Abstract
It has been demonstrated that obesity is an independent risk factor for worse outcomes in patients with COVID-19. Our objectives were to investigate which classes of obesity are associated with higher in-hospital mortality and to assess the association between obesity and systemic inflammation. This was a retrospective study which included consecutive hospitalized patients with COVID-19 in a tertiary center. Three thousand five hundred thirty patients were included in this analysis (female sex: 1579, median age: 65 years). The median body mass index (BMI) was 28.8 kg/m2. In the overall cohort, a J-shaped association between BMI and in-hospital mortality was depicted. In the subgroup of men, BMI 35–39.9 kg/m2 and BMI ≥40 kg/m2 were found to have significant association with higher in-hospital mortality, while only BMI ≥40 kg/m2 was found significant in the subgroup of women. No significant association between BMI and IL-6 was noted. Obesity classes II and III in men and obesity class III in women were independently associated with higher in-hospital mortality in patients with COVID-19. The male population with severe obesity was the one that mainly drove this association. No significant association between BMI and IL-6 was noted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-021-04260-z.
Keywords: COVID-19, SARS-CoV-2, Novel coronavirus, Obesity, Inflammation, IL-6, Mortality, Risk factor, Observational study
Introduction
Coronavirus disease 2019 (COVID-19) has claimed more than 1.25 million human lives since December 2019 when the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged [1]. Almost 50 million people have had a confirmed SARS-CoV-2 infection to date [1], the clinical spectrum of which ranges from asymptomatic infection to severe COVID-19 with critical illness [2].
Older age and male sex, along with chronic diseases such as hypertension, hyperlipidemia, diabetes, heart failure, coronary artery disease, cerebrovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease, have been shown to be associated with higher risk for severe disease and worse overall outcomes in patients with COVID-19 [3–8].
Studies have consistently demonstrated that obesity is an independent risk factor for hospitalization, severe disease, and death in patients with COVID-19 [9–17]. However, it remains unclear whether all classes of obesity are associated with worse outcomes or whether this is specific to severe obesity. In addition, it is unclear whether the association of obesity with worse outcomes is sex-specific.
The pathogenetic mechanisms involved in COVID-19 infection have not been fully elucidated, but it is known that a major cause of disease severity and death is an excessive inflammatory host response to SARS-COV-2 that is associated with high levels of circulating cytokines, such as interleukin-6 (IL-6) [18–21]. Obesity is considered a state of enhanced chronic inflammation [22].
Therefore, predisposition to systemic hyper-inflammation has been hypothesized to be one of the main mechanisms by which obesity leads to worse outcomes in COVID-19 [23–28]. Nevertheless, there is paucity of large observational data connecting obesity to systemic inflammation in patients with COVID-19.
Our primary objective in this large single-center retrospective analysis was to investigate which classes of obesity are associated with higher in-hospital mortality in patients with COVID-19 and whether or not this is sex-specific. Our secondary objective was to assess the association between body mass index (BMI) and systemic inflammation as indicated by IL-6 level.
Materials and methods
Study design and patient population
We conducted a retrospective cohort study at Montefiore Medical Center, a tertiary academic institution in the Bronx, New York. Patients ≥18 years of age who presented to the emergency room and were admitted to the inpatient medicine service or the intensive care unit (ICU) with laboratory-confirmed COVID-19 from March 10th, 2020, to May 1st, 2020 were included. All included patients had a SARS-CoV-2-positive result in real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of nasopharyngeal swab results. We excluded patients who met any one of the following exclusion criteria: (i) patients <18 years old, (ii) patients who tested negative for COVID-19, and (iii) patients who were still hospitalized at the time of data collection. The study was approved by the institutional review board (IRB) of the Albert Einstein College of Medicine with a waiver of informed consent (IRB number 2020-11794).
Data sources
Study data were obtained from electronic health records (Epic systems, Verona, WI). The extracted data included age, sex, BMI, tobacco use, history of hypertension, hyperlipidemia, diabetes, coronary artery disease, heart failure, cerebrovascular disease, peripheral artery disease, chronic obstructive pulmonary disease, asthma, chronic kidney disease, end-stage renal disease, liver cirrhosis, human immunodeficiency virus infection (HIV), other elements of the Charlson Comorbidity Index, vital signs, complete blood count, basic metabolic panel, C-reactive protein (CRP), IL-6 (obtained within 24 hours from admission), radiographic findings, systemic treatment with steroids or IL-6 inhibitors, intubation-mechanical ventilation, death, hospital discharge, and length of stay. The data were processed and analyzed without any personal identifiers to maintain patient confidentiality as per the Health Insurance Portability and Accountability Act (HIPAA).
Outcomes and statistical analysis
Patients were classified into six groups based on BMI: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), class I obesity (BMI 30 to <35 kg/m2), class II obesity (BMI 35 to <40 kg/m2), and class III obesity (BMI ≥40 kg/m2). The primary endpoint was in-hospital mortality. Secondary endpoints were the need for intubation and development of severe pneumonia. The latter was defined per the American Thoracic Society guidelines for community-acquired pneumonia [29].
Continuous data are presented as median with interquartile range (IQR) and categorical data as absolute and relative frequencies. The ANOVA test was used to compare the continuous variables, while chi-square was used for discrete variables. A logistic regression model was used to identify baseline variables associated with in-hospital mortality, intubation, and severe pneumonia. BMI 18.5–24.9 kg/m2 was used as a reference in order to perform dichotomous comparisons with patients in the other BMI groups. Five multivariate models with different definitions of our variable of interest were presented for robustness: model 1, BMI ≥30 kg/m2, age, sex, and all the variables with significant univariate associations (p value < 0.05); model 2, BMI ≥35 kg/m2, age, sex, and all the variables with significant univariate associations (p value < 0.05); model 3, BMI ≥40 kg/m2, age, sex, and all the variables with significant univariate associations (p value ≤ 0.05); model 4, BMI classified in six groups (<18.5 kg/m2; 18.5–24.9 kg/m2; 25–29.9 kg/m2; 30 to 34.9 kg/m2; 35–39.9 kg/m2; ≥ 40 kg/m2), age, sex, and all the variables with significant univariate associations (p value < 0.05); and model 5, the variables of model 4 and IL-6. Age and IL-6 were treated as continuous variables. Results of logistic regression are given as the odds ratio (OR) with the 95% confidence interval (CI). The threshold of statistical significance was p < 0.05. All analyses were performed using STATA software (version 14.1; STATA Corporation, College Station, TX, USA).
