Abstract
Objective:
Visual memory (ViM) declines early in Alzheimer’s disease (AD). However, it is unclear whether ViM impairment is evident in the preclinical stage and relate to markers of AD pathology. We examined the relationship between ViM performance and in-vivo markers of brain pathology in individuals with autosomal dominant AD (ADAD).
Methods:
Forty-five cognitively unimpaired individuals from a Colombian kindred with the Presenilin 1 (PSEN1) E280A ADAD mutation (nineteen carriers and twenty-six non-carriers) completed the Rey-Osterrieth complex figure immediate recall test, a measure of ViM. Cortical amyloid burden and regional tau deposition in the entorhinal cortex (EC) and inferior temporal cortex (IT) were measured using 11C-Pittsburgh compound B positron emission tomography (PET) and 11F-Flortaucipir PET, respectively.
Results:
Cognitively unimpaired carriers and non-carriers did not differ on ViM performance. Compared to non-carriers, unimpaired mutation carriers had higher levels of cortical amyloid and regional tau in both the EC and IT. In cognitively unimpaired carriers, greater cortical amyloid burden, higher levels of regional tau, and greater age were associated with worse ViM performance. Only a moderate correlation between regional tau and ViM performance remained after adjusting for verbal memory scores. None of these correlations were observed in non-carriers.
Conclusions:
Results suggest that AD pathology and greater age are associated with worse ViM performance in ADAD before the onset of clinical symptoms. Further investigation with larger samples and longitudinal follow-up is needed to examine the utility of ViM measures for identifying individuals at high risk of developing dementia later in life.
Keywords: Alzheimer’s disease, amyloid, tau, Rey-Osterrieth complex figure, visual memory, ADAD, preclinical, neuropsychological testing
INTRODUCTION
Recent advances in biomarkers have led to the definition of a preclinical stage of Alzheimer’s Disease (AD), in which pathophysiologic changes (e.g., accumulation of amyloid in the neocortex and tau in the medial temporal lobe) occur many years before the onset of cognitive impairment (Jack et al., 2018; Jack et al., 2010). Given that individuals in the preclinical stage represent a group at high risk for cognitive decline, there is a need to characterize the cognitive performance of individuals in this stage of the disease and how cognitive performance relates with the earliest pathological changes in AD.
The study of families with autosomal dominant AD (ADAD) offers a unique opportunity to characterize the preclinical stage of AD, as carriers of ADAD are virtually guaranteed to develop the disease, allowing the investigation of the earliest cognitive changes of AD (Fuller et al., 2019). Studies conducted in ADAD have suggested that changes in verbal episodic memory (VeM) can be observed approximately 10 years before the onset of clinical impairment (Aguirre-Acevedo et al., 2016; Bateman et al., 2012). In addition, it has been shown that greater cortical amyloid and higher levels of regional tau are associated with worse VeM performance in cognitively unimpaired ADAD carriers (Quiroz et al., 2018).
Evidence suggests that accumulation of pathology in early stages of AD involves brain pathways and structures that support memory processing (Schultz et al., 2018; Sperling et al., 2019). It has been observed, for example, that in cognitively unimpaired ADAD mutation carriers the earliest sites of tau accumulation involve regions of the medial temporal lobe (e.g., the entorhinal cortex) and inferior temporal lobe (Quiroz et al., 2018). In addition, results from human lesion studies and functional neuroimaging studies in cognitively normal adults suggest that these structures and their projections play a critical role not only in VeM but also in visual memory (ViM) processing (Barbeau, Pariente, Felician, & Puel, 2011; Braskie, Small, & Bookheimer, 2009; Fiebach, Rissman, & D’Esposito, 2006; Ranganath, Cohen, Dam, & D’Esposito, 2004).
In contrast to VeM, ViM and its relationship with markers of AD pathology has been less explored in preclinical ADAD. Studies conducted in subjects at risk of developing sporadic AD – in which ViM was included within a larger memory composite – indicated that relative to amyloid-negative older adults, amyloid-positive cognitively normal older adults exhibited greater memory impairment (Lim et al., 2018; Pike et al., 2007). However, it is unclear whether changes in ViM can be observed in individuals with ADAD who are still cognitively unimpaired, and whether these changes are associated with tau pathology, which has been closely associated with the onset of cognitive decline (Quiroz et al., 2018).
The Rey-Osterrieth Complex Figure test recall (ROCF-r) (Rey, 2003) is among the most widely used tests of ViM. The alterations observed in this test have been well documented in patients with mild cognitive impairment (MCI) (Lekeu et al., 2010; Peter et al., 2018) and mild dementia (Salimi et al., 2018), but it is less known how ROCF-r performance relates to in-vivo markers of AD pathology in preclinical stage of AD.
The present study was conducted with young, cognitively unimpaired Presenilin1 (PSEN1) E280A mutation carriers from the world’s largest ADAD kindred who have previously shown to have accumulation of AD pathology and changes in VeM many years before the median age of onset of MCI in this cohort (Aguirre-Acevedo et al., 2016; Fleisher et al., 2015; Quiroz et al., 2018). In this study we extend previous results by exploring whether ViM impairment is evident in preclinical ADAD, and whether it is related to in-vivo markers of AD pathology. We hypothesized that greater in-vivo AD pathology and greater age would be related to worse ROCF-r performance in cognitively unimpaired mutation carriers.
