Abstract
Objective
To determine the differences in outcomes of adult patients with ataxia initially evaluated for paraneoplastic cerebellar degeneration (PCD) as inpatients or outpatients.
Methods
In this retrospective cohort analysis, diagnosis, workup, and functional outcomes based on the change in the modified Rankin Scale (mRS) score were compared between patients with ataxia who underwent workup for PCD initially as inpatients vs outpatients between March 2011 and June 2018 at Rush University Medical Center.
Results
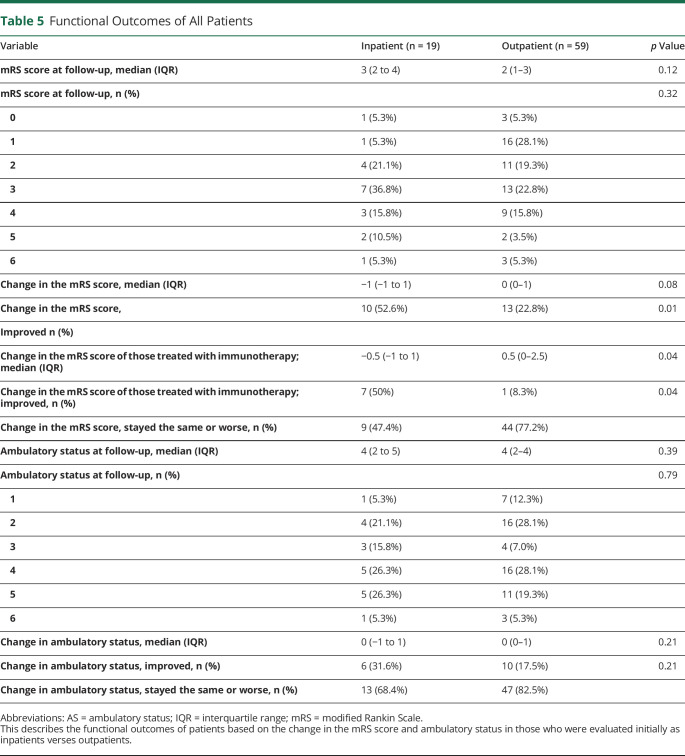
There were 78 patients included in the analysis; 59% were women, and the average age at symptom onset was 57 ± 19.5 years. Nineteen patients (24.3%) underwent evaluation as inpatients and 59 (75.6%) as outpatients. Admitted patients were more likely to receive immunotherapy (73.7% vs 20.3%, p < 0.0001) and received it faster than outpatients (0.40 months for inpatients, interquartile range [IQR] 0.03–1 months, vs 6.6 months for outpatients, IQR 2–11.7 months; p = 0.01). A greater percentage of inpatients improved based on the mRS score compared with those who underwent evaluation as outpatients (52.63% vs 22.81%, p = 0.01).
Conclusions
More patients improved from baseline in the inpatient cohort.
Classification of Evidence
This study provides Class III evidence that for patients undergoing initial evaluation for PCD, patients undergoing inpatient evaluation have better outcomes compared with those undergoing outpatient evaluation.
Paraneoplastic cerebellar degeneration (PCD) is rare. Paraneoplastic neurologic syndromes affect 1% of patients with cancer overall, but the prevalence may be as high as 10% of patients with certain malignancies, such as lymphoma.1 However, neurologists routinely consider PCD as part of their differential diagnosis for subacute ataxia in adults, which progresses over weeks to months.2,3 Although this diagnosis may signify underlying malignancy, once diagnosed, it opens the possibility for additional treatment options. Navigating the evaluation of ataxia can be challenging as the number of possible diagnostic tests continues to expand as new genetic and autoimmune biomarkers are discovered.
