Abstract
Improving the health status of people with chronic kidney disease (CKD) through physical activity (PA) or exercise interventions is challenging. One of the gaps in the process of translating the general public PA activity guidelines as well as the CKD-specific guidelines into routine clinical practice is the lack of systematic recording and monitoring of PA and physical function attributes, which can also be used to develop individualized and measurable plans of action to promote PA for health. We aim to present an overview of key considerations for PA, physical function and health-related quality of life (HRQoL) evaluation in people with CKD, with the aim of encouraging health professionals to integrate assessment of these outcomes in routine practices. Physical inactivity and impaired physical function, sometimes to the extent of physical and social disability levels, and subsequently lower perceived HRQoL, are highly prevalent in this population. Enhanced PA is associated with better physical function that also translates into multiple health benefits. Breaking the vicious circle of inactivity and physical dysfunction as early as possible in the disease trajectory may confer huge benefits and enhanced life satisfaction in the longer term. With this in mind, the importance of PA/exercise interventions in CKD to improve HRQoL is also summarized.
Keywords: chronic kidney disease, exercise, health-related quality of life, physical activity, physical functioning
PHYSICAL INACTIVITY HAS AN IMPORTANT NEGATIVE IMPACT FOR PATIENTS
Physical activity (PA) is any body movement produced by muscles that results in increased energy expenditure. Exercise is a subset of PA that is planned, structured, repetitive and purposeful. PA is a generic term, of which exercise is the major component [1]. The PA level is lower in chronic kidney disease (CKD) patients at any stage compared with healthy counterparts [2]. Both peritoneal dialysis (PD) and haemodialysis (HD) patients have been found to be sedentary, with HD patients having fewer steps measured by a pedometer, with half of the steps on HD days compared with PD patients [3]. A study reported that the duration of PA of HD patients per day was 42.7 min and the number of steps was ˂3950 [4, 5]. Another study showed how sedentary HD patients are, showing that patients were in a sitting or reclining posture 73.7 ± 12.9% of the day, so almost 18 h/day [6, 7].
Several studies have found associations between the amount of PA and survival in patients on HD. One study found superior survival in patients with greater PA time [4]. Similarly, another study concluded that mortality risk was lower for regular (≥1 time/week) versus non-regular (<1 time/week) exercisers [8]. Hishii et al. [7] found that sedentary behaviour (relative time that subjects were in a sitting or reclining posture according to accelerometer data) was an important factor for all-cause mortality, especially as measured over the total days or the non-dialysis days (dialysis is per se a treatment that requires a sitting or reclining posture).
Literature also supports that PA has a positive impact on health-related quality of life (HRQoL). The number of steps measured with a pedometer presented a significant inverse correlation with the level of distress caused by one’s perceived bodily dysfunction, and distress was significantly higher in patients with lower PA [5]. Hishii et al. [6, 7] found that patient’s sedentary behaviour was negatively associated with HRQoL and that there was a clinical impact of sedentary behaviour on HRQoL even after adjusting for confounding factors such as sex, duration of HD, age and history of diabetes mellitus.
It seems that the PA level also has an impact on physical function measured through physical performance tests (PPTs), as a study showed that patients on HD or PD with impaired PA measured through the adjusted activity score (AAS) of the Human Activity Profile (HAP) scale (<53 points) had worse physical function (walking capacity measured with the 6-min walking test, gait speed in 4 m, sit-to-stand-to-sit capacity, functional mobility as measured through the short physical performance battery) compared with patients who were moderately active or active (53–94 points) [9].
WHAT MEASUREMENT TOOLS TO MEASURE PHYSICAL ACTIVITY?
The literature describes different methods of measuring PA. A measurement tool used to measure patients’ habitual PA is the uniaxial accelerometer [4]. An accelerometer is a device that obtains objective information on PA patterns as it can continuously measure the intensity, duration and frequency of activities. The vector magnitude in the vertical direction is divided into different grades of 0, 0.5 or 1–9, with each grade reflecting the intensity of the PA. Grades 1–9 represent activities ranging from gentle walking to running [4]. Other articles have measured activity with a three-dimensional accelerometer and data were expressed as a net vector magnitude of the acceleration in the three axes. The vector’s magnitude was summed up over 7 days and expressed as a daily average (arbitrary units) [2, 10]. Hishii et al. [6, 7] reported that participants wore the device for 2 weeks and they defined sedentary behaviour as energy expenditure at ≤1.5 metabolic equivalents (METs) in a sitting or reclining posture.
Another PA measurement tool is the pedometer, which was used from 6 or 7 days [3, 5, 11] to 1 month [12] in dialysis patients to measure the mean number of steps per day.
