Abstract
Dysregulation of immune responses has been linked to the generation of immunoglobulin G (IgG) autoantibodies that target human β1ARs and contribute to deleterious cardiac outcomes. Given the benefits of β-blockers observed in patients harboring the IgG3 subclass of autoantibodies, we investigated the role of these autoantibodies in human β1AR function. Serum and purified IgG3(+) autoantibodies from patients with onset of cardiomyopathy were tested using human embryonic kidney (HEK) 293 cells expressing human β1ARs. Unexpectedly, pretreatment of cells with IgG3(+) serum or purified IgG3(+) autoantibodies impaired dobutamine-mediated adenylate cyclase (AC) activity and cyclic adenosine monophosphate (cAMP) generation while enhancing biased β-arrestin recruitment and Extracellular Regulated Kinase (ERK) activation. In contrast, the β-blocker metoprolol increased AC activity and cAMP in the presence of IgG3(+) serum or IgG3(+) autoantibodies. Because IgG3(+) autoantibodies are specific to human β1ARs, non–failing human hearts were used as an endogenous system to determine their ability to bias β1AR signaling. Consistently, metoprolol increased AC activity, reflecting the ability of the IgG3(+) autoantibodies to bias β-blocker toward G-protein coupling. Importantly, IgG3(+) autoantibodies are specific toward β1AR as they did not alter β2AR signaling. Thus, IgG3(+) autoantibody biases β-blocker toward G-protein coupling while impairing agonist-mediated G-protein activation but promoting G-protein–independent ERK activation. This phenomenon may underlie the beneficial outcomes observed in patients harboring IgG3(+) β1AR autoantibodies.
INTRODUCTION
β-Adrenergic receptors (βARs) are among the most well-studied proto-typical 7-trans-membrane receptors and are powerful regulators of cardiac function (Rockman et al., 2002; Vasudevan et al., 2011a). Among the βARs, β1- and β2ARs are highly expressed in the myocardium (Rockman et al., 2002). Catecholamine binding to the βARs results in G-protein coupling, leading to adenylate cyclase (AC) activation, mediating the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signal cascade (Wallukat, 2002). Activation of the cAMP-PKA cascade alters calcium cycling, resulting in increased myocardial contractility. Although β1- and β2ARs play a key role in myocardial contraction, there is also increasing appreciation that beyond contraction, they may have distinct roles to play in phenotypic outcomes (Liaudet et al., 2014; Dungen et al., 2020). Increasing evidence from in vivo and in vitro studies shows functional divergence between β1AR and β2ARs. Cellular studies show that chronic catecholamine stimulation leads to cardiomyocyte apoptosis through activation of β1ARs, while β2ARs may mediate cardioprotective signaling (Milano et al., 1994; Steinberg, 2018). Consistently, cardiomyocyte overexpression of β1ARs leads to maladaptive cardiac remodeling, while overexpression of β2ARs results in a hypertrophic response, reflecting their divergent roles (Engelhardt et al., 1999; Steinberg, 2018). However, β1AR down-regulation (loss of cell surface receptors) and desensitization (inability to be activated by catecholamine) are among the key hallmark features of human heart failure (Port and Bristow, 2001; Steinberg, 2018).
Dilated cardiomyopathy (DCM) is one of the most commonly observed phenotypes of heart failure associated with progressive loss in ventricular function, wherein idiopathic DCM represents pathogenesis without a specific known cause (Wynne and Braunwald, 1988; Magnusson et al., 1994). Patients with idiopathic DCM have a diagnosis of diverse etiologies and varied presentations; however, dysregulation of the immune system is considered to be one of the central players in the pathogenesis of cardiomyopathy. The human immune system is a complex multicellular regulated defense mechanism that is characterized by interindividual variability in response to a similar stress/injury. In healthy homeostatic conditions, it is designed to discriminate between self and foreign components and clear components deemed to be foreign (Crampton et al., 2010; Mann, 2011; Kaya et al., 2012). However, when this regulatory control is lost, it leads to pathological circumstances wherein self-components are attacked, resulting in autoimmune disease (Crampton et al., 2010; Mann, 2011; Kaya et al., 2012). Thus, autoantibodies generated against the self-antigens exacerbate and may accelerate disease progression (Crampton et al., 2010; Mann, 2011; Kaya et al., 2012).
Circulating autoantibodies to myocardial antigens have been identified in heart failure, and increasing evidence suggests that they may be critically linked to heart failure pathogenesis (Kaya et al., 2012). Autoantibodies against β1AR have been observed in patients with heart failure (30–40%) and are positively associated with the phenotype (Magnusson et al., 1990, 1994; Jahns et al., 1999b; Iwata et al., 2001; Stork et al., 2006; Nagatomo et al., 2009; Baba, 2010). Studies have shown that β1AR autoantibody stabilizes the receptor in an active form prolonging its activation mimicking catecholamines (Deubner et al., 2010). Furthermore, β1AR autoantibodies can elevate the L-type Ca2+ current increasing in vitro contractility (Christ et al., 2001). This elevated and prolonged activation is observed during the hypersympathetic state associated with deleterious cardiac remodeling and DCM. Although β-blockers ameliorate the signaling from the sympathetic overdrive, their role in up-regulation of βARs is thought to underlie the worsening outcomes of heart failure due to binding by β1AR autoantibodies. However, recent studies have shown myocardial recovery in patients who are on β-blockers and harbor β1AR autoantibodies belonging to the immunoglobulin G (IgG3) subclass (Nagatomo et al., 2018).
β1AR autoantibodies belong to the IgG class of immunoglobulins that can be further subclassified into IgG1, 2, 3, and 4 (Schur, 1988; Vidarsson et al., 2014). Given the observed clinical benefits of harboring IgG3 subclass of β1AR autoantibodies (Nagatomo et al., 2018), studies in the current article determine the role of these autoantibodies in modulating β1AR function/signaling. Serum from patients containing IgG3-positive (IgG3[+]), -negative (IgG3[−]), or purified β1AR autoantibodies were used in cellular studies. HEK 293 cells stably expressing human β1AR were pretreated with β1AR autoantibodies followed by β1AR-selective agonist (dobutamine [Dob]) or antagonist/blocker (metoprolol [Meto]). Given the specificity of the autoantibody toward human β1ARs, we used non–failing human heart samples as an endogenous system to assess AC activity in the presence of β1AR autoantibodies. Our studies show that the IgG3 subclass of β1AR autoantibodies markedly attenuates agonist-mediated G-protein signaling by promoting enhanced β-arrestin binding, while preserving G-protein—independent Extracellular Regulated Kinase (ERK) activation. Uniquely, β1AR autoantibodies bias β-blocker signal toward G-protein coupling, unraveling a distinctive signaling role for the IgG3 subclass of β1AR autoantibodies.
