Abstract
Context
Recombinant human growth hormone (rhGH) is approved for treatment of pediatric growth hormone deficiency (GHD), with greatest growth responses observed in those with severe GHD. Orally administered GH secretagogues (GHS) may be useful treatment in patients with moderate GHD. Distinguishing children with severe vs moderate GHD could identify children who would be better treated with rhGH or GHS.
Objectives
Evaluate baseline insulin-like growth factor-I (IGF-I) and stimulated peak GH response as predictors of 12-month height velocity (HV) in children with GHD.
Design
Data on children with GHD were analyzed in a legacy data base (GeNeSIS data).
Participants
514 naïve to rhGH-treatment, prepubertal children with idiopathic isolated GHD for whom stimulated GH, baseline serum IGF-I, and first-year HV during rhGH treatment data are available.
Outcome Measures
Children with severe or moderate GHD were categorized based on GH and IGF-I data and evaluated based on baseline auxologic and hormone profiles and first-year growth response to rhGH.
Results
Cohorts of severe and moderate GHD were 81/514 (15.8%) and 433/514 (84.2%). Cohorts differed significantly with regard to indicators of GHD [eg, baseline height SD score (SDS), height SDS minus target height SDS, HV, HV SDS, and change in height SDS during rhGH treatment]. Multiple regression analysis showed IGF-I and stimulated GH were significant predictors of HV independent of other known variables. Expected first-year HV in moderate GHD was 8.3 cm/y.
Conclusions
The combination of peak GH to GH stimulation testing and baseline IGF-I concentration are predictive enrichment markers for annualized HV responses to rhGH therapy.
Keywords: recombinant human growth hormone, rhGH, GeNeSIS, GH deficiency, GHD, insulin-like growth factor-I, IGF-I
Growth hormone deficiency (GHD) in children is a well-recognized medical condition leading to physical disability and psychological distress associated with short stature starting during childhood and persisting throughout adult life [1-4]. The amount of growth hormone (GH) secreted by children with GHD covers a range, from zero in the most severe cases to measurable but subnormal quantities in milder disease [5,6]. The condition is rare and is therefore defined as an “orphan” disease by Food and Drug Administration, European Medicines Agency, and other regulatory authorities. The only currently approved therapy for GHD is recombinant human growth hormone (rhGH), which must be given by daily subcutaneous injection. Nonadherence to this therapy is a widely recognized issue [7-9]. Ibutamoren (LUM-201, Lumos, Austin, TX, USA), previously MK-0677 (Merck and Company, Rahway, NJ, USA) is an orally bioavailable small molecule that was instrumental in the cloning of the GH secretagogue receptor [10,11]. The GH secretagogue receptor was used to identify ghrelin as the natural ligand for this receptor [12]. Ibutamoren increases the release of endogenous GH in adults [13] and in children more mildly affected by GHD [14,15] and has the potential to stimulate childhood growth over the longer term, attenuating the physical and psychological symptoms associated with short stature. Internal feedback systems limit the secretion of GH upon administration of such a GH-releasing agent [16], potentially preventing safety issues associated with exposure to excessive levels of GH. Because it is administered orally rather than by daily injection, ibutamoren treatment could increase adherence to a regimen of growth-promoting therapy, easing the burden of the disease on patients and families. Results of a previous study found no statistically significant difference in annualized height velocity (HV) after 6 months of treatment with ibutamoren or daily rhGH injections when moderate GHD was defined as a baseline insulin-like growth factor-I (IGF-I) > 30 µg/L and a peak GH to single dose ibutamoren ≥5 µg/L [17]. Consequently, baseline serum IGF-I and GH response to a single dose of the investigative drug were used to identify a subset of patients with moderate GHD. In addition, that study showed a peak GH response to a single dose of ibutamoren >5 µg/L was equivalent to a GH response in a standard stimulation test of >2 µg/L [17]. In planning for a comparative trial of different doses of ibutamoren vs standard doses of injected rhGH, data illustrating the effect of rhGH in a large population of children who participated in the Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS; Eli Lilly and Company, Indianapolis, IN, USA) were evaluated. Since theoretically GH secretagogues require a functionally intact pituitary gland, the analysis focused on patients with idiopathic isolated GHD (IGHD) excluding those with organic GHD or combined pituitary hormone deficiency. The objective of this analysis was (i) to corroborate the criteria for moderate GHD determined from the previous study [17] in an independent cohort and (ii) to obtain an average value of first-year annualized HV attained with standard rhGH therapy in this subset as a comparator for ibutamoren treatment. Such an approach would strengthen the evidence required by providers, patient families, and others to evaluate the effectiveness of the oral GH secretagogue in comparison with that of currently standard rhGH therapy.
Patients and Methods
Source of data
Data from GeNeSIS (Clinical Trial Registry Number: NCT01088412) were analyzed to serve as the independent cohort. Anonymized data of individual participants were made available by Lilly on the platform www.clinicalstudydatarequest.com after approval by an independent review committee.
Patients and data collection
GeNeSIS was a prospective, open-label, observational research program conducted in 30 countries at more than 800 study sites between 1999 and 2015. The main objective was to investigate safety and effectiveness of rhGH treatment (Humatrope®; Eli Lilly and Company, Indianapolis, IN, USA) in pediatric patients with growth failure. Data were collected by investigators according to their standard practices and entered on case report forms. Data included etiology of GHD; demographic, auxologic, and biochemical data; status of other pituitary hormones; and information on abnormal brain or pituitary imaging [18,19]. Bone age was mostly determined according to Greulich-Pyle, and data obtained by the Tanner-Whitehouse method were converted as described before [20]. Baseline data were collected before initiation of GH treatment, and follow-up data were typically collected at 6-month intervals. The plausibility of reported data was reviewed using scatter plots, and sites were queried to comment on or to correct implausible data. The diagnosis of GHD in an individual patient was accepted as provided by the investigator, irrespective of results of GH or IGF-I testing. If several GH stimulation test results were reported, the highest GH peak value was used in statistical analyses. In the final cohort (N = 514) the following standard GH stimulation tests were performed: arginine (152; 29.6%), clonidine (151; 29.4%), insulin tolerance (92; 17.9%), L-dopa (71; 13.8%), and glucagon (48; 9.3%). GH measurements were performed locally. Serum IGF-I concentrations were determined either centrally (University Children’s Hospital, Giessen, Germany, or Esoterix, Calabasas Hills, CA, USA) using an insulin-like growth factor-binding protein-blocked radioimmunoassay [21] or at local laboratories and converted to central laboratory equivalent values using previously reported algorithms [22]. The vast majority of IGF-I values, those from Giessen (for non-US countries) and those from Esoterix (for US sites) did not require conversion. IGF-I SD scores (SDS) were calculated using the reference values of the central laboratory [23].