Results
In total, 3530 patients admitted with COVID-19 were included in this analysis (female sex = 1579, BMI <18.5 kg/m2 = 82, BMI 18.5–24.9 kg/m2 = 814, BMI 25–29.9 kg/m2 = 1162, BMI 30–34.9 kg/m2 = 809, and BMI ≥ 35 kg/m2 = 663). The median BMI was 28.8 (IQR 24.9–33.2) kg/m2. The median age of the entire cohort was 65 (55–76) years, with significant differences among the four groups [BMI < 18.5 kg/m2: 76 (66–83); 18.5–24.9 kg/m2: 72 (61–81); BMI 25–29.9 kg/m2: 67 (57–77); BMI 30–34.9 kg/m2: 62 (53–72); BMI ≥ 35 kg/m2: 59 (49–68), p < 0.001]. 6.2% of patients were active or former smokers. Hypertension, hyperlipidemia, and diabetes were prevalent in 62.9%, 47.4%, and 39.8% of our patients, respectively. 8.1% had a history of heart failure, while 9.2% had a history of asthma and 4.8% history of COPD. 14.9% had a history of chronic kidney disease and 4.3% had ESRD. Coronary artery disease was prevalent in 7.2% of patients. Detailed baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of patients
| Characteristics | All patients | BMI <18.5 | BMI 18.5-24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | p-value |
|---|---|---|---|---|---|---|---|
| N=3530 |
n=82 (a) |
n=814 (b) |
n=1162 (c) |
n=809 (d) |
n=663 (e) |
||
| Male sex - no (%) | 1951 (55.27) | 43 (52.44) | 500 (62.29)de | 700 (60.24)de | 421 (52.04)bce | 280 (42.23)bcd | <0.001 |
| Age - median (IQR) | 65 (55–76) | 76 (66–83)cde | 72 (61–81)cde | 67 (57–77)abde | 62 (53–72)abce | 59 (47–68)abcd | <0.001 |
| Tobacco use - no (%) | 216 (6.17) | 5 (6.10) | 62 (7.69) | 67(5.82) | 43(5.36) | 39(5.94) | 0.350 |
| History - no (%) | |||||||
| Hypertension | 2199 (62.85) | 46 (56.10) | 496 (61.54) | 736 (63.89) | 494 (61.60) | 427 (64.99) | 0.336 |
| Hyperlipidemia | 1659 (47.41) | 37 (45.12) | 395 (49.01) | 574 (49.83)e | 376 (46.88) | 277 (42.16)c | 0.026 |
| Diabetes | 1392 (39.78) | 18 (21.95)cde | 296 (36.72) | 471 (40.89)a | 328 (40.90)a | 279 (42.47)a | 0.002 |
| CKD | 522 (14.92) | 17 (20.73) | 142 (17.62) | 170 (14.76) | 108 (13.47) | 85 (12.94) | 0.037 |
| Asthma | 323 (9.15) | 2 (2.44)e | 49 (6.02)de | 81 (6.97)e | 83 (10.26)e | 108 (16.29)abcd | <0.001 |
| Heart failure | 282 (8.06) | 6 (7.32) | 73 (9.06) | 86 (7.47) | 61 (7.61) | 56 (8.52) | 0.716 |
| CAD | 253 (7.23) | 5 (6.10) | 65 (8.06) | 87 (7.55) | 60 (7.48) | 36 (5.48) | 0.373 |
| COPD | 169 (4.79) | 3 (3.66) | 56 (6.88)c | 39 (3.36)b | 45 (5.56) | 26 (3.92) | 0.004 |
| CVA or TIA | 160 (4.57) | 4 (4.88) | 44 (5.46) | 54 (4.69) | 36 (4.49) | 22 (3.55) | 0.438 |
| ESRD | 149 (4.26) | 6 (7.32) | 47 (5.83) | 45 (3.91) | 26 (3.24) | 25 (3.81) | 0.050 |
| PAD | 138 (3.94) | 6 (7.32) | 32 (3.97) | 49 (4.25) | 31 (3.87) | 20 (3.04) | 0.383 |
| HIV | 78 (2.23) | 4 (4.88) | 24 (2.98) | 19 (1.65) | 18 (2.24) | 13 (1.98) | 0.154 |
| Cirrhosis | 41 (1.16) | 0 (0.00) | 12 (1.47) | 19 (1.64) | 7 (0.87) | 3 (0.45) | 0.114 |
| Charlson Comorbidity Index median (IQR) | 3 (1–4) | 4 (3–5)cde | 4 (2–5)cde | 3 (2–4)abde | 2 (1–4)abce | 2 (1–4)abcd | <0.001 |
(1) p-values refer to chi-square test/ANOVA, and the letters denote the columns with which a statistically significant pairwise comparison exists (Bonferroni’s method); (2) age in years; (3) BMI in kg/m2
BMI body mass index, CKD chronic kidney disease, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, TIA transient ischemic attack, ESRD end-stage renal disease, PAD peripheral artery disease, HIV human immunodeficiency virus infection
Patients with severe obesity were more likely to develop severe pneumonia (BMI <18.5 kg/m2: 13.4%; BMI 18.5–24.9 kg/m2: 17.9%; BMI 25–29.9 kg/m2: 18.9%; BMI 30–34.9 kg/m2: 21.4%; BMI ≥ 35 kg/m2: 25.5%; p = 0.001) and to undergo intubation (BMI <18.5 kg/m2: 6.1%; BMI 18.5–24.9 kg/m2: 10.3%; BMI 25–29.9 kg/m2: 12.7%; BMI 30–34.9 kg/m2: 16.4%; BMI ≥ 35 kg/m2: 18.6%; p < 0.001). In-hospital outcomes and data on length of stay are presented in Table 2.
Table 2.