Self-reported PA is another method. PA questionnaires are commonly used to assess activity in the general population, as they are inexpensive and simple to administer, and to promote PA in HD patients, a simple and effective questionnaire is greatly needed [5]. In patients on dialysis, questionnaires are correlated with accelerometry (gold standard) and therefore are valid tools to measure PA level [10]. Three questionnaires were chosen in a validity study because they had been previously validated among older persons or persons with CKD and could be completed in ≤20 min. The Standford 7-day PA Recall (PAR) Questionnaire is a self-reported questionnaire that collects information about the time spent performing various levels of activity during the previous 7 days [13]. A MET value is assigned to sleep and four levels of PA (light, moderate, hard and very hard). Caloric expenditure is estimated from the MET values and PA estimated with this instrument is reported as kcal/kg/day. The PA Scale for the Elderly (PASE) is designed to measure activity during the previous week based on the participants’ responses to a series of questions [14]. The HAP [15] is a self-administered scale designed to survey participation in common physical activities across a broad range of energy requirements. It comprises a list of 94 daily activities, with the AAS being more commonly used to report PA level. The AAS classifies patients as having an impaired PA level (<53 points), moderately active (53–74 points) or active (>74 points). The HAP correlated better than the PASE with accelerometry [10]. One study found that in Stage 3–5 CKD (patients with end-stage renal disease on HD), the AAS correlated better than accelerometry with physical performance [2].
The International PA Questionnaire (IPAQ) score is converted to a MET (min/day of PA). A recent study reported the validity of the IPAQ-Chinese version (IPAQ-C; short version with nine items) by correlating results with pedometry data [5]. The authors found a moderate correlation between the data, but when stratified by sex, the correlation was only found for male patients.
As a single tool, we would recommend the HAP (AAS) for dialysis patients, as it is inexpensive and simple to administer and it has proved to discriminate patients with worse physical function [9].
HOW MUCH PHYSICAL ACTIVITY?
The literature reports how much PA is suggested for patients, as measured by different tools. A prospective observational study followed a sample of 202 Japanese HD patients for 7 years. A multivariable analysis was performed to estimate the independent prognostic effect of PA time (analysed through accelerometry) on survival after adjustment for confounders (age, comorbidity, among others). Patients were categorized into two PA groups by a PA cut-off value of 50 min/day, since this value predicted whether the HD patients could reach the gait speed obtained by healthy counterparts [4]. They concluded that younger age and a lower comorbidity score were found in more active patients and they found superior survival in patients with ≥50 min of PA time per day. Regarding the pedometer, Cobo et al. [3] considered sedentary those with ˂5000 steps/day as a general threshold applicable to the adult population, so the recommendation for patients on dialysis treatment would be to walk above this threshold. Another easy approach is to measure PA through a self-administered questionnaire on physical exercise frequency, answering the question ‘How often do you exercise or do physical activity during your leisure time?’. The recommended answer would be to exercise at least once a week, since a study reported that mortality risk was lower for ‘regular’ (≥1 time/week) versus ‘non-regular’ (<1 time/week or never) exercisers [8]. This study also concluded that mortality risk tended to decrease as exercise frequency increased (1 time/week versus 6–7 times/week). Regarding the HAP (AAS), the threshold for any patient undertaking dialysis treatment would be to reach at least 53 points (moderately active) [9], since a recent cross-sectional study showed that patients with ˂53 points showed impaired functional capacity as measured with commonly used physical function tests.
Table 1 summarizes the main measurement tools and the minimal amount of PA reported and the time frame reported in the literature. These suggestions must be taken cautiously and future meta-analysis from randomized controlled trials (RCTs) is needed to validate them.
Table 1.
Indicative suggestions for PA amount and measurement tools
| Measurement tools | Time frame | Minimal amount |
|---|---|---|
| Diaries/self reports | ||
| Self-report questionnaires | ||
| HAP average activity score [9] | One single session | 53 points |
| PASE [10, 14] | One single session |
|
| IPAQ-C total PA [5] | One single session |
|
| PAR [16] | One single session | 38.4 (5.8) SD |
| Frequency of exercise or PA [8] | One single session | Once a week |
| Devices | ||
| Accelerometer uniaxial or 3D [2, 7] | 1–2 weeks | 50 min of PA/day |
| Pedometer [3, 11, 12] | 6–30 days | 5000 steps/day |
The above suggestions include different tools that could be used to measure PA according to the literature. Questionnaires are simple and cheap, and devices are objective measures. A single measurement is enough to report the PA level.
ASSESSMENT OF PHYSICAL FUNCTION FOR PEOPLE WITH CKD
A framework for physical function assessment
According to the World Health Organization, health is defined as not just the absence of disease, but the harmonious balance between physical, mental and social dimensions of life. The physical aspect of health can be characterized and assessed by ‘symptoms’ experienced, such as pain, fatigue, breathlessness and stiffness, and by ‘physical function indicators’, such as level of participation in PA and one’s perceived ability or measured capacity in carrying out various physical tasks ranging from self-care [activities of daily living (ADL)] to more challenging and vigorous activities that require increasing degrees of mobility, balance, strength or endurance. An adequate level of physical function is therefore essential for independent living. This applies to all people but becomes even more important in people with CKD, in whom the effects of underlying disease pathophysiology, accelerated ageing and physical inactivity frequently result in levels of impairment, functional limitations and/or disability that negatively impact on all aspects of life and ultimately survival [17].
