Abstract
Purpose of Review
With the advent of precision medicine and demand for genomic testing information, we may question whether it is time to offer genetic testing to our patients with Parkinson disease (PD). This review updates the current genetic landscape of PD, describes what genetic testing may offer, provides strategies for evaluating whom to test, and provides resources for the busy clinician.
Recent Findings
Patients with PD and their relatives, in various settings, have expressed an interest in learning their PD genetic status; however, physicians may be hesitant to widely offer testing due to the perceived low clinical utility of PD genetic test results. The rise of clinical trials available for patients with gene-specific PD and emerging information on genotype-phenotype correlations are starting to shift this discussion about testing.
Summary
By learning more about the various genetic testing options for PD and utility of results for patients and their care, clinicians may become more comfortable with widespread PD genetic testing in the research and clinical setting.
Genetic testing is not part of the routine evaluation of individuals with Parkinson disease (PD) and is rarely offered in late-onset PD.1 Yet, approximately 5% of the population with adult-onset PD who are of European descent carries major PD-associated pathogenic variants specifically in either the glucocerebrosidase (GBA) or leucine-rich repeat kinase (LRRK2) genes.2 In individuals of Ashkenazi Jewish and North African Berber descent, the prevalence of variants in these genes approaches 40%.3 An association between PD genetic status and specific phenotypic features has been reported in multiple studies.4,5 In some cases, identification of PD variants may aid in diagnosis and management and prediction of disease course. In addition, knowledge of genetic status is a prerequisite for enrollment in interventional research studies including clinical trials evaluating the genetic pathogenesis of PD. Therefore, we now ask whether we should include genetic testing more frequently in the clinical setting.6

PD Genetics Basics
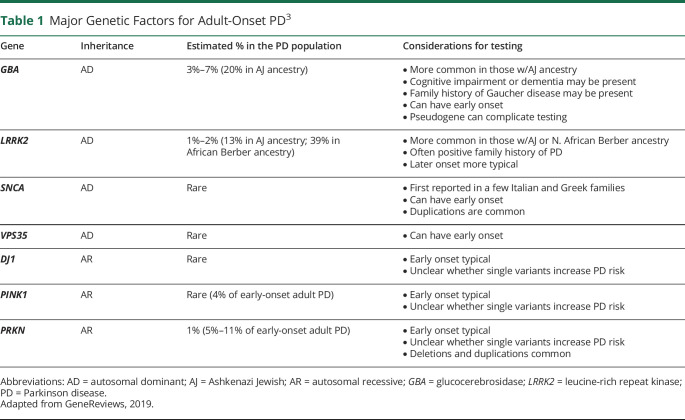
A greater awareness of the importance of genetic factors in the causation of both simplex (sporadic) and familial PD has evolved over the past 3 decades. We now know that major pathogenic variants can be found in about 5%–10% of individuals with PD and that PD arises from a complex interplay of multiple genetic and nongenetic factors.3,7 In the adult-onset PD population, the most likely major pathogenic variants identified are in LRRK2, GBA, and PRKN, but other less frequent genetic factors may also be identified (table 1). Variants in the first described PD-associated gene, alpha-synuclein (SNCA), are very rare in most populations, but are important to the understanding of PD pathogenesis, because the encoded protein, alpha-synuclein, is abundantly expressed in Lewy bodies.8 In general, later-onset PD (age >50 years) is associated with autosomal dominant variants (relating to PD risk) such as those in the LRRK2 or GBA genes, whereas PRKN, PINK1, and DJ1 variants are usually found in individuals with earlier-onset PD (age <40 years) and follow autosomal recessive inheritance. In addition to these major pathogenic variants, over 90 susceptibility loci that may contribute to overall PD risk have been described through genome-wide association studies.9,10
Table 1.
