Abstract
There is increasing evidence showing the health benefits of physical activity, such as better survival and possibly even a slower decline in kidney function, in people with chronic kidney disease (CKD). There is convincing evidence that exercise training improves physical function measured as aerobic capacity, muscle endurance strength and balance at all ages and all stages of CKD. In fact, long-term adherence to well-designed and adequately monitored exercise training programmes is high. In general, patients express interest in exercise training and are motivated to improve their physical function and health. A growing number of nephrologists regard physical activity and exercise training as beneficial to patients with CKD. However, many feel that they do not have the knowledge to prescribe exercise training and suppose that patients are not interested. Patients state that support from healthcare professionals is crucial to motivate them to participate in exercise training programmes and overcome medical, physical and psychological barriers such as frailty, fatigue, anxiety and fear. Equally important is the provision of funding by healthcare providers to ensure adequate prescription and follow-up by trained exercise physiologists for this important non-pharmacological treatment.
Keywords: adherence, barriers, chronic kidney disease, dialysis, exercise training, GFR, physical activity, physical function, mortality, motivators
INTRODUCTION
A growing number of nephrologists around the world regard physical activity and exercise training as an integral part of the care of patients with chronic kidney disease (CKD). The Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Management of CKD recommends that ‘people with CKD be encouraged to undertake physical activity compatible with cardiovascular health and tolerance (1D)’ [1]. For clinicians, level 1 means that ‘most patients should receive the recommended course of action’, for policymakers it means that ‘the recommendation can be evaluated as a candidate for developing a policy or performance measure’ and for patients it means ‘most people in your situation would want the recommended course of action and only a small proportion would not’. However, the evidence rating was D, which is very low, implying ‘the estimate of effect is very uncertain, and often will be far from the truth’.
Now, nearly 10 years later, there is more evidence, including several larger randomized controlled clinical trials and a number of meta-analyses. This overview will describe the effects of exercise training on factors such as mortality, morbidity, progression of kidney disease and physical function in patients with CKD Stages 3–5D. It will also discuss adherence to exercise training programmes and facilitators and barriers that affect motivation.
MORTALITY AND SELF-REPORTED PHYSICAL ACTIVITY
Physical activity affects survival and health in the general population. There is similar evidence from observational studies in patients with CKD. Generally, people with CKD Stage ≥3 have lower levels of physical activity than their healthy counterparts [2]. In a recent systematic review studying non-dialysis-dependent (NDD) patients with CKD ranging from Stage 1 to 5 as well as renal transplant recipients, reduced physical activity was associated with higher mortality [3]. Observational studies comprising people with CKD at any stage have shown that higher levels of physical activity are associated with lower mortality [2, 4, 5]. In the Dialysis Outcomes and Practice Patterns Study, an exercise frequency of more than one session per week was associated with lower mortality risk [6].
MORTALITY AND MEASURED PHYSICAL FUNCTION
Recently a systematic review in NDD patients with CKD found that aerobic capacity and muscle endurance in the lower extremities, notably gait speed and ability to stand up from a chair, were associated with increased all-cause mortality [3]. Several studies have reported an association between lower gait speed and decreased muscular endurance in the legs and higher mortality in people with CKD Stages 2–5D [7, 8]. In one study in patients on haemodialysis (HD), the peak volume of oxygen (VO2) was found to be a strong predictor of survival [9].
PHYSICAL FUNCTION AND PHYSICAL ACTIVITY DETERIORATE AS GLOMERULAR FILTRATION RATE (GFR) DECLINES
As GFR declines, physical function deteriorates. This relationship is consistent whichever measure of physical function is used. Several cross-sectional studies found significant negative relationships between GFR and maximal exercise capacity [10], walking distance [11], strength, balance and fine motor skills [12]. A recent large cross-sectional study in patients with CKD ranging from Stage 1 to 5D showed that self-reported physical inactivity is highly prevalent across all stages of CKD, with 66% reporting insufficient physical activity in Stages 1 and 2 to >90% of patients on dialysis [13].
RELATIONSHIP BETWEEN PHYSICAL FUNCTION, PHYSICAL ACTIVITY AND KIDNEY FUNCTION
Not only does physical function deteriorate as CKD progresses, but low physical function might also affect the rate of progression. One meta-analysis studied the effects of exercise training on GFR and found that exercise training improved estimated GFR (eGFR) by 2.16 mL/min/1.73 m2 [14]. However, this improvement was driven by three studies comprising 8–14 patients exercising for 3–12 months; there were no within-group effects. Another meta-analysis showed that exercise increased eGFR by 2.62 mL/min/1.73 m2; however, this increase was only found in studies with an observation period <3 months. In studies with an observation period of 3–12 months, there was no group effect of exercise training on eGFR [15]. The largest study included in the meta-analysis is the Landmark study, a randomized controlled trial (RCT) comprising 72 patients who completed the 12-month study period with unchanged eGFR in both the exercise and control arms [16]. The Renal Exercise (RENEXC) trial, a RCT with two active arms comprising 112 patients who completed 12 months of exercise training, reported a decline in measured GFR of 1.8 mL/min/1.73 m2 in both groups with no between-group difference. Interestingly, the endurance and resistance group showed a significant reduction in the urine albumin:creatinine ratio compared with the endurance and balance group; there was a between-group difference [17].
Observational studies have found an association between higher levels of self-reported physical activity and a slower rate of eGFR decline [4, 18] as well as a dose-dependent relationship between the level of physical activity and loss of eGFR. A level of physical activity >150 min/week was related with the slowest decline in eGFR, while the highest degree of inactivity was related with the fastest decline [4, 18]. Measured physical function has also been found to be associated with a decline in eGFR. A higher fitness level in US veterans was associated with a lower risk of acquiring CKD [19]; handgrip strength (HGS) and the 1-min step test in the upper 50th percentile were associated with a slower progression to end-stage kidney disease (ESKD) [20].
To summarize, there are indications that exercise training slows the progression of GFR. However, for conclusive evidence, longitudinal prospective studies need to be performed. Moreover, GFR should be measured rather than estimated in order to eliminate a confounding effect of increased muscle mass. On the one hand, exercise can increase muscle mass, as has been shown in NDD CKD patients at Stages 3A–5 [21]; on the other hand, in observational studies, highly active people will have a higher muscle mass and may falsely be classified as having CKD based on eGFR. Finally, an observation period of at least 12 months is recommended.
EFFICACY OF SUPERVISED EXERCISE TRAINING
Characteristics of the meta-analyses referred to and an overview of the reported effects of different exercise training regimes on various measures of physical function are given in Tables 1 and 2. The reviews comprise studies with in-centre group training, intradialytic (ID) training, home- or gym-based training, aerobic training only, resistance training only and a combination of both.