METHODS
Participants
Forty-five cognitively unimpaired individuals from a Colombian kindred with the Presenilin 1 (PSEN1) E280A mutation (nineteen carriers and twenty-six age-matched non-carriers) were recruited from the Colombian Alzheimer’s Prevention Initiative (API) registry (Tariot et al., 2018). The clinical and preclinical courses of this cohort have been well characterized. Mutation carriers develop MCI around 44 years old [95% CI 43–45] and dementia due to AD around the age of 49 [95% CI 49–50] (Acosta-Baena et al., 2011). In mutation carriers, high levels of cortical amyloid burden is observed at an average age of 28 years (Fleisher et al., 2012); and elevated regional tau levels in the medial temporal lobe (e.g. entorhinal and inferior temporal cortices) at an average age of 38 years (Quiroz et al., 2018).
In this cross-sectional study, we included cognitively unimpaired individuals who had: (1) a Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) score of 26 or greater; (2) a Clinical Diagnostic Rating Scale (Morris, 1993) score of 0; and (3) a Functional Assessment Staging Test (Reisberg, 1988) score lower than 3 points. Patients with dementia or with other neurological or psychiatric disorders, such as clinical depression or anxiety, were excluded from the study.
This study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the Ethical Research Committee of University of Antioquia in Colombia and Massachusetts General Hospital (MGH) in Boston. All participants provided written informed consent. Participants and researchers were blind to genetic status.
Procedures
All the participants completed a comprehensive neuropsychological evaluation in Medellín, Colombia at the University of Antioquia and travelled to Boston, USA to undergo PET imaging at MGH. The data of this study are part of the Colombia-Boston (COLBOS) project, a longitudinal study aimed at studying biomarkers and cognitive changes in preclinical ADAD.
Visual Memory: Immediate Recall of Rey-Osterrieth Complex Figure test (ROCF-r)
We measured ViM using the ROCF-r (Rey, 2003). The ROCF test assesses both visuospatial and constructional abilities, as well as ViM. The test consists of a copy (ROCF-c), and immediate recall phase. Participants are first asked to copy a complex geometrical figure and immediately after the copy phase they are asked to recall as much as possible. The maximum score for each phase is 36, and the scores were based on previously established criteria (Rey, 2003). In this study, we analyzed the percent retention of the ROCF (ROCF-pr) in which the recall total score is divided by copy score and multiplied by 100. In contrast to the recall total score, ROCF-pr could be a more useful measure to detect alterations in ViM, given that it removes the effect of execution level from the copy phase (Boone, Lesser, Hill-gutierrez, Berman, & D’Elia, 1993).
In order to compare ViM with VeM, participants completed the word list memory test from the Colombian-normed Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-m) (Aguirre-Acevedo et al., 2007). In this study we included the word list learning and the word list free recall. The maximum number of correct responses of the learning phase and the recall phase is 30 and 10, respectively.
The ROCF-c and CERAD-m scores were transformed to Z scores based on the normative data of a larger sample of Colombians whom were not ADAD mutation carriers (Torres et al., 2019). We also calculated a CERAD-m total score from the average of the Z scores of each subtest. The percent retention’s Z score was calculated based on the data from the non-carriers in this present study, as Colombian norms were not available. Finally, a visual-verbal composite was calculated by averaging the Z scores of the ROCF-pr and of the two CERAD-m subtests. All neuropsychological tests were administered in Spanish, which was the primary language of all participants. The administration and scoring of these cognitive tests were carried out by clinical neuropsychologists of at the Neurosciences Group at the University of Antioquia in Medellín, Colombia.
Brain Imaging
The procedures for the imaging acquisition and processing have been previously described in detail (Quiroz et al., 2018). In brief, cortical amyloid was measured using carbon 11C-Pittsburgh compound B (PiB) positron emission tomography (PET). PiB PET data were expressed as the distribution volume ratio (DVR) and the cerebellar gray matter was used as the reference tissue. PiB PET retention was measured using a cortical regions of interest (ROIs) conformed by frontal, lateral temporal, retrosplenial cortices. Regional Tau levels were assessed using 18F-Flortaucipir (FTP) PET imaging. The FTP binding was expressed in Free-Surfer ROIs as standardized uptake value ratio (SUVR) and the cerebellar grey matter was used as the reference, as previously described (Quiroz et al., 2018). In this study, we examined tau levels in the bilateral entorhinal (EC) and inferior temporal (IT) cortices – regions which were characterized as the earliest sites of tau accumulation in this ADAD cohort (Quiroz et al., 2018). PET data were partial volume corrected.
Statistical Analysis
Demographic, clinical, memory and biomarkers data were analyzed using descriptive measures. Mann-Whitney U and chi-square tests were used to test differences between cognitively unimpaired carriers and non-carriers. Non-parametric effect sizes (r) were calculated by dividing Z score by the squared root of the sample size. Spearman correlations were used to explore the relation among memory scores (ROCF-pr, CERAD-m and visual-verbal composite), in vivo PET, and age. Non-parametric partial correlations were performed between ViM and biomarkers adjusting for VeM and age. Confidence intervals were calculated for the correlation coefficients. Alpha values were set at p < .05. All statistical analyses were carried out on SPSS 24.0 statistical software (IBM, 2016).
RESULTS
Demographics and clinical data
Demographics and clinical data are shown in Table 1. Cognitively unimpaired mutation carriers and non-carriers did not differ on age, level of education, gender distribution, and clinical or neuropsychological test scores. The median age of carriers was 35 years – approximately 9 years younger than the median age of MCI onset in this ADAD cohort (Acosta-Baena et al., 2011).
Table 1.