Major Genetic Factors for Adult-Onset PD3
The genetics of PD is complex, and there may be hundreds of pathogenic variants described for an associated gene that can present as point mutations or deletions/duplications. Variants in the same gene may differ in how they influence disease risk and clinical effect, and it appears that the background ancestral genotype may also matter in disease expression.3 Intriguingly, PD pathogenic variants do not always behave as expected: Both LRRK2 and GBA dominant variants have markedly reduced clinical penetrance, requiring other modifiers, and autosomal recessive gene variants, such as in PRKN and PINK1, may confer an increased risk of PD for those individuals who carry only 1 variant in the heterozygous state.3,5 Pedigrees for familial or simplex forms of PD may not follow well-defined mendelian patterns due to incomplete penetrance, variable expressivity, phenocopies, and even small family size.11 GBA variants are unique in that most, when present on both chromosomes (biallelic), are associated with the autosomal recessive lysosomal storage disorder Gaucher disease. Astute physicians reported in the 1990s that both patients with Gaucher disease and their GBA carrier relatives were at an increased risk of PD. There is interest to increase clinician and patient awareness about this unusual PD-Gaucher connection because Gaucher disease can have reproductive implications for patients and their relatives planning families.12
Assessment of the Utility of PD Genetic Testing
For the Clinician
Physicians may perceive that PD genetic testing has low clinical utility, reserved only for patients with strong family histories or early-onset PD. This perspective is beginning to shift with the development of targeted therapeutics for certain genetic forms of PD, but there still may be differing views of whether genetic testing is ready for inclusion in standard clinical care.6 There is relatively little guidance on genetic testing in PD from large professional groups: The European Federation of Neurological Societies published recommendations 10 years ago that were focused on the use of molecular testing for the diagnosis of neurogenetic disorders including PD. They include the following: (1) for Europeans, LRRK2 testing in cases suggestive of autosomal dominant inheritance, (2) LRRK2 G2019S testing in Ashkenazi Jewish or North African Berber Arabs with PD, regardless of family history, and (3) PRKN, PINK1, and DJ-1 testing in cases suggestive of recessive inheritance and/or in sporadic cases of early-onset PD. These recommendations and other statements that have been previously published are now outdated.11,13
We propose that it is time to consider more widespread PD genetic testing for the following reasons: By testing a heterogeneous group of patients with PD, we can begin to delineate genetic subgroups who may benefit from targeted treatment and provide them with more specific clinical information regarding disease course. Targeted clinical trials are now available for patients with PD who have GBA or LRRK2 pathogenic variants, and trials for those with other gene variants may be emerging in the future. We are also learning that PD genetic testing may inform treatment beyond the individual patient; insight into genetic forms of PD has and continues to provide critical information about potential therapies for the larger PD population. In a recent study, it was observed that wild-type LRRK2 activity was enhanced in brain tissue of patients with idiopathic PD, resulting in activation of its substrate in dopamine neurons and subsequent downstream events that could be associated with neurodegeneration.14