In the interests of standardizing physical function-related research and practice, we recommend the adoption of the International Classification Framework of Functioning, Disability and Health’s classifications and terminology for the description of physical functioning [18]. This approach advocates that physical function assessments should be ‘grouped’ (and reported) according to their ability to describe physiological impairment at the level of body structures and functions (often achieved in relation to physical function via exercise tolerance testing); function limitations of the individual at the level of activities and primarily described via objective PPTs and disability (participation) experiences of the individual within their sociocultural and environmental context (largely via self-reported functional status assessments).
Most researchers and health practitioners who manage people with CKD would agree that no matter what domain/dimension of physical function one measures, the overall consensus is the same: physical dysfunction and inactivity are severe and prevalent in all ages and CKD stages compared with normative data. Deterioration of physical function starts early in the disease process [19] and may result in the rapid onset of severe physical frailty and disability, especially in the elderly CKD Stage 5 population [20].
Physical function indicators are increasingly recognized as a key characteristic of the clinical picture and life profile of CKD sufferers and have been shown to be strong determinants of clinically important but also patient-relevant outcomes of mortality [17], morbidity, hospitalizations and life participation (https://songinitiative.org/projects/). Yet physical function assessment does not form part of the routine clinical monitoring and evaluation of people with CKD. That this situation persists in 2020 is surprising, as we believe a case already exists for the regular assessment and promotion of physically active lifestyles to enhance clinical management of the CKD patient [21, 22]. Therefore the utility of physical function assessment in CKD can be summarized as follows:
people with CKD Stages 3–5 with better scores in measures of physiological impairment [maximal oxygen consumption (VO2max), muscle strength], functional limitations (gait speed, sit-to-stand performance, PA levels) and in patient-reported disability outcomes (ADL) are characterized by longer event-free survival, better mental health, less frequent hospitalizations and fewer functional limitations. A VO2max >17.5 mL/kg/min predicted significantly longer survival over 3.5 years. Gait speed >1.3 m/s was associated with an 18–26% reduced risk of rapid kidney function decline in CKD Stages 2–4. Poor timed up and go performance was associated with a 7-fold increased likelihood of ADL disability in elderly CKD Stage 5 patients. Walking 5–6 times/week reduced the risk of death or dialysis by 50% over a year in CKD Stages 2–4. Physical frailty was associated with a 2.5 times increased risk of death or dialysis in middle-aged people with CKD Stages 2–4. A patient-reported physical function composite score (PCS) from the 36-item ShortForm Health Survey (SF-36) questionnaire indicated that a 1-point increase corresponded to a 2% reduction in mortality rate, whereas patients with an SF-36 PCS <25 had a 93% increased risk of dying and a 56% increased risk of hospitalization [23, 24];
physical function outcomes are responsive to subclinical changes in underlying altered physiological processes (e.g. poor nutritional status, frailty and muscle wasting, which sometimes are hard to accurately assess) and therapeutic interventions such as exercise rehabilitation or lifestyle behaviour changes. Appropriately designed and implemented PA programmes can induce clinically meaningful improvements in some physical function outcomes (VO2max, PCS from the SF-36 and related questionnaires and gait speed and distance walked) [24–26], suggesting that routine evaluation of physical function can be used not only as a means to characterize prognosis and adverse clinical risk, but also to monitor progress towards optimized levels of well-being for a given individual. Routine monitoring of physical function and sharing this information with the people concerned can also be used to motivate patients to actively engage with PA to maintain/enhance physical function [24]
What tests?
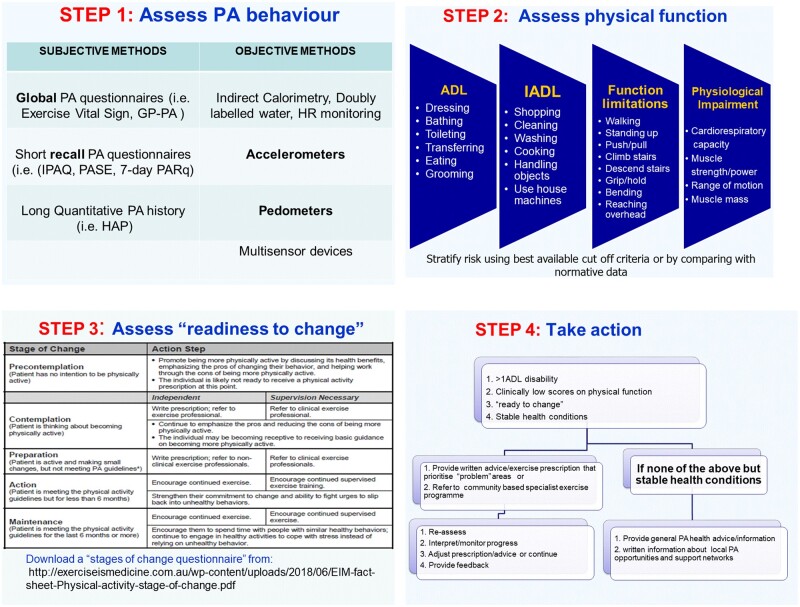
One of the gaps in the process of translating the general public PA activity guidelines as well as the CKD-specific guidelines [21, 22] into routine clinical practice is the lack of systematic recording and monitoring of PA and physical function attributes that can be used to develop individualized and measurable plans of action to promote PA for health. Clinical guidelines state that all CKD patients should have at least one session with a health professional to assess physical function and to advise on health-enhancing PA. Given the evidence that inactivity, physical dysfunction and physical frailty are prevalent and severe, we suggest that all CKD Stages 3b–5 patients should have at least one session with an exercise/rehabilitation specialist to assess PA behaviour, physical function and readiness to change PA behaviour, with the aim that all patients receive written advice and specific goals/targets and exercise prescription or referral and information about local PA/exercise opportunities that they can self-manage and that outcomes should be documented on medical records for monitoring purposes (Figure 1).