Table 1.
Descriptive data of recent systematic reviews and meta-analyses of the effects of exercise training in patients with NDD CKD and ESKD
| Meta-analyses | Studies, n |
Participants, n (men/women) |
Age (years), rangea | ESKD Interdialytic exercise |
ESKD ID exercise |
NDD CKD exercise |
Duration (months), rangeb |
||
|---|---|---|---|---|---|---|---|---|---|
| At home | In-centre | At home | In-centre | ||||||
| Vanden Wyngaert et al. [14] | 11 | 362 | 51–72 | NA | NA | NA | Yes | Yes | 3–12 |
| Heiwe and Jacobson [22] | 41 | NA | 36–71 | Yes | Yes | Yes | Yes | Yes | 2–18 |
| Barcellos et al. [23] | 59 | 2665 | NA | Yes | Yes | Yes | Yes | Yes | 2–12 |
| Andrade et al. [24] | 7 | 243 (153/90) | NA | NA | NA | Yes | NA | NA | 3–12 |
| Bogataj et al. [25] | 33 | 1274 | 40–70 | Yes | Yes | Yes | NA | NA | 2–10 |
| Clarkson et al. [26] | 27 | 1156 | 36–70 | Yes | Yes | Yes | NA | NA | 2–6 |
| Ferrari et al. [27] | 50 | 2062 | NA | NA | NA | Yes | NA | NA | NA |
| Huang et al. [28] | 20 | 677 | 30–70 | Yes | Yes | Yes | NA | NA | 2–12 |
| Lu et al. [29] | 21 | 898 (382/516) | 45–71 | Yes | Yes | Yes | NA | NA | NA |
| Pu et al. [30] | 27 | 1215 (723/492) | 53 | NA | NA | Yes | NA | NA | 2–12 |
| Scapini et al. [31] | 31 | 1251 | NA | NA | Yes | Yes | NA | NA | 2–12 |
| Young et al. [32] | 8 | 354 | 41–70 | NA | NA | Yes | NA | NA | 3.5 |
aAge, given as range, of participants in the individual studies of the meta-analysis (except in the review by Pu [30] where mean age was given). bDuration of exercise intervention in the individual studies of the meta-analysis (except in the review by Young et al. [32], where the mean duration of the exercise intervention was given). Interdialytic exercise: exercise on non-dialysis days; ID exercise: exercise during HD; NA: no data available or not studied.
Table 2.
Overview of effects of exercise training in patients with NDD CKD and patients with ESKD (comprises HD and PD) from recent systematic reviews and meta-analyses
| Meta-analyses | Type of exercise | Aerobic capacity |
Muscular endurance STS ESKD |
Muscle strength |
||||
|---|---|---|---|---|---|---|---|---|
| Peak VO2 |
6MWT |
Leg |
HGS |
|||||
| NDD CKD | ESKD | ESKD | NDD CKD | ESKD | ESKD | |||
| Vanden Wyngaert et al. [14] |
Aerobic Combined |
Increase Increase |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
| Heiwe and Jacobson [22] |
Aerobic Combined Resistance |
Increase Increase Increase |
Increase Increase Increase |
= = = |
NA NA NA |
Increase Increase Increase |
Increase Increase Increase |
NA NA NA |
| Barcellos et al. [23] |
Aerobic Combined Resistance |
Increase Increase Increase |
Increase Increase Increase |
NA NA NA |
NA NA NA |
Increase Increase Increase |
Increase Increase Increase |
NA NA NA |
| Andrade et al. [24] |
Aerobic Combined |
NA NA |
Increase Increase |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
| Bogataj et al. [25] |
Aerobic Combined |
NA NA |
Increase Increase |
Increase Increase |
Increase Increase |
NA NA |
NA NA |
NA NA |
| Clarkson et al. [26] |
Aerobic Combined Resistance |
NA NA NA |
NA NA NA |
Increase = Increase |
Increase Increase Increase |
NA NA NA |
NA NA NA |
Increase NA NA |
| Ferrari et al. [27] |
Aerobic Combined Resistance |
NA NA NA |
Increase Increase NA |
Increase = Increase |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
| Huang et al. [28] |
Aerobic Combined Resistance |
NA NA NA |
Increase NA Increase |
Increase = = |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
| Lu et al. [29] |
Aerobic Combined Resistance |
NA NA NA |
NA NA NA |
Increase = Increase |
Increase Increase Increase |
NA NA NA |
= = Increase |
Increase = Increase |
| Pu et al. [30] |
Aerobic Combined Resistance |
NA NA NA |
Increase Increase Increase |
Increase Increase Increase |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
| Scapini et al. [31] |
Aerobic Combined Resistance |
NA NA NA |
Increase = Increase |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
| Young et al. [32] | Aerobic | NA | = | Increase | NA | NA | NA | NA |
Increase: statistically significant effect of exercise training; =: no effect of exercise training, NA: no data available or not studied.
In NDD CKD, there were no data available for aerobic capacity measured as 6MWT, except in Heiwe and Jacoobson’s [22] review, where no effect was reported.
In NDD CKD, there were no data available for muscular endurance measured as STS, strength measured as HGS or balance.
In ESKD, there were no data available for balance except in Clarkson et al.’s [26] review, where no effect was reported.
NDD CKD
We found three meta-analyses including NDD patients with CKD. Two of the reviews provided the number of patients in each study, comprising a total of 357 and 354 patients, respectively [14, 23]. All meta-analyses showed positive effects of exercise training on aerobic capacity measured as peak VO2. One review analysed the effect of exercise training on the 6-min walk test (6MWT) and found no effect [22]. Two reviews showed positive effects on leg muscle strength [14, 22].
To date there are three larger RCTs comprising a total of 330 patients. The Landmark study, with a total of 72 patients exercising for 12 months [33], the study by Rossi et al., with 107 patients exercising for 12 weeks, and the RENEXC trial, with 151 patients exercising for 12 months [17]. The Landmark study and Rossi et al. compared exercise training with standard care. The RENEXC trial had two active arms of intervention: endurance and resistance training or endurance and balance training. The study by Rossi et al. and the RENEXC trial were not included in the most recent meta-analysis by Vanden Wyngaert et al. [14]. The participants in these three studies had CKD Stages 3–5, with a mean age of 60–69 years. Only one study, the RENEXC trial, specified using intention-to-treat (ITT) analysis [17]. In the Landmark trial, the patients had 8 weeks of in-centre supervised training for 150 min/week, after which they were given individually adapted home-based programmes and were contacted regularly to monitor progress. In Rossi et al.’s study, patients had supervised in-centre exercise training twice a week. The RENEXC trial prescribed individually tailored gym- or home-based training for 150 min/week with regular telephone follow-up by the study physiotherapist every week for the first 3 months and every other week for the remaining study period. All studies showed an increase in the 6MWT. Rossi et al. and the RENEXC trial showed an improvement in the sit-to-stand (STS) test. The RENEXC trial showed improved muscle strength and endurance in the leg muscles, improved balance and improved fine motor skills, but no improvement in HGS. The Landmark trial showed an increase in metabolic equivalents (METs).