RESULTS
HEK 293 cells stably expressing human β1AR
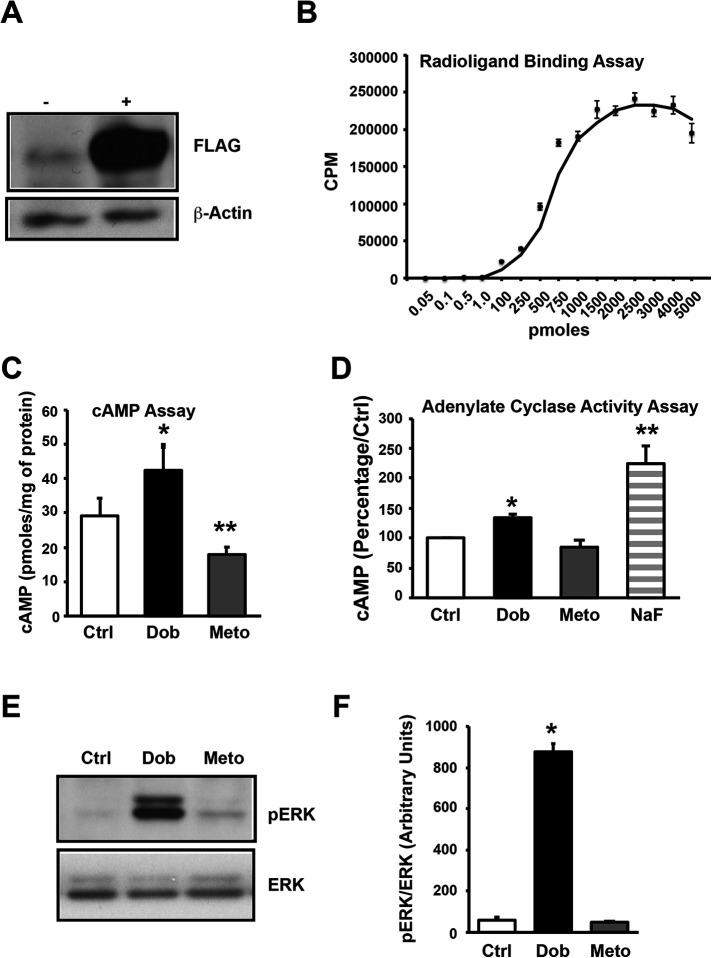
To understand how the IgG3 subclass of β1AR autoantibodies modulate the function of human β1AR, we generated the human embryonic kidney (HEK) 293 cell line stably expressing FLAG-tagged human β1AR (β1AR-HEK 293 cells). The expression of the FLAG-β1AR in the cells was tested by Western immunoblotting using anti-FLAG antibody. Immunoblotting of cell lysates showed robust expression of FLAG-β1AR in β1AR-HEK 293 cells (Figure 1A). Cell surface expression of the β1ARs was confirmed by radioligand binding using [125]I-cyanopindolol on plasma membranes isolated from β1AR-HEK 293 cells. The binding curve showed high levels of β1AR expression on the cell surface (Figure 1B). To determine whether overexpressed β1ARs were functional, cAMP generation was measured in β1AR-HEK 293 cells treated with either the β1AR-specific agonist Dob or -blocker Meto. The cAMP generation was significantly increased in cells treated with Dob and was dramatically suppressed with Meto, reflecting functional fidelity of overexpressed FLAG-β1ARs (Figure 1C). Given that G-protein coupling is a key tenet of β1AR function, we measured G-protein coupling by performing an AC assay using plasma membrane fractions. Dob treatment significantly increased AC activity, which was markedly suppressed with Meto (Figure 1D). Sodium fluoride (NaF) was used as a positive control to directly stimulate G-protein and to show the integrity of the G-protein–coupling complex in the isolated plasma membranes (Figure 1D). To further test for activation of downstream signaling, β1AR-HEK 293 cell lysates were immunoblotted for phospho-ERK (pERK) levels (Figure 1E). ERK phosphorylation was significantly increased by Dob stimulation, and Meto treatment did not appreciably alter ERK phosphorylation (Figure 1F). This human β1AR-overexpressing, HEK 293 cell line now provides the primer to determine the role of the IgG3 subclass of β1AR autoantibodies in modulating β1AR function.
FIGURE 1:
Generation and characterization of stable HEK cell line expressing human β1-adrenergic receptors (β1AR). (A) Parental HEK 293 cells and HEK 293 cells overexpressing FLAG-human-β1AR (HEK-β1AR) were lysed with NP-40 lysis buffer, and cell lysates (50 μg each) were subjected to SDS–PAGE and immunoblotted with anti-FLAG antibody. The blots were stripped and immunoblotted with anti–β-actin antibody as loading control. (B) Isolated plasma membranes from HEK-β1AR cells were used to perform the receptor-binding assay using 125I-cyanopindolol to show the expression of receptors on the cell membranes and binding curve establish very high expression of the receptors (n = 3). (C) HEK-β1AR cells were serum starved for 4 h and stimulated with 10 μM dobutamine (Dob) or 10 μM metoprolol (Meto) for 10 min. The cells were lysed and cAMP generation was measured using a cAMP assay kit. Bar graphs represent cumulative data (n = 3 independent experiments, and each experiment is performed in triplicate). * p ≤ 0.05 Ctrl vs. Dob, ** p ≤ 0.01 Meto vs. Dob/Ctrl. (D) Isolated membranes from HEK-β1AR cells were used to perform the adenylate cyclase (AC) assay in vitro to assess the function of the expressed receptors in the presence of Dob or Meto. The amount of cAMP generated by control is expressed as 100%, and percent changes in the generation of cAMP in treatments are shown (n = 3). * p ≤ 0.05 Ctrl vs. Dob, ** p ≤ 0.001 NaF vs. Ctrl/Dob/Meto. (E) HEK-β1AR cells were serum starved and stimulated with Dob or Meto. The cell lysates were subjected to Western immunoblotting with anti–phospho-ERK. The blots were stripped and immunoblotted with anti-ERK antibody as loading control. (F) Cumulative densitometric data are presented as bar graphs (n = 3). * p ≤ 0.0001, Dob vs. Ctrl/Meto.
Role of human serum containing β1AR autoantibodies on cAMP generation
To assess whether β1AR autoantibodies modulate β1AR function, we used the validated β1AR-HEK 293 cells for these studies. Human sera positive for IgG were collected and classified into IgG3(−) and IgG3(+) (classification described elsewhere [ Iwata et al., 2001; Nagatomo et al., 2009, 2011, 2016, 2018]). The β1AR-HEK 293 cells were treated with these sera and the cAMP generation measured. Treatment of the cells with IgG3(−) sera significantly increased cAMP, whereas IgG3(+) sera did not alter cAMP levels compared with untreated controls (Figure 2A). To test whether IgG3-specific sera containing β1AR autoantibody modulate β1AR responses to Dob or Meto treatment, cells were pretreated with IgG3(−) or IgG3(+) sera, and cAMP generation was measured following Dob or Meto treatment. Pretreatment of cells with IgG3(−) sera blunted the Dob-mediated cAMP generation, while Meto significantly decreased cAMP production (Figure 2B). Similarly, pretreatment with IgG(+) sera blocked cAMP generation following Dob (Figure 2C). However, surprisingly, Meto treatment resulted in a significant increase in cAMP generation in IgG3(+) sera–treated cells (Figure 2C). To further elucidate how serum containing IgG3(+) β1AR autoantibodies allows for differential G-protein coupling, the effect of Dob or Meto was assessed by measuring AC activity (which is a direct measure of G-protein coupling). Pretreatment of plasma membrane with human serum containing IgG3(−) autoantibodies decreased Dob-mediated AC activity but did not reach significance and significantly reduced AC activity by Meto treatment (Figure 2D; Supplemental Figure S1A). In contrast, pretreatment of plasma membranes with human serum containing IgG3(+) immunoglobulins significantly decreased Dob-mediated increase in AC activity (Figure 2D; Supplemental Figure S1B). Pretreatment with human serum containing IgG3(+) autoantibodies significantly decreased AC activity with Meto treatment (Figure 2D; Supplemental Figure S1B).
FIGURE 2:
Autoantibody (AAb)-positive human serum alters β1AR function. (A) HEK-β1AR cells were serum starved and treated with human serum negative for the IgG3 class of AAb (IgG3[−]) or positive for the IgG3 class of AAb (IgG3[+]) for 10 min. The cAMP was measured (n = 3–4 independent patient serum samples). * p ≤ 0.01 IgG3(−) vs. Ctrl/IgG3(+). (B) HEK-β1AR cells were serum starved, pretreated with IgG3(−) human serum for 30 min, and stimulated with dobutamine (Dob) or metoprolol (Meto). The cAMP was measured (n = 3 independent patients). * p ≤ 0.05 Meto vs. Ctrl. (C) HEK-β1AR cells were serum starved, pretreated with IgG3(+) human serum, and stimulated with Dob or Meto. The cAMP was measured (n = 4). * p ≤ 0.05 Meto vs. Ctrl/Dob. (D) Isolated membranes from HEK-β1AR cells were pretreated with IgG3(−) or IgG3(+) human serum, and AC activity was determined in the presence of Dob or Meto. The amount of cAMP generated by control is expressed as 100%. Percent changes in generation of cAMP following treatments were calculated, and Δ-change compared with control is represented; n = 4 (no serum), 5 (IgG3[−] serum), or 7 (IgG3[+] serum). ** p ≤ 0.05 Dob human serum vs. Ctrl human serum, * p ≤ 0.01 Meto human serum vs. Dob human serum.
