the three following parts with full score of 9: the selection of study groups (0–4 points), the comparability between the two groups (0–2 points), and the determination of either the exposure or the outcome of interest (0–3 points). Generally, studies scored above 5 were considered to be of high quality.
Statistical analysis
The systematic review and meta-analysis were registered at https://www.crd.york.ac.uk/PROSPERO/ (Review Registry 213733) and performed using RevMan version 5.3 and Stata 15. Odds ratio (OR) with its 95% confidence interval (CI) was chosen as an effect measure to evaluate the rates of pCR, response, T down-staging, N down-staging, R0 resection, sphincter preservation, local relapse and distant metastasis. All results would be investigated for statistical heterogeneity by I2 statistics. If there is considerable heterogeneity (I2 > 50%, P< 0.05) for an outcome, random-effect model would be used [23]. Nevertheless, a sensitivity analysis would be performed in each outcome by removing one of the included studies at a time. Forest plots were conducted to evaluate the publication bias with Begg’s [24] and Egger’s test [25].
Results
Basic characteristic of the included studies
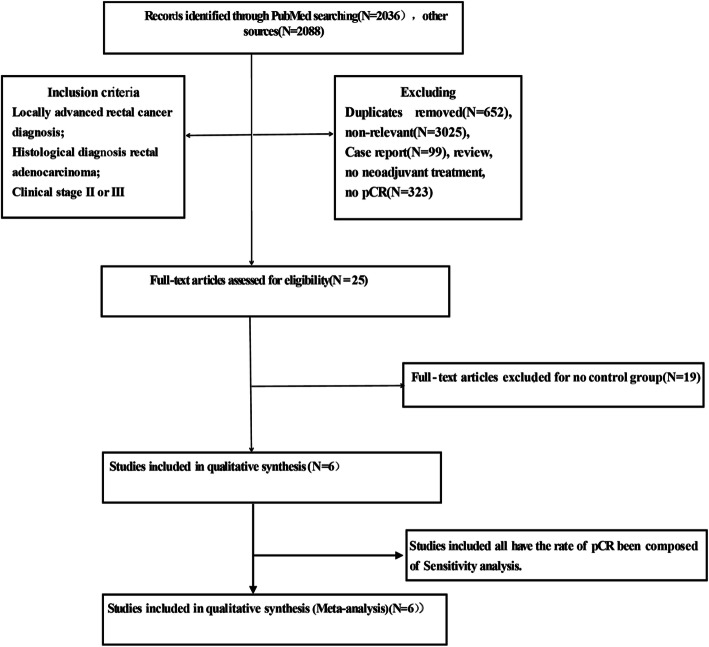
According to the predesigned searching strategy, 4124 records were identified by two independent reviewers, and then 4118 records were excluded based on the established inclusion and exclusion criteria. Finally, seven trials remained to be analyzed in this study (Fig. 1).
Fig. 1.
PRISMA flow diagram showing a selection of articles for meta-analysis
Totally, 12,812 patients were enrolled in this meta-analysis, including 677 patients in the NAC group, 12,135 patients in the NACRT group, respectively. Characteristics of each trial and score of each included study were depicted in Table 1. Briefly, there is only one randomized controlled trial (RCT) [29] eligible for this meta-analysis, and four of the six included studies came from Japan [17, 18, 26, 28]. Patients from China cohorts were much younger than those from other cohorts and had a higher proportion of T4 [29]. Of note, one [27] of the included studies was scored as five, one [18] as six, three [17, 26, 29] as seven, and one [28] as eight, respectively. In addition, the score of each item was depicted in Supplement Table 1.
Table 1.
