Abstract
Introduction
Despite the high volume of infections, some clinical aspects of this disease are still unknown. There are currently no studies in Colombia that describe the disease’s clinical and treatment aspects in detail.
Objective
Describe the characteristics and clinical management of a group of admitted patients with SARS-CoV-2 infection in a private clinic in Montería, Córdoba-Colombia.
Patients and methods
A descriptive observational study was carried out between May and August 2020 in 209 hospitalized patients with a confirmed diagnosis of COVID-19. Upon admittance, clinical, sociodemographic characteristics, comorbidities, and complications were analyzed. Additionally, the effect of the following medications was described: 1—antibiotics (cefepime, piperacillin, tazobactam, meropenem, vancomycin) + low molecular weight heparin (LMWH) + corticosteroids (dexamethasone–methylprednisolone) + colchicine. 2— Antibiotic + LMWH + corticosteroids. 3—LMWH + corticosteroids. 4—LMWH + corticosteroids + colchicine. 5—Other treatments (Tocilizumab).
Results
107 (51%) of the 209 patients with a confirmed diagnosis of COVID-19 passed away. The main comorbidities related to mortality of these hospitalized patients with COVID-19 were obesity and kidney disease (P < 0.05). The main complications associated with fatal outcomes in this group of patients were Acute Respiratory Distress Syndrome (ARDS) and sepsis (P < 0.05). Furthermore, it was evidenced that the colchicine combination showed a significant difference in reducing mortality in hospitalized patients compared to the other therapeutic regimens (P < 0.05).
Conclusion
A mortality rate of 51% was found attributable to several factors such as advanced age, obesity, kidney disease, and an average time in days of late consultation. The implementation of the colchicine combination could reduce the mortality rate in this disease.
Keywords: Mortality determinant, public health surveillance, COVID-19 drug treatment, therapeutics, Colchicine, Emerging infectious disease
Introduction
The Annual Report on Global Preparedness from the World Health Organization (WHO) for September 2019 [1] warned of the: “Imminent possibility of a pandemic caused by a lethal respiratory pathogen.” The warning became real in December 2019 with the appearance of a new Coronavirus in Wuhan, China. The new coronavirus quickly crossed geographic boundaries through Asia, Europe, and the Americas. The recent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has caused 6,000,000 infections and approximately 1,500,000 deaths [2], with a fatality rate of 3.6%. In Colombia, the number of infections is 1,225,490 cases and 34,761 deaths. The fatality rate is 3.3%. In the department of Córdoba, the number of infections is 27,704 people and 1600 deaths with a fatality rate of 7.6% [3].
The disease was unknown before its first outbreak in Wuhan, China. SARS-CoV-2 manifests in flu-like symptoms such as fever, cough, dyspnea, myalgia, and fatigue. Sudden loss of smell and taste is characteristic of Coronavirus disease 2019 (COVID-19) [4]. In severe cases, it is characterized by pneumonia, acute respiratory distress syndrome, sepsis, and septic shock, leading to death in around 3% of those infected [4]. Although there is currently a high volume of infections, some clinical aspects of the disease remain unknown. There are no studies in Colombia that describe, in detail, the clinical and treatment aspects of the disease.
The main goal of this study is to describe the characteristics and clinical management of a group of hospitalized patients with SARS-CoV-2 infection in a private clinic in Montería, Córdoba-Colombia.
Patients and Methods
Type of study and patients
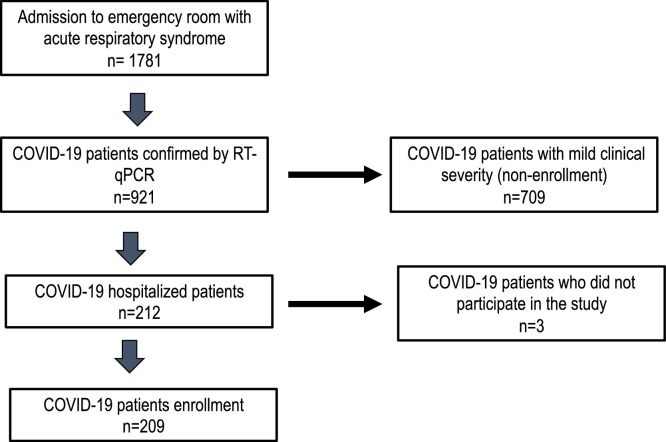
A descriptive observational study was carried out between May and August 2020 in patients with a confirmed diagnosis of COVID-19. Two hundred nine admitted patients with clinical manifestations compatible with SARS-CoV-2 infection were included. The clinical and sociodemographic characteristics, comorbidities, and complications were analyzed (Fig. 1 ).