Role of affinity-purified β1AR autoantibodies on cAMP generation
Because sera could contain factors that may potentially regulate β1ARs beyond β1AR autoantibodies, the autoantibodies were affinity purified to specifically determine their role in modulating Dob- or Meto- mediated signaling. Treatment of cells with the purified immunoglobulins, IgG3(−) or IgG3(+), alone did not alter cAMP generation (Supplemental Figure S2A) in contrast to the sera-alone treatment (Figure 2A). Pretreatment of cells with affinity-purified IgG3(−) immunoglobulins resulted in a slight increase in Dob-mediated cAMP generation and a significant decrease in cAMP with Meto (Figure 3A; Supplemental Figure S2B), consistent with IgG3(−) sera (Figure 2B). Interestingly, Dob-mediated cAMP generation was abrogated in cells following pretreatment with affinity-purified IgG3(+) immunoglobulins (Figure 3A; Supplemental Figure S2B) suggesting inhibition of G-protein coupling upon agonist binding. Surprisingly, Meto treatment reversed this inhibition, resulting in a significant generation of cAMP (Figure 3A; Supplemental Figure S2C). These observations show that both IgG3(−) and IgG3(+) samples have divergent effects with Dob-mediated or Meto-mediated cAMP generation. Importantly, the presence of IgG3(+) immunoglobulins uniquely modulates the β1AR blocker Meto to engage in G-protein pathways while contrastingly blocking the classical coupling of G-protein by the β1AR agonist Dob. To further understand how purified IgG3(+) β1AR autoantibodies allow for differential G-protein coupling, AC activity was assessed following Dob or Meto. Dob treatment in the presence of purified IgG3(−) autoantibodies increased AC activity but did not reach significance (Figure 3B; Supplemental Figure S2D). However, purified IgG3(−) immunoglobulins significantly increased AC activity with Meto treatment (Figure 3B; Supplemental Figure S2D). This is in contrast to cAMP data wherein pretreatment with IgG3(−) β1AR autoantibodies resulted in a modest Dob-mediated change in cAMP that did not reach significance, while cAMP levels were significantly reduced in the presence of Meto (Figure 2D). However, Meto treatment in the presence of purified IgG3(+) β1AR autoantibodies showed restoration of AC activity (Figure 3B; Supplemental Figure S2E) compared with the plasma membranes that were pretreated with human serum containing IgG3(+) autoantibodies (Figure 2D). The AC data are consistent with the increase in cAMP generation following treatment with the IgG3(+) human serum (Figure 2C) or affinity-purified IgG3(+) immunoglobulins (Figure 3A). These observations strengthen the findings that IgG3(+) β1AR autoantibodies impair Dob-mediated G-protein coupling, while surprisingly allowing Meto, a β-blocker, to mediate signals through engagement of G-protein–coupled pathways.
FIGURE 3:
Autoantibodies (AAb) alter β1AR function. (A) HEK-β1AR cells were serum starved, pretreated with affinity-purified IgG3(−) AAb for 30 min, and stimulated with Dob or Meto. The cAMP was measured (n = 9). Δ-change compared with control is represented. * p ≤ 0.05 metoprolol (Meto) vs. dobutamine (Dob) (IgG3[−]). ** p ≤ 0.05 Meto vs. Dob (IgG3[+]). $ p ≤ 0.01 Meto (IgG3[+]) vs. Meto (IgG3[−]). (B) Isolated membranes from HEK-β1AR cells were pretreated with IgG3(−) purified IgG or IgG3(+) purified IgG, and AC activity was determined in the presence of Dob or Meto. The amount of cAMP generated by vehicle treatment (control) is expressed as 100%. Percent changes in generation of cAMP following treatments were calculated, and Δ-change compared with control is represented; n = 4 (no AAb), 5 (purified IgG3[−] AAb), or 7 (purified IgG3[+] AAb). ** p ≤ 0.05 Meto IgG3(+) purified IgG vs. Dob IgG3(+) purified IgG.
IgG3(+) autoantibodies promote β-arrestin bias to β1AR agonist
To test whether reduced G-protein coupling to β1AR agonist in the presence of IgG3(+) β1AR antibodies is due to enhanced engagement of scaffolding protein β-arrestin, PRESTO-Tango β-arrestin recruitment assays were performed in HTLA cells (HEK cells expressing tTA-dependent luciferase reporter and a β-arrestin2-TEV fusion gene). The treatment of cells with human sera harboring either IgG3(−) or IgG3(+) autoantibodies did not alter the β-arrestin engagement (as measured by relative luminescence) to the β1AR complex (Figure 4A). However, Dob treatment of these cells resulted in a significant increase of luminescence specifically in cells pretreated with human sera containing IgG3(+) autoantibodies, reflecting the unique β-arrestin bias promoted by IgG3(+) autoantibodies. To directly test the role of IgG3(+) autoantibodies in promoting β-arrestin bias, the PRESTO-Tango assay was performed using affinity-purified autoantibodies. Treatment of cells with IgG3(−) or IgG3(+) β1AR autoantibodies by itself did not result in any significant recruitment of β-arrestin to β1AR complex as reflected by no appreciable changes in luminescence (Figure 4B). Notably, pretreatment of cells with IgG3(+) β1AR autoantibodies significantly increased Dob-mediated recruitment of β-arrestin to the receptor complex compared with no IgG or IgG3(−) autoantibodies (Figure 4B).
FIGURE 4:
Autoantibodies (AAb) alter β-arrestin recruitment. (A) HTLA cells (HEK cells stably expressing tTA-dependent luciferase reporter and a β-arrestin2-TEV fusion gene) were transfected with the ADBR1-Tango (β1AR) gene, pretreated with human sera negative for IgG3 AAb or positive for IgG3 AAb for 30 min, subjected to β-arrestin recruitment assay, and compared with no IgG control (n = 8). * p ≤ 0.001 dobutamine (Dob) IgG3(+) serum vs. Dob IgG3(−) serum and Dob no IgG. (B) HTLA cells (HEK cells expressing tTA-dependent luciferase reporter and a β-arrestin2-TEV fusion gene) were transfected with the ADBR1-Tango (β1AR) gene pretreated with affinity-purified IgG3(−) or IgG3(+) AAb for 30 min, subjected to the β-arrestin recruitment assay, and compared with no IgG control (n = 8). * p ≤ 0.001 Dob IgG3(+) AAb vs. Dob IgG3(−) AAb or Dob no IgG.
To further validate the role of IgG3(+) autoantibodies in biasing β1AR agonist toward arrestin, β-arrestin recruitment assays were performed. β1AR-HEK cells were transiently transfected with a β-arrestin2-GFP (green fluorescent protein) fusion construct to visualize the β-arrestin recruitment to the β1ARs using confocal microscropy. The cells were pretreated with IgG3(−) or IgG3(+) autoantibodies and stimulated with Dob, wherein no IgG treatment served as a control. The clearance of green fluorescence from the cytoplasm is used as a measure of β-arrestin recruitment (Figure 5). Pretreatment of cells with either IgG3(−) (Figure 5A, panel 3) or IgG3(+) (Figure 5A, panel 5) autoantibodies did not alter the β-arrestin recruitment and was similar to no IgG control (Figure 5A, panel 1). Dob-mediated β-arrestin recruitment was significantly increased when cells were pretreated with IgG3(+) autoantibodies (Figure 5A, panel 6) compared with the cells pretreated with IgG3(−) autoantibodies (Figure 5A, panel 4) or no IgG (Figure 5A, panel 2). Given the quantitative nature of increased β-arrestin recruitment to the β1AR complex following Dob in the presence of IgG(+) versus IgG(−) autoantibodies, we performed comprehensive plot profiles of the GFP fluorescence across the cells to assess the ratio of membrane (Mem) to cytoplasm (Cyt) fluorescence intensity across various cells (Figure 5B). Consistent with Dob mediating β-arrestin recruitment to the β1AR complex, cells pretreated with no IgG or IgG3(−) autoantibodies showed β-arrestin clearance from the cytoplasm in a significant percentage of GFP-expressing cells compared with no Dob treatment (Figure 5C, top and middle panels). A plot analysis summary showed that pretreatment of cells with IgG3(+) autoantibodies resulted in significantly higher changes in the fluorescence intensity (represented as percentage) characterized by cytoplasmic clearance of β-arrestin compared with no IgG or IgG3(−) autoantibodies (Figure 5D). Furthermore, pretreatment with IgG3(+) autoantibodies resulted in a significant percentage of cells showing changes in the total cell fluorescence (a reflection of loss in cytoplasmic fluorescence due to β-arrestin recruitment to the membrane) compared with no IgG or IgG3(−) autoantibodies (Figure 5E). These findings show that the human IgG3(+) β1AR autoantibodies blunt G-protein coupling and selectively enhance β-arrestin bias in response to the β1AR agonist, which may underlie their beneficial outcomes.
