Abstract
Context
We hypothesize, based on the degree of residual hypothalamic-pituitary function, that some, but not all, children with growth hormone deficiency (GHD) may have beneficial growth responses to the orally administered growth hormone (GH) secretagogue LUM-201.
Objective
To determine if pretreatment testing can identify predictive enrichment markers (PEM) for subjects with adequate residual function who are responsive to LUM-201.
Methods
We performed an analysis of a completed, randomized, placebo-controlled trial of LUM-201, a GH secretagogue receptor agonist, in which all randomized subjects had pretreatment testing.
This international multicenter study conducted in pediatric endocrinology clinics included 68 naïve-to-treatment, prepubertal children with established diagnoses of GHD. Outcome measures included the sensitivity, specificity, and predictive accuracy of potential markers to predict 6-month growth responses to oral LUM-201 and daily rhGH.
Results
Two PEM were identified for use in defining PEM-positive status: (1) baseline insulin-like growth factor I (IGF-I) concentration >30 ng/mL and (2) peak GH response of ≥5 ng/mL upon administration of single-dose LUM-201. PEM-positive status enriches a population for better growth responses to LUM-201. PEM-negative status enriches a population for better growth responses to rhGH.
Conclusion
Combined, the peak GH response to single-dose LUM-201 and the baseline IGF-I concentration are effective PEMs for 6-month growth responses to LUM-201 and rhGH in prepubertal children with GHD.
Keywords: predictive enrichment markers (PEM), growth hormone deficiency (GHD), GH, secretagogues, receiver operating characteristic (ROC), LUM-201
LUM-201 (ibutamoren, formerly MK-0677) is an oral growth hormone (GH) secretagogue that augments endogenous GH pulsatility [1]. GH secretagogues act by stimulation of the GHSR1a receptor (ghrelin receptor) in the hypothalamus and pituitary. Multiple GHSR1a actions include increased growth hormone–releasing hormone (GHRH) and decreased somatostatin secretion at the hypothalamus as well as potentiation of GHRH signaling and GH secretion by the somatotrophs [2, 3]. Oral administration of LUM-201 was shown to increase 24-hour GH production in the healthy elderly [1] and to produce GH and insulin-like growth factor I (IGF-I) responses in a subset of children and adults with GH deficiency [4, 5]. It is of considerable interest to develop methods that may prospectively identify subjects who may respond with a sustained increase in height velocity to oral GH secretagogues. Interest in GH secretagogues as noninjectable alternatives to daily recombinant human growth hormone (rhGH) was not supported by treatment results in early pediatric trials [6-8]. More recently, a reconsideration of data from early trials suggested that oral GH secretagogues may not be beneficial to all subjects within the growth hormone deficiency (GHD) spectrum. Inclusion of a broad spectrum of GHD subjects in early trials may have diminished overall treatment responses. In hindsight, those without adequate baseline hypothalamic-pituitary function could not have been expected to benefit. Fortunately, in a randomized, placebo-controlled study of LUM-201 in prepubertal children with GHD, along with several standard baseline values (including IGF-I), all subjects had a pharmacodynamic (PD) study done prior to treatment. That PD study included measurement of peak GH response to a single dose of LUM-201. We hypothesized that this PD result might form the basis for pretreatment identification of treatment responders. Accordingly, we have performed a post hoc analysis of that earlier study database to determine if the GH response to a single-dose LUM-201, test along with other baseline characteristics, can enrich a broad spectrum of pediatric GHD subjects for positive growth responses. The results of this post hoc analysis were positive and will be utilized in a predictive enrichment marker (PEM) strategy [9] for clinical development of LUM-201 as an alternative to injectable rhGH in a subpopulation of children with GHD.
Methods
Evaluation of potential PEM requires pretreatment measurement of PEMs, an active treatment period, and a posttreatment analysis to determine if pretreatment markers functioned to enrich the treatment population for positive treatment responses.
Data Collection
The study was conducted in accordance with the Declaration of Helsinki; institutional review board approvals were obtained and all applicable regulatory requirements in the participating countries were followed. Written consent for data collection, processing, and publication was provided by the parents or legal guardian for each child according to national laws and regulations.
Subject Population
This study was conducted among naïve-to-treatment, prepubertal children who were at least 4 years of age, with GHD with short stature (height standard deviation score [HT-SDS] < −2), slow height velocity (< 10th percentile for age and gender) and a bone age delay of at least 1 year. Bone films taken within 3 months prior to randomization were acceptable for baseline analysis. Bone age was ≤ 8 years for girls and ≤ 9 years for boys. Maximal GH responses to 2 standard GH stimulation tests of < 10 ng/mL and an absence of other growth-limiting conditions were required. Of those randomized, 68 completed the 6-month treatment period and were evaluable for efficacy (per protocol analysis). Each participant was randomized to receive treatment with either placebo or LUM-201. Based on prior observations that the children with more severe GHD might not respond to GH secretagogues [4, 5], subjects with pretreatment peak GH responses of <1.9 ng/mL to a single dose of LUM-201 were not randomized to receive treatment.
Study Drug Administration
LUM-201 was provided as an aqueous formulation containing 2.0 mg/mL of ibutamoren (LUM-201; with orange flavoring and sucrose). LUM-201 was administered as single, daily, oral doses (0.4 mg/kg/day [n = 22] or 0.8 mg/kg/day [n = 24]). After 6 months, 20 of the 22 subjects in the placebo group agreed to be switched to receive rhGH, 0.3 mg/kg/week, generally taken as 6 daily doses per week. Doses were periodically adjusted to current body weight. In practice, doses were done in increments of 5-kg body weight dose groups resulting in maximal dosing errors of −11% to −25% depending on actual body weight.