To summarize, exercise training is effective in increasing aerobic capacity and muscle endurance and strength in the lower extremities. However, there are few large long-term studies. Few studies have employed ITT to evaluate their results.
Maintenance dialysis
We found 11 meta-analyses comprising 11 438 patients with ESKD; most of the patients were on HD, but some studies included patients on peritoneal dialysis (PD). It is important to note that many reviews include the same studies in their analysis, so the actual number of patients who have participated in exercise training studies is considerably less. All meta-analyses reviewed ID training, i.e. during HD, and seven reviews also included interdialytic training, i.e. on non-dialysis days.
All found that exercise training increased aerobic capacity, measured with peak VO2 in eight reviews and the 6MWT in seven reviews. Three reviews reported positive effects of aerobic training only or combined training on peak VO2 [27, 28, 31]; one review found no effect of resistance training only on peak VO2 [31]. Three reviews found positive effects of aerobic only or resistance training only on the 6MWT [26, 27, 29]. Four reviews found no effect of combined training on the 6MWT [26–29]. Three reviews reported that all described exercise training modalities had a positive effect on muscle endurance measured with the STS test [25, 26, 29]. Two reviews reported an increase in leg muscle strength with all types of exercise training [22, 23], while one review found that only resistance training increased leg muscle strength [29].
Eighteen of the studies included in the meta-analyses comprised between 50 and 100 patients. We found four studies comprising >100 patients: Van Vilsteren et al. [35] with 103, Tao et al. [36] with 113, Manfredini et al. [37] with 296 and Bennett et al. [38] with 228. Van Vilsteren et al. [35] randomized 103 patients to strength training before the dialysis session followed by ID cycling two to three times a week for 12 weeks. Each patient also received four sessions of individual exercise counselling. They found significant improvements in muscle endurance measured with the STS test and leg muscle strength, but not in peak VO2. Tao et al. [36] conducted a nurse-supervised case management programme with person-to-person counselling. A total of 113 patients were randomized to 6 weeks of biweekly exercise training before dialysis, followed by a 6-week observation period or 6 weeks of biweekly exercise training before dialysis and weekly health and exercise counselling for the first 6 weeks followed by 6 weeks of biweekly counselling. The patients in the counselling group improved their general endurance, measured as gait speed, and their muscle endurance, measured with the STS test. There was a significant group effect for gait speed. Results were analysed using ITT. Bennett et al. [38] randomized 228 patients in clinic clusters using a step-wedged design in which patients were their own controls. They were prescribed 12–36 weeks of individualized resistance training during HD three times a week, of which one session each week was supervised. Patients improved their muscle endurance as measured with the STS test and the timed-up-and-go test.
The hitherto largest study is the EXerCise Introduction To Enhance Performance in Dialysis (EXCITE) trial comprising 296 patients on either HD or PD. Patients were randomized to usual care or 6 months of a home-based exercise (HBE) training programme consisting of walking a predefined number of steps per minute as determined by a metronome, which was increased regularly according to each patient’s capacity. Dialysis nurses were in daily contact with the patients to motivate them. The exercise group showed significant improvements in the 6MWT and STS test [37]. Results were analysed using ITT.
To summarize, most studies compared various forms of exercise training (in-centre or home-based, intra- or interdialytic) to a usual care control group. The meta-analyses report a positive effect of exercise training on aerobic capacity, muscle endurance and strength. There are a number of small studies with <20 participants and at least 16 studies with 50–80 patients. We found four studies with >100 patients. All studies showed positive effects on aerobic capacity irrespective of the test used. The largest studies measured muscle endurance and strength and found positive effects. Some studies analysed their data using ITT analysis, but most did not.
EFFICACY OF COUNSELLING
Several studies integrated some degree of counselling into their programmes. Tao et al. [36] showed significant effects of an integrated exercise prescription and counselling programme. An early study showed that patients with NDD CKD and ESKD increased their physical function with regular counselling by weekly telephone calls [39]. Both the RENEXC and EXCITE trials incorporated regular motivational contact by dedicated staff [17, 37].
EXERCISE TRAINING IN THE ELDERLY
One meta-analysis studied the effects of exercise training in people >60 years of age on dialysis, with inconclusive results [40]. An early study compared 12 weeks of in-centre individualized exercise training in patients with CKD Stages 4–5 and in healthy subjects with their respective sedentary counterparts; the average age was 75 years. Both the patients and the healthy subjects showed a similar increase in walking distance, muscle strength and endurance [41]. In a secondary analysis of the EXCITE trial comprising patients >65 years of age, 6 months of home-based walking improved the 6MWT and STS tests [42].
To summarize, older people with CKD Stages 4–5D benefit from individualized exercise training. In fact, for this group, exercise training can be especially important in order to preserve and increase functional ability and maintain personal autonomy.
EXERCISE TRAINING AND DIALYSIS EFFICACY
One meta-analysis showed increased dialysis efficacy as measured by Kt/Vurea after ID exercise [30]; another meta-analysis found this effect was specifically due to aerobic training [31].
WHERE AND WHEN TO EXERCISE?
To date, there are a large number of studies from around the world and from countries on every continent. For patients with NDD CKD, in-centre supervised exercise training has often been used initially to get patients accustomed to the training regime, after which individually tailored home-based training has been recommended. For patients with ESKD, ID training is most commonly prescribed, although interdialytic training, either in-centre or home based, is also recommended. There are several studies comprising 46–60 patients comparing ID with interdialytic in-centre or home-based training, with observation periods ranging from 4 to 6 months. Two of the studies found positive effects of exercise training on aerobic capacity in all groups [43, 44]; one study found no effect [45].
TYPE OF EXERCISE
The most common form of exercise training is aerobic training, such as cycling, walking and swimming. There are a few studies prescribing resistance training only, but many advocate a combination of endurance and resistance training using machines, weights or therabands. Balance in combination with aerobic training has been shown to be equally effective as a combination of resistance and aerobic training [17, 46]. Complemetary and alternative exercise for enhancing physical well-being has also been successfully employed in patients on HD. A RCT comprising 31 patients found that 12 weeks of yoga resulted in a non-significant improvement in self-assessed physical function [47]. A non-RCT comprising 172 patients found significantly less fatigue in the group practicing qigong compared with the control group after 6 months [48]. A systematic review studied the effects of inspiratory muscle training for a duration of 6–24 weeks in 134 patients and reported a significant improvement in the 6MWT [49].
