Abstract
The present opinion deals with an updated safety assessment of the food additive titanium dioxide (E 171) based on new relevant scientific evidence considered by the Panel to be reliable, including data obtained with TiO2 nanoparticles (NPs) and data from an extended one‐generation reproductive toxicity (EOGRT) study. Less than 50% of constituent particles by number in E 171 have a minimum external dimension < 100 nm. In addition, the Panel noted that constituent particles < 30 nm amounted to less than 1% of particles by number. The Panel therefore considered that studies with TiO2 NPs < 30 nm were of limited relevance to the safety assessment of E 171. The Panel concluded that although gastrointestinal absorption of TiO2 particles is low, they may accumulate in the body. Studies on general and organ toxicity did not indicate adverse effects with either E 171 up to a dose of 1,000 mg/kg body weight (bw) per day or with TiO2 NPs (> 30 nm) up to the highest dose tested of 100 mg/kg bw per day. No effects on reproductive and developmental toxicity were observed up to a dose of 1,000 mg E 171/kg bw per day, the highest dose tested in the EOGRT study. However, observations of potential immunotoxicity and inflammation with E 171 and potential neurotoxicity with TiO2 NPs, together with the potential induction of aberrant crypt foci with E 171, may indicate adverse effects. With respect to genotoxicity, the Panel concluded that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico‐chemical properties of TiO2 particles and the outcome of either in vitro or in vivo genotoxicity assays. A concern for genotoxicity of TiO2 particles that may be present in E 171 could therefore not be ruled out. Several modes of action for the genotoxicity may operate in parallel and the relative contributions of different molecular mechanisms elicited by TiO2 particles are not known. There was uncertainty as to whether a threshold mode of action could be assumed. In addition, a cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified. No appropriately designed study was available to investigate the potential carcinogenic effects of TiO2 NPs. Based on all the evidence available, a concern for genotoxicity could not be ruled out, and given the many uncertainties, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive.
Keywords: Titanium dioxide, E 171, CAS No 13463‐67-7
Summary
At the request of the European Commission, the Panel on Food Additives and Flavourings (FAF Panel) of EFSA provides an updated safety assessment of the food additive titanium dioxide (E 171) taking into account all new relevant data available to EFSA since the completion of its re‐evaluation in 2016. These include the data generated by a consortium of interested business operators (IBOs) in response to the follow‐up call launched by the European Commission further to the 2016 re‐evaluation by the EFSA Panel on Food Additive and Nutrient Sources added to Food (EFSA ANS Panel) under Regulation (EC) No 257/2010. New data retrieved from the published literature and considered to be in line with the data requirements specified in the 2018 EFSA ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain’ were also included.
The safety of E 171 was re‐evaluated by EFSA in 2016 under Regulation (EU) No 257/2010, as part of the re‐evaluation programme for food additives authorised in the EU before 20 January 2009. On the basis of the information available at that time, the EFSA ANS Panel considered that E 171 mainly consisted of micro‐sized TiO2 particles, with a nano‐sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E 171 were however highlighted, noting that no limits for the particle size of E 171 were set in the EU specifications. The ANS Panel concluded that, based on the data available at that time, E 171 when used as a food additive did not raise concern with respect to genotoxicity and that it was not carcinogenic after oral administration. Taking into account the presumed limited absorption of TiO2, the ANS Panel concluded that, based on a margin of safety (MoS) calculated from the no‐observed‐adverse‐effect level (NOAEL) of 2,250 mg TiO2/kg bw per day (identified from a carcinogenicity study in rats) and the exposure, calculated based on the reported use levels and analytical data, E 171 would not be of concern. However, given the toxicological data set at that time, the ANS Panel identified data gaps and uncertainties that required follow‐up by the European Commission by means of a call for data aimed at gathering information from interested business operators. In particular, in order to address concerns related to the lack of adequate data on reproductive and developmental toxicity, the ANS Panel recommended that an extended one‐generation reproduction toxicity (EOGRT) study be performed. An EOGRT study was commissioned by interested business operators and its study protocol was later amended to accommodate the investigation of additional parameters related to the occurrence and TiO2‐related induction of aberrant crypt foci (ACF) in the colon; these are preneoplastic lesions that had been reported by Bettini et al. (2017) shortly after the completion of the ANS Panel re‐evaluation of E 171.
Subsequent to the evaluation of data submitted by interested business operators, in 2019, the Panel recommended that the EU specifications for E 171 include the parameter of median minimum external dimension by particle number > 100 nm (measured by electron microscopy), which is equivalent to less than 50% of constituent particles by number with a minimum external dimension < 100 nm.
Based on the presence of a fraction of nanoparticles in E 171, the food additive falls under the scope of the EFSA Guidance on nanotechnology, which was broadened in its 2018 revision to also cover ‘a material that is not engineered as nanomaterial but contains a fraction of particles, less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm’.
For the reason given above, the proposed amendment to the specifications of the food additive E 171 in 2019 was accompanied by a recommendation from the Panel for a re‐assessment of the toxicological data set in line with the data requirements specified in the 2018 EFSA Guidance on nanotechnology.
Accordingly, the Panel considered that studies with TiO2 NPs were relevant in the current risk assessment of E 171. TiO2 particles in pristine E 171 likely form large agglomerates. When dispersion procedures are applied, these agglomerates may de‐agglomerate, resulting in increased numbers of ‘free’ nanoparticles. The extent of agglomeration and the number of ‘free’ nanoparticles present may be further affected by the conditions in food and the gastrointestinal tract (GIT) environment. The data available to EFSA showed that the percentage by number of constituent particles < 30 nm was in the order of 1% or less in samples of pristine E 171 or in E 171 extracted from foods analysed after dispersion. The Panel therefore considered that studies with TiO2 NPs < 30 nm were of limited relevance to the safety assessment of E 171.
In mice, E 171 has a low oral systemic availability, probably not greater than 0.5%. In studies in rats with TiO2 NPs, the oral systemic availability was also low (most probably < 1%) but higher than that of E171 and TiO2 NPs were detected in blood and tissues. For absorbed TiO2 particles, half‐lives of 200−450 days were estimated by the Panel.
Concerning general and organ toxicity, the Panel concluded that the available information in the literature did not indicate adverse effects with either E 171 up to a dose of 1,000 mg/kg bw per day or with TiO2 NP > 30 nm up to the highest dose tested of 100 mg/kg bw per day. No reliable studies were found in the literature addressing reproductive and developmental toxicity of E 171 and no effect was reported up to a dose of 1,000 mg/kg bw per day for TiO2 containing a fraction of nanoparticles. Concerning neurotoxicity, no reliable studies performed with E 171 were found in the literature. In studies with TiO2 NP > 30 nm, neurotoxic effects were observed at the only dose tested of 100 mg/kg bw per day in rats exposed in embryonal life and at the only dose tested of 500 mg/kg bw per day in rats exposed in adult life. In studies using TiO2 NPs < 30 nm, effects were seen at doses as low as 2.5 mg/kg bw per day. The findings in studies with E 171 on immunotoxicity and inflammation were considered inconsistent; in studies with TiO2 NPs > 30 nm effects were seen at a dose of 20 mg/kg bw per day whereas in studies with TiO2 NPs < 30 nm effects were observed at doses as low as 2.5 mg/kg bw per day.
Regarding the newly performed EOGRT study with E 171, the Panel concluded that there were no indications of general toxicity, no effect on thyroid or sex hormone levels, no effect on reproductive function and fertility in either male or female rats. Furthermore, no effects were observed on pre‐ and postnatal development. No effects on neurofunctional endpoints in F1 offspring were observed either. Concerning immunotoxicity, a marginal but statistically significant decrease in antigen‐induced IgM levels (−9%) in males of the F1 Cohort 3 only was noted, with no apparent dose‐response. However, the Panel noted that there were methodological shortcomings in the design of this part of the EOGRT study. Therefore, the Panel could not conclude on immunotoxicity. In a satellite group of that study, E 171 at doses up to 1,000 mg/kg bw per day did not induce ACF in the colon. The Panel considered that there was uncertainty regarding the extent of the internal exposure to TiO2 nanoparticles (present in E 171) across the range of tested doses.
The Panel considered that the effect of E 171 in producing ACF reported by Bettini et al. (2017) was not replicated in later investigations (EOGRT study and Blevins et al., 2019), but noted that the investigation by Blevins et al. had methodological limitations. Furthermore, it is unclear to what extent animals were exposed to TiO2 NPs in the EOGRT and in the study by Blevins et al. The Panel concluded that E 171 may induce ACF in male rats at a dose of 10 mg/kg bw per day when the test substance is pre‐dispersed and stabilised in a liquid medium preventing agglomeration of NPs prior to administration by gavage.
Concerning the genotoxicity studies, combining the available lines of evidence, the Panel concluded that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico‐chemical properties of TiO2 particles – such as crystalline form, size of constituent particles, shape and agglomeration state – and the outcome of in vitro or in vivo genotoxicity assays. The Panel concluded that several modes of action (MOA) may operate in parallel and the relative contributions of the different molecular mechanisms resulting in the genotoxicity of TiO2 particles are unknown. Based on the available data, no conclusion could be drawn as to whether the genotoxicity of TiO2 particles is mediated by a mode(s) of action with a threshold(s). Therefore, the Panel concluded that a concern for genotoxicity of TiO2 particles cannot be ruled out.
Concerning absorption and toxicity of TiO2 particles that are present in E 171, the Panel concluded that:
the absorption of TiO2 particles is low; however, they may accumulate in the body due to their long half‐life;
studies on general and organ toxicity, including the newly performed EOGRT study with E 171, did not indicate adverse effects up to a dose of 1,000 mg/kg bw per day. Also, no effects were seen in studies retrieved from the literature with TiO2 NP > 30 nm up to the highest dose tested of 100 mg/kg bw per day;
no effects on reproductive and developmental toxicity up to a dose of 1,000 mg/kg bw per day, the highest dose tested, were observed in the EOGRT study E 171. No other reliable studies were found in the literature addressing these effects with E 171;
some findings regarding immunotoxicity and inflammation with E 171 as well as neurotoxicity with TiO2 NPs may be indicative of adverse effects;
there are indications of an induction of ACF with E 171;
no studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available;
combining the available lines of evidence on genotoxicity, TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico‐chemical properties of TiO2 particles – such as crystalline form, size of constituent particles, shape and agglomeration state – and the outcome of either in vitro or in vivo genotoxicity assays;
a concern for genotoxicity of TiO2 particles that may be present in E 171 could not be ruled out;
several modes of action for the genotoxicity may operate in parallel. The relative contributions of different molecular mechanisms elicited by TiO2 particles are unknown and there is uncertainty as to whether a threshold mode of action could be assumed;
a cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified.
Overall, on the basis of all currently available evidence along with all the uncertainties, in particular the fact that genotoxicity concern could not be ruled out, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive.
This conclusion applies to E 171 as described in Commission Regulation (EU) No 231/2012 as well as to E 171 specified in the EFSA FAF Panel opinion in 2019.
1. Introduction
In the present opinion, the EFSA Food Additives and Flavourings (FAF Panel) provides an updated safety assessment of the food additive titanium dioxide (E 171) on the basis of newly available scientific evidence. The principles of the EFSA Guidance on nanotechnology (EFSA Scientific Committee, 2018a,b) have been followed in the assessment.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives. Only food additives that are included in the Union list, in particular in Annex II to that Regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No. 231/2012.
Titanium dioxide (E 171) is authorised for use as food additive (food colour) in the Union. Since titanium dioxide (E 171) was permitted in the Union before 20 January 2009, it belongs to the group of food additives which are subjected to a new risk assessment by the European Food Safety Authority (EFSA), according to Commission Regulation (EU) No 257/2010, and in line with the provision of Regulation (EC) No 1333/2008.
The re‐evaluation of titanium dioxide (E 171) as food additive was completed by EFSA in June 2016 and a scientific opinion was published on 14 September 2016. In that opinion, EFSA concluded, on the basis of the available evidence that titanium dioxide used as a food additive (E 171) did not raise a concern with respect to genotoxicity, was not carcinogenic after oral administration and exposure from the reported use/analytical levels would not be of concern. EFSA recommended that additional reproductive toxicity testing could be performed to enable EFSA to establish a health‐based guidance value (e.g. an accepted daily intake – ADI) for titanium dioxide (E 171). Therefore, the Commission issued in January 2017 a call for data requesting business operators to submit new reproductive toxicity data for titanium dioxide (E 171), as well as data addressing other recommendations made by EFSA concerning the specifications for titanium dioxide (E 171). In reply to this call for data, business operators committed to submitting by June 2020 data from a new extended one‐generation reproduction toxicity (EOGRT) study carried out according to the current OECD guidelines.
On 4 April 2017, the French Agency for Food, Environment and Occupational Health and Safety (ANSES) published an opinion on dietary exposure to nanoparticles of titanium dioxide assessing, in particular, the study of Bettini et al. (2017) and concluded that the data available do not bring into question the risk assessment performed by EFSA.
On 22 March 2018, the Commission requested EFSA to evaluate four new studies describing potential adverse health effect of titanium dioxide used as food additive (E 171). The EFSA opinion, published on 4 July 2018, concluded that the outcome of the four studies did not merit re‐opening the existing opinion of EFSA related to the safety of titanium dioxide (E 171) as food additive. In that opinion EFSA, however recommended that biomarkers for putative pre‐cancerous lesions in the colon should be examined, as additional parameters, in the reproductive toxicity study recommended by EFSA in 2016. Business operators have followed this recommendation and consequently the EOGRT study that is expected for submission in June 2020 will also cover the outcome of theses examinations.
On 15 April 2019, ANSES published a review of the risk related to the ingestion of food additive titanium dioxide (E 171) taking into account the most recent scientific studies available and referring to its earlier opinion of 2017. On 5 May 2019, the European Commission requested EFSA to assess the ANSES review and to indicate whether it includes any and major findings showing that titanium dioxide (E 171), when used as food additive, is of safety concern and thus whether it overrules the conclusion of the previous EFSA safety evaluations of titanium dioxide (E 171). EFSA was also requested to indicate whether the ANSES review identified additional uncertainties that could be addressed in the follow‐up work undertaken by the interested business operators.
On the 13 May 2019, the European Food Safety Authority (EFSA) published a statement on the review of the risk related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environment and Occupational Health Safety (ANSES). In that statement EFSA concluded that the ANSES opinion does not identify any major new finding that would overrule the conclusions made in the previous EFSA scientific opinion on the safety of titanium dioxide (E 171) as a food additive. The ANSES opinion reiterates the previously identified uncertainties and data gaps, which are currently being addressed in the context of the follow‐up activities originating from the previous EFSA evaluation and their recommendations. In addition, ANSES recommended further investigations of in vivo genotoxicity. EFSA considered that this recommendation should be revisited once the work on the physico‐chemical characterisation of the food additive titanium dioxide (E 171) is completed.
On the 7 August 2018, the European Commission requested EFSA to assess new data provided by interested food business operators in response to the call for data published as a follow‐up of the re‐evaluation of titanium dioxide (E 171), and addressing the uncertainties identified with respect to the characterisation of this food additive, including its particle size and particle size distribution. This led to the publication on 12 July 2019 of a scientific opinion on the proposed amendment of the specifications of titanium dioxide (E 171) with respect to the inclusion of the additional parameters related to its particle size distribution. In that opinion EFSA indicated that the conclusions made, and the uncertainties identified, in the previous EFSA assessment of the food additive titanium dioxide (E 171) remain valid. Moreover, EFSA indicated that the characterisation of titanium dioxide (E 171) does not provide a reason to revise the conclusion on the genotoxicity of titanium dioxide (E 171) drawn by EFSA in the previous opinions on titanium dioxide (E 171). Nevertheless, EFSA concluded that based on the proposed change in the specifications, the toxicological database on titanium dioxide (E 171) as a food additive should be revisited in line with the data requirements specified in the 2018 EFSA “Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain”. Based on this latest EFSA opinion, the specifications for the food additive titanium dioxide (E 171) in Commission Regulation (EU) No 231/2012 will be updated.
The legislation on food additives envisage that food additives should be kept under continuous observation and re‐evaluation whenever necessary in the light of new scientific information. Therefore, it is appropriate to ask EFSA to reassess the safety of the food additive titanium dioxide (E 171) taking into account all new relevant data available to EFSA since the completion of the re‐evaluation of titanium dioxide (E 171) as a food additive by EFSA in 2016, including the new EOGRT study recommended by EFSA. The new assessment should take into account the relevance of the data in line with the data requirements specified in the 2018 EFSA “Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain”.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2012, the European Commission requests the European Food Safety Authority (EFSA) to provide an updated scientific opinion as regards the safety of the food additive titanium dioxide (E 171).
In particular, EFSA is requested to reassess the safety of food additive titanium dioxide (E 171) taking into account all new relevant data available to EFSA since the completion of its re‐evaluation of titanium dioxide (E 171) as a food additive in 2016. These included the data generated by a consortium of interested business operators in response to the follow‐up call lunched by the European Commission further to the re‐evaluation of the food additive completed by EFSA in the context of Regulation (EC) No 257/2010, once available, as well as any new data retrieved from the published literature and considered to be in line with the data requirements specified in the 2018 EFSA “Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain”.
2. Data and methodologies
2.1. Data
The present evaluation is based on the following data:
Information from publications retrieved in the literature search (see Section 2.2).
Data submitted in response to the call for data from European Commission as follow‐up of the re‐evaluation of E 171 (Documentation provided to EFSA No 1, 2, 3, 4, 5 and 6).
Toxicokinetic and genotoxicity studies considered in the re‐evaluation of titanium dioxide (E 171) (EFSA ANS Panel, 2016).
Exposure data available in the re‐evaluation 2016 and additional relevant information that came available since then (see Section 4.4).
In vitro and in vivo studies reported in the OECD dossier (2016) and submitted to EFSA (Documentation provided to EFSA No 7, 8, 9 and 10).
In vitro genotoxicity studies submitted to EFSA (Documentation provided to EFSA No 14 and 15).
Food consumption data used to estimate the dietary exposure to titanium dioxide (E 171) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database1). Dietary data from the UK were included in the EFSA Comprehensive European Food Consumption Database for the period in which UK was a member of the European Union.
The Mintel's Global New Products Database (GNPD) was used to verify the use of titanium dioxide (E 171) in food and beverage products and food supplements within the EU's food market. The Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidance from the EFSA Scientific Committee, in particular the Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health (EFSA Scientific Committee, 2018a).
A literature search was performed following the approach described in Appendix A. Information on the criteria for inclusion and exclusion of publications based on information from the abstract and title, and kind of material used in the study is available in Appendix B.
Toxicokinetic and toxicity studies considered ‘included’ according to Appendix B were assessed for their relevance and reliability taking into account the criteria described in Appendix C.
Genotoxicity studies considered ‘included’ according to Appendix B were assessed taking into account the criteria described in Appendix D.
Nanoscale considerations for the assessment of the study design and study results in toxicity studies classified with reliability 1 and 2 (see Section 4.1), and genotoxicity studies (see Section 4.3), were assessed according to criteria described in Appendix E.
Dietary exposure to E 171 from its use as a food additive was estimated combining the food consumption data available within the Comprehensive Database with reported use levels submitted to EFSA (EFSA ANS Panel, 2016) and information extracted from a report of the Netherlands National Institute for Public Health and the Environment (RIVM) (Sprong et al., 2015). The exposure was estimated according to different exposure scenarios (EFSA ANS Panel, 2017). Uncertainties in the exposure assessment were identified and discussed (Section 4.4.3).
After receiving the mandate from the European Commission, the support from the cross‐cutting Working Group (ccWG) on Genotoxicity to review the evidence and conclude for the genotoxicity of E 171 was requested. Accordingly, the assessment of the data (referred to in Section 4.3) has been conducted independently by the ccWG Genotoxicity and submitted to the Panel for its consideration and decision.
3. Background information and previous evaluations
Titanium dioxide (E 171) is authorised as a food additive in the EU according to Annex II of Regulation (EC) No 1333/2008 and specifications have been defined in Commission Regulation (EU) No 231/20122
In June 2016, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS Panel) completed the re‐evaluation of the safety of the food additive E 171, performed under the frame of Regulation (EC) No 257/2010 (EFSA ANS Panel, 2016).
In that opinion, the ANS Panel had concluded that the food additive did not raise concerns with respect to genotoxicity and carcinogenicity but the ANS Panel was unable to establish a health‐based guidance value (HBGV) because of certain deficiencies identified in the available toxicological data set, in particular with respect to the investigation of potential reproductive toxicity. Another important source of uncertainty identified during the re‐evaluation concerned the characterisation of the material used as the food additive E 171.
The European Commission followed up on the recommendations issued by the ANS Panel and, in January 2017, published a call for data addressed to interested business operators and requesting their commitment to provide the data requested to reduce the uncertainties underpinning the conclusions of the ANS Panel opinion. These included data on the characterisation of the material and the performance of a new EOGRT study in rodents, to be conducted in accordance with the latest OECD Guidance applicable and with test material representative of the food additive on the EU market.
In January 2017, the publication of a study by Bettini et al. (2017) raised some concerns on the potential tumour promoting effect of dietary intake of E 171, which led to an opinion of the ANSES in April 2017 on the dietary exposure to nanoparticles of titanium dioxide (ANSES, 2017). In that opinion, the ANSES concluded that the data available did not put the 2016 EFSA assessment in question.
A subsequent scientific opinion was issued by the ANS Panel in June 2018, to address a request from the European Commission for the evaluation of four publications – among them, Bettini et al. (2017) previously considered by ANSES – raising concerns on the safety of E 171. Having reviewed these publications, the ANS Panel maintained the conclusions reached in 2016 but recommended the inclusion of biomarkers for putative pre‐cancerous lesions in the colon to be included in the ongoing EOGRT study as additional parameters to be investigated (EFSA ANS Panel, 2018).
In July 2018, the safety assessment of food additives was handed over from the EFSA ANS Panel to the newly constituted EFSA Food Additives and Flavourings (FAF) Panel.
In July 2018, the EFSA Scientific Committee published a Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health (EFSA Scientific Committee, 2018a) updating the 2011 Guidance Document on nanomaterials (EFSA Scientific Committee, 2011a), and clarifying that conventional materials containing a fraction of nanoparticles require specific risk assessment considerations, which were detailed in the document.
While the follow‐up activities for the generation of new data were ongoing, in April 2019, the French Government decided to take risk management action introducing a ban on foods containing the food additive E 171. The French decree, that entered into force on 1 January 2020, was based by the application of the precautionary principle to the latest advice issued by ANSES (2019). The ban on foods containing the food additive E 171 in France has been reconfirmed for the current year, pending the finalisation of the present assessment by EFSA.3
As a follow‐up to the re‐evaluation of E 171 completed by the ANS Panel, in 2018, the European Commission requested EFSA to assess a proposal for an amendment of the EU specifications for the food additive E 171 based on the data on particle size and particle size distribution that had been provided by the interested business operators in response to the first part of the European Commission call for data. The related scientific opinion was adopted by the FAF Panel in June 2019 and published shortly afterward (EFSA FAF Panel, 2019). The FAF Panel, while recommending the inclusion of additional parameters related to the particle size distribution in the EU specifications for E 171, also concluded that the toxicological database should be revisited in line with the data requirements specified in the 2018 EFSA ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain’ (EFSA Scientific Committee, 2018a). Scope of this guidance document is not only engineered nanomaterials but also those materials containing a fraction of particles that is less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm, a definition which could be applicable to the case of the food additive titanium dioxide (E 171).
Based on the recommendations from the 2019 scientific opinion of the FAF Panel, the European Commission made a proposal for amending the definition and specifications of E 171, introducing limits with respect to the particle size and particle size distribution in the food additive in Regulation (EU) No 231/2012. In October 2020, the European Parliament called on the Commission to withdraw its draft regulation, to apply the precautionary principle and to remove E 171 from the list of food additives authorised by the Union.4
In 2019, the Office for Risk Assessment and Research of the Netherlands Food and Consumer Product Safety Authority (NVWA) delivered an opinion on possible health effects of the food additive E 171 (NVWA, 2019). The opinion concluded that studies conducted since 2016 in mice and rats provide an indication of tumour promotion by E 171 in the intestinal tract but should be considered ‘exploratory’ since they were not conducted in accordance with OECD guidelines. With regard to the EOGRT study (ongoing at the time), the opinion concluded that an examination of immunotoxicological effects was important given recent studies, in addition to potential reprotoxicological effects. The opinion also concluded that an examination for potential promotion of colon cancer by E 171 should be examined but considered it doubtful whether the performance of an EOGRT study or chronic exposure test would be suitable test system. In addition to this, further research in humans was considered required to establish any relevance of experimental findings to man.
In parallel to the re‐evaluation of titanium dioxide as a food additive, the EFSA FEEDAP Panel was also evaluating the safety of titanium dioxide in feed for all animal species. This assessment has been put on hold, awaiting submission of the data requested as a follow‐up of the re‐evaluation of the food additive.5
The substance evaluation for titanium dioxide under REACH was started in 2018. The Competent Authority of France (the evaluating Member State Competent Authority) was appointed to carry out the evaluation. Currently the decision‐making is under the registrants’ comment review period.
The European Chemicals Agency (ECHA) Committee for Risk Assessment (RAC) concluded in its scientific opinion of 14 September 2017 that titanium dioxide met the criteria in Regulation (EC) No 1272/2008 for classification as a carcinogen in category 2 by inhalation (ECHA, 2017). The adopted harmonised classification and labelling was later included in an amendment of Regulation (EC) No 1272/2008 as indicated in Commission Delegated Regulation (EU) 2020/217.6 The new entry in Annex VI to Regulation (EC) No 1272/2008 applies to titanium dioxide in powder form containing 1% or more of particles with aerodynamic diameter ≤ 10 μm.
Titanium dioxide is widely used as an excipient in medicinal products, mainly as a colour/opacifier in oral and cutaneous dosage forms. Titanium dioxide for use in medicinal products needs to meet the requirements defined in the European Pharmacopoeia. Colouring matter should comply with the requirements of European Union Directive 2001/83/EC.7 Current EU legislation laying down specific purity criteria concerning colours for use in foodstuffs (Commission Regulation (EU) No 231/2012) also applies to medicinal products (as detailed in Directive 2009/35/EC8).
4. Assessment
Relevant studies with (i) the food additive titanium dioxide (E 171), (ii) titanium dioxide – other than E 171 – containing a fraction of particles < 100 nm (TiO2 (X% nano))9 or (iii) nano titanium dioxide (TiO2 NPs) have been evaluated when considered reliable, i.e. scoring 1 or 2 according to the approach described in Appendix C for toxicity or the approach described in Appendix D for genotoxicity. Unless otherwise indicated by the Panel (Appendices G, H), the constituent particles of these materials investigated in the assessed studies had a nearly spherical shape and the purity of the material was considered acceptable. The constituent particle size is indicated in the summaries below (Appendices G, H, J, K, L, M, N, O, P), followed by the analytical technique in parenthesis.
The characterisation of E 171 was previously evaluated by the Panel and it was concluded that, according to data received from interested business operators, less than 50% of constituent particles in E 171 have a minimum external dimension below 100 nm by number (EFSA FAF Panel, 2019)
Information on the physico‐chemical characteristics of the representative test materials considered relevant for the assessment of E 171, e.g. those from the JRC repository (NM‐100, NM‐102 and NM‐105) is available in the JRC report (Rasmussen et al., 2014)) and in specific publications (Taurozzi and Hackley, 2012). As opposed to commercially manufactured TiO2 materials such as ‘P25’ (AEROXIDE® TiO2 P 25) that are also applied as intralaboratory standards (Taurozzi and Hackley, 2012)), NM‐100 and NM‐102 originate from single batches of commercially manufactured TiO2 materials and have been demonstrated to be sufficiently homogeneous and stable to be used as reference materials in chemical analysis and toxicological testing. The Panel noted that NM‐105 is produced from a batch of the P25 material.
The Panel considered that studies performed with TiO2 NPs that predominantly consist of particles smaller than 30 nm (e.g. P25) are of limited relevance to the safety assessment of E 171, since in samples of E 171 and in E 171 extracted from foods the percentage by number of particles below 30 nm is in the order of 1% or less (Verleysen et al., 2020, 2021; Geiss et al., 2021; Appendix W). However, data from toxicity studies performed with TiO2 < 30 nm have been considered for completeness of the database and may be relevant with respect to whether a minimum limit for particle size should be included in the EU specifications for E 171.
In several studies described in the open literature, there was an inadequate description of the test material (size or crystalline form is not reported). In all cases, EFSA requested additional information about the test material by contacting the corresponding authors reported in the publications. No responses were received.
4.1. Toxicokinetic and toxicity studies from the literature search (January 2015–November 2020)
More than 11,000 publications retrieved according to the literature search (Appendix A) were screened based on the criteria agreed in advance (Appendix B). After the first screening based on the description of title and abstract of the publication, around 200 in vivo studies and 300 in vitro studies were identified as potentially relevant for the current assessment.
Information on the assessment of in vitro and in vivo genotoxicity studies is provided in Appendix D.
For the current toxicokinetic and toxicological assessment, it was decided to focus on in vivo studies (Appendix F). The relevance of the in vivo toxicity studies for the different key areas of concern (Table 1) was assessed following the approach described in Appendix C. Around 30 studies were considered relevant for hazard characterisation and 33 additional studies were considered only relevant for providing supporting evidence (Table 1).
Table 1.
Summary of the number of in vivo toxicity studies evaluated for their relevance and reliability
| Studies assessed for their relevance | Studies considered relevant | Studies considered with reliability 1 and 2 | ||
|---|---|---|---|---|
| Relevant for hazard characterisation | Relevant for providing supporting evidence | |||
| Studies examining toxicity effect in the liver, spleen and pancreas | 56 | 32 | 3 | 3 |
| Studies examining toxicity effect in heart and kidney | 27 | 10 | 3 | 2 |
| Studies examining other organs | 37 | 14 | 5 | 3 |
| Reproductive and developmental toxicity studies | 39 | 29 | 4 | 3 |
| Neurotoxicity and developmental neurotoxicity studies | 24 | 17 | 6 | 6 |
| Inflammation and immunotoxicity studies | 38 | 30 | 9 | 8 |
| Gut microbiota | 16 | 13 | 8 | |
In a second step, an assessment of the reliability of the relevant publications took place according to the criteria described in Appendix C. Publications were classified from 1 to 4 and only those publications considered sufficiently reliable with respect to their internal validity, i.e. the extent to which the design and conduct of a study are likely to have prevented bias (reliability 1 and 2) were further examined for the safety assessment of E 171 (Appendix H).
4.1.1. Toxicokinetic studies
The toxicokinetics of TiO2 was reviewed by the ANS Panel in the 2016 opinion on the re‐evaluation of E 171 (EFSA ANS Panel, 2016). According to the ANS Panel, the absorption of orally administered TiO2 is low. Its oral systemic availability (measured either as particles or as titanium) is estimated to be 0.02–0.1%, and the vast majority being eliminated unchanged in the faeces. The ANS Panel had noted that the small amount of orally ingested TiO2 appeared to be absorbed by Peyer's patches, a group of cells in the gut‐associated lymphoid tissue (GALT). It is subsequently distributed to various organs (by order of decreasing concentration: mesenteric lymph nodes, liver, spleen, kidney, lungs, heart and reproductive organs), from which the material disappears with variable half‐lives. The ANS Panel noted the potential for tissue accumulation based on the slow elimination of titanium from tissues after intravenous administration with calculated half‐lives ranging between 28 and 650 days in different organs (EFSA ANS Panel, 2016). Interpretation of these findings was, however, complicated by the extent of the variability in the background levels of Ti in animals and humans which also prevented the accurate determination of kinetic parameters such as the elimination half‐life.
The focus of the current updated assessment was to gather from the newly available evidence, any relevant information that could be used to refine the risk assessment and reduce the uncertainties identified by the ANS Panel in its earlier evaluation. In particular, the Panel examined whether new data from the published literature could provide better estimates of the oral systemic availability, the distribution in tissues and the elimination half‐life of TiO2 after oral administration.
The estimate of the oral systemic availability of TiO2 was updated by multiplying the reported concentration with the respective organ or tissue weights. Subsequently, the sum of the calculated amounts in the different organs was compared to the dose applied to estimate the percentage absorbed. Data were extracted only from those publications in which the analytical method used for the measurement of internal exposure was evaluated as reliable or reliable with some limitations (see Appendix C). In addition, two references from the previous ANS Panel opinion were also re‐examined because their results could contribute to a quantitative estimate of absorption (Geraets et al., 2014; Tassinari et al., 2014).
The issue of the variability in the environmental, dietary and tissue background levels of Ti in the studies, already flagged by the ANS Panel in its previous opinion, remains one of the main critical aspects to be taken into consideration when evaluating the toxicokinetics of TiO2. Challenges in the analytical determination of low concentrations of Ti in tissues, primarily related to detection limits, contamination issues and spectral interferences in inductively coupled plasma mass spectrometry (ICP‐MS) determination, further complicates obtaining accurate and reliable tissue concentrations and toxicokinetic data.
Further information on the description of the test materials, scoring for nanoscale considerations (NSC) and internal exposure for the following studies is reported in Appendix G.
Studies on E 171
Mice
One of the aims of the study of Talamini et al. (2019) was to investigate whether repeated administration of E 171 to mice would result in accumulation in tissues. Mice (n = 4) were administered 5 mg E 171/kg body weight (bw) per day, 3 days per week, for 3 weeks (nine treatments in 21 days, providing an average daily dose of 2 mg E 171/kg bw). On day 21, the animals were sacrificed and Ti concentration was measured by triple quadrupole ICP‐MS in tissues from four mice.
Total Ti10 concentrations in the stomach, small intestine, large intestine and liver were one order of magnitude greater than those in the other organs. Differences in Ti concentrations in lungs, spleen, stomach and small intestine of treated animals compared to controls were not statistically significant. The Ti concentration in liver (0.94 ± 0. 57 μg Ti/g tissue) and in the large intestine (1.07 ± 0.38 μg Ti/g tissue) was significantly higher in the treated animals than in the controls (ca. 0.2 μg Ti/g tissue, liver, and 0.6 μg/g tissue, large intestine). Ti concentrations in brain, kidney and testis were below the limit of quantification (LOQ) of 0.03 μg Ti/g tissue.
The Panel adopted a conservative approach and considered that all colon‐associated Ti was absorbed. On this basis, the Panel calculated that 0.1% of the total dose of TiO2 was absorbed in the Talamini et al. (2019) study.11
In the study of Coméra et al. (2020) in C57BL/6 mice, the processes associated with the absorption of TiO2 in the gastrointestinal tract (GIT) were investigated. In the first series of experiments, mice were given a single dose of a suspension of E 171 (40 mg/kg) in water by gavage, while control mice received water. Segments of the jejunum, ileum and colon were prepared 2, 4, 8 and 24 h after administration following intraluminal content recovery through gentle scraping. Particle diameters in tissues were measured by transmission electron microscopy (TEM). The existence of particles in gut tissue and blood was analysed by laser‐reflective confocal microscopy. ICP‐MS analysis was performed to measure Ti concentration. In the second series of experiments, performed under anaesthesia, a closed mid‐jejunal loop of 10 cm was pretreated with inhibitors of tight junctions, micropinocytosis, clathrin‐mediated endocytosis or raft‐dependent endocytosis. Thereafter, the loops were filled with sonicated E 171 (300 μg/mL) in buffer or buffer as control, followed by incubation for 30 min. Confocal microscopic evaluation was performed on cryosectioned tissue slices to detect TiO2 particles by light reflection. The same detection method was applied to examine the sizes of particles present in the E 171 water suspension prepared for the gavage, in the gastrointestinal (GI) luminal contents and in blood. In mice having received 40 mg E 171/kg, increases in reflective particle content was observed in the ileal and jejunal villi and colon crypts. In jejunal and ileal villi, maximal reflective particle content occurred 4 h after administration and returned to basal values at 8 h. In the colon, a small non‐significant increase in reflective particle content was observed at 4 h with normalisation at 8 h. The presence of Ti and O in particles was confirmed in jejunal goblet cells and enterocytes by transmission electron microscopy energy‐dispersive X‐ray spectroscopy (TEM‐EDX). In the Peyer's patches, a statistically significant increase in laser‐reflecting particles was found only at 8 h and not at 4 h. In blood, the number of particles significantly increased by 3.5‐ and 4.1‐fold at 4 and 8 h, respectively. In blood, the Ti concentrations remained below the limit of detection (LOD < 0.02 ng Ti/kg) at all time points. From the content in the intestines and the weight of the mice tissues, the authors calculated that approximately 0.007% of the Ti administered was present in the entire intestine at the 4 h time point. The authors concluded that the TiO2 was absorbed primarily/predominantly in the ileum, partly in jejunum with a small amount absorbed in the colon. The authors considered – based on surface area considerations – that TiO2 is primarily/predominantly absorbed in the area of the small intestinal villi and to a less extent, through Peyer's patches. From the ex vivo experiments, inhibiting the paracellular pathway reduced the absorption of TiO2. Blockers of transepithelial passage did not influence the absorption of TiO2 to a significant extent. As the absorption was not totally blocked by paracellular pathway inhibitors, the authors concluded that besides a paracellular pathway, endocytosis could also be involved in the transport of TiO2 from the intestinal lumen to blood.
The Panel noted that TiO2 can be taken up from the small intestine by the paracellular pathway and by endocytosis. Specialised cells in the Peyer's patches may also play a role in the uptake. The authors indicated that 0.007% of the total TiO2 dose at 4 h is present in the intestine. The Panel noted high agglomeration of TiO2 in the exposure medium.
In the study of Riedle et al. (2020a), primarily aimed at studying effects of E 171 on immune cells in Peyer's patches, C57BL/6 mice at 6 weeks of age were randomly exposed to 0 and ≈ 1, 10 and 100 mg E 171/kg bw per day for 6, 12 and 18 weeks. After 18 weeks, the presence of particles in the basal regions of Peyer's patches was examined via confocal microscopy and scanning electron microscopy (SEM) with EDX analysis. According to the authors, GI harvest and the method used to prepare tissue sections took care to avoid inadvertent contamination. Reflectance confocal microscopy was employed to examine for the presence of E 171 in Peyer's patches. Subsurface particles were identified by reflectance confocal microscopy which were rich in Ti based on SEM/EDX analyses. The lowest and mid‐dose groups (1 and 10 mg E 171/kg bw per day) showed very weak signals from impacted cells at the base of the Peyer's patch, whereas higher signals were observed in the highest dose group (100 mg E 171/kg bw per day). No quantitative information was given by the authors.
The Panel noted the uptake of TiO2 particles into cells in the Peyer's patches after 18‐week exposure to E 171.
Human
A study on the oral systemic availability of E 171 was performed by Pele et al. (2015) in eight volunteers (seven completed the study). Participants in the study ingested two capsules each containing 50 mg of E 171 with 250 ml water. Blood samples were taken at 0, 30 min and 1, 1.5, 2, 3, 6, 8 and 10 h after ingestion. Tea and coffee with milk and/or sugar were allowed from 2 h after ingestion. Blood drops were spread on a glass slide and protected from drying. Particles were detected by light microscopy with a 100‐fold and then 400‐fold magnification using a dark field condenser. This method could only be carried out in the samples of five out of the seven subjects due to blood clotting in the samples from two of the subjects. Reflectance grade (0, 1, 2 and 3) was used as a semiquantitative measure of the number of TiO2 particles. Total Ti concentration was measured in blood via isotope dilution analysis by high resolution (HR)‐ICP‐MS. Significant increases in positive signals by dark field microscopy were observed in blood films from 2 h onwards, with a peak at 6 h. Blood Ti concentrations were not statistically significantly different from the baseline value up to 3 h. The highest blood concentration of Ti determined in any participant was approximately 11 ng/mL (at 6 h). Thereafter the blood Ti‐concentration declined to about 5 ng/mL at 10 h after administration. Reflectance grades, indicating the number of particles in blood, correlated with the levels of Ti measured by HR‐ICP‐MS. The authors considered that two routes of particle uptake appear to exist in the human gut, one proximal (in the duodenum/jejunum) and one distal (Peyer's patch uptake in the ileum).
The Panel concluded that after oral administration of 100 mg E 171 in human volunteers, blood Ti concentration and the number of particles increased, demonstrating oral systemic availability of TiO2.
The study of Guillard et al. (2020) consisted of two parts. In the first part, Ti and TiO2 particle concentrations were measured in placentas collected from 22 women given birth at term without complications. Ti and TiO2 particle concentrations were also measured in 18 meconium samples of newborn infants. The placentas and the meconium samples used in this study were not related. Ti concentrations were measured by ICP‐MS and the morphology, size and chemical characterisation of the particles identified in the placental tissue sections and in the meconium samples were prepared for electron microscopy. Ti concentrations in the placentas ranged between 0.01 and 0.48 mg/kg, the median being 0.05 mg/kg. In only 9 of the 18 meconium samples, Ti concentrations were above the LOQ (0.01 mg/kg). The median concentration was 0.25 mg/kg. Scanning transmission electron microscopy coupled to energy dispersive X‐ray (STEM‐EDX) analysis in two placentae and two meconium samples confirmed the presence of TiO2 particles. The Panel noted that the meconium samples were collected within the first few days after birth.
In the second part of the study, a well‐established ex vivo human placenta perfusion model was used to quantify the transplacental transfer of TiO2 across the placenta. Integrity and functionality of the placental membranes were confirmed by antipyrine testing. The perfusion medium of a cotyledon contained either 15 μg E 171/mL (n = 13) or E 171 (n = 2) controls, with confirmed dispersion of the TiO2 particle. In two of the placentae, particles were identified and their size measured; 53% of the 34 particles sizes measured were above 100 nm and 47% below. Using confocal microscopy for particle visualisation in the fetal effluent and SEM‐EDX to ascertain the chemical nature of detected particles, it was shown that the number of particles in the fetal exudate increased for the first 40 min of the 1‐h placental perfusion. The number of particles (depicted as mean ± SEM of four to six independent experiments) was between 6 and 8 per microscopic field.
From these studies, the Panel considered that Ti is found in the placenta in low concentrations, indicating that TiO2 is systemically available, is distributed to the placenta and is capable of crossing placental membranes. The Panel estimated the amount of Ti in the placenta.12 The resulting total median amount of Ti in the placenta was 35.75 μg (19.5–84.5 μg, 25–75 percentiles).
From the ex vivo study, the Panel noted that the extent of TiO2 transfer is small and not measurable within the short experimental period, in conformity with the in vivo findings. However, particles were present in the fetal exudate and 53% of the 34 particles had a size above 100 nm and 47% below. The process by which TiO2 particles enter the meconium was not considered by the authors. Given the fact that the meconium collection period was up to 48 h after birth and that in 50% of the meconium samples, no Ti was measurable and no particles identified, the Panel considered that some of the infants would have been nursed before the meconium was collected. The Panel noted that the authors did not control for the possibility that TiO2 particles could have come from the diapers used to collect the meconium or from other sources, e.g. mothers’ milk.
Studies with TiO 2 NPs or TiO 2 other than E 171
Rat – Intravenous administration
In the study of Disdier et al. (2015), male Fisher rats were injected with a TiO2 NPs suspension (1 mg/kg), controls received saline buffer. From six controls and six treated animals, samples were taken at 30 min, 1, 2, 6 and 24 h, and 7, 28, 90 and 356 days following injection encompassing blood, liver, brain, spleen, kidney and lungs. Additional blood and brain samples were obtained at 5 and 15 min following injection. Ti concentrations in the samples were determined by ICP‐MS. In blood cells and plasma, the Ti concentration in the treated and control groups did not differ at any time point. In the brain, the Ti concentrations were higher than in the control in the first 24 h. From the next sampling time on (7 days), no difference in brain Ti concentrations existed between controls and treated animals. The amount of Ti in the brain was very low. In kidneys, the Ti concentration was several‐fold higher than in the control in the first 24 h. From 7 days on, the next sampling time, no difference existed between controls and treated animals. In spleen, liver and lung, the Ti concentrations declined over time, with the concentrations of Ti being higher in the treated group than in the control animals at 356 days after injection. The authors estimated that at 6 h about 44% of the total dose was in the liver, 10% in the lungs and 2% in the spleen.
The Panel estimated the half‐lives and the accumulation factors (R).13 The estimate for the half‐life was 83 days for the liver and lung and 350 days for the spleen. The Panel considered these estimates have high level of uncertainty. However, for the liver, the Panel calculated an accumulation factor of 134.7. The steady state for Ti/TiO2 NPs in the liver would be reached after approximately 1.5 years. These figures are roughly the same for the lungs. For the spleen, the accumulation factor would be 350. The steady state would be reached after 5 half‐lives which would be roughly 5 years.
The Panel noted that this study identifies a potential for TiO2 NP accumulation in the liver, lung and spleen.
Kreyling et al. (2017a) investigated the fate of an intravenously administered aqueous [48V]TiO2 nanoparticle suspension, primary particle size 7–10 nm, particle size in water/tissue 88 nm in female Wistar‐Kyoto rats. This preparation was obtained by irradiation of TiO2 NPs with a proton beam current of 5 μA. The radioactive product (1.0 MBq/mg (48V‐activity per TiO2 mass)) was used for the experiments lasting up to 24 h, whereas the product after irradiation for 5 days with a higher activity concentration of 2.35 MBq/mg was used for the experiment of 7 days and 28 days duration. At 1 h, 4 h, 24 h, 7 days and 28 days, four rats per time point were euthanised by exsanguination under anaesthesia. Blood, all organs, tissues and excreta were collected and 48V‐radioactivities were measured. The authors reported the nanoparticle quantities as percentages of the total intravenously injected 48V radioactivity, which was calculated as the sum of all measured radioactivities, including faecal and urinary samples, corrected for background and radioactive decay during the experiments (48V‐half‐live: 16.0 days). The percentage was the average over the group of four rats per time point. The detection limit was 0.2 Bq. The results of a separate intravenous study performed to investigate the absorption and biodistribution of soluble ionic 48V were used to correct 48V release from [48V]TiO2 nanoparticle. At 4 h following injection, 99.5% of the radioactive dose was found in the liver and at 28 days 88.9% of the dose was found in the liver. The spleen and the kidneys contained a few per cent of the dose (spleen between 2.5% and 4%, and kidneys between 0.05% and 0.2%). All other tissues had lower contents. The bones (including the marrow) and the remaining tissues contained 1% and 0.7%. 48V was excreted in urine, within 28 days the excretion amounted to roughly 1%, the highest amount being excreted on day 1. Excretion by the faeces, indicative of biliary excretion, amounted to 3% over 28 days.
The Panel did not estimate the half‐life of the [48V]TiO2 as the last measurement was at day 28 after administration when the decline in radioactivity was only 10% and the extrapolation would have a high degree of uncertainty. However, the data indicate that the half‐life is long and likely of the order of months rather than weeks.
The study by Geraets et al. (2014) has already been described in the EFSA opinion on the re‐evaluation of E 171 published in 2016. In the current opinion, the results of an analysis of the data with respect to an estimate of the whole body half‐life and the resulting accumulation factor are presented. Geraets et al. (2014) investigated the fate of four TiO2 nanomaterials after single and repeated (five times on consecutive days) intravenous administration. In the context of this evaluation, only NM‐100 and NM‐102 were considered of relevance for the assessment of E 171. The authors had reported half‐lives for several organs they had investigated (not for the whole body) and they reported on the body recovery. Recovery of Ti was measured 24 h after the last dosing, on day 2 (single administration) and 6 (repeated administration), respectively, and for both dosing schedules on day 90 by summing up the contents of all organs investigated.
From these data, the Panel estimated a half‐life for TiO2 NPs for the whole body of roughly 200 and 450 days, with an accumulation factor of 290 and 450, for NM‐100 and NM‐102, respectively, and the time to steady state as 3 and 5 years, for NM‐100 and NM‐102, respectively.
Rats – Oral administration
The study by Tassinari et al. (2014) has already been described in the EFSA opinion on the re‐evaluation of E 171 published in 2016 (EFSA ANS Panel, 2016). In the current opinion, the analysis of the data with respect to an estimate of absorption is presented. Three groups of Sprague–Dawley rats (n = 14 per dose group per sex) were dosed by gavage with TiO2 NPs 1 mg/kg bw per day or 2 mg/kg bw per day or with vehicle for 5 days. On day 6 (24 h after the last treatment), blood samples were taken under anaesthesia and organs were excised after sacrifice. Total Ti concentration was determined in tissues (uterus, ovary, testes, thyroid and adrenals) by inductively coupled plasma dynamic reaction cell mass spectrometry (ICP‐DRC‐MS), TiO2 NPs by SEM‐EDX in spleen slices and TiO2 NPs by single particle inductively coupled plasma mass spectrometry (spICP‐MS) in spleen homogenates. The limit of detection of ICP‐DRC‐MS for Ti determination in tissues was 0.009 μg/g. The Ti concentrations in the tissues investigated were not different from the controls with the exception of spleen and ovary in the 2 mg/kg bw dose group. Ti concentrations equal to 0.046 ± 0.008 μg/g (2 mg/kg bw per day) vs. 0.036 ± 0.009 μg/g (control) and 0.28 ± 0.07 μg/g (2 mg/kg bw per day) vs. 0.12 ± 0.04 (control) were measured in spleen (n = 8 animals per group) and in ovary (number of animals not mentioned), respectively. Agglomerates of TiO2 particles (diameter 200–400 nm) were identified by SEM‐EDX in spleen of the 2 mg/kg bw per day dose group. The mass concentration of such TiO2 particles was quantitatively determined by single‐particle ICP‐MS and was found to be in agreement with the total Ti concentration determined by ICP‐DRC‐MS.
The Panel concluded from this study that the absorption of the investigated material is low. Based on Ti determinations, the Panel calculated that 0.001% of the total TiO2 NP dose was present in the spleen and ovaries.
In the study of Hendrickson et al. (2016), TiO2 NPs were administered at a dose of 250 mg/kg bw per day to 6 rats (with 6 rats as controls) by gavage administration. After 28 days treatment, the animals were euthanised and blood was obtained from abdominal vein. Lungs, liver, spleen, brain, testicles, small intestine, heart, stomach and kidneys were harvested. Ti was measured by graphite furnace atomic absorption spectroscopy. TiO2 particles in tissues were detected by TEM and diffraction analysis. After 28 days, the Ti concentration was below the LOD in all tissues from the control group. In treated animals, the Ti concentration was the highest in liver, followed by spleen and small intestine and the lowest in kidney. It was below the level of detection in lungs, brain, testicles, heart and blood.
From these data, the Panel estimated the Ti amount of the body14 after 28 days of exposure. Body Ti amount was determined to be 3.4 μg. The oral systemic availability can be estimated from the body Ti amount of 3.4 μg divided by cumulative dose of 60 mg (daily Ti dose) × 28 days, which equates to 2.1 × 10−4% of the cumulative Ti dose. For those samples where Ti was below the LOD, the Ti amount was taken to be 0.5 LOD.
In the study of Ammendolia et al. (2017), male and female Sprague–Dawley rats (n = 10/sex per group) were treated with TiO2 NPs with doses of 0, 1 or 2 mg/kg bw per day for 5 consecutive days by gavage. TiO2 NPs were dispersed in water by sonication, performed on a daily basis. Twenty‐four hours after the last treatment, the rats were euthanised and small intestine was sampled. After removing any GI digestion residues, the tissue samples were characterised for their Ti levels by ICP‐DRC‐MS (LOD of 0.009 μg/g).
Ti levels in small intestine tissue (mean ± SD, n = 4) were 0.08 ± 0.02 μg/g in the control, 0.09 ± 0.02 μg/g in the dose group of 1 mg/kg and 0.13 ± 0.03 μg/g in the dose group of 2 mg/kg.
The Panel noted that results for Ti concentration were provided by four animals per dose group.
Based on Ti determinations and subtracting control levels of Ti, the Panel calculated that 0.01% of the total TiO2 NPs dose was present in the small intestine. As only measurements were made in the small intestine tissue, no estimate for systemic exposure could be made.
In the study of Kreyling et al. (2017b), female Wistar‐Kyoto rats received an aqueous [48V]TiO2 NPs suspension by gavage. Preparation and the procedure to calculate the concentrations are described in Kreyling et al. (2017a). After gavage, rats were kept separately to be able to collect individual urine and faeces. At 1 h, 4 h, 24 h and 7 days, four animals per time point were euthanised by exsanguination under 5% isoflurane anaesthesia. Blood, all organs, tissues and excreta were collected and 48V radioactivities were measured.
After gavage, most of the radioactivity was excreted in the faeces. Absorption was calculated as the fraction of the dose that could not be accounted for by the radioactive content of the intestinal tract plus faeces. About 0.6% of the applied dose was absorbed during the first h after gavage; the fraction still present in tissues after 7 days amounted to about 0.05% of the applied dose. The authors noted that the distribution patterns between animals were variable and that several data were below the LOD during the first 4 h. Measurable deposition could be observed only after 4 h in spleen, kidneys, heart and uterus. The retention maximum was reached in spleen, kidneys and heart at 24 h post‐dosing. In liver, lung and blood, nanoparticle retention declined from 4 h to 7 days. In brain, uterus and kidneys, the highest concentrations were observed at day 7. The peak concentration in liver and spleen was 12.5% (4 h) and 2.6% (24 h) of the absorbed dose, respectively. According to the authors, due the slow excretion kinetics, accumulation of systemically circulating particles in specific cells and organs is likely to occur in subjects chronically exposed to TiO2 NPs.
By means of the radiotracer method used, up to 50% of the suspended [48V]TiO2 NPs dose to be administered was demonstrated to be retained in syringes and cannulas, presumably due to adsorption on plastic surfaces. According to the authors, such effects are likely to occur to a variable extent in in vivo studies (depending on the materials used and their handling), are difficult to detect and might be one reason for variations in reported results.
Comparing the biodistribution of [48V]TiO2 nanoparticles retained after oral administration with that determined after intravenous injection (Kreyling et al., 2017a), the authors conclude that the kinetics patterns are very different and intravenous injection does not appear an adequate surrogate for assessing the biodistribution occurring after oral exposure to TiO2 NPs.
The Panel considered that this study demonstrates that the systemic availability of orally administered TiO2 NPs is 0.6%, based on the assumption that 48V is a faithful tracer for TiO2 NPs. The Panel further considered that this study shows significant differences in distribution between the routes of exposure.
In the study of Hendrickson et al. (2020), the isolated intestinal loop technique was used to administer 50 mg/kg bw TiO2 NPs to Wistar rats. Three hours after administration the isolated loop was cut out, in addition to the liver and spleen. The presence of particles in tissues was studied by TEM and diffraction analysis. Loose agglomerates were seen with a size of 100 nm and larger. By diffractions analyses, it was confirmed that the particles were TiO2. TiO2 NPs were detected on the surface and between the microvilli of the mucosal cells of the small intestine and also in the mucosal tissue. Nanoparticles were detected in the Peyer's patches, both as single nanoparticles and agglomerates of sizes ranging between 20 and 60 nm. In the liver, parenchymal tissue aggregates of TiO2 NPs (150–200 nm) and up to 300 nm were seen. In the spleen red pulp, single nanoparticles (20–30 nm), agglomerates (up to 100 nm) and conglomerates (up to 800 nm) could be observed.
The Panel noted that this study demonstrates the presence of TiO2 NPs, either as single particles or as agglomerates of variable size, in intestine, liver and spleen. However, no quantitative data were provided.
In the study of Chen et al. (2020a,b), 4‐week‐old Sprague–Dawley (n = 6/dose group) were administered daily doses of 0, 2, 10 and 50 mg TiO2 NPs/kg bw per day for 90 days by oral gavage, using a suspension in distilled water (sonicated and mixed before administration). Blood, liver, stomach, small intestine, colon, spleen, heart, lung, kidneys and testicles were harvested at day 91. Ti was determined in the organs and blood by high‐resolution ICP‐MS. In spleen and heart, the Ti concentrations were below the LOD (32 ng/g tissue). In blood, liver, intestine, lung, kidney and testicle, the measured concentration of Ti (ng/g tissue) was not statistically different in the treated groups compared with controls. In the colon, the concentration was higher in the 50 mg/kg bw per day group than in the controls and the groups treated with 2 and 10 mg/kg bw per day. The authors considered that the high concentration of Ti in the colon of the animals from the highest dose group was due to TiO2 nanoparticles attached on the surface of the colonic mucosa tissue and not in mucosa cells. The authors concluded that the absorption of TiO2 in this study was very low.
The Panel considered that TEM without chemical characterisation of the particles (e.g. by EDX) would lead to uncertainty on the identity of the particles.
Human
In the study of Heringa et al. (2018), Ti was measured using ICP‐HR‐MS in liver and spleen from 15 deceased human subjects (nine women and six men) who had donated their bodies for research and educational purposes. The LOD of the method was 10 ng/g tissue. The size of Ti‐containing particles in the organs was quantified using spICP‐MS, and the presence of TiO2 particles in the tissues was verified by SEM‐EDX. In 8 of the 15 liver samples and in 1 of the spleen samples, the total Ti concentration was below the LOD. The average concentration in samples where Ti could be determined was 40 ng/g in the liver and 80 ng/g in the spleen. The average particle size was 86–426 nm in the liver and 88–445 nm in the spleen, with the lower size being the lower size limit of detection. According to the authors, almost all Ti was present as particles, as the concentration of the particle‐Ti and the concentration of total‐Ti in the organs measured by an independent procedure was overlapping. The amount of Ti in the liver of females (including only the females in which concentrations were above LOD) was 56.0 ± 37 μg and that in males (including only the males in which concentrations were above LOD) was 72 ± 25 μg, calculated by the Panel.15 The amount of Ti in spleen (including only the females in which concentrations were above LOD) was 12 ± 17 μg in females and that in males (including only the males in which concentrations were above LOD) of 5.5 ± 4 μg. Summing the amounts in liver and spleen for both genders gives amounts of 83 ± 51 μg. The mean exposure to E 171 in the age group most representative of the human subjects is 200–2,800 μg/kg bw per day (non‐brand loyal scenario in the elderly; EFSA ANS Panel, 2016), corresponding to ~ 8.4 and 117 mg Ti per day. Assuming that the majority of body Ti is present in liver and spleen and that all body Ti is derived from dietary E 171, the Panel estimated that the oral systemic availability of E 171 in human would be below 1% at the most.
The Panel noted that nanoparticles of TiO2 can be present in liver and spleen in humans at low concentrations. Complete data (i.e. concentration in liver and spleen) were available only for a limited subset of the investigated subjects (50% of the subjects). Since the subjects were between 79 and 104 years of age, the Panel considered that steady‐state levels of TiO2 had been reached. From the comparison of the Ti body burden with the mean exposure in the non‐brand loyal scenario of exposure towards TiO2 in the elderly, the Panel concluded that the absorption of TiO2 as food additive in humans under normal life conditions would be low.
In the study of Peters et al. (2020), Ti content was measured using ICP‐HR‐MS in liver from 15 deceased human subjects, eight women and seven men, aged 64–97 years, who had donated their bodies for research and educational purposes. Further measurements in jejunum and ileum from seven women and five men were done. The LOD of the method was 0.01 μg/g tissue. The presence of nanoparticles in the tissues was verified by SEM‐EDX. In four liver samples, in two spleen samples and one kidney sample out of 15 samples, the total Ti concentration was below the LOD. In one jejunum sample out of 12 samples, the total Ti concentration was below the LOD. The average concentration in the liver samples where Ti could be quantified was 0.03 μg/g, that in the spleen samples was 0.06 μg/g, that in kidney 0.08 μg/g, that in jejunum 0.34 μg/g and that in ileum 0.43 μg/g. The particle sizes, measured by spICP‐MS, ranged between 50 and 500 nm in the different tissues, with 50 nm being the lower size detection limit. The TiO2 particle concentrations were considered by the authors to represent about 80% of the total Ti concentrations, showing that most of the Ti in these organs consisted of particulate material and that Ti content can be seen as a good surrogate for the presence of particles. For samples with concentrations above the LOD, the content in spleen amounted to 78 ± 5 μg in females and to 11 ± 13 μg in males. Using the same criterion (samples > LOD), the content in kidney amounted to 33 ± 34 μg in females and to 16 ± 13 μg in males. Summing up the content in liver, spleen and kidney for both genders, an average amount of 105 ± 83 μg is obtained. The high content of 262 ± 185 μg as the sum of the content in jejunum and ileum, obtained using the same calculation approach, is notable.
The Panel noted that Ti can be present in particulate form in liver, spleen, kidney, jejunum and ileum in humans at low concentrations.