Immune-mediated cerebellar ataxia (IMCA) includes PCD and other etiologies, such as MS, anti–GAD65 antibody–associated cerebellar ataxia, or postinfectious cerebellitis.4 Identification and treatment of the underlying malignancy is essential in patients with PCD, and these patients may warrant concomitant immunotherapy.5,6 Patients with nonparaneoplastic IMCA, such as anti–GAD65 antibody–associated cerebellar ataxia, have been shown to respond better to immunotherapy than patients with paraneoplastic IMCA.7 There is a limited timeframe during which the treatment of patients with IMCA can restore function, and thus, timely diagnosis is crucial.5 A growing body of evidence suggests that time is cerebellum and that earlier treatment of IMCA confers a better chance of recovery.4,7–9
When clinicians consider PCD as an etiology of ataxia, there is little evidence to guide whether patients should undergo inpatient or outpatient evaluation. Patients with PCD often present with relatively acute onset of balance deficits that may be associated with other debilitating features such as nausea, diplopia, dysphagia, and, at times, cognitive impairment.10 Providers must consider numerous factors in making a decision to admit patients for expedited evaluation, including the patient's disease severity, socioeconomic background, autonomy, safety, and potential outcomes. This study aims to assess the outcomes of patients with ataxia who underwent evaluation for PCD and to compare the outcomes of those who had their initial consultation and workup completed as inpatients vs outpatients. The hypothesis was that admitted patients with PCD would experience greater improvement in functional outcomes based on the change in the modified Rankin Scale (mRS) score.
Methods
Primary Research Question and Classification of Evidence
Do patients with suspected PCD who are admitted for initial workup experience a greater functional outcome compared with patients who undergo initial workup as an outpatient? This retrospective cohort analysis provides Class III evidence because of the retrospective observational design with baseline confounding.
Setting
This study is a retrospective cohort analysis, reviewing the electronic medical records of all patients older than 18 years with a diagnosis of ataxia who underwent evaluation for PCD at Rush University Medical Center (RUMC), Chicago, IL, between March 2011 and June 2018. RUMC is a tertiary medical center that evaluates patients with ataxia in outpatient general neurology clinics, subspecialty clinics, and as inpatients.
Definitions
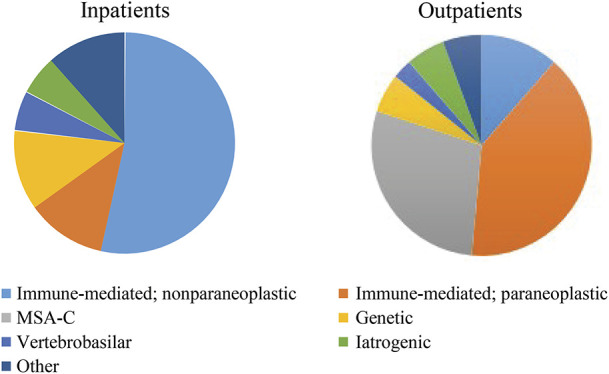
Ataxia was defined by motor dysfunction including documented imbalance, impaired coordination, limb and body tremor, dysarthria, and/or oculomotor abnormalities on examination.11 A probable or definitive diagnosis of PCD was determined clinically and was confirmed based on the diagnostic criteria outlined by Graus et al.10 A probable or definitive diagnosis of the etiology of the ataxia from all causes was determined by the patient's clinician, and subcategory designation was assigned by consensus of 3 investigators (N.W., D.H., and M.A.).6,7,12 For patients who had an identifiable etiology of ataxia, the diagnostic etiologies were categorized as immune mediated, with subgroup classification as paraneoplastic or nonparaneoplastic (such as cerebellitis, GAD65-associated ataxia, or multiple sclerosis); vascular; multiple system atrophy; genetic (confirmed by genetic testing); or other etiology (such as prion disease) (figure e-3, links.lww.com/NXI/A263). Acute time course was defined as the onset progressing over minutes to days, subacute lasting days to weeks, and chronic as progressing over months to years. The presence of antibodies was determined using standardized indirect, immunofluorescence tissue-based or cell-binding assays, such as the Mayo paraneoplastic or autoimmune encephalopathy panel, which were ordered at the time of clinical encounter. These panels collectively tested for antineuronal nuclear antibody type 1 (ANNA-1 or anti-Hu); antineuronal nuclear antibody type 2 (ANNA-2 or anti-Ri); antineuronal nuclear antibody type 3 (ANNA-3); antiglial and/or neuronal nuclear antibody type 1 (AGNA-1); Purkinje cell cytoplasmic (PCA type 1, 2, and Tr); striational (striated muscle); voltage-gated calcium channel (VGCC); collapsin response-mediator protein 5 (CRMP-5); acetylcholine receptor; amphiphysin; voltage-gated potassium channel; NMDA receptor; leucine-rich glioma inactivated 1 (LGI1); contactin-associated protein 2 receptor (CASPR2); glutamic acid decarboxylase (GAD65); and gamma-aminobutyric acid receptor (GABABR) antibodies. Immunologic therapy was defined as receiving an immunosuppressant or immunomodulatory medication, such as steroids, IV immunoglobulin (IVIG), rituximab, cyclophosphamide, and azathioprine.4 Oncologic therapy was defined as receiving a therapy, radiation, or surgery to treat an underlying malignancy. Symptomatic therapy of cerebellar motor dysfunction was defined as treatment for ataxia with medications such as riluzole, 4-aminopyridine, buspirone, amantadine, varenicline, and others as outlined by Zesiewicz et al.11 Follow-up time was defined as the last appointment within the study period with documentation of examination and functional status.
