Abstract
Background
There are controversial suggestions about steroid use to treat patients infected with COVID-19. Conclusive evidence regarding the use of steroids to treat COVID-19 is still lacking. This meta-analysis aimed to determine the mortality and severity associated with corticosteroid therapy compared to noncorticosteroid treatment in patients with COVID-19.
Methods
The information was collected from electronic databases: PubMed, CINAHL, the Cochrane Library, clinicaltrials.gov, and Google scholar through January 30, 2021. Risk ratios (RRs) with 95% confidence intervals (CIs) were performed using random effect models. Endnote citation manager software version X9 for Windows was utilized to collect and organize search outcomes (into relevant and irrelevant studies) and to remove duplicate articles.
Results
Thirty-two studies were included in the meta-analysis, including 14,659 COVID-19 patients. No significant differences in mortality between the steroid and nonsteroid treatment groups (RR = 0.95; 95% CI: 0.80–1.13; p = 0.57). There was no significant reduction in mortality in critically ill COVID-19 patients treated with corticosteroid (RR = 0.89; 95% CI: 0.62–1.27; p = 0.52). Significant differences were observed in severe disease conditions between the steroid and nonsteroid treatment groups (RR = 1.10; 95% CI, 1.03–1.19, p = 0.007).
Conclusion
There was no significant difference in all-cause mortality between the steroid and nonsteroid treatment users' of COVID-19 patients. There was no significant reduction of all-cause mortality in critically ill COVID-19 patients treated with corticosteroids.
1. Background
Coronavirus disease-19 (COVID-19) was first identified at the end of 2019 in Wuhan City, China. It rapidly spread in China and other countries throughout the world [1, 2]. In March 2020, the World Health Organization characterized the disease as pandemic [3]. As of May 21, 2020, more than five million confirmed cases have been documented and several death cases reported globally. It is affecting 213 countries and territories around the world and 2 international conveyances [4]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is identified as the cause of COVID-19 [5].
Currently, there is no drug confirmed by clinical trial to prevent or treat COVID-19. However, more than 300 active clinical treatment trials are under investigation [6]. Drugs that are already marketed for other conditions are being off-label used. For example, antimalarial medications chloroquine and hydroxychloroquine are widely used to treat COVID-19 [7]. A multinational registry analysis debunked the benefit of hydroxychloroquine or chloroquine when used alone or with a macrolide. The finding of the study showed that each of these regimens was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for the treatment of COVID-19 [8]. Remdesivir is another drug being used as a promising option for treating COVID-19 based on laboratory experiments [9].
Corticosteroids were widely used to treat severe acute respiratory syndrome coronavirus-1(SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS) during their outbreaks and are being used in patients with COVID-2019 [10]. Up to 70% of critically ill patients are receiving systemic corticosteroids. It is identified that patients treated with a corticosteroid had more clinical symptoms, a higher inflammation index, and more abnormalities on chest computed tomography [11]. A study showed that the use of high-dose corticosteroids increases the risk of death in patients with severe COVID-19 [12], indicating several controversial issues about the use of the steroid to treat patients infected with COVID-19. It is suggested that available evidence does not endorse the use of a steroid for COVID-19 patients, which may cause several side effects [13]. However, it is believed that short-term glucocorticoid therapy with small- or medium-dose could be beneficial for patients with severe conditions [14]. According to the World Health Organization (WHO) guidelines recommendation, glucocorticoids should only be used under clinical trial conditions [15]. Conclusive evidence regarding the use of the steroid to treat COVID-19 is still lacking. Therefore, this study aims to summarize the current evidence of the severity and mortality associated with steroid therapy for patients with COVID-19 which will support us in making the best decision in the management of the COVID-19.
2. Methods
2.1. Study Design
Analyses were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. This study was registered in PROSPERO with registration number CRD42020185773.
2.2. Search Strategy
The information was collected from electronic databases: PubMed, CINAHL, the Cochrane Library, clinicaltrial.gov, and Google scholar. There was no limitation applied to the language. The reference list of all identified articles was searched for additional studies. Then, an extensive list of search terms was prepared by the analysis of title, abstract, and keywords of retrieved articles. A full-scale search of databases in PubMed, CINAHL, the Cochrane Central Register of Controlled Trials, clinicaltrial.gov, and Google scholar from inception to January 30, 2021, was performed. No language restriction was imposed during the identification of studies. Flow diagram was used to summarize the number of studies identified, screened, excluded, and finally included in the study.