Conclusions
Overall, the Panel noted that the toxicokinetics of E 171 was addressed in three studies in mice and in two studies in humans.
In two studies in mice, the data enabled the derivation of estimates of internal exposure at 0.01% (Coméra et al., 2020) and 0.1% (Talamini et al., 2019) of the external dose, respectively. However, these estimates are based on Ti concentrations measured in a limited number of organs. Although it is uncertain to what extent TiO2 distributes to other organs, the Panel's estimates have always included the Ti amount in the liver, which accounted for about 12.5% of the Ti amount in the body (Kreyling et al., 2017b). The underestimation in body burden and absorption is therefore unlikely to be more than 5‐fold. The Panel noted that in mice, TiO2 can be taken up from the small intestine by the paracellular pathway and by endocytosis. Furthermore, in two of the studies (Coméra et al., 2020; Riedle et al., 2020a), uptake of TiO2 particles was demonstrated into M‐cells of the Peyer's patches, whereby the quantitative contribution to the systemic exposure seems to be low.
In humans, the Panel considered that after oral administration of 100 mg E 171, Ti concentration in blood increased ca. 5‐ to 10‐fold from 6 to 10 h post‐dosing (Pele et al., 2015), demonstrating some oral systemic availability. TiO2 particles were found in human placenta in low concentrations (Guillard et al., 2020), indicating that TiO2 is systemically available after ingestion and also can distribute to the placenta. In an ex vivo human placenta model, particles were transferred and the size distribution of the particles was similar to the E 171 present in the perfusate. The Panel noted that the extent of transfer across placental membranes was small.
The Panel noted that materials other than E 171, mainly TiO2 NPs, were investigated in rats and humans.
In rats, two intravenous studies (Disdier et al., 2015; Kreyling et al., 2017a) demonstrated long half‐lives and, hence the potential for accumulation. Together with data from an intravenous study (Geraets et al. (2014) already addressed in the EFSA opinion on the re‐evaluation of E 171 (EFSA ANS Panel, 2016), half‐lives of 83 days (for liver) and of 450 days (for whole body) were estimated and accumulation factors between 135 and 450. Based on these data, the steady state would be reached between 1.5 and 5 years.
Out of five oral rat studies, one provided an estimate for oral systemic availability of 0.0002% based on a limited number of organs (Hendrickson et al., 2016) and another study provided an estimate of 0.6% (Kreyling et al., 2017b). The Panel noted that in a study employing the model of isolated loop technique, the authors could provide data indicating the presence of TiO2 NPs either as single particles or as smaller and larger agglomerates in intestine, liver and spleen (Hendrickson et al., 2020). The other studies did not give data suitable to quantify absorption and/or accumulation. The Panel considered that in two studies analysing tissues from deceased subjects, deposition of Ti‐containing nanoparticles was observed in liver, spleen and kidney as well as in intestine. After quantification of the Ti amount in the organs and comparison with the estimated mean daily intake of E 171, the Panel concluded that the oral systemic availability of TiO2 NP ingested from a number of sources, including dietary exposure to E 171, would be low (less than 1% by mass).
In summary, the Panel considered that E 171 has a low oral systemic availability, probably not greater than 0.5%. It may pass the placenta and may be transferred to the fetus.
Furthermore, the Panel considered that rat studies with TiO2 NPs, consisting of nanoparticles with primary particle sizes between 7 and 90 nm, showed long half‐lives (roughly 200–450 days), a potential for accumulation (accumulation factor of 290 to 450) and long time to reach steady state (3–5 years) (Geraets et al., 2014; Disdier et al., 2015). The oral systemic availability of these materials was low (most probably < 1%) but higher than for E 171. In tissues from deceased subjects, TiO2 particles were identified in liver and spleen, the low Ti amount of the investigated organs indicating low oral systemic availability of TiO2 ingested from a number of sources, including dietary exposure to E 171.
4.1.2. Toxicity studies
Toxicity studies considered sufficiently reliable with respect to their internal validity (reliability 1 and reliability 2 according to Appendix C) are described in Appendix H, including details of the appraisals of their suitability for investigating the toxicological effects of nanoparticles.
The score for the assessment of suitability of the studies to investigate the toxicity of nanoparticles has been performed according to Appendix E and is independent from the score on internal validity.
General and organ toxicity
E 171
Mice
Talamini et al. (2019) (scoring 1 for NSC) examined the effect of exposing mice (n = 4) to 5 mg E 171/kg bw per day, 3 days per week, for 3 weeks (nine treatments in 21 days, providing an average daily dose of 2 mg E 171/kg bw). The exposure did not affect body weight, feed intake or organ weights. Some inflammatory biomarkers were changed in some of the tissues examined (stomach, gut, liver). Total area of ‘necroinflammatory’ foci in the liver of exposed mice (n = 4) was greater as compared to the area in the controls (n = 2). The Panel noted that this study was limited to one dose group, that the histological examination included a small number of mice and that the increase in ‘necroinflammatory’ area in the E 171 exposed mice was not accompanied by additional endpoints indicative of evidence for liver injury.
Rats
Talbot et al. (2018) (scoring 1 for NSC) examined the effect of exposing rats to E 171 (0, 0.1 and 10 mg/kg bw per day) for 60 days, with a focus on GIT microbial production of short‐chain fatty acids (SCFAs) and mucin O‐glycosylation. The Panel considered that the results indicated no effects of E 171 on SCFA production and mucus barrier in the gut at the highest dose tested of 10 mg/kg bw per day.
Han et al. (2020a) (scoring 2 for NSC) examined the effects of E 171 (0, 10, 100 or 1,000 mg/kg bw per day) for 90 days in rats. Although some changes were reported by the authors (6–10% higher feed intake of high‐dose males, an 8% decrease in relative lymphocyte count in low‐ and high‐dose males, respectively), overall, the Panel considered that gavage administration of E 171 at doses up to 1,000 mg/kg bw per day to rats for up to 90 days did not induce any adverse effects on clinical appearance, survival, body weight, feed intake, haematology, clinical chemistry, urinalysis, organ weights, or gross and microscopical pathology.
The Panel considered that E 171 had no adverse effects in a mouse study (Talamini et al., 2019) on parameters regarded as indicative of systemic toxicity (body weight, feed intake, organ weights or morphology of the examined tissues) given by gavage at a mean daily dose of 2 mg/kg be per day for 21 days. The Panel considered that E 171 was dispersed and administered such that exposure to small particles and NPs present in E 171 would have taken place. The Panel considered the reported enlargement of ‘necroinflammatory’ liver foci in the exposed mice deserved attention. However, the Panel could not conclude on the association of this finding with exposure to E 171, due to very limited number of livers examined. The Panel noted the absence of additional endpoints indicative of evidence for liver injury and the fact that these reported changes can variably occur as a background pathology in murine liver. In the rat, no signs of systemic toxicity were seen after gavage administration for 90 days at doses up amounting to 1,000 mg E 171/kg bw per day; the study had limitations for assessing the toxicological effects of the fraction of nanoparticles (Han et al., 2020a). E 171 did not affect caecal microbial production of SCFA in the caecum or mucus barrier in small and large intestine after 60 days of exposure via gavage at the highest dose tested of 10 mg/kg bw per day (Talbot et al., 2018).
TiO 2 NPs or TiO 2 containing a fraction of NPs
Mice
No studies available.
Rats
Vasantharaja et al. (2015) (scoring 2 for NSC) examined the effect of exposing rats to TiO2 NPs (0, 50 and 100 mg/kg bw per day) by gavage for 14 days on clinical chemistry parameters. Although some changes were reported by the authors, the Panel considered that the only major change was a decrease in serum triacylglycerols to 64% of the control levels in both treatment groups.
El‐Din et al. (2019) (scoring 4 for NSC) examined the effect of exposing rats to TiO2 NPs (0, 1,200 mg/kg bw per day) by gavage for 90 days on morphology of the heart ventricles. The authors reported histological changes in the myocardium of the treated group. However, due to the limited information reported, the Panel was not able to conclude on the relationship between the reported histopathological changes and treatment with TiO2 NPs.
Warheit et al. (2015a) (scoring 4 for NSC) examined the effect of exposing rats by gavage to two types of TiO2 (11% nanoparticles) at a single, unrealistically high‐dose level of 24,000 mg/kg bw per day for 29 days. Microscopic evaluation revealed the presence of the test substance in intestinal lymphoid tissue, considered by the authors as not adverse. The study examined all endpoints incorporated in an OECD TG 407 study. The Panel considered that no treatment‐related adverse effects on any of the endpoints were found; however, the Panel further noted that the scoring for NSC was 4 and there was no demonstration of internal exposure.
The Panel considered that there were no adverse effects on clinical chemistry parameters in rats exposed by gavage to TiO2 NPs at doses up to 100 mg/kg bw per day for 14 days (Vasantharaja et al., 2015). A potential adverse effect on the heart was reported after subchronic (90‐day) exposure to 1,200 mg TiO2 NPs/kg bw per day, but due to limited reporting no conclusion could be drawn (El‐Din et al., 2019). Gavage administration of 24,000 mg TiO2 (11% NPs)/kg bw per day for 29 days, without demonstrating of internal exposure, had no adverse effects on the endpoints required by the OECD TG 407 testing guideline (Warheit et al., 2015a).
TiO 2 NPs < 30 nm
Mice
Hu et al. (2015) examined the effects of gavage administration of TiO2 NPs (26 nm) (0, 64 and 320 mg/kg bw per day for 14 weeks) on the hormonal control of glycaemia in male CD‐1 mice. The authors reported that treated mice had increased fasting blood glucose levels from weeks 10. Impaired glucose tolerance was observed (without showing a dose response), but no changes in blood insulin or lipids could be detected. The Panel considered that the oral administration of TiO2 NPs at both doses, 64 and 320 mg/kg bw per day led to increases in fasting state plasma glucose, and also to increases in glucose levels in a glucose tolerance test without showing a dose response and without differences in plasma insulin levels, indicating inconsistency between the measured outcomes.
Yu et al. (2016) examined the effects of gavage administration of TiO2 NPs (5–6 nm) (0, 2.5, 5 and 10 mg/kg bw for 90 consecutive days) in female CD‐1(ICR) mice on the heart. Body weight gain was statistically significantly decreased in a dose‐dependent manner to 30.3% in all the TiO2 NPs groups. Based on the limited reporting, the Panel was not able to conclude on the decreased body weight gain and the relationship between the reported histological changes and treatment with TiO2 NPs.
Another study (Hong et al., 2016) examined the effects of gavage administration of TiO2 NPs (5–6 nm) (0, 2.5, 5 and 10 mg/kg bw for 90 consecutive days) in male CD‐1(ICR) mice on the liver. A dose‐dependent decrease in body weight gain was observed at all tested doses (approx. 5%, 5% and 7% decrease in BW gain compared to control at 2.5, 5 and 10 mg/kg bw per day, respectively), with statistically significant differences at the two highest doses. Relative liver weights increased by ~ 10–15% compared to control; however, absolute liver weights were unchanged. Histological alterations of the liver (lymphocyte infiltration and necrobiosis) were reported. Changes in the liver expression of inflammation‐related proteins were also found. The Panel noted that the histopathological data in the liver were not accompanied by any other confirmatory investigations (e.g. clinical chemistry) and considered the effects reported in this study as likely an hepatic inflammatory response to TiO2 NPs (5–6 nm).
Yang et al. (2017) examined the effects of gavage administration of TiO2 NPs (21 nm) (0, 250 and 500 mg/kg bw for 14 consecutive days) in male C57BL/6 mice on the liver. The only noteworthy effect was a 3‐fold increase of serum bilirubin (both total and indirect) at the highest dose, in the absence of inflammation, apoptosis, necrosis and molecular defects in bilirubin metabolism. The Panel noted structural changes in hepatocytes which were not quantified. However, these increases occurred in the absence of any changes in relative liver weight, changes in other serum markers for liver injury or quantitative histopathological changes in the liver. Changes in the hepatic expression of selected genes were considered as either incidental or adaptive, but not evidence of adversity.
Rats
Chen et al. (2015a) (scoring 2 for NSC) examined the effect of exposing rats to TiO2 NPs (24 nm) (0, 2, 10 and 50 mg/kg bw per day) with and without glucose (1.8 g/kg bw per day) for 30 and 90 days. Some haematological parameters were changed; however, the Panel considered that these were of no toxicological significance. The authors reported ‘oedema, fatty degeneration and necrosis’ in livers of the high‐dose group in the absence of serum enzyme activities reflecting liver injury. The Panel considered there were limitations in the reporting of histopathological changes in the liver and in the absence of changes in serum enzyme activities reflective of liver injury considered effects on the liver as not adverse.
Chen et al. (2015b) (scoring 1 for NSC) examined the effect of exposing rats to TiO2 NPs (24 nm) (0, 2, 10 and 50 mg/kg bw per day) with a focus on investigating effects on the cardiovascular system. Although some changes were reported by the authors, the Panel considered that gavage administration of TiO2 NP (24 nm) in doses up to 50 mg/kg bw per day to rats for up to 90 days did not induce any treatment‐related effects.
Grissa et al. (2015) (scoring 2 for NSC) administered TiO2 NPs (5–12 nm) in distilled water by daily gavage at doses of 0, 50, 100 or 200 mg/kg bw to rats for 60 days. The Panel considered the reported haematological changes to be of no toxicological significance.
Chen et al. (2020a) (scoring 2 for NSC) daily gavaged TiO2 NPs (29 nm) at doses of 0, 2, 10 or 50 mg TiO2 NPs/kg bw per day for 90 days to male rats. Starting from week 8, the 10 and 50 mg/kg bw per day groups showed a decrease in body weight gain of up to about 15%, with no effect on food intake. Serum levels of triglycerides (TGs) in the 10 and 50 mg/kg bw per day groups were statistically significantly lower than in the control group while serum total cholesterol, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol (LDL‐C) were not affected. The Panel considered that, while the change in body weight gain may be adverse, other reported changes were of no toxicological significance.
Chen et al. (2020b) (scoring 2 for NSC) examined the effect of exposing rats to TiO2 NPs (24 nm) (0, 2, 10 and 50 mg/kg bw per day) with and without glucose (1.8 g/kg bw per day) for 90 days. Effects were seen with TiO2 NPs (24 nm) on blood glucose levels, blood glycoproteins (glycated haemoglobin (HbA1c); glycated serum protein (GSP)), blood insulin, C‐peptide and an oral glucose tolerance test (OGTT; performed at day 90). The Panel considered the changes in blood glucose, HbA1c, GSP, insulin, C‐peptide, glucagon and glucose tolerance as either not test substance related or irrelevant for the safety evaluation of E 171.
Grissa et al. (2017) (scoring 3 for NSC) gave male rats control vehicle or 100 mg TiO2 NPs (5–10 nm)/kg bw per day by gavage for 8 weeks. The TiO2 NP‐treated group had a statistically significantly decreased body weight gain and serum cholesterol, glucose and TG concentrations were statistically significantly higher. The authors reported significant changes in plasma oxidative stress markers and an increase in plasma interleukin‐6 (IL‐6) compared to control. The Panel noted the changes in glucose levels which are potentially adverse, and considered that the changes in cholesterol and TG are of unclear toxicological relevance.
Hassanein and El‐Amir (2017) (scoring 3 for NSC) administered a single dose level of 150 mg/kg bw TiO2 NPs (21 nm) in 1% Tween 80 daily by gavage. The control group received 1% Tween 80. Based on the many flaws in the study reporting (e.g. descriptions of ‘histopathological’ lesions are unclear, some of the findings are not histopathological lesions, the number of lesions per organ and the number of animals with any lesions are not clearly stated), the Panel was not able to draw any conclusions.
Heo et al. (2020) (scoring 3 for NSC) performed a repeated‐dose 28‐day and a repeated‐dose 90‐day study in rats. TiO2 NPs (21 nm) in sodium phosphate buffer were administered by gavage at 0, 250, 500 and 1,000 mg/kg bw per day. No statistically significant treatment‐related differences with respect to body weight gain, food and water intake were observed. No mortality or clinical signs were detected during the exposure period of 28 and 90 days. No effects were detected in a functional observation battery in the last week of the 90‐day study. Ophthalmoscopic examination and urinalysis did not show statistically significant differences between the groups. Changes – circulating neutrophils and lymphocytes, blood urea nitrogen and blood Na – occurred without a clear dose response. No abnormal gross findings were found at necropsy in treated animals. Changes in some organ weights were considered unrelated to the treatment. On histopathological examination, differences between the control group and the 1,000 mg/kg bw per day group were found. The Panel considered that the reported changes were within the historical control normal range and therefore of no toxicological significance.
Concluding remarks
In mice, no adverse effects were observed up to 1,000 mg E 171/kg bw per day, the highest dose tested, in a 90‐day study (Han et al., 2020a; scoring 2 for NSC). In rats, toxicity studies with TiO2 NPs or TiO2 containing a fraction of nanoparticles, having different duration (14–90 days), no adverse effects were observed up to the highest dose tested (100 mg/kg bw per day, Vasantharaja et al. (2015) and scored 2 for NSC). Overall, no adverse effects associated with general toxicity were observed in rats orally exposed to E 171, TiO2 NPs or TiO2 containing nanoparticles.
In mice orally exposed to TiO2 NPs < 30 nm for up to 90 days, some effects were reported, which by their nature could be adverse. However, mild hyperbilirubinaemia was not accompanied by any changes in liver enzymes (Yang et al., 2017); the effect size of increased fasting glycaemia and impaired glucose tolerance (Hu et al., 2015) was not of toxicological relevance and not accompanied by changes in insulin or other changes in lipid metabolism and therefore was not of toxicological relevance. Histopathological changes were reported in the heart (Yu et al., 2016); however, these findings were not supported by incidences and severity scores. Histopathological findings indicating inflammation were reported in the liver, but investigations to confirm hepatic injury were not performed (Hong et al., 2016).
In rats orally exposed to TiO2 NPs < 30 nm, inconsistent and/or unexplained sex differences in some parameters were reported (e.g. hypobilirubinaemia in females (Chen et al. (2015a); heart rate and blood pressure changes in females (Chen et al. (2015b); leucocyte changes in females (Heo et al., 2020); higher absolute pituitary weights in males (Heo et al., 2020); lower blood insulin levels in females, lower C‐peptide levels in males and differences in blood concentrations compared to controls in a glucose tolerance test in males (Chen et al., 2020b). The Panel considered that the TG changes reported in several studies were likely incidental study findings since the reductions were seen in only one sex and without a clear dose response (Chen et al., 2015bb), lacked a clear dose response (Vasantharaja et al., 2015) or increased in a single dose study (Grissa et al., 2015)
The Panel considered that the effects reported in mouse studies with TiO2 NPs < 30 nm could be associated with accumulation of NPs in various tissues whereas inconsistent findings in rats were considered incidental.
Reproductive and developmental toxicity studies
E 171
No studies performed with E 171 and considered with reliability 1 and 2 have been identified in the literature search.
TiO 2 /TiO 2 NPs
Mice
No studies available.
Rats
From an oral prenatal developmental toxicity study in rats with five different TiO2 materials, TiO2 NPs or TiO2 containing a fraction of nanoparticles (Warheit et al., 2015b) (scoring 4 for NSC), no maternal and developmental effects were observed up to 1,000 mg TiO2 NPs/kg bw per day (the highest dose tested), when administered from gestation days (GDs) 6 to 15.
TiO 2 NPs < 30 nm
Mice
Karimipour et al. (2018) (scoring 2 for NSC) examined the effects of oral administration of TiO2 NPs (10–25 nm) at 100 mg/kg bw per day for 5 weeks on the histology of ovaries, oestrogen and malondialdehyde (MDA) serum levels (7 animals/group), fertility (10 animals/group) and IVF rates (10 animals/group) in female mice. Impairment of female fertility at the only dose tested was observed.
Khorsandi et al. (2016) (scoring 2 for NSC) examined the effects of oral administration of TiO2 NPs on testicular parameters in young adult male NMRI mice at doses of 0, 75, 100 and 300 mg/kg bw per day (8 animals/group) for 35 days. Dose‐dependent decreases in testis weight occurred from a dose of 100 mg/kg bw per day. At higher doses, additional testicular parameters were affected. The Panel considered that TiO2 NPs (size unknown) from 100 mg/kg bw per day had an effect on testis weight.
Khorsandi et al. (2017) (scoring 2 for NSC) administered TiO2 NPs (20–30 nm) by oral gavage at 300 mg/kg bw per day to eight young adult male NMRI mice for 35 days. The authors reported significant decreases in testis weight, circulating and testicular testosterone, testicular catalase (CAT) and superoxide dismutase (SOD) concentrations, sperm counts and sperm motility. Significant increases were found in the percentage of abnormal or degenerative spermatogenic tubules, germ cell apoptosis, testicular MDA concentration and in the percentage of sperm with abnormal morphology. The Panel considered that testicular toxicity was observed with TiO2 NPs (20–30 nm) at 300 mg/kg bw per day, the only dose tested.
Karimi et al. (2019) (scoring 2 for NSC) treated eight 6‐ to 8‐week‐old male NMRI mice daily by gavage with 50 mg TiO2 NPs (< 30 nm)/kg bw per day for 35 days. TiO2 NPs significantly reduced testis weight accompanied by reduced serum testosterone, reduced seminiferous tubule diameter and epithelium height and reduced the maturity of the germinal epithelium. Also, adverse findings were observed in reduced sperm counts, increased sperm abnormalities and reduced sperm motility. The Panel noted that 50 mg TiO2 NPs/kg bw per day, the only dose tested, resulted in adverse effects on the testis.
Lu et al. (2020) (scoring 4 for NSC) treated four groups of 15 male ICR mice, age 6–8 weeks daily by gavage with TiO2 NPs (7 nm) at doses of 0, 10, 50 or 100 mg/kg bw per day for 30 days. The authors report tight junction damage in the blood–testis barrier (BTB) at 50 and 100 mg/kg bw, though the histopathological pictures provided are hard to interpret. Serum testosterone was 50% decreased at the two highest doses tested. Sperm motility was dose‐relatedly reduced, accompanied by increased sperm malformation rates. The Panel considered that TiO2 NPs (7 nm), at 50 or 100 mg/kg bw per day, resulted in a dose‐related reduction of sperm motility and increased sperm malformations, accompanied by histological observations in the testis, changes in BTB‐related protein levels, changes in MAPK‐related mRNA levels and reduced circulating testosterone concentrations.
Rats
Lee et al. (2019) (scoring 3 for NSC) treated mated female Sprague–Dawley rats (12 females per group) with TiO2 NPs (21 nm) daily by gavage at dose levels of 0, 100, 300 and 1,000 mg/kg bw per day from GDs 6 to 19. There were no statistically significant differences in general clinical signs, body weight, organ weights (absolute and relative to body weight), macroscopic findings. Caesarean section parameters and fetal external and visceral examinations did not reveal any statistically significant differences. The Panel considered that no adverse maternal and developmental effects were reported with TiO2 NPs (21 nm) up to 1,000 mg/kg bw per day, the highest dose tested.
Concluding remarks
No reproductive or developmental toxicity studies performed with E 171 and considered sufficiently reliable with respect to their internal validity (see Appendix C) have been identified from the published literature.
No maternal and developmental effects were observed up to 1,000 mg/kg bw per day, the highest dose tested, in a single rat developmental toxicity study with five different TiO2 materials, TiO2 NPs or TiO2 containing a fraction of nanoparticles (Warheit et al., 2015a) (scoring 4 for NSC).
In mice, the effects of TiO2 NPs < 30 nm on the testis (decreased weight, decreased seminiferous tubule diameter, germ cell apoptosis) and sperm (decreased sperm counts and motility, increased percentage of abnormal spermatozoa) were observed in three studies (Khorsandi et al., 2016, 2017; Karimi et al., 2019) at doses ranging from 50 to 300 TiO2 NPs/kg bw per day. The lowest dose at which the effects were observed was 50 mg TiO2 NPs/kg bw per day (Karimi et al., 2019). In a mouse study by Lu et al. (2020), no effects were observed at the lowest dose tested, 10 mg/kg bw per day (scoring 4 for NSC). In rats, administration of TiO2 NPs (21 nm) did not show effects at any dose level in a developmental toxicity study up to 1,000 mg/kg bw per day (Lee et al., 2019, scoring 3 for NSC)).
Neurotoxicity and neurodevelopmental toxicity studies
E 171
No studies performed with E 171 and considered with reliability 1 and 2 have been identified.
TiO 2 NPs
Pregnant Wistar rats (n = 6/group) were administrated TiO2 NPs (< 100 nm) by gavage at 0 or 100 mg/kg bw per day from GD 2 to 21 (gestation group) or from postnatal days (PND) 2 to 21 (lactation group). In offspring (PND 1 in gestation group, PND 22 in lactation group), increased hippocampal apoptosis and reduced hippocampal neurogenesis after both gestational and lactational exposure (Ebrahimzadeh et al., 2017; scoring 3 for NSC) were observed.
Kandeil et al. (2019) (scoring 3 for NSC) dosed adult male albino rats (n = 20/group) by gavage with TiO2 NPs (90 nm, range 40–140 nm) at 0 or 500 mg/kg bw per day for 14 days. Adverse effects in CNS possibly related to oxidative stress were observed at the single dose tested of 500 mg/kg bw per day.
The Panel concluded that these data show that oral TiO2 NPs administered to rats during embryofetal and early postnatal development reduced hippocampal neurogenesis at 100 mg/kg bw per day, and that oral administration to adult rats produced adverse effects in the brain consistent with oxidative stress at 500 mg/kg bw per day.
TiO 2 NPs < 30 nm
A number of studies have reported adverse effects on both adult and developing mouse and rat brain after oral dosing with TiO2 NPs < 30 nm.
Adult mice
The most sensitive endpoint observed in adult mice was reduced volume of the hippocampus, and the polymorph layer of the dentate gyrus, and reduced density and total number of dentate gyrus granular cells at all doses tested (in males dosed for 35 days) (Rahnama et al., 2020; scoring 4 for NSC). The mice in this study (n = 20/group) were dosed by gavage with TiO2 NPs (21 nm) at 0, 2.5, 5 or 10 mg/kg bw per day.
Other studies using adult mice and a single dose of TiO2 NPs < 30 nm also reported adverse effects on the brain after oral dosing.
Zhang et al. (2020) (scoring 3 for NSC) dosed young adult male mice (probably n = 15/group) by oral gavage with TiO2 NPs (21 nm) at 0 or 150 mg/kg bw per day for 30 days. Treatment had no effect on body weight or histopathology of gut or brain, but significantly decreased the richness and evenness of gut microbiota, elevated gut HuC/D and TuJ1 and markedly reduced serotonergic markers Sstr1 and Sstr2 in gut but not in cerebral cortex, suggesting an effect on the enteric nervous system. However, gut–brain peptides secreted by endocrine cells and enteric neurons, and also inflammatory cytokines, were not affected by treatment. In the open field test, centre field activity was statistically significantly reduced by the treatment, consistent with anxiety‐like behaviour, but MWM learning and spatial memory were unaffected. The Panel considered that TiO2 NPs (21 nm) at 150 mg/kg bw per day, the only dose tested, altered gut microbiota, without pathological changes in small intestine and brain.
The Panel noted that the most sensitive endpoint in adult mice was reduced volume of hippocampus and dentate gyrus granular layer, and density and number of dentate gyrus granular cells observed with TiO2 NPs (21 nm) at 2.5 mg/kg bw per day, the lowest dose tested, in males dosed for 35 days (Rahnama et al., 2020).
Adult rats
In adult rats, the most sensitive endpoint was reduced brain cholinesterase activity (about 35–50%) and increased brain Na, K‐ATPase activity (about 2‐fold), observed with TiO2 NPs (21 nm) at all doses tested, in female albino rats dosed for 14 days, as reported by Canli et al. (2020) (scoring 4 for NSC). In this study, rats (n = 6/group) were dosed by gavage with TiO2 NPs (21 nm) at 0, 0.5, 5 or 50 mg/kg bw per day.
Grissa et al. (2016) (scoring 3 for NSC) reported reduced brain cholinesterase activity at 100 and 200 mg/kg bw per day, and reduced plasma cholinesterase activity at all doses tested, in male rats dosed for 60 days. In this study, rats were dosed by gavage with TiO2 NPs (5–10 nm) at 0, 50, 100 or 200 mg/kg bw per day. Brain cholinesterase activity was not affected at 50 mg/kg bw per day, in contrast to the significant reduction in brain cholinesterase activity at 0.5 mg/kg bw per day reported by Canli et al. (2020). This apparent 200‐fold difference in potency adds to uncertainty; possible contributory factors include differences in test substance dispersion and internal exposure between the two studies. The Panel noted that the reduced brain cholinesterase activity reported by Grissa et al. (2016) was not dose related (similar effect at 100 and 200 mg/kg bw per day doses, about 50% reduction), but plasma cholinesterase activity was dose‐dependently reduced at all doses (about 35% at 50 mg/kg bw per day, 50% at 100 and 200 mg/kg bw per day). The Panel noted that the method used to determine cholinesterase activity by Grissa et al. (2016) probably measures both acetylcholinesterase (AChE) and butyryl ChE (BChE) activity. In any case, TiO2 NPs reduced brain cholinesterase activity.
Grissa et al. (2020) (scoring 2 for NSC), using a similar study design (strain age and dosing regimen) as Grissa et al. (2016) and with TiO2 NPs (5–12 nm), reported reduced SOD and CAT, and increased NO and tumour necrosis factor‐alpha (TNF‐α) in brain frontal cortex at all doses tested (50, 100 and 200 mg/kg bw per day).
Other studies using adult rats and a single dose of TiO2 NPs also reported adverse effects on the brain after oral dosing.
Hassanein and El‐Amir (2017) (scoring 3 for NSC) dosed adult male Sprague–Dawley rats (n = 10/group) by gavage with TiO2 NPs (21 nm) at 0 or 150 mg/kg bw per day for 6 weeks. Increased total leucocyte, lymphocyte and neutrophil counts, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lipid peroxidation (LPO), TNF‐α and liver DNA damage by Comet assay, and decreased glutathione (GSH), and histopathological alterations in liver, brain, lung, kidney, heart and testes were reported at the only dose tested. However, with respect to neurotoxicity, the Panel noted that the tissue fixation method was not optimal for histology of brain tissue (non‐perfused formaldehyde fixation), therefore the Panel considered that artefactual brain findings could not be excluded.
The Panel noted that the most sensitive endpoint in adult rats was reduced (dose related) brain cholinesterase activity and increased brain Na/K‐ATPase activity, observed at 0.5 mg/kg bw per day (in females dosed for 14 days), the lowest of three doses tested, reported by Canli et al. (2020) with TiO2 NPs (21 nm). However, Grissa et al. (2016) reported reduced brain cholinesterase activity at 100 but not 50 mg/kg bw per day (in males dosed for 60 days with TiO2 NPs (5–10 nm)). This apparent 200‐fold difference in potency adds to uncertainty.
Mice developmental
In pre‐ and perinatal mice, the study by Zhou et al. (2017) (scoring 2 for NSC) reported inhibited dendritic outgrowth, increased autophagy and oxidative stress and reduced mitochondrial function, in ex vivo hippocampal CA1 neurons of the offspring of CD‐1 with TiO2 NPs (6–7 nm) at all doses tested. In this study, mice (n = 6/group) were dosed by gavage with TiO2 NPs (6–7 nm) at 0, 1, 2 or 3 mg/kg bw per day from GD 7 to PND 21.
The Panel noted that the only available study on developing mice (Zhou et al., 2017) reported inhibited dendritic outgrowth, increased autophagy and oxidative stress and reduced mitochondrial function, in ex vivo hippocampal neurons of the offspring with TiO2 NPs (6 = 7 nm) at all doses tested (1, 2 or 3 mg/kg bw per day).
Rats developmental
In pre‐ and perinatal rats, offspring passive avoidance behaviour was altered after maternal dosing during lactation with TiO2 NPs (10 nm) at 100 mg/kg bw per day (the only dose tested) (Mohammadipour et al., 2016) (scoring 3 for NSC). The same dose level increased offspring hippocampal apoptosis and reduced offspring hippocampal neurogenesis after maternal dosing during both gestation and lactation (Ebrahimzadeh et al., 2017; scoring 3 for NSC).
The Panel concluded that gestational and/or lactational maternal rat exposure to TiO2 NPs (10 nm) at 100 mg/kg bw per day altered passive avoidance behaviour, increased hippocampal apoptosis and reduced hippocampal neurogenesis in the offspring.
The Panel noted that the effects on brain structure and function reported in Mohammadipour et al. (2016) and Ebrahimzadeh et al. (2017) are mutually plausible, given that passive avoidance behaviour is related to hippocampal functioning (Eagle et al., 2016; Anacker et al., 2018).
Concluding remarks
No studies performed with E 171 and considered sufficiently reliable with respect to their internal validity (see Appendix C) have been identified from the published literature.
Oral TiO2 NPs dosed in rats during embryofetal and early postnatal development reduced hippocampal neurogenesis at 100 mg/kg bw per day exposure (Ebrahimzadeh et al., 2017), and dosed in adult rats produced adverse effects in the brain consistent with oxidative stress at 500 mg/kg bw per day (Kandeil et al., 2019). Both studies scored 3 for NSC.
After oral dosing with TiO2 NPs < 30 nm, adverse effects in both adult and developing mouse and rat brain were reported. Most of these effects are possibly related to oxidative stress. In mice, the Panel noted that the reduced volume of the polymorph layer of the hippocampal dentate gyrus and reduced density and number of dentate gyrus granular neurons reported by Rahnama et al. (2020) with TiO2 NPs (21 nm), is consistent with the behavioural effects reported by Zhang et al. (2020) also with TiO2 NPs (21 nm), i.e. increased open field anxiety‐like behaviour and unaffected spatial learning and memory. Ventral dentate gyrus is associated with anxiety behaviour, CA regions with spatial learning/memory (Eagle et al., 2016; Anacker et al., 2018). In adults rats, the most sensitive endpoint in the evaluated studies was reduced brain cholinesterase activity and increased brain Na/K‐ATPase activity with TiO2 NPs (21 nm) at a dose of 0.5 mg/kg bw per day in females dosed for 14 days (Canli et al., 2020).
The Panel noted that inhibition of cholinesterase activity by nanoparticles other than TiO2, both metal and plastic, has been reported in a number of species (Prüst et al., 2020). Since oxidative stress‐related inflammation is generally associated with increased and not decreased cholinesterase activity (Corrêa Mde et al., 2008; Vaknine and Soreq, 2020), it is unclear whether there is a link between TiO2‐induced oxidative stress and TiO2‐induced decrease in cholinesterase activity.
Overall for neurotoxicity, adverse effects were seen with TiO2 NPs < 30 nm. In mice, Zhou et al. (2017; scoring 3 for NSC), reported adverse effects (i.e. inhibited dendritic outgrowth, increased autophagy and oxidative stress and reduced mitochondrial function) in ex vivo hippocampal neurons of weanling mice after dosing TiO2 NPs (6–7 nm) during gestation and early lactation at a dose of 1 mg/kg bw per day, the lowest dose tested. In adult female rats (Canli et al., 2020; scoring 3 for NSC), adverse effects (reduced brain cholinesterase, and increased brain Na/K‐ATPase activity) were observed with TiO2 NPs (21 nm) at 0.5 mg/kg bw per day, the lowest of three doses tested, in a 14‐day study.
Inflammation and immunotoxicity studies
E 171
Mice
No adverse effects in the mouse study (Riedle et al., 2020a; scoring 1 for NSC) were observed up to the highest dose tested (100 mg/kg per day). Pinget et al. (2019), scoring 2 for NSC, found a reduction of colonic crypt length, in addition to an increase in colon macrophages and CD8 cells in IL‐10, TNF‐α and IL‐6 mRNA at doses of 10 and 50 mg/kg bw per day. Other studies in mice only included one dose where increased inflammatory parameters were observed at 5 mg/kg bw per day (Urrutia‐Ortega et al., 2016; scoring 1 for NSC) or 2 mg/kg bw per day (Talamini et al., 2019; scoring 1 for NSC). In Urrutia‐Ortega et al. (2016) study, it was also observed that 5 mg/kg bw per day alone had no effect on tumour formation but could potentiate intestinal tumour formation in mice exposed to azoxymethane/dextran sulfate sodium.
Rats
In Han et al. (2020a) study (scoring 2 for NSC), rats were exposed to E 171 at 10, 100 or 1,000 mg/kg bw per day per 90‐day, a statistically significantly decreased granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) plasma level of approximately 40% was observed at the highest dose. Whereas it is difficult to predict an adverse effect from such an intermediate endpoint, GM‐CSF is involved in haemopoiesis which may explain the modest but statistically significant decrease in immunoglobulin (Ig) M level (~ 10%).
In Bettini et al. (2017) study (scoring 1 for NSC), only one dose of E 171 was tested (10 mg/kg bw per day) and increased inflammatory parameters were observed. These results were not confirmed in another study (Blevins et al., 2019; scoring 3 for NSC), as no effects were observed up to 267 mg E 171/kg bw per day, the highest dose tested. However, the Panel noted that Blevins et al. (2019) study was scored 3 for NSC.
The Panel noted that the reported effects of E 171 on the immune system were variable. Certain studies with E 171 did not find an effect (Blevins et al., 2019; Riedle et al., 2020a), while others did, showing especially changes in parameters indicating inflammatory processes (Urrutia‐Ortega et al., 2016; Talamini et al., 2019).
TiO 2 orTiO 2 NPs
Two mouse studies performed with TiO2 NPs (Mohamed, 2015; Li et al., 2019) were scored 2 for NSC and a rat study (Hashem et al., 2020) was scored 3.
Mice
Both mice studies were short‐term studies, 5 days (Mohamed, 2015) and 7 days (Li et al., 2019). In Mohamed (2015), TiO2 NPs (47 nm) at doses of 5, 50 or 500 mg/kg bw per day were administered and this resulted in an inflammatory response in the stomach, which was already evident at the lowest dose tested, i.e. 5 mg/kg per day. In Li et al. (2019) study, no relevant effects were noted on the histology of the spleen with TiO2 NPs (25, 50 or 80 nm) at 1 mg/kg bw per day, the only dose tested.
Rats
In rats exposed to TiO2 at 20 or 40 mg/kg bw per day for 90 days, changes in haematological and immunological parameters were noted at the lowest dose tested, i.e. 20 mg/kg per day (Hashem et al., 2020).
TiO 2 NPs < 30 nm
Mice
In a 90‐day study (Yu et al., 2016) (scoring 2 for NSC), animals were exposed to TiO2 NPs (5–6 nm) at 2.5, 5 or 10 mg/kg bw per day. The Panel noted inflammatory mediators at all doses tested and corroborated by histopathological lesions.
In Li et al. (2018) (scoring 4 for NSC), a single dose of TiO2 NPs (20 nm) at 100 mg/kg bw per day for 28 days was tested. Zhang et al. (2020) (scoring 4 for NSC) administered TiO2 NPs (21 nm) at 150 mg/kg bw per day for 30 days. Both studies were focused on the effects of TiO2 NPs on the microbiota. No change in spleen histology or in local or systemic inflammatory parameters was observed by Li et al. (2018).
Rats
In a rat study (Chen et al., 2015a) (scoring 2 for NSC) animals were exposed to TiO2 NPs (24 nm) at 2, 10 or 50 mg/kg bw per day. An increase in leucocytes was observed which may suggest an inflammatory response at the highest dose tested. In another study in rats by the same group (Chen et al., 2019), scoring for NSC 2, histopathologically, reduced numbers of goblet cells were found as a result of exposure to 50 mg/kg bw per day, as well as inflammatory infiltration, while in serum increased IL‐6 expression was observed.
In Grissa et al. (2020) study (scoring 2 for NSC), rats were exposed to TiO2 NPs (5–12 nm) at 50, 100 or 200 mg/kg bw per day. The Panel noted changes in inflammatory markers at 100 and 200 mg/kg bw per day.
Concluding remarks
The Panel concludes that these studies indicate immune dysregulatory activity of E 171, evidenced by several immune‐related and inflammatory markers. These effects were not observed up 50 mg E 171/kg bw per day. In three single dose level studies with E 171, effects were noted at lower doses, i.e. 2, 5 and 10 mg/kg bw per day.
Effects of E 171 may, at least in part, stem from the activity of the fraction of the smaller TiO2 particles, as studies with these particles also indicate inflammatory effects of exposure to TiO2 NPs (5–6 nm) at 2.5 mg/kg per day.
Aberrant crypt foci
This endpoint was specifically requested to be investigated in the EOGRT study (see Section 4.2.6) as part of the follow‐up to the re‐evaluation of E 171 (EFSA ANS Panel, 2018). Therefore, studies reporting information on aberrant crypt foci (ACF) were collected from the literature search.
In total, there were two studies investigating this endpoint (Bettini et al., 2017; Blevins et al., 2019).
The study by Bettini et al. (2017) (scoring 1 for NSC) was previously reviewed by the ANS Panel (EFSA ANS Panel, 2018) and it has also been evaluated in the current assessment (Appendix H – immunotoxicity). The Panel considered that E 171 per se at a dose of 10 mg/kg bw per day may induce development of ACF in male rats. The Panel also noted that E 171 at a dose of 10 mg/kg bw per day increased the number of ACF initiated by a genotoxic carcinogen.
From the Blevins et al. (2019) (scoring 3 for NSC), the Panel noted that no changes in the number of ACF and aberrant crypts (ABC) were observed due to E 171 exposure alone, while the number of ACF increased not statistically significantly in the colon samples from animals exposed to E 171 and initiated with a carcinogen (dimethylhydrazine (DMH)) relative to a group exposed to the carcinogen alone. However, limitations in the pathological examination of ABC and ACF (sampled colon area limited; technical issues related to fixation) preclude a conclusion on potential for ABC and ACF formation. Dietary E 171, with or without treatment with DMH, had no effect on the length of the colonic glands examined or the number of goblet cells/unit.
The Panel considered that E 171 may induce ACF in male rats at a dose of 10 mg/kg bw per day.
Gut microbiota
A number of studies, considered to be reliable by the Panel, have examined or included analyses of GIT microbiota changes in response to oral exposure to E 171 (Pinget et al., 2019), TiO2 NPs (Li et al., 2019; Yan et al., 2020) and TiO2 NPs < 30 nm (Li et al., 2018; Chen et al., 2019, 2020a; Zhang et al., 2020; Zhao et al., 2020). Detailed description of references Li et al. (2018), Pinget et al. (2019), Chen et al. (2019), Li et al. (2019), Zhang et al. (2020) and Chen et al. (2020a), investigating also other relevant endpoints, are available in Appendix H.
E 171
Mice
Mice were administered E 171 via drinking water at doses of 0, 2, 10 and 50 mg E 171/kg bw per day for 3 weeks (Pinget et al., 2019) (scoring 2 for NSC) followed by examination of microbiota populations in faecal samples or the small intestine through 16S rRNA sequencing. Exposure to E 171 had minimal impact on their compositions but altered the release of bacterial metabolites.
Rats
No studies have been identified.
TiO 2 or TiO 2 NPs
Mice
In a 7‐day study, mice were administered by gavage with three types of TiO2 NPs (25 nm, 50 nm and 80 nm) at a dose of 1 mg/kg bw per day (Li et al., 2019; scoring 2 for NSC). The microbiota of the distal colonic content was analysed. According to the authors, observed effects in the colon (epithelial injury, suppressed expression levels of tight junction proteins and reduced thickness of the luminal mucus layer) were associated with altered gut microbiota composition.
In a 28‐day study, mice were administered via gavage with TiO2 (250 nm) or TiO2 NPs (25 nm) at doses of 10, 40 and 160 mg/kg bw per day (Yan et al., 2020; scoring 2 for NSC). Colon tissues were isolated under aseptic conditions and contents were excised for 16S rRNA analysis. The results showed that exposure to TiO2 or to TiO2 NPs led to changes in the composition of the gut microbiota, especially the microbiota associated with mucus.
TiO 2 NPs < 30 nm
Mice
Li et al. (2018) (scoring 4 for NSC) performed an analysis of gut microbiota through sampling of faeces and performing 16S rRNA sequencing. Animals were administered via gavage with two types of TiO2 NPs (20 nm anatase with near spherical shape and 15 nm rutile with edged with corners morphology) at a dose of 0 or 100 mg/kg bw per day for 28 days. The results showed that gut microbiota diversity was not affected by the treatments but it shifted their composition in a time‐dependent manner, with rutile NPs having a more pronounced influence than anatase NPs.
In Zhang et al. (2020) (scoring 4 for NSC), animals were administered by gavage with TiO2 NPs (21 nm) at a dose of 0 or 150 mg/kg bw per day for 30 days. According to the authors, the results showed that oral exposure to TiO2 NPs decreased the ‘richness and evenness’ of gut microbiota.
Rats
Rats were administered via gavage with TiO2 NPs (29 nm) at doses of 0, 2, 10, 50 mg/kg bw per day for 30 days (Chen et al., 2019) (scoring 2 for NSC). Changes in the gut microbiota and gut‐associated metabolism were analysed through 16S rRNA sequencing and faeces metabolomics, respectively. The results showed that exposure to TiO2 NPs led to structural and compositional changes in the gut microbiota over time and to alteration of metabolites in faeces.
In a 90‐day study (Chen et al., 2020a) (scoring 2 for NSC), animals were administered by gavage with TiO2 NPs (29 nm) at doses of 0, 2, 10 or 50 mg/kg bw per day. SCFAs in rat stool samples were analysed by targeted metabolomics. The results showed no change in the SCFAs content after exposure to TiO2 NPs.
In Zhao et al. (2020) (scoring 1 for NSC), weaning rats were administered via gavage with TiO2 NPs (25 nm) at doses of 0, 1, 50 and 100 mg/kg bw per day for 14 days. Faeces samples were collected and were used for microbiota analysis. The results indicated that the gut microbiota composition was altered by TiO2 NPs treatment.
Concluding remarks
The Panel noted that changes in GIT microbiota in response to exposure to other substances used as food additives were observed (Swidsinski et al., 2009a,b; Renz et al., 2012; Merga et al., 2014; Cani and Everard, 2015; Chassaing et al., 2015, 2017; Romano‐Keeler and Weitkamp, 2015; Lecomte et al., 2016; Nejrup et al., 2017; Holder and Chassaing, 2018; Jiang et al., 2018; Viennois and Chassaing, 2018; Marion‐Letellier et al., 2019). However, there is currently no consensus on when changes in GIT microbiota should be considered adverse.16 Accordingly, the Panel was unable to come to any conclusion regarding the effects of E 171 on GIT microbiota and related effects on health.
4.2. Data submitted to the call for data from the European Commission as follow‐up of the re‐evaluation of E 171
As mentioned in Section 3, an EOGRT study together with complementary reports (Documentation provided to EFSA No 1, 2, 3,4, 5 and 6) and additional clarifications (Documentation provided to EFSA No 11–13, 16–18) were submitted to EFSA.
The tested material corresponds to a commercial brand of E 171 for which data were submitted during the assessment of the amendment of the EU specifications for E 171 (sample E) (EFSA FAF Panel, 2019). Physico‐chemical characterisation by two other laboratories (Documentation provided to EFSA No 11; Verleysen et al., 2020) confirmed that the test substance, after applying sample dispersion protocols, consists almost exclusively of near‐spherical constituent particles with a median diameter in the order of 100 nm that are often agglomerated (i.e. 50% of the individual particles are at the nanoscale). The crystalline form of this E 171 is anatase.
E 171 was administered via the diet.17 The concentration was adjusted weekly using the food consumption values from the previous week to maintain a constant dose level in relation to the animals’ body weights. The actual E 171 intake for each test week was reported in mg kg body weight per day and calculated from the relative food consumption per day and the nominal E 171 concentration that was used in that test week. The titanium levels in the control and test diets were determined by inductively coupled plasma optical emission spectroscopy (ICP‐OES). The background level of titanium in the diet was 17 mg Ti/kg diet (mean value) and ranged 11–31 mg Ti/kg diet.
The EOGRT study was performed in male and female rats according to OECD TG 443 and good laboratory practice (GLP) compliance. In the F0 generation, E 171 was administered in the diet at doses of 0, 100, 300 or 1,000 mg/kg bw per day from 10 weeks prior to mating until weaning of the F1 generation. The F1 generation received these diets from weaning until PND 4 or 8 of the F2 generation. The F2 generation was exposed through the milk until the termination of the study on PND 4 or PND 8. Duration of dosing depended on the endpoints under evaluation in the different cohorts, with the longest duration of treatment up to 18 weeks. Endpoints included were consistent with those specified in OECD TG 443 guidance in order to ensure the investigation of reproductive and developmental toxicity of E 171 (except for balanopreputial separation, as noted below in the section ‘Growth and sexual development’). Table 2 describes parameters considered in the EOGRT study with the corresponding generation/cohort/number of animals for each.
Table 2.
Parameters considered in the EOGRT study
| Generation | F0 | F0 satellite | F1 pups | F1 1A | F1 1B | F1 2A | F1 2B | F1 3 | F2 |
|---|---|---|---|---|---|---|---|---|---|
| Number of animals/sex per group | 20 | 30 | 20 | 20 | 10 | 10 | 10 | ||
| Mortality | X | X | X | X | X | X | |||
| Clinical signs | X | X | X | X | X | ||||
| Body weight | X | X | X | X | X | X | X | ||
| Food consumption | X | X | X | X | X | ||||
| Water consumption | X | X | |||||||
| Haematology | X | X | |||||||
| Clinical biochemistry | X | X | |||||||
| Lymphocyte typing (spleen) | X | ||||||||
| Urinalysis | X | X | |||||||
| Sexual hormone levels | X | X | X | X | X | ||||
| Thyroid hormone levels | X | X | X | ||||||
| Sexual maturation | X | X | X | X | |||||
| Oestrous cycle data | X | ||||||||
| Sperm parameters | X | X | |||||||
| Necropsy | X | X | X | X | X | X | X | ||
| Histopathology | X | X | X | X | |||||
| Reproductive parameters | X | X | X | ||||||
| Pre‐postnatal development | X | X | X | ||||||
| Functional neurotoxicity observations | X | ||||||||
| Neurohistopathology | X | X | |||||||
| Lymphocyte typing (spleen) after KLH immunisation | X | X | |||||||
| Anti‐KLH IgM levels after KLH immunisation | X | ||||||||
| Aberrant crypt foci (ACF) scoring | X |
EOGRT: extended one‐generation reproductive toxicity; KLH: keyhole limpet haemocyanin; IgM: immunoglobulin M.
The scoring for NSC (dispersion and/or confirmation of internal exposure), according to Appendix E (high doses only, with no considerations on dispersion, and insufficient confirmation of exposure to the fraction of small particles) was 4.
4.2.1. Assessment of internal exposure
The Panel noted that the basal diet contained an amount of Ti equivalent to approx. 1.4 mg TiO2/kg bw per day and that there were variable measurable levels of Ti in blood and urine from control animals. The Panel considered that there were small dose‐related increases in Ti concentrations in blood and urine in E 171‐treated animals. In particular, the Ti blood concentration in cohort F2 at PND 4–7 was increased after correction for background. This is consistent with exposure via placenta and possibly milk, and thus, the dams must have taken up TiO2 as well. Data on Ti concentration in urine in parental animals may be not accurate due to possible contact with Ti‐containing faeces. The Panel considered that at least a small fraction of the Ti in the mothers’ diet was absorbed. Further information is available on Appendix I.
4.2.2. Clinical observation, body weight and food consumption
In the F0 parental animals, no premature death or changes in general behaviour or external appearance were noted in any treatment group of either sex. Pale faeces were noted at 300 and 1,000 mg E 171/kg bw per day in both males and females. No difference in food consumption, body weight or body weight gain between control and treatment groups was observed in males and females during pre‐mating, gestation and lactation. A slight but statistically significant increase in water consumption was recorded in females at 100 mg E 171/kg bw per day. Considering the lack of dose–response relationship, the temporary nature and the small magnitude, this effect was considered incidental and not‐treatment‐related.
In the F1 generation cohort 1A animals, no premature deaths or changes in general behaviour or external appearance were noted in any treatment group of either sex. Pale faeces were noted at 1,000 mg E 171/kg bw per day in both males and females. A slight decrease in body weight was noted in males at 300 mg E 171/kg bw per day, reaching statistical significance on test days 36 and 64. Body weight gain was not affected at any other dose. Considering the lack of a dose–response relationship, its temporary nature and small magnitude, the decrease in body weight was considered incidental and not treatment‐related. No other effects on body weight, body weight gain and food consumption were observed in males and females at any dose.
In the F1 generation cohort 1B animals, no premature deaths or changes in general behaviour or external appearance were noted in any treatment group of either sex. Pale faeces were noted at 1,000 mg E 171/kg bw per day in both males and females. A slight but statistically significant decrease in body weight was noted in males at 300 mg E 171/kg bw per day, on five non‐consecutive testing days. Body weight gain was not affected at any dose. Considering the lack of dose–response relationship, the temporary nature and the small magnitude, this effect was considered incidental and not treatment‐related. No other effects on body weight, body weight gain, food and water consumption were observed in males and females at any dose.
In the F1 generation cohorts 2A and 2B animals, no premature deaths or changes in general behaviour or external appearance were noted in any treatment group of either sex. Pale faeces were noted at 1,000 mg E 171/kg bw per day in both males and females. No effects on body weight or body weight gain were noted. Transient statistically significant but inconsistent changes in food consumption were noted in both sexes at all doses, but considering the lack of dose–response relationship, their temporary nature and small magnitude, these effects were considered incidental and not treatment‐related.
4.2.3. Clinical pathology
Haematology, biochemical analysis and urinalysis were performed on F0 and F1 cohort 1A generations.
Haematology – F0 generation
The only statistically significant haematological changes observed were in low‐dose (100 mg/kg bw per day) males for haemoglobin concentration and for the haematocrit value. These differences were marginal and not dose dependent. The study authors, therefore, concluded that there were no test substance‐related differences for the examined haematological parameters. The Panel agreed with this conclusion.
Haematology – F1 generation cohort 1 A
The only change observed was the absolute differential count of neutrophilic granulocytes, which was lower in all treated males compared to the control group; the difference attained statistical significance for low and high doses. The study authors considered this as an incidental finding, because a dose response relationship was lacking, the values were in the range of the historical control data from the laboratory, no statistically significant difference was recorded for the relative neutrophilic granulocyte count, and no differences for these endpoints were seen in females. The Panel noted that the total white blood cells (WBC) count was slightly but not statistically significantly lower in all treated males but the change was not dose related. Overall, the Panel agreed with the evaluation of the study authors.
Clinical biochemistry – F0
The only changes observed were decreases in serum LDL‐C levels in males, statistically significant only at 100 and 1,000 mg/kg bw per day; serum alkaline phosphatase levels increased in males, statistically significantly at 1,000 mg/kg bw per day and serum chloride levels decreased in females, statistically significantly only at 300 mg/kg bw per day.
Regarding the LDL‐C levels in males, the decreases were considered by the study author to be incidental as no dose–response relationship was evident. Additionally, no decreases were observed in females, in contrast, LDL‐C levels were statistically significantly increased at the low‐ and the high‐dose levels. The Panel agreed with this conclusion.
The moderate increase in alkaline phosphatase was considered by the authors as incidental. The Panel noted that there were no increases in serum AST and LDH and that serum ALT activity was only slightly increased to a level that was of no toxicologically relevance. Overall, the Panel agreed with the conclusion of the study authors.
The slight decrease in chloride level in females was considered by the authors as incidental, as no dose–response relationship was noted. The Panel agreed with this conclusion.
Clinical biochemistry – F1 cohort 1A
An increase in serum sodium/potassium ratio was noted in females at all doses, reaching statistical significance at 300 mg/kg bw per day.
The author considered this increase as incidental since the difference with respect to the control was only small, no dose–response relationship was noted and no changes were noted for the male animals. Furthermore, the observed changes were in the normal range of the historical control data of the laboratory, and no changes were seen in the serum sodium levels. No other test substance‐related changes were observed. The Panel agreed with this conclusion.
Urinalysis – F0
Regarding the examined urinalysis parameters, no test substance‐related differences were noted between the control group and all treatment groups in the F0 generation.
Urinalysis – F1 generation cohort 1A
The only changes observed were a statistically significant increase in pH value at all doses in females and an increase in urinary volume at all doses in females, reaching statistical significance at 1,000 mg/kg bw per day.
The author considered this increase as incidental since no dose–response relationship was noted. In addition, all values were in the range of the historical control data of the laboratory. The Panel agreed with the study authors that there were no treatment‐related effects.
4.2.4. Endocrine function
Thyroid hormones (T4, T3 and TSH)
Thyroid hormones (T4, T3 and thyroid‐stimulating hormone (TSH)) were measured in the F0 generation at necropsy in treatment week 20, in the F1 Pups (at PND 4 and PND 22) and in F1 cohort 1A (at PND 87–92).
In addition, in order to investigate the 24‐h circadian variations in hormone levels, an analysis of the variation in T4, T3 and TSH levels were conducted in satellite animals from the F0 control and high‐dose groups. Blood from the animals (five animals for each time point) was taken at 2‐h intervals pre‐dosing (treatment day 14/15) and after 10 weeks of dosing (treatment day 84/85).
F0 generation
According to the authors, there were no test substance‐related differences in T4, T3 and TSH levels between the control group and the treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F0 animals.
A slight but statistically significant increase in T3 level was noted in females at the high‐dose level (+ 11% compared to control, p ≤ 0.05). There were no other significant differences in T3, T4 and TSH this or any other dose group.
The mean and individual values from the animals in the control and the three treatment groups were within the same range of values observed in a satellite group monitored for circadian variations of hormones over a 24‐h period. The study authors considered the observed change to be incidental and not related to the test substance.
The Panel considered that there were no treatment‐related effects.
Satellite – Circadian cycles of thyroid hormones levels
Thyroid hormones are subject to substantial variation during the day, and overall no test substance‐related effect was noted between the F0 control and high‐dose groups for the examined time point after 10 weeks of treatment.
The Panel agrees with the study authors that there was no treatment‐related effect on the circadian variation of thyroid hormones in the F0 high‐dose group.
F1 Pups
According to the study authors, there were no test substance‐related differences in T4 on PND 4 and T4, T3 and TSH on PND 22) between the control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 pups.
The Panel agrees with the conclusions of the study authors that there were no treatment‐related effects on thyroid hormone levels.
F1 generation cohort 1 A
According to the study authors, there were no test substance‐related differences in T3, T4 and TSH at sacrifice at about PND 90 between the control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 cohort 1A animals.
The study authors reported statistically significantly lower mean T3 levels in low‐dose females (–13.2%) compared to controls. The finding was considered incidental as there was no dose relationship. Moreover, all mean and individual values were within the normal range of values associated with the circadian variation. The Panel agrees with the study authors.
Mean TSH was higher than controls in females in all three treatment groups (+ 58.5%, + 120.0% and + 120.4% for 100, 300 and 1,000 mg/kg bw per day, respectively, compared to control), but the increases did not reach statistical significance. According to the authors, lack of statistical significance was due to the variability of the individual values. Furthermore, no dose–response relationship was observed between the intermediate and the high‐dose groups.
Although a treatment‐related effect on F1 serum TSH levels cannot be excluded, the Panel considered that the observed effect was more likely incidental than a treatment‐related effect. This was because the higher mean serum TSH in treated the F1A (cohort 1A, PND 90) was not statistically significant, was within the normal control range and there were no concordant changes in T3/T4 (T3 was slightly but significantly decreased by 13% at 100 mg/kg only, with no significant changes in T4). In addition, there were no group differences in TSH in the F1 pups at weaning (PND 22).
Sex hormones
Fasting serum oestradiol, oestrone and testosterone levels were measured in parental F0 at sacrifice (study week 20), in F1 cohort 1A (PND 86–96), cohort 1B (PND 120–136), cohort 2A (PND 84–90) and cohort 2B (PND 21–23).
In addition, in order to investigate the 24‐h circadian variations in hormone levels, oestradiol and testosterone levels were examined in satellite F0 animals from the control and the high‐dose groups. Blood was withdrawn at 2‐h intervals for 24 h pre‐dosing (treatment day 14/15) and after 10 weeks of dosing (treatment day 84/85) (n = 5/time point).
F0 generation
According to the authors, there were no test substance‐related differences in serum oestradiol, oestrone and testosterone levels between the control and the treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either males or females.
Mean oestrone levels were higher in F0 males at the mid dose (+ 33.5% compared to control, p ≤ 0.01) and high dose (+47.4% compared to control, not statistically significant).