FIGURE 5:
Autoantibodies (AAb) alter membrane localization of β-arrestin. (A) HEK-β1AR cells transfected with plasmids containing the β-arrestin2-GFP fusion gene were plated on poly-l-lysine–coated coverslips, serum starved, pretreated with no IgG or affinity-purified IgG3(−) or IgG3(+) autoantibody, stimulated with Dob, fixed with 4% paraformaldehyde for 30 min, and mounted using prolong gold mountant with 4’,6-diamidino-2-phenylIndole (DAPI). The β-arrestin recruitment was assessed using confocal microscopy. Representative images of β-arrestin recruitment depicted by green fluorescence (bar = 10 μm). (B) Representative depiction of plot profile measurement of GFP fluorescence showing changes in fluorescence intensities from one edge to the other edge of the cell, which depicts the reduction of fluorescene intensity in the cytoplasm and increase at the plasma membrane following dobutamine (Dob treatment. (C) The relative ratio of plasma membrane (Mem) to cytoplasm (Cyt) intensity with Dob treatment in the presence of no IgG or IgG3(−) or IgG3(+) AAb (n = 3). * p<0.05 IgG3(+) vs. IgG3(−) AAb or no IgG. (D) Average changes (i.e,. clearance/loss) in cytoplasmic fluorescence intensity calculated over total fluorescence intensity of the cell reflecting β-arrestin recruitment, represented as percentage (∼ 30 cells/experiment [n = 3]). * p < 0.05 IgG3(+) AAb vs. no IgG or IgG3(−) AAb. (E) Percentage of cells showing effective β-arrestin recruitment following Dob in the presence of no IgG or IgG3(−) or IgG3(+) AAb (∼30 cells/experiment [n = 3]). *p < 0.05 IgG3(+) AAb vs. no IgG or IgG3(−) AAb.
IgG3(+) β1AR autoantibodies modulate downstream ERK activation
Activation of ERK is one of the key measures of biased downstream G-protein–independent signaling that is mediated by β-arrestins (Shenoy et al., 2006). Because IgG3(+) β1AR autoantibodies block G-protein coupling upon Dob, we assessed for changes in phospho-ERK following IgG3(−) or IgG3(+) sera or affinity-purified antibodies. Treatment of cells with either IgG3(+) or IgG3(−) serum by itself did not result in any significant difference in ERK activation (Figure 6, A–C). However, Dob or Meto treatment of cells in the presence of IgG3(−) or IgG3(+) sera showed no appreciable ERK activation (Figure 6, A–C). These data suggest that both IgG3(−) and IgG3(+) sera impair Dob-mediated ERK activation while Meto does not alter phospho-ERK status. Because sera may contain components that could alter β1AR function and signaling, we tested for ERK activation using affinity-purified β1AR autoantibodies. Treatment with both IgG3(−) and IgG3(+) autoantibodies activated ERK, and surprisingly Dob-mediated ERK phosphorylation was restored in cells pretreated with affinity-purified IgG3(−) or IgG3(+) β1AR autoantibodies (Figure 6, D and E; Supplemental Figure S3, A–C). However, Meto treatment did not activate ERK in the presence of IgG3(−) affinity-purified β1AR autoantibodies (Figure 6, D–E; Supplemental Figure S3, A–C) but significantly activated ERK in the presence of IgG3(+) autoantibodies. It is important to note that IgG3(+) autoantibodies that abrogate G-protein coupling with Dob (Figure 3B) do not impair ERK activation while Meto does activate ERK. This suggests that IgG3(+) autoantibodies could uniquely bias the agonist signaling toward pathways, reflecting a yet unappreciated role of IgG3(+) autoantibodies in regulating receptor function.
FIGURE 6:
Autoantibodies (AAb) alter β1AR signaling. (A) HEK-β1AR cells were serum starved for 4 h, pretreated with IgG3(−) or IgG3(+) human serum for 30 min, and stimulated with dobutamine (Dob) or metoprolol (Meto). The cell lysates were subjected to Western immunoblotting with anti–phospho-ERK antibody. The blots were stripped and immunoblotted with anti-ERK antibody as loading control. (B) Cumulative data for cells pretreated with IgG3(−) human serum (n = 3). (C) Cumulative data for cells pretreated with IgG3(+) human serum (n = 3). (D) HEK-β1AR cells were serum starved, pretreated with no IgG or affinity-purified IgG3(−) or IgG3(+) AAb and stimulated with Dob or Meto. The cell lysates were subjected to Western immunoblotting as above. (E) Cumulative data (n = 3). *p ≤ 0.05 Ctrl IgG3(−)/IgG3(+) vs. Ctrl no IgG. **p ≤ 0.01 Dob IgG3(−)/IgG3(+) vs. Dob no IgG. $ p ≤ 0.05 Meto IgG3(+) vs. Meto no IgG/IgG3(−).
β1AR autoantibodies alter AC activity in human cardiac membranes
To understand whether the observations made using a heterologous cell system is physiologically relevant, non–failing donor human hearts were used to isolate cardiac plasma membranes to determine AC activity in the presence or absence of purified IgG3 autoantibodies. Dob mediated a significant decrease of AC activity in the plasma membranes pretreated with IgG3(+) autoantibodies when compared with both IgG3(−) autoantibodies and no IgG control (Figure 7, A and B). However, divergent AC activity was observed in response to Meto following pretreatment with IgG3(−) or IgG3(+) autoantibodies. Meto mediated the inhibition of AC activity in the presence of IgG3(−) autoantibodies (Figure 7, A and C]). In contrast, the presence of IgG3(+) autoantibodies resulted in a significant increase in AC activity (Figure 7, A and C), reflecting their ability to mediate opposing effects selectively in response to the β1-blocker Meto. In the absence of IgG3 autoantibodies, the cardiac plasma membranes from non–failing human hearts responded classically to agonist Dob by activating AC activity and to Meto by inhibiting AC activity (Figure 7A). The inhibition of AC activity in the presence of β-agonist and the increase of AC activity in the presence of β-blocker when pretreated with IgG3(+) autoantibodies show a unique ability of this subclass of autoantibodies to preserve β1AR function that may underlie the benefits observed in patients harboring these autoantibodies (Nagatomo et al., 2018).
FIGURE 7:
Autoantibodies (AAb) alter β1AR function in human hearts. (A) Isolated membranes from donor human heart tissues were pretreated with no AAb, purified IgG3(−) AAb, or purified IgG3(+) AAb, and AC activity was determined in the presence of dobutamine (Dob) or metoprolol (Meto). The amount of cAMP generated by control is expressed as 100%. Percent changes in generation of cAMP following treatments were calculated, and Δ-change compared with control is represented (n = 4). *p ≤ 0.05 Dob IgG3(+) purified IgG vs. Dob IgG(−) purified IgG/no IgG. **p ≤ 0.005 Meto IgG3(+) purified IgG vs. Meto IgG3(−) purified IgG/no IgG. (B) AC activity determined as above. The Dob-mediated cAMP is expressed as percent over control. *p ≤ 0.05 IgG3(−) purified IgG vs. no IgG. **p ≤ 0.05 IgG3(+) purified IgG vs. IgG3(−) purified IgG/no IgG. (C) AC activity determined as above. The Meto-mediated cAMP is expressed as percent over control. *p ≤ 0.05 IgG3(+) purified IgG vs. IgG3(−) purified IgG/no IgG.