Characteristics and treatments of the included studies
| Author/year | Country | Arm | No. of Pts | Treatment regimens | Type of surgery | Duration | Design | Median age (years) | Clinical T category (T2/T3/T4) | Clinical N category (N0/N1/N2/N3) | Clinical stage (I/II/III/IV) | Follow-up (months) | pCR | R0 | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matsumoto, 2015 [17] | Japan | NAC | 15 | FOLFOX: 6 cycles, IRIS: 3 cycles, FOLFIRI: 5 cycles | LAR (12), APR/ASR (2), ISR (1) | 2005–2010 | Retrospective | 64 (56–68) | 0/12/3 | NA | NA | 44.2 (30.7–59.5) | 2 | 15 | 7 |
| NACRT | 109 | NA | LAR (75), APR/ASR (22), ISR (3) | 67 (61–74) | 0/92/17 | NA | NA | 0 | 105 | ||||||
| Sakuyama, 2016 [26] | Japan | NAC | 44 | FOLFOX: 6 cycles | ISR (34), other (10) | 2001–2014 | Retrospective | 57.4 (28–76) | 0/38/6 | 5/16/7/16 | 0/5/35/4 | NA | 4 | NA | 7 |
| NACRT | 44 | 5FU+RT (45 Gy/25 F) | ISR (44) | 56 (27–77) | 9/35/0 | 27/10/6/1 | 6/19/29/0 | 9 | NA | ||||||
| Okuyama, 2018 [18] | Japan | NAC | 27 | SOX+cetuximab, SOX+mFOLFOX6 | LAR (19), APR (8) | 2010–2016 | Retrospective | 66 (40–79) | 0/24/3 | 0/18/9/0 | NA | 45.4 | 1 | 26 | 6 |
| NACRT | 28 | 5FU+RT (45 Gy/25 F) | LAR (8), APR (20) | 68 (42–78) | 0/22/5 | 0/17/11/0 | NA | 4 | 26 | ||||||
| Sada, 2018 [27] | American | NAC | 410 | NA | NA | 2006–2010 | Retrospective | NA | NA | NA | NA | NA | 35 | NA | 5 |
| NACRT | 11614 | NA | NA | NA | NA | NA | NA | 1352 | NA | ||||||
| Sato, 2019 [28] | Japan | NAC | 16 | SOX:S1+Ox: 3 cycles | Laparoscopically | 2002–2016 | Retrospective | 67.5 (43–77) | NA | NA | NA | NA | 2 | NA | 8 |
| NACRT | 10 | 5FU+RT (40–45Gy) | Laparotomy | 66 (53–71) | NA | NA | NA | 0 | NA | ||||||
| Deng, 2019 [29] | China | NAC | 165 | mFOLFOX6 | NA | 2010–2015 | RCT | 54.1 | 1/114/50 | 46/76/43/0 | 0/46/119/0 | NA | 10 | 136 | 7 |
| NACRT | 330 | 5FU/mFOLFOX6+RT (46–50.4 Gy/23–25 F) | NA | 54.1/52.1 | 11/206/113 | 67/172/91 | 0/67/263/0 | 61 | 262 |
Note: No. of Pts Number of patients, RCT randomized controlled trial, pCR pathologic complete response, NAC neoadjuvant chemotherapy without radiation, NACRT neoadjuvant chemoradiotherapy, 5FU 5-fluorauracil, OX oxaliplatin, RT radiotherapy, FOLFOX fluorouracil, leucovorin, and oxaliplatin, IRIS irinotecan, tegafurgimeracil-oteracil potassium, FOLFIRI fluorouracil, leucovorin, and irinotecan, SOX S-1+ oxaliplatin, S-1 tegafurgimeracil-oteracil potassium, mFOLFOX6 modified infusional fluorouracil, leucovorin, and oxaliplatinm, XELOX capecitabine + oxaliplatinm, APR, abdominoperineal resection, ISR intersphincteric resection, LAR low anterior resection, ASR abdominosacral resection, NOS Newcastle-Ottawa Scale, NA not available
Regimens of NAC and NACRT in each included study were depicted in Table 2, which showed that differences existed among the different studies, especially for the regimen of NAC. Surgical techniques were also depicted in Table 2.
Table 2.
Outcomes of included studies
| Outcomes | Terms | HR (95%CI) | Heterogenicity | Heterogenicity | Effect size | Effect size2 |
|---|---|---|---|---|---|---|
| P | I2 (%) | Z | P | |||
| pCR | 6 | 0.62 (0.27, 1.41) | 0.01 | 66 | 1.15 | 0.25 |
| R0 Resection | 3 | 1.24 (0.78, 1.98) | 0.93 | 0 | 0.91 | 0.36 |
| sphincter preservation | 5 | 1.87 (1.24, 2.81) | 0.20 | 35 | 3.01 | 0.003 |
| Response | 4 | 0.46 (0.27, 0.76) | 0.02 | 68 | 3.02 | 0.003 |
| N downstaging rates | 3 | 1.20 (0.25, 5.79) | <0.001 | 90 | 0.23 | 0.82 |
| T downstaging rates | 3 | 0.67 (0.52, 0.87) | 0.36 | 2 | 2.99 | 0.003 |
| Local relapse rate | 3 | 1.12 (0.58, 2.14) | 0.86 | 0 | 0.33 | 0.74 |
| Distant metastases rate | 2 | 0.84 (0.31, 2.27) | 0.23 | 31 | 0.35 | 0.73 |
Note: pCR pathologic complete response, HR (95%CI), hazard rate (95% confidence interval)
Meta-analysis of tumor response to neoadjuvant treatment
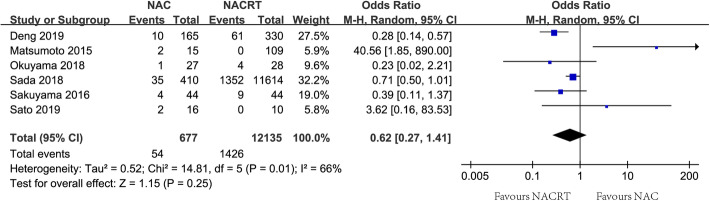
pCR as the primary endpoint was evaluated in six included studies [17, 18, 26–29], and a significant difference was observed among the included studies (I2=66%, P=0.01). The rate of pCR was lower in the NAC group than that in the NACRT group (8.0% vs. 11.8%), but there was no significant difference between groups of NAC and NACRT using a random-effect model (OR=0.62, 95%CI=0.27~1.41, Fig. 2). The rates of total response, T down-staging, and N down-staging were evaluated in the included studies of four [18, 26, 27, 29], three [26–28], and three [26–28], respectively. I2 for the meta-analysis of total response, T down-staging and N down-staging were 68% (P=0.02), 2% (P=0.36), and 90% (P< 0.01), respectively. The pooled OR for the rate of response was not in favor of NAC using a random-effect model (31.6% vs. 42.5%, OR=0.63, 95%CI=0.46~0.85, Table 2). The inferiority of NAC to NACRT was also found in the pooled OR for the rate of T down-staging using a fixed-effect model (16.4% vs. 20.1%, OR=0.67, 95%CI=0.52~0.87, Table 2), but there was no significant difference in the pooled OR for the rate of N down-staging (46.6% vs. 56.3%, OR=1.20, 95%CI=0.25~5.79, Table 2).