The search terms used were ((“covid 19” [All Fields] OR “covid 19” [MeSH Terms] OR “covid 19 vaccines” [All Fields] OR “covid 19 vaccines” [MeSH Terms] OR “covid 19 serotherapy” [All Fields] OR “covid 19 serotherapy” [Supplementary Concept] OR “covid 19 nucleic acid testing” [All Fields] OR “covid 19 nucleic acid testing” [MeSH Terms] OR “covid 19 serological testing” [All Fields] OR “covid 19 serological testing” [MeSH Terms] OR “covid 19 testing” [All Fields] OR “covid 19 testing” [MeSH Terms] OR “sars cov 2” [All Fields] OR “sars cov 2” [MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2” [All Fields] OR “ncov” [All Fields] OR “2019 ncov” [All Fields] OR ((“coronavirus” [MeSH Terms] OR “coronavirus” [All Fields] OR “cov” [All Fields]) AND steroidal” [All Fields] OR “steroidals” [All Fields] OR “steroidic” [All Fields] OR “steroids” [MeSH Terms] OR “steroids” [All Fields] OR “steroid” [All Fields]) AND (“mortality” [MeSH Terms] OR “mortality” [All Fields] OR “mortalities” [All Fields] OR “mortality” [MeSH Subheading]) AND (“cohort studies” [MeSH Terms] OR (“cohort” [All Fields] AND “studies” [All Fields]) OR “cohort studies” [All Fields] OR (“cohort” [All Fields] AND “study” [All Fields]) OR “cohort study” [All Fields])) NOT (“review” [Publication Type] OR “review literature as topic” [MeSH Terms] OR “review” [All Fields]).
2.3. Study Selection
Two reviewers independently carried out a literature search and examined relevant studies and sequentially screened their titles and abstracts for eligibility. The full texts of potentially eligible studies were retrieved. Disagreements were solved in a discussion. A screening guide was used to ensure that all review authors reliably apply the selection criteria.
2.4. Inclusion and Exclusion Criteria
Randomized controlled trials (RCTs), observational studies, prospective and retrospective comparative cohort studies, and case-control studies were eligible for the review. The review considered all articles comparing corticosteroid with noncorticosteroid treatment for patients with the diagnosis of COVID-19. Nonhuman studies and studies that did not report mortality and severity data were excluded from the review. The outcome considered was mortality, death within the hospital (all-cause mortality) of COVID 19 patients. The other outcome studied was the number of severe cases in the two groups (corticosteroid versus noncorticosteroid treatment groups).
2.5. Methodological Quality Assessment
Selected papers were assessed by two independent reviewers for methodological validity before inclusion in the review. Observational studies were assessed using Newcastle–Ottawa Scale (NOS), which consists of three domains: (1) subject selection, (2) comparability of the study groups, and (3) assessment of outcome(s). A score of 0–9 was allocated to each study. A standardized Cochrane risk of bias tool was used for the randomized controlled trial (RCT). Any disagreements that arise between the reviewers were resolved through discussion.
2.6. Data Extraction
Two reviewers independently extracted data from the studies using a predesigned format prepared. Data that were extracted include first author, a region of study, included population, study design, sample size, comparator group, patient status, age, gender, interventions, mortality, and severity. Severe cases were characterized as patients admitted to ICU or on invasive mechanical ventilation.
2.7. Data Analysis and Statistical Methods
To perform a meta-analysis, Review Manager 5.4 (Copenhagen: The Cochrane Collaboration, 2014) was used. The outcome variables were calculated using the Mantel Haenszel formula. Risk ratios (RRs) were reported with 95% confidence intervals (CIs) for the variables. The p value was two-tailed, and the statistical significance was set at ≤0.05. Heterogeneity was assessed with the Q-statistic test and the I2 test. The I2 statistic measured the percentage of total variation across the studies due to clinical or methodological heterogeneity instead of chance. The significant Q statistics (p < 0.05) indicated heterogeneity across the studies; thus, a random effect model was utilized. Substantial heterogeneity was represented by I2 for >50%.
3. Results and Discussion
The search returned a total of 1419 (PubMed: 85, Google Scholar: 1290, clinicaltrials.gov: 24, Cochrane Library: 1, and other sources: 19) citations, of which 40 were duplicates. According to the exclusion criteria, 1292 citations were excluded after the title and abstract screening. Figure 1 shows the study selection processes.
Figure 1.
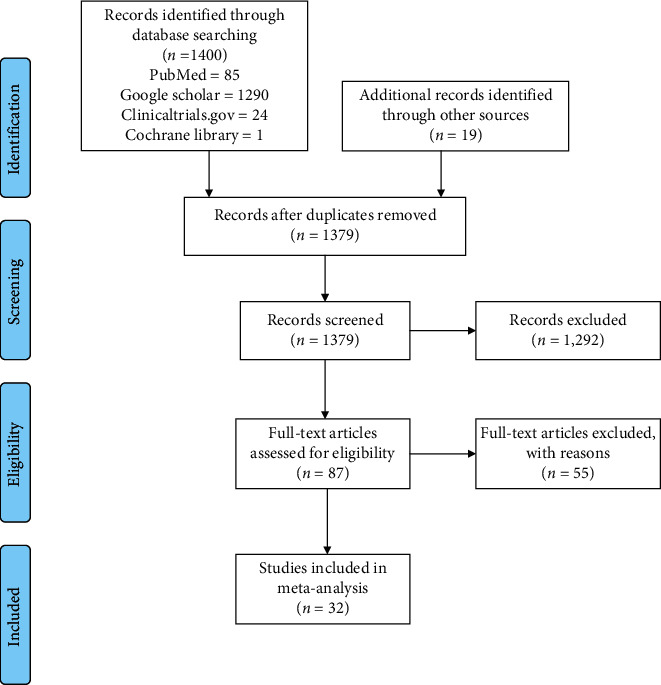
PRISMA study flow diagram.