Patients
To identify all relevant patients, electronic medical records were searched for patients diagnosed with ataxia based on ICD-10 codes (R 27.0 and G 11.9) who also underwent testing for serum and/or CSF neuronal and glial antibody testing ordered for suspected PCD at RUMC. Patients were excluded from the analysis if they did not have an examination documenting ataxia in their medical records. Demographic data including age, sex, race/ethnicity, clinical features at presentation, and baseline severity of illness defined by the mRS score were recorded. The time between symptom onset, presentation, diagnosis, and treatment were recorded. Collected data were stored in a deidentified manner in a password-protected database. Based on documentation in the medical records, the principal investigator (N.W.) divided patients into 2 cohorts: those who underwent initial evaluation as an inpatient and those who underwent evaluation in an outpatient clinical setting.
The primary outcome assessed was the change in the mRS score from baseline to follow-up (dichotomized as improved or worsened/stayed the same). The mRS score for each patient was determined by review of the records by a movement disorders trained neurologist (M.A.), who was blinded to patients' group designation and visit chronology (e.g., if the visit was a baseline or follow-up visit) to minimize bias. The mRS score was designed to measure disability following stroke, but has been used in many retrospective studies assessing the outcomes of patients with PCD.7,13,14
Secondary outcomes included change in ambulatory status, as outlined by Jones et al. , which is a descriptive 5-point scale rating patients from having a normal gait (1) to being wheelchair dependent or bedbound (5).7 In regard to diagnostic evaluation, the frequency of physician ordering, test completion, and the results of the following diagnostic tests were analyzed: neuronal and glial autoantibody testing, MRI of the brain, MRI of the spine, lumbar puncture, CT of the chest, CT of the abdomen, CT of the pelvis, and PET-CT. Other secondary outcomes included type of therapy received and the timing between symptom onset, presentation, diagnosis, and treatments. Treatments analyzed included immunologic therapy, oncologic therapy, and symptomatic therapies.
Statistical Analysis
Baseline demographic, clinical, diagnostic, and outcomes of those who underwent initial workup as inpatient vs outpatient were compared using a t-test or Mann-Whitney U test to assess differences in continuous variables and a χ2 test or Fisher exact test to assess differences between categorical variables. Missing data were excluded from the analysis. A p value <0.05 was considered statistically significant. Data were analyzed between November 2018 and March 2019. All statistical analyses were performed using SAS version 9.4 (Cary, NC).
Standard Protocol Approvals, Registrations, and Patients Consents
The research protocol was approved by Rush University Hospital IRB (17080806-IRB01).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator with IRB approval.
Results
Baseline Characteristics
Of the 81 patients identified, 78 patients met the inclusion criteria for ataxia based on physical examination in the medical records and were included in the analysis: 19 patients (24.4%) underwent initial evaluation for ataxia as an inpatient, and 59 (75.6%) underwent workup as an outpatient (figure e-1, links.lww.com/NXI/A263). Three patients were excluded for not having documentation of ataxia on physical examination in the medical records. Two of the 59 patients were lost to follow-up in the outpatient cohort, but available data were included in the statistical analysis.