β1AR autoantibodies do not alter β2AR signaling
To test whether β1AR autoantibodies are specific to only modulating β1AR signaling, we used HEK 293 cells stably expressing β2AR (β2AR-HEK 293 cells) (Supplemental Figure S4) (Shenoy et al., 2006; Vasudevan et al., 2011b, 2013). β2AR-HEK 293 cells were pretreated with either no sera or IgG3(−) or IgG(+) sera. Following pretreatment, the cells were stimulated with isoproterenol (Iso, βAR agonist) or ICI (an inverse β2AR antagonist) and immunoblotting was performed to assess phospho-β2AR as a measure of activation. There was a significant increase in β2AR phosphorylation following Iso treatment with either no sera or IgG3(−) or IgG(+) sera (Figure 8, A–D). Similarly, IgG(−) or IgG(+) sera did not alter the phosphorylation state of β2ARs upon ICI treatment (Figure 8, A, top panel, and B–D). These studies show that IgG3(−) or IgG(+) sera do not alter β2AR responses to Iso. FLAG immunoblotting was performed as the loading control (Figure 8A, bottom panel). To further show that IgG(−) or IgG(+) sera do not alter downstream signaling, phospho-ERK was assessed following pretreatment with sera and stimulation with Iso or ICI. Pretreatment with either IgG3(−) or IgG3(+) sera did not affect Iso-mediated ERK activation or alter ICI-dependent responses (Figure 8, E–H). Together these data suggest that sera containing β1AR autoantibodies from either the IgG3(−) or the IgG3(+) family of immunoglobulins do not affect the activation/downstream signaling of β2ARs, reflecting the specificity of these autoantibodies to selectively modulate human β1AR signaling.
FIGURE 8:
Autoantibodies (AAb)-positive human serum does not alter β2AR signaling. (A) HEK 293 cells overexpressing FLAG-human-β2AR (HEK-β2AR) were serum starved for 4 h, pretreated with no IgG or IgG3(−) or IgG3(+) human serum for 30 min, stimulated with 10 μM isoproterenol (Iso) or 10 μM ICI for 10 min, and lysed with NP-40 lysis buffer, and cell lysates (50 μg each) were subjected to SDS–PAGE and immunoblotted with anti–phospho-β2AR antibody. The blots were stripped and immunoblotted with anti-FLAG antibody as loading control. The cell lysates were subjected to Western immunoblotting with anti–phospho-β2AR. The blots were stripped and immunoblotted with anti-FLAG antibody as loading control. (B) Cumulative data for cells pretreated with no IgG3 human serum (n = 3). *p ≤ 0.01 Iso vs. Ctrl/ICI. (C) HEK-β2AR cells were serum starved, pretreated with IgG3(−) human serum, and stimulated with Iso or ICI. The cell lysates were subjected to Western immunoblotting as above (n = 3). *p ≤ 0.01 Iso vs. Ctrl/ICI. (D) HEK-β2AR cells were serum starved, pretreated with IgG3(+) human serum, and stimulated with Iso or ICI. The cell lysates were subjected to Western immunoblotting as above (n = 4). *p ≤ 0.01 Iso vs. Ctrl/ICI. (E) HEK-β2AR cells were serum starved, pretreated with no IgG or IgG3(−) or IgG3(+) human serum, and stimulated with Iso and ICI. The cell lysates were subjected to Western immunoblotting with anti–phospho-ERK. The blots were stripped and immunoblotted with anti-ERK antibody as loading control. (F) Cumulative data for cells pretreated with no IgG human serum (n = 3). *p ≤ 0.01 Iso vs. Ctrl/ICI. (G) Cumulative data for cells pretreated with IgG3(−) human serum (n = 3). *p ≤ 0.05 Iso vs. Ctrl/ICI. (H) Cumulative data for cells pretreated with IgG3(+) human serum (n = 4). *p ≤ 0.05 Iso vs. Ctrl/ICI.
DISCUSSION
β1AR autoantibodies have been found in 30–95% of patients with a diagnosis of idiopathic dilated cardiomyopathy (DCM) (Wallukat et al., 1991; Limas et al., 1992), and higher percentages are consistently associated with patients requiring a left ventricular assist device (LVAD) (Dandel et al., 2012; Youker et al., 2014). Such an association suggests a role for β1AR autoantibodies in the progression of heart failure. However, recent studies on patients enrolled in the IMAC-2 (Intervention in Myocarditis and Acute Cardiomyopathy-2) study found favorable myocardial outcomes in patients with β1AR autoantibodies belonging to the IgG3 subclass (Nagatomo et al., 2016, 2018). This suggests that the IgG3 subclass of β1AR autoantibodies may uniquely regulate β1AR function, providing benefits compared with the known deleterious role of the IgG class of β1AR autoantibodies. Using IgG3(−) or IgG3(+) β1AR autoantibody-containing sera and affinity-purified β1AR autoantibodies, we show that the IgG3(+) subclass of the antibodies blunt β1AR response to the β1AR agonist Dob by enhancing the recruitment of β-arrestin. However, the IgG3(+) subclass of β1AR autoantibodies modulates the β1AR-blocker Meto-dependent signaling. Surprisingly IgG3(+) β1AR autoantibodies increase AC activity and cAMP levels with Meto treatment, suggesting a yet unappreciated role of the IgG3(+) subclass of β1AR autoantibodies that could now bias the Meto signal endogenously. Such a unique role for the IgG3(+) human β1AR autoantibodies in modulating β1AR G–protein coupling by Meto was validated using cardiac plasma membranes from a non–failing donor human heart sample that endogenously harbors β1ARs. This unique signaling mechanism may potentially underlie the beneficial outcomes observed in patients.
Autoantibodies classically belong to the IgG class of immunoglobulins, which are one of the most abundant among the five classes (IgM, IgE, IgG, IgA, and IgD) found in human serum. Previous studies have identified that β1AR autoantibodies belonging to the IgG class of immunoglobulins can modulate human β1AR responses (Jahns et al., 1999a,b; Iwata et al., 2001; Kaya et al., 2012), and observations have implied that nonspecific IgG deposition in human failing myocardium was associated with poorer outcomes (Youker et al., 2014). However, the use of the IgG class of β1AR autoantibodies isolated from patients with DCM showed varied responses in terms of their ability to modulate β1AR internalization and cAMP generation (Bornholz et al., 2013), highlighting that specificity of the antibody is the key differentiating factor in regulation of β1AR signaling. The IgG class of immunoglobulins are further divided into four subclasses, namely, IgG1, IgG2, IgG3, and IgG4 (Schur, 1988; Vidarsson et al., 2014). Thus, the variability in the observed responses to IgG isolates from different patients could potentially be due to changes in the relative abundance of these subclasses, which may differentially alter β1AR responses and cardiac pathogenic outcomes. Consistent with this concept, recent studies show that patients who harbor the subclass of IgG3-enriched β1AR autoantibodies have significant myocardial recovery compared with non-IgG3(+) patients (Nagatomo et al., 2018). This suggests that heart failure progression and exacerbation in patients may depend on the representation of the IgG subclass of β1AR autoantibodies. In this regard we do recognize the concerns/issues associated with the reliability of enzyme-linked immunosorbent assay (ELISA)-based methodologies in identifying IgG3 subtypes (Wenzel et al., 2018). However, our findings point to direct evidence of distinct downstream β1AR signaling in samples identified by this method.
Previous cellular studies show that pretreatment with many of the β1AR autoantibody IgG isolates from patients positively modulates agonist Iso coupling as measured by cAMP (Bornholz et al., 2013). This suggests that the β1AR autoantibody is able to mediate a receptor conformation that allows for elevated generation of cAMP beyond the levels generated by Iso alone (Bornholz et al., 2013). This supported the concept that the IgG class of β1AR autoantibodies may lead to a chronic elevation in cAMP levels due to a hypersympathetic state exacerbating the heart failure outcomes (Jahns et al., 1999b; Iwata et al., 2001; Kaya et al., 2012). In contrast, our studies show that sera containing IgG(+) β1AR autoantibodies or affinity-purified IgG3(+) β1AR autoantibodies impair agonist-mediated activation of β1ARs as measured by cAMP generation and AC activity. This suggests that the presence of the IgG3 subclass of β1AR autoantibodies may chronically reduce cAMP levels that underlie beneficial outcomes of myocardial recovery observed in these sets of patients (Nagatomo et al., 2018). This supports the evolving idea that subclass members (IgG1, IgG2, IgG3, and IgG4) of the IgG family of β1AR autoantibodies may have differential effects on β1AR function as described by our studies on the IgG3 subclass of β1AR autoantibodies and substantiated by studies showing variable cAMP response to pan-IgG β1AR autoantibodies (Bornholz et al., 2013).