3.1. Characteristics of the Included Studies
The present meta-analysis included 28 observational studies [12, 17–43] and 4 RCTs [44–47]. These studies included 14,659 patients with the diagnosis of COVID-19 who received corticosteroids (5,830 patients) or noncorticosteroids (8,829 patients). Tables 1 and 2 list the characteristics of the included studies. All eligible studies were published in 2020/21. Individual assessment of the risk of bias was presented in Table 3.
Table 1.
Characteristics of included studies.
| S.No | Authors | Study type | Sample size | Number of control | Patient status | Country | Follow-up period |
|---|---|---|---|---|---|---|---|
| 1 | Wang et al. [19] | Retrospective study | 46 | 20 | COVID-19 with pneumonia | Wuhan Union Hospital, China | January 20 to February 25, 2020 |
| 2 | Fadel et al. [17] | Multicenter quasi-experimental study | 213 | 81 | COVID-19 | Five hospitals in Michigan, USA | March 12, 2020 through March 27, 2020 |
| 3 | Wu et al. [21] | Retrospective cohort study | 201 | 139 | COVID-19 with pneumonia | Wuhan Jinyintan Hospital in China | December 25, 2019, to January 26, 2020 |
| 4 | Li et al. [12] | Ambispective cohort study | 548 | 207 | COVID-19 | Tongji Hospital, China | January 26, to March 3, 2020. |
| 5 | Zhou et al. [23] | Multicenter, retrospective cohort study | 191 | 134 | COVID-19 | Wuhan, China | Dec 29, 2019, to Jan 31, 2020 |
| 6 | Shang et al. [18] | Multicenter, retrospective, observational study | 416 | 220 | COVID-19 | Hubei province, China | Dec 27, 2019, to Feb 17, 2020 |
| 7 | Yang et al. [22] | Retrospective observational study | 52 | 22 | SARS-CoV-2 pneumonia | Wuhan, China | December, 2019, to Jan 26, 2020 |
| 8 | Huang et al. [24] | Prospective cohort study | 41 | 32 | COVID-19 | Wuhan, China | Dec 16, 2019, to Jan 2, 2020 |
| 9 | Guan et al. [27] | Retrospective cohort study | 1099 | 895 | COVID-19 | China | December 11, 2019, to January 31, 2020. |
| 10 | Zhao et al. [26] | Retrospective cohort study | 91 | 12 | COVID-19 | Jingzhou Central Hospital, China | January 16, 2020, to February 10, 2020. |
| 11 | Ling et al. [25] | Retrospective cohort study | 66 | 61 | COVID-19 | Shanghai, China | January 20, to February 10, 2020 |
| 12 | Horby et al. [44] | Randomized controlled trial | 6425 | 4321 | COVID-19 | United Kingdom | March 9, to June 8, 2020 |
| 13 | Angus et al. [45] | Randomized controlled trial | 384 | 101 | COVID-19 | REMAP-CAP multicenter (Australia, Canada, France, Ireland, The Netherlands, New Zealand, the United Kingdom, and the United States) | March 9 to August 12, 2020 |
| 14 | Borie et al. [28] | Cohort study | 171 | 63 | COVID-19 | Paris | March 27 to April 10, 2020 |
| 15 | Dequin et al. [46] | Randomized controlled trial | 149 | 73 | Critically Ill patients with COVID-19 | France | March 7 to June 29, 2020 |
| 16 | Falcone et al. [43] | Prospective observational study | 315 | 174 | COVID-19 and pneumonia | University Hospital of Pisa | March 4–April 30, 2020 |
| 17 | Fernández-Cruz et al. [29] | Retrospective controlled cohort study | 463 | 67 | COVID-19 and pneumonia | Spain | 4 March 2020 to 7 April 2020 |
| 18 | Jeronimo et al. [47] | Randomized controlled trial | 393 | 199 | COVID-19 | Brazil | 18 April to 16 June 2020 |
| 19 | Krishnan et al. [30] | Retrospective observational study | 152 | 136 | COVID-19 and pneumonia | USA | March 10, to April 15, 2020 |
| 20 | Li et al. 2020 [31] | Multicenter, retrospective study | 294 | 111 | Critically ill COVID-19 patients | Hubei, China | Between December 30, 2019 and February 19, 2020 |
| 21 | Papamanoli et al. [32] | Retrospective cohort | 447 | 294 | severe COVID-19 pneumonia | New York, USA | 1 March to 15 April 2020 |
| 22 | Tomazini et al. [48] | Randomized controlled trial | 299 | 148 | Acute respiratory distress syndrome and COVID-19 | Brazil | April 17 to July 21, 2020 |
| 23 | You et al. [33] | Retrospective cohort study | 343 | 225 | COVID-19 | China | February 1 to March 31, 2020 |
| 24 | Rodríguez-Baño et al. [34] | Retrospective cohort study | 778 | 583 | COVID-19 | Spain | February 2 to March 31, 2020 |
| 25 | Ma et al. [35] | Multicenter retrospective cohort study | 72 | 25 | COVID-19 | China | January 2020 to March 2020 |
| 26 | Lu et al. [36] | Retrospective cohort study | 62 | 31 | Critically ill COVID-19 | China | January 25 to February 25, 2020 |
| 27 | Cao et al. [37] | Retrospective cohort study | 102 | 51 | COVID-19 | China | January 3 and February 1, 2020 |
| 28 | Nelson et al. [38] | Retrospective cohort study | 117 | 69 | COVID-19 pneumonia | USA | March 1, 2020 and April 12, 2020 |
| 29 | Bani-Sadr et al. [39] | Prospective cohort study | 257 | 85 | COVID-19 pneumonia | France | 3 March 2020 and 14 April 2020 |
| 30 | Salton et al. [40] | Multicenter observational study | 173 | 90 | severe COVID-19 pneumonia | Italy | February 27 to May 21, 2020 |
| 31 | Mikulska et al. [41] | Observational single-center study | 196 | 66 | COVID-19 pneumonia | Italy | NR |
| 32 | Majmundar et al. [42] | Retrospective cohort study | 205 | 145 | COVID-19 pneumonia | USA | March 15 to April 30, 2020 |
Table 2.