The study was conducted in accordance with the Declaration of Helsinki; institutional review board approvals were obtained; and all applicable regulatory requirements in the participating countries were followed. Written consent for data collection, processing, and publication was provided by the parents or legal guardian for each child according to national laws and regulations.
Final cohort for analysis
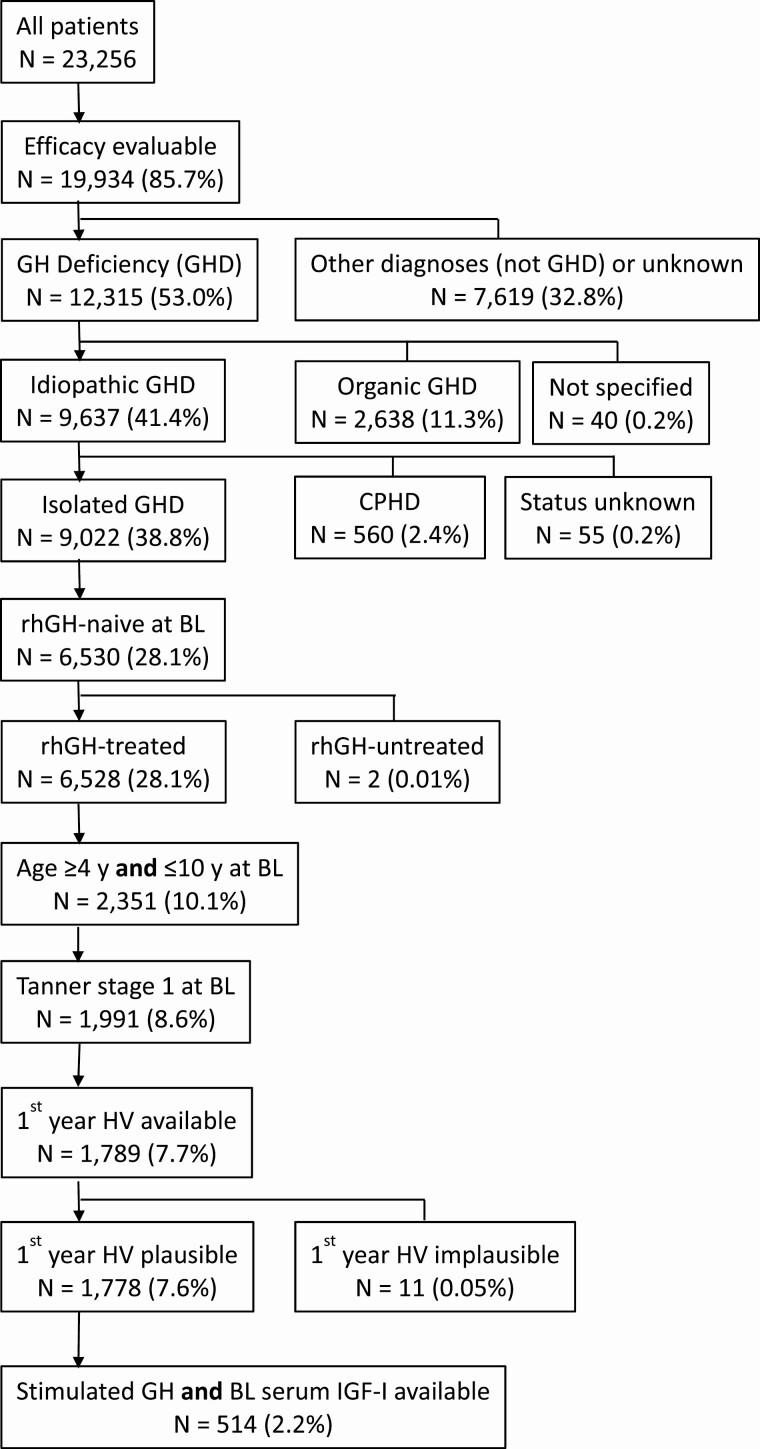
Only patients with a primary diagnosis of GHD were included, while patients with additional growth-limiting disorders (eg, Turner syndrome, SHOX deficiency, short for gestational age) were excluded. The large number of patients was substantially reduced by a stringent selection process, applying criteria intended to ensure the final study cohort included the patients of primary interest. Patients were considered suitable for the overall analysis if the following information had been reported (“efficacy evaluable” criteria): visit 1 provided sex, age, diagnosis, current height, previous rhGH treatment (yes/no), and GH dose. The resulting cohort was further stepwise limited by the following criteria: GHD, primary diagnosis idiopathic GHD. If idiopathic GHD was the highest prioritized diagnosis, patients with organic GHD (congenital GHD including, for instance, genetic defects, abnormal pituitary development, or certain clinical syndromes; acquired GHD including intracranial tumors, surgery, irradiation, and others) were automatically excluded by applying the GeNeSIS diagnostic scheme. Details on the GeNeSIS diagnostic scheme can be obtained upon request. After having identified the patients with idiopathic GHD without additional growth disorders, further selection steps followed: IGHD, GH-naïve at baseline, GH-treated, chronological age ≥4 y and ≤10 y at baseline, pubertal stage 1 (B1 in girls, G1 in boys) at baseline, first-year annualized HV (cm/y) available and plausible, stimulated maximum GH and baseline serum IGF-I available (Fig. 1).
Figure 1.
Patient disposition. The percentages refer to the total number of patients in GeNeSIS. The variable “efficacy evaluable” comprises a minimum set of variables that had been defined a priori to be indispensable for sensible analyses of rhGH treatment response such as “Visit 1 provided” sex, age, diagnosis, current height, rhGH treatment (yes/no), and rhGH dose.
Statistics
Age- and sex-based SDS values for height [24], annualized HV [25], body mass index (BMI) [26], target height according to Tanner, bone age, and serum IGF-I were calculated as described previously [18,19]. Results are presented as mean ± SD unless otherwise stated. Comparisons among groups were performed by 1-way analysis of variance for continuous variables and by chi-square test for categorical variables. For factors influencing response to rhGH, multiple linear regression analysis was performed with first-year annualized HV as the response variable and varied sets of exploratory variables. The baseline characteristics examined included chronological age, sex, weight SDS, BMI SDS, height SDS, difference between height and target height SDS, dose per week, serum IGF-I, and maximum GH (continuous or categorical by the cutoff). Sex was not a significant predictor and was excluded from subsequent modeling. Weight SDS was also removed from the models because of high collinearity with height SDS, and BMI SDS was selected instead. Model diagnostics were carried out by examining residuals, leverages, and Cook’s distances; 3 influential outliers were thus excluded from the analyses. Both standardized and unstandardized regression parameter estimates were reported. All analyses were conducted using SAS 9.4 (Cary, NC, USA). A P-value of less than 0.05 was considered statistically significant.