In-hospital outcomes
| Outcomes - no. (%) | All patients | BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | p-value |
|---|---|---|---|---|---|---|---|
| n=3530 |
n=82 (a) |
n=814 (b) |
n=1162 (c) |
n=809 (d) |
n=663 (e) |
||
| In-hospital mortality | 963 (27.28) | 33 (40.24)de | 250 (30.71)d | 317 (27.28) | 198 (24.47)ab | 165(24.89)a | 0.002 |
| Severe pneumonia | 719 (20.37) | 11 (13.41) | 146 (17.94)e | 220 (18.93)e | 173 (21.38) | 169(25.49)bc | 0.001 |
| Intubation | 493 (13.97) | 5 (6.10)e | 84 (10.32)de | 148 (12.74)e | 133 (16.44)b | 123(18.55)abc | <0.001 |
| Length of stay | 6 (3–10) | 6 (3–9) | 6 (3–10) | 6 (3–10) | 6 (3–9) | 6 (3–10) | 0.669 |
(1) p-values refer to chi-square test/ANOVA, and the letters denote the columns with which a statistically significant pairwise comparison exists (Bonferroni’s method); (2) BMI in kg/m2; (3) length of stay in median (IQR) days
BMI body mass index, IQR interquartile range
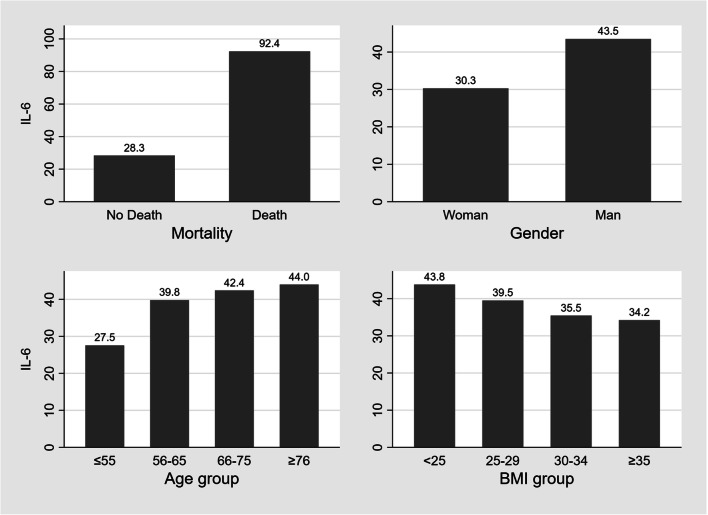
Median IL-6 was 38.1 (16–80) pg/mL. No significant association between BMI group and IL-6 level was noted (BMI < 18.5 kg/m2: 45.7 pg/mL; BMI 18.5–24.9 kg/m2: 43.4 pg/mL; BMI 25–29.9 kg/m2: 39.5 pg/mL; BMI 30–34.9 kg/m2: 35.5 pg/mL; BMI ≥ 35 kg/m2: 34.2 pg/mL; p = 0.714). The levels of inflammatory markers are presented in Table 3. A sensitivity analysis was performed after exclusion of patients that received steroids or IL-6 inhibitors during the hospitalization. The median IL-6 in this cohort (N=2389) was lower compared to the total cohort (32.3 vs. 38.1 pg/mL). Similar to the initial cohort, no significant association between BMI group and IL-6 level was noted (Supplementary table 1). A further descriptive analysis demonstrated that (a) deceased patients had significantly higher median IL-6 level compared to median IL-6 levels in survivors (92.4 vs. 28.4 pg/mL, p <0.001); (b) men had significantly higher median IL-6 levels than women (43.5 vs. 30.3 pg/mL, p = 0.037); (c) median IL-6 levels gradually increased while the age groups increased, but this association was not statistically significant (p= 0.062); and (d) median IL-6 levels gradually decreased while the BMI group increased, but this association was not statistically significant (p= 0.729) (Fig. 1). The areas under the curves (AUC) for all inflammatory markers predicting mortality were also computed and ranged from 0.387 to 0.921 (Supplementary Table 2).
Table 3.
Levels of inflammatory markers
| Lab result - median (IQR) | Missing data | All patients | BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | p-value |
|---|---|---|---|---|---|---|---|---|
|
n=82 (a) |
n=814 (b) |
n=1162 (c) |
n=809 (d) |
n=369 (e) |
||||
| IL-6 | 1956 | 38.1 (16–80) | 45.7 (22.4–89.1) | 43.4 (18.6–91.7) | 39.5 (16.9–85.9) | 35.5 (14.6–73.5) | 34.2 (13.2–71.6) | 0.714 |
| CRP - initial | 543 | 10.6 (4.6–18.6) | 9.2 (4.2–18.3) | 9.6 (4.2–18.2) | 11.7 (5.5–19.9)d | 9.4 (4.3–17.3)c | 11.2 (5–18.9) | 0.013 |
| CRP - maximum | 23 | 11.5 (2.9–22.5) | 10.2 (2.9–19.4) | 10.1 (2.2–21.1) | 13 (3.4–23.1) | 11.2 (2.8–22) | 11.6 (3.3–23.3) | 0.053 |
| CRP - final | 547 | 5.8 (2.5–13.1) | 6.6 (3–16.5) | 6 (2.8–14.2) | 6.2 (2.6–13.7) | 5.4 (2.1–12.15) | 5(2.1–11.2) | 0.316 |
| WBC - initial | 281 | 7.2 (5.5–9.9) | 7.5 (5.9–11.2)cde | 7.2 (5.4–10.6) | 7.4 (5.6–10.2)a | 7 (5.3–9.4)a | 7.3 (5.4–9.5)a | <0.001 |
| Neutrophils - initial | 283 | 5.5 (3.8–8) | 5.7 (4.2–8.9) | 5.6 (3.8–8.8)de | 5.6 (4.1–8.3)d | 5.2 (3.7–7.5)bc | 5.5 (3.8–7.5)b | <0.001 |
| Lymphocytes - initial | 282 | 1 (0.7–1.4) | 0.8 (0.6–1.3)bcde | 0.9 (0.6–1.3)a | 1 (0.7–1.3)a | 1 (0.7–1.4)a | 1.1 (0.7–1.4)a | 0.005 |
(1) p-values refer to ANOVA, and the letters denote the columns with which a statistically significant pairwise comparison exists (Bonferroni’s method); (2) BMI in kg/m2, IL-6 in pg/mL, CRP in mg/dL, WBC in 103/μL, neutrophils 103/μL, lymphocytes in 103/μL
BMI body mass index, IQR interquartile range, CRP C-reactive protein, IL-6 interleukin, WBC white blood cell count
Fig. 1.
The Association of median IL-6 level and Survival Status, Sex, Age, and BMI. Notes: (1) BMI in kg/m2, IL-6 in pg/mL, age in years. Abbreviations: BMI=body mass index, IL-6=interleukin 6
Logistic regression analyses
In-hospital mortality
Univariate associations with in-hospital mortality were examined for the available baseline demographic and clinical characteristics and are presented in Table 4. In the multivariable analysis (Table 5, panel A), BMI ≥30 kg/m2 (model 1, OR: 1.27; 95% CI: 1.07–1.51; p = 0.006), BMI ≥ 35 kg/m2 (model 2, OR: 1.49; 95% CI: 1.20–1.85; p < 0.001), and BMI ≥ 40 kg/m2 (model 3, OR: 1.68; 95% CI: 1.23–2.29; p = 0.001) all were found to have significant associations with higher in-hospital mortality. When BMI was analyzed based on conventional classification (<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, 35–39.9 kg/m2, ≥ 40 kg/m2) compared with reference group 18.5–24.9 kg/m2, patients with BMI of 35–39.9 kg/m2 and ≥ 40 kg/m2 were found to have significantly higher in-hospital mortality (model 4, OR: 1.44; 95% CI: 1.06–1.96; p = 0.020 and OR: 1.92; 95% CI: 1.35–2.72; p < 0.001, respectively). Another multivariate analysis was performed, which included age, sex, BMI, and the Charlson Comorbidity Index (instead of individual baseline comorbidities). This analysis replicated the findings of the main analysis and is presented in Supplementary Table 3. A J-shaped association between BMI and risk for death was demonstrated (Fig. 2).