Characteristics of included studies.
| S. No | Authors | Median age (IQR) in years | Gender | Intervention | No. of patients | |||
|---|---|---|---|---|---|---|---|---|
| Mortality | Severe cases | |||||||
| Control group | Intervention group | Control group | Intervention group | |||||
| 1 | Wang et al. [19] | 54 (48–64) | 26 (57%) males | Methylprednisolone (n = 26) | 1 | 2 | NR | NR |
| 2 | Fadel et al. [17] | 62 (51–62) | 109 (51.2%) male | Methylprednisolone (n = 132) | 21 | 18 | 21 | 27 |
| 3 | Wu et al. [21] | 51 (43–60) | 128 (63.7%) men | Methylprednisolone (n = 62) | 21 | 23 | NR | NR |
| 4 | Li et al. [12] | 60 (48–69) | 279 (50.9%) male | Systemic corticosteroids (n = 341) | NR | NR | 73 | 196 |
| 5 | Zhou et al. [23] | 56 (46–67) | Male 119 (62%) | Corticosteroids (n = 57) | 28 | 26 | NR | NR |
| 6 | Shang et al. [18] | 49 (36–61) | 197 (47%) males | Corticosteroid therapy (n = 196) | 8 | 43 | 62 | 77 |
| 7 | Yang et al. [22] | 59·7 | 35 (67%) males | Glucocorticoids (n = 30) | 16 | 16 | 22 | 30 |
| 8 | Huang et al. [24] | 49 (41–58) years | 30 [73%] males | Use of corticosteroid (n = 9) | NR | NR | 7 | 6 |
| 9 | Guan et al. [27] | 47 years | 639 males | Systemic glucocorticoids(n = 204) | 10 | 5 | 96 | 77 |
| 10 | Zhao et al. [26] | 46 years | 49 males | Glucocorticoid (n = 79) | 1 | 1 | 5 | 25 |
| 11 | Ling et al. [25] | 44 (34–62) years | 38 males | Glucocorticoid (n = 5) | 0 | 0 | NR | NR |
| 12 | Horby et al. [44] | 66.1 years | 4088 males | Dexamethasone (n = 2104) | 1065 | 454 | 683 | 324 |
| 13 | Angus et al. [45] | Mean age, 60 years | 29% female | Hydrocortisone (n = 283) | 33 | 78 | 101 | 283 |
| 14 | Borie et al. [28] | Median (IQR): 67.1 (56.7–78.1) | Female 48 (28.1%) | Methyl-prednisolone (n = 108) | 25 | 32 | 63 | 108 |
| 15 | Dequin et al. [46] | Mean age, 62.2 years | 30.2% women | Hydrocortisone (n = 76) | 20 | 11 | 73 | 76 |
| 16 | Falcone et al. [43] | Median age was 70 (IQR, 57–80) | (76.2%) males | Steroids (n = 141) | 43 | 27 | NR | NR |
| 17 | Fernández-Cruz et al. [29] | Mean age 66.75 years | 317 males | Steroids (n = 396) | 16 | 55 | 0 | 58 |
| 18 | Jeronimo et al. [47] | Mean age (SD) 55 ± 15 yrs | 139 females | Methylprednisolone (n = 194) | 76 | 72 | NR | NR |
| 19 | Krishnan et al. [30] | 68 years (IQR 58–75) | 95 males | Oral steroids n = 16 | 82 | 10 | 136 | 16 |
| 20 | Li et al. [31] | 66 yrs (56–75) | 197 (67%) males | Corticosteroids, n = 183 | 49 | 97 | 111 | 183 |
| 21 | Papamanoli et al. [32] | Mean age 61.5 yrs | Females 156 | Methylprednisolone, n = 153 | 146 | 71 | 294 | 153 |
| 22 | Tomazini et al. [48] | Mean age 61.4 yrs | Females 112 | Dexamethasone (n = 151) | 91 | 85 | 148 | 151 |
| 23 | You et al. [33] | Mean age 53.8 | Female 157 | Methylprednisolone (n = 118) | 1 | 14 | 9 | 58 |
| 24 | Rodríguez-Baño et al. [34] | Age 71 yrs | Female 226 | Corticosteroids (n = 195) | 62 | 30 | NR | NR |
| 25 | Ma et al. [35] | Age 60 (13.8) yrs | Female 32 (44%) | Corticosteroid group (n = 47) | 2 | 2 | 25 | 47 |
| 26 | Lu et al. [36] | 57 (50–69) yrs | Male 32 | Steroid (n = 31) | 5 | 12 | 31 | 31 |
| 27 | Cao et al. [37] | Age, years 54(37–67) | Female 49 | Methylprednisolone Sodium (n = 51) | 6 | 11 | NR | NR |
| 28 | Nelson et al. [38] | Age 61.5 (46–69) | Male 80 | Methylprednisolone n = 48 | 29 | 15 | 69 | 48 |
| 29 | Bani-Sadr et al. [39] | Age 71 yrs | Male 135 | Corticosteroids n = 172 | 17 | 31 | 12 | 9 |
| 30 | Salton et al. [40] | Age 65.75 yrs | Male 120 | Methylprednisolone (n = 83) | 21 | 6 | 90 | 83 |
| 31 | Mikulska et al. [41] | Age mean 67.5 yrs | Male 132 | Methylprednisolone (n = 130) | 22 | 14 | NR | NR |
| 32 | Majmundar et al. [42] | Age, mean 57.61 | Male 153 | Corticosteroids (N = 60) | 34 | 8 | 0 | 0 |
NR: not reported.
Table 3.
Methodological quality assessment.
