PRACTICAL IMPLICATIONS
In this case, the distribution of flortaucipir (FTP) PET uptake reflects the clinical manifestation and neuroanatomical distribution of Pick disease (PiD) pathology. FTP-PET imaging may be helpful in predicting tau burden at autopsy in patients with underlying PiD neuropathology.
A 48-year-old, right-handed woman presented with a 2.5-year history of difficulties forming and finding words. At evaluation, she had a moderate phonetic-predominant apraxia of speech (AOS)1 and mild agrammatic aphasia. She developed increasing impulsivity, distractibility, and inappropriate behaviors, but AOS remained the predominant clinical feature. She met the criteria for progressive AOS and behavioral variant frontotemporal dementia. At 5.5 years postonset, she was mute, yet her motor function was largely preserved. Autopsy examination, 7 years postonset, revealed Pick disease (PiD) pathology.
PiD is a neurodegenerative disease characterized by focal deposition of hyperphosphorylated 3-repeat (3R) tau in the brain. Molecular neuroimaging with various ligands, via PET, affords in vivo views of accumulated abnormal tau. The utility of one such ligand, flortaucipir ([18F]AV-1451; FTP), in 3R-tauopathies is unclear, as autoradiography studies, expected to detect tau burden ex vivo, have found absent-to-minimal binding in PiD and other non-Alzheimer disease tauopathies.2–5 Importantly, some studies have shown in vivo signal of unknown etiology. To date, no FTP-PET-autopsy studies have been published for 3R-tauopathies. This case assessed the relationship between FTP-PET uptake and (1) tau burden at autopsy and (2) measures of neurodegeneration from hypometabolism on [18F]fluorodeoxyglucose (FDG) PET and atrophy on MRI. Correlative autoradiography was also performed.
Methods
The patient underwent neurologic, neuropsychological, speech, and language assessments, as previously described,1 with 3T volumetric head MRI, and FDG, Pittsburgh Compound B (PiB), and FTP-PET scans. Data from the visit 13 months before death, with subsequent autopsy and autoradiography evaluation,2 were utilized. She provided written consent to participate in this Mayo Clinic Institutional Review Board–approved study.
Tau burden at autopsy was abstracted as previously described.6 FTP- and FDG-PET uptake and gray matter thickness for available regions of interest (ROIs) (amygdala, anterior cingulate, Broca area, middle frontal, orbitofrontal, inferior parietal, primary and supplementary motor, and anterior and superior temporal) were computed. To create standard uptake ratios (SUVRs), FTP-PET uptake in each ROI was divided by cerebellar crus gray matter uptake, and FDG-PET uptake was divided by the pons uptake. A global PiB SUVR was generated, with cerebellar crus gray matter as a reference region (amyloid positivity > 1.48). PET scans were coregistered to the MRI in SPM12. ROIs were localized using the Mayo Clinic Adult Lifespan Template atlas (nitrc.org/projects/mcalt/). Spearman rank correlations were calculated using JMP software (v.14.1.0), α = 0.05.
Results
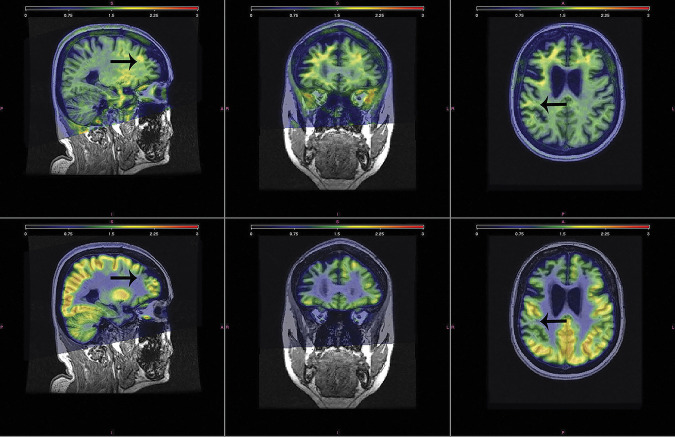
FTP-PET revealed increased signal across the right greater than left temporal and frontal lobes, the putamen, pallidum, amygdala, entorhinal cortex, and anterior cingulate. FDG-PET similarly demonstrated asymmetric, right greater than left, bilateral frontal, and anterior temporal lobe hypometabolism. Regions of reduced metabolism were noted in the regions with the highest FTP uptake (figure 1). The PiB-PET scan was negative, with a global SUVR of 1.30.
Figure 1. PET Scans.