Demographic data and clinical evaluation.
| Unimpaired carriers | Non-carriers | Carriers versus Non-carriers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 19 | n = 26 | ||||||||||
| Median | IQR | Median | IQR | U | p value | ||||||
| Age (years) | 35.00 | 30.00 | 40.00 | 36.00 | 29.75 | 39.25 | 233.50 | .76a | |||
| Education (years) | 11.00 | 6.00 | 13.00 | 11.00 | 6.50 | 13.25 | 241.50 | .90a | |||
| Gender (F:M)* | 11:8 | 12:14 | .60b | .44b | |||||||
| MMSE score | 29.00 | 28.00 | 29.00 | 29.00 | 28.00 | 29.25 | 191.50 | .17a | |||
| Semantic Fluency | 21.00 | 17.00 | 25.00 | 19.00 | 17.00 | 22.00 | 184.00 | .15a | |||
| Naming score | 14.00 | 13.00 | 14.00 | 14.00 | 12.75 | 14.00 | 218.50 | .48a | |||
| Praxis CERAD | 11.00 | 10.00 | 11.00 | 11.00 | 10.00 | 11.00 | 229.50 | .65a | |||
| Zung Depression Scale | 24.00 | 20.00 | 28.00 | 21.50 | 20.00 | 25.75 | 219.50 | .52a | |||
| GAI | 1.00 | .00 | 6.00 | 1.00 | .00 | 4.00 | 217.50 | .49a | |||
| FAST | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 214.00 | .20a | |||
Note: Values are expressed as median and interquartile range (IQR) with the exception of gender.
MMSE: Mini Mental State Examination; GAI: Geriatric Anxiety Inventory; FAST: Functional Assessment Staging, CERAD: Consortium to Establish a Registry for Alzheimer’s Disease.
U: Mann-Whitney U test.
chi-square test (χ2)
p values were calculated through Mann-Whitney U test.
p values were calculated through chi-square test (χ2).
In-vivo markers of AD pathology
Compared with non-carriers, cognitively unimpaired carriers had greater cortical amyloid and regional tau burden, consistent with a previous report (Quiroz et al., 2018). Biomarker data are shown in Table 2.
Table 2.
Performance of each group on memory tests and biomarker data
| Unimpaired carriers | Non-carriers | Carriers versus Non-carriers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 19 | n = 26 | ||||||||
| Median | IQR | Median | IQR | U | p-value | Effect size (r) | |||
| Memory Test (z scores) | |||||||||
| ROCF-pr | 0.21 | −1.02 | 0.71 | 0.18 | −0.92 | 0.67 | 240.00 | .87 | − |
| CERAD-m | 0.48 | −0.71 | 1.17 | 0.64 | 0.3 | 1.28 | 196.00 | .24 | - |
| Visual-Verbal composite | 0.33 | −0.48 | 0.94 | 0.65 | 0.05 | 0.87 | 215.00 | .46 | |
| Biomarkers | |||||||||
| Cortical Amyloid (DVR) | 1.51 | 1.34 | 1.74 | 1.12 | 1.08 | 1.16 | 17.00 | < .001 | .79 |
| Entorhinal Tau (SUVR) | 1.48 | 1.12 | 1.77 | 1.03 | 0.97 | 1.15 | 98.00 | < .001 | .51 |
| Inferior Temporal Tau (SUVR) | 1.28 | 1.19 | 1.32 | 1.19 | 1.05 | 1.27 | 157,00 | .04 | .31 |
Note: Values are expressed as median and interquartile range (IQR).
ROCF: Rey-Osterrieth Complex Figure test. ROCF-pr: ROCF percent retention. CERAD: Consortium to Establish a Registry for Alzheimer’s Disease. CERAD-m: CERAD word list memory Test.
DVR: distribution volume ratio; SUVR = standardized uptake value ratio.
U: Mann-Whitney U test.
p values were calculated through Mann-Whitney U test.
ViM and VeM
There were no significant differences between cognitively unimpaired carriers and non-carriers on the ROCF-pr, or CERAD-m, and the visual-verbal composite. In contrast with the non-carriers, mutation carriers showed greater variability in these measures. For further reference, see Table 2.
Association among cortical amyloid, regional tau, ViM, and VeM
In cognitively-unimpaired mutation carriers, greater mean cortical PiB retention was related to worse performance on ROCF-pr, CERAD-m, and the visual-verbal composite. Similarly, higher levels of FTP binding in EC and IT were associated with lower performance on ROCF-pr and the visual-verbal composite. The correlations for CERAD-m scores were only observed with tau in the EC, but not in the IT. As shown in Figure 1, the relation between VeM and ViM with the in vivo PET measurements shared a comparable distribution in the scatter plots. Table 3 lists the correlations between markers of AD pathology, ViM, and VeM. None of these associations were observed in non-carriers.
Figure 1.