| Newcastle–Ottawa scale (NOS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Selection | Comparability | Outcome | Quality score | |||||
| A | B | c | d | e | f | g | h | ||
| Fadel et al. [17] | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 8 | |
| Guan et al. [27] | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Huang et al. [24] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Li et al. [12] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Ling et al. [25] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Shang et al. [18] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Yang et al. [22] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Wang et al. [19] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Wu et al. [21] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Zhao et al. [26] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Zhou et al. [23] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Borie et al. [28] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Falcone et al. [43] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Fernández-Cruz et al. [29] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Krishnan et al. [30] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Li et al. [31] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Papamanoli et al. [32] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| You et al. [33] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Rodríguez-Baño et al. [34] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Ma, Q et al. [35] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Lu et al. [36] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Cao et al. [37] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Nelson et al. [38] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Bani-Sadr et al. [39] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Salton et al. [40] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Mikulska et al. [41] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Majmundar et al. [42] | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
|
| |||||||||
| Cochrane risk of bias tool | |||||||||
| I | J | k | l | m | n | ||||
| Horby et al. [44] | Low risk of bias | Low risk of bias | High risk of bias | Unclear risk of bias | Low risk of bias | Low risk of bias | |||
| Angus et al. [45] | Low risk of bias | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | |||
| Dequin et al. [46] | Low risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | |||
| Jeronimo et al. [47] | Low risk of bias | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | |||
a: representativeness of the exposed cohort, b: selection of the nonexposed cohort, c: ascertainment of exposure, d: demonstration that the outcome of interest was not present at the start of the study, e: comparability of cohorts based on the design or analysis, f: assessment of outcome, g: follow-up long enough for outcomes to occur, h: adequacy of follow-up of the cohort; i: random sequence generation (selection bias), j: allocation concealment (selection bias), k: blinding of participants and personnel (performance bias), l: blinding of outcome assessment (detection bias), m: incomplete outcome data (attrition bias), n: selective reporting (reporting bias).
3.2. Mortality Associated with Steroid Use in Patients with COVID-19
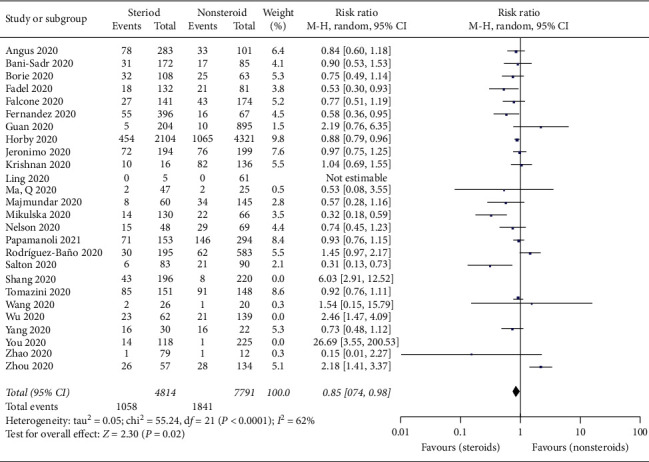
In the 26 included studies [17–19, 21–23, 25–30, 32–35, 37–42, 44, 45, 47, 48] with 13,565 patients, there were no significant differences in mortality between the steroid and nonsteroid treatment groups (RR = 0.95; 95% CI: 0.80–1.13; p = 0.57, I2 = 78%, p < 0.0001) (Figure 2). The sensitivity analysis showed that the exclusion of three studies [18, 21, 33] changed the above conclusion.