Fig. 2.
Forest plot of pathological complete response rate between groups of NAC and NACRT
Meta-analysis of R0 resection and sphincter preservation
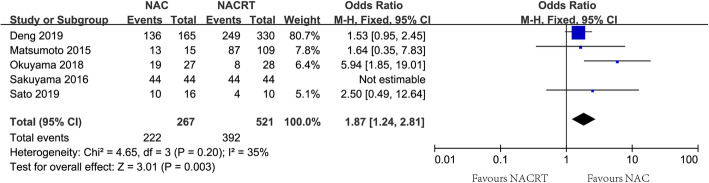
The rate of R0 resection was evaluated in three included studies [17, 18, 29], and no heterogeneity was found (I2=0, P=0.93). Using a fixed-effect model, there was no significant difference in the pooled rate of R0 resection between groups of NAC and NACRT (85.5% vs. 84.2%, OR=1.24, 95%CI=0.78~1.98, Table 2). The rate of sphincter preservation as the secondary outcome was evaluated in five included studies [17, 18, 26, 28, 29], and significant heterogeneity was not observed among the included studies (I2=35%, P=0.20). The pooled OR for the rate of preservation was in favor of NAC using a fixed-effect model (83.1% vs. 75.2%, OR=1.87, 95%CI=1.24~2.81, Fig. 3).
Fig. 3.
Forest plot of sphincter preservation between groups of NAC and NACRT
Meta-analysis of local relapse and distant metastasis
The rate of local relapse was evaluated in four included studies [17, 18, 29], and no heterogeneity was found among the included studies (I2=0, P=0.86). The rate of local relapse was comparable between groups of NAC and NACRT (7.2% vs. 6.4%), and the pooled OR was 1.12 (95%CI=0.58~2.14, Table 2) using a fixed-effect model. The rate of distant metastasis was evaluated in two included studies [17, 18], and significant heterogeneity was not found among the included studies (I2=31%, P=0.23). The rate of distant metastasis was lower in the NAC group than that in the NACRT group (14.3% vs. 20.4%), but the pooled OR for the rate of distant metastasis was 0.84 (95%CI=0.31~2.27, Table 2) using a fixed-effect model.
Complications
Complications were evaluated in three included studies, which were depicted in Table 3. Generally, there was no significant difference in each included study.
Table 3.
Complications of the included studies
| Study | Treatment | Patients | Related complications |
|---|---|---|---|
| Matsumoto, 2015 [17] | NAC | 15 | Grade 3–4 adverse events: 2 neutropenia and 1 diarrhea |
| NACRT | 109 | NA | |
| Okuyama, 2018 [18] | NAC | 27 |
Grade 3–4 adverse events: no; Frequent toxic events:grade 1 neutropenia |
| NACRT | 28 |
Grade 3–4 adverse events: no; Frequent toxic events: grade 1 diarrhea |
|
| Deng, 2019 [29] | NAC | 152 | Grade 3/4 toxicities: leukopenia 9; neutropenia 15; nausea/vomiting 4; diarrhea 12 |
| NACRT | 292 | Grade 3/4 toxicities: leukopenia 48; neutropenia 40; nausea/vomiting 13; diarrhea 35; radiation dermatitis 54; radiation proctitis 35; |
Note: NAC neoadjuvant chemotherapy without radiation, NACRT neoadjuvant chemoradiation therap, NA not available
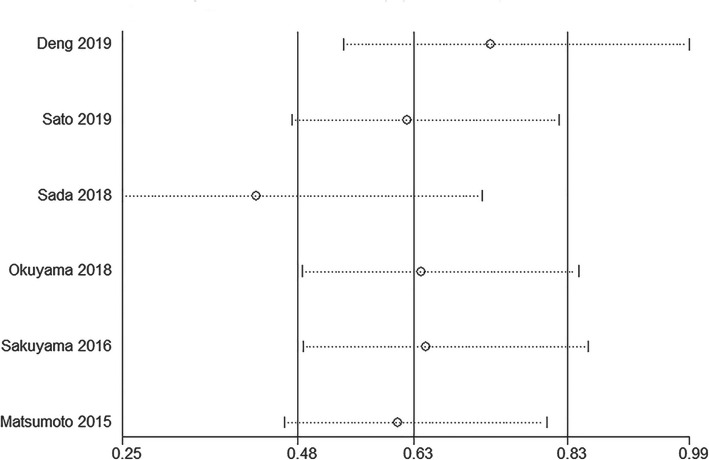
Sensitivity analysis
Sensitivity analysis was conducted for the rate of pCR. Result showed that the pooled OR would not change greatly by removing any single included study (Fig. 4), which indicated that the result was robust.