Single-Dose LUM-201 Test
Prior to treatment and following an overnight fast, subjects received a single oral dose of LUM-201 (0.8 mg/kg) and serum samples for GH response were collected prior to the dose and at 30-, 60-, 90-, and 120-minutes post-dose. The peak GH response for each subject was used as the result of the test.
GH and IGF-I Assays
GH and IGF-I assays were performed at Esoterix (Calabasas Hills, California). GH was measured by a standard double-antibody radioimmunoassay, with a lower detection limit of 0.3 ng/mL, an intra-assay coefficient of variation (CV) of 3.4% to 10%, and an interassay CV of 7.2% to 13% for GH levels ranging from 0.92 to 8.9 ng/mL. IGF-I was determined by a competitive-binding radioimmunoassay, after acid ethanol extraction, with a lower limit of detection of 10 ng/mL, an intra-assay CV of 4.6% to 20%, and an interassay CV of 6.3% to 28% for IGF-I levels ranging from 24 to 580 ng/mL.
Statistical Analyses
As a test of the mechanism of action, the GH peak response to single-dose LUM-201 was the primary candidate for a PEM for 6-month annualized height velocities (AHV). Considering subjects with 6-month AHV above a specified threshold as the positive growth response, receiver operating characteristic (ROC) analyses were performed to determine sensitivity, specificity, and predictive accuracy at each level of GH peak. In this study of pediatric GHD, the 6-month AHV was significantly greater for both LUM-201 doses over placebo (see “Results”), but the 0.8 mg/kg/day group had the higher mean growth response and so was used for these ROC analyses. Accordingly, the median AHV (6.85 cm/year) for the 0.8 mg/kg/day dose group, including all 24 children, was used as the growth cutoff value for ROC analyses. Predictive enrichment was assessed by comparing the 6-month AHV for PEM-positive and PEM-negative subjects. Baseline IGF-I can be a useful tool in GHD diagnostics [10]. Serum IGF-I as a potential second PEM was assessed by a process of iterative filtering, that is, determining if an IGF-I cutoff value further improved the predictive enrichment achieved by the ROC-selected marker. Comparisons of optimal cutoff points for the standard GH stimulation tests and the single-dose LUM-201 test were performed by calculating positive and negative agreement rates at various cutoff values of these tests [11]. Baseline clinical characteristics of PEM-positive and PEM-negative populations were compared.
Results
The baseline characteristics of the study dosing arms are shown in Table 1. The mean age for the 0.4 mg/kg/day group was greater than for the other 2 groups, and there was a trend toward taller stature in the 0.8 mg/kg/day LUM-201 group, but all other characteristics were generally well-balanced between the groups.
Table 1.
Baseline Characteristics by Treatment Groups (N = 68). Values are Medians, Interquartile Ranges and Absolute Ranges. All Subjects were Prepubertal at Baseline. Significance for Between-Group Differences were Tested by One-Way Anova
| Number Evaluable | Age (years) | Bone Age (years) | HT-SDS | Height Velocity cm/yr | Maximal GH in 2 stimulation tests (ng/mL) | Peak GH to single-dose LUM-201 (ng/mL) | IGF-I (ng/mL) | |
|---|---|---|---|---|---|---|---|---|
| All subjects | 68 | 9.2 (7.2,10.8) (3.7, 14.3) |
8.5 (5.9, 10.4) (1.8, 10.5) |
−3.3 (−4.5, −2.5) (−7.0,−1.7) |
4.4 (3.5, 5.1) (0.1, 7.6) |
5.4 (1.8, 7.6) (0.8, 10) |
15 (3.5, 49) (1.9, 103) |
51 (24 111) (10, 231) |
| Placebo | 22 | 8.5 (5.9, 10.4) (3.7, 14) |
5.5 (4.0, 7.8) (1.8, 10) |
−3.4 (−4.1, −2.5) (−7.0, −1.7) |
4.4 (3.5, 5.1) (1.1, 7.6) |
6.4 (3.0, 8.4) (1.2, 10) |
26 (5.5, 56) (2.2, 103) |
56.5 (23 113) (10, 180) |
| LUM-201 0.4 mg/kg/d | 22 | 10.6 (9.0, 12.0) (5.3, 14.3) |
6.7 (4.5, 7.8) (2.3, 10) |
−3.8 (−5.3,−2.9) (−6.9, −1.8) |
3.6 (2.7,4.5) (0.1, 5.9) |
3.4 (1.7, 7.3) (0.8, 8.9) |
9.4 (2.9, 39) (1.9, 81) |
48.5 (23, 96) (13, 231) |
| LUM-201 0.8 mg/kg/d | 24 | 8.3 (7.1, 10.1) (5.1, 12.4) |
6.5 (5.0, 8.0) (2.7, 10.5) |
−2.8 (−3.7,−2.3) (−6.0, −1.8) |
3.8 (3.5,4.5) (1.1, 5.4) |
5.9 (1.6,7.5) (1.1, 9.4) |
13 (3.9, 52) (1.9, 102) |
50 (29 122) (15, 281) |
| P value for between-group differences | 68 | 0.025 | 0.35 | 0.08 | 0.11 | 0.2 | 0.48 | 0.94 |
| Placebo switch to daily rhGH | 20 | 9.0 (6.6, 10.8) (4.1, 14.5) |
5.9 (4.2, 8.0) (2.0, 10.5) |
−3.5 (−4.2,−2.7) (−6.7, −1.6) |
4.6 (3.4,5.6) (0.1, 6.8) |
6.3 (3.0, 7.8) (1.2, 10) |
21 (4.6, 43) (2.2, 103) |
73 (24, 118) (10, 139) |
Abbreviations: ANOVA, analysis of variance; GH, growth hormone; HT-SDS, height standard deviation score; IGF-I, insulin-like growth factor I.