Results
Patients
The independent cohort that was analyzed after the filtering process identified patients considered a priori the most suitable candidates for ibutamoren therapy (Fig. 1). The authors believe that the diagnosis of idiopathic IGHD was the most important diagnostic category for identifying the patients of interest, accounting for 73.3% of all patients with GHD (9022 of 12 315 patients). Only rhGH treatment-naïve patients at baseline who actually received treatment were eligible (72.4% of idiopathic IGHD and 53.0% of all GHD). To avoid the interference caused by patients undergoing a pubertal growth spurt and the high HV of patients at a very young age, the study population was further limited to patients with baseline chronological age between 4 and 10 years and prepubertal stage (Tanner 1), which further reduced the cohort (30.5% of rhGH-treated naive idiopathic IGHD, 16.2% of all GHD). Because the primary endpoint was first-year annualized HV, only patients for whom this variable was available and plausible were included (27.2% of rhGH-treated naive idiopathic IGHD patients, 14.4% of all GHD patients). To test the hypothesis that stimulated maximum GH and baseline serum IGF-I are suitable markers for determining the severity of GHD, we focused finally on the cohort where both variables were available and plausible (N = 514, 7.9% of rhGH-treated naive idiopathic IGHD patients, 4.2% of all GHD patients). Based on a previous study [17], we hypothesized that either stimulated maximum GH < 2 µg/L or baseline serum IGF-I ≤ 30 µg/L indicate severe GHD in contrast to moderate GHD predicted by the combined markers GH ≥ 2 µg/L and IGF-I > 30 µg/L. Cohort sizes of these 2 subgroups were 81/514 (15.8%) with severe GHD and 433/514 (84.2%) with moderate GHD.
Demographics at baseline
Baseline characteristics before start of rhGH treatment for all patients and for the proposed subgroups are shown in Table 1. The total number of boys [313/514 (61%)] was greater than that of girls [201/514 (39%)]. The individual GHD subgroups were also predominantly male; however, the difference was less marked in the moderate GHD cohort. Chronological age was similar for the subgroups. Bone age was significantly more retarded in the severe vs the moderate GHD group when adjusted for chronological age. Height SDS was more diminished in the severe GHD cohort (−3.01 vs −2.58; P < 0.0001), while target height SDS was less diminished (−0.52 vs −0.75; P = 0.0146); consequently, the difference between the patients’ height SDS and their target (mid-parental) height SDS was, on average, greater (−2.49 vs −1.82; P < 0.0001). Pretreatment annualized HV was not different between the 2 cohorts. BMI SDS was significantly lower in the severe GHD group (−1.36 vs −0.37; P < 0.0001). The stimulated maximum GH peaks and baseline serum IGF-I values (concentrations or means of SDS) were different between both groups, as expected. The values complied with the predefined cutoffs of these parameters. The mean GH dose was slightly greater in the severe GHD group (0.21 mg/kg/week vs 0.20 mg/kg/week; P < 0.0200), which lay well in the recommended dose ranges of 0.18 to 0.25 mg/kg/week (Europe) and 0.18 to 0.3 mg/kg/week (USA).
Table 1.
Baseline characteristics of the final study group and the cohorts with severe or moderate GH deficiency
| All | Severe GHD IGF-I ≤ 30µg/L or GH < 2 µg/L | Moderate GHD IGF-I > 30µg/L and GH ≥ 2 µg/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean | SD | n | Mean | SD | n | Mean | SD | P |
| Male/female (%) | 514 | 61/39 | 81 | 72/28 | 433 | 59/41 | 0.0349a | |||
| Chronological age (year) | 514 | 7.05 | 1.7 | 81 | 6.80 | 1.7 | 433 | 7.10 | 1.7 | 0.1535 |
| Bone age (year) | 380 | 5.04 | 2.0 | 60 | 4.39 | 2.3 | 320 | 5.17 | 1.9 | 0.0049 |
| Bone age—chronological age (year) | 380 | -2.14 | 1.2 | 60 | -2.52 | 1.6 | 320 | -2.07 | 1.1 | 0.0072 |
| Bone age SDS | 380 | -2.67 | 1.5 | 60 | -3.15 | 1.8 | 320 | -2.57 | 1.4 | 0.0044 |
| Height SDS | 514 | -2.65 | 0.8 | 81 | -3.01 | 1.0 | 433 | -2.58 | 0.7 | <0.0001 |
| Target height SDS | 492 | -0.71 | 0.8 | 80 | -0.52 | 0.8 | 412 | -0.75 | 0.8 | 0.0146 |
| Height SDS − target height SDS | 492 | -1.93 | 1.0 | 80 | -2.49 | 1.1 | 412 | -1.82 | 0.9 | <0.0001 |
| Pretreatment HV (cm/y) | 363 | 4.80 | 1.3 | 54 | 4.99 | 1.6 | 309 | 4.76 | 1.3 | 0.2352 |
| Pretreatment HV SDS | 363 | -1.29 | 1.4 | 54 | -1.09 | 1.7 | 309 | -1.32 | 1.3 | 0.2562 |
| Body mass index SDS | 510 | -0.53 | 1.4 | 80 | -1.36 | 1.7 | 430 | -0.37 | 1.3 | <0.0001 |
| Maximum GH peak (µg/L)b | 514 | 7.40 | 4.8; 10.5 | 81 | 5.10 | 1.4; 9.3 | 433 | 7.73 | 5.4; 10.8 | <0.0001b |
| IGF-I (µg/L)b | 514 | 80 | 49; 109 | 81 | 22 | 11; 43 | 433 | 86 | 60; 114 | <0.0001b |
| IGF-I SDS | 509 | -1.72 | 1.7 | 79 | -4.13 | 2.3 | 430 | -1.28 | 1.2 | <0.0001 |
| rhGH dosage weekly (mg/kg/wk) | 507 | 0.20 | 0.045 | 80 | 0.21 | 0.053 | 427 | 0.20 | 0.043 | 0.0200 |
P values for comparing severe and moderate GHD groups were calculated by one-way ANOVA unless specified otherwise.
Abbreviations: ANOVA, analysis of variance; GH, growth hormone; GHD, growth hormone deficiency; IGF-I, insulin-like growth factor-I; HV, height velocity; rhGH, recombinant human growth hormone; SDS, SD score.
a P-value by chi-square test.
b Because of skewed distribution of serum GH and IGF-I concentrations, data are shown as median, first quartile, and third quartile. P-value calculated using Wilcoxon rank sum test.