Table 4.
Univariate analysis
| Variable | Hospital mortality | Severe pneumonia | Intubation |
|---|---|---|---|
| OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | |
| Male sex | 1.25 (1.07–1.45) p=0.004 | 1.34 (1.13–1.58) p=0.001 | 1.34 (1.11–1.63) p=0.003 |
| Age per 10 years | 1.64 (1.55–1.75) p<0.001 | 1.08 (1.03–1.14) p=0.004 | 0.99 (0.93–1.04) p=0.609 |
| BMI ≥25(2) | 0.75 (0.64–0.89) p=0.001 | 1.28 (1.05–1.55) p=0.015 | 1.64 (1.29–2.10) p<0.001 |
| BMI ≥30(2) | 0.80 (0.68–0.93) p=0.003 | 1.35 (1.14–1.59) p<0.001 | 1.62 (1.34–1.96) p<0.001 |
| BMI ≥35(2) | 0.86 (0.71–1.04) p=0.125 | 1.44 (1.18–1.76) p<0.001 | 1.54 (1.23–1.92) p<0.001 |
| BMI ≥40(2) | 0.91 (0.69–1.19) p=0.477 | 1.38 (1.05–1.82) p=0.023 | 1.47 (1.08–2.00) p=0.015 |
| BMI <18.5(3) | 1.52 (0.95–2.42) p=0.078 | 0.71 (0.37–1.37) p=0.307 | 0.56 (0.22–1.43) p=0.229 |
| 25–29.9(3) | 0.85 (0.69–1.03) p=0.097 | 1.07 (0.85–1.35) p=0.575 | 1.27 (0.95–1.69) p=0.101 |
| 30–34.9(3) | 0.73 (0.59–0.91) p=0.005 | 1.24 (0.97–1.59) p=0.081 | 1.71 (1.28–2.29) p<0.001 |
| 35–39.9(3) | 0.73 (0.55–0.96) p=0.026 | 1.56 (1.16–2.10) p=0.003 | 1.96 (1.39–2.78) p<0.001 |
| ≥ 40(3) | 0.77 (0.57–1.04) p=0.094 | 1.57 (1.14–2.15) p=0.006 | 2.00 (1.38–2.90) p<0.001 |
| Congestive heart failure | 2.07 (1.62–2.66) p<0.001 | 1.34 (1.01–1.78) p=0.041 | 0.83 (0.57–1.20) p=0.320 |
| Coronary artery disease | 1.54 (1.18–2.02) p=0.002 | 1.11 (0.82–1.52) p=0.494 | 0.91 (0.63–1.33) p=0.638 |
| Diabetes | 1.27 (1.09–1.48) p=0.002 | 1.35 (1.14–1.59) p<0.001 | 1.17 (0.96–1.41) p=0.120 |
| Chronic kidney disease | 1.95 (1.61–2.37) p<0.001 | 1.64 (1.33–2.03) p<0.001 | 1.21 (0.94–1.56) p=0.142 |
| ESRD | 1.32 (0.93–1.87) p=0.122 | 1.73 (1.21–2.48) p=0.003 | 1.01 (0.63–1.61) p=0.982 |
| COPD | 2.34 (1.71–3.20) p<0.001 | 1.40 (0.98–2.00) p=0.062 | 1.18 (0.77–1.80) p=0.440 |
| Tobacco use | 1.39 (1.03–1.86) p=0.029 | 1.22 (0.88–1.68) p=0.237 | 1.03 (0.69–1.52) p=0.889 |
| Hypertension | 1.23 (1.05–1.43) p=0.011 | 1.02 (0.86–1.20) p=0.861 | 0.85 (0.70–1.03) p=0.095 |
| Hyperlipidemia | 1.28 (1.10–1.48) p=0.001 | 1.04 (0.88–1.23) p=0.643 | 0.90 (0.74–1.09) p=0.293 |
| Asthma | 1.00 (0.77–1.29) p=0.988 | 1.05 (0.79–1.39) p=0.749 | 1.03 (0.74–1.42) p=0.881 |
| HIV | 0.91 (0.55–1.53) p=0.732 | 1.26 (0.75–2.12) p=0.389 | 1.23 (0.67–2.25) p=0.499 |
| Cirrhosis | 1.90 (1.02–3.56) p=0.044 | 1.83 (0.94–3.55) p=0.074 | 1.27 (0.56–2.89) p=0.565 |
| Peripheral vascular disease | 2.05 (1.45–2.89) p<0.001 | 1.45 (0.98–2.13) p=0.061 | 1.04 (0.64–1.69) p=0.874 |
| CVA or TIA | 1.68 (1.21–2.33) p=0.002 | 1.17 (0.81–1.72) p=0.393 | 0.71 (0.43–1.19) p=0.206 |
| Interleukin-6 per 10 pg/mL | 1.06 (1.03–1.09) p<0.001 | 1.03 (1.01–1.05) p=0.001 | 1.02 (1.01–1.04) p=0.002 |
(1) BMI in kg/m2; (2) dichotomous variables, reference groups: BMI <25, <30, <35, and <40 kg/m2, respectively; (3) ordinal variable, reference group: BMI 18.5–24.9 kg/m2; (4) age was analyzed as a continuous variable, and 10 years was used as a unit of time for easier interpretation of OR (95% CI)
OR odds ratio, CI confidence interval, BMI body mass index, ESRD end-stage renal disease, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus infection, CVA cerebrovascular accident, TIA transient ischemic attack
Table 5.