Figure 2.
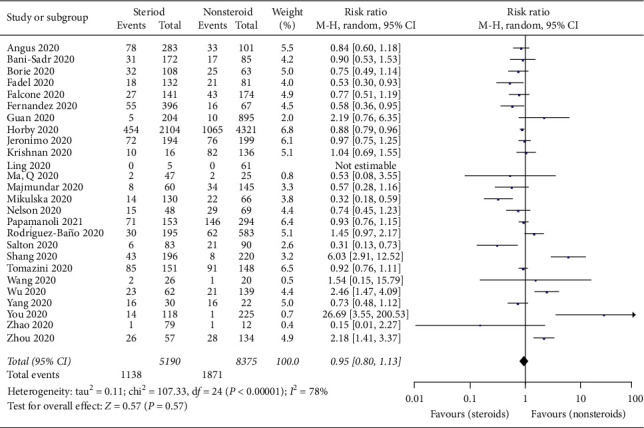
Forest plot for mortality of COVID-19 patients taking steroids versus nonsteroids.
3.3. Mortality Associated with Steroid Use in Patients with Critically Ill COVID-19 Patients
In the 5 included studies [22, 31, 36, 44, 46] with 1,564 critically ill COVID-19 patients, there were no significant differences in mortality between the steroid and nonsteroid treatment groups (RR = 0.89; 95% CI: 0.62–1.27; p = 0.52, I2 = 78%, p = 0.001) (Figure 3).
Figure 3.
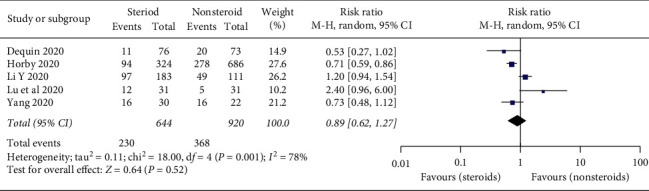
Forest plot for mortality associated with steroid use in patients with critically ill COVID-19 patients.
3.4. Severity Associated with Steroid Use in Patients with COVID-19
Twenty-three studies reported the severity data of the COVID-19 patients. In the included studies [12, 17, 18, 22, 24, 26–33, 35, 36, 38–40, 42, 44–46, 48] with 12,473 patients, there were significant differences in severe disease condition between the steroid and nonsteroid treatment groups (RR = 1.10; 95% CI, 1.03–1.19, p = 0.007) (Figure 4). There was significant heterogeneity among the studies (I2 = 99% p < 0.001); the random-effects model was used.
Figure 4.
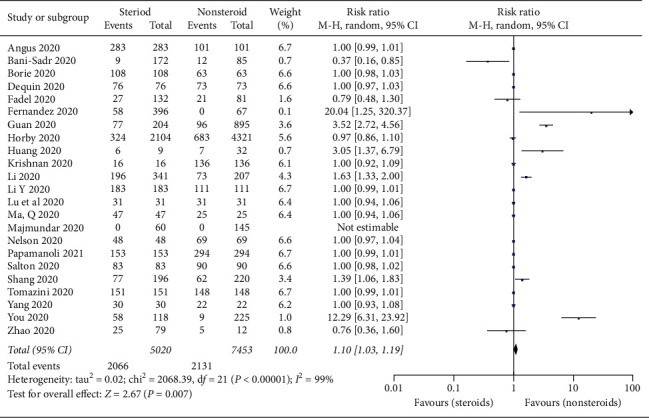
Forest plot for severe events of COVID-19 patients taking steroids versus nonsteroids.
3.5. Publication Bias
To assess the small-study effect and publication bias, the regression-based Egger's test was performed. The funnel-plot analysis showed a symmetrical shape for mortality (Figure 5), indicating no publication bias; Egger's test indicated nonsignificant small-study effects (p = 0.570). The funnel-plot analysis showed asymmetrical shape for severe cases (Figure 6), indicating publication bias; Egger's test indicated significant small-study effects for severe cases (p = 0.027).
Figure 5.
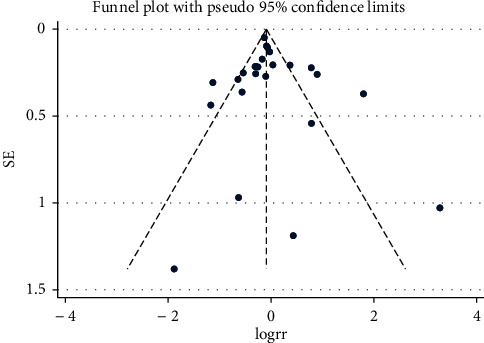
Funnel plot for mortality.
Figure 6.
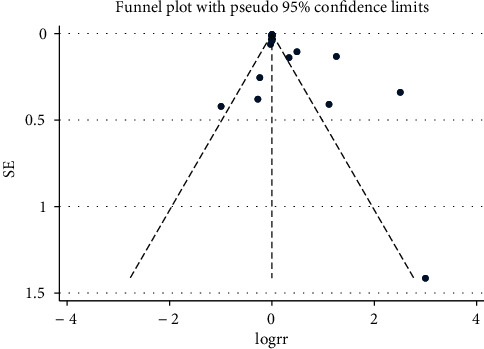
Funnel plot for severe cases.