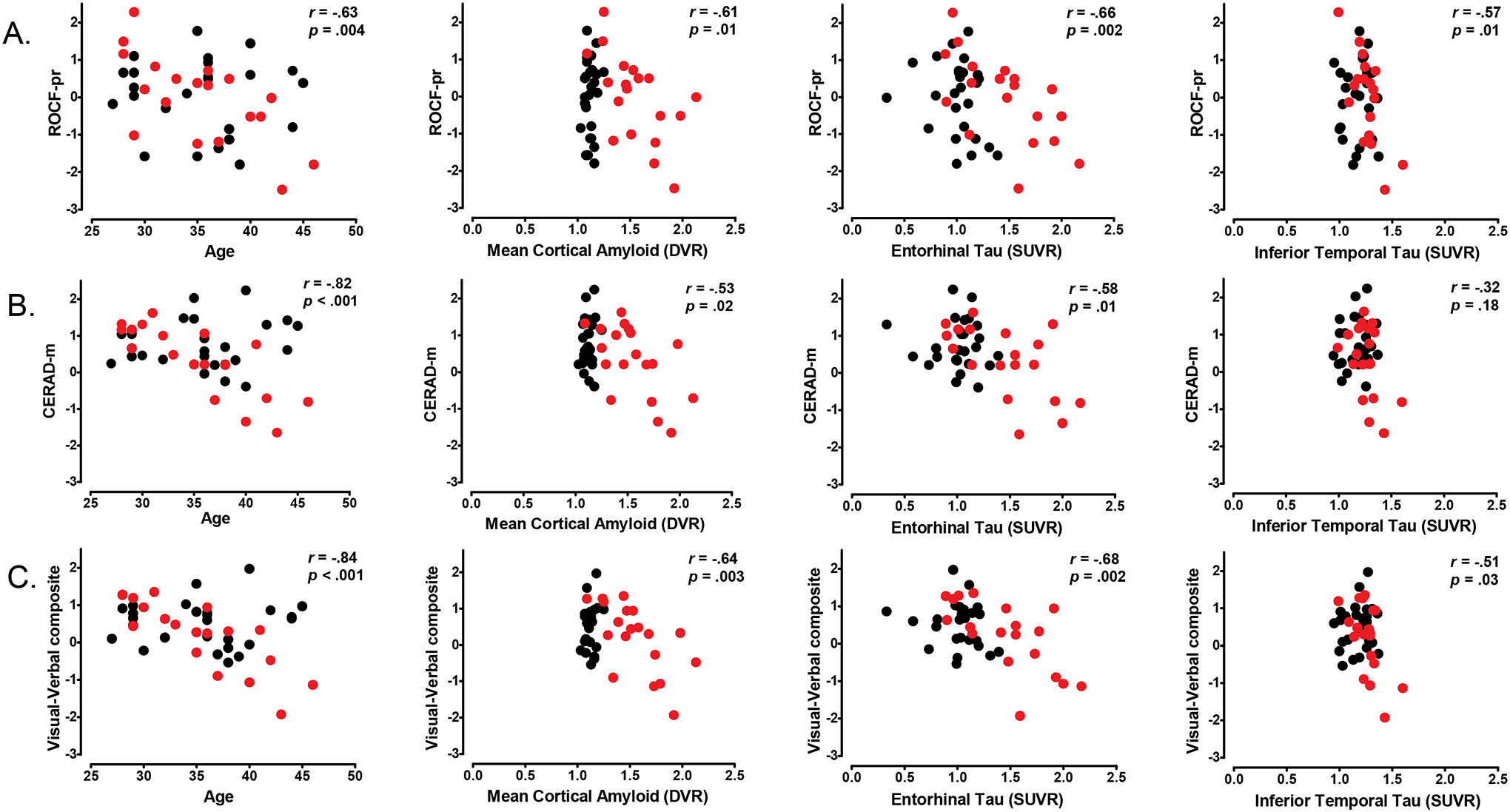
Correlations among in-vivo biomarkers, visual and verbal memory and age. Scatter plots show the negative correlations between in-vivo biomarkers and (A) ROCF-pr and (B) CERAD-m, and (C) Visual-Verbal composite. Memory test are expressed as Z-score. Black circles represent non-carriers, red circles represent cognitively unimpaired PSEN1 mutation carriers. Statistical values included correspond to the correlation coefficients in the mutation carriers. Abbreviations: ROCF: Rey-Osterrieth Complex Figure test; ROCF-pr: ROCF percent retention; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CERAD-m: CERAD word list memory test; DVR: distribution volume ratio; SUVR: standardized uptake value ratio.
Table 3.
Correlations among visual and verbal memory, age and in-vivo biomarkers.
| Unimpaired carriers | Non-carriers | ||||||
|---|---|---|---|---|---|---|---|
| n = 19 | n = 26 | ||||||
|
rs (p value) [95% CI] |
|||||||
| ROCF-pr | CERAD-m | Visual-Verbal composite | ROCF-pr | CERAD-m | Visual-Verbal composite | ||
| Age |
−.63 (.004) [−0.84, −0.25] |
−.82 (< .001) [−0.93, −0.58] |
−.84 (< .001) [−0.94, −0.62] |
−.09 (.66) [−0.46, 0.31] |
.02 (.93) [−0.37, 0.40] |
−.09 (.66) [−0.46, 0.31] |
|
| Cortical Amyloid (DVR) |
−.61 (.01) [−0.83, −0.22] |
−.53 (.02) [−0.79, −0.10] |
−.64 (.003) [−0.85, −0.26] |
.12 (.56) [−0.28, 0.48] |
.15 (.48) [−0.25, 0.51] |
.15 (.47) [−0.25. 0.51] |
|
| Entorhinal Tau (SUVR) |
−.66 (.002) [−0.86, −0.29] |
−.58 (.01) [−0.82, −0.17] |
−.68 (.002) [−0.87. −0.33] |
−.22 (.28) [−0.56, 0.18] |
−.03 (.89) [−0.41, 0.36] |
−.11 (.60) [−0.48, 0.29] |
|
| Inferior Temporal Tau (SUVR) |
−.57 (.01) [−0.81, −0.16] |
−.32 (.18) [−0.68, 0.16] |
−.51 (.03) [−0.78, −0.07] |
.06 (.76) [−0.34, 0.44] |
.19 (.34) [−0.21, 0.54] |
.15 (.44) [−0.24, 0.52] |
|
Note: The correlations were calculated through Spearman’s rho (rs).