Fig. 1.
Flowchart of admitted patients in the study.
Study site
This study was carried out in the State of Córdoba in the Caribbean northwest region. The capital of the State is Montería, with a population of 505,000 inhabitants. The study took place in a private third-level clinic, specialized in the comprehensive management of patients with onco-hematological diseases named Instituto Medico de Alta Tecnologia – IMAT. The clinic had 105 beds for the care of patients with COVID-19. The clinic's building was divided to face the pandemic into two sectors to safely and adequately separate patients with COVID-19 from other patients and healthy people.
Pharmacological treatments
The following drugs were used: 1—broad-spectrum antibiotics (cefepime, piperacillin-tazobactam, meropenem, vancomycin) + low molecular weight heparin (LMWH) + corticosteroids (dexamethasone–methylprednisolone) + colchicine. 2—Antibiotic + LMWH + corticosteroids. 3—LMWH + corticosteroids. 4—LMWH + corticosteroids + colchicine. 5—Other treatments (Tocilizumab). Broad-spectrum antibiotics were prescribed to patients with suspected bacterial coinfection. Coinfections were diagnosed by radiography or chest tomography, isolation of the germ from respiratory specimens, blood cultures, and molecular detection with PCR. Corticosteroids were administered to patients in need of supplemental O2, with partial oxygen saturation (SpO2) less than 92% [5]. Antithrombotic prophylaxis with low molecular weight heparin (LMWH) was administered to all patients diagnosed with SARS-CoV-2 infection, and therapeutic doses of LMWH were administered to patients with Dimer D > 1000 ng/ml, severe disease, and suspected or confirmed venous thromboembolic events. Colchicine was given for 20 days to all patients with SARS-CoV-2 infection, as long as they did not present gastrointestinal intolerance or a history of hypersensitivity [6]. According to the Colombian consensus’s recommendations for the care, diagnosis, and management of SARS-CoV-2 infection [7], tocilizumab was given.
Inclusion and exclusion criteria
The patients had to be admitted to the hospital ward and meet the criteria for COVID-19 disease classified as moderate, severe, or critical [7,8]. The moderate disease was one with clinical or radiological evidence of pneumonia with clinical of pneumonia (fever, cough, dyspnea, tachypnea) without signs of severe pneumonia, with SpO2 ≥ 90% in room air. Severe disease was one that demonstrated clinical evidence of pneumonia, plus one of the following findings: respiratory rate >30 breaths/min; severe shortness of breath; o SpO2 < 90% in ambient air. The critical disease was considered if it met Acute Respiratory Distress Syndrome (ARDS) criteria, sepsis, or septic shock [7,8].
The exclusion criteria were the following: patients’ clinical history with the loss of clinical and demographic information more significant than 10%. Patients with a mild diagnosis of Covid-19 disease. Symptomatic patients based on the COVID-19 case definition criteria without evidence of viral pneumonia or hypoxia [7,8]. Patients admitted to hospital for the treatment of diseases other than Covid-19.
Ethical aspects
The consent for the treatment of the patients or relatives was obtained, and they were privately categorized. The work was endorsed by the Ethics Committee of the Instituto de Investigación Biológicas del Trópico (IIBT) and Clínica IMAT. This study was under strict alignment with international ethical standards given by the World Health Organization and the Pan American Health Organization, supported by The Helsinki's Declaration, and Colombian legislation, resolution number 008430 of 1993 of the Ministry of Health of Colombia.