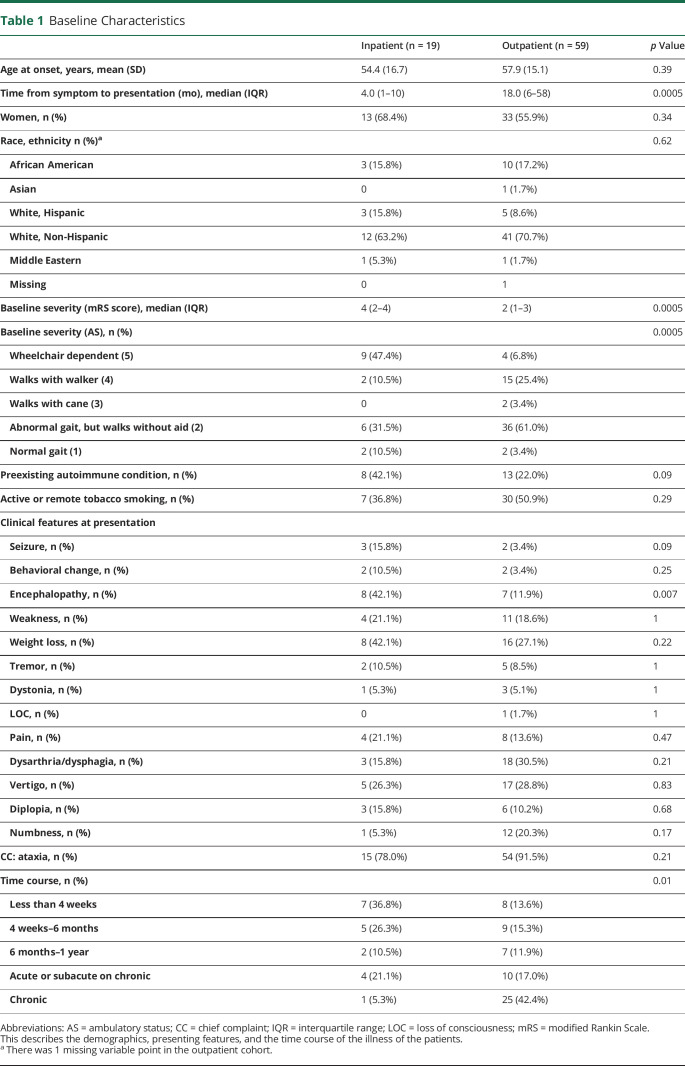
There was no difference between age, sex, and race between the 2 groups (table 1). The majority of the patients evaluated were women (59.0%). Admitted patients were more likely to be clinically severe, encephalopathic, present to care sooner after symptoms developed, and have a more acute time course (table 1). The mRS score of patients who underwent workup for ataxia as an inpatient had higher (worse) mRS scores on presentation than those who underwent workup as an outpatient (median mRS score 4, interquartile range [IQR] 2–4 vs median mRS score 2, IQR 1–3; p = 0.0005). Patients who were admitted for initial evaluation were also more severe based on ambulatory status at the time of presentation. For example, 47.4% of the patients evaluated as inpatients were wheelchair dependent compared with 6.8% of the patients evaluated as outpatients (p = 0.0005). Admitted patients presented sooner after their symptoms started (median 4.0 months, IQR 1–10 months, vs median 18.0 months, IQR 6–58 months). Admitted patients had a more rapid time course than patients who were evaluated as outpatients (table 1). Also, admitted patients were more likely to present with encephalopathy in addition to ataxia compared with the outpatient cohort (42.1% vs 11.9%, p = 0.007).
Table 1.