Multivariate analysis for the primary and secondary outcomes
| Panel A: Mortality | |||||
|---|---|---|---|---|---|
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| n=3499 | n=3499 | n=3499 | n=3499 | n=1564 | |
| OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | |
| Male sex | 1.57 (1.33–1.85) p < 0.001 | 1.58 (1.34–1.87) p < 0.001 | 1.56 (1.32–1.84) p < 0.001 | 1.61 (1.36–1.90) p < 0.001 | 1.43 (1.09–1.88) p=0.010 |
| Age per 10 years | 1.74 (1.62–1.87) p < 0.001 | 1.75 (1.63–1.88) p < 0.001 | 1.74 (1.62–1.86) p < 0.001 | 1.77 (1.64–1.90) p < 0.001 | 1.77 (1.57–1.99) p < 0.001 |
| BMI ≥30 | 1.27 (1.07–1.51) p=0.006 | ||||
| BMI ≥35 | 1.49 (1.20–1.85) p < 0.001 | ||||
| BMI ≥40 | 1.68 (1.23–2.29) p=0.001 | ||||
| BMI <18.5(2) | 1.38 (0.82–2.30) p=0.221 | 0.86 (0.33–2.22) p=0.753 | |||
| 25–29.9(2) | 1.07 (0.86–1.32) p=0.543 | 1.17 (0.83–1.65) p=0.363 | |||
| 30–34.9(2) | 1.17 (0.92–1.49) p=0.195 | 1.40 (0.96–2.05) p=0.077 | |||
| 35–39.9(2) | 1.44 (1.06–1.96) p=0.020 | 1.36 (0.81–2.26) p=0.243 | |||
| ≥ 40(2) | 1.92 (1.35–2.72) p < 0.001 | 1.81 (1.05–3.13) p=0.033 | |||
| Interleukin-6 per 10pg/mL | 1.06 (1.03–1.09) p < 0.001 | ||||
| Panel B: Severe pneumonia | |||||
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| n=3499 | n=3499 | n=3499 | n=3499 | n=1564 | |
| OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | |
| Male sex | 1.49 (1.25–1.78) p < 0.001 | 1.48 (1.25–1.77) p < 0.001 | 1.43 (1.21–1.70) p < 0.001 | 1.52 (1.28–1.82) p < 0.001 | 1.43 (1.09–1.89) p=0.011 |
| Age per 10 years | 1.12 (1.05–1.19) p<0.001 | 1.11 (1.05–1.18) p < 0.001 | 1.09 (1.03–1.16) p=0.002 | 1.14 (1.07–1.21) p < 0.001 | 1.06 (0.96–1.17) p=0.215 |
| BMI ≥30 | 1.57 (1.31–1.87) p < 0.001 | ||||
| BMI ≥35 | 1.71 (1.38–2.11) p < 0.001 | ||||
| BMI ≥40 | 1.61 (1.21–2.15) p=0.001 | ||||
| BMI <18.5(2) | 0.71 (0.36–1.38) p=0.311 | 0.20 (0.01–4.88) p=0.325 | |||
| 25–29.9(2) | 1.13 (0.89–1.44) p=0.312 | 1.21 (0.82–1.79) p=0.336 | |||
| 30–34.9(2) | 1.44 (1.11–1.87) p=0.006 | 1.69 (1.13–2.53) p=0.011 | |||
| 35–39.9(2) | 1.97 (1.44–2.69) p < 0.001 | 1.61 (0.96–2.70) p=0.073 | |||
| ≥40(2) | 2.10 (1.49–2.97) p < 0.001 | 2.29 (1.34–3.92) p=0.002 | |||
| Interleukin-6 per 10pg/mL | 1.03 (1.01–1.05) p=0.001 | ||||
| Panel C: Intubation | |||||
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| n=3530 | n=3530 | n=3530 | n=3530 | n=1574 | |
| OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | |
| Male sex | 1.48 (1.21–1.81) p < 0.001 | 1.44 (1.18–1.76) p < 0.001 | 1.39 (1.14–1.69) p=0.001 | 1.51 (1.23–1.85) p < 0.001 | 1.45 (1.07–1.97) p=0.016 |
| Age per 10 years | 1.05 (0.98–1.12) p=0.137 | 1.03 (0.97–1.10) p=0.341 | 1.02 (0.96–1.08) p=0.604 | 1.07 (1.00–1.14) p=0.057 | 0.99 (0.90–1.10) p=0.912 |
| BMI ≥30 | 1.77 (1.44–2.18) p < 0.001 | ||||
| BMI ≥35 | 1.68 (1.32–2.13) p < 0.001 | ||||
| BMI ≥40 | 1.58 (1.15–2.18) p=0.005 | ||||
| BMI <18.5(2) | 0.57 (0.22–1.46) p=0.243 | 0.15 (0.00–4.87) p=0.281 | |||
| 25–29.9(2) | 1.31 (0.98–1.75) p=0.066 | 1.53 (0.98–2.41) p=0.064 | |||
| 30–34.9(2) | 1.88 (1.38–2.56) p < 0.001 | 2.09 (1.31–3.35) p=0.002 | |||
| 35–39.9(2) | 2.26 (1.56–3.26) p < 0.001 | 1.84 (1.02–3.31) p=0.041 | |||
| ≥40(2) | 2.43 (1.63–3.61) p < 0.001 | 2.42 (1.33–4.41) p=0.004 | |||
| Interleukin-6 per 10pg/mL | 1.02 (1.01–1.04) p=0.001 | ||||
(1) BMI in kg/m2; (2) reference group, BMI 18.5–24.9 kg/m2; (3) mortality is adjusted for the variables found to have significant univariate associations with congestive heart failure, CAD, diabetes, CKD, COPD, tobacco use, hypertension, hyperlipidemia, cirrhosis, PAD, and CVA, or TIA; the multivariate associations of these variables with mortality are presented in the Supplementary table 4; (4) severe pneumonia is adjusted for the variables found to have significant univariate associations with congestive heart failure, diabetes, CKD, tobacco use, and ESRD; (5) age was analyzed as a continuous variable, and 10 years was used as a unit of time for easier interpretation of OR (95% CI)
OR odds ratio, CI confidence interval, BMI body mass index, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, PAD peripheral artery disease, CVA cerebrovascular disease, TIA transient ischemic attack, ESRD end-stage renal disease
Fig. 2.