ROCF: Rey-Osterrieth Complex Figure test. ROCF-pr: ROCF percent retention. CERAD: Consortium to Establish a Registry for Alzheimer’s Disease. CERAD-m: CERAD word list memory test. CI: confidence interval.
VeM and ViM were positively associated (r = .39, p = .01, 95% CI, [0.11, 0.61]) across all participants. ViM was related to tau pathology, even after controlling for VeM (EC: r = −.49, p = .04, 95% CI, [−0.77, −0.04]; IT: r = −.50, p = .04, 95% CI, [−0.78, −0.05]). A moderate negative correlation between ROCF-pr and cortical PiB retention was observed when controlling for VeM, but this was not significant (r = −.44, p = .07, 95% CI, [−0.75, 0.02]).
Association among age, cortical amyloid, regional tau, ViM, and VeM
We found that greater age in cognitively unimpaired carriers was associated with lower scores in ROCF-pr, CERAD-m and the visual-verbal composite (Table 3). This association was not observed in non-carriers. When we examined the correlations between markers of AD pathology with both ViM and VeM adjusting for age, we did not find any significant associations (ROCF-pr: PiB: r = −.25, p = .33, 95% CI, [0.63, 0.23]; EC tau: r = −.40, p = .10, 95% CI, [−0.72, 0.07]; IT tau: r = −.30, p = .22, 95% CI, [−0.67, 0.18]; CERAD-m: PiB: r = .31, p = .22, 95% CI, [−0.17, 0.67]; EC tau: r = −.02, p = .93, 95% CI, [−0.47, 0.44]; IT tau: r = .37, p = .13, 95% CI, [−0.10, 0.71]; Visual-verbal composite: PiB: r = .06, p = .81, 95% CI, [−0.40, 0.50]; EC tau: r = −.23, p = .35, 95% CI, [−0.62, 0.25]; IT tau: r = −.01, p = .98, 95% [CI, −0.46, 0.45]).
ViM vs. visuo-spatial abilities
We used the ROCF copy to examine whether these associations with age and brain pathology were specific to VIM versus visuo-spatial abilities. No significant correlations were observed between ROCF-c and age (mutation carriers: r = −.06, p = .80, 95% CI, [−0.50, 0.41]). There was no relation between ROCF-c and brain pathology in either group (mutation carriers: PiB: r = .16, p = .52, 95% CI, [−0.32, 0.57]; EC tau: r = −.21, p = .39, 95% CI, [−0.61, 0.27]; IT tau: r = −.11, p = .65, 95% CI, [−0.54, 0.36]).
DISCUSSION
The present study examined the relation between ViM and in-vivo AD pathology in preclinical ADAD. To this end, we administered the ROCF-r test and measured cortical amyloid and regional tau using PET in cognitively unimpaired PSEN1 E280A mutation carriers and non-carrier family members who belong to a large Colombian cohort with ADAD due to the PSEN1 E280A mutation. Our results showed that greater cortical amyloid burden, higher levels of regional tau, and older age were associated with lower ViM performance in cognitively unimpaired mutation carriers.
Our findings showed that the cognitively unimpaired mutation carriers performed similarly to non-carriers on the ROCF-r. Most of the studies in ADAD or subjects at risk of developing sporadic AD have used VeM measures to evaluate memory function (Jansen et al., 2018; Mormino et al., 2017; Papp, Rentz, Orlovsky, Sperling, & Mormino, 2017; Sperling et al., 2013; Storandt, Balota, Aschenbrenner, & Morris, 2014; Wang et al., 2015), and those that have included ViM measures yield mixed findings, probably due to methodological differences. For example, cross-sectional studies, similar to ours, have not found differences on ViM and visual learning tasks between cognitively healthy, amyloid-positive (on PET) adults when compared with amyloid-negative adults (Hollands et al., 2015; Johnson et al., 2014). Longitudinal studies, however, have suggested that relative to amyloid-negative individuals, cognitively normal amyloid-positive older adults exhibited a faster decline on ViM and VeM over thirty-six months of follow-up (Lim et al., 2014), as well as a greater rate of multidomain cognitive decline, including in ViM (Petersen et al., 2016; Zhao et al., 2018). Though we did not find differences between cognitively unimpaired carriers and non-carriers on median ViM performance in our cross-sectional study, future longitudinal studies with larger samples are needed to evaluate the trajectory of ViM change across the ADAD disease continuum.
In our study we found a relationship between markers of AD pathology and ViM measures only in cognitively unimpaired carriers. Higher levels of neocortical amyloid were associated with lower ViM performance, consistent with previous reports showing that in contrast to amyloid-negative older adults, amyloid-positive cognitively normal older adults show greater memory impairment, assessed through a composite of visual and verbal memory tasks (Lim et al., 2018; Pike et al., 2007). Additionally, the progression of ViM and VeM deficits were greater in amyloid-positive apolipoprotein E (APOE) ε4 allele carriers (Lim et al., 2018). Furthermore, past research has suggested that greater amyloid deposition in the precuneus is associated with lower ViM scores in cognitively normal subjects with high PIB retention (Ossenkoppele et al., 2014). The combination of amyloid deposition and neurodegeneration was also associated with greater ViM decline, in addition to other cognitive domains (Zhao et al., 2018).