β-Blockers are known to block βAR responses to agonist and have been identified by their ability to inhibit the generation of cAMP following agonist treatment (Kenakin, 2004; Wisler et al., 2007). Because autoantibodies mediate a β1AR conformation that drives the receptors to generate cAMP in response to agonist (Bornholz et al., 2013), we used Meto, a β1AR selective blocker (antagonist) (Bristow, 1997; Prakash and Markham, 2000). It has been recognized that β-blockers alprenolol or carvedilol can bias the downstream β1AR signaling toward G-protein–independent ERK activation, while simultaneously blocking G-protein coupling (Shenoy et al., 2006; Wisler et al., 2007; Kim et al., 2008). While Meto by itself blocks G-protein coupling, the presence of IgG3(+) β1AR autoantibodies surprisingly results in G-protein coupling and cAMP generation. More intriguingly, IgG3(+) β1AR autoantibodies markedly impair agonist Dob-mediated G-protein coupling by selectively enhancing the binding of β-arrestin to the β1AR, desensitizing the receptor while remarkably preserving ERK activation. This observation suggests that IgG3(+) β1AR autoantibodies uniquely bias agonist signaling towards the G-protein–independent pathway while blocking the G-protein–dependent pathway. In this context, recent studies have shown that the β-blocker carvedilol mediates a unique β1AR conformation that selectively allows for inhibitory G-protein coupling promoting a G-protein–independent β-arrestin–dependent pathway (Wang et al., 2017). Thus, it is possible that IgG3(+) β1AR autoantibodies may bind to the β1ARs promoting Gi coupling and β-arrestin signaling that may underlie the beneficial outcomes observed in patients harboring IgG3(+) β1AR autoantibodies (Nagatomo et al., 2018).
Patients harboring the IgG class of β1AR autoantibodies are known to have worse patient outcomes (Jahns et al., 1999b; Iwata et al., 2001; Kaya et al., 2012), but the contrasting observation of benefits observed with the IgG3 subclass suggests differential modulation/engagement of β1ARs to mediate beneficial signals (Nagatomo et al., 2018). The unique ability of the IgG3 subclass of β1AR autoantibodies to bias β1AR signaling through the G-protein–independent ERK pathway may underlie the beneficial outcomes in patients with this antibody (Nagatomo et al., 2018). It is known that β-blockers inhibit G-protein coupling but also simultaneously initiate signals through β-arrestin–dependent, G-protein–independent mechanisms (Shenoy et al., 2006; Wisler et al., 2007; Kim et al., 2008). Because β-blocker treatment has positive outcomes in heart failure patients, the biased G-protein–independent, β-arrestin–dependent signaling initiated by β-blockers is considered beneficial (Wisler et al., 2007; Kim et al., 2008). However, the uniqueness of the IgG3(+) β1AR autoantibodies lies in their ability to modulate β1AR signaling to inhibit the classical agonist-mediated G-protein coupling, while mediating the beneficial β-arrestin pathway preserving G-protein–independent ERK activation. Given the hypersympathetic state of patients with heart failure, the presence of the IgG3(+) β1AR autoantibodies would allow for preferential engagement of the G-protein–independent pathway, reflected in the myocardial recovery of patients harboring this subclass of β1AR autoantibodies (Nagatomo et al., 2018). Our current studies are focused on determining whether the IgG3(+) subclass of β1AR autoantibodies engages the β-arrestin–dependent epidermal growth factor receptor (EGFR) transactivation pathways to mediate ERK activation while blocking G-protein coupling.
Although one of the limitations of the study is the availability of IgG3(+) patient samples, we still have provided direct G-protein–coupling evidence to demonstrate the distinctive ability of the IgG3(+) subclass of the β1AR autoantibodies in modulating β1AR signaling (Figure 9). Another limitation in our study is the availability of human tissues/cells endogenously expressing human β1ARs as the autoantibodies have selectivity toward human β1ARs. Comprehensive protein expression pattern analysis using GeneCard showed appreciable expression of β1ARs in the human kidney, brain, and heart. Therefore, we have used non–failing human heart samples to show that the human IgG3(+) β1AR autoantibodies uniquely modulate human cardiac β1AR signaling to the β1-blocker Meto, given that the functional outcome in the heart is directly affected by the presence of this subclass of autoantibodies.
FIGURE 9:

Illustration depicting signaling mechanism of IgG3(+) autoantibodies (AAb) generated against human β1AR. (A) Agonist-mediated β1AR signaling. (B) Antagonist-mediated β1AR signaling. (C) Agonist-mediated β1AR signaling modulated by IgG3(+) AAb. (D) Antagonist-mediated β1AR signaling modulated by IgG3(+) AAb.
These observations tempt us to speculate about the unique beneficial modulatory role IgG3(+) β1AR autoantibodies may have in patients with cardiomyopathy and may even extend to any hyperadrenergic states as a counterregulatory response. Thus, IgG3(+) β1AR autoantibodies in the presence of high levels of circulating β-blocker would allow for moderate coupling to G-proteins while with higher levels of circulating catecholamines they would impair G-protein coupling but importantly allow for G-protein–independent signaling. These signaling outcomes modulated by IgG3(+) β1AR autoantibodies could have physiological significance given that G-protein–independent signaling is considered beneficial in conditions of sympathetic overdrive (Noma et al., 2007; Carr et al., 2016). Because cardiomyopathy is associated with sympathetic overdrive, the presence of IgG3(+) β1AR autoantibodies would now selectively engage the G-protein–independent signaling pathway that potentially underlies the benefits observed in patients harboring this subclass of autoantibodies (Nagatomo et al., 2018). In summary, we present here a mechanistic pilot study that lays the foundation for 1) performing comprehensive analysis using a larger cohort of patients harboring IgG3(+) β1AR autoantibodies, 2) using the subclass of IgG3(+) β1AR autoantibodies as a potential therapeutic strategy as patients carrying IgG3(+) β1AR autoantibodies have significantly better outcomes than IgG3(−) patients, and 3) confirming the safety and benefits of IgG3(+) β1AR autoantibodies as a unique immunomodulatory strategy in hyperadrenergic states (Nagatomo et al., 2018).
MATERIALS AND METHODS
Request a protocol through Bio-protocol.
Stable cell lines
HEK cells stably overexpressing FLAG-β1AR (FLAG-β1AR-HEK 293) were used in the experiments. A stable cell line was developed in-house by clonal selection using G418 (geneticin) as antibiotic selection after transfecting HEK 293 cells with the mammalian expression vector plasmid (pcDNA3.1(−)) containing Flag-tagged β1AR cDNA (gift from Yang K. Xiang, University of California, Davis, CA). HEK 293 cells stably expressing β2AR (FLAG-β2AR-HEK 293) were a gift from Robert J. Lefkowitz, Duke University, Durham, NC (Shenoy et al., 2006).
Human heart tissues and plasma samples
Deidentified non–failing donor human heart tissues were obtained from the Cleveland Clinic’s Tissue Bank following approved Institutional Review Board (IRB) protocol and consent. Plasma samples from patients with a clinical diagnosis of chronic systolic heart failure were collected following written informed consent in an IRB-approved protocol as previously described (Nagatomo et al., 2016).
Cell culture
For optimal growth of cells, MEM supplemented with 10% FBS and 5% penicillin–streptomycin was used. Cells were incubated at 37°C and 5% CO2.
Cell treatments
The cells were serum starved with serum-free MEM for 4 h before pretreatment and stimulation. HEK-β1AR and β2AR cells were pretreated with IgG3(+) or (−) human serum or affinity-purified IgG3(+) or (−) immunoglobulins (autoantibodies) for 30 min. Following pretreatment, β1AR cells were treated with specific agonist Dob or specific β1-blocker Meto. HEK-β2AR cells were treated with specific agonist Iso or specific inverse agonist ICI following pretreatment. The whole cell extracts were prepared by lysing the cells in NP-40 lysis buffer (20 mM Tris-HCl, pH 7.4; 137 mM NaCl; 1% NP-40; 20% glycerol; 1 mM phenyl methyl sulfonyl fluoride (PMSF); 2 μg/ml leupeptin; and 1 μg/ml protinin).