3.6. Sensitivity Analysis
Sensitivity analysis for between-study heterogeneity and analytical methods was performed to show the robustness of the finding. The analysis showed that the result was stable except for the exclusion of three studies [18, 21, 33] that changed the conclusion (Figure 7).
Figure 7.
Sensitivity analysis: forest plot for mortality of COVID-19 patients taking steroids versus nonsteroids.
The rationale for the use of corticosteroids is to decrease the host inflammatory responses in the lungs, which may lead to acute lung injury and acute respiratory distress syndrome [6]. Because of the high amount of cytokines induced by COVID-19 infection, corticosteroids were used frequently for the treatment of patients with severe illness, for possible benefit by reducing inflammatory-induced lung injury [24]. There were potential harms and a lack of proven benefit for corticosteroid cautions against their routine use in patients with COVID-19 [6].
Our analysis demonstrated that the mortality rate was comparable in COVID-19 patients treated with corticosteroids versus noncorticosteroids. However, in patients with critically ill COVID-19 patients, even though nonsignificant (RR = 0.89; 95% CI: 0.62–1.27; p = 0.52), a lower mortality rate in corticosteroid treatment groups was observed. Evidence suggests that cytokine storm, a hyperinflammatory state resembling secondary hemophagocytic lymphohistiocytosis (HLH), is a contributing factor in COVID-19-associated mortality [49].
In our finding, there were no significant differences in mortality between the corticosteroid and noncorticosteroid treatment groups in critically ill COVID-19 patients. The initial clinical trial results from the United Kingdom (UK) showed that dexamethasone, a corticosteroid, can be lifesaving for patients who are critically ill with COVID-19. For patients on ventilators, the treatment was shown to reduce mortality by about one-third, and for patients requiring only oxygen, mortality was cut by about one-fifth, according to preliminary findings shared with the World Health Organization (WHO) [50].
The most commonly invoked rationale for giving steroids in patients with severe COVID-19 is to modulate the destructive inflammatory immune response that occurs with advancing disease. The guidelines recommend against using corticosteroids to try to modulate the immune system in mechanically ventilated COVID-19 patients without acute respiratory distress syndrome (ARDS) but do recommend steroids in those with COVID-19 and ARDS [51]. Our finding demonstrates that there is an association between severe COVID-19 and corticosteroid therapy (RR = 1.10; 95% CI, 1.03–1.19, p = 0.007).
A previous meta-analysis in persons with SARS-CoV-2, SARS-CoV, or MERS-CoV (Middle East respiratory syndrome coronavirus) infection indicated that corticosteroid did not significantly reduce the risk of death [52]. Another meta-analysis that included 5 studies indicated a lack of benefit of corticosteroid therapy on mortality in critically ill patients with COVID-19 [53]. In addition, a review that included 15 studies with coronavirus-infected patients (2 studies with COVID-19 patients) showed that corticosteroid treatment was associated with higher mortality and critical patients were more likely to require corticosteroids therapy [54].
Some of the limitations of this meta-analysis are as follows: the included studies are retrospective cohort studies, there is a high degree of between-study heterogeneity, and mortality may be influenced by other therapeutic options.
4. Conclusion
No significant differences in mortality between the corticosteroid and noncorticosteroid treatment groups were observed. There was no significant reduction in mortality in critically ill COVID-19 patients treated with corticosteroids. More randomized clinical trials are needed to further verify this conclusion.
Abbreviations
- ARDS:
Acute respiratory distress syndrome
- CI:
Confidence interval
- HLH:
Hemophagocytic lymphohistiocytosis
- ICU:
Intensive care unit
- MERS-CoV:
Middle east respiratory syndrome coronavirus
- NOS:
Newcastle–Ottawa scale
- PRISMA:
Preferred reporting items for systematic reviews and meta-analyses
- RCT:
Randomized controlled trial
- RR:
Risk ratio
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus 2
- UK:
United Kingdom
- WHO:
World Health Organization.
Data Availability
The data presented are properly cited and can be obtained from already published original research articles, which are available on electronic databases.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Guan W.-J, Ni Z.-Y, Hu Y., et al. Clinical characteristics of 2019 novel coronavirus infection in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization; Coronavirus Disease, May 2020, https://www.who.int.