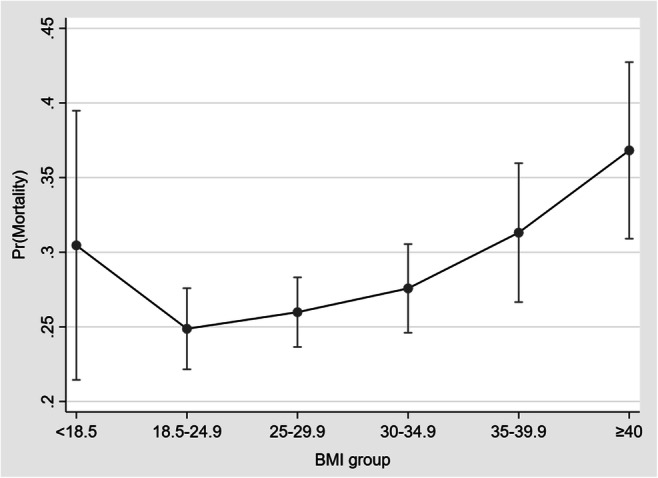
The average predicted probabilities (marginal effects) with 95% CIs of mortality per BMI group. Abbreviation: BMI=body mass index (expressed in kg/m2)
Severe pneumonia
Univariate associations with the outcome of severe pneumonia were examined for available baseline demographic and clinical characteristics and are presented in Table 4. In the multivariable analysis (Table 5, panel B), BMI ≥30 kg/m2 (model 1, OR: 1.57; 95% CI: 1.31–1.87; p < 0.001), BMI ≥ 35 kg/m2 (model 2, OR: 1.71; 95% CI: 1.38–2.11; p < 0.001), and BMI ≥ 40 kg/m2 (model 3, OR: 1.61; 95% CI: 1.21–2.15; p = 0.001) were found to have significant associations with the development of severe pneumonia. When BMI was analyzed based on the conventional classification (<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, 35–39.9 kg/m2, ≥ 40 kg/m2) compared with reference group 18.5–24.9 kg/m2, the 30–34.9 kg/m2, 35–39.9 kg/m2, and ≥ 40 kg/m2 groups were found to have significant associations with the development of severe pneumonia (model 4, OR: 1.44; 95% CI: 1.11–1.87; p = 0.006, OR: 1.97; 95% CI: 1.44–2.69; p < 0.001, and OR: 2.10; 95% CI: 1.49–2.97; p < 0.001, respectively).
Intubation
Univariate associations with the outcome of intubation were examined for the available baseline demographic and clinical characteristics and are presented in Table 4. In the multivariable analysis (Table 5, panel C), BMI ≥30 kg/m2 (model 1, OR: 1.77; 95% CI: 1.44–2.18; p < 0.001), BMI ≥ 35 kg/m2 (model 2, OR: 1.68; 95% CI: 1.32–2.13; p < 0.001), and BMI ≥ 40 kg/m2 (model 3, OR: 1.58; 95% CI: 1.15–2.18; p = 0.005) were found to have significant associations with a need for intubation. When BMI was analyzed based on the conventional classification (<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, 35–39.9 kg/m2, ≥ 40 kg/m2) compared with reference group 18.5–24 kg/m2, the 30–34.9 kg/m2, 35–39.9 kg/m2, and ≥40 kg/m2 groups were found to have significant associations with need for intubation (model 4, OR: 1.88; 95% CI: 1.38–2.56; p < 0.001, OR: 2.26; 95% CI: 1.56–3.26; p < 0.001, and OR: 2.43; 95% CI: 1.63–3.61; p < 0.001, respectively).
Subgroup analysis
A sex-based subgroup analysis for the outcome of mortality was performed and is presented in Table 6. In the subgroup of men, BMI 35–39.9 kg/m2 (model 1, OR: 1.99; 95% CI: 1.31–3.00; p < 0.001) and BMI ≥40 kg/m2 (model 1, OR: 2.26; 95% CI: 1.33–3.84; p < 0.001) were found to have significant association with higher in-hospital mortality, while only BMI ≥40 kg/m2 (model 1, OR: 2.26; 95% CI: 1.33–3.84; p < 0.001) was found significant in the subgroup of women. Similar results were demonstrated when IL-6 was added to the model (model 2).
Table 6.
Sex-based subgroup analysis for the outcome of mortality
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| n=1567 | n=1932 | n=683 | n=881 | |
| OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | OR (95% CI), p-value | |
| Age per 10 years | 1.62 (1.45–1.81) p < 0.001 | 1.91 (1.73–2.10) p < 0.001 | 1.52 (1.27–1.82) p < 0.001 | 1.98 (1.69–2.33) p < 0.001 |
| BMI <18.5(2) | 1.92 (0.89–4.12) p=0.094 | 1.01 (0.51–2.00) p=0.975 | 1.14 (0.21–6.19) p=0.879 | 0.71 (0.23–2.14) p=0.539 |
| 25–29.9(2) | 1.19 (0.83–1.69) p=0.349 | 1.00 (0.76–1.31) p=0.997 | 1.12 (0.65–1.94) p=0.685 | 1.18 (0.75–1.85) p=0.469 |
| 30–34.9(2) | 1.08 (0.74–1.58) p=0.680 | 1.25 (0.91–1.72) p=0.161 | 1.02 (0.57–1.81) p=0.956 | 1.74 (1.05–2.89) p=0.033 |
| 35–39.9(2) | 1.09 (0.69–1.74) p=0.712 | 1.99 (1.31–3.00) p=0.001 | 1.07 (0.51–2.25) p=0.858 | 1.63 (0.82–3.24) p=0.162 |
| ≥40(2) | 1.72 (1.05–2.81) p=0.031 | 2.26 (1.33–3.84) p=0.002 | 1.35 (0.64–2.86) p=0.435 | 2.28 (0.96–5.42) p=0.062 |
| Interleukin-6 per 10 pg/mL | 1.04 (1.00–1.09) p=0.079 | 1.08 (1.03–1.12) p < 0.001 | ||
(1) BMI in kg/m2; (2) reference group, BMI 18.5–24.9 kg/m2; (3) mortality is adjusted for the variables found to have significant univariate associations with congestive heart failure, CAD, diabetes, CKD, COPD, tobacco use, hypertension, hyperlipidemia, cirrhosis, PAD, CVA, or TIA; the multivariate associations of these variables with mortality are presented in the Supplementary table 5; (4) age was analyzed as a continuous variable, and 10 years was used as a unit of time for easier interpretation of OR (95% CI)
OR odds ratio, CI confidence interval, BMI body mass index, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, PAD peripheral artery disease, CVA cerebrovascular disease, TIA transient ischemic attack
Interleukin-6
IL-6 analyzed as a continuous variable per 10 pg/mL was found to have significant univariate associations with higher mortality, development of severe pneumonia, and need for intubation (Table 4, OR: 1.06; 95% CI: 1.03–1.09, p < 0.001; OR: 1.03; 95% CI: 1.01–1.05, p = 0.001; and OR: 1.02; 95% CI: 1.01–1.04, p = 0.002; respectively). The significant associations remained after the multivariate adjustments (Table 5).