ROC Analysis of Single-Dose LUM-201 Test
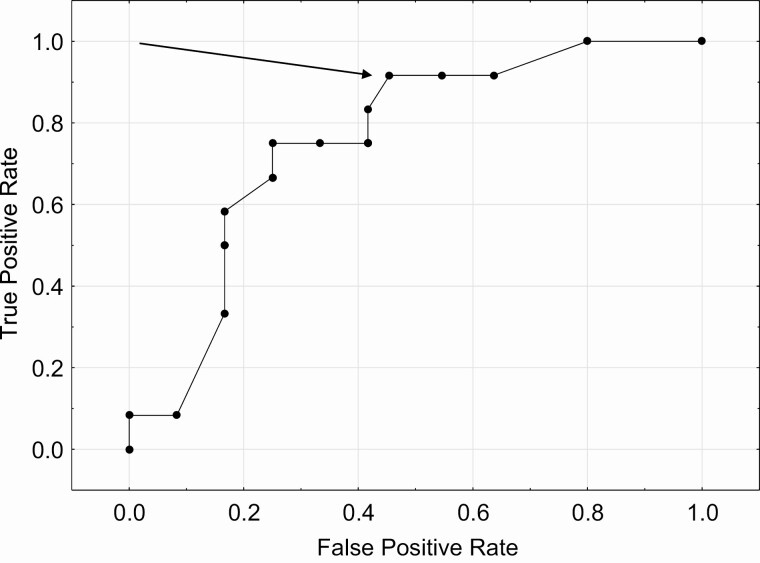
Using an AHV of ≥6.85 cm/year (the median value for the 0.8 mg/kg/day LUM-201 group with all subjects included), the ROC analysis comparing true positive rate (TPR) (sensitivity) to the false positive rate (FPR) (1 − specificity) yielded an area under the curve of 0.75. TPR is defined as the proportion of subjects with positive tests and positive growth responses among all with positive growth responses. FPR is defined as the proportion of subjects with positive test results among all with negative growth responses. Predictive accuracy is the proportion of subjects with true test results among all who were tested. The ROC analysis for the single-dose LUM-201 test is shown in Fig. 1. The arrow designates the point at which TPR (sensitivity) and FPR (1 − specificity) are optimized to produce the highest value of predictive accuracy [12]. The peak GH value at which this occurs is 5 ng/mL (TPR [sensitivity] = 0.92, FPR = 0.50, predictive accuracy 0.71, specificity 0.5).
Figure 1.
ROC analysis for prediction of 6-month AHV to 0.8 mg/kg/day LUM-201 treatment with the GH peak from the single-dose LUM-201 test. The arrow designates the point at which TPR (sensitivity) and FPR (1 − specificity) are optimized to produce the highest value of predictive accuracy.
Annualized Height Velocities
The 6-month AHVs for all subjects randomized to a dosing group and completing the 6-month treatment period (per protocol analysis) are shown in Table 2. After completing a 6-month double-blind placebo treatment, 20 of the 22 subjects completed an additional 6-month treatment period taking subcutaneous injections of rhGH (0.3 mg/kg/week). Both LUM-201 groups had increased AHV as compared with placebo (P = 0.0046 for the 0.4 mg/kg/day group and P < 0.0001 for the 0.8 mg/kg/day group). AHV for the rhGH treatment was greater than in either of the LUM-201 groups (P < 0.0001).
Table 2.
AHV in All Subjects by Treatment Group
| Treatment group | ||||
|---|---|---|---|---|
| Placebo (n = 22) | LUM-201 | LUM-201 | rhGH | |
| 0.4 mg/kg/day (n = 22) | 0.8 mg/kg/day (n = 24) | 0.3 mg/kg/week (n = 20) | ||
| Mean (SD) AHV at 6 months (cm/year) | 4.5 (1.4) | 6.0 (1.9) | 6.9 (1.9) | 11.1 (4.0) |
| P value versus placeboa | NA | 0.0046 | < 0.0001 | < 0.0001 |
| P value versus rhGHa | < 0.0001 | < 0.0001 | < 0.0001 | NA |
Abbreviations: AHV, annualized height velocity; NA, not applicable.
Values are means (SD).
aT-tests were used to test statistical significance.
Evaluation of Baseline IGF-I Concentration as a Second PEM
IGF-I secretion is primarily stimulated by GH; thus, IGF-I may serve as a biomarker for the severity of GH deficiency. A process of iterative filtering was used to determine if a specific baseline IGF-I concentration could further improve mean 6-month AHV when used in conjunction with peak GH from the single-dose LUM-201 test. For subjects with peak GH ≥5 ng/mL (determined by ROC to provide the highest value of predictive accuracy), as the mean IGF-I cutoff was increased from 0 to 30 ng/mL, the mean AHV for the 0.8 mg/kg LUM-201 group increased from 7.5 to 7.7 cm/year and the mean AHV for daily rhGH decreased from 9.6 to 8.8 cm/year. At an IGF-I cutoff level of 30 ng/mL, the between–treatment group difference in AHV was minimized (1.1 cm/year). Above the IGF-I cutoff of 30 ng/mL, the improvement in between-group difference was minimal but excluded an increasingly larger number of subjects from the analysis.