The study authors considered that these differences were not test substance‐related, because the individual oestrone values were in the same range as controls or only marginally higher for the mid dose (control range: 5.8–13.5 pg/mL vs. mid dose: 8.7–14.0 pg/mL).
The authors further noted a single high oestrone level of 36.4 pg/mL for one animal in the high‐dose group and considered it as incidental.
The Panel agreed with the study authors, that there were no treatment‐related effects on serum oestradiol, oestrone and testosterone levels in F0.
Satellite F0 animals – Circadian cycles of sexual hormone levels
Sex hormones are subject to substantial circadian variation and overall, no test substance‐related effects were noted between the control and high‐dose groups for any of the examined time points after 10 weeks of treatment.
The Panel agreed with the study authors that there were no treatment‐related effects on the circadian range of serum oestradiol and testosterone levels in F0 animals.
F1 generation cohort 1A
The study authors reported no test substance‐related differences in serum oestradiol, oestrone and testosterone levels between control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 cohort 1A animals at sacrifice on about PND 90.
An increase in oestrone levels in males was noted, reaching statistical significance at the high‐dose level (+ 14.4%, + 45.0% and + 51.6% compared to control for 100, 300 and 1,000 mg/kg bw per day, respectively). The study authors considered this finding as incidental because the measured values in the mid‐ and high‐dose groups were in the same range as those in the cohort 2A control and low‐dose groups of animals of comparable age (about PND 90). The finding was therefore attributed to the inherent variability in the oestrone concentration and not related to the test substance.
The Panel agreed with the study authors’ conclusions and additionally noted that the significantly higher mean serum oestrone level seen in 1,000 mg/kg bw per day males of the F1 cohort 1A was an isolated finding, not seen in the cohort 1B; there were no test substance‐related effects on serum oestradiol, oestrone and testosterone in F1 cohort 1A.
F1 generation cohort 1B
According to the authors, there were no test substance‐related differences in serum oestradiol, oestrone and testosterone between F1 cohort 1B control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 cohort 1B at sacrifice on about PND 90.
Mean oestradiol levels were lower than controls in mid‐ and high‐dose males (−18.1% and −14.8%, respectively), statistically significant for the mid‐dose group only. The study authors considered this finding as incidental because in cohort 1B control mean oestradiol was anomalously high; the mean values in cohort 1B mid‐ and high‐dose males were actually higher (not lower) than the normal circadian range in F0, cohort 1A and cohort 2A. The finding was therefore attributed to increased concentrations of oestradiol in the control group of cohort 1B and was therefore considered to be incidental and of no toxicological relevance.
Mean testosterone levels were higher than controls in mid‐ and high‐dose cohort 1B females (+ 20.4% and + 12.3% compared to control for 300 and 1,000 mg/kg bw per day, respectively), reaching statistical significance for the high‐dose group only. The authors considered this finding to be incidental because all the values in the mid‐ and high‐dose females of cohort 1 B were within or only slightly above the normal circadian range. The authors further noted that female testosterone levels in cohort 1B controls were slightly lower than those in F0 and cohorts 1A and 2A controls.
The Panel agreed with the study authors that these are isolated findings, not related to treatment, and that there were no test substance‐related effects on serum oestradiol, oestrone and testosterone in F1 cohort 1B.
F1 generation cohort 2A
According to the authors, there were no test substance‐related differences in serum oestradiol, oestrone and testosterone) between control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 cohort 2A at sacrifice on PND 84–90.
Non‐statistically significant differences were noted by the study authors for mean serum oestrone and testosterone levels in F1 cohort 2A males and oestradiol in females. Some variations in hormone levels were observed, but these variations were within the normal circadian range (with the exception of oestrone, whose circadian variation was not measured).
The Panel agreed with the study authors that these are isolated findings, not related to treatment, and that there were no test substance‐related effects on serum oestradiol, oestrone and testosterone in F1 cohort 2A.
F1 generation cohort 2B
According to the study authors, there were no test substance‐related differences in serum oestradiol, oestrone and testosterone between control and treatment groups (100, 300 or 1,000 mg E 171/kg bw per day) in either male or female F1 cohort 2B at sacrifice on PND 21–23.
Mean serum testosterone levels were significantly lower than controls in mid‐dose females (−31%). Lower mean testosterone levels, albeit non‐statistically significant, were also seen in mid‐ and high‐dose males and in low‐ and high‐dose females, without a dose–response relationship. Some of the individual values in all dose groups were below the circadian range determined in satellite animals during the study. The Panel considered that these findings were incidental.
It is noted that lower testosterone levels, albeit non‐statistically significant, were also seen in treated cohort 2A females, but again without a dose–response relationship. The Panel therefore considered that this finding was incidental, and that there were no test substance‐related effects on serum oestradiol, oestrone and testosterone in F1 cohort 2B.
Overall conclusions on effects on hormone levels
Overall, the Panel concluded that, although there were occasional statistically significant differences between control and treated groups, there were no consistent treatment‐related effects on T4, T3, TSH, oestradiol, oestrone and testosterone levels in F0 or F1 animals, and that therefore there was no evidence for a treatment‐related effect on thyroid or sex hormone levels.
4.2.5. Pathology
All or part of the animals – depending on the cohort to which they were assigned – were subjected to an extensive macroscopic and microscopic examination, in accordance with OECD TG 443. Necropsy of the F0 generation (dams and males) was scheduled (shortly) after weaning of the F1 animals; necropsy of the F1 generation at the end of the dosing period (PND 86–96, cohort 1A), after behavioural testing (PND 84–90, cohort 2A) or on PND 21/22/23 (cohort 2B). At necropsy, animals were sacrificed under CO2 anaesthesia and by exsanguination from abdominal aorta and were further dissected and examined macroscopically for any abnormalities or pathological changes, with special attention given to the reproductive system and GI tract. Organ weights were recorded.
Pathology examination was performed in accordance with OECD TG 443.18 This guideline indicates that in principle the control and high‐dose groups must be examined and, when adverse effects are observed in the high‐dose group, then the mid‐ and low‐dose groups should additionally be examined. In males, qualitative stages of spermatogenesis and histopathology of interstitial testicular structure were studied on one testicle and one epididymis. In females, primordial and small growing follicles were evaluated quantitatively together with the corpora lutea on one ovary. Fresh bone marrow was obtained from the femur (3 air‐dried smears/animal: all groups).
Tissues examined for neurohistopathology were selected from F1 cohorts 2A and 2B (control and high‐dose group), i.e. brain [olfactory bulbs, cerebral cortex, hippocampus, basal ganglia, thalamus, hypothalamus, mid‐brain (tectum, tegmentum and cerebral peduncles), brainstem and cerebellum]. From the F1 cohort 2A, the eye with optic nerve and retina, muscle (skeletal), nerve (sciatic) and spinal cord (3 sections) were also studied. Neurohistopathology evaluation included a qualitative and semi‐quantitative microscopic inspection of the selected nervous tissues focussing on: (i) alterations in gross size or shape of olfactory bulbs, cerebrum or cerebellum; (ii) in the relative size of various brain regions (resulting, e.g. from loss or persistence of normally transient populations of cells or axonal projections); (iii) areas of apoptosis or necrosis, clusters or dispersed populations of ectopic, disoriented or malformed neurons or alterations in the relative size of various layers of cortical structures (indicative of alterations in proliferation, migration and differentiation); (iv) alterations in patterns of myelination; and (v) enlargement of ventricles, stenosis of cerebral aqueduct and thinning of cerebral hemispheres. Finally, a quantitative evaluation, i.e. linear morphometry, was performed (cohort 2A, control and high‐dose group) in cerebral cortex, mid‐brain, brainstem and cerebellum.
All animals survived until the scheduled necropsy.
Gross pathology – including organ weights – of F0 (all groups; dams and males) and F1 generations (cohorts 1A, 2A and 2B; all groups; males and females) with special attention to the reproductive system and GI tract, did not identify any test substance‐related effects. In one animal from the high‐dose group (F0, male), ‘white fine granular deposit’ was observed at the mucosa of the duodenum but this was considered by the authors to be of no toxicological relevance.
The histopathological examination of F0 and F1 generations and comparison of the high‐dose group and the control group did not identify any test substance related changes, either in the F0 generation (dams and males) or the F1 generation cohort 1A (males and females).
No test substance‐related histological changes were observed in the testis and epididymis in all animals in the control and high‐dose groups (F0 and F1 generation cohort 1A). This included an absence of any effects on any spermatogenic phases (proliferative, meiotic and spermiogenic). Furthermore, in the females, no statistically significant differences were observed for the total mean of primordial and small growing follicles as well as corpora lutea for the high‐dose group, when compared to control group.
Qualitative and quantitative neurohistopathological examination was performed on cohorts 2A and 2B.
No test substance‐related abnormalities were found in the cohort 2A high‐dose group animals during qualitative examination. The only abnormality observed appeared in an animal of the control group (eye/optic nerve: slight unilateral retrobulbar haemorrhage). The Panel noted that evaluation of sections in cohort 2A from brain level 119 containing variable amounts of olfactory bulbs indicated no abnormalities.
Subarachnoid haemorrhages were observed in animals of both sexes in both the control and high‐dose groups of cohort 2B. This was attributed to the preparation and processing of the brain and therefore artefacts. The Panel agreed with this conclusion.
Quantitative neuropathology assessment of cohort 2A and comparison of total means of all measurements in the brain sections examined (cerebrum, mid‐brain, cerebellum and brain stem) did not reveal any pathological changes indicative of toxicity for both sexes.
From the results of gross pathology of all animals and the (quantitative) microscopic assessment of animals of the control and high‐dose groups of the F0 (dams and males) and F1 generations (cohorts 1A, 2A and B; males and females), the Panel concluded that there were no test‐substance related effects.
4.2.6. Aberrant crypt foci examination in satellite F0 animals
Although not a requirement in the OECD TG 443, an evaluation of ACF in the colon of satellite F0 animals (10/sex per group) treated with 0, 100, 300 and 1,000 mg E 171/kg bw per day and terminated after weaning was undertaken. The Panel noted that the design of the study did not include a positive control group (e.g. treatment with a known GIT tumour initiator such as DMH) for the development of ACF.
The colon was excised, opened longitudinally and the contents removed by rinsing with a 0.9% NaCl solution. Thereafter, the tissue was divided in parts of a suitable size for fixation by immersion in 5% buffered formalin. A blind examination of these samples stained with 0.5% (w/v) methylene blue in water was performed under a stereomicroscope at 50x magnification for presence of ACF. The study pathologist followed the definition of ACF given by Shwter et al. (2016), i.e. ‘foci containing more than 2 ABCs’.
No ACF were found in the colons of the control and the treated groups that would fulfil the above definition. A mildly increased morphological variability (increased size and intensity of the staining of a small portion) of the crypts in the two caudal parts of colon was observed in seven animals (males: 1/10, 0/10, 1/10, 1/10; females: 1/10, 0/10, 1/10, 2/10 in the control, low‐, mid‐ and high‐dose groups, respectively). These changes were assessed by the study pathologist as inconsistent with the appearance and definition of ACF presented in Shwter et al. (2016). Based on the description in the report, the Panel agreed with this conclusion. Furthermore, the incidence of these single crypts observed in the mid and high doses was not significantly different from the control.
The additional submission of data by the interested party (Documentation provided to EFSA No 15) included photomicrographs of mildly increased variability in crypt morphology from all seven animals. In this submission, a re‐examination was extended to an additional randomly selected nine control animals (4M and 5F) and eight high‐dose group animals (3M and 5F). A transmitted light microscope instead of an incident light microscope was used to improve visualisation. Among these additional animals, a mild increased variability in crypt morphology was observed in eight of the nine (4M and 4F) controls and six of the eight (3M and 3M) high‐dose animals.
Based on the evaluation of the data from the first and additional submission, the Panel considered that oral exposure to E 171 at doses up to 1,000 mg/kg bw per day did not induce ACF in the colon.
4.2.7. Reproductive and developmental toxicity
Evaluation of sexual function and fertility
Male fertility
An overview of results for male fertility parameters is reported in Table 3. No statistically significant or dose‐related effects on sperm motility, total spermatids/gram testis, percentage of abnormal spermatozoa and male mating index were observed in the F0. The slight decrease in the number of successful matings at 300 and 1,000 mg/kg bw per day appears unrelated to the male partners, as all males that failed to impregnate their females showed normal sperm motility and sperm counts. Only one of the high‐dose males was found to have a lower testicular spermatid content (50% of the group mean), a finding that was also associated with a slightly lower testis weight (85% of the group mean). The number of abnormal sperm was low in all dose groups and remained below 2% in the few males in which abnormal sperm were found. The Panel noted that the epidydimal sperm parameters were not evaluated; this is a deviation from the OECD TG 443. The Panel considered that this deviation has no effect on the final conclusion of the study.
Table 3.
Male fertility parameters of F0 and F1 (cohort 1A)
| F0 | F1 (cohort 1A) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dietary dose (mg/kg bw per day) | Control | 100 | 300 | 1,000 | Control | 100 | 300 | 1,000 |
| No. of males in study | 24 | 24 | 24 | 24 | 20 | 20 | 20 | 20 |
| No. of fertile malesa | 24 | 23 | 22 | 22 | – | – | – | – |
| No. of males evaluated | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Testis weight (right), g | 1.85 | 1.88 | 1.88 | 1.74 | 1.74 | 1.72 | 1.62 | 1.65 |
| Spermatids/g testis (× 106) | 106.2 | 106.4 | 102.5 | 103.2 | 106.1 | 107.9 | 107.5 | 112.0 |
| % motile spermatozoa | 72.0 | 71.6 | 70.7 | 70.7 | 73.5 | 74.4 | 73.8 | 72.0 |
| No. of males with some abnormal spermatozoa (< 2%)b | 6 | 4 | 0 | 3 | 0 | 0 | 0 | 0 |
bw: body weight.
Number of males inducing pregnancy in female partners.
Calculated by the Panel based on individual data.
There were no effects on any of the sperm endpoints in the cohort 1A.
Female fertility
An overview of results for female fertility parameters is reported in Table 4. No effects on mean oestrus cycle duration were noted in F0 and F1 (cohort 1B) parental generations and all F0 females in the control, 100, 300 and 1,000 mg/kg bw per day groups mated. In the F1 generation 2 and 3 animals from the mid‐ and the high‐dose groups, respectively, were erroneously removed from the study, before mating had been unequivocally confirmed. All other females mated, except one F1 female in the 100 mg/kg bw per day group. With few exceptions, mating occurred at the first oestrus after the females were housed with males. No effects of treatment were observed.
Table 4.
Female fertility and litter data of F0 and F1 (cohort 1B)
| Dietary dose (mg/kg bw per day) | F0 | F1 (cohort 1B) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 300 | 1,000 | Control | 100 | 300 | 1,000 | |
| No. of females paired | 24 | 24 | 24 | 24 | 20 | 20 | 20 | 20 |
| No. of females mated | 24 | 24 | 24 | 24 | 20 | 19 | 18 | 17 |
| No. of females pregnant | 24 | 23 | 22 | 22 | 20 | 19 | 17 | 17 |
| Live litters | 24 | 23 | 21 | 21 | 20 | 19 | 17 | 16 |
| Total litter loss | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Mean oestrous cycle duration (days) | 4.6 | 4.5 | 4.5 | 4.4 | 4.4 | 4.4 | 4.2 | 4.5 |
| Precoital interval > 4 daysa | 0 | 1 | 1 | 1 | 2 | 0 | 3 | 0 |
| Duration of pregnancy > 23 daysa | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Mean duration of pregnancy (days) | 22.6 | 22.7 | 22.8 | 22.9 | 22.6b | 22.6b | 22.3b | 22.5b |
| Implantation sites/dam | 14.7 | 15.5 | 15.6 | 15.3 | 15.6 | 16.0 | 16.2 | 15.3 |
| Total number of implantation sites | 294 | 309 | 311 | 306 | 311 | 304 | 275 | 260 |
| Post‐implantation loss | 38 | 24 | 48 | 30 | 20 | 29 | 16 | 28 |
| Post‐implantation loss (mean per dam, %) | 17.5 | 8.1 | 17.0 | 11.8 | 6.7 | 10.1 | 6.0 | 12.8 |
| Litters with > 2 post‐implantation lossesa | 4 | 3 | 9 | 4 | 2 | 3 | 1 | 3 |
| Live litters with > 1 stillborna | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Live litter size | 12.7 | 14.3 | 13.5 | 13.7 | 14.6b | 14.4b | 15.3b | 13.7b |
| Litter weight at birth | 87.3 | 97.5 | 95.3 | 94.6 | 97.7b | 97.2b | 97.2b | 96.4b |
Calculated by the Panel based on individual data.
Calculated by the Panel based on individual data and including all 21 pregnant animals with live litters in the study.
The pregnancy rate was slightly lower in the F0 generation at 300 and 1,000 mg/kg bw per day (100, 96, 92 and 92%). As this finding was not confirmed in the F1 generation (100, 95, 94 and 100%) the Panel considered it as incidental and not treatment‐related. No effects were noted on pregnancy duration, number of implantation sites and post‐implantation loss. Although they occurred in the mid‐and high‐dose groups, three single total litter losses, either from total resorption of all embryos or from death of the litter during or shortly before birth, were not considered to be due to treatment. This is because the two F0 dams had unusually small litters of two pups each, which were stillborn, and the F1 dam showed total resorptions of eight implants at necropsy after failing to litter. Live litter sizes and litter weights were comparable to control values in all dose groups in the F0 and the F1 generation.
Conclusions on sexual function and fertility
No effects of E 171 on sexual function and fertility were observed in either males or females.
Evaluation of developmental toxicity
Pre‐ and postnatal lethality, structural abnormalities
No treatment‐related pre‐ or postnatal loss was observed in the F0 and F1 generations. The average litter size at birth in all dose groups was comparable, or even higher than in the control group. The sex ratio was unaffected (see Table 5).
Table 5.
Offspring data for F1 and F2 generations
| Dietary dose (mg/kg bw per day) | F1 | F2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 300 | 1,000 | Control | 100 | 300 | 1,000 | |
| No of pups delivered | 309 | 332 | 286 | 294 | 292 | 276 | 261 | 233 |
| Live‐born pups | 304a | 317a | 283a | 287a | 291 | 274 | 259 | 233 |
| Stillborn/dead pups on PND 1 | 5a | 15a | 3a | 7a | 1 | 2 | 2 | 0 |
| % males | 52.0a | 52.4a | 49.1a | 51.6a | 54.6 | 54.7 | 48.8 | 49.4 |
| Litter size PND 1 | 12.7a | 13.8a | 13.5a | 13.7a | 14.6 | 14.4 | 15.2 | 13.7 |
| Litter size PND 4 (pre‐cull) | 12.3a | 13.2a | 13.1a | 13.2a | 12.9 | 14.1 | 14.9 | 13.4 |
| Litter size PND 4 (post‐cull) | 8.8a | 9.5a | 9.9a | 9.8a | – | – | – | – |
| Litter size PND 21 | 8.3a | 9.1a | 9.6a | 9.5a | – | – | – | – |
| Total litter loss PND 2–4 | 0a | 0a | 0a | 0a | 1 | 0 | 0 | 0 |
| Litters with pup loss > 1 PND 2–4 | 1a | 1a | 2a | 2a | 3 | 1 | 0 | 1 |
| Litters with pup loss > 0 PND 5–21 | 4a | 4a | 3a | 3a | – | – | – | – |
Calculated by the Panel based on individual data for F1.
No external or internal abnormalities were detected in F1 and F2 pups at termination.
Growth and sexual development
An overview of the results related to growth and sexual development for the F1 and F2 generations is reported in Table 6. No treatment‐related effects were observed in birth weights and growth of the pups. There were no indications for any androgenic and/or oestrogenic effects on the male and female anogenital distance (AGD) and the retention of nipples in males. The Panel noted that instead of examining balanopreputial separation as required by the OECD TG 443 the laboratory examined balanopreputial gland cleavage which does not comply with the OECD TG 443 and cannot be considered a measure of puberty in males.
Table 6.
Pup body weight development and attainment of puberty
| Dietary dose (mg/kg bw per day) | F1 | F2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 300 | 1,000 | Control | 100 | 300 | 1,000 | |
| Birth weight males (g) | 7.19a | 7.26a | 7.25a | 7.22a | 7.01 | 6.94 | 6.59 | 6.87 |
| PND 4 | 9.96a | 9.86a | 9.83a | 9.77a | 9.68 | 9.58 | 8.94 | 9.34 |
| PND 14 | 32.34a | 30.76a | 30.61a | 31.41a | – | – | – | – |
| PND 21 | 53.49a | 51.92a | 49.56a | 51.02a | – | – | – | – |
| AGD/body weight cubed PND 4 | 17.0a | 16.5a | 17.1a | 17.5a | 15.92 | 15.38 | 15.87 | 15.53 |
| Nipple retention PND 13b | 0 | 2 | 3 | 0 | – | – | – | – |
| Age (PND) at balanopreputial gland cleavage C1Ac | 22.3 | 22.6 | 22.1 | 22.2 | – | – | – | – |
| Body weight (g) on day of balanopreputial gland cleavage C1A | 59.1 | 60.4 | 55.4 | 57.3 | ||||
| Age (PND) at balanopreputial gland cleavage C1Bc | 22.3 | 22.4 | 22.2 | 22.4 | ||||
| Body weight (g) on day of balanopreputial gland cleavage C1B | 59.2 | 58.4 | 56.4 | 58.4 | ||||
| Birth weight females (g)a | 6.80a | 6.88a | 6.96a | 6.82a | 6.55 | 6.55 | 6.22 | 6.59 |
| PND 4 | 9.30a | 9.41a | 9.62a | 9.34a | 9.17 | 9.15 | 8.66 | 9.02 |
| PND 14 | 30.99a | 29.43a | 29.87a | 29.98a | – | – | – | – |
| PND 21 | 50.67a | 49.52a | 48.48a | 49.18a | – | – | – | – |
| AGD/body weight PND 4 | 8.2a | 8.4a | 8.5a | 8.5a | 7.70 | 7.64 | 7.52 | 7.70 |
| Age (PND) at vaginal opening C1A | 33.6 | 32.8 | 32.8 | 32.8 | – | – | – | – |
| Body weight (g) on day of vaginal opening C1A | 123.0 | 117.8 | 112.4d | 117.5 | ||||
| Age (PND) at vaginal opening C1B | 33.2 | 32.7 | 32.9 | 32.6 | ||||
| Body weight (g) on day of vaginal opening C1B | 120.6 | 120.2 | 114.2 | 117.1 | ||||
bw: body weight; PND: postnatal day; AGD: anogenital distance.
Calculated by the Panel for the pups of F0 dams based on individual data.
Calculated by the Panel based on individual data; Litters with > 0 male pups with more than 2 retained nipples.
The Panel noted that this test as conducted by the laboratory does not comply with the OECD TG 443 and cannot be considered a measure of puberty in male.
p ≤ 0.05 (ANOVA and Dunnett).
The mean age at vaginal opening was comparable between control and treated groups. The statistically significant lower body weight on the day of vaginal opening in cohort 1A at 300 mg/kg bw per day was not considered to be biologically relevant due to the slightly higher litter sizes in all treated groups, which would have given a growth advantage to the control group.
Conclusions on developmental toxicity
No effects of E 171 on pre‐ and postnatal development were observed. Data on the attainment of puberty in males (i.e. an appropriate assessment of the timing of the balanopreputial separation) were missing; however, given the lack of any other treatment‐related effects on other parameters, the Panel does not consider this to be critical in this case.
4.2.8. Neurofunctional screening
Male and female F1 cohort 2A offspring were tested for auditory startle response between PND 23 and 25, and for a functional observation battery including grip strength evaluation and for quantitative locomotor activity between PND 58 and 64.
No differences in the response to an auditory startle stimulus were observed between the control and all the tested doses.
Compared to controls, an increase in hindlimb splay was observed in females, reaching statistical significance at 100 and 1,000 mg/kg bw per day. A statistically significant increase in mean forelimb grip strength was noted at 300 mg/kg bw per day in both males and females.
To check whether the significant differences in grip strength and hindlimb splay could be due to systematic bias in group testing order, the testing order was checked (Documentation provided to EFSA No 11). The Panel considered that there was no systematic bias in group testing order and that this was therefore not a plausible explanation for the observed group differences.
Grip strength and hindlimb splay belong to the same domain of neurological function, i.e. motor function and/or sensory–motor coordination. However, the effects observed (i.e. increase in hindlimb splay and increase in mean forelimb grip strength) seem to point in opposite directions when it comes to muscle strength. In particular, an increase in hindlimb splay can be interpreted as muscular weakness whereas an increase in mean forelimb grip strength could be indicative of myotonia. The Panel noted that the effects observed were not correlated to any other changes (e.g. alterations in muscle tone, righting reflex, gait, wire manoeuvre, posture). No dose response was observed for any of these endpoints or for the two functional measurements, indicating that the likelihood of an association with test substance is low. No other changes in the functional observation battery measurements or locomotor activity were noted. Furthermore, there were no notable histopathological findings in brain or in peripheral nerve (sciatic). Based on all the above considerations, the Panel considered that the effects on grip strength and hindlimb splay were not treatment‐related. However, the Panel noted that quantitative information on peripheral nerves was not available.
Overall, the Panel considered that E 171 had no adverse effects on neurofunctional endpoints in F1 cohort 2A offspring at the doses used.
4.2.9. Developmental Immunotoxicity
Effects on developmental immunotoxicity were determined in the F1 cohort 3 animals through an examination of their ability to raise an antibody response to a foreign antigen. Animals are sensitised and the primary IgM antibody response to the sensitising antigen, in this case to keyhole limpet haemocyanin (KLH) antigen, is measured. The ability of the test compound to modulate serum anti‐KLH antibody titre is taken as indicative of a developmental immunotoxic effect. A KLH‐immunised control group also exposed to a known immunosuppressant (i.e. cyclophosphamide (CY)), resulting in at least 50% inhibition in serum IgM anti‐KLH titre, is crucial for the verification of assay performance.
These data can be considered in combination with additional data related to potential immunotoxic effects. For example, in the F1 cohort 1A animals, the following parameters – even if generally not regarded as having the predictive power of functional tests such as the KLH assay – may contribute to the general assessment for immunotoxicity: weight and histopathology of the spleen, thymus and lymph nodes, as well as bone marrow histopathology, total and differential peripheral WBC count and splenic lymphocyte subpopulation distribution.
T‐cell-dependent anti‐KLH response (KLH assay)
The determination of serum anti KLH‐IgM antibodies was performed in F1 cohort 3 (10/sex per group, PND 53–61) using an enzyme‐linked immunosorbent assay (ELISA). The animals were sacrificed 5 days after intravenous bolus injection (tail vein) of KLH, blood was withdrawn and the level of anti‐KLH IgM was measured in serum. In addition, satellite animals of F1 (10/sex, PND 55) were immunised with KLH and treated with CY (single administration of 40 mg/kg bw by gavage on the same day of KLH treatment) to provide a positive control (for an inhibition of immune response).
A slight, but statistically significant decrease in the antigen specific IgM level was measured at the highest dose tested (1,000 mg/kg bw per day) in males only (–9%) and without an apparent dose response.
In addition, the Panel noted that treatment with CY was not performed at the same time as the rest of F1 cohort 3, without a separate control for the CY response, conducted at the same time (Documentation provided to EFSA No 11). Since the results from the CY positive control were not valid, the sensitivity of the test was not demonstrated.
It was noted that the assay conditions may have not been optimal resulting in an apparent low antibody response to KLH when compared to literature (Gore et al., 2004), as also pointed out by the study authors (Documentation provided to EFSA No 11).
The study authors considered that all tested animals in the study had a weak immunogenic response to KLH that was insufficient to identify a T‐cell‐dependent immunotoxic effect of E 171. The study authors therefore considered that no conclusion can be drawn on the effect of E 171 on the developing immune system.
The Panel agreed with the conclusion of the study authors.
Assessment of pathology, haematology and splenic lymphocyte subpopulations
At necropsy, pathology of lymphoid organs, haematology and lymphocyte subpopulations in the spleen were investigated. The spleens of all animals were cut in two, and histopathology was performed on one of the spleen halves of the F1 cohort 1A animals only. From the remaining half spleen from animals of F1 cohort 1A (20/sex per group, PND 87–96) and from the spleens of the animals of F1 cohort 3 (10/sex per group, PND 53–61), single cell suspensions were prepared for the analysis of the subpopulation of lymphocytes. The following lymphocyte subpopulations were determined via flow cytometry analysis (FACS): T cells, T helper cells, T suppressor/cytotoxic cells, NK cells and B cells.
The Panel noted that haematology, spleen weight and histopathology of lymphoid organs in animals from F1 cohort 1A did not indicate any dose‐related effects. As regards the splenic lymphocyte subpopulation analysis, no statistically significant differences were observed in the percentage of T cells, T helper cells, T suppressor/cytotoxic cells, NK cells and B cells of any of the treated groups compared to control in both sexes. The study authors concluded that no test substance‐related effect was observed on the proportion of the examined lymphocyte subtypes.
The Panel agreed with the study author conclusion that the splenic lymphocyte subpopulations in this cohort were not affected. However, the Panel considered that an isolated observation in F1 cohort 1A is not sufficient to conclude on immunotoxicity.
According to the study authors, when compared to animals of F1 cohort 1A, F1 cohort 3 animals showed a shift in the lymphocyte subpopulation that indicated activation of the immune system by injection of KLH, and concluded that increased B‐cell proliferation may have led to the production of antigen‐specific antibodies. In F1 cohort 3 animals, no differences in the relative size of the lymphocyte subpopulations were observed between the control group and the E 171‐treated groups, after immunisation of the animals with KLH. The study authors argued that the B‐cell shift in F1 cohort 3 was caused by KLH immunisation, supported by the fact that there was no such shift found for the positive control animals that were sensitised to KLH and treated with CY.
The study authors have acknowledged that the positive control of F1 cohort 3, i.e. the effect of CY on the antibody response to KLH, has not met with expectations. However, the authors considered that KLH induced an immune reaction, and that this response was influenced by CY in the way it would be expected (i.e. a shift in T‐/B‐cell populations in the spleen). According to the study authors, KLH would increase the percentage of splenic B cells and decrease the percentage of T cells. Therefore, they concluded that the immune response was affected by CY but was not adversely affected by the TiO2 test substance.
The Panel did not agree with the conclusion of the study authors that a shift to B cells by KLH was substantiated. The Panel considered that it is incorrect to compare the groups of F1 cohort 1A and of F1 cohort 3 because the groups of animals of F1 cohort 3 had a different age than that of the animals in F1 cohort 1A at the time of sacrifice (PND 87–96 vs. PND 53–61, respectively). In addition, the FACS analyses on the splenic cell suspensions were not all performed in the same round of analysis but were performed separately, while it is known that this may have influenced staining and subsequent quantification.
The study authors suggested that even if the positive CY control did not perform as expected, the data still indicate there is no effect of E 171 on sensitisation to KLH. However, the Panel did not agree with this conclusion and overall considered that the data did not allow to conclude on developmental immunotoxicity with respect to E 171.
4.2.10. Uncertainty
The Panel identified the following uncertainties regarding the EOGRT study with respect to its validity to fully identify all potential adverse effects of E 171 when used as a food additive:
The extent to which the particle size distribution of the E 171 used in the EOGRT study is reflective of the particle size distributions of E 171 when added to foods.
The extent to which the particle size distribution of E 171 in transit through the GIT in the EOGRT study was affected by the concentration in the diet (i.e. dose).
The selected test material was representative of E 171 containing a large proportion (around 50% by number) of constituent particles below 100 nm (E 171 sample E reported in EFSA FAF Panel, 2019). The particle size distribution of the E 171 in samples of the test diet was also analysed after applying a sample dispersion protocol that aims to extract E 171 particles from the feed matrix ((Documentation provided to EFSA No 16) and the results show that the particle size distribution of the constituent particles was similar to that of pristine E 171 after dispersion (EFSA FAF Panel, 2019; Verleysen et al., 2020).
However, neither of these procedures were considered by the Panel to reliably determine the particle size distribution of E 171 in the feed. The Panel acknowledges that methods for determining particle size distributions in complex foods and feeds in situ are not currently available. Accordingly, the Panel considers that the extent to which the particle size distribution of the E 171 used in the EOGRT study is sufficiently reflective of the particle size distributions of E 171 when added to foods remains uncertain.
The interested business operator considered that mixing of two dry components (feed and E 171) was the best possible option to retain the particle size distribution properties of the original E 171 sample, and that the use of liquid dispersion would add further superfluous unknowns (Documentation provided to EFSA No 11).
The Panel considered that E 171 has a broad size distribution of constituent particles (from about 40 to 250 nm); considered that in dry form, this size distribution of the constituent particles is expected to be stable and further, that homogenous mixing of E 171 with dry diet is a pragmatic approach to adopt in terms of performing an animal study over an extended time frame such as the EOGRT study. The Panel considered this approach to be representative of some uses of E 171 in food (e.g. E 171 in confectionary coatings and fillings and in ready to use sauces ((Documentation provided to EFSA No 11)). However, the Panel also noted that this approach may not be fully representative for all uses of E 171 in food since liquid dispersion of E 171 was reported to be used, potentially along with additional processes, to reduce the formation of agglomerates in suspension in some products (e.g. incorporation of E 171 into a tablet coating or capsule (Documentation provided to EFSA No 17).
The Panel considered that investigations of Ti levels in tissues would have reduced uncertainty regarding dose dependency of internal exposure. However, the Panel noted that the EOGRT study demonstrated unequivocally low levels of internal exposure to TiO2 in animals that were fed a diet prepared by addition of E 171 to dry feed.
Dispersed NPs show a greater tendency to agglomerate when suspended in liquid media at higher concentrations. This concentration effect on agglomeration and/or resistance to de‐agglomeration may also exist in the GIT at high‐dose levels (Appendix E). The Panel therefore considered that there remains an uncertainty regarding the effects of dose levels/concentrations in feed and the extent to which agglomeration occurred in the GIT. However, the Panel considered the propensity for this agglomeration is likely reduced when exposure is via feed rather than through bolus gavage administration of E 171.
4.3. Genotoxicity
The genotoxicity of TiO2 was reviewed by the ANS Panel in the 2016 opinion on the re‐evaluation of the food additive (E 171) (EFSA ANS Panel, 2016). According to the data provided by interested parties and from the literature at that time, TiO2 as E 171 was not considered to be a nanomaterial according to the EU Recommendation on the definition of a nanomaterial (i.e. ‘a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm’). Therefore, the ANS Panel had considered that the data on TiO2 as a nanomaterial were not directly applicable to the evaluation of the food additive. The EFSA ANS Panel (2016) had noted mixed results in in vitro genotoxicity studies, which had provided some evidence of in vitro genotoxicity for TiO2 micro‐ and nanoparticles. The ANS Panel had considered that most positive results were reported under experimental conditions associated with the induction of oxidative stress (as shown by increased 8‐oxo‐7,8‐dihydro‐2’‐deoxyguanosine (8‐oxodG), LPO and reactive oxygen species (ROS) generation), and that the genotoxic effects observed mainly concerned indicator assays (comet and H2AX histone phosphorylation), which in some studies were shown not to be associated with permanent chromosome damage such as chromosome breaks visualised as micronuclei (MN).
In vivo, overall negative results were obtained in genotoxicity studies with microsized TiO2 pigment. Limited evidence of genotoxicity, if any, was provided by studies with orally administered TiO2 NPs. Limited or no indication of the genotoxicity of TiO2 NPs was provided by studies using an intravenous route of administration, which allows maximum exposure of target tissues. Overall, the ANS Panel had concluded that the use of E 171 as a food additive did not raise a concern with respect to genotoxicity (EFSA ANS Panel, 2016).
Upon request by the European Commission, in 2018, the ANS Panel had evaluated four new studies on the potential toxicity of E 171. One of these studies (Proquin et al., 2017) investigated the potential genotoxicity of three different TiO2 test materials in two human colon cancer cells lines. The study authors reported genotoxic activity in vitro of E 171 in a comet assay and in a cytokinesis‐block MN assay. The ANS Panel had noted that, in view of the composition of the tested E 171 (containing around 40% of nanoparticles by number), and of the available evidence of genotoxicity in vitro of both TiO2 microparticulate (MP) and TiO2 NPs, a genotoxic activity of E 171 under in vitro conditions could already be anticipated. In this respect, the ANS Panel also had noted that overall negative results were obtained in studies in vivo with both TiO2 NPs and TiO2 MP (EFSA ANS Panel, 2016), indicating that TiO2 MP and TiO2 NPs, and consequently E 171, did not raise concern for in vivo genotoxicity (EFSA ANS Panel, 2018).
In 2019, EFSA published a statement on the review of the risks related to the exposure to E 171 performed by ANSES (2019). With respect to genotoxicity, the ANSES opinion made reference to a systematic review of in vitro genotoxicity studies on nano titanium dioxide (Charles et al., 2018). According to the authors of this review, the majority of the publications analysed had shown that the in vitro genotoxic effect of TiO2 NPs was linked to a secondary mechanism consequent to oxidative stress, although primary genotoxic effects could not be excluded. The only additional in vivo study identified (Jensen et al., 2019) was considered by ANSES to have methodological limitations that reduced the reliability of the negative results observed. According to the ANSES opinion, although there were no studies showing direct interaction of E 171 with the DNA and/or the mitotic apparatus, a direct effect of E 171 on genetic material or other molecules interacting with the genetic material could not be excluded. ANSES recommended further investigation of in vivo genotoxicity. Overall, EFSA noted that the new genotoxicity studies assessed in the ANSES's opinion did not add new elements to the previous conclusions by the EFSA ANS Panel and did not provide any reason to revise the conclusion on genotoxicity of E 171, previously drawn by the EFSA ANS Panel (2016, 2018). EFSA considered that a review of the overall genotoxicity database may be needed once the characterisation of titanium dioxide used as the food additive E 171 is completed (see EFSA, 2019).
The focus of the current updated assessment was to gather any relevant information from the newly available evidence that could be used to refine the risk assessment and to reduce the uncertainties identified by the ANS Panel in its earlier evaluation. In particular, the FAF Panel examined whether new data from the published literature could provide new evidence on the potential genotoxicity of E 171. To this aim, a literature search was performed as reported in Appendices A and B. Based on new information regarding the constituent particle size distribution of E 171 (EFSA FAF Panel, 2019) and on the updated EFSA Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health (EFSA Scientific Committee, 2018a) also the references from the previous ANS Panel opinion (2016) have been considered in the present assessment. In addition, data submitted in the context of the NANOGENOTOX project, 2013 (Documentation provided to EFSA No 7, 8, 9 and 10), publications reported in the OECD dossier (OECD, 2016) and documentation provided by interested business operators (IBOs) (Documentation provided to EFSA No. 14 and 15) have been included in the current assessment using the same appraisal criteria applied to the newly published data.
Genotoxicity studies considered for this assessment are:
in vitro and in vivo studies retrieved from the literature search (Appendices J, K),
in vitro and in vivo studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016) (Appendices L, M),
in vitro and in vivo studies reported in the OECD (2016) ((published papers and results from NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7, 8, 9 and 10)) (Appendices N, O) and
in vitro studies submitted by IBOs (Documentation provided to EFSA No 14 and 15) (Appendix P)
studies considered relevant for the assessment of E171 in addition to studies considered in the re‐evaluation of E171 (EFSA ANS Panel, 2016) or retrieved in the updated literature search (see Section 2.2)
The score for NSC related to specific aspects in the study design (dispersion and/or confirmation of internal exposure) for all studies has been performed according to Appendix E.
The genotoxicity studies have been assessed using a scoring system for reliability based on criteria published by Klimisch et al. (1997) as explained in Appendix D. In a second step, the relevance (high, limited, low) of study results was assessed based on reliability of the study, some general aspects e.g. genetic endpoint, route of administration and status of validation of the assay and specific NSC (see Appendix D).
Genotoxicity studies evaluated as of low relevance have not been further considered in the assessment. The assessment of in vitro and in vivo studies of high and limited relevance is reported in the following sections.
4.3.1. Gene mutation
In vitro gene mutation studies
Fourteen studies investigated the ability of TiO2 NPs to induce gene mutations in mammalian cultured cells. Seven of these studies were considered of high or limited relevance and consequently were taken into account in the assessment of genotoxicity (Appendices J, L, N). Positive results were reported in two hypoxanthine‐guanine phosphoribosyl transferase (HPRT) studies performed in Chinese hamster V79 lung fibroblasts (Chen et al., 2014; Jain et al., 2017) and in a Spi– gene mutation assay in primary embryo fibroblasts from gpt delta transgenic mouse (Xu et al., 2009). On the contrary, no mutagenic effect was observed in two HPRT assays, one in V79‐4 cells (a cell line derived from V79) (Kazimirova et al., 2020) and one in Chinese hamster ovary cells CHO‐K1 (Wang et al., 2011). In a mouse lymphoma assay (Demir et al., 2017), no statistically significant increase over the untreated control was reported and the global evaluation factor (GEF) was never exceeded; however, there was a statistically significant concentration–effect relationship in all six experiments performed, therefore the overall result was considered equivocal. Negative results were reported in another mouse lymphoma study ((NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7 and 8)).
In addition, the data set included seven bacterial reverse mutation studies, plus one recently submitted by industry. However, all these studies were considered of low relevance, due to limitations in the penetration of particles through the bacterial cell wall and the lack of internalisation in bacteria (EFSA Scientific Committee, 2018a).
In vivo gene mutation studies
The induction of gene mutations was investigated in vivo in six studies considered of high or limited relevance (Appendices K, M, Table 7). All studies were performed with TiO2 NPs (< 30 nm).
Table 7.
Summary table of test results of in vivo gene mutation studies
| Study design | Test material | Results | Reliability/Relevance | Reference |
|---|---|---|---|---|
| Oral | ||||
| C57BL/6Jp un/p un mice In vivo DNA deletion assay in the p un locus Drinking water for 10 days to pregnant mice; 500 mg/kg | NSC: 2, TiO2 NPs (P25), 15–24 nm | Positive | 2/limited | Trouiller et al. (2009) |
| Intraperitoneal injection | ||||
| Male B6C3F1 mice Pig‐a gene mutation assay in peripheral blood reticulocytes and in total red blood cells; 0.5, 5.0 and 50 mg/kg bw per day for 3 days | NSC: 1, TiO2 NPs, anatase, ellipsoidal shape (TEM), minor axes 12.1 ± 3.2 nm | Negative | 2/limited | Sadiq et al. (2012) |
| Intravenous injection | ||||
| Gene mutation in gpt, Spi– (liver) and pig‐a (erythrocytes) Delta transgenic C57BL/6J mice; 2, 10 and 50 mg/kg bw for 4 weeks | NSC: 1, TiO2 NPs (P25), 15–24 nm | Negative | 1/limited | Suzuki et al. (2016) |
| Gene mutation in gpt, Spi– (liver) Delta transgenic C57BL/6J mice; 0, 2, 10 and 50 mg/kg bw for 4 weeks | NSC: 1, TiO2 NPs (P25), 15–24 nm | Negative | 2/limited | Suzuki et al. (2020) |
| LacZ mutation assay in liver and spleen C57BL/6 transgenic mice; 0, 10 and 15 mg/kg bw, i.v. on 2 days | NSC: 1, TiO2 NPs (NM‐102), 21–22 nm | Negative | 1/limited | Louro et al. (2014) |
| Intratracheal instillation | ||||
| Male Sprague–Dawley rats Pig‐a gene mutation assay in peripheral blood reticulocytes and in total red blood cells; 3 endotracheal instillation over 8 days; 0.5, 2.5 and 10 mg/kg (a total particle surface area lung deposition of 87, 437 and 1,700 cm2/lung) | NSC: 1 TiO2 NPs (P25), 15–24 nm | Negative | 2/limited | Relier et al. (2017) |
NSC: nanoscale considerations; NP: nanoparticle; i.v.: intravenous.
There are three in vivo gene mutation assays that were performed in mice with intravenous application, all with negative results. TiO2 NPs (< 30 nm) were administered to male gpt Delta transgenic C57BL/6J mice at doses up to 50 mg/kg bw once a week for 4 weeks, then Pig‐a mutation was analysed in erythrocytes and gpt and Spi– mutation in liver (Suzuki et al., 2016). In another assay performed in the same laboratory the gpt and Spi– genes were investigated in liver 90 days after the last injection (Suzuki et al., 2020). Louro et al. (2014) treated intravenously C57BL/6 transgenic mice and analysed LacZ mutations in liver and spleen.
Pig‐a gene mutation was analysed also in two studies in peripheral blood reticulocytes and in total red blood cells: one in male B6C3F1 mice, after intraperitoneal administration (Sadiq et al., 2012); one in male Sprague–Dawley rats after intratracheal administration (Relier et al., 2017). In both these studies, no mutagenic effects were reported.
In contrast, Trouiller et al. (2009) found a significant increase in large DNA deletions in an eyespot assay performed in fetuses after a 10‐day administration of TiO2 (P25) suspended in drinking water to pregnant C57BL/6Jpun/pun mice.
Concluding remarks
Several in vitro studies demonstrated the ability of TiO2 NPs to induce gene mutations in cultured mammalian cells. One in vivo study indicated the induction of large DNA deletions, however four other studies, that investigated different molecular targets suitable for identification of point mutations and small deletions, gave consistently negative results. Overall, the available experimental data do not confirm the potential of TiO2 NPs (< 30 nm) to induce gene mutations in vivo.
4.3.2. Induction of micronuclei/chromosomal aberrations in vitro and in vivo
In vitro micronuclei/chromosomal aberrations
Fifty‐six in vitro studies on MN frequency and structural chromosomal aberrations (CAs) in different cell lines were available for the evaluation ((44 papers, NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7 and 8) and industry (Documentation provided to EFSA No 15)) (Appendices J, L, N, P). Of 56 studies, 43 were classified as of high or limited relevance and further considered in the assessment (Figure 1).
Figure 1.
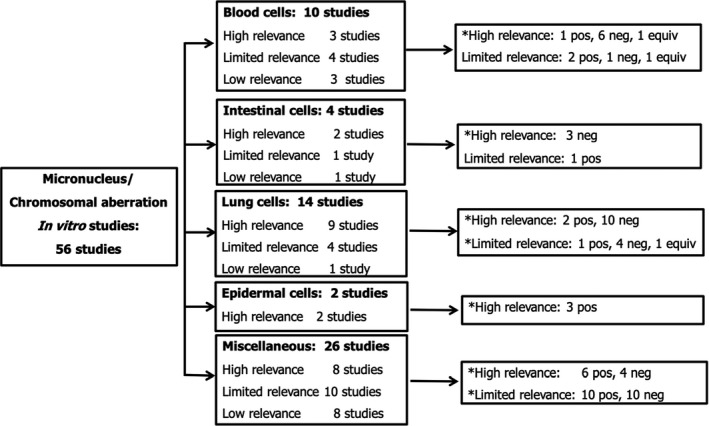
- *: Some of the studies used more than one test material and in these cases, the results are reported separately.
Three out of seven studies performed with primary human lymphocytes to which high or limited relevance was assigned showed positive results. In a study, classified of high relevance, a concentration‐dependent increase of MN frequency was observed in peripheral blood lymphocytes from healthy subjects and colon cancer patients (Kurzawa‐Zegota et al., 2017). Positive results in cultures of human peripheral lymphocytes were also reported in two studies with limited relevance (Turkez and Geyikoglu, 2007; Kang et al., 2008). Negative or equivocal results were described in four studies classified at high or limited relevance (NANOGENOTOX Project 2013 Documentation provided to EFSA No 7 and 8; Tavares et al., 2014; Andreoli et al., 2018; Osman et al., 2018)).
Three out of four studies performed with intestinal cells were considered relevant. One study, classified at high relevance, showed negative results with MN assays in Caco‐2 cells exposed at different concentrations of TiO2 NPs (Zijno et al., 2015). The outcome of this study is consistent with the results reported in the same cell line by the NANOGENOTOX Project, 2013 (Documentation provided to EFSA No 7 and 8). A single study showed concentration dependent increase of MN frequency in human colon adenocarcinoma (HCT116) cell line (Proquin et al., 2017).
Thirteen studies performed with lung cells were classified as relevant. Four out of five studies available in human lung epithelial cells (BEAS‐2B) were negative with MN tests after exposure at different concentrations of TiO2 NPs and for different times ((NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7 and 8); Vales et al., 2015; Di Bucchianico et al., 2017; Zijno et al., 2020). In Falck et al. (2009), negative (rutile, 5,000 nm) and equivocal (anatase, < 25 nm) results were reported. Positive results with the MN test were reported in BEAS‐2B cells only using a treatment medium that minimised the nanoparticle agglomeration (Prasad et al., 2013). Inconsistent results were reported in studies in human lung carcinoma cell line (A549). Two out of five studies were evaluated as positive (Srivastava et al., 2013; Stoccoro et al., 2017). Negative results were reported in two studies ((NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7 and 8); Brandão et al., 2020) classified at high relevance and in a study with limitations (Jugan et al., 2012). Negative results with the CA test were described in a Chinese hamster lung cell line (CHL/IU cells) (Nakagawa et al., 1997).
Two studies in human epidermal cell lines (A431, NHEK) to which high relevance was assigned were positive ((NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7 and 8); Shukla et al., 2011).
Twenty‐six studies carried out in various other types of cell lines of different origin, reporting results on MN frequency or on structural CAs were evaluated: eight of them were classified of high relevance and ten of limited relevance. The differences in the results observed in different studies could not be attributed to a certain parameter such as the crystalline form, particle size, degree of aggregation, treatment medium used, concentrations applied and treatment time.
The Panel noted that around 60% of the available results were obtained with TiO2 NPs < 30 nm. The majority of in vitro MN or CA tests gave negative results, regardless of the size of the tested particles (55% for TiO2 NPs < 30 nm and 67% for TiO2 NPs > 30). A single study tested E 171 in intestinal cells and reported positive results (Proquin et al., 2017).
In vivo micronuclei/chromosomal aberrations
Twenty‐six in vivo studies addressing the potential of TiO2 to induce MN and structural CA through various routes of exposure were available for the evaluation. After a screening for reliability and relevance of the results (Appendices K, M), 15 studies, ranked as of high (one study) and limited (14 studies) relevance, were selected for further consideration in the assessment (Table 8, Figure 2).
Table 8.
Summary table of test results of micronucleus and chromosomal aberrations in vivo studies. Studies within the same route of exposure are ordered by the size of the tested material
| Study design | Test material | Results | Reliability/Relevance | Reference |
|---|---|---|---|---|
| Oral | ||||
| MN in bone marrow rats 10–200 mg/kg per bw 30 days | NSC: 2, TiO2 NPs, Anatase 75 ± 15 nm | Negative | 2/limited | Chen et al. (2014) |
| MN and CA in bone marrow mice 200 and 500 mg/kg per bw 90 days | NSC: 4, TiO2 NPs, crystalline form unknown, mean 58 nm | Positive | 2/limited | Chakrabarti et al. (2019) |
| MN in bone marrow mice 10, 50, 100 mg/kg per bw 14 days | NSC: 1, TiO2 NPs Anatase, 20–50 nm | Positive | 1/high | Shukla et al. (2014) |
| CA in bone marrow mice 0.2–0.8 mg/kg per bw 28 days | NSC: 2, TiO2 NPs, Rutile, 21–31 nm | Positive | 2/limited | Manivannan et al. (2020) |
| MN in bone marrow mice 50, 100 and 200 mg/kg bw 60 days | NSC: 2, TiO2 NPs Anatase, 5–12 nm | Positive | 2/limited | Grissa et al. (2015) |
| Intraperitoneal injection | ||||
| MN assay in peripheral blood and in bone marrow mice 250, 500 and 1,000 mg/kg (1st exp.), 500, 1,000 and 1,500 mg/kg bw (2nd exp.) | NSC: 3, TiO2 Anatase > 100 nm | Equivocal | 2/limited | Shelby et al. (1993) |
| Chromosomal aberration in bone marrow mice 625–2,500 mg/kg bw Single administration | NSC: 3, TiO2, Anatase, > 100 nm | Negative | 2/limited | Shelby and Witt (1995) |
| MN in bone marrow mice 500–2,000 mg/kg bw 5 days | NSC: 1 TiO2 NPs, anatase/rutile, 44 nm | Positive | 1/limited | El‐Ghor et al. (2014) |
| MN in bone marrow mice 0.1–3 g/kg bw Single administration | NSC: 2, TiO2 NPs, Rutile, 28.88 nm (XRD) and 5–45 nm (TEM) | Positive | 2/limited | Lotfi et al. (2016) |
| MN in bone marrow mice 9.38–150 mg/kg bw 5 days | NSC: 2, TiO2 NPs Anatase, < 30 nm | Positive | 1/limited | Fadoju et al. (2019) |
| MN in bone marrow mice 10, 100 and 500 mg/kg bw Single administration | NSC: 2, TiO2 NPs Anatase, 20.17 nm (XRD) and 1–25 nm (TEM) | Equivocal | 2/limited | Zirak et al. (2016) |
| MN assay in peripheral blood reticulocytes Mice 0.5, 5.0 and 50 mg/kg bw/day, for 3 days | NSC: 1, TiO2 NPs, Anatase, ellipsoidal shape (TEM), minor axes 12.1 ± 3.2 nm | Negative | 2/limited | Sadiq et al. (2012) |
| Intravenous injection | ||||
| MN test in bone marrow PCE and reticulocytes Rats 5 mg/kg bw Single administration | NSC: 2, TiO2 NPs (NM‐105), 15–24 nm | Positive | 2/limited | Dobrzynska et al. (2014) |
| MN in peripheral blood reticulocytes Transgenic mice 2–50 mg/kg bw per week for 4 consecutive weeks | NSC: 1, TiO2 NPs (P25) 15–24 nm | Negative | 2/limited | Suzuki et al. (2016) |
| MN test in peripheral blood reticulocytes Transgenic mice 10 and 15 mg/kg bw 2 days | NSC: 1, TiO2 NPs (NM‐102), 21–22 nm | Negative | 2/limited | Louro et al. (2014) |
NSC: nanoscale considerations, MN: micronucleus assay, CA: chromosomal aberration assay, XRD: X‐ray diffraction; TEM: transmission electron microscopy, PCE: polychromatic erythrocytes; bw: body weight.
Figure 2.
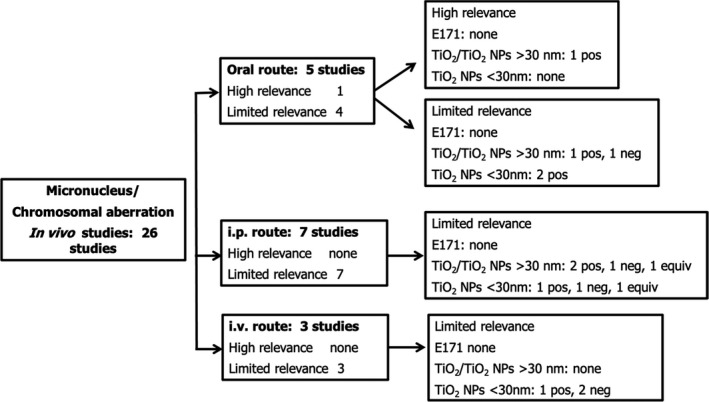
In vivo micronucleus and chromosomal aberration studies – Summary of test results
The five studies by the oral route were given higher weight. All these studies entailed repeated gavage administration of TiO2 NPs (from 14 to 90 daily treatments): of these, four were evaluated as positive for the induction of MN (Shukla et al., 2014; Grissa et al., 2015; Chakrabarti et al., 2019) or structural CAs (Manivannan et al., 2020) in mice. One (Chen et al., 2014) tested negative in the rat bone marrow MN assay, although some evidence of bone marrow exposure was provided by the concurrent analysis of H2AX foci.
Supporting evidence was provided by studies via intraperitoneal and intravenous injection.
Seven studies using the intraperitoneal route were considered. Increased incidences of MN in mouse bone marrow were observed after single (Lotfi et al., 2016) or repeated (El‐Ghor et al., 2014; Fadoju et al., 2019) injections of TiO2 NPs. Negative results were reported in a MN assay in mouse peripheral blood after three daily injections of TiO2 NPs (Sadiq et al., 2012), and in a CA assay in bone marrow cells of mice after single injection of TiO2 (> 100 nm) (Shelby and Witt, 1995). Equivocal results were reported in two studies (Shelby et al., 1993; Zirak et al., 2016).
Out of three intravenous studies, sufficiently reliable for consideration, one study was positive. The study was performed in rats, with single administration of TiO2 NPs. Increased incidence of MN was observed in bone marrow polychromatic erythrocytes (but not in reticulocytes) (Dobrzynska et al., 2014). Two other studies in mice, with two daily (Louro et al., 2014) or four weekly injection (Suzuki et al., 2016) did not report any significant increase of MN in peripheral blood reticulocytes.
In summary, the in vivo studies – one of them of high relevance and the others of limited relevance – were predominantly positive, independently of the route of exposure. Discrepant results were reported in some studies using comparable dose ranges, species and endpoint, which cannot be traced to size or other specificities of the test material. Rather, it is possible that differences in handling of TiO2 NPs, and dispersion protocols, which were insufficiently reported for most studies, were important variability factors.
Concluding remarks
Overall, based on the available lines of evidence, the Panel considered that ‐ on balance ‐ TiO2 NPs have the potential to induce MN/CA. The Panel noted that a significant portion of the studies was performed using TiO2 NPs < 30 nm, however some positive results were observed with TiO2 particles > 30 nm and no clear dependence of the particle size on positive effects in MN/CA assay was observed.
4.3.3. In vitro and in vivo Comet assay
In vitro Comet assay
One hundred and forty‐two in vitro studies (reported in 68 publications) using Comet assays in different cell lines were available for the evaluation, including data provided by the NANOGENOTOX Project (Appendices J, L, N). The NANOGENOTOX report summarises results from several laboratories who investigated TiO2 NPs in various cell lines ((NANOGENOTOX Project, 2013 Documentation provided to EFSA No 7, 8 and 10)). One hundred and six studies (reported in 43 publications plus NANOGENOTOX project, 2013) were classified as of high or limited relevance and further considered in the assessment (Figure 3). The range of TiO2 particle size tested was from 2.3 nm to 5 μm.
Figure 3.
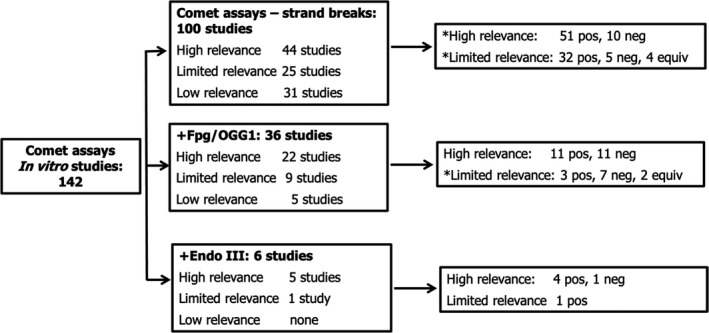
- *: Some of the studies used more than one test material and in these cases, the results are reported separately.
Of all the studies analysed, the great majority were performed on human cell lines originating from colon epithelium (Caco‐2, HT‐29 or a co‐culture of both), blood or lung tissue. Other human cells used were derived from liver, lymphoid, endothelial, epidermal, kidney or macrophage cell lines. Rodent cells from rat, mouse or hamster were also used.
The majority of studies ((NANOGENOTOX project, 2013 Documentation provided to EFSA No 7, 8 and 10); Zijno et al., 2015; Proquin et al., 2017; Schneider et al., 2017; Garcia‐Rodriguez et al., 2018; Brown et al., 2019; Murugadoss et al., 2020) were positive on Colon cancer cells (Caco‐2, HT‐29 alone or in co‐culture) showing an increase in DNA damage, i.e. strand breaks or strand breaks and formamidopyrimidine DNA glycosylase (Fpg)‐sensitive sites (Fpg detects oxidised purines). Some of the studies have been found to be negative (Dorier et al., 2017), or equivocal (Vila et al., 2018).
All five studies performed on human peripheral blood mononuclear cells (PBMC) were positive, most of them for strand breaks (Demir et al., 2013; Cowie et al., 2015; Kurzawa‐Zegota et al., 2017; Andreoli et al., 2018; Kazimirova et al., 2019) and also for Fpg‐ and Endo III‐sensitive sites (Demir et al., 2013). One from these studies showed a negative response in some donors (Kazimirova et al., 2019).