FIGURE 1.
Proposed steps for the implementation of physical function assessment in clinical practice.
The choice of assessment tools will largely be determined by the individual’s overall health status and willingness to collaborate, staff expertise and equipment availability. It is also important to note that for some patients, functional capacity assessment and especially determination of peak exercise capacity may be contraindicated [24, 27]. Furthermore, the choice of the type and specific protocol for physical function assessment should be based on the primary purpose of the assessment (diagnostic, exercise training prescription and risk stratification) and should also take into consideration the minimum standards of clinimetric utility (published validity, reliability and ideally responsiveness where data available) and normative data.
Summary information, extracted from the research and practice literature, about the different types of physical function tests indicated for use in clinical practice with CKD patients is provided in Table 2.
Table 2.
Selection of evidence-based physical function outcome measures for use in people in all stages of CKD for routine physical function assessment
| Physical function domain | Measurement outcomes | End-points (when to terminate physical function testing) |
Comments |
|---|---|---|---|
| Physiological impairment | |||
| Incremental shuttle walk |
Distance (m) and speed (m/s) BP/HR Angina scales RPE scales |
No increases in BP with increasing workload BP >220/110 mmHg Symptoms such as dizziness, angina, lack of responsiveness to oral and/or visual signs Patient’s request Equipment failure |
Familiarization session should be provided Reproducibility information available [28] |
| Absolute dynamic muscle strength | Maximum weight lifted in a continuous fashion; once, 1 maximum repetition (1-RM), 3 times (3-RM) or 5 times (5-RM) |
Patient’s request Inability to continue due to adverse symptom development |
Familiarization sessions may be required Whole body and muscle group–specific warm-up sessions are required Muscle function–related measures are strong independent predictors of disease progress and survival Reproducibility information available [28] Normative data and cut-off criteria available [28, 29] Cut-off points for Frailty phenotype [29] Cut-off points for sarcopaenia [30] |
| Relative dynamic muscle strength | Maximum number of repetitions performed as a percent of RM | ||
| Hand grip strength | kg/m/s or maximum kg achieved | ||
| Function limitations | |||
| Incremental shuttle walk | Walking distance (m) |
Patient’s request Inability to continue due to adverse symptom development |
Reproducibility information available [29] Familiarization sessions may be required Assessor and patient friendly Quick and inexpensive Minimum interference and inconvenience for patient Normative data and cut off criteria available [29–32] |
|
6-min walk test Gait speed over 3, 4 or 7 m |
Gait speed (m/s) Gait speed (m/s) |
||
| 3-m timed up and go test | Total time (s) | ||
| Sit-to-stand tests |
Time (s) it takes to complete 5 (STS-5) or 10 (STS-10) complete STS transfers or STS-60 (total number of complete STS transfers achieved in 60 s) |
||
| Disability (ADL) | |||
| PCS from SF-36 or from KDQOL | PCS (0–100) |
Composite score made up of a range of perceived physical abilities for ADL and symptoms KDQOL also reports on a range of symptoms and burdens specific to kidney disease (https://www.rand.org/pubs/papers/P7994.html) Normative data available Widely used in the CKD population |
|
| DASI index [33] | Total score from 0 to 58.2 |
All widely used in various chronic conditions and limited DASI- and KATZ-based data exist in CKD All subject to ceiling effects by higher functioning patients |
|
| Rivermead mobility index [34] | Total score from 0 to 15 | ||
| Katz index [35] | Total score from 0 to 6 | ||
| Barthel index [36] | Total score from 0 to 100 or in modified version 0–20 | ||
BP, blood pressure; HR, heart rate; RPE, ratings of perceived exertion; DASI: Duke Activity Status Index.