Changes During rhGH treatment
Mean height SDS at 1 year of rhGH treatment (Table 2) was not significantly different between the severe and moderate GHD cohorts due to a greater mean increase in the severe GHD group (0.86 vs 0.61; P < 0.0001). This finding corresponds with the greater mean first-year HV and HV SDS of the severe GHD cohort vs the moderate cohort. Although mean BMI SDS increased more in the severe GHD cohort (0.22 vs 0.02; P = 0.0054), it was still significantly smaller than in the moderate GHD cohort (−1.15 vs −0.34; P < 0.0001). Starting from very low values at baseline, mean IGF-I and IGF-I SDS values in the severe GHD group ended up in the low normal range after 1 year of rhGH treatment whereas in the moderate GHD group, IGF-I concentrations were higher at baseline by definition and ended up at greater concentrations. The change in IGF-I SDS was greater in the severe GHD group (3.01 vs 1.35, P < 0.0001).
Table 2.
Changes in relevant variables during one-year of rhGH treatment in the final study group and the severe vs moderate GHD cohorts
| All | Severe GHD IGF-I ≤ 30 µg/L or GH < 2 µg/L | Moderate GHD IGF-I > 30 µg/L and GH ≥ 2 µg/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean | SD | n | Mean | SD | n | Mean | SD | P |
| First-year rhGH dose (mg/kg/week) | 514 | 0.21 | 0.06 | 81 | 0.22 | 0.06 | 433 | 0.21 | 0.06 | 0.0562 |
| Height SDS at 1 year | 513 | −2.02 | 0.8 | 81 | −2.16 | 0.9 | 432 | −2.00 | 0.7 | 0.0767 |
| First-year change in height SDS | 513 | 0.65 | 0.4 | 81 | 0.86 | 0.5 | 432 | 0.61 | 0.3 | <0.0001 |
| Height SDS at 1 year − target height SDS | 492 | −1.29 | 1.0 | 80 | −1.65 | 1.0 | 412 | −1.22 | 1.0 | 0.0004 |
| First-year HV (cm/y) | 514 | 8.50 | 1.8 | 81 | 9.62 | 2.6 | 433 | 8.29 | 1.6 | <0.0001 |
| First-year HV SDS | 512 | 3.00 | 2.0 | 80 | 4.22 | 2.8 | 432 | 2.77 | 1.7 | <0.0001 |
| BMI SDS at 1 year | 511 | −0.47 | 1.2 | 81 | −1.15 | 1.5 | 430 | −0.34 | 1.1 | <0.0001 |
| First-year change in BMI SDS | 507 | 0.05 | 0.6 | 80 | 0.22 | 0.7 | 427 | 0.02 | 0.6 | 0.0054 |
| IGF-I at 1 year (µg/L)a | 311 | 153 | 103;214 | 52 | 85 | 54; 137 | 259 | 162 | 117;217 | <0.0001 |
| First-year change in IGF-I (µg/L)a | 310 | 74 | 37; 107 | 52 | 53 | 28; 83 | 257 | 78 | 43;111 | 0.0064 |
| IGF-I SDS at 1 year | 127 | −0.09 | 1.6 | 30 | −1.05 | 1.8 | 97 | 0.20 | 1.3 | <0.0001 |
| First-year change in IGF-I SDS | 127 | 1.74 | 1.7 | 30 | 3.01 | 2.7 | 97 | 1.35 | 1.0 | <0.0001 |
P values for comparing severe and moderate GHD groups were calculated by one-way ANOVA unless specified otherwise.
Abbreviations: BMI, body mass index; GHD, growth hormone deficiency; HV, height velocity; IGF-I, insulin-like growth factor-I; rhGH, recombinant human growth hormone; SDS, SD score.
a Because of skewed distribution of serum IGF-I concentrations, data are shown as median, first quartile, and third quartile. P-value calculated using Wilcoxon rank sum test.
GH, IGF-I, and height velocity
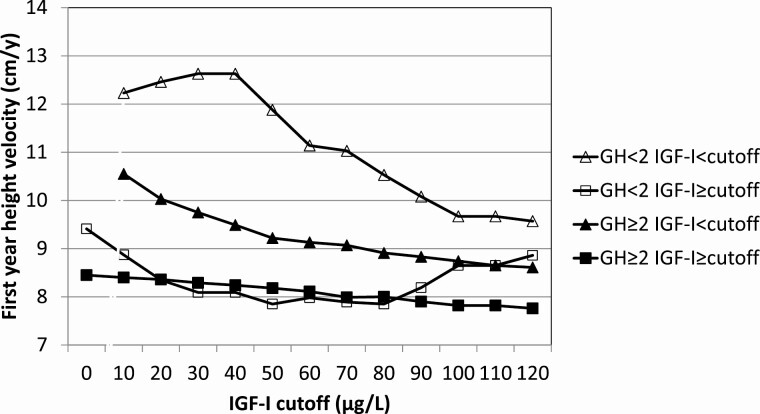
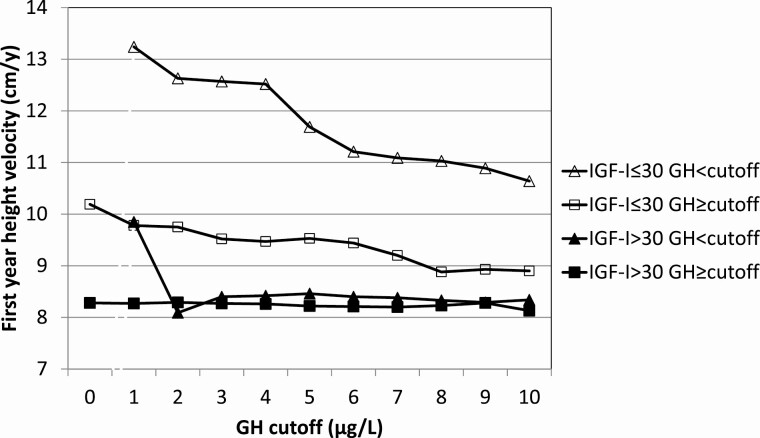
To further confirm the combined cutoffs for stimulated maximum GH and serum IGF-I concentration as positive enrichment markers, we divided the population into 2 stimulated GH categories (<2 µg/L or ≥2 µg/L) and varied IGF-I cutoffs from 0 to 120 µg/L. First-year annualized HV was chosen as the dependent response variable (Table 3). Evidently, low GH or low IGF-I result in a better growth response with a maximum at GH < 2 µg/L and IGF-I < 30 µg/L (mean HV = 12.6 cm/y). In patients with low GH (<2 µg/L) and IGF-I above the cutoff, mean annualized HV was clearly smaller and decreased further with increasing baseline IGF-I cutoff, but to a smaller degree. In patients with greater GH (≥2 µg/L) but IGF-I below the cutoffs (0-120 µg/L), mean HV was greater and decreased further from 10.6 to 8.6 cm/y. Mean annualized HV was smallest in patients with greater GH (GH ≥ 2 µg/L) and decreased slightly with IGF-I above increasing cutoffs (8.5-7.8 cm/y). At GH ≥ 2 µg/L and IGF-I cutoff >30 µg/L, the first-year HV was 8.3 ± 1.6 cm/y. When serum IGF-I was divided into 2 categories (≤30 µg/L or >30 µg/L) and cutoffs for stimulated GH maximum varied between 0 and 10 µg/L, a similar picture was obtained (Table 4). Mean HV was greatest in the low IGF-I group (≤30 µg/L) and decreased with increasing stimulated GH cutoff values. In the high IGF-I group (>30 µg/L), mean HV was practically independent of the GH cutoff values; for GH greater than the increasing cutoffs, mean annualized HV remained around 8.3 cm/y. A visual impression of the dependence of the mean first-year HV on the choice of cutoff values for stimulated GH maximum and baseline serum IGF-I is given in Figures 2 and 3.