Two studies performed with human lymphoblastoid TK6 cells, showed DNA damage after exposure to TiO2 particles (Cowie et al., 2015; El Yamani et al., 2017) and two studies were negative (Magdolenova et al., 2012; Woodruff et al., 2012).
Fourteen studies (nine with high and five with limited relevance) used a lung model (cell lines A549, BEAS‐2B, 16HBE14o, HBE). The majority of these studies showed positive results regarding strand breaks (Falck et al., 2009; Karlsson et al., 2009; Jugan et al., 2012; NANOGENOTOX project, 2013 Documentation provided to EFSA No 7, 8 and 10; Prasad et al., 2013; Cowie et al., 2015; Wang et al., 2015; Biola‐Clier et al., 2017; El Yamani et al., 2017; Stoccoro et al., 2017; Murugadoss et al., 2020; Zijno et al., 2020)) as well as oxidised DNA lesions (Di Bucchianico et al., 2017; El Yamani et al., 2017; Stoccoro et al., 2017; Zijno et al., 2020). Only two studies were negative for strand breaks (Vales et al., 2015; Di Bucchianico et al., 2017).
A number of other Comet assay studies were performed with various human cell types such as HepG2, THP‐1, BeWo b30 placenta, HEK293, cerebral endothelial cells, HeLa, HUVECs, THP‐1, TH‐1, GM07492, MCF‐7, L‐02 human fetus hepatocytes, NHEK normal keratinocytes, HEp‐2 derived from HeLa, A431 keratinocytes, EUE human embryonic epithelial cells. The majority of these studies showed positive results (Osman et al., 2010; Shukla et al., 2011, 2013; Demir et al., 2013; NANOGENOTOX project, 2013 Documentation provided to EFSA No 7, 8 and 10); Cowie et al., 2015, Shi et al., 2015; Ferraro et al., 2016; Brown et al., 2019; Liao et al., 2019; Murugadoss et al., 2020; Kumar et al., 2020); however, some of them demonstrated negative results (Woodruff et al., 2012; Franchi et al., 2015; Sramkova et al., 2019; Elje et al., 2020) or were equivocal (Magdolenova et al., 2012; Brzicova et al., 2019).
The Panel also evaluated in vitro comet assay studies that were conducted on cells from monkey, rat, mouse or hamster origin showing a similar pattern of response, the majority of studies were positive (Nakagawa et al., 1997; Barillet et al., 2010; Guichard et al., 2012; Cowie et al., 2015; Stoccoro et al., 2016; Jain et al., 2017; Brown et al., 2019; Chakrabarti et al., 2019). Three from four different types of TiO2 tested in mouse lymphoma L5178Y cells by Nakagawa et al. (1997) were negative (anatase 21 nm, rutile 255 nm and rutile 420 nm) and one was positive (anatase 255 nm). In a study of Brown et al. (2019), E 171 was positive for strand breaks in all studied cell lines, and positive for oxidised DNA lesions only in one of them (HepG2) (Brown et al., 2019).
The Panel noted that around 57% of the available results were obtained with TiO2 NPs < 30 nm. No clear dependence of the positive effects on the particle size in the comet assay was observed. The majority of in vitro comet assay gave positive results, regardless of the size of the tested particles (87% positive findings for TiO2 particles > 30 nm and 78% positive findings for TiO2 NPs < 30 nm). Five studies of high or limited relevance investigated, by the in vitro Comet assay, the effect of E 171 treatment; 4 studies were positive for strand breaks and 1 negative.
In vivo Comet assay
The ability of TiO2 to induce single‐strand breaks (SSBs) and Fpg‐sensitive sites has been investigated by the in vivo comet assay. Thirty‐four studies published between 2015 and 2020, in addition to 10 previously considered by the ANS Panel (EFSA ANS Panel, 2016), have been evaluated in the current assessment. Eighteen studies out of 44 were classified as of high or limited relevance (Appendices K, M, Table 9 and Figure 4).
Table 9.
Summary table of test results in vivo Comet assay. Studies within the same route of exposure are ordered by the size of the tested material
| Study design | Test material | Results | Reliability/Relevance | Reference |
|---|---|---|---|---|
| Oral | ||||
| Rat; 50 and 500 mg/kg bw; once/week, 10 weeks; analysis: 24 h | NSC: 2, E 171 Anatase, three size groups of particles: 135, 305, 900 nm (TEM image) | Negative liver, lung | 1/high | Jensen et al. (2019) |
| Mice; 40 and 200 mg/kg bw per day; 7 days; analysis 24 h after the last dose | NSC: 3, TiO2, Anatase 160 nm | Positive BM Negative liver, brain | 2/limited | Sycheva et al. (2011) |
| Rat; 10 mg/kg bw per day; 7 days | NSC: 1, E 171: 118 nm Anatase (range 20–340 nm) and TiO2 NPs NM‐105) 15–24 nm | Negative Peyer's patch cells (both materials) | 2/limited | Bettini et al. (2017) |
| Mice; 10, 50, 250 μg/mouse; analysis: 3 days after treatment | NSC: 1, TiO2, Anatase 117 nm; and TiO2 NPs, anatase 17 nm | Positive Blood leucocytes | 2/limited | Murugadoss et al. (2020) |
| Mice; 10, 50, 100 mg/kg bw per day; 14 days; analysis: 24 h | NSC: 1, TiO2 NPs, Anatase, 20–50 nm | Positive Liver | 1/high | Shukla et al. (2014) |
| Rat; 0.5 mg/kg bw per day; 45 days; analysis: immediate | NSC: 1, TiO2 NPs, crystalline form unknown, 42 nm | Negative Blood and liver | 2/limited | Martins et al. (2017) |
| Mice; 0.2, 0.4, 0.8 mg/kg bw per day; 28 days; analysis: immediate | NSC: 2, TiO2 NPs, Rutile, 25 nm | Positive Liver, BM, spleen, thymus, lymph nodes | 2/limited | Manivannan et al. (2020) |
| Mice; 500, 1,000, 2,000 mg/kg bw per day; 7 days; analysis: day 8 | NSC: 4, TiO2 NPs, Anatase, 10–25 nm | Positive Liver and kidney | 2/limited | Shi et al. (2015) |
| Rat; 50, 100, 200 mg/kg bw per day; 60 days; analysis: 24 h | NSC: 2, TiO2 NPs, Anatase, 5–12 nm | Positive Leucocytes | 2/limited | Grissa et al. (2015) |
| Intraperitoneal injection | ||||
| Mice; 500, 1,000, 2,000 mg/kg bw per day 5 days; analysis: 24 h | NSC: 1; TiO2 NPs, Anatase/rutile 44 nm | Positive BM, liver, brain | 2/limited | El‐Ghor et al. (2014) |
| Mice; 50 mg/kg bw per day; 3 days; analysis: immediate | NSC: 1; TiO2 NPs Anatase 8.9–15.3 nm | Positive Liver +OGG1/EndoIII: lung and liver | 2/limited | Li et al. (2017b) |
| Intravenous injection | ||||
| Rat; 5 mg/kg bw; single dose; analysis: 24 h, 1 and 2 weeks | NSC: 2, TiO2 NPs (NM‐105), 15–24 nm | Negative Leucocytes (BM) | 2/limited | Dobrzynska et al. (2014) |
| Mice; 2,10, 50 mg/kg bw per week; 4 weeks; analysis: 3 days | NSC: 1, TiO2 NPs (P25), 15–24 nm | Negative Liver | 2/limited | Suzuki et al. (2016) |
| Rats; 0.59 mg/kg bw (single); analysis: 24 h, 1, 2, 4 weeks | NSC: 1, TiO2 NPs (NM‐105), 15–24 nm | Positive PBMC (only at 24 h) | 2/limited | Kazimirova et al. (2019) |
| Rat; 5, 25, 50 mg/kg bw per week; once/week, 4 weeks; analysis: immediate | NSC: 1, TiO2 NPs, Anatase 10–20 nm | Positive Brain | 2/limited | Meena et al. (2015b) |
| Intratracheal instillation | ||||
| Rat; 0.5, 2.5, 10 mg/kg bw (3 times every 4 days, over 8 days); analysis 2 h and 35 days | NSC: 1, TiO2 NPs (P25), 15–24 nm | Positive Lung (2 h, 35 days), liver (2 h, 35 days), blood (35 days) | 2/limited | Relier et al. (2017) |
| Mice; 18, 54, 162 μg/mouse (single); analysis: 1, 3 and 28 days | NSC: 1, TiO2 NPs, Rutile, 10 nm | Positive, Lung (28 days), liver (3–28 days); (negative: BAL) | 2/limited | Wallin et al. (2017) |
| Rat; 1, 5 mg/kg bw (single); 0.2, 1 mg/kg bw (once/week) x 5 weeks; analysis: 3 and 24 h | NSC: 1, TiO2 NPs, Anatase, 5 nm | Negative Lung | 1/limited | Naya et al. (2012b) |
BAL: bronchoalveolar lavage cells; NSC: nanoscale considerations, NP: nanoparticle; PBMC: peripheral blood mononuclear cells.
Figure 4.
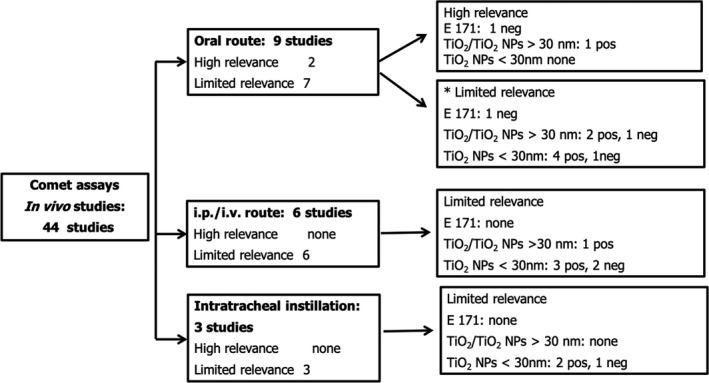
- *: Some of the studies used more than one test material and, in these cases, the results are reported separately.
Among the nine gavage studies selected for the assessment, six were positive (Sycheva et al., 2011; Shukla et al., 2014; Grissa et al., 2015; Shi et al., 2015; Manivannan et al., 2020; Murugadoss et al., 2020) and three were negative (Bettini et al., 2017; Martins et al., 2017; Jensen et al., 2019) including the two studies performed with E 171 (Bettini et al., 2017; Jensen et al., 2019). To identify possible factors responsible for the different outcomes of the assays, the Panel took into consideration physico‐chemical characteristics of TiO2 NPs (crystalline form, size of constituent particles, shape and agglomeration state), time of exposure, doses and target tissues.
The crystalline form, the size and the agglomeration state of TiO2 NPs have all been hypothesised to play a role in the genotoxic potential. The majority of the comet assays (in which the crystalline form is indicated) contained > 90% TiO2 in the anatase form. Only one study used pure rutile (Manivannan et al., 2020). No obvious correlation could be identified between specific physico‐chemical properties of the TiO2 particles and the outcome of the assays.
Neither the time of exposure, which ranged from a few days (3–7 days) up to several weeks (4–10 weeks) nor the administered TiO2 particles doses discriminated positive from negative results. In addition, the Panel calculated a cumulative dose by integrating dose and time of treatment (Table 9). This factor alone, however, appeared not to be the main determinant of the assay results.
Comet assays were performed on several target tissues. Negative results were reported for the lung (Jensen et al., 2019) and in the immune cells of the jejunal and ileal Peyer's patches (Bettini et al., 2017) and brain (Sycheva et al., 2011). Contradictory results were reported for leucocytes (Grissa et al., 2015; Martins et al., 2017; Murugadoss et al., 2020) and liver (Sycheva et al., 2011; Shukla et al., 2014; Shi et al., 2015; Martins et al., 2017; Jensen et al., 2019). Positive results were observed for bone marrow (Sycheva et al., 2011; Manivannan et al., 2020), thymus and afferent lymph node (Manivannan et al., 2020) as well as in the kidney (Shi et al., 2015) and spleen (Manivannan et al., 2020). The Panel noted that the majority of the positive results were obtained from organs of the reticulo‐endothelial system.
Additional information was provided by four studies with intravenous administration of TiO2 NPs (anatase or anatase/rutile being anatase > 80%, < 30 nm size). Negative results were reported in liver with TiO2 NPs (P25) (Suzuki et al., 2016), while positive ones were reported in the brain with TiO2 NPs (anatase, 10–20 nm) (Meena et al., 2015b). Using the same test item (NM‐105, a mixture of anatase and rutile), experimental conditions and harvest time, positive results were observed from peripheral blood leucocytes (Kazimirova et al., 2019) whereas leucocytes from the bone marrow were negative (Dobrzynska et al., 2014).
Intraperitoneal administration of TiO2 NPs induced SSBs in the liver, lung, brain and bone marrow in a large range of doses and a relatively short time of exposure (50–2,000 mg/kg bw for 3–5 days) (El‐Ghor et al., 2014; Li et al., 2017b).
Two studies using intratracheal instillation were negative with no DNA damage observed in bronchoalveolar lavage (BAL) (Wallin et al., 2017) and lung (Naya et al., 2012) (single and chronic exposures up to 5 weeks in the range of 1–5 mg/kg bw). In contrast, Wallin et al. (2017) and Relier et al. (2017) reported positive results in liver and lung. The induction of DNA damage in liver following intra‐tracheal instillation demonstrates a systemic effect which is possibly triggered by an inflammatory response observed in the lung.
Concluding remarks
Based on the results of the in vitro and in vivo comet assays, the Panel concluded that TiO2 particles have the potential to induce DNA damage. The Panel noted that a significant portion of the studies were performed using TiO2 NPs < 30 nm, however some positive results were also observed with TiO2 particles > 30 nm and no clear dependence of the particle size on positive effects in Comet assay was observed.
4.3.4. DNA Binding
The ability of TiO2 NPs (anatase, 5 nm) to bind DNA in vivo was investigated by Li et al., 2010. Binding to genomic DNA prepared from livers of TiO2 NPs‐treated ICR mice (by i.p. 5, 10, 50, 100 and 150 mg/kg bw per day for 14 days) was investigated by UV–Vis absorption spectroscopy, circular dichroism (CD), extended X‐ray absorption fine structure (EXAFS) spectroscopy and gel electrophoresis. A dose‐dependent increase in the content of TiO2 NPs in liver DNA was identified by ICP‐MS. CD spectroscopy indicates that changes in the DNA conformation occur in the 50–150 mg/kg bw per day dose range. In addition, EXAFS spectroscopy indicates that nano‐anatase TiO2 NPs can be bound with the oxygen or phosphorus atoms of the nucleotide and nitrogen atoms of base pairs in DNA.
After intranasal administration (300 μg/rat per day for 45 days), the interaction between TiO2 particles and liver DNA extracted from Sprague–Dawley rats was investigated by UV–Vis absorption spectrometry, atomic force microscopy (AFM), TEM, micro‐synchrotron radiation X‐ray fluorescence (m‐SRXRF) and gel electrophoresis (Jin et al., 2013). The analysed TiO2 particles were a) nano‐anatase (d < 25 nm); b) micro‐rutile (d < 5 μm); c) a mixture of 5–10% rutile and 90–95% anatase (d < 100 nm). DNA binding was observed with the TiO2 NPs anatase a) and TiO2 NPs rutile/anatase mixture c) but not with micro rutile b). According to the authors TiO2 NPs anatase can insert itself between DNA base pairs or covalently bind to DNA nucleotide via P–O–Ti–O bond. The Panel noted that evidence for covalent binding was not provided since a decrease in UV absorption can also be explained by non‐covalent interactions.
Assessment of in vitro DNA binding capacity of TiO2 NPs (< 100 nm) by UV–Vis spectroscopy identified an hyperchromic effect, probably due to strong stacking interactions between human genomic DNA and TiO2 NPs (Patel et al., 2016, 2017). In addition, fluorescence emission spectra of intercalated ethidium bromide and human genomic DNA incubated with increasing concentrations of TiO2 NPs indicate that these NPs also intercalate DNA strands. The authors suggest that all these results are due to electrostatic interactions between DNA and TiO2 NPs leading to conformational changes in DNA. A strong binding affinity of TiO2 NPs with human genomic DNA was identified by fluorescence spectroscopy (binding constant: 4.1 × 106 M−1). TiO2 NPs (14 nm) interaction with calf thymus DNA was studied by UV–Vis spectroscopy and molecular docking analysis (Ali et al., 2018). The hyperchromic behaviour observed by UV–Vis spectrometry confirms unwinding of double‐stranded DNA. The DNA binding constant was found to be 5.4 × 103 M−1. Molecular docking analysis revealed a selective binding of TiO2 NPs with A‐T bases in minor groove of DNA.
Hekmat et al. (2013) investigated the structural changes in calf thymus DNA caused by the combined exposure to TiO2 NPs anatase (< 10 nm) and doxorubicin (DOX) as well as by the single components. UV–Vis and CD spectrometry, thermal denaturation and fluorescence emission spectra demonstrated that there is an interaction of TiO2 NPs with DNA leading to changes in the secondary structure of the DNA helix.
By using a similar approach, the same group investigated the structural changes in calf thymus DNA induced by a combined treatment of TiO2 NPs anatase (< 10 nm) + paclitaxel (PTX) in comparison to single exposures to either compound. Upon addition of TiO2 NPs to the solution of DNA, hyperchromism was observed, indicating the formation of a complex between DNA and TiO2 NPs (Hekmat et al., 2020).
The ability of TiO2 NPs (21 nm) to interact with DNA (from salmon sperm) was confirmed by other analytical techniques (capillary electrophoresis coupled with UV and Fourier transform infrared spectroscopy). Electrostatic interactions of TiO2 NPs via the sugar‐phosphate backbone were demonstrated both with double‐stranded and single‐stranded DNA, with the last one showing stronger interactions (Alsudir and Lai, 2017).
The Panel noted that the interaction between TiO2 NPs and DNA resulted in spectrally contrasting effects when examined by UV–VIS spectrometry in vitro (hyperchromicity) or after exposure in vivo (hypochromicity).
In conclusion, there is evidence, from both in vitro and in vivo studies, for interaction(s) of TiO2 NPs with DNA. However, due to the techniques employed, the precise nature of these interactions, i.e. whether involving covalent or non‐covalent binding, could not be established.
4.3.5. Other studies
γH2AX foci and other markers of DNA Damage
The induction of γH2AX foci, a marker of DNA double‐strand breaks, by TiO2 particles was investigated in four in vitro and two in vivo studies.
Kathawala et al. (2015) reported a slight but statistically significant increase in the percentage of cells with γH2AX foci in human primary epidermal keratinocytes and a concomitant increase in the intracellular ROS. Toyooka et al. (2012) reported a concentration‐dependent increase in the induction of γH2AX foci in A549 human lung carcinoma cells both with micro‐ and (more pronounced) with nanoparticles and a concomitant induction of double‐strand breaks, as detected by biased sinusoidal field gel electrophoresis (BSFGE). On the contrary, Jugan et al. (2012) in the same cell line observed no similar effect on the induction of γH2AX foci. Exposure to TiO2 particles (12, 24 and 142 nm) did not significantly increase the amount of γH2AX foci in the nuclei of NRK‐52E rat kidney cells (Barillet et al., 2010).
In vivo, a dose‐dependent increase in cells with γH2AX foci was reported in the bone marrow of C57BL/6Jpun/pun mice by Trouiller (Trouiller et al., 2009). Another study (Chen et al., 2014) also reported a similar result in the bone marrow of Sprague–Dawley male rats, in the absence of MN induction.
Overall, the induction of γH2AX foci, a marker of DNA double‐strand breaks, was observed in two out of four in vitro studies and was also reported in two in vivo studies.
A ToxTracker assay, that includes the analysis of the expression of genes responsive to DNA damage and oxidative stress, was negative for all the endpoints in primary mouse embryonic fibroblasts (Brown et al., 2019).
Limited relevance was assigned to all of these studies.
Oxidised DNA bases
The induction of 8‐oxodG (as a marker of oxidation‐induced DNA damage) by TiO2 particles was investigated in five in vitro studies (to which limited relevance was assigned), four of which were positive.
Significant increases of 8‐oxodG in DNA (measured by HPLC with electrochemical detection) were reported in human peripheral blood mononuclear cells (PBMC, mixed population of lymphocytes and monocytes) after treatment with TiO2 NPs and TiO2 particles covering the nano and micro range (anatase, rutile and a mixture of anatase and rutile) (Andreoli et al., 2018). Also in human colon carcinoma Caco‐2 cells a significant increase in basal levels of 8‐oxodG compared to control was reported after treatment with anatase NPs (20–60 nm) (Zijno et al., 2015).
A treatment with TiO2 NPs (21 ± 9 nm) increased the level of 8‐oxodG lesions (HPLC–MS/MS) in two different human cell lines: BEAS‐2B normal bronchial lung cells and A549 alveolar carcinoma lung cells (Biola‐Clier et al., 2017). In another study using A549 alveolar carcinoma lung cells, the only oxidised base detected in cells exposed to TiO2 NPs (anatase 12 nm, P25 and rutile 21 nm) was 8‐oxodG whereas other lesions such as thymidine glycols, 5‐(hydroxymethyl)‐2’‐deoxyuridine, 5‐formyl‐2’‐deoxyuridine or 8‐oxo‐7,8‐dihydro‐2’‐deoxyadenosine were either below the detection limit or were not produced in higher frequency upon treatment. No induction of oxidised bases were observed with TiO2 (anatase, 140 nm) in this study (Jugan et al., 2012)
In contrast, no significant increase of the 8‐oxodG (HPLC–MS/MS) level was reported in a study (Dorier et al., 2017) in which a co‐culture of Caco‐2 colon and HT‐29 human colon cancer cells was treated with the additive E 171 and two different TiO2 NPs (12 ± 3 nm and 24 ± 6 nm).
In an in vivo study, oxidation‐induced DNA damage was investigated by measuring the level of 8‐oxodG in DNA isolated from livers of TiO2 NP‐treated and untreated mice (Trouiller et al., 2009). Administration of TiO2 NPs (P25) with drinking water at a dose of 100 mg/kg bw per day for 5 days resulted in a 1.5‐fold increase of the 8‐oxodG level in NP‐treated mice, compared to the control group.
Another in vivo study on the induction of 8‐oxodG (Rehn et al., 2003) was not considered relevant for this assessment because it was based on the analysis of lung cells after intratracheal instillation.
Overall, based on the available in vitro studies, nano‐ and microparticles of anatase and rutile seem to have a potential to induce oxidation of DNA resulting in 8‐oxodG.
Reactive oxygen species
The induction of ROS, such as superoxide radicals, hydroxyl radicals and hydrogen peroxide, was investigated in many in vitro and in vivo studies in parallel with investigation of DNA damage by comet and MN assays.
There are several in vitro studies in which the induction of ROS by TiO2 particles has been observed in different cell lines (Kang et al., 2008; Xu et al., 2009; Shukla et al., 2011, 2013; Wang et al., 2011, 2019; Guichard et al., 2012; Jugan et al., 2012; Saquib et al., 2012; Kathawala et al., 2015; Khan et al., 2015; Shi et al., 2015; Tomankova et al., 2015; Jain et al., 2017; Dorier et al., 2017; Liao et al., 2019; Santonastaso et al., 2019). In some of these studies, it was shown that the generation of ROS was inhibited by the addition of antioxidants (Kang et al., 2008; Xu et al., 2009) or SOD (e.g. Wang et al., 2011). In the study performed by Guichard et al. (2012), the induction of ROS in SHE cells was higher with TiO2 NPs (anatase and rutile < 100 nm) than with TiO2 anatase particles of 160 nm size and rutile particles of 530 nm size.
In contrast, no induction of intracellular ROS (evaluated by flow cytometry) was observed in an in vitro study in BEAS‐2B normal bronchial lung cells after one week of exposure to anatase TiO2 NPs (NM‐102) (Vales et al., 2015) and no significant increase of ROS was observed with E 171 at concentrations of 0.143 or 1.43 μg/cm2 in human colon carcinoma Caco‐2 cells (Proquin et al., 2017).
In vivo, increased levels of ROS, NO, MDA, IFN‐γ, TNF‐α and activation of NF‐κB and decreased levels of SOD and GSH‐Px as well as expression of apoptosis markers (p53, Bax, Bcl‐2 and cyto c) were observed in rats exposed to TiO2 NPs (anatase) by intravenous injection at doses up to 50 mg/kg bw once a week for 4 weeks (Meena et al., 2015b). TiO2 NPs induced increased ROS levels also after intratracheal instillation of mice (Danielsen et al., 2020). Likewise, exposure of mice to TiO2 NPs (anatase), administered by gavage at doses of up to 100 mg/kg bw per day for 14 days, resulted in increased levels of ROS (Shukla et al., 2014). In a study in which TiO2 NPs (anatase) were administered by gavage to mice at doses of 500, 1,000 and 2,000 mg/kg bw per day for 7 days, increased levels of ROS were observed at 1,000 and 2,000 mg/kg bw in a dose‐dependent manner in liver and kidney (Shi et al., 2015). However, no changes in the ROS levels were observed in these organs at 500 mg/kg bw, compared to the control group. The administration of TiO2 NPs at the dose of 1,000 mg/kg bw to Nrf2(‐/-) mice (in which Nrf2, a regulator for the expression of antioxidant genes, was knocked out) resulted in higher increases of the ROS levels in liver and kidney than the administration of the same dose to wild type mice (Shi et al., 2015).
Epigenetic DNA methylation
There are some studies in which epigenetic DNA methylation has been investigated.
A decrease of global DNA methylation was observed with TiO2 NPs (P25) in several mammalian cell cultures, whereas promotor methylation of several specific genes was increased in a study performed by Pogribna et al. (2020). In this study, altered expression levels of several genes involved in the regulation of DNA methylation were also observed (Pogribna et al., 2020). In a human lung carcinoma cell line A549, TiO2 NPs (P25) induced a statistically significant demethylation after 72 h of exposure while no effect was observed after 48 h (Stoccoro et al., 2017). A significant reduction of genomic DNA methylation levels after treatment with TiO2 NPs (60 nm) was observed in the human lung carcinoma cell line A549 and a human bronchial epithelial cell line in a study by Ma et al. (Ma et al., 2017). Bidirectional changes in methylation of some specific loci were observed in vitro by (Emi et al., 2020) with TiO2 particles (1 μm).
Epigenetic modifications, particularly in gene promoter regions, have been shown to result in changes in the expression of DNA repair genes. Biola‐Clier et al. (2017) studied overall DNA methylation and specific methylation of DNA repair gene promoters after TiO2 NPs (21 nm) exposure. They found that 31 out of 44 upstream DNA regulators for genes involved in several DNA repair pathways including nucleotide excision repair, base excision repair, mismatch repair and double‐strand break repair were downregulated.
These studies might be worth to address epigenetic endpoints. They were considered as supporting information for the evaluation of genotoxicity.
Cell transformation
Statistically significant increases in the frequency of morphologically transformed Balb/c 3T3 cells (mouse embryo fibroblasts) were observed after treatment with TiO2 NPs (P25) (Stoccoro et al., 2016). In a further cell transformation assay with the same cell line, a significant induction of transformed colonies (foci type III) was observed with rutile (micro‐ and nanosized), whereas no significant effects were observed with anatase (micro and nanosized) (Uboldi et al., 2016). Increased number of colonies growing in soft agar has been observed with TiO2 NPs (anatase, 21 and 50 nm) in human embryonic kidney cells and in mouse embryonic fibroblasts (NIH/3T3). The effect was concentration‐related and statistically significant at 1,000 μg/ml but not at 10 and 100 μg/mL and also not with microparticles (Demir et al., 2015). In another study, a statistically significant concentration‐dependent increase in the number of colonies growing on soft‐agar in human bronchial epithelial cells (BEAS‐2B cells) was shown for both the total of colonies and medium‐large size colonies after exposure to TiO2 NPs (anatase, 22 nm) at concentrations up to 20 μg/mL (Vales et al., 2015).
The cell transformation assays provide information on initial steps of carcinogenesis that may include both genotoxic and non‐genotoxic events. Results from cell transformation assays are considered to be of limited relevance for the evaluation of genotoxicity. Their relevance for the assessment of carcinogenicity is also limited.
4.3.6. Mode of Action
Numerous studies, both in vitro and in vivo, indicate that TiO2 particles, over a wide range of sizes, induce DNA strand breaks, oxidatively generated DNA lesions and chromosomal damage.
Several mechanisms have been proposed to explain the genotoxicity associated with TiO2 NPs exposure. They invoke DNA damage by reactive oxygen and nitrogen species (ROS/RNS) which may be triggered by inflammation, by intrinsic ability of TiO2 NPs or via induction of mitochondrial dysfunction. There is also some evidence that TiO2 NPs:DNA binding, possibly via electrostatic interactions, may alter DNA secondary structure.
In vivo exposure to TiO2 NPs can be associated with an inflammatory response. This occurs independently of the route of administration (Saber et al., 2012; El‐Ghor et al., 2014; Shukla et al., 2014; Meena et al., 2015b; Shi et al., 2015; Relier et al., 2017; Wallin et al., 2017; Murugadoss et al., 2020). This response is characterised by increased macrophage and neutrophil infiltration, the release of inflammatory mediators (chemokines, cytokines) and the increased production of ROS and oxidative stress markers (Dankovic et al., 2007; Olmedo et al., 2008; Chen et al., 2009). Following oral administration, the induction of DNA strand breaks was associated with an inflammatory response (Shukla et al., 2014). Along the same lines, intravenous administration of TiO2 NPs induced an increase in MN frequency associated with an inflammatory response (Kumar et al., 2016).
Co‐administration of TiO2 NPs and chlorophillin, a free‐radical scavenger, was associated with a reduction in DNA strand breaks and chromosomal damage (El‐Ghor et al., 2014). In addition, TiO2 NPs induced more DNA strand breaks in mice defective in the Nrf2 transcription factor (Nrf2‐/-) than in their wild type counterparts. Since Nrf2 regulates antioxidant and inflammatory responses (Shi et al., 2015), both sets of observations are consistent with a role of ROS/RNS and inflammation in TiO2 NPs‐associated DNA damage, which is reflected in positive findings in several genotoxicity studies. The Panel noted, however, that there is no simple correlation between the extent of TiO2 NP‐induced inflammation and DNA damage since an inflammatory response without genotoxic effects can also be observed (e.g. Saber et al., 2012).
An alternative mechanism invokes the intrinsic ability of TiO2 NPs to generate ROS/RNS, i.e. to generate reactive radicals also in an acellular system (Knaapen et al., 2004). This hypothesis is supported by experimental evidence coming from investigations with acellular systems (Gilmour et al., 1997; Fenoglio et al., 2009). Intracellular superoxide can also be formed by interaction of TiO2 NPs with proteins to form a TiO2 NPs corona (Jayaram and Payne, 2020). Additional potential sources of ROS include activation of cytoplasmic NADPH oxidases (Bedard and Krause, 2007) and damage to the mitochondrial membrane (Ghosh et al., 2013). With regard to the latter possibility, TiO2 NPs (< 100 nm) have been shown to be localised in liver mitochondria (Louro et al., 2014) and to induce mitochondrial swelling, promote membrane fluidity and increase ROS generation (Kathawala et al., 2015; Barkhade et al., 2019).
In vitro studies in different experimental models using blood, GI tract, liver, lung and other organs and tissues, showed an association between TiO2 induced DNA strand breaks/chromosome damage and oxidative stress, measured as increased ROS level and/or decreased level of antioxidants (Turkez and Geyikoglu, 2007; Shukla et al., 2011, 2013; Prasad et al., 2013; Srivastava et al., 2013; Proquin et al., 2017; Stoccoro et al., 2017; Liao et al., 2019; Tables on in vitro comet assay in Appendices J, L, N)
Additionally, in vitro studies indicated that TiO2 NPs exposure induced the well‐known premutagenic DNA lesion produced by ROS, i.e. 8‐oxodG (Shukla et al., 2011, 2013; Jugan et al., 2012; Demir et al., 2013; Stoccoro et al., 2016, 2017; Di Bucchianico et al., 2017; El Yamani et al., 2017; Schneider et al., 2017; Andreoli et al., 2018; Zijno et al., 2020). However, a lack of association between DNA strand breaks and DNA 8‐oxodG levels has also been reported. Indeed, increased DNA 8‐oxodG levels in Caco‐2 cells (Zijno et al., 2015) and human lung cells (Bhattacharya et al., 2009) were not associated with increased DNA strand breaks. Moreover, no firm conclusions can be drawn from the few in vivo studies investigating the level of TiO2 NPs‐induced oxidatively damaged DNA. No changes in the levels of liver fpg‐sensitive sites were found in intravenous‐treated Ogg1‐/- mice that are defective in the repair of DNA 8‐oxodG (Asare et al., 2016), whereas oral exposure increased levels of oxidatively damaged liver DNA (Trouiller et al., 2009; Shukla et al., 2014).
TiO2 NPs‐induced production of ROS may also trigger the formation of DNA double‐strand breaks as measured by ?H2AX foci in vitro (Kathawala et al., 2015; Wang et al., 2019). This property is reflected in an increase of CA and MN frequency in several in vitro studies (Turkez and Geyikoglu, 2007; Osman et al., 2010; Shukla et al., 2011; Prasad et al., 2013; Srivastava et al., 2013; Kurzawa‐Zegota et al., 2017; Proquin et al., 2017; Stoccoro et al., 2017; Liao et al., 2019). In some of these studies the increased MN frequency was correlated with DNA damage measured by the comet assay (Osman et al., 2010; Shukla et al., 2011, 2013; Prasad et al., 2013; Kurzawa‐Zegota et al., 2017; Proquin et al., 2017; Stoccoro et al., 2017; Liao et al., 2019). In addition, six in vivo studies investigated both the induction of DNA strand breaks and chromosomal damage in the same experimental setting. Four of these studies provided concordant positive results for both endpoints (El‐Ghor et al., 2014; Shukla et al., 2014; Grissa et al., 2015; Manivannan et al., 2020) indicating a potential clastogenic mode of action.
An indirect confirmation of an association between TiO2 NPs exposure and induction of DNA double‐strand breaks comes from the positive result reported in an in vivo DNA deletion assay in the pun locus (eye‐spot assay) by Trouiller et al. (2009). The eye‐spot assay detects deletions of one copy of a duplicated 70‐kb DNA fragment within the pun locus and is considered an experimental method suitable for the detection of homologous recombination events (Karia et al., 2013). Homologous recombination can be associated with the repair of double‐strand breaks or the processing of a blocked DNA replication fork. Therefore, a positive result in the eye‐spot assay could reflect the ability of TiO2 NPs to cause DNA breaks, consistently with the findings reported in the comet and in MN/CA studies.
On the other hand, no evidence of induction of gene mutations in vivo was reported in four different studies analysing target genes that are considered suitable for detecting a wide range of molecular events, including point mutations, small and large deletions (Pig‐a, gpt, Spi and lacZ mutations).
Studies applying kinetochore staining (Rahman et al., 2002) or FISH analysis (Stoccoro et al., 2017) indicate that MN induction by TiO2 NPs predominantly occurs via clastogenic events.
TiO2 NPs have been found in the nucleus either as single particles or as agglomerates (Jugan et al., 2012; Ahlinder et al., 2013; Shukla et al., 2013; Louro et al., 2014; Jain et al., 2017). Some studies failed to demonstrate internalisation of TiO2 particles into nuclei (Singh et al., 2007; Di Virgilio et al., 2010). In contrast, Ahlinder et al. (2013) demonstrated nuclear uptake by two techniques – TEM and Raman spectroscopy. Most of TiO2 uptake studies were performed in vitro on TiO2 NPs smaller than 30 nm. Bettini et al. (2017) reported that after administration of E 171, TiO2 particles could be internalised into nucleus. In addition, TiO2 particles were identified in the nuclei closely associated with the phosphorus‐positive chromatin signal (Bettini et al., 2017).
The mechanisms underlying nuclear internalisation is to a large extent unknown. Generally, NPs may be transported to the cell nucleus through nuclear pores by passive diffusion, or interaction with transport receptors (Panté and Kann, 2002; Terry et al., 2007). They also could enter the nucleus during the cell division (Kim et al., 2012). According to Ahlinder et al. (2013), their findings provide evidence of possible direct interactions between TiO2 NPs and DNA as a cause of genotoxicity.
Following nuclear internalisation, TiO2 NPs might interact with proteins involved in the control of chromosome segregation and the spindle apparatus. Chromosome mis‐segregation due to disturbance of mitotic progression has been described in NIH 3T3 cells following long‐term exposure to TiO2 NPs (Huang et al., 2009) and an interaction of TiO2 particles in E 171 with the centromere region during mitosis has been reported in colon HCT116 cells (Proquin et al., 2017). While aneugenicity seems to be a plausable effect for TiO2 particles present in the nucleus, the MN studies with centromere staining suggest that clastogenicity is the predominant mode of action.
A limited number of studies indicated that TiO2 NPs can interact with DNA. The authors of these studies conclude that binding of TiO2 NPs to DNA occurs via electrostatic and/or other kinds of non‐covalent interaction leading to minor alterations in DNA conformation and secondary structure (Hekmat et al., 2013; Jin et al., 2013; Patel et al., 2016; Alsudir and Lai, 2017; Ali et al., 2018; 2020). However, the Panel considered that while there is limited understanding of the non‐covalent interactions with DNA, there is no evidence of covalent binding to DNA.
4.3.7. Conclusions
Overall, combining the available lines of evidence, the Panel concluded that TiO2 particles had the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations.
No clear correlation was observed between the physico‐chemical properties of TiO2 NPs, such as crystalline form, size of constituent particles, shape and agglomeration state, and the outcome of either in vitro or in vivo genotoxicity assays.
There is some evidence for internalisation of TiO2 nanoparticles in the nucleus and mitochondria.
There is evidence for several modes of action for genotoxicity that may operate in parallel:
Direct interaction of TiO2 nanoparticles with DNA (there is no proof for covalent binding).
Direct formation of reactive (oxygen) species due to intrinsic properties of TiO2 nanoparticles.
Reactive (oxygen) species formation via TiO2 particles‐induced inflammation.
Reactive (oxygen) species formation via interference of TiO2 nanoparticles with mitochondrial function.
Additionally, there are indications that TiO2 particles may:
induce epigenetic modifications affecting the expression of genes involved in the maintenance of genome function (e.g. downregulation of some genes involved in DNA repair pathways).
interact with proteins involved in the control of chromosome segregation and the spindle apparatus.
The relative contribution of the modes of action mentioned above to the genotoxicity elicited by TiO2 particles is unknown and there is uncertainty on whether a threshold mode of action could be assumed. Even if it was assumed that all modes of action would be indirect, the available data would not allow identification of a threshold dose. Therefore, the Panel concluded that a concern for genotoxicity of TiO2 particles that may be present in E 171 cannot be ruled out. A cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified.
4.4. Exposure
4.4.1. Authorised uses and use levels
Maximum levels of E 171 have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, E 171 is authorised in four food categories at quantum satis (QS), in addition it is listed among the food colours of Group II authorised at QS in other 44 food categories. Table 10 lists the 48 food categories in which E 171 is authorised.
Table 10.
MPLs of E 171 in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exceptions | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.4 | Flavoured fermented milk products including heat‐treated products | Group II | QS | |
| 01.5 | Dehydrated milk as defined by Directive 2001/114/EC | Group II | Except unflavoured products | QS |
| 01.6.3 | Other creams | Group II | Only flavoured creams | QS |
| 01.7.1 | Unripened cheese, excluding products falling in category 16 | Group II | Only flavoured unripened cheese | QS |
| 01.7.3 | Edible cheese rind. | Group II | QS | |
| 01.7.4 | Whey cheese | Group II | QS | |
| 01.7.5 | Processed cheese | Group II | Only flavoured processed cheese | QS |
| 01.7.6 | Cheese products, excluding products falling in category 16 | Group II | Only flavoured unripened products | QS |
| 01.8 | Dairy analogues, including beverage whiteners | Group II | QS | |
| 03 | Edible ices | Group II | QS | |
| 04.2.4.1 | Fruit and vegetable preparations, excluding compote | Group II | Only mostarda di frutta | QS |
| 04.2.4.1 | Fruit and vegetable preparations, excluding compote | E 171 | Only seaweed based fish roe analogues | QS |
| 04.2.5.3 | Other similar fruit or vegetable spreads | Group II | Except crème de pruneaux | QS |
| 05.2 | Other confectionery including breath‐refreshening microsweets | Group II | QS | |
| 05.3 | Chewing gum | Group II | QS | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group II | QS | |
| 06.3 | Breakfast cereals | Group II | Only breakfast cereals other than extruded, puffed and/or fruit‐flavoured breakfast cereals | QS |
| 06.5 | Noodles | Group II | QS | |
| 06.6 | Batters | Group II | QS | |
| 06.7 | Pre‐cooked or processed cereals | Group II | QS | |
| 07.2 | Fine bakery wares | Group II | QS | |
| 08.3.3 | Casings and coatings and decorations for meat | Group II | Except edible external coating of pasturmas | QS |
| 09.2 | Processed fish and fishery products, including molluscs and crustaceans | Group II | only surimi and similar products and salmon substitutes based on Theragra chalcogramma, Pollachius virens and Clupea harengus | QS |
| 09.2 | Processed fish and fishery products, including molluscs and crustaceans | E 171 | Only fish paste and crustacean paste | QS |
| 09.2 | Processed fish and fishery products, including molluscs and crustaceans | E 171 | Only precooked crustacean | QS |
| 09.2 | Processed fish and fishery products, including molluscs and crustaceans | E 171 | Only smoked fish | QS |
| 09.3 | Fish roe | Group II | Except Sturgeons’ eggs (caviar) | QS |
| 12.2.2 | Seasonings and condiments | Group II | Only seasonings, for example, curry powder, tandoori | QS |
| 12.4 | Mustard. | Group II | QS | |
| 12.5 | Soups and broths | Group II | QS | |
| 12.6 | Sauces | Group II | Excluding tomato‐based sauces | QS |
| 12.7 | Salads and savoury‐based sandwich spreads | Group II | QS | |
| 12.9 | Protein products, excluding products covered in category 1.8 | Group II | QS | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group II | QS | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group II | QS | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group II | QS | |
| 14.1.4 | Flavoured drinks | Group II | Excluding chocolate milk and malt products | QS |
| 14.2.3 | Cider and perry | Group II | Excluding cidre bouché | QS |
| 14.2.4 | Fruit wine and made wine | Group II | Excluding wino owocowe markowe | QS |
| 14.2.5 | Mead | Group II | QS | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group II | Except spirit drinks as defined in Article 5(1) and sales denominations listed in Annex II, paragraphs 1–14 of Regulation (EC) No 110/2008 and spirits (preceded by the name of the fruit) obtained by maceration and distillation, Geist (with the name of the fruit or the raw material used), London Gin, Sambuca, Maraschino, Marrasquino or Maraskino and Mistrà | QS |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group II | QS | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group II | QS | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group II | QS | |
| 15.2 | Processed nuts | Group II | QS | |
| 16 | Desserts, excluding products covered in categories 1, 3 and 4 | Group II | QS | |
| 17.1 | Food supplements supplied in a solid form, excluding food supplements for infants and young children | Group II | QS | |
| 17.2 | Food supplements supplied in a liquid form, excluding food supplements for infants and young children | Group II | QS |
MPL: maximum permitted level.
Annex II part B1 lists all authorised food colours and according to Annex II part E, all food colours are authorised for use for the decorative colouring of egg shells, therefore E 171 is also authorised on the shell of eggs (FC 10.1 and 10.2).
E 171 is not authorised according to Annex III to Regulation (EC) No 1333/2008.
4.4.2. Exposure data
Reported use levels or data on analytical levels of E 171
Data on the occurrence of E 171 in food were collected at the time of the re‐evaluation of E 171 by the ANS Panel by means of a call for data launched in 2013.20 In response to this call, 61 use levels and 28 analytical results on E 171 were submitted to EFSA by industry and Member States, respectively (EFSA ANS Panel, 2016). These use levels cover 14 food categories.
Levels of E 171 in food have been reported in scientific publications (Lomer et al., 2000; Chen et al., 2013; Weir et al., 2012; Peters et al., 2014; Rompelberg et al., 2016; Kim et al., 2018; Lim et al., 2018; Taboada‐López et al., 2019). Most of these publications are reporting on the development of new methodologies for the analysis of E 171 in food. Foods usually analysed are chewing‐gum, coatings, fine bakery wares, beverage whiteners, flavoured drinks and sauces. Most of the analysed foods correspond to food categories already considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016). It has been noted that some of the use levels reported in scientific publications refer to food categories for which E 171 is not authorised in the EU. Since most of the publications are from outside Europe, it is uncertain if products in which E 171 has been analysed, are available on the European market.
In addition, in a report issued by the Dutch National Institute for Public Health and the Environment (RIVM) (Sprong et al., 2015), data on use levels submitted by industry in the Netherlands, as well as analytical data afterwards also published by Rompelberg et al. (2016) are reported.
For the current exposure assessment, the Panel considered, in addition to the data collected during the EFSA call for data in 2013, use levels reported by industry in the Netherlands in order to increase the number of food categories having data from 14 to 16, since two additional food categories were covered (Sprong et al., 2015). Levels used for estimating the dietary exposure assessment of E 171 are presented in Appendix Q.
Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information on over 3.6 million food and beverage products of which more than 1,300,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 member countries plus Norway and the UK presented in the Mintel's GNPD.21
For the purpose of this Scientific Opinion, the Mintel's GNPD22 was used for checking the labelling of food and beverage products and food supplements for E 171 within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, E 171 was labelled on more than 13,000 products of which 5,300 products were published in the database between January 2016 and February 2021. Since 2016, the number of food products labelled with E 171 is decreasing each year.
Appendix R lists the percentage of the food products labelled with E 171 out of the total number of food products per food subcategory according to the Mintel's GNPD food classification for the period January 2016–February 2021.22 The percentages ranged from less than 0.1% in many food subcategories to 52%. The highest percentages are for the subcategories ‘Sticks, Liquids & Sprays’ (52.2%), ‘gum’ (39%), ‘mixed assortments’ (20.9%), ‘lollipops’ (14.6%), ‘vitamins and dietary supplements’ (13.9%). The average percentage of food products in the EU labelled as containing E 171 was 1% of all the food products included in the subcategories in Mintel in which E 171 is listed.
As can be seen in Appendix R, some foods are labelled with E 171 because the additive is authorised in an ingredient of the food. This is the case for instance for ‘spoonable yoghurt’ (FC 01.4) and ‘chocolate tablets’ (FC 05.1) which contain dragées (i.e. confectionary) authorised to contain titanium dioxide (E 171) as belonging to the FC 05.4. For a few other food categories in which the use of E 171 is not authorised, foods were found labelled as containing E 171 (e.g. nectars, sucrose, tea, bread and bread products). In these categories, the number of foods labelled with titanium dioxide (E 171) is low (approximately 35 products in total).
Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level.23 Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011).
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons may not be appropriate. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 40 different dietary surveys carried out in 23 European countries (Table 11). Since more dietary surveys are available in the EFSA comprehensive database compared to 2016, more countries are now considered in each population group. As the 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011), in the present assessment, it was not estimated for infants from Italy and France, for toddlers from Belgium and Italy and for adolescents from Estonia.
Table 11.
Population groups considered for the exposure estimates of E 171
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Portugal, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Spain, Sweden, UK |
The term ‘toddlers’ in the Comprehensive Database (EFSA, 2011) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Comprehensive Database (EFSA, 2011).
Consumption records were codified according to the FoodEx2 classification system (EFSA, 2015). Nomenclature from the FoodEx classification system was linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform the exposure assessments. In practice, the FoodEx2 food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of E 171
The food categories for which occurrence data of E 171 are available were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx2 classification system), at the most detailed level possible (EFSA, 2015).
Data on the food supplements (FC 17) do not indicate which is the form (liquid or solid) of the product. Therefore, the levels were assigned to the whole FC 17.
The EFSA Comprehensive European Food Consumption Database considered in the current assessment is different from the one used in the re‐evaluation of E 171 (EFSA ANS Panel, 2016) and allows to consider FC 05.4 for which reported use level were available at that time.
16 food categories were taken into account as occurrence data were available (Appendix Q). For relevant food categories, the refinements considering the restrictions/exceptions when as set in Annex II to Regulation No 1333/2008 were applied.
4.4.3. Exposure estimates
Dietary exposure to E 171 from its use as a food additive
The Panel estimated the chronic dietary exposure to E 171 for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. The methodology to estimate dietary exposure to E 171 in the current assessment and the different scenarios – maximum level exposure assessment scenario, refined exposure assessment scenarios (brand‐loyal and non‐brand‐loyal) and food supplements consumers only exposure assessment scenario – are described in the approach for the refined exposure assessment of food additives under re‐evaluation (EFSA ANS Panel, 2017).
Table 12 summarises the estimated exposure to E 171 from its use as a food additive in six population groups (Table 11) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix S.
Table 12.
Summary of dietary exposure to E 171 from its use as a food additive in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Maximum level exposure assessment scenario | ||||||
|
0.06–3.6 0.2–15.8 | 0.9–12.8 2.9–31.4 | 1.9–11.5 5.9–31.3 | 1.3–6.2 4.0–18.6 | 0.7–6.7 2.4–15.9 | 0.4–4.9 1.9–12.7 |
| Refined exposure assessment scenarios | ||||||
| Brand‐loyal scenario | ||||||
|
0.05–3.5 0.1–14.3 | 0.8–10.0 2.6–28.0 | 1.7–9.7 5.2–25.4 | 1.1–5.0 3.3–14.9 | 0.6–5.5 2.0–13.1 | 0.4–4.2 1.7–10.4 |
| Non‐brand‐loyal scenario | ||||||
|
0.03–2.9 0.1–9.9 | 0.6–6.0 1.9–27.5 | 0.9–6.9 2.5–23.7 | 0.6–3.6 1.6–13.2 | 0.3–3.8 1.2–9.5 | 0.2–2.8 0.9–7.1 |
bw: body weight.
In the maximum level exposure assessment scenario, mean exposure to E 171 from its use as a food additive ranged from 0.06 mg/kg bw per day in infants to 12.8 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 0.2 mg/kg bw per day in infants to 31.4 mg/kg bw per day in toddlers.
In the brand‐loyal refined exposure assessment scenario, mean exposure to E 171 from its use as a food additive ranged from 0.05 mg/kg bw per day in infants to 10.0 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 0.1 mg/kg bw per day in infants to 28.0 mg/kg bw per day in toddlers. In the non‐brand‐loyal scenario, mean exposure ranged from 0.03 mg/kg bw per day in infants to 6.9 mg/kg bw per day in children. The 95th percentile of exposure ranged from 0.1 mg/kg bw per day in infants to 27.5 mg/kg bw per day in toddlers.
It can be noted that for infants, toddlers, children and adolescents, the maximum of the range of dietary exposure calculated is higher than in the 2016 EFSA ANS opinion. This is due to one dietary survey which was recently included in the EFSA Comprehensive database.
For the food supplements consumers only (results reported in Appendix T), mean exposure to E 171 from its use as a food additive ranged from 0.8 mg/kg bw per day for adults to 11.7 mg/kg bw per day for children. The 95th percentile ranged from 3.1 mg/kg bw per day for the elderly to 41 mg/kg bw per day for children.
The main food categories contributing to the exposure to E 171 are presented in Appendix U. In the non‐brand‐loyal exposure assessment scenario, the main contributing food categories for infants, toddlers and adolescents were fine bakery wares, soups and broths and sauces; they were soups and broths, sauces and salads and savoury‐based sandwich spreads for children, adults and the elderly. Processed nuts were also a main contributing food category for adults and the elderly (Appendix U).
An exposure estimate for E 171 considering data from scientific publications (Weir et al., 2012; Taboada‐López et al., 2019) was also performed. The six additional food categories considered were unripened cheeses (FC 01.7.1), processed cheeses (FC 01.7.5), breakfast cereals (FC 06.3), rice (FC 06.7), surimi (FC 09.2) and snacks (FC 15.1). Levels of E 171 for these foods were based on analytical data and were low compared to use levels reported for the other food categories. It is uncertain if products from these additional food categories are available on the European market, and therefore, the relevance of the data is unclear. However, even taking these six additional food categories into consideration resulted in only a small increase in the calculated dietary exposure to E 171 (up to an additional 0.01 mg/kg bw per day for some populations groups) (Appendix V). Some publications reported levels of E 171 in foods where E 171 is not authorised to be added in the EU (e.g. raw cows milk, fresh milk, long‐life milk, plain yoghurt (Rompelberg et al., 2016); chocolate products, ripened cheese,(Weir et al., 2012) and seafood products (Yin et al., 2017). As these are not authorised uses in the EU, these levels were not considered in the above assessment.
Uncertainty analysis
In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 13.
Table 13.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Methodology used to estimate high percentiles (95th) long‐term (chronic) exposure based on data from food consumption surveys covering only a few days | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
Occurrence data:
|
+ +/– |
| The 16 food categories which were taken into account in the refined exposure assessment scenarios out of all authorised food categories (N = 48), corresponded to different percentage, depending on the food categories (32%–96% of the amount (gram of foods by body weight) of food consumption documented in the EFSA Comprehensive Database) | – |
| Food categories included in the exposure assessment: no data for certain food categories which were therefore not considered in the exposure estimates (n = 32/48) | – |
Maximum level exposure assessment scenario:
|
+ |
Refined exposure assessment scenarios:
|
+/– |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
E 171 is authorised in four food categories and listed among the food colours of Group II authorised in other 44 food categories (Table 10).
In the current exposure assessment, 16 food categories were considered in the maximum level and in the refined scenarios. These food categories are the main food categories in terms of consumption levels. The Panel calculated that out of the foods authorised to contain E 171 according to Annex II to Regulation (EC) No 1333/2008, 32–96% of the amount of food consumed (by weight) per population group was reported to potentially contain E 171 as a food additive.
The Panel noted that information from the Mintel's GNPD (Appendix R) indicated that 49 out of 86 food subcategories, categorised according to the Mintel's GNPD nomenclature, in which E 171 was labelled were included in the current exposure assessment. These 49 food subcategories represented approximately 95% of the food products labelled with E 171 in the database. Furthermore, the percentage of foods per subcategory labelled to contain E 171 was maximally about 52% in sticks, liquids and sprays (on average, the percentage of food products in the EU labelled as containing E 171 was 1% of all the food products included in the subcategories in Mintel in which E 171 is listed) (Appendix R), while in the assessment, it was assumed that 100% of the foods belonging to an authorised food category contained the food additive.
Given these observations, the Panel considered overall that the uncertainties identified resulted in an overestimation of the exposure to E 171 from its use as a food additive according to Annex II to Regulation No 1333/2008 for all scenarios.
Oral exposure via other sources
E 171 is widely used as an excipient in medicinal products (Section 3). No information on the extent and level of use of E 171 in medicinal products was made available to EFSA and, therefore, its exposure from this use could not be considered. Exposure to TiO2 via cosmetics (e.g. toothpaste) is not considered in this opinion.
4.4.4. Exposure to TiO2 NPs from the use of E 171
Number‐based particle size distributions of a series of pristine E 171 food additives and of E 171 particles extracted from food products have been reported (EFSA FAF Panel, 2019; Verleysen et al., 2020, 2021; Geiss et al., 2021). In these publications, the size and shape of isolated constituent particles and of constituent particles in aggregates and agglomerates were measured by image analysis of representative transmission electron micrographs using ellipse fitting. The short (a‐axis) and long axes (c‐axis) of the ellipse fitted to the 2D projection of each constituent particle was measured as a proxy of its minimal and maximal external dimension, respectively. Assuming that the particles are prolate ellipsoids (a axis = b axis < c axis), the volume (V) of each particle was estimated as V = 4/3·π·a2 c and the mass (M) of each particle was calculated as M = V ϕ, with ϕ = the density of the particles (3.89 g/cm3 for anatase and 4.32 g/cm3 for rutile particles). More information on the analysis of E 171 is available in the report of a project focusing on the development of analytical methodologies that allow identification and characterisation of nanoparticles in food additives in their pristine state and in simple food matrices (Verleysen et al., 2021). Using this methodology, the mass and number percentages of particles in different samples of E 171 were calculated (also Appendix W; reported in Verleysen et al., 2021).
Taking into account the available data (Verleysen et al., 2021), it can be presumed that the mass of constituent particles below 100 nm could be up to 30%, where the mean of the 12 analysed samples is 25%. The Panel noted that different types of E 171 are used in food and the percentage by number of constituent particles below 100 nm can range from 5% (rutile) to around 50% (EFSA FAF Panel, 2019). The use levels of the different types of E 171 are unknown, and therefore, it is not possible to estimate accurately the exposure to nanoparticulate TiO2 from the use of E 171 (Table 12).
5. Uncertainty considerations
The Panel, after evaluating the scientific evidence available, has identified uncertainties related to the following points:
The size distribution of the particles in marketed E 171 that consumers are exposed to, related to the different types of E 171, as presented in the EFSA FAF Panel (2019) opinion.
The processes used by industry when using E 171 in food and to what extent these processes may affect the degree of agglomeration and thus internal exposure.
State of agglomeration i.e. presence of ‘free’ (non‐agglomerated) particles of tested material in GIT of the animals and its effect on absorption.
Representativity of different tested materials used in toxicity and genotoxicity studies for the food additive E 171 when used in food.
Differences in the physico‐chemical properties of the different tested materials and the extent of their impact on the observed results.
Interference in the measurements of Ti/TiO2 in blood, tissues or organs with the most widely used analytical technique, i.e. ICP‐MS, and its impact on the reliability of tissue concentration data.
Confidence in the limited kinetic data as the basis for estimating half‐lives and accumulation and for assessment of internal exposure and, related to that, the extent of systemic availability.
None of the rodent studies were sufficiently long to cover the time needed for reaching the steady state for accumulation and this impacted the interpretation of the study results.
Relative contribution of different molecular mechanisms leading to the production of ROS resulting in the genotoxicity of TiO2 (inflammation, interaction with mitochondria, intrinsic potential of TiO2 to generate ROS).
Several modes of action for the genotoxicity may operate in parallel. The relative contributions of different molecular mechanisms elicited by TiO2 particles are unknown; it is unclear if a threshold mode of action could be assumed.
Nature of the interactions between DNA and TiO2 particles leading to conformational changes in DNA
Due to large amount of information that needed reviewing in the limited time available, a structured uncertainty analysis in line with the EFSA Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b) was not possible. However, the Panel took a conservative approach in reaching the final conclusions.
In relation to the exposure assessment (Section 4.4.3), the Panel considered that the uncertainties identified resulted in an overestimation of the exposure to E 171 from its use as a food additive according to Annex II to Regulation No 1333/2008 for all scenarios.
6. Discussion
The safety of E 171 was re‐evaluated by EFSA in 2016 in the frame of Regulation (EU) No 257/2010, as part of the re‐evaluation programme for food additives authorised in the EU before 20 January 2009 (EFSA ANS Panel, 2016). On the basis of the information available at that time, the EFSA ANS Panel considered that E 171 mainly consisted of micro‐sized TiO2 particles, with a nano‐sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E 171 were however highlighted, noting that no limits for the particle size of E 171 were set in the EU specifications. The ANS Panel concluded that, based on the data available at that time, E 171 when used as a food additive did not raise concern with respect to genotoxicity and that it was not carcinogenic after oral administration. Taking into account the presumed limited absorption of TiO2, the ANS Panel concluded that, based on a margin of safety (MoS) calculated from the no‐observed‐adverse‐effect level (NOAEL) of 2,250 mg TiO2/kg bw per day (identified from a carcinogenicity study in rats) and the exposure, calculated based on the reported use levels and analytical data, E 171 would not be of concern. However, given the toxicological data set at that time, the ANS Panel identified data gaps and uncertainties that required follow‐up by the European Commission by means of a call for data aimed at gathering information from IBOs. In particular, in order to address concerns related to the lack of adequate data on reproductive and developmental toxicity, the ANS Panel recommended that an EOGRT study be performed. An EOGRT study was commissioned by IBOs and its study protocol was later amended to accommodate the investigation of additional parameters related to the occurrence and TiO2‐related induction of ACF in the colon, which are preneoplastic lesions that had been reported by Bettini et al. (2017) shortly after the completion of the ANS Panel re‐evaluation of E 171.
Subsequent to the evaluation of data submitted by IBOs on the characterisation of E 171 used as a food additive in the EU, the Panel recommended that the EU specifications for E 171 include the parameter of median minimum external dimension by particle number should be higher than 100 nm, measured by electron microscopy, which is equivalent to less than 50% of constituent particles by number with a minimum external dimension below 100 nm (EFSA FAF Panel, 2019).