Unlike VeM studies (Gordon et al., 2019; Quiroz et al., 2018), to our knowledge no previous studies have explored the specific relationship between ViM and regional tau PET in preclinical ADAD. In our study, we found that higher levels of regional tau were significantly related to lower ViM performance in cognitively unimpaired ADAD carriers. Whereas the correlations between CERAD-m and regional tau were only present with EC tau, but not with IT tau, the correlations between ROCF-pr with regional tau were observed in both the EC and IT. The IT cortex and its connections with other regions of the ventral visual pathway has been proposed as a structure that plays a role in ViM processing (Kravitz, Saleem, Baker, Ungerleider, & Mishkin, 2013); consequently, our results do not exclude the possibility that tau accumulation in the IT cortex might contribute to the negative correlation found with ViM in cognitively unimpaired carriers. It is important to note that although the relation between CERAD-m and IT tau was not significant, the association shared a comparable distribution to that shown between ROCF-pr and lT tau (see Figure 1). Therefore, our results suggest that the performance of both types of memory are related to regional tau in preclinical stage, aligning with the hypothesis that tau accumulation is strongly linked to cognitive impairment in AD (Gordon et al., 2019; Pontecorvo et al., 2017; Quiroz et al., 2018).
Considering that the performance of ViM and VeM were related our sample, we conducted additional analyses to explore whether the relations between ViM and tau are specific or moderated by VeM. Our results showed that the association between ROCF-pr and regional tau remained after adjusting for VeM, but the degree of this relation was moderate. These findings suggest that regional tau accumulation might play a role in ViM performance in the preclinical stage of AD.
In line with the previous results, we also found that the scores of the visual-verbal composite were negatively related to both amyloid and regional tau. In contrast with the results of the individual memory tests, the visual-verbal composite might better depict the relation between episodic memory performance and the earliest changes of accumulation of AD pathology. Taken as a whole, our findings suggest that accumulation of brain pathology (amyloid and tau) is related to early changes observed in both VeM and ViM in preclinical AD.
Our findings also suggest that age plays an important role in the relation between memory performance and pathology burden in ADAD, as no relationships survived partial correction by age; the strong effect of age on these correlations is likely due to the fact that in this and other ADAD mutation cohorts, age is considered as a proxy for disease progression (Acosta-Baena et al., 2011). As carriers progress in age closer to the median age of onset of MCI in this cohort (44 years old; Acosta-Baena et al., 2011), it is more likely that decline in ViM and VeM will be evident and that there will be increasing rates of amyloidosis and tau deposition.
It is important to highlight that our findings have clinical implications for the evaluation of memory in the preclinical stage of AD. In this study, we focused on the ROCF-r – probably one of the most widely used tests of ViM in clinic and research contexts. The total score of this test has proven to have good psychometric properties in adults, the elderly population, and patients with memory impairment, with internal reliability greater than 0.8 and interrater reliability > .9 (Berry, Allen, & Schmitt, 1991; Tupler, Welsh, Asare-Aboagye, & Dawson, 1995). Additionally, the ROCF-r test has large studies of normative data, including the data used in this study which were derived from a larger cohort of non-carrier Colombians who belong to families at risk for ADAD due to E280A mutation (Torres et al., 2019). Additionally, this test has been shown to be a sensitive instrument for the detection of ViM impairment in patients with MCI (Lekeu et al., 2010; Peter et al., 2018) and mild dementia (Salimi et al., 2018). Even though we did not find differences on ViM performance between carriers and non-carriers (probably because of the cross-sectional design and limited sample size) we found significant associations between ViM and in-vivo markers of AD pathology. In our study we included ROCF-pr because unlike the immediate recall total score, this one removes the effect of execution level from the copy phase (i.e., it controls for the effect of a potentially poor copy of the figure). Percent retention scores have also been included in other ViM tests and have been studied in healthy elderly adults and in patients with memory impairment (Boone et al., 1993; Schoenberg et al., 2008). Consequently, we provide evidence that the ROCF-pr test might also be a useful ViM assessment measure in the preclinical stage of AD and should be used along with VeM tests and other measures.
LIMITATIONS, STRENGHTS AND FUTURE DIRECTIONS
Our study has some limitations. First, the sample size of the carrier and non-carrier groups was small, which could impact the ability to accurately characterize statistical relationships. Second, this study was conducted with participants with a single ADAD mutation, bringing into question the generalization of the results to sporadic late-onset AD and other ADAD mutations. Third, our cross-sectional design does not allow us to capture the longitudinal progression of AD pathology and ViM. However, as mentioned before, our baseline data are part of a longitudinal study (the COLBOS ADAD biomarker study) in which cognitively unimpaired carriers and non-carriers are being followed to characterize changes in biomarkers and cognitive performance across the preclinical and early clinical stages of ADAD. Although our findings are based on a small sample size compared with studies in healthy older adults, the opportunity to capitalize on the homogeneity of this group of cognitively unimpaired carriers who are destined to develop the AD clinical syndrome with virtually 100% certainty (and non-carrier family members) to study the preclinical stage of the disease should not be understated.
CONCLUSIONS
We demonstrated that worse ViM performance is associated with greater cortical amyloid burden, higher levels of regional tau and greater age in cognitively unimpaired carriers who were, on average, 9 years away from the mean age of MCI onset. The association between ViM and regional tau was present not only in the EC but also in the IT cortex, one of the main structures that has early vulnerability to AD pathology. In general, these results are similar to other research which has examined verbal memory in the preclinical stage of the disease. It is necessary to conduct further research with larger samples and longitudinal follow-up in order to explore the usefulness of ViM measures to identify individuals at high risk of developing dementia later in life.