Table 3.
First-year height velocity (cm/y) during rhGH treatment in low (<2 µg/L) and high (≥2 µg/L) stimulated GH groups according to different baseline serum IGF-I concentrations (N = 514)
| Baseline IGF-I < cutoff | Baseline IGF-I ≥ cutoff | |||||
|---|---|---|---|---|---|---|
| IGF-I cutoff (µg/L) | AHV mean (cm/y) | AHV SD (cm/y) | n | AHV mean (cm/y) | AHV SD (cm/y) | n |
| GH < 2 | ||||||
| 0 | — | — | — | 9.41 | 2.96 | 31 |
| 10 | 12.23 | 3.06 | 5 | 8.87 | 2.67 | 26 |
| 20 | 12.46 | 2.40 | 8 | 8.35 | 2.36 | 23 |
| 30 | 12.63 | 2.31 | 9 | 8.09 | 2.06 | 22 |
| 40 | 12.63 | 2.31 | 9 | 8.09 | 2.06 | 22 |
| 50 | 11.88 | 2.45 | 12 | 7.85 | 2.08 | 19 |
| 60 | 11.14 | 3.21 | 14 | 7.98 | 1.83 | 17 |
| 70 | 11.03 | 3.12 | 15 | 7.89 | 1.84 | 16 |
| 80 | 10.53 | 3.13 | 18 | 7.85 | 1.88 | 13 |
| 90 | 10.08 | 3.28 | 20 | 8.19 | 1.83 | 11 |
| 100 | 9.67 | 3.30 | 23 | 8.65 | 1.56 | 8 |
| 110 | 9.67 | 3.30 | 23 | 8.65 | 1.56 | 8 |
| 120 | 9.57 | 3.27 | 24 | 8.86 | 1.56 | 7 |
| GH ≥ 2 | ||||||
| 0 | — | — | — | 8.45 | 1.71 | 483 |
| 10 | 10.55 | 2.19 | 11 | 8.40 | 1.66 | 472 |
| 20 | 10.03 | 2.39 | 26 | 8.36 | 1.62 | 457 |
| 30 | 9.75 | 2.30 | 50 | 8.29 | 1.56 | 433 |
| 40 | 9.49 | 2.06 | 79 | 8.24 | 1.55 | 404 |
| 50 | 9.22 | 1.81 | 123 | 8.18 | 1.59 | 360 |
| 60 | 9.13 | 1.76 | 158 | 8.11 | 1.58 | 325 |
| 70 | 9.07 | 1.75 | 202 | 7.99 | 1.52 | 281 |
| 80 | 8.91 | 1.75 | 238 | 8.00 | 1.53 | 245 |
| 90 | 8.83 | 1.83 | 284 | 7.90 | 1.33 | 199 |
| 100 | 8.74 | 1.77 | 327 | 7.82 | 1.37 | 156 |
| 110 | 8.65 | 1.77 | 363 | 7.82 | 1.31 | 120 |
| 120 | 8.61 | 1.77 | 389 | 7.76 | 1.22 | 94 |
Abbreviations: AHV, annualized height velocity; GH, growth hormone; IGF-I, insulin-like growth factor-I.
Table 4.
First-year height velocity (cm/y) during rhGH treatment in low (≤30 µg/L) and high (>30 µg/L) IGF-I groups according to different stimulated GH peak concentrations (N = 514)
| Baseline GH < cutoff | Baseline GH ≥ cutoff | |||||
|---|---|---|---|---|---|---|
| GH Cutoff (µg/L) | AHV Mean (cm/y) | AHV SD (cm/y) | n | AHV Mean (cm/y) | AHV SD (cm/y) | n |
| IGF-I ≤ 30 | ||||||
| 0 | — | — | — | 10.19 | 2.51 | 59 |
| 1 | 13.24 | 1.98 | 7 | 9.78 | 2.29 | 52 |
| 2 | 12.63 | 2.31 | 9 | 9.75 | 2.30 | 50 |
| 3 | 12.57 | 2.04 | 13 | 9.52 | 2.21 | 46 |
| 4 | 12.52 | 1.97 | 14 | 9.47 | 2.21 | 45 |
| 5 | 11.69 | 2.47 | 18 | 9.53 | 2.25 | 41 |
| 6 | 11.21 | 2.52 | 25 | 9.44 | 2.24 | 34 |
| 7 | 11.09 | 2.49 | 31 | 9.20 | 2.16 | 28 |
| 8 | 11.03 | 2.36 | 36 | 8.88 | 2.18 | 23 |
| 9 | 10.89 | 2.38 | 38 | 8.93 | 2.28 | 21 |
| 10 | 10.64 | 2.43 | 44 | 8.90 | 2.35 | 15 |
| IGF-I > 30 | ||||||
| 0 | — | — | — | 8.28 | 1.58 | 455 |
| 1 | 9.85 | 0.96 | 5 | 8.27 | 1.58 | 450 |
| 2 | 8.09 | 2.06 | 22 | 8.29 | 1.56 | 433 |
| 3 | 8.40 | 1.89 | 36 | 8.27 | 1.56 | 419 |
| 4 | 8.42 | 1.77 | 68 | 8.26 | 1.55 | 387 |
| 5 | 8.46 | 2.07 | 118 | 8.22 | 1.37 | 337 |
| 6 | 8.40 | 1.93 | 167 | 8.21 | 1.34 | 288 |
| 7 | 8.38 | 1.83 | 213 | 8.20 | 1.33 | 242 |
| 8 | 8.33 | 1.78 | 246 | 8.23 | 1.31 | 209 |
| 9 | 8.29 | 1.71 | 291 | 8.28 | 1.33 | 164 |
| 10 | 8.34 | 1.69 | 334 | 8.13 | 1.22 | 121 |
Abbreviations: AHV, annualized height velocity; GH, growth hormone; IGF-I, insulin-like growth factor-I.