Fig. 4.
Sensitivity analysis of the pathological complete response
Publication bias
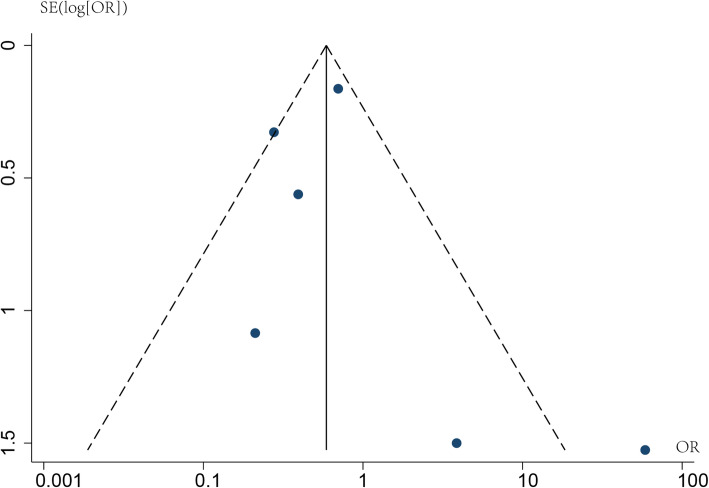
Publication bias was evaluated for the rate of pCR. Asymmetry was not observed in the funnel plot for the rate of pCR (Fig. 5), and the P value of Egger’s and Begg’s tests were 0.452 and 0.568, respectively.
Fig. 5.
Funnel plot about publication bias of pathological complete response
Discussion
NACRT followed by surgery has been the standard treatment for LARC according to the guidelines of the National Comprehensive Cancer Network (NCCN) [2], but its long-term efficacy including both OS and DFS has never been confirmed [5, 6]. On the other hand, previous clinical trials have identified the clinical efficacy of NAC alone [30–43] which challenged the current neoadjuvant modality. This was the first meta-analysis comparing the clinical efficacy of NAC and NACRT for patients with LARC. Six studies including 12,812 patients were identified to be eligible in this meta-analysis. Results showed that NAC was not inferior to NACRT in terms of pCR, R0 resection, local relapse, and distant metastasis, but was superior to NACRT in terms of sphincter preservation.
pCR is one of the most important indexes to evaluate the efficacy of neoadjuvant treatments. Compared with neoadjuvant radiotherapy or chemotherapy alone, NACRT has a higher rate of pCR [44], although it remains unsatisfactory. But no significant difference was observed in terms of pCR between groups of NAC and NACRT in this meta-analysis (8.0% vs. 11.8%, OR=0.62, 95%CI=0.27~1.41), mainly because more intensive chemotherapy regimens and more courses were administrated in the group of NAC. Nonetheless, the advantage of NACRT over NAC in terms of the total response rate and T down-staging was confirmed in this meta-analysis (16.4% vs. 20.1%, OR=0.67, 95%CI=0.52~0.87). N down-staging is an advantage of NAC, but in this meta-analysis, there was no significant difference between groups of NAC and NACRT. Hence, we recommend NACRT as the first-line treatment for LARC patients, if they are present with a more advanced T stage or unwilling to receive surgery.
Sphincter preservation is well concerned by surgeons and patients, especially for those with lower LARC. Sphincter preservation is also one of the initial aims of neoadjuvant treatments, but NARCT was reported to be associated with an increased risk of fistula and worse anal function [12, 45]. In this meta-analysis, NAC is found to be superior to NACRT in terms of sphincter preservation (83.1% vs. 75.2%, OR=1.87, 95%CI=1.24~2.81). Hence, NAC would be recommended first for patients with a strong willingness to preserve anal.