Effect of PEM Status on AHV
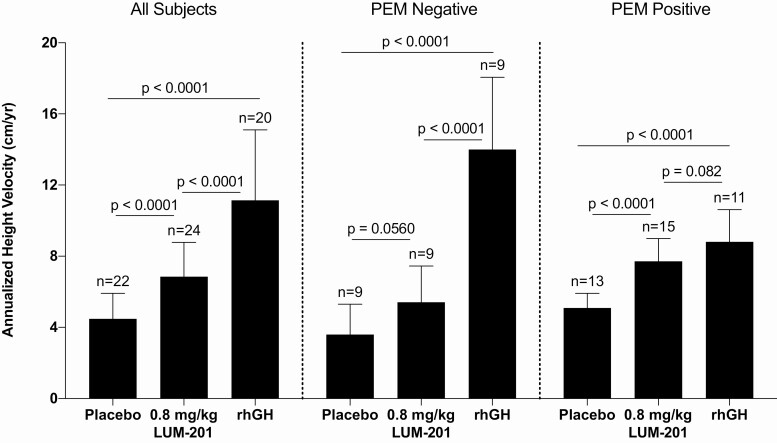
Taken together, the use of dual PEM markers where PEM-positive is defined as a single-dose GH peak ≥5 ng/mL and a baseline IGF-I concentration >30 ng/mL had substantial enrichment effects on AHV (Table 3; Fig. 2). No effect by PEM status was observed for the 0.4 mg/kg/day LUM-201 dosing group, which had the fewest positive growth responses. In contrast, PEM-positive status enriched height velocity responses in the 0.8 mg/kg/day LUM-201 group (7.7 ± 1.3 vs 5.4 ± 2.0 cm/year; P = 0.0025) while PEM-negative status enriched height velocity responses in the rhGH group (14.0 ± 4.1 vs 8.8 ± 1.8 cm/year; P = 0.0013).
Table 3.
Effect of PEM on Mean (SD) AHV by Treatment and PEM Groups
| Dose | All subjects | PEM-positive peak GH ≥5 ng/mL and baseline IGF-I > 30 ng/mL | PEM-negative peak GH <5 ng/mL and/or baseline IGF-I ≤ 30 ng/mL | P value PEM-positive vs PEM-negative a |
|---|---|---|---|---|
| LUM-201 | 6.0 (1.9) | 6.2 (1.8) | 5.8 (2.1) | 0.60 |
| 0.4 mg/kg/day | n = 22 | n = 12 | n = 10 | |
| LUM-201 | 6.9 (1.9) | 7.7 (1.3) | 5.4 (2.0) | 0.0025 |
| 0.8 mg/kg/day | n = 24 | n = 15 | n = 9 | |
| rhGH | 11.4 (4.0) | 8.8 (1.8) | 14.0 (4.1) | 0.0013 |
| 0.3 mg/kg/week | n = 20 | n = 11 | n = 9 |
Abbreviations: AHV, annualized height velocity; GH, growth hormone; IGF-I, insulin-like growth factor I; PEM, predictive enrichment marker; rhGH, recombinant human growth hormone.
aPaired t-tests were used to test for statistical significance.
Figure 2.
Effect of predictive enrichment markers (PEM) on annualized height velocities (AHV) (means and SD) by treatment and PEM groups. T-tests were used to test for statistical significance. The AHV is shown for all children in the left panel, where AHV is increased in response to LUM-201 versus placebo, but the response is greater with rhGH treatment. In the middle panel, the AHV in the PEM-negative children is not increased with LUM-201 treatment but with rhGH treatment is even greater than in the all-subject group. In contrast, in the right panel in PEM-positive children, both LUM-201 and rhGH increase AHV but the response to the 2 treatments is not different.
Comparison of GH Responses to Standard Testing and to Single-dose LUM-201
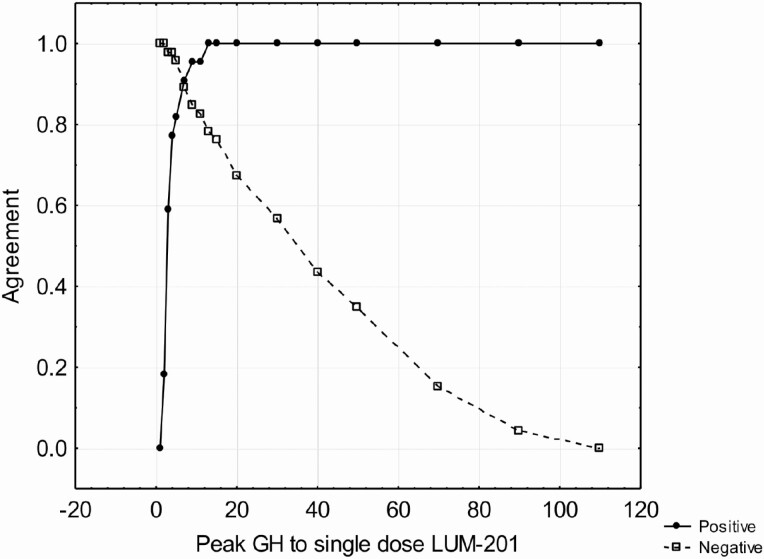
For eligibility, each randomized subject was to have a maximal GH response <10 ng/mL to two standard stimuli among arginine, clonidine, glucagon, insulin or L-dopa. Tests were performed using standard medical practices of the investigators. The maximal GH from these tests were compared to the maximal GH response to single-dose LUM-201 by comparing rates of positive agreement (both test results above a specified cutoff) and negative agreement (both tests below a specified cutoff). The agreement of GH peak to single-dose LUM-201 to a standard GH cutoff is shown in Fig. 3. The intersection of the 2 functions identifies the peak GH to single-dose LUM-201 that best corresponds to the specified GH response to standard stimuli.