Another advancement is the growing body of PD literature about how genotype exerts an effect on clinical phenotype.4,5,15 As we gather data from large genetic cohorts, differing clinical profiles are emerging for PD-associated genes such as LRRK2 and GBA, with variant-specific subgroups becoming delineated.5 Compared with LRRK2 variant carriers or patients with idiopathic PD, individuals who carry GBA variants are prone to earlier onset, cognitive impairment including Lewy body dementia, and postural instability gait disturbance.3,16 Carriers of severe vs mild GBA variants (variant severity historically based on Gaucher disease classification) are more likely to have these adverse clinical outcomes as well.5 New data about genotype-phenotype correlations may inform prognostic information provided to the patient and their families or help shape forward-thinking treatment plans. Another way in which determining genetic variant status may be helpful is in guiding treatment for deep brain stimulation (SBS) therapy. The effects of subthalamic nucleus DBS in GBA mutation carriers are currently being investigated, as those with GBA variants appear to have more cognitive impairment and be at risk of worse outcomes following the procedure.17,18
For the Patient
The large amount of internet-accessible information allows for patients to be actively involved in the direction of their care. It is also an easy way for testing companies and initiatives to directly recruit or inform patients of genetic testing and clinical trials.19,20 Evidence suggests that there is large public interest within the PD population to gather more personal genetic data.20,21 Approximately 10,000 individuals who have PD had enrolled in 23andMe direct-to-consumer (DTC) testing by 2017 (23andMe Blog).22 In many cases, patients have a strong curiosity about their genetic makeup and are proactive in seeking out testing for themselves. In a study of US patients with PD, 83% stated that they would undertake genetic testing for PD, if it was offered. In this group, there was also a high interest in learning about the risks, benefits, and effects of genetic testing.21
Patients may seek out genetic information for a variety of reasons that may be addressed, to some extent, by genetic testing. Some patients are seeking a better understanding of why they developed PD, if their PD is inherited, or additional information to make their diagnosis feel more definitive. Some individuals may seek this information to learn reproductive risks that can be used for family planning. Advantages of genetic testing frequently mentioned by patients are the ability to better inform relatives about their PD risk, learning more about oneself, and aiding research efforts.21,23 In addition, affected individuals recognize that genetic test results could affect their decisions about obtaining insurance and guide financial planning.21 Published genetic counseling outcomes in other settings suggest that genetic test results paired with genetic counseling can serve to empower patients, reduce anxiety and guilt in some, and provide greater perceived personal control.21,24–26
Evaluation Strategies for PD Genetic Testing in the Clinical Setting
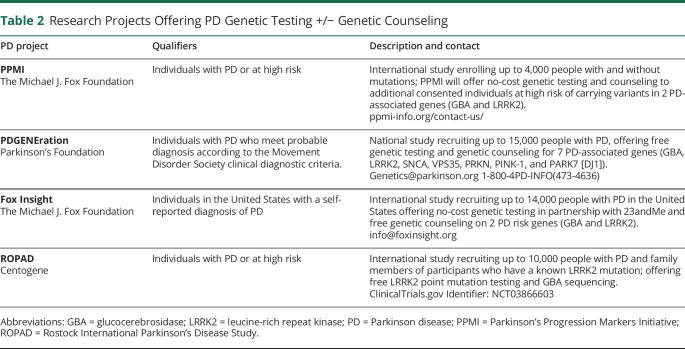
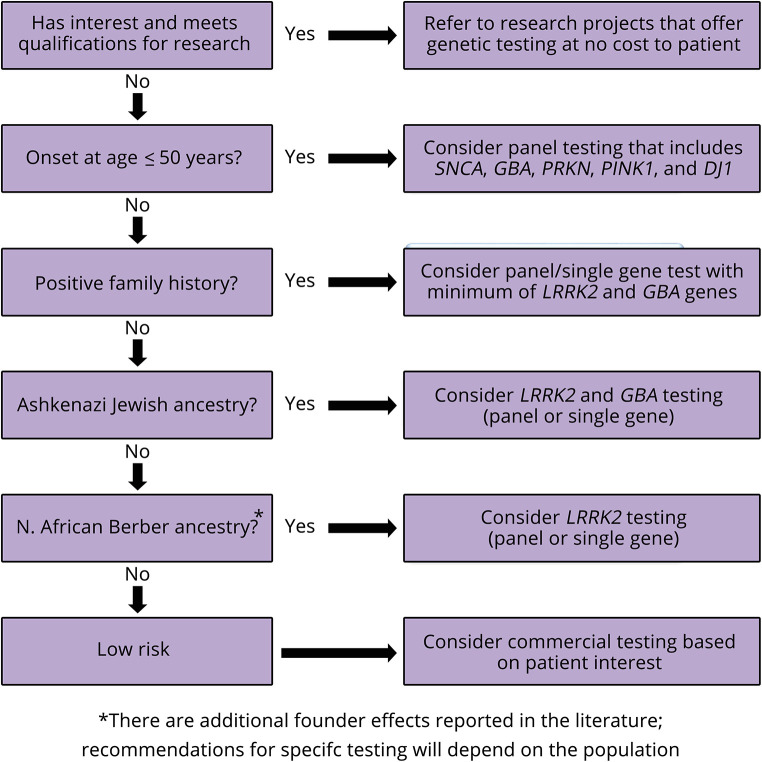
It is not a trivial question to ask who should have PD genetic testing.11 With the recent availability of no-cost genetic testing to patients and families with PD, we recommend that all patients be referred to ongoing research projects based on their interest and qualifications (table 2). There has been a consensus that PD commercial genetic testing may be reasonable to consider in patients with 1 or more of the following (figure):13
Earlier onset of the disorder
Positive family history suggestive of autosomal dominant (a first-degree relative or second-degree relatives in different generations affected) or autosomal recessive inheritance (siblings affected and/or consanguinity present)
High-risk ethnicity such as Ashkenazi Jewish or North African Berber
Table 2.
Research Projects Offering PD Genetic Testing +/− Genetic Counseling
Figure. Proposed Parkinson Disease Genetic Testing Pathway.