In the era of total mesorectal excision (TME), local relapse is no longer fatal with the rate of 11% [5, 46]. In this meta-analysis, NAC exhibited equivalent efficacy in terms of local relapse (7.2% vs. 6.4%). On the contrary, distant metastasis is still the dominant cause for treatment failure, which is reported to be as high as 25% to 30% [6, 47, 48]. In this meta-analysis, we found that the rate of distant metastasis was lower in the NAC group than that in the NACRT group (14.3% vs. 20.4%), although it lacked statistical significance in the pooled OR (OR=0.84, 95%CI=0.31~2.27). Considering that distant metastasis was only evaluated in two included studies and both of them were from Japan [17, 18], where lateral lymph node dissection (LLND) was conducted routinely in the procedure of surgery, subgroup analysis stratified by LLND or not should be expected in future.
The advantages of NAC also lie on its low toxicity and high compliance. Generally, NAC but not NACRT could not cause severe fibrosis, which often increases the difficulty of surgery and risk of the fistula. Radiotherapy toxicity such as radiation colitis is inevitable, although most of the radiation colitis is mild and transient [49–51]. In addition, patients receiving NAC are more likely to receive adjuvant chemotherapy, compared with those receiving NACRT [52–54]. What is important, NAC could offer a chance for patients with early local recurrence to receive a salvage curative radiotherapy.
However, there were several limitations in this meta-analysis. First, most of the studies (5/6) were retrospective, which indicated that recalling bias and selection bias were hard to avoid. Second, in this meta-analysis, we included studies from 2011 to 2019, during which staging systems on rectal cancer have experienced a few changes on N staging [2]. According to the 8th (American Joint Committee on Cancer (AJCC) staging system, tumor deposits are defined as N1c if no regional lymph nodes are positive; while it was not referred in the 7th AJCC staging system. However, it might not bring substantial changes to our research, because LARC include Tany N+ or T3/4Nany. Third, most of the studies (4/6) were from Japan, where LLND was conducted routinely, which would weaken the conclusion of this meta-analysis. Forth, the NAC regimens were largely different for each included study, and the optimum regimen has not been reached. The last but not the least, data on the adjuvant radiotherapy was not available, which would weaken the conclusion of this meta-analysis, although adjuvant radiotherapy is not routinely used in clinic.
Conclusion
With the current data, we concluded that NAC could be taken as a reasonable alternative to CRT in LARC, and it should be given priority to recommend for patients with T2/3, and strong willingness to sphincter preservation. Nonetheless, NACRT should be recommended firstly if patients were present with T4, high-risk factors including positive circumferential margin involvement, and were not prepared to perform surgery. In future, more intensive NAC regimens including targeted drugs and/or immune therapies are expected, and identifying patients who would be benefited from each of the neoadjuvant modalities is the key.
Supplementary Information
Additional file 1: Table S1 Assessment of methodological quality of included studies for meta-analysis based on the Newcastle-Ottawa Scale for cohort studies.
Additional file 2: Table S2 Search strategy in PubMed.
Acknowledgements
None.
Abbreviations
- NACRT
Neoadjuvant chemoradiotherapy
- LARC
Locally advanced rectal cancer
- NAC
Neoadjuvant chemotherapy
- OR
Odds ratio
- CI
Confidence interval
- CRT
Concurrent chemoradiotherapy
- CRC
Colorectal cancer
- SEER
Surveillance, Epidemiology, and End Results
- AEs
Adverse events
- FOFLOX
Oxaliplatin, fluorouracil (5-FU), and leucovorin
- PCR
Pathological complete response
- OS
Overall survival
- DFS
Disease-free survival
- NOS
Newcastle-Ottawa Scale
- RCT
Randomized controlled trial
- NCCN
National Comprehensive Cancer Network
- TME
Total mesorectal excision
- LLND
Lateral lymph node dissection
- AJCC
American Joint Committee on Cancer
- IRIS
Irinotecan, tegafurgimeracil-oteracil potassium
- FOLFIRI
Fluorouracil, leucovorin and irinotecan
- SOX
Oxaliplatin and tegafurgimeracil-oteracil potassium
- mFOLFOX6
Modified infusional fluorouracil, leucovorin, and oxaliplatin
- XELOX
Capecitabine oxaliplatin
- APR
Abdominoperineal resection
- ISR
Intersphincteric resection
- LAR
Low anterior resection
- ASR
Abdominosacral resection
Authors’ contributions
Huaqin Lin and Xiaohong Zhong acquisition of data, analyzing and interpretation of data; Lei Wang drafting the article; Lingdong Shao and Junxin Wu, designing, revising, and guiding the study. The authors read and approved the final manuscript.
Funding
This research was funded by the Fujian Province Natural Science Foundation, grant numbers 2017 J01260; the Fujian Medical Innovation Project, grant number 2015-CX-8; the Joint Funds for the Innovation of Science and Technology, Fujian Province, grant number 2017Y9074; the Key Clinical Specialty Discipline Construction Program of Fujian, China, the National Clinical Key Specialty Construction Program; and Fujian Province Finance Department Project (No. (2015) 1249); Startup Fund for scientific research, Fujian Medical University (Grant number:2019QH1198).