Figure 3.
Positive and negative agreement rates for peak GH to single-dose LUM-201 to maximal GH in 2 standard stimulation tests. GHD defined as maximal GH < 3 ng/mL and optimal cutoff for peak GH at 7 ng/mL (positive agreement 0.91, negative agreement 0.89). Cutoff values for the single-dose LUM-201 that best correspond to other levels in the standard GH stimulation tests are shown in Table 4.
Table 4.
Positive and Negative Agreement Rates for GH Peak to Single-Dose LUM-201 to Selected Values of the Standard GH Stimulation Tests
| Maximal GH to standard stimulation tests cutoff value (ng/mL) | Peak GH to single-dose LUM-201 cutoff value (ng/mL) | Positive agreement | Negative agreement |
|---|---|---|---|
| <2 | 5 | 0.85 | 0.94 |
| <3 | 7 | 0.91 | 0.89 |
| <5 | 15 | 0.86 | 0.85 |
| <7 | 20 | 0.80 | 0.80 |
Abbreviations: GH, growth hormone; GHD, growth hormone deficiency.
Baseline Characteristics of Subjects by PEM Status
By correlational matrices and multiple regression analyses, some of the subject baseline characteristics were strongly correlated to the PEMs identified in these analyses. For example, maximal GH to standard stimulation tests was correlated to peak GH to single-dose LUM-201 (r = 0.68, P < 0.0001), maximal GH to standard stimulation tests was correlated to baseline IGF-I (r = 0.58, P < 0.0001), and peak GH to single-dose LUM-201 was correlated to baseline IGF-I (r = 0.85, P < 0.0001). It was therefore of some interest to retrospectively test if PEM status was associated with any differences in the baseline clinical characteristics of these subjects. The results are shown in Table 5. As compared to similar-age subjects who are PEM-negative, PEM-positive subjects are taller, have higher pretreatment height velocities (P = 0.02), less delay in bone age (P < 0.001), and higher GH responses to standard stimuli (P < 0.001). It appears clear that PEM-positive status, as defined by the GH peak to single-dose LUM-201 and baseline IGF-I, is associated with a more moderate degree of GH deficiency as defined by growth, bone age, and standard GH stimulation test parameters.
Table 5.
Baseline Characteristics by PEM Status
| Parametera | All subjects | PEM-positive | PEM-negative | P value for difference between PEM-positive and PEM-negativeb |
|---|---|---|---|---|
| n = 68 | n = 40 | n = 28 | ||
| Age | 9.2 (7.2, 10.8) | 9.1 (7.0, 10.5) | 9.9 (7.4, 11.6) | 0.51 |
| HT-SDS | −3.3 (−4.5, −2.5) | −2.6 (−3.1, −2.2) | −4.6 (−5.3, −3.8) | <0.00001 |
| Pretreatment Height Velocity (cm/year) | 4.0 (3.2, 4.6) | 4.3 (3.7, 4.8) | 3.3 (2.8, 4.4) | 0.02 |
| Difference between chronological and bone age | 2.3 (1.6, 4.1) | 1.9 (0.9, 2.8) | 4.0 (2.1, 5.0) | <0.001 |
| Standard GH stimulation test result (ng/mL) | 5.4 (1.8, 7.6) | 7.1 (5.1, 8.8) | 1.7 (1.3, 3.5) | <0.0001 |
Abbreviations: GH, growth hormone; HT-SDS, height standard deviation score; PEM, Predictive Enrichment Marker
amedians and interquartile ranges
b P value for difference between PEM-positive an PEM-negative groups by one-way ANOVA
Discussion
Within this cohort of naïve-to-treatment, prepubertal children with GHD, the mean AHVs for the 2 LUM-201 treatment arms were significantly greater than for the placebo-treated arm but less than for the daily rhGH treatment arm when the PEMs identified in this study were not employed. Both the placebo and LUM-201 treatment arms contained subjects with minimal ability to respond to orally administered GH secretagogue. A sustained improvement in growth in children with GHD requires sustained and sufficient increases in GH and IGF-I, but this may not be possible in all LUM-201-treated subjects, particularly those with the most severe degrees of GHD [4, 5]. The peak GH response in the single-dose LUM-201 test is a subject-specific test of each subject’s response to LUM-201 and therefore a test of the mechanism of the drug’s action. Peak GH was found to be positively correlated to the maximal GH response in 2 standard GH stimulation tests and to baseline IGF-I concentrations. As such, the degree of GHD can also be informed by the single-dose LUM-201 test. The peak GH in the single-dose LUM-201 test is lowest in the most severe forms of GHD and more elevated in moderate GHD as defined by the GH stimulation test and baseline IGF-I. Furthermore, under the conditions of this study, the peak GH in the single-dose test and the baseline IGF-I concentration have been shown to be effective PEMs for 6-month AHV. There was a significant increase in AHV among the 0.8 mg/kg/day treated LUM-201 subjects when both markers were positive (PEM-positive: peak GH >5 ng/mL and baseline IGF-I >30 ng/mL) compared with PEM-negative. In contrast, the opposite effect was seen with rhGH treatment. With rhGH, the AHV for daily rhGH-treated subjects was decreased in PEM-positive subjects and increased in PEM-negative subjects. These data suggest that oral administration of LUM-201 might be reserved for pediatric subjects with more moderate GHD, whereas children with more severe GHD would be better served with rhGH. The use of PEMs identified in this study, if confirmed prospectively, may identify the more appropriate modality prior to treatment. When positive, the 2 PEMs, peak GH to single-dose LUM-201 and baseline IGF-I, indicate a more moderate form of GHD, and this observation is extended by a consideration of other pretreatment characteristics. As compared to