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Health- Office of the Director (DP5OD019833 [YTQ]), MGH ECOR Clafin Distinguished Scholar Award [YTQ], MGH Physician/Scientist Development Award [YTQ], COLCIENCIAS (Colombia) [FLR]. The authors thank the Colombian families for contributing their valuable time and effort, without which this study would not have been possible. We also thank David Aguillón, Claudia Ramos, Francisco Piedrahita and Alex Navarro from Grupo de Neurociencias, Universidad de Antioquia in Medellín, Colombia, as well as Enmanuelle Pardilla-Delgado, Arabiye Artola and Diana Múnera from the Massachusetts General Hospital in Boston, MA, for helping coordinate visits to Boston.
Disclosure Statement
Dr. Lopera was supported by COLCIENCIAS-Colombia (111565741185), and Genentech/Roche /API COLOMBIA GN28352. Dr. Quiroz received funding from NIH for RO1AG054671, DP5OD019833, and from MGH. Mr. Fox-Fuller reports NRSA support from the National Institute on Aging (1F31AG06215801A1). Drs. Bocanegra, Baena, Guzmán-Vélez and Vila-Castelar, have no competing interests.
REFERENCES
- Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, … Lopera F (2011). Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol, 10(3), 213–220. doi: 10.1016/S1474-4422(10)70323-9 [DOI] [PubMed] [Google Scholar]
- Aguirre-Acevedo DC, Gómez RD, Moreno S, Henao-Arboleda E, Motta M, Muñoz C, … Lopera F (2007). [Validity and reliability of the CERAD-Col neuropsychological battery]. Rev Neurol, 45(11), 655–660. [PubMed] [Google Scholar]
- Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, … Jaimes F (2016). Cognitive Decline in a Colombian Kindred With Autosomal Dominant Alzheimer Disease: A Retrospective Cohort Study. JAMA Neurol, 73(4), 431–438. doi: 10.1001/jamaneurol.2015.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EJ, Pariente J, Felician O, & Puel M (2011). Visual recognition memory: a double anatomo-functional dissociation. Hippocampus, 21(9), 929–934. doi: 10.1002/hipo.20848 [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, … Network DIA (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med, 367(9), 795–804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DTR, Allen RS, & Schmitt FA (1991). Rey-Osterrieth complex figure: Psychometric characteristics in a geriatric sample. Clinical Neuropsychologist, 5(2), 143–153. doi: 10.1080/13854049108403298 [DOI] [Google Scholar]
- Boone KB, Lesser IM, Hill-gutierrez E, Berman NG, & D’Elia LF (1993). Rey-osterrieth complex figure performance in healthy, older adults: Relationship to age, education, sex, and IQ. Clinical Neuropsychologist, 7(1), 22–28. doi: 10.1080/13854049308401884 [DOI] [PubMed] [Google Scholar]
- Braskie MN, Small GW, & Bookheimer SY (2009). Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Hum Brain Mapp, 30(12), 3981–3992. doi: 10.1002/hbm.20823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, & D’Esposito M (2006). Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron, 51(2), 251–261. doi: 10.1016/j.neuron.2006.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, … Reiman EM (2012). Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol, 11(12), 1057–1065. doi: 10.1016/S1474-4422(12)70227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gutierrez Gomez M, Langois CM, … Reiman EM (2015). Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol, 72(3), 316–324. doi: 10.1001/jamaneurol.2014.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fuller JT, Cronin-Golomb A, Gatchel JR, Norton DJ, Guzmán-Vélez E, Jacobs HIL, … Quiroz YT (2019). Biological and Cognitive Markers of Presenilin1 E280A Autosomal Dominant Alzheimer’s Disease: A Comprehensive Review of the Colombian Kindred. J Prev Alzheimers Dis, 6(2), 112–120. doi: 10.14283/jpad.2019.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Christensen J, Dincer A, Flores S, Keefe S, … Benzinger TLS (2019). Tau PET in autosomal dominant Alzheimer’s disease: relationship with cognition, dementia and other biomarkers. Brain, 142(4), 1063–1076. doi: 10.1093/brain/awz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands S, Lim YY, Buckley R, Pietrzak RH, Snyder PJ, Ames D, … Maruff P (2015). Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis, 43(2), 677–686. doi: 10.3233/JAD-140678 [DOI] [PubMed] [Google Scholar]
- IBM. (2016). IBM SPSS Statistics for Windows, Version 24.0 In. Armonk, NY: IBM Corp. [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, … Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol, 9(1), 119–128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Tijms BM, Fagan AM, Hansson O, Klunk WE, … Group ABS (2018). Association of Cerebral Amyloid-β Aggregation With Cognitive Functioning in Persons Without Dementia. JAMA Psychiatry, 75(1), 84–95. doi: 10.1001/jamapsychiatry.2017.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, … Sager MA (2014). Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol Aging, 35(3), 576–584. doi: 10.1016/j.neurobiolaging.2013.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, & Mishkin M (2013). The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci, 17(1), 26–49. doi: 10.1016/j.tics.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekeu F, Magis D, Marique P, Delbeuck X, Bechet S, Guillaume B, … Salmon E (2010). The California Verbal Learning Test and other standard clinical neuropsychological tests to predict conversion from mild memory impairment to dementia. J Clin Exp Neuropsychol, 32(2), 164–173. doi: 10.1080/13803390902889606 [DOI] [PubMed] [Google Scholar]