Figure 2.
Dependence of first-year HV on stimulated maximum GH (<2 µg/L vs ≥2 µg/L) and varying baseline IGF-I cutoffs.
Figure 3.
Dependence of first-year HV on baseline IGF-I (≤30 µg/L vs >30 µg/L) and varying baseline GH cutoff.
Multiple regression analysis
HV during rhGH treatment in patients with GHD is influenced by a number of parameters. To assess the value of stimulated maximum GH and baseline serum IGF-I as markers for rhGH response in comparison with other potential markers, we conducted a series of multiple linear regression analyses with first-year HV as the response variable and a variety of exploratory variables including stimulated maximum GH and baseline IGF-I (continuous or categorical according to the cutoffs), sex, baseline chronological age, baseline bone age, baseline height or height SDS, baseline BMI SDS, target height or target height SDS, and GH dose. In general, the models were significant (Pr|>|t|<|0.0001) and of acceptable quality. Depending on the set of exploratory variables, chronological age, BMI SDS, and rhGH dose contributed significantly to growth prediction. However, IGF-I (absolute, SDS, or categorical) was the most powerful predictor of first-year HV in all models, with an inverse relationship to HV. Relevant parameters of the best model are shown in Table 5. The single most important predictor was the categorical variable of baseline IGF-I > 30 µg/L with a standardized estimate of −0.2982. The second most important predictor was baseline chronological age, followed by GH dose, baseline BMI SDS, baseline height SDS minus target height SDS, and maximum GH ≥ 2 µg/L. Baseline height SDS was not a significant predictor. The significance of the combined markers stimulated GH ≥ 2 µg/L and baseline IGF-I > 30 µg/L was verified by this multivariate analysis (Table 5).
Table 5.
Multiple regression analysis: final model for prediction of first-year height velocity
| Variable | Parameter estimate | Standard error | Pr > |t| | Standardized estimate |
|---|---|---|---|---|
| Intercept | 10.6721 | 0.6349 | <0.0001 | 0 |
| BL IGF-I > 30 µg/L (= 1)a | −1.5835 | 0.2326 | <0.0001 | −0.2982 |
| Maximum GH ≥ 2 µg/L (= 1) | −0.7749 | 0.2881 | 0.0074 | −0.1117 |
| BL chronological age (y)a | −0.2112 | 0.0406 | <0.0001 | −0.2131 |
| BL height SDSa | −0.1238 | 0.1250 | 0.3225 | −0.0520 |
| BL height SDS − target height SDS | −0.2404 | 0.0962 | 0.0128 | −0.1322 |
| BL body mass index SDS | 0.2114 | 0.0525 | <0.0001 | 0.1730 |
| rhGH dose (mg/kg/wk) | 7.0316 | 1.6539 | 0.0001 | 0.1763 |
| Model, analysis of variance | ||||
| R2 | 0.2290 | |||
| Adjusted R2 | 0.2176 | |||
| F value | 20.11; Pr > F: <0.0001 |
Note that baseline serum IGF-I and stimulated maximum GH concentrations are included as categorical variables defined by the stated cutoffs.
Abbreviations: BL, baseline; GH, growth hormone; IGF-I, insulin-like growth factor-I; rhGH, recombinant human growth hormone; SDS, SD score.
a BL = baseline, at start of rhGH treatment.
Discussion
Examination of the GeNeSIS data corroborates the use of baseline IGF-I concentration and stimulated GH as meaningful indicators of the severity of GH and the response to treatment. Using cut-off values comparable to those determined in a companion study [17], baseline IGF-I concentration ≤30 ng/mL and stimulated GH < 2 are independent indicators of good annualized HV to rhGH in children with GHD. In the IGF-I cut-off groups, subjects with lower IGF-I concentration had higher annualized HV than those with higher IGF-I; annualized HV decreases as the IGF-I cut-off is increased. In the GH cut-off groups, annualized HV is highest in the low GH group and, again, HV decreases with increasing IGF-I. The IGF-I and GH cutoffs identified in this study also serve to segregate the study population into severe and moderate GHD groups. Those with baseline IGF-I concentration ≤30 ng/ml or GH < 2 have more severe GHD and are found to have lower height SDS, greater differences in chronological and bone age, greater differences in height SDS and target height SDS, and lower pretreatment height velocities. Moreover, the group with severe GHD has greater first-year annualized HV when treated with daily injections of rhGH.
Ibutamoren is a GHS receptor agonist that was studied in the late 1990s as a potential therapy for pediatric GHD. At the time, study results suggested a lack of efficacy when compared to rhGH [14,27]. Re-examination of the data in 2018 confirmed the findings from these studies. When data from all children studied were combined, the growth data during the first 6 months of rhGH or ibutamoren therapy showed that daily injected rhGH was superior to once-daily oral ibutamoren therapy. However, when only the data from children with severe GHD were considered, the efficacy of rhGH treatment was even more pronounced. In contrast, among children with moderate GHD, the difference in 6-month HV between the ibutamoren- and rhGH-treated patients was not statistically significant [17]. Based on this observation, the data were retrospectively examined, and 2 criteria were selected to evaluate the effect of treatment based on the severity of GHD in individual children. We hypothesized that a peak GH response to a single test dose of 0.8 mg/kg ibutamoren orally and baseline serum IGF-I could distinguish between the children who had moderate GHD and those who were severely deficient [17]. As there were no data on the response to a test dose of ibutamoren in the GeNeSIS data base, we also performed a receiver operator curve analysis from the data of acute response to ibutamoren and compared the results to those of either serum IGF-I or standard GH stimulation tests. We determined that a cutoff of 5 µg/L in the ibutamoren stimulation test was equivalent to a peak response to a standard GH stimulation test of 2 µg/L [17]. For serum IGF-I, a conservative cutoff of 30 µg/L was tested as a way to distinguish between moderate and severe GHD.
Since this was a retrospective analysis and the number of children in the study [17] was limited, we wanted to confirm this approach, in particular the GH and IGF-I cutoffs, in an independent large cohort by studying typical indicators of the severity of GHD in children treated with rhGH. When the GeNeSIS data base was made accessible to investigators, courtesy of Eli Lilly and Company through clinicalstudydatarequest.com, we were given permission to interrogate the data base. This paper presents the results of that interrogation. Theoretically, responsiveness to an oral GH secretagogue requires functionally intact somatotrophs in the pituitary. It is unlikely that this prerequisite is fulfilled in patients with an organic etiology of GHD such as genetic defect, abnormal pituitary development, intracranial tumor, certain clinical syndromes, central nervous system malformations, irradiation, and other causes. Most of these etiologies cause combined pituitary hormone deficiencies. Therefore, we focused on patients with idiopathic IGHD, which accounts for the great majority of patients with GHD [4,28,29].