THE EFFECTS OF EXERCISE TRAINING ON HRQOL IN PATIENTS WITH CKD
HRQoL is a multidimensional concept that includes several domains focused on physical, mental, emotional and social functioning and reflects the impact of health status on individual well-being. Several studies have shown that CKD per se affects patients’ HRQoL, as there is an association between the severity of CKD and the self-reported HRQoL [37]. On the other hand, low HRQoL was found to be associated with clinical outcomes, frequent hospitalization and decreased survival in dialysis patients [38]. Van Loon et al. [39] showed that HRQoL is an indicator of poor outcome in HD patients, as sedentary lifestyle, low HRQoL and reduced VO2max were associated with increased mortality risk among HD patients. Thus HRQoL is considered an important outcome in CKD and, according to the Kidney Disease Outcomes Quality Initiative clinical practice guidelines 2002, is recommended to be repeatedly measured in clinical practice [40]. The tools used for HRQoL assessment and its determinants can either be generic or disease specific. They can be self- or interviewer administered. In clinical practice, the combination of multiple instruments may provide more reliable results. Studies that focus on the assessment of HRQoL in CKD patients have most frequently used the SF-36 and the Kidney Disease Quality of Life Short Form as instruments, since they are well-validated measures of general health and physical functioning and are translated into many languages [41–48] (Table 3).
Table 3.
Assessment of HRQoL in CKD patients: the questionnaires most commonly used
| Generic questionnaires | Content |
|---|---|
| Karnofsky Performance Scale Index [41] | A scale assessing functional impairment scoring using three states (conditions) that describe different levels of performance from 100 (normal state) to 0 (dead): A (100–80%): Able to carry on normal activity and to work. No special care is needed. B (70–50%): Unable to work. Able to live at home, care for most personal needs. A varying degree of assistance is needed. C (40–0%): Unable to care for self. Requires equivalent of institutional or hospital care. Disease may be progressing rapidly |
| Nottingham Health Profile [42] | 38 items to measure six dimensions: physical mobility, energy, emotional reactions, sleep, pain and social isolation |
| SF-36 [43] | 36 items in eight domains with two composite scores: PCS and mental composite score (MCS) and a question about self-evaluation of change in health during the past year (reported health), which does not belong to any score or dimension or the total SF-36 score |
| Sickness Impact Profile [44] | 136 items to measure 12 dimensions: sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behaviour, emotional behaviour and communication |
| Schedule for Evaluation of Individual Quality of Life [45] | Interview-based instrument that focuses on the domains and factors that determine each patient’s quality of life |
| Disease-specific questionnaires | |
| KDQOL Questionnaire [46] | 36-item health survey with multi-item scales targeted at concerns of individuals with kidney disease and on dialysis |
| KDQOL-SF [47] | 43 kidney disease–targeted items and 36 items that provide a measure of physical and mental health. Three composite scores (PCS, MCS and kidney disease component summary score) |
| Parfrey Test [48] | 24 items with two dimensions (physical and emotional) |
HRQOL was found to be a significant independent risk factor for CKD progression [49]. In dialysis patients, renal replacement therapies in combination with the presence of other chronic diseases, such as cardiovascular diseases and diabetes, malnutrition and sarcopaenia, frailty or disability, pain/discomfort and low physical functioning are known factors that have a negative impact on HRQoL [49, 50]. Moreover, depression and anxiety are found to be associated with poor HRQoL [51]. Additionally, a sedentary lifestyle and the lack of PA affect thhe patient’s biopsychosocial health and independence and are associated with lower levels of physical functioning and physical domains of HRQoL [39].
Exercise rehabilitation programmes are focused on improving each patient’s physical and psychosocial functioning to their optimal level. Research reports from the early 1980s through 2000 clearly showed that an outpatient aerobic exercise training programme lasting from 16 to 24 weeks can decrease anxiety and depression and improve physical function and the burden of disease and thus enhance overall HRQoL results [52]. An outpatient exercise training programme is an effective way to improve the quality of social interaction since patients exercise in groups. Later on, intradialytic exercise training programmes gained ground as a more convenient and flexible way of training that increases compliance with the exercise programme. Several meta-analyses of RCTs concluded that an intradialytic exercise programme lasting from 8 to 48 weeks can improve HD adequacy, exercise capacity and the physical domain of HRQoL [53]. However, after intradialytic exercise training, no improvement was noted in the SF-36 mental component summary level [53]. The exercise regimens differed widely between the studies in terms of exercise type, intensity, frequency and duration.
The majority of the studies that have examined the effects of intradialytic exercise training on HRQoL have used an aerobic programme at a moderate intensity. There is a discrepancy in the results from a resistance exercise training programme during HD on HRQoL. Although Segura-Ortí et al. [54] reported that resistance intradialytic training did not affect HRQoL, there is evidence that a combination of resistance training plus oral supplementation can improve some aspects of HRQoL, such as self-reported physical functioning [55] and general health perceptions and social function [56]. On the other hand, interventions that included both intradialytic aerobic and resistance exercises reported favourable physical HRQoL changes [25].
The important role of PA in improving HRQoL in CKD patients is well recognized [57]. A 6-month home-based, low-intensity exercise programme was found to improve several domains of HRQoL in HD patients, and specifically cognitive function and quality of social interaction [58]. Similarly, a 12-week home-based either aerobic or mixed-type exercise programme resulted in improvements of several domains of HRQoL exclusively in PD patients [59].