Specific demographic and clinical features can be useful in guiding testing strategies to obtain a high yield from a commercial test; however, we suggest that they should not be rigid qualifications. PD variant carriers are often clinically indistinguishable from noncarriers and, if identified, could qualify for clinical trials. Furthermore, we are learning more about how PD variant frequencies vary within populations and age groups, and there may be additional bottleneck/founder effects not fully recognized.27,28 Thus, broader genetic testing with PD gene panels is increasingly being considered.
The commercial genetic testing strategy as outlined above is based on the perspective that it is worth testing a patient if there is likely to be a positive test result. In this case, testing should be recommended if the patient is interested and the results are likely to be medically actionable. Even if a patient appears low risk, they may benefit from testing if they are interested in qualifying for clinical trials. There can be other reasons to consider testing including patient concern and personal interest. Anxiety about risk to offspring can strongly drive a patient's need to know genetic information. Testing in this group of individuals can be offered, following a full discussion of test limitations, including the low likelihood of positive results and possible out-of-pocket costs.
Which Type of PD Genetic Testing and Where?
There is a wide variety of genetic testing types available in the research and clinical settings:
1. Single gene targeted variant testing
2. Single gene sequencing (and/or deletion/duplication analysis)
3. Multigene targeted variant panel
4. Multigene sequencing panel (and/or deletion/duplication analysis)
5. Whole-exome sequence (WES) or whole-genome sequence (WGS) testing
The decision of which type of test to order will depend on the individual patient and setting. The conservative and historical approach to genetic testing is to order targeted single gene tests in a serial fashion based on the highest probability of a positive return. Genetic testing has rapidly changed from this approach to large panel testing for many disorders due to reduced cost and improved efficiency. Depending on the type of PD genetic testing panel (options can range from a specific PD panel to a neurodegenerative and/or dementia panel), the testing scope can vary from several genes to 25+. At this time, it is cumbersome to order single gene or 2 gene tests for PD through commercial laboratories. A disadvantage of PD panels is that they greatly vary in what variants they include, not all include major PD-associated genes, or they may include less well-established genes. For instance, not all commercial PD gene panels include the GBA gene; thus, it is important to assess what specifically is being tested by a laboratory. Most companies provide an easily accessible list of queried genes for a particular panel on their website. Physicians may want to consider a larger neurodegenerative panel that casts a wider net if a patient and/or family presents with atypical or overlapping features with other neurodegenerative disorders such as amyotrophic lateral sclerosis, frontotemporal dementia, other dementias, or dystonia. Expanding the number of genes increases the likelihood of incidental findings, results that are ambiguous including variants of uncertain significance, or information that may be difficult to interpret in a clinical context.
More comprehensive genetic testing, such as WES and WGS, are options for which the clinician does not need to determine which genes need to queried. Results are filtered by bioinformatic algorithms and are not necessarily comprehensive. The yield and utility of genomic testing in PD has not been carefully evaluated.3,29
PD genetic testing most likely to be appropriate includes (1) panels with full gene sequencing, typically via next-generation sequencing that detect DNA substitutions, insertions and small deletions and (2) optional testing via copy number analysis that detects larger duplications and deletions such that can occur with SNCA and PRKN. It is important to consider cost to patients because insurance companies may not cover PD genetic testing. National Center for Biotechnology Information houses The Genetic Testing Registry (ncbi.nlm.nih.gov/gtr/), which provides updated lists of commercial laboratories, including their website links, for all types of genetic testing including PD testing.
Free genetic testing and personalized genetic counseling may be available through ongoing research projects offered by various foundations such as the Michael J. Fox Foundation, Parkinson's Foundation, and other groups (table 2). It is important to stay aware of testing through research opportunities because patients are often highly motivated to participate, and they may receive no-cost PD genetic testing with genetic counseling. Cost is a common barrier to obtaining PD genetic testing.1 A few limitations of research testing include variability of genes tested and if the testing is performed in a non-Clinical Laboratory Improvement Amendments laboratory.
DTC companies offer a variety of genetic tests from targeted testing of LRRK2 G2019S and GBA N370S to whole-genome screening. DTC testing may or may not be overseen by medical professionals and often does not include genetic counseling. Although this testing is affordable and accessible, it can vary greatly in quality and information that is relevant for PD. False-positive and false-negative results may be more commonly encountered compared with results from a clinical laboratory,30 and there is variability in which PD genes and variants are tested. Genetic counseling regarding the benefits and limitations of DTC testing can be especially beneficial to patients before and after testing.