In this study, severe GHD was defined by either IGF-I ≤ 30 µg/L or maximum GH < 2 µg/L, while moderate GHD was defined by IGF-I > 30 µg/L and GH ≥ 2µg/L. Both cohorts differed significantly at baseline in height SDS and height SDS minus target height SDS, which are considered typical indicators of the severity of GHD [30]. As expected by definition, stimulated GH and baseline serum IGF-I were also significantly different between both groups. P-values were provided, because in the severe GHD cohort the logical connector for GH and IGF-I is “or,” which may allow for substantial overlap of IGF-I or GH values with the moderate GHD cohort. Growth responses to rhGH treatment such as annualized HV, HV SDS, and change in height SDS were significantly greater in the severe GHD cohort, consistent with reports from other studies [31-34].
Using first-year annualized HV as a surrogate marker of rhGH responsiveness, it was evident that low stimulated maximum GH and low baseline serum IGF-I indicated good response to rhGH treatment, although the response decreased with increasing values of either parameter. This is consistent with reports from other studies [32-36]. In conclusion, these data confirm that the combined use of stimulated GH peak and baseline IGF-I with the applied cutoffs allows for identifying children with moderate GHD who may be candidates for ibutamoren testing and potentially treatment.
A relevant question is whether stimulated maximum GH and serum IGF-I are still significant indicators of severe or moderate GHD, if other contributing variables are included. A series of multivariate analyses of annualized HV from 514 children treated with rhGH indicated that age, rhGH dose, the difference between patient and target height SDS, BMI SDS, GH stimulation test result, and baseline IGF-I concentration were all independent indicators. These findings are consistent with reports from other studies in prepubertal children with idiopathic GHD [31,33-39]. Based on our analysis, prepubertal children aged 4 to 10 years with moderate idiopathic IGHD (stimulated GH ≥ 2 µg/L and IGF-I > 30 µg/L) have the potential to grow on average 8.3 cm/y in the first year of rhGH treatment. Data from the KIGS registry of patients with idiopathic GHD, not necessarily IGHD, including children at younger ages (1-10 years) and a GH cutoff of 5 µg/L have a similar mean first-year HV (8.6 cm/y) [36]. We hypothesize that this HV will be true both for treatment with daily rhGH injections or with once daily oral ibutamoren treatment.
An unexpected finding from this analysis was that catch-up growth decreased from >12 cm/y for patients with severe GHD to 8.3 cm/y for patients with moderate GHD, where moderate GHD was defined by peak GH to a stimulation test of 2 µg/L and serum IGF-I of 30 µg/L or greater.
A weakness of the GeNeSIS data was that the diagnosis of GHD was not subject to a stringent protocol: GH stimulation tests were not standardized, and GH measurements were not performed by the same assay in a central laboratory. This is typical for a “real-world” observational study. On the other hand, a strength of these data is that IGF-I measurements were performed in central laboratories or data were converted to central laboratory data. Regarding the primary objective of the current study, corroborating the peak GH and IGF-I cutoff values of the twin study [17], another weakness may be that the mean rhGH doses were different. The dose-dependence of GH treatment has in fact been demonstrated in various studies [40,41], but its role may only be moderate [42]. The mean dose in the twin study was 0.30 mg/kg/week (maximum approved US dose) whereas in the current study it was 0.20 mg/kg/week. This difference may cause slightly different growth responses. In this context, however, it should be kept in mind that dose-response relationships in biology commonly follow logit laws resulting in S-shaped curves (effect linear vs dose logarithmic). Therefore, a 30% decline in dose may have only a moderate effect and is unlikely to affect the study objective.
In conclusion, baseline IGF-I and stimulated GH alone or together are significant indicators of the degree of pediatric GHD, independent of other markers. In children with idiopathic IGHD, their combined use with cutoffs >30 µg/L for IGF-I and ≥2 µg/L for GH are predictive enrichment markers to segregate HV responses to rhGH therapy and can identify patients with moderate GHD who qualify for oral GH secretagogue testing and treatment. Notably, children with the most severe GHD grow at highest HV in response to rhGH treatment. Our working hypothesis is that children with moderate GHD will grow at similar rates in response to either daily injections of rhGH or oral ibutamoren.
Acknowledgments
The authors thank the patients, their families and the contributing physicians and site study coordinators for their participation and engagement in this study.
Author Contributions: WFB, GMB, and MOT contributed to the scientific literature search. MOT, WFB, GMB, and JCM contributed to the study design. WFB, GMB, MOT, MTD, and HC contributed to data analyses. All authors provided critical revision of the manuscript.
Additional Information
Disclosures: GeNeSIS was sponsored by Eli Lilly and Company (Lilly, Indianapolis, IN, USA). The sponsor did not impose any impediment, directly or indirectly. WFB is a former employee and a current stockholder of Lilly. WFB and GMB are nonequity-holding consultants to Lumos Pharma. MOT is a consultant to Lumos Pharma, and has equity interests in Lumos Pharma. MOT is an inventor of patents 9 763 919 and 10 105 352 assigned to Lumos Pharma. WFB is a nonequity-holding consultant to Lumos Pharma and a former employee and a current stockholder of Eli Lilly and Company. MTD and JCM are employees of Lumos Pharma and have equity interests in Lumos Pharma. HC declares no conflict of interest.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Magnusson PK, Gunnell D, Tynelius P, Davey Smith G, Rasmussen F. Strong inverse association between height and suicide in a large cohort of Swedish men: evidence of early life origins of suicidal behavior? Am J Psychiatry. 2005;162(7):1373-1375. [DOI] [PubMed] [Google Scholar]
- 2. Chaplin JE, Kriström B, Jonsson B, et al. Improvements in behaviour and self-esteem following growth hormone treatment in short prepubertal children. Horm Res Paediatr. 2011;75(4):291-303. [DOI] [PubMed] [Google Scholar]
- 3. Geisler A, Lass N, Reinsch N, et al. Quality of life in children and adolescents with growth hormone deficiency: association with growth hormone treatment. Horm Res Paediatr. 2012;78(2):94-99. [DOI] [PubMed] [Google Scholar]
- 4. Grimberg A, DiVall SA, Polychronakos C, et al. ; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society . Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361-397. [DOI] [PubMed] [Google Scholar]
- 5. Shalet SM, Toogood A, Rahim A, Brennan BM. The diagnosis of growth hormone deficiency in children and adults. Endocr Rev. 1998;19(2):203-223. [DOI] [PubMed] [Google Scholar]