Based on the presence of a fraction of nanoparticles in E 171, the food additive falls under the scope of the EFSA Guidance on nanotechnology, which was broadened in its 2018 revision to cover also ‘a material that is not engineered as nanomaterial but contains a fraction of particles, less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm’ (EFSA Scientific Committee, 2018a).
For the reason given above, the proposed amendment to the specifications of the food additive E 171 (EFSA FAF Panel, 2019) was accompanied by a recommendation by the Panel for a re‐assessment of the toxicological data set in line with the data requirements specified in the EFSA Guidance on nanotechnology (EFSA Scientific Committee, 2018a).
Scientific criteria for implementing the provisions of the EFSA Guidance on nanotechnology (EFSA Scientific Committee, 2018a) and specific information on the characteristics of TiO2 nanoparticles were considered when preparing the advice from the ccWG Nano. The advice elaborated on the NSC and adaptations related to specific aspects of study design with TiO2 which are adequate for a hazard identification and hazard characterisation of small particles, including nanoparticles (Appendix E). Following this advice (Appendix E), toxicokinetic and toxicity studies were scored for NSC (dispersion and/or confirmation of internal exposure). The confidence for assessing the toxicological effects of the fraction of small particles, including nanoparticles was as follows:
Scoring 1 for NSC: the study is suitable.
Scoring 2 for NSC: the study has some limitations.
Scoring 3 for NSC: the relevance of the results cannot be verified.
Scoring 4 for NSC: the relevance of the results is low.
As mentioned above, the characterisation of E 171 was previously evaluated by the Panel who noted that E 171 currently contains nanoparticles at different percentages (EFSA FAF Panel, 2019). Moreover, from samples of E 171 or in E 171 extracted from foods the percentage by number of particles below 30 nm is in the order of 1% or less (Verleysen et al., 2020, 2021; Geiss et al., 2021, Appendix W) Therefore, the Panel considered that studies performed with TiO2 NPs that predominantly consist of particles smaller than 30 nm (e.g. P25) are of limited relevance to the safety assessment of E 171. However, data from toxicity studies performed with TiO2 < 30 nm have been considered for completeness of the database and may be relevant with respect to whether a minimum limit for particle size should be included in the EU specifications for E 171.
The Panel considered that E 171 has a low oral systemic availability, probably not higher than 0.5% (Section 4.1.2). It may pass the placenta and be transferred to the fetus. Rat studies showed long half‐lives for TiO2 NP (7–90 nm) (roughly 200–450 days), a potential for accumulation (accumulation factor of 290–450) and a long time to reach steady state (3–5 years) could be estimated from these studies. The oral systemic availability of TiO2 NP was low (most probably < 1%) but higher than for E 171. In tissues from deceased subjects, TiO2 particles were identified in liver, spleen, kidney and intestinal tissues. The low Ti amount of the investigated organs indicated low oral systemic availability of TiO2 ingested from a number of sources, including dietary exposure to E 171.
The Panel noted that none of the studies were sufficiently long to cover the time needed for reaching the steady‐state for accumulation. Therefore, the Panel considered that this could contribute to the uncertainty for the interpretation of the toxicological findings.
In mice, no adverse effects associated with general toxicity were observed up to 1,000 mg E 171/kg bw per day, the highest dose tested, for dosing durations up to 90 days (Han et al., 2020a, scoring 2 for NSC). Also in rats, no adverse effects associated with general toxicity were observed in the EOGRT study with E 171 (Documentation provided to EFSA No 1) (scoring 4 for NSC) at doses up to 1,000 mg/kg bw per day (Section 4.2). In rat toxicity studies with TiO2 NPs or TiO2 containing a fraction of nanoparticles, having different duration (14–90 days), no adverse effects were observed up to the highest dose tested (from 40 to 100 mg/kg bw per day). Overall, in a weight of evidence consideration, no adverse effects associated with general toxicity were observed in rats orally exposed to E 171, TiO2 NPs or TiO2 containing nanoparticles.
In mice orally exposed to TiO2 NPs < 30 nm for up to 90 days, some effects were reported, which by their nature could be adverse. However, mild hyperbilirubinaemia was not accompanied by any in liver enzymes (Yang et al., 2017); the effect size of increased fasting glycaemia and impaired glucose tolerance (Hu et al., 2015) was not accompanied by changes in insulin or other changes in lipid metabolism and therefore was not of toxicological relevance. Histopathological changes were reported in the heart (Yu et al., 2016), however, these findings were not supported by incidences and severity scores. Histopathological findings indicating inflammation were reported in the liver but investigations to confirm hepatic injury were not performed (Hong et al., 2016).
In rats orally exposed to TiO2 NPs < 30 nm, inconsistent and/or unexplained sex differences in some parameters were reported (e.g. hypobilirubinaemia in females (Chen et al., 2015a); heart rate and blood pressure changes in females (Chen et al., 2015b); leucocyte changes in females (Heo et al., 2020); higher absolute pituitary weights in males (Heo et al., 2020); lower blood insulin levels in females, lower C‐peptide levels in males and differences in blood concentrations compared to controls in a glucose tolerance test in males (Chen et al., 2020b).
The Panel considered that the effects reported in mouse studies with TiO2 NPs < 30 nm could be associated with accumulation of NPs in various tissues, whereas inconsistent findings in rats were considered incidental.
No effects of E 171 on sexual function and fertility in either male or female rats, and on pre‐ and postnatal developmental were observed in the EOGRT study with E 171 (Documentation provided o EFSA No 1, scoring 4 for NSC) up to 1,000 mg/kg bw per day, the highest dose tested (Section 4.2.7). No other reproductive or developmental toxicity studies performed with E 171 have been identified from the published literature that were considered sufficiently reliable (see Appendix H). No maternal and developmental effects were observed up to 1,000 mg/kg bw per day, the highest dose tested, in a single rat developmental toxicity study with five different TiO2 materials, TiO2 NPs or TiO2 containing a fraction of nanoparticles (Warheit et al., 2015b) (scoring 4 for NSC).
In mice, the effects of TiO2 NPs < 30 nm on the testis (decreased weight, decreased seminiferous tubule diameter, germ cell apoptosis) and sperm (decreased sperm counts and motility, increased percentage of abnormal spermatozoa) were observed in three studies (Khorsandi et al., 2016, 2017; Karimi et al., 2019) at doses ranging from 50 to 300 TiO2 NPs mg/kg bw per day. In a mouse study by Lu et al. (2020), no effects were observed at the lowest dose tested, 10 mg/kg bw per day (scoring 4 for NSC). In rats, administration of TiO2 NPs (21 nm) did not show effects at any dose level in a developmental toxicity study up to 1,000 mg/kg bw per day (Lee et al., 2019) (scoring 3 for NSC).
No neurotoxicity studies performed with E 171 have been identified from the published literature that were considered sufficiently reliable (see Appendix C). Based on the results of the EOGRT study in rats with E 171 (Documentation provided to EFSA No 1, scoring 4 for NSC), the Panel considered that E 171 had no adverse effects on neurofunctional endpoints in F1 cohort 2A offspring up to 1,000 mg/kg bw per day (Section 4.2.8).
TiO2 NPs orally administered to rats during embryofetal and early postnatal development reduced hippocampal neurogenesis with TiO2 NPs (< 100 nm) at 100 mg/kg bw per day exposure (Ebrahimzadeh et al., 2017), and dosed to adult rats produced adverse effects in the brain consistent with indications of oxidative stress with TiO2 NPs (90 nm) at 500 mg/kg bw per day (Kandeil et al., 2019).
After oral dosing with TiO2 NPs < 30 nm, adverse effects in both adult and developing mouse and rat brain were observed in studies identified from the published literature. Most of these effects are possibly related to oxidative stress. In mice, the Panel noted that the reduced volume of the polymorph layer of the hippocampal dentate gyrus and reduced density and number of dentate gyrus granular neurons reported by Rahnama et al. (2020, scoring 4 for NSC) with TiO2 NPs (21 nm), is consistent with the behavioural effects reported by Zhang et al. (2020, scoring 3 for NSC), i.e. increased open field anxiety‐like behaviour and unaffected spatial learning and memory. Ventral dentate gyrus is associated with anxiety behaviour, CA regions with spatial learning/memory (Eagle et al., 2016; Anacker et al., 2018). In adults rats, the most sensitive endpoint in the evaluated studies was reduced brain cholinesterase activity and increased brain Na/K‐ATPase activity with TiO2 NPs (21 nm) at a dose of 0.5 mg/kg bw per day in females dosed for 14 days (Canli et al., 2020, scoring 4 for NSC).
The Panel noted that inhibition of cholinesterase activity by nanoparticles other than TiO2, both metal and plastic, has been reported in a number of species (Prüst et al., 2020). Since oxidative stress‐related inflammation is generally associated with increased and not decreased cholinesterase activity (Corrêa Mde et al., 2008; Vaknine and Soreq, 2020), it is unclear whether there is a link between TiO2‐induced oxidative stress and TiO2‐induced decrease in cholinesterase activity.
For neurotoxicity, adverse effects were seen with TiO2 NPs < 30 nm. In mice, Zhou et al. (2017), reported adverse effects (i.e. inhibited dendritic outgrowth, increased autophagy and oxidative stress and reduced mitochondrial function) in ex vivo hippocampal neurons of weanling mice after dosing TiO2 NPs (6–7 nm) during gestation and early lactation at a dose of 1 mg/kg bw per day, the lowest dose tested. In adult female rats (Canli et al., 2020), adverse effects (reduced brain cholinesterase activity, and increased brain Na/K‐ATPase activity) were observed with TiO2 NPs (21 nm) at 0.5 mg/kg bw per day, the lowest of three doses tested, in a 14‐day study. The Panel noted that Canli et al. (2020) was scored 4 for NSC.
In the immunotoxicity cohort of the EOGRT study with E 171 (Documentation provided to EFSA No 1, scoring 4 for NSC), a slight, but statistically significant decrease (–9%) in antigen specific IgM level was measured at the highest dose tested (1,000 mg/kg bw per day) in males, but without an apparent dose response. Due to technical limitations in this part of the study, it is currently not possible to conclude on the developmental immunotoxicity of E 171 (Section 4.2.9).
From other studies identified from the published literature with E 171, the Panel concludes that these studies suggest an immune dysregulatory activity of E 171, evidenced by several immune‐related and inflammatory markers.
In the Han et al. (2020a) 90‐day study, effects on GM‐CSF and IgM and were observed following exposure to E 171, however the Panel noted the lack of a dose response, the magnitude of the effect was small that did not allow a firm conclusion given the natural variability in the parameters measured. It should be noted that in three single dose level studies with E 171 inflammatory effects were noted at lower doses, i.e. 2, 5 and 10 mg/kg per day, respectively, in rats (Bettini et al., 2017) and in mice (Urrutia‐Ortega et al., 2016; Talamini et al., 2019).
Effects of E 171 may, at least in part, stem from the activity of the fraction of the smaller TiO2 particles, as studies with these particles also indicate inflammatory effects of exposure to TiO2 NPs (5–6 nm) at 2.5 mg/kg per day (Yu et al., 2016).
Although not a requirement in the OECD TG 443, an evaluation of ACF in the colon of satellite F0 animals was investigated in the EOGRT study (Section 4.2.6). From this study (scoring 4 for NSC), the Panel considered that oral exposure to E 171 at doses up to 1,000 mg/kg bw per day did not induce ACF in the colon. Two additional studies reporting information on ACF were identified from the literature search. From the study by Bettini et al. (2017) (scoring 1 for NSC), previously reviewed by the ANS Panel (EFSA ANS Panel, 2018), the Panel considered that E 171 at a dose of 10 mg/kg bw per day may induce ACF per se. In addition, E 171 enhanced ACF formation after pretreatment with a genotoxic carcinogen (i.e. DMH) in rats. From a more recent study (Blevins et al., 2019) (scoring 3 for NSC), the Panel noted that no changes in the number of ACF and ABC were observed due to E 171 exposure alone. However, limitations in the pathological examination of ABC and ACF (sampled colon area limited; technical issues with fixation) precluded a conclusion by the Panel on any potential for ABC and ACF formation. Dietary E 171, with or without treatment with DMH, had no effect on the length of the colonic glands examined or the number of goblet cells/unit.
Overall, the Panel noted that the effect of E 171 alone (without prior initiation) in producing ACF reported by Bettini et al., has not been replicated in later investigations (EOGRT and Blevins et al., 2019), but one of these investigations (Blevins et al., 2019) had methodological limitations. Furthermore, it is unclear to what extent animals were exposed to NPs in the EOGRT and Blevins et al. (2019). The Panel considered that E 171 may induce ACF in male rats at a dose of 10 mg/kg bw per day when it is dispersed in test vehicle preventing agglomeration of NPs prior to administration. The Panel noted that there is literature indicating that ACFs may be a risk factor for human colorectal cancer (Anderson et al., 2012; Drew et al., 2018; Quintanilla et al., 2019; Hong et al., 2019; Clapper et al., 2020; Kowalczyk et al., 2020; Siskova et al., 2020).
No new publications on chronic toxicity or carcinogenicity have been identified in the literature search. During the re‐evaluation of E 171 in 2016, the ANS Panel had evaluated a carcinogenicity study in mice and rats (NCI, 1979), performed with TiO2 mixed with the diet. The ANS Panel had concluded that the study indicated that TiO2 was not carcinogenic in rats and mice. However, in the current opinion, the Panel considered that this study was not appropriate to ascertain the absence of a potential to elicit chronic toxicity and carcinogenicity by TiO2 nanoparticles.
A number of studies, considered to be reliable by the Panel, have examined or included analyses of GIT microbiota changes in response to exposure to E 171, TiO2 NPs and TiO2 NPs < 30 nm. There is no consensus on quantifying the extent of GIT microbiota changes and when such changes should be considered adverse. Therefore, the Panel was unable to come to any conclusion regarding the effects of E 171 on GIT microbiota and related effects on health.
Combining the available lines of evidence, the Panel considered that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. The Panel noted that the largest particle sizes resulting in genotoxicity after oral exposure were TiO2 (160 ± 59 nm) in an in vivo comet assay (Sycheva et al., 2011) and TiO2 NPs (58 ± 8 nm) in in vivo MN and CA assays (Chakrabarti et al., 2019). Negative results were observed in two studies with particles of similar or larger size, i.e. in an in vivo MN assay with TiO2 NPs (75 ± 15 nm) (Chen et al., 2014) and in an in vivo comet assay with E 171 reported by the authors to be distributed in three size groups of particles as observed from the TEM images (135 ± 46 nm, 305 ± 61, 900 ± 247 nm) but without quantitative information of each side group (Jensen et al., 2019). The Panel noted that the ranges of the particles sizes in studies resulting in a positive or negative outcome were overlapping, the number of negative studies performed with large particles is low. Accordingly, a cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified. The Panel also considered that no clear correlation was observed between other physico‐chemical properties of TiO2 NPs, such as crystalline form, shape and agglomeration state and the outcome of genotoxicity assays.
The relative contribution of the MOAs, which may operate in parallel, to the resulting genotoxicity of TiO2 is unknown and there is uncertainty on whether a thresholded mode of action could be assumed. Even if it was assumed that all potential modes of action would be indirect, the available data would not allow identification of a threshold dose. Therefore, the Panel concluded that a concern for genotoxicity of TiO2 particles cannot be ruled out.
Considering the technical data, including heterogeneous information on particle size of E 171, as provided by IBOs during the re‐evaluation of this food additive (EFSA ANS Panel, 2016), the ANS Panel considered that the highest reported percentage value of 3.2% of nanoparticles (< 100 nm) by mass, could be used as an estimate of NPs in E 171. In contrast, the current data (Verleysen et al., 2020) suggest E 171 can contain about 30% of nanoparticles < 100 nm by mass. Therefore, in the current assessment, the Panel took into consideration the principles established in the EFSA Guidance on Nanotechnology (2018). In addition, a literature review was performed for the current assessment that also captured studies performed specifically with TiO2 NPs. Data on the potential genotoxicity of TiO2 – that was not previously identified as relevant based on the available information on maximum percentage value of nanoparticles present in E 171 for the 2016 re‐evaluation of E 171 – were also included in the current assessment.
7. Conclusions
Concerning the content of nanoparticles in E 171, the Panel considered that:
-
–
according to the Regulation EU (No) 231/2012, there is currently no limitation for the content of nanoparticles in E 171.
-
–
according to data received from interested business operators, less than 50% of constituent particles in E 171 have a minimum external dimension below 100 nm by number (EFSA FAF Panel, 2019).
-
–
the percentage by number of constituent particles below 30 nm was in the order of 1% or less in samples of pristine E 171 or in E 171 extracted from foods analysed after dispersion.
-
–
TiO2 particles in pristine E 171 likely form large agglomerates. When dispersion procedures are applied, these agglomerates may deagglomerate, resulting in increased numbers of ‘free’ nanoparticles. The extent of agglomeration and number of ‘free’ nanoparticles present may be further affected by the conditions in food and the GIT environment.
Accordingly, the Panel concluded that studies with TiO2 nanoparticles were relevant in the current risk assessment of E 171. However, studies performed with TiO2 NPs that predominantly consisted of particles smaller than 30 nm were considered to be of limited relevance.
Concerning absorption and toxicity of TiO2 particles that are present in E 171, the Panel concluded that:
the absorption of TiO2 particles is low, however they can accumulate in the body due to their long half‐life;
studies on general and organ toxicity, including the newly performed EOGRT study with E 171, did not indicate adverse effects up to a dose of 1,000 mg/kg bw per day. Also, no effects were seen in studies retrieved from the literature with TiO2 NP > 30 nm up to the highest dose tested of 100 mg/kg bw per day;
no effects on reproductive and developmental toxicity up to a dose of 1,000 mg/kg bw per day, the highest dose tested, were observed in the EOGRT study with E 171. No other reliable studies were found in the literature addressing these effects with E 171;
some findings regarding immunotoxicity and inflammation with E 171 as well as neurotoxicity with TiO2 NPs may be indicative of adverse effects;
there are indications of the induction of aberrant crypt foci with E 171;
no studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available;
combining the available lines of evidence on genotoxicity, TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico‐chemical properties of TiO2 particles – such as crystalline form, size of constituent particles, shape and agglomeration state – and the outcome of either in vitro or in vivo genotoxicity assays;
a concern for genotoxicity of TiO2 particles that may be present in E 171 could not be ruled out
several modes of action for the genotoxicity may operate in parallel. The relative contributions of different molecular mechanisms elicited by TiO2 particles are unknown and there is uncertainty whether a threshold mode of action could be assumed;
a cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified.
Overall, on the basis of all currently available evidence along with all the uncertainties, in particular the fact that genotoxicity concern could not be ruled out, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive.
This conclusion applies to E 171 as described in Commission Regulation (EU) No 231/2012 as well as to E 171 specified in the EFSA FAF Panel (2019).
Documentation provided to EFSA
LPT (Laboratory Pharmacology and toxicology), 2020a. Interim Report ‐ EOGRT study of Titanium dioxide E171‐E in rats by oral administration via the diet. Report No. 36222. Submitted by the European Commission on 27 May 2020; updated version of the report submitted by Titanium Dioxide Manufacturers Association on 1st September 2020
LPT (Laboratory Pharmacology and toxicology), 2020b. Dietary Analysis ‐ EOGRT study of Titanium dioxide E 171‐E in rats by oral administration via the diet. Report No. 36222, Submitted by the European Commission on 14 September 2020.
Global Pathology Support D.V, 2020. Pathology assessment ‐ EOGRT study of Titanium dioxide E 171‐E in rats by oral administration via the diet (Draft amendment to final pathologist report). Test site reference no. 523/LPT Report No. 36222, Submitted by the European Commission on 15 September 2020; Draft amendment to final pathologist report submitted by Titanium Dioxide Manufacturers Association on 16 November 2020; update submitted 08 January 2021.
Fraunhofer Institute for Molecular Biology an Applied Ecology, 2020a. Determination of titanium in rat blood and urine of an EOGRT study with Titanium Dioxide E 171‐E. Study number: U‐EBR-244/6-27, Submitted by the European Commission on 16 September 2020.
Fraunhofer Institute for Molecular Biology an Applied Ecology, 2020b. Validation: Digestion of Titanium dioxide E 171‐E in rat blood samples and determination of dissolved titanium in digested samples. Submitted by the European Commission on 16 September 2020.
Fraunhofer Institute for Molecular Biology an Applied Ecology, 2020c. Validation: Digestion of Titanium dioxide E 171‐E in rat urine samples and determination of dissolved titanium in digested samples. Submitted by the European Commission on 16 September 2020.
Finish Institute of occupational health (FIOH), 2013. Facilitating the safety evaluation of manufactured nanomaterials by characterising their potential genotoxic hazard Project Coordinator French Agency for Food, Environmental and Occupational Health & Safety (ANSES) report. In vitro testing strategy for nanomaterials including database. Final report, March 2013. Submitted by FIOH, October 2020.
French Agency for Food, Environmental and Occupational Health & Safety (ANSES), 2013. Nanogenotox, Deliverable 5: In vitro testing strategy for nanomaterials including database. Final report, March 2013. Submitted by ANSES, October 2020.
National Research Centre for the Working Environment (NRCWE), 2013 NANOGENOTOX work package six report, Comet and Micronucleus in vivo data. Submitted by NRCWE, November 2020.
Federal Institute for Risk Assessment (BfR) 2012. BfR study reports of the comet for TiO2 NP as part of the work package five of the Nanogenotox project. Submitted by BfR, November 2020.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 16th and 18th November 2020.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 7 January 2021.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 12 January 2021.
BioReliance, 2020a. Bacterial Reverse Mutation Assay. Submitted by Titanium Dioxide Manufacturers Association, 12 January 2021.
BioReliance, 2020b. In Vitro Mammalian Cell Micronucleus Assay in Human Peripheral Blood Lymphocytes (HPBL). Submitted by Titanium Dioxide Manufacturers Association, 12 January 2021.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 22 January 2021.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 29 January 2021.
Additional clarifications submitted in response to a request from EFSA. Submitted by Titanium Dioxide Manufacturers Association, 09 February 2021.
Abbreviations
- 8‐oxodG
8-oxo‐7,8-dihydro‐2’‐deoxyguanosine
- ABC
aberrant crypt
- ACF
aberrant crypt foci
- AChE
acetylcholinesterase
- ADI
accepted daily intake
- AFM
atomic force microscopy
- AGD
anogenital distance
- ALB
albumin
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- ANOVA
analysis of variance
- ANSES
French Agency for Food, Environment and Occupational Health and Safety
- AOM
azoxymethane
- AST
aspartate aminotransferase
- AUC
area under the curve
- BAL
bronchoalveolar lavage
- BChE
butyrylcholinesterase
- BSFGE
biased sinusoidal field gel electrophoresis
- BTB
blood–testis barrier
- BUN
blood urea nitrogen
- bw
body weight
- CA
chromosomal aberration
- CAC
colitis‐associated colorectal cancer
- CAT
catalase
- ccWG
cross‐cutting Working Group
- CD
circular dichroism
- CK
creatine kinase
- COX
cycloxygenase
- CREA
creatinine
- CY
cyclophosphamide
- DBP
diastolic blood pressure
- DG
dentate gyrus
- DMH
dimethylhyrazine
- DOX
doxorubicin
- DSS
dextran sulfate sodium
- ECHA
European Chemicals Agency
- EFSA
ANS EFSA Panel on Food Additives and Nutrient sources added to Food
- ELISA
enzyme‐linked immunosorbent assay
- ENA‐78
epithelial neutrophil‐activating protein‐78
- EOGRT
extended one‐generation reproduction toxicity
- EXAFS
extended X‐ray absorption fine structure
- FACS
flow cytometry analysis
- FAF
Food Additives and Flavourings
- FCS
food categorisation system
- FFA
free fatty acid
- Fpg
formamidopyrimidine DNA glycosylase
- GALT
gut‐associated lymphoid tissue
- GC–MS/MS
gas chromatography–tandem mass spectrometry
- GD
gestation day
- GEF
global evaluation factor
- GI
gastrointestinal
- GIT
gastrointestinal tract
- GLB
globulin
- GLP
Good laboratory practice
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- GNPD
Global New Products Database
- GPx
glutathione peroxidase
- GSH
glutathione
- GSP
glycated serum protein
- H&E
haematoxylin and eosin
- HBDH
α‐hydroxybutyrate dehydrogenase
- HBGV
health‐based guidance value
- HDL
high‐density lipoprotein
- HDL‐C
high‐density lipoprotein cholesterol
- HMC
hydroxypropyl methylcellulose
- HPLC
high‐performance liquid chromatography
- HPRT
hypoxanthine‐guanine phosphoribosyl transferase
- HR
heart rate
- IBO
interested business operator
- ICP‐AES
inductively coupled plasma atomic emission spectroscopy
- ICP‐DRC-MS
inductively coupled plasma dynamic reaction cell mass spectrometry
- ICP‐MS
inductively coupled plasma mass spectrometry
- ICP‐OES
inductively coupled plasma optical emission spectroscopy
- Ig
immunoglobulin
- IL‐6
interleukin‐6
- i.p.
intraperitoneal
- i.v.
intravenous
- JAK2
Janus protein tyrosine kinase 2
- KLH
keyhole limpet haemocyanin
- LDH
lactate dehydrogenase
- LDL
low‐density lipoprotein
- LDL‐C
low‐density lipoprotein cholesterol
- LOD
limit of detection
- LOQ
limit of quantification
- LPO
lipid peroxidation
- m‐SRXRF
micro‐synchrotron radiation X‐ray fluorescence
- MALDI‐TOF
matrix‐assisted laser desorption/ionization time‐of-flight
- MDA
malondialdehyde
- MMP
mitochondrial membrane potential
- MN
micronuclei
- MoS
margin of safety
- MP
microparticulate
- MPL
maximum permitted level
- MS/MS
tandem mass spectrometry
- MWM
Morris water maze
- NBT
nitro blue tetrazolium
- NGAL
neutrophil gelatinase‐associated lipocalin
- NOAEL
no‐observed‐adverse‐effect level
- NSC
nanoscale considerations
- NVWA
Netherlands Food and Consumer Product Safety Authority
- OECD
glycated serum protein
- OFT
open field test
- OGTT
oral glucose tolerance test
- PA
passive avoidance
- PBMC
peripheral blood mononuclear cell
- PC
protein carbonylation
- PCE
polychromatic erythrocyte
- PGC‐1α
PPAR‐gamma coactivator 1 alpha protein
- PND
postnatal day
- PPAR‐γ
peroxisome proliferator‐activated receptor‐gamma
- PTX
paclitaxel
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- QS
quantum satis
- RAC
Committee for Risk Assessment
- RP
reference point
- SBP
systolic blood pressure
- SCFA
short‐chain fatty acid
- SEM
scanning electron microscopy
- SOD
superoxide dismutase
- spICP‐MS
single particle inductively coupled plasma mass spectrometry
- SSB
single‐strand break
- STAT6
signal transducers and activators of transcription 6
- STEM‐EDX
Scanning transmission electron microscopy coupled to energy dispersive X‐ray
- SOCS1
suppressors of cytokine signalling
- TAS
total antioxidant status
- TBAR
total antioxidant capacity
- TBARS
thiobarbituric acid reactive substances
- TBILI
total bilirubin
- TC
total cholesterol
- T/D
transformation/dissolution
- TEM
transmission electron microscopy
- TEM‐EDX
transmission electron microscopy energy‐dispersive X‐ray spectroscopy (
- TG
triglyceride
- TNF‐α
tumour necrosis factor‐alpha
- TP
total protein
- T‐SOD
total superoxide dismutase
- UA
uric acid
- WBC
white blood cells
- XRD
X‐ray diffraction
Appendix A – Search methodology for the literature search
Appendix B – Criteria for inclusion and exclusion applied to screening of publication retrieved in the literature search
Appendix C – Approach for assessing toxicokinetic and toxicity studies
Appendix D – Approach for assessing genotoxicity studies
Appendix E – Advice from EFSA ccWG Nanotechnology: Nanoscale considerations for the assessment of the study design and study results of TiO2 toxicity studies
Appendix F – List of in vivo toxicokinetic and toxicity studies retrieved from the literature search
1.
Appendix A–F can be found in the online version of this output (in the ?Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2021.6585
Appendix G – Description of test materials, scoring for nanoscale consideration and internal exposure for toxicokinetic studies
1.
| Reference | Test material | Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E | Internal exposure |
|---|---|---|---|
| Talamini et al. ( 2019 ) | E 171 (35% nano), anatase, 201 nm in suspension (NTA) | 1 | Quantitative analysis in tissues with methodology reliable with some limitations |
| Coméra et al. ( 2020 ) | E 171, anatase, 20–340 nm (118 nm) (TEM); 44.7% particles < 100 nm | 1 | Quantitative analysis in tissue; methodology reliable with some limitations |
| Riedle et al. ( 2020 ) | E 171, anatase, 119 nm (TEM) | 1 | Qualitative analysis in tissues, methodology reliable with some limitations |
| Pele et al. ( 2015 ) | E 171, anatase, d50 = 260 nm | 1 | Qualitative analysis in blood, methodology highly reliable |
| Guillard et al. ( 2020 ) | E 171, anatase, 20–440 nm (mean 104.9 nm (SEM‐EDX), 55% nanoparticles | 1 | Quantitative analysis in tissues, methodology reliable with some limitations |
| Disdier et al. ( 2015 ) | TiO2 NPs, P25 (15–24 nm) | 1 | Quantitative analysis in blood and tissues, methodology reliable with some limitations |
| Kreyling et al. ( 2017a ) | TiO2 NPs, anatase, 50 nm (TEM), purity unknown | 1 | Quantitative analysis in blood and tissues; methodology highly reliable |
| Geraets et al. ( 2014 ) | NM‐100 (50–150 nm) and NM‐102 (21–22 nm) | 1 | Quantitative analysis in tissues; methodology highly reliable |
| Tassinari et al. ( 2014 ) | TiO2 NPs, anatase, two different morphologies: spherical shape with a size 20–60 nm (TEM) and irregular 60–40 nm (TEM) | 1 | Quantitative analysis in tissues; methodology highly reliable |
| Hendrickson et al. ( 2016 ) | TiO2 NPs, anatase, 20–25 nm (TEM) | 1 | Quantitative analysis in blood and tissue, methodology reliable with some limitations |
| Ammendolia et al. ( 2017 ) | TiO2 NPs, anatase, spherical shape 20–60 nm and irregular shape 40x60 nm (TEM) | 1 | Quantitative analysis in tissues, methodology highly reliable |
| Kreyling et al. ( 2017b ) | TiO2 NPs, anatase, 50 nm (TEM), purity unknown | 1 | Quantitative analysis in blood and tissue, methodology highly reliable |
| Hendrickson et al. ( 2020 ) | TiO2 NPs, rutile, needle‐ or rod‐like shape, 5 × 30 nm (TEM) | 1 | Particles identified in tissues by EM, methodology reliable with some limitations |
| Chen et al. ( 2020a , b ) | TiO2 NPs, anatase, 29 nm (SEM) | 1 | Quantitative analysis in blood and tissue, methodology reliable with some limitations |
| Heringa et al. ( 2018 ) | TiO2 particles | Not experimental study | Quantitative analysis in tissue, methodology highly reliable |
| Peters et al. ( 2020 ) | TiO2 particles | Not experimental study | Quantitative analysis in tissue, methodology highly reliable |
NTA: nanoparticle tracking analysis; SEM: scanning electron microscopy: TEM: transmission electron microscopy.
Appendix H – Description of toxicity studies classified with reliability 1 and 2
1.
General and organ toxicity studies
E 171
Mice
Talamini et al. ( 2019 )
Test material: E 171 (35% nano), anatase, 201 nm in suspension (NTA).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure examined: quantitative analysis in tissues with methodology reliable with some limitations.
Eight‐week‐old male NFR mice were randomly divided into two groups (22 animals/group). One group was orally treated with 5 mg E 171/kg bw, freshly dispersed in water. The other group was treated with water (vehicle control). E 171 or water was slowly dripped with a pipette into the mice mouths, allowing each drop to be swallowed. Mice were treated 3 days/week for 3 weeks for a total of nine treatments in 21 days. According to the authors, this treatment schedule resulted in the mice receiving an average daily dose of 2 mg E 171/kg body weight (bw).
On day 21, three days after the last dose, mice were anaesthetised with 5% isoflurane and blood was collected in heparinised tubes from the retro‐orbital plexus. They were then killed by cervical dislocation and liver, spleen and whole intestine excised for either histological examination (4/group); superoxide determinations after first snap freezing in dry ice and storing at –80°C (4/group) or gene expression analyses (10/group).
A statistically significant increase in ‘necroinflammatory area’ was observed in treated mouse liver based on bright‐field haematoxylin and eosin (H&E) images acquired with an Aperio Scanscope System CS2 microscope and an ImageScope program (the areas occupied by necroinflammatory foci in the liver were manually identified by three different operators blinded to treatment allocation).
Statistically significant increases in apparent superoxide levels were reported in stomach (~ 50%) and whole intestine (~ 25%) – but not liver – according to the authors, through nitro blue tetrazolium (NBT) reduction.
Statistically significant increases in IL‐1β – but not TNF‐α or IL‐10 – mRNA transcripts were reported in both stomach (~ 75%) and whole intestine tissues (~ 75%), but not liver tissues. A statistically significant reduction in liver IL‐10 mRNA transcript expression was observed (~ 40%). These data are based on quantitative real‐time polymerase chain reaction (qRT‐PCR) methodology.
The Panel noted that this study was limited to one dose group and that the increase in necroinflammatory area was not accompanied by additional endpoints indicative of evidence for liver injury. Furthermore, the Panel noted that the histological examination of the liver was performed only on two male mice in the control group and four male mice in the group exposed to E 171. Considering that this type of inflammatory foci (inflammatory cell infiltrates with adjacent necrotic hepatocytes with distinct eosinophilic cytoplasm) of variable size are known to be present in not‐treated mice, a conclusion of the relation to the treatment of these lesions cannot be drawn. The biochemical changes in the stomach and small intestine were considered indicative for increases in oxidative stress and inflammation and adaptive, but not evidence of adversity.
Rats
Talbotet al.( 2018 )
Two test materials: E 171, anatase, 118 nm (TEM), 45% nanoparticles; 2) TiO2NPs (NM‐105), anatase/rutile, 15–24 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure not examined.
Adult male Wistar rats (175–200 g) were randomly divided into three groups (n = 8) and daily administered by gavage E 171 (10 mg/kg bw per day), NM‐105 (10 mg/kg bw per day) or vehicle (water) for 7 days. In the second series of experiments, rats were randomly divided into three groups (n = 10) and exposed for 60 days to E 171 at doses of 10 mg/kg bw per day or 0.1 mg/kg bw per day, through the drinking water, or water alone for the control animals. The stock suspensions of E 171 or TiO2 NPs in water were prepared fresh prior to each experiment. At the end of study rats were sacrificed and the caecal contents collected for analysis of short‐chain fatty acids (SCFAs) and tissues from the small intestine and distal colon sampled for mucin O‐glycosylation. SCFAs were assessed by gas–liquid chromatography. Jejunal and/or ileal and distal colonic mucosa were scraped and the mucins solubilised and purified, permethylated oligosaccharides were analysed by matrix‐assisted laser desorption/ionisation time‐of‐flight (MALDI‐TOF) mass spectrometry.
The Panel considered that the results indicate that TiO2 did not modify caecal short‑chain fatty acid profiles (involved in the regulation of intestinal mucin MUC2 expression) and gut mucin O‑glycosylation patterns (it influences the cohesive properties of mucus and hence its protective function), indicating the absence of a mucus barrier impairment. The absence of mucin O‐glycan alterations indicates that the protective function of mucus against particle uptake remained intact, even after subchronic oral exposure to E 171 (NM‐105 was not tested at 60 days).
Han et al. ( 2020a )
Test material: E 171, anatase, 150 nm (dynamic light scattering (DLS)),
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure: quantitative analysis in tissues; methodology not reliable.
Sprague–Dawley rats (10/sex per group) received by gavage 0, 10, 100 or 1,000 mg E 171/kg bw per day for 90 day. The study was conducted according to OECD TG 408. No mortality was observed. Clinical appearance and body weights of the treated males and females were comparable with those in the controls. Feed intake in high‐dose males was statistically significantly increased from day 11 onwards with exception for days 39 and 74, the difference to controls ranging from 6% to 10%. The statistically significant increase in feed intake was also seen in the low‐dose males on days 11, 18 and 88 (+8%, +5% and +7%, respectively). Haematological examination revealed a slight but statistically significant decrease in relative lymphocyte count in low‐ and high‐dose males (8% at each dose). No differences relative to controls were reported in all treated groups of both sexes in clinical chemistry, absolute and relative organ weights or type and incidence of macroscopic and histopathological findings. In the stomach from high‐dose rats (sex not informed), the test material was observed on the surface and in mucosa. The authors referred to this finding as ‘E 171 accumulation in the stomach wall’. Analysis of Ti amount in the colon revealed a higher amount of the element in the high‐dose group relative to control, while Ti amount in the kidney and spleen were similar to those in the controls.
The Panel noted that oral administration of E 171 in doses amounting to 1,000 mg/kg bw per day to rats had no adverse effects on general toxicity endpoints.
TiO 2 NPs or TiO 2 containing a fraction of nanoparticles
Rats
Warheit et al. ( 2015a )
Test material: TiO2 (11% nano), rutile, d50 = 173 nm (TEM), purity unknown. Two samples were tested, one was a research grade and the other a commercial grade of the pigment; both having the same physico‐chemical characterisation.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
In a 28‐day repeated dose study (OECD TG 407), two groups of young adult male rats (n = 5/group) were gavaged with TiO2 (11% nano) as a suspension (4 mL/100 g bw) at 24,000 mg/kg bw per day. A control group (n = 5) was administered nanopure water (vehicle). Clinical pathology endpoints were evaluated after the end of the 29‐day treatment.
No clinical signs of toxicity, and no effects on body weight or nutritional and clinical pathology parameters were observed. No adverse effects on organ weights (adrenal glands, brain, epididymides, heart, kidneys, liver, spleen, testes and thymus) and no gross or microscopic changes were reported. The authors reported (data not shown) that the evidence of the test substance was observed in intestinal lymphoid tissue of the treated group through microscopic examination but this was not considered to be an adverse effect. They concluded that the no‐observed‐adverse‐effect level (NOAEL) was 24,000 mg/kg bw per day, the only dose tested.
The Panel noted that a 90‐day toxicity study (OECD TG 408) with TiO2 (21% nano) rutile coated with Al2O3 is also described in this publication. Since this tested material was TiO2 coated with Al2O3 and was not representative of E 171, the data pertaining to this material was not considered relevant for the assessment of E 171 (according to Appendix B).
Vasantharaja et al. ( 2015 )
Test material: TiO2 NPs, anatase/rutile, < 100 nm, purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Adult male Wistar rats divided in groups (n = 6) received TiO2 NPs suspended in 0.9% saline by gavage at doses of 0, 50 and 100 mg/kg bw per day for 14 consecutive days.
Serum was obtained at the end of the experiment and different clinical biochemistry parameters were measured. Total protein (TP), albumin (ALB), globulin (GLB), cholesterol, triglycerides (TGs) and high‐density lipoprotein (HDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin (TBILI), blood urea nitrogen (BUN), uric acid (UA) and creatinine (CREA) as well as glucose
The following changes were observed: glucose increased in the treated groups (1.17‐ and 1.19‐fold, at 50 and 100 mg/kg bw, respectively; cholesterol increased (1.25‐ and 1.5‐fold, respectively), with mild elevation of HDL (1.13‐fold) at the high dose; TG decreased to 64% of the control in both groups no significant difference in ALB, GLB and TBILI. TP changes while statistically significant, were both very limited (< 10% change) and not dose dependent (with a decrease to 92% of the control at the low dose and an increase to 1.06% at the high dose).
Serum AST levels were significantly increased only at the high treatment dose by ca 10%, while ALT levels were unchanged. A decrease (by ca. 10%) of ALP activity was observed in both the treated groups. BUN increased at both doses (1.17‐ and 1.21‐fold at low and high doses, respectively) and UA increased by 1.3‐ and 1.5‐fold, at low and high doses, respectively, but the statistical significance of these changes was either absent or weak (p < 0.05). There was no change of CREA.
The Panel noted a single major change, reduction of serum TG in both the treated groups. The Panel considered that no adverse effects of toxicological significance were observed.
El‐Din et al. ( 2019 )
Test material: TiO2 NPs, anatase/rutile, < 100 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
In a study investigating whether curcumin could ameliorate cardiotoxic (and genotoxic) effect of TiO2 NPs male albino rats (strain not informed; n = 8/group) received by gavage either saline (vehicle control A), corn oil (vehicle control B), 200 mg curcumin/kg bw in corn oil, 1,200 mg TiO2 NPs/kg bw per day in saline or 200 mg curcumin/kg bw per day in corn oil and 1,200 mg TiO2 NPs/kg bw per day in saline for 90 days (El‐Din et al., 2019). Additionally, an untreated control group (n = 8) was included. In this assessment only the results from the TiO2 NPs and the control groups are presented. Histopathological examinations were made on heart ventricular tissue, accompanied by some immunohistochemical staining. Photomicrographs of the H&E‐stained samples revealed wide intercellular spaces between cardiomyocytes, vacuolation of cytoplasm, deeply stained homogenous acidophilic cytoplasm with fading striation or pale acidophilic material indicative of oedema in some cardiomyocytes, severely congested capillaries, extravasation, pyknotic and peripherally displaced nuclei of cardiomyocytes, prominent mononuclear cell infiltration of the tissue in the TiO2 NPs group. Immunohistochemically stained specimens of the heart ventricle revealed a statistically significant increased per cent areas for collagen and anti‐3‐nitrotyrosine staining parts and a statistically significantly decreased per cent area for anti‐desmin in the TiO2 NPs group as compared to the untreated or vehicle control groups. The authors concluded that TiO2 NPs could cause cardiac toxicity. The Panel noted that the number of stained samples examined per group were not reported and information of blinding of samples was not given. Based on the limited reporting of the morphological changes in the heart ventricular tissue, the Panel was not able to conclude on the relationship between the reported histopathological changes and treatment with TiO2 NPs.
TiO 2 NPs < 30 nm
Mice
Huet al.( 2015 )
Test material: TiO2 NPs, anatase, 25.6 nm (TEM, SEM), purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure examined: quantitative analysis in blood and tissues; methodology with important flaws
In a study designed to explore potential endocrine effects of TiO2 NPs, 6‐week‐old CD‐1 male mice (25.37 ± 0.71 g; n = 10/group) were acclimatised for 7 days and provided with autoclaved water and rodent diet ad libitum then randomly divided into three groups: control (PBS vehicle), 64 mg TiO2 NPs/kg bw per day and 320 mg TiO2 NPs/kg bw per day and dosed daily for 14 weeks by gavage.
Body weights of mice and food intakes were determined every week and biweekly, respectively.
Every 2 weeks, mice were fasted for 16 h prior to daily administration of TiO2 NPs and blood was collected from the tail vein to measure the plasma glucose level. If plasma glucose was increased in comparison to control group, mice plasma glucose was tested for 4 weeks. After 14 weeks, blood was also collected from the heart.
Ti concentration was measured in organs (liver lobes, spleen, middle part of small intestine, gastrocnemius muscle, middle part of the kidney and pancreas) using ICP‐OES, and Ti particles in the tissue were detected by EDXA. The Panel noted that the analytical technique (ICP‐OES) was not appropriate for a reliable Ti quantification in tissues.
Ti concentrations were in both the treated groups increased in all organs, with no significant difference between the two dose groups. Ti concentration in kidney and small intestine was the highest. No significant difference in body weights or food intake was observed between the 3 groups. However, plasma glucose levels (tail vain) were increased from week 10 in both TiO2 NPs groups. The plasma glucose concentration in heart blood at termination was also statistically significantly increased. There were no changes in the concentrations of plasma insulin, blood TG, free fatty acid (FFA), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C) or total cholesterol (TC) in any group. Levels of total superoxide dismutase (T‐SOD) and glutathione (GSH) were significantly reduced and the level of MDA was significant increased for both dose group in serum and liver. The authors concluded that oral exposure to TiO2 NPs increased reactive oxygen species. No apoptotic pancreatic β‐cells were observed.
In an oral glucose tolerance test (OGTT), a significant increase in plasma glucose levels was observed in the treated animals compared to control group, but no differences in plasma insulin levels at 30, 60 and 120 min were observed.
At week 14, western blot results showed that the phosphorylation of IRS1 (Ser307) was increased and phosphorylation of Akt (Ser473) was reduced in the liver in both the dose groups. The serum levels of tumour necrosis factor‐alpha (TNF‐α) were significantly increased for both groups compared to control. IL‐6 was significantly increased in the serum of the high‐dose group. Phosphorylation of JNK1 and p38 MAPK was significantly increased in the liver in both dose groups.
According to the authors, this study indicates that oral administration of TiO2 NPs increases plasma glucose in mice, in fasting state and after OGTT without showing a dose response.
According to the authors, TiO2 NPs increased ROS by activating an inflammatory response and MAPK pathways, which induce the insulin resistance and cause an increased plasma glucose levels in mice.
The Panel considered that the oral administration of TiO2 NPs (25.6 nm) at both doses, 64 and 320 mg/kg bw per day leads to increases in fasting state plasma glucose, and also to increases in glucose levels in a glucose tolerance test without showing a dose response and without differences in plasma insulin levels, indicating inconsistency between the measured outcomes.
Yu et al. ( 2016 )
Test material: TiO2 NPs, anatase, 5–6 nm (further information on characterisation from Hu et al., 2011).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
In a study assessing effects of TiO2 NPs on the heart, female CD 1 (ICR) mice (initial body weight 20 ± 2 g, n = 20/group) received by gavage 0, 2.5, 5 or 10 mg TiO2 NPs/kg bw per day for 90 days. The endpoints evaluated were body weight gain, absolute and relative heart weight (for 20 mice per group), morphology of the heart, activities of ATPases and protein expression of CAMK II, NCX‐1, α‐1AR and β‐1AR in the heart tissue by ELISA (each parameter for 5 mice per group). The results for endpoints related to the immunological system are not included. Body weight gain was statistically significantly decreased in a dose‐dependent manner up to 30.3% in all TiO2 NPs groups. Mean absolute heart weights were comparable with those of the control group but the relative heart weights were increased with increasing dose and the difference to the control group reached statistical significance at the high dose. Microscopic examination of H&E‐stained heart tissue revealed fragmentation, interstitial spaces or disordered myocardial fibre arrangement, fatty degeneration or necrosis at all doses, and cardiomyocyte hypertrophy, myocardial haemorrhage or hyperaemia and focal inflammatory cell infiltration in myocardial interstitium at mid‐ and high‐dose groups. There were statistically significant decreases in the activities of cardiac Na+/K+‐ATPase and Ca2+/Mg2+‐ATPse at all doses and of Ca2+‐ATPase in mid and high doses. The decreases were dose related. The decreases at the high dose were approximately 25%, 26% and 23% for Ca2+‐ATPase, Na+/K+‐ATPase and Ca2+/Mg2+‐ATPase, respectively, as estimated by the Panel. The Panel noted that reporting of morphological changes in the heart was based solely on the presented photomicrographs from the heart sections but the incidence and severity scores of the morphological changes were not presented. Based on this limited reporting, the Panel was not able to conclude on the decreased body weight gain and the relationship between the reported histological changes and treatment with TiO2 NPs (5–6 nm). The Panel further noted that the decreases in activities of ATPases were not indicative for an impaired cardiac pump function (Akera and Brody, 1978; Dostanic et al., 2004; Griffiths et al., 2009; Figtree et al., 2012).
Hong et al. ( 2016 )
Test material: TiO2 NPs, anatase, 5–6 nm (further information on characterisation from Hong et al., 2014).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
CD‐1 (ICR) male mice were divided into four groups (n = 40) and administered TiO2 NPs by gavage at doses of 0, 2.5, 5 and 10 mg/kg bw per day for 90 days.
A dose‐dependent decrease in body weight gain was observed at all tested doses (approx. 5%, 5% and 7% decrease in body weight gain compared to control at 2.5, 5 and 10 mg/kg bw per day, respectively), with statistically significant differences at the two highest doses. Relative liver weights increased by ~ 10–15% compared to control however, absolute liver weights were unchanged. According to the authors, histopathology ‘showed dose‐related severity of liver injury, such as hyperaemia, lymphocyte infiltration and necrosis’. However, the Panel noted that quantitative data (incidence and severity) were not reported and there may be operator bias in selection of images.
According to the authors, the inflammatory response in the liver was demonstrated by dose‐dependent increases in protein expression of cycloxygenase (COX, ca. 30% and 100% at 5 and 10 mg/kg, respectively), neutrophil gelatinase‐associated lipocalin (NGAL, by ca. 50 and 80%) and epithelial neutrophil‐activating protein‐78 (ENA‐78, by ca. 23%), and decreased protein expression of peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ, to ca. 29 and 27% of control). The decreased expression of PPAR‐gamma coactivator 1 alpha protein (PGC‐1α, to ca. 82 and 61% of control) indicates, according to the authors, possible decreased capacity in mitochondrial metabolism.
The ET‐1 and P2X7 levels were not significantly different among the four groups. Western blot analysis of biomarkers of inflammation showed increased expression of IL‐6, Janus protein tyrosine kinase 2 (JAK2), signal transducers and activators of transcription 6 (STAT6), COX, NGAL and ENA‐78, and decreased expression of suppressors of cytokine signalling (SOCS1), PPAR‐γ and PGC‐1α. According to the authors, ‘the JAK–STAT pathway may play an important role in nano‐TiO2‐induced liver injury’.
The Panel noted that the histopathological data in the liver was not accompanied by any other confirmatory investigations (e.g. clinical chemistry) and considered the effects reported in this study as likely an hepatic inflammatory response to TiO2 NPs (5–6 nm).
Yang et al. ( 2017 )
Test material: TiO2 NPs, mixture (anatase and rutile), 21 nm (the Panel noted that from the product number catalogue indicated in the publication, it is P25).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure examined: particles identified in liver; methodology with important flaws.
Male 5‐ to 7‐week‐old C57/Bl6 mice (22–25 g, 5/group) were treated by gavage once daily for 14 days with either vehicle control (0.5% CMC‐Na, not defined), 250 mg TiO2 NPs/kg bw or 500 mg TiO2 NPs/kg bw. The Panel noted there was no randomisation of animals in this study.
After 14 days, blood was collected from the retro‐orbital vessel. Mice were then killed by CO2 asphyxiation. Two samples of each liver from three mice in each group were examined via TEM, with the remainder either fixed for histopathological examination or frozen for later analyses of gene expression.
There were no significant effects of TiO2 NPs on relative liver weight, serum ALT, AST, ALP and total bile acid levels. Conjugated bilirubin (direct) and bilirubin (indirect) serum levels were statistically significantly elevated ~ 2.5‐ to 3‐fold in the highest dose group. However, no histopathological changes were observed in the liver (livers from three randomly selected mice from each experimental group were examined).
The authors reported increases in particles, the number of mitochondria and oedema of the endoplasmic reticulum in hepatocytes based on analysis by TEM in both the dose groups. However, none of these data were analysed quantitatively.
qRT‐PCR and western blotting on liver tissues identified significant increases in the expression of selected genes (Oatp1, Mrp3, Cyp2b10 and Cyp2c37) with increasing dose of TiO2 NPs (21 nm).
The Panel noted the increases in serum bilirubin levels along with structural changes in hepatocytes which were not quantified. However, these increases occurred in the absence of any changes in relative liver weight, changes in other serum markers for liver injury or quantitative histopathological changes in the liver. Changes in the hepatic expression of selected genes were considered as either spontaneous or adaptive, but not evidence of adversity.
Rats
Chen et al. ( 2015a )
Test material: TiO2 NPs, anatase 24 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Four‐week‐old healthy Sprague–Dawley rats (n = 10 per group) were randomised into experimental and control groups, with five male and five female rats in each group. Suspensions in ultrapure water of TiO2 NPs (0, 2, 10 and 50 mg/kg bw per day), glucose (1.8 g/kg bw per day) and TiO2 NPs (0, 2, 10, 50 mg/mg bw per day) + glucose (1.8 g/kg bw per day) were gavaged daily for 30 or 90 consecutive days. The animals were kept under standard environmental conditions. In this assessment only the results from the TiO2 NPs and the control groups are presented.
During the experiments, no significant changes in the body weight and food intake of the exposed rats were found and no mortality was observed.
After 30 days or 90 days, animals were weighed and sacrificed. Blood samples were collected for haematological and clinical chemistry investigations. The liver, kidney, spleen, testicle, ovary and heart were collected.
The relative kidney weight was increased in male rats only in the 2 and 10 mg/kg bw per day groups, not in the 50 mg/kg bw per day group; the increase being statistically significant, but biologically not relevant (about 10%). No difference in heart body weight was recorded after 30 or 90 days.
No effect was seen on the relative heart weight. No adverse effect on heart rate and blood pressure was noted. Some laboratory parameters were changed (α‐hydroxybutyrate dehydrogenase (HBDH) decreased at 90 days in males in the dose group of 50 mg/kg bw per day, CK decreased at 90 days in females in the dose group of 10 mg/kg bw per day, serum BUN increased at 90 days in males the dose group of 10 mg/kg bw per day without increased serum creatinine, serum creatinine decreased at 90 days in males the dose group of 50 mg/kg bw per day), however, the Panel considered of no toxicological relevance.
Relative organ weight of liver and spleen were unchanged in all groups.
No histopathological findings were reported, except for the liver for which the authors reported ‘edema, fatty degeneration and necrosis were evident in all the 50 mg/kg bw per day treated groups’. However, the images shown are too small to independently assess the reported findings. In addition, serum enzyme activities reflecting liver injury (the aminotransferases ALT, AST and ALT/AST) were unchanged in females or males exposed to TiO2 NPs, irrespective of the dose, and this does not confirm the histopathological changes reported by the authors. Mostly random, modest changes in total bilirubin values were observed across the control and treated groups of both sexes. The authors considered that decreased total bilirubin values in the female groups treated with 10 and 50 mg TiO2 NPs/kg bw per day was a sign of liver dysfunction, but the Panel did not agree since there is no liver injury which is known to result in hypobilirubinaemia.
The Panel noted that no TiO2 measurements were performed in blood or tissues. The Panel considered that no adverse effects were identified in this study up to a dose of 50 mg TiO2 NPs (24 nm)/kg bw per day.
Chenet al.( 2015b )
Test material: TiO2 NPs, anatase, 24 nm (TEM) (physico‐chemical properties were described in detail at Chen et al., 2015a).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure was not examined.
In a study investigating effects of TiO2 NPs on the cardiovascular system, Sprague–Dawley rats (n = 10/sex per group) received by gavage 0, 2, 10 or 50 mg TiO2 NPs/kg bw per day for 30 or 90 days (5/sex per group terminated at each time point). Body weight was recorded weekly and feed intake every 3–4 days. The heart rate (HR), systolic and diastolic blood pressure (SBP and DBP) were monitored at the start, weekly up to 30 days and every second week thereafter (no information was given about the number of animals per group used for the measurements and whether there were the same animals used repeatedly). At each termination, body and heart weights were recorded and blood (from abdominal aorta) was collected for measurements of activities of creatine kinase (CK), lactate dehydrogenase (LDH) and HBDH as biomarkers of cardiac damage, for clinical chemistry parameters (gluvodr, TGs, TC, HDL‐C, LDL‐C and absolute leucocyte (WBC) count, TNF‐α) and IL‐6 as indicators of inflammatory response in the body. Heart tissue samples were taken for histological examination. Treatment with TiO2 NPs at all doses had no effect on body weight, feed intake or the relative heart weight. Statistically significant changes in heart rate and blood pressure relative to the control group were recorded in mid‐ and high‐dose females as increased DBP on day 47 and decreased SBP on day 57 (no data shown to evaluate a dose response), and mid‐dose males as a decreased HR on day 88. The area under curve for SBP was statistically significantly lower for mid‐dose females. According to the authors, TiO2 NPs could induce changes in HR and blood pressure. The Panel noted that each of these transient changes were limited to one sex and considered them not to be treatment related. Statistically significant differences relative to control in biomarkers of cardiac injury were decreased serum activities of LDH and HBDH in high‐dose males in the absence of an apparent dose response relationship and of CK in mid‐dose females after 90 days of the treatment. According to the authors, these findings suggested that TiO2 NPs at high doses could induce cardiac impairment detectable at the level of blood molecular markers. The Panel noted that clinical diagnostic increases in activities of the CK and LDH are regarded as indicative of myocardial injury and increased serum activity of HBDH may reflect renal, red blood cell and/or myocardial damage. No increase in activities of the three biomarkers is in agreement with no histopathological changes in the heart. Values of clinical chemistry parameters were comparable to controls at two terminations with exception of not dose related statistically significantly lower TG in mid‐ and high‐dose males on day 90. Statistically significant increases relative to the control group were reported for total leucocyte count and granulocyte count in high‐dose females after 90 days and in total leucocyte count, lymphocyte and monocyte counts in high‐dose males after 30 days. The concentration of TNF‐α was statistically significantly higher relative to the control in mid‐dose females after 30 days. The concentration of IL‐6 was statistically significantly increased in mid‐dose females and in mid‐ and high‐dose males after 30 days. This, according to the authors, indicated that TiO2 NPs at high doses could induce inflammatory response in rats. The Panel noted that the changes in WBC, TNF‐α and IL‐6 were not time‐ or dose‐related. Overall, the Panel considered that gavage administration of TiO2 NP (24 nm) in doses up to 50 mg/kg bw per day to rats for up to 90 days did not induce any treatment‐related effects on the evaluated endpoints.
Grissa et al. ( 2015 )
Test material: TiO2 NPs, anatase, 5–12 nm (TEM, XRD).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Four‐month‐old healthy male Wistar rats were provided a commercial pelleted diet and provided drinking water ad libitum. The rats were weighed and randomised into three experimental and one control group (n = 6 per group). Rats were daily administered fresh suspensions of TiO2 NPs in distilled water by gavage at doses of 0 (distilled water control), 50, 100 or 200 mg/kg bw for 60 days. Body weight changes, feed and water consumption, activity and mortality were monitored daily.
No data on feed consumption, body weight or mortality were reported.
After 60 days, animals were anaesthetised and blood samples were collected by cardiac puncture for haematological parameters, blood smears (and comet assay). Rats were then euthanised. The haematological results indicated statistically significant dose‐related decreases in RBC (up to 28%), HCT (up to 23%) and haemoglobin (up to 28%) in exposed animals although the decreases in the last parameter were not dose dependent. Mean corpuscular volume (up to 29%), platelets (up to 42%), mean platelet volume (up to 30%) and WBC (up to 235%) were statistically significantly and dose‐dependently increased in exposed animals. The reported data fails to indicate the number of animals used in each investigation. According to the authors, animals exposed to 100 or 200 mg/kg bw TiO2 NPs (5–12 nm) per day had poikilocytotic (abnormally shaped) hyperchromatic RBCs and abnormally shaped nuclei and hyper‐segmented nuclei in lymphocytes and neutrophils.
The Panel considered the changes to be of no toxicological significance.
Grissa et al. ( 2017 )
Test material: TiO2 NPs, anatase, 5–10 nm (the Panel noted that this material is mentioned as E 171, however, the same material produced by AZ tech (Italy) is described in Grissa et al. (2015) as paint pigment in ceramics).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Four‐month‐old male Wistar albino rats (n = 6 per group) were randomly divided into control and a TiO2 NPs (5–10 nm) groups and maintained under control conditions (12‐h light cycle, temperature and humidity). The control group received 10 ml/kg bw distilled water and the treated rats received 100 mg TiO2 NPs/kg bw in suspension in distilled water by gavage, daily for 8 weeks.
During the 8‐week treatment, all animals were observed daily for clinical signs and mortality, body weights were evaluated weekly. After 8 weeks and 24 h after the last TiO2 NPs dose, the animals were weighed and anaesthetised with ether and blood samples were collected by cardiac puncture.
A statistically significantly reduced body weight gain in the treated animal group was observed compared to the control. Serum cholesterol (Chol), glucose (Glu) and TG concentrations were statistically significantly higher in treated rats than in controls (Chol: 2.41 ± 0.11 mmol/L and 1.53 ± 0.02 mmol/L; Glu: 13.37 ± 0.18 mmol/L and 8.18 ± 0.33 mmol/L; TG: 0.69 ± 0.1 mmol/L and 0.34 ± 0.11 mmol/L, treated vs. control).