- Lim YY, Kalinowski P, Pietrzak RH, Laws SM, Burnham SC, Ames D, … Maruff PT (2018). Association of β-Amyloid and Apolipoprotein E ε4 With Memory Decline in Preclinical Alzheimer Disease. JAMA Neurol, 75(4), 488–494. doi: 10.1001/jamaneurol.2017.4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, … Group AR (2014). Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain, 137(Pt 1), 221–231. doi: 10.1093/brain/awt286 [DOI] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio R, Quiroz YT, … Sperling RA (2017). Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement, 13(9), 1004–1012. doi: 10.1016/j.jalz.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Madison C, Oh H, Wirth M, van Berckel BN, & Jagust WJ (2014). Is verbal episodic memory in elderly with amyloid deposits preserved through altered neuronal function? Cereb Cortex, 24(8), 2210–2218. doi: 10.1093/cercor/bht076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KV, Rentz DM, Orlovsky I, Sperling RA, & Mormino EC (2017). Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: The PACC5. Alzheimers Dement (N Y), 3(4), 668–677. doi: 10.1016/j.trci.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Sandkamp R, Minkova L, Schumacher LV, Kaller CP, Abdulkadir A, & Klöppel S (2018). Real-world navigation in amnestic mild cognitive impairment: The relation to visuospatial memory and volume of hippocampal subregions. Neuropsychologia, 109, 86–94. doi: 10.1016/j.neuropsychologia.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, … Jack CR (2016). Association of Elevated Amyloid Levels With Cognition and Biomarkers in Cognitively Normal People From the Community. JAMA Neurol, 73(1), 85–92. doi: 10.1001/jamaneurol.2015.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, … Rowe CC (2007). Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain, 130(Pt 11), 2837–2844. doi: 10.1093/brain/awm238 [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Devous MD, Navitsky M, Lu M, Salloway S, Schaerf FW, … investigators F-A--A (2017). Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain, 140(3), 748–763. doi: 10.1093/brain/aww334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz YT, Sperling RA, Norton DJ, Baena A, Arboleda-Velasquez JF, Cosio D, … Johnson KA (2018). Association Between Amyloid and Tau Accumulation in Young Adults With Autosomal Dominant Alzheimer Disease. JAMA Neurol, 75(5), 548–556. doi: 10.1001/jamaneurol.2017.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, & D’Esposito M (2004). Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci, 24(16), 3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B (1988). Functional assessment staging (FAST). Psychopharmacol Bull, 24(4), 653–659. [PubMed] [Google Scholar]
- Rey A (2003). Rey, Test de copia y reproducción de memoria de figuras geométricas complejas. Madrid: TEA ediciones. [Google Scholar]
- Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, & Burrell JR (2018). Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimers Dement (Amst), 10, 66–74. doi: 10.1016/j.dadm.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg MR, Duff K, Beglinger LJ, Moser DJ, Bayless JD, Mold J, … Adams RL (2008). Retention rates on RBANS memory subtests in elderly adults. J Geriatr Psychiatry Neurol, 21(1), 26–33. doi: 10.1177/0891988707311030 [DOI] [PubMed] [Google Scholar]
- Schultz SA, Gordon BA, Mishra S, Su Y, Perrin RJ, Cairns NJ, … Benzinger TLS (2018). Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol Aging, 72, 177–185. doi: 10.1016/j.neurobiolaging.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, … Group A-AS (2013). Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging, 34(3), 822–831. doi: 10.1016/j.neurobiolaging.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, … Johnson KA (2019). The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol, 85(2), 181–193. doi: 10.1002/ana.25395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Balota DA, Aschenbrenner AJ, & Morris JC (2014). Clinical and psychological characteristics of the initial cohort of the Dominantly Inherited Alzheimer Network (DIAN). Neuropsychology, 28(1), 19–29. doi: 10.1037/neu0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Lopera F, Langbaum JB, Thomas RG, Hendrix S, Schneider LS, … Initiative A. s. P. (2018). The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical. Alzheimers Dement (N Y), 4, 150–160. doi: 10.1016/j.trci.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VL, Vila-Castelar C, Bocanegra Y, Baena A, Guzmán-Vélez E, Aguirre-Acevedo DC, … Lopera F (2019). Normative data stratified by age and education for a Spanish neuropsychological test battery: Results from the Colombian Alzheimer’s prevention initiative registry. Appl Neuropsychol Adult, 1–15. doi: 10.1080/23279095.2019.1627357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, Welsh KA, Asare-Aboagye Y, & Dawson DV (1995). Reliability of the Rey-Osterrieth Complex Figure in use with memory-impaired patients. J Clin Exp Neuropsychol, 17(4), 566–579. doi: 10.1080/01688639508405146 [DOI] [PubMed] [Google Scholar]
- Wang F, Gordon BA, Ryman DC, Ma S, Xiong C, Hassenstab J, … Network DIA (2015). Cerebral amyloidosis associated with cognitive decline in autosomal dominant Alzheimer disease. Neurology, 85(9), 790–798. doi: 10.1212/WNL.0000000000001903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tudorascu DL, Lopez OL, Cohen AD, Mathis CA, Aizenstein HJ, … Snitz BE (2018). Amyloid β Deposition and Suspected Non-Alzheimer Pathophysiology and Cognitive Decline Patterns for 12 Years in Oldest Old Participants Without Dementia. JAMA Neurol, 75(1), 88–96. doi: 10.1001/jamaneurol.2017.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]