Finally, another source of PD genetic testing, specifically GBA testing, is through preconception or prenatal screening for the detection of carriers of Gaucher disease. Pan-ethnic or universal carrier screening is now commonly offered in the United States to couples contemplating pregnancy or pregnant.31
Approaching PD Genetic Testing With Your Patient
It is important to become comfortable with PD genetics topics and familiar with the different types of PD testing before approaching genetic testing with patients. Genetic counselors can be useful resources for the neurologist in this regard. Clinicians will want to ascertain a patient's interest, motivations, and goals and discuss with them the implications of a positive or negative test result. Practical aspects of testing to help the patient explore include but are not limited to:
1. Psychological effects of learning genetic information about their PD
2. Implications for family members
3. Possible guilt about transmitting a genetic risk factor
4. Misperceptions such as a negative result eliminates PD risk to the family
5. What genetic data are available to them now and in the future, and who owns the genetic data and how will it be used?
6. Genetic privacy and discrimination concerns
Some patients may be hesitant to include genetic results into their medical records due to worries about potential insurance discrimination. Patients and their families often focus on this concern, so clinicians should be knowledgeable about the federal Genetic Information Nondiscrimination Act and state laws surrounding genetic information. It can be reassuring to mention that the actual number of genetic discrimination cases reported in the literature is small.32
Detailed informed consent should be the first step in the genetic testing process, which should cover all the benefits, risks, and limitations of a particular test, including what will and will not be tested. Whether to test may not be decided in a single visit, but may require dialogue over time before a decision is made. It can be important to stress to patients that choosing not to undergo genetic testing may be the most appropriate decision for them.
Once test results are ordered and received, an appointment should be scheduled that ensures sufficient time for questions. Results disclosure should include the following basic components:
1. Type of result and method of testing
2. Limitations of the testing (not all genes or variants may have been tested)
3. Clinical correlations (note a patient may think a negative result means that they do not have PD)
4. Implications of results to the patient and family
5. Clinical trial and other research eligibility
Both negative and positive results provide important information to the patient and family. Negative results should not be quickly glossed over but explained in the context of the medical and family histories. Positive results will require more time for disclosure and counseling, sometimes requiring discussions across multiple visits. It can be stressed to the patient and family that identification of a variant associated with PD in an unaffected individual does not guarantee that an individual will develop PD, and a negative result does not eliminate all genetic risks or nongenetic risks of PD.
Where to Go for Additional PD Genetics and Testing Information
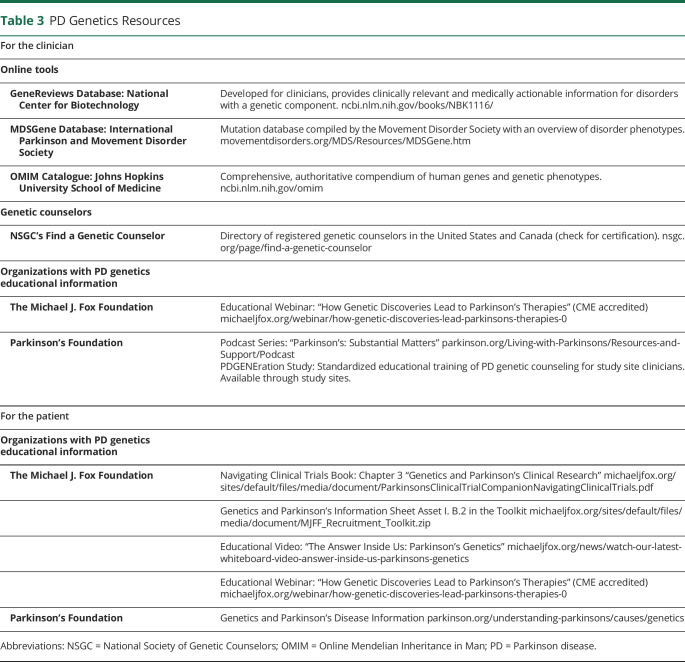
Genetic results need to be interpreted to determine clinical relevance. Usually, genetic test reports issued from laboratories provide some results interpretation and references for the clinician. Additional resources to help in interpretation and guide discussions with patients include various online tools and, if needed, a referral to a genetic counselor in person or remotely (table 3). PD foundations and other entities continue to develop educational resources for both patients and their health care providers (table 3).