Availability of data and materials
All the data for this article can be found on PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All the authors of the article agreed to be published in the journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huaqin Lin, Lei Wang and Xiaohong Zhong contributed equally to this work.
Contributor Information
Lingdong Shao, Email: lingdongshao@163.com.
Junxin Wu, Email: junxinwufj@aliyun.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18(7):806–815. doi: 10.6004/jnccn.2020.0032. [DOI] [PubMed] [Google Scholar]
- 3.National Health Commission of the People’s Republic of China. Zhonghua Wai Ke Za Zhi. 2020;58(8):561–85. 10.3760/cma.j.cn112139-20200518-00390. [DOI] [PubMed]
- 4.Wang R, Zhao D, Liu YJ, et al. Prognostic significance of preoperative radiotherapy in stage II and III rectal cancer patients: A Strobe-compliant study of SEER 18 registries database (1988-2011) Neoplasma. 2019;66(6):995–1001. doi: 10.4149/neo_2019_190112N36. [DOI] [PubMed] [Google Scholar]
- 5.van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 7.Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, Weitz J, Koch M. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20(13):4169–4182. doi: 10.1245/s10434-013-3198-9. [DOI] [PubMed] [Google Scholar]
- 8.Allegra CJ, Yothers G, O'Connell MJ, Beart RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A, Evans LS, Bahary N, Soori G, Eakle JF, Robertson JM, Moore DF, Jr, Mullane MR, Marchello BT, Ward PJ, Sharif S, Roh MS, Wolmark N. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J Natl Cancer Inst. 2015;107(11):djv248. doi: 10.1093/jnci/djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azria D, Doyen J, Jarlier M, Martel-Lafay I, Hennequin C, Etienne P, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah J, Mahé M, Bécouarn Y, Dupuis O, Lledo G, Seitz J, Bedenne L, Gourgou-Bourgade S, Juzyna B, Conroy T, Gérard J. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405-PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate-risk rectal cancer. Ann Oncol. 2017;28(10):2436–2442. doi: 10.1093/annonc/mdx351. [DOI] [PubMed] [Google Scholar]
- 10.Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah JF, Mahé MA, Bécouarn Y, Dupuis O, Lledo G, Montoto-Grillot C, Conroy T. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer. 2011;117(16):3703–3712. doi: 10.1002/cncr.25943. [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg M, Glimelius B, Graf W, Påhlman L. Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum. 1998;41(5):543–551. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- 13.Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Adverse Effects of Preoperative Radiation Therapy for Rectal Cancer: Long-Term Follow-Up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23(34):8697–8705. doi: 10.1200/JCO.2005.02.9017. [DOI] [PubMed] [Google Scholar]
- 14.Peeters KCMJ, van de Velde CJH, Leer JWH, Martijn H, Junggeburt JMC, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CAM. Late Side Effects of Short-Course Preoperative Radiotherapy Combined With Total Mesorectal Excision for Rectal Cancer: Increased Bowel Dysfunction in Irradiated Patients—A Dutch Colorectal Cancer Group Study. J Clin Oncol. 2005;23(25):6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 15.Hong YS, Kim SY, Lee JS, Nam BH, Kim KP, Kim JE, Park YS, Park JO, Baek JY, Kim TY, Lee KW, Ahn JB, Lim SB, Yu CS, Kim JC, Yun SH, Kim JH, Park JH, Park HC, Jung KH, Kim TW. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J Clin Oncol. 2019;37(33):3111–3123. doi: 10.1200/JCO.19.00016. [DOI] [PubMed] [Google Scholar]
- 16.Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Ströbel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–989. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Hasegawa S, Zaima M, Inoue N, Sakai Y. Outcomes of Neoadjuvant Chemotherapy without Radiation for Rectal Cancer. Dig Surg. 2015;32(4):275–283. doi: 10.1159/000430469. [DOI] [PubMed] [Google Scholar]
- 18.Okuyama T, Sameshima S, Takeshita E, Yoshioka R, Yamagata Y, Ono Y, Tagaya N, Noie T, Oya M. Therapeutic effects of oxaliplatin-based neoadjuvant chemotherapy and chemoradiotherapy in patients with locally advanced rectal cancer: a single-center, retrospective cohort study. World J Surg Oncol. 2018;16(1):105. doi: 10.1186/s12957-018-1403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 20.Banzi R, Cinquini M, Gonzalez-Lorenzo M, Pecoraro V, Capobussi M, Minozzi S. Quality assessment versus risk of bias in systematic reviews: AMSTAR and ROBIS had similar reliability but differed in their construct and applicability. J Clin Epidemiol. 2018;99:24–32. doi: 10.1016/j.jclinepi.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 21.de Campos-Lobato LF, Stocchi L, da Luz MA, Geisler D, Dietz DW, Lavery IC, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18(6):1590–1598. doi: 10.1245/s10434-010-1506-1. [DOI] [PubMed] [Google Scholar]