DURATION AND DOSE OF EXERCISE
Most studies prescribed 20–60 min of exercise training two to three times a week. Some studies following the World Health Organization’s recommendations prescribed a weekly exercise dose of 150 min of moderate-intensity activity with muscle-strengthening activities involving major muscle groups at least twice a week [50].
When prescribing exercise dose and intensity it is important to acknowledge that many patients with CKD, both NDD and ESKD, can be severely deconditioned, weak and frail and might only be able to manage short sessions of physical activity consisting of up to 10 min/day to begin with. However, it is important to emphasize that even small doses of physical activity confer significant health benefits and that even 40 or 90 min of physical activity per week has been shown to reduce all-cause mortality in people with chronic conditions [51]. Moreover, inactive people can have the greatest benefit from low- to moderate-intensity physical activity [51]. Thus it is important to encourage patients to be physically active, but the dose and intensity of activity must be adjusted to their level of functional ability and physical function.
PARTICIPATION AND ADHERENCE
In order to be effective, an exercise training programme must attract a majority of available participants and must be constructed so that they are motivated to persevere. In this section, exercise training studies will be evaluated according to (i) the proportion of the total number of patients treated in the facility willing to participate; (ii) adherence to therapy, described as the number of possible sessions performed according to prescribed duration and intensity; and (iii) adherence to the programme, described as the number of patients who dropped out due to ‘lack of interest’. An overview of these results is presented in Table 3 for patients with NDD CKD and Table 4 for patients with ESKD.
Table 3.
Participation and adherence to exercise training in recent studies in patients with NDD CKD
| Studies | Study design | Type of exercise | Study duration ( months) | Exercise location | Exercise duration (min) per week or per session and number of weekly sessions (n/week) | Number screened | Screening failures, n | Number randomized, n (men/women) | Number completed, n (men/women) | Possible sessions completed (%)a | Total dropouts, (dropouts due to death, TX, illness, moving)a, n | Overall adherence(adherence excluding death, TX, illness)a, (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hellberg et al. [17] |
RCT two treatment arms: (i) AE + RE (ii) AE + balance CKD 3–5 |
CE | 12 | HBE or CBE |
AE: 60 min/week RE or balance: 90 min/week 3–5/week |
217 | NA |
151 (98/53) |
112 (NA) |
66% of patients completed prescribed sessions | 39 (NA) | 74 (NA) |
| Howden et al. [31, 33] |
RCT CKD 3 + 4 |
CE | 12 |
CBE: 2 months HBE: 10 months |
150 min/week NA |
90 | 3 |
83 (52/31) |
72 (NA) |
70% in CBE 57% in HBE |
6 (4) | 86 (98) |
| Aoike et al. [52] |
RCT three arms CKD 3 + 4 |
AE | 6 |
HBE CBE |
30–40 min/session 3/week |
59 | NA |
45 (NA) |
40 (27/13) |
NA | 5 (NA) | 89 (NA) |
|
Ikizler et al. |
RCT four arms 2 × 2 factorial design, CKD3 + 4 |
AE | 4 | HBE |
30 min/session 3/week |
122 | 6 |
111 (64/47) |
92 (NA) |
85% of patients completed >50% of sessions, 49% completed >75% of sessions |
19 (13) | 82 (95) |
| Headley et al. [51, 54] |
RCT CKD 3, only Diabetes or hypertension |
AE | 4 | CBE |
40 min/session 3/week |
1116 | 86 |
51 (NA) |
46 (30/16) |
97% | 5 (4) | 90 (98) |
| Rossi et al. [32, 54] |
RCT CKD 3 + 4 |
CE | 3 | CBE |
AE: 60 min/session RE: 2/week |
404 | NA |
119 (56/51) |
94 (NA) |
73% of patients completed 100% of sessions, 27% completed 50% of sessions |
25 (5) | 79 (83) |
| Kirkman et al. [32, 55] |
RCT CKD 3–5 |
AE | 3 | CBE |
45 min/session 3/week |
384 | 196 |
36 (22/9) additional 27 individuals enrolled into a healthy control arm |
31 (NA) |
92% | 5 (3) | 86 (94) |
| Watson et al. [32, 54] |
RCT CKD 3b–4 |
RE | 2 | CBE |
NA 3/week |
2349 | 365 |
38 (56/51) |
33 (NA) |
92% | 5 (4) | 87 (97) |
Between 2010 and 2020, 11 RCT trials were conducted, from which 8 studies comprising ≥35 patients were selected. The studies are ranked according to study duration.
AE: aerobic exercise; RE: resistance exercise; CE: combined exercise; screening failures: patients declining participation due to a lack of interest.
When not given, extracted and/or calculated from the data provided.
Table 4.