According to the authors, oxidative stress markers SOD, total antioxidant status (TAS) and catalase (CAT) in plasma were significantly decreased and changes in the lipid peroxidation (LPO) potential were observed in treated rats compare to control. Furthermore, plasma IL‐6 was significantly increased in treated rats compare to control.
The Panel noted the changes in glucose levels which are potentially adverse, and considered that the changes in cholesterol and TGs are of unclear toxicological relevance.
Hassanein and El‐Amir ( 2017 )
Test material: TiO2 NPs, 21 nm [no further information].
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Male Sprague–Dawley rats, weighing 160–200 g, maintained under standard environmental conditions, provided standard feed and water ad libitum. After 2 weeks of acclimatisation, the rats were randomly divided into 6 groups (n = 10 rats/group) that received TiO2 NPs only for 6 weeks. TiO2 NPs (150 mg/kg bw) in 1% Tween 80 was administered daily by gastric intubation. The control group received 1% Tween 80 (0.5 mL/rat). After 6 weeks of treatment, the animals were terminated and samples from the brain, kidney, liver, lung, heart and testes were stored in 10% buffered formalin for histopathology and prepared for H&E staining. A complete blood count, including erythrocyte count (RBC), haemoglobin (Hb) and total and differential leucocytes (WBC, Lym, Monocy, Neutro), eosinophils (EOS) were performed for each sample. Activities of ALT and AST in the serum samples, serum LPO, total antioxidant capacity (TBAR), glutathione (GSH) and TNF‐α were measured by appropriate methods. Furthermore, serum testosterone was determined.
According to the authors, RBC, Hb, WBC, Monocy and EOS were not different compared with control, whereas the Lym and Neutro were statistically significantly increased by treatment with TiO2 NPs. No units were given for Lym, Monocy and Neutro. Hence, the Panel could not conclude on these data. ALT increased 2.5‐fold, AST 2‐fold, LPO 2‐fold, TNF‐α 5‐fold, whereas TBAR decreased 2‐fold, GSH 4‐fold and testosterone 5.5‐fold.
In all organs investigated (liver, brain, lung, heart, testis, kidney) ‘histopathological’ lesions were reported in either 4–6 (++) or 7–10 (+++) rats per treated group, with no lesions found in the control group. The reported findings in the liver were congestion, vacuolar degeneration, mononuclear infiltration in the portal area, focal necrosis with mononuclear infiltration; in the brain: haemorrhage, congestion of choroid plexus blvs, chromatolysis, neuronal degeneration, perivascular lymphocytic cuffing; in the lung: congestion, thrombosis, hyalinisation of the blood vessels wall, hyperplasia of peribronchial lymphoid aggregation; in the heart: vacuolar degeneration and myocardial necrosis; in the testis: congestion and coagulative necrosis; in the kidney: congestion and perivascular mononuclear infiltration. It is not mentioned whether the pathologist performing the histopathology was blinded or whether there was a blinded second reading of the slides. The Panel considered that the descriptions of ‘histopathological’ lesions are unclear; some of the findings are not histopathological lesions. In addition, the organs in which lesions are reported, the number of lesions per organ and the number of animals with any lesions are not clearly stated.
The Panel noted that only one dose of 150 mg TiO2 NPs (21 nm)/kg bw has been tested. Based on the many flaws in the study reporting, the Panel was not able to draw any conclusions.
Heo et al. ( 2020 )
Test material: TiO2 NPs (P25), anatase/rutile, 15–24 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Healthy pathogen‐free young adult male and non‐pregnant female Sprague–Dawley rats (n = 140) were used. For a repeated‐dose 28‐day study, animals were randomly divided into four groups (n = 5/sex) following a 6‐day acclimatisation period. For the repeated‐dose 90‐day study, the animals were randomly assigned to four groups (n = 15/sex) following a 6‐day acclimatisation period. While 10 animals in each group were terminated for toxicity evaluation after the repeated‐dose 90‐day study, the remaining 5 animals underwent a 28‐day recovery period. No information on the type of diet was provided. The suspensions of TiO2 NPs (15–24 nm) in sodium phosphate buffer were daily administered in the morning based on the body weight of the animals by gavage TiO2 NPs at concentrations of 0, 250, 500 and 1,000 mg/kg bw per day with a dosing volume of 10 ml/kg bw.
Food and water intake were recorded before the first administration and then weekly during the study. No statistically significant treatment‐related differences with respect to body weight gain, food and water intake were observed. No mortality and clinical signs were detected during the exposure period of 28 and 90 days. No effects were detected in the functional observations battery in the last week of the 90‐day study.
Ophthalmoscopic examination and urinalysis did not show statistically significant differences between the groups. Reductions in the number of circulating neutrophils in the 500 mg/kg bw female group and increased numbers of circulating lymphocytes in all three female groups (without a clear dose–response pattern) were reported. These changes were not observed in males. These differences did not persist in the animals in the recovery group and were considered by the authors as spontaneous and not treatment‐related. BUN levels were statistically significantly reduced in the 1,000 mg/kg bw female group and blood Na levels were increased in the 1,000 mg/kg bw per day male group.
There were no abnormal gross findings at necropsy in treated animals with or without recovery. Higher absolute pituitary weights in the 1,000 mg/kg bw per day male group, lower absolute uterine weights in the 1,000 mg/kg bw per day female group and higher relative liver weights found in the 1,000 mg/kg bw per day female group. Therefore, the Panel considered these changes as unrelated to the treatment. On histopathological examination, lesions were observed in the kidney, thymus, heart and lung in controls and in the 1,000 mg/kg bw per day dose group in both sexes. The observations did not differ significantly between the control group and the 1,000 mg/kg bw per day group and were not considered to be associated with the treatment by the authors.
The Panel considered that the reported changes were within the historical control range and of no toxicological significance.
Chen et al. ( 2020a )
Test material: TiO2 NPs, anatase, 29 nm (STEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Three‐week‐old healthy male Sprague–Dawley rats were randomly divided into groups of six animals receiving daily by gavage TiO2 NPs (29 nm) at doses of 0, 2, 10 or 50 mg/kg bw per day for 90 days. Animals were provided a commercial pelleted diet and provided deionised water ad libitum. Behaviour and mortality were monitored daily. Body weights were assessed every 7 days and at termination, while the food intake was recorded every 3–4 days. Blood from the abdominal aorta was taken at termination. In addition, SCFAs in stool samples were analysed by targeted metabolomics using gas chromatography–tandem mass spectrometry (GC–MS/MS).
No mortality was observed during the study. Starting from week 8, the 10 and 50 mg/kg bw per day groups showed decreased body weight gains up to about 15%, while food intake was not different between the groups
Serum levels of triglycerides in the 10 and 50 mg/kg bw per day groups were statistically significantly lower than in the control group while serum TC, HDL‐C and LDL‐C were not affected. In an untargeted metabolomic analysis, 343 of 1,837 lipophilic metabolites were differentially expressed between controls and the 50 mg/kg bw per day group.
No statistically significant differences in organ weights for the heart, spleen, liver, kidney, lung, stomach and testis were observed. No further results were reported.
Six SCFAs in faeces, including acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid and hexanoic acid, did not change after exposure to TiO2 NPs.
The Panel considered that, while the change in body weight gain may be adverse, other reported changes were of no toxicological significance.
Chen et al. ( 2020b )
Test material: TiO2 NPs, anatase 24 nm (SEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Three‐week‐old Sprague–Dawley rats were fed a commercial pellet diet and deionised water ad libitum. After 1 week of acclimation, rats were weighed and randomly divided into experimental and control groups. Rats (n = 5/sex per group) were daily administered by gavage with 0, 2, 10 or 50 mg/kg bw per day with TiO2 NPs (24 nm) with or without 1.8 g/kg bw glucose in 1 mL of water for 90 consecutive days.
Body weights were determined weekly and there were no significant differences in body weight gains in any group during the study.
After fasting overnight, blood was drawn from the tail vein of rats at 9, 16, 23, 30, 44, 58, 72 and 90 days to monitor blood glucose levels. Although differences were seen in treated and control rats, these were only in female rats and at occasional time points. The authors calculated the area under the curve (AUC) of blood glucose and determined that the female rats treated with glucose alone and female rats treated with glucose with either 2 or 10 mg/kg bw per day TiO2 NPs had slight but significantly reduced AUCs. After 90 days, animals were weighed and killed. Blood samples were collected from the abdominal aorta and the levels of blood glycoproteins, including glycated haemoglobin (HbA1c) and glycated serum protein (GSP) were also measured. Rats treated with glucose and TiO2 NPs (10 and 50 mg/kg bw per day groups) had a significantly reduced levels of HbA1c in female rats. Male rats treated with glucose and TiO2 NPs (2 mg/kg bw group) had a significantly reduced level of GSP.
Blood insulin, C‐peptide and glucagon levels were also determined at a single undeclared time point in the study by a radioimmunoassay. Considering only those effects in which there appeared to be a potential dose–response effect of TiO2 NPs (no dose response effects were seen when glucose was co‐administered with TiO2 NPs), blood insulin levels were statistically significantly lower than control in females in the 10 or 50 mg/kg bw per day TiO2 NPs groups, but there was no effect in males. In males, C‐peptide was significantly lower in the 50 mg/kg bw per day TiO2 NPs group, but no such effect was seen in females. No clear dose–response effect was seen on glucagon levels.
Prior to termination, an OGTT was performed at day 90. The rats were fasted overnight for 16 h and subsequently challenged with glucose alone at 2 g/kg bw via gavage. Blood glucose levels were determined at 0 h (pre‐glucose treatment) and at 30, 60, 90 and 120 min (post‐glucose treatment). Although differences were seen in blood glucose concentrations, these were only in male rats (at 30 and 60 min after glucose challenge in the 2 mg/kg bw per day TiO2 NPs group and at 60 min after glucose challenge in the 50 mg/kg bw per day TiO2 NPs + glucose group). Only rats treated chronically with both glucose and 50 mg/kg bw per day TiO2 NPs showed a significant increase in AUC for blood glucose in the glucose tolerance tests.
Pancreata were excised, fixed and processed for histopathological examination by a pathologist who was blinded to the treatment group and dosing regimen. No pathological changes associated with TiO2 NP administration were observed.
In the absence of any clear dose–response effects for TiO2 NPs (24 nm) and the occasional nature of these changes, the Panel considered the changes in blood glucose, HbA1c, GSP, insulin, C‐peptide, glucagon and glucose tolerance as either not test substance related or irrelevant for the safety evaluation of TiO2 NPs.
Reproductive and developmental toxicity studies
TiO 2 NPs or TiO 2 containing a fraction of nanoparticles
Rats
Warheit et al. ( 2015b )
In this study, there were five different test materials relevant for E 171: 1) anatase/rutile (89/11%) (uf‐1), d50 = 43 nm (XSDC), d50 = 23 nm (TEM), irregular; 2) anatase (100% nano) (uf‐2), d50 = 42 nm (XSDC), d50 = 19 nm (TEM), irregular; 3) rutile (100% nano) (uf‐3), d50 = 47 nm (XSDC), d50 = 22 nm (TEM), rod‐like; 4) anatase (27% nano) (pg‐1), d50 = 153 nm (XSDC), d50 = 120 nm (TEM), irregular; 5) rutile (11% nano) (pg‐2), d50 = 195 nm (XSDC), d50 = 165 nm (TEM), irregular. The purity of each tested material was not reported but the most abundant metals were analysed by inductively coupled plasma atomic emission spectroscopy (ICP‐AES).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
These studies report prenatal developmental toxicity studies with different tested materials in pregnant rats, performed in accordance with OECD TG 414. The test substances were formulated in sterile water. Dosing formulations were collected and analysed near the beginning and end of the dosing period for analyses. These analyses confirmed that the formulations were at the targeted concentrations, were uniformly mixed and were stable under the experimental conditions used during the study.
In three studies, time‐mated pregnant Sprague–Dawley, Crl:CD(SD), rats (n = 22/group) were daily exposed to TiO2 (uf‐1, uf‐3 and pg‐1) by gavage on GDs 6–20. In three additional studies, pregnant Wistar rats (n = 22–23/group) were daily exposed to TiO2 (uf‐2 and pg‐2) by gavage from GDs 5 to 19. The dose levels used in the studies were 0, 100, 300 or 1,000 mg/kg bw per day. The dose volume was 5 mL/kg bw per day. Clinical signs were recorded at least daily. Body weight and feed intake were measured at regular intervals. Sprague–Dawley rats were killed for a caesarean section on GD 21 and Wistar rats on GD 20. Gross necropsy included gross examination of the dam, counting of the number of corpora lutea, implantation sites, resorptions, live and dead fetuses, fetal sex and weight. Fetal pathological external, visceral and skeletal examinations were performed in order to identify any abnormalities. At 1,000 mg uf‐1/kg per day, mean fetal sex ratio and the means for male and female fetuses per litter were statistically significantly different from the control group means. The mean number of male fetuses was 7.2 compared with 5.5 male fetuses for the concurrent control group; the test facility historical control group data ranged at that time from 5.2 to 7.4. The mean number of female fetuses was 4.8 compared with 6.7 for the concurrent control group; the test facility historical control group data ranged at that time from 5.8 to 8.3. Mean fetal sex ratio of the 1,000 mg uf‐1/kg bw per day group was 60% (males/females) compared with a sex ratio of 46% in the concurrent control group; the test facility historical control group data ranged at that time from 43% to 53%. Apart from some incidental changes in body weight and feed intake, no other changes were observed in the dams or the fetuses in these studies. The authors concluded that there were no significant toxicological or developmental effects in females or fetuses at any of the dose levels or compounds tested and considered the NOAEL for each compound to be 1,000 mg/kg bw per day, the highest dose tested.
The ANS Panel (EFSA ANS Panel, 2016) agreed with the authors. The Panel agreed with both the author and ANS Panel conclusions.
TiO 2 NPs < 30 nm
Mice
Khorsandi et al. ( 2016 )
Test material: TiO2 NPs (no further information, the Panel assumed that the test material is the same as in Khorsandi et al., 2017).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
The study examined the effects of oral administration of TiO2 NPs on testis volume, seminiferous tubules, interstitial tissue and total Leydig cell numbers in male NMRI mice that were 6‐ to 8‐week‐old at the start of the study. TiO2 NPs dispersed in BSA solution were administered by oral gavage at doses of 0, 75, 100 and 300 mg/kg bw per day to randomly selected groups of 8 animals for 35 days. The authors did not provide data on the general toxicity of the treatment nor for the systemic absorption of the test substance. One day after the last administration blood samples for the determination of circulating testosterone were taken and the mice sacrificed by cervical dislocation under ether anaesthesia. Endpoints included body and testis weight and volume at termination, testicular testosterone concentration and testicular histology and morphometry after fixation in Bouin's solution. While body weight was unaffected by treatment, the authors reported dose‐dependent decreases in testis weight from a dose of 100 mg/kg bw per day. The Panel noted that there is a discrepancy between the statement in the text that a statistically significant decrease was only observed at 300 mg/kg bw per day, and the presentation in Table 1 of the publication that indicates significant differences to the control in both the mid‐ and high‐dose groups. These groups also showed decreases in serum and testicular testosterone levels, the diameter and total volume of seminiferous tubules, the height of the spermatogenic epithelium and total Leydig cell numbers. Contrarily, the total volume of the interstitial tissue was found to be increased.
The Panel considered that TiO2 NPs (size unknown) at 100 mg/kg bw per day had an effect on testis weight.
Khorsandiet al. ( 2017 )
Test material: TiO2 NPs, 20–30 nm (AFM), crystalline form unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
This single dose level study examined the effects of quercetin on the TiO2‐induced damage on reproductive parameters in male NMRI mice. In this assessment, only the results from the TiO2 NPs and the control groups are discussed. TiO2 NPs were dispersed in BSA solution and administered by oral gavage at a dose of 300 mg/kg bw per day to a randomly selected group of 8 animals for 35 days. Treatment was started after the animals had been given oral saline for 7 days. Controls received saline throughout. The authors did not provide data on the general toxicity of the treatment nor for the systemic absorption of the test substance. One day after the last administration, blood samples for the determination of circulating testosterone were taken and the mice sacrificed by cervical dislocation under ether anaesthesia. Endpoints included body and testis weight, testicular testosterone, MDA, SOD and CAT concentrations, testicular histology and apoptosis assessment by a TUNEL assay after fixation in Bouin's solution, as well as cauda epididymis sperm counts, motility and morphology. The authors reported significant decreases in testis weight, circulating and testicular testosterone, testicular CAT and SOD concentrations, sperm counts and sperm motility. Significant increases were found in the percentage of abnormal or degenerative spermatogenic tubules, germ cell apoptosis, testicular MDA concentration and in the percentage of sperm with abnormal morphology. Body weight in the TiO2 NPs group was similar to the control at termination.
The Panel considered that testicular toxicity was observed with TiO2 NPs (20–30 nm) at a dose of 300 mg/kg bw per day, the only dose tested.
Karimipour et al. ( 2018 )
Test material: TiO2 NPs, anatase, 10–25 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
The study examined the effects of oral administration of TiO2 NPs for 5 weeks on the histology of ovaries, oestrogen and MDA serum levels (7 animals/group), fertility (10 animals/group) and IVF rates (10 animals/group) in female mice (presumably NMRI). TiO2 NPs dispersed in phosphate buffered saline with 0.5% Tween 80 were administered by gavage at a dose of 100 mg/kg bw per day. Mice were randomly assigned to the control and treatment group. General endpoints for toxicity such as body weight development, food consumption, clinical signs and systemic absorption of TiO2 were not examined/reported. The authors reported a significantly decreased pregnancy rate (70% vs. 100% in the control group), a 20% decrease in litter size and increases in circulating oestrogen (20%) as well as MDA (25%). They observed degeneration and reduction of follicles, cyst formation and impairment of follicular development in the ovaries of the TiO2 NPs group but presented no quantitative data. The in vivo findings were supported by a lower number of oocytes isolated from the exposed group and a higher percentage of developmental arrest before the blastocyst stage after in vitro fertilisation. The authors suggest that the observed effects could be the consequence of an indirect effect of TiO2 NPs through the generation of increased ROS levels.
The Panel considered that the study shows an impairment of female fertility at a dose of 100 mg TiO2 NPs (10–25 nm)/kg bw per day.
Karimi et al. ( 2019 )
Test material: TiO2 NPs, 68 nm (DLS; no direct information on constituent particle size, the Panel considered the majority of constituent particles to be below 30 nm), crystalline form and purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Four groups of eight 6‐ to 8‐week‐old male NMRI mice were daily treated by gavage with either (1) saline for 42 days, (2) 200 mg/kg bw per day curcumin for 42 days, (3) with saline for 7 days followed by 50 mg TiO2 NPs/kg bw per day for 35 days or (4) the simultaneous combination of exposures to curcumin and TiO2 NPs as in (2) and (3). In this assessment, only the results from the TiO2 NPs and control groups are presented Testicular damage was studied. The authors do not report on general health or clinical signs, nor is there data showing systemic absorption of TiO2. TiO2 NPs at 50 mg/kg significantly reduced testis weight in the presence of a non‐significant trend towards lower body weight, accompanied by reduced serum testosterone to around 30% of control levels. Similarly, seminiferous tubule diameter and epithelium height were affected by TiO2 NPs treatment. TiO2 NPs treatment significantly reduced the maturity of the germinal epithelium, as determined by the Johnsen's scoring system. Similar significant adverse findings were observed in reduced sperm counts, increased sperm abnormalities and reduced sperm motility.
The Panel noted that 50 mg TiO2 NPs (< 30 nm)/kg bw per day resulted in adverse effects on the testis.
Lu et al. ( 2020 )
Test material: TiO2 NPs, anatase, 7 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure not examined.
Four groups of 15 male ICR mice, age 6–8 weeks, were daily treated by gavage with TiO2 NPs at doses of 0, 10, 50 or 100 mg/kg bw per day for 30 days.
Animals were fasted for 10 h before each administration. Effects were studied on the blood–testis barrier (BTB), MAPK signalling pathways, serum testosterone and oestradiol levels and sperm parameters. The authors did not report on general health and clinical signs, nor is there data showing systemic absorption of TiO2. Through TEM, the authors report tight junction damage in the BTB at 50 and 100 mg/kg bw per day, though the histopathological pictures provided are hard to interpret. BTB‐related proteins F‐actin, ZO‐1 and claudin‐11 were dose‐relatedly significantly increased up to 2‐fold at the high dose, with no significant changes observed in connexin‐43 and occludin. F‐Actin was significantly increased at all doses tested. As to elements of MAPK signalling pathways, ERK and JNK mRNA expression was slightly but significantly increased in testis tissue at the high dose only. No effect was found on p38 mRNA expression. Serum testosterone was 50% decreased at the two highest doses tested, accompanied by a slight but significant reduction in oestradiol at the same doses. Sperm counts were unaffected by exposure, but sperm motility was dose‐relatedly reduced from around 70% in controls to around 50% at the high dose, accompanied by increased sperm malformation rates from 3% in controls to 8% at the high dose, both effects being statistically significant at the two highest doses.
The Panel considered that TiO2 NPs (7 nm), at 50 or 100 mg/kg bw per day, resulted in a dose‐related reduction of sperm motility and increased sperm malformations, accompanied by histological observations in the testis, changes in BTB‐related protein levels, changes in MAPK‐related mRNA levels and reduced circulating testosterone concentrations.
Rats
Lee et al. ( 2019 )
Test material: TiO2 NPs (the Panel noted that from the description of the tested material, it corresponds to P25 (15–24 nm)).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure examined: quantitative analysis in blood/tissues; methodology reliable with some limitations.
Mated Sprague–Dawley rats (12 females per group) were daily administered by gavage TiO2 NPs at dose levels of 0, 100, 300 and 1,000 mg/kg bw per day from GD 6 to 19. The study was designed in accordance to OECD Guideline 414 and in compliance with GLP. The dose volume was 10 mL/kg bw per day. Clinical signs were recorded at least daily. Body weight and feed intake were measured at regular intervals. Rats were killed for a caesarean section on GD 20. Gross necropsy included gross examination of the dam, counting of the number of corpora lutea, implantation sites, resorptions, live and dead fetuses, fetal sex and weight. Fetal pathological external, visceral and skeletal examinations were performed in order to detect abnormalities. In addition, the following organs were weighed and the absolute and relative organ weights were presented: adrenal glands, brain, heart, kidney liver, pituitary gland, spleen, ovaries (right, left) and thymus.
There were no statistically significant differences in general clinical signs, body weight, organ weights (absolute and relative to body weight), macroscopic findings, apart from a statistically significant decrease in food intake of the females of the high‐dose group. The authors considered that this decrease did not have toxicological significance since it was minimal and there was no correlated decreased body weight or body weight gain during the study period. The Panel agreed with this conclusion.
Caesarean section parameters and fetal external and visceral examinations did not reveal any statistically significant differences. The only difference seen was a small but statistically significant increase (4%) in the number of ossification centres in the metatarsals of both hindlimbs of the fetuses of 100 mg/kg bw per day group. The Panel agreed with the authors that this could be considered as an incidental finding as no other related effects were observed.
The Panel considered that no adverse effects were reported with TiO2 NPs (21 nm) up to 1,000 mg/kg bw per day, the highest dose tested.
Neurotoxicity and neurodevelopmental toxicity studies
TiO 2 NPs
Rats
Ebrahimzadehet al. ( 2017 )
Test material: TiO2 NPs, anatase, < 100 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Pregnant Wistar rats (n = 6/group, randomly assigned) were administered by gavage TiO2 NPs in distilled water vehicle (volume not reported) at doses of 0 or 100 mg/kg bw per day from GD 2 to 21 (gestation group) or from PND 2 to 21 (lactation group).
Endpoints were quantification of hippocampal apoptosis (TUNEL, RT‐PCR) and neurogenesis (DCX‐positive cells) in CA1‐3 and dentate gyrus (DG) on PND 1 (gestation group) or PND 21 (lactation group) (n = 6: 1 male/litter, 6 litters/group; unclear if TUNEL and DCX n = 3/group).
Both gestational and lactational exposure significantly increased hippocampal apoptosis in CA1‐3 and DG, and reduced neurogenesis in CA1‐3.
The Panel agreed with the author's conclusion that exposure with TiO2 NPs at 100 mg/kg bw per day during pregnancy and lactation increased apoptosis and reduced neurogenesis in the hippocampus of the offspring.
Kandeil et al. ( 2019 )
Test material: TiO2 NPs, 90 nm (range 40–140 nm) (TEM), crystalline form and purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Adult male albino rats (n = 20/group, randomly distributed) were administered by gavage TiO2 NPs in distilled water (volume not reported) at doses of 0 or 500 mg/kg bw per day for 14 days.
The main endpoints examined were clinical signs, brain homogenate dopamine and 5‐hydroxytryptamine, cholinesterase activity, oxidative stress markers (GSH, SOD, MDA, total antioxidant capacity (TAC), TOS, OSI = TOS/TAC), inflammatory and apoptosis markers (IL‐1β, TNF‐α, caspase‐3, Fas; rf2, N N QO1 and INOS gene expression), cerebral mitochondrial viability (MTT), cerebral DNA fragmentation (diphenylamine colorimetric) and prefrontal cortex histopathology (blinded quantitative).
The TiO2 NPs group showed decreased physical activity, passive behaviour, loss of appetite and tremors. Treatment statistically significantly reduced brain GSH, SOD, TAC, Nrf2 and NQO1 expression; significantly reduced cerebral mitochondrial viability; increased DA and 5‐HT; increased cholinesterase activity; increased MDA, TOS and OSI levels; increased IL‐1β, TNF‐α, caspase‐3, Fas; INOS expression; increased cerebral DNA fragmentation; and prefrontal cortical congestion, pericellular oedema, perivascular oedema and pyknosis.
The Panel considered that these data show that oral TiO2 NPs can induce CNS toxicity at 500 mg/kg bw per day, possibly related to oxidative stress.
TiO 2 NPs < 30 nm
Mice
Zhouet al. ( 2017 )
Test material: TiO2 NPs, anatase, 6–7 nm (TEM, XRD, Hu et al., 2011).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure: quantitative measured in tissues; methodology not reliable.
Pregnant or lactating CD‐1 (ICR) mice (n = 6/group, randomly assigned) were administered by gavage TiO2 NPs in 0.5% w/v aqueous hydroxypropylmethylcellulose vehicle (volume not reported) at doses of 0, 1, 2 or 3 mg/kg bw per day from ‘prenatal day 7’, presumed to be GD 7, although possibly GD 14, to PND 21.
Endpoints in primary cultures of hippocampal CA1 neurons harvested after PND 21 from the pups were: neuron ultrastructure (by TEM), dendrite morphology (by SEM), mitochondrial membrane potential (MMP; fluorescence), ROS, MDA, protein carbonylation (PC), ATP levels, apoptosis‐ and autophagy‐related factors (ELISA, western blot) (n = 5/group per endpoint).
The Panel noted that the method applied to randomise the pups from 6 dams/group into 30 pups/group is not reported. It is also unclear whether CA1 neurons were harvested from all 30 pups/group.
The authors reported that the Ti amount in hippocampus was dose‐dependently increased at all doses. The Panel noted that the description of the analytical method was insufficient to establish its reliability and tissue concentrations were expressed with incorrect measurement units.
At all doses, there were dose‐dependent decreases in dendritic growth (primary dendrite length, significantly reduced by 24.71%, 63.82% and 77.99% at low, mid and high doses, respectively), MMP (significantly reduced by 17.14%, 36.57% and 51.43% at low, mid and high doses), and concentrations of MDA, PC and ATP (nmol/mg protein).
At all doses, apoptosis‐ and autophagy‐related factors (rH2AX, Cytc, caspase 3, PI3K3C, Beclin 1 and c‐Jun; LC3I, LC3II, JNK and p‐JNK) were significantly and dose‐relatedly increased, and anti‐apoptosis‐related protein Bcl‐2 was significantly and dose‐relatedly decreased.
Tissue ROS was significantly increased at mid and high doses only (to the same level, no dose response).
Treatment‐related neuronal ultrastructural changes were reported (narrative only, no quantitative data), including mitochondrial swelling, carina disappearance, nucleus shrinkage, chromatin marginalisation, anomalous nuclear membrane and dilation of endoplasmic reticulum accompanied by the emergence of autolysosomes and the formation of vacuoles. The Panel noted that TiO2 NPs (6–7 nm), at all doses tested, inhibited dendritic outgrowth, reduced mitochondrial function and increased autophagy and oxidative stress markers, in ex vivo hippocampal CA1 neurons after dosing during gestation and early lactation.
Zhanget al.( 2020 )
Test material: TiO2 NPs, 21 nm (TEM), crystalline form and purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Young adult (age 7 weeks) male C57BL/6J mice (n = 30 randomised into two groups; presumably n = 15/group but not reported) were administered by gavage TiO2 NPs in vehicle (2% heat‐inactivated mouse serum, volume not reported) at doses of 0 or 150 mg/kg bw per day for 30 days.
Main endpoints were small intestine and brain (hippocampus and cerebral cortex) histopathology, gut microbiota (faecal bacterial 16S rRNA gene sequencing), gut and cerebral cortex transcriptomics and functional tests (open field test (OFT), Morris water maze (MWM)). It is noted that the number of animal tested is not reported, but results for some endpoints show n = 10/group.
Treatment had no effect on body weight or histopathology of gut or brain, but significantly decreased the richness and evenness of gut microbiota (decreased Shannon's diversity, chao, observed species and elevated Simpson's diversity) and elevated gut HuC/D and TuJ1, suggesting an effect on the enteric nervous system.
Serotonergic markers Sstr1 and Sstr2 were markedly reduced in the gut but not in the cerebral cortex. Gut–brain peptides secreted by endocrine cells and enteric neurons, and also inflammatory cytokines, were not affected by treatment.
OFT centre field activity was markedly reduced by treatment, consistent with anxiety‐like behaviour, but MWM learning and memory were unaffected.
The Panel considered that these data show that TiO2 NPs (21 nm) can markedly alter the mouse gut microbiota, without pathological changes in small intestine and brain. Spatial learning and memory were not affected but centre field activity in open field testing was decreased at the only dose tested of 150 mg/kg bw per day, consistent with increased anxiety. The Panel is aware of ongoing research on relationships between gut microbiota and anxiety (Yang et al., 2019)
Rahnamaet al. ( 2020 )
Test material: TiO2 NPs, 21 nm (TEM), crystalline form and purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure not examined.
Effects of TiO2 NPs were investigated on stereological parameters (volume of brain region and number of neurons) in the hippocampal DG and on the qualitative morphology of hippocampal granular neurons in adult mice.
Adult male mice (n = 20, 10–12 weeks old), food and water ad libitum, were randomly divided over four groups (no further information reported) and administered daily by gavage a suspension of TiO2 NPs at doses of 0 (saline control), 2.5, 5 or 10 mg/kg bw per day for 35 days.
After formalin perfusion fixation, brains were removed and the two hemispheres carefully divided. One hemisphere (chosen randomly) was used for stereology; the other for qualitative morphological examination of volume appearance of the hippocampus, DG and sublayers (H&E systematic sections; and silver Golgi staining for neuronal dendrite appearances (dendritic length and branching). The total volume of the hippocampus, DG (including also the different sublayers) was estimated using the Cavalieri principle and H&E‐stained systematically sampled sections. The numerical density of DG granular cells was estimated with physical dissector. The morphology of DG granular cells was studied using qualitative silver nitrate Golgi staining. The Panel noted that in the Methods section, the correct formula for volume estimation according to the Cavalieri principle is missing; instead, the formula for numerical density estimation using physical dissector is erroneously given twice.
Compared to the saline control group, TiO2 NPs induced dose‐related reductions in the total volume of the hippocampus (statistically significant in the low‐, mid‐ and high‐dose groups), of the DG (statistically significant in the mid‐ and high‐dose groups), including the volume of its molecular and granular layers (both statistically significant in the mid‐ and high‐dose groups) and the volume of the polymorph layer (statistically significant in the low‐, mid‐ and high‐dose groups). In addition, there was a dose‐related reduction in the numerical density of DG granular cells, as well as a dose‐related reduction in the total number of granular cells of the DG (both statistically significant for the low‐, mid‐ and high‐dose groups).
According to the authors, qualitative examination of the morphology of the hippocampus and DG granular cells supported the measured reduction in volume of hippocampus and DG and suggested that the length of the dendrites of the granular cells appeared shorter and the number of branches reduced. The Panel noted that in Figure 9, photographs of five groups are shown (instead of four), illustrating the morphological appearances of the hippocampus/DG. The Panel noted that the results show both a control and a sham without describing these controls in the methods.
The Panel agreed with the authors that the results of the (unbiased) stereological data will contribute to the understanding of memory and learning disorders resulting from exposure to TiO2 NPs.
The Panel noted that TiO2 NPs (21 nm) at all doses tested lead to a reduced volume of the hippocampus, and the polymorph layer of the DG, and on reduced numerical density and total number of granular cells of the DG.
Rats
Grissaet al. ( 2016 )
Test material: TiO2 NPs, anatase, 5–10 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Adult male Wistar rats (n = 6/group, randomly assigned) were administered by gavage TiO2 NPs at doses of 0, 50, 100 or 200 mg/kg bw per day in 10 mL water vehicle/kg bw for 60 days.
The main study endpoints were necropsy body and brain weight, plasma and brain IL‐6, whole brain homogenate cholinesterase activity, cerebral cortex GFAP‐positive cells (by immunohistochemistry, counted in ‘areas that had a maximum of positive cells’, n = 3/group)
Body weights were unaffected by treatment, but relative brain weight was dose‐dependently decreased, significantly at 100 and 200 mg/kg bw per day. Plasma IL‐6 was increased at all doses (no dose response). Brain IL‐6 was dose‐dependently increased significantly at 100 and 200 mg/kg bw per day. Plasma cholinesterase activity was statistically significantly and dose‐dependently reduced at all doses (by about 35%, 50% and 50% at 50, 100 and 200 mg/kg bw per day, respectively). The Panel noted the methodology of the authors did not indicate whether plasma cholinesterase activity represented acetylcholinesterase or butyrylcholinesterase or both. Brain cholinesterase activity was reduced at 100 and 200 mg/kg bw per day only (at both doses by about 50%, i.e. no dose response). Cerebral cortex GFAP‐positive cell counts were dose‐dependently increased at 100 and 200 mg/kg bw per day. The Panel noted that TiO2 NPs (5–10 nm) reduced plasma cholinesterase activity and increased plasma IL‐6 at all dose tested levels, and reduced brain cholinesterase activity at doses of 100 and 200 mg/kg bw per day. The Panel noted reduced plasma cholinesterase activity and increased plasma IL‐6 at all doses and reduced brain cholinesterase activity at 100 mg/kg bw per day.
Mohammadipour et al. ( 2016 )
Test material: TiO2 NPs, anatase, 10 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Wistar rat dams (n = 6/group, randomisation not reported) were administered by gavage TiO2 NPs in distilled water (volume not reported) at doses of 0 or 100 mg/kg bw per day.
To avoid possible dermal and oral transfer, the animals’ cages were cleaned daily. Thus, according to the authors, the exclusive and significant way of offspring exposure to TiO2 nanoparticles was through maternal milk.
Offspring were weaned and housed 5/cage from PND 21, and learning/memory tested from PND 60.
Endpoints were learning and spatial recognition memory in Morris water maze (MWM) and two‐compartment light/dark shock passive avoidance (PA) tests.
MWM acquisition path length and latency were initially higher in the treated group on days 1–3, but normal on days 4–5. Performance in the memory probe trial was unaffected by treatment.
In the PA test, latency to enter the dark compartment was significantly lower in the treated than the control group at 1 and 24 but not 48 h post‐shock; total time spent in the dark compartment was significantly higher than in controls at all three time points post‐shock.
The authors concluded that TiO2 NPs dosing of lactating mothers impairs memory and learning of pups in adulthood. However, the Panel noted that MWM acquisition (days 4 and 5) and retention probe trial) were normal, and the reduced latency to dark compartment entry post‐shock was transient (1 and 12, not 48 h). The Panel considered that these data do not show that maternal exposure to TiO2 NPs (10 nm) during lactation impairs learning and memory in the offspring.
Hassanein and El‐Amir ( 2017 )
Test material: TiO2 NPs, 21 nm [no further information].
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
Adult male Sprague–Dawley rats (n = 10/group, randomly assigned) were daily administered by gavage TiO2 NPs in 1% Tween 80 (0.5 mL/rat) at doses of 0 or 150 mg/kg bw per day for 6 weeks.
The only reported endpoint relevant to neurotoxicity was brain histopathology.
Reported findings were haemorrhage, congestion of choroid plexus ‘blvs’ (the Panel interpreted this as blood vessels), chromatolysis, neuronal degeneration and perivascular lymphocytic cuffing, each in at least 4 of 10 treated animals.
The Panel noted that effects on brain histopathology were observed at a dose of 150 mg/kg bw per day TiO2 NPs (21 nm), the only dose tested, although the tissue fixation method was not optimal for histology of brain tissue (non‐perfused formaldehyde fixation).
Canli et al. ( 2020 )
Test material: TiO2 NPs, anatase, 21 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure: qualitative analysis in tissues; methodology with important flaws.
Adult female albino rats (n = 6/group, randomisation not reported) were administered by gavage TiO2 NPs at doses of 0, 0.5, 5 or 50 mg/kg bw per day for 14 days.
Main endpoints were liver thiobarbituric acid reactive substances (TBARS) and GSH, ATPase activity in supernatant of homogenised kidney, intestine and brain, and cholinesterase activity in brain homogenate supernatant. TEM images of TiO2 NP in liver, kidney and brain were recorded. Body and organ weights were not reported.
The authors reported that TEM demonstrated the presence of TiO2 particles in the liver, kidney and brain which ‘seemed dose dependent’). The Panel noted that verification of the elemental composition of the particles of interest was not performed. There was one death at 0.5 mg/kg bw per day group (no further details reported), but no other notable clinical signs (e.g. ‘eye colour, feeding habits, activity’). Treatment had no effect on liver total, reduced or oxidised glutathione (tGSH, rGSH or GSSG) or the ratio between reduced and oxidised glutathione (GSH/GSSG ratio), or on kidney and intestine ATPase activity. Brain Na/K‐ATPase activity was significantly increased (approximately 2‐fold) at 0.5 and 5 mg/kg bw per day, Mg‐ATPase and total ATPase activity at 5 mg/kg bw per day. Brain cholinesterase activity was significantly reduced at all doses (by about 50%, 35% and 50% at 0.5, 5 and 50 mg/kg bw per day, respectively, i.e. no dose response).
The Panel noted that reduced brain cholinesterase activity was observed at all dose levels tested, while increased brain Na/K‐ATPase activity was observed only at the lowest dose tested of 0.5 mg/kg bw per day and at the highest dose tested of 50 mg/kg bw per day of TiO2 NPs (21 nm).
Grissaet al.( 2020 )
Test material: TiO2 NPs, anatase, 5–12 nm (TEM, XRD).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure: quantitative in tissues; methodology with important flaws.
Male Wistar rats were administered by gavage (aqueous suspension; volume of 10 mL/kg bw) with TiO2 NPs (five times/week; 8 weeks) after being randomised over 4 groups (n = 8/group): 0 (control), 50, 100 and 200 mg TiO2 NPs/kg bw per day. Clinical signs and mortality were recorded daily. Rats were euthanised the day after treatment. The brain was removed and weighed and frontal lobes isolated (right one was formalin‐fixed; part of left homogenised). Titanium amount was quantified in the homogenate.
Brain frontal lobe histopathology, biomarkers of oxidative stress (antioxidant enzyme activity (SOD), glutathione peroxidase (GPx), TAS, CAT, TBARS and inflammatory markers nitric oxide (NO) and TNF‐α) were examined.
The amount of titanium in brain after intragastric administration of TiO2 NPs for 8 weeks was significantly and dose‐dependently elevated in all dose groups compared to controls (in which no titanium was detected). However, the Panel noted that the measured Ti levels in tissues were below the LOQ.
Brain histopathology was reported at mid and high doses (qualitative description only): lymphoid infiltration, probably due to cerebral inflammation and hypersensitivity, vascular congestion, cerebral oedema, proliferation of glial cells, some cell necrosis and neuron cells cleavaged into filamentous shapes. At low dose, authors reported ‘quasi‐normal histological structure, suggesting no abnormal pathological changes in the cerebral cortex’.
SOD and CAT activities were significantly decreased at all doses, and GPx activity and total antioxidative status (TAS) at mid and high doses. Inflammatory biomarkers: NO was significantly increased at mid and high dose, and TNF‐α at high dose. Lipid peroxidation: TBARS was significantly increased at mid and high doses. Apoptosis (by TUNEL) was significantly increased at the high dose.
The Panel noted that reduced activity of brain frontal lobe SOD and CAT were observed at all doses of TiO2 NP (5–12 nm).
Inflammation and immunotoxicity studies
E 171
Mice
Urrutia‐Ortegaet al.( 2016 )
Test material: E 171, particles below and above 100 nm, crystalline form not reported.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure: qualitative analysis in tissues, methodology reliable with some limitations.
BALB/c male mice of 4‐ to 6‐week‐old after 1 week of acclimation were randomly divided in to four groups (n = 6): a) control, b) E 171 group, c) chemically colitis‐associated colorectal cancer (CAC) group and d) CAC + E 171 group. The E 171 group of mice received an administration by gavage of 5 mg/kg bw per day for 5 days/week during 10 weeks. The CAC group received a single intraperitoneal dose of 12.5 mg/kg bw azoxymethane (AOM) and 2% dextran sulfate sodium (DSS) in the third, sixth and ninth week in water ad libitum. The CAC + E 171 group received a single dose of AOM and DSS in the same scheme of CAC group, and in addition, received administration of E 171 by gavage according the same scheme as the E 171 group. The control group received a single intraperitoneal injection of saline solution. After 11 weeks, mice were sacrificed and the colon, kidney, liver, spleen, lung tissue and blood samples collected. The colon was dissected, opened and fixed in paraformaldehyde. For histological examination, sections were stained with H&E (blinding is not mentioned). Tumour progression was evaluated by the detection of COX2, β‐catenin and Ki67, while NF‐κB was used as a key marker of inflammation in colon tissue by immunohistochemistry. Goblet cells were identified by counting Alcian blue‐stained blue cells. In colon homogenates tissue levels of IL‐2, TNF‐α, IFN‐γ, IL‐10 and GM‐CSF were determined by Bio‐Plex multiplex MAGPIX.
Mice body weight and consumption of food and water remained unchanged by administration to E 171 by gavage. Regarding organ weights, the spleen, liver and lung weights remained unchanged, with no histological abnormalities in these organs. Mice from group CAC + E 171 group had a decrease of 74% in kidney weight; however, E 171 administration had no effect on this parameter. BUN and serum creatinine, used as kidney function markers, however, remained without changes (data not shown).
Regarding tumour formation and inflammation, this study aimed to investigate the impact of oral E 171 intake on the enhancement of colorectal tumour formation in a CAC mouse model. Results obtained indicate that E 171 alone was unable to induce tumour formation, but dysplastic alterations were observed in the distal colon with a statistical significant enhancement of tumour formation in CAC + E 171 group vs. CAC group (p < 0.01). Some E 171 particles were internalised in colonic cells of the E 171 and CAC + E 171 groups, and both groups showed a decrease in goblet cells in the distal colon. CAC tumour progression markers including COX2, Ki67 and b‐catenin indicated that E 171 exacerbates tumour progression and p65 NF‐κB, a key regulator of inflammation was also induced by E 171 administration in CAC group. In the E 171 group, despite the absence of tumour formation, a slight statistical significant increase in COX2, Ki67 and b‐catenin, and p65 NF‐κB were observed. Regarding cytokines in colon tissue, no changes, despite enhanced the p65‐NF‐κB expression, were found in the E 171 alone and CAC groups. Furthermore, a statistically significant decreases of IL‐2, TNF‐α, INF‐g and IL‐10 were observed in the CAC + E 171 group when compared to the E 171 group, probably as a consequence of colon tissue damage.
The Panel considered that the results suggest that while E 171 (5 mg/kg bw per day) alone administered for 10 weeks has no effect on tumour formation, it can potentiate intestinal tumour formation in mice exposed to AOM/DSS.
Talamini et al. ( 2019 )
Test material: E 171 (35% nano), anatase, 201 nm in suspension (NTA).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure examined: quantitative analysis in tissues with methodology reliable with some limitations.
Eight‐week‐old male NFR mice were housed in pathogen‐free animal rooms. Mice were randomly divided in to two groups (22 animals/group). One group was orally treated with 5 mg E 171/kg bw, freshly dispersed in water, and control animals received water. Mice were treated 3 days/week for 3 weeks. According to this treatment schedule, mice received an average daily dose of approximately 2 mg/kg bw per day. On day 21, three days after the last dose, mice were sacrificed and lung, liver, stomach, spleen, kidney, brain, testes and whole intestine were excised. At autopsy, the organs were sampled for each mouse, fixed in formalin, processed, embedded in paraffin and sections cut and stained with H&E or further processed for immunohistochemical analyses. Immunohistochemical staining for tissue monocytes/macrophages was performed utilising an anti‐F4/80 specific antibody. Necroinflammatory foci in the liver were manually identified by three different operators blinded to treatment allocation and quantified using ImageScope software. Cytokine mRNA expression was evaluated by quantitative real‐time PCR in stomach, intestine and liver. The levels of IL‐1b, TNF‐α and IL‐6 in plasma samples were analysed with the MESO QuickPlex SQ 120, while SDF‐1 (stromal‐cell‐derived factor‐1) was determined using a ELISA kit.
This study aimed to investigate whether TiO2 is deposited in the digestive system and internal organs and whether there are any molecular and cellular alterations associated with an inflammatory response. Regarding inflammation, to assess whether TiO2 accumulation and/or the production of superoxide in target organs may lead to histological changes, H&E and F4/80 staining were performed. Neither overt structural and morphological histological alterations nor significant recruitment of monocytes/macrophages were observed in the stomach and whole intestine in E 171‐treated animals. Neither disruption of crypt structure nor atypical epithelial cell proliferation in colon was observed. No changes in spleen histology were mentioned.
In the liver, increased size of necroinflammatory foci infiltrated with F4/80 monocytes/macrophages was noted three days after the last E 171 dose (n = 4) vs. controls (n = 2), while no changes were observed in stomach or intestine of treated animals. Liver histological findings are not supported by ROS production data or cytokine levels. An increased ROS production (superoxide anion) was observed in the stomach and in the intestine, with no changes in the liver (n = 4), of treated animals. Regarding cytokine mRNAs, as assessed by quantitative real‐time PCR in stomach, whole intestine and liver, data were not striking: a very modest increase of IL‐1b was observed in stomach and whole intestine of animals exposed to E 171, a decrease in TNF‐α was observed in the whole intestine but in the stomach, with no changes in liver. A slight decrease in IL‐10 was observed in the liver, which was not associated with an increase in pro‐inflammatory cytokines (IL‐1b or TNF‐α). mRNA data were provided for n = 10 for IL‐1b and TNF‐α, n = 5 for IL‐10. The levels of serum cytokines were not consistent with tissue mRNA expression. As an increase in IL‐6 and SDF1, with no changes in TNF‐α or IL‐1b, were observed in treated animals (n = 5).
The Panel considered that analyses, which were limited to few animals (reduced power/data attrition), showed some evidence for modest inflammation which cannot be clearly identified as adverse.
Riedle et al. ( 2020 )
Test material: E 171, anatase, 119 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure: qualitative analysis in tissues, methodology reliable with some limitations.
C57BL/6 mice aged 6 weeks were randomly assigned to one of four diets and exposed to 0 and ≈ 1, 10 and 100 mg E 171/kg bw per day. Mice were housed conventionally and feed intake and body weight recorded biweekly. At 6, 12 and 18 weeks, six mice per group were euthanised and the GI tracts harvested. Ileal tissues containing the most distal Peyer's patch were excised and snap frozen. Peyer's patches from 18 weeks feeding were examined by confocal microscopy and SEM with EDX analysis. Remaining Peyer's patches were collected and enzymatically digested to form single‐cell suspensions for analysis by flow cytometry after immunostaining for CD4, CD45R and CD8a for lymphocytes or CD11b, CD11c and CD8a for myelocytes.
The Panel considered that this study demonstrates that particles in E 171 administered via the diet are taken up by basal cells of intestinal lymphoid follicles. The Panel considered that the parameters investigated did not show an effect on the immune system or inflammation.
Pinget et al. ( 2019 )
Test material: E 171, anatase, 30–300 nm (SEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E , was 2.
Internal exposure not examined.
Five to six male C67Bl/6JAusb mice were exposed by drinking water to E 171 (0, 2, 10, 50 mg/kg bw per day) for 4 weeks. Histological analysis of the gut revealed a reduction of colonic crypt length. An increase in colon macrophages and CD8 cells were observed by FACS analysis in cell suspensions prepared from colon, and increased mRNA encoding for IL‐10, TNF‐α and IL‐6 was detected by RT‐PCR in RNA extracted form colon tissue.
Rats
Bettiniet al.( 2017 )
Two test materials: 1) E 171, anatase, 20–340 nm (118 nm) (TEM); 44.7% particles < 100 nm; 2) TiO2 NPs (NM‐105), anatase/rutile, 15–24 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 1.
Internal exposure: qualitative measurement in tissues, methodology reliable with some limitations.
Adult Wistar rats were administered by gavage 10 mg E 171/kg bw per day for one week or 100 days. In addition, a group of animals were exposed to TiO2 NP (NM‐105) 10 mg/kg bw per day for one week. Titanium was detected in the immune cells of Peyer's patches. Effects were not noted in the spleen, but in Peyer's patches dendritic cell percentage were increased, as measured by flow cytometry of cells isolated from tissue samples. This effect was transient, as it was observed 7 days after exposure, but not at 100 days. The percentage of regulatory T cells and T‐helper (Th) cells were significantly decreased at both time points in E 171 exposed animals. Stimulation of immune cells isolated from Peyer's patches showed a decrease in T‐helper (Th)‐1 IFN‐γ secretion, while splenic Th1/Th17 inflammatory responses sharply increased, as measured in cells taken from exposed rats, stimulated in vitro with anti CD3/CD28 antibodies. Regarding the effects of TiO2 NP, similar to E 171 an increase in the percentage of dendritic cells in Peyer's patches was observed with no decrease in the percentage of Tregs. Stimulation of immune cells isolated from Peyer's patches showed a decrease in T helper (Th)‐1 IFN‐γ secretion, while splenic Th1/Th17 inflammatory responses sharply increased, as measured in cells taken from exposed rats, stimulated in vitro with anti CD3/CD28 antibodies. Regarding intestinal mucosal inflammation, E 171 for one week did not initiate intestinal inflammation, but a 100‐day E 171 treatment promoted colon microinflammation evidenced by significantly increased IL‐1β, IL‐8 and TNF‐α expression in the colon. In the same samples, increased IL‐10 was also observed. Data on the effects of TiO2 NP on intestinal mucosa were not presented. In this study, changes (aberrant crypts) were examined in the colon after staining with methylene blue. The authors did not explicitly define an aberrant crypt foci (ACF) but the Panel presumed it was 1‐or more aberrant crypts/ACF. The authors defined a ‘large ACF’ as consisting of more than three aberrant crypts per ACF. In this study, changes (aberrant crypts) in the colon were examined. The authors provided morphological data demonstrating aberrant crypts after staining with methylene blue. The authors did not explicitly define an aberrant crypt foci (ACF) but the Panel presumed it was 1‐or more aberrant crypts/ACF. The authors defined a ‘large ACF’ as consisting of more than three aberrant crypts per ACF.
In the first experiment, in 12 male rats pretreated with a single injection (180 mg/kg intraperitoneal in isotonic saline) of the genotoxic carcinogen dimethylhydrazine (DMH) there were on average per colon approximately 470, 190 and 30 aberrant crypts, ACF and large ACF, respectively, after 100 days. In DMH pretreated rats also subsequently (7 days later) exposed to either 0.2 or 10 mg/kg bw per day E 171 in drinking water (12 rats/group), there was a statistically significant increase per colon in number of aberrant crypts and large ACF and a statistically non‐significant increase in total number of ACF in the high‐dose group compared to DMN only controls. No statistically significant differences were observed between the low‐dose and control groups. The incidence of ACF was not reported.
In the second experiment, male rats received either drinking water (12 controls) or 10 mg E 171/kg bw per day in drinking water (n = 11) for 100 days. No ACF were observed in the colons of controls but four rats in the treated group developed one to three ACF per colon (which in three rats consisted of 1–3 aberrant crypts/ACF with the remaining rat having 12 aberrant crypts in an ACF). The increase in the incidence of rats with ACF (4/11 vs. 0/12 in the control group) was statistically significant.
Regarding inflammation, the Panel considers that these data indicate that E 171 has pro‐inflammatory potential at the systemic level, paralleled by the development of an inflammatory microenvironment in the intestinal mucosa.
The Panel considered that E 171 alone at a dose of 10 mg/kg bw per day may induce development of ACF in male rats. The Panel also noted that E 171 at a dose of 10 mg/kg bw per day increased the number of ACF initiated by a genotoxic carcinogen.
Blevins et al. ( 2019 )
Test material: E 171, anatase, 110–115 nm (SEM), 36% particles < 100 nm.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure not examined.
Six‐week‐old male Wistar Han IGS (Crl:WI (Han)) rats were exposed to E 171 in a standard diet at a concentration of 0, 40, 400 or 5,000 mg/kg diet (equal to 1.8, 4.8, 31.4, 374 mg/kg bw per day) for 7 and (equal to 1.3, 3.5, 22.4 or 267 mg/kg bw per day24) 100 days. There were two different studies, one study was performed over 7 days (n = 5/group) and the other over 100 days (n = 15/group). The two studies were performed at different Institutions, with the 7‐day study performed twice whereas the 100 day study was performed once. For the 7‐day studies, rats were randomised into 4 groups of 5 animals and the data from the two studies were pooled. Total food and water consumption were determined at the end of each study. Body weights were determined at the start and end of the 7‐day exposure period at the time of euthanasia. For the 100‐day study, animals were randomised into 8 groups of 15 animals each. At the start of the study, animals in groups 5–8 were treated with a single intraperitoneal injection of a sterile dose of 180 mg/kg bw dimethylhydrazine (DMH) dihydrochloride while groups 1–4 were treated with vehicle only. Seven days after intraperitoneal injection, dietary administration of 0, 40, 400 or 5,000 mg/kg diet E 171 was started and continued for 100 days. Body weights were determined weekly beginning on day 0 of the study and just prior to euthanasia. Food consumption was determined weekly beginning with administration of the E 171 supplemented diets. Water consumption was determined during weeks 3, 8 and 13 of the study.
No significant changes in food intakes or body weights or liver and spleen weights were found and no mortality was observed. A trend towards increased food consumption in rats of the high E 171 group was observed. According to the authors, dietary E 171 produced no general signs of overt toxicity at the highest dose tested, over 100 days.
The objectives of the study were to evaluate the acute (7 days) and subchronic (100 days) effects of dietary E 171 exposure on the immune system of the GI tract and periphery as well as to evaluate effects of the subchronic exposure either alone or after pre‐administration of a known intestinal genotoxic carcinogen, DMH. Concerning the latter an examination of colon for presence of aberrant crypt foci (ACF) and of aberrant crypt (ABC) was included. Concerning the effects on the immune system, the following parameters were investigated: phenotyping of immune cells (i.e. CD103+ DC, total and activated T helper cells, total and activated Treg cells) and inflammatory cytokines [(IL‐1α, IL‐1β, IL‐6, interferon γ (IFNγ), IL‐12p70, IL‐17A, IL‐18, IL‐33. CCL2/MCP‐1, CXCL1/KC (IL‐8), GM‐CSF and tumour necrosis factor α (TNF‐α)).
Following the 7‐ and 100‐day feeding periods, rats were euthanised and measurements of inflammatory cytokines (using the LEGENDplex rat inflammation Panel) and phenotyping of immune cells (by flow cytometry) in the periphery and GI tract were performed. Peyer's patches, peripheral blood mononuclear cells (PBMC) and spleen cells were analysed for inflammatory and regulatory T‐cell responses directly ex vivo or after in vitro stimulation with anti‐ratCD3 (5 μg/ml) and anti‐rat CD28 (5 μg/ml) for 4 days. Histopathology, ACF, ABC and goblet cell evaluations were performed on rats in the 100‐day study. All tissues were collected from well‐defined areas, and measurements, procedures and evaluations were performed in a standardised and blinded manner.
CD103+ dendritic cells (DC) were evaluated in the gut, Peyer's patches, spleen and in peripheral blood over time period. No change in the percentage of CD103+ DC in peripheral blood, spleen or Peyer's patches due to acute or subchronic dietary E 171 consumption alone was observed.
The total percentage of CD4+ T helper cells, the percentage of T helper cells expressing CD25, an indicator of T helper activation, and the percentage of Treg cells (CD4+FoxP3+) and activated Treg (CD4+CD25+FoxP3+), critical mediators of local and systemic inflammation, which could lead to a low level inflammatory response in the absence of increased inflammatory cells, were quantified in peripheral blood, spleen and Peyer's patches. Dietary E 171 exposure did not change the frequency of CD4+ T helper cells systemically or in intestinal Peyer's patches. In addition, there was no detectable impact on the percentage of activated CD4+ T helper cells or on the percentage of Treg cells either peripherally or locally in the Peyer's patches of treated rats fed for 7 or 100 days. Collectively, these results suggest that E 171 consumption does not alter T‐cell‐mediated mechanisms of immune control, either promoting inflammatory CD4+T helper cell activation or in reducing the percentage of anti‐inflammatory Treg cells.
Regarding the effects on cytokines, data presented suggest that dietary E 171 does not induce inflammation peripherally or in the GI tract at both time points. In addition, studies were conducted to explore the possibility that E 171 might alter the effector cytokine profile of T helper cells in lymphoid tissue or circulation, which may not be manifest without T cell‐specific stimuli. Lymphocytes were isolated from peripheral blood, spleen and Peyer's patches and activated ex vivo with anti‐CD3/anti‐CD28 for 4 days to induce T helper cell cytokine production. No effects of E 171 exposure on any of the induced cytokines produced from ex vivo stimulated T helper cells were observed.
In the 100‐day study, all animals were treated with E 171, some groups were initiated with 180 mg/kg bw DMH before the start of the dietary exposure to E 171 and an additional control initiated with DMH was also included. The same parameters as described above were evaluated, with some differences observed. A modest increase in the relative spleen weight in 22.4 mg E 171/kg bw per day + DMH compared to not initiated animals, an increase in IL‐17A in colon (22.4 mg E 171/kg bw per day + DMH) and IL‐12p70 in plasma (3.5 mg E 171/kg bw per day + DMH), with no dose‐related effects, were observed. There were no changes in spleen cellularity across any of the treated groups. No changes were observed in the percentage of CD103+ DC, CD4+ T helper cells or total or activated Treg in peripheral blood, spleen or Peyer's patches in animals exposed to E 171 + DMH compared to animals treated with only DMH.
According to the authors, there were no treatment related histopathological changes in the duodenum, jejunum, ileum, spleen, liver, lung and testes in animals exposed only to E 171. Rats that were initiated with DMH only and those which received 171 in the diet after the initiation displayed several histopathological abnormalities. There were two invasive adenocarcinomas in one animal in the 1.3 mg E 171/kg bw per day + DMH group, and single adenomas in one animal in the 3.5 mg E 171/kg bw per day E 171 + DMH group and in one animal in the 22.4 mg E 171/kg bw per day + DMH group. There were no other histopathological changes in the large intestines of the other animals treated with DMH. One rat in the 1.3 mg E 171/kg bw per day + DMH group and one rat in the 22.4 mg E 171/kg bw per day + DMH group had subpleural lymphocytes in the lung, but without any evidence of acute inflammatory changes or hyperplasia.