Table 3.
PD Genetics Resources
Conclusion
It is imperative to stay abreast of the genetic testing opportunities for patients as knowledge about the genetic aspects of PD relevant to clinical practice rapidly advances. This need will continue as more genes and genetic mechanisms involved in the pathogenesis of PD are discovered. As we look to the future, we hope to provide a framework for the expansion of genetic testing to more patients with PD and their relatives. The goals are to meet patient and family needs for genetic information, refine treatments, and provide opportunities for patients to participate in the growing number of targeted therapeutic trials.
TAKE-HOME POINTS
→ Knowledge is increasing about how genetics contributes to PD etiology and clinical expression.
→ There is greater demand and interest in genetic testing among consumers, driving genetic testing availability.
→ The time has arrived to consider more widespread PD genetic testing in the clinical setting, within the context of fully informed consent.
→ Genetic testing should be accompanied by pretest and posttest counseling.
→ There are multiple research initiatives available that may offer no-cost genetic testing and genetic counseling, as well as ongoing clinical trials for patients with PD-associated gene variants.
Acknowledgments
The authors extend their appreciation to all their many patients, families, and research participants who have helped move genetic testing for Parkinson disease forward and who have taught them so much about the importance of quality genetic testing and counseling for Parkinson disease.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
L. Cook, J. Schulze, C. Kopil, T. Hastings, A. Naito, J. Wojcieszek, and K. Payne report no disclosures relevant to the manuscript. R.N. Alcalay's research is supported by the NIH, the DoD, the Parkinson's Foundation, and the Michael J. Fox Foundation; he has received consultation fees from Genzyme/Sanofi, resTORbio, and Roche. C. Klein serves as a medical consultant to Centogene for genetic testing for movement disorders and dementia, except Parkinson disease. R. Saunders-Pullman receives funding from NIH NS-107016 and the Bigglesworth Family Foundation and is the Bachmann Strauss Chair. She has served as a consultant to Denali Therapeutics (over 2 years ago). T. Simuni has served as a consultant for Acadia, AbbVie, Adamas, Anavex, Aptinyx, Allergan, Accorda, Denali, NeuroDerm, Neurocrine, Revance, Sanofi, Sunovion, Teva, Takeda, Voyager, US World Meds, Parkinson's Foundation, and the Michael J. Fox Foundation for Parkinson's Research; has served as a speaker and received an honorarium from Acadia, Adamas, and Teva; is on the scientific advisory boards of NeuroDerm, Sanofi, and Michael J. Fox Foundation; and has received research funding from the NINDS, Parkinson's Foundation, The Michael J. Fox Foundation, Biogen, Roche, NeuroDerm, Sanofi, and Sun Pharma. T. Foroud has received research funding from the Parkinson's Foundation and The Michael J. Fox Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Late-BreakingAbstractsPublicationFile.pdf [internet]. Available at: mdscongress.org/Congress-Branded/Congress-2019-Files/2019Late-BreakingAbstractsPublicationFile.pdf. Accessed October 5, 2019. [Google Scholar]
- 2.Lill CM. Genetics of Parkinson's disease. Mol Cell Probes 2016;30:386–396. [DOI] [PubMed] [Google Scholar]
- 3.Cook Shukla L, Schulze J, Farlow J, Pankratz ND, Wojcieszek J, Foroud T. Parkinson Disease Overview [internet]. Seattle: University of Washington; 2019. [Google Scholar]
- 4.Gan-Or Z, Amshalom I, Kilarski LL, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 2015;84:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CY, Alcalay RN. Genetic forms of Parkinson's disease. Semin Neurol 2017;37:135–146. [DOI] [PubMed] [Google Scholar]
- 6.Genetic testing for GBA and LRRK2 mutations [internet]. Available at: movementdisorders.org/MDS/Scientific-Issues-Committee-Blog/Genetic-Testing-for-GBA-and-LRRK2-Mutations.htm. Accessed October 5, 2019. [Google Scholar]
- 7.Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna) 2017;124:901–905. [DOI] [PubMed] [Google Scholar]