Several factors contribute to the improvement of HRQoL domains after exercise training in CKD patients (Figure 2). Several studies have found a positive strong correlation between improvements in aerobic capacity and HRQoL scores [59–61]. Exercise training can reverse fatigue, frailty and disability in CKD. The favourable effects of exercise training on functional performances such as endurance, gait, postural control and muscular strength can increase patients’ mobility, vitality and physical domains of HRQoL. That makes them more independent and able to participate in several ADL, increasing their socialization and life satisfaction [52]. A Cochrane systematic review also revealed significant improvements in physical fitness, muscular functioning, walking capacity, cardiovascular function and HRQoL in HD patients, especially after aerobic exercise programmes [62].
FIGURE 2.
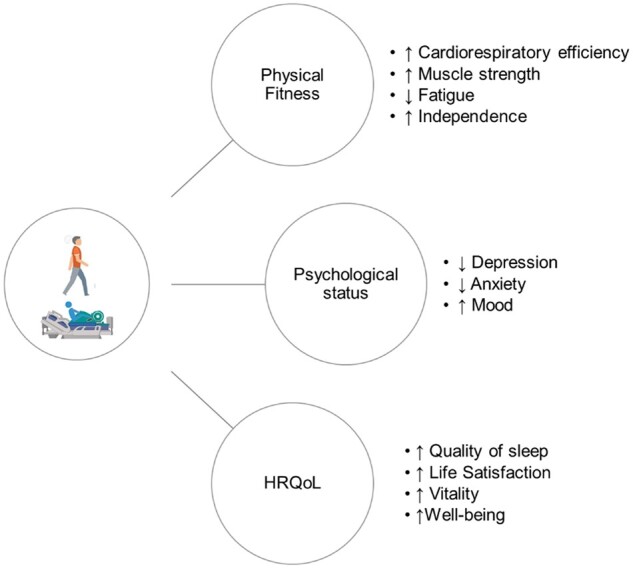
Effects of regular PA/exercise training on physical functioning and HRQoL in CKD patients.
Also, a recent meta-analysis showed that exercise interventions can improve the mood disorders that might be triggered by a reduction in physical function, especially depression and anxiety in CKD patients [51]. Similarly, a 1-year intradialytic exercise training programme was found to be effective in improving the functional limitations, cardiac autonomic dysfunction and psychological distress that are known disorders in HD patients [63].
Quality of sleep is another significant parameter that affects patient’s HRQoL and can be improved by exercise training. Corrêa et al. [64] showed that a 3-month intradialytic resistance training programme can improve the clinical status of HD patients by improving their sleep quality, oxidative and inflammatory parameters. This is in line with previous reports that exercise training can improve stress and inflammation biomarkers in patients with CKD [65].
Educating patients about the benefits of regular exercise will help them better manage the discomfort they face in everyday life and obtain a near-normal life.
CONCLUSION
Adequate and varied PA participation is a prerequisite for adequate physical function for normal life participation, perceived satisfaction and general feelings of well-being. Maintenance of functional independence and prevention of disability is a priority in all clinical practice guidelines for the management of people in all stages of CKD. Exercise-based therapies are effective in restoring and further improving physical function, and subsequently HRQoL. We propose that PA and physical function should be routinely assessed and monitored in the CKD population and we have suggested a range of tools for their assessment as currently supported by research and practice-based evidence. Further research is required to establish cut-off points for the minimum amount of PA and the physical function level that anchor different risk classifications for broader health outcomes such as mortality, optimal HRQoL and disability.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. McArdle WD, Katch FI, Katch VL.. Exercise Physiology: Energy, Nutrition, and Human Performance. Baltimore, MD: Williams & Wilkins, 1996. [Google Scholar]
- 2. Segura-Orti E, Gordon PL, Doyle JW. et al. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res 2018; 27: 579–596 [DOI] [PubMed] [Google Scholar]
- 3. Cobo G, Gallar P, Gama-Axelsson T. et al. Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol 2015; 28: 503–510 [DOI] [PubMed] [Google Scholar]
- 4. Matsuzawa R, Matsunaga A, Wang G. et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2012; 7: 2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lou X, Li Y, Shen H. et al. Physical activity and somatic symptoms among hemodialysis patients: a multi-center study in Zhejiang, China. BMC Nephrol 2019; 20: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hishii S, Miyatake N, Nishi H. et al. Relationship between sedentary behavior and health-related quality of life in patients on chronic hemodialysis. Acta Med Okayama 2018; 72: 395–400 [DOI] [PubMed] [Google Scholar]
- 7. Hishii S, Miyatake N, Nishi H. et al. Relationship between sedentary behavior and all-cause mortality in Japanese chronic hemodialysis patients: a prospective cohort study. Acta Med Okayama 2019; 73: 419–425 [DOI] [PubMed] [Google Scholar]
- 8. Tentori F, Elder SJ, Thumma J. et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 2010; 25: 3050–3062 [DOI] [PubMed] [Google Scholar]
- 9. Junque Jimenez A, Esteve Simo V, Andreu Periz L. et al. The relationship between physical activity levels and functional capacity in patients with advanced chronic kidney disease. Clin Nurs Res 2020; doi: 10.1177/1054773820907757 [DOI] [PubMed] [Google Scholar]
- 10. Johansen KL, Painter P, Kent-Braun JA. et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int 2001; 59: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 11. Masuda R, Imamura H, Mizuuchi K. et al. Physical activity, high-density lipoprotein cholesterol subfractions and lecithin: cholesterol acyltransferase in dialysis patients. Nephron Clin Pract 2009; 111: c253–9 [DOI] [PubMed] [Google Scholar]