PEM-negative subjects, PEM-positive subjects are taller, have greater pretreatment height velocities and less delay in bone age, and have higher GH responses to standard GH stimulation tests (Table 5). The maximal GH in 2 standard stimulation tests was also evaluated as a potential PEM. In this cohort, with a range of stimulated GH results limited to 0.8 to 10.0 ng/mL, the maximum predictive accuracy was 0.67 at a stimulated GH value ≥2 ng/mL). A predictive accuracy of 0.6 occurred when stimulated GH was used as a solitary predictor for 1-year growth responses to rhGH in the National Cooperative Growth Study [13] that permitted evaluation of a greater range of GH stimulation results. Stimulated GH results are of greater value when included as one of multiple predictors in multivariate models for growth responses to rhGH [14-16]. The maximal GH response to standard GH stimulation tests and baseline serum IGF-I are correlated (r = 0.576, P < 0.0001). However, there is a stronger correlation (r = 0.85, P < 0.00001) between the peak response to LUM-201 and serum IGF-I in the same children. Serum IGF-I reflects the effects of GH acting on the liver to release IGF-I. In healthy subjects, serum IGF-I is strongly correlated with spontaneous GH secretion assessed by 24-hour studies
[17]. In addition, GH acts at the epiphyseal plate to stimulate growth by stimulating clonal expansion of chondrocytes, which also increase local IGF-I levels to act synergistically to facilitate growth [18, 19]. Serum IGF-I levels are low in children with severe GH deficiency. Since there is a stronger correlation between peak GH after a single dose of LUM-201 and baseline serum IGF-I than the correlation between standard stimulation tests and IGF-I, it is likely that the LUM-201 test is measuring endogenous GH reserve and thus serves as a more important predictor of the likelihood of a beneficial therapeutic response to treatment with LUM-201. In naïve-to-treatment, prepubertal children with GHD, age and markers for the severity of GHD are generally predictive of growth responses with rhGH. Younger children exhibit greater growth rates than older children. Lower values of baseline HT-SDS, maximal GH on standard stimulation tests, and baseline IGF-I generally indicate more severe GHD and predict greater growth responses to rhGH [14-16, 20-25]. In the present study, comparisons of AHV between the 0.8 mg/kg/day LUM-201 and daily rhGH arms are valid because age and markers of GHD severity are well-balanced with no significant between-group differences. Treatment of moderate GHD with rhGH, as here defined by the 2 PEMs and in the KIGS study using a GH stimulation test result between 5 and 10 ng/mL [14, 15], are expected to achieve a mean first-year height velocity of approximately 8.5 to 9.0 cm/year, but actual height velocities would be expected to vary depending on subject age, GH dose, severity of GHD, and other factors. In a retrospective evaluation of data from the GeNeSIS study, prepubertal children aged 4 to 10 years with moderate idiopathic GHD grew on average 8.3 cm/year in the first year of rhGH treatment [26]. Although the null hypothesis test of no significant difference in AHV between PEM-positive 0.8 mg/kg/day LUM-201 and rhGH treatment groups did not reach statistical significance
(P = 0.082), there were higher AHV in the 0.8 mg/kg/day group than the 0.4 mg/kg/day group and both LUM-201 groups had higher AHV than placebo. These comparisons suggest the presence of a dose-response curve in this subset and that a LUM-201 dose higher than the highest used here (0.8 mg/kg/day) may be required to achieve first-year height velocity targets comparable to daily rhGH.
Over the dosing range used in this study (0.4-0.8 mg/kg/day), LUM-201 was generally well tolerated. Full safety data are provided in the supplementary materials [27]. “Increased appetite” was the most frequently reported drug-related clinical adverse event, with the 2.5- to 3-fold higher incidence rate reported for the LUM-201 treatment groups distinguishing it from the placebo group. Increased appetite is frequently observed concurrent with catch-up growth [28], and orally administered GH secretagogues may also stimulate appetite in children with GHD [29]. In this context, increased appetite observed with LUM-201 treatment may be a consequence of the intended therapeutic goal and not just an adverse event.
Multiple years of treatment with daily injections of rhGH impose a significant treatment burden on children and their caregivers. Over a period of 15 years, reports have consistently substantiated incomplete treatment adherence with daily injections. No single factor accounts for poor adherence; rather, several reasons, alone or in combination, have been reported by the nonadherent population [30, 31]. Nonadherence has consequences for treatment outcomes [32-34]. Height velocity is dependent on adherence; height velocities decrease as dosing adherence falls [35, 36]. To lessen the treatment burden of daily rhGH injections, rhGH fusion products and analogues are in development that may permit weekly (or longer) dosing intervals [37-40]. An orally administered GH secretagogue such as LUM-201 at doses that result in noninferior growth rates and exhibit a comparable safety profile when compared with injectable GH products might minimize the treatment burden and optimize treatment adherence.