Immunoblotting
Whole cell lysates were resolved using SDS–PAGE and immunoblotted for respective proteins. The following primary antibodies were used for the study; α-Flag (1:2000), α-β-actin (Sigma-Aldrich; 1:25,000), α-pERK (Cell Signaling; 1:2000), α-ERK (Cell Signaling; 1:2000), and α-pβ2AR (Santa Cruz Biotechnology; 1:1000). Appropriate horseradish peroxidase (HRP)-conjugated secondary antibody was used, and chemiluminescence signals were assessed. Densitometry analysis was performed using ImageJ software.
Plasma membrane isolation
The plasma membrane was isolated using a previously described protocol (Naga Prasad et al., 2001). Briefly, the treated cells were scraped using osmotic lysis buffer (5 mM Tris-HCl, pH 7.4; 5 mM EDTA; 1 mM PMSF; 2 μg/ml leupeptin, and 1 μg/ml aprotinin) and homogenized by Dounce homogenizer. Cardiac tissue samples from non–failing human hearts were homogenized in osmotic lysis buffer using Polytron homogenizer. This was followed by toggling the samples for 10 min. The intact cells and nuclei were removed by centrifuging these samples at 2500 rpm for 5 min. The collected supernatant was centrifuged at 17,500 rpm for 30 min to obtain the plasma membrane as pellet. All the above steps were performed at 4°C. The pellet was resuspended in ice cold binding buffer (75 mM Tris-HCl, pH 7.5; 2 mM EDTA; and 12.5 mM MgCl2) to perform receptor binding and the AC assay.
Receptor-binding assay (βAR density assay)
The expression of the βAR receptors on plasma membrane was determined using a radioligand-binding assay with βAR specific radioligand 125I-cyanopindolol (Ferguson et al., 1996; Naga Prasad et al., 2001). This assay was performed by incubating 20 μg of plasma membrane at 37°C for 1 h and assessing nonspecific binding in the presence of the β1AR-specific β-blocker Meto.
cAMP assay
The cAMP generation assay was performed using whole cell lysates. The assay was done using the standard manufacturer’s protocol. The Catchpoint cAMP fluorescence assay kit from Molecular Devices (San Jose, CA) was used to measure the cAMP levels (Vasudevan et al., 2013).
Adenylate cyclase assay
G-protein coupling was measured using 20 μg of plasma membrane for the AC assay and by measuring the generated cAMP using a standard procedure as previously reported (Vasudevan et al., 2011b).
β-Arrestin2 recruitment assay
Measurement of β-arrestin2 recruitment was performed on HTLA cells, which are HEK 293 cells stably expressing a tTA-dependent luciferase reporter and a β-arrestin2-TEV fusion gene (gift from the laboratory of Bryan L. Roth, University of North Carolina, Chapel Hill, NC) following the protocol as previously described (Kroeze et al., 2015). Briefly, HTLA cells were seeded on a poly-l-lysine–coated 96-well, white wall clear-bottom plate (Corning;#Cat-356651) at a density of ∼30,000 cells/well maintained in 100 µl DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 μg/ml puromycin, and 100 μg/ml hygromycin B in a humidified atmosphere at 37°C in 5% CO2 (day 1).The following day (day 2), cells were transfected with the ADRB1-Tango plasmid (Roth lab PRESTO-Tango GPCR Kit;addgene #Cat 1000000068) using the Lipofactamine 3000 transfection kit (Invitrogen; Cat #L3000008). On day 4, human sera negative/positive for IgG3 autoantibodies or affinity-purified IgG3(−)/IgG3(+) autoantibodies diluted in assay buffer (20 mM Hank’s Balance Salt Solution (HEPES) and 1× HBSS, pH 7.4) was added (10 µl of 10× concentration) to the respective wells of the 96-well plate. Following incubation for 30 min, 10 µM Dob was added (25 µl of 5× concentration). On day 5, spent medium and drug solutions were removed from the wells by aspiration, and 100 μl of Bright-Glo solution (Promega; #Cat E2620) diluted fivefold in assay buffer was added to each well. After incubation for 10 min at room temperature, luminescence was measured in a FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices). Relative luminescence units (RLU) were documented and exported into GraphPad Prism for the analysis of data.
Confocal microscopy
HEK-β1AR cells transfected with plasmids containing the β-arrestin2-GFP fusion gene were plated on poly-l-lysine–coated coverslips, serum starved, pretreated with no IgG, affinity-purified IgG3(−), or IgG3(+) autoantibodies, stimulated with Dob, fixed with 4% paraformaldehyde for 30 min, and mounted using prolong gold mountant. Samples were visualized using sequential line excitation at 488 and 405 nm for green and blue, respectively. Quantitation of the fluorescence intensity of the cells and plot profile measurements were done using ImageJ software.
ELISA and IgG purification from plasma sample
The ELISA and IgG purification was described in detail previously (Nagatomo et al., 2009, 2016). Briefly, fusion protein containing the second extracellular loop of the human β1AR (amino acids 197–222) was synthesized (as the target peptide) and (1 μg) coated on the microtiter plates. Patient serum (100 μl) (at dilutions starting from 1:20) was added to the coated wells of the microtiter plate, and following multiple washes the bound HRP-conjugated antibodies detected by incubation with streptavidin. The assay color was developed by incubation with H2O2 and tetramethylbenzidine, and the reaction was stopped by 2 N H2SO4. The optical reading was determined at 450 nm, and the positive reaction was defined as ≥2.5 times the background. Following the identification of IgG3(+) patient samples, β1AR autoantibodies were purified using a MabTrap Kit (GE #17-1128-01), based on the manufacturer’s protocol. The kit has a binding capacity of 25 mg IgG/ml medium. Briefly the purification began with first collecting the protein from the plasma sample. This was done by adding the collected plasma to a syringe containing 1.5 ml binding buffer (20 mM sodium phosphate, pH 7.0). A 0.45 μm filter was used to flush 1 ml of the binding buffer through the syringe to get a total volume of 2.5 ml. This was followed by purification of the obtained protein, where first the column was equilibrated with binding buffer. The samples were then slowly added to the column using a syringe at a speed of ∼1 drop/2–10 s (0.2-1 ml/min). The samples were collected and reloaded back to the column. This step was repeated four times. This was followed by washing the samples with binding buffer until no materials appeared in the effluent. The samples were then eluted with ∼3–5 ml of elution buffer (100 mM glycine-HCl, pH 2.7), and the eluate was neutralized in a collection tube containing 375 μl of neutralizing buffer (100 mM Tris-HCl, pH 9.0). The columns were reconditioned using 5 ml binding buffer. The final step was the enrichment of the obtained IgG. The collected samples were passed through a 10 kDa Amicon Ultra tube containing 10 ml of phosphate-buffered saline. This was centrifuged at 4000 × g for 30 min. Finally, ∼200 μl of samples from the above steps was centrifuged at 14,000 × g for 30 min using a 3 kDa Amicon Ultra 0.5 ml device to collect the enriched IgG. The enriched IgG3(−) or IgG3(+) autoantibodies were then diluted to a final concentration of 0.5 μg/μl and used for the outlined mechanistic studies.
Statistical analysis
All data are expressed as mean ± SEM (n ≥ 3 experiments performed under identical conditions). Analysis of variance was used for multiple comparisons of the data. Statistical analyses were performed using GraphPad Prism, and the significance between the treatments was determined by Student’s t test. A p value less than <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We acknowledge a Cleveland Clinic Research Programs Committee Award to W.H.W.T. for funding of the clinical study that procured the samples. This work was supported by National Institutes of Health grants R01 HL103931 to W.H.W.T., R01 HL126827 to W.H.W.T. and S.L.H., R01 HL089473 and RO1 HL128382 to S.V.N.P., and P01 HL147823 to S.L.H. and American Heart Association (AHA) Transformational Project Award 18TPA34170554 to M.L.M. Y. N. was supported by a Postdoctoral Research Fellowship from the Myocarditis Foundation. P.P.S. was supported by a Postdoctoral Research Fellowship from the AHA. We thank Wendy Sweet and Christine S. Moravec for providing non–failing donor heart tissue samples.