- 4. COVID-19 Coronavirus Pandemic, April 2020, https://www.worldometers.info/coronavirus/
- 5.Rubin S. J., Falkson S. R., Degner N. R., Blish C. Clinical characteristics associated with COVID-19 severity in California. Journal of Clinical and Translational Science. 2020;5(1):1–4. doi: 10.1017/cts.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders J. M., Monogue M. L., Jodlowski T. Z., Cutrell J. B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 7.Rome B. N., Avorn J. Drug evaluation during the COVID-19 pandemic. New England Journal of Medicine. 2020;382(24):2282–2284. doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
- 8.Mehra M. R., Desai S. S., Ruschitzka F., Patel A. N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020;395(10240):p. 1820. doi: 10.1016/s0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z., Wang X., Cao D., Sun R., Li C., Li G. Rapid review for the anti-coronavirus effect of remdesivir. Drug Discoveries & Therapeutics. 2020;14(2):73–76. doi: 10.5582/ddt.2020.01015. [DOI] [PubMed] [Google Scholar]
- 10.Russell C. D., Millar J. E., Baillie J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/s0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zha L., Li S., Pan L., Tefsen B., Li Y., French N. Impact of corticosteroid treatment in patients with coronavirus disease 2019. Medical Journal of Australia. 2020;1 doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Xu S., Yu M., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. The Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu S., Zhou Q., Huang L., et al. Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis. Annals of Translational Medicine. 2020;8(10):p. 627. doi: 10.21037/atm-20-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease Is Suspected, World Health Organization, March 2020, https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf.
- 16.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadel R., Morrison A., Vahia A., et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clinical Infectious Diseases. 2020;71(16):2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang J., Du R., Lu Q., et al. The treatment and outcomes of patients with COVID-19 in Hubei, China: a multi-centered, retrospective, observational study. Lancet. 2020 [Google Scholar]
- 19.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. 2020.
- 20.Yam L. Y.-C., Lau A. C.-W., Lai F. Y.-L., et al. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. Journal of Infection. 2007;54(1):28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling Y., Xu S.-B., Lin Y.-X., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chinese Medical Journal. 2020;133(9):1039–1043. doi: 10.1097/cm9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X.-Y., Xu X.-X., Yin H.-S., et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infectious Diseases. 2020;20:1–8. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.-J., Ni Z.-Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borie R., Savale L., Dossier A., et al. Glucocorticoids with low-dose anti-IL1 anakinra rescue in severe non-ICU COVID-19 infection: a cohort study. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243961.e0243961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Cruz A., Ruiz-Antorán B., Muñoz-Gómez A., et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrobial Agents and Chemotherapy. 2020;64(9) doi: 10.1128/aac.01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan S., Patel K., Desai R., et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. Journal of Clinical Anesthesia. 2020;67 doi: 10.1016/j.jclinane.2020.110005.110005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Meng Q., Rao X., et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Critical Care (London, England) 2020;24(1):p. 698. doi: 10.1186/s13054-020-03429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papamanoli A., Yoo J., Grewal P., et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. European Journal of Clinical Investigation. 2021;51(2) doi: 10.1111/eci.13458.e13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You X., Wu C. H., Fu Y. N., et al. The use of methylprednisolone in COVID-19 patients: a propensity score matched retrospective cohort study. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244128.e0244128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Baño J., Pachón J., Carratalà J., et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021;27(2):244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Q., Qi D., Deng X. Y., et al. Corticosteroid therapy for patients with severe novel Coronavirus disease 2019. European Review for Medical and Pharmacological Sciences. 2020;24(15):8194–8201. doi: 10.26355/eurrev_202008_22508. [DOI] [PubMed] [Google Scholar]
- 36.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Critical Care (London, England) 2020;24(1):p. 241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao J., Tu W.-J., Cheng W., et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clinical Infectious Diseases. 2020;71(15):748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson B. C., Laracy J., Shoucri S., et al. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bani-Sadr F., Hentzien M., Pascard M., et al. Corticosteroid therapy for patients with COVID-19 pneumonia: a before-after study. International Journal of Antimicrobial Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106077.106077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salton F., Confalonieri P., Meduri G. U., et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infectious Diseases. 2020;7(10) doi: 10.1093/ofid/ofaa421.ofaa421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikulska M., Nicolini L. A., Signori A., et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237831.e0237831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majmundar M., Kansara T., Lenik J. M., et al. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238827.e0238827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falcone M., Tiseo G., Barbieri G., et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infectious Diseases. 2020;7(12) doi: 10.1093/ofid/ofaa563.ofaa563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horby P., Lim W. S., Emberson J., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. 2020.
- 45.Angus D. C., Derde L., Al-Beidh F., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dequin P.-F., Heming N., Meziani F., et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeronimo C. M. P., Farias M. E. L., Val F. F. A., et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomazini B. M., Maia I. S., Cavalcanti A. B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Assessment of Evidence for COVID-19-Related Treatments, June 2020, https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx.
- 50. World Health Organization, WHO Welcomes Preliminary Results about Dexamethasone Use in Treating Critically Ill COVID-19 Patients, June 2020, https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients.
- 51.Bruce J. Despite guidelines, controversy remains over corticosteroids in COVID-19. June 2020. https://www.medscape.com/viewarticle/931505.
- 52.Li H., Chen C., Hu F., et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–1511. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gangopadhyay K. K., Mukherjee J., Sinha B., Ghosal S. The role of corticosteroids in the management of critically ill patients with coronavirus disease 2019 (COVID-19): a meta-analysis. 2020. [DOI]
- 54.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. Journal of Infection. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented are properly cited and can be obtained from already published original research articles, which are available on electronic databases.