- 6. Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone-IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf). 2010;72(6):721-728. [DOI] [PubMed] [Google Scholar]
- 7. Desrosiers P, O’Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005;2 Suppl 3:327-331. [PubMed] [Google Scholar]
- 8. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143-154. [DOI] [PubMed] [Google Scholar]
- 9. Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. Plos One. 2011;6(1):e16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patchett AA, Nargund RP, Tata JR, et al. Design and biological activities of L-163 191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92(15):7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974-977. [DOI] [PubMed] [Google Scholar]
- 12. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656-660. [DOI] [PubMed] [Google Scholar]
- 13. Chapman IM, Pescovitz OH, Murphy G, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab. 1997;82(10):3455-3463. [DOI] [PubMed] [Google Scholar]
- 14. Codner E, Cassorla F, Tiulpakov AN, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clin Pharmacol Ther. 2001;70(1):91-98. [DOI] [PubMed] [Google Scholar]
- 15. Cassorla F, Bright GM, Do MT, Thorner MO. OR10-03 Augmentation of pulsatile endogenous GH secretion and height velocity in pediatric growth hormone deficiency: Mechanism of action for the oral GH secretagogue LUM-201. Paper presented at: Annual Meeting of the Endocrine Society; March 2020, San Francisco (cancelled). [Google Scholar]
- 16. Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bright GM, Do MT, McKew J, Blum WF, Thorner MO. Development of a predictive enrichment marker strategy for the oral GH secretagogue LUM201 for children with growth hormone deficiency. J Endocr Soc. 2021. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deal C, Hasselmann C, Pfäffle RW, et al. Associations between pituitary imaging abnormalities and clinical and biochemical phenotypes in children with congenital growth hormone deficiency: data from an international observational study. Horm Res Paediatr. 2013;79(5):283-292. [DOI] [PubMed] [Google Scholar]
- 19. Blum WF, Deal C, Zimmermann AG, et al. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with idiopathic isolated GH deficiency. Eur J Endocrinol. 2014;170(1):13-21. [DOI] [PubMed] [Google Scholar]
- 20. Wit JM, Rekers-Mombarg LT, Cutler GB, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect. J Pediatr. 2005;146(1):45-53. [DOI] [PubMed] [Google Scholar]
- 21. Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 1994;4(Suppl 1):11-19. [PubMed] [Google Scholar]
- 22. Boettcher C, Schaefer M, Wudy SA, Blum WF. Cross-calibration of IGF-I and IGFBP-3 assays to obtain comparable values. Hormone Res. 2006;65:178. [Google Scholar]
- 23. Blum WF, Schweizer R. Insulin-like growth factors and their binding proteins. In: Ranke MB, ed. Diagnostics of Endocrine Function in Children and Adolescents. 3rd ed. Karger; 2003:155-199. [Google Scholar]
- 24. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45-60. [DOI] [PubMed] [Google Scholar]
- 25. Preece MA. Evaluation of growth and development. In: Holliday MA, Barratt TM, Avner ED, Kogan BA, ed. Pediatric Nephrology. 3rd ed. Williams and Wilkins; 1994:378-396. [Google Scholar]
- 26. Cole TJ. A chart to link child centiles of body mass index, weight and height. Eur J Clin Nutr. 2002;56(12):1194-1199. [DOI] [PubMed] [Google Scholar]
- 27. Yu H, Cassorla F, Tiulpakov A, et al. OR24-6, A double-blind, placebo-controlled efficacy trial of an oral growth hormone (GH) secretagogue (MK-0677) in GH deficient (GHD) children. Program, Annual Meeting of The Endocrine Society, June 24-27, 1998, New Orleans. [Google Scholar]
- 28. August GP, Lippe BM, Blethen SL, et al. Growth hormone treatment in the United States: demographic and diagnostic features of 2331 children. J Pediatr. 1990;116(6):899-903. [DOI] [PubMed] [Google Scholar]
- 29. Pfäffle R, Land C, Schönau E, et al. Growth hormone treatment for short stature in the USA, Germany and France: 15 years of surveillance in the genetics and neuroendocrinology of short-stature international study (GeNeSIS). Horm Res Paediatr. 2018;90(3):169-180. [DOI] [PubMed] [Google Scholar]
- 30. Ranke MB, Wit JM. Growth hormone—past, present and future. Nat Rev Endocrinol. 2018;14(5):285-300. [DOI] [PubMed] [Google Scholar]
- 31. Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84(4):1174-1183. [DOI] [PubMed] [Google Scholar]
- 32. Wikland KA, Kriström B, Rosberg S, Svensson B, Nierop AF. Validated multivariate models predicting the growth response to GH treatment in individual short children with a broad range in GH secretion capacities. Pediatr Res. 2000;48(4):475-484. [DOI] [PubMed] [Google Scholar]
- 33. Kriström B, Wikland KA. Growth prediction models, concept and use. Horm Res. 2002;57(Suppl 2):66-70. [DOI] [PubMed] [Google Scholar]
- 34. Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG; American Norditropin Clinical Trials Group . Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87(1):90-98. [DOI] [PubMed] [Google Scholar]
- 35. Cohen J. Genomics: a little gene xeroxing goes a long way. Science. 2007;317(5844):1483. [DOI] [PubMed] [Google Scholar]
- 36. Ranke MB, Lindberg A; KIGS International Board . Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95(3):1229-1237. [DOI] [PubMed] [Google Scholar]
- 37. Wit JM, Deeb A, Bin-Abbas B, Al Mutair A, Koledova E, Savage MO. Achieving optimal short- and long-term responses to paediatric growth hormone therapy. J Clin Res Pediatr Endocrinol. 2019;11(4):329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blethen SL, Compton P, Lippe BM, Rosenfeld RG, August GP, Johanson A. Factors predicting the response to growth hormone (GH) therapy in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 1993;76(3):574-579. [DOI] [PubMed] [Google Scholar]
- 39. Wit JM, Ranke MB, Albertsson-Wikland K, et al. Personalized approach to growth hormone treatment: clinical use of growth prediction models. Horm Res Paediatr. 2013;79(5):257-270. [DOI] [PubMed] [Google Scholar]
- 40. Kriström B, Aronson AS, Dahlgren J, et al. Growth hormone (GH) dosing during catch-up growth guided by individual responsiveness decreases growth response variability in prepubertal children with GH deficiency or idiopathic short stature. J Clin Endocrinol Metab. 2009;94(2):483-490. [DOI] [PubMed] [Google Scholar]
- 41. Albertsson-Wikland K, Kriström B, Lundberg E, et al. Growth hormone dose-dependent pubertal growth: a randomized trial in short children with low growth hormone secretion. Horm Res Paediatr. 2014;82(3):158-170. [DOI] [PubMed] [Google Scholar]
- 42. Ranke MB, Lindberg A, Martin DD, et al. The mathematical model for total pubertal growth in idiopathic growth hormone (GH) deficiency suggests a moderate role of GH dose. J Clin Endocrinol Metab. 2003;88(10):4748-4753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.