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences version 21 (SPSS). The univariate analysis for the qualitative variables resulted through the calculation of absolute and relative frequencies. In quantitative variables, measures of central tendency were calculated. Moreover, the normality of the quantitative variables was determined by applying the Kolmogórov–Smirnov test. The Bivariate analysis was performed through Pearson’s chi-square test. Multivariate analysis was performed through binomial logistic analysis. The significance of the P-value was set to <0.05 for all analyses performed.
Results
Two hundred and nine patients with a confirmed diagnosis of COVID-19 were analyzed. One hundred out of the 209 were in the Intensive Care Unit, and the remaining 109 were treated in the general hospitalization ward. In total, 51% perished as a consequence of the infection. The patient’s median age was 60 years old, in which 61% of them were males, and 8.6% were health care workers. The patients’ primary clinical manifestation was respiratory distress, which was observed in 66.5% of patients. In individuals who died with COVID-19, respiratory distress was 83.3% (P < 0.0001). Supplementary critical clinical manifestations were fever (61.7%), cough (58.8%), and adynamic (25.3%). Regarding comorbidities, it was noted that 47% suffered arterial hypertension, 25.3% diabetes mellitus, 18.6% of patients had a history of solid tumors, 17.2% cardiovascular disease, 14.8 % kidney disease in various stages, and 14.3% were obese. Moreover, kidney disease and obesity were related to higher mortality in this group of patients (P < 0.05) (Table 1 ).
Table 1.
Sociodemographic and clinical characteristics of patients with COVID-19.
| Characteristics |
Hospital discharge |
P value | |||
|---|---|---|---|---|---|
| Deceased (%) | Alive (%) | Total (%) | |||
| Symptoms | Respiratory distress | 85 (83.3) | 54 (49.5) | 139 (66.5) | <0.0001 |
| Fever | 60 (58.8) | 69 (63.3) | 129 (61.7) | 0.085 | |
| Cough | 67 (65.7) | 56 (51.3) | 123 (58.8) | 0.25 | |
| Fatigue or adynamic | 25 (24.5) | 28 (25.6) | 53 (25.3) | 0.5 | |
| Headache | 10 (9.8) | 23 (21.1) | 33 (15.8) | 0.009 | |
| Diarrhea | 18 (17.6) | 15 (13.8) | 33 (15.8) | 0.67 | |
| Odynophagia | 11 (10.8) | 15 (13.8) | 26 (12.4) | 0.94 | |
| Dysgeusia | 7 (6.8) | 10 (9.2) | 17 (8.1) | 0.74 | |
| Myalgia | 7 (6.8) | 10 (9.2) | 17 (8.1) | 0.39 | |
| Anosmia | 8 (7.8) | 9 (8.2) | 17 (5.7) | 0.72 | |
| Sore throat | 2 (2) | 10 (9.2) | 12 (2.8) | 0.014 | |
| Rhinorrhea | 5 (4.9) | 1 (1) | 6 (2.4) | 0.11 | |
| Other symptoms | 30 (29.4) | 24 (22) | 54 (25.8) | 0.46 | |
| Comorbidities | Arterial hypertension | 52 (51) | 48 (44) | 100 (47) | 0.82 |
| Diabetes | 31 (30.3) | 22 (20.2) | 53 (25.3) | 0.22 | |
| Solid tumors | 23 (22.5) | 16 (14.7) | 39 (18.6) | 0.28 | |
| Cardiovascular disease | 17 (16.6) | 19 (17.4) | 36 (17.2) | 0.6 | |
| Kidney disease | 28 (27.4) | 3 (2.8) | 31 (14.8) | <0.0001 | |
| Obesity | 24 (23.5) | 6 (5.5) | 30 (14.3) | 0.001 | |
| COPD | 17 (16.6) | 12 (11) | 29 (13.8) | 0.21 | |
| Other | 29 (28.4) | 28 (25.7) | 57(27.2) | 0.39 | |
| Hypothyroidism | 10 (9.8) | 5 (4.8) | 15 (7.2) | 0.37 | |
| Hematological tumors | 6 (5.8) | 9 (8.3) | 15 (7.2) | 0.036 | |
| Asthma | 5 (4.9) | 3 (2.8) | 8 (3.8) | 0.165 | |
| Clinical complications | ARSD | 92 (90.1) | 10 (9.2) | 102 (48.8) | <0.0001 |