Participation and adherence to exercise training in recent studies in patients on dialysis
| Studies | Study design | Type of exercise | Study duration (months) | Exercise location | Session duration (min), number of weekly sessions | Number screened (source population), n | Screening failures, n | Number randomized (men/women), n | Number completed (men/women), n | Possible sessions completea (%) | Total dropouts (dropout due to death, TX, illness, moving)a, n | Overall adherence (adherence excluding death, TX, illness)a (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anding et al. [32, 57] | Clinical trial, no control group | CE |
12 (5 years) |
CBE-ID |
AE: 30 min RE: 30 min 2/week |
72 (72) |
26 |
Patients starting with exercise: 46 (24/22) |
1 year: 36 5 years : 20 (NA) |
87/71/39 (high/moderate /low adherence) |
1 year: 10 (8) 5 years: 26 (23) |
1 year: 78 (96) 5 years: 43 (93) |
| Jeong et al. [32, 58] |
RCT Two intervention arms, one control: (i) Exercise + Protein supplement (ii) Protein supplement (iii) Control |
AE | 12 | CBE-ID |
40 min 3/week |
337 | 109 |
138 (80/58) |
101 (NA) |
79 |
37 (28) Exercise + protein: 20 (13) Protein and control: 17 (15) |
73 (95) Exercise + protein: 59 (86) |
| Bennett et al. [32, 38] | RCT, stepped-wedge cluster | RE | 3, 6 and 9 | CBE-ID |
Duration: NA 3/week |
304 (NA) |
9 |
228 (NA) |
113 (NA) |
>33 | 115 (73) | 50 (82) |
| Manfredini et al. [32, 37] |
RCT HD and PD |
AE | 6 | HBE |
20 min (2 × 10 min) 3/week |
497 (714) |
196 |
296 (NA) |
227 (151/76) |
83 | 69 (32) | 77 (89) |
|
Bohm et al. |
RCT Two intervention arms: (i) AE, CBE-ID (ii) AE, HBE |
AE | 6 |
CBE-ID HBE |
CBE-ID: 52–130 min HBE: NA 3/week |
191 (NA) |
107 |
60 (40/20) |
43 (NA) |
CBE-ID: 53 HBE: 58 |
17 (10) | 72 (88) |
| Konstantinidou et al. [32, 44] |
RCT Three intervention arms, one control: (i) CE, HBE (ii) CE, CBE-ID (iii) AE, HBE (iv) Control group |
CE | 6 |
CBE-NDD CBE-ID HBE |
60 min 3/week |
120 (120) |
NA |
58 (NA) |
48 (NA) |
NA | 10 (2) | 83 (97) |
| Ortega-Perez de Villar et al. [32, 43] |
RCT Two intervention arms: (i) CE, CBE-ID (ii) CE, HBE |
CE | 4 |
CBE-ID HBE |
60 min 3/week |
63 | NA |
46 (29/17) |
23 (NA) |
CBE-ID: 81 HBE: 53 |
23 (18) | 50 (89) |
|
Tao et al. |
RCT Two intervention arms: (i) CE (ii) RE |
CE | 3 |
CBE-BD + HE |
CBE-BD: 20 min 2/week HE: 2–3/week |
466 (750) |
76 |
113 (59/54) |
107 (NA) |
NA | 6 (4) | 95 (98) |
| van Vilsteren et al. [32, 35] | RCT | CE | 3 |
CBE-ID + CBE-BD |
AE: 50 min RE: 20 min 2–3/week |
128 (128) |
7 |
103 (NA) |
96 (64/32) |
AE: 91 RE: 62 |
7 (2) | 93 (95) |
| Abdelaal et al. [46] |
RCT Two intervention arms one control group: (i) AE (ii)RE (iii)control |
AE, RE | 3 | CBE-NDD |
AE : 30–45 min RE : 30 min 3/week |
87 (NA) |
6 |
66 (36/30) |
66 (NA) |
NA | 0 (0) | 100 (100) |
|
Cheema et al. |
RCT | RE | 3 | CBE-ID | 3/week |
77 (142) |
28 |
49 (34/15) |
44 (NA) |
85 | 5 (4) | 90 (98) |
| Greenwood et al. [60] |
Uncontrolled cohort study HD, CKD 3–4, TX |
CE | 3 |
CBE-NDD + HBE |
60 min 3/week |
263 | 132 |
131 (NA) |
77 (NA) |
>50 | 54 (NA) | 59 (NA) |
| Uchiyama et al. [61] |
RCT PD |
CE | 3 | HBE |
AE: 30 min, 3/week RE: ≈30 min 2/week |
68 | 7 |
47 (35/12) |
44 (NA) |
AE: 52 RE: 76 |
3 (3) | 94 (94) |
|
Wu et al. [62] |
RCT Two intervention arms: (i) AE (ii) Stretching |
AE | 3 | CBE-ID |
15 min 3/week |
NA (NA) |
NA |
69 (NA) |
65 (55/10) |
NA | 4 (3) | 94 (99) |
The studies are ranked according to study duration. Studies selected were prospective interventional studies with a duration ≥3 months. Only studies with ≥45 randomized patients were included. Eleven of the studies were conducted between 2010 and 2020. Three older studies from 2002, 2005 and 2007 were included, as they fulfilled the selection criteria.
RCT= randomized controlled trial; HD= hemodialysis; PD= peritoneal dialyis; TX= transplantation, AE= aerobic exercise; RE= resistance exercise; CE= combined exercise; CBE-BD = centre-based exercise before dialysis; CBE-NDD: centre-based exercise on nondialysis days; CBE-ID= centre-based exercise intradialytic that is during hemodialysis; HBE= home-based exercise.Source population: number of all patients on dialysis in study dialysis units; screening failures: patients declining participation due to a lack of interest.
when not given extracted and/or calculated from the data provided.
Proportion of patients willing to participate
There is a considerable range in the literature between the percentage of randomized patients and the number of patients screened or the source population (i.e. all CKD patients treated in the study facility; see Tables 3 and 4). The source population and number of screened patients are either equal [35, 44, 57], differ substantially [36, 37, 53] or the numbers are not provided. Moreover, whether patients who did not meet the inclusion/exclusion criteria were excluded before or after the screening procedure differs from study to study. In studies in dialysis patients, the proportion of randomized patients in the source population ranged from 15% [36] to 40% [37] to 80% [35]; when the entire source population was invited to participate, 48–80% agreed [35, 44, 57]. In studies screening patients with NDD CKD, the differences regarding participation are even more pronounced, ranging from 5% [63] to 16% [56] to 70% [17] to ˃90% [53]. In the studies with low participation rates, there is either a pre-selection of patients due to strict inclusion/exclusion criteria or a high percentage of patients who were not interested. In some studies, the number of screening failures, defined as patients declining participation due to a lack of interest, equals or exceeds the number of randomized patients [54–56, 58, 60]. However, there are a considerable number of studies with patients on dialysis as well as with NDD CKD characterized by a relatively high percentage of randomized patients compared with screened patients/source population and a low number of screening failures [17, 34, 35, 37, 38, 43, 44, 52, 53, 57, 61, 64, 65].
Adherence to prescribed therapy
The attendance rate to the prescribed exercise sessions and the quality of exercise, defined as being performed according to the prescribed duration and intensity, are important for the sustainability of an exercise programme. The percentage of possible training sessions completed in the studies is shown in Tables 3 and 4. Most long-term studies (>6 months) showed attendance rates of >60% [17, 33, 37, 57, 58].
The quality of exercise was rarely reported. In some studies it was self-reported using patient questionnaires. In centre-based training, objective measurements such as weekly training volume for strength training [55] or mean power per session for aerobic training [57] were reported; in home-based training, metronomes and/or pedometers were used [37, 66].
Adherence to the programme
Interestingly, the dropout rates were relatively low, especially if non-motivational dropouts due to death, transplantation (TX) or illness were excluded. In the latter case, adherence was often >80% (>60% without any exclusions), even for studies running >6 months [17, 33, 45, 57, 58, 67]. The EXCITE trial, which had broad inclusion criteria for HBE in patients on dialysis, reported an adherence of 89% (77% without exclusions) [37]. In short-term studies in patients on dialysis there was a tendency towards higher dropout rates with exercise on non-dialysis days for home-based as well as for centre-based training [44, 60]. In NDD CKD patients, however, both home-based training and a mixture of home- and centre-based exercise [17, 33] showed high adherence rates.