Baseline Characteristics
Diagnostic Evaluation
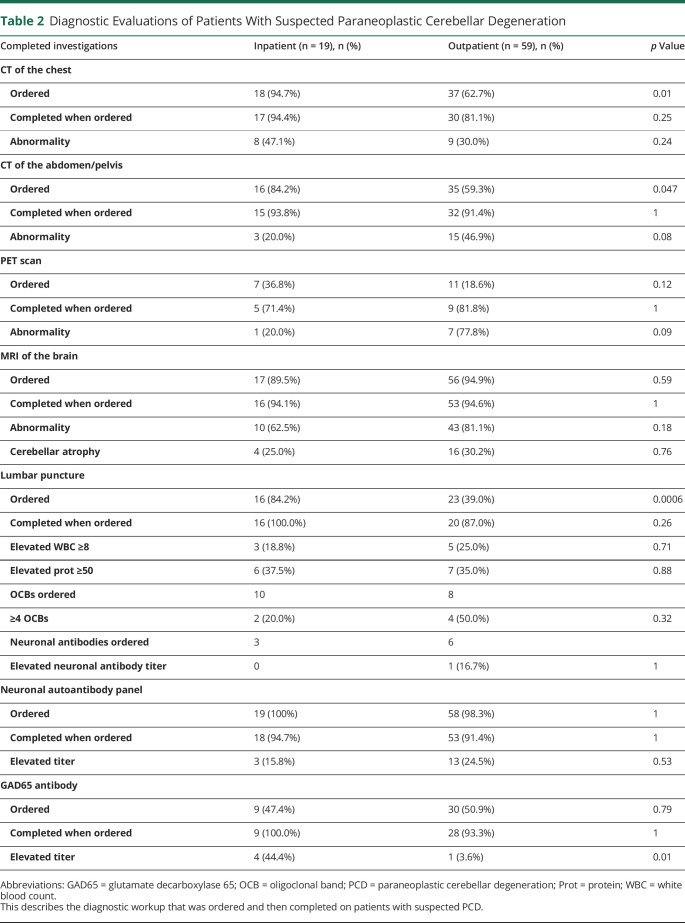
Patients evaluated as inpatients were more likely to undergo a lumbar puncture (84.2% vs 39.0%, p = 0.0006), CT of the chest (94.7% vs 62.7%, p = 0.01), and CT of the abdomen/pelvis (84.2% vs 59.3%, p = 0.047) than those evaluated initially as outpatients (table 2). Nearly all patients had an MRI of the brain (89.5% of patients evaluated as inpatients vs 94.9% of patients evaluated as outpatients, p = 0.59), and cerebellar atrophy was seen in a minority of these patients (25.0% of inpatients vs 30.2% of outpatient cohort, p = 0.76). An elevated serum neuronal or glial autoantibody titer was present in 16/71 (22.5%) of all of the patients who completed the serum paraneoplastic panel and autoimmune encephalopathy panels. The most common abnormally elevated antibody was P/Q type VGCC antibody in the patients who were eventually diagnosed with PCD (figure e-2, links.lww.com/NXI/A263 and table e-1, links.lww.com/NXI/A264). GAD65 antibody was the second most common abnormally elevated antibody overall, but was not elevated in any patients diagnosed with PCD in this cohort.
Table 2.
Diagnostic Evaluations of Patients With Suspected Paraneoplastic Cerebellar Degeneration
Diagnostic Outcomes and Etiologies
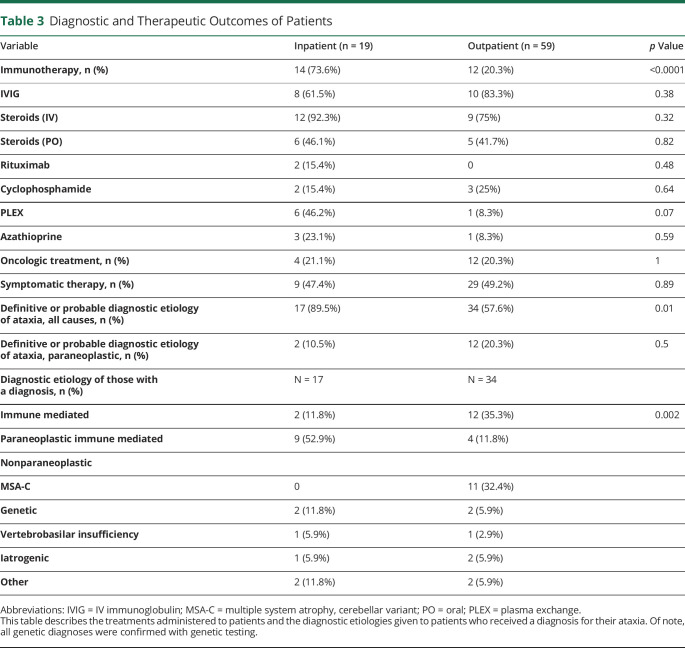
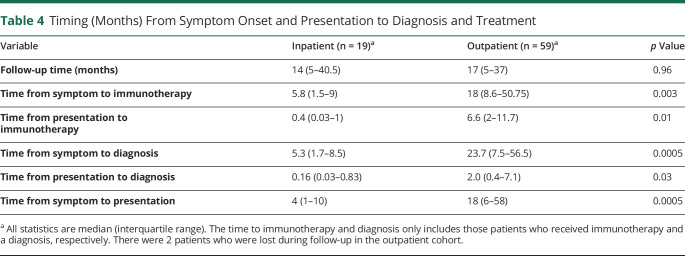
Patients who were admitted for initial workup were more likely to receive a diagnosis for their ataxia compared with outpatients (89.5% vs 57.6%; p = 0.01), and those who received a diagnosis received it faster after presentation than those who underwent outpatient workup (median 0.16 months, IQR 0.03–0.83 months vs median 2.0 months, IQR 0.4–7.1 months; p = 0.03; Tables 3 and 4). Follow-up times between those evaluated as an inpatient or outpatient were similar (median follow-up 14 months [IQR 5–40.5 months] vs 17 months [IQR 5–37 months], p = 0.96; table 4). In both inpatients and outpatients alike, only a minority of the patients was ultimately diagnosed with PCD (2/17 [10.5%] of the inpatients vs 12 [20.3%] of the outpatients; p = 0.5; table 3). Of the patients who received a definitive or probable diagnosis for their ataxia, the most common diagnosis made in the inpatient cohort was nonparaneoplastic IMCA 9/17 (52.9%), and the most common diagnosis in the outpatient cohort was PCD 12/34 (35.3%; table 3). No patients were diagnosed with a neurodegenerative disease in the inpatient cohort, but in the outpatient cohort, 11/59 (18.6%) of patients were diagnosed with multiple systems atrophy, with predominant cerebellar ataxia.
Table 3.
Diagnostic and Therapeutic Outcomes of Patients
Table 4.
Timing (Months) From Symptom Onset and Presentation to Diagnosis and Treatment