| Sepsis | 72 (70.6) | 10 (9.2) | 82 (39.23) | <0.0001 | |
| Bacterial co-infection | 31 (30.4) | 13 (11.9) | 44 (21.1) | 0.004 | |
| Viral co-infection | 1 (1) | 1 (1) | 2 (1) | 0.97 | |
| Hospitalization | Average in days between symptoms and hospitalization | 9.5 (7.5) | 8.8 (6.0) | 9.1 (6.8) | 0.054 |
| Hospital floor | 28(13) | 81(39) | 109 (52) | <0.0001 | |
| Intensive care unit | 79(38) | 21(10) | 100 (48) | <0.0001 | |
Concerning complications, it was evidenced that 48.8% of those admitted presented Acute Respiratory Distress Syndromes (ARDS), and 39.23% sepsis. These complications were present in 90.1% and 70.6% of the deceased patients (P < 0.0001). Other palpable complications were bacterial coinfection (21.1 %); the most frequent microorganisms involved in pulmonary bacterial coinfection were: Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterobacter cloacae. The Rhinovirus/Enterovirus viral agents were the most associated with pneumonia (Table 1). A multivariate analysis was carried out to study symptoms, comorbidities, and clinical complications related to the deceased COVID-19 patients. The analysis of the variables projected that symptoms such as respiratory distress are related to a higher rate of mortality by COVID-19 (P < 0.05 [OR 3.5, 95% CI 1.7-7.2]). Furthermore, this analysis showed that comorbidities as obesity and mainly kidney disease could be considered as risk factors related to high fatality rates in these patients (P < 0.0001 [OR 16 95% CI 4.4-60]). On the other hand, the analysis carried out on clinical complications also showed that ARDS and sepsis were two variables with sufficient statistical corroboration linked to fatal outcomes in individuals infected by SARS-CoV-2 (P < 0.05). Likewise, ADRS could be linked to an increase of almost 36 times in the mortality rate (OR 36, 95% CI 12-102) (Table 2 ).
Table 2.
Association between symptoms, comorbidities and clinical complications with hospitalization by COVID-19 (multivariate analysis).
| Variables |
Hospitalization by
COVID-19 |
||
|---|---|---|---|
| P value | OR, 95%CI | ||
| Symptoms | Respiratory distress | 0.001 | 3.5 (1.7-7.2) |
| Fever | 0.43 | 0.8 (0.4-1.5) | |
| Cough | 0.28 | 1.4 (0.74-2.8) | |
| Fatigue or adynamic | 0.36 | 0.72 (0.35-1.5) | |
| Headache | 0.055 | 0.4 (0.15-1) | |
| Diarrhea | 0.34 | 1.5 (0.6-3.6) | |
| Odynophagia | 0.56 | 0.74 (0.27-2) | |
| Dysgeusia | 0.61 | 0.66 (0.13-3.2) | |
| Myalgia | 0.42 | 0.63 (0.21-1.9) | |
| Anosmia | 0.74 | 1.3 (0.3-5.5) | |
| Sore throat | 0.24 | 0.35 (0.6-2.1) | |
| Rhinorrhea | 0.079 | 11 (0.8-175) | |
| Comorbidities | Arterial hypertension | 0.82 | 0.47 (0.4-1.5) |
| Diabetes | 0.43 | 1.3 (0.63-2.9) | |
| Solid tumors | 0.25 | 1.6 (0.7-3.6) | |
| Cardiovascular disease | 0.64 | 0.8 (0.35-1.9) | |
| Obesity | <0.0001 | 6.2 (2.2-17) | |
| Kidney disease | < 0.0001 | 16 (4.4-60) | |
| COPD | 0.27 | 2.1 (0.57-7.6) | |
| Hypothyroidism | 0.46 | 0.62 (1.8-2.2) | |
| Hematological tumors | 0.088 | 2.52 (0.5-1.2) | |
| Asthma | 0.9 | 1 (0.9-1.05) | |
| Clinical complications | ARDS | <0.0001 | 36 (12-102) |
| Bacterial co-infection | 0.054 | 0.26 (0.64-1.02) | |
| Viral co-infection | 0.9 | 0.94 (0.13-68) | |
| Sepsis | 0.004 | 5.6 (1.8-18) | |
COPD: chronic obstructive pulmonary disease; ARDS: acute respiratory distress syndrome.