Regarding colonic ACF and ABC, according to the authors, a technical issue was encountered in that much of the epithelial surface of the sampled colon (proximal, middle and distal) was obscured when observed by light microscopy. The authors’ reported that they were unable to examine the entire surface of the colon samples. The results for the areas of epithelium that were examined indicated an increase in ACF/cm2 and ABC/cm2 in groups initiated with DMH compared to the groups that were not initiated with DMH. E 171 treatment administered after DMH did not result in statistically significant increases in ACF or ABC. No change in the number of ACF and ABC were observed due to E 171 exposure alone. The Panel noted a considerable variability in the results, which may mask possible effects. Furthermore, the Panel noted that the examination for presence of ACF and ABC was not performed on the whole colon but was limited to three 2 cm long samples (one from the proximal, mid‐portion and the distal parts). Dietary E 171, with or without treatment with DMH, had no effect on the length of the colonic glands examined or the number of goblet cells/unit.
Overall, the Panel considered that this study indicates that acute and subchronic dietary intake of E 171 resulted in no significant effects on either peripheral or GI tract immune homeostasis as evidenced by immune cell phenotyping or inflammatory cytokine analysis.
Limitations in the pathological examination for ABC and ACF (sampled colon area limited; delayed fixation) preclude a conclusion on potential for ABC and ACF formation.
Han et al. ( 2020a )
Test material: E 171, anatase, 150 nm (DLS).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure: quantitative analysis in tissues; methodology not reliable.
A 90‐day study was conducted according OECD TG 408. E 171 (10 rats/sex/dose) was administered daily by gavage for 90 days to Sprague–Dawley rats (0, 10, 100 and 1,000 mg/kg bw per day). Clinical signs of all rats were observed daily during the study period.
Regarding immunological parameters: blood levels of immunoglobulin (Ig) A, IgE, IgG, IgM and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) were measured. In addition, total and differential white blood cell (WBC) counts and immune organ weights and histology were also evaluated. There were no changes in the weight of immune organs or their histology. The proportion of lymphocytes slightly decreased (by 9%) in male but not female rats administered the highest E 171 dose, without an apparent dose response relationship. According to the study authors, the level of GM‐CSF was reduced by 41% in females only at the highest dose of 1,000 mg/kg bw per day. In males there was also a decrement in GM‐CSF level (approximately by 30%), that was slightly less pronounced than in females, and due to a higher variability in the controls, did not gain statistical significance. A reduction in the levels of IgM was observed in both sexes at the highest dose tested, i.e. by 12% in females and by 9% in males, but there were no clear dose response relationships. Transcriptomics also showed that immune response‐related microRNAs were most strongly affected by E 171 exposure, which may support the effect observed at the high dose.
The Panel noted the lack of a dose response, the magnitude of the effect was small that did not allow a firm conclusion given the natural variability in the parameters measured.
TiO 2 or TiO 2 NPs
Mice
Mohamed ( 2015 )
Test material: TiO2 NPs, rutile/anatase (77/22%), 47 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure: quantitative measurement in tissues, methodology with important flaws
Male Swiss Webster mice, aged 10–12 weeks, were housed in plastic cages under standard lighting conditions (12‐h day/night cycle) with free access to water and food. Animals were randomly divided into four groups: the negative control group (treated with distilled water); and three experimental groups (15 mice per group) administered by gavage with TiO2 NPs suspensions at the doses of 5, 50 or 500 mg/kg bw per day for 5 days. Animals from each group were sacrificed at 24 h, 7 days and 14 days after the last treatment. According to the authors, routine histopathology showed dose‐dependent effects in the stomach. At 5 mg/kg, submucosal oedema was noted after 24 h that developed into ulcerations and mucosal necrosis after one and two weeks, respectively. After exposure to 50 mg/kg bw per day, submucosal vasculitis, massively degenerated glands and both mucosal and submucosal necrosis was evident after 24 h, 7 days and 14 days, respectively. Submucosal congested blood vessels, focal areas of leucocytic cell infiltrations and necrotic glands with mononuclear cell infiltrations (i.e. the highest grade of damage) were seen with TiO2 NPs at 500 mg/kg bw per day at all time points.
The Panel noted that histopathological changes were not sufficient reported as no incidences and/or severity score were provided.
The Panel considered that these data suggest an inflammatory response in the stomach after short‐term bolus exposure to TiO2 NPs (47 nm).
Li et al. ( 2019 )
Three test materials: (1) TiO2 NPs, anatase, 25 nm; (2) TiO2 NPs, anatase, 50 nm; (3) TiO2 NPs, anatase, 80 nm. Purity not reported for none of them.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2 (TiO2 NPs 25 nm) and 2 ((TiO2 NPs 50–80 nm).
Internal exposure: quantitative analysis in tissues, methodology with important flaws.
Male C57BL/6 mice (8 weeks old) were randomly divided into four groups (n = 28): control, TiO2 NPs (25 nm), TiO2 NPs (50 nm) and TiO2 NPs (80 nm). Mice were administered by gavage TiO2 NPs suspended in PBS with a dose of 1 mg/kg bw per day for seven consecutive days. The control group was given an equal volume of PBS. Fresh faeces were collected 2 h after dosing every day, and the distal colonic contents from each mouse were collected 24 h after the last dosing, for subsequent analysis.
Following 3‐day or 7‐day TiO2 NPs administration, 6 mice per group were randomly selected and sacrificed for analysis. Fresh brain, heart, spleen, liver, kidney, heart, serum and faeces were collected and the samples were weighed.
Mice were randomly selected and sacrificed for the macroscopic and histological examination of tissues. Pathological examinations of the colon were performed in a blinded manner.
The purpose of this study was to study the interaction between TiO2 NPs of different dimensions, gut microbiota and intestinal barrier function.
Results indicate that short‐term ingestion of TiO2 NPs (25 nm) (1 mg/kg bw per day for 7 days) led to colonic epithelial injury, reduced expression levels of tight junction proteins and reduced thickness of the ‘luminal mucus layer’. This was associated with altered gut microbiota composition, with reduction in number of Bifidobacterium compared with controls.
Regarding immunological organs, histological findings were not reported.
The Panel considered that this study provides no relevant information on immunological effects of TiO2 NPs.
Rats
Hashem et al. ( 2020 )
Test material: TiO2 (from Sigma, no information on constituent particle size distribution nor crystalline form).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 3.
Internal exposure not examined.
Adult male Wistar rats were housed in steel mesh cages at 21–24 °C, 50–60% relative humidity and a 12 ‐h light‐dark cycle. The rats were weighed and randomly distributed into three groups, that were gavaged with 0.5% hydroxypropyl methylcellulose (HMC), or TiO2 suspended in HMC at doses of 20 or 40 mg/kg bw per day for 90 consecutive days.
At the end of treatment, the body weight of low‐ and high‐dose groups was 25% lower than that of the control group. Standard haematology using automated counting revealed a statistically significantly dose‐dependent leucopenia and thrombocytopenia, as well as eosinophilia and neutrophilia. In the spleens that were evaluated histopathologically in a blinded fashion, statistically significantly dose‐dependent alterations were observed that included lymphoid necrosis, white pulp expansion and increased numbers of macrophages. A marked increase in CD4+ and CD8+ immunolabelling was noted in the spleen. In addition, IgG and IgM measured by ELISA were statistically significantly elevated in TiO2‐treated rats. Phagocytic activity measured by a modified colorimetric nitro blue tetrazolium assay as well as lysozyme expression and nitric oxide levels measured by ELISA were significantly reduced following TiO2 exposure. Lymphocyte proliferation in response to PHA, measured by a lymphocyte transformation assay and using MTT as a proxy for cell number was statistically significantly reduced.
The Panel concluded that these findings taken together indicate that TiO2‐induced haematological and immunological alterations after exposure for 90 days at all dose tested.
TiO 2 NPs < 30 nm
Mice
Yu et al. ( 2016 )
Test material: TiO2 NPs, anatase, 5–6 nm (further information on characterisation from Hu et al., 2011).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
CD‐1 (ICR) female mice were housed in stainless steel cages in a ventilated animal room at 24 ± 2°C with a relative humidity of 60 ± 10% and a 12‐h light/dark cycle and were randomly divided in 4 groups of 20 animals each. Control (treated with 0.5% w/v HPMC) and three experimental groups (2.5, 5 and 10 mg/kg bw TiO2 NPs suspensions in HPMC) were administered daily by gavage for 90 days. After 90 days, the mice were weighed and then anaesthetised. After sacrifice, the hearts were excised, weighed and cryopreserved at –80°C.
Inflammatory lesions and tissue damage were seen histopathologically, and were more pronounced at the mid and high doses. It was not reported whether the histopathology was performed blinded, but the results were corroborated with objective measures. The expression of NF‐κB, and of pro‐inflammatory cytokines TNF‐α, IL‐1β, IL‐6 and IFN‐γ expression were increased in a dose‐dependent fashion (statistically significant increase up to 1.8‐fold compared with the control); expression of the NF‐κB inhibitor I‐κB was decreased in a dose‐dependent fashion (statistically significant decrease up to 1.55‐fold compared with the control), as evidenced by western blotting.
The Panel considered that these data indicate an effect of TiO2 NPs (5–6 nm) exposure at all dose levels tested, as evidenced by histopathological lesions, corroborated by intermediate endpoints indicating disturbance of intracellular ion homeostasis that were adrenergic receptors in the heart. These lesions are accompanied by increases in the expression of intermediate inflammatory endpoints.
The Panel noted effects on inflammatory mediators with TiO2 NPs (5–6 nm) at all doses tested and corroborated by histopathological lesions.
Li et al. ( 2018 )
Two test materials: 1) TiO2 NPs, anatase, 20 nm (in water, DLS); 2) TiO2 NPs, rutile, edged with corners morphology (SEM), 15 nm (in water, DLS).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure: quantitative measurement in tissues, methodology with important flaws.
Male C57BL/6 mice, 8 weeks old, were kept in a humidity‐controlled room on a 12‐h/12‐h light–dark cycle with food and water available ad libitum. The animals were randomly divided into 3 groups (n = 10 mice per group). TiO2 NPs (either rutile or anatase) suspended in distilled water were administered by gavage at a dose of 100 mg/kg bw per day for 28 days, whereas the control group received an equal volume of distilled water. There were no effects on body weight. Whereas particles were observed in the spleen, examination of H&E‐stained samples of the spleen revealed no histopathological changes. No histopathological changes were seen recorded in other tissues examined (the lung, jejunum, kidney, liver or brain). In colon, the increased length of villi was increased and irregularly arranged epithelial cells were reported irregularly arranged after exposure to TiO2 NPs.
Phylogenetic analysis was performed by PCR of faecal DNA, extracted using the FastDNA® Spin Kit for Stool (MP Biomedicals, Santa Ana, USA) and amplified by barcoded composition primers flanking the V4/V5 regions of the 16S rRNA gene. Rutile NPs had a more pronounced influence on the gut microbiota than anatase NPs. The most influenced phylum was Proteobacteria, which was significantly increased by rutile NPs but not by anatase NPs. At the genus level, Rhodococcus was enriched by rutile NPs, Prevotella was significantly decreased by both the TiO2 NPs.
The Panel considered that these data support an effect of TiO2 NPs on the microbiota, but as no immunological parameters other than the histopathology of the spleen were included in this study, any consequence(s) associated with these changes in terms of inflammation and the immune system remain uncertain.
Zhang et al. ( 2020 )
Test material: TiO2 NPs, 21 nm (TEM), crystalline form and purity unknown.
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 4.
Internal exposure not examined.
C57BL/6J mice, aged 7 weeks, were maintained in the controlled conditions with temperature 23 ± 1°C and humidity 55 ± 10% on a 12‐h light/dark cycle, with free access to a standard rodent diet and tap water. Thirty mice were randomly distributed over a vehicle group. Mice were treated with either vehicle or a suspension of TiO2 NPs at 150 mg/kg bw per day by gavage for 30 days. Microbiota were evaluated by 16S ribosomal RNA (rRNA) gene sequencing in the faecal samples. Total genomic DNA was extracted from the faecal samples and the bacterial 16S rRNA were amplified by PCR. Subsequently PCR products were quantified using the QuantiFluor™‐ST Blue Fluorescence Quantification. The results show that oral exposure to TiO2 NPs resulted in significantly changed richness and composition of the gut microbiota. No changes in parameters indicating inflammation (IL‐6 and IL‐1β) in either intestines or brain were observed.
The Panel considered that exposure to TiO2 NPs (21 nm) leads to changes in the microbiota composition, but the study does not indicate a local or systemic inflammatory action.
Rats
Chen et al. ( 2015a )
Test material: TiO2 NPs, anatase 24 nm (TEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Four‐week‐old healthy Sprague–Dawley rats were randomised into experimental and control groups, with 5 male and 5 female rats in each group. Suspensions in ultrapure water of TiO2 NPs (0, 2, 10 and 50 mg/kg bw per day), glucose (1.8 g/kg bw per day) and TiO2 NPs (0, 2, 10 and 50 mg/mg bw per day) + glucose (1.8 g/kg bw per day) were gavaged daily for 30 or 90 consecutive days.
Regarding the effects on spleen and white blood cells in animals exposed to TiO2 NPs alone, no significant histopathological changes were observed in the spleen in all groups. On the contrary, increases in white blood cells parameters (white blood cell counts and granulocytes) were observed in female rats after exposure to TiO2 NPs 50 mg/kg bw per day for 90 days and among male rats exposed to TiO2 NPs 50 mg/kg bw per day for 30 (white blood cells counts, lymphocytes, monocytes absolute numbers and in the percentage of lymphocytes and granulocytes) and 90 days (percentage of monocytes); and a decrease in the while blood cells at 90 days in rats exposed to 10 mg/kg bw per day. The Panel considered that the increase in leucocytes may suggest an inflammatory response induced by TiO2 NPs (24 nm) at the highest dose tested (50 mg/kg bw per day).
Chen et al. ( 2019 )
Test material: TiO2 NPs, anatase, 29 nm (SEM).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure not examined.
Three‐week‐old male Sprague–Dawley rats, 6 per group, were administered via gavage with TiO2 NPs at doses of 0, 2, 10, 50 mg/kg bw per day for 30 days. Morphology of colon was evaluated by routine histopathology and TEM. Serum levels of IL‐6 and TNF‐α were measured, as well as oxidative stress markers in colon tissue homogenates.
Histopathologically, reduced numbers of goblet cells were found as a result of exposure, as well as inflammatory infiltration, while in serum increased IL‐6 expression was observed.
Grissa et al. ( 2020 )
Test material: TiO2 NPs, anatase, 5–12 nm (TEM, XRD).
Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure), assigned according to Appendix E, was 2.
Internal exposure: quantitative in tissues; methodology with important flaws.
Male Wistar rats were administered by gavage (aqueous suspension; volume of 10 mL/kg bw) with TiO2 NPs (five times/week; 8 weeks) after being randomised over 4 groups (n = 8/group): 0 (control), 50, 100 and 200 mg TiO2 NPs/kg bw per day. One day after the last TiO2 NPs treatment, rats were euthanised and fresh brain from each rat was excised and weighed. A part of the frontal lobe of the left cerebral hemispheres was homogenised for biochemical assays, and the other part of the frontal lobe was used for the quantification of Ti. TNF‐α and nitric oxide levels were measured using commercially available kits.
A statistically significant dose‐related increase in the level of NO in 100 and 200 mg/kg bw per day TiO2 NPs groups was observed together with a statistically significant increase in brain TNF‐α in 200 mg/kg bw per day TiO2 NPs group. The increase was dose‐related for both parameters.
The Panel noted changes for the above‐mentioned inflammatory markers at doses starting from 100 mg TiO2 NPs (5–12 nm)/kg bw per day.
Appendix I – Analysis of Ti‐concentration in blood and urine in the EOGRT study
1.
Sample collection
Urine in F2 pups was collected via sterile bladder punctuation, whereas the urine in F0 and cohort 1A and 1B animals were collected by using metabolic cages. The applicant assumes that the Ti‐levels in urine that were collected via metabolic cages was the result of contamination of urine from faeces in the metabolic cages.
Analysis Ti‐concentration in blood and urine
Blood and urine were microwave digested with sulfuric acid, followed by analysis with ICP‐MS/MS. A comparison between analysis by ICP‐MS/MS and ICP‐OES was performed and it was concluded the use of H2SO4 in sample preparation did not result in interferences in the ICP‐MSMS analysis. Background concentrations in blood and urine were determined and used to correct the Ti‐concentrations. The instrumental LOD varied between 0.001 and 0.014 μg/L for blood, and between 0.002 and 0.026 μg/L for urine. The instrumental LOQ varied between 0.004 and 0.043 μg/L for blood, and between 0.006 and 0.077 μg/L for urine. Method LODs and LOQs were estimated to be substantially greater.
Assessment of background concentration in blood and urine
The background concentrations in rat blood method blanks varied between 0.008 and 0.046 μg/L, and between 0.010 and 0.036 μg/g for urine. As uncorrected median Ti concentrations in blood for exposed animals ranged between 0.01 and 0.542 μg/g, and between 0.034 and 1.017 μg/g for urine, the background consisted of a considerable fraction of the Ti determined in many blood and urine samples. Together with the considerable variability in background concentrations and the magnitude of the estimated method LODs and LOQs, this suggests there is some uncertainty in the Ti‐concentrations of blood and urine, especially in the lower values.
Kinetic profile
For cohort F2, the Ti‐concentration blood (n = 10/group) was increased in dose group 300 mg/kg bw/d and further increased in dose group 1,000 mg/kg bw/d. The Ti‐concentration in blood for dose group 1,000 mg/kg bw/d in cohort F0 seems to be slightly increased, whereas cohorts 1A and 1B did not show a clear increase in Ti blood levels. The Panel noted that there are a few rats that showed an increased blood concentration that are considered outliers.
Urine (n = 10/group) Ti‐levels generally increase in the exposed groups as compared to the control group. The variation between animals is large. For cohort F2, urine concentrations are low and there is not a clear increase with dose.
Increased Ti‐levels in blood indicate that some Ti from the diet must have been systemically available (Figure I.1). Also Ti in urine suggests that Ti from the diet is absorbed, but for the cohorts F0, 1A and 1B, contamination of urine via faeces caused by the sampling of the excreta could have contributed to the Ti concentration in urine.
Figure I.1.
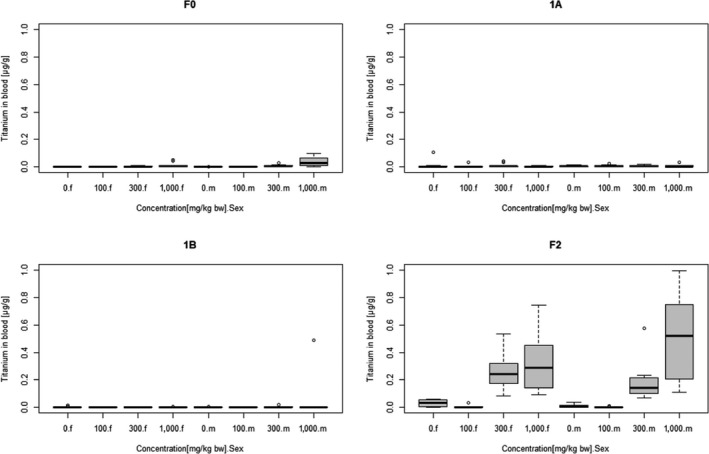
Boxplots comparing Ti levels in blood (μg/g blood) for treatment concentration and sex combinations for each generation and cohort (Documentation provided to EFSA No 18) (for further explanation see text above)
An increase in Ti concentrations in blood in the F2 cohort (dose group 300 and 1,000 mg/kg bw per day) at post‐natal day 4–7 has been noted. The Panel considered that the pups may have been exposed to TiO2 via inhalation of dust from powdered feed containing E171. However, the Panel concluded that the pups were primarily exposed to E171 in utero (i.e. via the placenta) and that pup Ti blood levels were able to accumulate to detectable levels soon after birth due to limited foetal excretion. Accordingly, although the blood concentrations of Ti in F1 females were not measurable, the Panel concluded that E171 was systemically available.
Small particles generally do not remain long in the blood stream (Landsiedel et al., 2012; ISO/TR, 2019). They are taken up by tissues rich in macrophages, such as liver and spleen. The blood kinetics therefore have limited value for assessing the toxicokinetic behaviour. Yet, the presence of Ti in blood suggests that some of the TiO2 particles in E 171 were absorbed from the GI tract.
It should be noted that cohort F2 showed the highest blood concentration and the lowest urine concentration (Figure I.2). The low urine concentration as compared to the other cohorts may be explained by the manner of collecting urine, i.e. bladder punctuation for cohort F2 vs. metabolic cages for the other cohorts. In the latter case, contamination from faeces may have occurred.
Figure I.2.
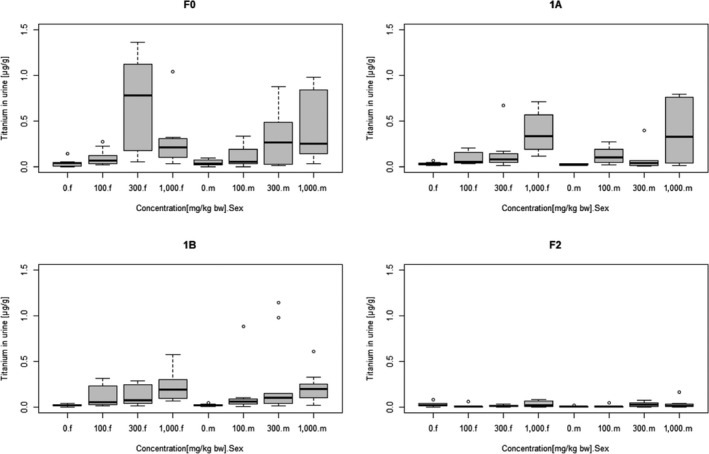
Boxplots comparing Ti levels in urine (μg/g blood) for treatment concentration and sex combinations for each generation and cohort (Documentation provided to EFSA No 18)
The Panel considered that the blood concentrations in the F2 pups on PND 4–7 at concentrations clearly above the LOQ indicate intrauterine exposure and hence internal exposure of the F1 mothers. The percentage of the systemically available fraction is not known and the variability of the concentration data is high: Nevertheless, the F2 pup blood concentrations showed a dose‐dependent increase, suggesting that the systemically available fraction in the F1 mothers also increased and that absorption from their GIT did not decrease with increasing dose of E 171 (i.e. any agglomeration of E 171 with increasing dose did not impact on absorption).
Appendix J – New in vitro genotoxicity studies
Appendix K – New in vivo genotoxicity studies
Appendix L – In vitro genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
Appendix M – In vivo genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
Appendix N – In vitro genotoxicity studies from OECD dossier (OECD, 2016)
Appendix O – In vivo genotoxicity studies from OECD dossier (OECD, 2016)
Appendix P – Genotoxicity studies submitted by Interested Business Operators
Appendix Q – Concentration levels of E 171 used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix R – Number and percentage of food products labelled with E 171 out of the total number of food products present in the Mintel GNPD per food subcategory between 2016 and 2021
Appendix S – Summary of total estimated exposure of E 171 from its use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix T – Summary of total estimated exposure of E 171 from its use as a food additive for the food supplements consumers only exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix U – Main food categories contributing to exposure to E 171 using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Appendix V – Summary of total estimated exposure of E 171 from its use as a food additive considering reported use levels and analytical data (mg/kg bw per day)
1.
Appendix J–V can be found in the online version of this output (in the ‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2021.6585
Appendix W – Reported data on the analysis of pristine E 171 (Verleysen et al., 2020 , 2020)
1.
Number‐ and mass‐based percentages of TiO2 particles smaller than threshold x are reported in Tables W.1 and W.2
Table W.1.
Number percentage of particles
| x (nm) | Number % of particles with minimum Feret diameter smaller than threshold x | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | E 171‐Aa | E 171‐Ba | E 171‐Ca | E 171‐Da | E 171‐Ea | E 171‐Fa | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 6 | 1 | 2 | 2 | 8 | 3 | 1 | 2 | 1 | 1 | 2 | 0 |
| 50 | 13 | 4 | 5 | 5 | 16 | 7 | 3 | 6 | 3 | 1 | 6 | 2 |
| 60 | 22 | 9 | 12 | 13 | 25 | 15 | 6 | 15 | 9 | 2 | 14 | 3 |
| 70 | 36 | 21 | 24 | 25 | 37 | 29 | 11 | 29 | 19 | 4 | 24 | 7 |
| 80 | 50 | 35 | 39 | 38 | 51 | 44 | 20 | 44 | 31 | 8 | 40 | 9 |
| 90 | 63 | 50 | 54 | 52 | 63 | 58 | 30 | 58 | 44 | 13 | 54 | 14 |
| 100 | 74 | 64 | 67 | 65 | 73 | 71 | 40 | 70 | 56 | 18 | 65 | 20 |
| 110 | 81 | 75 | 77 | 74 | 80 | 79 | 50 | 79 | 67 | 24 | 75 | 28 |
| 120 | 87 | 83 | 85 | 82 | 86 | 85 | 60 | 85 | 75 | 31 | 83 | 35 |
| 130 | 92 | 89 | 90 | 89 | 91 | 90 | 68 | 90 | 82 | 38 | 88 | 43 |
| 140 | 94 | 92 | 94 | 93 | 94 | 94 | 77 | 93 | 88 | 44 | 91 | 51 |
| 150 | 97 | 95 | 96 | 96 | 96 | 96 | 83 | 95 | 92 | 50 | 95 | 59 |
| 160 | 98 | 97 | 98 | 97 | 98 | 97 | 88 | 97 | 95 | 57 | 97 | 67 |
| 170 | 98 | 98 | 99 | 99 | 98 | 98 | 91 | 98 | 97 | 65 | 98 | 76 |
| 180 | 99 | 99 | 99 | 99 | 99 | 99 | 93 | 99 | 98 | 72 | 99 | 81 |
| 190 | 99 | 100 | 100 | 100 | 99 | 99 | 96 | 99 | 99 | 76 | 99 | 87 |
| 200 | 100 | 100 | 100 | 100 | 100 | 100 | 97 | 100 | 99 | 83 | 100 | 91 |
| 250 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 100 | 99 |
| 300 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98 | 100 | 100 |
| 400 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 500 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Sample claimed to be the same of the E 171 for which data were evaluated in EFSA FAF Panel (2019).
Table W.2.
Mass percentage of particles
| x (nm) | Mass % of particles with minimum Feret diameter smaller than threshold x | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | E 171‐Aa | E 171‐Ba | E 171‐Ca | E 171‐Da | E 171‐Ea | E 171‐Fa | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 60 | 3 | 1 | 2 | 2 | 3 | 2 | 0 | 2 | 1 | 0 | 2 | 0 |
| 70 | 7 | 4 | 5 | 5 | 7 | 6 | 1 | 6 | 3 | 0 | 4 | 0 |
| 80 | 14 | 9 | 11 | 10 | 13 | 12 | 3 | 12 | 7 | 0 | 10 | 1 |
| 90 | 23 | 18 | 20 | 18 | 22 | 21 | 6 | 20 | 13 | 1 | 18 | 2 |
| 100 | 33 | 29 | 32 | 29 | 31 | 32 | 10 | 30 | 20 | 2 | 27 | 3 |
| 110 | 44 | 41 | 43 | 39 | 41 | 43 | 15 | 41 | 30 | 3 | 38 | 6 |
| 120 | 54 | 52 | 56 | 50 | 52 | 52 | 23 | 50 | 39 | 5 | 49 | 9 |
| 130 | 64 | 63 | 66 | 64 | 61 | 62 | 30 | 59 | 49 | 8 | 58 | 13 |
| 140 | 71 | 70 | 75 | 72 | 70 | 73 | 40 | 68 | 59 | 11 | 66 | 19 |
| 150 | 80 | 79 | 81 | 82 | 78 | 78 | 49 | 73 | 69 | 15 | 75 | 27 |
| 160 | 84 | 84 | 88 | 87 | 84 | 84 | 58 | 80 | 77 | 20 | 82 | 35 |
| 170 | 88 | 90 | 92 | 91 | 88 | 88 | 65 | 85 | 84 | 27 | 86 | 47 |
| 180 | 92 | 93 | 95 | 95 | 92 | 92 | 70 | 90 | 89 | 34 | 90 | 55 |
| 190 | 94 | 96 | 96 | 96 | 95 | 95 | 77 | 94 | 92 | 40 | 93 | 65 |
| 200 | 95 | 98 | 99 | 98 | 96 | 97 | 82 | 96 | 95 | 49 | 96 | 74 |
| 250 | 100 | 99 | 100 | 100 | 100 | 100 | 95 | 100 | 100 | 75 | 99 | 96 |
| 300 | 100 | 100 | 100 | 100 | 100 | 100 | 98 | 100 | 100 | 88 | 100 | 100 |
| 400 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99 | 100 | 100 |
| 500 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Sample claimed to be the same of the E 171 for which data were evaluated in EFSA FAF Panel (2019).
Supporting information
Search methodology for the literature search
Criteria for inclusion and exclusion applied to screening of publication retrieved in the literature search
Approach for assessing toxicokinetic and toxicity studies
Approach for assessing genotoxicity studies
Advice from EFSA ccWG Nanotechnology: Nanoscale considerations for the assessment of the study design and study results of TiO2 toxicity studie
List of in vivo toxicokinetic and toxicity studies retrieved from the literature search
New in vitro genotoxicity studies
New in vivo genotoxicity studies
In vitro genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
In vivo genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
In vitro genotoxicity studies from OECD dossier (OECD, 2016)
In vivo genotoxicity studies from OECD dossier (OECD, 2016)
Genotoxicity studies submitted by Interested Business Operators
Concentration levels of E 171 used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Number and percentage of food products labelled with E 171 out of the total number of food products present in the Mintel GNPD per food subcategory between 2016 and 2021
Summary of total estimated exposure of E 171 from its use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Summary of total estimated exposure of E 171 from its use as a food additive for the food supplements consumers only exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to E 171 using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of total estimated exposure of E 171 from its use as a food additive considering reported use levels and analytical data (mg/kg bw per day)
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wölfle D, Corsini E, Cubadda F, De Groot D, FitzGerald R, Gunnare S, Gutleb AC, Mast J, Mortensen A, Oomen A, Piersma A, Plichta V, Ulbrich B, Van Loveren H, Benford D, Bignami M, Bolognesi C, Crebelli R, Dusinska M, Marcon F, Nielsen E, Schlatter J, Vleminckx C, Barmaz S, Carfí M, Civitella C, Giarola A, Rincon AM, Serafimova R, Smeraldi C, Tarazona J, Tard A and Wright M, 2021. Scientific Opinion on the safety assessment of titanium dioxide (E171) as a food additive. EFSA Journal 2021;19(5):6585, 130 pp. 10.2903/j.efsa.2021.6585
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00262
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Ana Campos Fernandes, Laura Ciccolallo, Esraa Elewa, Galvin Eyong, Christina Kyrkou, Irene Munoz, Giorgia Vianello, the members of the SCER Cross‐cutting WG nanotechnologies: Jacqueline Castenmiller, Mohammad Chaudhry, Roland Franz, David Gott, Stefan Weigel and the former member of the SCER Cross‐cutting WG Genotoxicity Maciej Stepnik. The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 25 March 2021
Appendices A, B, C, D, E, F, J, K, L, M, N, O, P and Q‐V are available under the Supporting Information section .
Notes
Available online: https://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Arrêté du 21 décembre 2020 portant suspension de la mise sur le marché des denrées contenant l'additif E 171 (dioxyde de titane ‐ TiO2) ‐ Légifrance (legifrance.gouv.fr).
2020/2795(RPS) – 8/10/2020 – Objection pursuant to Rule 112(2) and (3) and (4)(c): Specifications for titanium dioxide (E 171) (europa.eu).
EFSA‐Q‐2010‐01522.
Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation. C/2019/7227. OJ L 44, 18.2.2020, p. 1–14. ELI: http://data.europa.eu/eli/reg_del/2020/217/oj
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. OJ L 311, 28.11.2001, p. 67–128.
Directive 2009/35/EC of the European Parliament and of the Council of 23 April 2009 on the colouring matters which may be added to medicinal products. OJ L 109, 30.4.2009, p. 10–13.
Where X refers to the percentage of particles < 100 nm as described in the publication.
Total Ti corresponds to the analytical determination of the Ti element from all sources after digestion of tissues and application of appropriate analytical techniques and may be used as a proxy estimate for TiO2 mass concentration. In the current opinion, the term titanium (Ti) is used to indicate total Ti.
The content of Ti in the liver and the large intestine was estimated by multiplying the measured concentrations with the organ weights (Ronald et al., 1997). To obtain the percentage of the dose, the sum of both contents was related to the dose applied.
The content of Ti in placenta was estimated by multiplying the measured concentrations with the organ weight of 650 g (Valentin, 2002).
The authors did not give numbers for the tissue concentration but depicted their results in a figure with bars from which a rough estimate could be made at which time the concentration was 50% of the concentration after the end of distribution (24 h). Accumulation was estimated by using the textbook formula with ke = ln 2/half‐life with the assumption that the same dose would be applied every day.
The content of Ti in the tissues was calculated by multiplying the measured concentrations with the organ weights (Schoeffner, 1999). In the cases where the concentrations were reported as being below the LOD, the LOD was used. To receive the percentage of dose, the sum of the contents was related to the dose applied.
The content of titanium in the liver and the spleen was calculated by multiplying the measured concentrations with the organ weights for men (liver 1800 g, spleen 150 g) and for women (liver 1400 g, spleen 130 g) (Valentin, 2002.)
EFSA has launched a thematic Art. 36 grant to review the state of the art and critically appraise the evidence, technologies and models (in vitro/in silico/in vivo) available. This thematic grant should yield a roadmap to advance research to address risk assessment needs to account for effects on/by gut microbiota in humans and domestic animals (https://www.efsa.europa.eu/sites/default/files/documents/art36/gpefsaenco202002/Call%20for%20Proposals_Thematic%20Grant.pdf
The appropriate amount of E 171 and diet (ssniff® R‐Z V1320) were mixed with an impact mill to produce a premix. The premix was further added to diet, mixed with a mixer for 15 min and the mixture was freshly prepared once a week.
Selected tissues were preserved in a suitable fixative and further processed for histopathology where appropriate: adrenal gland; bone; bone marrow (femur); brain (cerebrum, cerebellum, pons); colon; duodenum; epididymis; eye with optic nerve; heart (3 levels – right and left ventricle, septum); ileum; jejunum; kidney; liver, lymph node, lungs (with main stem bronchi and bronchioles); lymph node (cervical and mesenteric); mammary gland; muscle (skeletal); nerve (sciatic); oesophagus; Peyer's patches; pituitary; prostate; rectum; seminal vesicles with coagulating glands; spinal cord (3 sections); spleen; stomach; testicle; thymus, thyroid (including parathyroids); trachea (including larynx); ureter and urinary bladder.
Brain level 1 refers to the first coronal section of the brain containing the olfactory bulb following a rostral to caudal trimming.
Missing Cyprus, Luxembourg and Malta.
http://www.gnpd.com/sinatra/home/ accessed on 8/2/2021.
February 2020 release. Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
As calculated by the Panel and taking the mean of the two periods given in the paper: 1.5, 3.9, 25.5, 294 or 1.1, 3, 19, 236 mg/kg bw per day for weeks 1‐10 or 11‐15, respectively (groups 1–4) and 1.5, 4.1, 25.7, 300 or 1.1, 3.1, 19.2, 237 mg/kg bw per day for weeks 1–10 or 11–15, respectively (groups 5–8).
References
- Ahlinder L, Ekstrand‐Hammarström B, Geladi P and Osterlund L, 2013. Large uptake of titania and iron oxide nanoparticles in the nucleus of lung epithelial cells as measured by Raman imaging and multivariate classification. Biophysical Journal, 105, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akera T and Brody TM, 1978. The role of Na+, K+‐ATPase in the inotropic action of digitalis. Pharmacological Reviews, 29, 187–220. [PubMed] [Google Scholar]
- Ali K, Abul QF, Dwivedi S, Abdel‐Salam EM, Ansari SM, Saquib Q, Faisal M, Al‐Khedhairy AA, Al‐Shaeri M and Musarrat J, 2018. Titanium dioxide nanoparticles preferentially bind in subdomains IB, IIA of HSA and minor groove of DNA. Journal of Biomolecular Structure and Dynamics, 36, 2530–2542. [DOI] [PubMed] [Google Scholar]
- Ali SA, Rizk MZ, Hamed MA, Aboul‐Ela EI, El‐Rigal NS, Aly HF and Abdel‐Hamid AZ, 2019. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: effect of dose and particle size. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 24, 492–498. [DOI] [PubMed] [Google Scholar]
- Alsudir S and Lai EPC, 2017. Electrosteric stabilization of colloidal TiO2 nanoparticles with DNA and polyethylene glycol for selective enhancement of UV detection sensitivity in capillary electrophoresis analysis. Analytical and Bioanalytical Chemistry, 409. [DOI] [PubMed] [Google Scholar]
- Ammendolia MG, Iosi F, Maranghi F, Tassinari R, Cubadda F, Aureli F, Raggi A, Superti F, Mantovani A and De Berardis B, 2017. Short‐term oral exposure to low doses of nano‐sized TiO2 and potential modulatory effects on intestinal cells. Food and Chemical Toxicology, 102, 63–75. [DOI] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B and Hen R, 2018. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature, 559, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Swede H, Rustagi T, Protiva P, Pleau D, Brenner BM, Rajan TV, Heinen CD, Levine JB and Rosenberg DW, 2012. Aberrant crypt foci as predictors of colorectal neoplasia on repeat colonoscopy. Cancer Causes & Control, 23, 355–361. [DOI] [PubMed] [Google Scholar]
- Andreoli C, Leter G, De Berardis B, Degan P, De Angelis I, Pacchierotti F, Crebelli R, Barone F and Zijno A, 2018. Critical issues in genotoxicity assessment of TiO2 nanoparticles by human peripheral blood mononuclear cells. Journal of Applied Toxicology: JAT, 38, 1471–1482. [DOI] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail), 2017. Relatif à une demande d'avis relatif à l'exposition alimentaire aux nanoparticules de dioxyde de titane. Referral No. 2017‐SA-0020.
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail), 2019. Relatif aux risques liés à l'ingestion de l'additif alimentaire E 171. Referral No. 2019‐SA-0036.
- Asare N, Duale N, Slagsvold HH, Lindeman B, Olsen AK, Gromadzka‐Ostrowska J, Meczynska‐Wielgosz S, Kruszewski M, Brunborg G and Instanes C, 2016. Genotoxicity and gene expression modulation of silver and titanium dioxide nanoparticles in mice. Nanotoxicology, 10, 312–321. [DOI] [PubMed] [Google Scholar]
- Azim SA, Darwish HA, Rizk MZ, Ali SA and Kadry MO, 2015. Amelioration of titanium dioxide nanoparticles‐induced liver injury in mice: possible role of some antioxidants. Experimental and Toxicologic Pathology: Official Journal of the Gesellschaft fur Toxikologische Pathologie, 67, 305–314. [DOI] [PubMed] [Google Scholar]
- Bajic V, Spremo‐Potparevic B, Zivkovic L, Cabarkapa A, Kotur‐Stevuljevic J, Isenovic E, Sredojevic D, Vukoje I, Lazic V, Ahrenkiel SP and Nedeljkovic JM, 2017. Surface‐modified TiO2 nanoparticles with ascorbic acid: antioxidant properties and efficiency against DNA damage in vitro. Colloids and surfaces B, Biointerfaces, 155, 323–331. [DOI] [PubMed] [Google Scholar]
- Barillet S, Simon‐Deckers A, Herlin‐Boime N, Mayne‐L'Hermite M, Reynaud C, Cassio D, Gouget B and Carriere M, 2010. Toxicological consequences of TiO2, SiC nanoparticles and multi‐walled carbon nanotubes exposure in several mammalian cell types: an in vitro study. Journal of Nanoparticle Research, 12, 61–73. [Google Scholar]
- Barkhade T, Mahapatra KS and Banerjee I, 2019. Study of mitochondrial swelling, membrane fluidity and ROS production induced by nano‐TiO2 and prevented by Fe incorporation. Toxicol. Res, 8, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat N, Lopes VR, Scholermann J, Jensen LD and Cristobal S, 2015. Vascular toxicity of ultra‐small TiO2 nanoparticles and single walled carbon nanotubes in vitro and in vivo. Biomaterials, 63, 1–13. [DOI] [PubMed] [Google Scholar]
- Bedard K and Krause K‐H, 2007. The NOX family of ROS‐Generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews, 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Bettini S, Boutet‐Robinet E, Cartier C, Comera C, Gaultier E, Dupuy J, Naud N, Tache S, Grysan P, Reguer S, Thieriet N, Refregiers M, Thiaudiere D, Cravedi JP, Carriere M, Audinot JN, Pierre FH, Guzylack‐Piriou L and Houdeau E, 2017. Food‐grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports, 7, 40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya K, Davoren M, Boertz J, Schins RPF, Hoffmann E and Dopp E, 2009. Titanium dioxide nanoparticles induce oxidative stress and DNA‐adduct formation but not DNA‐breakage in human lung cells. Particle and Fibre Toxicology, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biola‐Clier M, Beal D, Caillat S, Libert S, Armand L, Herlin‐Boime N, Sauvaigo S, Douki T and Carriere M, 2017. Comparison of the DNA damage response in BEAS‐2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis, 32, 161–172. [DOI] [PubMed] [Google Scholar]
- Blevins LK, Crawford RB, Bach A, Rizzo MD, Zhou J, Henriquez JE, Khan D, Sermet S, Arnold LL, Pennington KL, Souza NP, Cohen SM and Kaminski NE, 2019. Evaluation of immunologic and intestinal effects in rats administered an E 171‐containing diet, a food grade titanium dioxide (TiO2). Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão F, Fernández‐Bertólez N, Rosário F, Bessa MJ, Fraga S, Pásaro E, Teixeira JP, Laffon B, Valdiglesias V and Costa C, 2020. Genotoxicity of TiO2 nanoparticles in four different human cell lines (A549, HEPG2, A172 and SH‐SY5Y). Nanomaterials, Basel, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Danielsen PH, Derr R, Moelijker N, Fowler P, Stone V, Hendriks G, Moller P and Kermanizadeh A, 2019. The mechanism‐based toxicity screening of particles with use in the food and nutrition sector via the ToxTracker reporter system. Toxicology in vitro: an international journal published in association with BIBRA, 61. [DOI] [PubMed] [Google Scholar]
- Brzicova T, Javorkova E, Vrbova K, Zajicova A, Holan V, Pinkas D, Philimonenko V, Sikorova J, Klema J, Topinka J and Rossner P Jr, 2019. Molecular responses in THP‐1 macrophage‐like cells exposed to diverse nanoparticles. Nanomaterials (Basel, Switzerland). 9 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD and Everard A, 2015. Keeping gut lining at bay: impact of emulsifiers. Trends in Endocrinology and Metabolism, 26, 273–274. [DOI] [PubMed] [Google Scholar]
- Canli EG, Gumus C, Canli M and Ila HB, 2020. The effects of titanium nanoparticles on enzymatic and non‐enzymatic biomarkers in female Wistar rats. Drug and Chemical Toxicology, 1–9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Goyary D, Karmakar S and Chattopadhyay P, 2019. Exploration of cytotoxic and genotoxic endpoints following sub‐chronic oral exposure to titanium dioxide nanoparticles. Toxicology and Industrial Health, 35, 577–592. [DOI] [PubMed] [Google Scholar]
- Charles S, Jomini S, Fessard V, Bigorgne‐Vizade E, Christophe Rousselle C and Michel C, 2018. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology, 12, 357–374. 10.1080/17435390.2018.1451567 [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE and Gewirtz AT, 2015. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, de Wiele TV, De Bodt J, Marzorati M and Gewirtz X, 2017. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Dong X, Zhao J and Tang G, 2009. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitoneal injection. Journal of Applied Toxicology, 29, 330. [DOI] [PubMed] [Google Scholar]
- Chen X‐X, Cheng B, Yang Y‐X, Cao A, Liu J‐H, Du L‐J, Liu Y, Zhao Y and Wang H, 2013. Characterization and preliminary toxicity assay of nano‐titanium dioxide additive in sugar‐coated chewing gum. Small (Weinheim an der Bergstrasse, Germany), 9, 1765–1774. 10.1002/smll.201201506 [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Y, Ba T, Li Y, Pu J, Chen T, Song Y, Gu Y, Qian Q, Yang J and Jia G, 2014. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicology Letters, 226, 314–319. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Y, Zhuo L, Chen S, Zhao L, Chen T, Li Y, Zhang W, Gao X, Li P, Wang H and Jia G, 2015a. Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration. Nanomedicine: Nanotechnology, Biology, and Medicine, 11, 1633–1642. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Y, Zhuo L, Chen S, Zhao L, Luan X, Wang H and Jia G, 2015b. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicology Letters, 239, 123–130. [DOI] [PubMed] [Google Scholar]
- Chen Z, Han S, Zhou D, Zhou S and Jia G, 2019. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut‐associated metabolism in vivo. Nanoscale, 11, 22398–22412. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zheng P, Han S, Zhang J, Li Z, Zhou S and Jia G, 2020a. Tissue‐specific oxidative stress and element distribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale, 12, 20033–20046. [DOI] [PubMed] [Google Scholar]
- Chen Z, Han S, Zheng P, Zhou D, Zhou S and Jia G, 2020b. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague‐Dawley rats. Nanoscale, 12, 5973–5986. [DOI] [PubMed] [Google Scholar]
- Clapper ML, Chang WL and Cooper HS, 2020. Dysplastic aberrant crypt foci: biomarkers of early colorectal neoplasia and response to preventive intervention. Cancer Prevention Research (Philadelphia, Pa.), 13, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coméra C, Cartier C, Gaultier E, Catrice O, Panouille Q, El Hamdi S, Tirez K, Nelissen I, Théodorou V and Houdeau E, 2020. Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food‐grade TiO2 particles: an in vivo and ex vivo study in mice. Part Fibre Toxicol, 17, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa Mde C, Maldonado P, da Rosa CS, Lunkes G, Lunkes DS, Kaizer RR, Ahmed M, Morsch VM, Pereira ME and Schetinger MR, 2008. Oxidative stress and erythrocyte acetylcholinesterase (AChE) in hypertensive and ischemic patients of both acute and chronic stages. Biomedicine & Pharmacotherapy, 62, 317–324. [DOI] [PubMed] [Google Scholar]
- Cowie H, Magdolenova Z, Saunders M, Drlickova M, Correia CS, Halamoda KB, Gombau L, Guadagnini R, Lorenzo Y, Walker L, Fjellsbo LM, Huk A, Rinna A, Tran L, Volkovova K, Boland S, Juillerat‐Jeanneret L, Marano F, Collins AR and Dusinska M, 2015. Suitability of human and mammalian cells of different origin for the assessment of genotoxicity of metal and polymeric engineered nanoparticles. Nanotoxicology, 9, 57–65. [DOI] [PubMed] [Google Scholar]
- Cupi D and Baun A, 2016. Methodological considerations for using umu assay to assess photo‐genotoxicity of engineered nanoparticles. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 796, 34–39. [DOI] [PubMed] [Google Scholar]
- Danielsen PH, Knudsen KB, Strancar J, Umek P, Koklic T, Garvas M, Vanhala E, Savukoski S, Ding Y, Madsen AM, Jacobsen NR, Weydahl IK, Berthing T, Poulsen SS, Schmid O, Wolff H and Vogel U, 2020. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicology and Applied Pharmacology, 386. [DOI] [PubMed] [Google Scholar]
- Dankovic D, Kuempel E and Wheeler M, 2007. An approach to risk assessment for TiO2 . InhALTion Toxicology, 19, 205–212. [DOI] [PubMed] [Google Scholar]
- Dekanski D, Spremo‐Potparevic B, Bajic V, Zivkovic L, Topalovic D, Sredojevic DN, Lazic V and Nedeljkovic JM, 2018. Acute toxicity study in mice of orally administrated TiO2 nanoparticles functionalized with caffeic acid. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 115, 42–48. [DOI] [PubMed] [Google Scholar]
- Demir E, Burgucu D, Turna F, Aksakal S and Kaya B, 2013. Determination of TiO2, ZrO2, and Al2O3 nanoparticles on genotoxic responses in human peripheral blood lymphocytes and cultured embyronic kidney cells. Journal of Toxicology and Environmental Health, Part A: Current Issues, 76, 990–1002. [DOI] [PubMed] [Google Scholar]
- Demir E, Akca H, Turna F, Aksakal S, Burgucu D, Kaya B, Tokgun O, Vales G, Creus A and Marcos R, 2015. Genotoxic and cell‐transforming effects of titanium dioxide nanoparticles. Environmental Research, 136, 300–308. [DOI] [PubMed] [Google Scholar]
- Demir E, Creus A and Marcos R, 2017. Titanium dioxide and zinc oxide nanoparticles are not mutagenic in the mouse. Fresenius Environmental Bulletin, 26, 1001–1016. [Google Scholar]
- Di Bucchianico S, Cappellini F, Le Bihanic F, Zhang Y, Dreij K and Karlsson HL, 2017. Genotoxicity of TiO2 nanoparticles assessed by mini‐gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis, 32, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio AL, Reigosa M, Arnal PM, Fernandez L and de Mele M, 2010. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminium oxide nanoparticles in Chinese hamster ovary (CHO‐K1) cells. Journal of Hazardous Materials, 177, 711–718. [DOI] [PubMed] [Google Scholar]
- Disdier C, Devoy J, Cosnefroy A, Chalansonnet M, Herlin‐Boime N, Brun E, Lund A and Mabondzo A, 2015. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood‐brain barrier clearance and brain inflammation in rat. Particle and Fibre Toxicology, 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynska MM, Gajowik A, Radzikowska J, Lankoff A, Dusinska M and Kruszewski M, 2014. Genotoxicity of silver and titanium dioxide nanoparticles in bone marrow cells of rats in vivo. Toxicology, 315, 86–91. [DOI] [PubMed] [Google Scholar]
- Donner EM, Myhre A, Brown SC, Boatman R and Warheit DB, 2016. In vivo micronucleus studies with 6 titanium dioxide materials (3 pigment‐grade & 3 nanoscale) in orally‐exposed rats. Regulatory Toxicology and Pharmacology: RTP, 74, 64–74. [DOI] [PubMed] [Google Scholar]
- Dorier M, Brun E, Veronesi G, Barreau F, Pernet‐Gallay K, Desvergne C, Rabilloud T, Carapito C, Herlin‐Boime N and Carriere M, 2015. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco‐2 gut epithelial cells. Nanoscale, 7, 7352–7360. [DOI] [PubMed] [Google Scholar]
- Dorier M, Beal D, Marie‐Desvergne C, Dubosson M, Barreau F, Houdeau E, Herlin‐Boime N and Carriere M, 2017. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology, 11, 751–761. [DOI] [PubMed] [Google Scholar]
- Dorier M, Tisseyre C, Dussert F, Beal D, Arnal ME, Douki T, Valdiglesias V, Laffon B, Fraga S, Brandao F, Herlin‐Boime N, Barreau Rabilloud T and Carriere M, 2019. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co‐culture of Caco‐2 and HT29‐MTX intestinal cells. Mutation Research, 845. [DOI] [PubMed] [Google Scholar]
- Dostanic I, Schultz Jel J, Lorenz JN and Lingrel JB, 2004. The alpha 1 isoform of Na, K‐ATPase regulates cardiac contractility and functionally interacts and co‐localizes with the Na/Ca exchanger in heart. Journal of Biological Chemistry, 279, 54053–54061. [DOI] [PubMed] [Google Scholar]
- Drew DA, Mo A, Grady JJ, Stevens RG, Levine JB, Brenner BM, Anderson JC, Forouhar F, O'Brien MJ, Devers TJ and Rosenberg DW, 2018. Proximal aberrant crypt foci associate with synchronous neoplasia and are primed for neoplastic progression. Molecular Cancer Research, 16, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG and Bertram TA, 1997. Effects of particle exposure and particle‐elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis, 18, 423–430. [DOI] [PubMed] [Google Scholar]
- Du X, Gao S, Hong L, Zheng X, Zhou Q and Wu J, 2019. Genotoxicity evaluation of titanium dioxide nanoparticles using the mouse lymphoma assay and the Ames test. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 838, 22–27. [DOI] [PubMed] [Google Scholar]
- Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K, Rosenkranz HS and Simmon VF, 1985. Reproducibility of microbial mutagenicity assays: II. Testing of carcinogens and noncarcinogens in Salmonella typhimurium and Escherichia coli . Environmental MutageneisMutagenesis, 7(Suppl 5), 1–248. [DOI] [PubMed] [Google Scholar]
- Eagle AL, Wang H and Robison AJ, 2016. Sensitive assessment of hippocampal learning using temporally dissociated passive avoidance task. Bio Protoc, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimzadeh BA, Mohammadipour A, Fazel A, Haghir H, Rafatpanah H, Hosseini M and Rajabzadeh A, 2017. Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl‐2 levels, induces apoptosis and decreases neurogenesis. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie, 69, 329–337. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemical Agency), 2011. Guidance on information requirements and chemical safety assessment. Chapter R.4: Evaluation of available information. https://echa.europa.eu/documents/10162/13643/information_requirements_r4_en.pdf/d6395ad2-1596-4708-ba86-0136686d205e
- ECHA (European Chemicals Agency), 2017. Committee for risk assessment RAC opinion proposing harmonised classification and labelling at EU level of titanium dioxide. EC Number: 236‐675-5.