The proportion of men in the studies was higher than women, ranging from 65% to 85%, as seen in Tables 3 and 4 [17, 60]. The studies screened in the present review did not present data enabling an analysis of whether the study participants’ sex influenced the impact of and adherence to exercise training.
BARRIERS AND FACILITATORS TO EXERCISE TRAINING
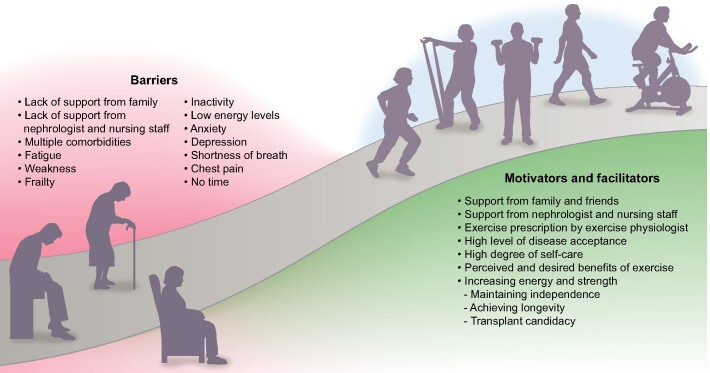
Already in 1991 investigators described a high adherence rate to self-care and medical management, as well as having a support group encouraging exercise, as important factors for high adherence to exercise training in patients with ESKD. Negative factors were long-term inactivity and weakness due to old age, coupled with a belief that exercise would not help [68]. Figure 1 depicts barriers and facilitators of exercise training.
FIGURE 1:
Barriers, facilitators and motivators affecting interest and willingness to engage in exercise training.
Disease acceptance and positive expectations
Recent studies confirm that patients with high levels of disease acceptance and higher expectations concerning the outcome of exercise were more prone to adhere to the prescription of physical activity [69, 70]. One qualitative study reported that a majority of patients with CKD felt that exercise would make them feel better and have a positive impact on their health [71]. These findings were corroborated in a large quantitative study comprising patients on HD and PD in which the top two desired benefits from exercise training were improved energy and strength [72]. Older patients’ top priority was maintaining independence, while longevity and transplant candidacy were the most important motivators for younger patients [72]. A recent study in patients with ESKD participating in a pedometer intervention showed improved physical function was their prime motivator [73].
Support
Support from family, friends, peers and from healthcare professionals has been identified as a main motivating factor [74–76]. In facilities providing an exercise physiologist, she/he is perceived as a primary source of support. Patients’ sense of physical ability, body confidence and self-esteem was enhanced by the technical competence, caring and esteem conveyed by the exercise physiologist [77]. Of great importance is that a lack of advice and support from the nephrologist, as well as from the dialysis nurses, is experienced as a major barrier [74, 77]. This experience is corroborated by attitudes expressed by nephrologists and dialysis nurses stating that they did not have time to discuss exercise with patients, did not believe that it was their role to provide advice on exercise training, did not have the knowledge to prescribe exercise or did not believe that exercise was important [77–80]. Some expressed a firm belief that patients lacked interest in exercise and that their compliance to exercise would be low [78, 80]. These attitudes resulted in the healthcare professionals not even asking patients about their exercise patterns [79].
Interestingly, the physicians’ and nursing staff’s own patterns of physical activity affect whether they recommend exercise to their patients. One study reported a highly significant relationship between CKD primary care physicians’ own level of physical activity and their readiness to recommend exercise training to their patients [81].
Medical, physical, psychological and temporal barriers
Many patients experience having too many medical problems as a barrier to physical activity [72, 74, 78, 79]. Predominant physical barriers comprise fatigue, tiredness, low energy levels, weakness, shortness of breath and chest pain [72, 74, 78, 79]. Frailty and a low level of physical function, often in combination with old age, are also perceived to be significant barriers. These patients risk being housebound and thus are physically inactive [69]. External conditions such as cold weather, hot weather and unsafe neighbourhoods are other barriers [74, 82]. Psychological and existential barriers are lack of motivation, sadness, depression, anxiety, feeling of helplessness and a fear of getting hurt [78–80, 83]. Another important barrier is the time consumed by maintenance dialysis [78].
PREFERRED TYPE OF EXERCISE AND LOCATION
One study reported that patients, irrespective of age or dialysis modality, preferred a combination of aerobic and strength exercises [72]. Despite the focus on ID exercise in most studies, a recent study found that 73% of the patients stated that their preferred exercise location was their home, followed by their neighbourhood or the gym. Less than one in four preferred to exercise at their HD unit [72]. Being able to exercise close to home was identified as a facilitator in another study [75].
CONCLUSION
There is growing and convincing evidence that physical activity affects mortality and that exercise training improves physical function in patients with CKD. Many studies show a high adherence rate to exercise training programmes. Patients are increasingly aware of the beneficial effects of exercise training but express the need for support. This safe and efficacious non-pharmacological treatment modality requires the involvement of nephrologists and nurses. The structural support of healthcare providers is necessary in order to provide on-site exercise physiologists to prescribe and monitor individualized exercise training programmes.
ACKNOWLEDGEMENTS
We would like to thank research librarian Matthias Bank at the Faculty of Medicine, Lund University for excellent help in searching for articles for this overview.
CONFLICT OF INTEREST STATEMENT
Neither author has any financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications or opinions stated including pertinent commercial or other sources of funding for the individual authors or for the associated departments or organizations, personal relationships, or direct academic competition.