As a standard course of treatment given to all admitted patients, the following was the most common combination of antibiotics: antibiotics, low molecular weight heparin, corticosteroids, and colchicine. Fifty-two percent of the patients who received this combination were discharged alive, and this combination was related to reducing the mortality in the total cohort of patients (P < 0.05 [OR 0.26 95% CI 0.08-0.71]) (Table 3 ).
Table 3.
Comparison between mortality and the pharmacological treatments used in patients hospitalized with COVID-19 (multivariate analysis).
| Severity | Treatment | Deceased (%) | Alive (%) | Total (%) | P value | OR, 95% CI |
|---|---|---|---|---|---|---|
| Critic n = 112 | Antibiotics + LMWH + corticosteroids | 15 (93.8) | 1 (6.2) | 16 (14.3) | 0.0087** | 15 (3.04–271.2) |
| Antibiotics + LMWH + corticosteroids + colchicine | 45 (83.3) | 9 (16.7) | 54 (48.2) | 0.32 | 0.3 (0.017–2) | |
| LMWH + corticosteroids | 10 (90.1) | 1 (0.9) | 11 (9.8) | 0.78 | 0.667 (0.024–18.2) | |
| LMWH + corticosteroids + colchicine | 7 (87.5) | 1 (12.5) | 8 (7.1) | 0.61 | 0.47 (0.017–12.9) | |
| Other treatment | 17 (73.9) | 6 (26.1) | 23 (20.5) | 0.14 | 0.19 (0.009–1.28) | |
| Moderate n = 87 | Antibiotics + LMWH + corticosteroids | 1 (25) | 3 (75) | 4 (4.6) | 0.34 | 0.333, (0.016–2.6) |
| Antibiotics + LMWH + corticosteroids + colchicine | 2 (4.7) | 41 (95.3) | 43 (49.4) | 0.16 | 0.146 (0.010–3.66) | |
| LMWH + corticosteroids | 1 (12.5) | 7 (87.5) | 8 (9.2) | 0.59 | 0.43 (0.013–13.33) | |
| LMWH + corticosteroids + colchicine | 1 (20) | 4 (80) | 5 (5.7) | 0.86 | 0.75 (0.022–24.66) | |
| Other treatment | 4 (14.8) | 23 (85.2) | 27 (31) | 0.61 | 0.52 (0.05–12) | |
| Severe n = 10 | Antibiotics + LMWH + corticosteroids | 2 (66.7) | 1 (33.3) | 3 (30) | 0.57 | 2 (0.191–4.3) |
| Antibiotics + LMWH + corticosteroids + colchicine | 1 (50) | 1 (50) | 2 | 0.71 | 0.5 (0.008–2.3) | |
| LMWH + corticosteroids | 0 | 2 (100) | 2 (20) | 0.997 | 0 (NA–1472) | |
| LMWH + corticosteroids + colchicine | 0 | 1 (100) | 1 (10) | 0.998 | 0 (NA–NA) | |
| Other treatment | 1 (50) | 1 (50) | 2 (20) | 0.711 | 0.5 (0.008–2.35) | |
| All patients hospitalized n = 209 | Antibiotics + LMWH + corticosteroids | 18 (78.3) | 5 (21.7) | 23 (11) | 0.0113* | 3.6 (1.437–10.9) |
| Antibiotics + LMWH + corticosteroids + colchicine | 48 (48) | 51 (52) | 99 (47.4) | 0.014* | 0.26 (0.08-0.71) | |
| LMWH + corticosteroids | 11 (52.4) | 10 (47.6) | 21 (10) | 0.08 | 0.3 (0.07-0.09) | |
| LMWH + corticosteroids + colchicine | 8 (57.1) | 6 (42.8) | 14 (6.7) | 0.18 | 0.37 (0.08-0.57) | |
| Other treatment | 22 (42.3) | 30 (57.7) | 52 (24.9) | 0.006* | 0.2 (0.06-0.6) |
NA: not apply.