Discussion
Our study investigated whether there is an association of obesity in men and women with in-hospital outcomes in 3530 consecutive patients who were hospitalized due to COVID-19 in a tertiary medical center in the Bronx, New York. In addition, our study attempted to assess the role of systemic inflammation using IL-6 levels as a surrogate in the outcomes of obese inpatients with COVID-19. The main findings can be summarized as follows: (1) obesity classes II and III, but not class I, appeared to be an independent risk factor for in-hospital death in men with a stronger association as obesity class increases. (2) Obesity class III, but not classes I and II, appeared to be an independent risk factor for in-hospital death in women. (3) The outcomes of in-hospital death, male sex, and increasing age were associated with higher IL-6 level, but there was no clear association between BMI and IL-6 level.
One of the main findings of our study is that obesity is associated with increased in-hospital mortality in patients with COVID-19. A J-shaped association between BMI and mortality was demonstrated with a lower predicted probability of death in the normal BMI range. BMI <18.5 kg/m2 seemed to be associated with increased predicted probability of mortality. It is known that underweight has been associated with higher all-cause mortality. However, it should be mentioned that number of patients in this group was too small to draw definite conclusions [30]. Subgroup analysis revealed that the male population with severe obesity (obesity classes II and III) is the one that mainly drives this association. While several studies have repeatedly shown a strong association of severe obesity with increased mortality in patients with COVID-19 [11, 12, 17, 31–33], only Tartof et al. in a retrospective study from California that involved almost 7000 patients have previously demonstrated that obesity in men is probably a stronger risk factor than obesity in women [10]. Similar to our findings, analysis of the overall cohort revealed that obesity classes II and III, but not class I, were independently associated with in-hospital death; the subgroup analysis also revealed that obesity might play a more profound role in risk for death in male patients compared to female patients [10].
The different pattern of adipose tissue distribution between sexes might help explain the disproportionate impact of severe obesity in the outcomes of men compared to women. Men tend to have more adipose tissue distributed in the central or abdominal region, also known as the android phenotype, whereas women tend to have more peripherally distributed subcutaneous fat, also known as the gynoid phenotype [34–36]. The android phenotype carries greatest risk for metabolic disorders [34–36]. The gynoid phenotype may have a less inflammatory phenotype [37]. In contrast, visceral fat has been shown to demonstrate greater pro-inflammatory characteristics than subcutaneous fat [38]. In addition, excessive visceral adipose tissue has a negative impact on lung function by reducing chest wall compliance, forced expiratory volume, and forced vital capacity [39].
One of the hypotheses of this study was that obesity increases the risk for death in patients with COVID-19 mainly via predisposition to systemic hyper-inflammation, which is a well-described possible mechanism in the COVID-19 literature [40–43]. The association between systemic inflammation and obesity in our population was assessed mainly via IL-6 level. IL-6 is a member of the pro-inflammatory cytokine family that induces the expression of variety of proteins responsible for acute inflammation and exerts a pleiotropic effect on inflammation, immune response, tissue regeneration, hematopoiesis, and metabolism [44–46]. Rapid production of IL-6 contributes to host defense during tissue injury and infection, but its excessive release may lead to hyper-inflammation [45, 46]. Abdominal visceral adiposity has been associated with significantly higher IL-6 concentrations regardless of race or sex [47]. Our findings suggest that higher IL-6 levels correlate well with male sex and increasing age, two of the strongest risk factors for death in patients with COVID-19 [11, 41, 42, 48, 49]. It was also demonstrated that IL-6 seems to be a strong predictor for in-hospital death, a finding that is supported by the existing literature [41, 50–53]. However, contrary to our hypothesis, we found no correlation between higher BMI and IL-6 level. In fact, a trend towards higher IL-6 level in patients with normal BMI was noted. Similar to our finding, a significantly smaller study from New York revealed no significant difference in IL-6 level among patients from different BMI groups [54].
The latter finding of our study suggests that either predisposition to systemic hyper-inflammation may be less important pathophysiologic mechanism behind worse outcomes in patients with severe obesity or that this effect is not strongly mediated by IL-6 and other factors may play a role. Several possible pathogenetic mechanisms have been described through which obesity independently increases the risk for worse outcomes in patients with COVID-19. First, severe obesity may adversely affect lung function by directly altering the mechanical properties of the lungs and chest wall via accumulation of fat in the mediastinum, abdominal, and thoracic cavities, thereby leading to reduction of functional residual capacity [55]. Second, central obesity leads to increased work of breathing by augmenting the airway resistance and also results in decreased diaphragmatic excursion in supine patients thereby compromising ventilation [56, 57]. Third, obesity has been shown to be a state of chronic low-grade inflammation due to a poorly understood interplay between adipocytes and immune system cells that results in impaired immune function [22]. Fourth, obesity is associated with a perturbed intestinal microbiota that otherwise would directly prevent the invasion of pathogens including SARS-CoV-2 [58–62]. Fifth, there is a causal association between obesity and venous thromboembolism, which is also a common manifestation of COVID-19 [63, 64]. Finally, the fact that ACE2 is also expressed in adipose tissue, mainly in visceral fat, suggests that severely obese individuals can host significantly higher viral load leading to local inflammation at the ectopic fat tissue level and making this population more prone to develop severe COVID-19 [65, 66] and this effect may not be easily measurable by measuring systemic cytokine levels.
To our knowledge, our study is one of the largest to date demonstrating the differences in the impact of obesity in the in-hospital outcomes between men and women with COVID-19 and the largest study to measure the association of BMI with systemic inflammation, as indicated by IL-6, in patients with COVID-19. Our study has several limitations. First, this was a retrospective design involving electronic medical records, which is suboptimal compared to a prospective study utilizing a more accurate follow-up assessment. Second, the rapidly changing management of patients with COVID-19 infection might have affected our results, although it is highly unlikely that could have significantly affected the associations of interest.
In conclusion, in this large cohort of hospitalized patients with COVID-19, we found that obesity classes II and III in men and obesity class III in women were associated with higher in-hospital mortality even after adjusting for other pertinent potentially confounding factors, including but not limited to hypertension, diabetes, coronary artery disease, and chronic kidney disease. No significant association between BMI and IL-6 was noted. Particular attention should be paid in protecting the population living with severe obesity from SARS-CoV-2 with priority to vaccination access, remote work, telemedicine, and other measures given the higher risk of adverse outcomes once they are diagnosed with COVID-19. In addition, patients with severe obesity diagnosed with COVID-19 should be treated with particular attention given the high risk for worse outcomes. While we recognize the limitations, we hope that our study will contribute to the understanding of the different impact of obesity in various subgroups of patients with COVID-19 and the pathogenetic mechanisms that lead to worse outcomes. Further studies are needed to confirm our data, and pilot clinical trials would be useful to assess whether pharmacotherapy targeting the visceral adipose tissue may improve outcomes.