- 12. Oishi D, Koitabashi K, Hiraki K. et al. Physical activity is associated with serum albumin in peritoneal dialysis patients. Adv Perit Dial 2012; 28: 148–152 [PubMed] [Google Scholar]
- 13. Blair SN, Haskell WL, Ho P. et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985; 122: 794–804 [DOI] [PubMed] [Google Scholar]
- 14. Washburn RA, Smith KW, Jette AM. et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993; 46: 153–162 [DOI] [PubMed] [Google Scholar]
- 15. Fix A, Daughton D.. Human Activity Profile (HAP) Manual. Odessa, Ukraine: Psychological Assessment Resources, ; 1986 [Google Scholar]
- 16. Johansen KL, Chertow GM, Ng AV. et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 2000; 57: 2564–2570 [DOI] [PubMed] [Google Scholar]
- 17. MacKinnon HJ, Wilkinson TJ, Clarke AL. et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Ther Adv Chronic Dis 2018; 9: 209–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koufaki P, Mercer TH.. Assessment and monitoring of physical function for people with chronic kidney disease. Adv Chronic Kidney Dis 2009; 16: 410–419 [DOI] [PubMed] [Google Scholar]
- 19. Greenwood S, Lindup H, Taylor K. et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant 2012; 27(Suppl 3): 126–134 [DOI] [PubMed] [Google Scholar]
- 20. Tamura MK, Covinsky KE, Chertow GM. et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinical Practice Guideline on Management of Patients with Diabetes and Chronic Kidney Disease Stage 3b or Higher. https://www.era-edta.org/en/erbp/wp-content/uploads/sites/7/2020/05/DiabetesEnglish.pdf (29 December 2020, date last accessed) [DOI] [PubMed]
- 22. K/DOQI Work Group. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. http://www.ajkd.org/article/S0272-6386(05)00092-2/fulltext#Regularphysicalactivity (29 December 2020, date last accessed)
- 23. Painter P, Roshanravan B.. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 615–623 [DOI] [PubMed] [Google Scholar]
- 24. Koufaki P, Greenwood S, Painter P. et al. The BASES expert statement on exercise therapy for people with chronic kidney disease. J Sports Sci 2015; 33: 1902–1907 [DOI] [PubMed] [Google Scholar]
- 25. Gomes Neto M, de Lacerda FFR, Lopes AA. et al. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil 2018; 32: 1189–1202 [DOI] [PubMed] [Google Scholar]
- 26. Clarkson MJ, Bennett PN, Fraser SF. et al. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol 2019; 316: F856–F872 [DOI] [PubMed] [Google Scholar]
- 27. Greenwood S, Koufaki P.. Physical activity, function, and exercise-based rehabilitation for dialysis patients. In: Allen R, Nissenson MD, Richard N, Fine MD (eds). Handbook of Dialysis Therapy, 5th edn. Philadelphia: : Elsevier, 2016 [Google Scholar]
- 28. Wilkinson T, Xenophontos S, Gould DW. et al. Test–retest reliability, validation, and “minimal detectable change” scores for frequently reported tests of objective physical function in patients with non-dialysis chronic kidney disease. Physiother Theory Pract 2019; 35: 565–576 [DOI] [PubMed] [Google Scholar]
- 29. Fried LP, Tangen CM, Walston J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 30. Cruz-Jentoft JA, Baeyens JP, Bauer JM. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Ageing 2010; 39: 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steffen TM, Hacker TA, Mollinger L.. Age- and gender-related test performance in community-dwelling elderly people: six-minute, walk test, Berg balance scale, timed up & go test, and gait speeds. Phys Ther 2002; 82: 128–137 [DOI] [PubMed] [Google Scholar]
- 32. Hadjiioannou I, Wong K, Lindup H. et al. Test–retest reliability for physical function measures in patients with chronic kidney disease. J Ren Care 2020; 46: 25–34 [DOI] [PubMed] [Google Scholar]
- 33. Hlatky MA, Boineau RE, Higginbotham MB. et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989; 64: 651–654 [DOI] [PubMed] [Google Scholar]
- 34. Collen FM, Wade DT, Robb GF. et al. The Rivermead Mobility Index: a further development of the rivermead motor assessment. Int Disabil Stud 1991; 13: 50–54 [DOI] [PubMed] [Google Scholar]
- 35. Katz S, Ford AB, Moskowitz RW. et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. J Am Med Assoc 1963; 195: 94–99 [DOI] [PubMed] [Google Scholar]
- 36. Mahoney FI, Barthel D.. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 56–61 [PubMed] [Google Scholar]
- 37. Nguyen NTQ, Cockwell P, Maxwell AP. et al. Chronic kidney disease, health-related quality of life and their associated economic burden among a nationally representative sample of community dwelling adults in England. PLoS One 2018; 13: e0207960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Østhus TB, Preljevic V, Sandvik L. et al. Mortality and health related quality of life in prevalent dialysis patients: comparison between 12-items and 36-items short form health survey. Health Qual Life Outcomes 2012; 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Loon IN, Bots ML, Boereboom FTJ. et al. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol 2017; 18: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Briggs J. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–266 [PubMed] [Google Scholar]