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 2. Beddhu S, Baird BC, Zitterkoph J. et al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009; 4: 1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacKinnon HJ, Wilkinson TJ, Clarke AL. et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Ther Adv Chronic Dis 2018; 9: 209–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen IR, Wang SM, Liang CC. et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol 2014; 9: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke AL, Zaccardi F, Gould DW. et al. Association of self-reported physical function with survival in patients with chronic kidney disease. Clin Kidney J 2019; 12: 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tentori F, Elder SJ, Thumma J. et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 2010; 25: 3050–3062 [DOI] [PubMed] [Google Scholar]
- 7. Roshanravan B, Robinson-Cohen C, Patel KV. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellberg M, Wiberg EM, Simonsen O. et al. Small distal muscles and balance predict survival in end-stage renal disease. Nephron Clin Pract 2014; 126: 116–123 [DOI] [PubMed] [Google Scholar]
- 9. Sietsema KE, Amato A, Adler SG. et al. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 2004; 65: 719–724 [DOI] [PubMed] [Google Scholar]
- 10. Clyne N, Jogestrand T, Lins LE. et al. Factors limiting physical working capacity in predialytic uraemic patients. Acta Med Scand 2009; 222: 183–190 [DOI] [PubMed] [Google Scholar]
- 11. Roshanravan B, Patel KV, Robinson-Cohen C. et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis 2015; 65: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hellberg M, Höglund P, Svensson P. et al. Decline in measured glomerular filtration rate is associated with a decrease in endurance, strength, balance and fine motor skills. Nephrology (Carlton) 2017; 22: 513–519 [DOI] [PubMed] [Google Scholar]
- 13. Wilkinson TJ, Clarke AL, Nixon DGD. et al. Prevalence and correlates of physical activity across kidney disease stages: an observational multicentre study. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz235 [DOI] [PubMed] [Google Scholar]
- 14. Vanden Wyngaert K, Van Craenenbroeck AH, Van Biesen W. et al. The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3–4: a systematic review and meta-analysis. PLoS One 2018; 13: e0203662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Wang Y, Xiong L. et al. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: evidence from a meta-analysis. BMC Nephrol 2019; 20: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howden EJ, Leano R, Petchey W. et al. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 2013; 8: 1494–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hellberg M, Höglund P, Svensson P. et al. Randomized controlled trial of exercise in CKD—the RENEXC study. Kidney Int Rep 2019; 4: 963–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson-Cohen C, Littman AJ, Duncan GE. et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 2014; 25: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kokkinos P, Faselis C, Myers J. et al. Exercise capacity and risk of chronic kidney disease in US veterans: a cohort study. Mayo Clin Proc 2015; 90: 461–468 [DOI] [PubMed] [Google Scholar]
- 20. Tsai YC, Chen HM, Hsiao SM. et al. Association of physical activity with cardiovascular and renal outcomes and quality of life in chronic kidney disease. PLoS One 2017; 12: e0183642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Hellberg M, Hellmark T. et al. Sarcopenia, muscle mass and plasma myostatin after 12 months of exercise training in patients with CKD: a sub-study of RENEXC—a randomized controlled trial. Nephrol Dial Transplant 2019; 34: a183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heiwe S, Jacobson SH.. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 23. Barcellos FC, Santos IS, Umpierre D. et al. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 2015; 8: 753–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrade FP, Rezende PdS, Ferreira TdS . et al. Effects of intradialytic exercise on cardiopulmonary capacity in chronic kidney disease: systematic review and meta-analysis of randomized clinical trials. Sci Rep 2019; 9: 18470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bogataj Š, Pajek M, Pajek J. et al. Exercise-based interventions in hemodialysis patients: a systematic review with a meta-analysis of randomized controlled trials. J Clin Med 2019; 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clarkson MJ, Bennett PN, Fraser SF. et al. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol 2019; 316: F856– F8–72. [DOI] [PubMed] [Google Scholar]
- 27. Ferrari F, Helal L, Dipp T. et al. Intradialytic training in patients with end-stage renal disease: a systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J Nephrol 2020; 33: 251–266 [DOI] [PubMed] [Google Scholar]
- 28. Huang M, Lv A, Wang J. et al. Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am J Nephrol 2019; 50: 240–254 [DOI] [PubMed] [Google Scholar]
- 29. Lu Y, Wang Y, Lu Q.. Effects of exercise on muscle fitness in dialysis patients: a systematic review and meta-analysis. Am J Nephrol 2019; 50: 291–302 [DOI] [PubMed] [Google Scholar]
- 30. Pu J, Jiang Z, Wu W. et al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 2019; 9: e020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scapini KB, Bohlke M, Moraes OA. et al. Combined training is the most effective training modality to improve aerobic capacity and blood pressure control in people requiring haemodialysis for end-stage renal disease: systematic review and network meta-analysis. J Physiother 2019; 65: 4–15 [DOI] [PubMed] [Google Scholar]
- 32. Young HML, March DS, Graham-Brown MPMet al. . Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2018; 33: 1436–1445 [DOI] [PubMed] [Google Scholar]
- 33. Howden EJ, Coombes JS, Strand H. et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis 2015; 65: 583–591 [DOI] [PubMed] [Google Scholar]
- 34. Rossi AP, Burris DD, Lucas FL. et al. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol 2014; 9: 2052–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Vilsteren MCBA, de Greef MHG, Huisman RM.. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 36. Tao X, Chow SK, Wong FK.. A nurse-led case management program on home exercise training for hemodialysis patients: a randomized controlled trial. Int J Nurs Stud 2015; 52: 1029–1041 [DOI] [PubMed] [Google Scholar]
- 37. Manfredini F, Mallamaci F, D’Arrigo G. et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 2017; 28: 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bennett PN, Fraser S, Barnard R. et al. Effects of an intradialytic resistance training programme on physical function: a prospective stepped-wedge randomized controlled trial. Nephrol Dial Transplant 2016; 31: 1302–1309 [DOI] [PubMed] [Google Scholar]
- 39. Fitts SS, Guthrie MR, Blagg CR.. Exercise coaching and rehabilitation counseling improve quality of life for predialysis and dialysis patients. Nephron 1999; 82: 115–121 [DOI] [PubMed] [Google Scholar]
- 40. Matsuzawa R, Hoshi K, Yoneki K. et al. Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep 2017; 2: 1096–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heiwe S, Tollbäck A, Clyne N.. Twelve weeks of exercise training increases muscle function and walking capacity in elderly predialysis patients and healthy subjects. Nephron 2001; 88: 48–56 [DOI] [PubMed] [Google Scholar]
- 42. Baggetta R, D’Arrigo G, Torino C et al.. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr 2018; 18: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ortega-Pérez de Villar L, Martínez-Olmos FJ, Pérez-Domínguez FB. et al. Comparison of intradialytic versus home-based exercise programs on physical functioning, physical activity level, adherence, and health-related quality of life: pilot study. Sci Rep 2020; 10: 8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konstantinidou E, Koukouvou G, Kouidi E. et al. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 2002; 34: 40–45 [DOI] [PubMed] [Google Scholar]