Supplementary information
(PDF 88 kb)
Acknowledgments
Availability of data and material
Not applicable
Code availability
Not applicable
Author contribution
Drs. Guerson-Gil, Palaiodimos, Leider, and Brandt contributed to the study conception and design. Material preparation and data collection were performed by Drs. Guerson-Gil, Palaiodimos, Chamorro-Pareja, and Andrei Assa. Statistical analysis was performed by Dimitrios Karamanis. The first draft of the manuscript was written by Drs. Guerson-Gil and Palaiodimos. Critical review and editing of the manuscript were performed by Drs. Guerson-Gil, Palaiodimos, Kokkinidis, Kishore, and Brandt. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization, 2020. https://www.who.int/emergencies/diseases/novelcoronavirus-2019?gclid=Cj0KCQjw1a6EBhC0ARIsAOiTkrECxfD263wxharZbXDYl0R3aGW1qmgCect3GOUjZWjzOPmYev-By0QaAqJKEALw_wcB
- 2.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 3.Palaiodimos L, Chamorro-Pareja N, Karamanis D, Li W, Zavras PD, Chang KM, Mathias P, & Kokkinidis DG (Accepted/In press). Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. Hormones. 10.1007/s42000-020-00246-2 [DOI] [PMC free article] [PubMed]
- 4.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kragholm K, Andersen MP, Gerds TA, Butt JH, Ostergaard L, Polcwiartek C et al (2020) Association between male sex and outcomes of coronavirus disease 2019 (Covid-19) - a Danish nationwide, register-based study. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 6.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and SMOKING in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, Li Z, Shaw SF, Caparosa SL, Nau CL, Saxena T, Rieg GK, Ackerson BK, Sharp AL, Skarbinski J, Naik TK, & Murali SB (2020) Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern med 173(10):773–781. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed]
- 11.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Foldi M, Farkas N, Kiss S, Zadori N, Vancsa S, Szako L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring) 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamer M, Gale CR, Kivimaki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A. 2020;117(35):21011–21013. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chetboun MaR, Violeta and Labreuche, Julien and Simonnet, Arthur and Wallet, Florent and Caussy, Cyrielle and Antonelli, Massimo and Artigas, Antonio and Goma, Gemma and Meziani, Ferhat and Helms, Julie and Mylonakis, Eleftherios and Levy, Mitchell and Kalligeros, Markos and Latronico, Nicola and Piva, Simone and Cerf, Charles and Neuville, Mathilde and Klouche, Kada and Larchar, Romaric and Tamion, Fabienne and Occhiali, Emilie and Snacken, Morgane and Preiser, Jean-Charles and Kontar, Loay and Riviere, Antoine and Silva, Stein and Sarton, Benjamine and Krouchi, Raphael and Dubar, Victoria and Palaiodimos, Leonidas and Karamanis, Dimitrios and Perche, Juliette and L'Her, Erwan and Busetto, Luca and Dicker, Dror and Lev, Shaul and Duhamel, Alain and Jourdain, Mercèdes and Pattou, Francois (2020) Association of body mass index and other metabolic risk factors with pneumonia outcomes in critically ill patients with coronavirus disease-19: an international multicenter retrospective cohort study. 10.2139/ssrn.3667634
- 18.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 23.Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity (Silver Spring) 2020;28(7):1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319(1):E105–E1E9. doi: 10.1152/ajpendo.00198.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhaes KG. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol (Lausanne) 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID-19: Molecular mechanisms linking both pandemics. Int J Mol Sci. 2020;21(16):5793. doi: 10.3390/ijms21165793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya I, Ghayor C, Perez Dominguez A, Weber FE. From influenza virus to novel corona virus (SARS-CoV-2)-the contribution of obesity. Front Endocrinol (Lausanne) 2020;11:556962. doi: 10.3389/fendo.2020.556962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020;36:e3325. doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 29.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettit NN, MacKenzie EL, Ridgway JP, Pursell K, Ash D, Patel B, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19. Obesity (Silver Spring) 2020;28(10):1806–1810. doi: 10.1002/oby.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakeshbandi M, Maini R, Daniel P, Rosengarten S, Parmar P, Wilson C, et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes. 2020;44(9):1832–1837. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15(12):2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta. 2014;1842(3):377–392. doi: 10.1016/j.bbadis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinnick KE, Hodson L. Challenging metabolic tissues with fructose: tissue-specific and sex-specific responses. J Physiol. 2019;597(14):3527–3537. doi: 10.1113/JP277115. [DOI] [PubMed] [Google Scholar]
- 38.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790(10):1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiol Thorax. 2008;63(7):649–654. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 40.Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117(16):271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69(9):1857–1863. doi: 10.2337/dbi19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;44(8):1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uciechowski P, Dempke WCM. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98(3):131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17(5):1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J et al (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. [DOI] [PMC free article] [PubMed]
- 50.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH et al (2020) Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol [DOI] [PMC free article] [PubMed]
- 53.McElvaney OJ, Hobbs BD, Qiao D, McElvaney OF, Moll M, McEvoy NL, et al. A linear prognostic score based on the ratio of interleukin-6 to interleukin-10 predicts outcomes in COVID-19. EBioMedicine. 2020;61:103026. doi: 10.1016/j.ebiom.2020.103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, et al. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity (Silver Spring) 2020;28(9):1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson RA, Pride NB, Thomas EL, Fitzpatrick J, Durighel G, McCarthy J, et al. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol (1985) 2010;108(6):1605–1612. doi: 10.1152/japplphysiol.01267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 57.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 59.Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindstrom S, Germain M, Crous-Bou M, Smith EN, Morange PE, van Hylckama VA, et al. Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian Randomization study. Hum Genet. 2017;136(7):897–902. doi: 10.1007/s00439-017-1811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konstantinos G Kyriakoulis DGK, Kyprianou IA, Papanastasiou CA, Archontakis-Barakakis P, Doundoulakis I, Bakoyiannis C, Giannakoulas G, Palaiodimos L (2020) Venous thromboembolism in the era of COVID-19. Phlebology 1-9 [DOI] [PubMed]
- 65.Zhang Y, Somers KR, Becari C, Polonis K, Pfeifer MA, Allen AM, Kellogg TA, Covassin N, & Singh P (2018) Comparative expression of renin-angiotensin pathway proteins in visceral versus subcutaneous fat. Front Physiol 9:1370. 10.3389/fphys.2018.01370 [DOI] [PMC free article] [PubMed]
- 66.Iannelli A, Favre G, Frey S, Esnault V, Gugenheim J, Bouam S, et al. Obesity and COVID-19: ACE 2, the missing tile. Obes Surg. 2020;30(11):4615–4617. doi: 10.1007/s11695-020-04734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 88 kb)
Data Availability Statement
Not applicable