- 8.Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med 2012;2:a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed X, Bandrés-Ciga S, Blauwendraat C, Cookson MR. The role of monogenic genes in idiopathic Parkinson's disease. Neurobiol Dis 2019;124:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blauwendraat C, Reed X, Krohn L, et al. Genetic modifiers of risk and age at onset in GBA associated Parkinson's disease and Lewy body dementia. Brain 2020;143:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med 2012;2:a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook L, Schulze J. Connecting Gaucher and Parkinson disease: considerations for clinical and research genetic counseling settings. J Genet Couns 2017;26:1165–1172. [DOI] [PubMed] [Google Scholar]
- 13.Harbo HF, Finsterer J, Baets J, et al. EFNS guidelines on the molecular diagnosis of neurogenetic disorders: general issues, Huntington's disease, Parkinson's disease and dystonias. Eur J Neurol 2009;16:777–785. [DOI] [PubMed] [Google Scholar]
- 14.Di Maio R, Hoffman EK, Rocha EM, et al. LRRK2 activation in idiopathic Parkinson's disease. Sci Transl Med 2018;10:eaar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinh J, Zeldenrust FMJ, Huang J, et al. Genotype-phenotype relations for the Parkinson's disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord 2018;33:1857–1870. [DOI] [PubMed] [Google Scholar]
- 16.Malek N, Weil RS, Bresner C, et al. Features of GBA-associated Parkinson's disease at presentation in the UK Tracking Parkinson's study. J Neurol Neurosurg Psychiatry 2018;89:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lythe V, Athauda D, Foley J, et al. GBA-associated Parkinson's disease: progression in a deep brain stimulation cohort. J Parkinsons Dis 2017;7:635–644. [DOI] [PubMed] [Google Scholar]
- 18.Ligaard J, Sannæs J, Pihlstrøm L. Deep brain stimulation and genetic variability in Parkinson's disease: a review of the literature. NPJ Parkinsons Dis 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foroud T, Smith D, Jackson J, et al. Novel recruitment strategy to enrich for LRRK2 mutation carriers. Mol Genet Genomic Med 2015;3:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbrugge J, Rumbaugh M, Cook L, et al. The promise and pitfalls of facebook advertising: a genetic counselor's perspective. J Genet Couns 2018;27:326–328. [DOI] [PubMed] [Google Scholar]
- 21.Maloney KA, Alaeddin DS, von Coelln R, et al. Parkinson's disease: patients' knowledge, attitudes, and interest in genetic counseling. J Genet Couns 2018;27:1200–1209. [DOI] [PubMed] [Google Scholar]
- 22.New Genetic Associations for Parkinson's Disease Identified [internet]. 23andMe Blog. 2017. Available at: blog.23andme.com/23andme-research/new-genetic-associations-parkinsons-disease-identified/. Accessed October 6, 2019.
- 23.Falcone DC, Wood EM, Xie SX, Siderowf A, Van Deerlin VM. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. J Genet Couns 2011;20:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllister M, Dearing A. Patient reported outcomes and patient empowerment in clinical genetics services. Clin Genet 2015;88:114–121. [DOI] [PubMed] [Google Scholar]
- 25.Smit AK, Newson AJ, Best M, et al. Distress, uncertainty, and positive experiences associated with receiving information on personal genomic risk of melanoma. Eur J Hum Genet 2018;26:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury AR, Patrick-Miller L, Egleston BL, et al. Returning individual genetic research results to research participants: uptake and outcomes among patients with breast cancer. JCO Precision Oncol 2018;2. doi: 10.1200/po.17.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan MMX, Malek N, Lawton MA, et al. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson's disease study. Brain 2019;142:2828–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velez-Pardo C, Lorenzo-Betancor O, Jimenez-Del-Rio M, et al. The distribution and risk effect of GBA variants in a large cohort of PD patients from Colombia and Peru. Parkinsonism Relat Disord 2019;63:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schormair B, Kemlink D, Mollenhauer B, et al. Diagnostic exome sequencing in early-onset Parkinson's disease confirms VPS13C as a rare cause of autosomal-recessive Parkinson's disease. Clin Genet 2018;93:603–612. [DOI] [PubMed] [Google Scholar]
- 30.Tandy-Connor S, Guiltinan J, Krempely K, et al. False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet Med 2018;20:1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazareth SB, Lazarin GA, Goldberg JD. Changing trends in carrier screening for genetic disease in the United States. Prenat Diagn 2015;35:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet 2016;61:275–282. [DOI] [PubMed] [Google Scholar]