Some limitations exist to the interpretation of the current data. The PEM cutoff values were established using then-available assays and may differ when contemporary assays are used. Results of pubertal exams are not available for the end-of-treatment visit: subjects entering puberty may have influenced the comparison of height velocities with rhGH and LUM-201. A dose-response curve for rhGH in children with GHD and ISS is well documented [25, 41]. The rhGH dose used (43 mcg/kg/d) is higher than used in many regions: treatment differences between rhGH and LUM-201 may be exaggerated. Multivariate analysis of height velocity was used to determine if GH response to LUM-201 and IGF-I were reasonable candidates to evaluate as predictive enrichment markers. Data on some potential covariates (eg, midparental height), used in most treatment predictions models [14, 15, 42, 43] were not available and could have affected the results. IGF-I is here analyzed only as concentration because reliable calculators for IGF-I SDS were not available for this population at the time of this study (1996-1998). IGF-I and IGF-I SDS are under evaluation in the ongoing LUM-201 study (NCT04614337). Also, a limitation of the current report is that it is based on a retrospective analysis. This is being addressed in a clinical trial where prepubertal children with GHD are being studied using 3 doses of LUM-201 compared with a standard dose of daily subcutaneous injections of rhGH. In this study, peak GH response to a single dose of LUM-201 and baseline IGF-I concentrations proved to be significant PEMs for height velocity responses to LUM-201 over 6 months of treatment. Higher GH and IGF-I results (PEM-positive, indicative of moderate GHD) predict better responses to LUM-201. Further, subjects with lower values for either or both markers (PEM-negative, indicative of severe GHD) seem better served by injectable rhGH. This conclusion is corroborated by a retrospective evaluation of data from the GeNeSIS study, in which lower values of stimulated GH and baseline IGF-I predict greater growth responses to daily rhGH [26]. Future studies will determine if predictive accuracy is maintained for longer periods of treatment and if the cutoff points identified for peak GH to single-dose LUM-201 and baseline IGF-I are reproducible when applied prospectively in a second, independent PGHD population.
Acknowledgments
The authors wish to acknowledge the participants and institutions who conducted the Merck 020 study: H. Yu, University of Qindao, China; F. Cassorla University de Chile, Santiago, Chile; A. Tiulpakov, Russian Academy of Medical Sciences, Moscow, Russia; Y.-F. Shi, Beijing University Medical College, Beijing, China; N. Setian, Instituto di Crianca, Sao Paolo, Brazil; B. Bercu, University of South Florida, Tampa, Florida; A. Arango, Universidad Pontifica Bolivariana, Medellin, Columbia; G. Kletter, University of Washington, Seattle, Washington; O. Pescovitz, Indiana University, Indianapolis, Indiana; J. DiMartino, Albert Einstein College of Medicine New York, NY; D. Krupa, M. Cambria, and M.A. Bach, Merck and Company, Rahway, NJ.
Glossary
Abbreviations
- AHV
annualized height velocity
- CV
coefficient of variation
- FPR
false positive rate
- GH
growth hormone
- GHD
growth hormone deficiency
- GHRH
growth hormone–releasing hormone
- HT-SDS
height standard deviation score
- IGF-I
insulin-like growth factor I
- PEM
predictive enrichment marker
- PD
pharmacodynamic
- rhGH
recombinant human growth hormone
- ROC
receiver operating characteristic
- TPR
true positive rate
Additional Information
Disclosures: G.M.B. and M.O.T. are consultants to Lumos Pharma and M.O.T. has equity interests in Lumos Pharma. M.O.T. is an inventor of patents 763 9199 763 919 and 10 105 35210 105 352 assigned to Lumos Pharma. W.F.B. is a non-equity-holding consultant to Lumos Pharma, and a former employee and a current stockholder of Eli Lilly and Company. M.T.D. and J.C.M. are employees of Lumos Pharma and have equity interests in Lumos Pharma.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith RG, Van der Ploeg LH, Howard AD, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18(5):621-645. [DOI] [PubMed] [Google Scholar]
- 3. Patchett AA, Nargund RP, Tata JR, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92(15):7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Codner E, Cassorla F, Tiulpakov AN, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clin Pharmacol Ther. 2001;70(1):91-98. [DOI] [PubMed] [Google Scholar]
- 5. Chapman IM, Pescovitz OH, Murphy G, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab. 1997;82(10):3455-3463. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka T, Hasegawa Y, Yokoya S, Nishi Y. Increased secretion of endogenous GH after treatment with an intranasal GH-releasing peptide-2 spray does not promote growth in short children with GH deficiency. Clin Pediatr Endocrinol. 2014;23(4):107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pihoker C, Badger TM, Reynolds GA, Bowers CY. Treatment effects of intranasal growth hormone releasing peptide-2 in children with short stature. J Endocrinol. 1997;155(1):79-86. [DOI] [PubMed] [Google Scholar]
- 8. Yu H, Cassorla F, Tiulpakov A, et al. OR24-6, A double-blind, placebo-controlled efficacy trial of an oral growth hormone (GH) secretagogue (MK-0677) in GH deficient (GHD) children. Program, Annual Meeting of The Endocrine Society, New Orleans, June 24-27, 1998. 1998. [Google Scholar]
- 9. Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) March 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products
- 10. Binder G, Reinehr T, Ibáñez L, et al. GHD diagnostics in Europe and the US: an audit of national guidelines and practice. Horm Res Paediatr. 2019;92(3):150-156. [DOI] [PubMed] [Google Scholar]
- 11. Garcia JM, Biller BMK, Korbonits M, et al. Macimorelin as a diagnostic test for adult GH deficiency. J Clin Endocrinol Metab. 2018;103(8):3083-3093. [DOI] [PubMed] [Google Scholar]
- 12. Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bright GM, Julius JR, Lima J, Blethen SL. Growth hormone stimulation test results as predictors of recombinant human growth hormone treatment outcomes: preliminary analysis of the national cooperative growth study database. Pediatrics. 1999;104(4 Pt 2):1028-1031. [PubMed] [Google Scholar]