- 22.Wells G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses [C]// Symposium on Systematic Reviews: Beyond the Basics. 2014. [Google Scholar]
- 23.Siddaway AP, Wood AM, Hedges LV. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu Rev Psychol. 2019;70(1):747–770. doi: 10.1146/annurev-psych-010418-102803. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naoki Sakuyama MK, Kawano S, Akimoto T, Saito N, Ito M, Ochiai A. Histological differences between preoperative chemoradiotherapy and chemotherapy for rectal cancer: a clinicopathological study. Pathol Int. 2016;66:273–280. doi: 10.1111/pin.12409. [DOI] [PubMed] [Google Scholar]
- 27.Sada YH, Tran Cao HS, Chang GJ, Artinyan A, Musher BL, Smaglo BG, Massarweh NN. Prognostic value of neoadjuvant treatment response in locally advanced rectal cancer. J Surg Res. 2018;226:15–23. doi: 10.1016/j.jss.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Miura T, Morohashi S, Sakamoto Y, Morohashi H, Yoshida T, Hakamada K. Comparable regional therapeutic effects between neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy for locally advanced lower rectal cancer in terms of histopathological analysis. Mol Clin Oncol. 2019;10(6):619–624. doi: 10.3892/mco.2019.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Y, Pan C, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer Final Results of the Chinese FOWARC Trial. J Clin Oncol. 2019;37(34):3223–3233. doi: 10.1200/JCO.18.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cienfuegos JA, Rodríguez J, Baixauli J, et al. Neoadjuvant chemotherapy without radiotherapy for patients with locally advanced rectal cancer. Oncologic outcomes. Rev Esp Enferm Dig. 2020;112(1):16–22. doi: 10.17235/reed.2019.6454/2019. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Huang M, Cai Y, Wang L, Xiao J, Lan P, Hu H, Wu X, Ling J, Peng J, Chen D, Kang L, Zhang Y, Ren D, Wang H, Chen S, Lin F, Zheng J, Zhou Z, Wang J, Deng Y. Neoadjuvant Chemotherapy With mFOLFOXIRI Without Routine Use of Radiotherapy for Locally Advanced Rectal Cancer. Clin Colorectal Cancer. 2019;18(4):238–244. doi: 10.1016/j.clcc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura J, Hasegawa J, Kato T, Yoshioka S, Noura S, Kagawa Y, et al. Phase II trial of capecitabine plus oxaliplatin (CAPOX) as perioperative therapy for locally advanced rectal cancer. Cancer Chemother Pharmacol. 2018;82(4):707–716. doi: 10.1007/s00280-018-3663-z. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi M, Yamada T, Shinji S, Yokoyama Y, Takahashi G, Iwai T, et al. Feasibility of Neoadjuvant FOLFOX Therapy Without Radiotherapy for Baseline Resectable Rectal Cancer. In Vivo. 2018;32(4):937–943. doi: 10.21873/invivo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glynne-Jones R, Hall MR, Lopes A, Pearce S, Goh V, Bosompem S, Bridgewater J, Chau I, Wasan H, Moran B, Melcher L, West NP, Quirke P, Wong WL, Beare S, Hava N, Duggan M, Harrison M. BACCHUS: A randomised non-comparative phase II study of neoadjuvant chemotherapy (NACT) in patients with locally advanced rectal cancer (LARC) Heliyon. 2018;4(9):e00804. doi: 10.1016/j.heliyon.2018.e00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koike J, Funahashi K, Yoshimatsu K, Yokomizo H, Kan H, Yamada T, Ishida H, Ishibashi K, Saida Y, Enomoto T, Katsumata K, Hisada M, Hasegawa H, Koda K, Ochiai T, Sakamoto K, Shiokawa H, Ogawa S, Itabashi M, Kameoka S. Efficacy and safety of neoadjuvant chemotherapy with oxaliplatin, 5-fluorouracil, and levofolinate for T3 or T4 stage II/III rectal cancer: the FACT trial. Cancer Chemother Pharmacol. 2017;79(3):519–525. doi: 10.1007/s00280-017-3243-7. [DOI] [PubMed] [Google Scholar]
- 36.Hata T, Takahashi H, Sakai D, Haraguchi N, Nishimura J, Kudo T, Chu M, Takemasa I, Taroh S, Mizushima T, Doki Y, Mori M. Neoadjuvant CapeOx therapy followed by sphincter-preserving surgery for lower rectal cancer. Surg Today. 2017;47(11):1372–1377. doi: 10.1007/s00595-017-1527-5. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa S, Goto S, Matsumoto T, Hida K, Kawada K, Matsusue R, Yamaguchi T, Nishitai R, Manaka D, Kato S, Kadokawa Y, Yamanokuchi S, Kawamura J, Zaima M, Kyogoku T, Kanazawa A, Mori Y, Kanai M, Matsumoto S, Sakai Y. A Multicenter Phase 2 Study on the Feasibility and Efficacy of Neoadjuvant Chemotherapy Without Radiotherapy for Locally Advanced Rectal Cancer. Ann Surg Oncol. 2017;24(12):3587–3595. doi: 10.1245/s10434-017-5967-3. [DOI] [PubMed] [Google Scholar]