Abbreviations used:
- AC
adenylate cyclase
- βAR
β-Adrenergic receptor
- cAMP
cyclic adenosine monophosphate
- DCM
dilated cardiomyopathy
- EGFR
epidermal growth factor receptor
- ELISA
enzyme linked immunosorbent assay
- ERK
Extracellular Regulated Kinase
- GFP
green fluorescent protein
- HEK
human embryonic kidney
- IgG
immunoglobulin G
- IMAC
Intervention in Myocarditis and Acute Cardiomyopathy.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E20-06-0394) on February 3, 2021.
REFERENCES
- Baba A (2010). Targeted autoantibodies in apheresis treatment against severe heart failure. Jpn J Apheresis 29, 187–193. [Google Scholar]
- Bornholz B, Weidtkamp-Peters S, Schmitmeier S, Seidel CA, Herda LR, Felix SB, Lemoine H, Hescheler J, Nguemo F, Schafer C, et al. (2013). Impact of human autoantibodies on beta1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res 97, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow MR (1997). Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol 80, 26L–40L. [DOI] [PubMed] [Google Scholar]
- Carr R 3rd, Schilling J, Song J, Carter RL, Du Y, Yoo SM, Traynham CJ, Koch WJ, Cheung JY, Tilley DG, et al. (2016). beta-Arrestin-biased signaling through the beta2-adrenergic receptor promotes cardiomyocyte contraction. Proc Natl Acad Sci USA 113, E4107–E4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, Ravens U (2001). Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol 33, 1515–1525. [DOI] [PubMed] [Google Scholar]
- Crampton SP, Voynova E, Bolland S (2010). Innate pathways to B-cell activation and tolerance. Ann NY Acad Sci 1183, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandel M, Wallukat G, Englert A, Lehmkuhl HB, Knosalla C, Hetzer R (2012). Long-term benefits of immunoadsorption in beta(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur J Heart Fail 14, 1374–1388. [DOI] [PubMed] [Google Scholar]
- Deubner N, Berliner D, Schlipp A, Gelbrich G, Caforio AL, Felix SB, Fu M, Katus H, Angermann CE, Lohse MJ, et al. (2010). Cardiac beta1-adrenoceptor autoantibodies in human heart disease: rationale and design of the Etiology, Titre-Course, and Survival (ETiCS) Study. Eur J Heart Fail 12, 753–762. [DOI] [PubMed] [Google Scholar]
- Dungen HD, Dordevic A, Felix SB, Pieske B, Voors AA, McMurray JJV, Butler J (2020). beta1-Adrenoreceptor autoantibodies in heart failure: physiology and therapeutic implications. Circ Heart Fail 13, e006155. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ (1999). Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96, 7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE 3rd, Colapietro AM, Barak LS, Menard L, Caron MG (1996). Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271, 363–366. [DOI] [PubMed] [Google Scholar]
- Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S (2001). Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 37, 418–424. [DOI] [PubMed] [Google Scholar]
- Jahns R, Boivin V, Siegmund C, Boege F, Lohse MJ, Inselmann G (1999a). Activating beta-1-adrenoceptor antibodies are not associated with cardiomyopathies secondary to valvular or hypertensive heart disease. J Am Coll Cardiol 34, 1545–1551. [DOI] [PubMed] [Google Scholar]
- Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F (1999b). Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation 99, 649–654. [DOI] [PubMed] [Google Scholar]
- Kaya Z, Leib C, Katus HA (2012). Autoantibodies in heart failure and cardiac dysfunction. Circ Res 110, 145–158. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2004). Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25, 186–192. [DOI] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA (2008). Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA 105, 14555–14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, Sciaky N, Roth BL (2015). PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 22, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaudet L, Calderari B, Pacher P (2014). Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail Rev 19, 815–824. [DOI] [PubMed] [Google Scholar]
- Limas CJ, Goldenberg IF, Limas C (1992). Assessment of immune modulation of beta-adrenergic pathways in human dilated cardiomyopathy: influence of methodologic factors. Am Heart J 123, 967–970. [DOI] [PubMed] [Google Scholar]
- Magnusson Y, Marullo S, Hoyer S, Waagstein F, Andersson B, Vahlne A, Guillet JG, Strosberg AD, Hjalmarson A, Hoebeke J (1990). Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest 86, 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J (1994). Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation 89, 2760–2767. [DOI] [PubMed] [Google Scholar]
- Mann DL (2011). The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res 108, 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ (1994). Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science 264, 582–586. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA (2001). Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem 276, 18953–18959. [DOI] [PubMed] [Google Scholar]
- Nagatomo Y, Baba A, Ito H, Naito K, Yoshizawa A, Kurita Y, Nakamura I, Monkawa T, Matsubara T, Wakabayashi Y, et al. (2011). Specific immunoadsorption therapy using a tryptophan column in patients with refractory heart failure due to dilated cardiomyopathy. J Clin Apher 26, 1–8. [DOI] [PubMed] [Google Scholar]
- Nagatomo Y, Li D, Kirsop J, Borowski A, Thakur A, Tang WH (2016). Autoantibodies specifically against beta1 adrenergic receptors and adverse clinical outcome in patients with chronic systolic heart failure in the beta-blocker era: the importance of immunoglobulin G3 subclass. J Card Fail 22, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo Y, McNamara DM, Alexis JD, Cooper LT, Dec GW, Pauly DF, Sheppard R, Starling RC, Tang WH, for the IMAC-2 investigators (2018). Myocardial recovery in patients with systolic heart failure and autoantibodies against beta1-adrenergic receptors. J Am Coll Cardiol 69, 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo Y, Yoshikawa T, Kohno T, Yoshizawa A, Baba A, Anzai T, Meguro T, Satoh T, Ogawa S (2009). A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail 15, 224–232. [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, et al. (2007). Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117, 2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port JD, Bristow MR (2001). Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol 33, 887–905. [DOI] [PubMed] [Google Scholar]
- Prakash A, Markham A (2000). Metoprolol: a review of its use in chronic heart failure. Drugs 60, 647–678. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ (2002). Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212. [DOI] [PubMed] [Google Scholar]
- Schur PH (1988). IgG subclasses. A historical perspective. Monogr Allergy 23, 1–11. [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ (2006). beta-Arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281, 1261–1273. [DOI] [PubMed] [Google Scholar]
- Steinberg SF (2018). beta1-Adrenergic receptor regulation revisited. Circ Res 123, 1199–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, Jahns R (2006). Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J 152, 697–704. [DOI] [PubMed] [Google Scholar]
- Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV (2011a). Regulation of beta-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle 10, 3684–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Naga Prasad SV (2011b). Inhibition of protein phosphatase 2A activity by PI3Kgamma regulates beta-adrenergic receptor function. Mol Cell 41, 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, Chandrasekharan UM, Young D, Feldman AM, Sen S, et al. (2013). Gbetagamma-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor alpha-induced cardiac beta-adrenergic receptor dysfunction. Circulation 128, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidarsson G, Dekkers G, Rispens T (2014). IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G (2002). The beta-adrenergic receptors. Herz 27, 683–690. [DOI] [PubMed] [Google Scholar]
- Wallukat G, Morwinski M, Kowal K, Forster A, Boewer V, Wollenberger A (1991). Autoantibodies against the beta-adrenergic receptor in human myocarditis and dilated cardiomyopathy: beta-adrenergic agonism without desensitization. Eur Heart J 12(Suppl D), 178–181. [DOI] [PubMed] [Google Scholar]
- Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S, Rockman HA (2017). Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nat Commun 8, 1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel K, Schulze-Rothe S, Muller J, Wallukat G, Haberland A (2018). Difference between beta1-adrenoceptor autoantibodies of human and animal origin—limitations detecting beta1-adrenoceptor autoantibodies using peptide based ELISA technology. PLoS One 13, e0192615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ (2007). A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA 104, 16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne J, Braunwald E (1988). The cardiomyopathies and myocarditides. In: Heart Disease, ed. E. Braunwald, Philadelphia, PA: WB Saunders, 1410–1457. [Google Scholar]
- Youker KA, Assad-Kottner C, Cordero-Reyes AM, Trevino AR, Flores-Arredondo JH, Barrios R, Fernandez-Sada E, Estep JD, Bhimaraj A, Torre-Amione G (2014). High proportion of patients with end-stage heart failure regardless of aetiology demonstrates anti-cardiac antibody deposition in failing myocardium: humoral activation, a potential contributor of disease progression. Eur Heart J 35, 1061–1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.