Top panel shows the triplanar view of the SUVR FTP-PET scan, highlighting uptake predominantly in the white matter. Analogous view of the SUVR FDG-PET scan is shown in the bottom panel, demonstrating reduced metabolism in corresponding regions with the highest FTP uptake (example noted with arrows). FDG = fluorodeoxyglucose; FTP = flortaucipir; SUVR = standard uptake ratio.
Regional FTP-PET SUVRs showed correlations with FDG-PET (r = −0.88, p = 0.0008), gray matter thickness (r = 0.70, p = 0.0358), and tau burden at autopsy (r = 0.89, p = 0.0188) across ROIs. FTP autoradiography revealed absent-to-minimal binding in areas where dense 3R-tau was observed on immunohistochemistry (figure 2).
Figure 2. Immunohistochemistry, Autoradiography, and Scatter Plots of Relative Relationships.
(A) Scatter plots of the relationship between FTP-PET SUVR (2-compartment partial volume corrected) and tau burden at autopsy, FDG-PET SUVR, and MR thickness in the available regions of interest. (B) ARG (unblocked and blocked) and correlative IHC with PHF-1 in the amygdala. There was absent-to-minimal displaceable ARG binding corresponding to regions where 3 R-tau burden was dense on IHC. (C) Similarly shows ARG and IHC with PHF-1 in the temporal region, where there was also absent-to-minimal displaceable ARG binding corresponding to regions where 3 R-tau burden was dense on IHC. Tau burden assessed with phospho-tau CP13; gift from Peter Davies. ARG = autoradiography; FDG = fluorodeoxyglucose; FTP = flortaucipir; IHC = immunohistochemistry; MR = magnetic resonance; SUVR = standard uptake ratio.
Discussion
This study describes in vivo FTP-PET imaging and autoradiography in a case of autopsy-confirmed PiD. Moderate FTP uptake was seen, similar to previous in vivo reports3,4; the magnitude of uptake was comparable to that seen in 4R-tauopathies and lower relative to Alzheimer disease. Regional FTP uptake showed a strong correlation with underlying, quantitatively measured 3R-tau burden at autopsy, possibly mediated by stronger relationships with neurodegeneration. Although statistically significant, the relationship between FTP uptake and autopsy burden must be taken with caution, given the small areas sampled. Past research has yielded mixed findings in 4R-tauopathies, with a strong correlation between FTP-PET uptake and autopsy findings6 and weaker7 or absent6 relationships between FTP-PET and other measures of neurodegeneration (e.g., atrophy).
There was absent-to-minimal displaceable autoradiography in regions with tau burden on immunohistochemistry, similar to past findings in both 3R- and 4R-taupathies.2–5 Given the known tau burden at autopsy in this case, there is discordance between autoradiography and both autopsy and FTP-PET. Autoradiography may not wholly reflect FTP-PET results due to methodological decisions (e.g., tissue preparation methods) or contributions of perfusion to observed FTP signal.
Although limited by the sampling of 1 hemisphere due to standard operating procedures (less-affected left hemisphere), this study details the multimodal manifestation of the neuronal pathophysiology of PiD and provides insight into the relationship between in vivo FTP uptake and tau deposition at autopsy. Further research is needed to understand the relationship between FTP and 3R-tau pathologies and determine whether FTP is an appropriate biomarker for such tauopathies, particularly as uptake meaningfully reflected the clinical manifestation of PiD neuropathology in this case.
Acknowledgment
The authors extend their gratitude to this patient and her family for their time and dedication to their research program. They thank AVID Radiopharmaceuticals, Inc., for their support in supplying [18F]AV-1451 precursor, chemistry production advice and oversight and FDA regulatory cross-filing permission and documentation needed for this work.
Appendix. Authors

Study Funding
This study was funded by the NIH and National Institute on Deafness and Other Communication Disorders grants R01 DC010367 (PI: Josephs), R01 DC012519 (PI: Whitwell), R21 NS094684 (PI: Josephs), and R01 DC014942 (PI: Josephs).
Disclosure
All authors receive research support from the NIH. V.J. Lowe serves on scientific advisory boards for Bayer Schering Pharma and Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA and NCI). Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang 2018;184:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer's disease, primary tauopathies, and other dementias. Alzheimers Dement 2016;12:1116–1124. [DOI] [PubMed] [Google Scholar]
- 4.Aguero C, Dhaynaut M, Normandin MD, et al. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathol Commun 2019;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, Whitwell J, Tacik P, et al. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 2016;132:931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan CT, Irwin DJ, Nasrallah I, et al. Multimodal evaluation demonstrates in vivo (18)F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 2016;132:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]