- 38.Bensignor T, Brouquet A, Dariane C, Thirot-Bidault A, Lazure T, Julié C, Nordlinger B, Penna C, Benoist S. Pathological response of locally advanced rectal cancer to preoperative chemotherapy without pelvic irradiation. Colorectal Dis. 2015;17(6):491–498. doi: 10.1111/codi.12879. [DOI] [PubMed] [Google Scholar]
- 39.AlGizawy SM, Essa HH, Ahmed BM. Chemotherapy Alone for Patients With Stage II/III Rectal Cancer Undergoing Radical Surgery. Oncologist. 2015;20(7):752–757. doi: 10.1634/theoncologist.2015-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa J, Nishimura J, Mizushima T, Miyake Y, Kim HM, Takemoto H, Tamagawa H, Noura S, Fujii M, Fujie Y, Kato T, Miwa H, Takemasa I, Ikeda M, Yamamoto H, Sekimoto M, Nezu R, Doki Y, Mori M. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73(5):1079–1087. doi: 10.1007/s00280-014-2417-9. [DOI] [PubMed] [Google Scholar]
- 41.Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H, Ohmiya N, Goto H, Nagino M. Neoadjuvant Oxaliplatin and Capecitabine and Bevacizumab without Radiotherapy for Poor-risk Rectal Cancer: N-SOG 03 Phase II Trial. Japanese J Clin Oncol. 2013;43(10):964–971. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 42.Derwinger K, Kodeda K, Swartling T, Kalebo P, Carlsson G, Gustavsson B. A phase I/II study of neoadjuvant chemotherapy with Pemetrexed (Alimta) in rectal cancer. Eur J Surg Oncol. 2011;37(7):583–588. doi: 10.1016/j.ejso.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, Watanabe M, Kitagawa Y. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol (EJSO). 2010;36(11):1061–1065. doi: 10.1016/j.ejso.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34(27)):3300–3307. doi: 10.1200/JCO.2016.66.6198. [DOI] [PubMed] [Google Scholar]
- 45.Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, Carlsen E, Tveit KM. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76(4):1005–1011. doi: 10.1016/j.ijrobp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 47.Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6(36):38658–38666. doi: 10.18632/oncotarget.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12(8):637–645. doi: 10.1245/ASO.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg PA, Nicholls RJ, Porter NH, Love S, Grimsey JE. Long-term results of a randomised trial of short-course low-dose adjuvant pre-operative radiotherapy for rectal cancer: reduction in local treatment failure. Eur J Cancer. 1994;30A(11):1602–1606. doi: 10.1016/0959-8049(94)00312-s. [DOI] [PubMed] [Google Scholar]
- 50.Kapiteijn E, Kranenbarg EK, Steup WH, et al. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165(5):410–420. doi: 10.1080/110241599750006613. [DOI] [PubMed] [Google Scholar]
- 51.Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75(9):2269–2275. doi: 10.1002/1097-0142(19950501)75:9<2269::aid-cncr2820750913>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Merkow RP, Bentrem DJ, Mulcahy MF, Chung JW, Abbott DE, Kmiecik TE, Stewart AK, Winchester DP, Ko CY, Bilimoria KY. Effect of Postoperative Complications on Adjuvant Chemotherapy Use for Stage III Colon Cancer. Annals of Surgery. 2013;258(6):847–853. doi: 10.1097/SLA.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 53.Haynes AB, You YN, Hu CY, Eng C, Kopetz ES, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Chang GJ. Postoperative chemotherapy use after neoadjuvant chemoradiotherapy for rectal cancer: Analysis of Surveillance, Epidemiology, and End Results-Medicare data, 1998-2007. Cancer. 2014;120(8):1162–1170. doi: 10.1002/cncr.28545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamnagerwalla M, Tay R, Steel M, Keck J, Jones I, Faragher I, Gibbs P, Wong R. Impact of Surgical Complications Following Resection of Locally Advanced Rectal Adenocarcinoma on Adjuvant Chemotherapy Delivery and Survival Outcomes. Dis Colon Rectum. 2016;59(10):916–924. doi: 10.1097/DCR.0000000000000659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Assessment of methodological quality of included studies for meta-analysis based on the Newcastle-Ottawa Scale for cohort studies.
Additional file 2: Table S2 Search strategy in PubMed.
Data Availability Statement
All the data for this article can be found on PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science.