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA Supporting Publication 2015;12(5):EN‐804, 90 pp. 10.2903/sp.efsa.2015.EN-804 [DOI]
- EFSA (European Food Safety Authority), 2019. statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA Journal 2019;17(6):5714, 11 pp. 10.2903/j.efsa.2019.5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food), 2016. Re‐evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal 2016;14(9):4545, 83 pp. 10.2903/j.efsa.2016.4545 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food), 2017. Approach followed for the refined exposure assessment as part of the safety assessment of food additives under re‐evaluation. EFSA Journal 2017;15(10):5042, 9 pp. 10.2903/j.efsa.2017.5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food), 2018. Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA Journal 2018;16(7):5366, 27 pp. 10.2903/j.efsa.2018.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additive and Flavourings), 2019. Scientific opinion on the proposed amendment of the EU specification for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution. EFSA Journal 2019;17(7):5760, 23 pp. 10.2903/j.efsa.2019.5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011a. Scientific Opinion on Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA Journal 2011;9(5):2140, 36 pp. 10.2903/j.efsa.2011.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011b. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA Journal 2018;16(7):5327, 95 pp. 10.2903/j.efsa.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 35 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2020. Public consultation on the draft EFSA Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. Available online: https://www.efsa.europa.eu/en/consultations/call/public-consultation-draft-efsa-guidance-technical-requirements [DOI] [PMC free article] [PubMed]
- El Yamani N, Collins AR, Runden‐Pran E, Fjellsbo LM, Shaposhnikov S, Zienolddiny S and Dusinska M, 2017. In vitro genotoxicity testing of four reference metal nanomaterials, titanium dioxide, zinc oxide, cerium oxide and silver: towards reliable hazard assessment. Mutagenesis, 32, 117–126. [DOI] [PubMed] [Google Scholar]
- El‐Bassyouni GT, Eshak MG, Barakat IAH and Khalil WKB, 2017. Immunotoxicity evaluation of novel bioactive composites in male mice as promising orthopaedic implants. Central‐European Journal of Immunology, 42, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Din EAA, Mostafa HE, Samak MA, Mohamed EM and El‐Shafei DA, 2019. Could curcumin ameliorate titanium dioxide nanoparticles effect on the heart? A histopathological, immunohistochemical, and genotoxic study. Environmental Science and Pollution Research International, 26, 21556–21564. [DOI] [PubMed] [Google Scholar]
- Elespuru R, Pfuhler S, Aardema MJ, Chen T, Doak SH, Doherty A, Farabaugh CS, Kenny J, Manjanatha M, Mahadevan B, Moore MM, Ouédraogo G, Stankowski LF Jr and Tanir JY, 2018. Genotoxicity assessment of nanomaterials: recommendations on best practices, assays, and methods. Toxicological Sciences, 164, 391–416. 10.1093/toxsci/kfy100 [DOI] [PubMed] [Google Scholar]
- El‐Ghor AA, Noshy MM, Gala A and Mohamed HRH, 2014. Normalization of nano‐sized TiO2‐induced clastogenicity, genotoxicity and mutagenicity by chlorophyllin administration in mice brain, liver, and bone marrow cells. Toxicological Sciences, 142, 21–32. [DOI] [PubMed] [Google Scholar]
- Elje E, Mariussen E, Moriones OH, Bastús NG, Puntes V, Kohl Y, Dusinska M and Rundén‐Pran E, 2020. Hepato(geno)toxicity assessment of nanoparticles in a HEPG2 liver spheroid model. Nanomaterials, Basel, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnagar AMB, Ibrahim A and Soliman AM, 2018. Histopathological effects of titanium dioxide nanoparticles and the possible protective role of N‐acetylcysteine on the testes of male albino rats. International Journal of Fertility & Sterility, 12, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi T, Rivera LM, Tripathi VC, Yano N, Ragavendran A, Wallace J and Fedulov A, 2020. Transcriptomic and epigenomic effects of insoluble particles on J774 macrophages. Epigenetics, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda LM, Hagar H, Mohamed AM and Ali HM, 2018. Quercetin and idebenone ameliorate oxidative stress, inflammation, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in rat liver. Dose‐Response : A Publication of International Hormesis Society, 16, 1559325818812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda LM, Ali HM, Mohamed AM and Hagar H, 2019. Prophylactic administration of carnosine and melatonin abates the incidence of apoptosis, inflammation, and DNA damage induced by titanium dioxide nanoparticles in rat livers. Environmental Science and Pollution Research International. [DOI] [PubMed] [Google Scholar]
- Fadoju O, Ogunsuyi O, Akanni O, Alabi O, Alimba C, Adaramoye O, Cambier S, Eswara S, Gutleb AC and Bakare A, 2019. Evaluation of cytogenotoxicity and oxidative stress parameters in male Swiss mice co‐exposed to titanium dioxide and zinc oxide nanoparticles. Environmental Toxicology and Pharmacology, 70. [DOI] [PubMed] [Google Scholar]
- Falck GCM, Lindberg HK, Suhonen S, Vippola M, Vanhala E, Catalan J, Savolainen K and Norppa H, 2009. Genotoxic effects of nanosized and fine TiO2 . Human and Experimental Toxicology, 28, 339–352. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhang Y, Jiang M, Mo Y, Wan R, Jia Z, Tollerud DJ, Zhang X and Zhang Q, 2015. Up‐regulation of Gadd45αalpha after exposure to metal nanoparticles: the role of hypoxia inducible factor 1αalpha. Environmental Toxicology, 30, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio I, Greco G, Livraghi S and Fubini B, 2009. Non‐UV‐induced radical reactions at the surface of TiO2 nanoparticles that may trigger toxic responses. Chemistry–A European Journal, 15, 4614–4621. [DOI] [PubMed] [Google Scholar]
- Ferraro D, Anselmi‐Tamburini U, Tredici IG, Ricci V and Sommi P, 2016. Overestimation of nanoparticles‐induced DNA damage determined by the comet assay. Nanotoxicology, 10, 861–870. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Keyvan Karimi G, Liu CC and Rasmussen HH, 2012. Oxidative regulation of the Na(+)‐K(+) pump in the cardiovascular system. Free Radical Biology and Medicine, 53, 2263–2268. [DOI] [PubMed] [Google Scholar]
- Franchi LP, Manshian BB, de Souza TA, Soenen SJ, Matsubara EY, Rosolen JM and Takahashi CS, 2015. Cyto‐ and genotoxic effects of metallic nanoparticles in untransformed human fibroblast. Toxicology in Vitro: An International Journal Published in ASSOCIATION with BIBRA, 29, 1319–1331. [DOI] [PubMed] [Google Scholar]
- Franz P, Bürkle A, Wick P and Hirsch C, 2020. Exploring flow cytometry‐based micronucleus scoring for reliable nanomaterial genotoxicity assessment. Chemical Research in Toxicology. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Heinrich U, George M, Hendee L, Phillips DH and Lewtas J, 1994. Formation of DNA adducts in rat lung following chronic inhALTion of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis, 15, 1291–1299. [DOI] [PubMed] [Google Scholar]
- Garcia‐Rodriguez A, Vila L, Cortes C, Hernandez A and Marcos R, 2018. Effects of differently shaped TiO2NPs (nanospheres, nanorods and nanowires) on the in vitro model (Caco‐2/HT29) of the intestinal barrier. Particle and Fibre Toxicology, 15, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Senaldi C, Bucher G, Verleysen E, Waegeneers N, Brassinne F, Mast J, Loeschner K, Vidmar J, Aureli F, Cubadda F, Raggi A, Iacoponi F, Peters R, Undas A, Müller A, Meinhardt AK, Walz E, Gräf V and Barrero‐Moreno J, 2021. Particle size analysis of pristine food‐grade titanium dioxide and E 171 in confectionery products: Interlaboratory testing of a single‐particle inductively coupled plasma mass spectrometry screening method and confirmation with transmission electron microscopy. Food Control, 120, 107550. 10.1016/j.foodcont.2020.107550. PMID: 33536722; PMCID: PMC7730118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, Verharen HW, Brandon EF and de Jong WH, 2014. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol, 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Chakrabortyb A and Mukherjeea A, 2013. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. Journal of Applied Toxicology. [DOI] [PubMed] [Google Scholar]
- Gilmour P, Brown DM, Beswick PH, Benton E, MacNee W and Donaldson K, 1997. Surface free radical activity of PM10 and ultrafine titanium dioxide: a unifying factor in their toxicity? The Annals of Occupational Hygiene, 41, 32–38. [Google Scholar]
- Gore ER, Gower J, Kurali E, Sui JL, Bynum J, Ennulat D and Herzyk DJ, 2004. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology, 197, 23–35. [DOI] [PubMed] [Google Scholar]
- Griffiths PJ, Isackson H, Pelc R, Redwood CS, Funari SS, Watkins H and Ashley CC, 2009. Synchronous in situ ATPase activity, mechanics, and Ca2+ sensitivity of human and porcine myocardium. Biophysical Journal, 97, 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa I, Elghoul J, Ezzi L, Chakroun S, Kerkeni E, Hassine M, El Mir L, Mehdi M, Ben Cheikh H and Haouas Z, 2015. Anemia and genotoxicity induced by sub‐chronic intragastric treatment of rats with titanium dioxide nanoparticles. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 794, 25–31. [DOI] [PubMed] [Google Scholar]
- Grissa I, Guezguez S, Ezzi L, Chakroun S, Sallem A, Kerkeni E, Elghoul J, El Mir L, Mehdi M, Cheikh HB and Haouas Z, 2016. The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environmental Science and Pollution Research International, 23, 20205–20213. [DOI] [PubMed] [Google Scholar]
- Grissa I, Ezzi L, Chakroun S, Mabrouk A, Saleh AB, Braham H, Haouas Z and Cheikh HB, 2017. Rosmarinus officinalis L. ameliorates titanium dioxide nanoparticles and induced some toxic effects in rats’ blood. Environmental Science and Pollution Research International, 24, 12474–12483. [DOI] [PubMed] [Google Scholar]
- Grissa I, ElGhoul J, Mrimi R, Mir LE, Cheikh HB and Horcajada P, 2020. In deep evaluation of the neurotoxicity of orally administered TiO2 nanoparticles. Brain Research Bulletin, 155, 119–128. [DOI] [PubMed] [Google Scholar]
- Guichard Y, Schmit J, Darne C, Gate L, Le Goutet M, Rousset D, Rastoix O wrobel R, Witschger O, Martin A, Fierro V and Binet S, 2012. Cytotoxicity and genotoxicity of nanosized and microsized titanium dioxide and iron oxide particles in Syrian hamster embryo cells. Annals of Occupational Hygiene, 56, 631–644. [DOI] [PubMed] [Google Scholar]
- Guillard A, Gaultier E, Cartier C, Devoille L, Noireaux J, Chevalier L, Morin M, Grandin F, Lacroix MZ, Coméra C, Cazanave A, de Place A, Gayrard V, Bach V, Chardon K, Bekhti N, Adel‐Patient K, Vayssière C, Fisicaro P, Feltin N, de la Farge F, Picard‐Hagen N, Lamas B and Houdeau E, 2020. Basal Ti level in the human placenta and meconium and evidence of a materno‐foetal transfer of food‐grade TiO2 nanoparticles in an ex vivo placental perfusion model. Part Fibre Toxicol, 17, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot C and Spalla O, 2013. Stabilization of TiO2 nanoparticles in complex medium through a pH adjustment protocol. Environmental Science and Technology, 15, 47. [DOI] [PubMed] [Google Scholar]
- Gurr JR, Wang AS, Chen CH and Jan KY, 2005. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology, 15, 66–73. [DOI] [PubMed] [Google Scholar]
- Hackenberg S, Friehs G, Kessler M, Froelich K, Ginzkey C, Koehler C, Scherzed A, Burghartz M and Kleinsasser N, 2011. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environmental and Molecular Mutagenesis, 52, 264–268. [DOI] [PubMed] [Google Scholar]
- Haleem AM, Abbas RH, Jawad MA and Alberaqdar F, 2019. Cytotoxic effects of titanium dioxide nanaoparticles synthesized by laser technique on peripheral blood lymphocytes and hep‐2 Cell Line. Toxicology and Environmental Health Sciences, 11, 219–225. [Google Scholar]
- Han HY, Yang MJ, Yoon C, Lee GH, Kim DW, Kim TW, Kwak M, Heo MB, Lee TG, Kim S, Oh JH, Lim HJ, Oh I, Yoon S and Park EJ, 2020a. Toxicity of orally administered food‐grade titanium dioxide nanoparticles. Journal of Applied Toxicology. [DOI] [PubMed] [Google Scholar]
- Han B, Pei Z, Shi L, Wang Q, Li C, Zhang B, Su X, Zhang N, Zhou L, Zhao B, Niu Y and Zhang R, 2020b. TiO2 nanoparticles caused DNA damage in lung and extra‐pulmonary organs through ROS‐ activated FOXO3a signaling pathway after intratracheal administration in rats. Int J Nanomedicine, 15, 6279–6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem MM, Abo‐El-Sooud K, Abd‐Elhakim YM, Badr YA, El‐Metwally AE and Bahy‐El-Dien A, 2020. The long‐term oral exposure to titanium dioxide impaired immune functions and triggered cytotoxic and genotoxic impacts in rats. Journal of Trace Elements in Medicine and Biology, 60. [DOI] [PubMed] [Google Scholar]
- Hassanein KM and El‐Amir YO, 2017. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague‐Dawley rats. Pathology, Research and Practice, 213, 13–22. [DOI] [PubMed] [Google Scholar]
- Hekmat A, Saboury AA, Divsalar A and Seyedarabi A, 2013. Structural effects of TiO2 nanoparticles and doxorubicin on DNA and their antiproliferative roles in T47D and MCF7 cells. Anti‐Cancer Agents in Medicinal Chemistry, 13. [DOI] [PubMed] [Google Scholar]
- Hekmat A, Afrough M, Tackallou SH and Ahmad F, 2020. Synergistic effects of Titanium dioxide nanoparticles and Paclitaxel combination on the DNA structure and their antiproliferative role on MDA‐MB‐231 cells. Journal of Nanoanalysis, 7, 152–165. [Google Scholar]
- Hendrickson OD, Pridvorova SM, Zherdev AV, Klochkov SG, Novikova OV, Shevtsova EF, Bachurin SO and Dzantiev BB, 2016. Size‐dependent differences in biodistribution of titanium dioxide nanoparticles after sub‐acute intragastric administrations to rats. Current Nanoscience, 12, 228–236. [Google Scholar]
- Hendrickson OD, Platonova TA, Piidvorova SM, Zherdev AV, Gmoshinsky IV, Vasilevskaya LS, Shumakova AA, Hotimchenko SA and Dzantiev BB, 2020. Electron‐microscopic investigation of the distribution of titanium dioxide (rutile) nanoparticles in the rats’ small intestine mucosa. Liver, and Spleen Current Nanoscience, 16, 268–279. [Google Scholar]
- Heo MB, Kwak M, An KS, Kim HJ, Ryu HY, Lee SM, Song KS, Kim IY, Kwon JH and Lee TG, 2020. Oral toxicity of titanium dioxide P25 at repeated dose 28‐day and 90‐day in rats. Part Fibre Toxicol, 17, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa MB, Peters RJB, Bleys R, van der Lee MK, Tromp PC, van Kesteren PCE, van Eijkeren JCH, Undas AK, Oomen AG and Bouwmeester H, 2018. Detection of titanium particles in human liver and spleen and possible health implications. Particle and Fibre Toxicology, 15, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder MK and Chassaing B, 2018. Impact of food additives on the gut‐brain axis. Physiology & Behavior. [DOI] [PubMed] [Google Scholar]
- Hong BY, Ideta T, Lemos BS, Igarashi Y, Tan Y, DiSiena M, Mo A, Birk JW, Forouhar F, Devers TJ, Weinstock GM and Rosenberg DW, 2019. Characterization of mucosal dysbiosis of early colonic neoplasia. NPJ Precis Oncol, 3, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Wang L, Zhao X, Yu X, Sheng L, Xu B, Liu D, Zhu Y, Long Y and Hong F, 2014. Th2 factors may be involved in TiO₂ NP‐induced hepatic inflammation. Journal of Agriculture and Food Chemistry, 62, 6871–6878. [DOI] [PubMed] [Google Scholar]
- Hong J, Hong FS, Ze YG and Zhang YQ, 2016. The nano‐TiO2 exposure can induce hepatic inflammation involving in a JAK‐STAT signalling pathway. Journal of Nanoparticle Research, 18, 9. [Google Scholar]
- Hu R, Zheng L, Zhang T, Gao G, Cui Y, Cheng Z, Cheng J, Hong M, Tang M and Hong F, 2011. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. Journal of Hazardous Materials, 191, 32–40. [DOI] [PubMed] [Google Scholar]
- Hu H, Guo Q, Wang C, Ma X, He H, Oh Y, Feng Y, Wu Q and Gu N, 2015. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species‐induced insulin resistance in mice. Journal of Applied Toxicology: JAT, 35, 1122–1132. [DOI] [PubMed] [Google Scholar]
- Huang S, Chueh PJ, Lin YW, Shih TS and Chuang SM, 2009. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano‐TiO2 long‐term exposure. Toxicology and Applied Pharmacology, 241, 2. [DOI] [PubMed] [Google Scholar]
- Hufnagel M, Schoch S, Wall J, Strauch BM and Hartwig A, 2020. Toxicity and gene expression profiling of copper‐ and titanium‐based nanoparticles using air‐liquid interface exposure. Chemical Research in Toxicology, 33, 1237–1249. [DOI] [PubMed] [Google Scholar]
- ISO/TR, , 2019. Nanotechnologies ‐ considerations for performing toxicokinetic studies with nanomaterials. ISO/TR 22019;2019, IDT. [Google Scholar]
- Ivett JL, Brown BM, Rodgers C, Anderson BE, Resnick MA and Zeiger E, 1989. Chromosomal aberrations and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. IV. Results with 15 chemicals. Environmental and Molecular Mutagenesis, 14, 165–187. [DOI] [PubMed] [Google Scholar]
- Jain AK, Senapati VA, Singh D, Dubey K, Maurya R and Pandey AK, 2017. Impact of anatase titanium dioxide nanoparticles on mutagenic and genotoxic response in Chinese hamster lung fibroblast cells (V‐79): the role of cellular uptake. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 105, 127–139. [DOI] [PubMed] [Google Scholar]
- Jayaram DT and Payne CK, 2020. Intracellular generation of superoxide by TiO2 nanoparticles decreases histone deacetylase 9 (HDAC9), an epigenetic modifier. Bioconjugate Chem, 31, 1354–1361. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO, WHO Expert Committee on Food Additives), 2006. Titanium dioxide: Chemical and Technical Assessment. First draft prepared by P.M. Kuznesof, reviewed by M.V. Rao. [Google Scholar]
- Jensen DM, Lohr M, Sheykhzade M, Lykkesfeldt J, Wils RS, Loft S and Moller P, 2019. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food‐grade titanium dioxide and vegetable carbon particles. Mutagenesis, 34, 203–214. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zhao M, Zhang H, Li Y, Liu M and Feng F, 2018. Antimicrobial emulsifier‐ glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis and systemic low‐grade inflammation in low‐fat diet fed mice. Molecular Nutrition and Food Research, 1700547, 1–11. [DOI] [PubMed] [Google Scholar]
- Jin C, Tang Y, Fan XY, Ye XT, Li XL, Tang K, Zhang YF, Li AG and Yang YJ, 2013. In vivo evaluation of the interaction between titanium dioxide nanoparticle and rat liver DNA. Toxicology and Industrial Health, 29. [DOI] [PubMed] [Google Scholar]
- Jugan M‐L, Barillet S, Simon‐Deckers A, Herlin‐Boime N, Sauvaigo S, Douki T and Carriere M, 2012. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology, 6, 501–513. [DOI] [PubMed] [Google Scholar]
- Kada T, Hirano K and Shirasu Y, 1980. Screening of environmental chemical mutagens by the rec‐assay system with Bacillus subtilis. In: de Serres FJ and Hollaender A (eds.). Chemical Mutagens. Principles and Methods for their Detection. Vol 6. Plenum Press, New York, NY. pp. 149–173. [Google Scholar]
- Kandeil MA, Mohammed ET, Hashem KS, Aleya L and Abdel‐Daim MM, 2019. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2‐NPs)‐induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environmental Science and Pollution Research International. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Kim BM, Lee YJ and Chung HW, 2008. Titanium dioxide nanoparticles trigger p53‐mediated damage response in peripheral blood lymphocytes. Environmental and Molecular Mutagenesis, 49, 399–405. [DOI] [PubMed] [Google Scholar]
- Karia B, Martinez JM and Bishop AJR, 2013. Induction of homologous recombination following in utero exposure to DNA‐damaging agents. DNA Repair (Amst), 12, 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi S, Khorsandi L and Nejaddehbashi F, 2019. Protective effects of Curcumin on testicular toxicity induced by titanium dioxide nanoparticles in mice. JBRA Assisted Reproduction, 23, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimipour M, Zirak JM, Ahmadi A and Jafari A, 2018. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. International Journal of Reproductive Biomedicine (Yazd, Iran), 16, 397–404. [PMC free article] [PubMed] [Google Scholar]
- Karlsson HL, Gustafsson J, Cronholm P and M€oller L, 2009. Size‐dependent toxicity of metal oxide particles – a comparison between nano‐ and micrometer size. Toxicology Letters, 188, 112–118. [DOI] [PubMed] [Google Scholar]
- Kathawala MH, Yun Z, Chu JJH, Ng KW and Loo SCJ, 2015. TiO2‐nanoparticles shield HPEKs against ZnO‐induced genotoxicity. Materials & Design, 88, 41–50. [Google Scholar]
- Kazimirova A, Baranokova M, Staruchova M, Drlickova M, Volkovova K and Dusinska M, 2019. Titanium dioxide nanoparticles tested for genotoxicity with the comet and micronucleus assays in vitro, ex vivo and in vivo. Mutation Research, 843, 57–65. [DOI] [PubMed] [Google Scholar]
- Kazimirova A, El Yamani N, Rubio L, García‐Rodríguez A, Barancokova M, Marcos R and Dusinska M, 2020. Effects of titanium dioxide nanoparticles on the hprt gene mutations in V79 hamster cells. Nanomaterials, Basel, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Naqvi AH and Ahmad M, 2015. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicology Reports, 2, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorsandi L, Orazizadeh M, Mansouri E, Hemadi M and Moradi‐Gharibvand N, 2016. Morphometric and stereological assessment of the effects of titanium dioxide nanoparticles on the mouse testicular tissue. Bratislavske lekarske listy, 117, 659–664. [DOI] [PubMed] [Google Scholar]
- Khorsandi L, Orazizadeh M, Moradi‐Gharibvand N, Hemadi M and Mansouri E, 2017. Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environmental Science and Pollution Research International, 24, 5595–5606. [DOI] [PubMed] [Google Scholar]
- Kim JA, Aberg C and Dawson KA, 2012. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nature Nanotechnology, 7, 62–68. [DOI] [PubMed] [Google Scholar]
- Kim N, Kim C, Jung S, Park Y, Lee Y, Jo J, Hong M, Lee S, Oh Y and Jung K, 2018. Determination and identification of titanium dioxide nanoparticles in confectionery foods, marketed in South Korea, using inductively coupled plasma optical emission spectrometry and transmission electron microscopy. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 35, 1238–1246. [DOI] [PubMed] [Google Scholar]
- Klawonn X, et al., 2017 a‐f. Study summaries reported in the registration dossier under the REACH Regulation. Available online: https://echa.europa.eu/es/registration-dossier/-/registered-dossier/15560/4/9
- Klimisch HJ, Andreae M and Tillmann U, 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regulatory Toxicology and Pharmacology, 25, 1–5. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJA, Albrecht C and Schins RPF, 2004. Inhaled particles and lung cancer. Part A. Mechanisms. International Journal of Cancer, 109, 799–809. [DOI] [PubMed] [Google Scholar]
- Kowalczyk M, Orłowski M, Klepacki Ł, Zinkiewicz K, Kurpiewski W, Kaczerska D, Pesta W, Zieliński E and Siermontowski P, 2020. Rectal aberrant crypt foci (ACF) as a predictor of benign and malignant neoplastic lesions in the large intestine. BMC Cancer, 20, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Holzwarth U, Haberl N, Kozempel J, Hirn S, Wenk A, Schleh C, Schaffler M, Lipka J, Semmler‐Behnke M and Gibson N, 2017a. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: part 1. Nanotoxicology., 11, 434–442. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Holzwarth U, Schleh C, Kozempel J, Wenk A, Haberl N, Hirn S, Schaffler M, Lipka J, Semmler‐Behnke M and Gibson N, 2017b. Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: part 2. Nanotoxicology, 11, 443–453. [DOI] [PubMed] [Google Scholar]
- Kumar S, Meena R and Paulraj R, 2016. Role of macrophage (M1 and M2) in titanium‐dioxide nanoparticle‐induced oxidative stress and inflammatory response in rat. Applied Biochemistry and Biotechnology, 180, 1257–1275. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hussain A, Bhushan B and Kaul G, 2020. Comparative toxicity assessment of nano‐ and bulk‐phase titanium dioxide particles on the human mammary gland in vitro. Human and Experimental Toxicology, 39, 1475–1486. [DOI] [PubMed] [Google Scholar]
- Kurzawa‐Zegota M, Sharma V, Najafzadeh M, Reynolds PD, Davies JP, Shukla RK, Dhawan A and Anderson D, 2017. Titanium dioxide nanoparticles induce DNA damage in peripheral blood lymphocytes from polyposis coli, colon cancer patients and healthy individuals: an ex vivo/in vitro study. Journal of Nanoscience and Nanotechnology, 17, 9274–9285. [Google Scholar]
- Landsiedel R, Fabian E, Ma‐Hock L, van Ravenzwaay B, Wohlleben W, Wiench K and Oesch F, 2012. Toxico‐/biokinetics of nanomaterials. Archives of Toxicology, 86, 1021–1060. [DOI] [PubMed] [Google Scholar]
- Lazic V, Vukoje I, Milicevic B, Spremo‐Potparevic B, Zivkovic L, Topalovic D, Bajic V, Sredojevic D and Nedeljkovic JM, 2019. Efficiency of the interfacial charge transfer complex between TiO2 nanoparticles and caffeic acid against DNA damage in vitro: a combinatorial analysis. Journal of the Serbian Chemical Society, 84, 539–553. [Google Scholar]
- Lecomte M, Couedelo L, Meugnier E, Plaisancie P, Letisse M, Berengere B, Gabert L, Penhoat A, Durand A, Pineau G, Joffre F, Géloën A, Vaysse C, Laugerette F and Michalski MC, 2016. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet in high fat fed mice. Molecular Nutrition and Food Research, 60, 606–620. [DOI] [PubMed] [Google Scholar]
- Lee J, Jeong JS, Kim SY, Park MK, Choi SD, Kim UJ, Park K, Jeong EJ, Nam SY and Yu WJ, 2019. Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Particle and Fibre Toxicology, 16, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ma L and Wang J, 2010. Interaction Between Nano‐Anatase TiO2 and Liver DNA from Mice In Vivo. Nanoscale Research Letters, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Doak SH, Yan J, Chen DH, Zhou M, Mittelstaedt RA, Chen Y, Li C and Chen T, 2017a. Factors affecting the in vitro micronucleus assay for evaluation of nanomaterials. Mutagenesis, 32, 151–159. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan J, Ding W, Chen Y, Pack LM and Chen T, 2017b. Genotoxicity and gene expression analyses of liver and lung tissues of mice treated with titanium dioxide nanoparticles. Mutagenesis, 32, 33–46. [DOI] [PubMed] [Google Scholar]
- Li J, Yang S, Lei R, Gu W, Qin Y, Ma S, Chen K, Chang Y, Bai X, Xia S, Wu C and Xing G, 2018. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale, 10, 7736–7745. [DOI] [PubMed] [Google Scholar]
- Li XB, Zhang YS, Li B, Cui J, Gao N, Sun H, Meng QT, Wu SS, Bo JZ, Yan LC, Wu J and Chen R, 2019. Prebiotic protects against anatase titanium dioxide nanoparticles‐induced microbiota‐mediated colonic barrier defects. Nanoimpact, 14, 9. [Google Scholar]
- Liao F, Chen L, Liu Y, Zhao D, Peng W, Wang W and Feng S, 2019. The size‐dependent genotoxic potentials of titanium dioxide nanoparticles to endothelial cells. Environmental Toxicology, 34, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Lim JH, Bae D and Fong A, 2018. Titanium dioxide in food products: quantitative analysis using icpms and raman spectroscopy. Journal of Agricultural and Food Chemistry, 66, 13533–13540. [DOI] [PubMed] [Google Scholar]
- Linnainmaa K, Kivipensas P and Vainio H, 1997. Toxicity and cytogenetic studies of ultrafine titanium dioxide in cultured rat liver epithelial cells. Toxicology In Vitro, 1997, 329–335. [DOI] [PubMed] [Google Scholar]
- Lomer MC, Thompson RP, Commisso J, Keen CL and Powell JJ, 2000. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. Analyst, 125, 2339–2343. [DOI] [PubMed] [Google Scholar]
- Lotfi A, Zirak RG, Moghadam MS and Pazooki N, 2016. Effects of the interaction of nanorutile TiO2 with vincristine sulfate on chromosomal abnormalities in vivo. International Journal of Advanced Biotechnology and Research, 7, 1083–1093. [Google Scholar]
- Louro H, Tavares A, Vital N, Costa PM, Alverca E, Zwart E, de Jong W, Fessard V, Lavinha J and Silva MJ, 2014. Integrated approach to the in vivo genotoxic effects of a titanium dioxide nanomaterial using LacZ plasmid based transgenic mice. Environmental and Molecular Mutagenesis, 55, 500–509. 10.1002/em.21864 [DOI] [PubMed] [Google Scholar]
- Lu P‐J, Ho I‐C and Lee T‐C, 1998. Induction of sister chromatid exchanges and micronuclei by titanium dioxide in Chinese hamster ovary‐K1 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 414, 15–20. [DOI] [PubMed] [Google Scholar]
- Lu T, Ling C, Hu M, Meng X, Deng Y, An H, Li L, Hu Y, Wang H, Song G and Guo S, 2020. Effect of nano‐titanium dioxide on blood‐testis barrier and MAPK signaling pathway in male mice. Biological Trace Element Research. [DOI] [PubMed] [Google Scholar]
- Ma Y, Guo YS, Wu S, Lv ZQ, Zhang Q and Ke YB, 2017. Titanium dioxide nanoparticles induce size‐dependent cytotoxicity and genomic DNA hypomethylation in human respiratory cells. Rsc Advances, 7, 23560–23572. [Google Scholar]
- Magdolenova Z, Bilani D, Pojana G, Fjellsbø LM, Hudecova A, Hasplova K, Marcomini A and Dusinska M, 2012. Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. Journal of Environmental Monitoring, 14, 455. [DOI] [PubMed] [Google Scholar]
- Manivannan J, Banerjee R and Mukherjee A, 2020. Genotoxicity analysis of rutile titanium dioxide nanoparticles in mice after 28 days of repeated oral administration. Nucleus‐India, 63, 17–24. [Google Scholar]
- Marion‐Letellier R, Amamou A, Savoye G and Ghosh S, 2019. Inflammatory bowel diseases and food additives: to add fuel on the flames!. Nutrients, 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins ADC Jr, Azevedo LF, de Souza Rocha CC, Carneiro MFH, Venancio VP, de Almeida MR, Antunes LMG, de Carvalho Hott R, Rodrigues JL, Ogunjimi AT, Adeyemi JA and Barbosa F Jr, 2017. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co‐exposed to silver and titanium dioxide nanoparticles. Journal of Toxicology and Environmental Health. Part A, 80, 1156–1165. [DOI] [PubMed] [Google Scholar]
- Meacock G,Taylor KDA, Knowles MJ and Himonides A, 1997. The improved whitening of minced cod flesh using dispersed titanium dioxide. Journal of the Science of Food and Agriculture, 73, 221È225 [Google Scholar]
- Meena R, Kajal K and Paluraj R, 2015. a. Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male Wistar rat. Applied Biochemistry and Biotechnology, 175, 825–840. [DOI] [PubMed] [Google Scholar]
- Meena R, Kumar S and Paulraj R, 2015. b. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. Journal of Nanoparticle Research, 17, 14. [Google Scholar]
- Merga Y, Campbell BJ and Rhodes JM, 2014. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Digestive Diseases, 32, 475–483. [DOI] [PubMed] [Google Scholar]
- Miller BM, Pujadas E and Gocke E, 1995. Evaluation of the micronucleus test in vitro using Chinese hamster cells: results of four chemicals weakly positive in, in vivo micronucleus test. Environmental and Molecular Mutagenesis, 26, 240–247. [DOI] [PubMed] [Google Scholar]
- Minigalieva IA, Katsnelson BA, Privalova LI, Sutunkova MP, Gurvich VB, Shur VY, Shishkina EV, Valamina IE, Makeyev OH, Panov VG, Varaksin AN, Bushueva TV, Sakhautdinova RR, Klinova SV, Solovyeva SN and Meshtcheryakova EY, 2018. Combined Subchronic Toxicity of Aluminum (III), Titanium (IV) and Silicon (IV) Oxide Nanoparticles and Its Alleviation with a Complex of Bioprotectors. International Journal of Molecular Sciences, 19, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzynska J, Berthing T, Ravn‐Haren G, Jacobsen NR, Weydahl IK, Loeschner K, Mortensen A, Saber AT and Vogel U, 2018. Primary genotoxicity in the liver following pulmonary exposure to carbon black nanoparticles in mice. Particle and Fibre Toxicology, 15, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HR, 2015. Estimation of TiO2 nanoparticle‐induced genotoxicity persistence and possible chronic gastritis‐induction in mice. Food and Chemical Toxicology: AN International Journal Published for the British Industrial Biological Research Association, 83, 76–83. [DOI] [PubMed] [Google Scholar]
- Mohamed HR and Hussien NA, 2016. Genotoxicity studies of titanium dioxide nanoparticles (TiO2NPs) in the brain of mice. Scientifica, 2016, 6710840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadipour A, Hosseini M, Fazel A, Haghir H, Rafatpanah H, Pourganji M and Bideskan AE, 2016. The effects of exposure to titanium dioxide nanoparticles during lactation period on learning and memory of rat offspring. Toxicology and Industrial Health, 32, 221–228. [DOI] [PubMed] [Google Scholar]
- Mohamed Magdy Badr El Dine F, Samie Mohamed Hussein HA and Abdelmawla Ghazala R, 2018. Evaluation of epigenetic changes of liver tissue induced by oral administration of Titanium dioxide nanoparticles and possible protective role of Nigella Sativa oil, in adult male albino rats. Nanomedicine Journal, 5, 192–198. [Google Scholar]
- Møller P, Jantzen K, Løhr M, Andersen MH, Jensen DM, Roursgaard M, Danielsen PH, Jensen A and Loft S, 2018. Searching for assay controls for the Fpg‐ and hOGG1‐modified comet assay. Mutagenesis, 33, 9–19. 10.1093/mutage/gex015. [DOI] [PubMed] [Google Scholar]
- Moran‐Martinez J, del Rio‐Parra RB, Betancourt‐Martinez ND, Garcia‐Garza R, Jimenez‐Villarreal J, Nino‐Castaneda MS, Nava‐Rivera LE, Umana JAF, Carranza‐Rosales P and Perez‐Vertti RDA, 2018. Evaluation of the coating with TiO2 nanoparticles as an option for the improvement of the characteristics of NiTi archwires: histopathological, cytotoxic, and genotoxic evidence. Journal of Nanomaterials, 11. [Google Scholar]
- Mottola F, Iovine C, Santonastaso M, Romeo ML, Pacifico S, Cobellis L and Rocco L, 2019. NPs‐TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials (Basel, Switzerland), 9 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugadoss S, Brassinne F, Sebaihi N, Petry J, Cokic SM, Van Landuyt KL, Godderis L, Mast J, Lison D, Hoet PH and van den Brule S, 2020. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part Fibre Toxicol, 17, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhr BC and Caspary WJ, 1991. Chemical mutagenesis at the thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31 coded compounds in the National Toxicology Program. Environmental and Molecular Mutagenesis, 18, 51–83. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Wakuri S, Sakamoto K and Tanaka N, 1997. The photogenotoxicity of titanium dioxide particles. Mutation Research, 394, 125–132. [DOI] [PubMed] [Google Scholar]
- Naya M, Kobayashi N, Ema M, Kasamoto S, Fukumuro M, Takami S, Nakajima M, Hayashi M and Nakanishi J, 2012. In vivo genotoxicity study of titanium dioxide nanoparticles using comet assay following intratracheal instillation in rats. Regulatory Toxicology and Pharmacology, 62, 1–6. [DOI] [PubMed] [Google Scholar]
- NCI , 1979. National Toxicology Program. Bioassay of titanium dioxide for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser., 97, 1–123. [PubMed] [Google Scholar]
- Nejrup RG, Licht TR and Hellgren LI, 2017. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ‐free mice. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L, Shao M, Liu Y, Hu J, Li R, Xie H, Zhou L, Shi L, Zhang R and Niu Y, 2017. Reduction of oxidative damages induced by titanium dioxide nanoparticles correlates with induction of the Nrf2 pathway by GSPE supplementation in mice. Chemico‐Biological Interactions, 275, 133–144. [DOI] [PubMed] [Google Scholar]
- NVWA (Netherlands Food and Consumer Product Safety Authority), 2019. Opinion of BuRO on possible health effects of the food additive titanium dioxide (E171). Trcvwa/2019/4476/EN.
- OECD (Organisation for Economic Co‐operation and Development), 2005. Manual for the investigation of HPV chemicals. Chapter 3.1 Guidance for Determining the Quality of Data for the SIDS Dossier (Reliability, Relevance and Adequacy). Available online: http://www.oecd.org/chemicalsafety/risk-assessment/49191960.pdf
- OECD (Organisation for Economic Co‐operation and Development), 2014. Report on statistical issues related to OECD Test Guidelines (TGs) on genotoxicity, ENV/JM/MONO(2014)12.
- OECD , 2016. Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. Titanium dioxide: summary of the dossier. Vol No. 73. (Organisation for Economic Co‐operation and Development). ENV/JM/MONO(2016) 25. [Google Scholar]
- Olmedo DG, Tasat DR, Evelson P, Guglielmotti MB and Cabrini Cabrini RL, 2008. Biological response of tissues with macrophagic activity to titanium dioxide. Journal of Biomedical Materials Research, 84A, 1087. [DOI] [PubMed] [Google Scholar]
- Osman IF, Baumgartner A, Cemeli E, Fletcher JN and Anderson D, 2010. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp‐2 cells. Nanomedicine, 5, 1193–1203. [DOI] [PubMed] [Google Scholar]
- Osman IF, Najafzadeh M, Sharma V, Shukla RK, Jacob BK, Dhawan A and Anderson D, 2018. TiO2 NPs induce DNA damage in lymphocytes from healthy individuals and patients with respiratory diseases‐an ex vivo/in vitro study. Journal of Nanoscience and Nanotechnology, 18, 544–555. [DOI] [PubMed] [Google Scholar]
- Panté N and Kann M, 2002. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Molecular Biology of the Cell, 13, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Patel P, Sachin B, Undre SR, Pandya MS and Sonal B, 2016. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. Journal of Molecular Liquids, 213. [Google Scholar]
- Patel S, Patel P and Bakshi SR, 2017. Titanium dioxide nanoparticles: an in vitro study of DNA binding, chromosome aberration assay, and comet assay. Cytotechnology, 69, 245–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pele LC, Thoree V, Bruggraber S, Koller D, Thompson RP, Lomer MC and Powell JJ, 2015. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Particle and Fibre Toxicology, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RJB, Van Bemmel G, Herrera‐Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, Tromp PC, Oomen AG, Rietveld AG and Bouwmeester H, 2014. Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. Journal of Agricultural and Food Chemistry, 62, 6285–6293. [DOI] [PubMed] [Google Scholar]
- Peters RJB, Oomen AG, van Bemmel G, van Vliet L, Undas AK, Munniks S, Bleys RLAW, Tromp PC, Brand W and van der Lee M, 2020. Silicon dioxide and titanium dioxide particles found in human tissues. Nanotoxicology, 14, 420–432. [DOI] [PubMed] [Google Scholar]
- Phillips LG and Barbano DM, 1997. The influence of fat substitutes based on protein and titanium dioxide on the sensory properties of lowfat milks. Journal of Dairy Sciences, 80, 2726. [DOI] [PubMed] [Google Scholar]
- Pichot R, Duffus L, Zafeiri I, Spyropoulos F and Norton IT, 2015. Particle‐Stabilized Food Emulsions. Particle‐Stabilized Emulsions and Colloids. Formation and Applications, RSC. [Google Scholar]
- Pinget G, Tan J, Janac B, Kaakoush NO, Angelatos AS, O'Sullivan J, Koay YC, Sierro F, Davis J, Divakarla SK, Khanal D, Moore RJ, Stanley D, Chrzanowski W and Macia L, 2019. Impact of the food additive titanium dioxide (E171) on gut microbiota‐host interaction. Frontiers in Nutrition, 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittol M, Tomacheski D, Simoes DN, Ribeiro VF and Santana RMC, 2018. Evaluation of the toxicity of silver/silica and titanium dioxide particles in mammalian cells. Brazilian Archives of Biology and Technology, 61, 14. [Google Scholar]
- Pogribna M, Koonce NA, Mathew A, Word B, Patri AK, Lyn‐Cook B and Hammons G, 2020. Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology, 1–20. [DOI] [PubMed] [Google Scholar]
- Prasad RY, Wallace K, Daniel KM, Tennant AH, Zucker RM, Strickland J, Dreher K, Kligerman AD, Blackman CF and DeMarini DM, 2013. Effect of treatment media on the agglomeration of titanium dioxide nanoparticles: impact on genotoxicity, cellular interaction, and cell cycle. ACSNano, 3, 1929–1942. [DOI] [PubMed] [Google Scholar]
- Proquin H, Rodriguez‐Ibarra C, Moonen CG, Urrutia Ortega IM, Briede JJ, de Kok TM, van Loveren H and Chirino YI, 2017. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano‐sized fractions. Mutagenesis, 32, 139–149. [DOI] [PubMed] [Google Scholar]
- Prüst M, Meijer J and Westerink RHS, 2020. The plastic brain: neurotoxicity of micro‐ and nanoplastics. Part Fibre Toxicol, 17, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla I, López‐Cerón M, Jimeno M, Cuatrecasas M, Zabalza M, Moreira L, Alonso V, Rodríguez de Miguel C, Muñoz J, Castellvi‐Bel S, Llach J, Castells A, Balaguer F, Camps J and Pellisé M, 2019. Rectal aberrant crypt foci in humans are not surrogate markers for colorectal cancer risk. Clin Transl Gastroenterol, 10, e00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG and Schiffmann D, 2002. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environmental Health Perspectives, 110, 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnama S, Hassanpour A, Yadegari M, Anvari M and Hosseini‐sharifabad M, 2020. Effect of titanium dioxide nanoparticles on the stereological parameters of the dentate gyrus and the morphology of granular hippocampal neurons in mice. International Journal of Morphology, 38, 1623–1630. [Google Scholar]
- Ranjan S and Ramalingam C, 2016. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environmental Chemistry Letters, 14, 487–494. [Google Scholar]
- Rasmussen K, Mast J, De Temmerman P, Verleysen E, Waegeneers N, Van Steen F, Pizzolon J, De Temmerman L, Van Doren E, Jensen K, Birkedal R, Levin M, Nielsen S, Koponen I, Clausen P, Kofoed‐Sørensen V, Kembouche Y, Thieriet N, Spalla O, Giuot C, Rousset D, Witschger O, Bau S, Bianchi B, Motzkus C, Shivachev B, Dimowa L, Nikolova R, Nihtianova D, Tarassov M, Petrov O, Bakardjieva S, Gilliland D, Pianella F, Ceccone G, Spampinato V, Cotogno G, Gibson P, Gaillard C and Mech A, 2014. Titanium Dioxide, NM‐100, NM‐101, NM‐102, NM‐103, NM‐104, NM‐105: characterisation and physico‐chemical properties. JRC86291. 10.2788/79760 [DOI]
- Rehn B, Seiler F, Rehn S, Bruch J and Maier M, 2003. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicology and Applied Pharmacology, 189, 84–95. [DOI] [PubMed] [Google Scholar]
- Relier C, Dubreuil M, Lozano Garcìa O, Cordelli E, Mejia J, Eleuteri P, Robidel F, Loret T, Pacchierotti F, Lucas S, Lacroix G and Trouiller B, 2017. Study of TiO2 P25 nanoparticles genotoxicity on lung, blood, and liver cells in lung overload and non‐overload conditions after repeated respiratory exposure in rats. Toxicological Sciences, 156, 2. [DOI] [PubMed] [Google Scholar]
- Renz H, Brandtzaeg P and Hornef M, 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Reviews‐Immunology, 12, 9–23. [DOI] [PubMed] [Google Scholar]
- Riedle S, Wills JW, Miniter M, Otter DE, Singh H, Brown AP, Micklethwaite S, Rees P, Jugdaohsingh R, Roy NC, Hewitt RE and Powell JJ, 2020a. A murine oral‐exposure model for nano‐ and micro‐particulates: demonstrating human relevance with food‐grade titanium dioxide. Nano‐Micro Small, 16, 2000486. [DOI] [PubMed] [Google Scholar]
- Rizk MZ, Ali SA, Hamed MA, El‐Rigal NS, Aly HF and Salah HH, 2017. Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomedicine, Pharmacotherapy Biomedecine, Pharmacotherapie, 90, 466–472. [DOI] [PubMed] [Google Scholar]
- Rizk MZ, Ali SA, Kadry MO, Fouad GI, Kamel NN, Younis EA and Gouda SM, 2020. C‐reactive protein signaling and chromosomal abnormalities in nanotoxicity induced via different doses of TiO2 (80 nm) BOOST LIVER FUNCTION. Biological Trace Element Research. [DOI] [PubMed] [Google Scholar]
- Romano‐Keeler J and Weitkamp JH, 2015. Maternal influences on fetal microbial colonization and immune development. Pediatric Research, 77, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompelberg C, Heringa MB, van Donkersgoed G, Drijvers J, Roos A, Westenbrink S, Peters R, van Bemmel G, Brand W and Oomen AG, 2016. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology, 10, 1404–1414. [DOI] [PubMed] [Google Scholar]
- Ronald P, Brown RP, Michael D, Delp MD, Stan L, Lindstedt SL, Lorenz R, Rhomberg LR and Beliles RP, 1997. Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and Industrial Health, 13, 407–484. [DOI] [PubMed] [Google Scholar]
- Saber AT, Jensen KA, Jacobsen NR, Birkedal R, Mikkelsen L, Møller P, Loft S, Wallin H and Vogel U, 2012. Inflammatory and genotoxic effects of nanoparticles designed for inclusion in paints and lacquers. Nanotoxicology, 6, 453–471. [DOI] [PubMed] [Google Scholar]
- Sadiq R, Bhalli JA, Yan J, Woodruff RS, Pearce MG, Li Y, Mustafa T, Watanabe F, Pack LM, Biris A, Khan QM and Chen T, 2012. Genotoxicity of TiO2 anatase nanoparticles 383 in B6C3F1 male mice evaluated using Pig‐a and flow cytometric micronucleus assays. Mutation Research, 745, 65–72. [DOI] [PubMed] [Google Scholar]
- Santonastaso M, Mottola F, Colacurci N, Iovine C, Pacifico S, Cammarota M, Cesaroni F and Rocco L, 2019. In vitro genotoxic effects of titanium dioxide nanoparticles (n‐TiO2) in human sperm cells. Molecular Reproduction and Development, 86, 1369–1377. [DOI] [PubMed] [Google Scholar]
- Santonastaso M, Mottola F, Iovine C, Cesaroni F, Colacurci N and Rocco L, 2020. In vitro effects of titanium dioxide nanoparticles (TiO(2)NPs) on cadmium chloride (CdCl(2)) genotoxicity in human sperm cells. Nanomaterials, Basel, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib Q, Al‐Khedhairy AA, Siddiqui MA, Abou‐Tarboush FM, Azam A and Musarrat J, 2012. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicology In Vitro, 26, 351–361. [DOI] [PubMed] [Google Scholar]
- Schneider T, Westermann M and Glei M, 2017. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Archives of Toxicology, 91, 3517–3527. [DOI] [PubMed] [Google Scholar]
- Schoeffner DJ, 1999. Organ weights and fat volume in rats as a function of strain and age. Journal of Toxicology and Environmental Health Part A, 56, 449–462. [DOI] [PubMed] [Google Scholar]
- Shwter AN, Abdullah NA, Alshawsh MA, El‐Seedi HR, Al‐Henhena NA, Khalifa SA and Abdulla MA, 2016. Chemopreventive effect of Phaleria macrocarpa on colorectal cancer aberrant crypt foci in vivo . Journal of Ethnopharmacology, 193, 195–206. [DOI] [PubMed] [Google Scholar]
- Setyawati MI, Khoo PKS, Eng BH, Xiong S, Zhao X, Das GK, Tan TTY, Loo JSC, Leong DT and NG KW, 2013. Cytotoxic and genotoxic characterization of titanium dioxide, gadolinium oxide, and poly(lactic‐co‐glycolic acid) nanoparticles in human fibroblasts. Journal of Biomedical Materials Research Part A, 101A, 633–640. [DOI] [PubMed] [Google Scholar]
- Shelby MD and Witt KL, 1995. Comparison of results from mouse bone marrow aberration and micronucleus test. Environmental and Molecular Mutagenesis, 25, 302–313. [DOI] [PubMed] [Google Scholar]
- Shelby MD, Erexson GL, Hook GJ and Tice RR, 1993. Evaluation of a three‐exposure mouse bone marrow micronucleus protocol: results with 49 chemicals. Environmental and Molecular Mutagenesis, 21, 160–179. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhang JH, Jiang M, Zhu LH, Tan HQ and Lu B, 2010. Synergistic genotoxicity caused by low concentration of titanium dioxide nanoparticles and p, p’‐DDT in human hepatocytes. Environmental and Molecular Mutagenesis, 51, 192–204. [DOI] [PubMed] [Google Scholar]
- Shi Z, Niu Y, Wang Q, Shi L, Guo H, Liu Y, Zhu Y, Liu S, Liu C, Chen X and Zhang R, 2015. Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. Journal of Hazardous Materials, 298, 310–319. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S and Dhawan A, 2011. ROS‐mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicology In Vitro, 25, 231–241. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Kumar A, Gurbani D, Pandey AK, Singh S and Dhawan A, 2013. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology, 7, 48–60. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Kumar A, Vallabani NV, Pandey AK and Dhawan A, 2014. Titanium dioxide nanoparticle‐induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine (Lond), 9, 9. [DOI] [PubMed] [Google Scholar]
- Singh S, Shi T, Duffin R, Albrecht C, van Berlo D, Höhr D, Fubini B, Martra G, Fenoglio I, Borm PJ and Schins RP, 2007. Endocytosis, oxidative stress and IL‐8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicology and Applied Pharmacology, 222, 141–151. [DOI] [PubMed] [Google Scholar]
- Siskova A, Cervena K, Kral J, Hucl T, Vodicka P and Vymetalkova V, 2020. Colorectal adenomas‐genetics and searching for new molecular screening biomarkers. International Journal of Molecular Sciences, 21, 3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong C, Bakker M, Niekerk M and Vennemann F, 2015. Exposure assessment of the food additive titanium dioxide (E 171) based on use levels provided by the industry. RIVM Letter report, 2015‐0195. [Google Scholar]
- Sramkova M, Kozics K, Masanova V, Uhnakova I, Razga F, Nemethova V, Mazancova P, Kapka‐Skrzypczak L, Kruszewski M, Novotova M, Puntes VF and Gabelova A, 2019. Kidney nanotoxicity studied in human renal proximal tubule epithelial cell line TH1. Mutation Research‐Genetic Toxicology and Environmental Mutagenesis, 845, 9. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Rahman Q, Kashyap MP, Singh AK, Jain G, Jahan S, Lohani M, Llantow M and Pant AB, 2013. Nanotitanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Human and Experimental Toxicology, 32, 153–166. [DOI] [PubMed] [Google Scholar]
- Stoccoro A, Di Bucchianico S, Uboldi C, Coppede F, Ponti J, Placidi C, Blosi M, Ortelli S, Costa AL and Migliore L, 2016. A panel of in vitro tests to evaluate genotoxic and morphological neoplastic transformation potential on Balb/3T3 cells by pristine and remediated titania and zirconia nanoparticles. Mutagenesis, 31, 511–529. [DOI] [PubMed] [Google Scholar]
- Stoccoro A, Di Bucchianico S, Coppede F, Ponti J, Uboldi C, Blosi M, Delpivo C, Ortelli S, Costa AL and Migliore L, 2017. Multiple endpoints to evaluate pristine and remediated titanium dioxide nanoparticles genotoxicity in lung epithelial A549 cells. Toxicology Letters, 276, 48–61. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miura N, Hojo R, Yanagiba Y, Suda M, Hasegawa T, Miyagawa M and Wang RS, 2016. Genotoxicity assessment of intravenously injected titanium dioxide nanoparticles in gpt delta transgenic mice. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 802, 30–37. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miura N, Hojo R, Yanagiba Y, Suda M, Hasegawa T, Miyagawa M and Wang RS, 2020. Genotoxicity assessment of titanium dioxide nanoparticle accumulation of 90 days in the liver of gpt delta transgenic mice. Genes Environ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Loening‐Baucke V and Herber A, 2009a. Mucosal flora in Crohn's disease and ulcerative colitis – an overview. Journal of Physiology and Pharmacology, 60(supplement 6), 61–71. [PubMed] [Google Scholar]
- Swidsinski A, Ung V, Sydora BC, Loening‐Baucke V, Doerffel Y, Verstraelen H and Fedorak RN, 2009b. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflammatory Bowel Diseases, 15, 359–364. [DOI] [PubMed] [Google Scholar]
- Sycheva LP, Zhurkova VS, Iurchenkoa VV, Daugel‐Dauge NO, Kovalenko MA, Krivtsova EK and Durnev A, 2011. Investigation of genotoxic and cytotoxic effects of micro‐ and nanosized titanium dioxide in six organs of mice in vivo. Mutation Research, 726, 8–14. [DOI] [PubMed] [Google Scholar]
- Taboada‐López MV, Herbello‐Hermelo P, Domínguez‐González R, Bermejo‐Barrera P and Moreda‐Piñeiro A, 2019. Enzymatic hydrolysis as a sample pre‐treatment for titanium dioxide nanoparticles assessment in surimi (crab sticks) by single particle ICP‐MS. Talanta, 195, 23–32. [DOI] [PubMed] [Google Scholar]
- Talamini L, Gimondi S, Violatto MB, Fiordaliso F, Pedica F, Tran NL, Sitia G, Aureli F, Raggi A, Nelissen I, Cubadda F, Bigini P and Diomede L, 2019. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology, 13, 1087–1101. [DOI] [PubMed] [Google Scholar]
- Talbot P, Radziwill‐Bienkowska JM, Kamphuis JBJ, Steenkeste K, Bettini S, Robert V, Noordine ML, Mayeur C, Gaultier E, Langella P, Robbe‐Masselot C, Houdeau E, Thomas M and Mercier‐Bonin M, 2018. Food‐grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O‐glycosylatioand short‐chain fatty acid synthesis in vivo: implications for gut barrier protection. Journal of Nanobiotechnology, 16, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari R, Cubadda F, Moracci G, Aureli F, D'Amato M, Mauro Valeri M, De Berardis B, Raggi A, Mantovani A, Passeri D, Rossi M and Francesca Maranghi F, 2014. Oral, short‐term exposure to titanium dioxide nanoparticles in Spragues‐Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology, 8, 654–662. [DOI] [PubMed] [Google Scholar]
- Taurozzi JS and Hackley VA, 2012. Preparation of a nanoscale TiO2 aqueous dispersion for toxicological or environmental testing. National Institute of Standard and Technology Special Publication. 1200‐3, 11 pages (June 2012).
- Tavares AM, Louro H, Antunes S, Quarre S, Simar S, De Temmerman P‐J, Verleysen E, Mast J, Jensen KA, Norppa H, Nesslany F and Silva MJ, 2014. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi‐walled carbon nanotubes in human lymphocytes. Toxicology In Vitro, 28, 60–69. [DOI] [PubMed] [Google Scholar]
- Tennant RW, Margolin BH, Shelby MD, Zeiger E, Haseman JK, Spalding J, Caspary W, Resnick M, Stasiewicz S, Anderson B and Minor R, 1987. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science, 236, 933–941. [DOI] [PubMed] [Google Scholar]
- Terry LJ, Shows EB and Wente SR, 2007. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science, 318, 1412–1416. [DOI] [PubMed] [Google Scholar]
- Tomankova K, Horakova J, Harvanova M, Malina L, Soukupova J, Hradilova S, Kejlova K, Malohlava J, Licman L, Dvorakova M, Jirova D and Kolarova H, 2015. Reprint of: cytotoxicity, cell uptake and microscopic analysis of titanium dioxide and silver nanoparticles in vitro. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 85, 20–30. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Amano T and Ibuki Y, 2012. Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 742, 84–91. [DOI] [PubMed] [Google Scholar]
- Trouiller B, Reliene R, Westbrook A, Solaimani P and Schiestl RH, 2009. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Research, 69, 8784–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H and Geyikoglu F, 2007. An in vitro blood culture for evaluating the genotoxicity of titanium dioxide: the responses of antioxidant enzymes. Toxicology and Industrial Health, 23, 19–23. [DOI] [PubMed] [Google Scholar]
- Uboldi C, Urban P, Gilliland D, Bajak E, Valsami‐Jones E, Ponti J and Rossi F, 2016. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicology In Vitro: An International Journal Published in Association with BIBRA, 31, 137–145. [DOI] [PubMed] [Google Scholar]
- Urrutia‐Ortega IM, Garduno‐Balderas LG, Delgado‐Buenrostro NL, Freyre‐Fonseca V, Flores‐Flores JO, Gonzalez‐Robles A, Pedraza‐Chaverri J, Hernandez‐Pando R, Rodriguez‐Sosa M, Leon‐Cabrera S, Terrazas LI, van Loveren H and Chirino YI, 2016. Food‐grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 93, 20–31. [DOI] [PubMed] [Google Scholar]
- Vaknine S and Soreq H, 2020. Central and peripheral anti‐inflammatory effects of acetylcholinesterase inhibitors. Neuropharmacology, 168. [DOI] [PubMed] [Google Scholar]
- Valentin J, 2002. International Commission on Radiological Protection (ICRP). Basic anatomical and physiological data for use in radiological protection: reference values. In: Valentin J (ed.). Annals of the ICRP. ICRP Publication, Oxford, 89. [Google Scholar]
- Vales G, Rubio L and Marcos R, 2015. Long‐term exposures to low doses of titanium dioxide nanoparticles induce cell transformation, but not genotoxic damage in BEAS‐2B cells. Nanotoxicology, 9, 568–578. [DOI] [PubMed] [Google Scholar]
- Vasantharaja D, Ramalingam V and Reddy GA, 2015. Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomedicine Journal, 2, 46–53. [Google Scholar]
- Verleysen E, Waegeneers N, Brassinne F, De Vos S, Jimenez IO, Mathioudaki S and Mast J, 2020. Physicochemical characterization of the pristine E171 food additive by standardized and validated methods. Nanomaterials (Basel), 10, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleysen E, Waegeneers N, De Vos S, Brassinne F, Ledecq M, Van Steen F, Andjelkovic M, Janssens R, Mathioudaki S, Delfosse L, Machiels R, Cheyns K and Mast J, 2021. Physicochemical characterization of nanoparticles in food additives in the context of risk identification. EFSA supporting publication 2021:EN‐9992. 31 pp. 10.2903/sp.efsa.2021.EN-9992 [DOI]
- Viennois E and Chassaing B, 2018. First victim, later aggressor: how the intestinal microbiota drives the proinflammatory effects of dietary emulsifiers? Gut Microbes, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila L, Garcia‐Rodriguez A, Marcos R and Hernandez A, 2018. Titanium dioxide nanoparticles translocate through differentiated Caco‐2 cell monolayers, without disrupting the barrier functionality or inducing genotoxic damage. Journal of Applied Toxicology: JAT, 38, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Wallin H, Kyjovska ZO, Poulsen SS, Jacobsen NR, Saber AT, Bengtson S, Jackson P and Vogel U, 2017. Surface modification does not influence the genotoxic and inflammatory effects of TiO2 nanoparticles after pulmonary exposure by instillation in mice. Mutagenesis, 32, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Sanderson BJS and Wang H, 2007. Cyto‐ and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutation Research, 628, 99–106. [DOI] [PubMed] [Google Scholar]
- Wang S, Hunter LA, Arslan Z, Wilkerson MG and Wickliffe JK, 2011. Chronic exposure to nanosized, anatase titanium dioxide is not cyto‐ or genotoxic to Chinese hamster ovary cells. Environmental and Molecular Mutagenesis, 52, 614–622. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui H, Zhou J, Li F, Wang J, Chen M and Liu Q, 2015. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non‐small cell lung cancer A549 cells. Environmental Science and Pollution Research International, 22, 5519–5530. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Y, Wang J, Nie Y, Chen S, Hei TK, Deng Z, Wu L, Zhao G and Xu A, 2017. Amplification of arsenic genotoxicity by TiO2 nanoparticles in mammalian cells: new insights from physicochemical interactions and mitochondria. [DOI] [PubMed]
- Wang H, Ni J, Guo X, Zhou T, Ma X, Xue J and Wang X, 2018. Shelterin differentially respond to oxidative stress induced by TiO2‐NPs and regulate telomere length in human hepatocytes and hepatocarcinoma cells in vitro. Biochemical and Biophysical Research Communications, 503, 697–702. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang J, Liu Y, Nie Y, Si B, Wang T, Waqas A, Zhao G, Wang M and Xu A, 2019. Aging‐independent and size‐dependent genotoxic response induced by titanium dioxide nanoparticles in mammalian cells. Journal of Environmental Sciences (China), 85, 94–106. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL and Sayes CM, 2007. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicology Letters, 171, 99–110. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Brown SC and Donner EM, 2015a. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food and Chemical Toxicology, 84, 208–224. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Boatman R and Brown SC, 2015b. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment‐different grade & 3 nanoscale) demonstrate an absence of effects in orally‐exposed rats. Regulatory Toxicology and Pharmacology, 73, 887–896. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K and von Goetz N, 2012. Titanium dioxide nanoparticles in food and personal care products. Environmental Science and Technology, 46, 2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RS, Li Y, Yan J, Bishop M, Jones MY, Watanabe F, Biris AS, Rice P, Zhou T and Chen T, 2012. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. Journal of Applied Toxicology, 32, 934–943. [DOI] [PubMed] [Google Scholar]
- Xu A, Chai Y, Nohmi T and Hei TK, 2009. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gpt delta transgenic MEF cells. Particle and Fibre Technology, 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shi H, Ruth M, Yu H, Lazar L, Zou B, Yang C, Wu A and Zhao J, 2013. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS ONE, 8, e70618. 10.1371/journal.pone.0070618. Collection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wang D, Li K, Chen Q, Lai W, Tian L, Lin B, Tan Y, Liu X and Xi Z, 2020. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environmental Toxicology and Pharmacology, 80. [DOI] [PubMed] [Google Scholar]
- Yang J, Luo M, Tan Z, Dai M, Xie M, Lin J, Hua H, Ma Q, Zhao J and Liu A, 2017. Oral administration of nano‐titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environmental Toxicology and Pharmacology, 49, 112–118. [DOI] [PubMed] [Google Scholar]
- Yang S, Xiong F, Chen K, Chang Y, Bai X, Yin W, Gu W, Wang Q, Li J and Chen G, 2018. Impact of titanium dioxide and fullerenol nanoparticles on caco‐2 gut epithelial cells. Journal of Nanoscience and Nanotechnology, 18, 2387–2393. [DOI] [PubMed] [Google Scholar]
- Yang B, Wei J, Ju P and Chen J, 2019. Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. Gen Psychiatr, e100056, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Zhao W, Liu R, Liu R, Wang Z, Zhu L, Chen W and Liu S, 2017. TiO2 particles in seafood and surimi products: attention should be paid to their exposure and uptake through foods. Chemosphere, 188, 541–547. [DOI] [PubMed] [Google Scholar]
- Yu X, Hong F and Zhang YQ, 2016. Cardiac inflammation involving in PKCε or ERK1/2‐activated NF‐κB signalling pathway in mice following exposure to titanium dioxide nanoparticles. Journal of Hazardous Materials, 313, 68–77. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jiang X, Cheng S, Fan J, Qin X, Wang T, Zhang Y, Zhang J, Qiu Y, Qiu J, Zou Z and Chen C, 2020. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Archives of Toxicology, 94, 1173–1190. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tang Y, Chen L, Lv S, Liu S, Nie P, Aguilar ZP and Xu H, 2020. Restraining the TiO2 nanoparticles‐induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicology and Environmental Safety, 206. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hong F, Tian Y, Zhao X, Hong J, Ze Y and Wang L, 2017. Nanoparticulate titanium dioxide‐inhibited dendritic development is involved in apoptosis and autophagy of hippocampal neurons in offspring mice. Toxicology Research, 6, 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijno A, De Angelis I, De Berardis B, Andreoli C, Russo MT, Pietraforte D, Scorza G, Degan P, Ponti J, Rossi F and Barone F, 2015. Different mechanisms are involved in oxidative DNA damage and genotoxicity induction by ZnO and TiO2 nanoparticles in human colon carcinoma cells. Toxicology in Vitro: An International Journal Published In Association With BIBRA, 29, 1503–1512. [DOI] [PubMed] [Google Scholar]
- Zijno A, Cavallo D, Di Felice G, Ponti J, Barletta B, Butteroni C, Corinti S, De Berardis B, Palamides J, Ursini CL, Fresegna AM, Ciervo A, Maiello R and Barone F, 2020. Use of a common European approach for nanomaterials’ testing to support regulation: a case study on titanium and silicon dioxide representative nanomaterials. Journal of Applied Toxicology. [DOI] [PubMed] [Google Scholar]
- Zirak RG, Lotfi A and Moghadam MS, 2016. Effects of the interaction of nanoanatase TiO2 with bleomycin sulfate on chromosomal abnormalities in vivo. International Journal of Advanced Biotechnology and Research, 7, 1094–1108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search methodology for the literature search
Criteria for inclusion and exclusion applied to screening of publication retrieved in the literature search
Approach for assessing toxicokinetic and toxicity studies
Approach for assessing genotoxicity studies
Advice from EFSA ccWG Nanotechnology: Nanoscale considerations for the assessment of the study design and study results of TiO2 toxicity studie
List of in vivo toxicokinetic and toxicity studies retrieved from the literature search
New in vitro genotoxicity studies
New in vivo genotoxicity studies
In vitro genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
In vivo genotoxicity studies considered in the re‐evaluation of E 171 (EFSA ANS Panel, 2016)
In vitro genotoxicity studies from OECD dossier (OECD, 2016)
In vivo genotoxicity studies from OECD dossier (OECD, 2016)
Genotoxicity studies submitted by Interested Business Operators
Concentration levels of E 171 used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Number and percentage of food products labelled with E 171 out of the total number of food products present in the Mintel GNPD per food subcategory between 2016 and 2021
Summary of total estimated exposure of E 171 from its use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Summary of total estimated exposure of E 171 from its use as a food additive for the food supplements consumers only exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to E 171 using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of total estimated exposure of E 171 from its use as a food additive considering reported use levels and analytical data (mg/kg bw per day)