Treatment Outcomes
Patients who underwent inpatient workup were more likely to receive immunotherapy than outpatients (73.6% vs 20.3%; p < 0.0001; table 3). In addition, of the patients who received immunotherapy, those who underwent inpatient workup received treatment in a shorter time frame from presentation than outpatients (median of 0.4 months, IQR 0.03–1 months vs 6.6 months, IQR 2–11.7 months; p = 0.01, table 4). Steroids were the most commonly prescribed immunosuppressant, followed by IVIG and plasma exchange. Patients evaluated as an inpatient or outpatient received similar rates of oncologic (21.1% vs 20.3%, p = 1.0) and symptomatic therapy (47.4% vs 49.2%, p = 0.89).
Functional Outcomes
A greater proportion of patients who were admitted for workup of ataxia improved, based on the mRS score, compared with those who underwent evaluation as an outpatient (52.6% vs 22.8%, p = 0.01, table 5). Of the patients who were treated with immunotherapy, the median change in the mRS score improved in the inpatient cohort and worsened in the outpatient cohort (−0.5 [IQR −1 to 1] vs 0.5 [0–2.5], p = 0.04).
Table 5.
Functional Outcomes of All Patients
Discussion
This study showed that for patients with ataxia with suspected paraneoplastic syndrome, inpatient admission to the hospital was associated with improved outcomes. Patients admitted for evaluation of ataxia with suspected PCD were more likely to receive a diagnosis in an expedited fashion. Likewise, they were more likely to receive immunotherapy and to receive it faster. Patients who were admitted for initial evaluation in this study received immunotherapy over 15 times faster than the outpatients. It has been shown that the delay of treatment affects the outcome of patients, particularly those with immune-mediated neurologic diseases.5
Although there are potential benefits to an inpatient admission, almost all of the patients in this study had outpatient follow-up. For those who do not have a malignancy identified during hospitalization, these patients may need additional testing after discharge.7 It is important to note that there are a number of diagnostic tests that can be helpful in identifying occult malignancy, such as mammogram or PET-CT, but these tests may be challenging to obtain as inpatients in the United States.15 Collaboration between inpatient and outpatient providers is important to facilitate ongoing care and treatment between settings. Although there are benefits to expedited inpatient evaluation and treatment noted in this study, the cost-effectiveness of this approach remains unknown. More work is needed to refine the diagnostic approach to more quickly diagnose and treat patients with suspected PCD.