- 45. Bohm C, Stewart K, Onyskie-Marcus J. et al. Effects of intradialytic cycling compared with pedometry on physical function in chronic outpatient hemodialysis: a prospective randomized trial. Nephrol Dial Transplant 2014; 29: 1947–1955 [DOI] [PubMed] [Google Scholar]
- 46. Abdelaal AAM, Abdulaziz EM.. Effect of exercise therapy on physical performance and functional balance in patients on maintenance renal hemodialysis: randomized controlled study. J Exerc Rehabil 2019; 15: 472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Birdee GS, Rothman RL, Sohl SJ. et al. Feasibility and safety of intradialysis yoga and education in maintenance hemodialysis patients. J Ren Nutr 2015; 25: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu CY, Han HM, Huang MC. et al. Effect of qigong training on fatigue in haemodialysis patients: a non-randomized controlled trial. Complement Ther Med 2014; 22: 244–250 [DOI] [PubMed] [Google Scholar]
- 49. de Medeiros AIC, Fuzari HKB, Rattesa C. et al. Inspiratory muscle training imprives respiratory muscle strength, functional capacity and quality of life in patients with chronic kidney disease: a systematic review. J Physiother 2017; 63: 76–83 [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Physical activity. https://www.who.int/news-room/fact-sheets/detail/physical-activity (23 February 2018, date last accessed)
- 51. Warburton DE, Bredin SS.. Reflections on physical activity and health: what should we recommend? Can J Cardiol 2016; 32: 495–504 [DOI] [PubMed] [Google Scholar]
- 52. Aoike DT, Baria F, Kamimura MA. et al. Home-based versus center-based aerobic exercise on cardiopulmonary performance, physical function, quality of life and quality of sleep of overweight patients with chronic kidney disease. Clin Exp Nephrol 2018; 22: 87–98 [DOI] [PubMed] [Google Scholar]
- 53. Alp Ikizler T, Robinson-Cohen C, Ellis C. et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol 2018; 29: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Headley S, Germain M, Wood R. et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 2014; 64: 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kirkman DL, Mullins P, Junglee NA. et al. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle 2014; 5: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watson EL, Greening NJ, Viana JL. et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis 2015; 66: 249–257 [DOI] [PubMed] [Google Scholar]
- 57. Anding K, Bär T, Trojniak-Hennig J. et al. A structured exercise programme during haemodialysis for patients with chronic kidney disease: clinical benefit and long-term adherence. BMJ Open 2015; 5: e008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeong JH, Biruete A, Tomayko EJ. et al. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int 2019; 96: 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheema B, Abas H, Smith B. et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis 2007; 50: 574–584 [DOI] [PubMed] [Google Scholar]
- 60. Greenwood SA, Lindup H, Taylor K. et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant 2012; 27: iii126– iii–34. [DOI] [PubMed] [Google Scholar]
- 61. Uchiyama K, Washida N, Morimoto K. et al. Home-based aerobic exercise and resistance training in peritoneal dialysis patients: a randomized controlled. Sci Rep 2019; 9: 2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu Y, He Q, Yin X. et al. Effect of individualized exercise during maintenance haemodialysis on exercise capacity and health-related quality of life in patients with uraemia. J Int Med Res 2014; 42: 718–727 [DOI] [PubMed] [Google Scholar]
- 63. Headley S, Germain M, Milch C. et al. Exercise training improves HR responses and V̇O2peak in predialysis kidney patients. Med Sci Sports Exerc 2012; 44: 2392–2399 [DOI] [PubMed] [Google Scholar]
- 64. Bogataj Š, Pajek J, Buturović Ponikvar J. et al. Kinesiologist-guided functional exercise in addition to intradialytic cycling program in end-stage kidney disease patients: a randomised controlled trial. Sci Rep 2020; 10: 5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Howden EJ, Coombes JS, Leano R. et al. Effect of exercise and lifestyle intervention on left ventricular and vascular function in patients with chronic kidney disease: a randomised controlled trial. Eur Heart J 2012; 33: 51522096090 [Google Scholar]
- 66. Sheshadri A, Kittiskulnam P, Lazar AA. et al. A walking intervention to increase weekly steps in dialysis patients: a pilot randomized controlled trial. Am J Kidney Dis 2020; 75: 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aoike DT, Baria F, Kamimura MA. et al. Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol 2015; 47: 359–367 [DOI] [PubMed] [Google Scholar]
- 68. Williams A, Stephens R, McKnight T. et al. Factors affecting adherence of end-stage renal disease patients to an exercise programme. Br J Sports Med 1991; 25: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hornik B, Duława J.. Frailty, quality of life, anxiety, and other factors affecting adherence to physical activity recommendations by hemodialysis patients. Int J Environ Res Public Health 2019; 16: 1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bogataj Š, Pajek M, Ponikvar JB. et al. Outcome expectations for exercise and decisional balance questionnaires predict adherence and efficacy of exercise programs in dialysis patients. Int J Environ Res Public Health 2020; 17: 3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clarke AL, Young HM, Hull KL. et al. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant 2015; 30: 1885–1892 [DOI] [PubMed] [Google Scholar]
- 72. Moorman D, Suri R, Hiremath S. et al. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol 2019; 14: 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sheshadri A, Kittiskulnam P, Delgado C. et al. Association of motivations and barriers with participation and performance in a pedometer-based intervention. Nephrol Dial Transplant 2020; 35: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kendrick J, Ritchie M, Andrews E.. Exercise in individuals with CKD: a focus group study exploring patient attitudes, motivations, and barriers to exercise. Kidney Med 2019; 1: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Parsons TL, Bohm C, Poser K.. “A learned soul to guide me”: the voices of those living with kidney disease inform physical activity programming. Physiother Can 2018; 70: 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang J, Bennett P.. The perception of people with chronic kidney disease towards exercise and physical activity: a literature review. Renal Soc Aust J 2019; 15: 97–104 [Google Scholar]
- 77. Thompson S, Tonelli M, Klarenbach S. et al. A qualitative study to explore patient and staff perceptions of intradialytic exercise. Clin J Am Soc Nephrol 2016; 11: 1024–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fiaccadori E, Sabatino A, Schito F. et al. Barriers to physical activity in chronic hemodialysis patients: a single-center pilot study in an Italian dialysis facility. Kidney Blood Press Res 2014; 39: 169–175 [DOI] [PubMed] [Google Scholar]
- 79. Regolisti G, Maggiore U, Sabatino A et al.. Interaction of healthcare staff's attitude with barriers to physical activity in hemodialysis patients: a quantitative assessment. PLoS One 2018; 13: e0196313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Michou V, Kouidi E, Liakopoulos V. et al. Attitudes of hemodialysis patients, medical and nursing staff towards patients’s physical activity. Int Urol Nephrol 2019; 51: 1249–1260 [DOI] [PubMed] [Google Scholar]
- 81. Morishita Y, Numata A, Miki A. et al. Primary care physicians' own exercise habits influence exercise counseling for patients with chronic kidney disease: a cross-sectional study. BMC Nephrol 2014; 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Han M, Ye X, Preciado P. et al. Relationships between neighborhood walkability and objectively measured physical activity levels in hemodialysis patients. Blood Purif 2018; 45: 236–244 [DOI] [PubMed] [Google Scholar]
- 83. Delgado C, Johansen KL.. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant 2012; 27: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]