Statistical differences; 122 (58%) received antimicrobial treatment, 157 (75%) LMWH.
According to the clinical severity, the impact of treatments was evaluated; however, the present work did not show statistical differences regarding their use (P > 0.05). Critical patients under treatment without colchicine showed higher mortality than those who received colchicine combination (P < 0.05 [OR15 (3.04–271.2]).
Discussion
The present study is the first one of its class in the Republic of Colombia that describes the clinical and treatment aspects of a group of patients with COVID-19. In general, deceased patients tended to be older, with a median age in our population more significant than the ones shown in Asian cohorts [4,9,10], the United States [11], and some Latin American countries [12,13]. In the group of patients that did not survive, the predominant symptom was dyspnea; a behavior correlated with a more significant rate of complications and mortality is higher than its presentation in other series of hospitalized patients in cohorts from Asia and Europe [14,15] but is similar to those found in the United States and Latin America [12,13,16].
This high mortality rate is perhaps due to the delayed reporting of symptoms by the affected patients. (Average 9.1 days), which has been evidenced as an adverse prognostic factor [17]. We believe that delay in attention is correlated to misinformation given at the beginning of this pandemic in our region. People’s perception of the disease was misled when national authorities placed several restrictions to contain the virus, flatten the curve, and relieve the health care system. People believed they would get sicker at hospitals than at home. The misinformation and fear negatively impacted timely clinical care [16]. As described in some international media [18], this infodemic could impact morbidity and mortality. This topic should be evaluated with other studies that consider social factors, race, religion, educational level, and gender [[19], [20], [21]].
On the other hand, comorbidities such as kidney disease and obesity seem to be, according to studies in The United States, China, and Spain [14,22,23]. However, because the clinic is a regional referral center for cancer patients, the proportion of patients with solid tumors and hematological tumors was higher (25.8%). Cancer comorbidity could have influenced the results, being associated with a higher fatality rate in cancer patients [24].
In the multivariate analysis, the variable of obesity behaved as a factor that increases mortality risk and enters agreements with other findings in additional cohorts. It is an independent risk and prognostic factor for the greater severity of the disease [25,26]. In obese patients, it has been proposed that the low metabolic and cardiorespiratory reserve may lead to immune hyperreactivity and greater severity of the disease [27]. Chronic kidney disease (CKD) was associated with a progressive increase in the rate of hospital-diagnosed COVID-19 [28] and has significantly higher mortality than patients without kidney disease [29], behaving as a significant independent predictor of COVID-19 mortality [30]. We found a greater risk of mortality in the group of patients with chronic kidney disease, similar results found in New York, the United States, and Mexico City [31].
The most frequently encountered complications were sepsis and ARDS; the multivariate analysis showed an increased risk of mortality in patients who presented these complications [32]. Ninety percent of the deceased patients presented ARDS, which leads to a more significant number of days of ventilation and short-term mortality [33] similar to that published by Tzotzos et al. [34], who describe results of a global survey of studies that report ARDS associated with COVID-19.
The combination of broad-spectrum antibiotics, low molecular weight heparin, corticosteroids, and colchicine were included. Considering that approximately half of the patients were admitted to the ICU, antibiotics were consistent with those described in other series [10,35,36]. However, the present study had bacterial coinfection at a higher rate (58%) than those reported by Lansburri et al. [37], who reported 7% of patients hospitalized in the general ward and 14% of those hospitalized in the ICU had bacterial coinfection. In previous studies, 90% of the patients received empirical antibiotic treatment [38].
LMWH was administered in prophylactic doses according to weight in all patients with confirmed SARS-CoV-2 infection based on data suggesting a procoagulant pattern [39]. Likewise, in patients with hypercoagulability associated with severe inflammation states [40] with an increase in the incidence of venous thromboembolism [[41], [42], [43]]. According to other our finding related to LMWH is that its administration reduces mortality in patients with complicated forms of this disease [44,45].
Based on the evidence at the date of the RECOVERY study [5], glucocorticoids in all patients who needed supplemental oxygen were started. Based on clinical benefit data found in the GRECCO study [6], colchicine was used in 54.2% of the hospitalized patients with good gastrointestinal tolerance. In the multivariate analysis, colchicine showed a reduction in the risk of mortality. In other words, patients treated with the colchicine combination had less chance of dying than those who received the combination without colchicine (Table 3).