- 14. Ranke MB, Lindberg A; KIGS International Board . Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95(3):1229-1237. [DOI] [PubMed] [Google Scholar]
- 15. Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84(4):1174-1183. [DOI] [PubMed] [Google Scholar]
- 16. Wit JM, Ranke MB, Albertsson-Wikland K, et al. Personalized approach to growth hormone treatment: clinical use of growth prediction models. Horm Res Paediatr. 2013;79(5):257-270. [DOI] [PubMed] [Google Scholar]
- 17. Blum WF, Albertsson-Wikland K, Rosberg S, Ranke MB. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. J Clin Endocrinol Metab. 1993;76(6):1610-1616. [DOI] [PubMed] [Google Scholar]
- 18. Isaksson OG, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987;8(4):426-438. [DOI] [PubMed] [Google Scholar]
- 19. Yakar S, Werner H, Rosen CJ. Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol. 2018;61(1):T115-T137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blethen SL, Compton P, Lippe BM, Rosenfeld RG, August GP, Johanson A. Factors predicting the response to growth hormone (GH) therapy in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 1993;76(3):574-579. [DOI] [PubMed] [Google Scholar]
- 21. Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG; American Norditropin Study Group . Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. 2007;92(7):2480-2486. [DOI] [PubMed] [Google Scholar]
- 22. Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352-357. [DOI] [PubMed] [Google Scholar]
- 23. Kriström B, Dahlgren J, Niklasson A, Nierop AF, Albertsson-Wikland K. The first-year growth response to growth hormone treatment predicts the long-term prepubertal growth response in children. BMC Med Inform Decis Mak. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kriström B, Wikland KA. Growth prediction models, concept and use. Horm Res. 2002;57(Suppl 2):66-70. [DOI] [PubMed] [Google Scholar]
- 25. Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG; American Norditropin Clinical Trials Group . Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87(1):90-98. [DOI] [PubMed] [Google Scholar]
- 26. Blum WF, Bright GM, Do MT, et al. Corroboration of Height Velocity Prediction Markers for rhGH with an Oral GH Secretagogue Treatment in Children with GHD. J Endocr Soc. February 25, 2021. doi:10.1210/jendso/bvab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bright GM, Do MT, McKew JC, et al. Supplementary data for: development of a predictive enrichment marker for oral GH secretagogue LUM-201 in children with growth hormone deficiency. figshare. Journal contribution. 10.6084/m9.figshare.13242284v.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yackobovitch-Gavan M, Gat-Yablonski G, Shtaif B, et al. Growth hormone therapy in children with idiopathic short stature - the effect on appetite and appetite-regulating hormones: a pilot study. Endocr Res. 2019;44(1-2):16-26. [DOI] [PubMed] [Google Scholar]
- 29. Mericq V, Cassorla F, Bowers CY, Avila A, Gonen B, Merriam GR. Changes in appetite and body weight in response to long-term oral administration of the ghrelin agonist GHRP-2 in growth hormone deficient children. J Pediatr Endocrinol Metab. 2003;16(7):981-985. [DOI] [PubMed] [Google Scholar]
- 30. Desrosiers P, O’Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005;2(Suppl 3):327-331. [PubMed] [Google Scholar]
- 31. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143-154. [DOI] [PubMed] [Google Scholar]
- 32. Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189-196. [DOI] [PubMed] [Google Scholar]
- 33. Mohseni S, Heydari Z, Qorbani M, Radfar M. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31(1):13-20. [DOI] [PubMed] [Google Scholar]
- 34. Farfel A, Shalitin S, Morag N, Meyerovitch J. Long-term adherence to growth hormone therapy in a large health maintenance organization cohort. Growth Horm IGF Res. 2019;44:1-5. [DOI] [PubMed] [Google Scholar]
- 35. Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. Plos One. 2011;6(1):e16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Dommelen P, Koledova E, Wit JM. Effect of adherence to growth hormone treatment on 0-2 year catch-up growth in children with growth hormone deficiency. Plos One. 2018;13(10):e0206009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Battelino T, Rasmussen MH, De Schepper J, Zuckerman-Levin N, Gucev Z, Sävendahl L; NN8640-4042 Study Group . Somapacitan, a once-weekly reversible albumin-binding GH derivative, in children with GH deficiency: A randomized dose-escalation trial. Clin Endocrinol (Oxf). 2017;87(4):350-358. [DOI] [PubMed] [Google Scholar]
- 38. Chatelain P, Malievskiy O, Radziuk K, et al. ; TransCon GH Working Group . A randomized phase 2 study of long-acting transcon GH vs daily GH in childhood GH deficiency. J Clin Endocrinol Metab. 2017;102(5):1673-1682. [DOI] [PubMed] [Google Scholar]
- 39. Murray PG, Stevens A, De Leonibus C, Koledova E, Chatelain P, Clayton PE. Transcriptomics and machine learning predict diagnosis and severity of growth hormone deficiency. JCI Insight. 2018;3(7).e93247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore WV, Nguyen HJ, Kletter GB, et al. A randomized safety and efficacy study of somavaratan (VRS-317), a long-acting rhGH, in pediatric growth hormone deficiency. J Clin Endocrinol Metab. 2016;101(3):1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sävendahl L, Battelino T, Brod M, et al. Once-weekly somapacitan vs daily GH in children with GH deficiency: results from a randomized phase 2 trial. J Clin Endocrinol Metab. 2020;105(4):e1847-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wikland KA, Kriström B, Rosberg S, Svensson B, Nierop AF. Validated multivariate models predicting the growth response to GH treatment in individual short children with a broad range in GH secretion capacities. Pediatr Res. 2000;48(4):475-484. [DOI] [PubMed] [Google Scholar]
- 43. Kriström B, Jansson C, Rosberg S, Albertsson-Wikland K. Growth response to growth hormone (GH) treatment relates to serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in short children with various GH secretion capacities. swedish study group for growth hormone treatment. J Clin Endocrinol Metab. 1997;82(9):2889-2898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.