- 41. Karnofsky D, Burchenal J.. The clinical evaluation of chemotherapeutic agents in cancer. In: Maclead C (ed). Evaluation of Chemotherapeutic Agents. New York: Columbia: University Press, 1949, 191–205 [Google Scholar]
- 42. Hunt SM, McKenna SP, McEwen J. et al. A quantitative approach to perceived health status: a validation study. J Epidemiol Commun Health 1980; 34: 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalantar-Zadeh K, Unruh M.. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol 2005; 37: 367–378 [DOI] [PubMed] [Google Scholar]
- 44. Bergner M, Bobbitt RA, Carter WB.. The sickness impact profile: development and final revision of a health status measure. Med Care 1981; 19: 787–805 [DOI] [PubMed] [Google Scholar]
- 45. Hickey AM, Bury G, O'Boyle CA. et al. A new short form individual quality of life measure (SEIQoL-DW): application in a cohort of individuals with HIV/AIDS. BMJ 1996; 313: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hays RD, Kallich JD, Mapes DL. et al. Development of the Kidney Disease Quality of Life (KDQOL) Instrument. Qual Life Res 1994; 3: 329–338 [DOI] [PubMed] [Google Scholar]
- 47. Hays RD, Kallich JD, Mapes DL. et al. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for use and Scoring. Santa Monica, CA: Rand, 1997 [Google Scholar]
- 48. Parfrey PS, Vavasour H, Bullock M. et al. Development of a health questionnaire specific for end-stage renal disease. Nephron 1989; 52: 20–28 [DOI] [PubMed] [Google Scholar]
- 49. Oh TR, Choi HS, Kim CS. et al. Association between health-related quality of life and progression of chronic kidney disease. Sci Rep 2019; 9: 19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kang SH, Do JY, Lee SY. et al. Effect of dialysis modality on frailty phenotype, disability, and health-related quality of life in maintenance dialysis patients. PLoS One 2017; 12: e0176814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferreira T, Ribeiro H, Ribeiro A. et al. Exercise interventions improve depression and anxiety in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol 2020; 10.1007/s11255-020-02612-w [DOI] [PubMed] [Google Scholar]
- 52. Kouidi Ε. Health-related quality of life in end-stage renal disease patients: the effects of renal rehabilitation. Clin Nephrol 2004; 61(Suppl 1): S60– S–71. [PubMed] [Google Scholar]
- 53. Pu J, Jiang Z, Wu W. et al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 2019; 9: e020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Segura-Ortí E, Kouidi E, Lisón JF.. Effect of resistance exercise during hemodialysis on physical function and quality of life: randomized controlled trial. Clin Nephrol 2009; 71: 527–537 [DOI] [PubMed] [Google Scholar]
- 55. Johansen K, Painter P, Sakkas G. et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 2006; 17: 2307–2314 [DOI] [PubMed] [Google Scholar]
- 56. Martin-Alemañy G, Valdez-Ortiz R, Olvera-Soto G. et al. The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrol Dial Transplant 2016; 31: 1712–1720 [DOI] [PubMed] [Google Scholar]
- 57. Mallamaci F, Pisano A, Tripepi G.. Physical activity in chronic kidney disease and the exercise introduction to enhance trial. Nephrol Dial Transplant 2020; 35: ii18–ii22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manfredini F, Mallamaci F, D’Arrigo G. et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 2017; 28: 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uchiyama K, Washida N, Morimoto K. et al. Home-based aerobic exercise and resistance training in peritoneal dialysis patients: a randomized controlled trial. Sci Rep 2019; 9: 2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kouidi E, Iacovides A, Iordanidis P. et al. Exercise renal rehabilitation program (ERRP): psychosocial effects. Nephron 1997; 77: 152–158 [DOI] [PubMed] [Google Scholar]
- 61. Ouzouni S, Kouidi E, Sioulis A. et al. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 2009; 23: 53–63 [DOI] [PubMed] [Google Scholar]
- 62. Heiwe S, Jacobson SH.. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 63. Kouidi E, Karagiannis V, Grekas D. et al. Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil 2010; 17: 160–167 [DOI] [PubMed] [Google Scholar]
- 64. Corrêa HL, Moura SRG, Neves RVP. et al. Resistance training improves sleep quality, redox balance and inflammatory profile in maintenance hemodialysis patients: a randomized controlled trial. Sci Rep 2020; 10: 11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Howden EJ, Fassett RG, Isbel NM. et al. Exercise training in chronic kidney disease patients. Sports Med 2012; 42: 473–488 [DOI] [PubMed] [Google Scholar]