Similar to previous assessments of paraneoplastic disorders, which largely rely on retrospective analyses, the limitations in this study arise from using medical record chart abstraction to identify the clinical presentation and outcomes.16 Because of limitations in abstracting information from the records, details of whether patients were offered inpatient admission and declined were not available. There were differences between the patient cohorts both at baseline and throughout their workup, introducing both selection and ascertainment biases. Those who were admitted presented earlier in their disease course and were more severe at presentation. The diagnostic workup differed, with inpatients undergoing more lumbar punctures and CT scans of the chest, abdomen, and pelvis. The ultimate etiology of the ataxia differed between the cohorts as well. PCD was more commonly diagnosed as an outpatient. Nonparaneoplastic immune-mediated ataxia, which is more amenable to treatment, was a more common diagnosis in the inpatient cohort. Given the nature of this study as pilot data, no correction was performed for multiple analyses. More research is needed to delineate which subgroup of patients with ataxia with suspected PCD would benefit most from inpatient admission. The generalizability of this study may also be limited due to the geographic and practice-based variation of treatment of neurologic paraneoplastic syndromes and other immune-mediated neurologic disorders.17
Patients admitted for the evaluation of a suspected PCD received a diagnosis and immunotherapy faster than their outpatient counterparts did. Despite the limitations, this study provides evidence supporting the use of initial expedited inpatient evaluation and treatment of patients with ataxia with a suspected paraneoplastic syndrome, particularly those who are severely functionally affected. Further research is needed to identify a systematic approach to the evaluation and treatment of patients with ataxia.
TAKE-HOME POINTS
→ Inpatient admission in patients with ataxia with suspected PCD was associated with improved outcome.
→ Patients admitted for evaluation of paraneoplastic cerebellar degeneration were more likely to receive a diagnosis and to receive a diagnosis faster than those evaluated initially as outpatients.
→ Patients admitted for evaluation of paraneoplastic cerebellar degeneration were more likely to receive immunotherapy and receive immunotherapy faster than those evaluated initially as outpatients.
Appendix. Authors
Study Funding
Michael J. Fox Edmond J. Safra Fellowship provided funding for the Movement Disorder Fellowship of the corresponding author.
Disclosure
N. Witek receives research funds from the Parkinson's Foundation. M. Afshari, Y. Liu, and B. Ouyang report no disclosures. D. Hall receives research funds from the NINDS, Parkinson's Foundation, AbbVie, and Biogen. She receives editorial funds from the American Academy of Neurology. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clinic Proc 2010;85:838–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rojas I, Graus F, Keime-Guibert F, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology 2000;55:713–715. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitoma H, Manto M, Hampe CS. Immune-mediated cerebellar ataxias: from bench to bedside. Cerebellum Ataxias 2017;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitoma H, Manto M, Hampe CS. Time Is Cerebellum. London: Cerebellum; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanri K, Okuma M, Sato S, et al. Prevalence of autoantibodies and the efficacy of immunotherapy for autoimmune cerebellar ataxia. Intern Med 2016;55:449–454. [DOI] [PubMed] [Google Scholar]
- 7.Jones AL, Flanagan EP, Pittock SJ, et al. Responses to and outcomes of treatment of autoimmune cerebellar ataxia in adults. JAMA Neurology 2015;72:1304–1312. [DOI] [PubMed] [Google Scholar]
- 8.Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol 2002;1:294–305. [DOI] [PubMed] [Google Scholar]
- 9.Blaes F, Strittmatter M, Merkelbach S, et al. Intravenous immunoglobulins in the therapy of paraneoplastic neurological disorders. J Neurology 1999;246:299–303. [DOI] [PubMed] [Google Scholar]
- 10.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zesiewicz TA, Wilmot G, Kuo SH, et al. Comprehensive systematic review summary: treatment of cerebellar motor dysfunction and ataxia: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlman S. Evaluation and Management of Ataxic Disorders: An Overview for Physicians. Minneapolis, MN: National Ataxia Foundation; 2016. [Google Scholar]
- 13.Samii A, Ryan-Dykes P, Tsukuda RA, Zink C, Franks R, Nichol WP. Telemedicine for delivery of health care in Parkinson's disease. J Telemed Telecare 2006;12:16–18. [DOI] [PubMed] [Google Scholar]
- 14.Keime-Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry 2000;68:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saif MW, Tzannou I, Makrilia N, Syrigos K. Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med 2010;83:53–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Ebright MJ, Li SH, Reynolds E, et al. Unintended consequences of Mayo paraneoplastic evaluations. Neurology 2018;91:e2057–e2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh A, Wesley SF. Practice current: when do you suspect autoimmune encephalitis and what is the role of antibody testing? Neurol Clin Pract 2018;8:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator with IRB approval.