Our work has some weaknesses. The study was conducted in a single hospital center, which probably introduced a selection bias and limited the findings’ extrapolation. Furthermore, a proportion of patients were admitted with severe disease, limiting the ability to evaluate the relationship between independent variables and mortality.
In conclusion, the present work reveals that during the pandemic’s maximum peak, mortality from COVID-19 was 51%. These data related to higher mortality would be attributed to several related factors such as the advanced age of the hospitalized population, comorbidities such as kidney disease and obesity, and an average time in late consultation days. Furthermore, the present study showed that the colchicine combination was reducing mortality in COVID-19 disease. However, our findings need to be confirmed with a bigger casuistic and in other populations.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
This research was approved by the ethics and research committee of Oncomedica S.A.
References
- 1.A world at risk: annual report on global preparedness for health emergencies. 2019. pp. 26–30.https://apps.who.int/gpmb/assets/annual_report/GPMB_annualreport_2019.pdf Available from: [Google Scholar]
- 2.https://www.worldometers.info/coronavirus/ [Internet]. Available from: https://www.worldometers.info/coronavirus/. [cited 12 August 2020].
- 3.INS . 2020. COVID-19 en Colombia. Available from: https://www.ins.gov.co/Noticias/Paginas/coronavirus-departamento.aspx [cited 19 November 2020] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(April (18)):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dexamethasone in hospitalized patients with covid-19 — preliminary report. N Engl J Med. 2020;(July) doi: 10.1056/NEJMoa2021436. Available from: [DOI] [Google Scholar]
- 6.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(June (6)) doi: 10.1001/jamanetworkopen.2020.13136. Available from: https://jamanetwork.com/ [cited 23 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra Trujillo CH. 2020. Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID-19 en establecimientos de atención de la salud Recomendaciones basadas en consenso de expertos e informadas en la evidencia. Vol. 24, Infectio. scieloco; pp. 36–49. [Google Scholar]
- 8.Clinical management of COVID-19 [Internet]. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19 [cited 6 Dec 2020].
- 9.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(March (11)):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(February (10223)):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of covid-19 in New York City. The New England journal of medicine. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz-Brizuela E., Villanueva-Reza M., González-Lara M.F., Tamez-Torres K.M., Román-Montes C.M., Díaz-Mejía B.A. Clinical and epidemiological characteristics of patients diagnosed with covid-19 in a tertiary care center in Mexico City: a prospective cohort study. Rev Investig Clin organo del Hosp Enfermedades la Nutr. 2020;72(3):165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 13.de Souza W.M., Buss L.F., da S Candido D, Carrera J-P, Li S, Zarebski AE. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat Hum Behav. 2020;4(August (8)):856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez E., Fontán-Vela M., Valencia J., Fernandez-Jimenez I., Álvaro-Alonso E.A., Izquierdo-García E. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10(November (11)) doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong S., Liu L., Lin F., Shi J., Han L., Liu H. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational study. BMC Infect Dis. 2020;20(October (1)):787. doi: 10.1186/s12879-020-05452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Nava G., Yanez-Bello M.A., Trelles-Garcia D.P., Chung C.W., Chaudry S., Khan A.S. Clinical characteristics and risk factors for mortality of hospitalized patients with COVID-19 in a community hospital: a retrospective cohort study. Mayo Clin Proc Innov Qual Outcomes. 2020 doi: 10.1016/j.mayocpiqo.2020.10.007. http://www.sciencedirect.com/science/article/pii/S2542454820302071 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J., Shi L.-X., Xie Y., Zhang Y.-J., Huang S.-P., Li J.-G. Analysis of factors affecting the prognosis of COVID-19 patients and viral shedding duration. Epidemiol Infect. 2020;148(June) doi: 10.1017/S0950268820001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID Misinformation Is Killing People – Scientific American [Internet]. Available from: https://www.scientificamerican.com/article/covid-misinformation-is-killing-people1/ [cited 30 November 2020].
- 19.Where Are All the Patients? Addressing Covid-19 Fear to Encourage Sick Patients to Seek Emergency Care | Catalyst non-issue content [Internet]. Available from: https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0193 [cited 30 November 2020].
- 20.Galvão J. Lancet Publishing Group; 2020. COVID-19: The Deadly Threat of Misinformation. Vol. 0, The Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roozenbeek J., Schneider C.R., Dryhurst S., Kerr J., Freeman A.L.J., Recchia G. Susceptibility to misinformation about COVID-19 around the world. R Soc Open Sci. 2020;7(October (10)) doi: 10.1098/rsos.201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(May (20)):2052–2059. doi: 10.1001/jama.2020.6775. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet (London, England) 2020;395 doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(July (7)):904–913. doi: 10.1016/S1470-2045(20)30310-7. Available from: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30310-7/fulltext#.X7Ymjem-yXU.mendeley [cited 19 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14(4):655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A., Garg A., Rout A., Lavie C.J. Association of obesity with more critical illness in COVID-19. Mayo Clinic Proc. 2020;95:2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattar N., McInnes I.B., McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(July (1)):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 28.Carlson N., Nelveg‐Kristensen K., Freese Ballegaard E., Feldt‐Rasmussen B., Hornum M., Kamper A. Increased vulnerability to Covid‐19 in chronic kidney disease. J Intern Med. 2021;(January) doi: 10.1111/joim.13239. Available from: https://pubmed.ncbi.nlm.nih.gov/33452733/ [cited 31 January 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozturk S., Turgutalp K., Arici M., Odabas A.R., Altiparmak M.R., Aydin Z. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35(December (12)):2083–2095. doi: 10.1093/ndt/gfaa271. Available from: https://pubmed.ncbi.nlm.nih.gov/33275763/ [cited 31 January 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed N.E., Benn E.K.T., Astha V., Okhawere K.E., Korn T.G., Nkemdirim W. Association between chronic kidney disease and COVID-19-related mortality in New York. World J Urol. 2021;(January) doi: 10.1007/s00345-020-03567-4. Available from: http://link.springer.com/10.1007/s00345-020-03567-4 [cited 31 January 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parra-Bracamonte G.M., Parra-Bracamonte F.E., Lopez-Villalobos N., Lara-Rivera A.L. Chronic kidney disease is a very significant comorbidity for high risk of death in patients with COVID-19 in Mexico. Nephrology. 2020 doi: 10.1111/nep.13827. Available from: https://pubmed.ncbi.nlm.nih.gov/33184959/ [cited 31 January 2021] [DOI] [PubMed] [Google Scholar]
- 32.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(July (7)):934–943. doi: 10.1001/jamainternmed.2020.0994. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit care Resusc J Australas Acad Crit Care Med. 2020;22(September (3)):200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(August (1)):516. doi: 10.1186/s13054-020-03240-7. https://pubmed.ncbi.nlm.nih.gov/32825837 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(March (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;(May) doi: 10.1093/cid/ciaa530. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. http://www.sciencedirect.com/science/article/pii/S0163445320303236 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C-Y, Chan K-G. Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J Infect. 2020;81(September (3)):e29–e30. doi: 10.1016/j.jinf.2020.06.077. https://pubmed.ncbi.nlm.nih.gov/32628960 2020/07/03. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(July (7)):1747–1751. doi: 10.1111/jth.14854. Available from: https://pubmed.ncbi.nlm.nih.gov/32302448/ [cited 23 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(July (7)):1738–1742. doi: 10.1111/jth.14850. Available from: https://pubmed.ncbi.nlm.nih.gov/32302438/ [cited 23 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(August (8)):1995–2002. doi: 10.1111/jth.14888. Available from: https://pubmed.ncbi.nlm.nih.gov/32369666/ [cited 23 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191(July):145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(June (6)):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayerbe L., Risco C., Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020;50(August (2)):298–301. doi: 10.1007/s11239-020-02162-z. https://pubmed.ncbi.nlm.nih.gov/32476080 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(May (5)):1094–1099. doi: 10.1111/jth.14817. Available from: https://pubmed.ncbi.nlm.nih.gov/32220112/ [cited 23 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]